Abstract
A novel bimetallic and reusable Y2ZnO4 nanocatalyst was synthesized by a simple coprecipitation method. The prepared nanocatalyst exhibited dual catalytic activity and was characterized using X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), energy-dispersive X-ray spectroscopy (EDX), and scanning electron microscopy (SEM). The average crystallite and grain sizes were found to be 17 ± 1 and 10 ± 2 nm, respectively. On the one hand, the catalytic activity of the nanocatalyst was studied for the Knoevenagel condensation reaction of aromatic aldehydes with active methylene compounds, such as ethyl cyanoacetate and malononitrile, under microwave irradiation and solvent-free conditions. On the other hand, the nanoparticles also showed faster photocatalytic activity against methyl orange (MO) compared to other dyes. The nanocatalyst was easily recoverable by a simple filtration method and was recycled without any significant loss of catalytic activity. The advantages of this nanocatalyst were a simple workup procedure, high reaction yields, solvent-free conditions, reusability, and a short reaction time under green reaction conditions.
Introduction
In recent years, nanocatalysts have increasingly attracted attention in the development of chemical reactions under green reaction conditions. Some of the progressive areas of green chemistry are the solvent-free and microwave conditions used for various types of organic reactions with nanocatalysts as heterogeneous catalysts. Metal oxides and, particularly, bimetallic oxide nanoparticles have several applications including as catalysts,1 photocatalysts,2 antimicrobial agents,3 materials with magnetic properties,4 and CT-DNA binding agents.5 The most attractive feature of nanoparticles lies in the field of green chemistry as they serve as green catalysts in organic reactions and have advantages such as easy separation from the reaction mixture by simple filtration, reusability, recyclability, and environmental friendliness.6,7
Many conventional organic reactions need high temperatures and long reaction times. However, microwave (MW) irradiation techniques accelerated the rate of organic reactions dramatically, and the reactions were accomplished within a shorter reaction time. Owing to these attractive features, microwave-assisted functional group transformations and chemical synthesis gained tremendous interest in the field of green and sustainable chemistry. On the other hand, avoiding toxic and volatile organic solvents is the most acceptable practice in green chemical synthesis, and accordingly, development of solvent-free reaction conditions is a highly desirable process in terms of cost and environmental benefits.8−21
The Knoevenagel condensation is one of the most important reactions in organic synthesis involving C–C bond-forming reactions. The applications of the Knoevenagel reaction are enormous, including the synthesis of fine chemicals, anticoagulants, antibacterial agents, fungicides, pesticides, etc.22−25 The Knoevenagal condensation is facilitated mainly in the presence of bases, a catalyst such as piperidine, pyridine, sodium ethoxide, and ammonia, and other organocatalysts.26 Some metal oxides also serve as catalysts in the Knoevenagal condensation, such as ZrO2,27 TiO2,28,29 Fe3O4,30 NiO,31,32 and CuO.33,34 However, most of the reported works have one or several drawbacks, such as the use of toxic organic solvents, long reaction times, and nonseparable, nonrecyclable, expensive, and toxic catalyst systems. Thus, it was desirable to develop an efficient catalytic approach for the Knoevenagel condensation under green reaction conditions. In continuation of our research work toward the development of efficient multifunctional and recyclable catalysts, we wish to report an ecofriendly and green protocol for the Knoevenagel condensation using the bimetallic oxide, Y2ZnO4, as a nontoxic nanocatalyst under microwave and solvent-free reaction conditions.
The efficiency of the nanocatalyst was further demonstrated by the photocatalytic degradation of a dye, methyl orange (MO), under green conditions. Pollutant dyes come from different sources, such as textile, paper, cosmetic, and plastic industries, and so on.35−37 Non-biodegradable dyes can cause a variety of diseases, such as skin rashes, allergies, and liver and kidney damage.38,39 Therefore, non-biodegradable dyes must be removed from drinking water.
In this work, we synthesized the Y2ZnO4 nanocatalyst using a simple coprecipitation method, and it was characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), energy-dispersive X-ray spectroscopy (EDX), and scanning electron microscopy (SEM). The nanoparticles were used as a photocatalyst against methyl orange (MO) and as a catalyst to perform the Knoevenagel reaction between aromatic aldehydes with active methylene compounds, such as ethyl cyanoacetate and malononitrile, under solvent-free, microwave (MW) conditions.
Green Context
Bimetallic oxide Y2ZnO4 was used as a nontoxic and recyclable nanocatalyst in both Knoevenagel condensation and photocatalytic dye degradation under green conditions. The use of microwave (MW) radiation dramatically enhanced the reaction rate, and the Knoevenagel reaction was accomplished in a shorter reaction time with high conversion and selectivity under solvent-free conditions. The described methodology has several advantages over conventional methods because in this green approach, the nanocatalyst is easily separable and recoverable from the product. This approach avoided the use of toxic organic solvents, and there was no formation of byproducts as waste in the Knoevenagel condensation. Moreover, degradation of the pollutant dye, methyl orange, was carried out using the nanoparticles as the catalyst by following the concept of green chemistry in the presence of sunlight.
Results and Discussion
XRD Analysis
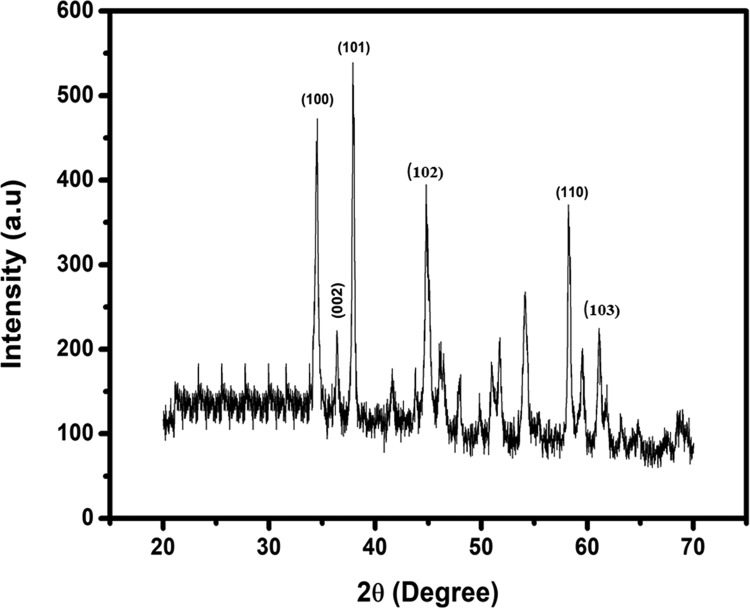
The XRD technique was used to determine and confirm the crystal structure of Y2ZnO4. The intense diffraction peaks observed at 34.63, 36.48, 37.90, 45.88, 58.30, and 63.25° were indexed to the (100), (002), (101), (102), (110), and (103) planes, respectively,3 as shown in Figure 1. The crystallite size of Y2ZnO4 was 17 ± 1 nm, measured using the Scherrer method.
Figure 1.
XRD pattern of Y2ZnO4.
SEM and EDX Analysis
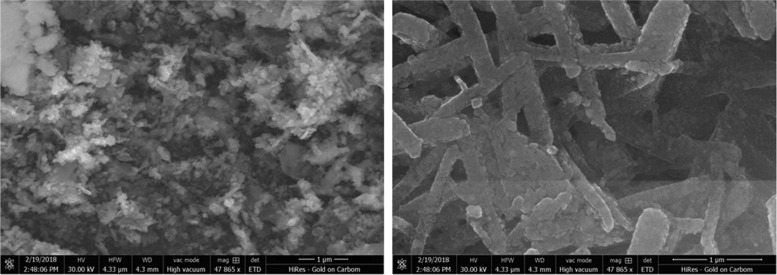
Scanning electron microscopy (SEM) studies were performed on Y2ZnO4 to gain information about the grain size. Figure 2 represents the SEM images of Y2ZnO4 at different resolutions. The SEM images show that the nanomaterials are rod-shaped, paddy-seed-like particles and that they are arranged in a well-ordered manner, with the average grain size of the nanoparticles being 10 ± 2 nm. The elemental description of the sample was obtained from energy-dispersive X-ray (EDX) analysis and the % elemental composition is shown in Figure S1 and Table S1. The peaks located at 1 and 2 keV are directly related to the characteristics of zinc and yttrium.
Figure 2.
SEM image of Y2ZnO4 at different resolutions.
FT-IR Analysis
The FT-IR spectrum of Y2ZnO4 is presented in Figure S2. The band observed at about 3436 cm–1 was characteristic of the H–O bending mode of the hydroxyl groups present on the surface due to moisture. The peak appearing at about 675 cm–1 was due to the bending vibration of the Zn–O bond.25b
Knoevenagel Condensation Using Y2ZnO4 as the Nanocatalyst
The optimization of the Knoevenagel condensation was performed using commercially available benzaldehyde 1a and ethyl cyanoacetate 2a in the presence of the synthesized nanocatalyst, Y2ZnO4.
Initially, we carried out the Knoevenagel reaction under solvent-free conditions at room temperature using 10 mol % Y2ZnO4 nanocatalyst and detected the formation of the Knoevenagel condensation product, 3a, as observed from the 1H NMR spectrum of the crude reaction mixture. However, at room temperature, the reaction was not completed after 5 h and the yield of the reaction was found to be poor (Table 1, entry 1). With this encouraging result, we carried out the optimization of the Knoevenagel condensation using Y2ZnO4 as the nanocatalyst and screened various reaction conditions, such as room temperature, catalyst loading, solvent-free conditions, effect of solvents, and conventional heating, followed by the effect of microwave (MW) radiation. The optimization results are summarized in Tables 1 and 2. When the reaction was performed at 70 °C, the yield of the condensation product 3a was improved to 56% using the same catalyst loading under solvent-free conditions (Table 1, entry 2). The Knoevenagel product, 3a, was confirmed by 1H NMR spectroscopic data and comparison with literature data.40−45 A decrease in reaction yield was noted when we performed the reaction using a lower catalyst loading (Table 1, entry 3). Next, we studied the effect of solvent on the catalytic reaction in the presence of 10 mol % nanocatalyst (Y2ZnO4). It was observed that the yield of product 3a was found to be better in ethanol and methanol (Table 1, entries 7 and 11) than in other solvents under heating conditions. However, conventional heating was found to be very slow and the reaction was not completed even after 5 h. To improve the efficiency of the Knoevenagel reaction and yield of 3a, we exploited the microwave (MW) effect on the catalytic reaction using the nanocatalyst (Y2ZnO4). In recent years, microwave-assisted synthesis has become highly attractive and useful for the development of green and sustainable processes.8 Subsequently, we further optimized the reaction under microwave irradiation, employing the same nanocatalyst (10 mol % Y2ZnO4) under solvent-free conditions. The optimization of the Knoevenagel reaction under microwave irradiation is shown in Table 2. When the reaction mixture was irradiated at 140 W, within a short period of time, the formation of 3a was observed and the NMR yield was found to be 70% (Table 2, entry 1).
Table 1. Optimization of the Knoevenagel Condensation Using Nanocatalyst Y2ZnO4a.
| entry | solvent | nanocatalyst (mol %) | time (h) | condition | yield (%)b |
|---|---|---|---|---|---|
| 1 | no solvent | Y2ZnO4 (10%) | 5 | RT | 41 |
| 2 | no solvent | Y2ZnO4 (10%) | 5 | 70 °C | 56 |
| 3 | no solvent | Y2ZnO4 (05%) | 5 | 70 °C | 48 |
| 4 | H2O | Y2ZnO4 (10%) | 5 | RT | ndc |
| 5 | H2O | Y2ZnO4 (10%) | 5 | 70 °C | ndc |
| 6 | CH3OH | Y2ZnO4 (10%) | 5 | RT | ndc |
| 7 | CH3OH | Y2ZnO4 (10%) | 5 | 70 °C | 42 |
| 8 | CH3CN | Y2ZnO4 (10%) | 5 | RT | ndc |
| 9 | CH3CN | Y2ZnO4 (10%) | 5 | 70 °C | ndc |
| 10 | C2H5OH | Y2ZnO4 (10%) | 5 | RT | ndc |
| 11 | C2H5OH | Y2ZnO4 (10%) | 5 | 70 °C | 62 |
Reaction conditions: Unless otherwise mentioned, the reaction was carried out with benzaldehyde 1a (0.530 mmol, 56.2 mg), ethyl cyanoacetate 2 (0.442 mmol, 50 mg), and nanocatalyst Y2ZnO4 (10 mol %, 0.044 mmol, 13.6 mg) at room temperature (RT).
Yield of 3a was determined by 1H NMR analysis.
Not determined (nd).
Table 2. Optimization of the Knoevenagel Condensation under Microwave (MW) Conditionsa.
| entry | nanocatalyst (mol %) | time (min) | conditiona | yield (%)b |
|---|---|---|---|---|
| 1 | Y2ZnO4 (10%) | 15 | MW 140 W | 70 |
| 2 | Y2ZnO4 (10%) | 15 | MW 280 W | 79 |
| 3 | Y2ZnO4 (10%) | 15 | MW 420 W | 92 |
| 4 | Y2ZnO4 (10%) | 10 | MW 420 W | 78 |
| 5 | Y2ZnO4 (20%) | 10 | MW 420 W | 86 |
Reaction conditions: Unless otherwise mentioned, the reaction was carried out with benzaldehyde 1a (0.530 mmol, 56.2 mg), ethyl cyanoacetate 2 (0.442 mmol, 50 mg), and nanocatalyst Y2ZnO4 (10 mol %, 0.044 mmol, 13.6 mg) under microwave (MW) and solvent-free conditions.
Yield of 3a was determined by 1H NMR analysis.
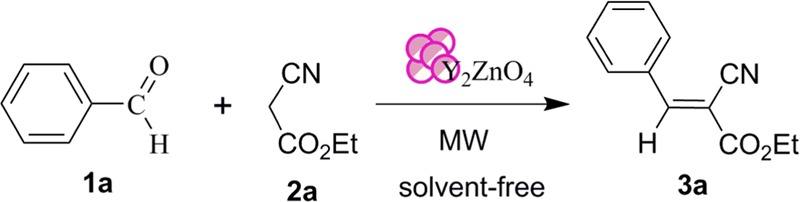
A variation in reaction yield was observed when the microwave irradiation power was varied. The reaction was found to be completed within 15 min when the microwave irradiation power was increased to 420 W, and the yield was increased to 92% (Table 2, entry 3). The effect of microwave irradiation power on the yield of 3a is depicted in Figure 3. Under the same reaction conditions, the yield of 3a was decreased when the reaction time was reduced to 10 min (Table 2, entry 4). Similarly, a reduced yield of 3a was observed when the catalyst loading was increased to 20 mol % (Table 2, entry 5). Under microwave irradiation, we achieved the best reaction condition at 420 W in the presence of 10 mol % nanocatalyst under solvent-free conditions (Table 2, entry 3). The effect of microwave irradiation power on the yield of 3a was studied, and we observed that the yield of 3a was increased by increasing the microwave power, as depicted in Figure 3.
Figure 3.
Effect of microwave irradiation power on the yield of product 3a.
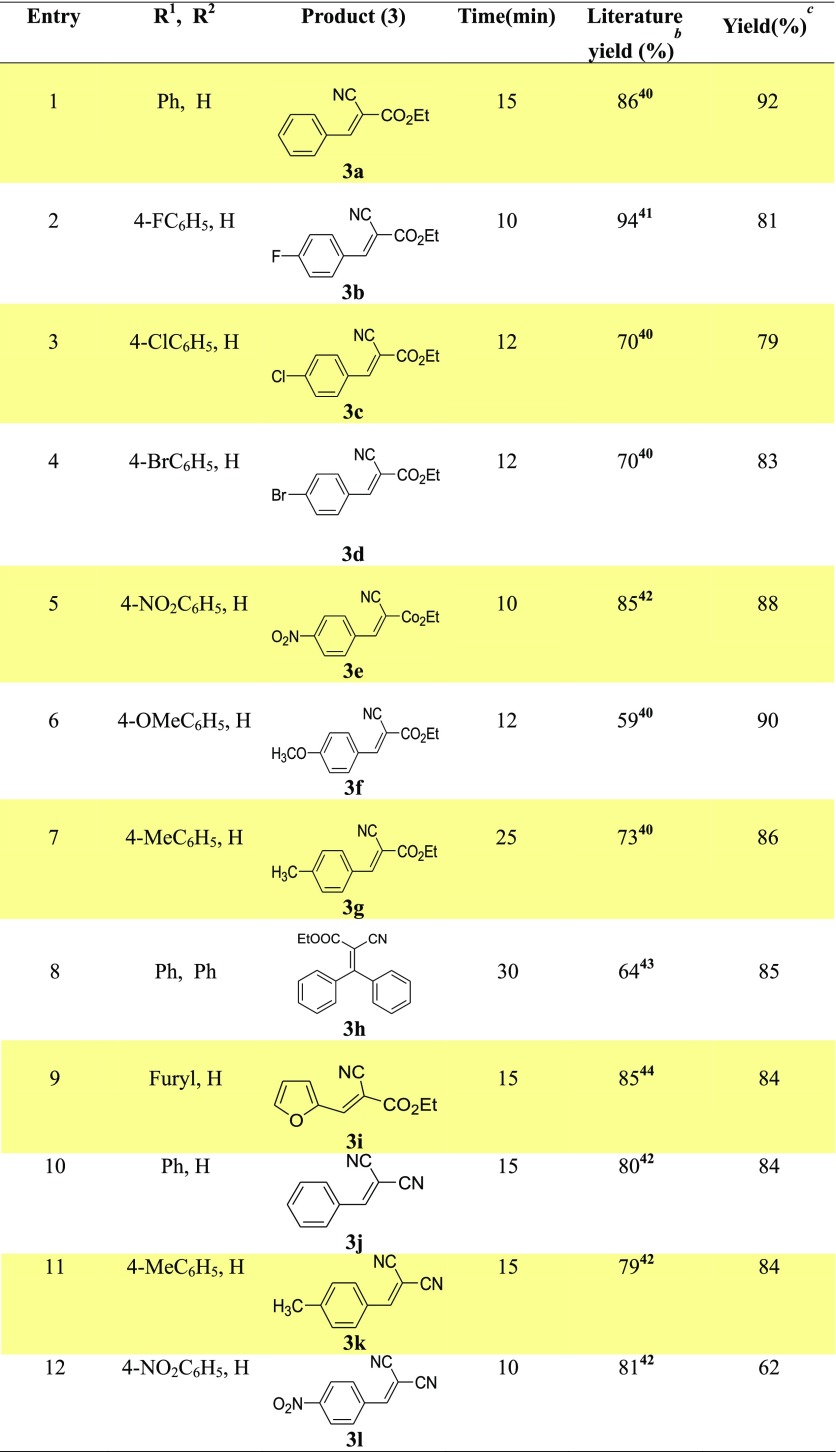
After the optimization of the reaction and determination of the best reaction condition using Y2ZnO4 as the nanocatalyst under microwave and solvent-free conditions, as shown in Table 2, entry 3, we next evaluated the scope and efficiency of the Knoevenagel condensation. Under the optimized condition, a range of aromatic carbonyl compounds 1 reacted smoothly with ethyl cyanoacetate 2a (R3 = CO2Et) and afforded the corresponding Knoevenagel condensation products (3a–l) with up to 92% yield within a short period of time (Table 3).
Table 3. Knoevenagel Condensation under the Optimized Conditiona.
Reaction conditions: Unless otherwise mentioned, the reaction was carried out with aromatic aldehyde 1 (0.530 mmol, 56.2 mg), ethyl cyanoacetate 2 (0.442 mmol, 50 mg), and nanocatalyst Y2ZnO4 (10 mol %, 0.044 mmol, 13.6 mg) under MW (420 W) and solvent-free conditions.
Literature yield.
Yield of 3 was determined by 1H NMR analysis.
No significant difference in the rate of reaction was found between various aromatic aldehydes containing electron-withdrawing and electron-donating groups under our reaction conditions. However, an aromatic ketone, such as benzophenone, reacted with a comparatively slower reaction rate to afford the corresponding Knoevenagel product, 3h (Table 3, entry 8). Under microwave conditions, heteroaromatic aldehyde furfural also reacted efficiently with ethyl cyanoacetate and afforded product 3i via the Knoevenagel condensation, as shown in Table 3, entry 9. Next, the substrate scope of the reaction was further evaluated using malononitrile as the active methylene compound. Under the optimized condition, aromatic carbonyl compounds reacted smoothly with malononitrile and afforded the corresponding Knoevenagel condensation products (3j–l) within a short period of time, as shown in Table 3 (entries 10–12). The structure of all products (3a–l) was determined by 1H NMR data and comparison with literature data.40−44 Compared to the reported methods, our method under microwave conditions is environmentally friendly and has several advantages including a short reaction time and solvent-free conditions with excellent conversion and yields. Moreover, the reaction does not require any additive or activators and the nanocatalyst used in the reaction is easily recoverable and recyclable.
Reusability of Nanocatalyst Y2ZnO4
After completion of the first run, diethyl ether was added to the reaction mixture and nanocatalyst Y2ZnO4 was recovered by simple filtration. The recovered catalyst was washed repeatedly with diethyl ether and dried at 100 °C in a hot air oven for reuse. The recyclability of the catalyst was studied for the Knoevenagel condensation under the optimized reaction condition. The catalyst recovered in the first run was reused in two more subsequent runs without any appreciable loss of catalytic activity (Figure 4).
Figure 4.
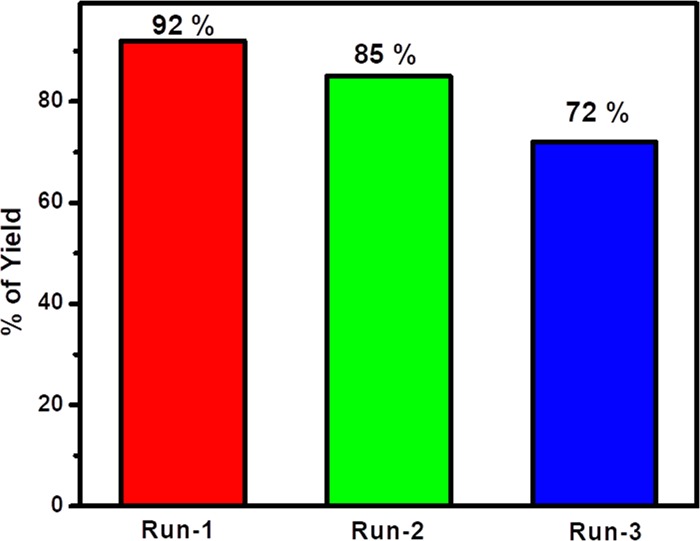
Recycling of nanocatalyst Y2ZnO4 in the Knoevenagel condensation.
Before and after the catalytic reaction, we studied the morphology of the Y2ZnO4 nanoparticle using SEM. After the reaction, the morphology was slightly changed, as shown in Figure 5; however, no loss of appreciable catalytic activity was observed in the subsequent runs. This is due to the high stability of nanocatalyst Y2ZnO4 under our reaction conditions.
Figure 5.
SEM image of nanocatalyst Y2ZnO4 after the reaction.
Mechanistic Pathway of the Knoevenagel Reaction
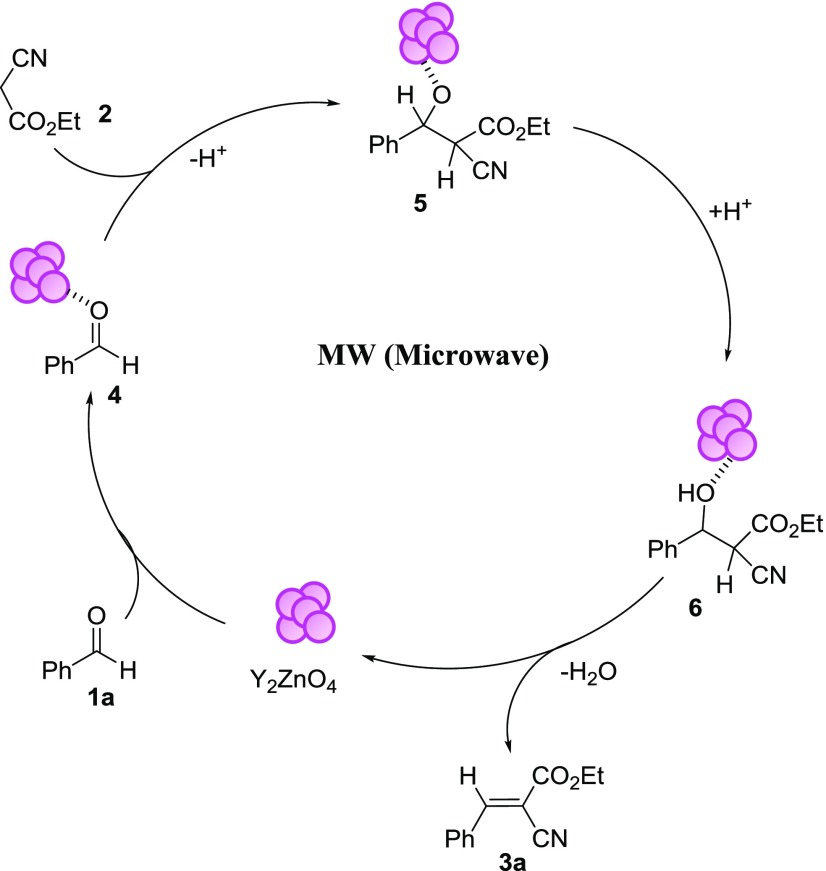
According to the literature6 and based on the above experimental facts, a possible reaction mechanism of the Knoevenagel reaction catalyzed by nanocatalyst Y2ZnO4 under microwave (MW) conditions is proposed in Scheme 1. In the first step, benzaldehyde 1a is activated by forming a complex between the carbonyl oxygen and the nanocatalyst. After that, the activated aldehyde complex 4 is attacked by the active methylene compound, ethyl cyanoacetate, generating an intermediate 5. Intermediate 5 is then protonated and activated by the nanocatalyst through coordination with a hydroxyl group, leading to the formation of complex 6. The elimination of water from intermediate 6 subsequently affords the corresponding product 3a. The high catalytic activity observed in this reaction is probably due to the large surface area of the nanoparticles.
Scheme 1. Proposed Mechanism for the Knoevenagel Condensation Catalyzed by Y2ZnO4.
Photocatalytic Activity
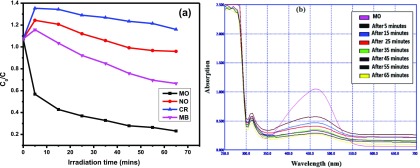
The photocatalytic activity of nanocatalyst Y2ZnO4 against different types of organic dyes, such as methyl orange (MO), methylene blue (MB), naphthol orange (NO), and congo red (CR), was studied in the presence of sunlight. The efficiency of a catalyst for the degradation of a dye is defined in terms of C/C0, where C and C0 represent the final and initial concentrations of the dye (Figure 6a). Among all dyes, MO was degraded faster compared with the other dyes and completely decolourized within 65 min in the presence of nanoparticles and sunlight (Figure 6a), whereas the other dyes, MB, NO, and CR, were not degraded. This is due to the lower energy band gap (2.7 eV) of nanocatalyst Y2ZnO4 calculated from optical spectra. According to Beer–Lambert’s law, the concentration of MO is linearly proportional to the intensity of the absorption peak of MO at 463 nm, and the efficiency of the nanocatalyst for degradation of MO can be calculated using the following eq 1
| 1 |
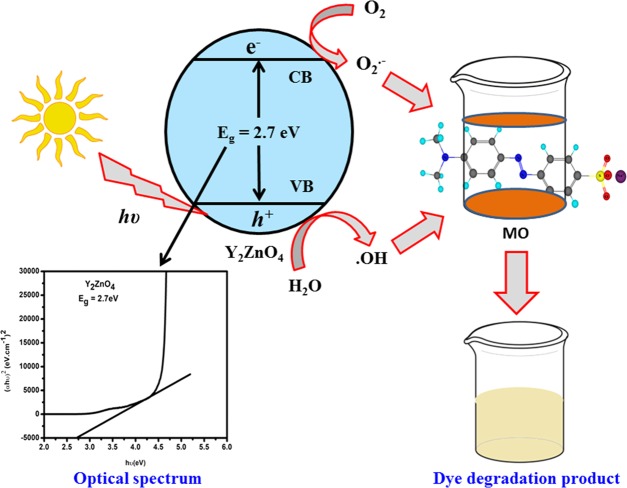
where At and A0 are the absorbance of the dye at time t and at the initial time, respectively. The degradation efficiency of nanocatalyst Y2ZnO4 is about 78.5% against MO and the corresponding rate constant (k) is about 3.939 × 10–4 s–1. The absorption spectra of methyl orange at a constant time interval were investigated and are shown in Figure 6b. The optical band gap was calculated according to our previous publication.46 The optical band gap value was 2.7 eV, which was supported by a literature review.3 The proposed mechanism pathway for the degradation of MO in the presence of the nanocatalyst and sunlight is shown in Figure 7.
Figure 6.
Photodegradation of different dyes by the nanocatalyst as a normalized change in the concentration as a function of irradiation time (a) and absorption spectra of the MO solution in the presence of the nanocatalyst under sunlight (b).
Figure 7.
Proposed mechanism for MO degradation in the presence of the nanocatalyst and sunlight.
Conclusions
In the above experiment, we synthesized a nanocatalyst Y2ZnO4 by a simple coprecipitation method. Nanocatalyst Y2ZnO4 has excellent dual catalytic activity; one of them is in the Knoevenagel reaction under microwave and solvent-free conditions to afford a range of substituted alkenes and the other one is as a photocatalyst for the degradation of MO. The reported method for the Knoevenagel reaction is commercially highly interesting because of several advantages, including mild reaction conditions, a green process, easy recovery and reusability of the catalyst, excellent yields, short reaction times, and an ecofriendly approach. The photocatalytic activity of Y2ZnO4 helps degrade the dye molecules and reduces harmful pollutants from the environment. Antimicrobial activity and other applications of Y2ZnO4 will be studied in further work.
Experimental Section
Materials and Apparatus
All required chemicals were purchased from Sigma-Aldrich and Merck. All chemicals were used without purification.
The Knoevenagel reaction was performed under microwave conditions. The microwave synthesis was carried out using a domestic microwave oven (2450 MHz, 1050 W) in a screw cap glass tube. The crystal structure of Y2ZnO4 was studied by X-ray diffraction (XRD) at room temperature using a D8 Advance Bruker, equipped with Cu Kα (1.54060 Å) as the incident radiation. The Scherrer equation was used for the calculation of crystal size. The Scherrer equation is D = Kλ/β cos θ, K = 0.9, D = crystal size (Å), λ = wavelength of Cu Kα radiation, and β = corrected half-width of the diffraction peak. The IR spectra were recorded on a Shimadzu FTIR-8400S spectrometer at room temperature. The fine structure of the prepared samples was analyzed by scanning electron microscopy (SEM) (Carl Zeiss Germany, Model Supra-40). Energy-dispersion X-ray spectroscopy (EDX) of nanoparticles was performed on a Sigma ZEISS, Oxford Instruments field emission microscope. The 1H NMR spectra were recorded on a JEOL-400 MHz spectrometer using chloroform-D (CDCl3) as the solvent and tetramethylsilane (TMS) as an internal standard. The chemical shifts are reported in ppm relative to TMS at δ 0.00 ppm. 1H NMR splitting patterns are designated as singlet (s), broad singlet (br. s), doublet (d), triplet (t), quartet (q), or multiplet (m).
Synthesis of Nanocatalyst Y2ZnO4
The total synthesis of the nanocatalyst was carried out by a coprecipitation method. Y(NO3)3·6H2O was dissolved in a minimum volume of distilled water (set-1). ZnO was also dissolved in minimum amounts of water and dil. HCl (set-2). Now, 20 mL of C2H5OH was added into set-1, and it was poured into set-2 with constant stirring with a magnetic stirrer for 30 min. A clear solution was obtained; then, NH4OH was added dropwise to maintain the pH (8–9), and a white gelatinous precipitate was produced, which was filtered. The precipitate washed with water and dried at about 600 °C for 2 h. The yield was 81.7% with respect to the starting materials.
General Procedure for the Knoevenagel Condensation Using Y2ZnO4 as the Nanocatalyst
A mixture of benzaldehyde 1a (0.530 mmol, 56.2 mg), ethyl cyanoacetate 2 (0.442 mmol, 50 mg), and Y2ZnO4 nanopowder (10 mol %, 0.044 mmol, 13.6 mg) was taken in a screw cap glass tube and subjected to microwave irradiation at 420 W. The reaction was monitored by thin layer chromatography (TLC) using 10% ethyl acetate in n-hexane. After 15 min, the reaction was found to be completed. Then, the glass tube was cooled and diluted with diethyl ether (5 mL). The catalyst was separated by filtration. The catalyst was washed thoroughly with diethyl ether (5 mL) and dried in a hot air oven for reuse. The combined ether was evaporated to dryness to obtain the crude product. The yield of the product was determined from the 1H NMR analysis of the crude reaction mixture. The yield of product 3a was found to be 92% (Table 3, entry 1). All products shown in Table 3 were synthesized according to the above procedure and were confirmed by 1H NMR spectral data and comparison with literature data (see the Supporting Information).
Dye Degradation
Rhodamine B (RB), methyl orange (MO), naphthyl orange (NO), and methylene blue (MB) were separately dissolved in water. Then, 10 mg of the nanocatalyst was soaked in 10 mL of dye solution and kept in sunlight. Furthermore, we examined the specific photocatalytic activity, and readings from the UV spectrometer were taken regularly.
Acknowledgments
This work was fully supported by Madhya Pradesh Council of Science & Technology, Govt. of India, Madhya Pradesh (File no. A/R&D/RP-2/Phy&Engg./2017-18/271), Science and Engineering Research Board (SERB), Govt. of India (File No. EMR/2017/000234), Indira Gandhi National Tribal University, Amarkantak, Madhya Pradesh, India, Dr. Harisingh Gour Vishwavidyalaya (Central University), Sagar, M.P., India, and Guru Ghasidas Vishwavidyalaya (Central University), Bilaspur, C.G.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b03875.
EDAX data, FT-IR data, and 1H NMR data (Figures S1–11 and Table S1) (PDF)
Author Contributions
All experiments and applications were performed by M.K.G., Dr. K.J., and S.K. under the supervision of Dr. T.K.G. and Dr. K.D. The manuscript was written by M.K.G. and reviewed by Dr. T.K.G. and Dr. K.D.
The authors declare no competing financial interest.
Supplementary Material
References
- Lakshminarayana B.; Mahendar L.; Ghosal P.; Sreedhar B.; Satyanarayana G.; Subrahmanyam C. Fabrication of Pd/CuFe2O4 hybrid nanowires: a heterogeneous catalyst for Heck couplings. New J. Chem. 2018, 42, 1646–1654. 10.1039/C7NJ04361A. [DOI] [Google Scholar]
- Jing L.; Xu Y.; Chen Z.; He M.; Xie M.; Liu J.; Xu H.; Huang S.; Li H. Different morphologies of SnS2 supported on 2D g-C3N4 for excellent and stable visible light photocatalytic hydrogen generation. ACS Sustainable Chem. Eng. 2018, 6, 5132–5141. 10.1021/acssuschemeng.7b04792. [DOI] [Google Scholar]
- Mote V. D.; Purushotham Y.; Shinde R. S.; Salunke S. D.; Dole B. N. Structural, optical and antibacterial properties of yttrium doped ZnO nanoparticles. Cerâmica 2015, 61, 457–461. 10.1590/0366-69132015613601932. [DOI] [Google Scholar]
- Andersen H. L.; Saura-Múzquiz M.; Granados-Miralles C.; Canévet E.; Lock N.; Christensen M. Crystalline and magnetic structure–property relationship in spinel ferrite nanoparticles. Nanoscale 2018, 10, 14902–14914. 10.1039/C8NR01534A. [DOI] [PubMed] [Google Scholar]
- Elmes R. B.; Orange K. N.; Cloonan S. M.; Williams D. C.; Gunnlaugsson T. Luminescent ruthenium(II) polypyridyl functionalized gold nanoparticles; their DNA binding abilities and application as cellular imaging agents. J. Am. Chem. Soc. 2011, 133, 15862–15865. 10.1021/ja2061159. [DOI] [PubMed] [Google Scholar]
- Ghomi J. S.; Akbarzadeh Z. Ultrasonic accelerated Knoevenagel condensation by magnetically recoverable MgFe2O4 nanocatalyst: A rapid and green synthesis of coumarins under solvent-free conditions. Ultrason. Sonochem. 2018, 40, 78–83. 10.1016/j.ultsonch.2017.06.022. [DOI] [PubMed] [Google Scholar]
- Lim C. W.; Lee I. S. Magnetically recyclable nanocatalyst systems for the organic reactions. Nano Today 2010, 5, 412–434. 10.1016/j.nantod.2010.08.008. [DOI] [Google Scholar]
- a Varma R. Solvent-free organic syntheses. using supported reagents and microwave irradiation. Green Chem. 1999, 1, 43–55. 10.1039/a808223e. [DOI] [Google Scholar]; b Peng Y.; Song G. Combined microwave and ultrasound accelerated Knoevenagel–Doebner reaction in aqueous media: a green route to 3-aryl acrylic acids. Green Chem. 2003, 5, 704–706. 10.1039/B310388A. [DOI] [Google Scholar]
- Gedye R.; Smith F.; Westaway K.; Ali H.; Baldisera L.; Laberge L.; Rousell J. The use of microwave ovens for rapid organic synthesis. Tetrahedron Lett. 1986, 27, 279–282. 10.1016/S0040-4039(00)83996-9. [DOI] [Google Scholar]
- Naeimi H.; Aghaseyedkarimi D. Fe3O4@SiO2·HM·SO3H as a recyclable heterogeneous nanocatalyst for the microwave-promoted synthesis of 2,4,5-trisubstituted imidazoles under solvent free conditions. New J. Chem. 2015, 39, 9415–9421. 10.1039/C5NJ01273B. [DOI] [Google Scholar]
- Sun D.; Wang B.; Wang H. M.; Li M. F.; Shi Q.; Zheng L.; Wang S. F.; Liu S. J.; Sun R. C. Structural transformations of Hybrid Pennisetum lignin: effect of microwave-assisted hydrothermal pretreatment. ACS Sustainable Chem. Eng. 2019, 7, 3073–3082. 10.1021/acssuschemeng.8b04695. [DOI] [Google Scholar]
- Egami H.; Tamaoki S.; Abe M.; Ohneda N.; Yoshimura T.; Okamoto T.; Odajima H.; Mase N.; Takeda K.; Hamashima Y. Scalable microwave-assisted Johnson–Claisen rearrangement with a continuous flow microwave system. Org. Process Res. Dev. 2018, 22, 1029–1033. 10.1021/acs.oprd.8b00185. [DOI] [Google Scholar]
- Ramesh K.; Basuli S.; Satyanarayana G. Microwave Assisted Domino Palladium Catalysis in Water: A Diverse Synthesis of 3,3′ Disubstituted Heterocyclic Compounds. Eur. J. Org. Chem. 2018, 2018, 2171–2177. 10.1002/ejoc.201800155. [DOI] [Google Scholar]
- Gawande M. B.; Shelke S. N.; Zboril R.; Varma R. S. Microwave-assisted chemistry: synthetic applications for rapid assembly of nanomaterials and organics. Acc. Chem. Res. 2014, 47, 1338–1348. 10.1021/ar400309b. [DOI] [PubMed] [Google Scholar]
- Hattori M.; Shimamoto D.; Ago H.; Tsuji M. AgPd@ Pd/TiO2 nanocatalyst synthesis by microwave heating in aqueous solution for efficient hydrogen production from formic acid. J. Mater. Chem. A 2015, 3, 10666–10670. 10.1039/C5TA01434D. [DOI] [Google Scholar]
- Villemin D.; Labiad B. Clay catalysis: dry condensation of barbituric acid with aldehydes under microwave irradiation. Synth. Commun. 1990, 20, 3333–3337. 10.1080/00397919008051567. [DOI] [Google Scholar]
- Qiu G.; Dharmarathna S.; Genuino H.; Zhang Y.; Huang H.; Suib S. L. Facile microwave-refluxing synthesis and catalytic properties of vanadium pentoxide nanomaterials. ACS Catal. 2011, 1, 1702–1709. 10.1021/cs200437x. [DOI] [Google Scholar]
- Praneeth N. V.; Paria S. Microwave-assisted one-pot synthesis of anisotropic gold nanoparticles with active high-energy facets for enhanced catalytic and metal enhanced fluorescence activities. CrystEngComm 2018, 20, 4297–4304. 10.1039/C8CE00654G. [DOI] [Google Scholar]
- Karmakar A.; Martins L. M.; Hazra S.; Guedes da Silva M. F. C.; Pombeiro A. J. Metal–organic frameworks with pyridyl-based isophthalic acid and their catalytic applications in microwave assisted peroxidative oxidation of alcohols and henry reaction. Cryst. Growth Des. 2016, 16, 1837–1849. 10.1021/acs.cgd.5b01178. [DOI] [Google Scholar]
- Qiu G.; Huang H.; Genuino H.; Opembe N.; Stafford L.; Dharmarathna S.; Suib S. L. Microwave-assisted hydrothermal synthesis of nanosized α-Fe2O3 for catalysts and adsorbents. J. Phys. Chem. C 2011, 115, 19626–19631. 10.1021/jp2067217. [DOI] [Google Scholar]
- Song C.; Li R.; Liu F.; Feng X.; Tan W.; Qiu G. Cobalt-doped todorokites prepared by refluxing at atmospheric pressure as cathode materials for Li batteries. Electrochim. Acta 2010, 55, 9157–9165. 10.1016/j.electacta.2010.09.003. [DOI] [Google Scholar]
- Martín-Acosta P.; Feresin G.; Tapia A.; Estévez-Braun A. Microwave-assisted organocatalytic intramolecular knoevenagel/hetero diels–alder reaction with O-(Arylpropynyloxy)-Salicylaldehydes: synthesis of polycyclic embelin derivatives. J. Org. Chem. 2016, 81, 9738–9756. 10.1021/acs.joc.6b01818. [DOI] [PubMed] [Google Scholar]
- Biswas P.; Ghosh J.; Maiti S.; Bandyopadhyay C. Microwave-induced domino-Knoevenagel-hetero Diels–Alder reaction—an easy route to di[1]benzopyrano[2,3-b:4′,3′-d] pyridine. Tetrahedron Lett. 2014, 55, 6882–6886. 10.1016/j.tetlet.2014.10.095. [DOI] [Google Scholar]
- Xiao B. X.; Jiang B.; Song X.; Du W.; Chen Y. C. Phosphine-catalysed asymmetric dearomative formal [4+2] cycloadditions of 3-benzofuranyl vinyl ketones. Chem. Commun. 2019, 55, 3097–3100. 10.1039/C9CC00386J. [DOI] [PubMed] [Google Scholar]
- a Peña R.; Martín P.; Feresin G. E.; Tapia A.; Machín F.; Estévez-Braun A. Domino synthesis of embelin derivatives with antibacterial activity. J. Nat. Prod. 2016, 79, 970–977. 10.1021/acs.jnatprod.5b01038. [DOI] [PubMed] [Google Scholar]; b Aghazadeh M.; Ghaemi M.; Nozad Golikand A.; Yousefi T.; Jangju E. Yttrium oxide nanoparticles prepared by heat treatment of cathodically grown yttrium hydroxide. ISRN Ceram. 2011, 2011, 542104 10.5402/2011/542104. [DOI] [Google Scholar]
- Zhou J. F.; Song Y. Z.; Lv J. S.; Gong G. X.; Tu S. Facile one-pot, multicomponent synthesis of pyridines under microwave irradiation. Synth. Commun. 2009, 39, 1443–1450. 10.1080/00397910802531930. [DOI] [Google Scholar]
- Ruys A. J.; Mai Y. W. The nanoparticle-coating process: a potential sol-gel route to homogeneous nanocomposites. Mater. Sci. Eng. A 1999, 265, 202–207. 10.1016/S0921-5093(98)01126-5. [DOI] [Google Scholar]
- Zhou C.; Liang G.; Gu A. Fabrication of variable frequency motors using polyester-imide-hybridized resins and hyperbranched polysiloxane coated nano-TiO2. J. Mater. Sci. 2015, 50, 7314–7325. 10.1007/s10853-015-9288-2. [DOI] [Google Scholar]
- Hsiung T. L.; Wang H. P.; Wang H. C. XANES studies of photocatalytic active species in nano TiO2–SiO2. Radiat. Phys. Chem. 2006, 75, 2042–2045. 10.1016/j.radphyschem.2005.11.021. [DOI] [Google Scholar]
- Xu H.; Cui L.; Tong N.; Gu H. Development of high magnetization Fe3O4/polystyrene/silica nanospheres via combined miniemulsion/emulsion polymerization. J. Am. Chem. Soc. 2006, 128, 15582–15583. 10.1021/ja066165a. [DOI] [PubMed] [Google Scholar]
- Ermakova M. A.; Ermakov D. Y.; Cherepanova S. V.; Plyasova L. M. Synthesis of Ultradispersed Nickel Particles by Reduction of High-Loaded NiO–SiO2 Systems Prepared by Heterophase Sol–Gel Method. J. Phys. Chem. B 2002, 106, 11922–11928. 10.1021/jp021231q. [DOI] [Google Scholar]
- Corrias A.; Mountjoy G.; Piccaluga G.; Solinas S. An X-ray Absorption Spectroscopy Study of the Ni K Edge in NiO–SiO2 Nanocomposite Materials Prepared by the Sol–Gel Method. J. Phys. Chem. B 1999, 103, 10081–10086. 10.1021/jp9927911. [DOI] [Google Scholar]
- Díaz G.; Perez-Hernandez R.; Gomez-Cortes A.; Benaissa M.; Mariscal R.; Fierro J. L. CuO–SiO2 sol–gel catalysts: characterization and catalytic properties for no reduction. J. Catal. 1999, 187, 1–14. 10.1006/jcat.1999.2578. [DOI] [Google Scholar]
- Armelao L.; Barreca D.; Bottaro G.; Mattei G.; Sada C.; Tondello E. Copper-Silica Nanocomposites Tailored by the Sol-Gel Route. Chem. Mater. 2005, 17, 1450–1456. 10.1021/cm048245o. [DOI] [Google Scholar]
- Chandraker S. K.; Ghosh M. K.; Lal M.; Ghorai T. K.; Shukla R. Colorimetric sensing of Fe3+ and Hg2+ and photocatalytic activity of green synthesized silver nanoparticles from the leaf extract of Sonchus arvensis L. New J. Chem. 2019, 43, 18175–18183. 10.1039/C9NJ01338E. [DOI] [Google Scholar]
- Yang L.; Li X.; Sun C. Y.; Wu H.; Wang C. G.; Su Z. M. A stable pillared-layer Cu (ii) metal–organic framework with magnetic properties for dye adsorption and separation. New J. Chem. 2017, 41, 3661–3666. 10.1039/C7NJ00389G. [DOI] [Google Scholar]
- Pathak S.; Ghosh M. K.; Ghorai T. K. Luminescence, Dye Degradation and DNA Binding Properties of a Dinuclear Nona Coordinated Y (III) Complex. ChemistrySelect 2018, 3, 13501–13506. 10.1002/slct.201801826. [DOI] [Google Scholar]
- Umamaheswari C.; Lakshmanan A.; Nagarajan N. S. Green synthesis, characterization and catalytic degradation studies of gold nanoparticles against congo red and methyl orange. J. Photochem. Photobiol., B 2018, 178, 33–39. 10.1016/j.jphotobiol.2017.10.017. [DOI] [PubMed] [Google Scholar]
- Mazaheri H.; Ghaedi M.; Azqhandi M. A.; Asfaram A. Application of machine/statistical learning, artificial intelligence and statistical experimental design for the modeling and optimization of methylene blue and Cd (II) removal from a binary aqueous solution by natural walnut carbon. Phys. Chem. Chem. Phys. 2017, 19, 11299–11317. 10.1039/C6CP08437K. [DOI] [PubMed] [Google Scholar]
- Wan J. P.; Jing Y.; Liu Y.; Sheng S. Metal-free synthesis of cyano acrylates via cyanuric chloride-mediated three-component reactions involving a cascade consists of Knoevenagel condensation/cyano hydration/esterification. RSC Adv. 2014, 4, 63997–64000. 10.1039/C4RA13826K. [DOI] [Google Scholar]
- Shen Y.; Yang B. Synthesis of α, β-Unsaturated Cyanoesters Promoted by Tri-N-Butylarsine. Synth. Commun. 1989, 19, 3069–3075. 10.1080/00397918908052703. [DOI] [Google Scholar]
- Cabello J. A.; Campelo J. M.; Garcia A.; Luna D.; Marinas J. M. Knoevenagel condensation in the heterogeneous phase using aluminum phosphate-aluminum oxide as a new catalyst. J. Org. Chem. 1984, 49, 5195–5197. 10.1021/jo00200a036. [DOI] [Google Scholar]
- Gholap A. R.; Paul V.; Srinivasan K. V. Novel Process for the Synthesis of Class I Antiarrhythmic Agent (±)-Cibenzoline and Its Analogs. Synth. Commun. 2008, 38, 2967–2982. 10.1080/00397910802006388. [DOI] [Google Scholar]
- Khan R. H.; Mathur R. K.; Ghosh A. C. Tellurium (IV) tetrachloride catalysed facile Knoevenagel reaction. Synth. Commun. 1996, 26, 683–686. 10.1080/00397919608086741. [DOI] [Google Scholar]
- Jain K.; Chaudhuri S.; Pal K.; Das K. The Knoevenagel condensation using quinine as an organocatalyst under solvent-free conditions. New J. Chem. 2019, 43, 1299–1304. 10.1039/C8NJ04219E. [DOI] [Google Scholar]
- Ghosh M. K.; Pathak S.; Ghorai T. K. Synthesis of Two Mononuclear Schiff Base Metal (M = Fe, Cu) Complexes: MOF Structure, Dye Degradation, H2O2 Sensing, and DNA Binding Property. ACS Omega 2019, 4, 16068–16079. 10.1021/acsomega.9b02268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.