Abstract
Differentiation of true tumor progression from treatment-related effects remains a major unmet need in caring for patients with glioblastoma. Here, we report how the intraoperative combination of MRI with18F-fluciclovine PET guided surgical sampling in 2 patients with recurrent glioblastoma.18F-Fluciclovine PET is FDA approved for use in prostate cancer and carries an orphan drug designation in glioma. To investigate its utility in recurrent glioblastoma, we fused PET and MRI images using 2 different surgical navigation systems and performed targeted stereotactic biopsies from the areas of high (“hot”) and low (“cold”) radiotracer uptake. Concordant histopathologic and imaging findings suggest that a combined18F-fluciclovine PET-MRI–guided approach can guide neurosurgical resection of viable recurrent glioblastoma in the background of treatment-related effects, which can otherwise look similar on MRI.
Keywords: 18F-fluciclovine, glioblastoma, positron emission tomography (PET), pseudoprogression
Glioblastoma (GB) invariably recurs, but surveillance with MRI remains problematic for the discrimination of tumor recurrence from treatment-related effects, that is, pseudoprogression or radionecrosis. Because chemoradiation treatment alone induces radiographic changes in approximately half of treated patients,1 radiographic progression without actual underlying disease progression is recognized as a major clinical dilemma in caring for patients with GB.2 Although pathological confirmation is the most reliable method to differentiate progressive disease from treatment effect, it is an invasive method; therefore, numerous noninvasive methods have been attempted to discriminate the 2 distinct entities, including diffusion-weighted imaging, various perfusion imaging techniques, and PET.3
To date, most clinicians rely on advanced MRI techniques such as dynamic susceptibility contrast (DSC) and dynamic contrast-enhanced MRI perfusion techniques to tease out a distinction between recurrent tumor vs treatment effect; however, there is considerable variability between institutions in optimal thresholds and a lack of standardization that limits the application of these techniques.4 As such, it is difficult to identify patients who can benefit from additional surgery, as in cases of disease recurrence, vs patients who are experiencing treatment efficacy, particularly in an era of multiple pharmacological options (ie, temozolomide, bevacizumab, targeted therapy, and promising immunotherapy trials).518F-Fluciclovine (Axumin®; Blue Earth Diagnostics), a synthetic amino acid PET tracer, has been used to identify cancer spread in prostate and breast cancer6,7 but its utility in GB is an area of active research. Its low uptake in normal brain, selective tumor uptake, and high lesion-to-background contrast profile (vs other commonly used tracers) make 18F-fluciclovine an attractive agent.8,9 We therefore investigated this tracer’s use and report 2 cases of patients undergoing surgery for recurrent GB that demonstrate the feasibility of 18F-fluciclovine PET plus MRI imaging to guide surgical sampling.
Case Presentation: Patient A
A 64-year-old woman with known GB (isocitrate dehydrogenase [IDH] wild type, O6-methylguanine-DNA methyltransferase [MGMT] promoter unmethylated) was referred to Neurosurgery when interval changes on MRI raised concern for possible disease progression vs treatment effect. Sixteen months previously, at the time of the initial diagnosis, she underwent craniotomy with gross total resection and made an excellent recovery. She enrolled at the University of Pennsylvania in the Adult Brain Tumor Consortium 1202 clinical trial, receiving adjuvant radiation (60 Gy in 30 fractions) with concurrent temozolomide and MK1775 (WEE1 inhibitor). Following radiation, she was treated with 2 monthly cycles of temozolomide and MK 1775, but was withdrawn from the study because of intolerance to the latter drug. She was thereafter maintained on monthly temozolomide cycles. The surveillance MRI at 16 months revealed increased thickening and nodularity of enhancement at the cavity margins and a new small satellite enhancing focus anteriorly (Fig. 1). The DSC perfusion MRI was degraded by susceptibility artifact secondary to blood products and therefore was nondiagnostic; however, given the progressive enhancement and possible neoplastic progression, our interdisciplinary tumor board recommended surgical resection. The patient met the criteria for the18F-fluciclovine PET study under way at the University of Pennsylvania (18F-Fluciclovine in Post-treatment Glioblastoma, NCT03990285). Five days prior to surgery, the patient underwent 60 minutes of dynamic brain PET-CT imaging after intravenous administration of 18F-fluciclovine (5.16 mCi, 191 MBq). Subsequently PET-CT images were reconstructed, and summed 30- to 40-minute postinjection PET images were fused with standard MRI images via surgical planning software (Brainlab).18F-fluciclovine uptake was quantified using the standardized uptake value (SUV) normalized to body weight (Table 1). Two areas of highest radiotracer uptake were identified preoperatively and sampled intraoperatively using stereotactic navigation during a left parietal craniotomy (Fig. 1). After surgery the patient awoke neurologically intact. Postoperative MRI confirmed a gross total resection of the contrast-enhancing tumor and nodules. Histological examination revealed that the 2 target nodules from the areas of increased radiotracer uptake were positive for recurrent glioma. Another specimen from the contrast-enhancing component but with low radiotracer uptake showed only atypical glial cells, consistent with treatment effect (Fig. 2). A neuropathologist assessed the amount of tumor semiquantitatively by estimating the density of tumor in each tissue specimen. In addition to describing the density of the neoplastic cells, the density estimation accounting for treatment-related changes such as macrophage infiltrates and vascular changes was also recorded. To reach a final tumor percentage, the density estimation was subsequently weighted by the percentage of each tissue specimen that consisted of tumor vs geographic necrosis, predominant reactive changes, or both (Table 1), similar to a method previously described.10
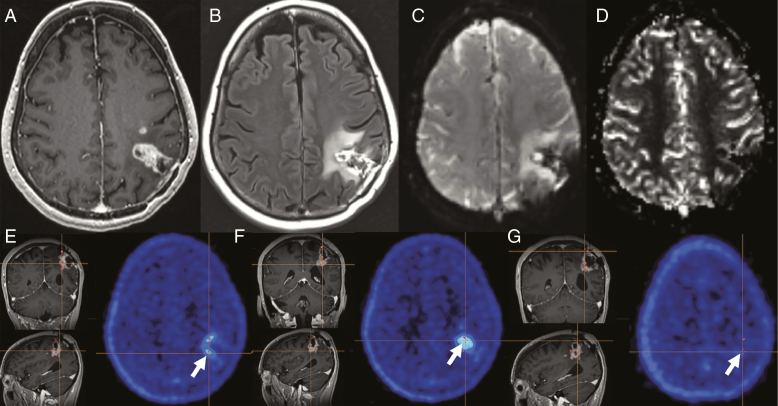
Fig. 1.
Case A. At 16 months after initial resection, A, T1 postcontrast MRI shows gradual progression of nodular enhancement around the surgical cavity with a satellite nodule anteriorly, and surrounding hyperintensity on B, fluid-attenuated inversion recovery. C, Source perfusion images demonstrate marked susceptibility artifact due to blood products at the resection site, making the D relative cerebral blood volume perfusion map nondiagnostic. E and F, Two 18F-fluciclovine PET–avid sites (biopsy 1 and biopsy 2) and G, 1 non–PET-avid site (biopsy 3), were sampled (white arrows). Intraoperative navigation was used to stereotactically identify the sites after registration of the images to the navigation software. E, F, and G, Orange navigation cross-hairs are faintly visible.
Table 1.
PET Intensity and Pathology Findings
| Specimen | SUVmean | SUVmax | Pathology Findings and Diagnosis |
|---|---|---|---|
| Patient A, mean brain SUVa (background) = 0.31 | Overall 10% tumor Final diagnosis: predominantly treatment-related changes with foci of recurrent/residual glioblastoma, IDH wild type | ||
| Biopsy 1 | 2.36 | 3.23 | 25% tumor |
| Biopsy 2 | 2.52 | 3.83 | 60% tumor |
| Biopsy 3 | 0.82 | 1.14 | 1% tumor |
| Patient B, mean brain SUV (background) = 0.60 | Overall 65% tumor Final diagnosis: recurrent/residual glioblastoma, IDH wild type, with treatment-related changes | ||
| Biopsy 1 | 3.71 | 6.09 | 50% tumor |
| Biopsy 2 | 4.05 | 5.19 | 70% tumor |
| Biopsy 3 | 3.40 | 4.07 | 30% tumor |
Abbreviations: IDH, isocitrate dehydrogenase; SUVmax, maximum standardized uptake value; SUVmean, mean standardized uptake value.
aSUV measurements are from 30- to 40-minute average images after PET radiotracer injection.
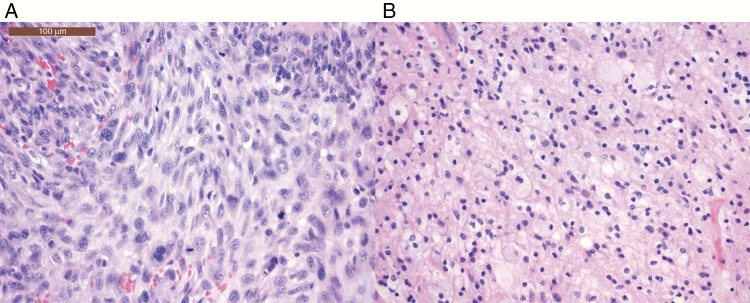
Fig. 2.
Case A. Images stained with hematoxylin and eosin compare A, biopsy 2, a “hot” PET-avid site, vs B, biopsy 3, a “cold” site, contrasting predominant findings of A, tumor recurrence vs B, treatment effect. Malignant cells with hyperchromatic chromatin and abundant mitotic activity are noted in A, whereas a high macrophage population is seen in B. Images taken at 20× objective (with a 10× eye piece, total magnification 200×). The scale bar in Figure 2A is 100 μm.
Case Presentation: Patient B
A 60-year-old woman with GB (IDH wild type, MGMT promoter unmethylated) was referred for neurosurgical opinion with concern for disease progression. Forty-six months previously she underwent a craniotomy followed by standard chemoradiation (Stupp regimen) and 1 year of adjuvant temozolomide. She presented to the Hospital of the University of Pennsylvania 44 months after initial diagnosis with acute right leg weakness. The MRI demonstrated 2 new areas of nodular enhancement along the posterior aspect of the resection cavity, with another satellite focus of enhancement posterior to the resection cavity (Fig. 3) Followed initially with active surveillance, an MRI 2 months later demonstrated interval-increased enhancement along margins of the left parietal resection cavity with marked elevation (6 times contralateral white matter) of relative cerebral blood volume (rCBV) on DSC perfusion imaging. This was concerning for GB recurrence, and surgery was recommended. Four days prior to surgery, the patient underwent 60 minutes of dynamic brain PET-CT imaging after intravenous administration of 4.38 mCi (162 MBq) of 18F-fluciclovine. Subsequently PET-CT images were reconstructed and the summed 30- to 40-minute postinjection images were merged with standard MRI images using surgical planning software (StealthStation, Medtronic). Areas of highest radiotracer uptake were identified in a way similar to that of the first patient and were sampled intraoperatively during a left parietal craniotomy (Fig. 3). After a near-total resection of enhancing tissue, the patient awoke neurologically intact. Pathologic assessment revealed dense areas of recurrence with some infiltration and with signs of treatment (Table 1).
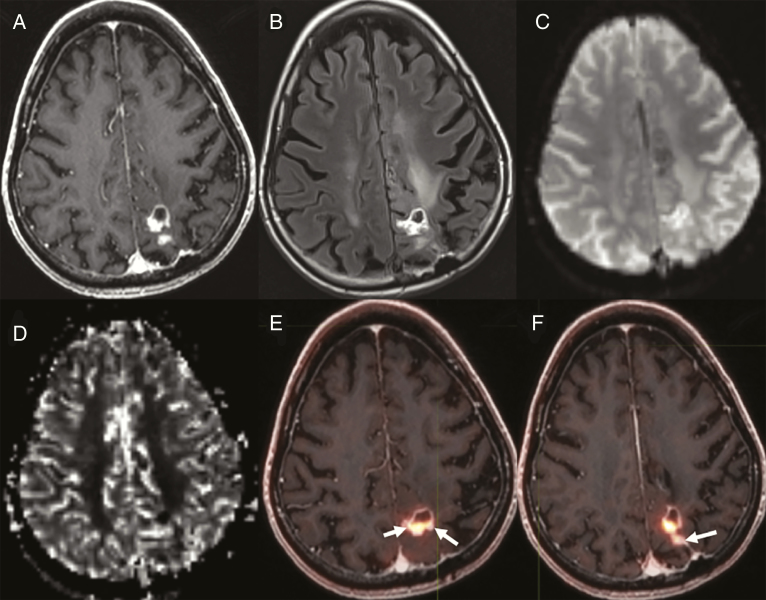
Fig. 3.
Case B. Nodular enhancement posterior to the resection cavity in left parietal lobe on A, T1 postcontrast, and with adjacent hyperintensity on B, fluid-attenuated inversion recovery images. C, Source perfusion image and relative cerebral blood volume (rCBV) map demonstrate D, elevated rCBV corresponding to the nodular and rim enhancement at A, left parietal surgical cavity. E and F, Three 18F-fluciclovine PET-avid biopsy sites are shown (E, small arrow, biopsy 1; larger arrow, biopsy 2; F, arrow, biopsy 3).
Ethical Standards
The patients provided informed consent to participate in “Multimodality 18F-fluciclovine PET, MRI and cell free circulating DNA to differentiate tumor progression from pseudoprogression in patients with glioblastoma” (IRB #832812, NCT03990285) approved by the University of Pennsylvania Institutional Review Board.
Discussion
In our 2 posttreatment GB patients,18F-fluciclovine PET–guided biopsy was feasible and correlated with histopathologic findings of tumor recurrence vs treatment effect. Both patients demonstrated high radiotracer uptake in the enhancing tissue compared to the normal brain background uptake. In both patients,18F-fluciclovine PET–targeted biopsies identified foci that showed high tumor content; in fact, the tumor content of at least 1 of the PET-avid foci exceeded the overall tumor percentage of the entire resection volume. In a discordant site, with relatively low18F-fluciclovine but with contrast enhancement, only minimal tumor was present on biopsy, underscoring the benefit of this molecular-based approach. This level of correlation between imaging and histopathological findings was not available with either standard gadolinium-based conventional MRI imaging or perfusion MRI.
Furthermore, in patient A, whose cavity revealed predominantly treatment effect (90%) and only 10% tumor in the total resected tissue, 1 of the PET-guided biopsies demonstrated approximately 60% tumor. GB dynamically evades treatment in part through clonal evolution.11 Therefore, it may be advantageous to selectively target the most active areas of neoplasia for histopathologic analysis to refine therapy. A sensitive, specific, clinically relevant radiological marker would identify and target foci of tumor recurrence in the background of treatment effect. These cases support the further investigation of 18F-fluciclovine as such a potential marker; a previous report of 18F-fluciclovine–guided biopsy in a patient with treatment-naive oligodendroglioma correlated radiotracer uptake with tumor grade, cell density, and Ki67 labeling index.12
Multiparametric MRI including postcontrast imaging is the most widely used single modality in surveillance of GB but its specificity to discriminate treatment-related changes from recurrent disease is imperfect, especially because patients frequently harbor a mixture of tumor progression and treatment effect, both of which may disrupt the blood-brain barrier to allow contrast extravasation.10 In addition, chronic blood products that cause susceptibility artifact and interfere with advanced MRI techniques such as DSC perfusion imaging frequently confound studies in posttreatment GB patients. However, the PET tracer approach described here enables analysis of biological activity beyond the disruption of the blood-brain barrier because it targets the specific use of nutrients via upregulation of amino acid transporters in the neoplastic cells.13,14
Because cell division and growth are hallmarks of neoplasia, amino acids, as integral substrates of protein and nucleic acid synthesis, are potential markers of neoplastic progression.18F-Fluciclovine was first evaluated in human clinical studies for patients with glioma, based on preclinical studies of gliosarcoma.15 The safety of 18F-fluciclovine PET in humans has been demonstrated in large urologic studies to detect biochemical recurrence of prostate cancer. This procedure exposes a patient to minimal radiation, and very limited toxicity was noted in a multicenter study of 596 patients. In these tumors,18F-fluciclovine is predominantly transported via 2 amino acid transporters, alanine-serine-cysteine transporter and L-amino acid transporter, with peak uptake 5 to 20 minutes after injection. Gliomas show upregulation of these same transporters, especially linked to transport of glutamine and leucine across cell membranes.16 The tracer has been approved by the FDA since 2016 for prostate cancer but also carries orphan designation for the diagnosis of glioma since April 2015, although it has not been approved for this use.17
Recently Bogsrud et al published a retrospective series of 21 patients with suspected recurrent high-grade glioma who received18F-fluciclovine PET imaging with no adverse tracer effects. Average SUVmax of the lesions was 8.3 ± 5.3, average SUVmean for normal brain background uptake was 0.34 ± 0.13, and therefore the median lesion-to-background ratio was 21.6 (range, 3.1-84.4). Of the 10 patients from whom tissue was obtained, all sites of 18F-fluciclovine signal correlated with disease recurrence and there was no case of definite treatment-related changes. Bogsrud and colleagues did, however, detect 4 cases in which a small (2-4 mm) satellite lesion was evident on PET that was absent on postcontrast MRI, suggesting a higher sensitivity of 18F-fluciclovine PET over contrast-enhanced MRI.8 Michaud and colleagues recently reported the evaluation of 18F-fluciclovine PET, MRI, and 11C-methionine in 27 patients with recurrent or progressive primary brain tumors. They found higher image contrast (and lower background uptake) with 18F-fluciclovine9 than with 11C-methionine; however, their series lacked a patient with confirmed treatment effect because all the patients were ultimately diagnosed with tumor recurrence. Our study is the first, to our knowledge, to use18F-fluciclovine PET to differentiate recurrent tumor from treatment effect in GB.
Small sample size limits our report, which should therefore be viewed as a preliminary feasibility study. A large prospective study is under way at our institution that will allow us to determine the predictive PET uptake thresholds to differentiate tumor recurrence from treatment effect.
Conclusion
We demonstrated the feasibility of 18F-fluciclovine PET-MRI–guided biopsy in posttreatment GB to distinguish areas of highest tumor recurrence from areas of predominant treatment effect. Our results in the 2 cases presented here support the continuing exploration of 18F-fluciclovine as a safe, potentially sensitive, clinically relevant biomarker of recurrent GB.9
Funding
This work was supported in part by Blue Earth Diagnostics [grant number Study BED-IIT-385].
Acknowledgments
Special thanks to Timothy Prior, Jessica Harsch, and Eileen Maloney-Wilensky of the Neurosurgery Clinical Research Division; Erin Schubert, Regan Sheffer, and Janet Reddin of the Nuclear Radiology Division; and Leeanne Lezotte, Sabrina Williams, and Lisa Desiderio of the Neuroradiology Research Core at the University of Pennsylvania.
Conflict of interest statement. Dr Nabavizadeh is a member of the Blue Earth Virtual Imaging Advisory Council. The other authors declare no financial or other conflicts of interest.
References
- 1. Fink J, Born D, Chamberlain MC. Pseudoprogression: relevance with respect to treatment of high-grade gliomas. Curr Treat Options Oncol. 2011;12(3):240–252. [DOI] [PubMed] [Google Scholar]
- 2. Rowe LS, Butman JA, Mackey M, et al. . Differentiating pseudoprogression from true progression: analysis of radiographic, biologic, and clinical clues in GBM. J Neurooncol. 2018;139(1):145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jang BS, Jeon SH, Kim IH, Kim IA.. Prediction of pseudoprogression versus progression using machine learning algorithm in glioblastoma. Sci Rep. 2018;8(1):12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patel P, Baradaran H, Delgado D, et al. . MR perfusion-weighted imaging in the evaluation of high-grade gliomas after treatment: a systematic review and meta-analysis. Neuro Oncol. 2017;19(1):118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okada H, Weller M, Huang R, et al. . Immunotherapy response assessment in neuro-oncology: a report of the RANO Working Group. Lancet Oncol. 2015;16(15):e534–e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bach-Gansmo T, Nanni C, Nieh PT, et al. . Multisite experience of the safety, detection rate and diagnostic performance of fluciclovine (18F) positron emission tomography/computerized tomography imaging in the staging of biochemically recurrent prostate cancer. J Urol. 2017;197(3 pt 1):676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ulaner GA, Goldman DA, Gönen M, et al. . Initial results of a prospective clinical trial of 18F-fluciclovine PET/CT in newly diagnosed invasive ductal and invasive lobular breast cancers. J Nucl Med. 2016;57(9):1350–1356. [DOI] [PubMed] [Google Scholar]
- 8. Bogsrud TV, Londalen A, Brandal P, et al. . 18F-fluciclovine PET/CT in suspected residual or recurrent high-grade glioma. Clin Nucl Med. 2019;44(8):605–611. [DOI] [PubMed] [Google Scholar]
- 9. Michaud L, Beattie BJ, Akhurst T, et al. . 18F-Fluciclovine (18F-FACBC) PET imaging of recurrent brain tumors [published online ahead of print August 15, 2019]. Eur J Nucl Med Mol Imaging. doi:10.1007/s00259-019-04433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bagley SJ, Schwab RD, Nelson E, et al. . Histopathologic quantification of viable tumor versus treatment effect in surgically resected recurrent glioblastoma. J Neurooncol. 2019;141(2):421–429. [DOI] [PubMed] [Google Scholar]
- 11. Osuka S, Van Meir EG. Overcoming therapeutic resistance in glioblastoma: the way forward. J Clin Invest. 2017;127(2):415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karlberg A, Berntsen EM, Johansen H, et al. . Multimodal 18F-fluciclovine PET/MRI and ultrasound-guided neurosurgery of an anaplastic oligodendroglioma. World Neurosurg. 2017; 108:989.e1–989.e8. [DOI] [PubMed] [Google Scholar]
- 13. Galldiks N, Dunkl V, Stoffels G, et al. . Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-L-tyrosine PET. Eur J Nucl Med Mol Imaging. 2015;42(5):685–695. [DOI] [PubMed] [Google Scholar]
- 14. Suchorska B, Jansen NL, Linn J, et al. ; German Glioma Network Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology. 2015;84(7):710–719. [DOI] [PubMed] [Google Scholar]
- 15. Shoup TM, Olson J, Hoffman JM, et al. . Synthesis and evaluation of [18F]1-amino-3-fluorocyclobutane-1-carboxylic acid to image brain tumors. J Nucl Med. 1999;40(2):331–338. [PubMed] [Google Scholar]
- 16. Savir-Baruch B, Zanoni L, Schuster DM. Imaging of prostate cancer using Fluciclovine. Urol Clin North Am. 2018;45(3):489–502. [DOI] [PubMed] [Google Scholar]
- 17. US Department of Health and Human Services. US Food and Drug Administration. Search Orphan Drug Designations and Approvals https://www.accessdata.fda.gov/scripts/opdlisting/oopd/. 2015. Accessed September 9, 2019.