Abstract
Clostridium perfringens type C causes severe and lethal necrotic enteritis (NE) in newborn piglets. NE is diagnosed through a combination of pathology and bacteriologic investigations. The hallmark lesion of NE is deep, segmental mucosal necrosis with marked hemorrhage of the small intestine. C. perfringens can be isolated from intestinal samples in acute cases but it is more challenging to identify pathogenic strains in subacute-to-chronic cases. Toxinotyping or genotyping is required to differentiate C. perfringens type C from commensal type A strains. Recent research has extended our knowledge about the pathogenesis of the disease, although important aspects remain to be determined. The pathogenesis involves rapid overgrowth of C. perfringens type C in the small intestine, inhibition of beta-toxin (CPB) degradation by trypsin inhibitors in the colostrum of sows, and most likely initial damage to the small intestinal epithelial barrier. CPB itself acts primarily on vascular endothelial cells in the mucosa and can also inhibit platelet function. Prevention of the disease is achieved by immunization of pregnant sows with C. perfringens type C toxoid vaccines, combined with proper sanitation on farms. For the implementation of prevention strategies, it is important to differentiate between disease-free and pathogen-free status of a herd. The latter is more challenging to maintain, given that C. perfringens type C can persist for a long time in the environment and in the intestinal tract of adult animals and thus can be distributed via clinically and bacteriologically inapparent carrier animals.
Keywords: Clostridium perfringens type C, necrotic enteritis, pigs
Introduction
Clostridium perfringens type C strains are defined by carrying the 2 typing toxin genes cpa (encoding for α-toxin or CPA) and cpb (encoding for β-toxin or CPB).51,56 In addition, some strains can carry the cpe gene, encoding for enterotoxin (CPE). Type C strains can produce several other toxins, which are however not used for the typing, such as β2-toxin (CPB2), perfringolysin (PFO), and the large clostridial toxin Tpel.2,56 C. perfringens type C causes severe, acute, necrotic enteritis (NE) in livestock and humans.25,69 Outbreaks of human type C enteritis were recorded in Germany after World War II,32 but to date, this disease has been reported only sporadically in developed countries.36,41 A similar disease was diagnosed more frequently in parts of Southeast Asia, particularly in the highlands of Papua New Guinea, where it was a frequent cause of childhood mortality until vaccination programs were initiated.24,33–35,52,63
C. perfringens type C more frequently causes enteritis in neonatal animals such as calves, sheep, goats, and particularly pigs.69,70 The disease in pigs occurs worldwide and is an economically important problem70 because it can lead to mortality rates of up to 100% in affected piglets. The first detailed reports on the pathology of the disease date back to the 1950–1960s.16–18,44,73,74 C. perfringens type C strains were determined as the causative agent and Koch’s postulates were soon fulfilled.4,9,22,23,30 Recent research has extended our knowledge about the pathogenesis of the disease, although several important aspects of it remain to be determined. Nevertheless, NE can be diagnosed easily in veterinary laboratories and can be prevented effectively by vaccination programs using commercial vaccines.
Clinical presentation of the disease
NE affects neonatal piglets from their first day of life until ~ 3 wk of age.72 The disease can spread rapidly in a herd, and mortality in affected piglets in nonvaccinated herds can reach 100%. The disease can occur in peracute-to-acute and subacute-to-chronic forms.16,17,21 Peracute and acute disease affect piglets mainly within the first 3 d after birth.70 In peracute cases, depression is followed rapidly by death without any further signs. Acute cases have hemorrhagic diarrhea shortly before death (Fig. 1A). Piglets with a more protracted subacute-to-chronic clinical course have nonhemorrhagic diarrhea, reduced growth, and emaciation (Fig. 2A, 2B). Affected animals usually die between the second and third week postpartum. In a retrospective study that included 142 cases of NE submitted over a period of 6 y, we did not find affected animals > 3 wk old.21
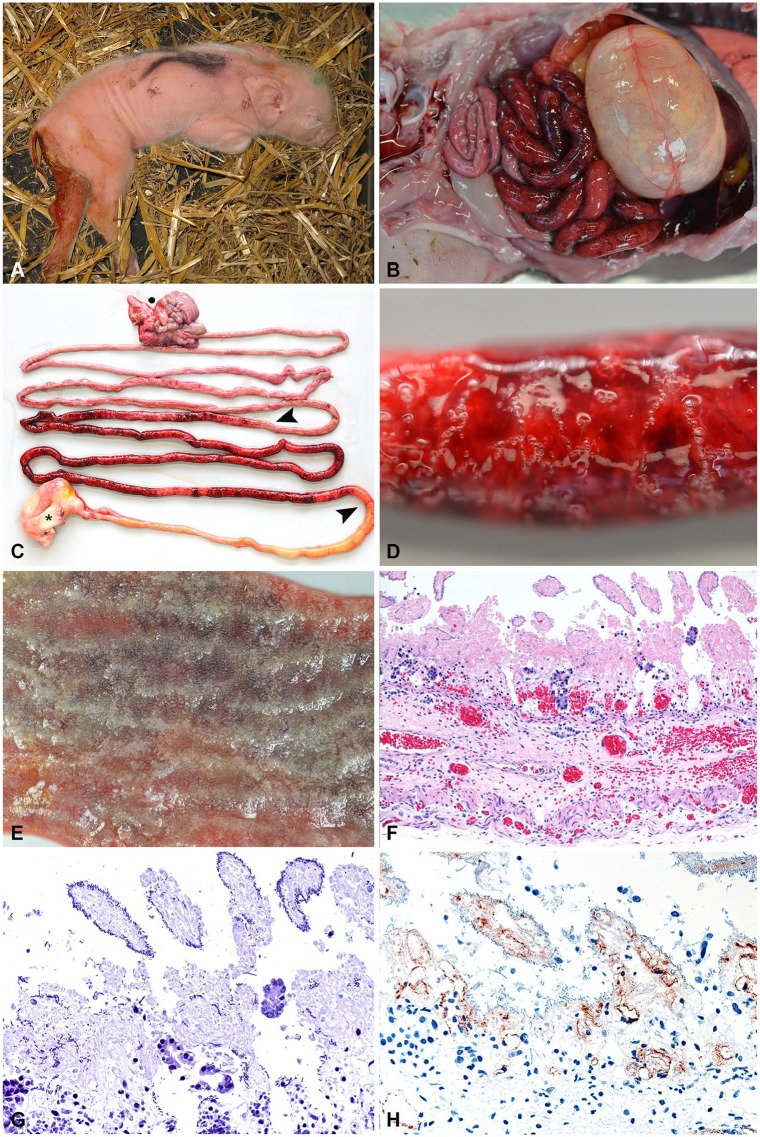
Figure 1.
Typical clinical and pathology presentation of acute necrotic enteritis (NE) in piglets. A. A 1-d-old piglet with hemorrhagic diarrhea. (Photo courtesy H. Nathues, Clinic for Swine, Vetsuisse Faculty, University of Bern.) B. Typical gross lesions of hemorrhagic enteritis in a 1-d-old piglet. C. Typical segmental hemorrhagic and necrotizing jejunitis (between arrowheads). Asterisk = stomach; dot = colon. D. Hemorrhagic intestinal wall with gas bubble formation. E. Mucosa of an acute case of NE. Note that intestinal villi appear white, which is a combination of necrosis and autolysis. Underlying hemorrhage is clearly visible. F. Typical histologic appearance of peracute-to-acute NE in a 1-d-old piglet that died spontaneously. Villi are necrotic and autolytic, with acute hemorrhage in deeper zones of the lamina propria and submucosa. Scant-to-absent inflammatory reaction. (Reprinted with permission.21) 200×. G. Gram stain of the same specimen as panel F depicting necrotic and autolytic villi covered with gram-positive rods. 400×. H. Immunohistochemical detection of Clostridium perfringens beta-toxin (CPB) in the vessels in the lamina propria and submucosa in an acute case of NE. (Reprinted with permission.42) 400×.
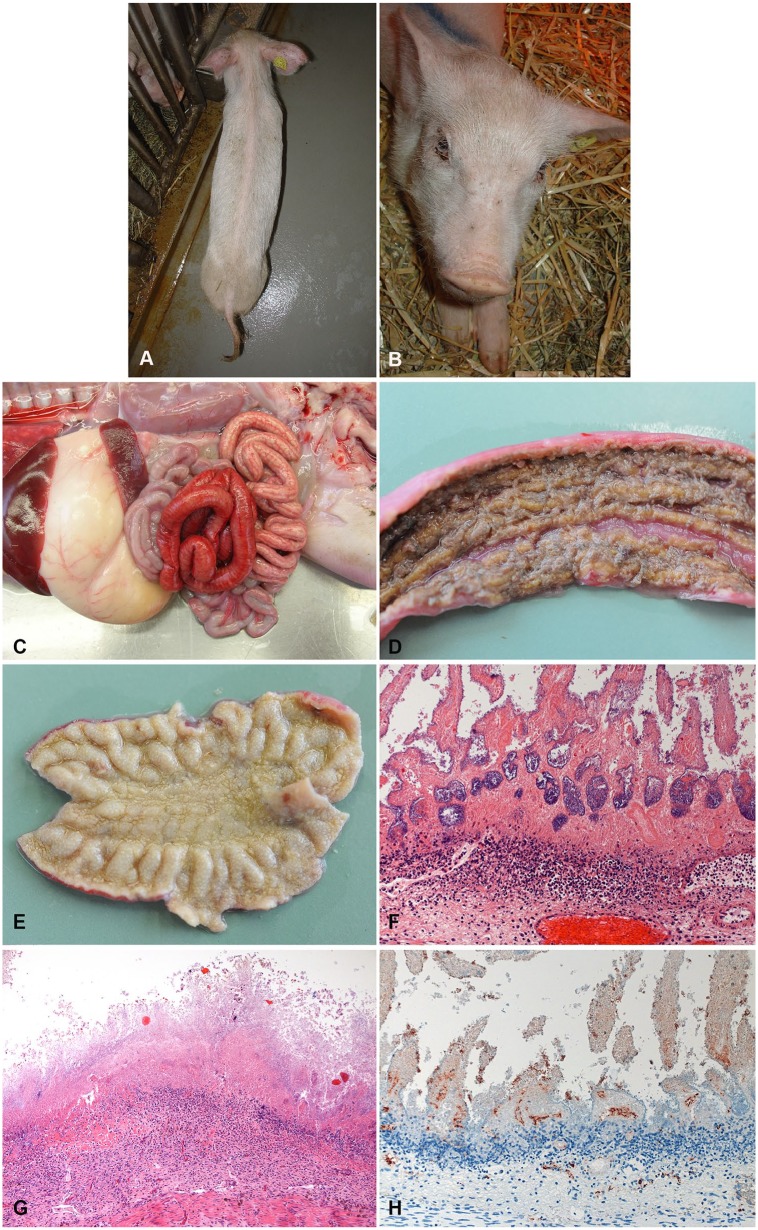
Figure 2.
Typical clinical and pathological presentation of subacute-to-chronic necrotic enteritis (NE) in piglets. A, B. External appearance of the same 3-wk-old, severely emaciated (A) and dehydrated (B) piglet. (Photo by N. Wollschläger, courtesy H. Nathues, Clinic for Swine, Vetsuisse Faculty, University of Bern.) C. Typical gross lesions of subacute NE in a 1-wk-old piglet. Segmental hemorrhage (but less severe than in acute case) and fibrin exudation (light red to yellow) in the small intestine. D. Mucosa of a subacute case of NE. Fibrinous pseudomembrane covering the necrotic mucosa. E. Mucosa of a chronic case of NE. Entire mucosa appears yellow because of deep necrosis and inflammatory reaction. F. Typical histologic appearance of the jejunum of a 1-wk-old piglet with subacute NE. Deep necrosis of mucosa and marked, “band-like” neutrophilic infiltration in underlying submucosa. (Reprinted with permission.42) 200×. G. Typical histologic appearance of the jejunum of a 2-wk-old piglet with chronic NE. Complete effacement of the architecture by deep necrosis of the mucosa. Marked inflammatory infiltration in submucosa. 200×. H. Immunohistochemical detection of Clostridium perfringens beta-toxin (CPB) in the vessels in the lamina propria and submucosa in an acute case of NE. 400×.
Intestinal lesions
At autopsy, the hallmark lesion of NE is segmental mucosal necrosis of the small intestine. In peracute and acute cases, macroscopic lesions are characteristic, with segmental mucosal necrosis and massive hemorrhage in the small intestine (Fig. 1B–1E).16,30,70,72 In most cases, lesions are confined to the jejunum (Fig. 1C); however, they can extend into the ileum. There are reports of lesions extending into the colon70; however, in our experience, this is exceptionally rare. Histologically, in animals that died of peracute-to-acute NE, small intestinal villi are necrotic, and there is extensive acute hemorrhage in the underlying lamina propria, submucosa, and muscularis, oftentimes extending into the serosa (Fig. 1F).21 Large numbers of gram-positive rods can be observed covering the outlines of necrotic villi (Fig. 1G). In addition, thrombosis and necrosis of small vessels in the lamina propria and submucosa is present consistently.4,16,23,30 There is either no, or only scant, infiltration by inflammatory cells in animals dying at this stage of the disease.21,42
CPB can be detected by immunohistochemistry (IHC) in the endothelial lining of vessels in all layers of the affected intestinal segment (Fig. 1E).42 Most animals will die spontaneously, and the necrotic tissue will rapidly be degraded by a combination of clostridial enzymes and autolysis from released cellular proteases and digestive enzymes in the intestine. Thus, dependent on the postmortem interval until autopsy, a certain degree of autolysis usually masks the necrotizing histologic lesions in the mucosa (Fig. 1F). This autolysis can destroy the architecture of the tissue and antigens present in it, thus IHC is not the method of choice for routine testing. Animals surviving the peracute-to-acute stage of NE do not gain weight and appear emaciated (Fig. 2A, 2B). They develop a marked intestinal inflammatory response after the initial mucosal necrosis. Macroscopic lesions are dominated by fibrinous pseudomembranes overlying the intestinal mucosal surface (Fig. 2C, 2D) and marked necrosis of the mucosa (Fig. 2C–2E), which can extend to transmural intestinal necrosis with fibrinous peritonitis.21 In this stage of the disease, there is less hemorrhage present in affected intestinal segments (Fig. 2C–2E).4,16,70
Histopathologically, marked infiltration of neutrophils demarcates the necrotic mucosa from underlying viable tissue (Fig. 2F, 2G). In contrast to acute cases, mixed populations of gram-positive and gram-negative bacteria, most likely representing overgrowth of commensal flora, are visible histologically in subacute-to-chronic cases. In subacute cases, IHC signals of CPB at the endothelium is markedly reduced compared to acute cases, most likely as a result of widespread vascular necrosis, phagocytosis of cellular debris by inflammatory cells, and proteolytic degradation of CPB (Fig. 2H).42 In chronic cases, IHC staining for CPB can be negative.42
Bacteriology
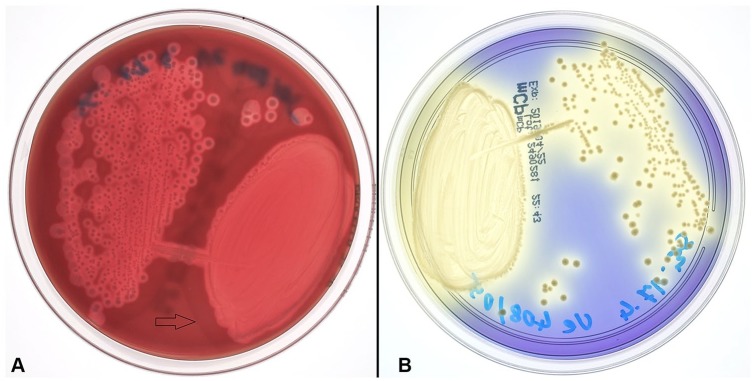
Although macroscopic and histologic lesions may be very characteristic for NE, bacterial culture is recommended to confirm a final diagnosis of C. perfringens type C enteritis. Culture can be performed from intestinal content or feces and should always be interpreted in light of pathology findings in affected animals, because the isolation of C. perfringens type C alone is not sufficient to establish a final diagnosis of type C enteritis. If samples are transported to the laboratory, transport media for anaerobes (e.g., Amies transport medium) is recommended. Although C. perfringens is a gram-positive, anaerobic, endospore-producing, rod-shaped bacterium, it is more aerotolerant than other Clostridium spp. Nevertheless, successful culture requires an anaerobic atmosphere and is preferably done on selective growth media because it can otherwise be overgrown by the normal, facultative, anaerobic intestinal flora.12,29,40 On blood agar, C. perfringens normally forms a characteristic double zone of hemolysis (Fig. 3). The inner zone of complete hemolysis is caused by PFO; the outer zone of incomplete hemolysis is caused by CPA. The cpa gene (encoding CPA) is considered part of the core genome, whereas pfoA (encoding PFO) is sometimes not present in environmental or food poisoning isolates.28
Figure 3.
Clostridium perfringens type C cultures. A. C. perfringens type C colonies after 24 h anaerobic incubation at 37°C on Brucella blood agar (BD). The outer zone of partial hemolysis is marked by an arrow. B. Typical yellow C. perfringens type C colonies after 24 h anaerobic incubation at 37°C on mCP agar (Thermo Fisher Scientific).
Various selective media can be used for isolation (e.g., tryptose–sulfite–cycloserine with egg yolk agar [TSC-EYA],31 blood agar with polymyxin B,43 or membrane C. perfringens agar plates [mCP; Thermo Fisher Scientific, Reinach, Switzerland]).12 At our institution, we routinely use mCP plates for isolation of C. perfrigens from feces. These plates contain a chromogenic medium in which suspected colonies of C. perfringens can be detected by their typical yellow appearance (Fig. 3). Identification can be achieved using various biochemical test panels, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, or PCR detection of plc (cpa), which is present in all types of C. perfringens.1,6,56 Given that C. perfringens type A can also be found in the intestine of healthy animals, it is important to determine the toxin type to establish a diagnosis. C. perfringens type C strains carry the cpa and cpb genes, with a few strains also carrying the cpe gene.56 The cpb gene is plasmid-borne and can be found on a variety of plasmids.13 The consensus cpb2 gene (encoding the β2-toxin), which is also often present in C. perfringens type C, was found to be located on additional plasmids.13 All plasmids have been found to contain the tcp locus, indicating the possibility of conjugative transfer; however, not all plasmid combinations can be maintained stably.29 Furthermore, an atypical cpb2 gene has been described26; the gene has been demonstrated in C. perfringens type A isolates from pigs but has so far not been clearly linked to disease.5,8
Classically, the presence of toxins was determined by animal inoculation. This method has been replaced by ELISAs, which detect the secreted toxins in samples such as stool samples or culture supernatants. PCR methods are now used routinely to detect toxin-encoding genes to identify C. perfringens isolates.43 Multiplex PCR methods for detection of toxin genes important for typing have been described.1,8,11 PCR amplification of toxin genes can be performed not only from culture isolates but also directly on DNA extracted from intestinal content, intestinal tissue, and even paraffin tissue blocks.21,61 PCR can be helpful in subacute-to-chronic cases or when an autopsy could not be performed immediately after the death of the animal. In such cases, identification of C. perfringens type C by culturing is more challenging because of the overgrowth of commensal bacteria including C. perfringens type A.70 Thus, if cultures are achieved in such cases, genotyping of several colonies is generally recommended to increase the chances of identifying the type C colonies.21
Commercial ELISA kits exist for the detection of CPB in intestinal contents. The toxin is, however, very sensitive to trypsin, pepsin, and possibly other proteases, which can lead to false-negative results if the samples are not fresh.38 Thus detection of CPB in intestinal samples of piglets with typical histologic lesions is diagnostic for type C enteritis, whereas lack of CPB detection does not preclude such a diagnosis.
Pathogenesis
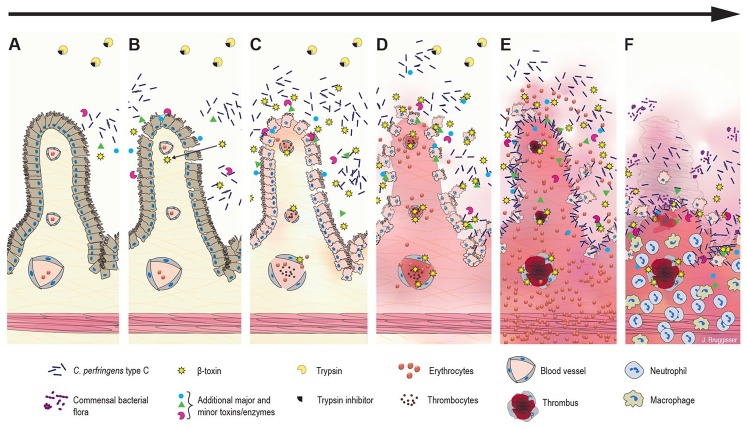
CPB was originally hypothesized to be the major virulence determinant of type C strains given the epidemiologic evidence of its association with the disease in pigs and humans.68,77 Experimental studies using genetic approaches and animal models of disease clearly demonstrated that CPB is the essential virulence factor of type C strains.60,78,79 Because of the rapid degradation of CPB by trypsin, high trypsin-inhibitor levels in the colostrum of the sow contribute to the disease.60,70 It has traditionally been hypothesized that CPB acts directly on small intestinal epithelial cells, leading to widespread epithelial necrosis, subsequent hemorrhage, and, in cases in which animals survive these initial effects, a marked inflammatory response.69 Recent research, however, depicts more complex pathogenesis (Fig. 4).
Figure 4.
Hypothesized pathogenesis of Clostridium perfringens type C enteritis in pigs. A. The disease starts with colonization, rapid proliferation of C. perfringens type C, and toxin secretion in the jejunal lumen. B. Initial epithelial damage or irritation could be caused by secreted toxins and/or the altered luminal environment. Trypsin inhibitors in the colostrum prevent degradation of C. perfringens beta-toxin (CPB), which can pass the epithelial barrier and diffuse into the lamina propria. C. CPB-induced damage in endothelial cells rapidly leads to increased permeability of vessels in the lamina propria with extravasation of fluid, plasma proteins, and erythrocytes. CPB additionally could affect platelet function and interfere with primary hemostasis promoting vascular leakage. The epithelial barrier is further disrupted by necrosis of epithelial cells. Blood components and necrotic cells accumulate in the intestinal lumen. D. Clostridial growth and toxin production accelerate. A vicious cycle of CPB-induced hemorrhage, tissue necrosis, accelerated clostridial growth, and toxin secretion develops. E. The peracute-to-acute stage of the lesion can develop within hours after initial effects: hemorrhage and necrosis rapidly progress from the initial site of damage in the jejunum. Next, there is widespread vascular thrombosis in the lamina propria and submucosa. In addition, toxins from the intestinal lumen can be absorbed into the systemic circulation and cause enterotoxemia. F. The subacute stage of the disease develops in cases in which the initial acute damage was less severe and did not cause death. Neutrophils infiltrate the zone under the necrotic mucosa. Additional bacteria present in the intestinal tract can proliferate, leading to mixed bacterial cultures.
The initial step of NE, as for any other clostridial enteric disease, is the overgrowth of pathogenic strains in the intestine.69 Newborn piglets are highly susceptible to an overgrowth of clostridia because of their incompletely developed intestinal flora. C. perfringens type C, when ingested early postpartum, rapidly outcompetes the developing intestinal flora,69 and, in cases of peracute-to-acute NE in 1- to 3-d-old piglets, it can be isolated in almost pure culture.
The next step, which is however incompletely understood, is most likely the initial alteration of the epithelial barrier, which, under normal circumstances, would inhibit diffusion of toxins and enzymes into underlying mucosal layers. Experimental studies showed that CPB itself is nontoxic to cultured porcine small intestinal epithelial cells, and, in this species, does not bind to small intestinal epithelial cells in vitro or in vivo.57,62 Studies using a rabbit intestinal loop model suggest intital epithelial damage elicited by CPB; however, binding of CPB to epithelial cells in this experimental model has not been investigated. In pigs, additional factors, such as toxins, enzymes, and/or accumulation of metabolites as a result of massive dysbacteriosis, are potentially important in the initial damage to the epithelial barrier. This, however, has not yet been demonstrated.
CPB could subsequently reach underlying tissue and cells, particularly the vascular endothelium, presumably by diffusion. In cultured endothelial cells, CPB disrupts the actin cytoskeleton, causing retraction of the cell borders, which rapidly progresses to cell shrinkage and cell death.3,14 Interestingly, early vascular endothelial damage and widespread vascular necrosis were documented by several authors investigating experimentally induced and naturally occurring lesions.16,33,45 Disruption of the endothelial barrier in the lamina propria of the jejunum will unequivocally lead to increased vascular permeability, edema, and hemorrhage. Morphologic evidence for this could, in fact, be demonstrated in early mucosal lesions in an infection model in piglets.62 In contrast to end-stage lesions in naturally affected animals,42 early lesions do not show signs of thrombosis,62 which could be expected to occur rapidly after endothelial damage. A reason for this could be that CPB also targets platelets and can alter their function, thus favoring the initial vascular leakage and hemorrhage.75 This would then lead to damage of the surrounding tissue and overlying epithelium and further increase the permeability of the intestinal epithelial barrier. Upon exposure to epithelial and other cells in vitro, C. perfringens type C can rapidly upregulate toxin secretion and proliferate even further.7,76 Overall, this would lead to a vicious cycle of bacterial proliferation, augmented toxin secretion, CPB-induced vascular damage, hemorrhage, and tissue necrosis. The end result is the rapidly progressing hemorrhagic necrosis observed in NE.
Given the massive destruction of the intestinal barrier, it is conceivable that toxemia contributes to the death of acutely affected animals. In particular, CPB toxemia was hypothesized to significantly contribute to the disease progression10,65; however, any toxin present in the intestinal lumen will readily be absorbed through the damaged mucosa and could thus act systemically. Importantly, besides nonspecific lesions such as pulmonary congestion and edema, specific C. perfringens–induced lesions in organs other than the small intestine are usually not observed in affected piglets.21
A proportion of animals survive the acute stage of the lesion and develop intestinal lesions characterized by marked neutrophil infiltration. A reason for this could be that initially lower infective doses of C. perfringens type C were taken up orally by the piglet. Alternatively, partial protection against overgrowth of C. perfringens may occur as a result of anti-clostridial antibodies in the colostrum of sows or an already partially developed intestinal flora, when animals get exposed to the pathogen several days after birth.
Although C. perfringens type C secretes many different toxins,56 CPB is essential for the pathogenesis of NE.60 It is synthesized as a 336 amino acid long, monomeric protein, which upon removal of a 27 amino acid signal sequence, has a molecular weight of 34.86 kDa.19 The protein is thermolabile and highly sensitive to proteolytic degradation, especially by trypsin.58,59 CPB belongs to the small beta–pore-forming toxins (β-PFT) of the α-hemolysin family.53 β-PFTs are secreted as water-soluble monomers, diffuse to the target cells where, depending on the toxin, they bind to protein receptors or lipid components of the plasma membrane, assemble into oligomeric complexes, and subsequently form functional transmembrane pores.20,46,47 These pores allow the efflux of K+ and the influx of Ca2+, Na+, and Cl− into the cells, which can lead not only to cellular swelling and lysis but also to intracellular signaling events, depending on the toxin dose and target cell affected by the toxin.
Many epithelial cell lines, including porcine and human intestinal epithelial cells, were shown to be resistant to CPB.39,46,57,64 Endothelial cells, platelets, and different leukocytic cell lines, however, were reported to be highly sensitive to CPB.3,49,62,75 This indicates that the action of CPB is cell type–specific and this might correlate with the expression of a cell type–specific receptor. Recent studies suggested the transmembrane protein P2X purinoreceptor 7 (P2RX7) as the putative receptor for CPB.48,50 This receptor is widely distributed on different cells including epithelial cells, which are however resistant to CPB. Thus, this receptor currently cannot sufficiently explain the cell type specificity of CPB. Further research is needed to unequivocally determine the molecular basis of the cell type specificity of CPB. Only a few studies have investigated potential roles of other toxins secreted by C. perfringens type C. In the rabbit ileal loop model, neither CPA nor PFO are required for the induction of typical intestinal lesions.60 However, the exact contribution of the various toxins secreted by C. perfringens type C strains to the pathogenesis of NE in pigs is not fully understood.65
Treatment, prophylaxis, and prevention strategies
C. perfringens can persist over long periods in the environment given its ability to form spores. Most likely, C. perfringens type C can also persist in the intestine of sows.69 We were able to detect C. perfringens type C several years after initial outbreaks of NE in pig herds that had since been disease free as a result of successful prevention programs.61
It has long been known that immunoprophylaxis can prevent NE effectively.4,15,27,71 Piglets are passively protected by the uptake of antibodies from the colostrum of vaccinated sows. C. perfringens vaccines for veterinary use are typically based on inactivated C. perfringens culture supernatants as immunogens. Such vaccines thus contain a mixture of antigens including all toxins that are secreted by the strain used for vaccine production. All C. perfringens type C vaccines have high amounts of inactivated CPB in common. To date, anti-CPB antibodies in sows and especially piglets are the best indicators of protection.55,67 It is, however, unclear exactly how vaccine-induced antibodies interfere with pathogenic mechanisms that occur in C. perfringens enteritis. C. perfringens type C has a very high proliferative capacity and secretes large amounts of toxins during its exponential growth phase.10,66 Potentially, in addition to simply neutralizing clostridial exotoxins, colostrum-derived antibodies might also prevent the massive proliferation of C. perfringens type C in the small intestine. For example, this could be achieved by inhibiting the effects of several secreted toxins and enzymes the pathogen uses (e.g., to obtain nutrition from its environment). How this would be achieved is, however, unknown. In any case, preventing disease unequivocally prevents the shedding of large amounts of C. perfringens type C into the environment. In combination with cleaning and sanitation procedures, it is thus possible to reduce the infection pressure on the farm.
The biology of C. perfringens type C and the possibility to prevent disease by vaccination has several considerations for prevention strategies to be used. First, the pathogen can be easily distributed by clinically and even bacteriologically inapparent carrier animals, in particular, gilts sold for restocking to other breeding and production farms. Negative bacterial cultures and even negative PCR results from fecal DNA extracts must be interpreted cautiously, given that they can be false negative because of very low numbers or intermittent shedding of C. perfringens type C in healthy adult carrier animals. It is thus important to keep breeding farms free of the pathogen. Under practical considerations, it would be ideal to monitor freedom of disease within the actual pig breeding farm in addition to ancillary breeding or production farms. This could be combined with repeated culturing and genotyping of C. perfringens from healthy and sick piglets in the herds under surveillance. Taken together, these measures require repeated testing of many animals and will be labor and cost intensive.
In nonvaccinated herds, introduction of C. perfringens type C into the herd will oftentimes only manifest as an acute outbreak of NE. In these cases, strict hygiene to reduce environmental contamination and thus infection pressure on newborn piglets is mandatory to reduce the immediate loss of piglets born shortly after the first cases of NE. Metaphylactic anitmicrobial treatment has also been used successfully to reduce losses during outbreaks37,71; however, in light of the emerging problem of antimicrobial resistance, this method should be avoided as much as possible.
Immediate vaccination will reduce mortality in litters of sows that can still develop sufficient amounts of colostral antibodies. According to the vaccination schemes recommended by manufacturers, this requires a minimum of 2 immunizations with an interval of several weeks before farrowing. We, however, repeatedly received feedback from practitioners, that, in particular, piglets from gilts are still affected despite correct vaccination in the herd (Posthaus, pers. obs., 2019). We have recently shown that despite following these recommended vaccination protocols, a proportion of gilts develop insufficient protective colostral anti-CPB antibody titers.54,55 This was in sharp contrast to multiparous sows that had been booster vaccinated repeatedly. This explains why piglets from vaccinated gilts can still be at risk of developing disease, especially in circumstances in which the infection pressure from the environment is relatively high. A simple adaptation of the vaccination scheme to 3 instead of 2 injections for the initial vaccination of gilts significantly improved colostral antibody titers and can therefore serve to increase the immunity of this particularly susceptible subpopulation of newborn piglets.54
The biology of C. perfringens type C, especially its tenacity in the environment and long-term persistence in the gastrointestinal tract of an adult animal, forces farmers and veterinarians to establish long-term vaccination programs to prevent the recurrence of NE. This implies that every gilt must receive basic immunization before or during its first pregnancy, and every multiparous sow must be administered a booster once during every pregnancy. Discontinuation or relaxation of such vaccination programs can lead to reoccurrence of NE on farms, even several years after the last outbreak.21,61,80 Farms that have established a vaccination program and remain free of NE, in turn, cannot of course be considered free of the pathogen.
Conclusion
NE in piglets, caused by C. perfringens type C, is a well-known disease with typical clinical signs and lesions. It can be diagnosed readily by a combination of well-established pathology and bacteriologic investigations. The disease can be prevented by strict and continuous vaccination programs using commercial vaccines combined with good sanitation practices. To implement prevention strategies for the swine industry, it is important to distinguish between the disease-free and pathogen-free status of pig breeding farms, because C. perfringens can persist in low numbers in the environment and the intestine of healthy adult pigs and thus be distributed by clinically and bacteriologically inapparent carrier animals. Recent research has greatly expanded our knowledge about the pathogenesis of the disease; however, many important aspects remain to be elucidated. In particular, additional knowledge about the mechanisms of immune protection might help to improve immunoprophylaxis for other, less well understood, clostridial enteric diseases.
Acknowledgments
We thank A. Schauer for English corrections. Photographs of clinical signs in piglets were provided by Prof. H. Nathues, Clinic for Swine, Vetsuisse Faculty, University of Bern.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: J. Burggisser is funded by the Swiss National Science Foundation (SNF). B. Tarek is funded by a University of Bern, International Student Grant.
ORCID iD: Horst Posthaus  https://orcid.org/0000-0002-4579-7493
https://orcid.org/0000-0002-4579-7493
References
- 1. Albini S, et al. Real-time multiplex PCR assays for reliable detection of Clostridium perfringens toxin genes in animal isolates. Vet Microbiol 2008;127:179–185. [DOI] [PubMed] [Google Scholar]
- 2. Amimoto K, et al. A novel toxin homologous to large clostridial cytotoxins found in culture supernatant of Clostridium perfringens type C. Microbiology 2007;153:1198–1206. [DOI] [PubMed] [Google Scholar]
- 3. Autheman D, et al. Clostridium perfringens beta-toxin induces necrostatin-inhibitable, calpain-dependent necrosis in primary porcine endothelial cells. PLoS One 2013;8:e64644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bergeland ME. Pathogenesis and immunity of Clostridium perfringens type C enteritis in swine. J Am Vet Med Assoc 1972;160:568–571. [PubMed] [Google Scholar]
- 5. Chan G, et al. The epidemiology of Clostridium perfringens type A on Ontario swine farms, with special reference to cpb2-positive isolates. BMC Vet Res 2012;8:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chean R, et al. Comparing the identification of Clostridium spp. by two matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry platforms to 16S rRNA PCR sequencing as a reference standard: a detailed analysis of age of culture and sample preparation. Anaerobe 2014;30:85–89. [DOI] [PubMed] [Google Scholar]
- 7. Chen J, et al. Host cell-induced signaling causes Clostridium perfringens to upregulate production of toxins important for intestinal infections. Gut Microbes 2014;5:96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farzan A, et al. An investigation into the association between cpb2-encoding Clostridium perfringens type A and diarrhea in neonatal piglets. Can J Vet Res 2013;77:45–53. [PMC free article] [PubMed] [Google Scholar]
- 9. Field HI, Goodwin RF. The experimental reproduction of enterotoxaemia in piglets. J Hyg (Lond) 1959;57:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fisher DJ, et al. Dissecting the contributions of Clostridium perfringens type C toxins to lethality in the mouse intravenous injection model. Infect Immun 2006;74:5200–5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garmory HS, et al. Occurrence of Clostridium perfringens beta2-toxin amongst animals, determined using genotyping and subtyping PCR assays. Epidemiol Infect 2000;124:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gobeli S, et al. Antimicrobial susceptibility of canine Clos-tridium perfringens strains from Switzerland. Schweiz Arch Tierheilkd 2012;154:247–250. [DOI] [PubMed] [Google Scholar]
- 13. Gurjar A, et al. Characterization of toxin plasmids in Clos-tridium perfringens type C isolates. Infect Immun 2010;78:4860–4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gurtner C, et al. Rapid cytopathic effects of Clostridium perfringens beta-toxin on porcine endothelial cells. Infect Immun 2010;78:2966–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hogh P. Experimental studies on serum treatment and vaccination against Cl. perfringens type C infection in piglets. Dev Biol Stand 1976;32:69–76. [PubMed] [Google Scholar]
- 16. Hogh P. Necrotizing infectious enteritis in piglets, caused by Clostridium perfringens type C. 3. Pathological changes. Acta Vet Scand 1969;10:57–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hogh P. Necrotizing infectious enteritis in piglets, caused by Clostridium perfringens type C. II. Incidence and clinical features. Acta Vet Scand 1967;8:301–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hogh P. The occurrence in Denmark of necrotizing enteritis in piglets caused by Clostridium perfringens type C. Bull Off Int Epizoot 1967;67:1351–1359. [PubMed] [Google Scholar]
- 19. Hunter SE, et al. Molecular genetic analysis of beta-toxin of Clostridium perfringens reveals sequence homology with alpha-toxin, gamma-toxin, and leukocidin of Staphylococcus aureus. Infect Immun 1993;61:3958–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iacovache I, et al. Pore formation: an ancient yet complex form of attack. Biochim Biophys Acta 2008;1778:1611–1623. [DOI] [PubMed] [Google Scholar]
- 21. Jäggi M, et al. Retrospective study on necrotizing enteritis in piglets in Switzerland. Schweiz Arch Tierheilkd 2009;151:369–375. [DOI] [PubMed] [Google Scholar]
- 22. Johannsen U, et al. Clostridium perfringens type C enterotoxemia (necrotizing enteritis) in suckling pigs. 1. Study of the experimental production of disease by Clostridium perfringens type C poisoning and infection (experimental set-up, clinical aspects, pathological findings). Arch Exp Veterinarmed 1986;40:811–825. [PubMed] [Google Scholar]
- 23. Johannsen U, et al. Clostridium perfringens type C enterotoxemia (necrotizing enteritis) of suckling pigs. 3. Light and electron microscopic studies of the pathology and pathogenesis of experimental Clostridium perfringens type C infection. Arch Exp Veterinarmed 1986;40:895–909. [PubMed] [Google Scholar]
- 24. Johnson S, et al. Enteritis necroticans among Khmer children at an evacuation site in Thailand. Lancet 1987;2:496–500. [DOI] [PubMed] [Google Scholar]
- 25. Johnson S, et al. Enterotoxemic infections. In: Rood JI, et al. , eds. The Clostridia: Molecular Biology and Pathogenesis. San Diego, CA: Academic Press, 1997:117–140. [Google Scholar]
- 26. Jost BH, et al. Atypical cpb2 genes, encoding beta2-toxin in Clostridium perfringens isolates of nonporcine origin. Infect Immun 2005;73:652–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kennedy KK, et al. Vaccination of pregnant sows with Clostridium perfringens type C toxoid. Vet Med Small Anim Clin 1977;72:1047–1049. [PubMed] [Google Scholar]
- 28. Kiu R, et al. Probing genomic aspects of the multi-host pathogen Clostridium perfringens reveals significant pangenome diversity, and a diverse array of virulence factors. Front Microbiol 2017;8:2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kiu R, Hall LJ. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg Microbes Infect 2018;7:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kohler B, et al. Studies of necrotizing enteritis of suckling piglets (Cl. perfringens typc C enterotoxemia) in industrialized sow breeding units. 3. Experimental reproduction of the disease. Arch Exp Veterinarmed 1979;33:313–333. [PubMed] [Google Scholar]
- 31. Kotsanas D, et al. Novel use of tryptose sulfite cycloserine egg yolk agar for isolation of Clostridium perfringens during an outbreak of necrotizing enterocolitis in a neonatal unit. J Clin Microbiol 2010;48:4263–4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kreft B, et al. Darmbrand (Enteritis necroticans). Eine historische und aktuelle Ubersicht [Necrotizing enterocolitis: a historical and current review]. Med Klin (Munich) 2000;95:435–441. German. [DOI] [PubMed] [Google Scholar]
- 33. Lawrence G. The pathogenesis of pig-bel in Papua New Guinea. P N G Med J 1979;22:39–49. [PubMed] [Google Scholar]
- 34. Lawrence G, et al. Prevention of necrotising enteritis in Papua New Guinea by active immunisation. Lancet 1979;1:227–230. [DOI] [PubMed] [Google Scholar]
- 35. Lawrence GW, et al. Impact of active immunisation against enteritis necroticans in Papua New Guinea. Lancet 1990;336:1165–1167. [DOI] [PubMed] [Google Scholar]
- 36. Li DY, et al. Enteritis necroticans with recurrent enterocutaneous fistulae caused by Clostridium perfringens in a child with cyclic neutropenia. J Pediatr Gastroenterol Nutr 2004;38:213–215. [DOI] [PubMed] [Google Scholar]
- 37. Luginbühl A. Die nekrotisierende Enteritis des Saugferkels durch Clostridium perfringens Typ C: I. Beobachtungen zu Klinik, Bekämpfung und Epidemiologie [The necrotizing enteritis by Clostridium perfringens type C in piglets: practical experience, management and epidemiology.] Schweiz Archiv Tierheilk 2002;144:263–273. German. [DOI] [PubMed] [Google Scholar]
- 38. Macias Rioseco M, et al. Freezing or adding trypsin inhibitor to equine intestinal contents extends the lifespan of Clostridium perfringens beta toxin for diagnostic purposes. Anaerobe 2012;18:357–360. [DOI] [PubMed] [Google Scholar]
- 39. Manich M, et al. Clostridium perfringens delta toxin is sequence related to beta toxin, NetB, and Staphylococcus pore-forming toxins, but shows functional differences. PLoS One 2008;3:e3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Markey B, et al. Clinical Veterinary Microbiology. 2nd ed. Edinburgh, UK: Elsevier, 2013. [Google Scholar]
- 41. Matsuda T, et al. Enteritis necroticans ‘pigbel’ in a Japanese diabetic adult. Pathol Int 2007;57:622–626. [DOI] [PubMed] [Google Scholar]
- 42. Miclard J, et al. Clostridium perfringens beta-toxin targets endothelial cells in necrotizing enteritis in piglets. Vet Microbiol 2009;137:320–325. [DOI] [PubMed] [Google Scholar]
- 43. Moller K, Ahrens P. Comparison of toxicity neutralization-, ELISA- and PCR tests for typing of Clostridium perfringens and detection of the enterotoxin gene by PCR. Anaerobe 1996;2:103–110. [Google Scholar]
- 44. Moon HW, Bergeland ME. Clostridium perfringens type C enterotoxemia of the newborn pig. Can Vet J 1965;6:159–161. [PMC free article] [PubMed] [Google Scholar]
- 45. Murrell TG. Pig-bel—case reports. P N G Med J 1966;9:68–71. [Google Scholar]
- 46. Nagahama M, et al. Biological activities and pore formation of Clostridium perfringens beta toxin in HL 60 cells. J Biol Chem 2003;278:36934–36941. [DOI] [PubMed] [Google Scholar]
- 47. Nagahama M, et al. Recent insights into Clostridium perfringens beta-toxin. Toxins (Basel) 2015;7:396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nagahama M, et al. Role of P2X7 receptor in Clostridium perfringens beta-toxin-mediated cellular injury. Biochim Biophys Acta 2015;1850:2159–2167. [DOI] [PubMed] [Google Scholar]
- 49. Nagahama M, et al. The p38 MAPK and JNK pathways protect host cells against Clostridium perfringens beta-toxin. Infect Immun 2013;81:3703–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Navarro MA, et al. Mechanisms of action and cell death associated with Clostridium perfringens toxins. Toxins (Basel) 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Petit L, et al. Clostridium perfringens: toxinotype and genotype. Trends Microbiol 1999;7:104–110. [DOI] [PubMed] [Google Scholar]
- 52. Poka H, Duke T. In search of pigbel: gone or just forgotten in the highlands of Papua New Guinea? P N G Med J 2003;46:135–142. [PubMed] [Google Scholar]
- 53. Popoff MR. Clostridial pore-forming toxins: powerful virulence factors. Anaerobe 2014;30:220–238. [DOI] [PubMed] [Google Scholar]
- 54. Richard OK, et al. Vaccination against Clostridium perfringens type C enteritis in pigs: a field study using an adapted vaccination scheme. Porcine Health Manag 2019;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Richard OK, et al. Application of an endothelial cell culture assay for the detection of neutralizing anti-Clostridium perfringens beta-toxin antibodies in a porcine vaccination trial. Toxins (Basel) 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rood JI, et al. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe 2018;53:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Roos S, et al. Binding studies on isolated porcine small intestinal mucosa and in vitro toxicity studies reveal lack of effect of C. perfringens beta-toxin on the porcine intestinal epithelium. Toxins (Basel) 2015;7:1235–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sakurai J, Duncan CL. Some properties of beta-toxin produced by Clostridium perfringens type C. Infect Immun 1978;21:678–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sakurai J, Fujii Y. Purification and characterization of Clos-tridium perfringens beta toxin. Toxicon 1987;25:1301–1310. [DOI] [PubMed] [Google Scholar]
- 60. Sayeed S, et al. Beta toxin is essential for the intestinal virulence of Clostridium perfringens type C disease isolate CN3685 in a rabbit ileal loop model. Mol Microbiol 2008;67:15–30. [DOI] [PubMed] [Google Scholar]
- 61. Schafer K, et al. Detection of Clostridium perfringens type C in pig herds following disease outbreak and subsequent vaccination. Vet Rec 2012;171:503. [DOI] [PubMed] [Google Scholar]
- 62. Schumacher VL, et al. Endothelial binding of beta toxin to small intestinal mucosal endothelial cells in early stages of experimentally induced Clostridium perfringens type C enteritis in pigs. Vet Pathol 2013;50:626–629. [DOI] [PubMed] [Google Scholar]
- 63. Shann F, et al. Enteritis necroticans in China. Lancet 1979;1:1083–1084. [DOI] [PubMed] [Google Scholar]
- 64. Shatursky O, et al. Clostridium perfringens beta-toxin forms potential-dependent, cation-selective channels in lipid bilayers. Infect Immun 2000;68:5546–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shrestha A, et al. Enterotoxic clostridia: Clostridium perfringens enteric diseases. Microbiol Spectr 2018;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Smedley JG, III, et al. The enteric toxins of Clostridium perfringens. Rev Physiol Biochem Pharmacol 2004;152:183–204. [DOI] [PubMed] [Google Scholar]
- 67. Solanki AK, et al. Clostridium perfringens beta toxin DNA prime-protein boost elicits enhanced protective immune response in mice. Appl Microbiol Biotechnol 2017;101:5699–5708. [DOI] [PubMed] [Google Scholar]
- 68. Songer JG. Clostridial diseases in domestic animals. In: Dürre J, ed. Handbook on Clostridia. Boca Raton, FL: Taylor & Francis, 2005:527–544. [Google Scholar]
- 69. Songer JG. Clostridial enteric diseases of domestic animals. Clin Microbiol Rev 1996;9:216–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Songer JG, et al. Clostridial enteric infections in pigs. J Vet Diagn Invest 2005;17:528–536. [DOI] [PubMed] [Google Scholar]
- 71. Springer S, et al. The control of necrotic enteritis in sucking piglets by means of a Clostridium perfringens toxoid vaccine. FEMS Immunol Med Microbiol 1999;24:333–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Straw BE, et al. Diseases of Swine. 8th ed. Oxford, UK: Blackwell Science, 1999. [Google Scholar]
- 73. Szabo S, Szent-Ivanyi T. Infectious necrotic enteritis in sucking pigs. II. Incidence and control of the disease in Hungary. Acta Vet Hung 1957;7:413. [Google Scholar]
- 74. Szent-Ivanyi T, Szabo S. Infectious necrotic enteritis of sucking pigs. I. Etiology and pathology. Acta Vet Hung 1956;6:217–229. [Google Scholar]
- 75. Thiel A, et al. Effect of Clostridium perfringens beta-toxin on platelets. Toxins 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Uzal FA, et al. Towards an understanding of the role of Clostridium perfringens toxins in human and animal disease. Future Microbiol 2014;9:361–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Uzal FA, McClane BA. Recent progress in understanding the pathogenesis of Clostridium perfringens type C infections. Vet Microbiol 2011;153:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Uzal FA, et al. Development and application of new mouse models to study the pathogenesis of Clostridium perfringens type C enterotoxemias. Infect Immun 2009;77:5291–5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vidal JE, et al. Effects of Clostridium perfringens beta-toxin on the rabbit small intestine and colon. Infect Immun 2008;76:4396–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wollschläger N, et al. Vorkommen von Clostridium perfringens Typ A und Typ C bei Saugferkeln in der schweizerischen Schweinepopulation [Occurrence of Clostridium perfringens type A and type C in piglets of the Swiss swine population]. Schweiz Arch Tierheilkd 2009;151:377–382. German. [DOI] [PubMed] [Google Scholar]