Abstract
We describe and illustrate herein a case of eosinophilic pulmonary granulomatosis (EPG) in a 16-mo-old, castrated male, Great Pyrenees crossbred dog. EPG appears to differ from eosinophilic pneumonias and eosinophilic bronchopneumopathy in dogs by the presence of nodules and masses in the lungs composed of eosinophils, macrophages, and various combinations of lymphocytes, plasma cells, neutrophils, and mast cells within fibrous tissue. Specific information from this dog was added to the information from the limited number of other dogs diagnosed with EPG reported in the veterinary literature, and the information analyzed. EPG appears to have no breed or sex predilection and tends to be a disease of younger dogs, often ≤ 3 y of age. Antemortem imaging of nodules and masses in the lungs, combined with cytologic evidence of eosinophils in the lesions, is highly suggestive of EPG, and the diagnosis can be confirmed postmortem. Concurrent eosinophilia and basophilia can be features of EPG, but their diagnostic value requires further investigation, as does the possible causal association with Dirofilaria immitis infection.
Keywords: dogs, eosinophilic granuloma, lung pathology
Eosinophils are prominent in various pulmonary diseases that have been described in dogs. These diseases include eosinophilic pneumonias, eosinophilic bronchopneumopathy, and canine eosinophilic pulmonary granulomatosis (EPG).4 EPG differs from the other eosinophil-rich entities by the presence of pulmonary nodules and masses composed of eosinophils, macrophages, and various combinations of lymphocytes, plasma cells, neutrophils, and mast cells within fibrous tissue. Some authors have suggested that some cases of EPG might represent an advanced stage of eosinophilic bronchopneumopathy.15 We describe and illustrate herein a case of EPG in a young dog, thereby contributing to the literature and assisting others with a diagnosis of EPG in the future. Also, we critically review the limited literature on EPG in dogs to promote an understanding of the pathogenesis of this condition.
A 16-mo-old, castrated male, Great Pyrenees crossbred dog was referred to the Veterinary Medical Centre (VMC) of the Western College of Veterinary Medicine (WCVM; University of Saskatchewan, Canada) because of a persistent cough, recent lethargy, anorexia; opacities in the lungs were noted in radiographs taken by the referring veterinarian. At ~9 mo of age, an episode of coughing and lethargy responded quickly to oral antibiotics. Then, a few weeks prior to referral, the dog was again reported to be coughing and anorexic, and partially responded to oral antibiotics, but never returned to normal.
At the VMC, the dog had an increased heart rate (180 beats/min), increased respiratory rate (60/min) and effort, and increased lung sounds that were more pronounced on the right. The results of serum biochemical analysis were unremarkable; a CBC revealed leukocytosis (49.1 × 109/L; reference interval [RI]: 4.9–15.4 × 109/L), eosinophilia (23.6 × 109/L; RI: 0.0–1.1 × 109/L), basophilia (1.5 × 109/L; RI: 0.0–0.1 × 109/L), and evidence of inflammation (segmented neutrophils 15.2 × 109/L, RI: 3.0–10.0; band neutrophils 1.0 × 109/L, RI: 0.0–0.1; monocytes 4.9 × 109/L, RI: 0.08–1.00). Radiographs confirmed lobulated opacities in the lungs and perihilar opacities (suspected to be enlarged tracheobronchial lymph nodes) that displaced the right cranial lobar bronchi. Cytology, using samples obtained by ultrasound-guided, fine-needle aspiration, revealed moderate numbers of variably preserved neutrophils, eosinophils and, possibly, degranulated eosinophils. A serum ELISA (SNAP 4Dx Plus test; IDEXX, Markham, Ontario) did not detect antibodies to Anaplasma phagocytophilum, Anaplasma platys, Borrelia burgdorferi, Ehrlichia canis, or Ehrlichia ewingii, and did not detect antigens of Dirofilaria immitis. Based on the available information, a working diagnosis of EPG was made, and the dog was treated with prednisone (50 mg, q24h), azathioprine (50 mg, q24h), and multiple doses of 10% fenbendazole suspension (12 mL, q24h). After 5 d of hospitalization, the dog was returned to the care of his owners, with somewhat improved health, but with restricted exercise recommended.
The dog was examined again 2 wk later and had improved clinically. Review of current and previous radiographs found that some of the smaller pulmonary opacities were no longer visible, the larger opacities were smaller, and the tracheobronchial lymph nodes were smaller. After another 6 wk, the pulmonary opacities, but not the tracheobronchial lymph nodes, were slightly smaller again. On a recheck CBC, there was mild regenerative anemia; leukocyte numbers were within RIs, except for mild lymphopenia, which was thought to be the result of corticosteroid therapy.
At ~19-mo-old, the dog was coughing, had leukocytosis (19.4 × 109/L) with neutrophilia and mild left shift (14.6 × 109/L segmented neutrophils and 0.4 × 109/L bands), eosinophilia (2.7 × 109/L), and basophilia (0.4 × 109/L). Compared to 2 mo earlier, pulmonary opacities and tracheobronchial lymph nodes were larger, and similar in size to when the dog was first examined at the WCVM; the dog was still being treated with prednisone at this time. Doxycycline (150 mg, q12h) and inhalant fluticasone propionate (125 µg, q6–8h) were added to the dog’s treatments. However, the dog’s condition deteriorated, with anorexia, reluctance to move, and dyspnea progressing to respiratory distress. Within a week, the dog was euthanized and submitted to Prairie Diagnostic Services (PDS), at the WCVM, for postmortem examination.
The postmortem findings were consistent with a diagnosis of EPG. Specifically, there were 4, coalescing, firm, pale masses in the right middle lung lobe. The largest mass was 18 × 12 × 12 cm. There were 3, closely associated, smaller masses measuring 9 × 4 × 3 cm, 7 × 4 × 3 cm, and 5 × 3 × 3 cm. All masses were firm, multinodular, and mottled red and white (pale). The cut surfaces of the masses were uniformly firm and pale, with areas of redness and small brown, dry foci that were interpreted as foci of necrosis (Fig. 1). The right cranial lobe was compressed and atelectatic. The tracheobronchial lymph nodes were, equivocally, mildly enlarged. No other masses or lesions of significance were evident in any other organs.
Figure 1.
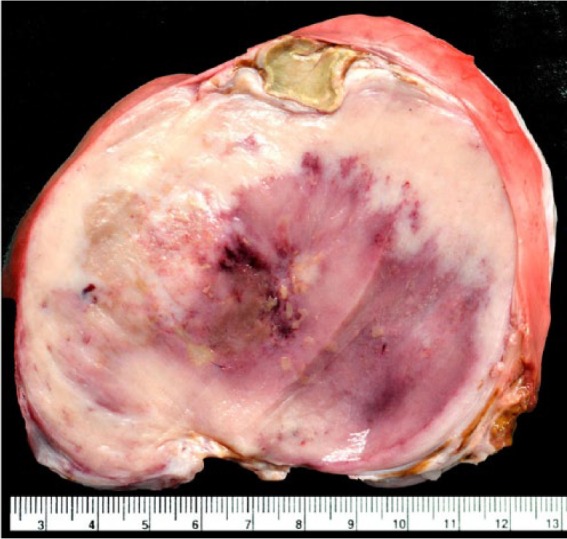
Eosinophilic pulmonary granulomatosis in a dog. The cut surface of a large, firm, pale mass in the right lung.
Histologically, the masses were composed of many polymorphonuclear cells, at least half of which were interpreted to be eosinophils. There were also many, moderate-to-large, variably shaped, mononuclear cells with moderate-to-large, round-to-oval nuclei, interpreted to be macrophages; and small numbers of neutrophils, lymphocytes, and plasma cells; all intermixed with, and separated and surrounded by, long, medium, and short, variably thick, bundles of collagen (Fig. 2). Fibroblasts were present as were several degenerate and necrotic cells, larger foci of necrotic cells, and well-demarcated foci of coagulative necrosis. In some areas, the fibrous tissue was more prominent than the mixed inflammatory cells. A moderately thick fibrous tissue capsule surrounded the masses. A mediastinal lymph node contained many lymphoid follicles. However, a large proportion of the node was effaced by fibrous tissue infiltrated by eosinophils, macrophages, and smaller numbers of neutrophils.
Figure 2.
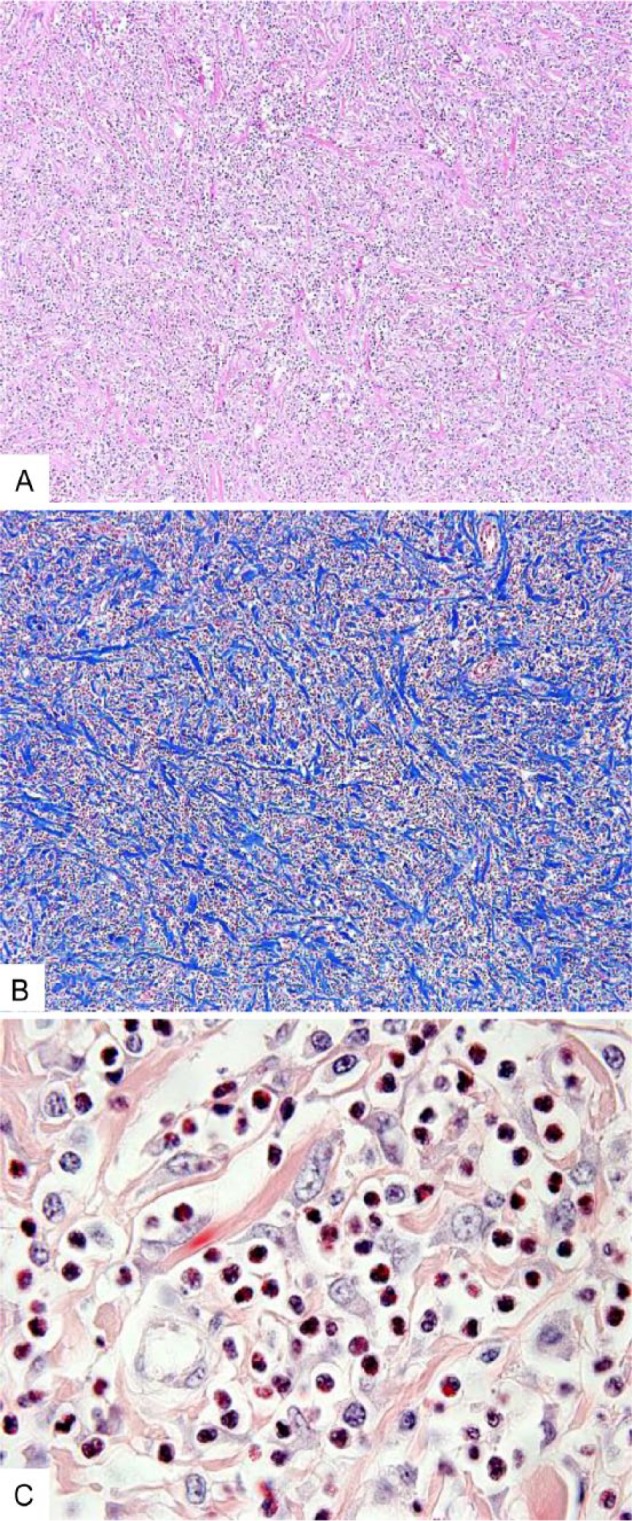
Eosinophilic pulmonary granulomatosis in a dog. A. Low-magnification photomicrograph of the mass. Many polymorphonuclear cells and other inflammatory cells are intermixed with bundles of collagen. H&E. Original objective 10×. B. Low-magnification photomicrograph of the mass highlighting the bundles of collagen. Masson trichrome. Original objective 10×. C. High-magnification photomicrograph of the mass demonstrating many eosinophils. Duffy. Original objective 100×.
We searched the veterinary literature for reports of EPG in dogs and selected information on signalment, clinical information, and pathology (Table 1).2,3,6–8,11,12,17 Our case was diagnosed as EPG, and selected information was added to Table 1. We excluded dogs for which there were no reports of nodules (defined as < 3-cm7) or masses (defined as > 3-cm7) in the lung, or which did not have histologic features of EPG, or both (shaded areas in Table 1). Thus, we included information from 26 dogs in further analysis.
Table 1.
Selected information regarding dogs with eosinophilic pulmonary granulomatosis reported in the veterinary literature (shaded cases were not included in analyses).
| Location of dog or authors | Date | Breed | Sex | Age (y) | Circulating eosinophils (×109/L) | Circulating basophils (×109/L) | Evidence of Dirofilaria immitis | Lung lesions | Regional lymph node lesions | Other lesions |
|---|---|---|---|---|---|---|---|---|---|---|
| Illinois3 | 1967 | Coonhound | ND | 4 | ND | ND | No. | Variably sized gray masses; up to 7.5 cm diameter. Macrophages, lymphocytes, plasma cells, and eosinophils within a dense fibrous stroma. | Enlarged thoracic lymph nodes contained plasma cells and occasional eosinophils. | 2 gray myocardial masses (4 × 1.2 cm, and 1.8 cm diameter). Similar cellular composition as in the lung. |
| Bristol, UK11 | 1979 | Fox Terrier | M | 2 | ND | ND | No. | 1 or more lobes infiltrated and consolidated by firm, pale tissue. Also, up to 3-cm nodules of pale tissue in other lobes of 2 dogs. Lesions = reticulum-like cells, eosinophils, lymphocytes, plasma cells. | Bronchial and mediastinal lymph nodes enlarged and replaced by firm, pale tissue. Cells closely resembling those present in lungs. | Up to 3 cm diameter, firm, pale tissue in liver and spleen of 2 dogs. |
| Springer Spaniel | M | 3 | ND | ND | No. | |||||
| Golden Retriever | F | 1.5 | 3–9* | ND | No. | |||||
| Southern Louisiana6 | 1983 | Afghan × Labrador Retriever cross | M | 4 | ND | ND | Numerous adults in pulmonary arteries. | 1–10 cm diameter, firm, pale nodules. Pronounced fibroplasia; epithelioid cells, macrophages, and eosinophils.** | No | Mild and likely not related to lesions in the lungs. |
| German Shepherd | M | 8 | 5.1 | ND | No. | Numerous gray-white nodules up to 5 cm diameter. Histologically as above.** | Bronchial lymph nodes slightly enlarged and edematous. | No. | ||
| Doberman Pinscher | F | 2 | 10.1 | 1.6 | Numerous adults in pulmonary arteries; “filarids” in arteries. | Multiple, firm nodules, 0.5–5 cm diameter. Histologically as above.** | No. | No. | ||
| Scottish Terrier | M | 8 | 10.1 | 1.0 | Numerous adults in pulmonary arteries; “filarids” in arteries. | Single, 7-cm mass removed during surgery. Histologically as above.** | No. | No. | ||
| Louisiana12 | 1986 | Boxer | SF | 1.5 | 4.0 | ND | No. | 4.5-cm mass in left caudal lobe. | Greatly enlarged tracheobronchial lymph nodes. | Right ventricular hypertrophy. |
| Great Dane | M | 8 | 10.5 | ND | Numerous adults in pulmonary arteries. | Variably sized, firm, yellow-tan-gray masses in all lobes; large mass in right caudal lobe. | Tracheobronchial lymph nodes slightly enlarged. | No. | ||
| Zürich, Switzerland17 | 1986 | German Shepherd | ND | 5 | ND | ND | No. | Multiple nodules up to 3 cm diameter; macrophages, eosinophils, mast cells, occasional giant cells. | Bronchopulmonary lymph nodes enlarged; plasma cells, macrophages, eosinophils. | No. |
| Georgia2 | 1988 | Alaskan Malamute | SF | 11 | 6.2 | 0.8 | No. | Foci of eosinophils, lymphocytes, and plasma cells. (Nodules not detected radiographically or grossly. No mention of macrophages.) | Hilar lymph nodes enlarged. | Eosinophilic tonsillitis, tracheitis, hepatitis, splenitis. |
| Beagle | M | 6 | 0.7 | 0.0 | Microfilaremia. | Disseminated eosinophilic granulomatous pneumonia. (No description of nodules radiographically or grossly.) | No. | Granulomatous tracheitis, gastroenteritis; eosinophilic hepatitis, splenitis, nephritis, lymphadenitis. | ||
| ND | M | 3 | 12.6 | 0.8 | ND | ND | ND | ND | ||
| German Shepherd | F | 8 | 6.4 | 0.8 | No. | Masses. One biopsied mass consisted of consolidation with macrophages, neutrophils, and many eosinophils. | Hilar and submandibular lymph nodes enlarged. | Pleural effusion and cranial mediastinal mass. | ||
| Pit Bull Terrier | SF | 7 | 0.4 | 2.6 | Several adults in right ventricle. | Large mass in right caudal lobe and EPGs (5 mm to 4 cm; macrophages, eosinophils, plasma cells). | Enlarged thoracic lymph nodes. | Right ventricular hypertrophy; ascites. | ||
| Siberian Husky × German Shepherd cross | M | 3 | 2.6 | 5.3 | “Positive” indirect fluorescent antibody test. | Radiographic evidence of right cranial lobe consolidation and masses. FNA = eosinophils and basophils with fewer plasma cells and macrophages. | Markedly enlarged hilar lymph nodes. | No. | ||
| German Shepherd cross | M | 7 or 8† | 1.3 | 0.0 | “Positive” indirect fluorescent antibody test. | Radiographic evidence of left caudal lobe consolidation and masses. FNA = eosinophils with fewer lymphocytes, macrophages, neutrophils. | Enlarged sternal lymph node. | Pleural effusion. Later, disseminated histiocytic lymphoma. | ||
| Chinese Shar Pei | F | 3 | 6.0 | 0.3 | No. | Disseminated eosinophilic granulomatous pneumonia with firm and nodular areas. Most alveoli filled with macrophages, eosinophils, and a few neutrophils. | No. | Eosinophilic granulomatous inflammation of heart, liver, spleen, and small intestine. | ||
| Cocker Spaniel | M | 1 | 0.5 | 0.0 | No. | Ill-defined radiographic masses in cranial lobes. Biopsy = macrophages, eosinophils, neutrophils. | No. | No. | ||
| ND | M | 5 | 25.1 | 0.0 | No. | Right cranial and middle lobes fused into a yellow-gray, solid, lobulated mass. Right caudal lobe consolidated. Parenchyma replaced by fibrous tissue, eosinophils, macrophages. | No. | No. | ||
| ND | SF | 7 | 4.7 | 0.0 | Several adults in right ventricle. | Nodules including a 10 × 12-cm mass in left caudal lobe composed of fibroplasia, macrophages, and areas of eosinophils. | No. | No. | ||
| Nummela, Finland8 | 2013 | Jack Russel Terrier | F | 2 | 3 and 4.9 | 0.7 and 1.6 | No. | 1.6-, 2.0-, and 2.6-cm masses, by radiography and CT. | No. | No. |
| Bologna, Italy or California7 | 2014 | Crossbreed | F | 4.5 | ND | ND | No. | < 3-cm nodules and > 3-cm masses, or both, in caudal lobes, detected by CT. Granulomatous and eosinophilic inflammatory cells detected via cytology, histology, or both. | Thoracic lymphadenopathy in 1 dog. | ND |
| Crossbreed | M | 3.5 | ND | ND | No. | |||||
| Crossbreed | M | 3 | ND | ND | No. | |||||
| German Shepherd | CM | 2.5 | ND | ND | No. | |||||
| Rottweiler | CM | 2.5 | Severe eosinophilia | ND | No. | |||||
| Abbott, Allen; Western Canada | 2020 | Great Pyrenees cross | CM | 1.5 | 23.6 | 1.5 | No. | 5-, 7-, 9-, and 18-cm, firm, pale masses in the right middle lobe. Fibrous tissue, many eosinophils and macrophages, with fewer neutrophils, lymphocytes, and plasma cells. | Mediastinal lymph node equivocally enlarged; same histologic changes as the lung. | No. |
CM = castrated male; CT = computed tomography; EPG = eosinophilic pulmonary granuloma; F = female; FNA = fine-needle aspirate; M = male; ND = not determined, not described, or appropriate test not performed; SF = spayed female.
Original values were reported to be ×103/mL, which is assumed to represent a typographical error and have been converted to ×103/µL.
Seven and 8 y of age were both reported (7.5 y was used in the calculation of the mean and median).
The 26 dogs with EPG included 17 purebreds and 7 crossbred dogs. The breed of 2 dogs was not reported. The diversity of the breeds reported does not suggest a breed predisposition. Although 6 of the dogs were German Shepherds or German Shepherd crossbred dogs, this may simply represent the popularity of that breed. Affected dogs included 15 males, 3 of which were castrated, and 9 females, 3 of which were spayed. The sex of 2 dogs was not reported. There does not appear to be a sex predilection for EPG in dogs.
The age of the 26 affected dogs ranged between 1 and 8 y, with mean and median ages of 4.2 and 3.3 y, respectively. Further, 13 of the dogs were ≤ 3 y old and 9 were ≤ 2.5 y old. These ages are important given that neoplasia is a differential diagnosis for any dog experiencing coughing and dyspnea, and found to have opacities (i.e., nodules and masses) in the lungs. However, dogs with primary lung cancer are, on average, 10–11 y of age at the time of diagnosis, and rarely < 6 y of age.4,10,19,20 Several reports did not provide hematologic data. Using published reference intervals9 (which are similar to those established by PDS, and provided above), at least 14 dogs with EPG reported in the literature had concurrent eosinophilia and 8 had basophilia. There are relatively few disease conditions associated with eosinophilia and even fewer associated with basophilia. However, one disease condition associated with both eosinophilia and basophilia is the presence of Dirofilaria immitis within the vascular system.16,18
Multiple reports have noted an association between the presence of D. immitis and EPG.2,6,12 However, further investigation is needed before suggesting that D. immitis is the cause of EPG. Of the 26 dogs with EPG reported in the literature, 18 had no evidence of D. immitis infection at the time EPG was diagnosed. It could be argued that 8 dogs with EPG had evidence of D. immitis infection and that, in the other 18 dogs, the absence of evidence of infection is not the same as “evidence of absence” of infection, either at the time of, or before, the diagnosis of EPG.
However, if the cases summarized in Table 1 are examined as 2 groups based on the date of reporting, divergence is apparent. Of the 19 cases of EPG reported before or during 1988, 8 had evidence of D. immitis infection, whereas 11 did not. Of the 7 cases of EPG reported during or after 2013, none of them had evidence of D. immitis infection even though the methods of detection of infection had improved and testing would have been more common. It is also important to note that heartworm prophylaxis was introduced during the 1980s and 1990s and has become common.1,5,13,14 Additionally, it has been suggested that there may be 2 forms of EPG in dogs, one that may be associated with D. immitis infection and another that is not.15
Antemortem detection of multiple nodules and masses in the lungs using diagnostic imaging, combined with cytologic evidence of eosinophils in the lesions, is highly suggestive of EPG. The usefulness of concurrent eosinophilia and basophilia in the diagnosis of EPG needs to be investigated, and the causal association of D. immitis infection and EPG is questioned and also needs to be further investigated.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Andrew L. Allen  https://orcid.org/0000-0001-5832-2350
https://orcid.org/0000-0001-5832-2350
References
- 1. Blair LS, Campbell WC. Efficacy of ivermectin against Dirofilaria immitis larvae in dogs 31, 60, and 90 days after infection. Am J Vet Res 1980;41:2108. [PubMed] [Google Scholar]
- 2. Calvert CA, et al. Pulmonary and disseminated eosinophilic granulomatosis in dogs. J Am Anim Hosp Assoc 1988;24:311–320. [Google Scholar]
- 3. Carroll JM, Simon J. Eosinophilic granuloma in a dog. J Am Vet Med Assoc 1967;150:526–528. [PubMed] [Google Scholar]
- 4. Caswell JL, Williams KJ. Respiratory system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed. Vol. 2 St. Louis, MO: Elsevier, 2016:465–591. [Google Scholar]
- 5. Clark JN, et al. Efficacy of ivermectin and pyrantel pamoate combined in a chewable formulation against heartworm, hookworm, and ascarid infections in dogs. Am J Vet Res 1992;53:517–520. [PubMed] [Google Scholar]
- 6. Confer AW, et al. Four cases of pulmonary nodular eosinophilic granulomatosis in dogs. Cornell Vet 1983;73:41–51. [PubMed] [Google Scholar]
- 7. Fina C, et al. Computed tomographic characteristics of eosinophilic pulmonary granulomatosis in five young dogs. Vet Radiol Ultrasound 2014;55:16–22. [DOI] [PubMed] [Google Scholar]
- 8. Katajavuori P, et al. Eosinophilic pulmonary granulomatosis in a young dog with prolonged remission after treatment. J Small Anim Pract 2013;54:40–43. [DOI] [PubMed] [Google Scholar]
- 9. Krimer PM. Generating and interpreting test results: test validity, quality control, reference values, and basic epidemiology. In: Latimer KS, ed. Duncan and Prasse’s Veterinary Laboratory Medicine. 5th ed. Ames, IA: Wiley, 2011:365–382. [Google Scholar]
- 10. López A, Martinson SA. Respiratory system, mediastinum, and pleurae. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 6th ed. St. Louis, MO: Elsevier, 2017:471–560. [Google Scholar]
- 11. Lucke VM, et al. A lymphomatoid granulomatosis of the lungs in young dogs. Vet Pathol 1979;16:405–412. [DOI] [PubMed] [Google Scholar]
- 12. Neer TM, et al. Eosinophilic pulmonary granulomatosis in two dogs and literature review. J Am Anim Hosp Assoc 1986;22:593–599. [Google Scholar]
- 13. Paul AJ, et al. Efficacy of ivermectin chewable tablets and two new ivermectin tablet formulations against Dirofilaria immitis larvae in dogs. Am J Vet Res 1991;52:1922–1923. [PubMed] [Google Scholar]
- 14. Paul AJ, et al. Efficacy of ivermectin against Dirofilaria immitis larvae in dogs 30 and 45 days after induced infection. Am J Vet Res 1986;47:883–884. [PubMed] [Google Scholar]
- 15. Reinero C. Interstitial lung diseases in dogs and cats, part II: known cause and other discrete forms. Vet J 2019;243:55–64. [DOI] [PubMed] [Google Scholar]
- 16. Stockham SL, Scott MA. Leukocytes. In: Fundamentals of Veterinary Clinical Pathology. 2nd ed. Ames, IA: Blackwell, 2008:53–106. [Google Scholar]
- 17. von Rotz A, et al. Eosinophilic granulomatous pneumonia in a dog. Vet Rec 1986;118:631–632. [DOI] [PubMed] [Google Scholar]
- 18. Webb JL, Latimer KS. Leukocytes. In: Latimer KS, ed. Duncan and Prasse’s Veterinary Laboratory Medicine. 5th ed. Ames, IA: Wiley, 2011:45–82. [Google Scholar]
- 19. Wilson DW. Tumors of the respiratory tract. In: Meuten DJ, ed. Tumors in Domestic Animals. 5th ed. Ames, IA: Wiley, 2017:467–498. [Google Scholar]
- 20. Withrow SJ. Tumors of the respiratory system. In: Withrow SJ, et al. , eds. Withrow & MacEwen’s Small Animal Clinical Oncology. 5th ed. St. Louis, MO: Elsevier, 2013:432–462. [Google Scholar]


