Abstract
The treatment landscape of metastatic renal cell carcinoma (mRCC) has been transformed with the advent of antiangiogenics, notably tyrosine kinase inhibitors (TKIs) targeting vascular endothelial growth factor receptor (VEGFR), and immune checkpoint inhibitors (ICIs). Both treatment options have improved outcomes of patients and modified the natural history of mRCC. Clinical investigations have focused on evaluating combination regimens containing ICIs and VEGFR-directed TKIs. Namely, the combinations of axitinib plus pembrolizumab (KEYNOTE-426) and axitinib plus avelumab (JAVELIN RENAL 101) have shown improved outcomes compared with sunitinib in treatment-naïve patients with mRCC. In this review, we discuss the clinical data of single-agent TKIs and ICIs in mRCC and the rationale for the combination ICIs and TKIs based on preclinical and clinical evidence. We also explore the current challenges for regimen selection and development of predictive biomarkers.
Keywords: angiogenesis, combination, immune checkpoint inhibitors, immunotherapy, renal cell carcinoma, tyrosine kinase inhibitors
Introduction
Around 400,000 cases of renal cell carcinoma are diagnosed worldwide every year with nearly a third having advanced-stage or metastatic disease at the time of diagnosis.1,2 A majority of patients are diagnosed with clear cell renal cell carcinoma, which is generally characterized by von Hippel–Lindau (VHL) gene inactivation and downstream upregulation of hypoxia-inducible factors, accounting for angiogenesis and proliferation. Tyrosine kinase inhibitors (TKIs) targeting angiogenesis through inhibition of vascular endothelial growth factor receptor (VEGFR) have transformed the prognosis of metastatic renal cell carcinoma (mRCC), as those were associated with substantial response rates and improved survival.3 However, most patients eventually developed drug resistance and disease progression while on therapy.4,5
Knowledge of the role of the immune system in carcinogenesis has led to a paradigm shift in the treatment approach to mRCC.6 Immune checkpoint inhibitors (ICIs), targeting tumor or immune cells surface receptors triggering immune tolerance, were shown to be effective in both pretreated and treatment-naïve patients with mRCC.7 Combination strategies have been developed to circumvent de novo or adaptive immune resistance mechanisms that can be encountered with monotherapies, aiming at a synergistic antitumor effect.8 In this review, we summarize clinical data for TKIs and ICIs in treatment-naïve mRCC, and provide an overview of the preclinical rationale and clinical data for the combination of TKIs and ICIs in mRCC. To inform treatment decision-making, we provide a comprehensive analysis of available data with regards to the choice of the regimen and the developing role of biomarkers.
Clinical data for TKIs in treatment-naïve mRCC
Antiangiogenic drugs can be classified according to three mechanisms of action: monoclonal antibodies that bind and deplete the VEGF ligand, monoclonal antibodies that bind to the VEGFR, and TKIs that block the intracellular domain of the VEGFR.9 Sunitinib and pazopanib, which target VEGFR were the first to be approved for the frontline treatment of patients with mRCC.9 Single-agent TKIs achieve objective response rates (ORR) of 20–47%, progression-free survival (PFS) of 8.4–11 months, and overall survival (OS) of 26.4–28.4 months.10,11 Cabozantinib was also approved in intermediate and high-risk patients based on the results of the Alliance A031203 CABOSUN trial, which compared cabozantinib and sunitinib in treatment-naïve mRCC patients.12 The study was not powered to observe an OS benefit, but cabozantinib achieved longer PFS (8.6 months versus 5.3 months; HR = 0.48, 95% CI 0.31–0.74) and higher ORR (20% versus 9%).12
Clinical data for ICI in treatment-naïve mRCC
ICIs include antibodies that target the interaction between programmed cell death protein (PD-1) and its ligand (PD-L1), as well as CTLA-4 and its ligand B7-CTLA-4 to prevent downregulation of cellular immune responses in the tumor microenvironment.13 Nivolumab, a PD-1 inhibitor, was initially evaluated in the CheckMate 025 trial, which enrolled pretreated mRCC with one or two antiangiogenic therapy.14 Nivolumab showed an OS benefit (25.0 versus 19.6 months; HR = 0.73, 98.5% CI 0.57–0.93) and a more tolerable safety profile (grade 3–4 adverse events 19% versus 37%) in comparison with everolimus.14 Based on these promising outcomes, the role of ICI has been evaluated in the frontline treatment of mRCC.
The KEYNOTE-427 study evaluated pembrolizumab in the frontline treatment of mRCC.15 This is a single-arm phase II study that included two cohorts (clear cell and non-clear cell mRCC). Cohort A enrolled 110 patients with clear cell mRCC only with 37.3% favorable risk, 47.3% intermediate risk, and 15.5% poor risk according to the International mRCC Database Consortium (IMDC) prognostic classification. After a median follow up of 22.6 months, pembrolizumab yielded an ORR of 36.4% with intermediate-/poor-risk patients achieving higher ORR compared with favorable-risk patients (39.7% versus 31.0%). Survival data showed a median PFS of 7.1 months, a 12-month OS of 88.2%, and a median OS that was not reached. Treatment-related adverse events occurred in 81.8% of patients, and included, most commonly, fatigue (29.1%) and pruritus (28.2%).15
The phase II trial CheckMate 016 evaluated the combination of nivolumab and ipilimumab in treatment-naïve patients with mRCC, and showed potent antitumor activity.16 The phase III CheckMate 214 trial validated this combination in patients with intermediate/poor risk mRCC.17 A total of 1096 patients were assigned to receive nivolumab plus ipilimumab or sunitinib (50 mg daily for 4 weeks every 6 weeks). Efficacy outcomes favored the combination arm for ORR (42% versus 27%, p < 0.001), OS (NR versus 26.6 months; HR = 0.66; 95% CI 0.54–0.80). PFS was longer with nivolumab plus ipilimumab than with sunitinib but did not reach statistical significance (8.2 months versus 8.3 months; HR = 0.77, 95% CI 0.65–0.9).18 A major caveat is that patients were accrued prior to approval of ICI for second-line treatment; subsequently, less than one-third of patients who received sunitinib and crossed-over to receive ICI as crossover were not allowed before approval. The combination arm was associated with a lower rate of grade 3–5 adverse events (47% versus 64%) but had a higher rate of treatment discontinuation due to adverse events (22% versus 12%). Of the 436 patients treated with nivolumab plus ipilimumab who had a treatment-related select, 35% received high-dose glucocorticoids.17 Exploratory analysis showed that OS was longer with nivolumab plus ipilimumab than with sunitinib among patients with PD-L1 expression <1% (HR = 0.73; 95% CI 0.56–0.96) and >1% (HR = 0.45; 95% CI 0.29–0.71). The ORR was higher with the combination arm across the PD-L1 expression level [PD-L1 expression <1%: 37% versus 28% (p = 0.03) and PD-L1 expression <1%: 58% versus 22% (p < 0.001)].17
Preclinical rationale for the combination of ICIs and TKIs in mRCC
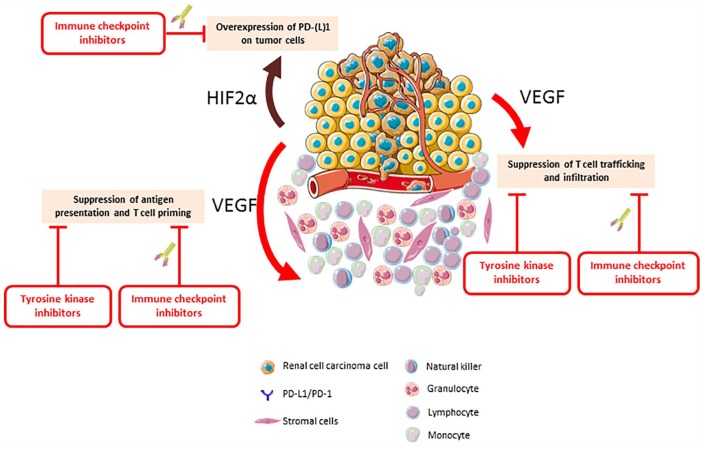
The most common genetic alteration associated with the development of RCC occurs in the VHL gene, which can be altered in up to 90% of cases.19 The major function of the VHL gene product is to regulate the levels of several intracellular proteins, including hypoxia-inducible factor 1 alpha and 2 alpha.20 These intracellular proteins serve as transcription factors by binding to the DNA, which results in the upregulation of pro-oncogenic genes, including genes involved in angiogenesis.20 As the new hallmarks of cancer recognize an active role for the immune system in carcinogenesis, further interest has developed in understanding the interaction of angiogenesis and immunosuppression (Figure 1), which seems to facilitate tumor development and progression.21,22 Indeed, pro-angiogenic factors may affect the immune contexture by a direct effect on immune cells or an indirect effect on the endothelium.23
Figure 1.
The interplay between the immune system and angiogenesis in renal cell carcinoma.
HIF2α, hypoxia-inducible factor alpha; PD-1, programmed cell death protein; PD-L1, programmed cell death protein ligand; VEGF, vascular endothelial growth factor.
Proangiogenic molecules bind cognate receptors expressed by immune cells, affecting the immune cells directly.23 VEGF inhibits the innate immune system by hampering the differentiation of monocytes into mature dendritic cells, and upregulating PD-L1 expression on dendritic cells.24–26 It increases the presence of myeloid-derived suppressor cells, which is characterized by immunosuppressive functions.27 VEGF also inhibits the adaptive immune system by blocking the differentiation of progenitor cells into CD8+ and CD4+ T cells.28 Moreover, VEGF upregulates the expression of immune checkpoint PD-1 and CTLA-4 on immune cells, which leads to T cell exhaustion and adverse outcomes.29–31 In contrast to the inhibitory effect on effector T cells, VEGF also increases the levels of regulatory T cells, maintaining an immunosuppressive context.32
Proangiogenic factors also induce changes in protein expression on endothelial cells that limit immune-cell tumor infiltration.33 Namely, the intercellular adhesion molecule 1 (ICAM1) is downregulated in endothelial cells purified from human RCC-derived samples.34 Consequently, endothelial cells become an impermeable barrier to immune cells that causes CD8+ T cells apoptosis and PD-L1/L2 upregulation.35,36 Proangiogenic molecules also lead to the formation of aberrant tumor vessels that can lead to disrupted immune-cell infiltration, poor perfusion, and hypoxia.37 Tumor hypoxia can lead to a wide range of defects that affect several components of the immune system, including effector T cells.38 In hypoxic conditions, cancer cells can recruit regulatory T cells and tumor-associated macrophages differentiate to an M2 phenotype, which can have immunosuppressive effects.39
As such, alterations in the VHL gene facilitate the oncogenic process through an immunosuppressive effect, potentially impacting concurrently T cell priming, trafficking and infiltration.31,40 Antiangiogenic drugs may restore the differentiation of dendritic cells, reduce the level of myeloid-derived suppressor cells and decreasing the levels of regulatory T cells.24,32,41,42 Antiangiogenic drugs can also lead to normalization of the tumor vasculature and hypoxia alleviation, which can have profound positive effects on immune-cell infiltration into tumors.39,43 As such, there is a strong rationale to combine ICIs and TKIs as preclinical models demonstrated the interest of such associations, with reports of synergistic antitumor effects framing the backdrop for the evaluation of these regimens in mRCC.44,45
Clinical data for the combination of ICIs and VEGF/VEGFR axis inhibitors in mRCC
The first clinical trials combining immunotherapy and antiangiogenics reported on the combination of bevacizumab, a monoclonal antibody targeting VEGF, and interferon.46,47 This combination showed a statistically significant improvement in PFS in comparison with interferon monotherapy (5.2 versus 8.5–10.2 months; p < 0.001). While differences in OS were not statistically significant between the two treatment arms, these data were the first to show the potential of the combination regimen in a phase III setting.46,47 The combination of ICI and VEGF/VEGFR axis inhibitors in mRCC has been evaluated in phase I studies and validated in phase III trials (Tables 1 and 2).
Table 1.
Summary of the phase I trials evaluating the combination of immune checkpoint inhibitors plus tyrosine kinase inhibitors in treatment-naïve mRCC.
| Regimen | Number of patients | Response rate | All grades treatment-related adverse eventsa | Grade 3–5 treatment-related adverse eventsb |
|---|---|---|---|---|
| Avelumab + axitinib JAVELIN Renal 10048 |
55 | ORR = 58% DCR = 78% |
All events 96% Diarrhea 58%, dysphonia 47%, hypertension 47%, fatigue 46%, PPE syndrome 31% |
All events 58% Hypertension 29%, amylase increase 8%, lipase increase 8%, increased ALT 7%, PPE syndrome 7% |
| Pembrolizumab + axitinib KEYNOTE-03549 |
52 | ORR = 73% DCR = 88% |
NR | All events 58% Hypertension 23%, diarrhea 10%, fatigue 10%, increased ALT 8% |
| Pembrolizumab plus cabozantinib NCT0314982250 |
8 | ORR = 25% DCR = 75% |
Fatigue 87.5%, weight loss 75%, anorexia 50%, diarrhea 50%, dysgeusia 50%, abnormal liver function tests 50% | Reversible posterior leukoencephalopathy syndrome 12.5%, hypertension 12.5%, anorexia 12.5%, confusion 2.5% |
| Pembrolizumab plus pazopanib NCT0201463651 |
25 | ORR = 10–60% DCR = NR |
NR | All events 80–90% |
| Pembrolizumab plus lenvatinib NCT0250109652 |
30 | ORR = 66.7% DCR = NR |
Diarrhea (83%), fatigue (70%), hypothyroidism (67%), stomatitis (63%), nausea (60%). | All events 70% |
| Nivolumab plus sunitinib NCT0147208153 |
33 | ORR = 52% DCR = 85% |
NR | All events 73% Increased ALT 18%, hypertension 15%, hyponatremia 15% |
| Nivolumab plus pazopanib NCT0147208153 |
20 | ORR = 45% DCR = 80% |
NR | All events 60% Increased ALT 20%, increased AST 20%, fatigue 15% |
| Nivolumab plus cabozantinib NCT0249620854 |
47/ 7 mRCC | NR | All events 96% Fatigue 70%, diarrhea 60%, abnormal liver function tests 60%, hypophosphatemia 45% |
All events 62% Increased lipase 17%, hypophosphatemia 15%, fatigue 6% |
| Nivolumab plus cabozantinib plus ipilimumab NCT0249620854 |
28/8 mRCC | NR | All events 96% Fatigue 71%, diarrhea 68%, hypophosphatemia 50%, abnormal liver function tests 43%. |
All events 71% Hypophosphatemia 21%, abnormal liver function tests 14%, increased lipase 14% |
Most common all grade treatment-related adverse events.
Most common grade3–5 treatment-related adverse events.
DCR, disease control rate; mRCC, metastatic renal cell carcinoma; NR, not reported; ORR: objective response rate; PPE, palmar–plantar erythrodysesthesia.
Table 2.
Phase II/III trials of the immune checkpoint-based regimens evaluated in treatment-naïve mRCC.
| Regimen | Nivolumab + ipilimumab CheckMate 21417,18 |
Avelumab + Axitinib JAVELIN RENAL 10155 |
Pembrolizumab + Axitinib KEYNOTE-42656 |
Atezolizumab + bevacizumab IMmotion 15157 |
|---|---|---|---|---|
| Number of patients | 550 | 442 | 432 | 454 |
| Pathology | Clear cell component | Clear cell component | Clear cell component | Clear cell component |
| IMDC risk score | ||||
| Favorable | 23% | 21% | 32% | 20% |
| Intermediate | 61% | 61% | 55% | 69% |
| Poor | 17% | 16% | 13% | 12% |
| Brain metastasis | BM were excluded | Stable BM were eligible | Stable BM were eligible | Stable BM were eligible |
| Primary endpoint | ORR, PFS and OS | PFS and OS in PD-L1 + patients | PFS and OS | PFS in PD-L1 + patients and OS in intent-to-treat patients |
| Median follow-up | 32.4 months | 9.9 months | 12.8 months | 16 months for PFS 24 months for OS |
| Median OS | Not reacheda | Not reached | Not reached | 33.6 months |
| Median PFS | 8.2 monthsa | 13.8 months | 15.1 months | 11.2 months |
| ORR | 42%* | 56% | 59.3% | 37% |
| Grade 3–4 adverse events | 47% | 71.2% | 62.9% | 40% |
Patients with favorable IMDC were excluded from the efficacy outcomes.
BM, brain metastasis; IMDC, International mRCC Database Consortium; mRCC, metastatic renal cell carcinoma; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
Atezolizumab plus bevacizumab
The phase III IMmotion151 trial compared the combination of atezolizumab plus bevacizumab to a sunitinib control group.58 At a median follow-up of 15 months, atezolizumab plus bevacizumab showed advantages in PFS (11.2 months versus 8.4 months; HR = 0.83, 95% CI 0.7–0.97) and ORR (37% versus 33%).59 In the intention-to-treat population, the median OS did not differ statistically between the two treatment arms (HR = 0.93, 95% CI 0.76–1.14).57 In the subset of patients who were PD-L1 positive, the combination arm achieved better PFS (11.2 months versus 7.7 months; HR = 0.74, 95% CI 0.57–0.96) and ORR (43% versus 35%), among whom complete response reached 9%.59
Nivolumab plus sunitinib/pazopanib
The combination of nivolumab with sunitinib or pazopanib was evaluated in a phase I study (ClinicalTrials.gov identifier: NCT01472081).53 The starting dose of nivolumab was 2 mg/kg intravenously every 3 weeks with planned escalation to 5mg/kg with the same schedule. The nivolumab plus sunitinib (50 mg for 4 weeks every 6 weeks) combination was administered in seven patients without dose-limiting toxicities. Subsequently, 19 additional patients including treatment-naïve patients were enrolled in this arm. Grade 3–4 adverse events were observed in 24 patients (73%) and led to treatment discontinuation in 8 patients (24%). The ORR was 52% in this arm.53 The nivolumab plus pazopanib (800 mg daily) combination was administered in 20 patients, among which four dose-limiting toxicities were reported, including abnormal liver functions in 3 patients and fatigue in 1 patient. Grade 3–4 related adverse events were observed in 12 patients (60%) and led to treatment discontinuation in 4 patients (20%). The ORR was 45% in this arm.53 Further development has been discontinued due to safety issues.
Avelumab plus axitinib
The combination of avelumab plus axitinib was initially evaluated in the phase Ib JAVELIN Renal 100 trial.48 This study enrolled treatment-naïve patients with mRCC who had undergone primary tumor resection. The six patients that were included in the dose-finding stage received axitinib monotherapy twice daily for 1 week, followed by intravenous avelumab every 2 weeks plus axitinib twice daily. After a median follow up of 17.4 months, grade 3–4 treatment-related adverse events, including hypertension, lipase concentration increase, mucosal inflammation, and proteinuria, were observed in four of six patients. Only grade 3 proteinuria was dose-limiting. The maximum tolerated dose was defined at avelumab 10 mg/kg every 2 weeks and axitinib 5 mg twice daily.48 The dose-expansion stage enrolled an additional 49 patients. This regimen achieved an ORR of 61.5%. Treatment-related adverse events were experienced in 53 patients of whom 32 had grade 3–5 adverse events.48
These promising outcomes were confirmed by the phase III JAVELIN Renal 101 trial.55 Treatment-naïve patients with mRCC were randomized to avelumab plus axitinib (n = 442) and sunitinib (n = 444). The coprimary endpoints were PFS per independent central review and OS in the PD-L1 positive patients, which constitutes 63% of the randomly selected population. PD-L1 positivity was defined by at least 1% expression on tumor-infiltrating immune cells using the SP263 immunohistochemistry assay. The secondary endpoints were PFS and OS in the overall population, also including the PD-L1 negative patients. PFS analysis were in favor of the combination arm in the PD-L1 positive patients (13.8 months versus 7.2 months; HR = 0.61, 95% CI 0.475–0.790) as well as the overall population (13.8 months versus 8.4 months; HR = 0.69, 95% CI 0.563–0.840) with a median follow up of 9.9 months. Subgroup analysis according to PD-L1 expression did not identify a PFS benefit in patients with PD-L1 negative status treated with the combination therapy (HR = 0.80; 95% CI 0.551–1.164). Regarding PFS, the findings across the IMDC risk groups favored the combination regimens in favorable risk (not reached versus 16.7 months; HR = 0.50, 95% CI 0.26–0.97), intermediate risk (13.3 months versus 7.9 months; HR = 0.64, 95% CI 0.47–0.88) and poor risk (5.6 months versus 2.8 months; HR = 0.53, 95% CI 0.30–0.93) groups. Regarding ORR, the findings across the IMDC risk groups favored combination regimens in the favorable risk (66% versus 38%), intermediate risk (50% versus 24%) and poor risk (31% versus 9%) groups. At the time of the study report, OS analysis was immature (HR = 0.78, 95% CI 0.554–1.084). The ORR was higher with the combination arm in the PD-L1 positive (62% versus 30%) and PD-L1 negative (47% versus 28%). Complete responders were seen in 3% in the combination arm and 2% of patients in the sunitinib arm. Treatment-related adverse events were similar in proportion between both study arms for all grade adverse events (95% versus 96%) and grade 3–4 (55% versus 55%).55
Pembrolizumab plus axitinib
The combination of pembrolizumab plus axitinib was initially evaluated in the phase Ib KEYNOTE-035 trial.49 This study enrolled treatment-naïve patients with mRCC that had undergone primary tumor nephrectomy. The 11 patients that were included in the dose-finding stage received axitinib monotherapy 5 mg twice daily plus intravenous pembrolizumab every 3 weeks. Three dose-limiting toxicities were reported, including one case of transient ischemic attack and two cases having axitinib-related toxicity. An additional 41 patients were enrolled in the dose-expansion phase. Grade 3–4 treatment-related adverse events, including hypertension, diarrhea, fatigue, and increased alanine aminotransferase concentration, occurred in 34 patients. This regimen achieved an ORR of 38%.49
The phase III KEYNOTE-426 trial compared the combination of pembrolizumab plus axitinib (n = 432) to sunitinib (n = 429) in treatment-naïve mRCC.56 The coprimary endpoints were PFS and OS in the overall population. Among the 822 patients that were evaluated for PD-L1 expression, 60.5% had a combined positive score of 1 or more (calculated by the ratio of the PD-L1 positive tumor cells, lymphocytes, and macrophages divided by the total number of tumor cells) using the 22C3 immunohistochemistry assay. After a median follow up of 12.8 months, the combination arm showed superiority in ORR (59.3% versus 35.7%), PFS (15.1 months versus 11.1 months; HR = 0.69, 95% CI 0.57–0.84) and OS (not reached versus not reached; HR = 0.53, 95% CI 0.38–0.74). Complete response was more commonly encountered in the combination arm (5.8% versus 1.9%). Treatment-related adverse events were almost similar between the study arms for all grade adverse events (96.3% versus 97.6%) and grade 3–5 (62.9% versus 58.1%).56
Pembrolizumab plus cabozantinib
The combination of pembrolizumab plus cabozantinib was evaluated in one phase Ib study (ClinicalTrials.gov identifier: NCT03149822).50 This dose-escalation study reported on the outcomes of eight pretreated patients with mRCC who had undergone primary tumor nephrectomy. Seven patients had MSKCC intermediate risk and one had MSKCC poor risk. No dose-limiting toxicities were observed. The best objective response was a partial response in two patients and stable disease in four patients. The remaining two patients experienced progressive disease. The maximum-tolerated dose was identified as cabozantinib 60 mg daily plus pembrolizumab 200 mg intravenously every 3 weeks.50 Based on these data, a 2-stage phase II study is expected to start recruiting in the near future.
Pembrolizumab plus pazopanib
The combination of pembrolizumab plus pazopanib was assessed in 20 patients enrolled in cohorts A and B assessing pazopanib 800 mg and 600 mg, respectively, both with pembrolizumab given 2 mg/kg every 3 weeks (ClinicalTrials.gov identifier: NCT02014636).51 Due to dose-limiting liver toxicity, five patients were enrolled in cohort C in order to investigate whether the sequential schedule of 9 weeks pazopanib run-in followed by pazopanib plus pembrolizumab would improve safety. Three patients of cohort C had dose-limiting toxicities (pneumonitis, bowel perforation, increased lipase). Grade 3–4 adverse events were observed in 90% of patients in cohorts A and B and in 80% of patients in cohort C. No grade 3–4 increased AST/ALT were reported in cohort C, while they were observed in 70% and 60% in cohorts A and B, respectively. The percentage of adverse events leading to treatment discontinuation reached as high as 80%. In view of its limited tolerability, the combination of pembrolizumab plus pazopanib was not investigated further. The ORR was 60%, 20%, and 10% in cohorts A, B, and C, respectively.51
Pembrolizumab plus lenvatinib
The combination of pembrolizumab and lenvatinib was evaluated in a phase I study of 12 treatment-naïve and 18 pretreated patients with mRCC (ClinicalTrials.gov identifier: NCT02501096).52 Lenvatinib (20 mg daily) plus pembrolizumab (200 mg every 3 weeks) was assessed as the maximum tolerated dose and recommended phase II dose in phase Ib. Grade 3–4 adverse events occurred in 21 (70%) patients, of which 4 (13%) discontinued treatment due to adverse events. Efficacy outcomes showed an ORR of 66.7% and a median PFS of 17.7 months.52 A phase III trial of lenvatinib plus pembrolizumab and lenvatinib plus everolimus versus sunitinib for the frontline treatment of mRCC is ongoing with a primary endpoint aiming at evaluating PFS (ClinicalTrials.gov identifier: NCT02811861).
Nivolumab plus cabozantinib with or without ipilimumab
The tolerability and efficacy of the combination nivolumab (3 mg/kg every 3 weeks) plus cabozantinib (40 mg daily) with or without ipilimumab (1 mg/kg every 3 weeks) has been evaluated in a cohort of 75 patients with genitourinary cancers (ClinicalTrials.gov identifier: NCT02496208).54 A total of 47 patients, including 7 with mRCC, were treated with nivolumab plus cabozantinib. All grade adverse events occurred in 96% including grade 3–4 adverse events in 62%; 28 patients, including 8 patients with mRCC were treated with nivolumab plus cabozantinib plus ipilimumab. All grade adverse events occurred in 96% including grade 3–4 adverse events in 71%. The ORR of the mRCC patients was 53.3%.54
The combination of cabozantinib to nivolumab plus ipilimumab is being assessed in two ongoing phase III trials: COSMIC313 (ClinicalTrials.gov identifier: NCT03937219) compares nivolumab and ipilimumab with or without cabozantinib in untreated mRCC, and PDIGREE (ClinicalTrials.gov identifier: NCT03793166) compares ipilimumab and nivolumab followed by nivolumab alone to nivolumab with cabozantinib in the same study population.
Nivolumab plus tivozanib
The combination of nivolumab and tivozanib was evaluated in a phase I study (ClinicalTrials.gov identifier: NCT03136627) of six patients with mRCC.60 Tivozanib was administered orally at two dose levels, 1.0 mg and 1.5 mg, once daily for 21 days every 28-day cycle in combination with nivolumab (240 mg every 14 days) intravenously. The most common adverse events were asthenia, reported in three patients and diarrhea, stomatitis, arthralgia, and dysphonia, all reported in two patients. Hypertension, elevations of liver enzymes, and the hand-foot syndrome were seen in one patient each. No immune-related adverse events were reported. There was no discernible difference between the two-dose cohorts.60
Atezolizumab plus cabozantinib
A phase Ib study is currently evaluating the combination of atezolizumab plus cabozantinib in multiple tumor types (ClinicalTrials.gov identifier: NCT03170960). The dose-escalation cohort includes RCC patients with or without prior systemic therapy. The expansion cohort 1 includes RCC patients with clear cell histology who have not received prior systemic therapy, and cohort 10 includes RCC subjects with nonclear cell histology who have had up to one prior TKI.
Discussion
The past 30 years have witnessed a transformation in the management of mRCC with the considerable expansion of treatment options.7 Currently, there are two major types of combination regimens approved in mRCC including a combination of two ICI and combinations of antiangiogenic therapy and an ICI.61,62 The combinations of nivolumab plus ipilimumab has become a standard of care in mRCC following the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) approval for patients with intermediate and poor-risk disease.7,61 Positive outcomes for the ICI and TKI combination are now validated in large phase III randomized trials leading to FDA and EMA approval in unselected treatment-naïve patients for pembrolizumab plus axitinib and avelumab plus axitinib. Several questions remain to be answered for better applicability in clinical practice.
The burning question is whether one ICI-based combination is superior to the other regimens (Table 2). The ICI combination, namely nivolumab plus ipilimumab, demonstrated an OS advantage over sunitinib and an 11% complete remission rate.18 The antiangiogenic drug plus ICI combination was reported to yield an OS benefit in the pembrolizumab plus axitinib trial whereas the avelumab plus axitinib trials did not show such an advantage at the time of analysis. This discrepancy may be favored by the higher proportion of good-risk patients in the pembrolizumab plus axitinib compared with the avelumab plus axitinib combination (21% versus 32%), which is reflected in the longer PFS in the control arm of the pembrolizumab plus axitinib trial (11.1 months versus 8.4 months) and higher percentage of complete remission (9% versus 4%).55,56 Using the IMDC dataset, patients treated with any ICI plus VEGF inhibitor (n = 113), and those treated with ipilimumab plus nivolumab (n = 75) had similar ORR (33% versus 40%; p = 0.4), time to treatment failure (14.3 months versus 10.2 months; HR = 0.71, 95% CI 0.46–1.12), time to next treatment (19.7 months versus 17.9 months; HR = 0.65, 95% CI 0.38–1.11), and OS (not reached for both treatment options; HR = 1.74, 95% CI 0.82–3.68).63,64 A total of 64 patients received second-line treatment. In patients receiving subsequent VEGF-based therapy, second-line response rates were lower, with ICI plus VEGF inhibitor compared with nivolumab plus ipilimumab (15% versus 45%; p = 0.04), but second-line time to treatment failure was not significantly different (3.7 months versus 5.4 months; p = 0.4).63,64 As such, the optimal combination regimen in the first line setting is yet to be defined, and more mature follow up may inform treatment decision.65
The second question concerns the treatment strategy of whether to use a combination or sequential therapies in the absence of randomized controlled trials addressing this issue.66 The first strategy may achieve longer-term responses and increase treatment-free intervals, whereas the second aims for tumor control and to limit the toxicity profile. A recent retrospective analysis reported 32 patients with mRCC treated with subsequent therapy after either atezolizumab plus bevacizumab (n = 20), ipilimumab plus nivolumab (n = 10), and axitinib plus avelumab (n = 2).67 All patients received one subsequent therapy including axitinib (n = 15), pazopanib (n = 9), sunitinib (n = 4), cabozantinib (n = 3), or nivolumab (n = 1). For the 26 patients with available responses, ORR was achieved in 27% and DCR in 77%. Median PFS for the first subsequent therapy was 7.9 months (95% CI, 4.5–11.3). The median PFS in second-line for patients previously treated with a combination of ICI plus antiangiogenic therapy was 7.9 months (95% CI 3.1–12.7), and was 9.3 months (95% CI 3.5–15.0) for patients previously treated with nivolumab plus ipilimumab (p = 0.732).67 Another report shows that patients receiving subsequent VEGF TKI monotherapy have similar ORR (13% versus 45%, p = 0.07) and time to treatment failure (5.5 months versus 5.4 months, p = 0.80) following ICI plus VEGFR inhibitors (n = 15) and nivolumab plus ipilimumab (n = 20).63 In the absence of randomized controlled trials comparing TKIs after ICI-based combinations, the guidelines recommend any VEGR TKI that has not been used in the first line.61
The lack of a decision-guiding biomarker is a major issue in the phase III trials of TKI plus ICI. The immunogenic phenotype of RCC has been initially attributed to high-affinity neoantigens consequent to indels and associated frameshift mutations.68 This data has not been consistent across the series, which limits its applicability in clinical practice.69 As such, the underlying biological basis of immunogenicity in RCC remains unidentified, which limits the development of clinically valid biomarkers. PD-L1 seemed the obvious biomarker that is reported in the majority of ICI trials.13 The strong correlation of PD-L1 expression with the Teff immune gene signature could explain a predictive role for ICI treatments.69 Interestingly, PD-L1 expression carries some predictive value for ICI monotherapy and ICI doublet but not TKI plus ICI.48,55,56 The biomarker role of PD-L1 is also limited by the lack of a standardized assessment as the pivotal trials used different kits and methods to evaluate PD-L1 expression (tumor or immune cells).70
The IMmotion150 phase II study suggests that the prediction of outcomes with antiangiogenic drugs and ICI is possible in treatment-naïve mRCC patients.59 A signature profile has been suggested according to the relative expression levels of angiogenesis, immune (including T-effector presence and function, IFN-γ response, checkpoint inhibitors, and antigen presentation), and myeloid inflammation associated genes.69 The combination of ICI plus antiangiogenic drug was enriched in TeffHigh, antiangiogenic drug monotherapy in highly angiogenic tumors (AngiogenesisHigh), ICI monotherapy in immunogenic tumors and low myeloid inflammation (TeffHigh MyeloidLow), and lesser in high myeloid inflammation (TeffHigh MyeloidHigh).69 The improved clinical outcome associated with ICI plus antiangiogenic drug compared with ICI monotherapy in the immune-suppressed TeffHigh MyeloidHigh subgroup suggests that the addition of an antiangiogenic drug to ICI overcomes innate inflammation-mediated resistance.69 Some of these findings were validated in the IMmotion151 trial, in which atezolizumab plus bevacizumab improved PFS over sunitinib in AngiogenesisLow and TeffHigh patients.71
A recent report of biomarker analysis from JAVELIN Renal 101 assessed the role of PD-L1 expression (Ventana SP263), CD8 expression (clone C8/144B), gene expression profiling (RNA sequencing), and mutation/polymorphisms (whole-exome sequencing).72 Patients whose tumors were PD-L1 positive, or contained greater numbers of CD8+ cells at the invasive margin, had extended PFS in the avelumab plus axitinib arm and reduced PFS in the sunitinib arm. A JAVELIN Renal 101 signature was established, comprising immune-related genes most significantly associated with PFS in the avelumab plus axitinib arm, but its broad applicability remains to be determined. Signatures from IMmotion150 were also evaluated in Javelin Renal 101; while elevated angiogenesis was associated with improved PFS with sunitinib, immune signatures from IMmotion150 could not demonstrate significant differences for PFS between the two arms. Finally, tumor mutational burden, as well as small insertions and deletions, did not distinguish patients with respect to PFS.72
Biomarker work for TKI and ICI combinations remain in its infancy. Several studies are ongoing to assess circulating biomarkers, including immune cells subpopulations and cytokines before and on-therapy.73 More accurate phenotyping of the microenvironment may better inform the immune contexture of tumors at a patient level,74 whereas single-cell RNA sequencing may identify precise states of activation and exhaustion of immune cell subtypes.75 These new developments in the realm of biomarkers could durably improve clinical decision-making for renal cell carcinoma patients.
Conclusion
In line with the concept that angiogenesis and evasion of immune destruction are hallmarks of cancer, the clinical development of VEGFR TKI and ICI was a success that translated into survival benefits in mRCC. However, patients with mRCC had limited benefits with single-agent therapies and are now living longer with combination therapies. The combination of ipilimumab and nivolumab was the first step in this direction. The present emphasis on combining TKI and ICI leads to favorable outcomes and durable responses, with OS benefit over sunitinib. Further challenges lie in identifying biomarkers that would guide treatment decisions in order to select the appropriate combination and avoid unnecessary toxicities.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: Elie Rassy and Ronan Flippot: none
Laurence Albiges: Consulting/advisory role: Novartis (Institution), Amgen (Institution), Bristol-Myers Squibb (Institution), Ipsen (Institution), Roche (Institution), Pfizer (Institution), Astellas Pharma (Institution), Merck (Institution), MSD (Institution), AstraZeneca (Institution), Exelixis (Institution), Corvus Pharmaceuticals (Institution), Peloton therapeutics (Institution). Research Funding: Bristol-Myers Squibb (Institution).
Contributor Information
Elie Rassy, Department of Cancer Medicine, Gustave Roussy, Université Paris-Saclay, Villejuif, France; Department of Medical Oncology, Saint Joseph University, Beirut, Lebanon.
Ronan Flippot, Department of Cancer Medicine, Gustave Roussy, Université Paris-Saclay, Villejuif, France.
Laurence Albiges, Department of Cancer Medicine, Gustave Roussy Institute, Université Paris-Saclay, 114 rue Edouard Vaillant, Villejuif, 94805, France.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Fisher R, Gore M, Larkin J. Current and future systemic treatments for renal cell carcinoma. Semin Cancer Biol 2013; 23: 38–45. [DOI] [PubMed] [Google Scholar]
- 3. Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med 2017; 376: 354–366. [DOI] [PubMed] [Google Scholar]
- 4. Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27(Suppl. 5): v58–v68. [DOI] [PubMed] [Google Scholar]
- 5. Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 2008; 8: 592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Curti BD. Immunotherapy in advanced renal cancer — is cure possible? N Engl J Med 2018; 378: 1344–1345. [DOI] [PubMed] [Google Scholar]
- 7. Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019; 30: 706–720. [DOI] [PubMed] [Google Scholar]
- 8. Hutson TE. Targeted therapies for the treatment of metastatic renal cell carcinoma: clinical evidence. Oncologist 2011; 16(Suppl. 2): 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Négrier S, Raymond E. Antiangiogenic treatments and mechanisms of action in renal cell carcinoma. Invest New Drugs 2012; 30: 1791–1801. [DOI] [PubMed] [Google Scholar]
- 10. Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009; 27: 3584–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013; 369: 722–731. [DOI] [PubMed] [Google Scholar]
- 12. Choueiri TK, Hessel C, Halabi S, et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): progression-free survival by independent review and overall survival update. Eur J Cancer 2018; 94: 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aoun F, Rassy EE, Assi T, et al. PDL-1/PD1 inhibitors: antibody or antinobody? Future Oncol 2017; 13: 1669–1671. [DOI] [PubMed] [Google Scholar]
- 14. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015; 373: 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Larkin JMG, Tykodi SS, Donskov F, et al. 949P-First-line pembrolizumab (pembro) monotherapy in advanced clear cell renal cell carcinoma (ccRCC): updated follow-up for KEYNOTE-427 cohort A. Ann Oncol 2019; 30(Suppl. 5): v381–v382. [Google Scholar]
- 16. Hammers HJ, Plimack ER, Infante JR, et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: the CheckMate 016 study. J Clin Oncol 2017; 35: 3851–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018; 378: 1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol 2019; 20: 1370–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nickerson ML, Jaeger E, Shi Y, et al. Improved identification of von Hippel-Lindau gene alterations in clear cell renal tumors. Clin Cancer Res 2008; 14: 4726–4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barry RE, Krek W. The von Hippel-Lindau tumour suppressor: a multi-faceted inhibitor of tumourigenesis. Trends Mol Med 2004; 10: 466–472. [DOI] [PubMed] [Google Scholar]
- 21. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 22. Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol 2011; 11: 702–711. [DOI] [PubMed] [Google Scholar]
- 23. Khan KA, Kerbel RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol 2018; 15: 310–324. [DOI] [PubMed] [Google Scholar]
- 24. Alfaro C, Suarez N, Gonzalez A, et al. Influence of bevacizumab, sunitinib and sorafenib as single agents or in combination on the inhibitory effects of VEGF on human dendritic cell differentiation from monocytes. Br J Cancer 2009; 100: 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med 1996; 2: 1096–1103. [DOI] [PubMed] [Google Scholar]
- 26. Curiel TJ, Wei S, Dong H, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 2003; 9: 562–567. [DOI] [PubMed] [Google Scholar]
- 27. Huang Y, Chen X, Dikov MM, et al. Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood 2007; 110: 624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ohm JE, Gabrilovich DI, Sempowski GD, et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood 2003; 101: 4878–4886. [DOI] [PubMed] [Google Scholar]
- 29. Gavalas NG, Tsiatas M, Tsitsilonis O, et al. VEGF directly suppresses activation of T cells from ascites secondary to ovarian cancer via VEGF receptor type 2. Br J Cancer 2012; 107: 1869–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Voron T, Colussi O, Marcheteau E, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med 2015; 212: 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ruf M, Moch H, Schraml P. PD-L1 expression is regulated by hypoxia inducible factor in clear cell renal cell carcinoma. Int J Cancer 2016; 139: 396–403. [DOI] [PubMed] [Google Scholar]
- 32. Adotevi O, Pere H, Ravel P, et al. A decrease of regulatory T cells correlates with overall survival after sunitinib-based antiangiogenic therapy in metastatic renal cancer patients. J Immunother 2010; 33: 991–998. [DOI] [PubMed] [Google Scholar]
- 33. Huang H, Langenkamp E, Georganaki M, et al. VEGF suppresses T-lymphocyte infiltration in the tumor microenvironment through inhibition of NF-κB-induced endothelial activation. FASEB J 2015; 29: 227–238. [DOI] [PubMed] [Google Scholar]
- 34. Griffioen AW, Damen CA, Martinotti S, et al. Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: the role of angiogenic factors. Cancer Res 1996; 56: 1111–1117. [PubMed] [Google Scholar]
- 35. Motz GT, Santoro SP, Wang LP, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med 2014; 20: 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pittet CL, Newcombe J, Prat A, et al. Human brain endothelial cells endeavor to immunoregulate CD8 T cells via PD-1 ligand expression in multiple sclerosis. J Neuroinflammation 2011; 8: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seaman S, Stevens J, Yang MY, et al. Genes that distinguish physiological and pathological angiogenesis. Cancer Cell 2007; 11: 539–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Palazón A, Aragonés J, Morales-Kastresana A, et al. Molecular pathways: hypoxia response in immune cells fighting or promoting cancer. Clin Cancer Res 2012; 18: 1207–1213. [DOI] [PubMed] [Google Scholar]
- 39. Movahedi K, Laoui D, Gysemans C, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res 2010; 70: 5728–5739. [DOI] [PubMed] [Google Scholar]
- 40. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013; 39: 1–10. [DOI] [PubMed] [Google Scholar]
- 41. Osada T, Chong G, Tansik R, et al. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother 2008; 57: 1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kusmartsev S, Eruslanov E, Kübler H, et al. Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J Immunol 2008; 181: 346–353. [DOI] [PubMed] [Google Scholar]
- 43. Huang Y, Goel S, Duda DG, et al. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res 2013; 73: 2943–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yasuda S, Sho M, Yamato I, et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin Exp Immunol 2013; 172: 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tian L, Goldstein A, Wang H, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 2017; 544: 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol 2008; 26: 5422–5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 2007; 370: 2103–2111. [DOI] [PubMed] [Google Scholar]
- 48. Choueiri TK, Larkin J, Oya M, et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): an open-label, dose-finding and dose-expansion, phase 1b trial. Lancet Oncol 2018; 19: 451–460. [DOI] [PubMed] [Google Scholar]
- 49. Atkins MB, Plimack ER, Puzanov I, et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol 2018; 19: 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Keeler ME, Kessler ER, Bernard B, et al. Pembrolizumab (pembro) and cabozantinib (cabo) in patients (pts) with metastatic renal cell carcinoma (mRCC): phase I results. J Clin Oncol 2019; 37(Suppl. 7): 600. [Google Scholar]
- 51. Chowdhury S, McDermott DF, Voss MH, et al. A phase I/II study to assess the safety and efficacy of pazopanib (PAZ) and pembrolizumab (PEM) in patients (pts) with advanced renal cell carcinoma (aRCC). J Clin Oncol 2017; 35(Suppl. 15): 4506. [Google Scholar]
- 52. Lee CH, Makker V, Rasco DW, et al. Lenvatinib + pembrolizumab in patients with renal cell carcinoma: updated results. J Clin Oncol 2018; 36(Suppl. 15): 4560. [Google Scholar]
- 53. Amin A, Plimack ER, Infante JR, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with sunitinib or pazopanib in patients (pts) with metastatic renal cell carcinoma (mRCC). J Clin Oncol 2014; 32(Suppl. 15): 5010. [Google Scholar]
- 54. Nadal RM, Mortazavi A, Stein M, et al. Results of phase I plus expansion cohorts of cabozantinib (Cabo) plus nivolumab (Nivo) and CaboNivo plus ipilimumab (Ipi) in patients (pts) with with metastatic urothelial carcinoma (mUC) and other genitourinary (GU) malignancies. J Clin Oncol 2018; 36(Suppl. 6): 515.29267131 [Google Scholar]
- 55. Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019; 380: 1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019; 380: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 57. Rini BI, Powles T, Atkins MB, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet 2019; 393: 2404–2415. [DOI] [PubMed] [Google Scholar]
- 58. Motzer RJ, Powles T, Atkins MB, et al. IMmotion151: a randomized phase III study of atezolizumab plus bevacizumab vs sunitinib in untreated metastatic renal cell carcinoma (mRCC). J Clin Oncol 2018; 36(Suppl. 6): 578. [Google Scholar]
- 59. Atkins MB, McDermott DF, Powles T, et al. IMmotion150: a phase II trial in untreated metastatic renal cell carcinoma (mRCC) patients (pts) of atezolizumab (atezo) and bevacizumab (bev) vs and following atezo or sunitinib (sun). J Clin Oncol 2017; 35(Suppl. 15): 4505. [Google Scholar]
- 60. Escudier B, Barthelemy P, Ravaud A, et al. Tivozanib combined with nivolumab: phase Ib/II study in metastatic renal cell carcinoma (mRCC). J Clin Oncol 2018; 36(Suppl. 6): 618. [Google Scholar]
- 61. Albiges L, Powles T, Staehler M, et al. Updated European association of urology guidelines on renal cell carcinoma: immune checkpoint inhibition is the new backbone in first-line treatment of metastatic clear-cell renal cell carcinoma. Eur Urol 2019; 76: 151–156. [DOI] [PubMed] [Google Scholar]
- 62. Rassy EE, Khoury Abboud RM, Ibrahim N, et al. The current state of immune checkpoint inhibitors in the first-line treatment of renal cancer. Immunotherapy 2018; 10: 1047–1052. [DOI] [PubMed] [Google Scholar]
- 63. Dudani S, Graham J, Wells C, et al. First-line (1L) immuno-oncology (IO) combination therapies in metastatic renal cell carcinoma (mRCC): preliminary results from the international metastatic renal cell carcinoma database consortium (IMDC). J Clin Oncol 2019; 37(Suppl. 7): 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dudani S, Graham J, Wells JC, et al. First-line immuno-oncology combination therapies in metastatic renal-cell carcinoma: results from the international metastatic renal-cell carcinoma database consortium. Eur Urol 2019; 76: 861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hahn AW, Klaassen Z, Agarwal N. et al. First-line Treatment of Metastatic Renal Cell Carcinoma: A Systematic Review and Network Meta-analysis. Eur Urol Oncol 2019; 2(6): 708–715. [DOI] [PubMed] [Google Scholar]
- 66. de Velasco G, Bex A, Albiges L, et al. Sequencing and Combination of Systemic Therapy in Metastatic Renal Cell Carcinoma. Eur Urol Oncol 2019; 2(5): 505–514. [DOI] [PubMed] [Google Scholar]
- 67. Barata PC, Gomez de Liano A, Mendiratta P, et al. Clinical outcome of patients (Pts) with metastatic renal cell carcinoma (mRCC) progressing on front-line immune-oncology based combination (IO-COMBO) regimens. J Clin Oncol 2018; 36(Suppl. 6): 613. [Google Scholar]
- 68. Turajlic S, Litchfield K, Xu H, et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol 2017; 18: 1009–1021. [DOI] [PubMed] [Google Scholar]
- 69. McDermott DF, Huseni MA, Atkins MB, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med 2018; 24: 749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhu J, Armstrong AJ, Friedlander TW, et al. Biomarkers of immunotherapy in urothelial and renal cell carcinoma: PD-L1, tumor mutational burden, and beyond. J Immunother Cancer 2018; 6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rini BI, Huseni M, Atkins MB, et al. LBA31Molecular correlates differentiate response to atezolizumab (atezo) + bevacizumab (bev) vs sunitinib (sun): results from a phase III study (IMmotion151) in untreated metastatic renal cell carcinoma (mRCC). Ann Oncol 2018; 29(Suppl. 8). DOI: 10.1093/annonc/mdy424.037. [DOI] [Google Scholar]
- 72. Choueiri TK, Albiges L, Haanen JBAG, et al. Biomarker analyses from JAVELIN Renal 101: Avelumab + axitinib (A+Ax) versus sunitinib (S) in advanced renal cell carcinoma (aRCC). J Clin Oncol 2019; 37(Suppl. 15): 101. [Google Scholar]
- 73. Krieg C, Nowicka M, Guglietta S, et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat Med 2018; 24: 144–153. [DOI] [PubMed] [Google Scholar]
- 74. Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 2018; 24: 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Young MD, Mitchell TJ, Vieira Braga FA, et al. Single-cell transcriptomes from human kidneys reveal the cellular identity of renal tumors. Science 2018; 361: 594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]