Abstract
Gas gangrene occurs in several animal species and is caused by one or more clostridial species. In horses, the disease is most often caused by Clostridium perfringens type A. Although Clostridium sordellii has been associated with gas gangrene in ruminants and humans, cases of the disease associated with this microorganism have not been described in horses, to our knowledge. We report herein 8 cases of gas gangrene caused by C. sordellii in horses. These cases were characterized by myonecrosis and cellulitis, associated with systemic changes suggestive of toxic shock. The diagnosis was confirmed by gross and microscopic changes combined with anaerobic culture, fluorescent antibody test, immunohistochemistry, and/or PCR. The predisposing factor in these cases was an injection or a traumatic skin injury. C. sordellii should be considered as a possible etiologic agent in cases of gas gangrene in horses.
Keywords: Clostridium sordellii, gas gangrene, horses, muscle, subcutaneous tissue
Introduction
Gas gangrene (formerly known as malignant edema) is a rapidly progressing infection of muscle and subcutaneous tissue produced by one or more clostridial species, characterized by severe myonecrosis and/or cellulitis in humans and several animal species.28,32 The pathogenesis of gas gangrene involves skin or mucosal wounds through which vegetative forms or spores of the clostridial species involved gain entry to the animal. At the port of entry, the organism multiplies rapidly and produces toxins that act locally and enter the bloodstream, producing toxic shock syndrome and multi-organ failure.22,29 Septicemia is also a common complication of the disease.8,28
Gas gangrene in horses is most often caused by Clostridium perfringens type A,23 although sporadic cases have been described in association with other clostridial species, including C. septicum, C. chauvoei, C. novyi, C. ramosum, C. sporogenes, and C. fallax.2,5,7,14,23,24,28,37 The majority of cases of equine gas gangrene described in the literature have been produced by a single clostridial species, although mixed infections with 2 or more clostridial species have been reported occasionally.15,23,27,37
Clostridium sordellii is one of the members of the gas gangrene complex and has been described as a cause of gas gangrene in humans,3,10,16,30 cattle,38 and sheep,20,35 and also in a series of cases of omphalitis in foals.22 However, to our knowledge, cases of gas gangrene associated with C. sordellii have not been described in horses.
C. sordellii is a gram-positive, anaerobic bacillus, which is a common inhabitant of soil29 and, rarely, the intestinal content of healthy animals. Most cases of clostridial gas gangrene, including those produced by C. sordellii, occur via contamination of wounds, including those associated with parturition and injections. Trauma-associated tissue necrosis generates local hypoxia, alkaline pH, and protein breakdown products required for clostridial proliferation.26 In humans, clostridial toxic shock is a rare syndrome occurring postpartum and post-abortion, characterized by tachycardia, hypotension, and lack of fever.39 The patients frequently progress to fatal toxic shock syndrome.12
Most strains of C. sordellii characterized to date encode sordellilysin (sdl), phospholipase C, and neuraminidase.9 In addition, some C. sordellii isolates may produce lethal toxin (TcsL) and/or hemorrhagic toxin (TcsH), both of which are considered the main virulence factors for toxic shock syndrome in humans.29,31 Although the role of these toxins in animal gas gangrene has not been determined, it is likely that the toxins play a role similar to that in human disease. We describe herein 8 equine cases of gas gangrene produced by C. sordellii.
Materials and methods
We searched the records of the California Animal Health and Food Safety Laboratory System (CAHFS) at the University of California in Davis for cases of horses submitted for autopsy between 1998 and 2019 that had a diagnosis of gas gangrene that was attributed to C. sordellii. This included 8 cases in which: 1) the horses had severe necrotizing cellulitis and/or myositis, 2) C. sordellii had been isolated from the affected muscle and/or detected intralesionally by immunohistochemistry, fluorescent antibody test, and/or PCR, and 3) the horses had died spontaneously or been euthanized because of severe clinical disease associated with this infection (Table 1). An autopsy was performed in all cases. Three horses died (cases 2, 4, and 5), and 2 were euthanized (cases 3 and 8). Information on the manner of death was not available in cases 1, 6, and 7.
Table 1.
Signalment, clinical history, main clinical signs, and affected region of 8 horses with gas gangrene caused by Clostridium sordellii.
| Case | Age (y) | Sex | Breed | Clinical history | Main clinical signs | Anatomic region affected |
|---|---|---|---|---|---|---|
| 1 | NR | NR | NR | Vaccination (EHV-1, influenza, tetanus) 3 d before onset | Local edema and pain | Left side neck and chest |
| 2 | 14 | F | Quarter Horse | Vaccination (rabies) 3 d before onset | Anorexia and seizures | Left gluteal region |
| 3 | 3 | F | Arabian | Injection (selenium-tocopherol, DMSO) 2 d before onset | Local pain, colic, and shock | Lumbar region and both thighs |
| 4 | 5 | F | Quarter Horse | NR | Sudden death | Both thighs |
| 5 | 20 | F | NR | Traumatic skin wound before onset (interval NR) | NR | Left thigh |
| 6 | 2 | M | Appaloosa | Traumatic skin wound 5 d before onset | NR | Left thigh |
| 7 | 19 | F | Quarter Horse | Chronic cellulitis of unknown origin and duration | Anorexia | Both thighs |
| 8 | 7 | F | Quarter Horse | Traumatic skin wound 7 d before onset | Local edema and pain | Right shoulder |
F = female; M = male; NR = not reported.
Samples of lung, liver, kidney, heart, skeletal muscle, stomach, small and large intestine, spleen, thymus, lymph node, uterus, ovary, adrenal gland, pituitary gland, thyroid gland, salivary gland, peripheral nerve, trachea, spinal cord, sciatic nerve, trigeminal ganglia, tongue, pancreas, urinary bladder, subcutaneous tissue, and/or the whole brain were collected in most cases and fixed in 10% neutral-buffered formalin (pH 7.2) for several days. The brains were then cut into ~0.5-cm thick slices, and fixed in fresh formalin for an additional 7–10 d; next, samples of parietal cortex, corpus striatum, thalamus, mid-brain at the level of rostral colliculi, pons, cerebellar peduncles, cerebellum, and medulla at the level of the obex were collected. All tissues were routinely processed to obtain 4-µm thick, hematoxylin and eosin–stained sections. In all cases, selected sections of subcutaneous tissue and muscle were also stained with Gram stain.
Samples of muscle and subcutaneous tissue from grossly affected areas, and multiple organs including one or more of liver, spleen, lung, skin, peripheral lymph nodes, peritoneal fluid, aqueous humor, and small intestinal and cecal content from most horses were collected aseptically, inoculated onto 5% sheep blood agar, and incubated aerobically and/or anaerobically at 37°C for 48 h (Table 2). Subsamples of most of these specimens were also inoculated into cooked meat medium and incubated anaerobically at 37°C for 48 h. All isolates were identified by conventional biochemical techniques.
Table 2.
Microbiologic and molecular findings in skeletal muscle of 8 horses with gas gangrene caused by Clostridium sordellii.
| Case | C. sordellii isolation | Gram stain | FAT |
C. sordellii PCR |
C. sordellii IHC | Other bacteria isolated | |||
|---|---|---|---|---|---|---|---|---|---|
| C. sordellii | C. chauvoei; C. septicum; C. novyi | sdl | tcsL | tcsH | |||||
| 1 | + | + | – | – | NP | NP | NP | NP | – |
| 2 | + | + | + | – | NP | NP | NP | NP | – |
| 3 | + | – | NP | NP | NP | NP | NP | – | C. perfringens |
| 4 | + | + | NP | NP | + | + | – | + | C. perfringens; Enterococcus spp. |
| 5 | + | + | NP | NP | NP | NP | NP | NP | Mixed flora; Streptococcus sp. gamma-hemolytic* |
| 6 | + | + | NP | NP | + | + | – | + | E. coli |
| 7 | + | + | + | – | NP | NP | NP | NP | – |
| 8 | + | NP | + | – | + | + | – | + | Mixed flora; Enterococcus spp. |
FAT = fluorescent antibody test; IHC = immunohistochemistry; + = positive; – = negative; NP = not performed.
Bacteria isolated from a muscle different from which C. sordellii was isolated.
Muscle smears of cases 1, 2, 7, and 8 were also subjected to direct fluorescent antibody test (FAT) for C. sordellii, C. chauvoei, C. novyi, and C. septicum as described previously22 (Table 2). Reference strains of the clostridial species mentioned above were used as controls for each FAT preparation.
Immunohistochemistry (IHC) was performed on formalin-fixed, paraffin-embedded (FFPE) sections of skeletal muscle and subcutaneous tissue of cases 3, 4, 6, and 8 as described previously.22 Briefly, a streptavidin–biotin kit was used according to the manufacturer’s instructions (LSAB-peroxidase K675; Dako, Carpinteria, CA). Primary rabbit polyclonal antibodies against C. sordellii (VMRD, Seattle, WA) were used. Positive controls consisted of muscle sections of a horse from which C. sordellii had been isolated. Negative controls consisted of sections incubated with normal rabbit serum instead of the primary antibody, and of muscle sections of a healthy horse from which no anaerobes had been isolated.
PCR for 3 genes specific to C. sordellii [i.e., sordellilysin (sdl), lethal toxin of C. sordellii (tcsL), and hemorrhagic toxin of C. sordellii (tcsH)] was performed on muscle from cases 4, 6, and 8. For this, three 5-μm thick scrolls of FFPE skeletal muscle were placed into 1.5-mL microcentrifuge tubes for dewaxing by adding 1 mL of xylene, followed by centrifugation for 2 min at 13,000 × g. The xylene was then removed, and the pellet was washed with 1 mL of 100% ethanol and centrifuged for 2 min at 13,000 × g. The ethanol was discarded, and the samples were air-dried at room temperature for 45 min. Then, DNA was extracted from dewaxed tissues (QIAamp DNA FFPE tissue kit; QIAGEN, Hilden, Germany) following the manufacturer’s instructions. The extracted DNA was used as template for conventional PCR detection of sdl, tcsL, and tcsH genes using the following sets of primers, respectively: 5’-CCATAAGTGGTGGTGCTTCG-3’ (sdlF) and 5’-TGATTGCAGCGTATAAGCAAAT-3’ (sdlR; 138 bp); 5’-GACCCAACGAAGAGTGGAGC-3’ (TcsLF) and 5’-TCAAGTGTACCAGCAGGAGC-3’ (TcsLR; 146 bp); 5’-GGGACACCTTCTGTAAGTGTAGG-3’ (TcsHF) and 5’-AGGTTCAACTGTATGCCCAACT-3’ (TcsHR; 133 bp). PCR was performed in a total volume of 25 μL containing 5 μL of extracted DNA, 0.25 μL of each primer (10 μM), 7 μL of nuclease-free water, and 12.5 μL of DreamTaq green PCR master mix 2× (Thermo Scientific, Waltham, MA), which contains DreamTaq DNA polymerase, 2× DreamTaq green buffer, dNTPs (0.4 mM each), and MgCl2 (4 mM). The following thermocycler profiles were used: 95°C for 4 min, 35 cycles at 95°C for 30 s, 54°C for 30 s, and 72°C for 1 min followed by a final extension step at 72°C for 5 min, and a final hold at 4°C. DNA extracted from the C. sordellii JGS6382 strain was used as positive control. This strain is positive for sdl, tcsL, and tcsH. Scrolls from the C. sordellii–negative skeletal muscle used for IHC (see above) and reactions in which nuclease-free water was used instead of DNA were used as negative controls. PCR amplicons were visualized in ethidium bromide–stained 1.5% agarose gels (Agarose SFR; Amresco, Solon, OH).
Results
In cases 1–3 and 5–8, there was a history of skin injury that was thought to be the portal of entry for C. sordellii. No information about a possible portal of entry was available in case 4. The skin injuries were injections (cases 1–3), trauma (5, 6, and 8), and chronic cellulitis (7). The clinical signs were observed 2–7 d before death and included local edema (cases 1 and 8), pain (1, 3, and 8), anorexia (2 and 7), seizures (2), and colic and shock (3). No information about clinical signs was available for cases 5 and 6. Case 4 was found dead without clinical signs being observed.
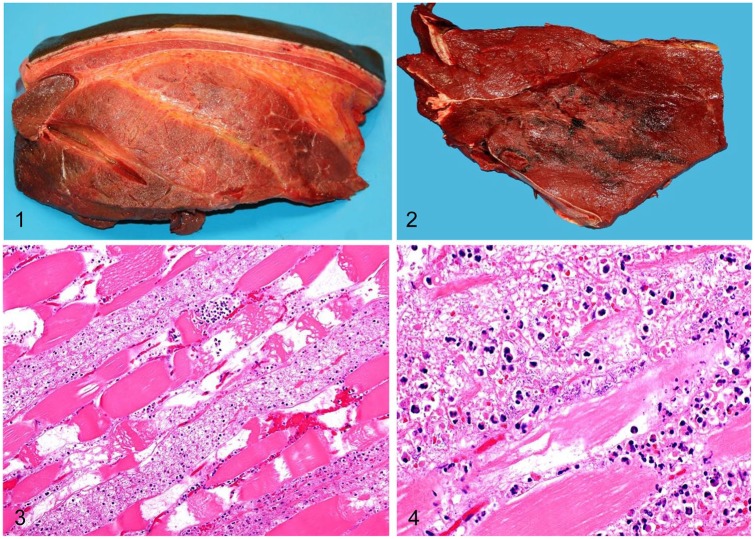
Grossly, the lesions involved muscle and/or subcutaneous tissue underneath areas of skin injuries except for one case (case 4), in which no skin lesions were seen. In all cases, the affected subcutaneous tissue had extensive, moderate-to-severe, foul-smelling, yellow, gelatinous edema, and hemorrhage, which frequently extended into the underlying musculature, separating muscle bundles (Fig. 1). The muscle of these areas was multifocally dark red with irregular pale areas, and was friable, soft, and dry, often with gas bubbles (Fig. 2). The lungs were diffusely congested and edematous, and had petechiae throughout the parenchyma and on the pleura. The heart had epicardial, myocardial, and subendocardial petechiae and ecchymoses that were most marked in the left and right ventricle, but were also observed in both atria. In addition, ascites, hydrothorax, and hydropericardium were observed in cases 1, 4, 6, and 8. Diffuse mucosal edema and subserosal petechiae were observed in the colon of cases 1, 2, 4, 5, and 8.
Figures 1–4.
Skeletal muscle from horses with gas gangrene produced by Clostridium sordellii. Figure 1. Severe subcutaneous and interstitial edema. Figure 2. Focally extensive necrosis and hemorrhage. Figure 3. Coagulative necrosis, hemorrhage, edema, and neutrophil infiltration. H&E. Figure 4. Neutrophil infiltration within necrotic fibers, and large numbers of intralesional rods. H&E.
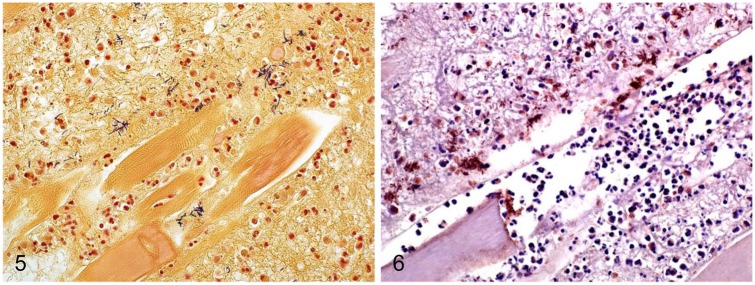
Microscopically, the lesions in skeletal muscle were similar in all animals. There was multifocal-to-coalescing necrosis of muscle fibers, characterized by a diffuse, dense, eosinophilic, and glassy appearance of the cytoplasm, with loss of cross-striations, fragmentation, vacuolation, hypercontraction bands, mineralization, karyorrhexis, and karyolysis (Figs. 2, 3). Multifocally, within the cytoplasm of the necrotic myofibers, there were moderate numbers of degenerate and viable neutrophils, and fewer macrophages (Fig. 4). The interstitium and fascia were expanded by moderate-to-severe hemorrhage, edema, fibrin, neutrophils, and fewer lymphocytes, plasma cells, and macrophages. The interstitium also had multifocal, large empty clear vacuoles with well-defined borders in cases 1–3 and 5–8, and large numbers of gram-positive rods, singly or in clusters (Fig. 5). These bacteria were ~5–7 µm × 0.8–1 µm, with parallel borders and round ends, and many of them had central or subterminal spores (Fig. 5). Fibrinoid, necrosuppurative vasculitis was observed in areas of muscle necrosis in cases 1 and 6. The subcutaneous tissue overlying the areas of myonecrosis in all cases showed pronounced expansion with edema, hemorrhage, fibrin, neutrophils, lymphocytes, plasma cells, and macrophages. The deep dermis was distended by fibrin, edema, and hemorrhage; blood vessels had multifocal and perivascular neutrophil infiltrates. In addition, cases 3, 5, and 7 had mild, multifocal myocardial necrosis, characterized by swollen myofibers with hypercontraction bands, which were surrounded by a mild neutrophilic and lymphoplasmacytic infiltrate. Multifocal, mild-to-severe interstitial hemorrhage was seen in endocardium, myocardium, and epicardium. The kidneys of cases 1–6 were congested, with homogeneous eosinophilic protein casts in the lumen of renal tubules. Acute proximal tubular necrosis was observed in cases 1 and 3.
Figures 5, 6.
Muscle from horses with gas gangrene produced by Clostridium sordellii. Figure 5. Clusters of gram-positive rods. Gram stain. Figure 6. C. sordellii stained by immunohistochemistry.
C. sordellii was isolated from muscle in all horses (Table 2). In addition, C. perfringens type A, Streptococcus spp., Enterococcus spp., Escherichia coli, and mixed aerobic and anaerobic flora were also isolated from affected muscle in cases 3–6 and 8. All 3 FFPE samples analyzed by PCR were positive for C. sordellii sdl and tcsL genes, but negative for the tcsH gene.
FAT for C. sordellii was positive in 3 of the 4 cases tested; FAT was negative for C. chauvoei, C. novyi, and C. septicum in the 4 cases. Sections of skeletal muscle and subcutaneous tissue from 3 of the 4 cases tested by C. sordellii IHC were positive (Fig. 6). The positive-stained bacteria were in the same location and had similar morphology to those described for the Gram-stained sections. Positive control tissues stained positively with FAT, and no staining was observed in any of the negative controls.
Discussion
We established a diagnosis of gas gangrene by C. sordellii on the basis of clinical history, gross and microscopic findings, and detection of the microorganism by bacterial culture, IHC, FAT, and/or PCR. Although Gram stain and IHC were negative in 1 case, C. sordellii was isolated from that animal, which, coupled with the gross and microscopic lesions, confirmed the diagnosis. It is likely that the sections used for IHC and Gram stain in that case were prepared from an area with low or no bacterial load, which resulted in negative IHC and Gram stain. The isolation of C. sordellii in pure culture from muscle of 3 horses and the supportive gross and microscopic lesions suggest that this microorganism can act as a primary pathogen to produce gas gangrene in horses. Although C. perfringens type A was isolated in only small numbers in 2 cases, it is possible that it acted synergistically with C. sordellii to produce gas gangrene. Other microorganisms that can produce similar lesions in horses (e.g., C. septicum, C. novyi, and C. chauvoei) were ruled out by culture and/or FAT.
Clostridial gas gangrene has been reported in horses previously.4,18,23,25,27,33,34 However, C. sordellii has not been reported associated with gas gangrene in horses to our knowledge. In a previous report of 37 cases of gas gangrene,23 C. perfringens type A was isolated in purity in 25 cases, and in combination with other clostridia in 4 cases. Based on those results, the authors concluded that C. perfringens type A is the most common cause of gas gangrene in horses; C. sordellii was not isolated from any case.23
In a 2003 study, lesions consisted of severe necrotizing fasciitis and myositis in the region of the inciting wound, coupled with splenic, hepatic, renal, and/or myocardial necrosis.23 In our cases, similar local and systemic lesions were observed, the latter suggesting that toxic shock syndrome also occurred. These lesions are similar to those described in cases of gas gangrene in several animal species.20,28
C. sordellii has been associated with multiple histotoxic infections in a variety of animals, including omphalitis in foals,22 gas gangrene in ruminants,20,35 emphysematous abomasitis in lambs,36 and metritis in sheep.6 This microorganism has also been blamed for sudden death syndrome in cattle38 and lions.11 Solid evidence for the role in the latter is, however, lacking. C. sordellii and its TcsL have also been suggested to be associated with equine atypical myopathy,31 a condition also affecting skeletal muscle.
In humans, C. sordellii has been associated with fulminant necrotizing omphalitis in babies,1,17,19 and endometritis and toxic shock syndrome in women.21 The cause of death of humans with C. sordellii infection is thought to be septic shock, including disseminated intravascular coagulation. The toxins generated by the microorganism at the site of infection are thought to spread systemically, leading to septic shock.13 The gross and microscopic findings described in the 8 horses of our study suggest that a similar mechanism of death occurred in these horses. In our study, a skin injury, either iatrogenic (injection) or accidental, was considered the portal of entry of the agent. This is consistent with most cases of gas gangrene reported previously in horses and other animal species.24,25,27,33,34
In humans, it is believed that 1 or 2 of the 2 main virulence factors of C. sordellii (TcsL and TcsH) are responsible for the main lesions and clinical signs observed in cases of gas gangrene.10 TcsL triggers apoptosis of endothelial cells, leading to vascular compromise, edema, and shock.12 The gene encoding TcsL was identified in the 3 cases available for PCR in our study, suggesting that this toxin might have been the main virulence factor responsible for these infections.
Acknowledgments
We thank J Beingesser for excellent technical assistance. SC Sacco was supported by the “Programa de Movilidad Académico-Científica (PROMAC 2018-2019)” of the National University of Litoral, Argentina. J Ortega was supported by “Ayudas a la movilidad investigadora CEU–Banco Santander”, Spain.
Footnotes
Declaration of conflicting interests: The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was funded by the California Animal Health and Food Safety Laboratory, University of California–Davis.
ORCID iDs: Sofia C. Sacco  https://orcid.org/0000-0003-0797-7968
https://orcid.org/0000-0003-0797-7968
Mauricio A. Navarro  https://orcid.org/0000-0002-7744-8052
https://orcid.org/0000-0002-7744-8052
Francisco A. Uzal  https://orcid.org/0000-0003-0681-1878
https://orcid.org/0000-0003-0681-1878
References
- 1. Adamkiewicz TV, et al. Neonatal Clostridium sordellii toxic omphalitis. Pediatr Infect Dis J 1993;12:253–257. [DOI] [PubMed] [Google Scholar]
- 2. Allen SD, et al. Clostridium. In: Manual of Clinical Microbiology. 7th ed Washington, DC: ASM Press, 1999:654–671. [Google Scholar]
- 3. Bouvet P, et al. Foot infection by Clostridium sordellii: case report and review of 15 cases in France. J Clin Microbiol 2015;53(Suppl 4):S1423–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bruehaus BA, et al. Clostridial muscle infections following intramuscular injections in the horse. J Equine Vet Sci 1983;3:42–46. [Google Scholar]
- 5. Choi YK, et al. Clostridium perfringens type A myonecrosis in a horse in Korea. J Vet Med Sci 2003;65:1245–1247. [DOI] [PubMed] [Google Scholar]
- 6. Clark S. Sudden death in periparturient sheep associated with Clostridium sordellii. Vet Rec 2003;153:340. [PubMed] [Google Scholar]
- 7. Coloe PJ, et al. Clostridium fallax as a cause of gas edema disease in a horse. J Comp Pathol 1983;3:597–601. [DOI] [PubMed] [Google Scholar]
- 8. Cooper BJ, Valentine BA. Muscle and tendon. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed Vol. 1 St. Louis, MO: Elsevier, 2016:230–232. [Google Scholar]
- 9. Couchman EC, et al. Clostridium sordellii genome analysis reveals plasmid localized toxin genes encoded within pathogenicity loci. BMC Genomics 2015;16:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cunniffe JG. Clostridium sordellii bacteraemia. J Infect 1996;33:127–129. [DOI] [PubMed] [Google Scholar]
- 11. de la Fe C, et al. Sudden death associated with Clostridium sordellii in captive lions (Panthera leo). Vet Pathol 2006;43:370–374. [DOI] [PubMed] [Google Scholar]
- 12. Elkbuli A, et al. Survival from Clostridium toxic shock syndrome: case report and review of the literature. Int J Surg Case Rep 2018;50:64–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gray SF, Dieudonne BE. Clostridium sordellii causing malignant edema in a trauma patient: a case report and review of literature. Pan Afr Med J 2018;30:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hagemoser WA, et al. Clostridium chauvoei infection in a horse. J Am Vet Med Assoc 1980;176:631–633. [PubMed] [Google Scholar]
- 15. Jeanes LV, et al. Clostridial myonecrosis in horses. Compend Contin Educ Pract Vet 2001;23:577. [Google Scholar]
- 16. Kimura AC, et al. Outbreak of necrotizing fasciitis due to Clostridium sordellii among blacktar heroin users. Clin Infect Dis 2004;38:87–91. [DOI] [PubMed] [Google Scholar]
- 17. Kosloske AM, Bartow SA. Debridement of periumbilical necrotizing fasciitis: importance of excision of the umbilical vessels and urachal remnant. J Pediatr Surg 1991;26:808–810. [DOI] [PubMed] [Google Scholar]
- 18. MacKay RJ, et al. Clostridium perfringens associated with a focal abscess in a horse. J Am Vet Med Assoc 1979;175:71–72. [PubMed] [Google Scholar]
- 19. Mason WH, et al. Omphalitis in the newborn infant. Pediatr Infect Dis J 1989;8:521–525. [DOI] [PubMed] [Google Scholar]
- 20. Morris WE, et al. Malignant oedema associated with blood-sampling in sheep. Aust Vet J 2002;5:280–281. [DOI] [PubMed] [Google Scholar]
- 21. Murray S, Wooltorton E. Septic shock after medical abortions with mifepristone (Mifeprex, RU 486) and misoprostol. CMAJ 2005;173:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ortega J, et al. Infection of internal umbilical remnant in foals by Clostridium sordellii. Vet Pathol 2007;44:269–275. [DOI] [PubMed] [Google Scholar]
- 23. Peek SF, et al. Clostridial myonecrosis in horses (37 cases 1985–2000). Equine Vet J 2003;35:86–92. [DOI] [PubMed] [Google Scholar]
- 24. Perdrizet JA, et al. Successful management of malignant edema caused by Clostridium septicum in a horse. Cornell Vet 1987;77:328–338. [PubMed] [Google Scholar]
- 25. Pfisterer BR, et al. Pathology in practice. J Am Vet Med Assoc 2019:254:681–683. [DOI] [PubMed] [Google Scholar]
- 26. Quinn PJ, et al. Clostridium species. In: Veterinary Microbiology and Microbial Diseases. 2nd ed West Sussex, UK: Wiley-Blackwell, 2011:241. [Google Scholar]
- 27. Rebhun WC, et al. Malignant edema in horses. J Am Vet Med Assoc 1985;187:732–736. [PubMed] [Google Scholar]
- 28. Silva ROS, et al. Gas gangrene (malignant edema). In: Uzal FA, et al. , eds. Clostridial Disease of Animals. Ames, IA: Wiley, 2016:243–254. [Google Scholar]
- 29. Songer G, Post K. The genus Clostridium. In: Veterinary Microbiology. Bacterial and Fungal Agents of Animal Diseases. St. Louis, MO: Saunders Elsevier, 2005:268. [Google Scholar]
- 30. Tsokos M, et al. Pathology of fatal traumatic and nontraumatic clostridial gas gangrene: a histopathological, immunohistochemical, and ultrastructural study of six autopsy cases. Int J Legal Med 2008;22:35–41. [DOI] [PubMed] [Google Scholar]
- 31. Unger-Torroledo L, et al. Lethal toxin of Clostridium sordellii is associated with fatal equine atypical myopathy. Vet Microbiol 2010;144:487–492. [DOI] [PubMed] [Google Scholar]
- 32. Uzal FA, et al. Animal models to study the pathogenesis of human and animal Clostridium perfringens infections. Vet Microbiol 2015;179:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Valberg SJ, McKinnon AO. Clostridial cellulitis in the horse: a report of five cases. Can Vet J 1984;25:67–71. [PMC free article] [PubMed] [Google Scholar]
- 34. Van Heerden J, Batha WS. Clostridial myositis in a horse. J S Afr Med Assoc 1982;53:211. [PubMed] [Google Scholar]
- 35. Vannelli SA, et al. Clostridium sordellii asociado a un caso de gangrene gaseosa ovina [Clostridium sordellii associated to a case of ovine gas gangrene]. Vet Arg 1996;12:420–422. Spanish. [Google Scholar]
- 36. Vatn S, et al. Sarcina-like bacteria, Clostridium fallax and Clostridium sordellii in lambs with abomasal bloat, haemorrhage and ulcers. J Comp Pathol 2000;122:193–200. [DOI] [PubMed] [Google Scholar]
- 37. Vengust M, et al. Preliminary evidence for dormant clostridial spores in equine skeletal muscle. Equine Vet J 2003;35:514–516. [DOI] [PubMed] [Google Scholar]
- 38. Williams BM. Clostridial myositis in cattle: bacteriology and gross pathology. Vet Rec 1977;100(Suppl 5):S90–91. [DOI] [PubMed] [Google Scholar]
- 39. Zane S, Guarner J. Gynecologic clostridial toxic shock in women of reproductive age. Curr Infect Dis Rep 2011;13:561–570. [DOI] [PubMed] [Google Scholar]