Abstract
Tetanus is a neurologic disease of humans and animals characterized by spastic paralysis. Tetanus is caused by tetanus toxin (TeNT) produced by Clostridium tetani, an environmental soilborne, gram-positive, sporulating bacterium. The disease most often results from wound contamination by soil containing C. tetani spores. Horses, sheep, and humans are highly sensitive to TeNT, whereas cattle, dogs, and cats are more resistant. The diagnosis of tetanus is mainly based on the characteristic clinical signs. Identification of C. tetani at the wound site is often difficult.
Keywords: animals, Clostridium tetani, diagnosis, tetanus, tetanus toxin
Introduction
Tetanus is a ubiquitous neurologic disease, characterized by spastic paralysis, that has a worldwide distribution in humans and animals. Tetanus is caused by the neurotoxin, tetanus toxin (TeNT). TeNT is produced by Clostridium tetani, an environmental soilborne, sporulating, anaerobic bacterium. Tetanus is not a transmissible disease. Infection and resultant clinical disease often result from wound contamination with C. tetani spores.7,28
Clostridium tetani
C. tetani is a gram-positive rod (0.3–0.6 μm wide and 3–12 μm long). Gram-positive staining is characteristic in young cultures, but C. tetani can lose the Gram coloration in cultures older than 24 h. Most strains of C. tetani have peritrichous flagella, which cause swarming growth on agar medium. However, some strains are non-flagellated and nonmotile. When cultured under anaerobic conditions, motile strains swarm over the entire surface of the agar leading to a transparent film. Discrete colonies (2–5 mm) can be obtained with media containing 3–4% agar. On blood agar, colonies are slightly raised, semi-translucent gray with irregular rough margins and surrounded by a narrow zone of hemolysis.52 C. tetani grows fairly well on the usual media containing peptones or tissue extracts.
C. tetani spores are round and terminal, giving a characteristic shape usually termed “drumstick.” Spore formation is variable according to the strain. At pH 7 or above and at temperatures near 37°C, sporulation starts within 24 h of culture and usually continues for 4–12 d. Sporulation does not occur above 41°C and is slow at pH <6. Sporulation depends on the composition of the culture medium.11 Spores generally survive moderate heating (75–80°C for 10 min) but usually are destroyed within 1 h at 100°C.
C. tetani spores can germinate under anaerobic or aerobic conditions in complex media such as liver broth. C. tetani spore germination occurs in a wide range of redox potential Eh from −100 mV to +580 mV, but subsequent vegetative growth cannot be obtained with Eh above +300 mV.26
The optimum temperature for growth is 37°C. Moderate growth of C. tetani is obtained at 30°C. Incubation at 25°C or 45°C induces no or poor growth. Moderate-to-abundant gas is produced in peptone–yeast extract–glucose medium according to the C. tetani strain.
Distribution of C. tetani in the environment
C. tetani is a ubiquitous organism that is commonly found in soil samples from all parts of the world. The frequency of its isolation varies according to the different investigations. Surveys in Japan, Canada, Brazil, and the United States have identified 30–42% positive samples.53 Several factors influence the different frequencies of C. tetani isolation from soil, including pH, temperature, moisture, and amount and type of organic materials. Thus, germination and multiplication of C. tetani preferentially favor neutral or alkaline soils, with temperatures >20°C and humidity reaching at least 15%.52
Geographical distribution of C. tetani shows a higher presence in warm humid regions, with the incidence of tetanus highest in West and Central Africa, Southeast Asia, India, the Pacific Islands, and the southern United States. In cooler regions of the world (e.g., Canada, Norway, England, Finland, Sweden, and others), the prevalence of C. tetani is lower.52 This bacterium can be detected in the intestine of animals but does not represent a significant part of the normal digestive flora. For example, the overall contamination rate of soil samples with C. tetani in the Durban, South Africa region, was estimated to be 28%. The recovery rate of C. tetani in 118 fecal samples from horses in this region was 5.9%.57 An investigation in southern India in 2009 showed that 2.6% of 115 soil samples contained C. tetani; this organism was not identified in 59 aquatic samples.49 In the Okinawa Prefecture in Japan, 18.6% of 290 soil samples in 1992 were positive for C. tetani.34 In Kanagawa Prefecture, another region of Japan, the prevalence of C. tetani was 22.9% from 35 soil samples. The contamination was higher in samples from mountains than from fields, private gardens, or public roads.27 A previous investigation showed a high prevalence of C. tetani in soil samples in Japan ranging from 20% in roadsides to 30% in school and hospital grounds, 53% in house yards, and 85% in fields, ponds, rivers, and wet shores.20 Excluding Japan, no country-wide systematic investigations of C. tetani in soil have been performed, to our knowledge, but because tetanus occurs worldwide, all soils are presumed to contain this pathogen.
Different surfaces and objects contaminated with soil particles, dust, or feces may contain C. tetani. Toxigenic strains of C. tetani have also been isolated in hospitals from catgut, cotton wool, dust and air samples, human skin, and wounds.11
C. tetani toxins
C. tetani produces a neurotoxin, TeNT, and a hemolysin called tetanolysin. Tetanolysin is a pore-forming toxin that belongs to the cholesterol-dependent cytolysin (CDC) family, the prototype of which is perfringolysin from C. perfringens. CDCs form large pores on the membrane surface of target cells, resulting in oligomerization of 40–70 toxin monomers and membrane damage. Tetanolysin may facilitate local tissue colonization and resistance to macrophages.32,58
TeNT is synthesized as a single-chain, 150-kDa protein that is poorly active. The toxin is proteolytically activated in the extra-bacterial medium either by clostridial proteases or by exogenous proteases from the host. The proteolytic cleavage occurs at the one-third N-terminal of the molecule. Active TeNT consists of a light chain (L; the N-terminal ~50 kDa) and a heavy chain (H; the C-terminal ~100 kDa). The L and H chains are linked by a disulfide bridge. The TeNT structure is similar to that of botulinum neurotoxin (BoNT) type E. The structures of BoNTs and TeNT have 3 distinct domains: 1) L chain containing α-helices and β-strands, and including the catalytic zinc-binding motif; 2) the N-terminal part of the H chain (HN) forming 2 unusually long and twisted α-helices; and 3) the C-terminal part of the H chain (HC) consisting of 2 distinct subdomains (HCN and HCC). The HC region is involved in the recognition of the membrane receptor and internalization of the toxin into the cell. Similar to BoNT/E, the L chain and HC domains are located on the same side of the translocation (HN) domain.39
Mode of action of the tetanus toxin and pathophysiology of tetanus
Tetanus occurs after the toxin is secreted by C. tetani that has contaminated a wound. Bacterially contaminated wounds can sometimes be small and difficult to find. Tetanus may even develop after wound healing. Contamination of wounds in parts of the body that are in contact with the soil are most at risk of producing tetanus. Examples include accidental wounds or punctures by prickly plants at the end of the limbs, the lower side of the trunk, and the abdomen. Umbilical infections are common predisposing factors for tetanus in newborns, notably in lambs and foals. Surgical wounds can be contaminated with C. tetani spores and are commonly observed with castration, tail docking, and ear surgery. Contaminated vaccinations or injection sites or contaminated wounds incurred during shearing are also common issues that have caused tetanus. Puerperal tetanus occurs after contamination of the vaginal mucosa and uterus during difficult delivery.10,14,19,38
Deep wounds with little exposure to air and the presence of necrotic tissue (ensuring anaerobic conditions) favor spore germination, C. tetani growth, and subsequent TeNT production. C. tetani is not an invasive bacterium and cannot enter healthy cells. However, the presence of damaged and lysed cells in a wound is a favorable substrate for C. tetani growth and toxin production. Tetanus is experimentally produced in laboratory animals by intramuscular injection of C. tetani spores in the presence of a necrotizing agent such as calcium chloride.
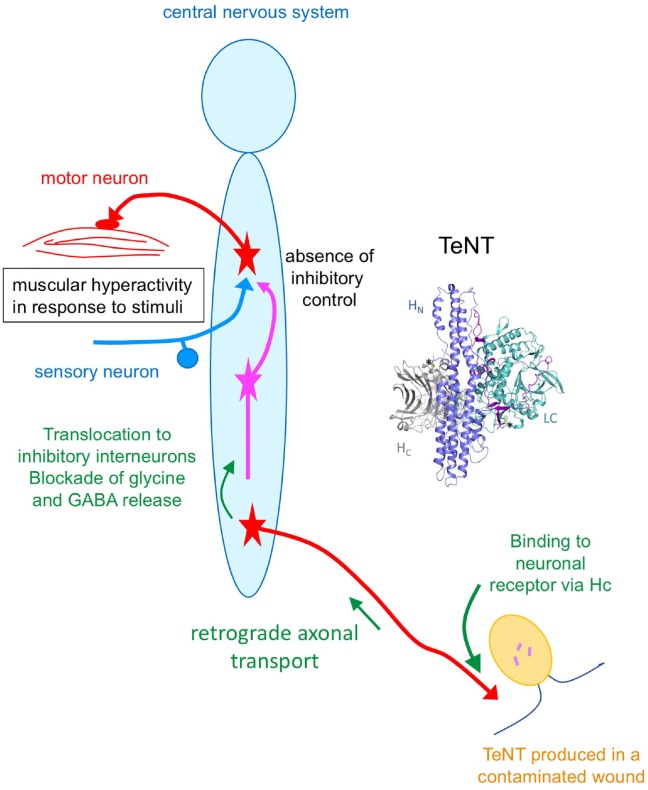
TeNT diffuses locally in the site of infection and interacts with demyelinated nerve endings. TeNT recognizes specific receptors on neuronal cell membranes consisting of a ganglioside part and a membrane protein. TeNT binds with a high affinity to gangliosides (preferentially GT1b and GD1b) by 2 carbohydrate-binding sites on the C-terminal domain of the toxin H chain.13,48 TeNT recognizes the extracellular matrix protein nidogen at the neuromuscular junction.6 However, the TeNT receptor on neuronal cell membranes is not yet known. TeNT bound to its receptors is internalized by receptor-mediated endocytosis. The toxin is then routed to endocytic vesicles that are not acidified. These vesicles undergo retrograde transport to the neuronal cell bodies in the central nervous system (CNS).8,9,15,16,36,37 In the CNS, TeNT is delivered to the extracellular space and enters the final target neurons that are inhibitory interneurons involved in the regulation of motor neuron activity. TeNT enters target inhibitory neurons via vesicles that are acidified, thus permitting the delivery of the L chain into the cytosol, where it inhibits the regulated release of glycine and gamma-aminobutyric acid (GABA). Acidification of the vesicle lumen triggers a conformational change of the neurotoxin and subsequent translocation of the L chain into the cytosol from the endocytic vesicle. The precise mechanism of L chain translocation into the cytosol is still a matter of debate. The disulfide bond between the L and H chains has a crucial role in the translocation process. The disulfide bond is reduced after translocation allowing the delivery of L chain in the cytosol.22–24,35 The TeNT L chain is a zinc-dependent metalloprotease that cleaves synaptobrevin of VAMP (vesicle-associated membrane protein), one of the 3 proteins of the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) complexes involved in the evoked release of neurotransmitter. Therefore, TeNT blocks the release of glycine and GABA, thus disrupting the negative controls exerted by the inhibitory interneurons onto the motor neuron, turning on excessive firing of the motor neurons, and ensuing muscle contraction (reviewed in previous reports40,44,45,47,50; Fig. 1). Thereby, tetanus is characterized by spastic paralysis and death by paralysis of diaphragm and respiratory muscles.
Figure 1.
Structure of tetanus toxin (TeNT) and schematic representation of its mode of action. HC = receptor binding domain; HN = translocation domain; LC = light chain or enzymatic domain containing the enzymatic site. TeNT is transported from contaminated wound to the central nervous system by retrograde axonal transport through motor neurons and possibly sensory neurons. In the spinal cord and brain, TeNT targets inhibitory interneurons blocking the release of glycine and GABA. This results in muscular hyperactivity subsequent to stimuli (e.g., touch, light, noise, temperature) as a result of the absence of inhibitory control.
Clinical signs
Although all animal species are susceptible to tetanus, there is considerable variability in susceptibility between species. The most susceptible species are the horse, guinea pig, monkey, sheep, mouse, goat, and human, whereas carnivores such as cats and dogs are less vulnerable, and birds are resistant (Table 1). Unlike sheep and goats, cattle are quite resistant. Interestingly, poikilothermic animals such as frogs are resistant to tetanus intoxication when maintained at low temperature (<18°C), despite large amounts of TeNT in the circulating body fluids, but susceptible when exposed to higher temperatures (≥27°C). The protective effects of cooling have been attributed to a retardation of the binding rate of TeNT on target neurons and inhibition of its action.52
Table 1.
Sensitivity of animal species to tetanus toxin. Relative minimum lethal doses compared to guinea pig lethal dose for various animal species, equivalent body weight basis (according to previous studies53,58).
| Species | Minimum guinea pig lethal dose |
|---|---|
| Horse | 0.5 |
| Guinea pig | 1 |
| Monkey | 2–4 |
| Sheep | 2 |
| Mouse | 2–6 |
| Goat | 12 |
| Cattle* | Unmeasurable low value |
| Rabbit | 4–900 |
| Dog | 300–600 |
| Cat | 960–7,200 |
| Goose | 6,000 |
| Pigeon | 6,000–24,000 |
| Hen | 180,000–360,000 |
| Human46 | 10 mouse lethal doses/kg |
| Human12 | 2.5 ng/kg |
Réthy LA. Unpublished.
Tetanus is characterized by hyperactivity of voluntary muscles leading to rigidity and tetanic spasms. Rigidity consists of tonic, involuntary, and prolonged muscle contractions, whereas spasms are shorter-lasting muscle contractions usually triggered by sensory stimulations (reflex spasms) such as touch, light, or noise. The signs of spastic paralysis are characteristic, and the diagnosis is often based on this clinical observation. The difficulty in diagnosing tetanus is often at the early onset of tetanus when misdiagnosis with other paralytic diseases such as myopathies can occur.1,7,54
Tetanus can appear as either a systemic (generalized) or localized disease process. In systemic tetanus, increasing spasticity of the muscles of mastication occurs initially, followed by progressive spastic paralysis of the muscles of the trunk, and upper and lower limbs. Rigidity of the masseter and temporal muscles (trismus) leads to the inability to open the mouth. Later, generalized tetanic spasms develop. Spastic paralysis of the extensors of the neck and back is accompanied by opisthotonus. Neonatal tetanus often occurs as a generalized form and is observed in newborns <1-mo-old.7
In localized tetanus, the muscles of the infected region become painful and then later spastic. Local tetanus is more likely to be observed in those animal species such as dogs that are relatively resistant to the toxin.1
The autonomic nervous system is also affected by episodes of tachycardia, hypertension, and sweating, alternating with bradycardia and hypotension. Death occurs because of respiratory failure as a result of the spastic paralysis of the diaphragm and laryngeal and other respiratory muscles. Clinical signs may last for weeks.7,28
Horses
Tetanus is common in non-immunized horses. Different forms of tetanus can be observed in horses. In the acute form, spastic paralysis rapidly spreads from the head (muscles of mastication, ears, third eyelid) to the respiratory muscles and then to the limbs. Generalized convulsions are accompanied by sweating. Death can occur in 1–2 d because of respiratory failure. In the subacute forms, signs develop in 1–3 wk. Some animals may recover. Hyperesthesia and prolapse of the third eyelid are common early signs. Eating and swallowing are difficult because of paralysis of the mastication muscles. The nostrils are often flared, and the ears are held stiffly in a vertical position. The muscles of the neck, back, and tail will be very tense, so that the tail is often raised vertically. The limbs become stiff, and the head is in an opisthotonic position. The body is in extension, with a global feature comparable to that of a wooden horse. The look of the horse is anxious and expresses pain. The face is tense because of trismus. Respiration is fast and respiratory movements painful. The pulse is normal; although, during the crisis of tetanic spasm, the pulse and respiratory rates are faster. Most cases end with the death of the horse. In a retrospective analysis of 176 tetanus cases in horses in Europe between 2000 and 2014, the mortality rate was 68.2%. Prognostic indicators of survival include voluntary eating of soft food and drinking.56 In localized tetanus, the muscle contractions are less intense and localized to a group of muscles such as those of a limb. This form can last several weeks, and the animals can recover.56
Sheep
Tetanus occurs mainly as sporadic cases, but outbreaks have been described in lambs and adult sheep.4 Newborn lambs can be affected by tetanus following soil contamination of the umbilicus. Dog bites, penetration of the oral mucosa by fibrous plant thorns, and surgical interventions such as castration and tail docking are also responsible for tetanus in sheep. A tetanus outbreak has been reported in sheep subsequent to ear tagging. In an outbreak in Brazil, 50 of 2,830 (1.7%) sheep treated subcutaneously with an anthelmintic contaminated with C. tetani developed clinical signs of tetanus.19 The predominant sign in sheep is stiffness of the limbs with a rigid gait. The tail is often stiff and straight. Feeding is difficult or impossible because of contraction of the masticatory muscles. The ears are stiff and in a horizontal position. The third eyelid is often prolapsed. As clinical signs progress, affected animals collapse into lateral recumbency. Other findings include tachycardia, dyspnea with dilated/flared nostrils, mild fever, teeth grinding, mild bloat, and anxiety. The mortality rate is 50%. Administration of penicillin G (20,000 IU/kg/5 d) can significantly reduce mortality.38
Cattle
In cattle, the signs are comparable to those observed in horses, but the muscular hyperactivity in response to stimuli is less pronounced. Affected animals initially develop overall stiffness with deviation and straightening of the tail to one side. Later, the head and neck go into extension. The ears become stiff and point backward; prolapse of the third eyelid is often observed. Bloat is common. Trismus is pronounced. In severe disease, the thoracic and abdominal muscles are spastic, and respiration is painful and loud. Severely affected animals die of respiratory failure in 5–9 d.19
Although less susceptible to tetanus than horses and sheep, outbreaks of tetanus in cattle have been reported (reviewed previously19). Common risk factors are accidental wounds, surgical castration, dehorning, and drug injections.14 An outbreak of tetanus in 6- to 8-mo-old calves occurred in Brazil in 2001 following subcutaneous administration of an anthelmintic (disophenol) contaminated with C. tetani; 297 of 4,504 (6.6%) cattle died 12–18 d after anthelmintic injection. In this case, calves displayed stiffness, rigidity of neck and limbs, bloat, straight pulled-back ears, and prolapse of the third eyelid. As the clinical disease progressed, the animals developed teeth grinding, hypersensitivity to environmental stimuli, tachycardia, hyperthermia, recumbency with extension of the limbs, convulsions, opisthotonus, and death.19
Swine
Tetanus in pigs is most often observed in piglets, which develop a generalized disease that is often fatal. Affected animals develop a stiff gait and have difficulty walking. Tetanic spasms often develop rapidly. Feeding is difficult or impossible because of trismus. The ears stand stiffly on the head. Respiration quickly becomes difficult with fast shallow breaths. Death often occurs rapidly as a result of respiratory failure.52
Dogs and cats
Tetanus is rare in dogs and cats. These 2 species (particularly cats) are resistant to TeNT. Often, clinical disease (particularly in cats) is localized and mild. The incubation period is usually 5–10 d. Spastic paralysis is observed in certain groups of muscles, causing trismus, stiff limbs, and stretching of the body because of large spinal extensor muscle spasm. Muscle spasms are relatively mild. The ears are usually erect and close together, and the skin of the forehead is often wrinkled because of muscle tension. Hyperthermia is common.1,54
In a retrospective series of 61 cases of tetanus in dogs, the source of the wound infection was identified in 58 of the 61 cases: 54% of wounds were in the thoracic limbs, 19% in the pelvic limbs (limb wounds were most often digital or nail wounds), 14% in the oral cavity mostly associated with teeth, and 13% in head, thorax, or abdomen including one post-surgical intervention (ovariohysterectomy). The mean duration between identification of wound and onset of signs was 15.2 d ranging from 0 to 30 d.51 The most common initial clinical signs are ocular and facial abnormalities. Signs progress rapidly to rigidity of localized muscles to generalized spastic paralysis including contracted facial musculature, trismus, retracted lips, wrinkled forehead, rictus grin, miosis, enophthalmos, prolapse of the third eyelid, erect ears, hyperextension of the limbs, stiff gait, then lateral decubitus and respiratory distress. The animals usually are hyperthermic and have low heart rate and low blood pressure.1,51,54 Young dogs are more likely to develop severe disease than older animals. Aberrant heart rate or blood pressure often suggests a poor prognosis.10 In the cohort of 61 cases, the mean survival time was 6.9 d, but ranged from 2 to 11 d.51 Mortality was 18–50%.5,10,51 Approximately half of surviving dogs develop sleep-associated disorders consisting of permanent muscular spasms including rapid eye movement and repeated episodes of vocalization.51 The differential diagnosis includes polymyositis, strychnine intoxication, spinal trauma, hypocalcemia, and meningoencephalitis.54
Diagnosis
The diagnosis of tetanus is essentially achieved by identification of the characteristic spastic paralytic signs. Strychnine intoxication shows a similar clinical picture and is the only condition that mimics tetanus. However, the presence of a suspected wound can support a diagnosis of tetanus. The onset of tetanus signs such as walking with a stiff gait can be confused with a myopathy.7,54 A diagnosis of focal tetanus in a dog can also be supported by electromyography.18
Identification of TeNT is often difficult and usually not detectable in biological samples. Very low levels of TeNT are needed for animals to develop signs of tetanus.3 The detection of TeNT in serum samples from humans or animals with tetanus is reported only rarely.17 Isolation of C. tetani from contaminated wounds is low.
Detection of C. tetani in contaminated wounds
Isolation of C. tetani at the point of entry consists of enrichment culture from tissues or exudate from the suspected wound. Enrichment cultures are performed in rich medium for anaerobic bacteria such as TGY (trypticase, 30 g/L; glucose 5 g/L; yeast extract 20 g/L; cysteine HCl 0.5 g/L; pH 7.5) or fortified cooked meat medium (FCMM; 12.5% cooked meat medium [Difco, Detroit, MI], 0.5% calcium carbonate, 1% ammonium sulfate, 1% yeast extract, 0.8% glucose, 0.5% soluble starch, and 0.1% cysteine–HCl, pH 7.6).55 Optionally, inoculated enrichment media are heated at 60°C for 30 min for spore selection. After 1–5 d incubation at 37°C in anaerobic conditions, C. tetani can be detected either by identification of TeNT in the enrichment culture supernatant or by detection of tent-containing clostridia. It is noteworthy that detection of C. tetani in suspected wounds is often problematic. For example, clostridia were cultured in only 4 of 2 wound samples from dogs with clinical signs of tetanus.51
Identification of TeNT in culture supernatant
TeNT can be detected in the culture supernatant by the mouse bioassay. Culture supernatant (0.2 mL) is injected intramuscularly in mice, and the animals are observed for typical spastic paralysis for 1–4 d. TeNT can be detected by ELISA with specific polyclonal or monoclonal antibodies.33 TeNT can be detected by its proteolytic activity towards its specific substrate VAMP using specific antibodies to the cleaved form of VAMP.31
Identification of tent-containing clostridia
A sensitive method of C. tetani detection consists of PCR based on tent identification. Several PCR methods specific to C. tetani including standard and real-time PCR have been described (Table 2). Bacterial DNA is commonly extracted from enrichment broth cultures or colonies on agar plates with commercial DNA extraction kits.2,25,41–43 In conventional PCR, the readout is performed by electrophoresis of DNA on an agar gel stained with ethidium bromide. More accurately, the sequencing of PCR products gives unambiguous results. Real-time PCR offers the advantage of being rapid and highly sensitive, with immediate readout of results.
Table 2.
Primers and PCR methods of Clostridium tetani detection in biological or environmental samples. Enrichment culture and subsequent DNA extraction are commonly used prior to PCR amplification.
| Primer | Sequence | PCR | Reference |
|---|---|---|---|
| P476 | ATGCCAATAACCATAAATAATTTTAGATATAG | Conventional PCR | 43 |
| P477 | TTCATCTTGAAATGGTTCTTCTG | ||
| F3 | GATAAAGATGCATCTTTAGGATT | LAMP PCR | 30,41 |
| B3 | TCTTCTTCATTATCAACCCAAC | ||
| FIP | AGTTGCTTGCAATTAATATATCCCTAGTAGGTACCCATAATGGTCA | ||
| BIP | AACATGTGATTGGTACTTTGTACCTTATGTGTCTATGGTGTGTTG | ||
| TET1 | CCTAGTTTCAAAACTTATTGGCTTATGTAA | Conventional PCR | 29 |
| TET2 | CATAATTCTCCTCCTAAATCTGTTAATGAT | ||
| Forward | CTGGATTGTTGGGTTGATAATG | Conventional PCR | 25,42 |
| Reverse | ATTTGTCCATCCTTCATCTGTAGG | ||
| TQ TET1 | CCTAGTTTCAAAACTTATTGGCTTATGTAA | Real-time PCR | 2 |
| TQ TET2 | CATAATTCTCCTCCTAAATCTGTTAATGATG |
LAMP = loop-mediated isothermal amplification.
A procedure including DNA extraction from soil samples with a commercial DNA extraction kit (SoilMaster DNA extraction kit; Epicentre Biotechnologies, Madison, WI) and real-time PCR without enrichment culture has been developed. The limit of detection was estimated to be 10 C. tetani in the sample.29
Tetanus serology
Antibodies against TeNT are often not detectable in nonvaccinated humans or animals that have recovered from naturally acquired tetanus. The low concentration of TeNT in natural disease does not usually induce an immune response. Surprisingly, tetanus cases have been observed in humans having antibody concentrations >0.01 IU/L, the minimal level considered as protective (reviewed previously21).
Prophylaxis, treatment, and control
Medical prevention consists of immunization with formaldehyde-inactivated TeNT. Two injections at a 3- to 4-wk interval are required to induce effective immunity. No specific treatment is available for tetanus. TeNT antibodies prevent free TeNT from entering neurons via the serum, but toxin that has been taken into neuronal cells cannot be accessed by antitoxin antibodies.7,12,56
Wound debridement and cleaning, antibiotic use, and injection of TeNT immunoglobulin are recommended when a risk of tetanus is suspected. Penicillin G and metronidazole are the antimicrobials of choice to treat infection but are ineffective against existing disease. Nonspecific treatment includes sedation and muscle relaxation, and supportive nursing care (feeding, maintaining hydration, and preventing soiling).
Standard treatment of tetanus in dogs consists of tetanus antitoxin administered intravenously, penicillin G (25 mg/kg/7 d), sedation with diazepam–acepromazine or chlorpromazine–phenobarbital, hospitalization in a dark and quiet room, and intravenous fluid therapy to maintain hydration.1,54
In neonates, good hygienic practices, notably disinfection of the umbilicus, are important in the prevention of tetanus. Surgical interventions (tail docking and castration) must be performed with sterilized materials and in appropriate conditions of hygiene.
Footnotes
Declaration of conflicting interests: The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Michel R. Popoff  https://orcid.org/0000-0001-9305-8989
https://orcid.org/0000-0001-9305-8989
References
- 1. Acke E, et al. Tetanus in the dog: review and a case-report of concurrent tetanus with hiatal hernia. Ir Vet J 2004;57:593–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akbulut D, et al. Wound botulism in injectors of drugs: upsurge in cases in England during 2004. Euro Surveill 2005;10:172–174. [PubMed] [Google Scholar]
- 3. Anderson JF. The influence of concentration (Gibson’s method) on the presence of tetanus toxin in blood serum. J Exp Med 1909;11:656–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aslani MR, et al. Outbreak of tetanus in lambs. Vet Rec 1998;142:518–519. [DOI] [PubMed] [Google Scholar]
- 5. Bandt C, et al. Retrospective study of tetanus in 20 dogs: 1988–2004. J Am Anim Hosp Assoc 2007;43:143–148. [DOI] [PubMed] [Google Scholar]
- 6. Bercsenyi K, et al. Tetanus toxin entry. Nidogens are therapeutic targets for the prevention of tetanus. Science 2014;346:1118–1123. [DOI] [PubMed] [Google Scholar]
- 7. Bleck TP. Tetanus: pathophysiology, management, and prophylaxis. Dis Mon 1991;37:545–603. [DOI] [PubMed] [Google Scholar]
- 8. Bohnert S, et al. Uptake and transport of clostridium neurotoxins. In: Alouf JE, Popoff MR, eds. The Comprehensive Sourcebook of Bacterial Protein Toxins. 3rd ed. Amsterdam: Elsevier Academic, 2006:390–408. [Google Scholar]
- 9. Bohnert S, Schiavo G. Tetanus toxin is transported in a novel neuronal compartment characterized by a specialized pH regulation. J Biol Chem 2005;280:42336–42344. [DOI] [PubMed] [Google Scholar]
- 10. Burkitt JM, et al. Risk factors associated with outcome in dogs with tetanus: 38 cases (1987–2005). J Am Vet Med Assoc 2007;230:76–83. [DOI] [PubMed] [Google Scholar]
- 11. Bytchenko B. Microbiology of tetanus. In: Veronesi R, ed. Tetanus: Important New Concepts. Amsterdam: Excerpta Medica, 1981:28–39. [Google Scholar]
- 12. Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, et al. , eds. 13th ed. Washington, DC: Public Health Foundation, 2015. [Google Scholar]
- 13. Chen C, et al. Gangliosides as high affinity receptors for tetanus neurotoxin. J Biol Chem 2009;284:26569–26577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coetzee JF, et al. A survey of castration methods and associated livestock management practices performed by bovine veterinarians in the United States. BMC Vet Res 2010;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deinhardt K, et al. Tetanus toxin is internalized by a sequential clathrin-dependent mechanism initiated within lipid microdomains and independent of epsin1. J Cell Biol 2006;174:459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deinhardt K, et al. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron 2006;52:293–305. [DOI] [PubMed] [Google Scholar]
- 17. Delbrassinne L, Vanderpas J. The mouse toxicity bioassay as a laboratory confirmation test for tetanus. Acta Clin Belg 2015;70:77–78. [DOI] [PubMed] [Google Scholar]
- 18. De Risio L, et al. Focal canine tetanus: diagnostic value of electromyography. J Small Anim Pract 2006;47:278–280. [DOI] [PubMed] [Google Scholar]
- 19. Driemeier D, et al. Outbreaks of tetanus in beef cattle and sheep in Brazil associated with disophenol injection. J Vet Med A Physiol Pathol Clin Med 2007;54:333–335. [DOI] [PubMed] [Google Scholar]
- 20. Ebisawa I, et al. Density and distribution of Clostridium tetani in the soil. Jpn J Exp Med 1986;56:69–74. [PubMed] [Google Scholar]
- 21. Ergonul O, et al. An unexpected tetanus case. Lancet Infect Dis 2016;16:746–752. [DOI] [PubMed] [Google Scholar]
- 22. Fischer A, Montal M. Crucial role of the disulfide bridge between botulinum neurotoxin light and heavy chains in protease translocation across membranes. J Biol Chem 2007;282:29604–29611. [DOI] [PubMed] [Google Scholar]
- 23. Fischer A, et al. Botulinum neurotoxin devoid of receptor binding domain translocates active protease. PLoS Pathog 2008;4:e1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Galloux M, et al. Membrane interaction of botulinum neurotoxin A translocation (T) domain. The belt region is a regulatory loop for membrane interaction. J Biol Chem 2008;283:27668–27676. [DOI] [PubMed] [Google Scholar]
- 25. Ganesh M, et al. Detection of Clostridium tetani in human clinical samples using tetX specific primers targeting the neurotoxin. J Infect Public Health 2016;9:105–109. [DOI] [PubMed] [Google Scholar]
- 26. Hachisuka Y, et al. The effect of oxidation-reduction potential on spore germination, outgrowth, and vegetative growth of Clostridium tetani, Clostridium butyricum, and Bacillus subtilis. Microbiol Immunol 1982;26:803–811. [DOI] [PubMed] [Google Scholar]
- 27. Haneda J, et al. [Distribution of Clostridium tetani in topsoil from Sagamihara, central Japan]. Kansenshogaku Zasshi 2006;80:690–693. Japanese. [DOI] [PubMed] [Google Scholar]
- 28. Hassel B. Tetanus: pathophysiology, treatment, and the possibility of using botulinum toxin against tetanus-induced rigidity and spasms. Toxins (Basel) 2013;5:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang SW, et al. The utilization of a commercial soil nucleic acid extraction kit and PCR for the detection of Clostridium tetanus and Clostridium chauvoei on farms after flooding in Taiwan. J Vet Med Sci 2013;75:489–495. [DOI] [PubMed] [Google Scholar]
- 30. Jiang D, et al. Rapid, sensitive, and specific detection of Clostridium tetani by loop-mediated isothermal amplification assay. J Microbiol Biotechnol 2013;23:1–6. [DOI] [PubMed] [Google Scholar]
- 31. Kegel B, et al. An in vitro assay for detection of tetanus neurotoxin activity: using antibodies for recognizing the proteolytically generated cleavage product. Toxicol In Vitro 2007;21:1641–1649. [DOI] [PubMed] [Google Scholar]
- 32. Keyel PA, et al. Macrophage responses to bacterial toxins: a balance between activation and suppression. Immunol Res 2011;50:118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kiessig ST, et al. A solid-phase enzyme immunoassay for the detection of tetanus toxin using human and murine monoclonal antibodies. J Basic Microbiol 1991;31:135–140. [DOI] [PubMed] [Google Scholar]
- 34. Kobayashi T, et al. [Distribution of Clostridium botulinum and Clostridium tetani in Okinawa Prefecture]. Kansenshogaku Zasshi 1992;66:1639–1644. Japanese. [DOI] [PubMed] [Google Scholar]
- 35. Koriazova LK, Montal M. Translocation of botulinum neurotoxin light chain protease through the heavy chain channel. Nat Struct Biol 2003;10:13–18. [DOI] [PubMed] [Google Scholar]
- 36. Lalli G, et al. The journey of tetanus and botulinum neurotoxins in neurons. Trends Microbiol 2003;11:431–437. [DOI] [PubMed] [Google Scholar]
- 37. Lalli G, Schiavo G. Analysis of retrograde transport in motor neurons reveals common endocytic carriers for tetanus toxin and neutrophin receptor p75NTR. J Cell Biol 2002;156:233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lotfollahzadeh S, et al. Tetanus outbreak in a sheep flock due to ear tagging. Vet Med Sci 2018:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Masuyer G, et al. The structure of the tetanus toxin reveals pH-mediated domain dynamics. EMBO Rep 2017;18:1306–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Meunier FA, et al. Molecular mechanism of action of botulinal neurotoxins and the synaptic remodeling they induce in vivo at the skeletal neuromuscular junction. In: Massaro J, ed. Handbook of Neurotoxicology. Vol. 1 Totowa, NJ: Humana Press, 2002:305–347. [Google Scholar]
- 41. Nagoba B, et al. Molecular methods for identification of Clostridium tetani by targeting neurotoxin. Methods Mol Biol 2017;1600:37–47. [DOI] [PubMed] [Google Scholar]
- 42. Plourde-Owobi L, et al. Molecular characterization of Clostridium tetani strains by pulsed-field gel electrophoresis and colony PCR. Appl Environ Microbiol 2005;71:5604–5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Popoff MR. Detection of toxigenic clostridia. In: Sachse K, Frey J, eds. PCR Detection of Microbial Pathogens. 1st ed. (Methods in Molecular Biology, Vol. 216) Totowa, NJ: Humana Press, 2002:137–152. [DOI] [PubMed] [Google Scholar]
- 44. Poulain B, et al. How do the botulinum neurotoxins block neurotransmitter release: from botulism to the molecular mechanism of action? Botulinum J 2008;1:14–87. [Google Scholar]
- 45. Poulain B, et al. Attack of the nervous system by clostridial toxins: physical findings, cellular and molecular actions. In: Alouf JE, Popoff MR, eds. The Sourcebook of Bacterial Protein Toxins. 3rd ed. Amsterdam: Elsevier Academic, 2006:348–389. [Google Scholar]
- 46. Rethy L, Rethy LA. Human lethal dose of tetanus toxin. Lancet 1997;350:1518. [DOI] [PubMed] [Google Scholar]
- 47. Rossetto O, et al. Tetanus neurotoxin. Toxicon 2013;66:59–63. [DOI] [PubMed] [Google Scholar]
- 48. Rummel A, et al. Two carbohydrate binding sites in the Hcc-domain of tetanus neurotoxin are required for toxicity. J Mol Biol 2003;326:835–847. [DOI] [PubMed] [Google Scholar]
- 49. Sathish S, Swaminathan K. Genetic diversity among toxigenic clostridia isolated from soil, water, meat and associated polluted sites in South India. Indian J Med Microbiol 2009;27:311–320. [DOI] [PubMed] [Google Scholar]
- 50. Schiavo G, et al. Neurotoxins affecting neuroexocytosis. Physiol Rev 2000;80:717–766. [DOI] [PubMed] [Google Scholar]
- 51. Shea A, et al. Association between clinically probable REM sleep behavior disorder and tetanus in dogs. J Vet Intern Med 2018;32:2029–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Smith LDS. Clostridium tetani In: The Pathogenic Anaerobic Bacteria. 2nd ed. (American Lecture Series no. 980.) Springfield IL: Charles C. Thomas, 1975:177–201. [Google Scholar]
- 53. Smith LDS, Williams BL. The Pathogenic Anaerobic Bacteria. 3rd ed. (American Lecture Series no. 1064.) Springfield, IL: Charles C. Thomas, 1984. [Google Scholar]
- 54. Sprott KR. Generalized tetanus in a Labrador retriever. Can Vet J 2008;49:1221–1223. [PMC free article] [PubMed] [Google Scholar]
- 55. Takeda M, et al. Characterization of the neurotoxin produced by isolates associated with avian botulism. Avian Dis 2005;49:376–381. [DOI] [PubMed] [Google Scholar]
- 56. van Galen G, et al. Retrospective evaluation of 155 adult equids and 21 foals with tetanus from Western, Northern, and Central Europe (2000–2014). Part 2: Prognostic assessment. J Vet Emerg Crit Care (San Antonio) 2017;27:697–706. [DOI] [PubMed] [Google Scholar]
- 57. Wilkins CA, et al. Occurrence of Clostridium tetani in soil and horses. S Afr Med J 1988;73:718–720. [PubMed] [Google Scholar]
- 58. Wright GP. The neurotoxins of Clostridium botulinum and Clostridium tetani. Pharmacol Rev 1955;7:413–465. [PubMed] [Google Scholar]