Abstract
Two putative zinc metalloproteases encoded by Clostridium perfringens have been implicated in the pathogenesis of necrotic enteritis, an economically significant poultry disease that is caused by this anaerobic bacterium. These proteases have ~64% amino acid identity and are encoded by the zmpA and zmpB genes. We screened 83 C. perfringens isolates by PCR for the presence of these genes. The first gene, zmpB, is chromosomally located and was present in all screened strains of C. perfringens, regardless of their origin and virulence. The second gene, zmpA, is plasmid-borne and was only found in isolates derived from chickens with necrotic enteritis. We describe the generation of insertionally inactivated mutants of both zmpA and zmpB in a virulent C. perfringens isolate. For each mutant, a significant (p < 0.001) reduction in virulence was observed in a chicken necrotic enteritis disease model. Examples of each mutant strain were characterized by whole genome sequencing, which showed that there were a few off-site mutations with the potential to affect the virulence of these strains. To confirm the importance of these genes, independently derived zmpA and zmpB mutants were constructed in different virulent C. perfringens isolates and shown to have reduced virulence in the experimental disease induction model. A zmpA–zmpB double mutant also was generated and shown to have significantly reduced virulence, to the same extent as the respective single mutants. Our results provide evidence that both putative zinc metalloproteases play an important role in disease pathogenesis.
Keywords: Clostridium perfringens; metalloproteases; mucin; necrotic enteritis, poultry
Introduction
Necrotic enteritis imposes a heavy economic burden on poultry producers and has been estimated to cost the world poultry industry U.S.$6 billion a year.39 The impact of necrotic enteritis has led to a surge in research on this disease4 and on the causative bacterium, Clostridium perfringens type G.31
The pore-forming toxin, NetB, is recognized as a major virulence factor, but there is still much to be learned about the pathogenesis of this complex, multifactorial disease.12,30 Few bacterial factors, other than NetB, have been shown to contribute to disease. A putative fimbrial operon has been shown to have a role in the adherence, colonization, and necrotic enteritis pathogenesis of C. perfringens.40,41 It has also been suggested that 2 zinc metalloproteases may have some role in the pathogenesis of necrotic enteritis.15,19
These 2 putative proteases belong to a family of M60-like/PF13402 domain–containing proteins25 that carry a classical HEXXH gluzincin–like motif that is responsible for zinc-binding and acts as the catalytic core.10 The proteins are usually secreted and are generally produced by bacteria that reside on or in the mucosal surfaces of their animal hosts. The proteins often also have accessory domains that have binding specificity for eukaryotic extracellular matrix molecules, specifically mucin, the primary constituent glycoprotein of mucosa.23–26,28 These M60-like proteases have been shown to degrade mucin and have been implicated in the disease processes of pathogens, especially enteric pathogens.23,24,28 Of the 2 putative zinc metalloproteases of this family implicated in necrotic enteritis, one, designated ZmpB, has been shown to be mucolytic.26
The ~190 kDa zinc metalloproteases from C. perfringens have been studied previously in the context of necrotic enteritis,15–17 and it was shown that there was an immune response to ZmpB after chickens were exposed to virulent C. perfringens strains.15 In subsequent vaccination trials, immune responses to this antigen were shown to be partially protective, and a role for the protease in disease was postulated.16,17 However, induction of a protective immune response does not necessarily indicate involvement in pathogenesis; for example, alpha-toxin can induce a degree of immune protection, but there is no evidence that it is involved in the pathogenesis of necrotic enteritis.6
Little relevant C. perfringens genomic data were available at the time of these earlier studies; therefore, it was not apparent that the virulent C. perfringens isolates generally carried 2 homologs of the protease, as has subsequently been shown for other virulent necrotic enteritis–causing strains of C. perfringens.20 The zmpB gene is located on the chromosome, and a homologous but distinct gene, referred to here as zmpA, is located within a plasmid-encoded region, NELoc-1.19 This conserved plasmid region also encodes the NetB toxin. It was not clear from these previous studies which zinc metalloprotease (they have 64% amino sequence identity) may be involved in the disease process, or if both (or neither) proteins have a role in disease.15,19
We report herein the prevalence of both zmpA and zmpB in C. perfringens isolates from different sources. We constructed several zmpA and zmpB mutants as well as a double mutant in necrotic enteritis–causing C. perfringens type G strains and determined their virulence in a chicken necrotic enteritis disease induction model. Our results showed that both putative zinc metalloproteases play an important role in disease pathogenesis.
Materials and methods
Bacterial strains and growth conditions
The growth conditions of strains used in our study (Table 1) were identical to those described previously,41 except that the brain–heart infusion (BHI) medium did not contain soluble starch. Growth curves were generated by inoculating 20 mL of BHI broth with 200 µL of an overnight culture and growth was monitored over 8 h, with 1-mL aliquots being taken every 2 h and the optical density at 600 nm determined. Growth curves were performed in triplicate.
Table 1.
Prevalence of zmpA and zmpB among Clostridium perfringens isolates.
| Strain | Source | netB | zmpB | zmpA | Reference | Strain | Source | netB | zmpB | zmpA | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NAG-NE1 | Australia, chicken with NE | – | + | + | 33 | CN1797 | Type C, 1947 | NT | + | – | BWC |
| EHE-NE3 | Australia, chicken with NE | + | + | + | 33 | JIR4036 D13 | Belgium, cattle | – | + | – | 41 |
| EHE-NE4 | Australia, chicken with NE | + | + | + | 33 | CN2065 | UK, sheep | – | + | – | BWC |
| EHE-NE5 | Australia, chicken with NE | + | + | + | 33 | CN3706 | UK, cattle | – | + | – | BWC |
| EHE-NE7 | Australia, chicken with NE | + | + | + | 33 | CN3686 | UK, pig | – | + | – | BWC |
| EHE-NE9 | Australia, chicken with NE | + | + | + | 33 | CN394 | UK, pig | – | + | – | BWC |
| EHE-NE13 | Australia, chicken with NE | + | + | + | 33 | JIR100 | Australia, pig. 1982 | – | + | – | 18 |
| EHE-NE14 | Australia, chicken with NE | + | + | + | 33 | 44025C03 | Belgium, bovine | – | + | – | 41 |
| EHE-NE15 | Australia, chicken with NE | + | + | + | 33 | #77885 | Country unknown, goat | NT | + | – | 41 |
| EHE-NE16 | Australia, chicken with NE | + | + | + | 33 | SM101 | Human food poisoning type F | – | + | – | 42 |
| EHE-NE17 | Australia, chicken with NE | + | + | + | 33 | CW459 | USA, pig | NT | + | – | 29 |
| EHE-NE18 | Australia, chicken with NE | + | + | + | 33 | Strain 13 | Human gas gangrene | – | + | – | 22 |
| EHE-NE20 | Australia, chicken with NE | + | + | + | 11 | PA1 | Australia, healthy chicken | – | + | – | 40 |
| EHE-NE21 | Australia, chicken with NE | + | + | + | 11 | PA2 | Australia, healthy chicken | – | + | – | 41 |
| EHE-NE22 | Australia, chicken with NE | + | + | + | 11 | PA3 | Australia, healthy chicken | – | + | – | 41 |
| NAG-NE23 | Australia, chicken with NE | – | + | + | 33 | PA4 | Australia, healthy chicken | – | + | – | 41 |
| NAG-NE24 | Australia, chicken with NE | – | + | + | 33 | PA5 | Australia, healthy chicken | – | + | – | 41 |
| NAG-NE25 | Australia, chicken with NE | – | + | + | 13 | PBS1 | Australia, healthy chicken | – | + | – | 41 |
| UNK-NE30 | Australia, chicken with NE | + | + | + | 13 | PBS2 | Australia, healthy chicken | – | + | – | 41 |
| NAG-NE31 | Australia, chicken with NE | + | + | + | 13 | PBS3 | Australia, healthy chicken | – | + | – | 41 |
| NAG-NE32 | Australia, chicken with NE | + | + | + | 13 | PBS4 | Australia, healthy chicken | – | + | – | 41 |
| BER-NE33 | Australia, chicken with NE | – | + | + | 13 | PBS5 | Australia, healthy chicken | – | + | – | 40 |
| SOM-NE34 | Australia, chicken with NE | + | + | + | 13 | PBD1 | Australia, healthy chicken | – | + | – | 40 |
| SOM-NE35 | Australia, chicken with NE | + | + | + | 13 | PBD2 | Australia, healthy chicken | – | + | – | 41 |
| WER-NE36 | Australia, chicken with NE | + | + | + | 35 | PBD3 | Australia, healthy chicken | – | + | – | 41 |
| TAM-NE42 | Australia, chicken with NE | – | + | + | Current study | PBD4 | Australia, healthy chicken | – | + | – | 41 |
| JGS4102 | USA, turkey with NE | + | + | + | 27 | PBD5 | Australia, healthy chicken | – | + | – | 41 |
| JGS4120 | USA, chicken with NE | – | + | – | 7 | PC1 | Australia, healthy chicken | – | + | – | 41 |
| JGS4122 | USA, chicken with NE | + | + | + | Songer,* pers. com., 2006 | PC2 | Australia, healthy chicken | – | + | – | 41 |
| JGS4125 | USA, chicken with NE | + | + | + | Songer,* pers. com., 2006 | PC3 | Australia, healthy chicken | – | + | – | 41 |
| 200302.1.1.Ba | Denmark, chicken with NE | + | + | + | 13 | PC4 | Australia, healthy chicken | – | + | – | 41 |
| 99.63206.34 | Denmark, chicken with NE | + | + | + | 13 | PC5 | Australia, healthy chicken | – | + | – | 40 |
| Strain 48 | Belgium, chicken with NE | – | + | – | 9 | M1 | Australia, healthy chicken | – | + | – | 40 |
| Strain 56 | Belgium, chicken with NE | + | + | + | 9 | M2 | Australia, healthy chicken | – | + | – | 41 |
| Strain 67 | Belgium, chicken with NE | + | + | + | 9 | M3 | Australia, healthy chicken | – | + | – | 41 |
| W1319 | Australia, chicken with NE | – | + | + | 33 | M4 | Australia, healthy chicken | – | + | – | 41 |
| ATCC 13124 | Human gas gangrene | – | + | – | 8 | M5 | Australia, healthy chicken | – | + | – | 41 |
| CN1884 | Type B | NT | + | – | BWC | LP1 | Australia, healthy chicken | – | + | – | 40 |
| CN2109 | Gas gangrene, type C | NT | + | – | BWC | LP2 | Australia, healthy chicken | – | + | – | 41 |
| CN462 | Goat, type D, 1942 | NT | + | – | BWC | LP3 | Australia, healthy chicken | – | + | – | 41 |
| ATCC27324 | Japan, type e | NT | + | – | 36 | LP4 | Australia, healthy chicken | – | + | – | 41 |
| ATCC10543 | Type A, 1948 | NT | + | – | 3 |
BWC = Burroughs Wellcome Collection; NE = necrotic enteritis; NT = not tested.
J.G. Songer, University of Arizona.
PCR for detection of zmpA and zmpB and for Sanger sequencing
Screening for zmpB and zmpA used primer combinations 1 and 2, respectively (Supplementary Table 1). PCR conditions were as follows: 95°C for 2 min, followed by 35 cycles of 95°C for 20 s, 50°C for 20 s, and 75°C for 1 min per kb of product size. The templates for PCR were generated from supernatants of boiled cell preparations. PCR primer combinations 3–9 were used for suicide and TargeTron vector construction or analysis, described below, and combinations 10–14 were used to produce amplicons for Sanger sequencing (Supplementary Table 1).
Targeted mutagenesis of zmpA and zmpB
The pALK1 vector was used as before11,41 for the generation of the suicide vector pBWP4. A 2,156-bp SpeI fragment of the region downstream of zmpA was generated by primer combination 3 (Supplementary Table 1), using EHE-NE18 genomic DNA as a template, and cloned into the SpeI site of pALK1, resulting in pBWP3. Similarly, a 2,535-bp BclI/NheI digested fragment of the region upstream of zmpA, generated by primer combination 4, was cloned into the BamHI/NheI sites of pBWP3, resulting in the final suicide vector, pBWP4. Successful mutagenesis using this construct resulted in 4,530 bp of zmpA being replaced with a 973-bp fragment containing the catP cassette, which encodes chloramphenicol and thiamphenicol resistance.34 After electroporation32 of pBWP4 into the target strain, colonies that grew on tryptose sulfite cycloserine (TSC) agar with thiamphenicol were selected for preliminary confirmation via PCR. Primer combination 5, which was designed to prime outside the genomic regions cloned into pBWP4, was used to show the expected reduction of the size of the amplified product from 9,456 bp to 5,917 bp in the mutants. Primer combinations 6 and 7 were then used to show that the catP cassette had inserted within zmpA.
The insertionally inactivated zmpB mutants were generated using TargeTron technology as before.5,41 The plc-targeted vector pJIR3562 was retargeted to zmpB using a BsrGI/HindIII fragment of the product of primer combination 8 cloned into a BsrGI/HindIII digestion of pJIR3562, resulting in pBWP5. The resultant plasmid was introduced into the target strain by electroporation and the transformants plated onto TSC agar with thiamphenicol. One colony was selected and grown in BHI with thiamphenicol overnight and plated onto TSC agar with erythromycin; erythromycin resistance becoming active only upon genomic insertion. Colonies were screened using primer combination 9 for successful insertion of the erythromycin resistance gene into zmpB. The strain was cured of pBWP5 via a single passage overnight in BHI broth.
High-throughput pyrosequencing
Strains EHE-NE18zmpB and EHE-NE18zmpA1 were subjected to whole genome sequencing (454 FLX genome sequencer; Roche, Hawthorn, Victoria, Australia) using a 3-kb paired-end protocol with Titanium chemistry, with average depths of coverage for the sequencing of 31.6× and 27.8×, respectively.
The resultant sequences from the 2 mutants were compared to the wild-type EHE-NE18 genome (gsMapper of the Newbler package v.2.6; Roche). Predicted differences were collated and then filtered based upon the parameters that any variation must be present in ≥ 10 reads, and that the variation must be supported by > 75% of reads covering that sequence. The sequence differences that were found to be either non-synonymous, or had the potential to disrupt promoter sequences, were confirmed via Sanger sequencing.
Necrotic enteritis disease induction model
A necrotic enteritis induction protocol was used as described previously.14 Briefly, unvaccinated 1-d-old Ross 308 birds were fed an antibiotic-free commercial starter feed. Animals were housed in 1.5 × 1.5-m pens with 3-cm deep wood shavings. On day 15, a wheat-based feed containing 50% (w/w) fishmeal was introduced. Feed mixed with a C. perfringens culture was fed to the birds on days 21 and 22, twice on each day. Birds were euthanized on day 23, and the mucosal surface of the intestines was examined for lesions of necrotic enteritis. Disease severity, on a scale of 0–6, was scored as described previously.11 Control animals had the same feed mixed with sterile broth. The assay was done in duplicate with 10–12 birds per group.
Ethics statement
All animal experiments were undertaken with the approval and oversight of the CSIRO Australian Animal Health Laboratory Animal Ethics Committee (Approval AEC 1517) and in accordance with national (Australian Code for the Care and Use of Animals for Scientific Purposes, 2013) and state (Victorian Prevention of Cruelty to Animals Act 1986 and Part 4 of the Prevention of Cruelty to Animals Regulations 2008) legislation.
Statistical analysis
All statistical analysis was undertaken using Prism v.5.02 (GraphPad Software, San Diego, CA). The nonparametric Kruskal–Wallis test with Dunn post-test was used to statistically compare disease outcome results across all groups.
Results
Prevalence of zmpA and zmpB
We screened 83 isolates of C. perfringens from Australia, United States, Belgium, Denmark, Japan, and the UK by PCR for the presence of zmpA and zmpB. Most of these strains were isolated from diseased or healthy chickens, many were NetB-positive, C. perfringens type G. Other strains included isolates from a variety of livestock species and humans, including representatives of each of the C. perfringens toxinotypes (Table 1). All strains were found to carry zmpB, regardless of their origin, whereas zmpA was found exclusively among strains isolated from birds suffering from necrotic enteritis (34 of 36 positive for zmpA).
The zmpA and zmpB single and double mutants display reduced virulence
Given that zmpA was specific to isolates from chickens suffering necrotic enteritis, we initially targeted this gene for mutagenesis in the virulent isolate EHE-NE18. Two independently derived isogenic zmpA mutants (EHE-NE18zmpA1 and EHE-NE18zmpA2) were generated by allelic exchange, replacing ~4.5 kb of zmpA with a catP cassette.4 These mutants were confirmed by PCR as described above.
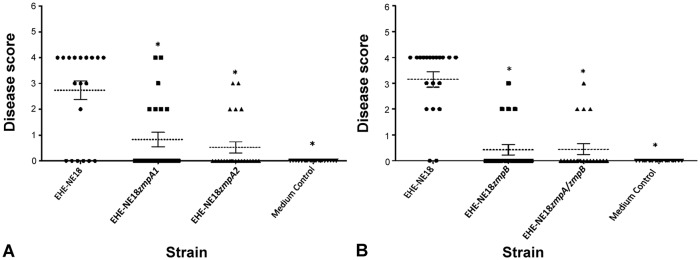
To investigate if zmpA plays a role in virulence, the 2 independent mutants were tested for their ability to cause disease in a chicken necrotic enteritis induction model. In 2 independent trials, the wild-type strain caused disease in 68% of experimentally inoculated birds. By contrast, the isogenic zmpA1 and zmpA2 mutants caused disease in 30% and 23% of birds, respectively (Fig. 1A). The average disease score was 2.74 ± 1.74 (disease data presented as mean ± standard deviation) in wild-type infected birds, and 0.83 ± 1.37 and 0.52 ± 1.04 in birds infected with the zmpA1 and zmpA2 mutants, respectively. The difference in severity of disease between the wild-type strain and the isogenic mutants was statistically significant (p < 0.001).
Figure 1.
Both zmpA and zmpB are required for virulence in EHE-NE18. A. The virulence of the wild-type EHE-NE18 strain and 2 independent zmpA mutants. B. The virulence of the EHE-NE18 wild-type, a zmpB mutant, and a zmpA–zmpB double mutant. The symbols represent the lesion severity for individual birds (n = 20–23 birds for each group), and the dotted line represents the mean lesion severity of that group. Error bars represent standard error of the mean. Lesion severity is scored as: 0 = no gross lesions; 1 = thin or friable walls; 2 = focal necrosis or ulceration (1–5 foci); 3 = focal necrosis or ulceration (6–15 foci); 4 = focal necrosis or ulceration (≥ 16 foci); 5 = patches of necrosis 2–3 cm long; 6 = diffuse necrosis typical of field cases.11 Medium control groups were inoculated with sterile broth only. Statistical analysis (Kruskal–Wallis with Dunn post-test) showed a significant difference (p < 0.001) between the positive control and other groups as denoted by an asterisk (*). Data represent combined results from 2 independent trials.
Given that there was a significant reduction in virulence, but not complete abolition, we postulated that the zmpB gene may have provided partial complementation of the zmpA mutants. Therefore, a zmpA–zmpB double mutant was constructed in strain EHE-NE18. Because this process required a different antibiotic resistance marker, we utilized TargeTron technology (MilliporeSigma, Burlington, MA) to insertionally inactivate the zmpB gene with the erythromycin resistance gene, ermB. However, given the nature of the TargeTron mutagenesis system, in which thiamphenicol is used for selection of the carrier plasmid that is subsequently lost after mutagenesis, it was necessary for selection purposes to generate a zmpB mutant and then to use that strain for the subsequent generation of the zmpA–zmpB double mutant. The zmpB mutant (EHE-NE18zmpB) was generated by TargeTron mutagenesis and was then used to generate the double mutant, EHE-NE18zmpAzmpB, by allelic exchange.
Both the zmpB mutant and its isogenic zmpA–zmpB double mutant were tested in the chicken disease model. The wild-type caused disease in 90% of infected animals with an average disease score of 3.15 ± 1.31; the zmpB mutant and its isogenic zmpA–zmpB double mutant caused disease in 19% (average disease score 0.43 ± 0.93) and 20% (average disease score 0.45 ± 0.94) of infected birds, respectively (Fig. 1B). There was a statistically significant (p < 0.001) reduction in disease incidence induced by both mutants, compared to the wild-type strain, indicating that the mutations reduced the virulence of the resultant strains.
Evidence that the phenotypes of the mutants were the result of the targeted mutations
We made numerous attempts to clone the wild-type zmpA and zmpB genes into the E. coli–C. perfringens shuttle vectors pJIR145721 and pJIR751,2 so that the mutations could be complemented. However, we were unable to generate full-length, mutation-free clones of either gene. This result was not entirely unexpected given that other researchers16,17 found that the zmpB gene was difficult to clone in its entirety. Accordingly, we used a different strategy to confirm that the inactivation of the zmpA and zmpB genes was responsible for the observed phenotypic changes in the mutants.
Growth assays of the wild-type and the zmpB and zmpA1 mutants were performed (Supplementary Fig. 1) to demonstrate that mutation of zmpA and/or zmpB did not alter the growth rate, and thereby negatively impact virulence. No statistically significant difference in growth rate was observed.
To confirm that the phenotypic changes resulted from the mutations, independent zmpA and zmpB mutants were constructed in different C. perfringens G strains. These strains were WER-NE36, another Australian virulent isolate, and strain 56, a virulent Belgian strain, both of which have the zmpA and zmpB genes. Mutants of zmpA were generated in both strains, and a zmpB mutant of strain 56 was obtained. Despite numerous attempts, no zmpB mutant could be generated in WER-NE36, for unknown reasons. These newly generated mutants were tested for virulence in the chicken disease model. The WER-NE36 wild-type caused disease in 80% of infected animals (average disease score 2.80 ± 1.67), whereas the isogenic zmpA mutant caused disease in only 29% of infected birds (average disease score 0.76 ± 1.30). Infection with the wild-type strain 56 resulted in disease in 85% of birds (average disease score 2.80 ± 1.44); the zmpB and zmpA mutants caused disease in 29% and 5% of birds (average disease scores 0.81 ± 1.25 and 0.09 ± 0.43, respectively). All 3 mutants displayed significantly reduced (p < 0.001) virulence compared to their respective wild-type strains (Fig. 2).
Figure 2.
zmpA and zmpB are required for virulence in strain 56 and zmpA in strain WER-NE36. A. The virulence of the wild-type WER-NE36 and its zmpA mutant. B. The virulence of the strain 56 wild-type and its zmpA and zmpB mutants. The symbols represent the lesion severity for individual birds (n = 20–22 birds for each group), and the dotted line represents the mean lesion severity of that group. Error bars represent standard error of the mean. Lesion severity scale is described in Figure 1. Medium control groups were inoculated with sterile broth only. Statistical analysis Kruskal–Wallis with Dunn post-test showed a significant difference (p < 0.001) between the positive control and other groups as denoted by an asterisk (*). Data represent combined results from 2 independent trials.
In addition, the whole genomes of both the EHE-NE18zmpB and EHE-NE18zmpA1 mutants were sequenced to enable comparison to the parent strain (GenBank BioProject PRJNA341531; BioSample SAMN05725818). The objective was to confirm the site of mutagenesis and to identify any secondary mutations that could potentially affect the virulence phenotype. From the draft assemblies of the genomes of the zmpA and zmpB mutants, putative genomic changes were identified and then further interrogated by a combination of manual inspection of the sequence data and additional Sanger sequencing.
In both mutant strains, 2 single-nucleotide polymorphisms (SNPs) were identified (Supplementary Table 2). In the zmpB mutant, apart from the ~1.8-kb insertion of the ermB cassette within zmpB, both SNPs led to conserved amino acid changes. One mutation was in a gene predicted to encode a methyltransferase and changed a leucine residue to an isoleucine residue, and the second mutation was in a gene predicted to encode an alcohol dehydrogenase, changing a glutamate to an aspartate residue.
In the zmpA1 mutant, apart from the ~4.5-kb deletion within zmpA and the insertion of the catP cassette,11 there were 2 SNPs in open reading frames; one of which resulted in an alanine to serine substitution in a gene that encoded a predicted queuine tRNA-ribosyltransferase, a protein not likely to have any role in necrotic enteritis virulence, with no previous studies having identified it as a possible virulence factor for any clostridial diseases. The second SNP also resulted in an amino acid change from alanine to serine in a predicted sigma-54–dependent regulatory gene. This gene, which we will refer to by its C. perfringens strain 13 ortholog, cpe2358, appears to encode an atypical response regulator, based on sequence similarity and BLAST analysis.1 The analysis of the predicted protein showed that it had a helix-turn-helix DNA-binding domain, a sigma-54–activating ATPase domain, a pair of PAS domains, and a phosphotransferase carrier domain (HPr). PAS domains act as signal sensor domains and are found in many signaling proteins.38 The HPr domain is present in signaling molecules, where it can act as a phosphoacceptor.37 Therefore, it appears likely that cpe2358 encodes a transcriptional regulator. The effect that a SNP in this gene may confer on the phenotype of the resultant strain is not known.
The sequence data confirmed that there was only a single insertion of the antibiotic resistance marker into the zmpA gene in EHE-NE18zmpA1, as expected for a mutant derived by allelic exchange. However, we found that a second erythromycin resistance gene had inserted into the genome of EHE-NE18zmpB. This second TargeTron insertion occurred in an intergenic region that was upstream of both genes on either side of the intergenic region. This pair of genes are referred to by their strain 13 orthologs: cpe2357 and cpe2358. The insertion had occurred at 243 and 233 bp upstream, respectively, of the predicted start codons. These genes encode a predicted phosphoenolpyruvate-protein phosphotransferase (cpe2357) and the predicted sigma-54–dependent regulatory protein (cpe2358) already described.
The occurrence in 2 independently derived protease mutants of 2 off-target mutations that potentially affected the same gene, cpe2358, was unexpected. Either what was observed was an improbable chance event, which seems unlikely, or the inactivation of either of these proteases exerted a selective pressure to mutate or change the expression or activity of cpe2358. To decipher which was the more likely scenario, we amplified, using several overlapping PCRs (Supplementary Table 1), the upstream region and coding region of cpe2358 and subjected the amplicons to Sanger sequencing in all 7 of our protease mutant strains and 3 wild-type strains. The logic was that if there was selective pressure present to mutate or change the expression or activity of cpe2358, then other off-target sequence changes would be found in the other mutants. We found that no SNPs were present in this gene region in any of the mutant strains, apart from where already reported. There were no equivalent mutations in the isogenic mutant EHE-NE18zmpA2, which displayed statistically similar levels of virulence as EHE-NE18zmpA1, suggesting that the reduction in virulence seen in EHE-NE18zmpA1 was not caused by the SNP in cpe2358. Taken together, the genetic analysis of the mutant strains gave us confidence that the changes in virulence observed for the mutant strains were the result of inactivation of the metalloprotease genes and not the off-target changes that were observed in these strains.
Discussion
Our study provides evidence that 2 putative zinc metalloproteases play a role in the ability of avian isolates of C. perfringens G to cause necrotic enteritis. Several experimental approaches, including the generation of isogenic mutants that were attenuated for virulence, the generation of equivalent mutants in different strains, and high-throughput pyrosequencing, when combined, indicated that the zmpA and zmpB zinc metalloprotease genes were both likely to be required for full virulence in our avian necrotic enteritis disease model.
Because it was not possible to clone either zmpA or zmpB as intact expressed genes suitable for complementation, we used 2 different approaches to show that the reduction in virulence in the various mutants was the result of the targeted mutation. First, mutants were generated in different strains and examined for their effect on virulence. Irrespective of the parent strain, all of the zmp mutations, in 7 independently derived mutants, had the same effect—attenuation of the ability to cause avian necrotic enteritis. Second, genomic sequencing confirmed the presence of the expected mutations, as well as screening for off-target effects. Of the 5 secondary mutations that were in putative genes or promoter regions (4 SNPs and 1 additional insertion) in the 2 mutants that were sequenced, 2 were conserved amino acid changes. Of the remaining mutations, one was not considered likely to influence virulence, being an alanine to serine substitution in a putative queuine tRNA-ribosyltransferase. Although it cannot be absolutely ruled out that these mutations may be influencing the virulence phenotype of these mutants, it seems unlikely, given that both sequenced protease mutants had a similar level of virulence yet contained different SNPs.
The zmpA mutation in EHE-NE18 significantly reduced virulence but did not totally abolish the ability to cause disease. The presence of a SNP in cpe2358 in EHE-NE18zmpA1 was deemed unlikely to be affecting virulence given that EHE-NE18zmpA2 lacked any such mutations in cpe2358 but caused a near identical level of disease. We hypothesized that zmpB may partially complement the zmpA mutant. Therefore, a zmpA–zmpB double mutant was generated and tested; however, it had the same level of virulence attenuation as the zmpA mutant. Taken on its own, this result would suggest that there was no partial complementation by ZmpB and that this protein did not play a role in disease. However, the EHE-NE18zmpB mutant was also attenuated for virulence, to an extent comparable to the zmpA mutants and the double mutant. This result was confirmed by the construction and analysis of a zmpB mutant in strain 56. The only source of doubt raised about the potential role of ZmpB in virulence was the unintended TargeTron insertion upstream of cpe2358 in the genome sequence of EHE-NE18zmpB. Also supporting the conclusion that ZmpB plays a role in disease were the virulence data generated for the strain 56 zmpB mutant that lacked any mutation in cpe2358 or its upstream region and produced disease levels equivalent to that of EHE-NE18zmpB.
Although it has been shown that ZmpB can degrade mucin, its exact affinity, in the context of the different types of mucin within the chicken intestinal mucosa, remains unknown, as does the precise target(s) of ZmpA. If the 2 proteins have different targets, we may have expected to see a cumulative impact upon virulence in the double mutant. Given the 64% amino acid sequence identity of ZmpA and ZmpB, it is possible that they have similar substrates and may be acting in a similar manner during necrotic enteritis. If so, it is difficult to understand why mutation of either gene would attenuate virulence so significantly and why only zmpA is preferentially associated with strains of C. perfringens isolated from diseased chickens. Perhaps the 2 putative proteases are involved in the same virulence-enhancing catabolic process, and loss of either protease is sufficient to halt the process, resulting in the observed reduction in virulence. What is clear from our experimental data is that both ZmpA and ZmpB are required for full virulence in the avian disease model, and further studies are required to determine the mode of action of these putative proteases and their precise role in the disease process.
We demonstrated that the zinc metalloprotease–encoding genes zmpA and zmpB are involved in necrotic enteritis in chickens. Together with the identification of a putative fimbrial operon that is required for virulence,41 it is clear that the pathogenesis of necrotic enteritis is a complex process that involves much more than the ability to produce the essential extracellular toxin, NetB. It is important that future studies focus on how these virulence factors, and potentially others that remain to be discovered, interact with each other and with the host to enable C. perfringens to cause avian necrotic enteritis.
Supplemental Material
Supplemental material, Supplemental_material for Two putative zinc metalloproteases contribute to the virulence of Clostridium perfringens strains that cause avian necrotic enteritis by Ben Wade, Anthony L. Keyburn, Volker Haring, Mark Ford, Julian I. Rood and Robert J. Moore in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank Honglei Chen for running the 454 pyrosequencing instrument. This work was supported by a PhD scholarship from CSIRO (B. Wade).
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with regards to the research, authorship, and/or publication of this article.
Funding: This research was conducted within the Poultry CRC, established and supported under the Australian Government’s Cooperative Research Centres Program, with partial support from project funding 07-15.
ORCID iD: Robert J. Moore  https://orcid.org/0000-0002-0776-5861
https://orcid.org/0000-0002-0776-5861
Supplementary material: Supplementary material for this article is available online.
References
- 1. Altschul SF, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 1997;25:3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bannam TL, Rood JI. Clostridium perfringens-Escherichia coli shuttle vectors that carry single antibiotic resistance determinants. Plasmid 1993;29:233–235. [DOI] [PubMed] [Google Scholar]
- 3. Boyd MJ, et al. The growth requirements of Clostridium perfringens (welchii) BP6K. J Biol Chem 1948;174:1013–1025. [PubMed] [Google Scholar]
- 4. Broom LJ. Necrotic enteritis; current knowledge and diet-related mitigation. Worlds Poult Sci 2017;73:281–292. [Google Scholar]
- 5. Chen Y, et al. Construction of an alpha toxin gene knockout mutant of Clostridium perfringens type A by use of a mobile group II intron. Appl Environ Microbiol 2005;71:7542–7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cooper KK, et al. Immunization with recombinant alpha toxin partially protects broiler chicks against experimental challenge with Clostridium perfringens. Vet Microbiol 2009;133:92–97. [DOI] [PubMed] [Google Scholar]
- 7. Cooper KK, Songer JG. Virulence of Clostridium perfringens in an experimental model of poultry necrotic enteritis. Vet Microbiol 2010;142:323–328. [DOI] [PubMed] [Google Scholar]
- 8. Eastoe JE, Long JE. The effect of nisin on the growth of cells and spores of Clostridium welchii in gelatine. J Appl Microbiol 1959;22:1–7. [Google Scholar]
- 9. Gholamiandekhordi AR, et al. Molecular and phenotypical characterization of Clostridium perfringens isolates from poultry flocks with different disease status. Vet Microbiol 2006;113:143–152. [DOI] [PubMed] [Google Scholar]
- 10. Hooper NM. Families of zinc metalloproteases. FEBS Lett 1994;354:1–6. [DOI] [PubMed] [Google Scholar]
- 11. Keyburn AL, et al. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect Immun 2006;74:6496–6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keyburn AL, et al. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog 2008;4:e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keyburn AL, et al. Association between avian necrotic enteritis and Clostridium perfringens strains expressing NetB toxin. Vet Res 2010;41:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keyburn AL, et al. Vaccination with recombinant NetB toxin partially protects broiler chickens from necrotic enteritis. Vet Res 2013;44:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kulkarni RR, et al. Clostridium perfringens antigens recognized by broiler chickens immune to necrotic enteritis. Clin Vaccine Immunol 2006;13:1358–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kulkarni RR, et al. Oral immunization of broiler chickens against necrotic enteritis with an attenuated Salmonella vaccine vector expressing Clostridium perfringens antigens. Vaccine 2008;26:4194–4203. [DOI] [PubMed] [Google Scholar]
- 17. Kulkarni RR, et al. A live oral recombinant Salmonella enterica serovar Typhimurium vaccine expressing Clostridium perfringens antigens confers protection against necrotic enteritis in broiler chickens. Clin Vaccine Immunol 2009;17:205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abraham LJ, et al. Hybridization analysis of the class P tetracycline resistance determinant from the Clostridium perfringens R-plasmid, pCW3. Plasmid 1988;19:113–120. [DOI] [PubMed] [Google Scholar]
- 19. Lepp D, et al. Identification of novel pathogenicity loci in Clostridium perfringens strains that cause avian necrotic enteritis. PLoS One 2010;5:e10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lepp D, et al. Identification of accessory genome regions in poultry Clostridium perfringens isolates carrying the netB plasmid. J Bacteriol 2013;195:1152–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lyras D, Rood JI. Conjugative transfer of RP4-oriT shuttle vectors from Escherichia coli to Clostridium perfringens. Plasmid 1998;39:160–164. [DOI] [PubMed] [Google Scholar]
- 22. Mahony DE, Moore TI. Stable L-forms of Clostridium perfringens and their growth on glass surfaces. Can J Microbiol 1976;22:953–959. [DOI] [PubMed] [Google Scholar]
- 23. Martens EC, et al. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol 2018;16:457–470. [DOI] [PubMed] [Google Scholar]
- 24. Belousov MV, et al. M60-like metalloprotease domain of the Escherichia coli YghJ protein forms amyloid fibrils. PLoS One 2018;13:e0191317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakjang S, et al. A novel extracellular metallopeptidase domain shared by animal host-associated mutualistic and pathogenic microbes. PLoS One 2012;7:e30287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Noach I, et al. Recognition of protein-linked glycans as a determinant of peptidase activity. Proc Natl Acad Sci U S A 2017;114:E679–E688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paredes-Sabja D, et al. Clostridium perfringens tpeL is expressed during sporulation. Microb Pathog 2011;51:384–388. [DOI] [PubMed] [Google Scholar]
- 28. Tapader R, et al. YghJ, the secreted metalloprotease of pathogenic E. coli induces hemorrhagic fluid accumulation in mouse ileal loop. Microb Pathog 2017;105:96–99. [DOI] [PubMed] [Google Scholar]
- 29. Rood JI, et al. Isolation and characterization of multiply antibiotic-resistant Clostridium perfringens strains from porcine feces. Antimicrob Agents Chemother 1978;13:871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rood JI, et al. NetB and necrotic enteritis: the hole movable story. Avian Pathol 2016;45:295–301. [DOI] [PubMed] [Google Scholar]
- 31. Rood JI, et al. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe 2018;53:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scott PT, Rood JI. Electroporation-mediated transformation of lysostaphin-treated Clostridium perfringens. Gene 1989;82:327–333. [DOI] [PubMed] [Google Scholar]
- 33. Sheedy SA, et al. Highly conserved alpha-toxin sequences of avian isolates of Clostridium perfringens. J Clin Microbiol 2004;42:1345–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sloan J, et al. Construction of a sequenced Clostridium perfringens-Escherichia coli shuttle plasmid. Plasmid 1992;27:207–219. [DOI] [PubMed] [Google Scholar]
- 35. Stanley D, et al. Changes in the caecal microflora of chickens following Clostridium perfringens challenge to induce necrotic enteritis. Vet Microbiol 2012;159:155–162. [DOI] [PubMed] [Google Scholar]
- 36. Stiles BG, Wilkins TD. Clostridium perfringens iota toxin: synergism between two proteins. Toxicon 1986;24:767–773. [DOI] [PubMed] [Google Scholar]
- 37. Sridharan S, et al. The HPr proteins from the thermophile Bacillus stearothermophilus can form domain-swapped dimers. J Mol Biol 2005;346:919–931. [DOI] [PubMed] [Google Scholar]
- 38. Taylor BL, Zhulin IB. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol Mol Biol Rev 1999;63:479–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wade B, Keyburn AL. The true cost of necrotic enteritis. World Poult 2015;31:16–17. [Google Scholar]
- 40. Wade B, et al. Binding of Clostridium perfringens to collagen correlates with the ability to cause necrotic enteritis in chickens. Vet Microbiol 2015;180:299–303. [DOI] [PubMed] [Google Scholar]
- 41. Wade B, et al. The adherent abilities of Clostridium perfringens strains are critical for the pathogenesis of avian necrotic enteritis. Vet Microbiol 2016;197:53–61. [DOI] [PubMed] [Google Scholar]
- 42. Zhao Y, Melville SB. Identification and characterization of sporulation-dependent promoters upstream of the enterotoxin gene (cpe) of Clostridium perfringens. J Bacteriol 1998;180:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_material for Two putative zinc metalloproteases contribute to the virulence of Clostridium perfringens strains that cause avian necrotic enteritis by Ben Wade, Anthony L. Keyburn, Volker Haring, Mark Ford, Julian I. Rood and Robert J. Moore in Journal of Veterinary Diagnostic Investigation