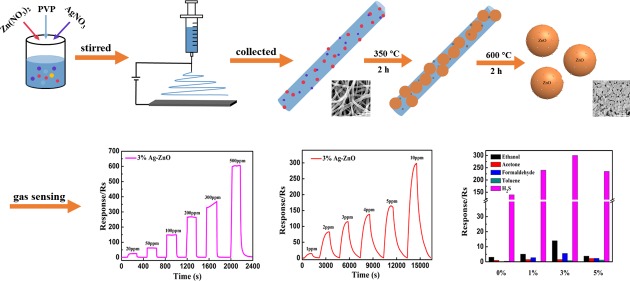
Abstract
Zinc oxide (ZnO) nanoparticles modified with uniformly dispersed silver (Ag) nanoparticles (Ag-ZnO) were prepared in one step by calcining precursor electrospun nanofibers. The molar ratios of Ag to Zn in the precursor solutions were 0, 1, 3, and 5%. The microstructure of the Ag-ZnO sensor was characterized by scanning electron microscopy and transmission electron microscopy. The existence of metallic Ag was confirmed by X-ray diffraction and X-ray photoelectron spectroscopy, and the gas sensing properties of Ag-ZnO were investigated. The results showed that the ZnO nanoparticles after Ag nanoparticles modification exhibited excellent gas sensing performance to ethanol and hydrogen sulfide (H2S). The optimal working temperature of the Ag-ZnO sensor significantly decreased for ethanol compared with pure ZnO. The 3% Ag-ZnO sensor exhibited the fastest response to ethanol with the response/recovery times of only 5 and 9 s, respectively. However, all the Ag-ZnO-based gas sensors showed a high response value to H2S, especially the 3% Ag-ZnO gas sensor exhibited a maximum response value of 298 at 10 ppm H2S. These results could be attributed to the spillover effect and electron sensitization effect of Ag nanoparticles, which led to more absorbed oxygen species and active sites, and thereby can further enhance the gas sensing performances of ZnO-based gas sensors.
1. Introduction
With the development of the quality of human life, people have put forward higher and higher requirements for a healthy living environment. The main source of pollution to the atmosphere is harmful gases, such as combustible and toxic gases.1−3 These harmful gases not only pollute the environment but also affect the health of humans in acute and chronic ways through respiratory and skin pollution.4,5 Some harmful gases may even lead to nerve paralysis, hypoxia, and even death.6−8 Therefore, the development of gas sensors for rapid and selective detection has aroused great interest in recent years.9−12 Among various gas sensors, semiconductor gas sensors such as tin oxide (SnO2), indium oxide (In2O3), and zinc oxide (ZnO) have attracted much more attention in the past few years due to their high reproducibility, thermal stability, and low cost.7,8,10,11
ZnO is an important multifunctional n-type semiconductor material with a wide direct band gap of approximately 3.37 eV, and it has been widely used as an active gas sensing material due to its fast response, short response/recovery time, excellent electrical performance, and long-term stability compared to other semiconductors.13−15 However, to further improve the gas sensing performance, ZnO has been modified with various noble metals such as Pt, Au, Ag, Pd, etc.16−19 The modification with Ag has often been used due to its low cost and simple preparation. Wang et al. prepared Ag-ZnO nanocomposites to achieve high selectivity to formaldehyde, and its response value increased from 4.67 to 170 under 100 ppm formaldehyde after Ag doping.20 Tai et al. prepared ZnO-Ag heterostructure nanoparticles and observed optimal light-assisted NO2 sensing performance with higher response and sensitivity.21 Algarni et al. reported the synthesis, characterization, and ethanol gas sensing application of Ag-doped ZnO nanoparticles, which showed a response of 32 for 200 ppm ethanol gas.22 Gao et al. fabricated Ag-doped ZnO nanoparticles, which showed good performance for CO gas sensing.23 Chung et al. reported the Ag nanoparticle-loaded ZnO-reduced graphene oxide hybrid, which exhibited an enhanced C2H2 sensing property with excellent repeatability and fast response/recovery times of 25/80 s.24 According to the above reported studies, it is crucial to prepare Ag nanoparticles, which can uniformly disperse on the surface of ZnO with the particle size smaller than 2Ld (Debye length) of ZnO (≈15 nm)25,26 that can accelerate the surface reactions and change the gas diffusivity assisted with the spillover effect of noble metals and further enhance the gas sensing performance.27 Therefore, it should be interesting to obtain uniformly dispersed Ag nanoparticles with much smaller size on the surface of ZnO. Many kinds of methods have been employed in recent years to prepare Ag-ZnO materials, such as coprecipitation, hydrothermal, solvothermal, electrospinning, and template method.28,29 Among these methods, electrospinning has been attracted more attention due to its simplicity, versatility, and low cost.28,29 Moreover, it can also allow uniform mixing of elements in the precursor solution and uniform distribution of elements (such as Ag) in the precursor fiber in advance compared with other methods of crystal growth.
In this study, the ZnO nanoparticles modified with uniformly dispersed Ag nanoparticles were prepared in one step by calcining precursor electrospun nanofibers (Figure 1a). The as-prepared four kinds of ZnO-based gas sensors were mainly studied for detecting ethanol and H2S gases. The results showed that the optimal working temperature of Ag-ZnO significantly decreased for ethanol compared with pure ZnO. Especially, the 3% Ag-ZnO sensor exhibited a rapid response to ethanol, and all the ZnO-based gas sensors showed a high response value to H2S. Finally, the mechanism of gas sensing was discussed in detail to get a deeper understanding of the importance of modification of Ag nanoparticles on the surface of ZnO nanoparticles.
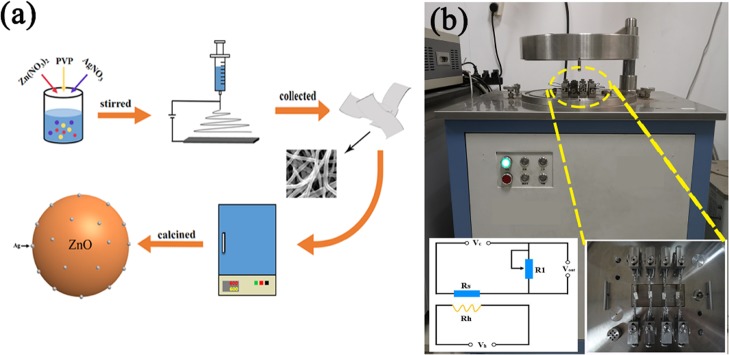
Figure 1.
(a) Schematic illustration of the preparation of Ag-ZnO nanoparticles and (b) actual photograph of CGS-4TPs gas sensing measurement system. (The inset is electric circuit diagram of sensor device.)
2. Results and Discussion
2.1. Material Characterization
The phase and crystal structure of the prepared pure ZnO nanoparticles and Ag-ZnO nanoparticles were characterized by XRD. As shown in Figure 2a, the XRD pattern of pure ZnO nanoparticles well matched with the standard JCPDS file card no. 36-1451 of the pure ZnO hexagonal wurtzite structure. No characteristic peaks of any other impurities were observed, indicating good phase purity of obtained ZnO nanoparticles. The XRD patterns of 1% Ag-ZnO, 3% Ag-ZnO, and 5% Ag-ZnO materials shown in Figure 2b–d revealed that all the additional three peaks indexed to JCPDS file card no. 65-2871 of Ag attributed to the lattice planes Ag(111), Ag(200), and Ag(220). The comparison among Figure 2a–d suggested that the formation of Ag in the calcination process had no influence on the crystal structure of ZnO.4 The diffraction peaks associated with Ag also increased gradually on increasing the modification amount of Ag.
Figure 2.
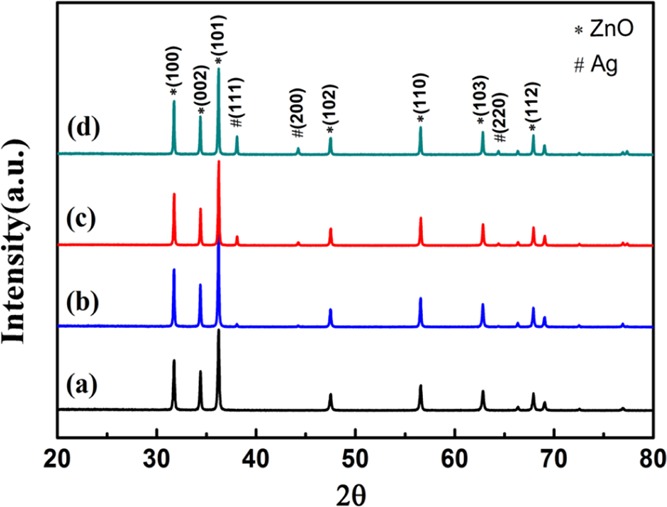
XRD patterns of (a) pure ZnO, (b) 1% Ag-ZnO, (c) 3% Ag-ZnO, and (d) 5% Ag-ZnO.
The micromorphology of pure ZnO and Ag-ZnO nanocomposites was observed by SEM. The precursor nanofibers of 3% Ag-ZnO with a diameter of 100 nm are shown in Figure 3a. Nanofibers no longer existed after calcination; rather, they changed into the nanoparticles of the hexagonal structure with a size of about 100 nm (Figure 3b–e). In addition, the size of the ZnO nanoparticles did not change after Ag modification; however, they were more uniform than the pure ZnO. Furthermore, the elemental composition of 3% Ag-ZnO was analyzed by the energy-dispersive spectrometer (EDS), which showed that only Zn, O, and Ag were present in the 3% Ag-ZnO (Figure 3f).
Figure 3.
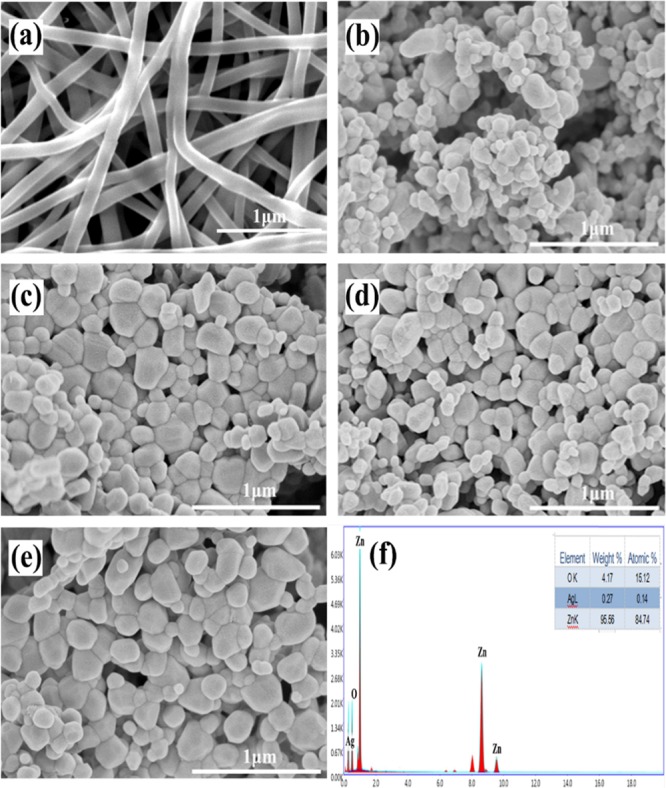
SEM images of precursor nanofibers of (a) 3% Ag-ZnO, (b) pure ZnO, (c) 1% Ag-ZnO, (d) 3% Ag-ZnO, and (e) 5% Ag-ZnO and (f) EDS spectrum of 3% Ag-ZnO (the inset is the amount of elements).
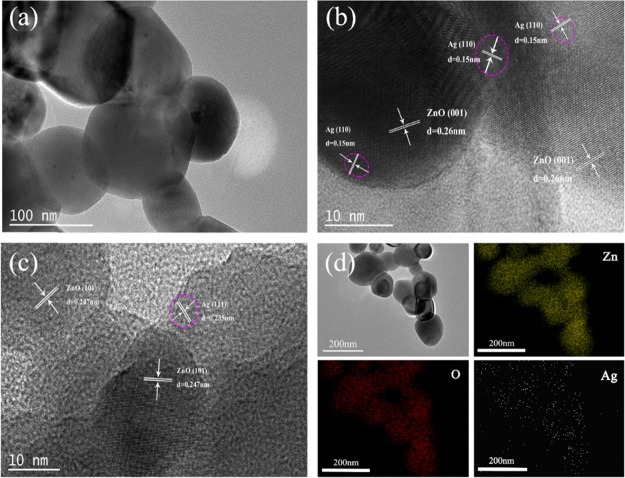
The nanostructure of 3% Ag-ZnO nanoparticles was characterized by TEM analysis. Figure 4 shows the typical TEM image of 3% Ag-ZnO nanoparticles, and it can be clearly seen that the size of as-prepared nanoparticles was around 100 nm after calcination and the size of Ag nanoparticles was found to be around 3–5 nm. HRTEM observation for the crystallinities of the ZnO and Ag is shown in Figure 4. The lattice fringe spacings of 0.247 and 0.26 nm correspond to the (101) and (001) planes, respectively, which were consistent with the slandered XRD pattern. The uniform distribution of Ag nanoparticles could be seen from the HRTEM images as confirmed by the lattice fringe spacings of 0.235 and 0.15 nm, which correspond to the (111) and (110) planes, respectively, which were in excellent agreement with the standard XRD pattern of Ag. Furthermore, the presence of Ag was also confirmed by the individual element mapping, which showed the presence of the uniformly distributed Ag element in the 3% Ag-ZnO nanoparticles along with Zn and O (Figure 4d). Moreover, the presence of three peaks in XRD at (111), (200), and (220) confirmed that the Ag was present in the metallic state. In addition, no characteristic peaks of any other impurities were observed except ZnO and Ag. The results of EDS also showed that the presence of Ag in the 3% Ag-ZnO nanoparticles in low quantity (0.27 wt %).
Figure 4.
(a) TEM images of 3% Ag-ZnO nanoparticles, (b, c) HRTEM image of 3% Ag-ZnO nanoparticles, and (d) Zn, O, and Ag elements mapping of 3% Ag-ZnO nanoparticles.
The surface elemental composition and chemical species of 3% Ag-ZnO nanoparticles were investigated by XPS and showed that all the peaks belong to Ag, Zn, O, and C elements (Figure 5a). The presence of the C 1s peak might be from the conductive adhesive in the measurement. Figure 5b shows the Zn 2p spectrum of 3% Ag-ZnO that contains two typical peaks of Zn 2p3/2 (1021.7 eV) and Zn 2p1/2 (1044.7 eV) with a splitting distance of 23.1 eV, indicating that the Zn element existed in the form of Zn2+.30,31 From Figure 5c, it could be observed that the O 1s spectrum was asymmetric; therefore, the O 1s spectrum was divided into two peaks located at 531.5 and 530 eV, indicating that the oxygen formed two different chemical bonds, which were attributed to the lattice oxygen of ZnO and oxygen of hydroxyl.32,33 In Figure 5d, two peaks centered at 367.4 and 373.4 eV could be attributed to Ag 3d5/2 and Ag 3d3/2, respectively. The two peak positions of Ag 3d shifted remarkably to lower binding energies compared with those of bulk Ag (Ag 3d5/2, 368.2 eV, and Ag 3d3/2, 374.2 eV).34 The shift in binding energy of Ag was mainly due to electron transfer from metallic Ag to ZnO crystals.35,36 When the metallic Ag nanoparticles and the ZnO nanoparticles contact with each other, they adjust the position of the corresponding Fermi level to the same value. Therefore, there are many free electrons on the new Fermi level of metallic Ag nanoparticles. Since the conduction band (CB) of the ZnO nanoparticles is vacant, the free electrons can tunnel into the CB, resulting in a higher valence state of Ag.35,37 In addition, the splitting distance of the two central peaks is 6 eV that also can give the evidence of metallic Ag modification,33 which is in excellent consistent with the XRD results.
Figure 5.
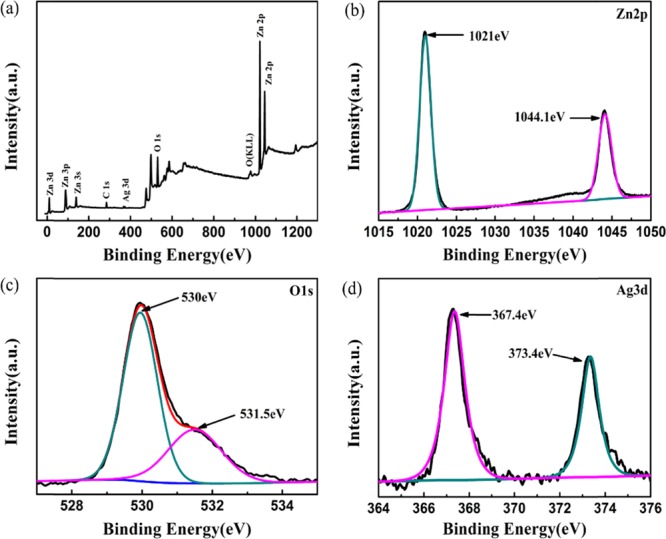
(a) XPS survey spectra and high-resolution spectra of (b) Zn 2p, (c) O 1s, and (d) Ag 3d in 3% Ag-ZnO.
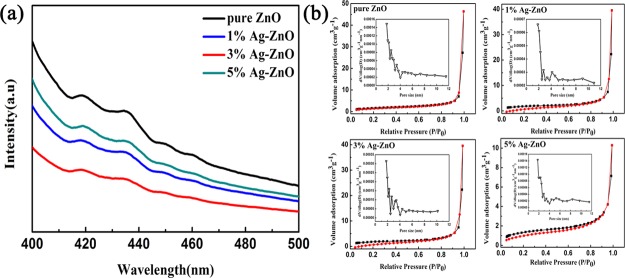
Figure 6 shows the PL spectrum of Ag-ZnO with an excitation wavelength of 400 nm to investigate the recombination of electron–hole pairs.38 The PL spectra intensity of Ag-ZnO nanoparticles decreased after Ag modification compared with pure ZnO. This can be attributed to the fact that Ag nanoparticles can effectively block the recombination of electron–hole pairs, which is better for adsorption of oxygen molecules on the surface of ZnO in gas sensing detection.39,40Figure 6b shows that the specific surface areas of pure ZnO, 1% Ag-ZnO, 3% Ag-ZnO, and 5% Ag-ZnO nanoparticles correspond to 6.03, 6.65, 4.69, and 4.97 m2/g, respectively. The type IV isotherms with H3 hysteresis loops indicated the presence of pores in the material,4 which were mainly the stacked pores among ZnO nanoparticles, as shown in the inset of Figure 6b.
Figure 6.
(a) PL spectra and (b) nitrogen adsorption-desorption isotherms of the as-prepared pure ZnO and Ag-ZnO nanoparticles. (The inset is the porosity distribution.)
2.2. Gas Sensing Performance of Fabricated Sensors to Ethanol
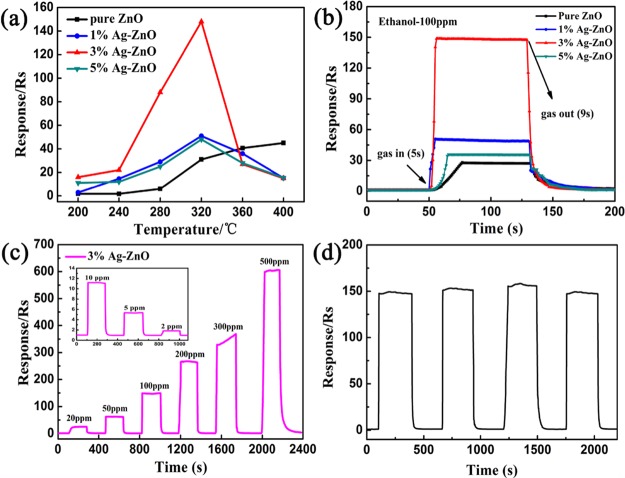
The optimal operating temperature is one of the major factors of the sensors to evaluate gas sensing performance. To obtain the optimal operating temperature of the sensors prepared with pure ZnO, 1% Ag-ZnO, 3% Ag-ZnO, and 5% Ag-ZnO, the responses of 100 ppm ethanol were studied under different temperatures, and the results are shown in Figure 7a. The optimal operating temperature of ZnO significantly decreased after Ag modification. The sensing response to ethanol increased with increasing temperature and reached the maximum value at 320 °C. However, the optimal temperature for pure ZnO could not be obtained due to the detection limit of the instrument. Figure 7a also shows that Ag-ZnO nanoparticles have a higher response value than pure ZnO nanoparticles, which could be attributed to the small size effect of Ag nanoparticles. When the average particle size of Ag is only 3–5 nm, which is smaller than 2Ld (the thickness of the depletion layer) of ZnO (≈15 nm),26 electron transit is controlled by the depletion layer and displays the excellent sensing performance. The small difference in the specific surface area also proved that the Ag modification on the surface of ZnO nanoparticles plays an important role in gas sensing performance. Interestingly, the specific surface area decreased, and no larger variation in the pore size was observed (Figure 6b inset) after Ag modification, but still, the gas sensing performance increased, which further suggested that the increase in the gas sensing performance was due to the small size effect of Ag nanoparticles.
Figure 7.
(a) Response of the sensors to 100 ppm ethanol under different operating temperatures (200–400 °C), (b) response/recovery curve of the sensors to 100 ppm ethanol at 320 °C, (c) response curves of the 3% Ag-ZnO sensor toward 20–500 ppm ethanol at 320 °C (the inset shows the response curves of the 3% Ag-ZnO sensor toward 2–10 ppm ethanol), and (d) the repeatability test of the 3% Ag-ZnO sensor toward 100 ppm ethanol at 320 °C.
Figure 7b shows the response/recovery time of Ag-ZnO sensors when they were exposed to 100 ppm ethanol at an optimum operating temperature of 320 °C. The results showed that the response/recovery times of the 3% Ag-ZnO sensor to 100 ppm ethanol were only 5 s/9 s, respectively. It showed the fastest response/recovery time compared with other sensors having different Ag modification amounts, and it was near about 5 times faster than pure ZnO (23 s/40 s).
The dynamic response and recovery characteristic curves to different concentrations of ethanol (2–500 ppm) under the optimal temperature are tested as shown in Figure 7c. The gas responses increased on increasing the gas concentration. Besides that, the low detection limit of the 3% Ag-ZnO sensor to ethanol was found to be 2 ppm with a response value of around 1.8 (shown in the Figure 7c inset). After this, the repeatability test of the 3% Ag-ZnO sensor for 100 ppm ethanol was performed under optimal operating temperature, and the results are shown in Figure 7d. Four successive response recovery cycles were provided at the optimal operating temperature, but there was only a slight variation in all the four cycles, which proved the good repeatability of the 3% Ag-ZnO gas sensor. The gas sensing performances of the 3% Ag-ZnO gas sensor in our study compared with other ZnO composites are organized in Table 1, and it exhibited a faster and higher response to ethanol.
Table 1. Comparison of Sensing Performances for Reported ZnO-Based Nanocomposites to Ethanol.
| materials | working temperature (°C) | response to ethanol | response/recovery time | refs |
|---|---|---|---|---|
| Ag-ZnO nanoparticles | 320 | 148 (100 ppm) | 5 s/9 s | our work |
| Ag-ZnO nanoparticles | 320 | 33 (200 ppm) | (22) | |
| Ag-ZnO thin films | 260 | 15 (100 ppm) | 5 s/27 s | (41) |
| Ag-ZnO nanoroads | 370 | 20 (20 ppm) | 15 s/20 s | (42) |
| Ag-ZnO nanowires | 300 | 26 (50 ppm) | 8 s/>20 s | (43) |
| Au-ZnO nanoparticles | 300 | 7 (5 ppm) | 13 s/>150 s | (44) |
| Pd-ZnO nanostructures | 300 | 47 (1000 ppm) | 3 s/26 s | (45) |
| Pt-ZnO nanoplates | 350 | 22 (15 ppm) | 80 s/150 s | (46) |
2.3. Gas Sensing Performance of Fabricated Sensors to H2S
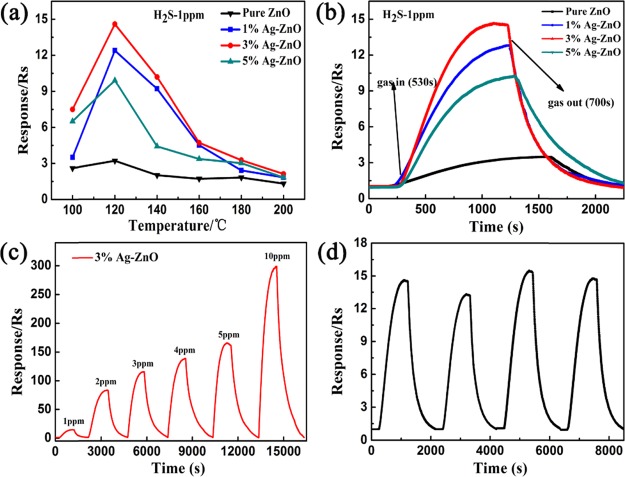
The responses to 1 ppm H2S under different temperatures were studied, and the results are shown in Figure 8a. All the Ag-ZnO sensors showed a relatively low optimal temperature of 120 °C. However, the 3% Ag-ZnO sensor displayed the highest response value, which was near 5 times higher than pure ZnO. All the samples showed a relatively low optimum temperature and high response compared with other reported ZnO-based nanocomposites (Table 2). From Figure 8b, it could be clearly seen that Ag-ZnO sensors displayed a similar response/recovery time to 1 ppm H2S measurement under the optimal operating temperature. The response/recovery time of the 3% Ag-ZnO sensor for H2S detection was found to be 530 s/700 s, respectively. In addition, the dynamic response and recovery characteristic curves to different concentrations of H2S (1–10 ppm) under the optimal temperature were tested and are shown in Figure 8c. It could be observed that the gas responses increased on increasing the gas concentration. The mechanism of H2S detection is discussed in detail in the following section: Mechanism of Gas Sensing. Moreover, response reproducibility and complete recovery are also important parameters for gas sensors. Figure 8d shows that the 3% Ag-ZnO sensor exhibited excellent repeatability in four successive response recovery cycles with similar response values and response/recovery times, and for each run, the response could basically restore its initial state.
Figure 8.
(a) Responses of Ag-ZnO sensors to 1 ppm H2S under different operating temperatures (100–200 °C), (b) response/recovery curve to 1 ppm H2S of the sensors at 120 °C, (c) response curves of the 3% Ag-ZnO sensor toward 1–10 ppm H2S at 120 °C, and (d) the repeatability test of the 3% Ag-ZnO sensor toward 1 ppm H2S at 120 °C.
Table 2. Comparison of Sensing Performances for Reported ZnO-Based Nanocomposites to H2S.
The responses of pure ZnO and Ag-modified ZnO sensors toward 10 ppm ethanol, acetone, formaldehyde, and toluene at 320 °C and H2S at 120 °C were measured, and the response histogram is shown in Figure 9. It could be clearly seen that all the prepared sensors exhibited a high response value for H2S. Besides that, Ag-ZnO sensors showed higher response value than a pure ZnO sensor to these five kinds of gases, which further proved the importance of Ag modification for enhancing the ZnO-based gas sensors.
Figure 9.
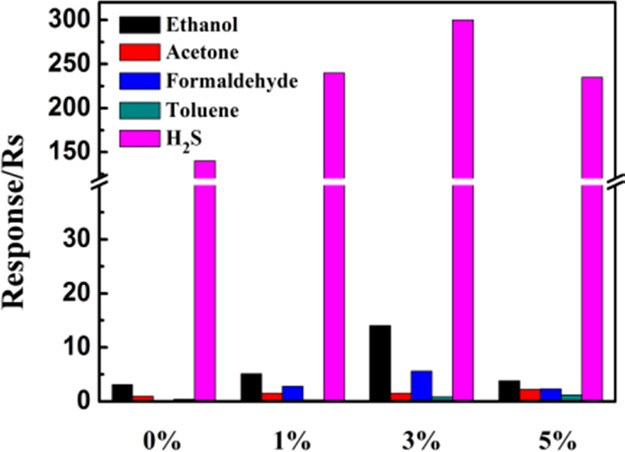
Response of pure ZnO, 1% Ag-ZnO, 3% Ag-ZnO, and 5% Ag-ZnO sensors exposed to 10 ppm of gases at their optimum operating temperature (H2S at 120 °C and ethanol, acetone, formaldehyde, and toluene at 320 °C).
2.4. Mechanism of Gas Sensing
Up to now, the most widely accepted gas sensing mechanism of semiconductor oxide is the model based on the electron transfer dynamics during an adsorption–oxidation–desorption process, which can change the resistance value of the sensors.51 The response of a typical n-type semiconductor greatly depends on the electron concentration. As shown in Figure 10a, when the gas sensor is exposed in the air, the oxygen molecules adsorb on the surface of ZnO nanoparticles and trap electrons from the conduction band (CB) of ZnO to form different oxygen species (O2–, O–, and O2–).52,53 The reduction of electron concentration leads to a thicker electron depletion layer of pure ZnO. For ZnO nanoparticles with Ag modification, more oxygen molecules can be transferred from the Ag nanoparticles to the surface of the ZnO nanoparticles due to the spillover effect of Ag. Successively, more electrons can be trapped from the CB of ZnO, and more oxygen species can be generated.54 In addition, the surface of metallic Ag can adsorb oxygen molecules and generate oxygen species due to its electron sensitization effect.55 Based on the above two effects, the Ag-ZnO nanoparticles can produce a thicker electron depletion layer compared with pure ZnO. This process is shown in Figure 10b.
Figure 10.
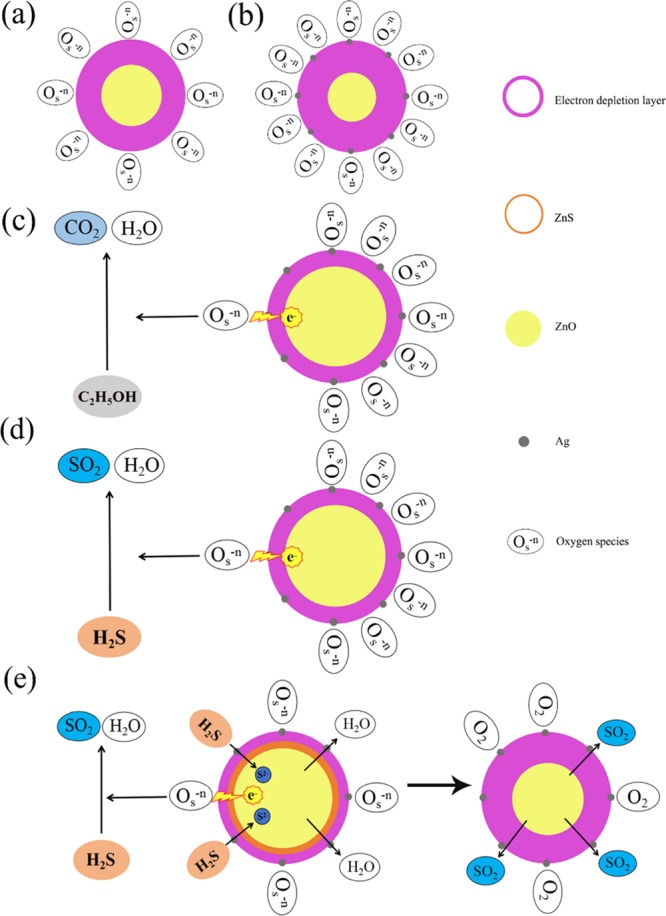
Schematic diagrams of the gas sensing mechanism of (a) pure ZnO, (b) Ag-ZnO in air, (c) Ag-ZnO in ethanol, (d) Ag-ZnO in low H2S concentration, and (e) Ag-ZnO in high H2S concentration.
When the ethanol and low concentration of H2S (lower than 2 ppm in our study) are injected into the chamber as the target gases, the ethanol and H2S molecules adsorb onto the material surface and can react with different oxygen species. The electrons can be then fed back to CB of ZnO to increase electron concentration, decreasing the thickness of electron depletion layer,56,57 and this can be described by the following equations (eqs 1 and 2) and is shown in Figure 10c,d.
| 1 |
| 2 |
However, when H2S reached in a certain concentration (higher than 2 ppm in our study), the H2S molecules might have not only reacted with the oxygen ions (O–) adsorbed on the surface (eq 2) but also have reacted with ZnO, which would have converted ZnO into ZnS; this can be described by eqs 3 and 4, and this mechanism of gas sensing are shown in Figure 10e. Since ZnO is an n-type semiconductor in which oxygen vacancies originate the carriers. The oxygen vacancies tend to be captured by the chemisorbed S generated due to the surface reactions. When the S diffuses into ZnO, it supplies excess carriers to the conduction band, which increase the electrical conductivity of ZnO.58 Therefore, the metallic ZnS could increase the conductivity and the surface active sites, which could further reduce potential barrier and resistance of the sensing material.47
| 3 |
| 4 |
Both kinds of gas sensing mechanisms for H2S detection were confirmed in our study, and the gas sensing results under different concentrations of H2S are shown in Figure 8b. It can be clearly seen that the response value jumped when the H2S concentration increased from 1 to 2 ppm but attained a steady growth process when the H2S concentration changed from 2 to 10 ppm. It could be concluded that the mechanism of gas sensing for H2S detection changes when the concentration of H2S increases from 1 ppm to 2 ppm.
3. Conclusions
The ZnO nanoparticles modified with uniformly dispersed Ag nanoparticles were obtained in one step by calcining of precursor electrospun nanofibers, and their gas sensing performance was tested. Compared with the pure ZnO sensor, Ag-ZnO sensors exhibited preferable gas sensing properties to ethanol and H2S. However, the 3% Ag-ZnO nanoparticle sensor exhibited the fastest response/recovery times to ethanol of about 5 and 9 s, respectively. Besides that, the Ag-ZnO-based nanoparticle sensors also showed a high response value to H2S, and the 3% Ag-ZnO sensor showed a maximum response value of 298 with 10 ppm H2S. In addition, the 3% Ag-ZnO sensor exhibited good repeatability in the gas sensing tests, which could be attributed to the spillover effect and electron sensitization effect of Ag that led to the generation of more oxygen species and active sites, which was beneficial for the adsorption of ethanol and H2S molecules on the surface of ZnO nanoparticles. Thus, ZnO nanoparticles modified with uniformly dispersed Ag nanoparticles provide a new approach for the development of gas sensors with high gas sensing performance.
4. Materials and Methods
4.1. Materials
Zinc nitrate hexahydrate (Zn(NO3)2·6H2O), silver nitrate (AgNO3), poly(vinyl pyrrolidone) (PVP, Mw=1300000), N,N-dimethylformamide (DMF), and ethanol (≥99.7%) were purchased from Sinopharm Chemical Reagent Co., Ltd. All chemicals were of analytical grade and used without any further purification. Ultrapure water was used throughout the experiments.
4.2. Preparation of Ag-ZnO Nanoparticles
ZnO nanoparticles with Ag modification (Ag-ZnO) were prepared in two steps (the scheme of preparation is shown in Figure 1a). In the first step, precursor nanofibers were prepared with an electrospinning technique. PVP (1.5 g), Zn(NO3)2·6H2O (2 g), and varied amounts of AgNO3 (corresponding to different molar ratios of Ag to Zn: 0, 1, 3, and 5%) were added into 24 mL of mixed solution of ethanol, N,N-dimethylformamide (DMF), and ultrapure water (v/v 2:1:1). This solution was then poured into a 10 mL plastic syringe, and a high voltage of 16 kV was applied between a stainless steel collector and needle tip at a distance of 16 cm. A feed rate of 0.04 mm/min was kept constant via a peristaltic pump. The obtained precursor nanofibers were collected on the alumina crucible. In the second step, precursor nanofibers were calcined in the muffle furnace, first at 350 °C for 2 h, and then the temperature was increased to 600 °C for another 2 h to remove organic matter thoroughly. The heating rate was maintained at 2 °C/min. The obtained Ag-ZnO nanoparticles were labeled as pure ZnO, 1% Ag-ZnO, 3% Ag-ZnO, and 5% Ag-ZnO for 0, 1, 3, and 5% molar ratios of Ag to Zn, respectively.
4.3. Characterization Techniques
The phase and crystallinity of the samples were analyzed by X-ray diffraction (XRD, Germany Bruker AXS D8 Advance). The morphologies of the samples were examined by scanning electron microscopy (SEM, USA FEI Sirion 200) and field emission transmission electron microscopy (FETEM, USA Tecnai G2 F20 S-TWIN). The surface elemental composition and chemical species were evaluated by X-ray photoelectron spectroscopy (XPS, USA Thermo Fisher Scientific Thermo ESCALAB 250Xi). The specific surface area was measured by nitrogen (N2) adsorption-desorption (BET, USA Micromeritics ASAP2460). The photoluminescence (PL) data were recorded by using an F-380 (Tianjin Gangdong Technology Development Co., Ltd. China) to explain the electron transfer situation.
4.4. Sensor Fabrication and Gas Sensing Measurement
A suitable amount of the as-prepared Ag-ZnO nanoparticles was mixed with anhydrous ethanol to form a slurry suspension and coated onto an alumina sheet by the screen printing method, which was supported by a pair of Ag electrodes at each end and dried naturally to remove the anhydrous ethanol.
The gas sensing properties were measured by a chemical gas sensor-4 temperature pressure (CGS-4TP) small intelligent gas sensing analysis system (Beijing Elliott Technology Co., Ltd., China) at room temperature. The photograph of the CGS-4TPs gas sensor system with the schematic of the circuit diagram of the gas sensing measurement setup is shown in Figure 1b. First, the alumina sheet was placed on the test platform and connected with a probe, and then the working temperature was set on the operation interface. After reaching the target working temperature, the base resistance (Ra) of the test sample in the background gas (in air) was analyzed. Then, the resistance (Rg) of the test sample under the target gas was analyzed, and when the resistance stabilized to the initial value in the background gas, the test was completed. In our work, the ethanol and H2S in calculated concentrations were introduced separately as the target gases into a 1.8 L testing chamber of the gas sensing test system by a microsyringe. The sensor response (Rs) is defined as the ratio of sensor resistance (Ra) in air to sensor resistance (Rg) in target gas. The response/recovery time is defined as the time required for the sensors to reach 90% of the change in total adsorption/desorption resistance, respectively.
Acknowledgments
The authors acknowledge supports from the National Natural Science Foundation of China (61704098), National Natural Science Foundation of China (61604089), National Natural Science Foundation of China (11904209), and Natural Science Foundation of Shandong Province (ZR2017BF025).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b04243.
TGA study in details (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Gouw J.; Warneke C. Measurements of volatile organic compounds in the earth’s atmosphere using proton-transfer-reaction mass spectrometry. Mass. Spectrom. Rev. 2007, 26, 223–257. 10.1002/mas.20119. [DOI] [PubMed] [Google Scholar]
- Qi G.; Zhang L.; Yuan Z. Improved H2S gas sensing properties of ZnO nanorods decorated by a several nm ZnS thin layer. Phys. Chem. Chem. Phys. 2014, 16, 13434–13439. 10.1039/C4CP00906A. [DOI] [PubMed] [Google Scholar]
- Najjar Y. S. H. Gaseous pollutants formation and their harmful effects on health and environment. Inno. Energy Polic. 2011, 1, 1–9. 10.4303/iep/E101203. [DOI] [Google Scholar]
- Feng Z.; Ma Y.; Natarajan V.; Zhao Q.; Ma X.; Zhan J. In-situ generation of highly dispersed Au nanoparticles on porous ZnO nanoplates via ion exchange from hydrozincite for VOCs gas sensing. Sens. Actuators, B 2018, 255, 884–890. 10.1016/j.snb.2017.08.138. [DOI] [Google Scholar]
- Tomer V. K.; Duhan S. Ordered mesoporous Ag-doped TiO2/SnO2 nanocomposite based highly sensitive and selective VOC sensors. J. Mater. Chem. A 2016, 4, 1033–1043. 10.1039/C5TA08336B. [DOI] [Google Scholar]
- Huang H.; Xu P.; Zheng D.; Chen C.; Li X. Sulfuration–desulfuration reaction sensing effect of intrinsic ZnO nanowires for high-performance H2S detection. J. Mater. Chem. A 2015, 3, 6330–6339. 10.1039/C4TA05963H. [DOI] [Google Scholar]
- Ramgir N. S.; Ganapathi S. K.; Kaur M.; Datta N.; Muthe K. P.; Aswal D. K.; Gupta S. K.; Yakhmi J. V. Sub-ppm H2S sensing at room temperature using CuO thin films. Sens. Actuators, B 2010, 151, 90–96. 10.1016/j.snb.2010.09.043. [DOI] [Google Scholar]
- Shinde V. R.; Gujar T. P.; Lokhande C. D. LPG sensing properties of ZnO films prepared by spray pyrolysis method: Effect of molarity of precursor solution. Sens. Actuators, B 2007, 120, 551–559. 10.1016/j.snb.2006.03.007. [DOI] [Google Scholar]
- Zhao Y.; Zou X.; Chen H.; Chu X.; Li G. Tailoring energy level and surface basicity of metal oxide semiconductor by rare-earth incorporation for high performance formaldehyde detection. Inorg. Chem. Front. 2019, 6, 1767–1774. 10.1039/C9QI00381A. [DOI] [Google Scholar]
- Zhao G.; Sun M.; Liu X.; Xuan J.; Kong W.; Zhang R.; Sun Y.; Jia F.; Yin G.; Liu B. Fabrication of CdS quantum dots sensitized ZnO nanorods/TiO2 nanosheets hierarchical heterostructure films for enhanced photoelectrochemical performance. Electrochim. Acta. 2019, 304, 334–341. 10.1016/j.electacta.2019.03.022. [DOI] [Google Scholar]
- Franke M. E.; Koplin T. J.; Simon U. Metal and metal oxide nanoparticles in chemiresistors: does the nanoscale matter?. Small 2006, 2, 36–50. 10.1002/smll.200500261. [DOI] [PubMed] [Google Scholar]
- Broza Y.; Zhou X.; Yuan M.; Qu D.; Zheng Y.; Vishinkin R.; Khatib M.; Wu W.; Haick H. Disease setection with molecular biomarkers: from chemistry of body fluids to nature-inspired chemical sensors. Chem. Rev. 2019, 119, 11761–11817. 10.1021/acs.chemrev.9b00437. [DOI] [PubMed] [Google Scholar]
- Yamazoe N.; Sakai G.; Shimanoe K. Oxide semiconductor gas sensors. Catal. Surv. Asia 2003, 7, 63–75. 10.1023/A:1023436725457. [DOI] [Google Scholar]
- Wang X.; Sun F.; Duan Y.; Yin Z.; Luo W.; Huang Y.; Chen J. Highly sensitive, temperature-dependent gas sensor based on hierarchical ZnO nanorod arrays. J. Mater. Chem. 2015, 3, 11397–11405. [Google Scholar]
- Xing X.; Yang Y.; Yan Z.; Hu Y.; Zou T.; Wang Z.; Wang Y. CdO-Ag-ZnO nanocomposites with hierarchically porous structure for effective VOCs gas-sensing properties. Ceram. Int. 2019, 45, 4322–4334. 10.1016/j.ceramint.2018.11.107. [DOI] [Google Scholar]
- Jing Z.; Zhan J. Fabrication and gas-sensing properties of porous ZnO nanoplates. Adv. Mater. 2008, 4547. 10.1002/adma.200800243. [DOI] [Google Scholar]
- Meng F.; Hou N.; Ge S.; Sun B.; Jin Z.; Shen W.; Kong L.; Guo Z.; Sun Y.; Wu H.; et al. Flower-like hierarchical structures consisting of porous single-crystalline ZnO nanosheets and their gas sensing properties to volatile organic compounds (VOCs). J. Alloy. Compd. 2015, 626, 124–130. 10.1016/j.jallcom.2014.11.175. [DOI] [Google Scholar]
- Runa A.; Zhang X.; Wen G.; Zhang B.; Fu W.; Yang H. Actinomorphic flower-like n-ZnO/p-ZnFe2O4 composite and its improved NO2 gas-sensing property. Mater. Lett. 2018, 225, 73–76. 10.1016/j.matlet.2018.04.087. [DOI] [Google Scholar]
- Chang S. J.; Weng W. Y.; Hsu C. L.; Hsueh T. J. High sensitivity of a ZnO nanowire-based ammonia gas sensor with Pt nano-particles. Nano Commun. Networks 2010, 1, 283–288. 10.1016/j.nancom.2010.09.005. [DOI] [Google Scholar]
- Xing X.; Xiao X.; Wang L.; Wang Y. Highly sensitive formaldehyde gas sensor based on hierarchically porous Ag-loaded ZnO heterojunction nanocomposites. Sens. Actuators, B 2017, 247, 797. 10.1016/j.snb.2017.03.077. [DOI] [Google Scholar]
- Zhang Q.; Xie G.; Xu M.; Su Y.; Tai H.; Du H.; Jiang Y. Visible light-assisted room temperature gas sensing with ZnO-Ag heterostructure nanoparticles. Sens. Actuators, B 2018, 259, 269–281. 10.1016/j.snb.2017.12.052. [DOI] [Google Scholar]
- Umar A.; Khan M.; Kumar R.; Algarni H. Ag-doped ZnO nanoparticles for enhanced ethanol gas sensing application. J. Nanosci. Nanotechnol. 2018, 18, 3557–3562. 10.1166/jnn.2018.14651. [DOI] [PubMed] [Google Scholar]
- Tang S.; Chen W.; Xu L.; Gao T. Fabrication of Ag-doped ZnO nanoparticle gas sensor and its application in detection of CO. Nanosci. Nanotech. Let. 2017, 9, 214–219. 10.1166/nnl.2017.2313. [DOI] [Google Scholar]
- Iftekhar Uddin A.; Phan D.; Chung G. Low temperature acetylene gas sensor based on Ag nanoparticles-loaded ZnO-reduced graphene oxide hybrid. Sens. Actuators, B 2015, 207, 362–369. 10.1016/j.snb.2014.10.091. [DOI] [Google Scholar]
- Suematsu K.; Shin Y.; Hua Z.; Yoshida K.; Yuasa M.; Kida T.; Shimanoe K. Nanoparticle cluster gas sensor: controlled clustering of SnO2 nanoparticles for highly sensitive toluene detection. Appl. Mater. Interfaces 2014, 6, 5319–5326. 10.1021/am500944a. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Zhu C. L.; Xiao G. Reduced-temperature ethanol sensing characteristics of flower-like ZnO nanorods synthesized by a sonochemical method. Nanotechnology 2006, 17, 4537–4541. 10.1088/0957-4484/17/18/002. [DOI] [PubMed] [Google Scholar]
- Suematsu K.; Watanabe K.; Tou A.; Sun Y.; Shimanoe K. Ultraselective toluene-gas sensor: nanosized gold loaded on zinc oxide nanoparticles. Anal. Chem. 2018, 90, 1959–1966. 10.1021/acs.analchem.7b04048. [DOI] [PubMed] [Google Scholar]
- Huang B.; Zhao C.; Zhang M.; Zhang Z.; Xie E.; Zhou J.; Han W. Doping effect of In2O3 on structural and ethanol-sensing characteristics of ZnO nanotubes fabricated by electrospinning. Appl. Surf. Sci. 2015, 349, 615–621. 10.1016/j.apsusc.2015.05.003. [DOI] [Google Scholar]
- Alali K. T.; Liu T.; Liu J.; Liu Q.; Fertassi M. A.; Li Z.; Wang J. Preparation and characterization of ZnO/CoNiO2 hollow nanofibers by electrospinning method with enhanced gas sensing properties. J. Alloy. Compd. 2017, 702, 20–30. 10.1016/j.jallcom.2017.01.167. [DOI] [Google Scholar]
- Koo A.; Yoo R.; Woo S. P.; Lee H.-S.; Lee W. Enhanced acetone-sensing properties of pt-decorated al-doped ZnO nanoparticles. Sens. Actuators, B 2019, 280, 109–119. 10.1016/j.snb.2018.10.049. [DOI] [Google Scholar]
- Deng S.; Han R.; Dong C.; Xiao X.; Wu J.; Wang Y. Flash synthesis of macro-/nanoporous ZnCo2O4 via self-sustained decomposition of metal–organic complexes. Mater. Lett. 2014, 134, 138–141. 10.1016/j.matlet.2014.07.086. [DOI] [Google Scholar]
- Kown K.; Shim M. γ-Fe2O3/II-IVsulfide nanocrystal heterojunctions. J. Am. Chem. Soc. 2005, 127, 10269–10275. 10.1021/ja051713q. [DOI] [PubMed] [Google Scholar]
- Ramgir N.; Mulla I.; Pillai V. Micropencils and microhexagonal cones of ZnO. J. Phys. Chem. B 2006, 110, 3995–4001. 10.1021/jp056629b. [DOI] [PubMed] [Google Scholar]
- Tarwal N. L.; Rajgure A. V.; Patil J. Y.; Khandekar M. S.; Suryavanshi S. S.; Patil P. S.; Gang M. G.; Kim J. H.; Jang J. H. A selective ethanol gas sensor based on spray-derived Ag–ZnO thin films. J. Mater. Sci. 2013, 48, 7274–7282. 10.1007/s10853-013-7547-7. [DOI] [Google Scholar]
- Liu H. R.; Shao G. X.; Zhao J. F.; Zhang Z. X.; Zhang Y.; Liang J.; Liu X. G.; Jia H. S.; Xu B. S. Worm-like Ag/ZnO core–shell heterostructural composites: fabrication, characterization, and photocatalysis. J. Phys. Chem. C 2012, 116, 16182–16190. 10.1021/jp2115143. [DOI] [Google Scholar]
- Yin Y.; Lia F.; Zhang N.; Ruan S.; Zhang H.; Chen Y. Improved gas sensing properties of silver-functionalized ZnSnO3 hollow nanocubes. Inorg. Chem. Front. 2018, 5, 2123–2131. 10.1039/C8QI00470F. [DOI] [Google Scholar]
- Zheng Y.; Zheng L.; Zhan Y.; Lin X.; Zheng Q.; Wei K. Ag/ZnO heterostructure nanocrystals: synthesis, characterization, and photocatalysis. Inorg. Chem. 2007, 46, 6980–6986. 10.1021/ic700688f. [DOI] [PubMed] [Google Scholar]
- Sarma B.; Sarma B. K. Fabrication of Ag/ZnO heterostructure and the role of surface coverage of ZnO microrods by Ag nanoparticles on the photophysical and photocatalytic properties of the metal-semiconductor system. Appl. Surf. Sci. 2017, 410, 557–565. 10.1016/j.apsusc.2017.03.154. [DOI] [Google Scholar]
- Aguirre M. E.; Rodríguez H. B.; San Román E.; Feldhoff A.; Grela M. A. Ag@ZnO core–shell nanoparticles formed by the timely reduction of Ag+ ions and zinc acetate hydrolysis in N,N-dimethylformamide: mechanism of growth and photocatalytic properties. J. Phys. Chem. C 2011, 115, 24967. 10.1021/jp209117s. [DOI] [Google Scholar]
- Zhong S.; Tian R.; Guo S.; Jiang W.; Wang J. Detonation-deposition approach to obtain Ag-ZnO photocatalyst using energetic Ag-Zn(NO3)2(N2H4)3 precursor. Appl. Surf. Sci. 2018, 448, 115–125. 10.1016/j.apsusc.2018.04.101. [DOI] [Google Scholar]
- Chen J.; Yan X.; Liu W.; Xue Q. The ethanol sensing property of magnetron sputtered ZnO thin films modified by Ag ion implantation. Sens. Actuators, B 2011, 160, 1499–1503. 10.1016/j.snb.2011.08.026. [DOI] [Google Scholar]
- Ding J.; Zhu J.; Yao P.; Li J.; Bi H.; Wang X. Synthesis of ZnO–Ag hybrids and their gas-sensing performance toward ethanol. Ind. Eng. Chem. Res. 2015, 54, 8947–8953. 10.1021/acs.iecr.5b01711. [DOI] [Google Scholar]
- Hastir A.; Kohli N.; Singh R. C. Ag doped ZnO nanowires as highly sensitive ethanol gas sensor. Mater. Today: Proc. 2017, 4, 9476–9480. [Google Scholar]
- Zhang J.; Liu X.; Wu S.; Cao B.; Zheng S. One-pot synthesis of Au-supported ZnO nanoplates with enhanced gas sensor performance. Sens. Actuators, B 2012, 169, 61–66. 10.1016/j.snb.2012.02.070. [DOI] [Google Scholar]
- Liang Y.-C.; Liao W.-K.; Deng X.-S. Synthesis and substantially enhanced gas sensing sensitivity of homogeneously nanoscale Pd- and Au-particle decorated ZnO nanostructures. J. Alloys Compd. 2014, 599, 87–92. 10.1016/j.jallcom.2014.01.167. [DOI] [Google Scholar]
- Daryakenari A. A.; Apostoluk A.; Delaunay J.-J. Effect of Pt decoration on the gas response of ZnO nanoparticles. Phys. Status Solidi C 2013, 10, 1–4. [Google Scholar]
- Na H. B.; Zhang X. F.; Deng Z. P.; Xu Y. M.; Huo L. H.; Gao S. Large-scale synthesis of hierarchically porous ZnO hollow tubule for fast response to ppb-Level H2S Gas. Appl. Mater. Interfaces 2019, 11, 11627–11635. 10.1021/acsami.9b00173. [DOI] [PubMed] [Google Scholar]
- Fu D.; Zhu C.; Zhang X.; Li C.; Chen Y. Two-dimensional net-like SnO2/ZnO heteronanostructures for high-performance H2S gas sensor. J. Mater. Chem. A 2016, 4, 1390–1398. 10.1039/C5TA09190J. [DOI] [Google Scholar]
- Shewale P. S.; Yu Y. S.; Kim J. H.; Bobade C. R.; Uplane M. D. H2S gas sensitive Sn-doped ZnO thin films: Synthesis and characterization. J. Anal. Appl. Pyrolysis 2015, 112, 348–356. 10.1016/j.jaap.2015.01.001. [DOI] [Google Scholar]
- Vuong N. M.; Chinh N. D.; Huy B. T.; Lee Y. I. CuO-decorated ZnO hierarchical nanostructures as efficient and established sensing materials for H2S gas sensors. Sci. Rep. 2016, 6, 26736–26748. 10.1038/srep26736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. F.; Sun Y. P.; Yin X.; Yin G. C.; Wang X. M.; Jia F. C.; Liu B. Effect of surfactants on the microstructures of hierarchical SnO2 blooming nanoflowers and their gas-sensing properties. Nanoscale Res. Lett. 2018, 13, 250. 10.1186/s11671-018-2656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadosaki M.; Sakai Y.; Tamura I.; Matsubara I.; Itoh T. Development of an oxide semiconductor thick film gas sensor for the detection of total volatile organic compounds. Electron. Commun. Jpn. 2010, 93, 34–41. 10.1002/ecj.10190. [DOI] [Google Scholar]
- Liu X.; Zhang J.; Wang L.; Yang T.; Guo X.; Wu S.; Wang S. 3D hierarchically porous ZnO structures and their functionalization by Aunanoparticles for gas sensors. J. Mater. Chem. 2011, 21, 349–356. 10.1039/C0JM01800G. [DOI] [Google Scholar]
- Guo J.; Zhang J.; Zhu M.; Ju D.; Xu H.; Cao B. High-performance gas sensor based on ZnO nanowires functionalized by Au nanoparticles. Sens. Actuators, B 2014, 199, 339–345. 10.1016/j.snb.2014.04.010. [DOI] [Google Scholar]
- David D.; Udo W.; Nicolae B. Current understanding of the fundamental mechanisms of doped and loaded semiconducting metal oxide-based gas sensing materials. ACS Sens. 2019, 4, 2228–2249. [DOI] [PubMed] [Google Scholar]
- Kolmakov A.; Klenov D. O.; Lilach Y.; Stemmer S.; Moskovits M. Enhanced Gas sensing by individual SnO2 nanowires and nanobelts functionalized with Pd catalyst particles. Nano Lett. 2005, 5, 667–673. 10.1021/nl050082v. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Liu X.; Wang L.; Yang T.; Guo X.; Wu S.; Wang S.; Zhang S. Synthesis and gas sensing properties of alpha-Fe(2)O(3)@ZnO core-shell nanospindles. Nanotechnology 2011, 22, 1855010. [DOI] [PubMed] [Google Scholar]
- Kim J.; Yong J. Mechanism study of ZnO nanorod-bundle sensors for H2S gas sensing. J. Phys. Chem. C 2011, 115, 7218–7224. 10.1021/jp110129f. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.