Abstract
Background: Cancer is a major health problem worldwide and the leading cause of death in many countries. Preclinical studies have shown the therapeutic anticancer effects of SH003, a novel herbal medicine containing Astragalus membranaceus, Angelica gigas, and Trichosanthes kirilowii. The present study investigated the maximum tolerated dose of SH003 in patients with solid cancers. Methods: This open-label, dose-escalation trial used the traditional 3 + 3 dose-escalation design. Patients with solid cancers were recruited and administered 1 to 4 tablets of SH003 thrice daily for 3 weeks according to the dose level. Adverse events were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE). Dose-limiting toxicities (DLTs) were defined as Grade 3 or higher adverse events based on CTCAE. The maximum tolerated dose was defined as the highest dose at which no more than 1 of 6 patients experienced DLT. Results: The present study enrolled 11 patients. A total of 31 adverse events occurred. According to the CTCAE, all the observed adverse events were grade 2 or less and no adverse events of grade 3 or more corresponding to DLT occurred. Conclusion: The study results indicated that the maximum tolerated dose of SH003 was 4800 mg/day. A Phase 2 study is required to determine the efficacy of SH003 in patients with cancer at a dose of 4800 mg/day or less.
Keywords: herbal medicine, maximum tolerated dose, SH003, phase 1, cancer, safety, first-in-human
Introduction
Cancer, a malignant growth or tumor resulting from the division of abnormal cells, is the second leading cause of death globally, accounting for 9.6 million deaths in 2018.1 Cancer incidence and mortality have risen rapidly worldwide, with an estimated 18.1 million new cancer cases.2 In Korea, there were 1.73 million patients with cancer in 2016, and if individuals live up to the life expectancy (82 years), they have a 36.2% chance of developing cancer.3 The socioeconomic cost of cancer increased from 14 trillion Korean Won in 2012 to 16.7 trillion Won in 2015.4,5 While large budgets have been funded and research and development is underway, cancer remains a challenge for mankind. The market for cancer treatment continues to grow, with treatments using herbal medicines recently receiving attention.6,7 Many herbal medicines have been investigated for the treatment of patients with cancer.
SH003 is a new herbal medicine containing Huang-Qi (Astragalus membranaceus), Dang-Gui (Angelica gigas), and Gua-Lou-Gen (Trichosanthes kirilowii). Each of these herbs has long been used in traditional medicine and several anticancer effects have been reported.8-10 Based on these findings and the theories of traditional medicine, studies have investigated the efficacy and mechanism of SH003 on cancer, reporting that SH003 suppresses breast cancer growth and metastasis by inducing autophagy11 and inhibiting STAT3-IL-6 signaling,12 repressing tumor angiogenesis by inhibiting VEGF-induced VEGFR2 activation,13 and inducing apoptotic cell death of prostate cancer cells by inhibiting ERK2-mediated signaling.14 SH003 also induces inhibition of HeLa cervical cancer cell growth by G1 phase cell cycle arrest.15 In the toxicity test, liver hypertrophy was observed in rats after administering SH003 for 13 weeks, but it was not observed in the 4-week recovery group: rats who stopped taking SH003 after 13 weeks of administration and observed for 4 weeks.11 Therefore, it was a reversible change with no toxicological significance. It is stated in the herbology textbook that the Dang-Gui should be used carefully for patients with diarrhea.16
Before evaluating its efficacy in humans for drug development, this Phase 1 dose-escalation study evaluated the maximum tolerated dose (MTD) of SH003 in patients with solid cancers.
Materials and Methods
Study Design and Ethical Approval
This Phase 1 dose-escalation study was conducted at Ajou University Hospital in Suwon, Republic of Korea, from March 2017 to April 2019. The present study was approved by the institutional review board (IRB) of the Ajou University Hospital (Reference: AJIRB-MED-CT1-16-311). Written informed consent was obtained by the investigator from each participant before their enrolment. The study protocol was previously published before study completion. We followed the methods described by Cheon et al.17
Patients
Patients with solid cancer treated at Ajou University Hospital who met the inclusion criteria and provided informed consent were recruited. The inclusion criteria included age 19 years and older; histologically or cytologically confirmed solid cancers; metastatic or unresectable cancers for which standard curative measures do not exist or are no longer effective; Eastern Cooperative Oncology Group Performance Status ≤2; estimated life expectancy of at least 12 weeks; no chemotherapy or surgery within the past 4 weeks; recovery levels of hemoglobin at ≥8 g/dL, platelet count ≥75 000/µL, and absolute neutrophil count ≥1500/µL; the ability to swallow tablets; the ability to understand the study; and willingness to sign a written informed consent document. The exclusion criteria were patients with known hypersensitivity to any study drug component, including A membranaceus, A gigas, and T kirilowii; with acute or chronic infections requiring treatment (active hepatitis A, B, and C viruses, human immunodeficiency virus, and tuberculosis); with estimated glomerular filtration rate <60 mL/min; with aspartate aminotransferase (AST), alanine aminotransferase (ALT), or total bilirubin ≥2.5 times the institutional upper limit of normal; with uncontrolled cardiovascular diseases (unstable angina, heart failure, myocardial infarction, or uncontrolled hypertension of 140/90 mm Hg or higher); with active cytomegalovirus infection within the past 4 weeks; and with a history of major surgery for cerebrovascular disease such as acute coronary syndrome, stroke, and so on, within the past year, as well as pregnant or lactating women and those with childbearing potential; patients who did not agree to either use effective means of contraception or not to donate sperm during the trial and up to 1 month after final administration; patients taking anticoagulants or anticonvulsants; patients with any psychological, sociological, or geographical conditions that could potentially interfere with their compliance with the study protocol; and patients who had participated in other clinical trials of medicine or medical devices within the past month.
Interventions
One tablet of SH003 (total 800 mg) included 400 mg of solid extracts from A membranaceus, A gigas, and T kirilowii (1:1:1) in a 30% ethanol extract. The pharmaceutical company Hanpoong Pharm & Foods Co Ltd (Jeonju, Republic of Korea) produced the SH003 in accordance with Korea Good Manufacturing Practice standards. SH003 contains the following chemical compounds: astragaloside I, II, IV, isomucronulatol 7-O-glucoside, calycosin-7-O-β-D-glucoside, pinitol, daucosterol 6′-palmitate, β-sitosterol, sucrose, formononetin, and so on of A membranaceus. Decursin, decursinol, decursinol angelate, demethylsuberosine, isoimperatorin, umbelliferone, nodakenin, and so on of A gigas. Trichosanthin, α-hydroxymethylserine, aspartic acid, threonine, serine, glutamic acid, citrulline, glycine, valine, tyrosine, glucose, galactose, and so on of T kirilowii. The major chemical compounds of SH003 are the following: A membranaceus is formononetin, astragaloside IV; A gigas is decursin and nodakenin; and T kirilowii is trichosanthin.16,18 Among them, formononetin and decursin are set as the marker compounds,18 and SH003 was also validated based on the contents of decursin and formononetin.
The dose-escalation rules for the traditional 3 + 3 design were adopted.19 This design recruits 3 patients into one cohort to evaluate toxicity and determine whether to proceed to the next cohort. Development of dose-limiting toxicities (DLTs) in more than 1 of 6 participants at a specific dose suggested that the MTD had been exceeded and further dose escalation was stopped. The dose increment followed the modified Fibonacci sequence. After confirming the safety of the preceding dose, the dose was increased twice by 100% of the preceding dose.
The participants received SH003 for 3 weeks. They orally ingested 1 to 4 tablets with water 3 times a day after meals for 3 weeks according to their dose level. Participants taking 1200, 2400, or 4800 mg/day were defined as Cohorts 1, 2, and 3, respectively.
In toxicity tests, the no observed adverse effect level of the investigational product was 2500 mg/kg in rats. Based on the Food and Drug Administration guidelines, the maximum recommended starting dose for adults was 2400 mg/day based on a safety factor of 10.15 Based on the toxicity and efficacy study results, the starting dose in this study was determined to be 1200 mg/day.10 The second and third cohort doses were 2400 and 4800 mg/day, respectively. During the study, the participants were prohibited from receiving other treatments for cancer, including chemotherapy and radiotherapy.
Outcome Measurements
This study aimed to determine the MTD by evaluating the DLT. The DLT was defined as Grade 3 or higher adverse events based on the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 from the National Cancer Institute (Bethesda, MD).20 The expected DLTs included diarrhea, increased ALT and/or AST levels, febrile neutropenia, and decreased platelet count. The MTD was defined as the highest dose at which no more than 1 of 6 patients experienced DLT. Additionally, adverse events were measured throughout the study period regardless of grade using the CTCAE. In terms of safety, vital signs, physical examination, hematologic, biochemical, and urine tests were measured.
Outcome Analysis
Maximum tolerated dose was defined as the dose just below the lowest dose level at which more than 1 out of 6 patients exhibited DLTs during the 4 weeks of the trial period. In the present study, the highest dose among the 3 dose groups (1200, 2400, or 4800 mg/day) with one patient or less experiencing DLT was defined as the MTD of SH003.
All analyses of data from the present study were descriptive, as the study included no inferential analysis or general hypothesis testing. Continuous variables were presented as medians and range, while categorical variables were presented as absolute and relative frequencies.
Results
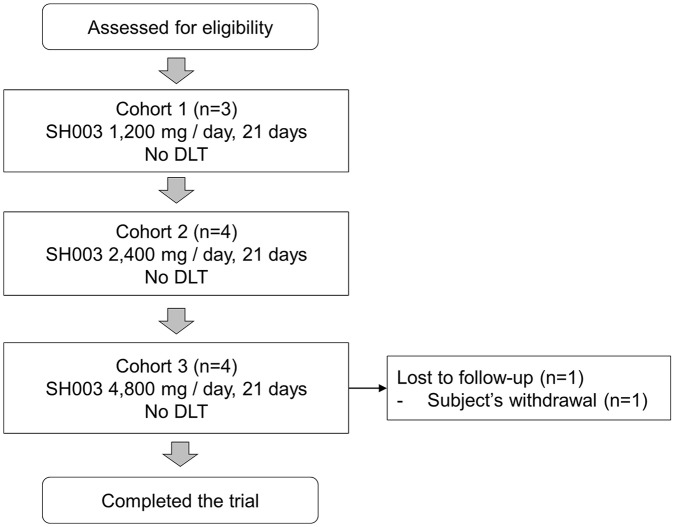
The present study enrolled a total of 11 patients. Three patients were enrolled in Cohort 1, 4 in Cohort 2, and 4 in Cohort 3. One of the participants in Cohort 3 withdrew her consent due to an adverse event. A detailed participant flowchart is presented in Figure 1. The first participant in Cohort 2 was enrolled before the safety assessment for Cohort 1 dose was completed. This was a protocol violation. To comply with the protocol, 3 participants were enrolled in Cohort 2 after the evaluation of Cohort 1 was completed. Therefore, a total of 4 participants were enrolled in Cohort 2. One participant in Cohort 3 withdrew consent after an adverse event occurred. The adverse event was diarrhea, and the severity was grade 2 according to CTCAE. Therefore, the participant was able to continue the clinical trial but discontinued to participate voluntarily. The participant was replaced by a new participant according to the study protocol. Therefore, a total of 4 participants were enrolled in Cohort 3.
Figure 1.
Participant flowchart.
Abbreviation: DLT, dose-limiting toxicity.
The characteristics for all participants and each cohort are shown in Table 1. The median age was 63 years, and there were 3 men and 8 women. The cancer types included cholangiocarcinoma (1 patient), colorectal cancer (6 patients), gastric cancer (2 patients), and lung cancer (2 patients).
Table 1.
Participants Characteristics.
| Total, n = 11 | Cohort 1, n = 3 (1200 mg/day) | Cohort 2, n = 4 (2400 mg/day) | Cohort 3, n = 4 (4800 mg/day) | |
|---|---|---|---|---|
| Age | ||||
| Median | 63 | 58 | 60 | 69.5 |
| Range | 45-82 | 50-62 | 45-77 | 64-82 |
| Sex | ||||
| Male | 3 | 0 | 2 | 1 |
| Female | 8 | 3 | 2 | 3 |
| Height | ||||
| Median (cm) | 165.8 | 167.3 | 169.85 | 157.9 |
| Range (cm) | 150.0-179.3 | 165.8-179.3 | 155-174 | 150-162 |
| Weight | ||||
| Median (kg) | 62.3 | 68.5 | 63.65 | 52.4 |
| Range (kg) | 40.7-89.2 | 64.2-89.2 | 52.2-79.0 | 40.7-62.0 |
| Type of cancer | ||||
| Cholangiocarcinoma | 1 | 1 | 0 | 0 |
| Colorectal cancer | 6 | 2 | 3 | 1 |
| Gastric cancer | 2 | 0 | 0 | 2 |
| Lung cancer | 2 | 0 | 1 | 1 |
The adverse events observed during the 4-week study period, including 3 weeks of medication and 1 week of follow-up, are shown in Table 2. A total of 21 kinds of adverse events were observed. Hot flashes and diarrhea were the most common, with 3 cases each. A total of 31 adverse events occurred in 12 cases in Cohort 1, 9 in Cohort 2, and 10 in Cohort 3. According to the CTCAE, all the observed adverse events were grade 2 or less with no adverse events of grade 3 or more corresponding to the DLT. In the present study, the dose was escalated to 4800 mg/day. Therefore, MTD of SH003 was determined to be 4800 mg/day, the highest dose for which DLT occurred in less than 1 of 6 participants.
Table 2.
Toxicity Results.
| Toxicity | Cohort 1 (n = 3), Grade ≤2/≥3 | Cohort 2 (n = 4), Grade ≤2/≥3 | Cohort 3 (n = 4), Grade ≤2/≥3 |
|---|---|---|---|
| Noncardiac chest pain | 1/0 | 0/0 | 0/0 |
| Hot flashes | 3/0 | 0/0 | 0/0 |
| Headache | 1/0 | 0/0 | 0/0 |
| Nausea | 1/0 | 0/0 | 1/0 |
| Back pain | 1/0 | 0/0 | 1/0 |
| Fatigue | 1/0 | 1/0 | 0/0 |
| Dyspepsia | 1/0 | 1/0 | 0/0 |
| Anorexia | 1/0 | 1/0 | 0/0 |
| Tumor pain | 1/0 | 0/0 | 0/0 |
| Laryngeal fistula | 1/0 | 0/0 | 0/0 |
| Dyspnea | 0/0 | 1/0 | 0/0 |
| Anxiety | 0/0 | 1/0 | 0/0 |
| Weight loss | 0/0 | 1/0 | 0/0 |
| Abdominal pain | 0/0 | 2/0 | 0/0 |
| Diarrhea | 0/0 | 1/0 | 2/0 |
| Aspartate aminotransferase increased | 0/0 | 1/0 | 1/0 |
| Generalized muscle weakness | 0/0 | 0/0 | 1/0 |
| Neoplasms benign | 0/0 | 0/0 | 1/0 |
| Constipation | 0/0 | 0/0 | 1/0 |
| Fever | 0/0 | 0/0 | 1/0 |
| Dizziness | 0/0 | 0/0 | 1/0 |
| Urinary urgency | 0/0 | 0/0 | 1/0 |
Discussion
This first-in-human phase 1 study of SH003 examined its MTD. The MTD is usually defined as the maximum dose that does not cause adverse events of grade 3 or more according to CTCAE in more than 2 of 6 participants.19 In the present study, no adverse events of grade 3 or more were observed; thus, 4800 mg/day, which was the highest dose administered, was determined to be the MTD of SH003.
A major reason for the interest in herbal medicines as complementary and alternative medicine is the expectation of fewer adverse events. A Korean medicine utilization survey reported that low rates of adverse events were the second most common reason for choosing herbal medicine.21 The present study showed that those expectations are consistent with reality. Because there are many kinds of herbal medicine and relatively few studies, it is difficult to say that they are safe; however, this study reported basic data on the safety of the combination of Huang-Qi, Dang-Gui, and Gua-Lou-Gen in patients with solid cancer. The hematological adverse events common in cytotoxic anticancer drugs were not observed in the present study. These results indicated that SH003 does not interfere with hematopoiesis. According to the theoretical framework of Korean medicine, Dang-Gui, which is a constituent of SH003, tonifies blood and is frequently used for the treatment of blood deficiency, which may cause symptoms such as anemia.16 Dang-Gui is useful in the treatment of cerebral infarction due to its anti-atherosclerosis, vasodilatation and improved microcirculation, and antiplatelet aggregation effects.22 Three cases of diarrhea, one of the expected adverse events, were observed, none of which were severe. Increased AST was observed in 2 participants but was not considered DLT because the severities were below grade 2. The lack of correspondence to the DLT may be due to the small sample size of the present study; therefore, diarrhea and liver function are safety variables that should continue to be of interest in further clinical trials. Hot flashes were also an adverse event occurring in 3 participants in the 1200 mg group and should, therefore, be of interest in future studies.
The present study administered SH003 alone; however, the results of experimental studies on the efficacy of the combined treatment of SH003 and anticancer drugs including doxorubicin and paclitaxel have been reported.23,24 We are also interested in combination therapy and have received investigational new drug approval from the Ministry of Food and Drug Safety (MFDS) for a Phase 1/2 clinical trial of combination therapy. Several preclinical studies have shown that SH003 has an anticancer effect on breast cancer.11,12,23,24 It has also been shown to have an anticancer effect on prostate cancer.14 Although it has not yet published, in vivo studies have shown that SH003 has an anticancer effect on lung cancer. Therefore, further researches on breast cancer, prostate cancer, or lung cancer can be planned.
The limitations of this study include the lack of pharmacokinetics (PK) data and that the dose was not increased to that at which DLTs occurred. Herbal medicine, unlike chemical products, includes various ingredients. The marker compounds of some medicinal herbs have been established for quality control; however, this number is small compared with the total number of medicinal herbs.25 As the ratio of marker compounds is small, limited information is available through PK study. Therefore, herbal medicines are exempted from the requirement for PK data submission to the MFDS during the development of new drugs. However, the metabolism of herbal medicines in the human body should be assessed.
To our knowledge, the present study is the first Phase 1 clinical trial of an herbal medicine conducted in Korea. Based on the results of this study, further exploratory studies can determine the efficacy of SH003 at a dose of 4800 mg/day or less in patients with cancer.
Acknowledgments
We would like to thank Hanpoong Pharm & Foods Co Ltd for providing investigational product support. We would also like to thank Prof Hyunwoo Lee of the Ajou University Hospital for his contributions.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number: HI11C2110 and HI18C2382). The management, analysis, and reporting of study was conducted independently by the study investigators.
Ethical Approval: The Institutional Review Board of the Ajou University Hospital approved the study (Reference AJIRB-MED-CT1-16-311).
Trial Registration: ClinicalTrials.gov: NCT03081819.
ORCID iD: Chunhoo Cheon  https://orcid.org/0000-0002-7078-0079
https://orcid.org/0000-0002-7078-0079
References
- 1. World Health Organization. Fact sheets: cancer. https://www.who.int/news-room/fact-sheets/detail/cancer. Accessed February 21, 2020.
- 2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [DOI] [PubMed] [Google Scholar]
- 3. National Cancer Information Center. National Cancer Center [in Korean]. http://www.cancer.go.kr. Accessed September 11, 2019.
- 4. Hyun KR, Lee SM. Analysis of socioeconomic costs of five major diseases. Health Insur Policy. 2014;13:91-107. [Google Scholar]
- 5. Hyun KR, Choi KC, Lee SM, Lee SY. Analysis of Socioeconomic Costs of Major Diseases for Establishment of Health Policy. Wonju, South Korea: National Health Insurance Service; 2017. [Google Scholar]
- 6. Yin SY, Wei WC, Jian FY, Yang NS. Therapeutic applications of herbal medicines for cancer patients. Evid Based Complement Alternat Med. 2013;2013:302426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peltzer K, Pengpid S. The use of herbal medicines among chronic disease patients in Thailand: a cross-sectional survey. J Multidiscip Healthc. 2019;12:573-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Auyeung KK, Han QB, Ko JK. Astragalus membranaceus: a review of its protection against inflammation and gastrointestinal cancers. Am J Chin Med. 2016;44:1-22. [DOI] [PubMed] [Google Scholar]
- 9. Choi HS, Cho SG, Kim MK, et al. Decursin in Angelica gigas Nakai (AGN) enhances doxorubicin chemosensitivity in NCI/ADR-RES ovarian cancer cells via inhibition of P-glycoprotein expression. Phytother Res. 2016;30:2020-2026. [DOI] [PubMed] [Google Scholar]
- 10. Li J, Li H, Zhang Z, Wang N, Zhang Y. The anti-cancerous activity of recombinant trichosanthin on prostate cancer cell PC3. Biol Res. 2016;49:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi YK, Cho SG, Choi YJ, et al. SH003 suppresses breast cancer growth by accumulating p62 in autolysosomes. Oncotarget. 2016;8:88386-88400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choi YK, Cho SG, Woo SM, et al. Herbal extract SH003 suppresses tumor growth and metastasis of MDA-MB-231 breast cancer cells by inhibiting STAT3-IL-6 signaling. Mediators Inflamm. 2014;2014:492173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choi HS, Kim MK, Lee K, et al. SH003 represses tumor angiogenesis by blocking VEGF binding to VEGFR2. Oncotarget. 2016;7:32969-32979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Choi YJ, Choi YK, Lee KM, Cho SG, Kang SY, Ko SG. SH003 induces apoptosis of DU145 prostate cancer cells by inhibiting ERK-involved pathway. BMC Complement Altern Med. 2016;16:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee KM, Lee K, Choi YK, Choi YJ, Seo HS, Ko SG. SH003induced G1 phase cell cycle arrest induces apoptosis in HeLa cervical cancer cells. Mol Med Rep. 2017;16:8237-8244. [DOI] [PubMed] [Google Scholar]
- 16. National College of Korean Medicine Publication Committee on Joint Textbook. Herbology. Seoul, Korea: Younglimsa; 2016. [Google Scholar]
- 17. Cheon C, Kang S, Ko Y, et al. Single-arm, open-label, dose-escalation phase I study to evaluate the safety of a herbal medicine SH003 in patients with solid cancer: a study protocol. BMJ Open. 2018;8:e019502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Institute of Food and Drug Safety Evaluation. The Korean herbal pharmacopoeia [in Korean]. http://www.nifds.go.kr. Accessed January 16, 2020.
- 19. Storer BE. An evaluation of phase I clinical trial designs in the continuous dose-response setting. Stat Med. 2001;20:2399-2408. [DOI] [PubMed] [Google Scholar]
- 20. US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) v4.03. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Accessed February 22, 2020.
- 21. National Development Institute of Korean Medicine. Report of Korean Medicine Utilization Survey—General Population, 2017. Seoul, Korea: National Development Institute of Korean Medicine; 2018. [Google Scholar]
- 22. Wu YC, Hsieh CL. Pharmacological effects of Radix Angelica sinensis (Danggui) on cerebral infarction. Chin Med. 2011;6:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Woo SM, Kim AJ, Choi YK, Shin YC, Cho SG, Ko SG. Synergistic effect of SH003 and doxorubicin in triple-negative breast cancer. Phytother Res. 2016;30:1817-1823. [DOI] [PubMed] [Google Scholar]
- 24. Choi HS, Cho SG, Kim MK, et al. SH003 enhances paclitaxel chemosensitivity in MCF-7/PAX breast cancer cells through inhibition of MDR1 activity. Mol Cell Biochem. 2017;426:1-8. [DOI] [PubMed] [Google Scholar]
- 25. Gang JS. Establishment of Marker Compounds and Content Criteria for Herbal Medicines [in Korean]. Osong, Korea: Ministry of Food and Drug Safety; 2014. [Google Scholar]