Abstract
Background
Congenital adrenal hyperplasia (CAH) is an autosomal recessive condition which leads to glucocorticoid deficiency and is the most common cause of adrenal insufficiency in children. In over 90% of cases, 21‐hydroxylase enzyme deficiency is found which is caused by mutations in the 21‐hydroxylase gene. Managing individuals with CAH due to 21‐hydroxylase deficiency involves replacing glucocorticoids with oral glucocorticoids (including prednisolone and hydrocortisone), suppressing adrenocorticotrophic hormones and replacing mineralocorticoids to prevent salt wasting. During childhood, the main aims of treatment are to prevent adrenal crises and to achieve normal stature, optimal adult height and to undergo normal puberty. In adults, treatment aims to prevent adrenal crises, ensure normal fertility and to avoid the long‐term consequences of glucocorticoid use. Current glucocorticoid treatment regimens can not optimally replicate the normal physiological cortisol level and over‐treatment or under‐treatment is often reported.
Objectives
To compare and determine the efficacy and safety of different glucocorticoid replacement regimens in the treatment of CAH due to 21‐hydroxylase deficiency in children and adults.
Search methods
We searched the Cochrane Inborn Errors of Metabolism Trials Register, compiled from electronic database searches and handsearching of journals and conference abstract books. We also searched the reference lists of relevant articles and reviews, and trial registries (ClinicalTrials.gov and WHO ICTRP).
Date of last search of trials register: 24 June 2019.
Selection criteria
Randomised controlled trials (RCTs) or quasi‐RCTs comparing different glucocorticoid replacement regimens for treating CAH due to 21‐hydroxylase deficiency in children and adults.
Data collection and analysis
The authors independently extracted and analysed the data from different interventions. They undertook the comparisons separately and used GRADE to assess the quality of the evidence.
Main results
Searches identified 1729 records with 43 records subject to further examination. After screening, we included five RCTs (six references) with a total of 101 participants and identified a further six ongoing RCTs. The number of participants in each trial varied from six to 44, with participants' ages ranging from 3.6 months to 21 years. Four trials were of cross‐over design and one was of parallel design. Duration of treatment ranged from two weeks to six months per treatment arm with an overall follow‐up between six and 12 months for all trials. Overall, we judged the quality of the trials to be at moderate to high risk of bias; with lack of methodological detail leading to unclear or high risk of bias judgements across many of the domains.
All trials employed an oral glucocorticoid replacement therapy, but with different daily schedules and dose levels. Three trials compared different dose schedules of hydrocortisone (HC), one three‐arm trial compared HC to prednisolone (PD) and dexamethasone (DXA) and one trial compared HC with fludrocortisone to PD with fludrocortisone. Due to the heterogeneity of the trials and the limited amount of evidence, we were unable to perform any meta‐analyses.
No trials reported on quality of life, prevention of adrenal crisis, presence of osteopenia, presence of testicular or ovarian adrenal rest tumours, subfertility or final adult height.
Five trials (101 participants) reported androgen normalisation but using different measurements (very low‐quality evidence for all measurements). Five trials reported 17 hydroxyprogesterone (17 OHP) levels, four trials reported androstenedione, three trials reported testosterone and one trial reported dehydroepiandrosterone sulphate (DHEAS). After four weeks, results from one trial (15 participants) showed a high morning dose of HC or a high evening dose made little or no difference in 17 OHP, testosterone, androstenedione and DHEAS. One trial (27 participants) found that HC and DXA treatment suppressed 17 OHP and androstenedione more than PD treatment after six weeks and a further trial (eight participants) reported no difference in 17 OHP between the five different dosing schedules of HC at between four and six weeks. One trial (44 participants) comparing HC and PD found no differences in the values of 17 OHP, androstenedione and testosterone at one year. One trial (26 participants) of HC versus HC plus fludrocortisone found that at six months 17 OHP and androstenedione levels were more suppressed on HC alone, but there were no differences noted in testosterone levels.
While no trials reported on absolute final adult height, we reported some surrogate markers. Three trials reported on growth and bone maturation and two trials reported on height velocity. One trial found height velocity was reduced at six months in 26 participants given once daily HC 25 mg/m²/day compared to once daily HC 15 mg/m²/day (both groups also received fludrocortisone 0.1 mg/day), but as the quality of the evidence was very low we are unsure whether the variation in HC dose caused the difference. There were no differences noted in growth hormone or IGF1 levels. The results from another trial (44 participants) indicate no difference in growth velocity between HC and PD at one year (very low‐quality evidence), but this trial did report that once daily PD treatment may lead to better control of bone maturation compared to HC in prepubertal children and that the absolute change in bone age/chronological age ratio was higher in the HC group compared to the PD group.
Authors' conclusions
There are currently limited trials comparing the efficacy and safety of different glucocorticoid replacement regimens for treating 21‐hydroxylase deficiency CAH in children and adults and we were unable to draw any firm conclusions based on the evidence that was presented in the included trials.
No trials included long‐term outcomes such as quality of life, prevention of adrenal crisis, presence of osteopenia, presence of testicular or ovarian adrenal rest tumours, subfertility and final adult height. There were no trials examining a modified‐release formulation of HC or use of 24‐hour circadian continuous subcutaneous infusion of hydrocortisone. As a consequence, uncertainty remains about the most effective form of glucocorticoid replacement therapy in CAH for children and adults.
Future trials should include both children and adults with CAH. A longer duration of follow‐up is required to monitor biochemical and clinical outcomes.
Plain language summary
Glucocorticoid replacement regimens in the treatment of 21‐hydroxylase deficiency congenital adrenal hyperplasia
Review question
Steroid replacement therapy is used for treating congenital adrenal hyperplasia (CAH) due to 21‐hydroxylase deficiency in children and adults; we looked at the evidence for how well different regimens work and how safe they are.
Background
CAH is a genetic disorder of the adrenal glands that affects the body's general health, growth, and development. Adrenal glands sit above the kidneys and are responsible for making the hormones cortisol and aldosterone. Cortisol helps to regulate blood sugar and blood pressure; and aldosterone is needed to control the salt concentration in the blood. The most common form of CAH is 21‐hydroxylase deficiency (more than 90% of cases). In a child with this type of CAH, the adrenal glands can not make enough cortisol and aldosterone. The glands overwork trying to make these hormones and end up making too many androgens (steroid hormones which regulate the development and maintenance of male characteristics in a person). Steroid medicines similar to cortisol are used to replace cortisol, and fludrocortisone (hormones that are similar to aldosterone) are the usual treatment for CAH due to 21‐hydroxylase deficiency.
There are many different schedules and formulations of steroid replacement therapies, e.g. daily, twice‐daily, three‐times daily, more than three‐times daily medications, modified‐release formulation of hydrocortisone or using a 24‐hour circadian continuous infusion of hydrocortisone under the skin. We wanted to know which is more effective in treating 21‐hydroxylase deficiency CAH in children and adults.
Search date
The evidence is current to: 24 June 2019.
Trial characteristics
The review included five trials (six references) comparing different steroid replacement regimens in 101 people with 21‐hydroxylase deficiency CAH. The number of people in each trial varied from six to 44 and they ranged in age from 3.6 months to 21 years. We also found six studies that are still ongoing.
Key results
All trials used an oral therapy, but with different daily schedules and dose levels of steroids. Three trials compared different dose schedules of hydrocortisone, one trial compared hydrocortisone to prednisolone and dexamethasone and one trial compared hydrocortisone with fludrocortisone to prednisolone with fludrocortisone. We found no trials using a modified‐release formulation of hydrocortisone or a continuous 24‐hour delivery under the skin of hydrocortisone.
Five trials reported androgen normalisation but using different measurements; none of these results showed any consistent and real difference between therapies. In one trial (26 participants) participants taking a higher dose of hydrocortisone reported a higher growth rate, but we are not sure whether this was directly due to the treatment. In a second trial (44 participants) comparing hydrocortisone to prednisolone we are unsure whether the therapies affect growth rate (due to the very low quality of the evidence).
No trials included long‐term outcomes for quality of life, preventing an adrenal crisis, presence of bone fragility, presence of testicular or ovarian adrenal rest tumours, difficulty in conceiving and final adult height.
Quality of the evidence
Many trials had a high or unclear overall risk of bias. There were problems with the quality of the evidence, which was judged to be very low for all outcomes we considered across all the trials. This was because the trials were only small and if they compared a second treatment after the first, they did not leave enough time for the effects of the first treatment to clear. Also, people taking part had previously been treated with different glucocorticoids.
Conclusions
There is not enough evidence to show which steroid replacement treatment schedule results in better outcomes or which is the most effective form of steroid replacement therapy in CAH for adults and children. Large, well‐designed trials are needed to assess the effectiveness and safety of different steroid replacement therapies for treating 21‐hydroxylase deficiency CAH in children and adults. A longer duration of follow‐up is needed to monitor biochemical and clinical outcomes.
Summary of findings
Background
Description of the condition
Congenital adrenal hyperplasia (CAH) represents a group of autosomal recessive conditions which lead to glucocorticoid deficiency. It is the most common cause of adrenal insufficiency in children, affecting 1 in 18,000 births in the UK (Khalid 2012), while in other populations, the incidence of CAH ranges from 1 in 5000 to 1 in 20,000 (Marumudi 2013; Riepe 2007). In more than 90% of cases, 21‐hydroxylase enzyme deficiency is found; this is caused by mutations in the 21‐hydroxylase gene (CYP21) (Marumudi 2013). In most forms of CAH, an enzyme defect blocks cortisol synthesis, thus impairing cortisol‐mediated negative feedback control of adrenocorticotrophic hormone (ACTH) secretion. Oversecretion of ACTH ensues, which results in overstimulation of the adrenals and causes them to enlarge (hyperplasia). This oversecretion also stimulates excessive synthesis of the adrenal products of those pathways unimpaired by an enzyme deficiency. Control of androgens is often highly variable and metabolic abnormalities such as obesity, hypercholesterolaemia, hypertension, insulin resistance and osteopenia have been reported (Marumudi 2013). The clinical forms of 21‐hydroxylase enzyme deficiency are typically categorised as classical 21‐hydroxylase deficiency CAH, which is the severe form, or non‐classic (NCAH), which is the mild or late‐onset form. Classical 21‐hydroxylase deficiency also has further subcategories of salt‐wasting (SW) or simple‐virilizing (SV) (also known as non‐salt wasting) forms, depending on the presence of aldosterone deficiency (Khalid 2012). Clinical manifestations in classical 21‐hydroxylase deficiency CAH are due to glucocorticoid deficiency, mineralocorticoid deficiency and androgen excess. The NCAH form is often under‐diagnosed and may be associated with hyperandrogenic symptoms presenting either in childhood (precocious puberty) or later in adulthood (acne, infertility) (Marumudi 2013). Biochemical diagnosis of CAH relies on the determination of 17 hydroxyprogesterone (17 OHP); the ACTH stimulation test is the diagnostic test for evaluating adrenal gland function and is used for the biochemical diagnosis of NCAH due to other enzyme deficiencies. A diagnosis of CAH is often further confirmed on genetic testing and urine steroid profiling.
Description of the intervention
The management of individuals with classical 21‐hydroxylase deficiency CAH involves the replacement of glucocorticoids (with oral glucocorticoids, including prednisolone and hydrocortisone), the suppression of ACTH and the replacement of mineralocorticoids to prevent salt wasting. Hydrocortisone is the preferred choice of glucocorticoid replacement in children with CAH, as prednisolone and dexamethasone are associated with growth suppression (Bonfig 2007; LWPES/ESPE 2002). The typical dosing of hydrocortisone in children is 10 to 15 mg/m² per day given in three divided doses. Hydrocortisone is rapidly absorbed from the intestine after oral intake. The bioavailability of hydrocortisone is greater than 90%, but it has a short time to maximum concentration (Tmax) of one to two hours and a short half life of 1.8 to two hours (Charmandari 2001). Hydrocortisone is highly protein‐bound and there is a high clearance rate with increasing dosage (Fuqua 2010). However, conventional hydrocortisone treatment is associated with reduced quality of life (QoL) and increased side effects on bone metabolism and cardiovascular risks (Debono 2009).
Several other regimens have been proposed to mimic the normal physiological endogenous cortisol levels, such as variable intravenous infusions of hydrocortisone (Merza 2006), multiple dosing of immediate‐release formulation of hydrocortisone tablets or suspension given four‐ to five‐times daily (Hindmarsh 2009; Hindmarsh 2014), dual release (immediate‐release tablet with sustained‐release core) hydrocortisone tablets (Johannsson 2009), modified‐release formulation tablets or suspension of hydrocortisone taken once (Verma 2010) or twice daily (Mallappa 2015) and a combination of hydrocortisone and prednisolone regimen (Ajish 2014).
During childhood, the main aims of medical treatment of 21‐hydroxylase deficiency CAH are to prevent adrenal crisis and to achieve normal stature, optimal adult height and to undergo normal puberty. In adulthood, the aims of medical treatment are to prevent adrenal crisis, ensure normal fertility and to avoid the long‐term consequences of glucocorticoid use.
How the intervention might work
Current treatment regimens for CAH with glucocorticoids cannot optimally replicate the normal physiological cortisol level (Merza 2006). Over‐treatment or under‐treatment of CAH is often reported in individuals who may be treated with different steroid treatment regimens. Conventional twice‐ or three‐times‐daily hydrocortisone replacement therapy does not replicate the normal circadian rhythm, as cortisol levels are always low in the early hours of the morning when endogenous cortisol levels are normally rising. This then drives a nocturnal rise in ACTH which increases the production of androgens (Charmandari 2001). Under‐treatment and the resulting excess production of androgens causes virilization, accelerated growth, advanced skeletal maturation and early epiphyseal fusion (Riepe 2007). Conversely, a longer‐acting cortisol regimen bears the risk of over‐treatment and if taken at night will expose individuals to high levels of steroid at the time of the cortisol nadir. Over‐treatment often leads to side effects such as obesity, hypertension and osteoporosis (Subbarayan 2014). In some regimens, reverse circadian rhythm pattern of hydrocortisone replacement has been used, where a higher dose is given at night in order to suppress overnight increases in ACTH rather than giving the highest dose of hydrocortisone in the morning.
Why it is important to do this review
There is no current standard treatment for 21‐hydroxylase deficiency CAH and physicians often customise treatment for each individual using various regimens. It remains unclear which treatment regimen is most effective (Riepe 2002). The pharmacokinetics and pharmacodynamics of currently available glucocorticoid regimens do not allow the matching of the hormonal fluctuations and the physiological requirements in people with 21‐hydroxylase deficiency CAH and there is much debate as to which is the most efficacious regimen used for the treatment of CAH (Merza 2006). This review aims to establish evidence of efficacy for the different treatment regimens of cortisol replacement in people with 21‐hydroxylase deficiency CAH and will separately examine trials comparing the different glucocorticoid replacement regimens in the treatment of 21‐hydroxylase deficiency CAH.
Objectives
To compare and determine the efficacy and safety of different glucocorticoid replacement regimens in the treatment of 21‐hydroxylase deficiency CAH in children and adults.
Methods
Criteria for considering studies for this review
Types of studies
We included any randomised controlled trials (RCTs) or quasi‐RCTs (where method of allocation is not truly random), of both parallel and cross‐over design (but not cluster RCTs), comparing different glucocorticoid replacement regimens in the treatment of 21‐hydroxylase deficiency CAH in children and adults.
Types of participants
Children and adults diagnosed with 21‐hydroxylase deficiency CAH according to the appropriate diagnostic criteria of the time. A child is defined as under 18 years of age.
Types of interventions
Any circadian, extended release or conventional hydrocortisone replacement regimen (no dose restrictions) of any duration for treating congenital adrenal hyperplasia in children and adults. We compared the following active interventions to each other if they were reported:
daily Plenadren® (an immediate‐release tablet with sustained‐release hydrocortisone core formulation);
daily Chronocort® (a modified‐release formulation of hydrocortisone);
twice‐daily Chronocort® (a modified‐release formulation of hydrocortisone);
three‐times daily conventional immediate‐release formulation of hydrocortisone;
more than three‐times daily conventional immediate‐release formulation of hydrocortisone;
24‐hour circadian continuous subcutaneous infusion of hydrocortisone;
combination of oral hydrocortisone and prednisolone regimen.
The mineralocorticoid replacement dosing will not be compared.
Types of outcome measures
Primary outcomes
QoL score (as assessed by Short Form Health Survey (SF‐36))
Androgen normalisation (defined by 17 OHP monitoring)
Prevention of adrenal crisis
Secondary outcomes
Presence of osteopenia (bone mineral density (BMD) measured by dual X‐ray absorptiometry (DEXA))
Presence of testicular or ovarian adrenal rest tumours
Subfertility (defined by history, evidence of adrenal progesterone hypersecretion, consequences of genital reconstructive surgery, secondary polycystic ovaries syndrome)
Final adult height in standard deviation score (SDS)
Search methods for identification of studies
The authors searched for all relevant published and unpublished trials without restrictions on language, year or publication status.
Electronic searches
The Cochrane Cystic Fibrosis and Genetic Disorders Group's Information Specialist conducted a search of the Group's Inborn Errors of Metabolism Trials Register using the keyword: congenital adrenal hyperplasia.
The Inborn Errors of Metabolism Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated with each new issue of the Cochrane Library), weekly searches of MEDLINE and the prospective handsearching of one journal ‐ Journal of Inherited Metabolic Disease. Unpublished work is identified by searching through the abstract books of the Society for the Study of Inborn Errors of Metabolism conference and the SHS Inborn Error Review Series. For full details of all searching activities for the register, please see the Cochrane Cystic Fibrosis and Genetic Disorders Group's website.
Date of the most recent search of the Group's Inborn Errors of Metabolism Trials Register: 24 June 2019.
The authors searched the following databases, registers and trial registries:
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 12) in the Cochrane Library (searched 17 December 2019);
MEDLINE Ovid (1946 to 17 December 2019);
HDAS Embase (1974 to 17 December 2019);
ISRCTN registry (www.isrctn.com; searched 17 December 2019);
US National Institutes of Health Ongoing Trials Register Clinicaltrials.gov (www.clinicaltrials.gov; searched 17 December 2019);
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch; searched 17 December 2019);
Health Canada’s Clinical Trial Database (health‐products.canada.ca/ctdb‐bdec/index‐eng.jsp; searched 17 December 2019);
NICE Evidence (www.evidence.nhs.uk; searched 17 December 2019).
For details of the search strategies, please see Appendix 1.
Searching other resources
The authors checked the bibliographies of included studies and also any relevant systematic reviews and health technology assessment (HTA) reports identified for further references to relevant trials. They also hand searched www.endocrine‐abstracts.org from 2001 to 2019.
Data collection and analysis
One author (KS) conducted an initial search and undertook an initial sift of the search results to identify potentially relevant articles. Both authors (KS and SN) then independently assessed the articles for eligibility.
Selection of studies
To determine the trials for further assessment, two review authors (SN, KS) independently scanned the abstract and title of every record retrieved. They reviewed the full texts of all potentially relevant articles.If there were any differences in opinion, the review authors planned to resolve these by consensus and with an independent advisor. If it was not possible to resolve a disagreement regarding trial selection, the review authors planned to add the article to those 'Awaiting assessment' and planned to contact the trial investigators for clarification. There was no disagreement on trial selection, therefore an independent advisor was not required.
Dealing with duplicate publications
In the case of duplicate publications and companion papers of a primary paper, the review authors extracted all available information from all publications. In cases of doubt, the review authors obtained the original publication (usually the oldest version) as a priority. We have listed all publications relating to each unique trial under a single trial ID.
Data extraction and management
The authors extracted data using the Cochrane Cystic Fibrosis and Genetic Disorders Review Group's trial selection, quality assessment and data extraction form, adapted to suit the outcomes of this review. They recorded trial design, trial participant characteristics, and outcome data and would have resolved any disagreements by discussion, or if required by a third party, if they had occurred. They also measured the inter‐rater agreement for trial selection using the kappa statistic (Cohen 1960). The review authors would have requested any relevant missing information from the trial investigators, if this had been required. The reviewers had planned to analyse the data from different interventions separately, but due to the data limitations were only able to report results narratively.
The review authors reported outcome data at the following intervals:
short term: less than 12 months;
medium term: one to five years;
long term: more than five years.
If trials reported multiple time points within the time frames described above (e.g. three months and six months) then the review authors presented any additional individual time points, but still described the results as 'short term', 'medium term' or 'long term' as appropriate.
Assessment of risk of bias in included studies
The authors used the Cochrane risk of bias tool to assess the following criteria: randomisation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; and selective reporting (Higgins 2011). They classified the risks as low, high or unclear and recorded each decision and their reasons for the judgement in a table; they also displayed the assessments in the overall risk of bias summary.
Measures of treatment effect
The authors planned to follow recommendations for data analysis set out in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). They have presented data in the graphs; however, they were not able to combine data to perform any meta‐analysis due to considerable heterogeneity in the trials.
For dichotomous outcomes, (such as presence of osteoporosis, testicular or ovarian tumours, hypertension, hypercholesterolaemia or obesity) the authors planned to analyse the treatment effect using risk ratios (RR), however, they were not able to undertake this due to the limited data. Considering the outcome measures listed, as the authors expected, most of the data collected for this review was continuous. They extracted means and standard deviations (SDs) from the trials where possible.
Unit of analysis issues
The authors implemented the following strategies to minimize the impact of non‐standard designs upon the conclusions of the review. Despite initially planning on considering cluster RCTs for this review and discussing this in the protocol, on reflection they decided that the trial design was not appropriate and amended the review's inclusion criteria.
The authors included cross‐over trials and analysed data from one of these in the review (Silva 1997). Although they planned to analyse only first‐arm data for cross‐over trials, the data available only allowed them to analyse the height data from the Silva trial using GIV analysis (Silva 1997). They entered the MD between treatment arms at six months into the GIV calculations and then derived the standard error (SE) from the published P value. The authors have reported results from the remaining three cross‐over trials narratively (German 2008; Nebesio 2016; Winterer 1985).
Dealing with missing data
If data were missing from the included trials, the review authors made every effort to contact the original trial authors to obtain these data. If data such as standard deviations (SDs) were missing, where appropriate, the authors planned to calculate these from SEs, CIs, T‐values or P values (if reported). In the event of large scale missing data or participant attrition, authors planned to carry out an intention‐to‐treat analysis but no trials reported participant attrition rates. Bias can result from the imperfect nature of estimates by imputation; to protect against this the authors carried out an available‐case analysis dealing with participants who completed the trial. If this occurred, the authors had planned to conduct sensitivity analysis but due to limited data this was not possible.
Assessment of heterogeneity
Authors planned to assess heterogeneity using the I² statistic, which gives insight into the level of variability within results that is due to heterogeneity as opposed to chance alone (Higgins 2003). The authors planned to assess heterogeneity in terms of overlapping percentage intervals: 0% to 40% (might not be important); 30% to 60% (may represent moderate heterogeneity); 50% to 90% (may represent substantial heterogeneity); and 75% to 100% (may represent considerable heterogeneity) (Deeks 2011). However, due to limited data, this was not possible.
Assessment of reporting biases
A comprehensive search strategy, including grey literature, protected against many forms of publication bias. The authors note, however, that this method alone would not prevent all possibility of publication bias infiltrating the review. Therefore, if the authors had identified and incorporated more than 10 trials, they would have further assessed publication bias using funnel plots. The authors planned to visually assess the funnel plots they generated to assess asymmetry and consider the descriptive characteristics of the plot. Further to visual inspection, authors also planned to assess funnel plot asymmetry using an adaptation of the linear regression model (Eggar 1997).
In order to examine possible selective reporting bias, the review authors requested trial protocols from two original trial authors but have received no response to date (Silva 1997; Winterer 1985).
Data synthesis
The authors have used fixed‐effect analyses in this review as a pooled effect estimate from a fixed‐effect meta‐analysis is normally interpreted as being the best estimate of the intervention effect. For future updates, where heterogeneity cannot be explained by the pre‐specified subgroup analyses, the authors plan to perform a sensitivity analysis using a random‐effects model. If a future meta‐analysis is not appropriate (e.g. a substantial level of heterogeneity), the review authors will present a narrative within the results section.
Subgroup analysis and investigation of heterogeneity
If sufficient data had been available and at least moderate heterogeneity had been present (as defined above), the authors planned to conduct subgroup analyses based on children (younger than 18 years of age) and adults (18 years and older) for the outcome final adult height within the different intervention categories. The achievement of final adult height for children with this condition is an important aspect of optimal management and the rationale for this subgroup analysis was a possible impact of steroid use in children on their delayed growth, puberty and their final height.
Lai previously showed that mean height after age of 18 years is significantly lower in boys previously treated with prednisolone (PD) versus placebo (Lai 2000). In adults, there are significant reductions in bone mineral density (BMD) associated with the use of PD (or equivalent) and fracture risk increased within three to six months of treatment initiation; this increased fracture risk was independent of the individual's age, gender and the underlying disease (van Staa 2002). However, due to limited data, the proposed subgroup analysis was not possible.
The authors do not propose any subgroup analyses by age for any other outcomes.
Sensitivity analysis
If authors had found data were incomplete, if they had needed to impute data, or if criteria limits were poorly defined (such as age ranges, or what constitutes 'standard care') they planned to undertake sensitivity analyses. If appropriate, they would have completed the meta‐analyses with and without the contentious data to assess its impact upon the overall findings. If the results of the meta‐analyses were not greatly altered, the robustness of the review would be increased. If results of the two analyses had differed greatly, then they would have interpreted the results of the review with caution.The authors planned to avoid this by conducting a sensitivity analysis at the participant level and incorporating adjustment using the intra‐class correlation coefficient (ICC).
If the authors had identified different levels of potential bias in trials, they would have conducted sensitivity analyses. If they judged some trials to contain potentially high or uncertain levels of bias, they would have omitted these from the analyses. This again would allow the reviewers to identify the impact of these trials upon the results of the analyses. If there had been no marked difference in results due to this omission, it would have strengthened the conclusions of the review by indicating that they were not in fact impacted by the potential bias of the trials.
If any heterogeneity could not be explained by the pre‐specified subgroup analyses, the authors would have performed a sensitivity analysis using a random‐effects model.
Summary of findings table
The authors presented a 'Summary of findings' table for each comparison to present the main findings of a review in a transparent and simple tabular format (Table 1; Table 2; Table 3; Table 4). In particular, to provide key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the primary and secondary outcomes listed below.
Summary of findings for the main comparison. HC (13.5 mg/m²/day to 15.5 mg/m²/day): high morning dose vs high evening dose for treating CAH.
| HC (13.5 mg/m²/day to 15.5 mg/m²/day) high morning dose compared with high evening dose for treating CAH | ||||||
|
Patient or population: people with CAH Settings: outpatients, tertiary centre Intervention: HC (13.5 mg/m²/day to 15.5 mg/m²/day): 50% daily dose in the morning followed by 25% at noon and 25% in the evening Comparison: HC (13.5 mg/m²/day to 15.5 mg/m²/day): 25% daily dose in the morning and 25% at noon followed by 50% in the evening | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| High evening dose | High morning dose | |||||
|
QoL Follow‐up: 4 weeks |
Outcome not reported. | NA | NA | NA | ||
|
Androgen normalisation Follow‐up: 4 weeks |
See comments | NA | 15 (1 cross‐over trial) |
⊕⊝⊝⊝a,b,c very low |
17 OHP (nmol/L) Results for 17 OHP are presented as medians (IQR); 44 (16 to 116) for the high morning dose compared to 33 (15 to 76) for the high evening dose. Androstenedione (nmol/L) Results for androstenedione are presented as medians (IQR); 1.80 (1.0 to 3.0) for the high morning dose compared to 1.90 (1.20 to 6.50) for the high evening dose. Testosterone (nmol/L) Results for testosterone are presented as medians (IQR); 0.70 (0.30 to 2.30) for the high morning dose compared to 1.1 (0.60 to 2.70) for the high evening dose. DHEAS (nmol/L) Results for DHEAS are presented as medians (IQR); 0.20 (0.20 to 0.60) for the high morning dose compared to 0.40 (0.20 to 0.70) for the high evening dose. |
|
|
Prevention of adrenal crisis Follow‐up: 4 weeks |
Outcome not reported. | NA | NA | NA | ||
|
Presence of osteopenia Follow‐up: 4 weeks |
Outcome not reported. | NA | NA | NA | ||
|
Presence of testicular or ovarian adrenal rest tumours Follow‐up: 4 weeks |
Outcome not reported. | NA | NA | NA | ||
|
Subfertility Follow‐up: 4 weeks |
Outcome not reported. | NA | NA | NA | ||
|
Final adult height Follow‐up: 4 weeks |
Outcome not reported | NA | NA | NA | ||
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 17 OHP: 17‐hydroxyprogesterone; CAH: congenital adrenal hyperplasia; CI: confidence interval; DHEAS: dehydroepiandrosterone sulphate; HC: hydrocortisone; IQR: interquartile range; NA: not applicable; QoL: quality of life. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a Downgraded once for high risk of bias due to incomplete outcome data and selective reporting. b Downgraded once due to potential risk of bias: unclear details related to methodological design. c Downgraded due to uncertainty: small sample size and wide IQR.
Summary of findings 2. HC (15 mg/m²/day) versus PD (3 mg/m²/day) versus DXA (0.3 mg/m²/day) for treating CAH.
| HC (15 mg/m²/day) versus PD (3.0 mg/m²/day) versus DXA (0.3 mg/m²/day) for treating CAH | ||||||
|
Patient or population: people with CAH Settings: outpatients, tertiary centre Intervention: HC (15 mg/m²/day) or PD (3.0 mg/m²/day) or DXA (0.3 mg/m²/day) Comparison: DXA (0.3 mg/m²/day) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| DXA | HC or PD | |||||
|
QoL Follow‐up: 6 weeks |
Outcome not reported. | NA | NA | NA | ||
|
Androgen normalisation Follow‐up: 6 weeks |
See comments. | NA | 9 (1 cross‐over trial) |
⊕⊝⊝⊝a,b,c,d very low |
17 OHP (nmol/L) There were lower levels of 17 OHP reported in the DXA group compared to HC (P < 0.001) and compared to PD (P < 0.001). Androstenedione (nmol/L) Androstenedione levels were significantly lower with DXA when compared to HC (P = 0.016) and PD (P = 0.002). |
|
|
Prevention of adrenal crisis Follow‐up: 6 weeks |
Outcome not reported. | NA | NA | NA | ||
|
Presence of osteopenia Follow‐up: 6 weeks |
Outcome not reported. | NA | NA | NA | ||
|
Presence of testicular or ovarian adrenal rest tumours Follow‐up: 6 weeks |
Outcome not reported. | NA | NA | NA | ||
|
Subfertility Follow‐up: 6 weeks |
Outcome not reported. | NA | NA | NA | ||
|
Final adult height Follow‐up: 6 weeks |
Outcome not reported. | NA | NA | NA | ||
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 17 OHP: 17‐hydroxyprogesterone; CAH: congenital adrenal hyperplasia; CI: confidence interval; DXA: dexamethasone; HC: hydrocortisone; NA: not applicable; PD: prednisolone; QoL: quality of life. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a Downgraded once for high risk of bias due to incomplete outcome data and selective reporting. b Downgraded once due to risk of bias: unclear details related to methodological design. c Downgraded due to uncertainty: small sample size so P values should be interpreted with caution. d Downgraded once for lack of applicability as included study only includes children so results are not applicable to adults.
Summary of findings 3. HC (10 mg/m²/day to 15 mg/m²/day) versus PD (2.4 mg/m²/day to 3.5 mg/m²/day) for treating CAH.
| HC (10 mg/m²/day to 15 mg/m²/day) versus PD (2.4 mg/m²/day to 3.5 mg/m²/day) for treating CAH | ||||||
|
Patient or population: people with CAH Settings: outpatients, tertiary centre Intervention: HC (10 mg/m²/day to 15 mg/m²/day) Comparison: PD (2.4 mg/m²/day to 3.5 mg/m²/day) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| PD | HC | |||||
|
QoL Follow‐up: 1 year |
Outcome not reported. | NA | NA | NA | ||
|
Androgen normalisation Follow‐up: 1 year |
17 OHP (nmol/L) The mean level of 17 OHP across the prepubertals and pubertals group in the control group was 1737.00 nmol/L. |
17 OHP (nmol/L) The mean level of 17 OHP was 1189.10 nmol/L higher (51.08 lower to 2429.28 higher) with HC. |
44 (1 trial) |
⊕⊝⊝⊝a,b,c very low |
17 OHP (nmol/L) Data reported separately for pubertal and pre‐pubertal participants. Neither individual result was significant; for the pre‐pubertal participants, MD 1436.00 nmol/L (95% CI ‐127.38 to 2999.38) and for pubertal participants, MD 770.00 nmol/L (95% CI ‐1266.86 to 2806.86). |
|
|
Androstenedione (nmol/L) The mean level of androstenedione across the prepubertals and pubertals group in the control group was 126.00 nmol/L |
Androstenedione (nmol/L) The mean level of androstenedione in the HC group was 57.75 nmol/L higher (11.19 higher to 104.31 higher). |
44 (1 trial) |
⊕⊝⊝⊝a,b,c very low |
Androstenedione (nmol/L) Data reported separately for pubertal and pre‐pubertal participants. Neither individual result was significant; for the pre‐pubertal participants, MD 63.00 nmol/L (95% CI ‐4.57 to 130.57) and for pubertal participants, MD 53.00 nmol/L (95% CI ‐11.25 to 117.25). |
||
|
Testosterone (nmol/L) The mean testosterone level across the prepubertals and pubertals group in the control group was 82.00 nmol/L. |
Testosterone (nmol/L) The mean testosterone level in the hydrocortisone group was 38.55 nmol/L higher (6.48 lower to 83.58 higher). |
44 (1 trial) | ⊕⊝⊝⊝a,b,c very low |
Testosterone (nmol/L) Data reported separately for pubertal and pre‐pubertal participants. Neither individual result was significant; for the pre‐pubertal participants, MD 35.00 nmol/L (95% CI ‐29.13 to 99.13) and for pubertal participants, MD 42.00 nmol/L (95% CI ‐21.24 to 105.24). |
||
|
Prevention of adrenal crisis Follow‐up: 1 year |
Outcome not reported. | NA | NA | NA | ||
|
Presence of osteopenia Follow‐up: 1 year |
Outcome not reported. | NA | NA | NA | ||
|
Presence of testicular or ovarian adrenal rest tumours Follow‐up: 1 year |
Outcome not reported. | NA | NA | NA | ||
|
Subfertility Follow‐up: 1 year |
Outcome not reported. | NA | NA | NA | ||
|
Final adult height Follow‐up: 1 year |
The mean height velocity in the PD group was 1.12. | The mean height velocity in the HC group was 0.26 higher (0.82 lower to 1.34 higher). | 44 (1 trial) |
⊕⊝⊝⊝a,b,c very low | The results are for height velocity which is a surrogate measure for final adult height. The ratio of bone age to chronological age (a further surrogate marker for final adult height) was also measured and results suggest no difference between the groups, MD 0.15 (95% CI ‐0.03 to 0.33). |
|
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 17 OHP: 17‐hydroxyprogesterone; CAH: congenital adrenal hyperplasia; CI: confidence interval; DXA: dexamethasone; HC: hydrocortisone; MD: mean difference; NA: not applicable; PD: prednisolone; QoL: quality of life. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a Downgraded once for high risk of bias due to incomplete outcome data and selective reporting. b Downgraded once due to risk of bias: unclear details related to methodological design. c Downgraded once due to imprecision: outcome was downgraded due to imprecision and uncertainty, very large CIs around the MD.
Summary of findings 4. HC (15 mg/m²/day) vs HC (25 mg/m²/day) with fludrocortisone (0.1 mg/day) for treating CAH.
| HC (15 mg/m²/day) vsus HC (25 mg/m²/day) with fludrocortisone (0.1 mg/day) for treating CAH | ||||||
|
Patient or population: people with CAH Settings: outpatients, tertiary centre Intervention: HC (15 mg/m²/day) Comparison: HC (25 mg/m²/day) with fludrocortisone (0.1 mg/day) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| HC with fludrocortisone | HC | |||||
|
QoL Follow‐up: 6 months |
Outcome not reported. | NA | NA | NA | ||
|
Androgen normalisation Follow‐up: 6 months |
See comments. | NA | 26 (1 cross‐over trial) |
⊕⊝⊝⊝a,b,c very low |
17 OHP (nmol/L) Results presented as medians (IQR) and split for prepubertal and pubertal participants. For prepubertals, the levels of 17 OHP were higher in the HC 15 mg/m²/day group, 113.7 (0.5 to 1207) compared to 11.5 (0.6 to 819.9) in the HC 25 mg/m²/day group. For the pubertal group, the levels of 17 OHP were lower in the 15 mg/m²/day group, 91.7 (6.8 to 453.0) compared to 314.2 (66.5 to 568.7) in the HC 25 mg/m²/day group. Androstenedione (nmol/L) Results show that for prepubertals androstenedione levels were higher in the HC 15 mg/m²/day group, 3.4 (0.5 to 40.2) compared to the HC 25 mg/m²/day group, 1.6 (0.1 to 31.8). For the pubertal group, androstenedione levels were lower in the HC 15 mg/m²/day group, 11 (6.1 to 41.9) compared to the HC 25 mg/m²/day group, 22.3 (10.5 to 47.5). Testosterone (nmol/L) Results show that testosterone levels for prepubertals were higher in the HC 15 mg/m²/day group, 2.5 (0.8 to 9.1) compared to the HC 25 mg/m²/day group, 2.3 (1.2 to 11.3). For the pubertal group, the levels of testosterone were lower in the HC 15 mg/m²/day group, 4.7 (3.9 to 6.9) compared to the HC 25 mg/m²/day group, 6.2 (3.5 to 9.2). |
|
|
Prevention of adrenal crisis Follow‐up: 6 months |
Outcome not reported. | NA | NA | NA | ||
|
Presence of osteopenia Follow‐up: 6 months |
Outcome not reported. | NA | NA | NA | ||
|
Presence of testicular or ovarian adrenal rest tumours Follow‐up: 6 months |
Outcome not reported. | NA | NA | NA | ||
|
Subfertility Follow‐up: 6 months |
Outcome not reported. | NA | NA | NA | ||
|
Final adult height Follow‐up: 6 months |
See comments. | The mean difference in height velocity between the 2 groups was 0.34 higher (0.27 higher to 0.41 higher). | NA | 26 (1 cross‐over trial) |
⊕⊝⊝⊝a,b,c very low | The results are for height velocity which is a surrogate measure for final adult height. |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 17 OHP: 17‐hydroxyprogesterone; CAH: congenital adrenal hyperplasia; CI: confidence interval; HC: hydrocortisone; IQR: interquartile range; NA: not applicable; QoL: quality of life. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a Downgraded once for high risk of bias due to incomplete outcome data and selective reporting. b Downgraded once due to potential risk of bias: unclear details related to methodological design. c Downgraded due to uncertainty: small sample size and wide IQR.
QoL score
Androgen normalisation
Prevention of adrenal crisis
Presence of osteopenia
Presence of testicular adrenal rest tumours (in boys)
Subfertility
Final adult height in SDS
The authors used GRADE to evaluate the quality of the evidence (Schünemann 2011a; Schünemann 2011b).The GRADE approach uses five considerations (trial limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for specific outcomes. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, inconsistency, imprecision of effect estimates or publication bias.
Results
Description of studies
Results of the search
The searches identified 1729 records; after initial screening 31 publications and 12 trial registry entries were selected for further examination. After screening the full texts of the selected papers and the registry entries, only five trials (six references), all of which were published as full papers, met the inclusion criteria; 23 studies (26 references) were excluded, two studies (one reference each) listed as 'Awaiting classification' and six trials (nine registry entries) were listed as ongoing (Figure 1).
1.
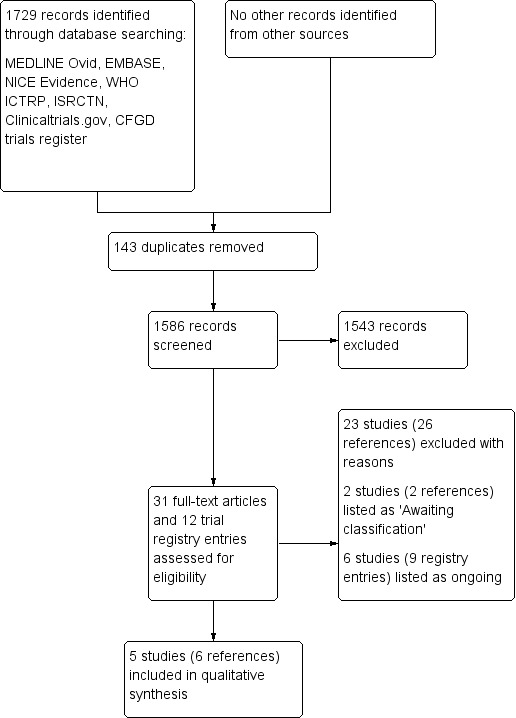
Study flow diagram.
Included studies
Five trials (n = 101) were included in the review (Caldato 2004; German 2008; Nebesio 2016; Silva 1997; Winterer 1985). There was a large variation between the trials identified in terms of design, intervention, duration of treatment and outcome measures. See Characteristics of included studies table for full details.
Trial design
Four trials were of cross‐over design (German 2008; Nebesio 2016; Silva 1997; Winterer 1985) and one was of parallel design (Caldato 2004). Duration of treatment ranged from two weeks (German 2008) to six months per treatment arm (Caldato 2004; Silva 1997) with an overall follow‐up between six and 12 months for all trials. Four trials were single centre (German 2008; Nebesio 2016; Silva 1997; Winterer 1985) and one was multicentre (two centres) (Caldato 2004). All trials were undertaken in tertiary endocrine centres.
Participants
All trials included both males and females with a confirmed diagnosis of CAH (n = 101). Sample size varied from six participants (Winterer 1985) to 44 participants (Caldato 2004). The age of participants ranged from 1.2 months (Silva 1997) to 21 years (Caldato 2004). Three trials were limited to children of both genders with confirmed diagnosis of CAH (German 2008; Nebesio 2016; Silva 1997).
Interventions
All trials employed an oral glucocorticoid replacement therapy, but with different daily schedules and dose levels (calculated by body surface area (BSA) mg/m²) as described below. Three trials compared different dose schedules of hydrocortisone (HC) (German 2008; Silva 1997; Winterer 1985), one three‐arm trial compared HC to PD and dexamethasone (DXA) (Nebesio 2016) and one trial compared HC with fludrocortisone to PD with fludrocortisone (Caldato 2004).
Of the three trials using HC, one trial compared two different regimens of three times daily HC of 13.5 mg/m²/day to 15.5 mg/m²/day where one group was given a morning high dose (50% of the daily HC in the morning followed by 25% at midday and 25% at night) and the second group was given an evening high dose (25% of daily HC in the morning and at midday followed by 50% at bedtime) (German 2008). A second trial compared HC 15 mg/m²/day to HC 25 mg/m²/day given as once‐daily doses (Silva 1997). The third trial compared five different regimens of three times daily HC with a total daily dose of 12.5 mg/m² as shown in the table (Winterer 1985).
| Group | Morning dose | Midday dose | Evening dose |
| 1 | full daily HC dose | placebo | placebo |
| 2 | 2/3 full daily HC dose | placebo | 1/3 full daily HC dose |
| 3 | 1/3 full daily HC dose | 1/3 full daily HC dose | 1/3 full daily HC dose |
| 4 | 1/3 full daily HC dose | placebo | 2/3 full daily HC dose |
| 5 | placebo | placebo | full daily HC dose |
A three‐arm trial compared three times daily HC with a total daily dose of 15 mg/m²/day to twice‐daily PD with a total daily dose of 3 mg/m²/day and to once‐daily DXA at a dose of 0.3 mg/m²/day (Nebesio 2016).
The remaining trial compared three times daily HC 10 mg/m² and fludrocortisone 0.1 mg/day versus 15 mg/m² to once‐daily PD 2.4 mg/m² to 3.5 mg/m² with fludrocortisone 0.1 mg/day (Caldato 2004).
Outcome measures
Five trials reported 17 OHP levels (Caldato 2004; German 2008; Nebesio 2016; Silva 1997; Winterer 1985). Four trials reported on adrenal androgens, such as testosterone (Caldato 2004; German 2008; Silva 1997), four trials reported on androstenedione (Caldato 2004; German 2008; Nebesio 2016; Silva 1997) and one trial reported on dehydroepiandrosterone sulphate (DHEAS) (German 2008). Three trials reported on growth and bone maturation (Caldato 2004; German 2008; Silva 1997). Two trials also reported on height velocity (Caldato 2004; Silva 1997).
No trial reported on our outcomes of QoL, prevention of adrenal crisis, presence of osteopenia or testicular or ovarian adrenal rest tumours or subfertility. However three trials reported outcomes not included in our review. One trial reported on sleep patterns and daytime activities (German 2008); a further trial reported on plasma ACTH, cortisol,11‐deoxycortisol,17‐hydroxysteroids and pregnanetriol (Winterer 1985); and a third trial reported on ACTH (Nebesio 2016).
Excluded studies
A total of 23 trials were excluded for a variety of reasons as stated in the tables (Characteristics of excluded studies). Nine trials were not RCTs or quasi‐RCTs (Ajish 2014; Bonfig 2007; Charmandari 2001; Mallappa 2015; Merza 2006; Neumann 2018; Panamonta 2003; PRENATAL DEX; Verma 2010) and the remaining trials did not have HC regimens as the intervention.
Studies awaiting classification
The search also identified two trials which are listed as 'Awaiting classification' pending full publication or further information from the investigators (Salud 2013; Silva 1994). Both seem to be RCTs and have relevant interventions and outcomes. However, one trial has only been published as a conference abstract; we have attempted to contact the authors for more information, but to date we have not received a reply (Salud 2013). We have only been able to access the abstract of the second RCT and will further evaluate when we are able to obtain the full article (Silva 1994).
Ongoing studies
Six ongoing trials were identified from trials registries (CareOnTIME; COCA; NCT01771328; NCT02716818; PULSES; RESTORE).
Trial design
Two trials are of cross‐over design (COCA; NCT01771328) and four are of parallel design (CareOnTIME; NCT02716818; PULSES; RESTORE). Duration of treatment ranged from six weeks (PULSES) to 52 weeks per treatment arm (RESTORE). All six trials were single centre and undertaken in tertiary endocrine centres.
Participants
Five trials included both males and females with a confirmed diagnosis of CAH (n = 546); one trial included only female participants (n = 40) (COCA). Sample size varied from 20 participants (NCT01771328) to 150 participants (CareOnTIME). The age of participants was 18 years and above in five trials and 16 years and above in one trial (RESTORE).
Interventions
All trials employed an oral glucocorticoid replacement therapy, but with different daily schedules and dose levels or mode of administration. One trial compared standard glucocorticoids (HC, cortisone, PD, prednisone, DXA) with dual release HC tablet administered in one dose per day (CareOnTIME). One three‐arm trial assessed the impact of physiological doses of HC, DXA or PD on biochemical parameters (COCA). A further trial examined a subcutaneous infusion of HC 10 mg/m²/24 hours as compared to cortisone acetate tablets (NCT01771328). A fourth trial compared a HC regimen with PD alone or PD plus HC and with DXA (NCT02716818). The PULSES trial compared HC with a placebo infusion to oral placebo and a HC infusion (PULSES). The final trial compared HC modified release doses of 5 mg, 10 mg and 20 mg with HC, DXA, PD or prednisone treatment (RESTORE).
Outcome measures
Five trials plan to report 17 OHP (COCA; NCT01771328; NCT02716818; PULSES; RESTORE). One trial plans to report androgen results such as testosterone and androstenedione (COCA). One trial plans to report on outcomes of QoL, fertility markers and hirsutism (RESTORE). Three trials plan to report on plasma ACTH ( COCA; NCT01771328; PULSES). Two trials plan to report bone markers such as P1NP and CTX (COCA; NCT01771328) and one to report the change in DEXA scan (NCT02716818).
None of the trials plan to report our outcomes of the prevention of adrenal crisis, testicular or ovarian adrenal rest tumours. However, four trials plan to report outcomes not included in our review: three trials plan to report on lipid profile (CareOnTIME; NCT01771328; PULSES) and one on body weight (RESTORE).
Risk of bias in included studies
We judged all trials as at unclear or high risk of bias in one or more 'Risk of bias' domains. These judgements are presented in a graph (Figure 2).
2.
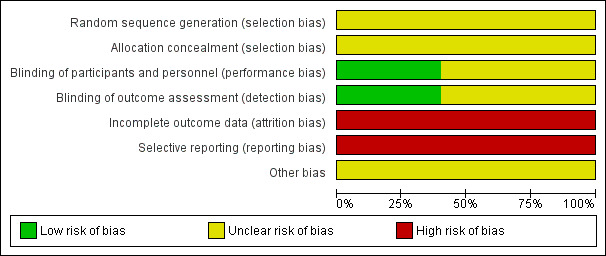
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sequence generation
All trials stated that allocation was randomised, but no trials described the method of randomisation used. We therefore judged the risk of bias due to the generation of the randomisation sequence as unclear in all trials (Caldato 2004; German 2008; Nebesio 2016; Silva 1997; Winterer 1985).
Allocation concealment
Concealment of allocation was not reported in any trial in which the randomisation procedure was carried out and all trials were deemed as having an unclear risk of bias (Caldato 2004; German 2008; Nebesio 2016; Silva 1997; Winterer 1985).
Blinding
Only two trials reported double blinding resulting in low risk of bias (Winterer 1985; Silva 1997). The remaining three trials did not report blinding of investigators or participants (Caldato 2004; German 2008; Nebesio 2016). We considered the risk of bias to be high if this aspect of trial quality was not discussed because the intervention was clearly different, therefore the blinding could not take place for the participants or caregivers.
Incomplete outcome data
No trials explicitly stated that an intention‐to‐treat analysis was performed or whether any participants deviated from the randomised group to which they were assigned. One trial reported that four out of nine participants recruited subsequently withdrew due to difficulties maintaining peripheral IV access therefore the risk of bias was high (Nebesio 2016). The remaining four trials did not report attrition rates, therefore the risk of bias was also deemed as high (Caldato 2004; German 2008; Silva 1997; Winterer 1985).
Selective reporting
We were unable to identify any selective reporting in the included trials, but did not have any access to the original trial protocols to definitely confirm this. We did contact the lead investigators of two trials, but have not received a reply to date (Caldato 2004; Nebesio 2016). We therefore concluded that there is a high risk of bias due to selective reporting for all trials (Caldato 2004; German 2008; Nebesio 2016; Silva 1997; Winterer 1985).
Other potential sources of bias
We were unable to identify any other potential sources of bias; but did not have any access to the original trial protocols to definitely confirm this. There was lack of information in all cross‐over trials with regards to lack of washout periods. We therefore concluded that there is an unclear risk from other potential sources of bias for all trials (Caldato 2004; German 2008; Nebesio 2016; Silva 1997; Winterer 1985).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Due to the heterogeneity of the included trials and the limited amount of evidence on patient‐important outcomes, we were unable to perform any meta‐analyses. We were unable to group data into the time points we had originally planned and so report results from the end of each trial. The duration of each trial is detailed in the table Characteristics of included studies.
The evidence grades stated for the outcomes reported in the summary of findings tables are based on GRADE (Data collection and analysis) and further details are provided in the tables (Table 1; Table 2; Table 3; Table 4).
Primary outcomes
1. QoL score
This outcome was not reported in any of the trials.
2. Androgen normalisation
All five reported on this outcome using different measurements. Five trials reported 17 OHP levels (Caldato 2004; German 2008; Nebesio 2016; Silva 1997; Winterer 1985), four trials reported androstenedione (Caldato 2004; German 2008; Nebesio 2016; Silva 1997), three trials reported testosterone (Caldato 2004; German 2008; Silva 1997), and one trial reported DHEAS (German 2008).
HC (13.5 mg/m²/day vs 15.5 mg/m²/day: high morning HC dose versus high evening HC dose
Androgen normalisation (determined by biochemical parameters such as 17 OHP, androstenedione, testosterone and DHEAS) was not significantly different between groups after four weeks of treatment in one cross‐over trial (n = 15) (German 2008) (very low‐quality evidence). These results were presented as median (interquartile (IQR) range) and so could not be analysed within RevMan.
Results for 17 OHP showed a median (IQR) 44.00 nmol/L (16.00 to 116.00) for the high morning HC dose group (n = 5) compared to 33.00 nmol/L (15.00 to 76.00) for the high evening HC dose group (n = 6). Androstenedione was reported as 1.80 nmol/L (1.0 to 3.0) in the high morning HC dose group compared to 1.90 nmol/L (1.20 to 6.50) in the high evening HC dose group. The median (IQR) testosterone was 0.70 nmol/L (0.30 to 2.30) in the high morning HC dose group compared to 1.1 nmol/L (0.60 to 2.70) high evening HC dose group. Finally, in the high morning HC dose group the median (IQR) DHEAS was 0.20 nmol/L (0.20 to 0.60) compared to 0.40 nmol/L (0.20 to 0.70) in the high evening HC dose group (German 2008).
HC (15 mg/m²/day) versus PD (3 mg/m²/day) versus DXA (0.3 mg/m²/day)
One trial (n = 27) compared HC 15 mg/m²/day in three doses (n = 9) to PD 3 mg/m²/day in two doses (n = 9) and to DXA 0.3 mg/m²/day in a daily single dose (n = 9) (Nebesio 2016) (very low‐quality evidence). After six weeks, DXA resulted in significantly lower levels of 17 OHP compared to HC (P < 0.001) and compared to PD (P < 0.001). Similarly, androstenedione levels were significantly lower with DXA when compared to HC (P = 0.016) and PD (P = 0.002). It was reported that HC and PD had similar adrenal hormone levels. Compared with PD, 17 OHP and androstenedione were suppressed more with HC and DXA treatment (Nebesio 2016)
HC (12.5 mg/m²/day) with placebo at five different schedules
Winterer (n = 8) compared five different HC and placebo dosing regimens with a total dose of 12.5 mg/m²/day in a cross‐over design; duration varied from four to six weeks for each dosing schedule. Treatment or placebo were given in different combinations at three time‐points across the day (morning, noon, evening). Investigators reported no significant difference in 17 OHP between the different dosing schedules (Winterer 1985).
As the results were presented graphically, we were not able to extract the data. The review authors have attempted to contact the trial authors to see if raw data are available.
HC (10 mg/m²/day to 15 mg/m²/day) versus PD (2.4 mg/m²/day to 3.5 mg/m²/day)
Caldato compared three times‐daily HC to once‐daily PD for one year (n = 44) (Caldato 2004). In the analysis, we have presented the data split as was reported in the paper (prepubertal participants and pubertal participants) and report total data here. Final values of 17 OHP were not significantly different between groups at one year, MD 1189.10 nmol/L (95% CI ‐51.08 to 2429.28) (Analysis 1.1) (very low‐quality evidence). However, there were significantly higher levels of androstenedione in the HC group, MD 57.75 nmol/L (95% CI 11.19 to 104.31) (Analysis 1.2) (very low‐quality evidence). Levels of testosterone showed no difference between HC or PD groups, MD 38.55 nmol/L (95% CI ‐6.48 to 83.58) (Analysis 1.3) (very low‐quality evidence).
1.1. Analysis.
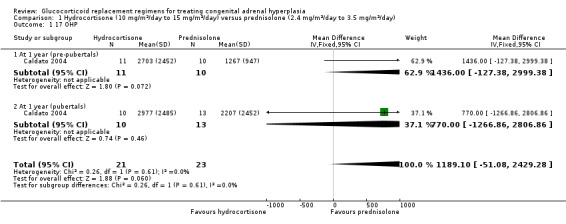
Comparison 1 Hydrocortisone (10 mg/m²/day to 15 mg/m²/day) versus prednisolone (2.4 mg/m²/day to 3.5 mg/m²/day), Outcome 1 17 OHP.
1.2. Analysis.
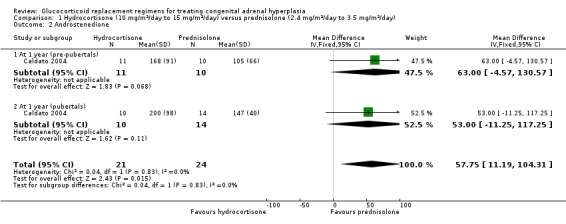
Comparison 1 Hydrocortisone (10 mg/m²/day to 15 mg/m²/day) versus prednisolone (2.4 mg/m²/day to 3.5 mg/m²/day), Outcome 2 Androstenedione.
1.3. Analysis.
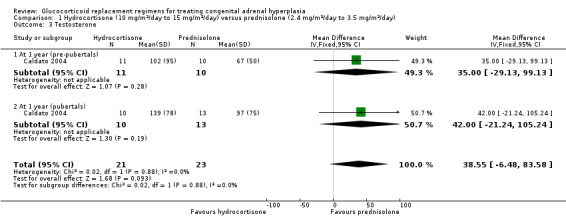
Comparison 1 Hydrocortisone (10 mg/m²/day to 15 mg/m²/day) versus prednisolone (2.4 mg/m²/day to 3.5 mg/m²/day), Outcome 3 Testosterone.
HC (15 mg/m²/day) versus HC (25 mg/m²/day) with fludrocortisone 0.1 mg/day
Silva compared once daily HC (15 mg/m²/day ) with once daily (25 mg/m²/day) HC plus fludrocortisone for six months using a cross‐over design (n = 26) (Silva 1997). Investigators reported the median (IQR) values separately for prepubertal and pubertal participants so we were not able to analyse the data in RevMan.
Median (IQR) levels of 17 OHP were more suppressed in the prepubertal group, with HC 25 mg/m²/day, 11.5 nmol/L (0.6 to 819.9) compared to HC 15 mg/m²/day 113.7 nmol/L (0.5 to 1207) (P < 0.05); however this was not true for the pubertal group, where levels in the HC 15 mg/m²/day group were lower than in the HC 25 mg/m²/day group, 91.7 nmol/L (6.8 to 453.0) versus 314.2 nmol/L (66.5 to 568.7). Likewise, androstenedione median (IQR) levels in prepubertal participants were more suppressed on HC 25 mg/m²/day, 1.6 nmol/L (0.1 to 31.8), compared to the HC 15 mg/m²/day group, 3.4 (0.5 to 40.2) (P < 0.05), Again, this was reversed for the pubertal participants where levels were lower in the HC 15 mg/m²/day group, 11 nmol/L (6.1 to 41.9), compared to the HC 25 mg/m²/day group, 22.3 nmol/L (10.5 to 47.5). No differences were noted in testosterone levels after six months in either prepubertal participants, HC 15 mg/m²/day group 2.5 nmol/L (0.8 to 9.1) versus HC 25 mg/m²/day group 2.3 nmol/L (1.2 to 11.3), or in pubertal participants HC 15 mg/m²/day group 4.7 nmol/L (3.9 to 6.9) versus HC 25 mg/m²/day group 6.2 nmol/L (3.5 to 9.2) (Silva 1997) (very low‐quality evidence for all androgen normalisation results).
3. Prevention of adrenal crisis
This outcome was not reported in any of the trials.
Secondary outcomes
1. Presence of osteopenia
This outcome was not reported in any of the trials.
2. Presence of testicular or ovarian adrenal rest tumours
This outcome was not reported in any of the trials.
3. Subfertility
This outcome was not reported in any of the trials.
4. Final adult height
Final adult height was not reported in any of the trials. However, the results of change in the ratio of bone age (BA) to chronological age (CA) and height velocity can be taken as surrogate markers for effects on final adult height since under‐treatment and the resulting excess production of androgens causes accelerated growth, advanced skeletal maturation and early epiphyseal fusion leading to reduced final adult height (Riepe 2007). Two trials reported data we can present for this outcome (Caldato 2004; Silva 1997).
HC (10 mg/m²/day to 15 mg/m²/day) versus PD (2.4 mg/m²/day to 3.5 mg/m²/day)
One trial (n = 44) found that once daily PD treatment after one year of treatment could lead to better control of bone maturation compared to HC especially in prepubertal children (Caldato 2004). Height SDS BA was significantly better for the PD group compared to the HC group, MD ‐0.81 (95% CI ‐1.47 to ‐0.15) (Analysis 1.4). No significant difference was found in height SDS CA between both groups, MD ‐0.14 (95% CI ‐0.99 to 0.71) (Analysis 1.5). Caldato also found that the ratio of BA/CA was higher in the HC group compared to the PD group at one year but the difference between groups was not significant, MD 0.15 (95% CI ‐0.03 to 0.33) (Analysis 1.6). After one year, Caldato (n = 34) reported that the absolute change in the ratio of BA/CA was higher in the HC group (‐0.64 to 0.01) compared to the PD group (0.37 to 0.11) (P < 0.02) (Caldato 2004). Growth velocity SDS was similar in both groups with small changes from baseline after one year in both groups, ‐0.4 in the PD group and 0.43 in the HC group and no statistical difference demonstrated, MD 0.26 (95% CI ‐0.82 to 1.34) (Analysis 1.7) (very low‐quality evidence). Final height (cm) also did not show any statistical difference between groups after one year, MD ‐0.17 cm (95% CI ‐0.87 to 0.52) (Analysis 1.8).
1.4. Analysis.
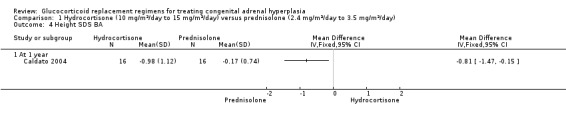
Comparison 1 Hydrocortisone (10 mg/m²/day to 15 mg/m²/day) versus prednisolone (2.4 mg/m²/day to 3.5 mg/m²/day), Outcome 4 Height SDS BA.
1.5. Analysis.
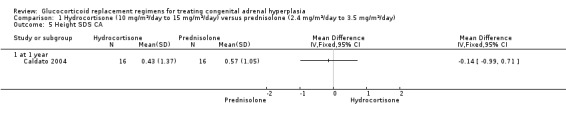
Comparison 1 Hydrocortisone (10 mg/m²/day to 15 mg/m²/day) versus prednisolone (2.4 mg/m²/day to 3.5 mg/m²/day), Outcome 5 Height SDS CA.
1.6. Analysis.
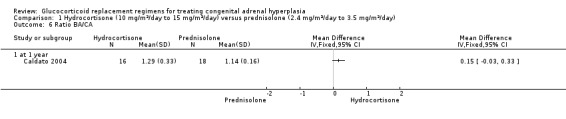
Comparison 1 Hydrocortisone (10 mg/m²/day to 15 mg/m²/day) versus prednisolone (2.4 mg/m²/day to 3.5 mg/m²/day), Outcome 6 Ratio BA/CA.
1.7. Analysis.
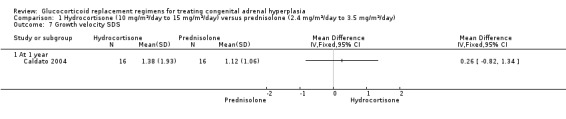
Comparison 1 Hydrocortisone (10 mg/m²/day to 15 mg/m²/day) versus prednisolone (2.4 mg/m²/day to 3.5 mg/m²/day), Outcome 7 Growth velocity SDS.
1.8. Analysis.
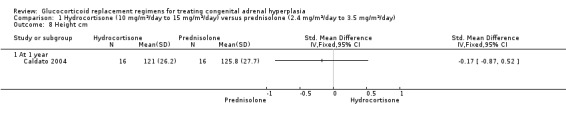
Comparison 1 Hydrocortisone (10 mg/m²/day to 15 mg/m²/day) versus prednisolone (2.4 mg/m²/day to 3.5 mg/m²/day), Outcome 8 Height cm.
HC (15 mg/m²/day) versus HC (25 mg/m²/day) with fludrocortisone 0.1 mg/day
Silva compared once daily HC (15 mg/m²/day) with plus fludrocortisone to once daily (25 mg/m²/day) HC plus fludrocortisone for six months in a cross‐over trial (n = 26) (Silva 1997).
Height velocity was significantly reduced (P = 0.03) in the higher dosing HC plus fludrocortisone regimen compared with the lower daily HC plus fludrocortisone regimen. Both the paper and our analysis reported a significantly greater increase in height for the 22 pre‐pubertal children while using 15 mg/m²/day as compared with 25 mg/m²/day, MD 0.34 (95% CI 0.27 to 0.41) (P < 0.00001) (Analysis 2.1) (very low‐quality evidence).
2.1. Analysis.
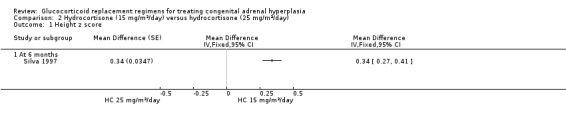
Comparison 2 Hydrocortisone (15 mg/m²/day) versus hydrocortisone (25 mg/m²/day), Outcome 1 Height z score.
We were not able to analyse the remaining data presented in the paper in RevMan and report them directly from the paper here. At the end of the trial, no significant differences were noted between groups in mean (SE) incremental growth hormone levels between the HC 15 mg/m²/day group 9.6 µg/L (2.0) and the HC 25 mg/m²/day group 6.9 µg/L (3.0). Investigators reported mean (SE) IGF‐1 levels split by age group and found no difference between doses for any age group; under six years of age, HC 15 mg/m²/day group (n = 16) 93.1 µg/L (14.9) compared to HC 25 mg/m²/day group (n = 15) 118.2 µg/L (20.6); for six to 12 years, HC 15 mg/m²/day group (n = 8) 320.0 µg/L (39.9) compared to HC 25 mg/m²/day group (n = 8) 310.0 µg/L (36.8); and for over 12 years, HC 15 mg/m²/day group (n = 2) 265.0 µg/L (25.0) compared to HC 25 mg/m²/day group (n = 2) 270.0 µg/L (30.0) (Silva 1997).
Discussion
The main goal in CAH therapy is to achieve physiological replacement of cortisol deficiency while suppressing adrenal androgen overproduction. The effectiveness of long‐term treatment is of major clinical relevance due to the high risk of effects from over treatment or under treatment. Outcomes such as final adult height have been disappointing and additional problems such as infertility and poor QoL are common (LWPES/ESPE 2002; Marumudi 2013).
Several different glucocorticoid replacement regimens and dosing schedules have been used, but it remains a challenge to reproduce the physiological circadian rhythm and pulsatile secretion of cortisol. In children, HC has been the preferred glucocorticoid replacement therapy due to potential concerns of growth suppression associated with longer acting glucocorticoids.
Summary of main results
There are five randomised controlled trials (n = 101) in both children and adults included in the review; four of these had a cross‐over design (German 2008; Nebesio 2016; Silva 1997; Winterer 1985) and one was of parallel design (Caldato 2004).
None of the included trials reported on the review's primary outcomes of QoL or prevention of adrenal crisis. All trials reported on androgen normalisation using a range of measurements: five trials reported 17 OHP levels (Caldato 2004; German 2008; Nebesio 2016; Silva 1997; Winterer 1985), three trials reported androstenedione (Caldato 2004; German 2008; Nebesio 2016;) two trials reported testosterone (Caldato 2004; German 2008), and one trial reported DHEAS (German 2008).
There were no significant differences in 17 OHP, testosterone, androstenedione or DHEA between a high morning dose of HC or a high evening dose after four weeks (German 2008). One trial found that HC and DXA treatment suppressed 17 OHP and androstenedione more than PD treatment after six weeks (Nebesio 2016). A further trial reported no significant difference in 17 OHP between the five different dosing schedules of HC at between four and six weeks (Winterer 1985). The trial comparing HC and PD found no differences in the values of 17 OHP, androstenedione and testosterone at one year (Caldato 2004). The trial of HC versus HC plus fludrocortisone found that at six months 17 OHP and androstenedione levels were more suppressed on HC alone but there were no differences noted in testosterone levels (Silva 1997).
With regards to the secondary outcomes, none of the included trials reported on the presence of osteopenia, of testicular or ovarian adrenal rest tumours or subfertility. While no trial reported final adult height two trials did report surrogate markers of height and growth (Caldato 2004; Silva 1997).
Caldato found that after one year of once‐daily PD treatment could lead to better control of bone maturation compared to HC especially in prepubertal children (Caldato 2004). Height SDS BA was significantly better for the PD group MD ‐0.81 (95% CI ‐1.47 to ‐0.15) (Analysis 1.4), but there was no significant difference between groups in height SDS CA, the ratio of BA/CA, growth velocity SDS or height (cm) at the end of the one‐year trial.
The six‐month Silva trial compared once daily HC 15 mg/m²/day with once daily HC 25 mg/m²/day with fludrocortisone 0.1 mg/day and found that height velocity was significantly reduced with the higher dose of HC with fludrocortisone (Silva 1997). Height z score was significantly higher with once‐daily HC 15 mg/m²/day than with once‐daily HC 25 mg/m²/day with fludrocortisone, MD 0.34 (95% CI 0.27 to 0.41) (Analysis 2.1). There were no differences noted in growth hormone or IGF1 levels.
Overall completeness and applicability of evidence
All the trials in this review were single‐centre with a variable duration of treatment ranging from four weeks to one year. It was unfortunate that there are no long‐term trials reporting outcomes beyond a trial duration of one year (six months for each treatment arm).
The five included trials reported on few of the review outcomes and used a variety of units of measurement, meaning we were unable to combine data in a meta‐analysis. Although 17 OHP and androstenedione are frequently used to monitor treatment, there is a great amount of variability in the measurements which hampers the usefulness of these tests. No trials included QoL outcomes and most trials did not report adverse events or serious adverse events. While there were differences reported in some growth outcomes, there were no differences noted in growth hormone or IGF1 levels indicating that laboratory markers are unreliable and clinical follow‐up is the most efficient way to evaluate adequacy of HC replacement. Since no trial presented data for final adult height, we reported a number of surrogate markers for this outcome.
Quality of the evidence
All the trials we included were small and had methodological weaknesses. There is relatively little replication of trials, with a single, small trial for each comparison. This weak evidence base makes it impossible to draw firm conclusions with confidence. Limitations included methodological quality, variations in intervention implementation, and outcomes.
This review addressed a diverse range treatment regimens with many trials at high or unclear risk of bias. Generally, the lack of methodological detail provided in the trial reports did not allow us to assess risk of bias across the trials, and led to many high or unclear 'Risk of bias' ratings across the various domains. In particular, details of the methods of random sequence generation, concealment of treatment allocation, and blinding of the outcome assessors were often not reported, and therefore, we could not accurately determine the risk of bias. While we judged only one trial to be at a low risk of selection bias, the remaining four had a high risk of selection bias. It was not clear ant trial whether there were attempts to blind outcome assessors; therefore, we judged an unclear risk of detection bias. Due to lack of access to trial registrations and protocols, or limited reporting of important review outcomes, or both, we judged none of the trials to be at a low risk of reporting bias. Overall the risk of bias was moderate to high.
The number of trials assessing different glucocorticoid regimens varied as well as the trial durations and It was difficult to draw overall conclusions from any single trial. Three trials were of a cross‐over design and we did not have the appropriate information to conduct comprehensive meta‐analyses, but trial authors have been contacted for further information in order that a more complete analysis can be carried out.
The evidence as assessed using GRADE, is very low quality for all outcomes assessed across all trials. This means that new, better quality research is highly likely to change the overall conclusions of this review.
Potential biases in the review process
We attempted to avoid bias by ensuring that we had identified all relevant trials through comprehensive systematic searching of the literature and contact with trial investigators to identify other trials both published and unpublished. We also attempted to contact all authors where the original publication did not provide sufficient data, and planned to incorporate that data into the review; unfortunately, we have not received any information to date. It was not possible to evaluate the overall possibility of publication bias as not all trials reported the same outcomes and overall the trials were too heterogeneous to combine. The GRADE approach assessments were made by two people, independently, and discrepancies resolved by discussion. Data extraction was completed independently by two authors without financial interest in the outcome.
Agreements and disagreements with other studies or reviews
Since this is the first review published on this topic, comparisons to previous published systematic reviews are not possible. Although there is no NICE guidance available for treating and managing CAH, there are international guidelines outlining principles of diagnosis and management of CAH, but they do not specify recommendations for treatment regimens (Speiser 2018). These guidelines recommend treatment with HC, but do not give details on specific timings or dose regimens. A maintenance dose of HC is recommended for children and adolescents with CAH who are still growing; while in adults with CAH, daily HC or long‐acting glucocorticoids plus mineralocorticoids, or a combination, is recommended as clinically indicated (Speiser 2018).
We were not able to include any long‐term RCTs which measured growth, but maintaining normal growth in CAH is a challenge and long‐term follow‐up trials have shown that individuals fail to achieve their predicted final adult heights (Mathews 2004; Merke 2000).
Authors' conclusions
Implications for practice.
The goal of glucocorticoid therapy in congenital adrenal hyperplasia (CAH) is to replace deficient cortisol and prevent the consequences of androgen excess whilst avoiding glucocorticoid over‐treatment. There are limited trials to date which compare the efficacy and safety of different glucocorticoid replacement regimens for treating of 21‐hydroxylase deficiency CAH in children and adults and no evidence to date of which regimen provides optimal outcomes. There were no trials on the modified‐release formulation of hydrocortisone or on the use of 24‐hour circadian continuous subcutaneous infusion of hydrocortisone. No common intervention characteristics for success were apparent among the trials included in this review.
The choice of a glucocorticoid regimen needs to be informed by an understanding of the adverse consequences and benefits of different regimens in individuals at different ages and stages of disease. We were unable to draw any firm conclusions based on the evidence that was reported in any of the included trials. It was unfortunate that no trials included long‐term outcomes such as quality of life, prevention of adrenal crisis, presence of osteopenia, presence of testicular or ovarian adrenal rest tumours, subfertility and final adult height. As a consequence, uncertainty remains about the most effective form of glucocorticoid replacement therapy for treating CAH for adults and children.
Implications for research.
This systematic review has identified the need for well‐designed, adequately‐powered, multi‐centred randomised controlled trials (RCTs) to assess the efficacy and safety of different glucocorticoid replacement regimens for treating 21‐hydroxylase deficiency CAH in children and adults. RCTs should be carried out over a longer duration (more than 10 years) to monitor biochemical and clinical outcomes and to provide further information on the long‐term effects on quality of life, final adult height, androgen normalisation, prevention of adrenal crisis, osteopenia, subfertility and presence of testicular or ovarian adrenal rest tumours. It is recognised that such long‐term RCTs are likely to be difficult to achieve due to either recruiting sufficient participants to allow for adequate power or due to maintaining follow‐up of participants until adult height is achieved.
It should be noted that while one trial reported differences in some growth outcomes, it did not report any differences in growth hormone or IGF1 levels, which indicates that laboratory markers are unreliable and that clinical follow‐up is the most efficient way to evaluate the adequacy of hydrocortisone replacement.
Notes
The protocol for this review was not compliant with Cochrane's Conflict of Interest Policy. A new author (Ashma Krishan) joined the team; she worked on the analysis and drafted the summary of findings tables.
Acknowledgements
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search methods – electronic searches
| Database | Strategy | Date last searched | Records identified |
| CENTRAL | #1 MeSH descriptor: [Adrenal Hyperplasia, Congenital] this term only [MeSH descriptor] #2 Adrenal Hyperplasia [all text] #3 hydroxylase deficiency [all text] #4 #1 AND #2 AND #3 #5 Mesh descriptor: [Glucocorticoids] this term only #6 (hydrocortisone or chronocort or plenadren or beclomethasone or betamethasone or budesonide or cortisone or dexamethasone or methylprednisolone or prednisolone or prednisone or triamcinolone) #7#5 or #6 #8 #4 and #7 [limit to trials only] |
17 December 2019 | 71 |
| MEDLINE Ovid | 1 randomized controlled trial.pt. 2 controlled clinical trial.pt. 3 randomized.ab. 4 placebo.ab. 5 drug therapy.fs. 6 randomly.ab. 7 trial.ab. 8 groups.ab. 9 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10 exp animals/ not humans.sh. 11 9 not 10 12 Adrenal Hyperplasia, Congenital/ 13 Adrenal Hyperplasia.af. 14 21‐hydroxylase deficiency.af. 15 12 or 13 or 14 16 Glucocorticoids/ 17 (hydrocortisone or chronocort or plenadren or beclomethasone or betamethasone or budesonide or cortisone or dexamethasone or methylprednisolone or prednisolone or prednisone or triamcinolone).af. 18 16 or 17 19 11 and 15 and 18 Lines 1‐11 are the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐maximizing version (2008 revision); Ovid format (Lefebvre 2011). |
17 December 2019 | 1042 |
| Embase HDAS (1974 to present) ‐ including RCT subject headings in filter | 1 ''CONGENITAL ADRENAL HYPERPLASIA''/ 2 (Adrenal Hyperplasia).af 3 ''STEROID 21 MONOOXYGENASE DEFICIENCY''/ 4 (21‐hydroxylase deficiency).af 5 (1 OR 2 OR 3 OR 4) 6 Exp GLUCOCORTICOID/ 7 (hydrocortisone OR chronocort OR plenadren OR beclomethasone OR betamethasone OR budesonide OR cortisone OR dexamethasone OR methylprednisolone OR prednisolone OR prednisone OR triamcinolone).af 8 6 OR 7 9 (''CROSSOVER PROCEDURE''/ OR ''DOUBLE BLIND PROCEDURE'' OR ''RANDOMIZED CONTROLLED TRIAL'' OR ''SINGLE BLIND PROCEDURE'').af 10 (random* OR factorial* OR crossover* OR (cross* ADJ over*) OR placebo*).af 11 ((doubl* ADJ3 blind*) OR (singl* ADJ3 blind*) OR assign* OR allocat* OR volunteer*).af 12 (9 OR 10 OR 11) 13 (5 OR 8 OR 12) Embase search strategy (Lefebvre 2011) |
17 December 2019 | 261 |
| ISRCTN (www.isrctn.com) |
[Basic Search] ''Adrenal hyperplasia'' OR ''21‐hydroxylase deficiency'' |
17 December 2019 | 25 |
| Clinicaltrials.gov (www.clinicaltrials.gov) |
[Advanced Search] Condition: '' congenital adrenal hyperplasia'' STUDY TYPE: Interventional Studies |
17 December 2019 | 76 |
| WHO ICTRP (apps.who.int/trialsearch) |
[Basic search] Adrenal hyperplasia OR 21‐hydroxylase deficiency |
17 December 2019 | 83 (76 trials) |
| Health Canada’s Clinical Trial Database (health‐products.canada.ca/ctdb‐bdec/index‐eng.jsp) | SEARCH 1 Medical condition: adrenal hyperplasia SEARCH 2: Medical condition: 21 hydroxylase deficiency |
17 December 2019 | 0 |
| NICE Evidence (www.evidence.nhs.uk) | ''adrenal hyperplasia'' OR ''21‐hydroxylase deficiency'' | 17 December 2019 | 134 |
Data and analyses
Comparison 1. Hydrocortisone (10 mg/m²/day to 15 mg/m²/day) versus prednisolone (2.4 mg/m²/day to 3.5 mg/m²/day).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 17 OHP | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 1189.10 [‐51.08, 2429.28] |
| 1.1 At 1 year (pre‐pubertals) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 1436.0 [‐127.38, 2999.38] |
| 1.2 At 1 year (pubertals) | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 770.0 [‐1266.86, 2806.86] |
| 2 Androstenedione | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 57.75 [11.19, 104.31] |
| 2.1 At 1 year (pre‐pubertals) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 63.0 [‐4.57, 130.57] |
| 2.2 At 1 year (pubertals) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 53.0 [‐11.25, 117.25] |
| 3 Testosterone | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 38.55 [‐6.48, 83.58] |
| 3.1 At 1 year (pre‐pubertals) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 35.0 [‐29.13, 99.13] |
| 3.2 At 1 year (pubertals) | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 42.0 [‐21.24, 105.24] |
| 4 Height SDS BA | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 At 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Height SDS CA | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 at 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Ratio BA/CA | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 at 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Growth velocity SDS | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 At 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Height cm | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 At 1 year | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Hydrocortisone (15 mg/m²/day) versus hydrocortisone (25 mg/m²/day).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Height z score | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 1.1 At 6 months | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Caldato 2004.
| Methods | RCT. Parallel design. Locaton: 2 tertiary centres in Brazil. Duration: 1 year. |
|
| Participants | 44 participants randomly assigned to groups: Group 1 n = 23; Group 2 n = 21. Age, mean (range): Group 1, 9.4 (1.6 ‐ 20) years; Group 2, 8.3 (1.2 ‐ 21) years Gender split: Group 1, 16 females, 7 males; Group 2, 18 females, 3 males. Tanner stage: Group 1, 10 pre‐pubertal (B1 and G1, according to Tanner’s classification), 8 pubertal (B2‐B4 and G2‐G4), and 5 post‐pubertal (adults, B5 and G5); Group 2, 11 prepubertal, 5 pubertal and 5 post‐pubertal. |
|
| Interventions |
Group 1: PD 2.4 ‐ 3.5 mg/m² BSA 1x daily. Group 2: HC 10 ‐ 15 mg/m² BSA 3x times daily. All participants received FC 0.1 mg/day. |
|
| Outcomes | Bone maturation ratio, height, weight, growth velocity, pubertal stage, plasma 17 OHP, androstenedione, serum testosterone and androstenedione values. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rate was not reported. |
| Selective reporting (reporting bias) | High risk | Did not have any access to the original trial protocols to definitely confirm this and we attempted to contact the investigators but did not receive a response. Not all outcome measures listed in the 'methods' section of the paper were fully reported in the results. |
| Other bias | Unclear risk | No other bias found. |
German 2008.
| Methods | Open‐label RCT. Cross‐over design. Location: single tertiary centre in Israel. Duration: 4 weeks ‐ 2 weeks for each treatment schedule; washout period was not stated in the paper. |
|
| Participants | 15 participants recruited. Age, mean (range): 10 (7.5 ‐ 14.5) years Gender split: 9 males, 6 females. Diagnosis: classical CAH due to 21‐hydroxylase deficiency (n = 14), 11‐hydroxylase deficiency (n = 1). |
|
| Interventions | Participants were randomised into 2 groups for their initial treatment schedule of 3 daily doses standardized to 0700 – 0800 h, 1300 – 1400 h and 2100 – 2200 h according to participants' age. Total HC dose ranged from 13.5 mg/m²/day to 15.5 mg/m²/day. Intervention 1: a high‐morning dose, when 50% of the daily HC was taken in the morning and 25% of the daily dose at other time‐points. Intervention 2: a high‐evening dose, when 50% was taken at bedtime and 25% of the daily dose at other time‐points. |
|
| Outcomes | 0800 h 17 OHP, testosterone, androstenedione, and DHEAS on the last day of each treatment; arousal number, total sleep time, sleep efficiency, and sleep latency. | |
| Notes | Outcomes measured but not presented in the review: arousal number, total sleep time, sleep efficiency and sleep latency. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised to treatment, but paper does not state how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation of concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessments. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rate was not reported. |
| Selective reporting (reporting bias) | High risk | We did not have any access to the original trial protocols to definitely confirm this and we attempted to contact the investigators but have not received a response. Not all outcome measures listed in the 'methods' section of the paper were fully reported in the results. |
| Other bias | Unclear risk | We did not have any access to the original trial protocols to definitely confirm this but there was no washout period described in the paper for this cross‐over trial. |
Nebesio 2016.
| Methods | RCT. Cross‐over design. Location: single tertiary centre in USA. Duration: 18 weeks, (6 weeks per treatment arm but no washout period). |
|
| Participants | Randomised 9 participants. Age, range: 4.8 to 11.6 years. Gender split: 4 males, 5 females. Diagnosis: CAH. |
|
| Interventions |
Group 1: HC 15 mg/m²/day in 3 doses. Group 2: PD 3 mg/m²/day in 2 doses. Group 3: DXA 0.3 mg/m²/day in a single dose. All treatment schedules lasted 6 weeks and then switched to another one |
|
| Outcomes | Mean ACTH, androstenedione, 17 OHP concentration, IGF‐1, GH, BMI, SNP. | |
| Notes | Outcomes reported but not presented in the review: ACTH and SNP. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 3 sequential 6‐week courses of treatment randomly assigned, but paper does not state how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation of concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcome assessments. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 participants withdrew due to difficulties with peripheral IV access but it did not state which groups these participants were in when they dropped out. |
| Selective reporting (reporting bias) | High risk | We did not have any access to the original trial protocols to definitely confirm whether this occurred and we attempted to contact the investigators but have not received a response.Not all outcome measures listed in the 'methods' section of the paper were fully reported in the results. |
| Other bias | Unclear risk | We did not have any access to the original trial protocols to definitely confirm this but there was no washout period described in the paper for this cross‐over trial. |
Silva 1997.
| Methods | RCT. Cross‐over design. Location: single tertiary centre in Brazil. Duration: 6 months per treatment arm, but washout period was not stated in paper. |
|
| Participants | 26 participants randomised. Age, median (range): 45.3 months (3.6 months to 15 years). Gender split: 8 males, 18 females. Diagnosis: CAH due to 21OH deficiency. |
|
| Interventions |
Group 1: HC 15 mg/m² 1x daily and FC 0.1 mg/day for 6 months. Group 2: HC 25 mg/m² 1x daily and FC 0.1 mg/day for 6 months. |
|
| Outcomes | 17 OHP, androstenedione, testosterone, GH, IGF‐1, growth pattern (height and weight). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Treatment randomly assigned, but paper does not state how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation of concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Each child was examined by the same physician who was blinded to the HC dosing schedule. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Each child was examined by the same physician who was blinded to the HC dosing schedule. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rate was not reported. |
| Selective reporting (reporting bias) | High risk | We did not have any access to the original trial protocols to definitely confirm whether this occurred and we attempted to contact the investigators but have not received a response. |
| Other bias | Unclear risk | We did not have any access to the original trial protocols to definitely confirm this but there was no washout period described in the paper for this cross‐over trial. |
Winterer 1985.
| Methods | RCT. Cross‐over design. Location: single tertiary centre in Brazil. Duration: 4 to 6 weeks of each dose schedule. |
|
| Participants | 8 participants randomised. Age, range: 21OH deficiency, 9 to 20 years; 11OH deficiency 6 to 12 years. Gender split: 7 males, 1 female. Diagnosis: 6 participants with 21‐hydroxylase deficiency, 2 participants with 11‐hydroxylase deficiency. |
|
| Interventions | HC (+2 participants were on FC). HC split across different timings (morning, noon, evening) and combined where necessary with placebo (it was not stated was the placebo was). Total dose per day was 12.5 mg/m². Group 1: morning dose: full daily dose of HC; noon dose: placebo; evening dose: placebo. Group 2: morning dose: 2/3 daily dose HC; noon dose: placebo; evening dose: 1/3 daily dose HC. Group 3: morning dose: 1/3 daily dose HC: noon dose: 1/3 daily dose HC; evening dose: 1/3 daily dose HC Group 4: morning dose: 1/3 daily dose HC; noon dose: placebo; evening dose: 2/3 daily dose HC. Group 5: morning dose: placebo; noon dose: placebo; evening dose: full daily dose of HC. |
|
| Outcomes | Plasma 17 OHP, cortisol or cortisol and 11‐deoxycortisol‐increase urine 17‐ketosteroids, 17‐hydroxysteroids, pregnanetriol. | |
| Notes | Outcomes measured but not presented in the review: cortisol or cortisol and 11‐deoxycortisol increase, urine 17‐ketosteroids, 17‐hydroxysteroids and pregnanetriol. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Treatment randomly assigned, but paper does not state how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation of concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported the trial was double blinded, but it was not clear who was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reported the trial was double blinded, but it was not clear who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rate was not reported |
| Selective reporting (reporting bias) | High risk | We did not have any access to the original trial protocols to definitely confirm whether this occurred and we attempted to contact the investigators but have not received a response. |
| Other bias | Unclear risk | We did not have any access to the original trial protocols to definitely confirm this but there was no washout period described in the paper for this cross‐over trial. |
17 OHP: 17‐hydroxyprogesterone ACTH: adrenocorticotrophic hormone BSA: body surface area CAH: congenital adrenal hyperplasia FC: fludrocortisone GH: growth hormone HC: hydroxycortisone IV: intravenous OH: hydroxylase PD: prednisolone RCT: randomised controlled trial SNP: single nucleotide polymorphism
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ajish 2014 | Not an RCT or quasi RCT. |
| Bonfig 2007 | Not an RCT or quasi RCT. |
| Charmandari 2001 | Not an RCT or quasi RCT. |
| ChiCTR‐TRC‐09000350 | Not relevant intervention. The effect of antisterone with angiotensin II and calcium ion antagonist in the treatment of hypertension with CAH. |
| Deuss 1985 | Not relevant intervention. The effect of a high and low dose of naloxone on ACTH was compared with placebo in people with Addison's disease, CAH, Cushing's disease and or Nelson's syndrome. |
| Johannsson 2009 | RCT, study of HC release in healthy volunteers, not people with CAH. |
| Kelch 1975 | Not relevant intervention. The aim was to investigate effects of glucocorticoid therapy, secretory patterns of HGH, FSH, LH and 17 OHP in people with CAH who were treated with or not treated with maintenance dose of HC. |
| Koenen 2009 | Not relevant intervention. The study assessed the effects of treatment with pioglitazone on insulin sensitivity and subcutaneous adipose tissue morphology and function in people with CAH who were insulin‐resistant. |
| Laue 1996 | Not relevant intervention. The pilot study to compare the effect of flutamide, testolactone, reduced‐dose HC and fludrocortisone versus hydrocortisone and fludrocortisone on normalizing linear growth, weight gain and bone maturation. |
| Mallappa 2015 | Not an RCT or quasi RCT. |
| Mathews 2004 | Not relevant intervention. A psychological study utilizing Edinburgh‐Crovitz Inventory and the performance of five tasks to measure hand preferences and, a dichotic listening task composed of consonant‐vowel nonsense syllables was a measure of language lateralization. |
| Merke 2000 | Not relevant intervention. Participants were treated with flutamide, testolactone, reduced does of HC and fludrocortisone. This regimen was compared to HC and fludrocortisone regimen. |
| Merza 2006 | Not an RCT. |
| NCT00001521 | Not relevant intervention. The study aims to determine if a combination of four drugs (flutamide, testolactone, reduced HC dose, and fludrocortisone) can normalize growth in children with CAH. |
| Neumann 2018 | Not an RCT |
| Panamonta 2003 | Not an RCT. |
| PRENATAL DEX | Not RCT. It is an evaluation of in utero dexamethasone on the cognitive development of children at risk of CAH. |
| Sarafoglou 2015 | Not relevant intervention. The study examined the absorption of an extemporaneously prepared HC suspension and compared it to tablets. |
| Spritzer 1990 | Not relevant intervention. Participants divided in 2 groups were treated with HC in order to suppress androgen secretion and cyproterone acetate antiandrogen therapy to inhibit peripheral androgen activity. |
| Turcu 2016 | Not relevant intervention. The study evaluates the safety and tolerability of the selective CRF1 receptor antagonist NBI‐77860 in women with classic 21‐hydroxylase deficiency and tested the hypothesis that CRF1 receptor blockade decreases early morning ACTH and 17 OHP in these people. |
| Verma 2010 | Not an RCT or quasi RCT. |
| Weise 2004 | Not relevant intervention. Participants were assigned to receive either an additional morning dose of HC or placebo, in addition to their usual glucocorticoid and mineralocorticoid replacement 1 hour before exercising. |
| Zhang 2011 | Not relevant intervention. Participants were randomised to different groups and received combinations of different antihypertensive medications at different doses. |
17 OHP: 17‐hydroxyprogesterone ACTH: adrenocorticotrpic hormone CAH: congenital adrenal hyperplasia CRF1: corticotropin‐releasing hormone receptor 1 HC: hydrocortisone RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Salud 2013.
| Methods | RCT, single‐blinded. Location: Phillipines. Duration: unclear. |
| Participants | 9 participants. Age: 16 (+11) months. Gender split: 3 males, 6 females. Diagnosis: CAH due to 21‐hydroxylase deficiency. |
| Interventions |
Arm 1: higher evening HC dose (n = 5). Arm 2: higher morning HC dose (n = 1). Arm 3: equal HC dose morning and evening (n = 3). |
| Outcomes | 17 OHP. |
| Notes | The full paper summarizing the results is not available. |
Silva 1994.
| Methods | RCT. Location: Brazil. Duration: not defined. |
| Participants | 13 participants. Age: children < 16 years. Gender split: not stated. Diagnosis: CAH due to 21OH deficiency. |
| Interventions | HC 20 mg/m² per day in divided doses. Arm 1: higher morning dose (n = 7). Arm 2: higher evening dose (n = 6). |
| Outcomes | 17 OHP, testosterone and androstenedione at the following time points: ‐24 h, baseline, 2 h, 4 h, and 6 h. |
| Notes | The full article summarizing the results is not available. |
17 OHP: 17‐hydroxyprogesterone CAH: congenital adrenal hyperplasia HC: hydrocortisone RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
CareOnTIME.
| Trial name or title | Congenital Adrenal Hyperplasia Once Daily Hydrocortisone Treatment (CareOnTIME). |
| Methods | Interventional Phase IV, open‐label RCT. Parallel design. Location: Naples, Italy. Duration: 6 months per treatment arm, but washout period was not stated in paper. |
| Participants | 150 participants. Age: > 18 years. Gender: both genders eligible. Diagnosis: CAH due to 21‐hydroxylase deficiency. |
| Interventions |
Arm 1: conventional glucocorticoid therapy is continued as before entering the study (immediate release HC, cortisone acetate, PD, prednisone, DXA). Arm 2: dual release HC oral tablets administered once‐daily in the fasting state. |
| Outcomes | Outcomes: change in LDL‐cholesterol, BMI, blood pressure, insulinaemia, glycated haemoglobin, bone mineral density, QoL, sex function in males/females, change in depression status, change in treatment compliance, changes in androgen levels, changes in sperm concentration, changes in ovarian follicle reserve. |
| Starting date | 11 August 2016. |
| Contact information | Professor Rosario Pivonello, MD, PhD. |
| Notes |
COCA.
| Trial name or title | COrticosteroid in Congenital Adrenal Hyperplasia (COCA) |
| Methods | Open‐label RCT. Cross‐over design. Location: Caen, France. Duration: 8 weeks. |
| Participants | 40 participants. Age: 18 years and above. Gender: females only eligible. Diagnosis: classical CAH due to 21OH deficiency. |
| Interventions |
Group 1: HC equivalent to physiological doses for each participant. Group 2: DXA equivalent to physiological doses for each participant. Group 3: PD equivalent to physiological doses for each participant. |
| Outcomes |
Primary outcomes: salivary 17 OHP, plasma ACTH, testosterone, androstenedione. Secondary outcomes: CTX, P1NP, bone alkaline phosphatase, blood glucose and insulin, lipid profile, QoL. |
| Starting date | August 2012. |
| Contact information | Yves Reznik, MD, PhD. |
| Notes |
NCT01771328.
| Trial name or title | Continuous Subcutaneous Hydrocortisone Infusion in Congenital Adrenal Hyperplasia (CAH) |
| Methods | Open‐label, Phase 2 RCT. Cross‐over design. Location: Haukeland University Hospital. Duration: 4 months (Arm 1) and 6 months (Arm 2). |
| Participants | 20 participants. Age: 18 ‐ 60 years. Gender: both females and males eligible. Diagnosis: classical CAH due to 21‐hydroxylase deficiency. |
| Interventions |
Arm 1: HC 10 mg/m²/24 hours subcutaneous infusion. Arm 2: cortisone acetate tablets. |
| Outcomes |
Primary: 17 OHP, ACTH, cortisol, bone markers, testosterone, androstenedione. Secondary: body weight, blood pressure. |
| Starting date | February 2013. |
| Contact information | Kristian Løvås, MD, PhD. |
| Notes |
NCT02716818.
| Trial name or title | Comparison of Chronocort® With Standard Glucocorticoid Therapy in Patients With Congenital Adrenal Hyperplasia |
| Methods | Phase 3, open‐label RCT. Location: USA. Duration: 6 months. |
| Participants | 122 participants. Age: > 18 years. Gender: females and males both eligible. Diagnosis: classical CAH due to 21‐hydroxylase deficiency. |
| Interventions |
Arm 1: HC. Arm 2: PD alone or PD and HC. Arm 3: DXA only or in combination with a different glucocorticoid. |
| Outcomes |
Primary: change from baseline 17 OHP. Secondary: change in DEXA scan. |
| Starting date | February 2016. |
| Contact information | Debbie Merke, MD. |
| Notes | Sponsored by Diurnal Ltd. |
PULSES.
| Trial name or title | Pulsed glucocorticoid replacement therapy for patients with adrenocortical insufficiency secondary to Addison’s disease and congenital adrenal hyperplasia ‐ the pulses study |
| Methods | Open‐label RCT. Parallel design. Location: Bristol, UK. Duration: 6 weeks. |
| Participants | 122 participants. Age: 18 ‐ 64 years. Gender: females and males both eligible. Diagnosis: classical CAH due to 21‐hydroxylase deficiency. |
| Interventions |
Arm 1: HC + placebo infusion. Arm 2: oral placebo + HC infusion. |
| Outcomes |
Primary: ACTH in all participants and 17 OHP in people with CAH using subcutaneous pulsatile HC replacement. Secondary: cognitive, EMA, metabolic profile. |
| Starting date | November 2014. |
| Contact information | Dr Georgina Russell. |
| Notes |
RESTORE.
| Trial name or title | Open‐label Comparison of Chronocort® Versus Standard Glucocorticoid Replacement Therapy (RESTORE). |
| Methods | Open‐label, Phase 3 RCT. Location: USA. Duration: 52 weeks. |
| Participants | 132 participants. Age: > 16 years. Gender: females and males both eligible. Diagnosis: classical CAH due to 21‐hydroxylase deficiency. |
| Interventions |
Arm 1: HC modified release at 5 mg, 10 mg, 20 mg. Arm 2: HC, DXA, PD or prednisone. |
| Outcomes |
Primary: 17 OHP. Secondary: markers of fertility, hirsutism, acne, HbA1c, body weight, QoL, metabolic screen. |
| Starting date | April 2018. |
| Contact information | Diurnal Ltd. |
| Notes |
17 OHP: 17‐hydroxyprogesterone ACTH: adrenocorticotrophic hormone BMD: bone mineral density BMI: body mass index CAH: congenital adrenal hyperplasia CTX: C‐terminal telopeptide DEXA: dual energy x‐ray absorptiometry DXA: dexamethasone HC: hydroxycortisone LDL: low‐density lipoprotein PD: prednisolone P1NP: N‐terminal propeptide of type I procollagen QoL: quality of life RCT: randomised controlled trial
Differences between protocol and review
Initial review stage
Since final adult height was not reported in any of the trials, we have included post hoc analyses such as change in the ratio of bone age to chronological age and height velocity which can be taken as surrogate markers for effects on final adult height. Similarly, we have presented IGF‐1 levels in a post hoc change as a surrogate marker for growth hormone levels; and also androstenedione and testosterone as a surrogate markers for androgen levels.
Authors decided that cluster RCTs were not eligible for inclusion in the review and amended the inclusion criteria.
Contributions of authors
| Roles and responsibilities | |
| TASK | WHO WILL UNDERTAKE THE TASK? |
| Protocol stage: draft the protocol | SMN |
| Review stage: select which trials to include (2 + 1 arbiter) | SMN and KS |
| Review stage: extract data from trials (2 people) | SMN and KS |
| Review stage: enter data into RevMan | KS |
| Review stage: carry out the analysis | SMN, AK and KS |
| Review stage: interpret the analysis | SMN and KS |
| Review stage: draft the final review | SMN, AK and KS |
| Update stage: update the review | SMN, AK and KS |
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
SMN: none known.
KS: I have received the travel grants and consultancy fees from Genzyme, Shire, Amicus, Alexion, Biomarin, Sobi and Nutricia; I have also received lecture fees from FYMCA Ltd, Biomarin and Nutricia. Furthermore, Biomarin sponsored our masterclass in November 2018. While Shire market drugs eligible for inclusion in this review, the travel grants and consultancy fees I have received from these companies relate to lysosomal storage disorders and other general metabolic disorders and not congenital adrenal hyperplasia.
AK: none known.
New
References
References to studies included in this review
Caldato 2004 {published data only}
- Caldato MC, Fernandes VT, Kater CE. One‐year clinical evaluation of single morning dose prednisolone therapy for 21‐hydroxylase deficiency. Arquivos Brasileiros de Endocrinologia e Metabologia 2004;48(5):705‐12. [CENTRAL: 527921; CRS: 5500135000001830; PUBMED: 15761542] [DOI] [PubMed] [Google Scholar]
German 2008 {published data only}
- German A, Suraiya S, Tenenbaum‐Rakover Y, Koren I, Pillar G, Hochberg Z. Control of childhood congenital adrenal hyperplasia and sleep activity and quality with morning or evening glucocorticoid therapy. Journal of Clinical Endocrinology and Metabolism 2008;93(12):4707‐10. [CENTRAL: 668871; CRS: 5500050000000559; PUBMED: 18782876] [DOI] [PubMed] [Google Scholar]
Nebesio 2016 {published data only}
- Nebesio TD, Renbarger JL, Nabhan ZM, Ross SE, Slaven JE, Li L, et al. Differential effects of hydrocortisone, prednisone, and dexamethasone on hormonal and pharmacokinetic profiles: a pilot study in children with congenital adrenal hyperplasia. International Journal of Pediatric Endocrinology 2016;2016:17. [CENTRAL: 1308802; CRS: 5500135000001835; PUBMED: 27688786] [DOI] [PMC free article] [PubMed] [Google Scholar]
Silva 1997 {published data only}
- Silva IN, Kater CE, Cunha CF, Viana MB. Randomised controlled trial of growth effect of hydrocortisone in congenital adrenal hyperplasia. Archives of Disease in Childhood 1997;77(3):214‐8. [CENTRAL: 145358; CRS: 5500135000001828; PUBMED: 9370898] [DOI] [PMC free article] [PubMed] [Google Scholar]
Winterer 1985 {published data only}
- Winterer J, Chrousos GP, Loriaux DL, Cutler GB. Effect of hydrocortisone dose schedule on adrenal steroid secretion in congenital adrenal hyperplasia. Annals of the New York Academy of Sciences 1985;458:182‐92. [CRS: 5500135000001837; PUBMED: 3879121] [DOI] [PubMed] [Google Scholar]
- Winterer J, Chrousos GP, Loriaux DL, Cutler GB. Effect of hydrocortisone dose schedule on adrenal steroid secretion in congenital adrenal hyperplasia. Journal of Pediatrics 1985;106(1):137‐42. [CENTRAL: 36478; CRS: 5500050000000555; PUBMED: 3871229] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ajish 2014 {published data only}
- Ajish TP, Praveen VP, Nisha B, Kumar H. Comparison of different glucocorticoid regimens in the management of classical congenital adrenal hyperplasia due to 21‐hydroxylase deficiency.. Indian Journal of Endocrinology and Metabolism 2014;18(6):815‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonfig 2007 {published data only}
- Bonfig W, Bechtold S, Schmidt H, Knorr D, Schwarz HP. Reduced final height outcome in congenital adrenal hyperplasia under prednisone: deceleration of growth velocity during puberty. Journal of Clinical Endocrinology and Metabolism 2007;92(5):1635‐9. [DOI] [PubMed] [Google Scholar]
Charmandari 2001 {published data only}
- Charmandari E, Matthews D, Johnston A, Brook CG, Hindmarsh PC. Serum cortisol and 17‐hydroxyprogesterone interrelation in classic 21‐hydroxylase deficiency: is current replacement therapy satisfactory?. Journal of Clinical Endocrinology and Metabolism 2001;86(10):4679‐85. [DOI] [PubMed] [Google Scholar]
ChiCTR‐TRC‐09000350 {published data only}
- ChiCTR‐TRC‐09000350. A clinical study of antisterone with angiotensin II and calcium ion antagonist in the treatment of hypertension with adrenal hyperplasia. www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR‐TRC‐09000350 (first received 19 March 2009).
Deuss 1985 {published data only}
- Deuss U, Allolio B, Kaulen D, Fischer H, Winkelmann W. Effects of high‐dose and low‐dose naloxone on plasma ACTH in patients with ACTH hypersecretion. Clinical Endocrinology 1985;22(3):273‐9. [CENTRAL: 37326; CRS: 5500050000000556; PUBMED: 2983909] [DOI] [PubMed] [Google Scholar]
Johannsson 2009 {published data only}
- Johannsson G, Bergthorsdottir R, Nilsson AG, Lennernas H, Hedner T, Skrtic S. Improving glucocorticoid replacement therapy using a novel modified‐release hydrocortisone tablet: A pharmacokinetic study. European Journal of Endocrinology 2009;161(1):119‐30. [DOI] [PubMed] [Google Scholar]
Kelch 1975 {published data only}
- Kelch RP, Spencer ML, Bacon GE. Effects of glucocorticoid therapy on growth hormone (HGH), gonadotropin (FSH and LH), and 17 OH progesterone (17OH P) secretion in congenital adrenal hyperplasia (CAH). Pediatric Research 1975;9(4):291. [Abstract no.: 207; CENTRAL: 194355; CRS: 5500050000000558; EMBASE: 1976067399] [Google Scholar]
Koenen 2009 {published data only}
- Koenen TB, Tack CJ, Kroese JM, Hermus AR, Sweep FC, Laak J, et al. Pioglitazone treatment enlarges subcutaneous adipocytes in insulin‐resistant patients. Journal of Clinical Endocrinology and Metabolism 2009;94(11):4453‐7. [CENTRAL: 730144; CRS: 5500135000001832; PUBMED: 19820024] [DOI] [PubMed] [Google Scholar]
- Kroese JM, Mooij CF, Graaf M, Hermus AR, Tack CJ. Pioglitazone improves insulin resistance and decreases blood pressure in adult patients with congenital adrenal hyperplasia. European Journal of Endocrinology 2009;161(6):887‐94. [CENTRAL: 732215; CRS: 5500135000001831; PUBMED: 19755409] [DOI] [PubMed] [Google Scholar]
- NCT00151710. Effects of pioglitazone in congenital adrenal hyperplasia. clinicaltrials.gov/show/NCT00151710 (first received 09 September 2005).
Laue 1996 {published data only}
- Laue L, Merke DP, Jones JV, Barnes KM, Hill S, Cutler GB. A preliminary study of flutamide, testolactone, and reduced hydrocortisone dose in the treatment of congenital adrenal hyperplasia. Journal of Clinical Endocrinology and Metabolism 1996;81(10):3535‐9. [CENTRAL: 131394; CRS: 5500135000001827; PUBMED: 8855797] [DOI] [PubMed] [Google Scholar]
Mallappa 2015 {published data only}
- Mallappa A, Sinaii N, Kumar P, Whitaker MJ, Daley L, Digweed D, et al. A Phase 2 study of chronocort, a modified‐release formulation of hydrocortisone, in the treatment of adults with classic congenital adrenal hyperplasia. Journal of Clinical Endocrinology and Metabolism 2015;100(3):1137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathews 2004 {published data only}
- Mathews GA, Fane BA, Pasterski VL, Conway GS, Brook C, Hines M. Androgenic influences on neural asymmetry: Handedness and language lateralization in individuals with congenital adrenal hyperplasia. Psychoneuroendocrinology 2004;29(6):810‐22. [CRS: 5500135000001836; PUBMED: 15110930] [DOI] [PubMed] [Google Scholar]
Merke 2000 {published data only}
- Merke DP, Keil MF, Jones JV, Fields J, Hill S, Cutler GB. Flutamide, testolactone, and reduced hydrocortisone dose maintain normal growth velocity and bone maturation despite elevated androgen levels in children with congenital adrenal hyperplasia. Journal of Clinical Endocrinology and Metabolism 2000;85(3):1114‐20. [CENTRAL: 276265; CRS: 5500050000000561; PUBMED: 10720048] [DOI] [PubMed] [Google Scholar]
Merza 2006 {published data only}
- Merza Z, Rostami‐Hodjegan A, Memmott A, Doane A, Ibbotson V, Newell‐Price J, et al. Circadian hydrocortisone infusions in patients with adrenal insufficiency and congenital adrenal hyperplasia. Clinical Endocrinology 2006;65(1):45–50. [DOI] [PubMed] [Google Scholar]
NCT00001521 {published data only}
- NCT00001521. Three drug combination therapy versus conventional treatment of children with congenital adrenal hyperplasia. clinicaltrials.gov/show/NCT00001521 (first received 04 November 1999).
Neumann 2018 {published data only}
- Neumann U, Whitaker MJ, Wiegand S, Krude H, Porter J, Davies M, et al. Absorption and tolerability of taste‐masked hydrocortisone granules in neonates, infants and children under 6 years of age with adrenal insufficiency. Clinical Endocrinology 2018;88(1):21‐9. [DOI] [PubMed] [Google Scholar]
Panamonta 2003 {published data only}
- Panamonta O, Thinkhamrop B, Kirdpon W, Pudtawaro LO, Sungsahachart D. Adrenocorticotropin stimulation test in congenital adrenal hyperplasia: comparison between standard and low dose test. Chotmaihet Thangphaet [Journal of the Medical Association of Thailand] 2003;86(7):634‐40. [CENTRAL: 559097; CRS: 5500050000000554; EMBASE: 2003380852; PUBMED: 12948258] [PubMed] [Google Scholar]
PRENATAL DEX {published data only}
- EUCTR2015‐003996‐32‐FR. Multicentric evaluation of in utero dexamethasone (DEX) on the cognitive development of children at risk of congenital adrenal hyperplasia ‐ PRENATAL DEX Study. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015‐003996‐32‐FR (first received 04 December 2015).
Sarafoglou 2015 {published data only}
- Sarafoglou K, Gonzalez‐Bolanos MT, Zimmerman CL, Boonstra T, Yaw Addo O, Brundage R. Comparison of cortisol exposures and pharmacodynamic adrenal steroid responses to hydrocortisone suspension vs. commercial tablets. Journal of Clinical Pharmacology 2015;55(4):452‐7. [CENTRAL: 1077775; CRS: 5500050000000560; EMBASE: 2015818168] [DOI] [PubMed] [Google Scholar]
Spritzer 1990 {published data only}
- Spritzer P, Billaud L, Thalabard JC, Birman P, Mowszowicz I, Raux‐Demay MC, et al. Cyproterone acetate versus hydrocortisone treatment in late‐onset adrenal hyperplasia. Journal of Clinical Endocrinology and Metabolism 1990;70(3):642‐6. [CENTRAL: 65998; CRS: 5500050000000557; PUBMED: 2137832] [DOI] [PubMed] [Google Scholar]
Turcu 2016 {published data only}
- Auchus RJ, Turcu AF, Spencer‐Segal JL, Farber RH, Luo R, Grigoriadis DE, et al. A pharmacokinetic and biomarker study of the corticotropin‐releasing factor receptor antagonist nbi‐77860 in adult females with classic, 21‐hydroxylase deficiency, congenital adrenal hyperplasia (CAH). Endocrine Reviews 2015;36(2 Suppl 1). [Google Scholar]
- Turcu AF, Spencer‐Segal JL, Farber RH, Luo R, Grigoriadis DE, Ramm CA, et al. Single‐dose study of a corticotropin‐releasing factor receptor‐1 antagonist in women with 21‐hydroxylase deficiency. Journal of Clinical Endocrinology and Metabolism 2016;101(3):1174‐80. [CENTRAL: 1168468; CRS: 5500135000001833; PUBMED: 26751191] [DOI] [PMC free article] [PubMed] [Google Scholar]
Verma 2010 {published data only}
- Verma S, Vanryzin C, Sinaii N, Kim MS, Nieman LK, Ravindran S, et al. A pharmacokinetic and pharmacodynamic study of delayed‐ and extended‐release hydrocortisone (Chronocort TM) versus conventional hydrocortisone (Cortef TM) in the treatment of congenital adrenal hyperplasia. Clinical Endocrinology 2010;72(4):441‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weise 2004 {published data only}
- Weise M, Drinkard B, Mehlinger SL, Holzer SM, Eisenhofer G, Charmandari E, et al. Stress dose of hydrocortisone is not beneficial in patients with classic congenital adrenal hyperplasia undergoing short‐term, high‐intensity exercise. Journal of Clinical Endocrinology and Metabolism 2004;89(8):3679‐84. [CENTRAL: 481372; CRS: 5500135000001829; PUBMED: 15292287] [DOI] [PubMed] [Google Scholar]
Zhang 2011 {published data only}
- Zhang J, Xu X, Chen Y, Han L, Liu H. Hypertension with adrenal hyperplasia of the different treatments. International Journal of Cardiology 2011;152:S21‐2. [CENTRAL: 1020865; CRS: 5500050000000563; EMBASE: 70573948] [Google Scholar]
References to studies awaiting assessment
Salud 2013 {published data only}
- Salud JLMF, Ramos‐Abad L. A pilot study on the effect of three hydrocortisone dosing regimen on 17 hydroxyprogesterone levels in patients with congenital adrenal hyperplasia. International Journal of Pediatric Endocrinology 2013;Suppl 1:P123. [Google Scholar]
Silva 1994 {published data only}
- Silva IN, Oliveira‐Junior DF, Simal CJ, Viana MB, Chagas AJ. Morning steroid profile in children with congenital adrenal hyperplasia under different hydrocortisone schedules. Indian Journal of Pediatrics 1994;61(4):341‐6. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
CareOnTIME {published data only}
- NCT03760835. Congenital Adrenal Hyperplasia Once Daily Hydrocortisone Treatment (CareOnTIME). clinicaltrials.gov/show/nct03760835 (first received 30 November 2018).
COCA {published data only}
- NCT02552251. COrticosteroid in congenital adrenal hyperplasia (COCA). clinicaltrials.gov/show/NCT02552251 (first received 17 September 2015).
NCT01771328 {published data only}
- EUCTR2011‐005822‐23‐NO. A trial comparing continuous subcutaneous hydrocortisone therapy with conventional oral glucocorticoid therapy in congenital adrenal hyperplasia. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2011‐005822‐23‐NO (first received 25 February 2013).
- NCT01771328. Continuous subcutaneous hydrocortisone infusion in congenital adrenal hyperplasia. clinicaltrials.gov/show/nct01771328 (first received 18 January 2013).
NCT02716818 {published data only}
- EUCTR2015‐000711‐40‐NL. Chronocort®, a slow release medicinal preparation of hydrocortisone, will be compared with currently used glucocorticoid replacement therapy in the treatment of congenital adrenal hyperplasia seeking to assess its safety, tolerability and effectiveness. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015‐000711‐40‐NL (first received 17 November 2015).
- NCT02716818. Comparison of chronocort® with standard glucocorticoid therapy in patients with congenital adrenal hyperplasia. clinicaltrials.gov/show/NCT02716818 (first received 23 March 2016).
PULSES {published data only}
- EUCTR2012‐001104‐37‐GB. Pulses study [Pulsed glucocorticoid replacement therapy for patients with adrenocortical insufficiency secondary to Addison’s disease and congenital adrenal hyperplasia ‐ the pulses study]. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2012‐001104‐37‐GB (first received 24 July 2014).
- ISRCTN67193733. The Pulses Study. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN67193733 (first received 03 December 2014).
RESTORE {published data only}
- NCT03532022. Open‐label Comparison of Chronocort® Versus Standard Glucocorticoid Replacement Therapy (RESTORE). clinicaltrials.gov/show/nct03532022 (first received 22 May 2018).
Additional references
Cohen 1960
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20(1):37–46. [Google Scholar]
Debono 2009
- Debono M, Price JN, Ross RJ. Novel strategies for hydrocortisone replacement. Best Practice & Research. Clinical Endocrinology & Metabolism 2009;23(2):221–32. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analysis. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Fuqua 2010
- Fuqua JS, Rotenstein D, Lee PA. Duration of suppression of adrenal steroids after glucocorticoid administration. International Journal of Pediatric Endocrinology 2010;2010:712549. [DOI: 10.1155/2010/712549] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hindmarsh 2009
- Hindmarsh PC. Management of the child with congenital adrenal hyperplasia. Best Practice & Research. Clinical Endocrinology & Metabolism 2009;23(2):193–208. [DOI] [PubMed] [Google Scholar]
Hindmarsh 2014
- Hindmarsh PC. Mimicking the circadian rhythm in glucocorticoid replacement. BMJ 2014;349:g5518. [DOI: 10.1136/bmj.g5518] [DOI] [PubMed] [Google Scholar]
Khalid 2012
- Khalid JV, Oerton JM, Dezateux C, Hindmarsh PC, Kelnar CJ, Knowles RL. Incidence and clinical features of congenital adrenal hyperplasia in Great Britain. Archives of Disease in Childhood 2012;97(2):101–6. [DOI] [PubMed] [Google Scholar]
Lai 2000
- Lai HC, FitzSimmons SC, Allen DB, Kosorok MR, Rosenstein BJ, Campbell PW, Farrell PM. Risk of persistent growth impairment after alternate‐day prednisone treatment in children with cystic fibrosis.. N Engl J Med. 23 March 2000;342(12):851‐9. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
LWPES/ESPE 2002
- Joint LWPES/ESPE CAH Working Group. Consensus statement on 21‐hydroxylase deficiency from The Lawson Wilkins Pediatric Endocrine Society and The European Society for Pediatric Endocrinology. Journal of Clinical Endocrinology and Metabolism 2002;87(9):4048‐53. [DOI] [PubMed] [Google Scholar]
Marumudi 2013
- Marumudi E, Khadgawat R, Surana V, Shabir I, Joseph A, Ammini AC. Diagnosis and management of classical congenital adrenal hyperplasia. Steroids 2013;78(8):741‐6. [DOI] [PubMed] [Google Scholar]
Riepe 2002
- Riepe FG, Krone N, Viemann M, Partsch CJ, Sippell WG. Management of congenital adrenal hyperplasia: results of the ESPE questionnaire. Hormone Research 2002;58(4):196‐205. [DOI] [PubMed] [Google Scholar]
Riepe 2007
- Riepe FG, Sippell WG. Recent advances in diagnosis, treatment, and outcome of congenital adrenal hyperplasia due to 21‐hydroxylase deficiency. Reviews in Endocrine and Metabolic Disorders 2007;8(4):349–63. [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH on behalf of the Cochrane Applicability and Recommendations Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Presenting results and ‘Summary of findings’ tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al on behalf of the Cochrane Applicability and Recommendations Methods Group and the Cochrane Statistical Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Speiser 2018
- Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, et al. Congenital adrenal hyperplasia due to steroid 21‐hydroxylase deficiency: an endocrine society clinical practice guideline. Journal of Clinical Endocrinology Metabolism 2018;103(11):4043‐88. [doi: 10.1210/jc.2018‐01865; PUBMED: 30272171] [DOI] [PMC free article] [PubMed] [Google Scholar]
Subbarayan 2014
- Subbarayan A, Dattani MT, Peters CJ, Hindmarsh PC. Cardiovascular risk factors in children and adolescents with congenital adrenal hyperplasia due to 21‐hydroxylase deficiency. Clinical Endocrinology 2014;80(4):471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Staa 2002
- Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid‐induced osteoporosis: a meta‐analysis.. Osteoporos Int. Oct 2002;13(10):777‐87. [DOI] [PubMed] [Google Scholar]


