Abstract
Clostridia can cause hepatic damage in domestic livestock, and wild and laboratory animals. Clostridium novyi type B causes infectious necrotic hepatitis (INH) in sheep and less frequently in other species. Spores of C. novyi type B can be present in soil; after ingestion, they reach the liver via portal circulation where they persist in phagocytic cells. Following liver damage, frequently caused by migrating parasites, local anaerobic conditions allow germination of the clostridial spores and production of toxins. C. novyi type B alpha toxin causes necrotizing hepatitis and extensive edema, congestion, and hemorrhage in multiple organs. Clostridium haemolyticum causes bacillary hemoglobinuria (BH) in cattle, sheep, and rarely, horses. Beta toxin is the main virulence factor of C. haemolyticum, causing hepatic necrosis and hemolysis. Clostridium piliforme, the causal agent of Tyzzer disease (TD), is the only gram-negative and obligate intracellular pathogenic clostridia. TD occurs in multiple species, but it is more frequent in foals, lagomorphs, and laboratory animals. The mode of transmission is fecal–oral, with ingestion of spores from a fecal-contaminated environment. In affected animals, C. piliforme proliferates in the intestinal mucosa, resulting in necrosis, and then disseminates to the liver and other organs. Virulence factors for this microorganism have not been identified, to date. Given the peracute or acute nature of clostridial hepatitis in animals, treatment is rarely effective. However, INH and BH can be prevented, and should be controlled by vaccination and control of liver flukes. To date, no vaccine is available to prevent TD.
Keywords: bacillary hemoglobinuria, black disease, Clostridium, hepatitis, infectious necrotic hepatitis, Tyzzer disease
Introduction
Clostridial hepatitis is a general term used to describe diseases produced by several clostridial species in which the liver is the most severely affected organ. In animals, there are 3 well-defined diseases that fulfill this criterion: infectious necrotic hepatitis (INH), caused by Clostridium novyi type B; bacillary hemoglobinuria (BH), caused by Clostridium haemolyticum; and Tyzzer disease (TD), caused by Clostridium piliforme. Although liver damage can occur in other clostridial diseases, such as Clostridium chauvoei–induced blackleg and gas gangrene produced by Clostridium septicum (and other clostridia), such damage occurs secondary to the primary lesions in other organs. We therefore review herein only INH, BH, and TD.
Infectious necrotic hepatitis
INH, sometimes referred to as “black disease,” is an acute toxemic disease of sheep produced by C. novyi type B.16,44 Sporadic cases have been also reported in cattle,28 goats,25 pigs,1,10,15 and horses.48
Etiology
C. novyi is a gram-positive, rod-shaped, motile, spore-forming, and strictly anaerobic bacterium.44 Low-oxygen levels stimulate spore germination, followed by proliferation and expression of several virulence factors.2 C. novyi is classified into 4 genotypes (A–D) based upon the production of 2 lethal toxins, alpha (TcnA) and beta (Table 1).61 In addition, other non-lethal toxins may be produced by all of the C. novyi types.43 C. novyi type A only encodes TcnA; this toxinotype causes gas gangrene in humans and animals, either as a primary agent or in combination with other pathogenic Clostridium spp.38,43 C. novyi type B encodes TcnA and beta toxin and is the etiologic agent of INH.44 C. novyi type C does not encode either of the 2 typing toxins, and is thus nontoxigenic and not associated with disease.42,61 C. novyi type D, which only produces beta toxin, is commonly known as C. haemolyticum and causes BH.43,45
Table 1.
Typing of Clostridium novyi.
| Type | Main toxin(s) |
Disease | |
|---|---|---|---|
| Alpha | Beta | ||
| A | +++ | − | Gas gangrene (humans and animals) |
| B | ++ | + | Infectious necrotic hepatitis |
| C | − | − | No known disease association |
| D (or C. haemolyticum) | − | +++ | Bacillary hemoglobinuria |
− = no toxin produced; + to +++ = increasing amount of toxin produced.
Production of TcnA is related to phage infection. A phage-cured C. novyi type B, which fails to produce TcnA, is able to produce it again after reinfection with the same phage,20 and infecting C. haemolyticum with the phage of C. novyi type A results in the production of TcnA.64 TcnA is a lethal, edematizing, and cytotoxic toxin related to the large clostridial glucosylating toxins, a group of high-molecular-weight proteins (250–300 kDa) that share many structural and functional features.56 This group also includes toxins A and B of Clostridium difficile, Clostridium perfringens large (TpeL), and lethal (TcsL) and hemorrhagic (TcsH) toxins of Clostridium sordellii.2,56
Pathogenesis
The spores of C. novyi type B may be found in soil and in feces of normal livestock, and they are highly resistant to adverse environmental conditions.4,47 After the spores are ingested by grazing animals, it is thought that they are absorbed from the intestine and reach the liver, spleen, and bone marrow, where they are phagocytized by local macrophages, remaining latent in the cytoplasm of these cells for up to several months.6,76 Experimentally, C. novyi type B spores were detected in the liver of sheep within 24 h of being administered orally.6
Although the literature on the pathogenesis of INH is scant, it is generally believed that migration of immature forms of flukes (mainly Fasciola hepatica) through the liver parenchyma induces tissue necrosis and local anaerobiosis, thus promoting the activation of C. novyi type B spores.5 This is followed by the production and release of TcnA and beta toxin.16,44 Like all large clostridial glucosylating toxins, TcnA enters cells by receptor-mediated endocytosis.56 This process starts with the interaction between the C-terminal domain of TcnA and an unidentified receptor located on the plasma membrane of target cells.56 Once within endocytic vesicles, acidification occurs, promoting the autolytic cleavage of the N-terminal catalytic domain and its translocation into the cytosol.2,56 In the cytosol, TcnA catalyzes the glucosylation of Rho- and/or Ras-GTPases from UDP-N-acetylglucosamine, leading to disruption of the actin cystoskeleton.17,56,65 TcnA also causes minor disruption of the vimentin and tubulin systems.51
Cytoskeletal disturbance results in cell death, loss of intercellular junctions, and increase in cell-barrier permeability.56 These effects occur in hepatocytes and endothelial cells, the damage in the latter being responsible for extensive leakage of blood-derived fluid into the connective tissues.16 The necrotizing and hemolytic beta toxin also contributes to hepatic necrosis.16,44 Because only small amounts of beta toxin are produced by C. novyi type B,41,72 the role of this toxin in INH is likely to be minor.
Epidemiology and clinical signs
INH affects animals of all breeds, sexes, and ages, with the highest incidence in individuals > 4 mo of age and usually in good nutritional condition.4,44 C. novyi type B may be part of the normal microbiome of soil and the gut of clinically healthy individuals, with the latter possibly spreading this microorganism in their feces.4 Farms with a history of INH commonly have a greater presence of C. novyi type B in the soil and in the livers of sheep, compared with farms with no such history.4 Furthermore, C. novyi type B spores originating from dead animals also contaminate pastures.4
In some areas, INH varies seasonally given its association with fascioliasis and the fluctuation in the concentration of encysted F. hepatica metacercariae on the pastures.53 Dry weather may force sheep to graze fluke-infested areas, particularly in poorly drained soils, increasing the risk for INH. However, cases of black disease have also been reported associated with periods of increased rainfall.53,67 The onset of frost or heavy snowfall significantly limits the incidence of the disease because the encysted metacercariae are killed by freezing.67
Despite its strong association with fascioliasis, INH can also occur in areas free of F. hepatica flukes. In these cases, the development of a hypoxic environment favorable to spore germination and bacterial growth in the liver has been suggested to be associated with abscesses, hepatocellular fatty change, plant toxins, telangiectasia, liver biopsy, and other parasites.19,21,44 Fasciola gigantica, Fascioloides magna, Cysticercus tenuicollis, Dicrocoelium dendriticum, and Thysanosoma actinoides have been mentioned, although not proved, to be possible predisposing factors for INH.16,25,31,44,60 As such, farms contaminated with any of these parasites may be considered at risk for developing INH. Farms that do not vaccinate cattle for INH are particularly at risk. Information about epidemiologic aspects of INH in other species, including cattle and horses, is scant, but it is assumed to be similar to that of sheep.
Clinical signs are rarely seen in sheep or cattle affected by INH given the peracute nature of the disease. In sheep, it is common to find several animals dead without premonitory signs.44,67 When clinical signs are observed, they are usually present for only a few hours, and are nonspecific. Affected animals lag behind the flock or are sternally recumbent for a short time before death. Other clinical manifestations may include drowsiness, anorexia, hyperthermia, tachycardia, and tachypnea.44,67 Cattle with INH have clinical signs similar to those described in sheep.
INH is rare in horses. The few reports available on the disease in this species suggest that the clinical course is of 12–72 h duration,48 and it is characterized by head tilt, ataxia, reluctance to move, abdominal pain, and, eventually, recumbency.73,82 Interestingly, and contrary to that which occurs in ruminants with INH, horses may sometimes exhibit jaundice, which can be readily observed in the mucous membranes and sclera.73,82 The reason for the occurrence of icterus in horses, but not ruminants, is unknown, but it may be attributed, at least in part, to an apparently increased susceptibility of horses to the action of the hemolytic beta toxin produced by C. novyi type B.44,48
In ruminants and horses with INH, changes in clinical pathology profiles include neutrophilia with a left shift, increase of several liver enzymes, and nonspecific changes associated with toxemia.5,14,44,48,68
Gross changes
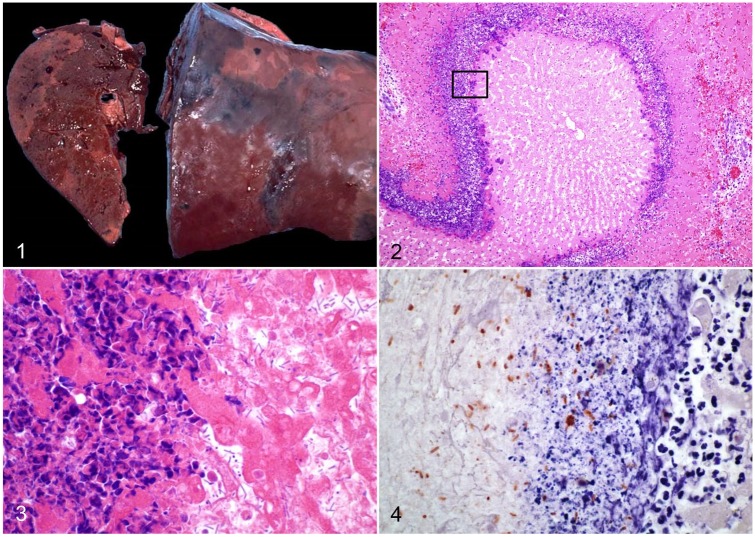
Rapid autolysis is commonly observed in carcasses of animals dying from INH, and unless an autopsy is performed soon after death, postmortem decomposition may mask the gross changes.67 In sheep, there is usually marked congestion of subcutaneous blood vessels, and hemorrhages, resulting in dark discoloration of the subcutis, from which the name “black disease” is derived.16,44,67,68 Subcutaneous edema may also be seen over the sternum, and in ventral abdominal muscles and the inguinal region.67 The abdominal cavity and pericardial sac contain variable amounts of straw-colored to serosanguineous fluid. Hemorrhages may be present on the pleura, endocardium covering papillary muscles, and mesentery.48 The most striking and pathognomonic lesion is found in the liver, which in sheep and cattle consists of multiple, or rarely single, gray-yellow, randomly distributed areas of necrosis, surrounded by a rim of intense hyperemia (Fig. 1). A thin coagulum of fibrin on the hepatic capsule may overlay necrotic areas. Occasionally, the classical necrotic foci may not be seen from the surface of the liver, and slicing of the organ is needed to detect them.16,67 Evidence of immature fluke infestation with necrotic and hemorrhagic tracts in the capsule and hepatic parenchyma is usually present.16 However, fluke-associated changes are not always seen at postmortem examination.
Figures 1–4.
Infectious necrotic hepatitis. Figure 1. Multiple, well-demarcated, randomly distributed, pale foci of necrosis in an ovine liver. Reproduced with permission from Navarro and Uzal, 2016.44 Figure 2. Large focus of coagulative necrosis in an equine liver, with an inflammatory cell rim. H&E. Figure 3. Higher amplification of the boxed area in Fig. 2. Large numbers of rod-shaped bacteria at the periphery of the necrotic area (at right), with a contiguous leukocytic infiltrate of viable and degenerate neutrophils (at left). H&E. Figure 4. Numerous immunopositive Clostridium novyi at the periphery of a necrotic hepatic focus. Indirect immunoperoxidase staining, as described in Nyaoke et al. 2018.48
In horses, a single focus of necrosis affecting the left hepatic lobe has been reported.48 In addition, icteric discoloration of the abdominal serosa as well as adipose and subcutaneous tissue may sometimes be observed in this species.48,82
Microscopic lesions
The microscopic appearance of the liver lesion is considered to be pathognomonic for both INH and BH (see below). It consists of focal or multifocal coagulative necrosis of hepatocytes, surrounded by a leukocytic rim composed mainly of degenerate and viable neutrophils, and a few lymphocytes, plasma cells, and macrophages (Fig. 2). Within the inner margin of this inflammatory rim, there are usually large numbers of large gram-positive rods, many of them with subterminal spores (Fig. 3).16,44 Other histologic changes may include necrotic tracts associated with immature liver fluke migration.16 Portal fibrosis, bile duct hyperplasia, and portal lymphocytic infiltrates, common features of chronic fascioliasis, may occur concurrently.44,54,55
Cases of equine INH may also exhibit segmental-to-diffuse fibrinoid necrosis of arteries and veins associated or not with fibrin thrombi and hemorrhage in various organs, including liver, lungs, and adrenal glands.48 In the brain, randomly distributed, perivascular extravasations of eosinophilic, high-protein edema may occur, presumably as a result of the endothelial tropism of TcnA.48
In horses, there may occasionally be cytoplasmic vacuolation of the renal tubular epithelium, with luminal protein casts, which are Okajima stain positive, consistent with hemoglobin.48
Diagnosis
Epidemiologic data, clinical signs, and gross and microscopic changes are generally sufficient to establish a presumptive diagnosis of INH. Other causes of sudden death, including, but not limited to, anthrax, blackleg, and C. perfringens type D enterotoxemia, should be excluded.72 At autopsy, the presence of acute focal or multifocal hepatocellular necrosis supports a presumptive diagnosis of INH in all susceptible species.5,44,48 However, confirmation of a diagnosis of INH must be based on demonstration of C. novyi type B. This can be achieved by isolation of C. novyi from the liver, followed by PCR typing of the isolate, or by direct PCR on fresh, frozen, or formalin-fixed, paraffin-embedded (FFPE) liver. A multiplex PCR has been developed for rapid identification of several clostridia by targeting the flagellin (fliC) gene of C. novyi type A, C. novyi type B, C. haemolyticum, C. septicum, and C. chauvoei.48,62 In addition, the detection of the tcnA gene by PCR is a simple method to directly assess the presence of the main virulence factor involved in INH.48 Immunohistochemistry (IHC) is useful to detect C. novyi in association with the characteristic hepatic lesions (Fig. 4),48,82 the lesion specificity of C. novyi being critical because type B spores may be present in the liver of some healthy animals.67 However, to date, available IHC techniques do not discriminate between the different types of C. novyi.
Prevention
In ruminants, given that most cases of INH are associated with liver fluke infestation, control of this parasitosis is vital to help prevent cases of INH. Preventive measures include drainage of soils, limiting access of animals to streams, canals, and ponds, and strategic treatment of animals with flukicides.67,68 In addition, all carcasses of animals dying from INH should be burned, buried, or removed from the premises in order to avoid the dispersal of C. novyi spores.67,68 Commercial multivalent vaccines composed of toxoids and/or bacterins are available against C. novyi type B and other pathogenic clostridia. The humoral response against toxins and, possibly some somatic antigens, results in solid immunity, although there may be differences in immunogenicity by vaccine and animal species.72 Vaccines can be given at any age, but it is usually recommended that the first vaccination be administered at 6 mo, followed by a booster 3–4 wk later.29,72 However, the duration of protection is short, and 2 annual boosters should be used in high-exposure areas.68,72 C. novyi is highly sensitive to penicillin and tetracyclines, but antibiotic treatment is rarely effective given the acute course of the disease and abundant toxin production in most cases of INH.14,68
Bacillary hemoglobinuria
BH is produced by C. haemolyticum, also known as C. novyi type D. It is an infectious, but not contagious, sporadic, and peracute disease that affects cattle primarily. However, sporadic cases have been reported in sheep, pigs, horses, and an elk.11,45,50,57,59
Etiology
C. haemolyticum is a large, soil-borne, anaerobic, gram-positive, sporulating rod. This bacterium is extremely fastidious, and it is known as one of the strictest pathogenic anaerobes.26,69 These bacteria develop oval, subterminal spores, and possess numerous peritrichous flagella, which confer motility.69 C. haemolyticum shares many biologic characteristics with C. novyi type B. In fact, and based on partial sequence analysis of the 16S rDNA gene, it has been proposed that both microorganisms may belong to a single species.61 The main difference between these 2 microorganisms is that TcnA is produced by C. novyi type B, but not by C. haemolyticum strains.42,61
The main virulence factor responsible for the pathogenicity of C. haemolyticum is beta toxin. The C. haemolyticum beta toxin gene consists of an open reading frame, which encodes a 399 amino acid protein containing a 27 amino acid signal sequence.27 The active beta toxin is a phospholipase C of ~43 kDa. The N-terminal portion contains a zinc-binding domain common to other bacterial phospholipases, and the C-terminal domain shows significant homology with the C-terminal domains of the phospholipases produced by C. perfringens (alpha toxin) and C. bifermentans.27 Beta toxin cleaves phosphatidylcholine by hydrolysis into phosphocholine and a diglyceride.37 Serologically, the beta toxin of C. haemolyticum is indistinguishable from the beta toxin produced by C. novyi type B.27,49
Pathogenesis
The mechanism by which C. haemolyticum causes BH is similar to that described for C. novyi type B in INH. An initial hepatic focus of necrosis, and the associated anaerobiosis, are essential for the spores of C. haemolyticum to germinate and proliferate in the liver, followed by the production and release of toxins.16,43 Anaerobic conditions have been observed in tissues as a trigger for these events, for example, when spores of a nonpathogenic Clostridium species introduced systemically in mice germinated only in the anaerobic environment of tumors and not in healthy tissue.36 In addition, the fact that C. haemolyticum spores have been isolated from the liver and the kidneys of healthy cattle supports this pathogenic mechanism for BH.70 Nevertheless, cases in which the initial triggering insult is not found in the liver of affected animals have been described.16,45 It may be hypothesized that those cases developed as a consequence of spore overload in the liver rather than activation of latent spores, but this has not been proved. As is the case in INH, the main predisposing factor for BH is the migration of F. hepatica through the liver parenchyma.16 Beta toxin is endotheliotoxic and hepatotoxic, which results in thrombosis and hepatocellular necrosis. Microvascular damage also results in hepatic edema and increased fluid in body cavities.27 Although thrombosis occurs in necrotic areas of the liver, this is probably a consequence rather than the cause of necrosis.16,45 This toxin also lyses erythrocytes, leading to hemoglobinemia and hemoglobinuria.27
Epidemiology and clinical signs
BH rarely affects young calves; most cases occur in animals > 1-y-old in good nutritional condition.72 The disease has low morbidity, but lethality is commonly very high.45 Cases of BH tend to be more frequent in animals recently introduced into infected pastures because native animals are believed to have some degree of natural immunity.52 Environmental conditions leading to BH usually involve poorly drained soils and semi-permanent anaerobic, alkaline (pH ≥ 8.0) wetlands, either naturally or artificially created, into which C. haemolyticum has been introduced.79 Given the strong association with fascioliasis, the epidemiologic features and dynamics of liver fluke infestation previously described for INH also apply to BH. In addition to F. hepatica and other less frequent causes of initial hepatic injury leading to INH as mentioned above, BH has also been associated with Fusobacterium necrophorum–induced hepatic abscesses and liver necrosis secondary to rumenitis produced by this bacterium.34 Although some of these predisposing factors (e.g., F. necrophorum) have not been described in cases of INH, it is possible that they are also associated with that disease.
Animals affected with BH have a rapid onset of clinical signs, which may last from 12 h to 4 d. There is sudden loss of appetite, and cessation of lactation, rumination, and defecation. Affected animals also have anorexia, depression, tachypnea, tachycardia, and fever, as well as icteric mucous membranes, blood-stained feces, and hemoglobinuria.21,66,75,81 Death occurs as a result of severe toxemia and hemolysis-induced hypoxia.43
Impaired hepatic function in BH leads to significant increases in plasma aspartate aminotransferase (AST) and gamma-glutamyl transferase (GGT) activities, and total bilirubin, and a reduction in the albumin-to-globulin ratio.32 Hemolysis results in severe anemia.32,43,66 Leukocyte counts vary from initial leukocytosis with a left shift to, in advanced stages, exhaustion leukopenia.32,52
Gross lesions
Carcasses of animals dying of BH are commonly in good nutritional condition but may exhibit signs of dehydration. Edema, petechiae, and ecchymoses are scattered throughout the subcutis, and adjacent fascia and muscle, being most evident on the ventral body wall.21,43,52 Mucous membranes are pale or exhibit mild-to-severe jaundice, the latter which may be prominent in the subcutaneous tissue. The perineal area and tail are stained by dark red, liquid feces.45,52 Variable degrees of jaundice, ecchymoses, and petechiae may be seen on the parietal and visceral peritoneum and the mesentery.52 The lungs are edematous, and the trachea and bronchi contain blood-tinged, frothy transudate. The pericardial sac and pleural cavity contain large amounts of hemoglobin-stained fluid.21,52
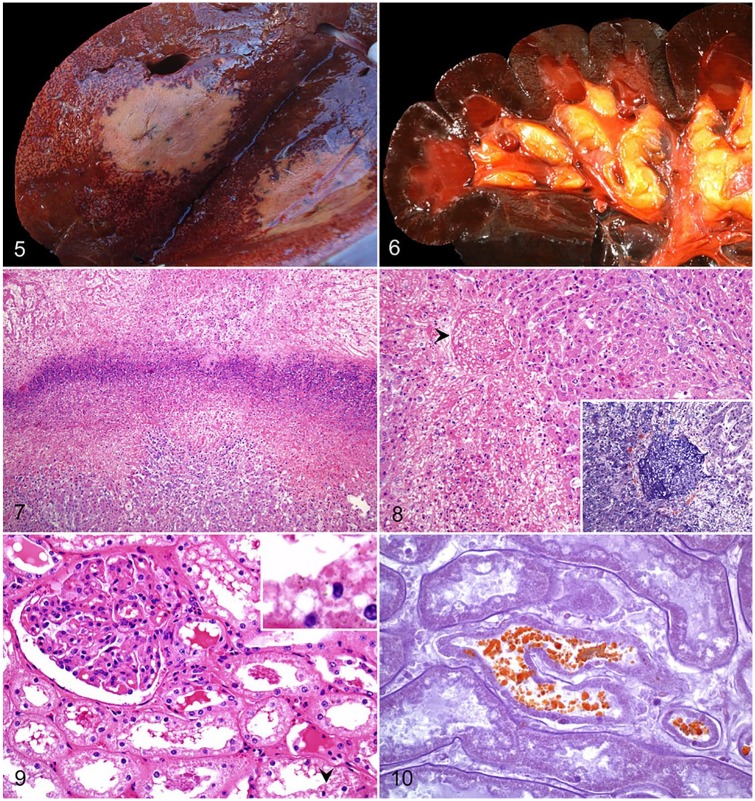
The characteristic, and pathognomonic, lesion is found in the liver. It generally consists of a single, wedge-shaped focus of necrosis with its base on the parietal surface of any of the hepatic lobes (Fig. 5).45 Multiple foci of hepatic necrosis are seen less frequently. These lesions affect up to ~30% of the hepatic parenchyma, are pale, soft-to-firm, and demarcated by a thin red-to-purple halo. Thrombosis is a common feature in small and medium-sized veins. Affected blood vessels are located within, or adjacent to, the necrotic lesions, and both portal and hepatic branches are involved.45 The rest of the liver is usually orange-tan, and may have a diffusely distributed, acinar pattern, suggestive of hypoxic degeneration (Fig. 5).21,43 Variable amounts of bright red blood clots may be found in the small and large intestine. There is splenomegaly, and the parenchyma has a gelatinous, purple appearance. The kidneys are dark brown to black (Fig. 6), friable, and may contain numerous petechiae. The urinary bladder is filled with dark red urine.43,45,52
Figures 5–10.
Bovine bacillary hemoglobinuria. Figure 5. Transverse section of liver with a single, large, well-demarcated area of necrosis, and an adjacent acinar pattern. Courtesy of Matias Liboreiro. Figure 6. The renal cortex and medulla are diffusely dark, and the papillary adipose tissue is icteric. Reproduced with permission from Navarro et al. 2016.43 Figure 7. A dense band of leukocytic infiltrate of degenerate neutrophils separates a zone of coagulative necrosis (top) from less severely affected liver (bottom). H&E. Figure 8. Thrombotic occlusion of a central hepatic vein (arrowhead). H&E. Inset: the thrombus is mainly composed of fibrin. Phosphotungstic acid hematoxylin. Figure 9. Renal cortical proximal convoluted tubular epithelium shows variable degrees of cytoplasmic vacuolation and nuclear hyperchromasia. Intracytoplasmic, eosinophilic protein droplets (arrowhead) and luminal casts are present. H&E. Inset: higher magnification of an intracytoplasmic droplet. Figure 10. Intratubular and intracytoplasmic granules shown in Fig. 9 stain orange-brown, consistent with hemoglobin. Okajima stain.
Microscopic lesions
The liver lesions in animals affected with BH are almost identical to those observed in INH (see above; Fig. 7). Thrombosis of hepatic vessels is a consistent feature in affected areas of the liver (Fig. 8), and it was traditionally thought to be the origin of the necrotic damage. However, hepatic infarction is unusual, probably the result of the dual hepatic blood supply, and thrombosis may be a consequence of the expanding necrotic damage, rather than its cause.16,45 Beyond the necrotic foci, areas of moderate-to-severe, probably hypoxia-induced, hepatic centrilobular necrosis are frequently seen. Caspase-3 activation seen in these areas suggests involvement of apoptosis as a mechanism of cell death.45 As previously described for INH, microscopic changes associated with liver fluke infestation may also be observed.43,54,55
Acute necrosis of proximal and distal convoluted tubules is a common feature in BH and is probably associated with the hypoxia of intravascular hemolysis and subsequent anemia seen in these cases. There is also Okajima-positive hemoglobin pigment in tubular epithelial cells and luminal hemoglobin casts (Figs. 9, 10).45 In the spleen, sinusoidal congestion and multifocal necrosis, infiltrated with degenerate and viable neutrophils, can be seen throughout the white and red pulp. In addition, signs of vascular damage, in the form of congestion and hemorrhages, are frequently seen in other tissues.45,52,78
Diagnosis
Given the many similarities between BH and INH, diagnostic criteria for these 2 diseases are concordant in many species. Epidemiologic information such as a history of non-vaccination, alkaline and poorly drained soils, history of fascioliasis, and clinical signs including sudden death, hemoglobinuria, and icterus, help facilitate a presumptive diagnosis, supported by gross and microscopic changes. The presence of a large focus of hepatic necrosis, typically single, together with Gram-stained impression smears containing numerous, large bacilli, is usually suggestive of BH or INH. However, the latter finding should be interpreted with caution given that these microorganisms are always present, and proliferate rapidly, as postmortem invaders.71
As mentioned previously, although IHC for C. novyi may support a presumptive diagnosis, currently available IHC techniques do not discriminate between C. novyi type B and C. haemolyticum. Given the fastidious nature of C. haemolyticum, failure to isolate the microorganism does not exclude a diagnosis of BH.43 Demonstration of C. haemolyticum by PCR on frozen samples of liver is the preferred method to achieve an etiologic diagnosis, particularly when attended by macroscopic and microscopic lesions compatible with BH, given that a small number of clinically healthy animals may carry C. haemolyticum in the liver.43,70 This testing should be complemented by excluding other pathogenic clostridial species.62 If frozen samples are not available, PCR on FFPE samples may be attempted, targeting short fragments of the C. haemolyticum beta toxin gene.45
Prevention
Preventive measures against BH are similar to those described above for INH, including the control and treatment of fascioliasis and other parasites, and the removal of dead animals from the premises. Vaccination with commercial bacterins or toxoids, in mono- and polyvalent preparations, is effective in reducing the incidence of the disease. In areas where the disease is more prevalent, the first 2 doses of vaccine, given 4–6 wk prior to the expected peak of occurrence, are recommended, in addition to the vaccination plan described for INH.43,72
Tyzzer disease
TD is an enterohepatic syndrome cause by C. piliforme. The disease occurs in numerous domestic, laboratory, and exotic species.3,13,33,35,39,40,46,58,63,74,83,84 The majority of cases have been documented in horses, particularly foals, and laboratory rabbits, mice, rats, and guinea pigs.12,22,24,30
Etiology
Originally termed Bacillus piliformis, the etiologic agent of TD was reassigned to the genus Clostridium and designated C. piliforme in 1993, based on 16S rRNA gene sequence analysis.18 Further analysis of this sequence revealed that C. piliforme has a close phylogenetic relationship to C. colinum.22 C. piliforme is an anaerobic, subterminally sporulating, filamentous, pleomorphic, and obligate intracellular bacterium.77 Vegetative forms are 0.3–0.6 μm diameter, with cell walls ~25 nm thick, and they have numerous peritrichous flagella of 10–15 nm diameter. Spores are 0.6–1.4 μm diameter and possess 90–250 nm thick coats.24,46 Although commonly reported to be gram-negative in tissue sections, C. piliforme may be gram-variable or gram-positive.18 Although virulence factors for C. piliforme have not been characterized, different isolates seem to cause different degrees of cytotoxicity, suggesting that various C. piliforme strains could induce different clinical forms of the disease.7,8,46 The bacterium can only be propagated in vitro using embryonated chicken eggs and selected cell lines,9 and given the difficulties in culturing C. piliforme, knowledge of the pathobiology of TD is limited.
Pathogenesis
The posited mode of infection in animals is by ingestion of C. piliforme spores from contaminated material. Experimental reproduction of the disease in foals by oral administration of feces from experimentally infected horses supports this hypothesis.74 Vegetative forms colonize and multiply in the intestinal mucosa of the ileum, cecum, and colon, inducing death of enterocytes. The bacterium then reaches the portal circulation and disseminates to the liver, myocardium, and other organs.8,24,40
Epidemiology and clinical signs
Young animals are the most susceptible to TD, particularly when immunocompromised. Foals are at higher risk when born to mares < 6 y old versus foals born to older mares, suggesting that different colostral quality may be an additional risk factor.23 In laboratory animals, the disease is mainly predisposed by immunosuppression, stress, high environmental temperatures, overcrowding, poor sanitation, changes in diet, and sulfonamide and corticosteroid administration.7,33 Fecal contamination of bedding with C. piliforme in laboratory rodents, and coprophagous oral transmission in foals, are considered important means of infection for TD.74 Intrauterine transmission has been experimentally produced in rats and mice treated with prednisolone, but vertical transmission does not seem to occur spontaneously.7,9 Outbreaks of the disease are usually of low morbidity and high lethality in affected animals.8,24 C. piliforme spores can survive in the environment for at least 5 y,7 and wild rodents and rabbits may act as carriers.33,46
The clinical presentation in cases of TD may vary across species but reflects hepatic and intestinal disease. Clinical signs include icterus, anorexia, weakness, depression, abdominal pain, watery diarrhea, dehydration, hypothermia, tachycardia, and recumbency.7,12 Neurologic manifestations, including ataxia, tremors, and head tilt, have been reported in a weaver bird and in free-ranging passerine birds.39,40 Sudden death, with no prior clinical signs, may occur in severe cases in rabbits and mice.7,8
Hematologic and biochemical findings reveal non-regenerative anemia, leukocytosis or leukopenia, metabolic acidosis, hypokalemia, hypoglycemia, hypoproteinemia, hyperbilirubinemia, and elevated alanine aminotransferase, AST, GGT, and sorbitol dehydrogenase activities in most domestic and laboratory species.12,24
Gross lesions
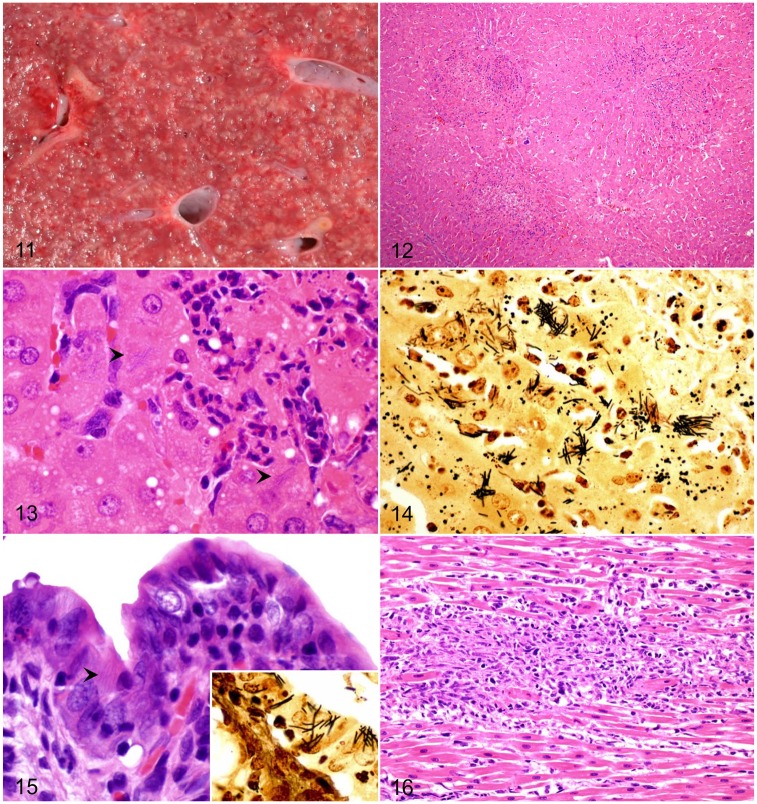
In all affected species, the disease is mainly characterized by hepatomegaly with numerous pale foci, 1–5 mm diameter, dispersed throughout the liver parenchyma (Fig. 11). Widely distributed icterus is present in most cases.24 There is marked mural edematous thickening of the ileum, cecum, and colon, with ecchymoses and petechiae found on the serosal surfaces. The content of the cecum and colon is watery to gelatinous.3,33 Single or multiple, pale, round-to-linear foci may be observed in the myocardium. These lesions, however, are most frequently observed only microscopically.24 The presence of lesions in liver, intestine, and heart constitute a diagnostic triad of lesions for TD, although their distribution is variable among different species.
Figures 11–16.
Tyzzer disease. Figure 11. Liver of a foal. Multiple pale necrotic foci distributed throughout the hepatic parenchyma of a foal. Figure 12. Randomly distributed, and variably sized, foci of coagulative necrosis are seen microscopically in the liver. H&E. Figure 13. Intracytoplasmic filamentous bacteria (arrowheads) in hepatocytes at the periphery of a necrotic focus. H&E. Figure 14. Intracytoplasmic bacteria shown in Fig. 13 are better visualized with a silver impression. Steiner stain. Figure 15. The lamina propria of the small intestine of a rabbit is infiltrated and enlarged by numerous mononuclear cells. Filamentous bacteria are observed in the cytoplasm of enterocytes (arrowhead). H&E. Inset: Intracytoplasmic filamentous bacteria in enterocytes are identified with a Steiner stain. Figure 16. Myocardial necrosis, with infiltration by neutrophils, macrophages, and lymphocytes in a rabbit. H&E.
Microscopic lesions
The characteristic microscopic hepatic lesion in TD is randomly distributed, well-demarcated, focal coagulative necrosis, infiltrated with large numbers of degenerate and viable neutrophils (Fig. 12). Formation of granulomas, composed of aggregates of macrophages, with occasional fibrosis and mineralization, may occur in chronic cases.24 Numerous filamentous bacilli are visible as bundles or crisscross patterns in the cytoplasm of viable hepatocytes at the margin of the necrotic areas (Figs. 13, 14).3,24,33
In young foals, hepatic lesions are most common, with minor involvement of the intestinal tract and myocardium; the latter 2 tissues are affected more frequently in rodents and lagomorphs.74 The affected segments of intestine are transmurally necrotic, with submucosal edema and hemorrhage. The mucosa and lamina propria are infiltrated by variable numbers of lymphocytes, plasma cells, and neutrophils, and the crypts are dilated with necrotic debris.3,35 Intracytoplasmic filamentous bacteria can be seen in enterocytes (Fig. 15).24,35
When present, lesions in the heart are characterized by multifocal myocardial necrosis and infiltration by variable numbers of neutrophils, macrophages, and lymphocytes (Fig. 16), with intra-sarcoplasmic filamentous bacteria.13,24,63 On occasion, these lesions may progress to granulomatous myocarditis.24
Microscopic changes in the central nervous system (CNS) have been reported in a few species including gerbils,80 a weaver bird,39 passerine birds,40 and a marmoset.84 CNS changes included regionally extensive areas of gliosis, neuropil rarefaction, and microabscess formation, mainly involving the cerebral cortex. At the periphery of these lesions, the characteristic filamentous bacteria may be found in neuronal perikarya.
Diagnosis
Diagnosis of TD relies on gross findings and histologic demonstration of hepatic, intestinal, and/or myocardial lesions. This is further supported by the presence of bacteria in the cytoplasm of infected cells. The bacteria are faintly stained in routine H&E sections; detection of C. piliforme can be achieved more precisely with special stains, particularly silver impregnation techniques, such as Steiner (Fig. 14),16 Giemsa, and periodic acid–Schiff stains.24 Although C. piliforme may be grown in tissue culture, and in the yolk sac of developing chicken embryos, the bacterium is very difficult to isolate from clinical or postmortem samples.74 Transmission electron microscopy may be attempted to visualize vegetative cells and spores in the cytoplasm of affected cells.33,46 DNA fragments of the C. piliforme 16S rRNA can be amplified by PCR to achieve an etiologic diagnosis,24 but, given its close phylogenetic proximity with C. colinum, and potentially other nonpathogenic clostridia,22 currently available PCR techniques for this pathogen must be interpreted in combination with the macroscopic and microscopic lesions compatible with TD.
Prevention
Given that fecal–oral transmission of C. piliforme spores is the main mode of infection in animals, preventive measures involve environmental hygiene.24 Control of potential carriers, such as wild mice and rats, is also an important preventive measure,33 together with adequate transfer of passive immunity soon after birth in foals.74 The avoidance of sudden changes in the diet of nursing mares has been proposed to control the disease in foals.74 Even though lethality is commonly high in cases of TD in foals, the disease has been treated successfully12 by promptly instituted intensive care, involving administration of ampicillin and gentamicin, in combination with intravenous nutritional support. In addition, attention to immunosuppressive events mentioned above should be taken into consideration. No commercially available vaccine is available for TD, to date.
Concluding remarks
INH, BH, and TD are the most prevalent clostridial hepatic diseases of animals and, although a presumptive diagnosis can be achieved by a combination of clinical, gross, and microscopic findings, confirmation relies on demonstration of the causative Clostridium by culture and/or PCR. However, the latter should be interpreted in light of clinicopathologic findings because C. novyi type B and C. haemolyticum can be present in tissues of clinically normal animals. The development of molecular techniques to detect each of the clostridia responsible for INH, BH, and TD has facilitated the diagnosis of these diseases and sometimes permits a definitive etiologic diagnosis.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Mauricio A. Navarro  https://orcid.org/0000-0002-7744-8052
https://orcid.org/0000-0002-7744-8052
References
- 1. Akiyama N, et al. Fatal Clostridium novyi type B infection in a sow. J Ag Res Quart 2017;51:85–89. [Google Scholar]
- 2. Aronoff DM, Kazanjian PH. Historical and contemporary features of infections due to Clostridium novyi. Anaerobe 2018;50:80–84. [DOI] [PubMed] [Google Scholar]
- 3. Artuković B, et al. Clinical and pathological findings of an outbreak of Tyzzer’s disease in a rabbit colony in Croatia. Veterinarski Arhiv 2010;80:761–770. [Google Scholar]
- 4. Bagadi HO, Sewell MM. An epidemiological survey of infectious necrotic hepatitis (black disease) of sheep in southern Scotland. Res Vet Sci 1973;15:49–53. [PubMed] [Google Scholar]
- 5. Bagadi HO, Sewell MM. Experimental studies on infectious necrotic hepatitis (black disease) of sheep. Res Vet Sci 1973;15:53–61. [PubMed] [Google Scholar]
- 6. Bagadi HO, Sewell MM. A study of the route of dissemination of orally administered spores of Clostridium novyi type B in guinea pigs and sheep. Res Vet Sci 1974;17:179–181. [PubMed] [Google Scholar]
- 7. Barthold SW, et al. , eds. Mouse. In: Pathology of Laboratory Rodents and Rabbits. 4th ed. Chichester, UK: Wiley, 2016:1–118. [Google Scholar]
- 8. Barthold SW, et al. , eds. Rabbit. In: Pathology of Laboratory Rodents and Rabbits. 4th ed. Chichester, UK: Wiley, 2016:253–324. [Google Scholar]
- 9. Barthold SW, et al. , eds. Rat. In: Pathology of Laboratory Rodents and Rabbits. 4th ed. Chichester, UK: Wiley, 2016:119–172. [Google Scholar]
- 10. Batty I, et al. Clostridium oedematiens: a cause of sudden death in sheep, cattle and pigs. Vet Rec 1964;76:1115–1116. [Google Scholar]
- 11. Bender LC, et al. Bacillary hemoglobinuria in a free-ranging elk calf. J Zoo Wildl Med 1999;30:293–296. [PubMed] [Google Scholar]
- 12. Borchers A, et al. Successful treatment and polymerase chain reaction (PCR) confirmation of Tyzzer’s disease in a foal and clinical and pathologic characteristics of 6 additional foals (1986–2005). J Vet Intern Med 2006;20:1212–1218. [DOI] [PubMed] [Google Scholar]
- 13. Brooks JW, et al. Clostridium piliforme infection in two farm-raised white-tailed deer fawns (Odocoileus virginianus) and association with copper toxicosis. Vet Pathol 2006;43:765–768. [DOI] [PubMed] [Google Scholar]
- 14. Cebra C, Cebra M. Diseases of the hematologic, immunologic, and lymphatic systems (multisystem diseases). In: Pugh DG, ed. Sheep & Goat Medicine. 1st ed Elsevier, 2002:359–391. [Google Scholar]
- 15. Corbould A, Munday B. Clostridium oedematiens and anthrax as a cause of sudden death in pigs. Vet Rec 1966;78:218–219. [Google Scholar]
- 16. Cullen JM, Stalker MJ. Liver and biliary system. In: Maxie MG, ed. Jubb, Kennedy, & Palmer’s Pathology of Domestic Animals. 6th ed. St. Louis, MO: Elsevier, 2016:258–352. [Google Scholar]
- 17. Donelli G, Fiorentini C. Bacterial protein toxins acting on the cell cytoskeleton. New Microbiol 1994;17:345–362. [PubMed] [Google Scholar]
- 18. Duncan AJ, et al. Assignment of the agent of Tyzzer’s disease to Clostridium piliforme comb. nov. on the basis of 16S rRNA sequence analysis. Int J Syst Bacteriol 1993;43:314–318. [DOI] [PubMed] [Google Scholar]
- 19. Duncan IF. Liver biopsy and black disease in a sheep. Aust Vet J 1984;61:272–273. [DOI] [PubMed] [Google Scholar]
- 20. Eklund MW, et al. Relationship of bacteriophages to alpha toxin production in Clostridium novyi types A and B. Infect Immun 1976;14:793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Erwin BG. Experimental induction of bacillary hemoglobinuria in cattle. Am J Vet Res 1977;38:1625–1627. [PubMed] [Google Scholar]
- 22. Feldman SH, et al. Ribosomal RNA sequences of Clostridium piliforme isolated from rodent and rabbit: re-examining the phylogeny of the Tyzzer’s disease agent and development of a diagnostic polymerase chain reaction assay. J Am Assoc Lab Anim Sci 2006;45:65–73. [PubMed] [Google Scholar]
- 23. Fosgate GT, et al. Risk factors for Clostridium piliforme infection in foals. J Am Vet Med Assoc 2002;220:785–790. [DOI] [PubMed] [Google Scholar]
- 24. Fresneda KC, Carvallo Chaigneau FR. Tyzzer’s disease. In: Uzal FA, et al. , eds. Clostridial Diseases of Animals. Hoboken, NJ: Wiley, 2016:281–291. [Google Scholar]
- 25. Hamid ME, et al. First report of infectious necrotic hepatitis (black disease) among Nubian goats in Sudan. Rev Elev Med Vet Pays Trop 1991;44:273–275. [PubMed] [Google Scholar]
- 26. Hatheway CL. Toxigenic clostridia. Clin Microbiol Rev 1990;3:66–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hauer PJ, et al. Cloning and molecular characterization of the beta toxin (phospholipase C) gene of Clostridium haemolyticum. Anaerobe 2004;10:243–254. [DOI] [PubMed] [Google Scholar]
- 28. Herbert TGG, Hughes LE. “Black disease” (infectious necrotic hepatitis) in a heifer. Vet Rec 1956;68:223–224. [Google Scholar]
- 29. Hjerpe CA. Clostridial disease vaccines. Vet Clin North Am Food Anim Pract 1990;6:222–234. [PubMed] [Google Scholar]
- 30. Hook RR, et al. Seroanalysis of Tyzzer’s disease in horses: implications that multiple strains can infect Equidae. Equine Vet J 1995;27:8–12. [DOI] [PubMed] [Google Scholar]
- 31. Hreczko I. Infectious necrotic hepatitis in sheep in South Australia, possibly associated with Cysticercus tenuicollis. Aust Vet J 1959;35:462–463. [Google Scholar]
- 32. Hussein HA, et al. Bacillary hemoglobinuria in dairy cows: clinical, hematological, biochemical, and pathological alterations. Comp Clin Pathol 2013;22:1137–1143. [Google Scholar]
- 33. Ikegami T, et al. Naturally occurring Tyzzer’s disease in a calf. Vet Pathol 1999;36:253–255. [DOI] [PubMed] [Google Scholar]
- 34. Janzen ED, et al. Bacillary hemoglobinuria associated with hepatic necrobacillosis in a yearling feedlot heifer. Can Vet J 1981;22:393–394. [PMC free article] [PubMed] [Google Scholar]
- 35. Langan J, et al. Tyzzer’s disease in a red panda (Ailurus fulgens fulgens). J Zoo Wildl Med 2000;31:558–562. [DOI] [PubMed] [Google Scholar]
- 36. Lemmon MJ, et al. Anaerobic bacteria as a gene delivery system that is controlled by the tumor microenvironment. Gene Ther 1997;4:791–796. [DOI] [PubMed] [Google Scholar]
- 37. Macfarlane MG. The biochemistry of bacterial toxins; the lecithinase activity of Clostridium haemolyticum toxin. Biochem J 1950;47:267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maclennan JD. The histotoxic clostridial infections of man. Bacteriol Rev 1962;26:177–276. [PMC free article] [PubMed] [Google Scholar]
- 39. Mete A, et al. Clostridium piliforme encephalitis in a weaver bird (Ploceus castaneiceps). J Vet Diagn Invest 2011;23:1240–1242. [DOI] [PubMed] [Google Scholar]
- 40. Mete A, et al. Tyzzer’s disease in free-ranging passerine birds in California, USA. J Wildl Dis 2017;53:938–941. [DOI] [PubMed] [Google Scholar]
- 41. Nakamura S, et al. Susceptibility to mitomycin C and lecithinase activities of Clostridium oedematiens (C. novyi) type B and D. J Med Microbiol 1975;8:289–297. [DOI] [PubMed] [Google Scholar]
- 42. Nakamura S, et al. Taxonomic relationships among Clostridium novyi types A and B, Clostridium haemolyticum and Clostridium botulinum type C. J Gen Microbiol 1983;129:1473–1479. [DOI] [PubMed] [Google Scholar]
- 43. Navarro M, et al. Bacillary hemoglobinuria. In: Uzal FA, et al. , eds. Clostridial Diseases of Animals. Hoboken, NJ: Wiley, 2016:265–274. [Google Scholar]
- 44. Navarro M, Uzal FA. Infectious necrotic hepatitis. In: Uzal FA, et al. , eds. Clostridial Diseases of Animals. Hoboken, NJ: Wiley, 2016:275–279. [Google Scholar]
- 45. Navarro MA, et al. Pathology of naturally occurring bacillary hemoglobinuria in cattle. Vet Pathol 2017;54:457–466. [DOI] [PubMed] [Google Scholar]
- 46. Neto RT, et al. Coinfection with Clostridium piliforme and Felid herpesvirus 1 in a kitten. J Vet Diagn Invest 2015;27:547–551. [DOI] [PubMed] [Google Scholar]
- 47. Nishida S, Nakagawara G. Isolation of toxigenic strains of Clostridium novyi from soil. J Bacteriol 1964;88:1636–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nyaoke AC, et al. Infectious necrotic hepatitis caused by Clostridium novyi type B in a horse: case report and review of the literature. J Vet Diagn Invest 2018;30:294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Oakley CL, et al. The toxins of Clostridium oedematiens (Cl. novyi). J Gen Microbiol 1947;1:91–107. [DOI] [PubMed] [Google Scholar]
- 50. Oaks JL, et al. Apparent Clostridium haemolyticum/Clostridium novyi infection and exotoxemia in two horses. J Vet Diagn Invest 1997;9:324–325. [DOI] [PubMed] [Google Scholar]
- 51. Oksche A, et al. Morphological and biochemical study of cytoskeletal changes in cultured cells after extracellular application of Clostridium novyi alpha-toxin. Infect Immun 1992;60:3002–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Olander HJ, et al. Bacillary hemoglobinuria: induction by liver biopsy in naturally and experimentally infected animals. Pathol Vet 1966;3:421–450. [DOI] [PubMed] [Google Scholar]
- 53. Osborne HG. Observations on the seasonal incidence of acute fascioliasis and infectious necrotic hepatitis (black disease) in sheep. Aust Vet J 1958;34:301–304. [Google Scholar]
- 54. Pacheco IL, et al. Fasciola hepatica induces Foxp3 T cell, proinflammatory and regulatory cytokine overexpression in liver from infected sheep during early stages of infection. Vet Res 2018;49:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pérez J, et al. Pathological and immunohistochemical study of the liver and hepatic lymph nodes of sheep chronically reinfected with Fasciola hepatica, with or without triclabendazole treatment. J Comp Pathol 2002;127:30–36. [DOI] [PubMed] [Google Scholar]
- 56. Popoff MR, Bouvet P. Clostridial toxins. Future Microbiol 2009;4:1021–1064. [DOI] [PubMed] [Google Scholar]
- 57. Randhawa SS, et al. An outbreak of bacillary haemoglobinuria in sheep in India. Trop Anim Health Prod 1995;27:31–36. [DOI] [PubMed] [Google Scholar]
- 58. Raymond JT, et al. Tyzzer’s disease in a neonatal rainbow lorikeet (Trichoglossus haematodus). Vet Pathol 2001;38:326–327. [DOI] [PubMed] [Google Scholar]
- 59. Records E, Huber M. Bacillus hemolyticum infection in a hog. J Am Vet Med Assoc 1931;78:863–865. [Google Scholar]
- 60. Robles CA, et al. Hepatitis infecciosa necrosante en ovinos merino de la patagonia argentina parasitados con Thysanosoma actinioides [Black disease in Merino sheep infected with Thysanosoma actinioides in Patagonia Region, Argentina]. Arch Med Vet 2000;32:93–99. Spanish. [Google Scholar]
- 61. Sasaki Y, et al. Phylogenetic positions of Clostridium novyi and Clostridium haemolyticum based on 16S rDNA sequences. Int J Syst Evol Microbiol 2001;51:901–904. [DOI] [PubMed] [Google Scholar]
- 62. Sasaki Y, et al. Phylogenetic analysis and PCR detection of Clostridium chauvoei, Clostridium haemolyticum, Clostridium novyi types A and B, and Clostridium septicum based on the flagellin gene. Vet Microbiol 2002;86:257–267. [DOI] [PubMed] [Google Scholar]
- 63. Sasseville VG, et al. Naturally occurring Tyzzer’s disease in cotton-top tamarins (Saguinus oedipus). Comp Med 2007;57:125–127. [PubMed] [Google Scholar]
- 64. Schallehn G, Eklund MW. Conversion of Clostridium novyi type D (C. haemolyticum) to alpha toxin production by phages of C. novyi type A. FEMS Microbiol Lett 1980;7:83–86. [Google Scholar]
- 65. Selzer J, et al. Clostridium novyi α-toxin-catalyzed incorporation of GlcNAc into rho subfamily proteins. J Biol Chem 1996;271:25173–25177. [DOI] [PubMed] [Google Scholar]
- 66. Shinozuka Y, et al. Bacillary hemoglobinuria in Japanese black cattle in Hiroshima, Japan: a case study. J Vet Med Sci 2011;73:255–258. [DOI] [PubMed] [Google Scholar]
- 67. Sinclair KB. Black disease: a review. Br Vet J 1956;112:196–200. [Google Scholar]
- 68. Smith GW. Black disease. In: Smith BP, ed. Large Animal Internal Medicine. 5th ed. St. Louis, MO: Mosby Elsevier, 2015:849–850. [Google Scholar]
- 69. Smith LD. Clostridium haemolyticum. In: The Pathogenic Anaerobic Bacteria. (American Lecture Series; Publication no. 1064.) Springfield, IL: Charles C. Thomas, 1975:271–280. [Google Scholar]
- 70. Smith LD, Jasmin AM. The recovery of Clostridium hemolyticum from the livers and kidneys of apparently normal cattle. J Am Vet Med Assoc 1956;129:68–71. [PubMed] [Google Scholar]
- 71. Snyder JH, Snyder SP. Bacillary hemoglobinuria. In: Smith BP, ed. Bacillary hemoglobinuria. 4th ed. St. Louis, MO: Elsevier, 2009:900–901. [Google Scholar]
- 72. Songer JG. Clostridium novyi (myonecrosis, black disease, and bacillary hemoglobinuria) and Clostridium septicum (braxy) infections. In: Food Animal Practice. Philadelphia, PA: Elsevier, 2009:58–61. [Google Scholar]
- 73. Sweeney HJ, Greig A. Infectious necrotic hepatitis in a horse. Equine Vet J 1986;18:150–151. [DOI] [PubMed] [Google Scholar]
- 74. Swerczek TW. Tyzzer’s disease in foals: retrospective studies from 1969 to 2010. Can Vet J 2013;54:876–880. [PMC free article] [PubMed] [Google Scholar]
- 75. Takagi M, et al. Successful treatment of bacillary hemoglobinuria in Japanese black cows. J Vet Med Sci 2009;71:1105–1108. [DOI] [PubMed] [Google Scholar]
- 76. Turner AW. Innocuity and latency of bacterial spores in the animal body, and the factors influencing their development, with special reference to the pathogenesis of black disease. Aust Vet J 1930;6:83–92. [Google Scholar]
- 77. Tyzzer EE. A fatal disease of the Japanese waltzing mouse caused by a spore-bearing bacillus (Bacillus piliformis, N. SP.). J Med Res 1917;37:307–338.5. [PMC free article] [PubMed] [Google Scholar]
- 78. Van Kampen KR, Kennedy PC. Experimental bacillaryhemoglobinuria. II. Pathogenesis of the hepatic lesion in the rabbit. Pathol Vet 1969;6:59–75. [DOI] [PubMed] [Google Scholar]
- 79. Van Ness GB, Erickson K. Ecology of bacillary hemoglobinuria. J Am Vet Med Assoc 1964;144:492–496. [PubMed] [Google Scholar]
- 80. Veazey RS, et al. Encephalitis in gerbils due to naturally occurring infection with Bacillus piliformis (Tyzzer’s disease). Lab Anim Sci 1992;42:516–518. [PubMed] [Google Scholar]
- 81. Vine N, et al. Bacillary haemoglobinuria in dairy cows. Vet Rec 2006;159:160. [DOI] [PubMed] [Google Scholar]
- 82. Whitfield LK, et al. Necrotic hepatitis associated with Clostridium novyi infection (black disease) in a horse in New Zealand. N Z Vet J 2015;63:177–179. [DOI] [PubMed] [Google Scholar]
- 83. Wobeser G, et al. Tularemia, plague, yersiniosis, and Tyzzer’s disease in wild rodents and lagomorphs in Canada: a review. Can Vet J 2009;50:1251–1256. [PMC free article] [PubMed] [Google Scholar]
- 84. Yoshida K, et al. Spontaneous Tyzzer’s disease with the central nerve involvement in a newborn common marmoset. J Vet Med Sci 2013;75:1119–1121. [DOI] [PubMed] [Google Scholar]