Abstract
Valproic acid (VP) is a very effective therapy for the management of generalized epilepsy. However, its use during pregnancy leads to increased risk of teratogenesis and cognitive malfunctioning in postnatal growing children. Antioxidants are used commercially as a palliative therapy. This study compares the different antioxidants effects on VP-induced teratogenicity. Pregnant female rats (n = 80) were divided into eight groups (n = 10) as follows: Group I, control group; Group II, disease group valproic acid (500 mg/kg); Groups III and IV, treated with 2000 and 8000 mg/kg vitamin C, respectively; Groups V and VI, treated with selenium 100 and 200 μg/kg dose, respectively; and Groups VII and VIII, administered grape seed extract 300 and 600 mg/kg, respectively. Groups III–VIII received valproic acid (500 mg/kg) along with their respective treatments. All treatments were given via an oral route. The fetuses were double stained, and levels of superoxide dismutase (SOD), catalase (CAT), nitrite, glutathione (GSH), and malondialdehyde (MDA) were estimated. Resorption rate was significantly reduced in Vit. C treated groups at both dose levels. Maternal death rate was decreased remarkably in all treatment groups. Vit. C at a high dose (8000 mg/kg) and grape seed at a high dose (600 mg/kg) significantly reduced the incidence of delayed cervical ossification. The values of MDA were significantly reduced in all groups except the Vit. C group (2000 mg/kg). However, no significant elevation was observed in the values of SOD, CAT, and GSH. The current study concluded that vitamin C at a high dose (8000 mg/kg) and grape seed extract at a high dose (600 mg/kg) had partially protected the fetuses exposed to VP.
1. Introduction
The term teratogenesis originated from the Greek word meaning “monster”. It is defined as a structural abnormality, functional or metabolic malfunctioning present at the time of birth of an infant leading to physical or mental disability or fatality.1 Drugs and chemicals can be labeled as teratogen on the basis of its potential to induce teratogenic effects. Research revealed that the teratogens are characterized according to their dose, route of administration, and gestational stage of exposure.2 Valproic acid (VP) is a known teratogen. It is an effective broad-spectrum anticonvulsant drug. It is also used in the treatment of bipolar disorder and migraine. Valproic acid is very effective in management of generalized epilepsy. However, its use during pregnancy leads to an increased risk of teratogenesis and cognitive malfunctioning in postnatal growing children.3 Exposure to valproic acid in pregnancy specifically during early embryonic stage corresponds with many fetal abnormalities including heart malfunctioning, skeletal anomalies, neural tube defects, and limb deformities.4 Some mechanisms are proposed as an etiology of VP-associated teratogenesis. In the current study, the focus was on the redox control of embryo toxicity. The most commonly reported fetal anomaly due to VP administration is neural tube defects. Previous studies suggested that VP administration during pregnancy increased the reactive oxygen species (ROS) production. These ROS damages the DNA double-stranded structure. It is repaired by homologous recombination (HR), which results in detrimental changes in the genetic makeup of the DNA. The irregulation in gene expression that causes dysfunctioning of the specified genes responsible for normal embryo development ultimately ends up with the neural tube defects in the fetus.5 The imbalance between the production and elimination of the ROS and nitrogenous species and loss of coordination between prevention and repair mechanisms are called oxidative/nitrosative stress. Nitrosative stress (NS) represented the generation of nitrogenous species that plays an important role in the progression of the diseases state.6 Antioxidants are the endogenous enzymes, natural substances, and drugs that counteract the harmful effects of oxidative/nitrosative stress.7 The current study was conducted to estimate the potential of selected antioxidants against the teratogenic effect of valproic acid. The selected antioxidants were vitamin C, selenium, and grape seed extract.
Vitamin C is a water-soluble vitamin and is an essential nutrient for humans. Vitamin C neutralizes the ROS production in oxidative stress. Its antioxidant mechanisms involve a direct reaction with aqueous peroxyl radicals or by indirectly restoring the fat-soluble antioxidant vitamins. These properties of vitamin C are most probably due to its chemical properties as a chelating and reducing agent.8 Another antioxidant selected in this study is selenium, which is an oligo element; it has diverse health benefits including its antioxidant activity, anti-inflammatory action, and reproduction and production of thyroid hormone. It is a trace element and very potent agent. On the basis of its antioxidant properties, it is used to reverse many pathological conditions associated with oxidative stress. The protective effects of selenium against hepatotoxicity, cytotoxicity, and genotoxicity are well-established.9 Few selenoproteins are termed as a selenoenzymes like glutathione peroxidase and thio redox in reductase, representing the basic antioxidant systems for the regulation of redox homeostasis at a cellular level.10
Grape seed is also a powerful antioxidant, which protects the body from development of pathological conditions secondary to oxidative stress. The major flavonoids in grape seed extract powder (GSEP) that contributes in its antioxidant property are proanthocyanidins. It is evident from the research that the antioxidant capacity of proanthocyanidins is 20 times higher than vitamin E and 50 times higher than vitamin C.11 The phytochemical components in grape seed powder extract are different in various kinds. GSEP contain anthocyanins, phenolic acids, proanthocyanidins, and stilbenes, all of these are strong antioxidants.12 It contains 11% protein, 35% fiber, 3% minerals, and 7% water. The range of the lipid content of GSEP is 7–20%.13 Many pharmacological activities of grape seed extract have been identified. It was found to be beneficial for diabetic patients by decreasing the paraoxonase activity in experimental rats.14 Multiple studies suggested that proanthocyanidins in GSEP successfully suppressed the inflammatory events.15 Regular consumption of GREP decreases the degenerative changes in the body.16 The antimicrobial activity of GSEP was determined against 43 strains of MRSA at 3 mg/mL GSEP equal to 20.7 μg/mL proanthocyanidin content. All bacterial strains were completely inhibited by GSEP.17 Due to the antioxidant property of GSEP, it is extensively used at both high- and low-dose levels in pregnant specimens to evaluate its protective effect against specified teratogens.18
2. Results and Discussion
Teratogenesis is a global problem especially with the emerging era of new drug development and investigation. The teratogenic study is considered as one of the fundamental part of toxicological evaluation of any drug, and it is important to reduce the births of malformed infants.19 The obscurity in getting data regarding the effect of drug on a developing fetus is due to limitations in inclusion of pregnant women in clinical trials.20 It has been reported that anticonvulsant drugs cause major fetal malformations called “anticonvulsant embryopathy”.21 Childbearing women are at major risk of embryopathy associated with anticonvulsants. Numerous studies suggested that valproic acid-induced fetal malformations are due to the formation of reactive oxygen species because, during the initial developmental stage, the embryonic antioxidant system is immature, and the embryo is more prone to damage by oxidative stress, which results in extensive fetal structural abnormalities.4 It has been established that supplementation with antioxidants reversed or reduced the incidence of valproic acid-induced embryo toxicity.22 The previous studies were further expanded in the present study, and specified antioxidants were administered to inhibit the VP-associated teratogenesis.
Table 1 shows that the values of malondialdehyde (MDA) were significantly reduced in all groups in comparison to the disease group except the low-dose vitamin C group (2000 mg/kg). This indicated that oxidative stress was reduced by the administration of specified antioxidants in combination with VP. However, activities of SOD, CAT, nitrite, and GSH were not significantly altered by the given antioxidants. The mechanism of teratogenicity of valproic acid was investigated by the estimation of levels of endogenous antioxidants. Malondialdehyde is used as an expedient biomarker for lipid peroxidation of fatty acids such as omega-3 and omega-6. This wide use of MDA is due to its simpler chemical reaction with thiobarbituric acid. It is the most convenient and reliable marker for the determination of oxidative stress in a variety of in vivo studies.23 In the present study, MDA values were significantly reduced, suggesting that malondialdehyde exerts a protective mechanism against VP-induced fetal toxicity (Table 1). Treatment agents along with valproic acid reduce somehow the levels of nitrite levels, so they inhibited the less nitrogen generating species (NOS) produced due to the imbalance between oxidative and nitrosative stress.
Table 1. Estimation of Biochemical Markers for Oxidative Stressa.
| treatment groups | SOD (μg/mg tissue) | CAT (μg/mg tissue) | GSH (μg/mg tissue) | MDA (n mole/mg of tissue) | nitrite (μg/mg protein) |
|---|---|---|---|---|---|
| N.C | 8.09 ± 1.11 | 216.55 ± 45.27 | 8.94 ± 0.79 | 2341.46 ± 2017.51 | 3.5 ± 0.5 |
| D.C V.P (500 mg/kg) | 7.38 ± 1.06 | 584.86 ± 223.90 | 71.56 ± 34.35 | 8650.38 ± 2708.84 | 5.6 ± 15.2 |
| V.P + Vit. C (500 mg + 2000 mg/kg) | 13.68 ± 2.18 | 1144.86 ± 143.11 | 4.82 ± 0.48 | 10166.80 ± 4193.76 | 5.2 ± 1.5 |
| V.P + Vit. C (500 mg + 8000 mg/kg) | 13.18 ± 1.04 | 189.29 ± 18.98 | 13.30 ± 3.10 | 80.80 ± 18.77* | 5.0 ± 2.5 |
| V.P + Se (500 mg + 100 μg/kg) | 10.88 ± 0.79 | 210.38 ± 27.04 | 11.56 ± 1.24 | 190.04 ± 60.02* | 4.9 ± 23.5 |
| V.P + Se (500 mg + 200 μg/kg) | 9.482 ± 1.65 | 180.98 ± 6.71 | 2.57 ± 0.44 | 60.34 ± 14.44* | 4.6 ± 15.2 |
| V.P + G.S (500 mg + 300 mg/kg) | 8.64 ± 1.85 | 101.63 ± 1.35 | 11.25 ± 4.30 | 329.38 ± 173.95* | 4.5 ± 1.8 |
| V.P + G.S (500 mg + 600 mg/kg) | 13.05 ± 0.36 | 183.91 ± 20.35 | 2.32 ± 0.70 | 75.64 ± 18.67* | 3.9 ± 3.6 |
Data are represented as mean ± SEM, n = 10; *P < 0.05 was given versus disease control, N.C = normal control, D.C = disease control, V.P = valproic acid, Vit. C = vitamin C, Se = selenium, and G.S = grape seeds.
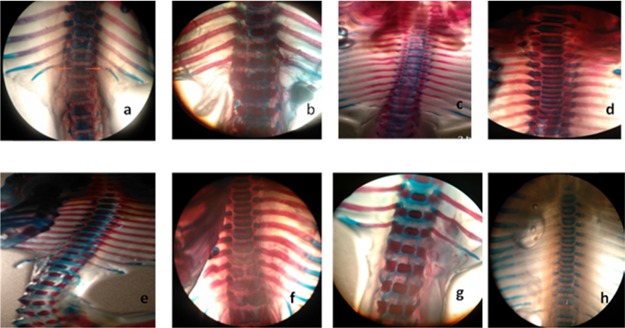
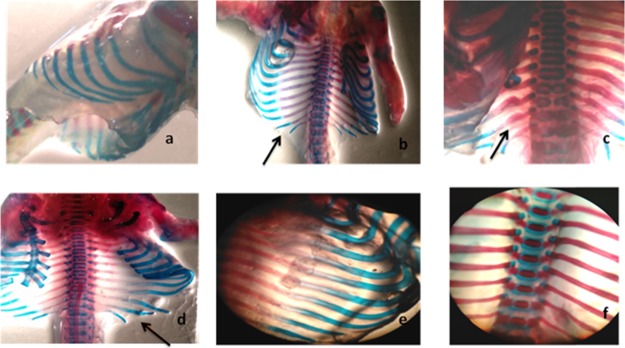
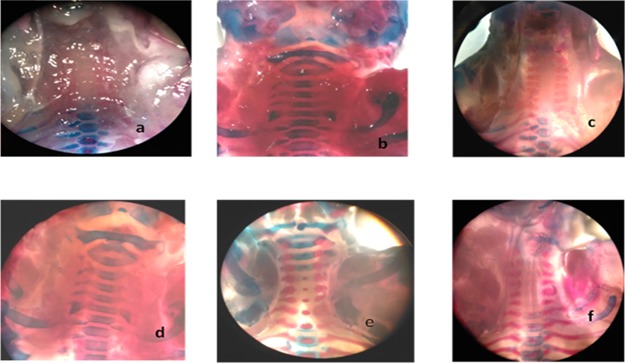
Skeletal anomalies were observed after double staining of fetal skeletons.24 In rodents, mostly spina bifida occulta is evident with valproic acid administration as compared to open neural tube defects such as spina bifida aperta.25 In the current study, our results indicated that spina bifida occulta was reduced by all specified antioxidants. Several specimens were presented with the normal vertebral arch (Table 2). The space between the cartilaginous ends of the vertebral arch of the fetus was measured, and spacing was reduced in all treatment groups except selenium at a low dose (10 μg/kg). Other skeletal anomalies such as rib malformations, cleft palate, and delayed cervical ossifications are also associated with VP-induced fetal toxicity.24 The vitamin C (8000 mg/kg) and grape seed (600 mg/kg) treatment group showed a protective effect against delayed cervical ossifications when compared to non-ossified cervical regions of the disease control group. Complete cervical ossification was observed in specimens received vitamin C (8000 mg/kg) and grape seed extract powder (600 mg/kg), which reflects upon the successful protective effect exerted by the specified antioxidants. The specimens were carefully examined under a dissecting microscope for cleft palate, spina bifida occulta, rib malformations, and cervical ossification (Figures 1–5). Vitamin C at a high dose (8000 mg/kg) and grape seed at a high dose (600 mg/kg) significantly reduced the incidence of delayed cervical ossification (Table 2), whereas rib malformation was significantly (P < 0.05) minimized by selenium at a high dose (200 μg/kg) and grape seed at a high dose (600 mg/kg) (Table 2). Moreover, co-administration of valproic acid with vitamin C (2000 + 8000 mg/kg), selenium (200 μg/kg), and grape seed (300 + 600 mg/kg) significantly (P < 0.05) reduced the distance between the cartilaginous ends of the vertebral arch, which is evident in the reduction of incidence of spina occulta in the stained specimens (Table 2).
Table 2. Incidence of Major Skeletal Malformations in Fetuses Treated with VP and the Protective Effect of Vit. C, Se, and Grape Seed Extracta.
| treatment groups | cleft palate | spina bifida occulta (μ) | rib malformation | delayed cervical ossification |
|---|---|---|---|---|
| N.C | 0.00 ± 0.00 | 40 ± 0.40 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| D.C V.P (500 mg/kg) | 0.40 ± 0.14 | 62.40 ± 0.77 | 0.45 ± 0.15 | 0.50 ± 0.14 |
| V.P + Vit. C (500 mg + 2000 mg/kg) | 0.11 ± 0.11 | 53.60 ± 2.28* | 0.30 ± 0.15 | 0.15 ± 0.10 |
| V.P + Vit. C (500 mg + 8000 mg/kg) | 0.05 ± 0.05 | 53.20 ± 2.35* | 0.20 ± 0.13 | 0.00 ± 0.00* |
| V.P + Se (500 mg + 100 μg/kg) | 0.25 ± 0.16 | 54.4 ± 2.36 | 0.40 ± 0.16 | 0.40 ± 0.16 |
| V.P + Se (500 mg + 200 μg/kg) | 0.20 ± 0.13 | 52.8 ± 2.60* | 0.00 ± 0.00* | 0.20 ± 0.13 |
| V + G.S (500 mg + 300 mg/kg) | 0.27 ± 0.14 | 51.8 ± 2.95* | 0.20 ± 0.13 | 0.30 ± 0.13 |
| V.P + G.S (500 mg + 600 mg/kg) | 0.11 ± 0.11 | 52 ± 2.95* | 0.00 ± 0.00* | 0.00 ± 0.00* |
All data are represented as mean ± SEM, n = 10; *P < 0.05 was given in comparison to the disease group.
Figure 1.
Spina bifida occulta represented the increased vertebral arch spacing of the lumbar region is evident for spina bifida occulta. (a) Disease group 62 μ. (b) Normal control 40 μ. (c, f) GSE at 300 mg/kg, 61 and 62 μ, respectively. (d, e) 60 μ, GSEP 600 mg/kg vertebral spacing 40 μ, no evidence of spina bifida. (g) Se low dose 58 μ. (h) Se low dose 54 μ.
Figure 5.
Rib malformation (black arrows). (a) Normal control group. (b) Disease group malformed. (c) GSEP at 300 mg/kg, wavy ribs. (d) Vit. C at 2000 mg/kg, fused ribs. (e) Vit. C at 8000 mg/kg, no rib malformation. (f) GSEP at 600 mg/kg, normal rib.
Figure 2.
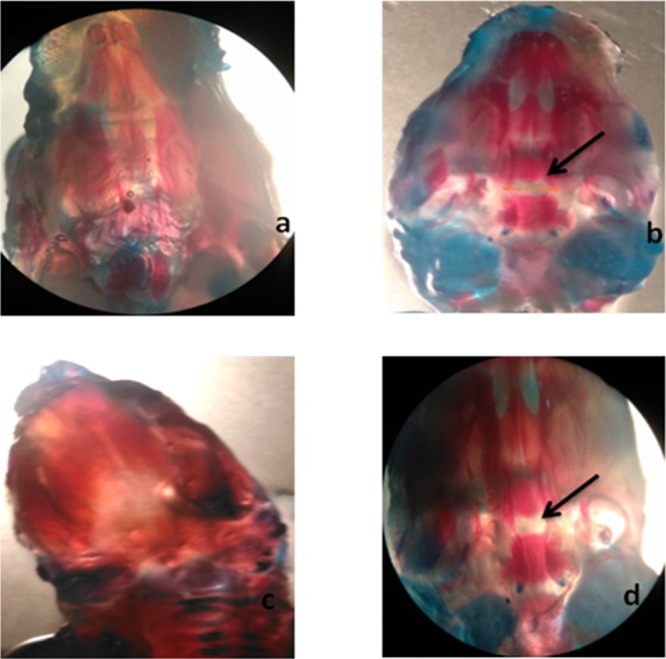
(a) Normal palate, control group. (b) Disease group (arrows indicate cleft palate). (c) GSEP at 600 mg/kg represents normal cleft palate. (d) Low-dose (300 mg/kg) GSEP depicting cleft palate.
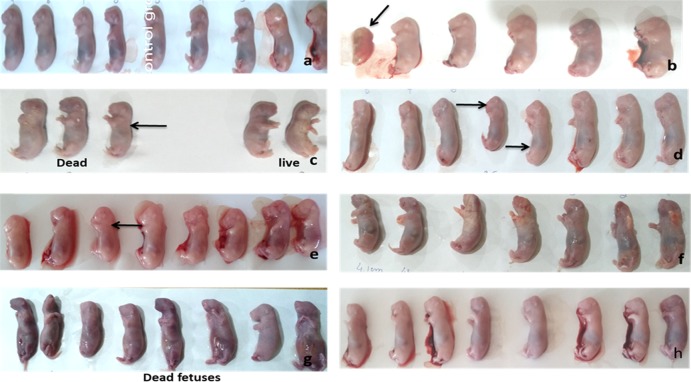
Figure 3.
Gross observation (black arrows show growth retardation). (a) Normal group. (b) Disease group. (c) Vit. C (2000 mg/kg) + VP. (d) VP + Se 100 μg/kg. (e) Se 100 μg/kg + VP. (f) VP + Vit. C (8000 mg/kg). (g) GSEP 300 mg/kg + VP. (h) GSEP 600 mg/kg + VP.
Figure 4.
Cervical ossification anterior view. (a, c) Specimens of the disease group shows non-ossified cervical vertebrae. (b) Normal control. (d) Se low-dose group represents non-ossified cervical vertebrae. (e) Vit. C low-dose incomplete ossification of cervical vertebrae. (f) GSEP at 600 mg/kg partial ossification of cervical vertebrae.
The dams were sacrificed on the 20th day of gestation and evaluated for maternal and developmental parameters. The valproic acid teratogenicity model was expressed with severe fetal toxicity. Spontaneous abortions were reported as a common event in women receiving antiepileptics.26 Vitamin C (200 + 8000 mg/kg) and selenium (10 μg/kg) reduced the rate of early and late resorptions. Dams were also observed for a number of abortions, which were reduced by selenium and grape seed extract powder. According to Wilson’s stages of rat embryo development, the most susceptible period for the onset of teratogenicity is organogenesis (Table 3).27
Table 3. Effect of Vitamin C (2000 + 8000 mg/kg b.w.), Selenium (100 μg + 200 μg/kg b.w.), and Grape Seed Powder (300 + 600 mg/kg b.w.) on Valproic Acid-Induced Teratogenesisa.
| treatment groups | early resorption | late resorption | abortion |
|---|---|---|---|
| N.C | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 |
| D.C V.P (500 mg/kg) | 1.4 ± 0.58 | 3.6 ± 1.04 | 0.6 ± 0.16 |
| V.P + Vit. C (500 mg + 2000 mg/kg) | 0.0 ± 0.00* | 0.6 ± 0.4* | 0.6 ± 0.16 |
| V.P + Vit. C (500 mg + 8000 mg/kg) | 0.0 ± 0.00** | 0.0 ± 0.00** | 0.2 ± 0.13 |
| V.P + Se (500 mg + 100 μg/kg) | 0.0 ± 0.00* | 1.6 ± 1.06 | 0.0 ± 0.00** |
| V.P + Se (500 mg + 200 μg/kg) | 0.8 ± 0.53 | 1.2 ± 0.8 | 0.2 ± 0.13 |
| V.P + G.S (500 mg + 300 mg/kg) | 1.2 ± 0.53 | 1.2 ± 0.8 | 0.2 ± 0.13 |
| V.P + G.S (500 mg + 600 mg/kg) | 0.2 ± 0.14 | 0.8 ± 0.53* | 0.0 ± 0.00** |
Data are presented as mean ± SEM, n = 10; **P < 0.01, *P < 0.05 were given in comparison to the disease control group. N.C = normal control, D.C = disease control, V.P = valproic acid, Vit. C = vitamin C, Se = selenium, and G.S = grape seed.
Delayed conception was also observed in many specimens. Figure 6 shows the fatty uterus of a dam presented with late conception. It has been reported that obesity has injurious effects on the reproductive system. One of the common reasons of infertility is the prevalence of obesity in both men and women.28 A study compared the changes in maternal weight with the weight of dams on day 1 of valproic acid administration and antioxidants. Significant alleviation in maternal weight was observed in vitamin C (8000 mg/kg) and grape seed (600 mg/kg) treated groups (Table 4).
Figure 6.
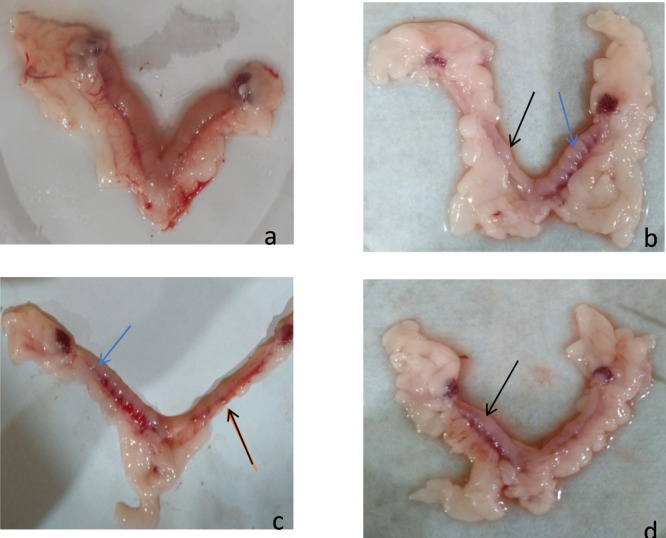
Representing early resorptions (black arrows) and late resorptions (blue arrows). (a) Control group. (b, d Group VII, low-dose GSE. (c) Group VIII, high-dose GSE.
Table 4. Weight Gain/Loss in Dams Receiving Valproic Acid in Combination with Antioxidants from Day 7 to Day 17 of Gestation and Weights on Alternative Days Are Mentioned as Days 1(7), 2(9), 3(11), 4(13), 5(15), and 6(17)a.
| maternal weight variation (g) | ||||||
|---|---|---|---|---|---|---|
| treatment groups | day 1 | day 2 | day 3 | day 4 | day 5 | day 6 |
| N.C | 170 ± 8.5 | 177 ± 7.98 | 182 ± 7.41 | 182 ± 6.41 | 187 ± 7.00 | 193 ± 6.41 |
| D.C V.P (500 mg/kg) | 180 ± 7.4 | 176 ± 7.42 | 189 ± 7.09 | 190 ± 7.64 | 188 ± 7.21 | 176 ± 5.51 |
| V.P + Vit. C (500 mg + 2000 mg/kg) | 165 ± 5.64 | 166 ± 2.00 | 184 ± 7.07 | 184 ± 7.07 | 196 ± 8.77 | 196 ± 8.77 |
| V.P + Vit. C (500 mg + 8000 mg/kg) | 158 ± 4.85 | 171 ± 4.72 | 166 ± 4.64 | 171 ± 5.95 | 182 ± 9.6 | 179 ± 27.81 |
| V.P + Se (500 mg + 100 μg/kg) | 144 ± 2.5 | 145 ± 1.26 | 114 ± 19.56*** | 119 ± 20.81*** | 127 ± 22.8** | 107 ± 29.4*** |
| V.P + Se (500 mg + 200 μg/kg) | 175 ± 2.31 | 196 ± 2.62 | 177 ± 3.33 | 173 ± 4.62 | 171 ± 4.27 | 165 ± 4.37 |
| V.P + G.S (500 mg + 300 mg/kg) | 147 ± 5.7 | 156 ± 2.35 | 169 ± 3.30 | 173 ± 3.17 | 175 ± 3.15 | 178 ± 3.88 |
| V.P + G.S (500 mg + 600 mg/kg) | 141 ± 5.46 | 158 ± 6.48 | 164 ± 7.38 | 144 ± 24.33* | 144 ± 24.41* | 149 ± 25.13 |
Data are represented as mean ± SEM, n = 10;*P < 0.05, **P < 0.01, ***P < 0.001maternal weight in comparison to day 1 of valproic acid administration.
Maternal and fetal lethality was reduced in all treatment groups (Table 5 and Figure 7). However, grape seed at a dose level of 300 + 600 mg/kg, vitamin C (200 mg/kg), and selenium (100 μg/kg) had not shown potential to reduce the fetal lethality induced by valproic acid. Fetuses were observed for gross malformations, and the crown rump length was significantly (P < 0.05) increased in grape seed (600 mg/kg). Moreover, at a high dose (600 mg/kg), grape seed has shown good potential (P < 0.01) to reduce the incidence of fetal growth retardation in comparison to other groups treated with antioxidants (Table 6). Treatment groups other than grape seed 600 (mg/kg) had not shown any significant effect on the crown rump length of fetuses. Fatty uterus of dams treated with grape seed extract was observed as shown in Figure 1. Accumulation of fat in the region of the uterus indicates the etiology behind the delayed conception of some dams.
Table 5. No. of Litters, No. of Live Fetuses, and Maternal Lethality Rate in Dams Exposed to Valproic Acid (500 mg/kg) Per Se and in Combination with Vit. C (2000 + 8000 mg/kg), Selenium (100 + 200 μg/kg), and Grape Seed (300 + 600 mg/kg)a.
| treatment groups | no. of litters | no. of live fetuses | maternal death rate |
|---|---|---|---|
| N.C | 8.1 ± 0.3 | 8.8 ± 0.44 | 0.0 ± 0.00 |
| D.C V.P (500 mg/kg) | 2.7 ± 1.11 | 3 ± 0.33 | 0.6 ± 0.16 |
| V.P + Vit. C (500 mg + 2000 mg/kg) | 3.3 ± 1.01* | 5.8 ± 1.2 | 0.2 ± 0.13a |
| V.P + Vit. C (500 mg + 8000 mg/kg) | 5.2 ± 0.95 | 8.6 ± 0.59a | 0.2 ± 0.13a |
| V + Se (500 mg + 100 μg/kg) | 4.4 ± 1.24 | 6.8 ± 1.02a | 0.2 ± 0.13a |
| V.P + Se (500 mg + 200 μg/kg) | 2.6 ± 1.08* | 6.3 ± 1.19 | 0.0 ± 0.00a |
| V.P + G.S (500 mg + 300 mg/kg) | 4.3 ± 1.20 | 3.7 ± 1.09 | 0.0 ± 0.00a |
| V.P + G.S (500 mg + 600 mg/kg) | 5.1 ± 1.02 | 6.8 ± 1.22 | 0.0 ± 0.00a |
Data are represented as mean ± SEM, n = 10; aP < 0.05 was given in comparison to the disease control group with the no. of live fetuses and maternal death rate. Mean ± SEM, *P < 0.05 was given in no. of litters compared with N.C.
Figure 7.
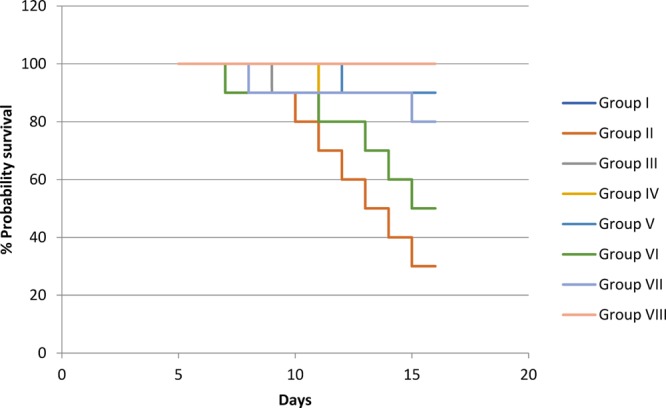
Kaplan–Meier survival curve of the treatment groups from days 5 to 15.
Table 6. Incidence of Gross Morphological Abnormalities in Rat Fetuses after Treatment with Valproic Acid and in Combination with Antioxidantsa.
| treatment groups | crown rump length (cm) | fetal growth retardation (cm) |
|---|---|---|
| N.C | 3.2 ± 0.4 | 0.0 ± 0.00 |
| D.C V.P (500 mg/kg) | 0.73 ± 0.15 | 1.11 ± 0.35 |
| V.P + Vit. C (500 mg + 2000 mg/kg) | 1.25 ± 0.27 | 0.4 ± 0.22 |
| V.P + Vit. C (500 mg + 8000 mg/kg) | 1.71 ± 0.32 | 0.2 ± 0.13* |
| V.P + Se (500 mg + 100 μg/kg) | 1.55 ± 0.25 | 0.2 ± 0.13* |
| V.P + Se (500 mg + 200 μg/kg) | 0.49 ± 0.09 | 0.5 ± 0.22 |
| V.P + G.S (500 mg + 300 mg/kg) | 1.58 ± 0.28 | 0.4 ± 0.26 |
| V.P + G.S (500 mg + 600 mg/kg) | 1.96 ± 0.37* | 0.0 ± 0.00** |
Data are represented as mean ± SEM, n = 10; *P < 0.05, **P < 0.01were given in comparison to the disease control group.
The current results demonstrated in general that the protective effect of vitamin C, selenium, and grape seed extract powder is incomplete, and more factors may be contributed. Moreover, the dosage levels of valproic acid and antioxidants are highly crucial as increased level or decreased levels of dose can result in damaging effects on the embryo. In this study, different antioxidants were compared to specify the most effective antioxidant to control the toxic effects of valproic acid on developing fetus.
3. Conclusions
Valproic acid possesses high potential of inducing teratogenesis; therefore, its embryo toxic effects were not fully reversed. In the present study, the protective effect of specified antioxidants were compared, and it is concluded that vitamin C at a high dose (8000 mg/kg b.w.) and grape seed extract at a high dose (600 mg/kg b.w.) had partially protected the fetuses exposed to valproic acid. It is suggested that a high dose of vitamin C and GSEP may protect the developing fetuses from the toxic effects of valproic acid. Moreover, the results of the current study support the proposed hypothesis that the teratogenic effect of valproic acid may be associated with the formation of reactive oxygen species and the specified antioxidants co-administered with valproic acid partially decrease the fetal growth retardation, incidence of spina bifida occulta, abortions, and fetal and maternal mortality rate.
4. Materials and Methods
4.1. Drugs and Chemicals
Vitamin C, selenium, and grape seed extract were purchased from General Nutrition Corporation (USA). Valproic acid was purchased from Abbott laboratories. Elman’s reagent, pyrogallol solution, and alcian blue were purchased from Oxford Lab Chemicals India. Alizarin red S was purchased from Omicron Sciences Limited UK. EDTA, carboxymethylcellulose, and Folin’s reagent were purchased from Sigma-Aldrich, USA.
4.2. Experimental Animals
Female albino rats were purchased from the University of Veterinary and Animal Sciences. All female rats were nulliparous with an average weight ranging from 130 to 160 g before conception. All experimental female rats were caged in the animal house of Riphah International University under 12 h light/dark cycles at 22 ± 2 °C temperature and 45–55% humidity with food and unlimited supply of water. All methods were approved by the research ethical committee of Riphah International University, Lahore, with an authorized number of REC/RIPS-LHR/013 ruled under the regulation of Institute of Laboratory Animal Resources, Commission on Life Sciences University, National Research Council (1996).
4.3. Experimental Protocol
The experiment was designed according to the OECD guidelines 414.29 Female rats were subjected to mating with healthy male rats. Three female rats were caged with one male rat until the vaginal plug was seen. The day when the vaginal plug was seen is labeled as 0 day of gestation.30 Pregnant female rats (n = 80) were divided into eight groups, each group contained 10 pregnant female rats. Group I: received CMC (0.5%) with no active drug treatment and served as a control group (N.C). Group II: disease group (D.G) with induced teratogenesis, 500 mg/kg valproic acid (V.P). Group III: treated with 2000 mg/kg vitamin C (Vit. C). Group IV: treated with 8000 mg/kg vitamin C. Group V: treated with selenium (Se, 100 μg/kg). Group VI: treated with selenium (200 μg/kg). Group VII: administered grape seed extract (300 mg/kg). Group VIII: administered grape seed extract (600 mg/kg). All the treatments were given via an oral route. Groups III–VIII received valproic acid prior to their respective treatment. All groups treated with their respective treatments from day 5 to day 15 of gestational period. C-section was done at the 19th day of pregnancy. The volume of each treatment administered orally was within the limits of the said guidelines. The doses for rats were calculated using the maximum human tolerable given in the literature.31
4.4. Gross Observations
Fetuses were carefully taken out after C-section of pregnant female rats; placenta was removed and kept separately. The crown rump length of each fetus was measured, each fetus was weighed, and numbers of live fetuses were counted. Fetuses were carefully observed for any deformity, for example, hematoma on the back bone or skull, limb deformity, and growth retardation. The weight of the placenta of each fetus was carefully measured.
4.5. Double Staining of Fetal Skeleton
Skeletons of fetuses of all groups were double stained with alcian blue and alizarin red.32 Rat fetuses were obtained after performing the C-section of female pregnant rats. These fetuses were kept in 4% normal saline for one night at 4 °C to macerate the skin. The fetal skin was removed carefully. All organs and muscles were carefully removed. The skinned fetuses were then kept in an acidic solution (pH 2.8) for about 24 h, and after that moisture from specimens was removed by keeping them in absolute ethanol for 6 h. When the specimens were dehydrated in ethanol, they were placed in a basic solution for at least 24–30 h, and the solution was changed during 30 h at least two times. The basic solution is for removing the adipose tissues from the specimens. KOH in the basic solution caused the bond annihilation in tissues.33 The stained specimens were cleared by placing them in a clearing solution for at least 6–8 h. The specimens were preserved in 1:1 70 % ethanol/ glycerin.
The stained specimens were studied under the dissecting microscope for the observation of cleft palate, bone ossification, spina bifida occulta, rib deformities, and limb formation. The distance between the cartilaginous ends of the vertebral arch of the lumbar and thoracic region was measured for the observation of spina bifida occulta. An ocular micrometer was fixed into the eyepiece of a dissecting microscope, and the distance between the vertebral arches in micrometer was measured. The value in micrometer was converted into the actual distance by multiplying it with the magnification (2×).34
Moreover, the interpretation of other skeletal deformities such as rib malformations, cervical ossification, and cleft palate was numbered as 1 if the specified malformation is present and 0 in the case of the absence of that specified malformation.
4.6. Biochemical Markers
4.6.1. Preparation of Tissue Homogenate
The pregnant female rats were anesthetized with chloroform.35 C-section was done to get the fetuses. Tissue homogenates of fetuses were prepared by a ratio of 1:10 in ice-cold phosphate buffer by using a tissue homogenizer (1 part of tissue and 10 parts of phosphate buffer). The homogenate was then centrifuged at 6000 rpm ± 4 °C for 10 min, and the supernatant was taken for performance of tests for analysis of biochemical markers.
4.6.2. Estimation of Superoxide Dismutase
In 0.1 mL of tissue homogenate, 0.1 mL of pyrogallol and 2.8 mL of potassium phosphate buffer (pH 7.4) were added. Absorbance on a spectrophotometer was measured at a wavelength of 325 nm.36 The following formula was used to calculate the value of SOD:
4.6.3. Catalase
Catalase activity was determined by the method in ref (36). A 0.05 mL solution of the fetal tissue homogenate was taken. To a 1.95 mL solution of phosphate buffer (50 mM, pH 7), 1 mL of 30 mM hydrogen peroxide solution was added. This reaction mixture was analyzed on a spectrophotometer for the measurement of absorbance. Absorbance of the reaction mixture was measured at 240 nm. The following formula was used for the calculation of catalase value:
where O.D is the change in absorbance/minute, and E is the extinction coefficient of hydrogen peroxide having a value of 0.071 mmol cm–1. Protein content was calculated by Lowry’s method.37
4.6.4. Estimation of Glutathione
One milliliter of the tissue homogenate was taken, and it was precipitated with 1 mL of 10% trichloroacetic acid. Four milliliters of phosphate buffer solution and 0.5 mL of DTNB reagent were added to an aliquot of the supernatant.38 Absorbance was measured at 412 nm. The following formula was used for estimation of GSH:
where DF is the dilution factor, which is 1, VU is the volume of the aliquot, Y is the absorbance at 412 nm, and BT is the tissue homogenate.
4.6.5. Estimation of Malondialdehyde
MDA level was calculated by the method in ref (38). One milliliter of the supernatant was taken, and it was added to 3 mL of TBA reagent. The mixture was shaken and left for 15 min. After that, the test tube containing the mixture was cooled down in an ice bath and then centrifuged. The upper layer was taken for measurement of absorbance. Absorbance of the reaction mixture was measured at 532 nm. The following formula was used for the calculation of the MDA value:
where VT is the total volume of the mixture, which is 4 mL, WT is the weight of the dissected brain, and VU is the aliquot volume.
4.6.6. Estimation of Nitrite Levels
The Griess reagent method was used for the estimation of nitrite. The supernatant of the tissue homogenate (1 mL) and Griess reagent (1 mL) were mixed thoroughly and incubated for 10 min at room temperature. Absorbance of the reaction mixture was measured at 546 nm. For the estimation of nitrite levels, a regression line of sodium nitrite was used.39
4.7. Statistical Analysis
Data are represented as mean ± SEM. One-way and two-way ANOVA was used. Dunnett and Bonferroni post-test were used, respectively. P < 0.05 was considered significant, P < 0.01 was moderately significant, and P < 0.001 was considered highly significant in comparison to the disease group.
Acknowledgments
This work was supported by the Riphah Institute of Pharmaceutical Sciences, Riphah International University, Lahore, Pakistan.
Author Contributions
M.S. and U.S. carried out the research and collected the data and wrote the manuscript. F.A. and B.A. conceptualized and supervised the study and approved the version to be published. A.A. formally analyzed the data. M.S. and F.A. captured all the figures and labeled them. Photograph in this manuscript courtesy of M.S. and F.A. Copyright 2019.
No funding is provided for this study.
The authors declare no competing financial interest.
Notes
All methods were approved by the research ethical committee of Riphah International University, Lahore with an authorized number of REC/RIPS-LHR/013 ruled under the regulation of Institute of Laboratory Animal Resources, Commission on Life Sciences University, National Research Council (1996).
References
- Schardein J.Chemically induced birth defects. CRC Press: 2000. [Google Scholar]
- Brent R. L. Environmental causes of human congenital malformations: the pediatrician’s role in dealing with these complex clinical problems caused by a multiplicity of environmental and genetic factors. Pediatrics 2004, 113, 957–968. [PubMed] [Google Scholar]
- Tomson T.; Battino D.; Perucca E. Valproic acid after five decades of use in epilepsy: time to reconsider the indications of a time-honoured drug. Lancet Neurol. 2016, 15, 210–218. 10.1016/S1474-4422(15)00314-2. [DOI] [PubMed] [Google Scholar]
- Tung E. W. Y.; Winn L. M. Valproic acid increases formation of reactive oxygen species and induces apoptosis in postimplantation embryos: a role for oxidative stress in valproic acid-induced neural tube defects. Mol. Pharmacol. 2011, 80, 979–987. 10.1124/mol.111.072314. [DOI] [PubMed] [Google Scholar]
- Defoort E. N.; Kim P. M.; Winn L. M. Valproic acid increases conservative homologous recombination frequency and reactive oxygen species formation: a potential mechanism for valproic acid-induced neural tube defects. Mol. Pharmacol. 2006, 69, 1304–1310. 10.1124/mol.105.017855. [DOI] [PubMed] [Google Scholar]
- Klandorf H.; Van Dyke K.. Oxidative and Nitrosative Stresses: Their Role in Health and Disease in Man and Birds, Oxidative Stress-Molecular Mechanisms and Biological Effects. InTech. InTech2012. [Google Scholar]
- Rahal A.; Kumar A.; Singh V.; Yadav B.; Tiwari R.; Chakraborty S.; Dhama K. Oxidative stress, prooxidants, and antioxidants: the interplay. BioMed Res. Int. 2014, 2014, 1. 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gendy K. S.; Aly N. M.; Mahmoud F. H.; Kenawy A.; El-Sebae A. K. H. The role of vitamin C as antioxidant in protection of oxidative stress induced by imidacloprid. Food Chem. Toxicol. 2010, 48, 215–221. 10.1016/j.fct.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A.; Basu A.; Ghosh P.; Biswas J.; Bhattacharya S. Protective effect of Selenium nanoparticle against cyclophosphamide induced hepatotoxicity and genotoxicity in Swiss albino mice. J. Biomater. Appl. 2014, 29, 303–317. 10.1177/0885328214523323. [DOI] [PubMed] [Google Scholar]
- Valdiglesias V.; Pásaro E.; Méndez J.; Laffon B. In vitro evaluation of selenium genotoxic, cytotoxic, and protective effects: a review. Arch. Toxicol. 2010, 84, 337–351. 10.1007/s00204-009-0505-0. [DOI] [PubMed] [Google Scholar]
- Shi J.; Yu J.; Pohorly J. E.; Kakuda Y. Polyphenolics in grape seeds—biochemistry and functionality. J. Med. Food 2003, 6, 291–299. 10.1089/109662003772519831. [DOI] [PubMed] [Google Scholar]
- Yang J.; Xiao Y.-Y. Grape phytochemicals and associated health benefits. Crit. Rev. Food Sci. Nutr. 2013, 53, 1202–1225. 10.1080/10408398.2012.692408. [DOI] [PubMed] [Google Scholar]
- Ma Z. F.; Zhang H. Phytochemical constituents, health benefits, and industrial applications of grape seeds: A mini-review. Antioxidants 2017, 6, 71. 10.3390/antiox6030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kıyıcı A.; Okudan N.; Gökbel H.; Belviranlı M. The effect of grape seed extracts on serum paraoxonase activities in streptozotocin-induced diabetic rats. J. Med. Food 2010, 13, 725–728. 10.1089/jmf.2009.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak M.-J.; Truong V. L.; Kang H.-S.; Jun M.; Jeong W.-S. Anti-inflammatory effect of procyanidins from wild grape (Vitis amurensis) seeds in LPS-induced RAW 264.7 cells. Oxid. Med. Cell. Longevity 2013, 2013, 409321. 10.1155/2013/409321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia E.-Q.; Deng G.-F.; Guo Y.-J.; Li H.-B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. 10.3390/ijms11020622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Habib A.; Al-Saleh E.; Safer A.-M.; Afzal M. Bactericidal effect of grape seed extract on methicillin-resistant Staphylococcus aureus (MRSA). J. Toxicol. Sci. 2010, 35, 357–364. 10.2131/jts.35.357. [DOI] [PubMed] [Google Scholar]
- Oueslati N.; Charradi K.; Bedhiafi T.; Limam F.; Aouani E. Protective effect of grape seed and skin extract against diabetes-induced oxidative stress and renal dysfunction in virgin and pregnant rat. Biomed. Pharmacother. 2016, 83, 584–592. 10.1016/j.biopha.2016.07.024. [DOI] [PubMed] [Google Scholar]
- Kalter H.Issues and Reviews in Teratology; Springer Science & Business Media, 2012, Vol. 2. [Google Scholar]
- Macklin R. Enrolling pregnant women in biomedical research. Lancet 2010, 375, 632–633. 10.1016/S0140-6736(10)60257-7. [DOI] [PubMed] [Google Scholar]
- Kluger B. M.; Meador K. J. In Teratogenicity of antiepileptic medications. Seminars in neurology; © Thieme Medical Publishers: 2008, pp 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C.-L.; Chen K.-C.; Lin P.-X.; Peng C.-C.; Peng R. Y. Resveratrol and vitamin E rescue valproic acid-induced teratogenicity: The mechanism of action. Clin. Exp. Pharmacol. Physiol. 2014, 41, 210–219. 10.1111/1440-1681.12205. [DOI] [PubMed] [Google Scholar]
- Ayala A.; Muñoz M. F.; Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longevity 2014, 2014, 1. 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegola E.; Broccia M. L.; Prati M.; Giavini E. Stage-dependent skeletal malformations induced by valproic acid in rat. Int. J. Dev. Biol. 1998, 42, 99–102. [PubMed] [Google Scholar]
- Ehlers K.; Stürje H.; Merker H.-J.; Nau H. Valproic acid-induced spina bifida: A mouse model. Teratology 1992, 45, 145–154. 10.1002/tera.1420450208. [DOI] [PubMed] [Google Scholar]
- Pittschieler S.; Brezinka C.; Jahn B.; Trinka E.; Unterberger I.; Dobesberger J.; Walser G.; Auckenthaler A.; Embacher N.; Bauer G.; Luef G. Spontaneous abortion and the prophylactic effect of folic acid supplementation in epileptic women undergoing antiepileptic therapy. J. Neurol. 2008, 255, 1926–1931. 10.1007/s00415-008-0029-1. [DOI] [PubMed] [Google Scholar]
- Altman P. L.; Dittmer D. S.. Growth, including reproduction and morphological development ;Federation of American Societies for Experimental Biology, 1962. [Google Scholar]
- Dağ Z. Ö.; Dilbaz B. Impact of obesity on infertility in women. J. Turk.-Ger. Gynecol. Assoc. 2015, 16, 111. 10.5152/jtgga.2015.15232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Aty A. M.; El-Dib M. A.; Badawy M. I. Toxicity of pesticide industrial wastewater to the green alga Scenedesmus obliquus: a case study. Pak. J. Biol. Sci. 2006, 9, 563–567. 10.3923/pjbs.2006.563.567. [DOI] [Google Scholar]
- ElMazoudy R. H.; Attia A. A.; El-Shenawy N. S. Protective role of propolis against reproductive toxicity of chlorpyrifos in male rats. Pestic. Biochem. Physiol. 2011, 101, 175–181. 10.1016/j.pestbp.2011.09.003. [DOI] [Google Scholar]
- Andrews K. Intraperitoneal injection (IP) in rats and mice SOP. UBC Animal Care Guidelines 2014, 1–6. [Google Scholar]
- Menegola E.; Broccia M. L.; Giavini E. Atlas of rat fetal skeleton double stained for bone and cartilage. Teratology 2001, 64, 125–133. 10.1002/tera.1055. [DOI] [PubMed] [Google Scholar]
- Kawamura S.; HIROHASHI A.; KATO T.; YASUDA M. Bone-staining technique for fetal rat specimens without skinning and removing adipose tissue. Congenital anomalies 1990, 30, 93–95. 10.1111/j.1741-4520.1990.tb00498.x. [DOI] [Google Scholar]
- Ehlers K.; Elmazar M. M. A.; Nau H. Methionine reduces the valproic acid-induced spina bifida rate in mice without altering valproic acid kinetics. J. Nutr. 1996, 126, 67–75. 10.1093/jn/126.1.67. [DOI] [PubMed] [Google Scholar]
- Steffey E. P.; Mama K. R.; Brosnan R. J.. 16 Inhalation Anesthetics. Veterinary Anesthesia and Analgesia: The Fifth Edition of Lumb and Jones; Wiley, 2015, 297. [Google Scholar]
- Hira S.; Saleem U.; Anwar F.; Ahmad B. Antioxidants Attenuate Isolation- and L-DOPA-Induced Aggression in Mice. Front. Pharmacol. 2018, 8, 945. 10.3389/fphar.2017.00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterborg J. H.The Lowry method for protein quantitation. In The protein protocols handbook; Springer: 2002, pp 7–9. [Google Scholar]
- Bhangale J. O.; Acharya S. R. Anti-Parkinson Activity of Petroleum Ether Extract of Ficus religiosa (L.) Leaves. Adv. Pharmacol. Sci. 2016, 2016, 9436106. 10.1155/2016/9436106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bais S.; Gill N. S.; Kumar N. Neuroprotective effect of Juniperus communis on chlorpromazine induced Parkinson disease in animal model. Chin. J. Biol. 2015, 2015, 542542. 10.1155/2015/542542. [DOI] [Google Scholar]