Abstract
Various neurodegenerative diseases and disorders as well as sleep deprivation seriously impact memory and cognition and can become life-threatening. Current medical techniques attempt to combat these detrimental effects mainly through the administration of neuromedicine. However, drug efficacy is limited by rapid dispersal of the drugs to off-target sites while the site of administration is prone to overdose. Many neuropathological conditions are accompanied by excessive reactive oxygen species (ROS) due to the inflammatory response. Accordingly, ROS-responsive drug delivery systems have emerged as a promising solution. To guide intelligent and comprehensive design of ROS-responsive drug delivery systems, this review article discusses the two following topics: (1) the biology of ROS in both healthy and diseased nervous systems and (2) recent developments in ROS-responsive, drug delivery system design. Overall, this review article would assist efforts to make better decisions about designing ROS-responsive, neural drug delivery systems, including the selection of ROS-responsive functional groups and disease-homing ligands.
Keywords: reactive oxygen species, neuroinflammation, neurological disorders, drug delivery
1. Introduction
Neuroinflammation is common to many neurodegenerative diseases within the central nervous system. As the body’s physiological response to defend itself from harmful stimuli, inflammation is beneficial to the central nervous system and contributes to neuronal repair, remyelination, and axon regeneration [1]. Although inflammation is a necessary and natural bodily response to disease and injury, it can also lead to unintended side effects such as the initiation and exacerbation of neurodegenerative diseases like Alzheimer’s disease [2], Parkinson’s disease [3], and epilepsy [4], among others [5]. Additionally, it can pose problems relating to rejection of medical devices, tissue necrosis, and impaired recovery [6,7]. Multiple inflammatory factors that are associated with neurodegeneration have been identified to mediate neurodegeneration; however, none of these are adequate therapeutic targets to combat the progression of neurodegenerative diseases [5].
Chemically reactive molecules containing oxygen known as reactive oxygen species (ROS) are key players in both the beneficial and damaging aspects of the immune response [8]. Typical ROS, including superoxide (O2−), hydrogen peroxide (H2O2), hypochlorous acid (HOCl), hydroxyl (•OH), alkoxyl (RO•), and peroxyl (ROO•) radicals, are commonly formed as byproducts of oxygen metabolism. They participate in a variety of signaling processes during neuronal development and function. However, ROS can cause unintended effects by non-specifically oxidizing essential molecules when they are produced in excess, even in the absence of inflammation.
Over the years, strategies to combat the neurological diseases and disorders have focused on the use of antioxidants and medicine that blocks cellular receptors and signaling pathways specific for specific diseases or disorders [9]. However, many of these treatments do not target the site of neuroinflammation, leading to dispersal of the drugs throughout the body. Therefore, in order to maintain sufficient concentrations of drugs at the diseased site, higher concentrations and additional drugs must be administered. This addition can lead to adverse side-effects such as overdose or anti-oxidative stress at the local site and unintended off-site reactions [10].
ROS-responsive drug delivery systems in the form of nano- or microparticles are currently being investigated for their potential to improve the therapeutic effects of drugs in response to excess ROS[11]. In general, these particles contain polymers that are functionalized with chemical groups that react with ROS. In an oxidative environment, particles swell to release their molecular cargo progressively, or burst to discharge the entire payload all at once. As these particles can release drug cargos in response to abnormally increased levels of ROS, ROS-responsive particles can potentially minimize the side-effects caused by off-target dispersal of therapeutic agents on the natural brain physiology.
With this motivation in mind, the scope of this review article is two-fold: (1) an overview of the role of ROS on the underlying biology of neuroinflammation and (2) a survey of current design approaches involving the use of ROS-responsive drug delivery systems for treating neurological diseases and disorders. This review may serve as a useful foundation for the design of future inflammation treatments that carefully consider the interplay between ROS, immune response, and various inflammatory diseases. ROS are not simply dangerous chemical species that must be eliminated. They are essential molecules that only cause problems when they are unregulated by extrinsic factors such as disease and injury. Therefore, the goal of any future medical treatment should be the balanced control of oxidation and anti-oxidation, not elimination of ROS. Modular responsive systems using biomaterials are well suited to achieve this end.
2. Endogenous production and physiological/pathophysiological role of ROS
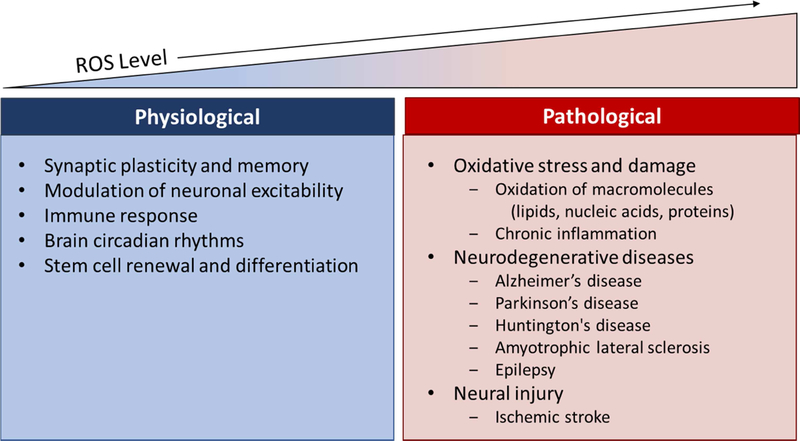
The brain is a metabolically demanding organ. Compared to other tissues in the human body, the brain consumes 20% of the oxygen in the body, even though it only comprises 2% of the total body weight [12]. Due to the extremely high metabolic rates, the brain in turn produces large concentrations of ROS slightly less than 10 μM [13]. ROS are generated in a wide range of normal physiological conditions and play a large role in a number of functions including synapse plasticity, learning and memory, and the immune response against pathogens [8,14,15]. When unregulated, excess ROS can lead to oxidative stress. High levels of ROS have been associated with a decline in cognitive function, as observed in some neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease (Fig. 1) [16–19]. To prevent excessive ROS production, cells produce a variety of antioxidant enzymes. In this section, we will discuss the main sources of ROS generation within the body, their involvement in normal function of the body, and the damaging effects of unregulated ROS generation.
Figure 1:
Physiological importance of ROS in the brain, and its pathological effects when unregulated.
2.1. Sources of ROS in the brain
The primary source of ROS generation is in the mitochondria, which are large cellular organelles involved in oxidative phosphorylation, a process which generates adenosine triphosphate (ATP) [20–22]. During this process, oxygen is consumed, and, in the final step, oxygen is reduced to H2O. Electrons are exchanged throughout this process by using a series of electron transporters composed of four complexes: complex I (NADH ubiquinone oxidoreductase), complex II (succinate ubiquinone oxidoreductase), complex III (ubiquinone-cytochrome c oxidase), and complex IV (cytochrome c oxidase).
Although the entire process is efficient, the electron transport chain is “leaky” in nature, and electrons often escape and directly react with O2 to produce the superoxide anion. The superoxide anion is quickly transformed into H2O2 by either manganese superoxide dismutase (MnSOD, also known as SOD-2) in the mitochondrial matrix or by copper/zinc superoxide dismutase (CuZnSOD, also known as SOD-1) in the mitochondrial intermembrane space. If H2O2 is not immediately converted into water, H2O2 can interact with transition metals to form highly toxic hydroxyl radicals [23]. H2O2 can also be used to catalyze the production of additional ROS (i.e. hypochlorous acids) [24,25].
Inhibition to individual components of the electron transport chain has provided valuable information for the identification of superoxide-producing sites. In isolated mitochondria, inhibition of complex I and complex III resulted in increased ROS generation [26]. In mitochondria of isolated nerve terminals in situ, inhibition of complex I, complex III, or complex IV increases ROS generation [26]. However, the influence of complexes III and IV in the production of ROS is debatable, as both complexes should be inhibited by over 70% for there to be an increase in ROS [26]. In contrast, for complex I, only 25–30% inhibition is required for ROS production [26,27].
Another component that generates ROS in the mitochondria is the monoamine oxidase enzyme (MAO) [28]. MAO is found in two forms (MAO-A and MAO-B) in the brain that differ in their inhibitor and substrate specificities; however, they are both capable of producing H2O2.[29] In particular, MAO-A is abundant in catecholaminergic neurons, which contain the neurotransmitters dopamine and norepinephrine [29]. MAO-B is also present, and preferentially expressed in serotoninergic and histaminergic neurons, as well as astrocytes [29,30]. MAOs typically catalyze a number of neurotransmitters to generate H2O2. These neurotransmitters include dopamine [31,32], serotonin [33,34], norepinephrine [34], and epinephrine [32].
In peroxisomes, β-oxidation of fatty acids also produces ROS, and is sometimes executed in coordination with the mitochondria.[35] Various substrates (i.e. very long fatty acid chains) are transported into the peroxisome and broken down by peroxisomal oxidases. These peroxisomal oxidases include acyl-CoA, urate, xanthine, D-amino acid, D-aspartate, L-alpha-hydroxy acid, and polyamine oxidases, among others. Another substrate, xanthine is broken down by xanthine oxidase to superoxide anions, which are scavenged by MnSOD and CuZnSOD.
Another source of ROS is the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase membrane-bound enzymic complex (NOX), which is primarily located in the phagosomes. Phagosomes defend the central nervous system against invading microorganisms and clear the debris from damaged cell through an oxidative burst of nitric oxide, H2O2, and superoxide anions. NADPH oxidase produces superoxide through the oxidation of NADPH in a two-step process. The reaction is initiated when an electron is transferred from NADPH to a flavin adenine dinucleotide (FAD). In the second step, the electron is transferred to a heme moiety, where it then interacts with molecular oxygen to form superoxide. Immunohistochemical studies on mouse and rat tissues identified higher concentrations of the NOX complex in the cortex and the hippocampus [36]. The NOX complex is present in cell bodies and dendrites and is localized at the synapses [36].
2.2. ROS function in normal physiological conditions
ROS are generated in response to a variety of growth factors, cytokines, and calcium signals, and is necessary for normal physiological conditions in the brain. In regulated amounts, ROS have been shown to be critical signaling molecules that are involved in synaptic plasticity [37], memory formation [15], the immune response [38], modulation of neuronal excitability [39,40], circadian rhythms [39–41], and neuron differentiation [42,43] (Fig. 1).
2.2.1. Synaptic plasticity and memory
Neurons form new sites of cell-cell communication (synapses) or change the efficacy of preexisting synapses in response to stimuli. This process, known as synaptic plasticity, especially long-lasting increase in synaptic strength or long-term potentiation (LTP) is thought to underlie learning and memory formation [15]. LTP studies in rodent hippocampus revealed that superoxide accumulates in hippocampal slices after the activation of N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors [37]. Both NMDA and AMPA are two key glutamate receptors that mediate fast excitatory synaptic transmission and are major players in synaptic plasticity.
The involvement of ROS in learning and memory comes from early studies on transgenic mice overexpressing SOD-1, which converts superoxide anions to H2O2 [44]. Using a Morris water maze test, these studies showed that SOD-1 overexpression impaired hippocampus-dependent spatial memory in mice. The authors proposed that excess H2O2 produced by SOD-1 overexpression potentially blocks the induction and/or expression mechanism of LTP. Consistent with this, incubation of hippocampal slices with superoxide scavengers has been shown to inhibit the induction of LTP [45]. Taken together, these findings support the notion that superoxide is necessary for LTP.
2.2.2. Immune response
Inflammation is part of the body’s natural protective response to remove harmful stimuli and begin the healing process. The generation of ROS by phagocytes such as neutrophils and macrophages plays a critical role in mediating inflammation [38]. Neutrophils provide the first line of defense against invading infections. Micro-organisms are ingested by the neutrophils and bombarded by high concentrations of ROS. This oxidative burst of ROS is generated by the NADPH oxidase (NOX) complex, which is rapidly activated by a number of soluble factors and stimuli which interact with cell-surface receptors [46,47]. Strong evidence further supports the role of ROS as secondary messengers, which regulates the production of cytokines and several inflammatory molecules. Bacterial lipopolysaccharides (LPS)-mediated ROS, for instance, has been shown to stimulate macrophages to release pro-inflammatory cytokine interleukin 1 (IL-1β) and tumor necrosis factor (TNF) [48].
2.2.3. Circadian rhythm
ROS levels have been shown to oscillate in different mammalian tissues depending on the period of day [39–41]. The circadian rhythm is orchestrated by the hypothalamic suprachiasmatic nucleus (SCN) of the brain. The redox state is relatively reduced in the daytime when neuronal activity is high, yet oxidized during nighttime, when neurons are relatively inactive [39]. The redox state can be measured by evaluating the ratio of redox-molecular pairs, such as NAD(P)+ / NAD(P)H, dehydroascorbic acid / ascorbic acid, or glutathione disulfide/glutathione. Melatonin, which helps maintain the body’s circadian rhythm, affects the activity of glutathione peroxidase, an antioxidative enzyme that eliminates hydrogen peroxide from the brain. Glutathione peroxidase prevents the formation of toxic hydroxyl radicals. Therefore, researchers have hypothesized that melatonin exerts neuroprotective effects in the brain by activating production of glutathione peroxidase [49]. In addition, circadian redox oscillations regulate neuronal excitability [39,40]. Musiek and coworkers further reported that deletion of the circadian clock transcriptional activators aryl hydrocarbon receptor nuclear translocator-like (Bmal1) deletion is associated with increases in brain ROS, and impairment in learning and memory [50].
2.2.4. Stem cell renewal and differentiation
H2O2 signaling is also essential for neural stem cell proliferation and differentiation. It was found that proliferating adult neural hippocampal progenitors have high endogenous ROS levels, specifically Nox2-derived H2O2, which regulate self-renewal and neurogenesis through the PI3K/Akt cell signaling pathway [42,43]. Adding low, non-toxic concentrations of H2O2 produced a larger increase in neural stem cells with a modest increase in overall cell proliferation [42]. These results suggest that H2O2 is both a product of active proliferation and a stimulant for proliferation. H2O2 has also been suggested to be an intracellular signal mediator for nerve growth factor (NGF)-induced neuronal differentiation [51]. Tsatmali and coworkers separated embryonic cortical neurons into populations with high and low levels of endogenous ROS, as measured using an ROS sensitive dye 5-(and-6)-chloromethyl-2′,7′dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCF-DA) [52,53]. Cells with low levels of ROS were multipotent neural progenitor cells which differentiated into neurons, oligodendrocytes, and astrocytes in clonal cultures. The high ROS-containing population were composed of neurons [53].
2.3. Damaging effects of unregulated ROS
ROS are generated continuously during oxidative metabolism; however, occasionally, increased production of ROS due to neuroinflammation or insufficient antioxidant activity can cause oxidative stress, leading to neurodegeneration, aging, and long-term side-effects [54,55].
Unregulated ROS results in damage to cells, which can lead to defects in the ubiquitin-proteasome systems, the presence of abnormal and aggregated proteins, and chronic inflammation. Superoxide radicals are highly reactive and can initiate pathological oxidative metabolism of biomacromolecules such as DNA, lipids, and proteins. In addition, the accumulation of H2O2 is also a concern, as the brain contains large quantities of iron and copper, which may catalyze the formation of hydroxyl radicals that can induce lipid peroxidation [56]. High levels of polyunsaturated fatty acids in the neuronal cell membrane draws further concern, as brain cells are susceptible to peroxidative damage [57]. Increasing evidence shows that the free radicals and their involvement in lipid peroxidation may be the primary cause for cerebral damage during ischemia [58]. Many of these non-specific oxidation of the biomacromolecules can lead to neuronal death.
In addition, persistent and high levels of ROS contribute to chronic inflammation through the activation of microglia, macrophage-like immune cells that participate in chronic inflammation [59]. Microglia play several important functions in the brain, including homeostasis, synaptogenesis, and regulation of the blood-brain barrier integrity [60]. However, microglia activation and proliferation after acute brain injury or chronic neurodegenerative diseases can increase the phagocytosis of healthy neurons and release various neurotoxic proinflammatory molecules [61,62].
2.3.1. Alzheimer’s disease
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by progressive and irreversible cognitive decline with synaptic loss and atrophy starting typically from the hippocampus, a brain region important for learning and memory. Two major molecular hallmarks of AD are extracellular amyloid plaques formed by aggregates of amyloid-β peptides and intracellular neurofibrillary tangles consisting of aggregates of hyperphosphorylated tau protein. Several studies have provided evidence for increased oxidative stress due to ROS in patients with AD [63–65]. Elevated levels of amyloid-β aggregates has been associated with increased oxidation of lipids, proteins, and nucleic acids in the hippocampi and cortices of AD patients [63]. Subbarao and coworkers found increased peroxidation, as demonstrated by the formation of thiobarbituric acid-reactive products (TBARs) from oxygen-containing free radicals, in the cerebral cortices of AD patients by up to 50% when compared to patients without AD [64]. Application of amyloid-β peptides at pathological concentration increases intracellular peroxides by three-fold in neuronal cell lines in vitro [65].
Various cellular binding sites and processes have been suggested to be key players for oxidative stress in AD pathology. An up to 2.5 fold increase in the expression of the receptor for advanced glycation endproducts (RAGE) was observed in AD patients, implicating RAGE in AD pathogenesis [66]. Binding of amyloid-β peptide to RAGE has been shown to promote microglial activation, generate ROS, and induce cytotoxic cellular stress [66], whereas inhibition of RAGE blocks the accumulation of amyloid-β peptides and preserves neuronal function early in the progression of AD [67,68]. In addition, Bamberger et al. identified a complex of microglial membrane receptors (α6β1 integrin, CD36, and CD47) which interact with amyloid-β fibrils [69]. Engagement of these receptor complexes activates the intercellular tyrosine kinase-based signaling cascades, leading to the generation of proinflammatory molecules and ROS.[69] Furthermore, NADPH oxidase-dependent production of superoxide in the microglia has been suggested to contribute to AD pathogenesis [70,71].
2.3.2. Parkinson’s disease
Parkinson’s disease is a neurodegenerative disorder of dopaminergic neurons in the substantia nigra, which results in severe and progressive impairment in motor control [72]. The pathological hallmark of Parkinson’s diseases includes protein aggregates which accumulate as Lewy bodies in dopaminergic neurons [72]. Multiple studies implicate oxidative damage in the pathogenesis of Parkinson’s disease. For instance, the brain tissue with Parkinson’s disease exhibits increased levels of malondialdehyde and cholesterol lipid hydroperoxides, markers for lipid peroxidation [73,74] as well as 8-hydroxy-2-deeoxyguanosine, a marker for oxidative damage to the DNA [75].
Deficiency of complex I in the mitochondrial electron transport chain has also been linked to the pathogenesis of Parkinson’s disease. In particular, inhibition of complex I of the electron transport chain with either rotenone or 1-methyl-4-phenyl-1,2,36-tetrahydropyridine (MPTP) causes degeneration of dopaminergic neurons, a key neuropathological feature of Parkinson’s disease [76,77]. Furthermore, rotenone inhibition was shown to enhance ROS generation, protein aggregation, and oxidative damage [78].
2.3.3. Huntington’s disease
Huntington’s disease (HD) is an inheritable neurodegenerative disorder, which causes psychiatric disturbances, emotional problems, and the inability to control thoughts and movements. It is caused by the mutation of the Huntingtin gene (HTT). The mutated Huntingtin gene (mHTT) was reported to cause high Ca2+ permeabilization into the mitochondria, leading to damage of mitochondrial DNA, mitochondria dysfunction, and elevated production of ROS [79]. In addition, severe defects in complexes II and III and deficiency in complex IV within the electron transport chain of the mitochondria were found in human HD brain, which contributed to oxidative stress [80]. Furthermore, elevated oxidative damage of DNA, proteins, and phospholipids were found in areas of degeneration in the brain with HD [81,82]. Protein carbonyls, a marker of oxidative stress, were increased in human brain postmortem samples obtained from the striatum and cortex of patients with HD when compared to samples of healthy age- and sex-matched controls [83].
2.3.4. Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is clinically characterized by progressive weakness, atrophy and spasticity of muscle tissue due to the degeneration of motor neurons in the spinal cord, brainstem, and cortex. While most cases of ALS are sporadic and due to unknown causes, about 5–10% are familial and of those, ~25% are associated with mutations in the copper-zinc superoxide dismutase gene (SOD1). Mutant SOD1 was shown to increase the production of ROS, as evidenced from oxidative damage to lipids and proteins in the mitochondria [84]. Malondialdehyde, a marker of lipid peroxidation, was increased in the cerebral cortex of mutant SOD1 mice [85]. Levels of vitamin E and malondialdehyde (a measure of lipid oxidation), were also increased [86]. It was suggested that mutant SOD1 accumulates and aggregates in the outer mitochondrial membrane, which clogs the transport of proteins and results in mitochondrial dysfunction [87]. Further studies showed that even in sporadic ALS there is oxidation of DNA and lipids, which suggests that oxidative stress may be involved in all types of ALS [88,89].
2.3.5. Epilepsy
Epilepsy is a brain disorder characterized by recurrent and unpredictable seizures that can accompany multiple comorbid conditions including emotional and cognitive impairments. Both clinical and experimental evidence have supported the hypothesis that inflammation may play roles in the pathophysiology of epilepsy [90–92]. Mitochondrial dysfunction has been reported in epilepsy patients and various animal models of chronic epilepsy and acute seizures [93–96]. A decrease in mitochondrial complex I activity has been observed in the rat cerebral cortex during the acute phase of seizures [93]. As a consequence, enhanced ROS could contribute to neuronal injury following epileptic seizures [93].
2.3.6. Ischemic stroke
During ischemic stroke, a region of the brain is suddenly deprived of blood flow. Early reperfusion, or restoration of the blood supply, is crucial. In the acute phases of ischemic stroke (minutes to hours), ROS and proinflammatory mediators are rapidly released from the neutrophils and microglia in the injured tissues [97,98]. In the subacute phase (hours to days), ROS accumulates, and the excess ROS amplifies the inflammatory responses, which can result in neuronal death and the disruption of the blood-brain barrier [99]. In response to tissue damage following stroke, iron is often released due to the lysis of red blood cells. This greatly enhances the production of toxic hydroxyl radicals [100,101]. While reperfusion of the ischemic area is necessary for survival, studies have shown that reperfusion also leads to the generation of mitochondrial ROS due to the accumulation of succinate, a citric acid cycle intermediate [102]. Various biomarkers for oxidative stress in stroke are summarized by Cherubini and coworkers [103].
3. ROS-responsive drug delivery systems
ROS-responsive drug carriers in the form of nano- or micro-sized particles can be designed to release their payloads in response to the high ROS level through increased or burst release. The drug molecules loaded into these particles can be small compounds or biomacromolecules. These loaded molecules can either influence the pathways relevant to neuropathology or treat inflammation by downregulating inflammatory pathways or scavenging ROS. These molecules include antioxidants and anti-inflammatory drugs such as indomethacin, ibuprofen, dexamethasone, and epigallocatechin gallate as well as neurological disease-specific drugs such as simvastatin and resveratrol, amongst others [104–110].
In terms of particle design, there is an ever-growing toolkit of chemistries from which researchers can select. Particles can be functionalized with chemical groups that can react with ROS to produce a charge and hydrophilicity change, bond cleavage, or even catalyze further reaction. Overall, these reactions can result in particle swelling, particle disassembly, or enhanced diffusion of drug molecules out of the particle. Depending on the end goal for the particle, researchers may select the mechanism for the particles’ functionality and subsequent drug delivery. For example, if the patient requires long-term control over inflammation, it would be more feasible to inject nanoparticles that react to ROS by swelling and increasing the drug release progressively rather than particles that completely dissociate on ROS exposure. Alternatively, particles can be designed to generate oxygen gas by decomposing H2O2. The resulting oxygen gas acts as a force to drive fast drug discharge [111,112]. Any of these effects may be achieved through appropriate selection of chemical building blocks used for the particle assembly. In this section, we discuss the major chemistries used in the fabrication of ROS-responsive particles.
3.1. Sulfide groups
Sulfide groups within a polymer are oxidized to sulfoxides when reacted with a range of ROS, including H2O2. When this transition to sulfoxides occurs, the sulfur containing part of the polymer becomes more hydrophilic. Therefore, particles made with polymers containing sulfide groups dissemble or swell in the environment at which hydrogen peroxide (or ROS) is upregulated [113]. One of the most prominent examples of this mechanism is polypropylene sulfide nanoparticles [114,119–122]. Since polypropylene sulfide is hydrophobic before being oxidized, the polymer can form particles that can encapsulate hydrophobic drug molecules, including curcumin, dexamethasone, and fluorocoxib A. Therefore, oftentimes the polypropylene sulfide may serve as the core of the nanoparticles while an outer shell consisting of a hydrophilic polymer such as polyethylene glycol stabilizes the particle in aqueous media. As a reference, polymersomes formed from copolymers with blocks of polyethylene glycol on either end of the polypropylene sulfide could be dissolved in 10% v/v H2O2 after 10 hours. Alternatively, the polypropylene sulfide can be stabilized by crosslinking neighboring polymers with disulfide bridge interactions after exposing pre-formed polypropylene sulfide particles to air [114]. Dissolution of these particles occurs over around a slightly longer time of 24 hours while being exposed to 10% v/v H2O2 because of the densely packed structure of the particles after air-drying.
These pure polypropylene sulfide nanoparticles were utilized by Mercazini et al. to mitigate inflammation during electrode-brain implantation using a rat model [121]. The 100 nm-diameter nanoparticles encapsulated dexamethasone (an antioxidant) were incorporated within a polyethylene oxide-coating layer that assists immobilization of the nanoparticles onto the surface of a flexible microelectrode. The loaded antioxidant was slowly released over the course of 9 days. As a consequence, the neuronal tissue integrated more seamlessly with the nanoparticle-coated electrodes over the course of one month. The result was supported by less inflammation in the tissue according to histology as well as less impedance measured by the electrode itself compared to the control containing the particle coating without loaded anti-oxidants.
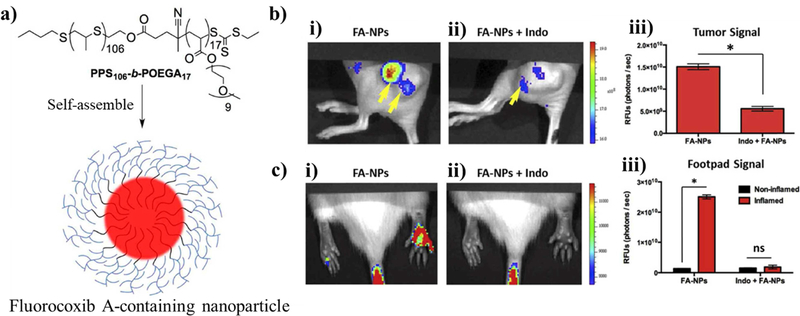
While few studies have used ROS-responsive polypropylene sulfide particles to treat neurodegenerative disorders, much can be learned for future delivery system design by surveying what has currently been done using these particles in other disease treatments complicated by overproduced ROS. For example, in a study reported by Uddin et al., poly(propylene sulfide)106-b-poly[oligo(ethylene glycol)9 methyl ether acrylate]17 nanoparticles with ~80 nm diameter were loaded with fluorocoxib A, a fluorescent inhibitor of the enzyme cyclooxygenase-2 which is overexpressed in sites of inflammation [123]. In an assay using Nile red, the particles started releasing the dye when exposed to 100 mM H2O2, but only showed full release of the payload using 1,000 mM H2O2 over 3 days. Normally, fluorocoxib A has poor water solubility, but encapsulating it within an ROS-responsive nanoparticle allowed for highly specific staining of inflammatory sites in mice. Inflammation was triggered in tumor-presenting mice using carrageenan (Figure 2). Therefore, since it is the inflammation that is being targeted in both of these cases rather than a specific disease, the particles should also respond to neurological inflammation.
Figure 2. Example particle utilizing sulfides groups as a ROS-responsive hydrophilicity switch.
(a) Schematic of the chemical structure of the diblock copolymer poly(propylene sulfide)106-b-poly[oligo(ethylene glycol)9 methyl ether acrylate]17 used to assemble a particle encapsulating Fluorocoxib A within its hydrophobic core. The particles were formed by the bulk solvent evaporation method. (b) Since the sulfide units preferentially go through a hydrophilic to hydrophobic transition at sites of inflammation, the particles selectively disassembled at sites of tumors (artificially created with tumor xenografts) releasing high levels of ROS as confirmed by fluorescent images within a mouse model. Additionally, the fluorescence of the images was reduced on the addition of indomethacin, confirming the Fluorocoxib A had bound to COX-2, which is overexpressed during inflammation. (c) The particle response to ROS was further quantified for carrageenan-induced inflamed and non-inflamed mice, using fluorescence and the indomethacin blocker. Reprinted (adapted) with permission from [110].Copyright 2016 Elsevier.
Other than polypropylene sulfide particles, nanoparticles can also incorporate ROS-responsive sulfide groups into their design [124]. For example, Cheng et al. coated the surface of mesoporous silica nanoparticles with phenyl sulfide groups by using phenyl sulfide (3-Aminopropyl)triethoxysilane (APTES) which can bind with silica [125]. The resulting 310 nm-diameter particles with a pore diameter of 3.4 nm were used to encapsulate and retain doxorubicin within the particle since external water is repelled by the hydrophobic pores under physiological conditions. However, in elevated H2O2 concentrations as low as 50 μM and up to 500 μM , the sulfide groups became oxidized, and therefore more hydrophilic, allowing water to wet the inner pores and release the encapsulated drug. Co-culture with normal human umbilical vein endothelial and MCF-7 breast cancer cells in vitro resulted in internalization of the particles. Interestingly, only cancerous MCF-7 cells produced enough internal ROS so that once the particles were endocytosed, they released doxorubicin. The doxorubicin release decreased the viability of cancer cells by 25%, but caused no change in the healthy cells. Since, ROS was the main trigger for this wettability switch within the particle pores, it would be interesting to see how similar particles respond to neuroinflammation that is often found in various neurological injuries, degeneration, and disorders.
Sulfides can also be combined with metal ions such as zinc to form ionic ZnS particles by mixing salts of sodium sulfide and zinc chloride in aqueous solution and, in turn, precipitating particles of around 10 nm-diameter [115–117,126]. Such particles release zinc into the surrounding solution when the sulfide is oxidized by ROS. Liu et al. embedded these ZnS nanoparticles within 400 nm-diameter poly(N-isopropylacrylamide-co-acrylic acid) microgels [118]. These microgels were mixed with a zinc-reactive fluorescent dye, fluozin-3, to detection inflammation, glucose and cholesterol. When the sulfides were oxidized, the Zn was released from the microgels and reacted with the surrounding fluozin-3 to create a fluorescent signal.
3.2. Mono-selenide and telluride-containing groups
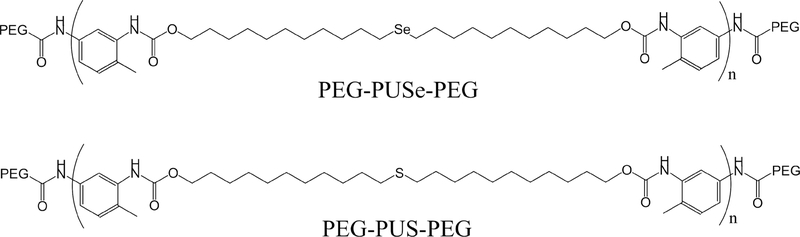
Particles containing Se and Te groups function in much the same way as sulfides, in that they undergo oxidization in response to ROS and change their hydrophilicity [127]. An important distinction between Se/Te and sulfides is that groups containing Se and Te atoms are more readily oxidized by ROS compared to sulfides. Therefore, the concentration of ROS required to cause these groups to change their oxidation state is substantially less than sulfides [128]. For example, in a solution of 0.1 % v/v H2O2, almost all the selenide groups are oxidized in a block copolymer of polyethylene glycol and poly(di-(1-hydroxylundecyl) selenide)-copolyethylene glycol (PEG-PUSe-PEG) according to X-ray photoelectron spectroscopy (XPS), whereas for the identical polymer containing poly(di-(1-hydroxylundecyl) sulfide as the middle group (PEG-PUS-PEG) only showed oxidation of ~30% of sulfide (Scheme 1) [129]. This difference in oxidation translates to a difference in drug release, since, using the same particles, the selenide-containing particle released ~72% of its doxorubicin cargos in 10 hours while the
Scheme 1. Molecular structure of PEG-PUSe-PEG and PEG-PUS-PEG.
sulfide-containing particle only released ~41%.
In addition to the amount of oxidation, particles containing selenide groups can be oxidized more quickly than equivalent particles containing sulfide groups, causing more rapid changes in the particle structure. This difference is highlighted in a study by Yu et al. where they synthesized three distinct poly(ε-caprolactone) (PCL)-based ~30 nm-diameter nanoparticles containing pendant functional groups of either thioether, phenyl sulfide or phenyl selenide groups [130]. They demonstrated that the oxidation rate for the nanoparticles in 0.5 mM H2O2 was much higher for the selenide-containing particle, allowing it to release ~30% of a fluorescent Nile red marker in 4 hours, while the sulfide-containing particles took ~15 hours to release the same amount.
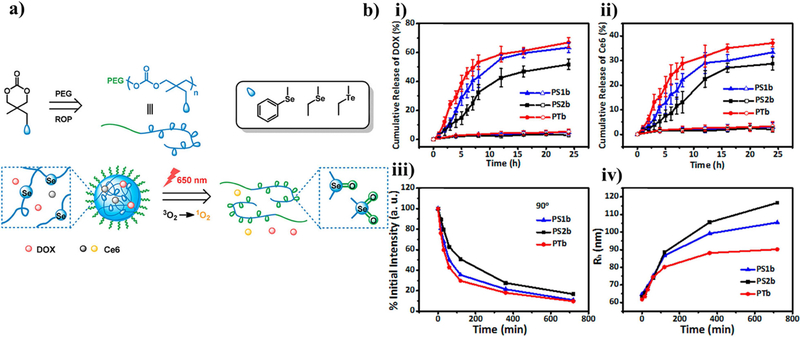
Tellurium has an even lower electronegativity than selenium, therefore it has a further increased sensitivity to ROS, albeit the difference is less extreme than the difference between selenium and sulfur. These differences in ROS sensitivity were quantified for differences in particle swelling and dissociation by using nanoparticles formed with block co-polymers of poly(ethylene glycol) and polycarbonate containing either selenide or telluride pendant groups. The polymers were synthesized via ring-opening polymerization (Figure 3a) [131]. The resulting 34 to 38 nm-diameter particles could be further sensitized by Ce6/light. Even without the photosensitizer, the particles could swell and release their drug in response to 0.1 mM H2O2, showing the most release in the particles containing Te, followed by Se, then phenyl Se. This result confirmed the researchers’ hypothesis that Te is more sensitive to oxidation than Se, and steric hinderance with the addition of a phenyl group to Se would further decrease its sensitivity to oxidation. When irradiated with light (650 nm, 100 mW/cm2), the sensitivity of the functional groups to oxidation by ROS was increased, leading to quicker drug release (Figure 3b). This could be useful in cases where the physician wants more control over the release rate of the drug in their patients by choosing whether or not to enhance the drug release rate with light.
Figure 3. Example particle utilizing selenium and tellurium-containing functional groups as a ROS-responsive hydrophilicity switch.
(a) Schematic of the chemical structure of the copolymers of PEG113 linked to polymers of either 5-(ethylselenyl)methyl-5-methyl-1,3-dioxan-2-one (PS1b), 5-methyl-5-(phenylselenyl)methyl-1,3-dioxan-2-one (PS2b), and 5-(ethyltellanyl)methyl-5-methyl-1,3-dioxan-2-one (PTb) assembled by the solvent evaporation method. ROS created by 650 nm light would oxidize the selenium or tellurium in the particles, causing a hydrophilic switch and subsequent disassembly. (b) The release of the drug doxorubicin and photosensitizer Ce6 from the particles in response to light (solid) or without light (open) was quantified by UV-vis spectroscopy at various time points. Additionally, the scattered light intensity and radii of the particles were measured to show the average dissolution of particles over time. Reprinted (adapted) with permission from [118]. Copyright 2018 American Chemical Society.
3.3. Diselenide groups
A related particle functionality is the use of diselenide bonds as an ROS-responsive unit. Rather than just changing the group hydrophilicity in response to ROS like in mono selenium-containing groups, oxidation of diselenide groups causes them to cleave after being converted into seleninic acid. Another marked difference in particles formed in this method instead of mono-selenium is that the bond cleavage of diselenide bonds causes a burst release of the particle payload.
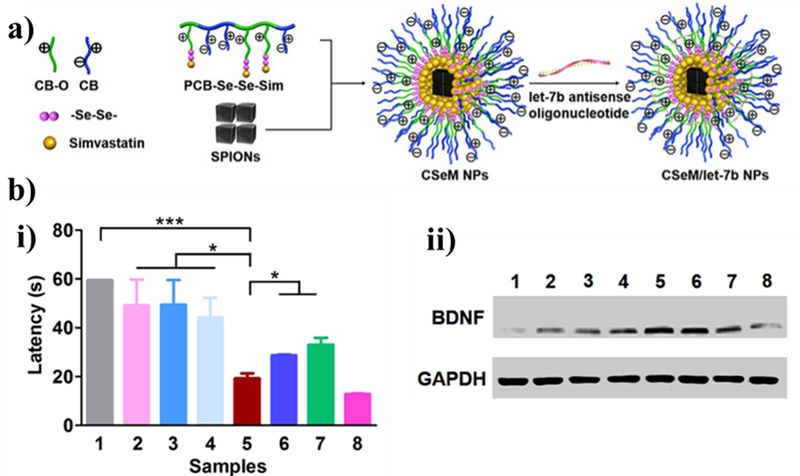
This strategy was used by Li et al. to treat Alzheimer disease model mice in vivo and inflammatory neural stem cells in vitro (Figure 4) [109]. This particle utilized two drugs simultaneously, namely simvastatin, which upregulates production of brain-derived neurotropic factor (BDNF) and improves spatial learning,[132] and lethal-7b antisense oligonucleotide (let-7b), which promotes neural stem cell differentiation. First, poly(carboxybetaine) was linked to simvastatin with a diselenide bond by reacting the polymer and simvastatin with disodium diselenide in a single batch then purified. The resulting polymer could spontaneously form 150 nm-diameter particles in solution at concentrations above 40 μg/mL and then encapsulate superparamagnetic iron oxide nanocubes (for tracking) and let-7b. Interestingly, neural stem cells treated with these particles and then implanted into Alzheimer disease model mice caused the mice to show remarkable improvement in memory using a Morris water maze test and in BDNF production.
Figure 4. Example particle utilizing diselenide bonds as a ROS-responsive cleavable site.
(a) Schematic of the chemical structure of the poly(carboxybetaine)-SeSe-simvastin nanoparticles containing superparamagnetic iron oxide nanocubes (SPIONs) for tracking and the antisense oligonucleotide let-7b (CSeM/let-7b NP). (b) Experimental results following injection of neuronal stem cells (NSCs) incubated with the nanoparticles for 4 hours to treat Alzheimer disease model mice (2xTg-AD). (i) The escape latency of the mice to complete a Morris water maze after 5 days of training with the maze once a day. (ii) Western blot of brain-derived neurotropic factor and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control after 7 days. Conditions: (1) saline, (2) NSCs alone, (3) let-7b antisense oligonucleotide-NSCs, (4) simvastatin-NSCs, (5) CSeM/let-7b NP-NSCs, (6) CH/M(encapsulated simvastin)/let-7b NP-NSCs, (7) CSeM/Control NP-NSCs, (8) wild. Reprinted (adapted) with permission from [96]. Copyright 2018 American Chemical Society.
Researchers have also formed particles using the diselenide ROS-responsive group by modifying a block copolymer of polyethylene glycol-b-polymethylacrylic acid (PEG-b-PMMA) particles by reacting the methacrylate groups with di-(4,1-hydroxybenzylene) diselenide to form PEG-b-PBSe [133]. The resulting phenyl diselenide groups in the core of the 130 nm nanoparticles were then cross-linked with neighboring polymer molecules using light (25 W, incandescent) to further stabilize the particles. The doxorubicin-loaded particles showed increased drug release when exposed to either concentrations as low as 100 μM H2O2 and complete release within 24 hours using 30 mM H2O2. Diselenide bonds can be reduced to selenol via reduction by glutathione [134]. Therefore, the particles could also release their entire cargo after 24 hours using 10 mM glutathione. The particles were then shown to degrade and deliver doxorubicin to kill cells exhibiting high ROS after being endocytosed while maintaining viability of healthy cells.
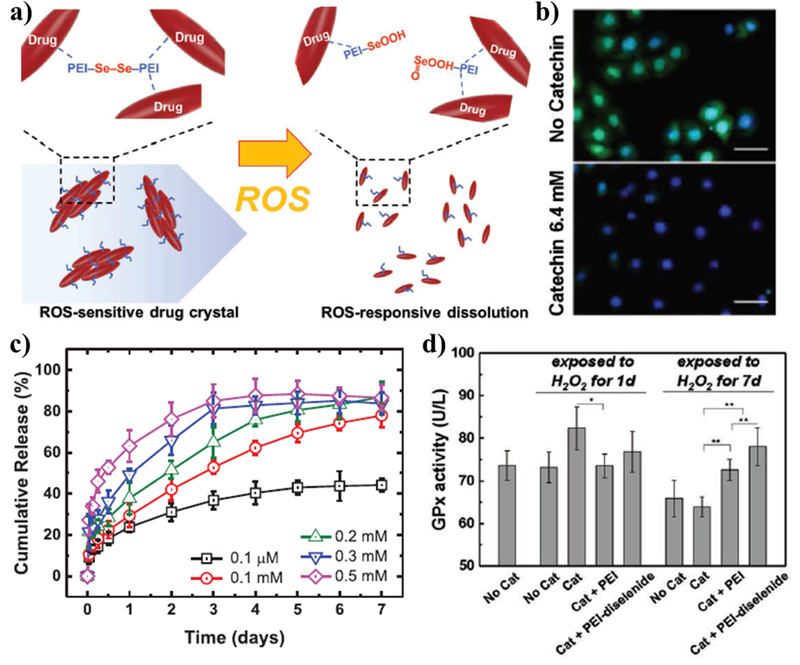
Diselenide linkers were also used to control the dissolution rate of antioxidizing catechin crystals so that they would dissolve in response to H2O2 [135]. Catechin is a polyphenolic flavonoid natural antioxidant that scavenges free radicals.[182] Kim et al. formed these ROS-responsive micro-crystals by crystalizing catechin in the presence of polyethyleneimine linked by diselenide bonds (Figure 5). The polyethyleneimine could form hydrogen bonds with the catechin crystals while the diselenide bonds were shown to cleave and release the drug over the course of 1 week in response to 0.1 mM H2O2 while a concentration of 0.1 μM only released 40% of the crystals after 1 week. These diselenide-stabilized crystals could prevent oxidative stress, as monitored by the heart rate of Daphnia magna in a solution of 0.5 mM H2O2. This was due to the progressive release of the catechin over a week, whereas free catechin could be metabolized quickly since most of the payload is released on the first day. Since catechin has been shown to mitigate inflammation due to cerebral ischemia,[183] this strategy could also be used to stabilize neuroinflammation.
Figure 5. Example drug crystal utilizing diselenide bonds as a ROS-responsive cleavable site.
(a) Schematic of the catechin drug crystals coated by polyethyleneimine (PEI) and crosslinked by ROS-reactive diselenide bonds. (b) Fluorescent images of C166 cells incubated in 0.2 mM H2O2 with oxidative stress marker (cellROX reagent) stained in green and cell nuclei (4′,6-diamidino-2-Phenylindole (DAPI)) stained in blue. (c) The amount of catechin released in various concentrations of H2O2 as measured by absorbance at 260 nm. (d) The glutathione peroxidase activity of C166 in response to incubation in solutions containing 0.2 mM H2O2 and either catechin crystals (Cat), PEI-coated crystals (Cat + PEI), or diselenide linked crystals (Cat + PEI-diselenide). Reprinted (adapted) with permission from [122]. Copyright 2019 John Wiley and Sons.
3.4. Thioketal bond
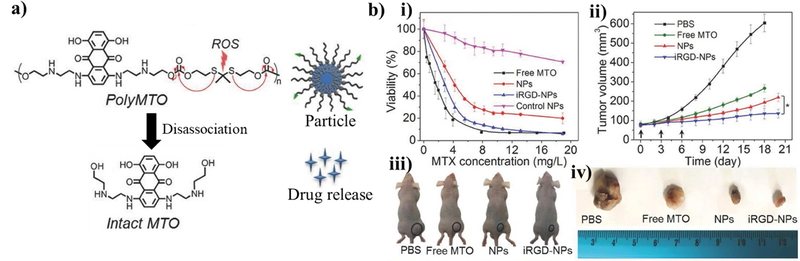
Thioketal bonds undergo cleavage in response to oxidation by ROS. Therefore, molecules linked with thioketal bonds can be used as poly-prodrugs or serve as the shell of burstrelease nanoparticles [136]. Thioketal bonds are also stable in acidic and basic solution. For example, the poly prodrug, poly(mitoxantrone), was formed by linking individual mitoxantrones, an anti-cancer drug, with thioketal linkers (Figure 6) [137]. The resulting poly-prodrug was encapsulated in 1,2-distearoyl-sn-glycerol-3-phosphoethanolamine-N-[methoxy(polyethylene glycol) (DSPE-PEG) nanoparticles that were functionalized with Arg-Gly-Asp (RGD) to enhance particle homing to tumors. Increased ROS levels cleaved the thioketal bonds, which released the individual mitoxantrone drug molecules. While this approach was used to treat tumors, an interesting future approach may be to deliver particle-encapsulated poly-prodrugs linked by thioketal bonds to sites of inflammation in the brain or nervous system.
Figure 6. Example particle utilizing thioketal bonds as a ROS-responsive cleavable site.
(a) Schematic of the chemical structure of the mitoxantrone (MTO) polymer made by linking individual MTO monomers with thioketal bonds to form the inner core of the particle. The outer shell consisted of 1,2-distearoylsn- glycero- 3- phosphoethanolamine- N- [methoxy(polyethylene glycol)- 3000] attached with the cell-homing peptide containing RGD sequence. The individual drug molecules could then be released from within the particle when the thioketal bonds are broken in response to surrounding ROS. (b) These particles were shown to target and eliminate tumor cells according to a viability assay and assessment of tumor volume in a mouse model. Reprinted (adapted) with permission from [124]. Copyright 2017 John Wiley and Sons.
The thioketal linkages can also be incorporated into the design of the particle itself so that the particle dissembles in response to ROS.[138,139] In such a study, Sun et al. synthesized a diblock copolymer of polyethylene glycol (PEG) and poly(ε-caprolactone) with a thioketal linkage separating the two blocks [140]. In this case the thioketal linkage’s purpose was two-fold: (1) the particle would dissemble and release the payload in response to ROS and (2) hydrophobic drug molecules could interact with the thioketal group via π-π stacking, enhancing the particle loading capacity. This approach resulted in fast cellular uptake and release of the drug cargo in cancer cells in response to the increased H2O2.
In some cases, the ROS concentration cleaves the thioketal bond at an insufficient rate. In such cases, researchers have combined photosensitizers with therapeutic agents for enhanced drug release rates and visualization [141]. This may be useful in persistent sites of inflammation. For example, PEGylated hyperbranched polyphosphates linked with thioketal groups showed enhanced release of doxorubicin when they encapsulated photosensitizer chlorin e6 and were irradiated by 660 nm light [142].
3.5. Boronic ester
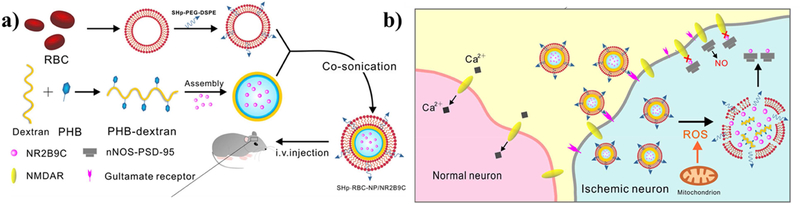
Much like the thioketal bond, boronic esters undergo cleavage due to oxidation[184]. Therefore, nanoparticles utilizing this bond are well suited for burst release of drugs in response to inflammation [143,144,147–149]. In fact, this chemistry has also been used to treat strokes, which have elevated amounts of ROS. Lv et al. engineered dextran nanoparticles modified with a boronic ester as a ROS-reactive component to encapsulate a neuroprotective agent NR2B9C (Figure 7) [150]. This particle was assembled by co-sonication of red blood cell membranes modified with a stroke-homing peptide (CLEVSRKNC) with boronic ester-modified dextran to form a 190 nm-diameter particle that could localize to stroke sites. This peptide specifically targets apoptotic neuronal cells at the site of focal cerebral ischemia in a rat model [185]. Interestingly, the particles could cross the blood-brain barrier compromised by ischemic injury induced by middle cerebral artery occlusion in a rat. Treated rats also had significantly less neurological deficits due to reperfusion, while the particle itself was not toxic according to a preliminary in vivo safety test.
Figure 7. Example particle utilizing boronic ester bonds as a ROS-responsive bond cleavage site.
(a) Schematic of the multilayered stroke-targeting nanoparticles made from an outer shell consisting of a red blood cell membrane modified with a stroke homing peptide (CLEVSRKNC), an inner shell consisting of the ROS-responsive dextran modified with boronic esters encapsulating a neuroprotective agent (NR2B9C). (b) Outline of the activity of the particle to use the stroke homing peptide, which targets glutamate receptors, to guide the particle to ischemic neurons. Following internalization, the ROS-cleavable boronic ester-modified dextran cell disassembles and releases the NR2B9C which interactions with N-methyl-d-aspartate receptors (NMDAR) to prevent toxic nitric oxide production within the cell. Reprinted (adapted) with permission from [137]. Copyright 2018 American Chemical Society.
In addition to the blood-brain barrier, there are additional barriers for DNA-based gene therapy using nanoparticles for treatment of neurological disease and inflammation, such as lysosomal trapping and serum instability. To this end, Xiang et al. surrounded a ROS-responsive, poly[(2-acryloyl)ethyl(p-boronic acid benzyl) diethylammonium bromide (B-PDEAEA) particle with a cationic lipid layer that overcomes these barriers, such as N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium/cholesterol (DOTAP/Chol) and 3β-[N-(N′,N′-dimethylaminoethane)-carbamoyl]cholesterol/dioleoylphosphatidylethanolamine (DC-Chol/DOPE) cationic lipid layers [151]. These cationic lipid layers aid in gene transfection and have good serum resistance. Therefore, the resulting particle could enter the cell, then dissemble the DNA/boronic ester-containing polymer polyplex in response to around 200 μM H2O2. The DNA is then released and could be internalized by the nucleus and undergo transcription. The bi-layered particles led to successful gene expression of a tumor suicide gene in an in vivo mouse model.
Boronic esters have also been applied to the design of particles to treat Alzheimer disease [152]. Yang et al. used boronic esters to link glucose as pore caps that would detach in response to ROS on mesoporous silica particles and release the loaded metal chelator, clioquinol. Clioquinol can bind Cu2+, a metal ion that induces amyloid-β aggregation. The mesoporous silica particles were also coated with gold nanoparticles, which mitigate amyloid-β fibrillization [153]. The particles were used to prevent neuronal cell death in response to amyloid-β complexes in vitro. The particles also brought the ROS content of these cells back to normal levels after an initial two-fold increase in ROS caused by the amyloid-β complexes.
Zhang et al. used cleavable boronic ester bonds to make a Nile red-loaded particle formed from a PEG-lipid conjugate that forms micelles [154]. These micelles showed good cytocompatibility and could be internalized by breast cancer cells. Interestingly, these micelles released the drug more rapidly as the concentration of H2O2 was increased from 10 – 200 mM. It is important to note that these concentrations are much higher than physiological concentrations that are closer to 10–50 μM during ROS signaling and up to 1 mM during oxidative stress [186,187].
Lux et al. formed benzylic ether boronic ester pendant backboned polymers that formed particles responsive to physiological ranges of H2O2 [145]. These particles could release 50% of their payload within 10 hours at 1 mM H2O2, but less than 20% at 0.1 mM H2O2. As a proof of concept, these particles, loaded with fluorescein diacetate, were able to selectively stain only active inflammatory neutrophils.
A hierarchical design is also useful for making particles that react to two different stimuli. Regions of inflammation in the brain and other organs commonly exhibit lower pH than normal around 5.5 pH[188,189]. Therefore, Feng et al. synthesized chemically modified cyclodextrins with boronic esters or acetal groups to make them responsive to ROS or pH, respectively[146]. These cyclodextrins can form complexes with drug molecules only in their unreacted forms and release their cargos once reacted with H2O2 or H+ ions. The cyclodextrins were enclosed in DSPE-PEG or Lecithin nanoparticles to facilitate delivery. These particles responded to physiologically relevant inflammatory conditions of pH of 5 and H2O2 concentration of 1 mM and successfully treated blood vessels exhibiting neointimal hyperplasia in a rat model.
3.6. Polyoxalate nanoparticles
Nanoparticles formed from polyoxalate can readily degrade in response to both H2O2 and water into biocompatible products [155–157]. Therefore, particles using this chemistry for inflammation treatment would be best used in locations where fast degradation is necessary because even in the absence of ROS, the particles will degrade. With regards to neurological inflammatory diseases, polyoxalate polymerized with vanillyl alcohol has attracted much attention recently [158–160]. Vanillyl alcohol is a natural product found in Gastrodia elata that has anti-inflammatory effects and has been used in treating brain ischemic injury [160]. Lee et al. made such particles with 500 nm-diameter in aqueous conditions despite the half-life of the particles being 36 hours [161]. These particles were shown to treat various types of inflammation including hind limb ischemia/reperfusion and hepatic inflammation in a mouse model. Another important highlight from this study was that since polyoxalate degrades quickly and into minimally toxic products, there was no harmful side-effects in any of the major organs tested.
3.7. MnO2 catalyst-based nanoparticles
Rather than incorporating cleavable or hydrophilicity switching functional groups into the nanoparticle-forming polymer, researchers have also began utilizing ROS-responsive catalysts such as MnO2 as the ROS-responsive component. These particles convert H2O2 into water and oxygen gas. So, they can be used to both decrease local ROS concentration as well as treat hypoxia in sites of inflammation [162,163]. Periera et al. showed that particles made from MnO2 and embedded in collagen can act as ROS-scavenging reactors that directly influence apoptotic pathways in inflamed cells [164]. However, it should be noted that direct injection of MnO2 nanoparticles at 87 μg/μL into the brain results in neuronal impairment and can increase inflammation [165,166]. Therefore, careful attention should be paid to the dosage and toxicity of related particles.
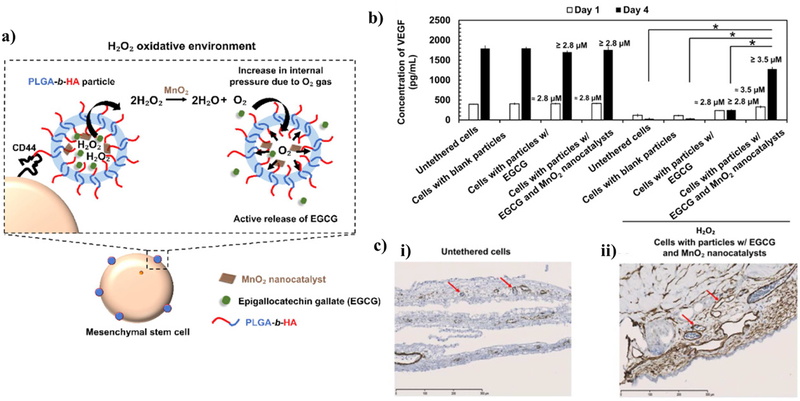
Recently, Seo et al, utilized MnO2 to assemble a particle that release drug cargos continuously in an environment with elevated levels of H2O2 [112]. In this study, water-dispersible MnO2 in a form of nano-sized sheets (10 nm length and 1 nm width) were incorporated into a drug-carrying poly(lactic-co-glycolic acid) (PLGA)-based particles at an encapsulation efficiency of 10% (about 4.35 μg nanosheets /1 mg PLGA). In media containing 0.2 mM H2O2, the MnO2 nanorods in the particle catalyzed decomposition of H2O2 up-taken by particles to produce O2 gas that acts as force to release antioxidizing epigallocatechin gallate (EGCG) cargo continuously. The ROS-responsive particles were effective to retain viability of brain cells in a rat brain slice explant exposed to an oxidative environment.
These catalytic MnO2 nanocatalysts were further used to promote release of EGCG from the particles tethered to adipose-derived mesenchymal stem cells and retain viability and secretory activity of stem cells. Teo et al. assembled nanoparticles of poly(d,l-lactide-co-glycolide)-hyaluronic acid loaded with MnO2 nanocatalysts and antioxidizing EGCG (Figure 8) [111]. The hyaluronic acid blocks of particles enabled the particles to adhere to CD44-expressing mesenchymal stem cells via specific CD44-hyaluronic acid bonds. In 200 μM H2O2-containing media, the particle released 1.5-fold larger EGCG cargos within the first hour. As a consequence, even in an oxidative environment, mesenchymal stem cells tethered with the anti-oxidizing nanoparticles exhibited a minimal increase in intracellular oxidative stress and secreted proangiogenic factors for revascularization while untreated stem cells lost viability. Since mesenchymal stem cells are being studied as a medicine to regenerate neural networks in inflammatory sites of neural injuries or degeneration, such ROS-responsive particles would potentially serve to improve the quality of stem cell therapies [190–192].
Figure 8. Example particle utilizing MnO2 nanocatalysts that catalyze the degradation of H2O2.
(a) Schematic of the poly(d,l-lactide-co-glycolide)-hyaluronic acid (PLGA-b-HA) particle that encapsulates MnO2 and an antioxidant. The particle is tethered to mesenchymal stem cells through the binding of hyaluronic acid and cell receptor CD44. When external H2O2 is catalyzed into O2 gas, the gas generates pressure that expels the payload. (b) The quantified secretion activity of stem cells as measured by VEGF production according to an enzyme-linked immunosorbent assay (ELISA) with and without exposure to 200 μM H2O2. (c) Histological stains of blood vessel formation enhanced by the stimulated stem cells using a chick chorioallantoic membrane (CAM) assay. Reprinted (adapted) with permission from [98]. Copyright 2019 Elsevier.
3.8. Cerium and ROS-scavenging particles
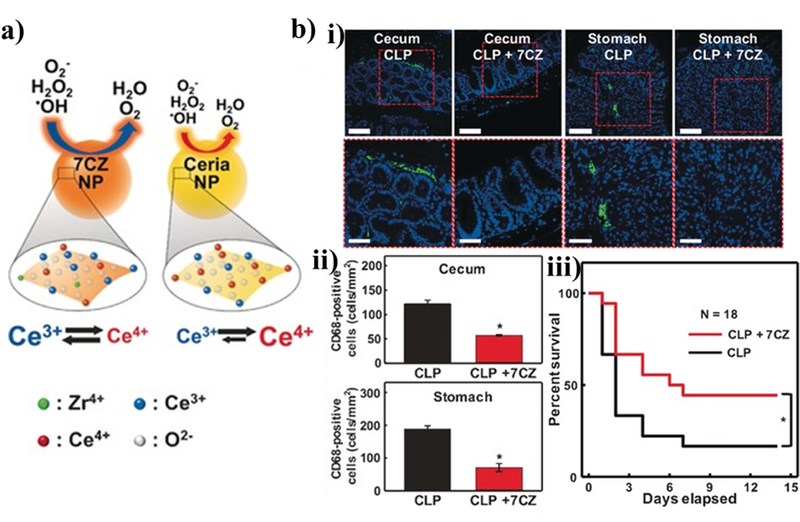
Rather than reacting with ROS, followed by subsequent dissociation or swelling, cerium (Ce) (III) nanoparticles have substantial utility in mitigating the local concentration by ROS. ROS react with the nanoparticles by oxidizing to Ce (IV) and scavenging stray ROS. These particles can be simply formed by precipitating out from cerium (III) acetate hydrate in aqueous solution with sonication. Since cerium oxide downregulates inflammatory biochemical pathways by decreasing lipopolysaccharide (LPS)-mediated cytokine release, Ce-based particles may useful in combination with other strategies to dampen ROS production [167,168]. In fact, Soh et al. used ceria-zirconia nanoparticles for sepsis treatment and combat dysregulated inflammation (Figure 9) [169]. These particles reduced ROS production during inflammation in a rat model which resulted in less immune cell recruitment and prolonged inflammation.
Figure 9. Example particle utilizing cerium as an ROS scavenger.
(a) Schematic of how the cerium containing particles are oxidized to scavenge excessive surrounding ROS to prevent cellular damage. The particles containing zirconia have a higher Ce (III) to Ce(IV) ratio than typical ceria nanoparticles and therefore are more readily oxidized and scavenge ROS more effectively. (b) The particles were used to treat an inflammation model in the cecum and stomach. CD68 positive cells, which are innate immune cells, were stained in green to assess the magnitude of the inflammatory response in a cecal ligation and puncture-induced bacteremia mouse model. In addition, the overall survival of the mice after infection and treatment was assessed. Reprinted (adapted) with permission from [172]. Copyright 2017 John Wiley and Sons.
Cerium-based nanoparticles have also been specifically used to reduce ROS generated by neuronal inflammation [170,171]. For example, Cimini et al. developed an amyloid-β-targeting nanoparticle to treat Alzheimer’s disease [172]. This was done with amine-functionalized cerium nanoparticles with a diameter of 3–5 nm. Bifunctional polyethylene glycol molecules were tethered onto the particles. Then, the other end of the polyethylene glycol chains were conjugated with anti-amyloid-β antibodies. The resulting particles significantly increased the survival of human neurons that were incubated with amyloid-β aggregates. The researchers propose that the particles downregulate the excessive production of an immature form of brain-derived neurotrophic factor that is typically produced by cells exposed to amyloid-β.
3.9. Other ROS-reactive chemistries
Oxidation is a common type of chemical reaction. Therefore, there are many chemistries that exhibit ROS-responsiveness. Most of these chemistries show similar reactivity: a functional group is oxidized by ROS, resulting in the particle dissociating and releasing its cargo. For instance, amino acrylate groups are cleaved via oxidation by ROS to release drug cargos like doxorubicin or DNA [176–178]. However, since amino acrylate groups are relatively stable compared to the aforementioned groups, additional ROS such as singlet oxygen are generated in solution by laser light to promote bond oxidation and cleavage. Rao et al. used an aminoacrylate-modified gadolinium-tetraazacyclododecanetetraacetic acid complex (Gd-DOTA) on the surface of the pores of mesoporous silica particles. Doxorubicin molecules encapsulated within particle were only released once the aminoacrylate group was cleaved by singlet oxygen generated by tissue-penetrating low energy light (660 nm) incident on polyethylene glycol-conjugated photosensitizer, chlorin e6. The Gd-DOTA complexed on the particles also served as a contrast agent for magnetic resonance imaging.
Also, melanin, a naturally occurring biopolymer associated with skin protection from UV light, can be used to form ROS-scavenging nanoparticles. 160 nm-diameter melanin-dopamine particles can be generated by allowing the dopamine component to self-polymerize in a solution of melanin in water, ethanol, and ammonia, encapsulating the melanin within the particles.[193] These particles were further coated with polyethylene glycol for better physiological stability by reacting the catechol groups in melanin with amine-terminated polyethylene glycol. The resulting polyethylene glycol-b-melanin particles were used as agents to scavenge ROS and active nitrogen species. As a result, Neuro 2A cells that were incubated with antimycin were protected against oxidative stress in vitro. The particles were further tested against the ischemic brain in a mouse model in vivo, and significantly reduced the infarct area [194].
Poly(vinylferrocene) or poly(ferrocenylsilane) particles that selectively swell when oxidized by H2O2 have been used for H2O2-responsive drug release [173,174]. This is because the ferrocene can convert from an uncharged to charged form when oxidized. Once oxidized, the particles become hydrophilic [175]. The 240 nm-diameter particles were formed by precipitating the poly(vinylferrocene)-b-poly(methyl methacrylate) dissolved in dichloromethane in the aqueous solution of sodium dodecyl sulfate. The resulting particles could swell in response to oxidation by a solution of 35% H2O2 and showed a more than two-fold increase in release of pyrene.
Similar to the abovementioned work on MnO2, it has been found that Prussian blue nanoparticles can catalyze the breakdown of H2O2 into oxygen gas and water. Prussian blue nanoparticles were made by injecting a K4[Fe(CN)6] solution into an aqueous Prussian blue and polyvinylpyrrolidone mixture. These particles were used as a magnetic resonance (MR) imaging contrast agent that enhances MR signal in response to ROS-producing inflammation [195]. Since oxygen generated from the catalysis of H2O2 is paramagnetic, sites of inflammation result in a greater contrast in the MR images [196]. Remarkably, the nanoparticles showed a detectable change in signal with as low H2O2 content as 5 μM . These particles were further utilized in vivo to detect inflammation in the liver of a mouse caused by lipopolysaccharide (LPS) treatment.
Another functional group that can be incorporated to make ROS-responsive nanoparticles is polylactic acid. Individual monomer units in polylactic acid can be cleaved by a variety of ROS, resulting in polymer degradation [197]. Shen et al. coated mesoporous silica nanoparticles with polylactic acid to form a ROS-degradable layer that prevents drug release when there is insufficient ROS [179]. These particles were further coated with a low-density lipoprotein receptor-binding peptide (Ac-[cMPRLRGC]c-NH2) that could aid particles in crossing the blood-brain barrier through receptor-mediated transcytosis [110]. By loading the particles with resveratrol, an antioxidant and neuroprotective drug, the researchers tested the particles for migration, drug release, and inflammation response using an in vitro rat blood-brain barrier model. The particles successfully normalized the inflammation response of microglia activated by lipopolysaccharide treatment.
The polysaccharide, hyaluronic acid, has also been used in ROS-responsive nanoparticle design [180]. Hyaluronic acid is degradable in the presence of ROS by oxidation of the ester linkages between monomer units [198]. For example, Lee et al. immobilized dopamine-modified hyaluronic acid onto the surface of gold nanoparticles [181]. The hyaluronic units were labeled with fluorescein molecules so that the particles could act as ROS-detecting particles via fluorescence. The particles reached their maximum fluorescence as a result of H2O2 concentrations of around 10 μM . These were used to detect lipopolysaccharide-induced inflammation in macrophage cells.
4. Current limitations and future prospects
While there are several examples of ROS-responsive drug delivery systems that specifically target neurodegenerative diseases [109,150,170,171,194], most current examples of ROS-responsive drug delivery systems have either simply been in vitro proof of concept designs [112,121,152,164,172,179] or been used to treat inflammation caused by cancerous cells. Therefore, there is a need for more research on ROS-responsive materials specifically tailored to treat neurodegenerative diseases. Fortunately, many neurodegenerative diseases and cancer share characteristics such as oxidative stress and leaky vasculature. To target neurodegenerative diseases more specifically, drug-carrying biomaterials could be engineered to target biomarkers of Alzheimer’s disease such as tau-protein, amyloid β, sulphatides, isoprostanes, and homocysteine [199] by presenting targeting ligands like the stroke homing peptide used by Lv et al[150]. Such targeting ligands could be peptides, proteins, or antibodies that are specific to a particular disease.
In addition, more research needs to be done on loading ROS-responsive drug delivery systems with drugs that treat specific neural injuries and neurodegenerative diseases. For instance, nanoparticle formulations (without ROS-responsiveness) that encapsulate or are coated with curcumin, quercetin, or the acetylcholinesterase inhibitor rivastigmine have been shown to aid in the treatment of Alzheimer’s disease [200–204]. On the other hand, molecules such as (i.e., apomorphine, bromocriptine, and L-dopa) would be useful for the treatment of Parkinson’s disease [205–207]. The effectiveness of these treatments would likely be improved by encapsulating these agents within ROS-responsive drug delivery systems.
Also, the brain is protected by the blood-brain barrier (BBB), which acts as a vital boundary between neural tissue and the circulating blood. In several pathologies including Alzheimer’s disease, Parkinson’s disease and stroke, the blood brain barrier (BBB) is impaired, resulting in higher permeability and molecular exchange across the boundary. However, the BBB still forms a barrier to molecular transport, albeit to a lessened extent. Therefore, the BBB can still pose serious challenges to the delivery of drugs. As a result, modification of nanoparticles with surfactants or ligands, as reviewed in [208], greatly enhances the ability for nanoparticles to cross the blood-brain-barrier. For example, transport across the BBB has been shown to be enhanced in particles engineered with antibodies for transferrin receptor-1 as well as polysorbate 80-coated poly(n-butyl cyanoacrylate) particles[209,210]. These findings will open new venues for advancing drug delivery systems to treat neurodegenerative diseases.
There are several other considerations for engineering future ROS-responsive drug delivery systems. Firstly, further research needs to be done to better understand the dynamic differences in ROS levels in healthy and diseased neuronal tissues. While attempts to measure the ROS level have been made, the true ROS levels that delineate healthy and diseased tissue are still unknown due to the large oxidative stress generated when excising brain tissue. Secondly, it is important to note that subtle differences in the pH caused by inflammation in neuronal injuries and neurodegenerative diseases could cause differences in swelling of the particles, and in turn, the drug release kinetics [211]. These differences in pH could slightly alter the redox state on ROS-responsive functional groups, which would change the ROS sensitivity of the groups and overall particle swelling behavior. To account for this, the quantity of ROS-responsive groups on particles that swell to release their cargo would need to be tuned appropriately. Finally, it is important to determine the correct dosage levels of the ROS-responsive drug delivery systems, as well as their biodistribution within the body, in order to prevent unintended toxicity and side effects.
5. Conclusion
Our aim is that readers can use this review as a guide towards the development of the next generation of ROS-responsive nanoparticles for the treatment of neurological disorders and diseases. By first overviewing the underlying biology and function of ROS production in both normal and diseased neuronal tissue, researchers may keep a holistic view of the biology while designing solutions to deliver drugs of interest in response to dysregulated ROS production. ROS-responsive nanoparticles are well suited to normalize oxidative stress that varies over time for improved neuronal healing and disease treatment, since they can vary the release rate of their molecular cargo. In particle design, researchers should consider the ROS levels, which vary with severity of inflammation and disease. Careful attention should also be paid to the dosage and selection of loaded drug molecules to mitigate the inflammation without causing anti-oxidant-induced stress. Also, several neurological disorders, such as Alzheimer disease and stroke, present specific conditions that can be targeted. The ability to tailor treatment via ROS level, drug efficacy, and disease-targeting moieties should guide the selection of the ROS-responsive functional group of the particle, encapsulated drug molecule, and particle surface ligands, respectively. Regarding inflammation in the central nervous system, researchers also need to account for the blood-brain barrier which can either block or allow for transport of the nanoparticles depending on particle surface functionalities. By taking these design conditions into account, future strategies of antioxidant and drug delivery systems will be both specific towards an application in treating neuroinflammation and effective to modulate ROS to healthy levels.
Table 1.
Overview of common ROS-reactive groups incorporated into particles and how they react
| Bond type | ROS-mediated reactions | References |
|---|---|---|
| Sulfides (Section 3.1) | 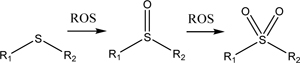 |
[113–126] |
| Selenide (Section 3.2) | 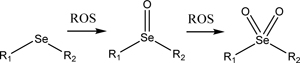 |
[127–130] |
| Telluride (Section 3.2) | 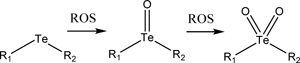 |
[131] |
| Diselenide (Section 3.3) | 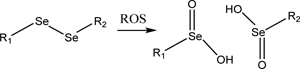 |
[109,132–135] |
| Thioketal (Section 3.4) | 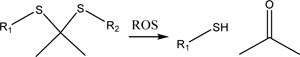 |
[136–142] |
| Boronic ester (Section 3.5) | 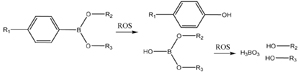 |
[143–154] |
| Polyoxalate (Section 3.6) | 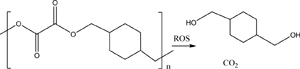 |
[155–161] |
| Manganese oxide (Section 3.7) | [111,112,162–166] | |
| Cerium (Section 3.8) | 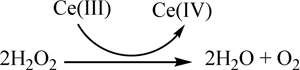 |
[167–172] |
| Ferrocene (Section 3.9) | 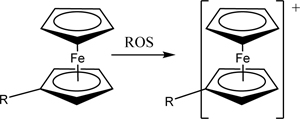 |
[173–175] |
| Amino acrylate (Section 3.9) | 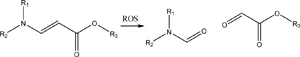 |
[176–178] |
| Polylactic acid (Section 3.9) | 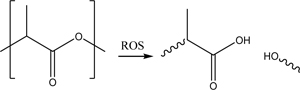 |
[110,179] |
| Hyaluronic Acid (Section 3.9) | 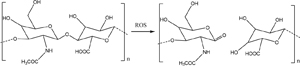 |
[180,181] |
Acknowledgements
This work was supported by the National Institutes of Health (Grant 1R01 HL109192 and 1 R21 HL131469 to H. J. K.; MH 117377 to M. U. J.; NINDS R01 NS083402 and R01 NS097610 to H. J. C.), the National Science Foundation (CBET-1403491 to H. J. K.; STC CBET-0939511 to H. J. K. and M. U. G.; Graduate Research Fellowship DGE – 1144245 to E. C. Q) and TechnipFMC (IL Reference #093250) to W. C. B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wee Yong V, Inflammation in Neurological Disorders: A Help or a Hindrance?, Neurosci. 16 (n.d.) 408–420. doi: 10.1177/1073858410371379. [DOI] [PubMed] [Google Scholar]
- [2].Heppner FL, Ransohoff RM, Becher B, Immune attack: the role of inflammation in Alzheimer disease, Nat. Rev. Neurosci 16 (2015) 358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- [3].Dias V, Junn E, Mouradian MM, The role of oxidative stress in parkinson’s disease, J. Parkinsons. Dis 3 (2013) 461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA, Glia and epilepsy: excitability and inflammation, Trends Neurosci. 36 (2013) 174–184. doi: 10.1016/J.TINS.2012.11.008. [DOI] [PubMed] [Google Scholar]
- [5].Kempuraj D, Thangavel R, Natteru PA, Selvakumar GP, Saeed D, Zahoor H, Zaheer S, Iyer SS, Zaheer A, Neuroinflammation Induces Neurodegeneration J Neurol. Neurosurg. Spine 1 (2016). [PMC free article] [PubMed] [Google Scholar]
- [6].Vishwakarma A, Bhise NS, Evangelista MB, Rouwkema J, Dokmeci MR, Ghaemmaghami AM, Vrana NE, Khademhosseini A, Engineering Immunomodulatory Biomaterials To Tune the Inflammatory Response, Trends Biotechnol. 34 (2016) 470–482. doi: 10.1016/J.TIBTECH.2016.03.009. [DOI] [PubMed] [Google Scholar]
- [7].Nathan C, Ding A, Nonresolving Inflammation, Cell. 140 (2010) 871–882. doi: 10.1016/J.CELL.2010.02.029. [DOI] [PubMed] [Google Scholar]
- [8].Wohlert S, Smart N, Bayon Y, Hunt J, Bryan N, Ahswin H, Reactive oxygen species (ROS) – a family of fate deciding molecules pivotal in constructive inflammation and wound healing, Eur. Cells Mater 24 (2016) 249–265. doi: 10.22203/ecm.v024a18. [DOI] [PubMed] [Google Scholar]
- [9].Kanwar JR, Sriramoju B, Kanwar RK, Neurological disorders and therapeutics targeted to surmount the blood-brain barrier, Int. J. Nanomedicine 7 (2012) 3259–3278. doi: 10.2147/IJN.S30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Poljsak B, Milisav I, The Neglected Significance of “Antioxidative Stress,” Oxid. Med. Cell. Longev 2012 (2012) 1–12. doi: 10.1155/2012/480895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang J, Zhang Y, Archibong E, Ligler FS, Gu Z, Leveraging H 2 O 2 Levels for Biomedical Applications, Adv. Biosyst 1 (2017) 1700084. doi: 10.1002/adbi.201700084. [DOI] [PubMed] [Google Scholar]
- [12].Siegel GJ, Basic neurochemistry : molecular, cellular, and medical aspects, Lippincott Williams & Wilkins, 1999. [Google Scholar]
- [13].Mueller S, Arnhold J, Fast and sensitive chemiluminescence determination of H2O2 concentration in stimulated human neutrophils, J. Biolumin. Chemilumin 10 (1995) 229–237. doi: 10.1002/bio.1170100406. [DOI] [PubMed] [Google Scholar]
- [14].Massaad CA, Klann E, Reactive oxygen species in the regulation of synaptic plasticity and memory., Antioxid. Redox Signal 14 (2011) 2013–54. doi: 10.1089/ars.2010.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Beckhauser TF, Francis-Oliveira J, De Pasquale R, Reactive oxygen species: Physiological and physiopathological effects on synaptic plasticity, J. Exp. Neurosci 2016 (2016) 23–48. doi: 10.4137/JEN.S39887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yang J, Yang J, Liang SH, Xu Y, Moore A, Ran C, Imaging hydrogen peroxide in Alzheimer’s disease via cascade signal amplification, Sci. Rep 6 (2016) 35613. doi: 10.1038/srep35613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Praticò D, Oxidative stress hypothesis in Alzheimer’s disease: a reappraisal, Trends Pharmacol. Sci 29 (2008) 609–615. doi: 10.1016/J.TIPS.2008.09.001. [DOI] [PubMed] [Google Scholar]
- [18].Jenner P, Oxidative stress in Parkinson’s disease, Ann. Neurol 53 (2003) S26–S38. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- [19].Emerit J, Edeas M, Bricaire F, Neurodegenerative diseases and oxidative stress, Biomed. Pharmacother 58 (2004) 39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- [20].Adam-Vizi V, Production of Reactive Oxygen Species in Brain Mitochondria: Contribution by Electron Transport Chain and Non–Electron Transport Chain Sources, Antioxid. Redox Signal 7 (2005) 1140–1149. doi: 10.1089/ars.2005.7.1140. [DOI] [PubMed] [Google Scholar]
- [21].Murphy MP, How mitochondria produce reactive oxygen species, Biochem. J 417 (2009) 1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zorov DB, Juhaszova M, Sollott SJ, Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release., Physiol. Rev 94 (2014) 909–50. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Winterbourn CC, Toxicity of iron and hydrogen peroxide: the Fenton reaction, Toxicol. Lett 82–83 (1995) 969–974. [DOI] [PubMed] [Google Scholar]
- [24].Kettle AJ, Winterbourn CC, Myeloperoxidase: a key regulator of neutrophil oxidant production Myeloperoxidase: a key regulator of neutrophil oxidant production, Redox Rep. 3 (1997) 3–15. doi: 10.1080/13510002.1997.11747085. [DOI] [PubMed] [Google Scholar]
- [25].Halliwell B, Gutteridge JMC, Biologically relevant metal ion-dependent hydroxyl radical generation An update, FEBS Lett. 307 (1992) 108–112. doi: 10.1016/0014-5793(92)80911-Y. [DOI] [PubMed] [Google Scholar]
- [26].Sipos I, Tretter L, Adam-Vizi V, Quantitative relationship between inhibition of respiratory complexes and formation of reactive oxygen species in isolated nerve terminals, J. Neurochem 84 (2002) 112–118. doi: 10.1046/j.1471-4159.2003.01513.x. [DOI] [PubMed] [Google Scholar]
- [27].Schapira AHV, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD, Mitochondrial Complex I Deficiency in Parkinson’s Disease, J. Neurochem 54 (1990) 823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- [28].Edmondson DE, Hydrogen Peroxide Produced by Mitochondrial Monoamine Oxidase Catalysis: Biological Implications, 2014. [DOI] [PubMed] [Google Scholar]
- [29].Westlund KN, Denney RM, Rose RM, Abell CW, Localization of Distinct Monamine Oxidase A and Monoamine Oxidase B Cell Populations in Human Brainstem, Neuroscience. 25 (1988) 439–456. [DOI] [PubMed] [Google Scholar]
- [30].Levitt P, Pintar JE, Breakefield XO, Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons, Proc. Natl. Acad. Sci 79 (2006) 6385–6389. doi: 10.1073/pnas.79.20.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schmiedeberg N’, O’carroll A-M, Fowler CJ, Phillips2 JP, Tobbia I, Tipton KF, The Deamination of Dopamine by Human Brain Monoamine Oxidase Specificity for the Two Enzyme Forms in Seven Brain Regions, 1983. [DOI] [PubMed] [Google Scholar]
- [32].Axelrod J, Metabolism of Epinephrine and Other Sympathomimetic Amines, Physiol. Rev 39 (2017) 751–776. doi: 10.1152/physrev.1959.39.4.751. [DOI] [PubMed] [Google Scholar]
- [33].Sjoerdsma A, Smith TE, Stevenson TD, Udenfriend S, Metabolism of 5-Hydroxytryptamine (Serotonin) by Monoamine Oxidase., Exp. Biol. Med 89 (1955) 36–38. doi: 10.3181/00379727-89-21707. [DOI] [PubMed] [Google Scholar]
- [34].Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Müller U, Aguet M, Babinet C, Shih JC, De Maeyer E, Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA, Science (80-. ). 268 (1995) 1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bonekamp NA, Völkl A, Fahimi HD, Schrader M, Reactive oxygen species and peroxisomes: Struggling for balance, BioFactors. 35 (2009) 346–355. doi: 10.1002/biof.48. [DOI] [PubMed] [Google Scholar]
- [36].Tejada-Simon MV, Serrano F, Villasana LE, Kanterewicz BI, Wu G-Y, Quinn MT, Klann E, Synaptic localization of a functional NADPH oxidase in the mouse hippocampus, Mol. Cell. Neurosci 29 (2005) 97–106. doi: 10.1016/J.MCN.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bindokas VP, Jordán J, Lee CC, Miller RJ, Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine., J. Neurosci 16 (1996) 1324–36. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Clark RA, Activation of the Neutrophil Respiratory Burst Oxidase, J. Infect. Dis 179 (1999) S309–S317. doi: 10.1086/513849. [DOI] [PubMed] [Google Scholar]
- [39].Wang TA, Yu YV, Govindaiah G, Ye X, Artinian L, Coleman TP, Sweedler JV, Cox CL, Gillette MU, Circadian Rhythm of Redox State Regulates Excitability in Suprachiasmatic Nucleus Neurons, Science (80-. ). 337 (2012) 839–842. doi: 10.1126/science.1222826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bothwell MY, Gillette MU, Circadian redox rhythms in the regulation of neuronal excitability, Free Radic. Biol. Med 119 (2018) 45–55. doi: 10.1016/J.FREERADBIOMED.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gillette MU, Wang TA, Brain Circadian Oscillators and Redox Regulation in Mammals, (n.d.). doi: 10.1089/ars.2013.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, Kornblum HI, Proliferative Neural Stem Cells Have High Endogenous ROS Levels that Regulate Self-Renewal and Neurogenesis in a PI3K/Akt-Dependant Manner, Cell Stem Cell. 8 (2011) 59–71. doi: 10.1016/J.STEM.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dickinson BC, Peltier J, Stone D, V Schaffer D, Chang CJ, Nox2 redox signaling maintains essential cell populations in the brain, Nat Chem Biol. 7 (2011) 106–112. doi: 10.1038/nchembio.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gahtan E, Auerbach JM, Groner Y, Segal M, Reversible impairment of long-term potentiation in transgenic Cu/Zn-SOD mice, Eur. J. Neurosci 10 (1998) 538–544. doi: 10.1046/j.1460-9568.1998.00058.x. [DOI] [PubMed] [Google Scholar]
- [45].Klann E, Roberson ED, Knapp LT, Sweatt JD, A role for superoxide in protein kinase C activation and induction of long-term potentiation., J. Biol. Chem 273 (1998) 4516–22. doi: 10.1074/JBC.273.8.4516. [DOI] [PubMed] [Google Scholar]
- [46].Lambeth JD, NOX enzymes and the biology of reactive oxygen, Nat. Rev. Immunol 4 (2004) 181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- [47].Sumimoto H, Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species, FEBS J. 275 (2008) 3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- [48].Hsu H-Y, Wen M-H, Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression., J. Biol. Chem 277 (2002) 22131–9. doi: 10.1074/jbc.M111883200. [DOI] [PubMed] [Google Scholar]
- [49].Barlow-Walden LR, Reiter RJ, Abe M, Pablos M, Menendez-Pelaez A, Chen L-D, Poeggeler B, Melatonin stimulates brain glutathione peroxidase activity, Neurochem. Int 26 (1995) 497–502. doi: 10.1016/0197-0186(94)00154-M. [DOI] [PubMed] [Google Scholar]
- [50].Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, Lee Y, Roh JH, OrtizGonzalez X, Dearborn JT, Culver JP, Herzog ED, Hogenesch JB, Wozniak DF, Dikranian K, Giasson BI, Weaver DR, Holtzman DM, FitzGerald GA, Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration, J. Clin. Invest 123 (2013) 5389–5400. doi: 10.1172/JCI70317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Suzukawa K, Miura K, Mitsushita J, Resau J, Hirose K, Crystal R, Kamata T, Nerve growth factor-induced neuronal differentiation requires generation of Rac1-regulated reactive oxygen species, J. Biol. Chem 275 (2000) 13175–13178. doi: 10.1074/jbc.275.18.13175. [DOI] [PubMed] [Google Scholar]
- [52].Tsatmali M, Walcott EC, Crossin KL, Newborn neurons acquire high levels of reactive oxygen species and increased mitochondrial proteins upon differentiation from progenitors, Brain Res. 1040 (2005) 137–150. doi: 10.1016/j.brainres.2005.01.087. [DOI] [PubMed] [Google Scholar]
- [53].Tsatmali M, Walcott EC, Makarenkova H, Crossin KL, Reactive oxygen species modulate the differentiation of neurons in clonal cortical cultures, Mol. Cell. Neurosci 33 (2006) 345–357. doi: 10.1016/J.MCN.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Barnham KJ, Masters CL, Bush AI, Neurodegenerative diseases and oxidative stress, Nat. Rev. Drug Discov 3 (2004) 205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- [55].Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P, Oxidative stress, aging, and diseases, Clin. Interv. Aging 13 (2018) 757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Oubidar M, Boquillon M, Marie C, Bouvier C, Beley A, Bralet J, Effect of intracellular iron loading on lipid peroxidation of brain slices, Free Radic. Biol. Med 21 (1996) 763–769. doi: 10.1016/0891-5849(96)00173-6. [DOI] [PubMed] [Google Scholar]
- [57].Benatti P, Peluso G, Nicolai R, Calvani M, Polyunsaturated Fatty Acids: Biochemical, Nutritional and Epigenetic Properties, J. Am. Coll. Nutr 23 (2004) 281–302. doi: 10.1080/07315724.2004.10719371. [DOI] [PubMed] [Google Scholar]
- [58].Kogure K, Arai H, Abe K, Nakano M, Free Radical Damage of the Brain Following Ischemia, Prog. Brain Res 63 (1985) 237–259. doi: 10.1016/S0079-6123(08)61987-1. [DOI] [PubMed] [Google Scholar]
- [59].Bordt EA, Polster BM, NADPH oxidase- and mitochondria-derived reactive oxygen species in proinflammatory microglial activation: a bipartisan affair?, Free Radic. Biol. Med 76 (2014) 34–46. doi: 10.1016/J.FREERADBIOMED.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].M.-E.` Tremblay V, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A, The Role of Microglia in the Healthy Brain, (2011). doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Neniskyte U, Neher JJ, Brown GC, Neuronal death induced by nanomolar amyloid β is mediated by primary phagocytosis of neurons by microglia., J. Biol. Chem 286 (2011) 39904–13. doi: 10.1074/jbc.M111.267583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Neher JJ, V Emmrich J, Fricker M, Mander PK, Théry C, Brown GC, Phagocytosis executes delayed neuronal death after focal brain ischemia, (n.d.). doi: 10.1073/pnas.1308679110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Butterfield DA, Lauderback CM, Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: Potential causes and consequences involving amyloid β-peptide-associated free radical oxidative stress, Free Radic. Biol. Med 32 (2002) 1050–1060. doi: 10.1016/S0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- [64].Subbarao KV, Richardson JS, Ang LC, Autopsy Samples of Alzheimer’s Cortex Show Increased Peroxidation In Vitro, J. Neurochem 55 (1990) 342–345. doi: 10.1111/j.1471-4159.1990.tb08858.x. [DOI] [PubMed] [Google Scholar]
- [65].Behl C, Davis JB, Lesley R, Schubert D, Hydrogen peroxide mediates amyloid β protein toxicity, Cell. 77 (1994) 817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- [66].Du Yan S, Chen X, Fu J, Chen M, Zhu H, Roher A, Slatteryt T, Zhaot L, Nagashimat M, Morsert J, Migheli A, Nawroth P, Stern D, Marie Schmidt A, Neurologica C, RAGE and amyloid-P peptide neurotoxicity in Alzheimer’s disease, n.d. [DOI] [PubMed] [Google Scholar]
- [67].Petrone A, Battaglia F, Wang C, Dusa A, Su J, Zagzag D, Bianchi R, Casaccia-Bonnefil P, Arancio O, Sap J, Luddy JS, Lue L, Walker DG, Roher A, Buttini M, Mucke L, Li W, Schmidt AM, Kindy M, Hyslop PA, Stern DM, Du Yan SS, Receptor protein tyrosine phosphatase alpha is essential for hippocampal neuronal migration and long-term potentiation., EMBO J. 22 (2003) 4121–31. doi: 10.1093/emboj/cdg399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B, RAGE mediates amyloid-β peptide transport across the blood-brain barrier and accumulation in brain, Nat. Med 9 (2003) 907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- [69].Bamberger ME, Harris ME, Mcdonald DR, Husemann J, Landreth GE, A Cell Surface Receptor Complex for Fibrillar-Amyloid Mediates Microglial Activation, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Bianca VD, Dusi S, Bianchini E, Dal Prà I, Rossi F, beta-amyloid activates the O-2 forming NADPH oxidase in microglia, monocytes, and neutrophils. A possible inflammatory mechanism of neuronal damage in Alzheimer’s disease., J. Biol. Chem 274 (1999) 15493–9. doi: 10.1074/JBC.274.22.15493. [DOI] [PubMed] [Google Scholar]
- [71].Shimohama S, Tanino H, Kawakami N, Okamura N, Kodama H, Yamaguchi T, Hayakawa T, Nunomura A, Chiba S, Perry G, Smith MA, Fujimoto S, Activation of NADPH Oxidase in Alzheimer’s Disease Brains, Biochem. Biophys. Res. Commun 273 (2000) 5–9. doi: 10.1006/BBRC.2000.2897. [DOI] [PubMed] [Google Scholar]
- [72].Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag A-E, Lang AE, Parkinson disease, Nat. Rev. Dis. Prim 3 (2017) 17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- [73].Dexter DT, Holley AE, Flitter WD, Slater TF, Wells FR, Daniel SE, Lees AJ, Jenner P, Marsden CD, Increased levels of lipid hydroperoxides in the parkinsonian substantia nigra: An HPLC and ESR study, Mov. Disord 9 (1994) 92–97. doi: 10.1002/mds.870090115. [DOI] [PubMed] [Google Scholar]
- [74].Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, Jenner P, Marsden CD, Basal Lipid Peroxidation in Substantia Nigra Is Increased in Parkinson’s Disease, J. Neurochem 52 (1989) 381–389. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- [75].Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, Jenner P, Halliwell B, Oxidative DNA Damage in the Parkinsonian Brain: An Apparent Selective Increase in 8-Hydroxyguanine Levels in Substantia Nigra, J. Neurochem 69 (2002) 1196–1203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- [76].Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT, Chronic systemic pesticide exposure reproduces features of Parkinson’s disease, Nat. Neurosci 3 (2000) 1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- [77].BEAL MF, Mitochondria, Oxidative Damage, and Inflammation in Parkinson’s Disease, Ann. N. Y. Acad. Sci 991 (2006) 120–131. doi: 10.1111/j.1749-6632.2003.tb07470.x. [DOI] [PubMed] [Google Scholar]
- [78].Moore DJ, West AB, Dawson VL, Dawson TM, Molecular Pathophysiology of Parkinson’s Disease, Annu. Rev. Neurosci 28 (2005) 57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- [79].Wang J-Q, Chen Q, Wang X, Wang Q-C, Wang Y, Cheng H-P, Guo C, Sun Q, Chen Q, Tang T-S, Dysregulation of mitochondrial calcium signaling and superoxide flashes cause mitochondrial genomic DNA damage in Huntington disease., J. Biol. Chem 288 (2013) 3070–84. doi: 10.1074/jbc.M112.407726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gu M, Gash MT, Mann VM, Javoy-Agid F, Cooper JM, Schapira AHV, Mitochondrial defect in Huntington’s disease caudate nucleus, Ann. Neurol 39 (1996) 385–389. doi: 10.1002/ana.410390317. [DOI] [PubMed] [Google Scholar]
- [81].Browne SE, Ferrante RJ, Beal MF, Oxidative Stress in Huntington’s Disease, Brain Pathol. 9 (2006) 147–163. doi: 10.1111/j.1750-3639.1999.tb00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Browne SE, Bowling AC, Macgarvey U, Baik MJ, Berger SC, Muquit MMK, Bird ED, Beal MF, Oxidative damage and metabolic dysfunction in Huntington’s disease: Selective vulnerability of the basal ganglia, Ann. Neurol 41 (1997) 646–653. doi: 10.1002/ana.410410514. [DOI] [PubMed] [Google Scholar]
- [83].Sorolla MA, Reverter-Branchat G, Tamarit J, Ferrer I, Ros J, Cabiscol E, Proteomic and oxidative stress analysis in human brain samples of Huntington disease, Free Radic. Biol. Med 45 (2008) 667–678. doi: 10.1016/j.freeradbiomed.2008.05.014. [DOI] [PubMed] [Google Scholar]
- [84].Mattiazzi M, D’Aurelio M, Gajewski CD, Martushova K, Kiaei M, Beal MF, Manfredi G, Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice., J. Biol. Chem 277 (2002) 29626–33. doi: 10.1074/jbc.M203065200. [DOI] [PubMed] [Google Scholar]
- [85].Ferrante RJ, Shinobu LA, Schulz JB, Matthews RT, Thomas CE, Kowall NW, Gurney ME, Beal MF, Increased 3-nitrotyrosine and oxidative damage in mice with a human copper/zinc superoxide dismutase mutation, Ann. Neurol 42 (1997) 326–334. doi: 10.1002/ana.410420309. [DOI] [PubMed] [Google Scholar]
- [86].Hall ED, Andrus PK, Oostveen JA, Fleck TJ, Gurney ME, Relationship of oxygen radical-induced lipid peroxidative damage to disease onset and progression in a transgenic model of familial ALS, J. Neurosci. Res 53 (1998) 66–77. doi:. [DOI] [PubMed] [Google Scholar]
- [87].Liu J, Lillo C, Jonsson PA, Vande Velde C, Ward CM, Miller TM, Subramaniam JR, Rothstein JD, Marklund S, Andersen PM, Brännström T, Gredal O, Wong PC, Williams DS, Cleveland DW, Toxicity of Familial ALS-Linked SOD1 Mutants from Selective Recruitment to Spinal Mitochondria, Neuron. 43 (2004) 5–17. doi: 10.1016/J.NEURON.2004.06.016. [DOI] [PubMed] [Google Scholar]
- [88].Ferrante RJ, Browne SE, Shinobu LA, Bowling AC, Baik MJ, MacGarvey U, Kowall NW, Brown RH, Beal MF, Evidence of Increased Oxidative Damage in Both Sporadic and Familial Amyotrophic Lateral Sclerosis, J. Neurochem 69 (2002) 2064–2074. doi: 10.1046/j.1471-4159.1997.69052064.x. [DOI] [PubMed] [Google Scholar]
- [89].Oteiza PI, Uchitel OD, Carrasquedo F, Dubrovski AL, Roma JC, Fraga CG, Evaluation of antioxidants, protein, and lipid oxidation products in blood from sporadic amiotrophic lateral sclerosis patients, Neurochem. Res 22 (1997) 535–539. doi: 10.1023/A:1027384432715. [DOI] [PubMed] [Google Scholar]
- [90].Vezzani A, Granata T, Brain Inflammation in Epilepsy: Experimental and Clinical Evidence, Epilepsia. 46 (2005) 1724–1743. doi: 10.1111/j.1528-1167.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- [91].Riazi K, Galic MA, Pittman QJ, Contributions of peripheral inflammation to seizure susceptibility: Cytokines and brain excitability, Epilepsy Res. 89 (2010) 34–42. doi: 10.1016/J.EPLEPSYRES.2009.09.004. [DOI] [PubMed] [Google Scholar]
- [92].Choi J, Nordli DR, Alden TD, DiPatri A, Laux L, Kelley K, Rosenow J, Schuele SU, Rajaram V, Koh S, Cellular injury and neuroinflammation in children with chronic intractable epilepsy, J. Neuroinflammation 6 (2009) 38. doi: 10.1186/1742-20946-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Folbergrová J, Ješina P, Haugvicová R, Lisý V, Houštěk J, Sustained deficiency of mitochondrial complex I activity during long periods of survival after seizures induced in immature rats by homocysteic acid, Neurochem. Int 56 (2010) 394–403. doi: 10.1016/J.NEUINT.2009.11.011. [DOI] [PubMed] [Google Scholar]
- [94].Lee YM, Kang HC, Lee JS, Kim SH, Kim EY, Lee SK, Slama A, Kim HD, Mitochondrial respiratory chain defects: Underlying etiology in various epileptic conditions, Epilepsia. 49 (2008) 685–690. doi: 10.1111/j.1528-1167.2007.01522.x. [DOI] [PubMed] [Google Scholar]
- [95].Kunz WS, Kudin AP, Vielhaber S, Blümcke I, Zuschratter W, Schramm J, Beck H, Elger CE, Mitochondrial complex I deficiency in the epileptic focus of patients with temporal lobe epilepsy, Ann. Neurol 48 (2000) 766–773. doi:. [DOI] [PubMed] [Google Scholar]
- [96].Waldbaum S, Patel M, Mitochondria, oxidative stress, and temporal lobe epilepsy, Epilepsy Res. 88 (2010) 23–45. doi: 10.1016/J.EPLEPSYRES.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT, Post-ischemic brain damage: pathophysiology and role of inflammatory mediators, FEBS J. 276 (2009) 13–26. doi: 10.1111/j.1742-4658.2008.06766.x. [DOI] [PubMed] [Google Scholar]
- [98].Jin R, Yang G, Li G, Inflammatory mechanisms in ischemic stroke: role of inflammatory cells, J. Leukoc. Biol 87 (2010) 779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG, Microglia Potentiate Damage to Blood–Brain Barrier Constituents, Stroke. 37 (2006) 1087–1093. doi: 10.1161/01.STR.0000206281.77178.ac. [DOI] [PubMed] [Google Scholar]
- [100].Selim MH, Ratan RR, The role of iron neurotoxicity in ischemic stroke, Ageing Res. Rev 3 (2004) 345–353. doi: 10.1016/J.ARR.2004.04.001. [DOI] [PubMed] [Google Scholar]
- [101].Wu J, Hua Y, Keep RF, Schallert T, Hoff JT, Xi G, Oxidative brain injury from extravasated erythrocytes after intracerebral hemorrhage, Brain Res. 953 (2002) 45–52. doi: 10.1016/S0006-8993(02)03268-7. [DOI] [PubMed] [Google Scholar]
- [102].Chouchani ET, Pell VR, Gaude E, Aksentijević D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord ENJ, Smith AC, Eyassu F, Shirley R, Hu C-H, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa ASH, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, Murphy MP, Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS, Nature. 515 (2014) 431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Cherubini A, Ruggiero C, Polidori MC, Mecocci P, Potential markers of oxidative stress in stroke, Free Radic. Biol. Med 39 (2005) 841–852. doi: 10.1016/J.FREERADBIOMED.2005.06.025. [DOI] [PubMed] [Google Scholar]
- [104].Monje ML, Toda H, Palmer TD, Inflammatory blockade restores adult hippocampal neurogenesis., Science. 302 (2003) 1760–5. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- [105].Chen H, Zhang SM, Hernán MA, Schwarzschild MA, Willett WC, Colditz GA, Speizer FE, Ascherio A, Nonsteroidal Anti-inflammatory Drugs and the Risk of Parkinson Disease, Arch. Neurol 60 (2003) 1059. doi: 10.1001/archneur.60.8.1059. [DOI] [PubMed] [Google Scholar]
- [106].Usmani OS, Belvisi MG, Patel HJ, Crispino N, Birrell MA, Korbonits M, Korbonits D, Barnes PJ, Theobromine inhibits sensory nerve activation and cough, FASEB J. 19 (2005) 231–233. doi: 10.1096/fj.04-1990fje. [DOI] [PubMed] [Google Scholar]
- [107].Kim D-H, Martin DC, Sustained release of dexamethasone from hydrophilic matrices using PLGA nanoparticles for neural drug delivery, Biomaterials. 27 (2006) 3031–3037. doi: 10.1016/J.BIOMATERIALS.2005.12.021. [DOI] [PubMed] [Google Scholar]
- [108].Cavet ME, Harrington KL, Vollmer TR, Ward KW, Zhang J-Z, Anti-inflammatory and anti-oxidative effects of the green tea polyphenol epigallocatechin gallate in human corneal epithelial cells, 2011. [PMC free article] [PubMed] [Google Scholar]
- [109].Li Y, Li Y, Ji W, Lu Z, Liu L, Shi Y, Ma G, Zhang X, Positively Charged Polyprodrug Amphiphiles with Enhanced Drug Loading and Reactive Oxygen Species-Responsive Release Ability for Traceable Synergistic Therapy, J. Am. Chem. Soc 140 (2018) 4164–4171. doi: 10.1021/jacs.8b01641. [DOI] [PubMed] [Google Scholar]
- [110].Malcor J-D, Payrot N, David M, Faucon A, Abouzid K, Jacquot G, Floquet N, Debarbieux F, Rougon G, Martinez J, Khrestchatisky M, Vlieghe P, Lisowski V, Chemical Optimization of New Ligands of the Low-Density Lipoprotein Receptor as Potential Vectors for Central Nervous System Targeting, J. Med. Chem 55 (2012) 2227–2241. doi: 10.1021/jm2014919. [DOI] [PubMed] [Google Scholar]
- [111].Teo JY, Seo Y, Ko E, Leong J, Hong Y-T, Yang YY, Kong H, Surface tethering of stem cells with H2O2-responsive anti-oxidizing colloidal particles for protection against oxidation-induced death, Biomaterials. 201 (2019) 1–15. doi: 10.1016/J.BIOMATERIALS.2019.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Seo Y, Leong J, Teo JY, Mitchell JW, Gillette MU, Han B, Lee J, Kong H, Active Antioxidizing Particles for On-Demand Pressure-Driven Molecular Release, ACS Appl. Mater. Interfaces 9 (2017) 35642–35650. doi: 10.1021/acsami.7b12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Jeong D, Bae B, Park S, Na K, Reactive oxygen species responsive drug releasing nanoparticle based on chondroitin sulfate–anthocyanin nanocomplex for efficient tumor therapy, J. Control. Release 222 (2016) 78–85. doi: 10.1016/J.JCONREL.2015.12.009. [DOI] [PubMed] [Google Scholar]
- [114].† Rehor Annemie, † and Hubbell Jeffrey A., †,‡ Tirelli Nicola*, Oxidation-Sensitive Polymeric Nanoparticles, (2004). doi: 10.1021/LA0478043. [DOI] [PubMed] [Google Scholar]
- [115].He W, Jia H, Cai J, Han X, Zheng Z, Wamer WG, Yin J-J, Production of Reactive Oxygen Species and Electrons from Photoexcited ZnO and ZnS Nanoparticles: A Comparative Study for Unraveling their Distinct Photocatalytic Activities, J. Phys. Chem. C 120 (2016) 3187–3195. doi: 10.1021/acs.jpcc.5b11456. [DOI] [Google Scholar]
- [116].Yu K-N, Yoon T-J, Minai-Tehrani A, Kim J-E, Park SJ, Jeong MS, Ha S-W, Lee J-K, Kim JS, Cho M-H, Zinc oxide nanoparticle induced autophagic cell death and mitochondrial damage via reactive oxygen species generation, Toxicol. Vitr 27 (2013) 1187–1195. doi: 10.1016/J.TIV.2013.02.010. [DOI] [PubMed] [Google Scholar]
- [117].Suyana P, Kumar SN, Kumar BSD, Nair BN, Pillai SC, Mohamed AP, Warrier KGK, Hareesh US, Antifungal properties of nanosized ZnS particles synthesised by sonochemical precipitation, RSC Adv. 4 (2014) 8439. doi: 10.1039/c3ra46642f. [DOI] [Google Scholar]
- [118].Liu Y, Wang Y-M, Sedano S, Jiang Q, Duan Y, Shen W, Jiang J-H, Zhong W, Encapsulation of ionic nanoparticles produces reactive oxygen species (ROS)-responsive microgel useful for molecular detection, Chem. Commun 54 (2018) 4329–4332. doi: 10.1039/C8CC01432A. [DOI] [PubMed] [Google Scholar]
- [119].Napoli A, Valentini M, Tirelli N, Müller M, Hubbell JA, Oxidation-responsive polymeric vesicles, Nat. Mater 3 (2004) 183–189. doi: 10.1038/nmat1081. [DOI] [PubMed] [Google Scholar]
- [120].Poole KM, Nelson CE, Joshi RV, Martin JR, Gupta MK, Haws SC, Kavanaugh TE, Skala MC, Duvall CL, ROS-responsive microspheres for on demand antioxidant therapy in a model of diabetic peripheral arterial disease, Biomaterials. 41 (2015) 166–175. doi: 10.1016/j.biomaterials.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Mercanzini A, Reddy ST, Velluto D, Colin P, Maillard A, Bensadoun J-C, Hubbell JA, Renaud P, Controlled release nanoparticle-embedded coatings reduce the tissue reaction to neuroprostheses, J. Control. Release 145 (2010) 196–202. doi: 10.1016/J.JCONREL.2010.04.025. [DOI] [PubMed] [Google Scholar]
- [122].Thomas SN, van der Vlies AJ, O’Neil CP, Reddy ST, Yu SS, Giorgio TD, Swartz MA, Hubbell JA, Engineering complement activation on polypropylene sulfide vaccine nanoparticles, Biomaterials. 32 (2011) 2194–2203. doi: 10.1016/J.BIOMATERIALS.2010.11.037. [DOI] [PubMed] [Google Scholar]
- [123].Uddin MJ, Werfel TA, Crews BC, Gupta MK, Kavanaugh TE, Kingsley PJ, Boyd K, Marnett LJ, Duvall CL, Fluorocoxib A loaded nanoparticles enable targeted visualization of cyclooxygenase-2 in inflammation and cancer, Biomaterials. 92 (2016) 71–80. doi: 10.1016/J.BIOMATERIALS.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Leong J, Chin W, Ke X, Gao S, Kong H, Hedrick JL, Yang YY, Disease-directed design of biodegradable polymers: Reactive oxygen species and pH-responsive micellar nanoparticles for anticancer drug delivery, Nanomedicine Nanotechnology, Biol. Med 14 (2018) 2666–2677. doi: 10.1016/J.NANO.2018.06.015. [DOI] [PubMed] [Google Scholar]
- [125].Cheng Y, Jiao X, Xu T, Wang W, Cao Y, Wen Y, Zhang X, Free-Blockage Mesoporous Anticancer Nanoparticles Based on ROS-Responsive Wetting Behavior of Nanopores, Small. 13 (2017) 1701942. doi: 10.1002/smll.201701942. [DOI] [PubMed] [Google Scholar]
- [126].Suyana P, Nishanth Kumar S, Madhavan N, Dileep Kumar BS, Nair BN, Mohamed AP, Warrier KGK, Hareesh US, Reactive oxygen species (ROS) mediated enhanced anti-candidal activity of ZnS–ZnO nanocomposites with low inhibitory concentrations, RSC Adv. 5 (2015) 76718–76728. doi: 10.1039/C5RA13316E. [DOI] [Google Scholar]
- [127].Ren H, Wu Y, Ma N, Xu H, Zhang X, Side-chain selenium-containing amphiphilic block copolymers: redox-controlled self-assembly and disassembly, Soft Matter. 8 (2012) 1460–1466. doi: 10.1039/C1SM06673K. [DOI] [Google Scholar]
- [128].Saravanakumar G, Kim J, Kim WJ, Reactive-Oxygen-Species-Responsive Drug Delivery Systems: Promises and Challenges, Adv. Sci 4 (2017) 1600124. doi: 10.1002/advs.201600124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Ma N, Li Y, Ren H, Xu H, Li Z, Zhang X, Selenium-containing block copolymers and their oxidation-responsive aggregates, Polym. Chem 1 (2010) 1609. doi: 10.1039/c0py00144a. [DOI] [Google Scholar]
- [130].Yu L, Zhang M, Du F-S, Li Z-C, ROS-responsive poly(ε-caprolactone) with pendent thioether and selenide motifs, Polym. Chem 9 (2018) 3762–3773. doi: 10.1039/C8PY00620B. [DOI] [Google Scholar]
- [131].Yu L, Yang Y, Du F-S, Li Z-C, ROS-Responsive Chalcogen-Containing Polycarbonates for Photodynamic Therapy, Biomacromolecules. 19 (2018) 2182–2193. doi: 10.1021/acs.biomac.8b00271. [DOI] [PubMed] [Google Scholar]
- [132].Han X, Yang N, Xu Y, Zhu J, Chen Z, Liu Z, Dang G, Song C, Simvastatin treatment improves functional recovery after experimental spinal cord injury by upregulating the expression of BDNF and GDNF., Neurosci. Lett 487 (2011) 255–9. doi: 10.1016/j.neulet.2010.09.007. [DOI] [PubMed] [Google Scholar]
- [133].Zhai S, Hu X, Hu Y, Wu B, Xing D, Visible light-induced crosslinking and physiological stabilization of diselenide-rich nanoparticles for redox-responsive drug release and combination chemotherapy, Biomaterials. 121 (2017) 41–54. doi: 10.1016/J.BIOMATERIALS.2017.01.002. [DOI] [PubMed] [Google Scholar]
- [134].Kice JL, Lee TWS, Oxidation-reduction reactions of organoselenium compounds. 1. Mechanism of the reaction between seleninic acids and thiols, J. Am. Chem. Soc 100 (1978) 5094–5102. doi: 10.1021/ja00484a031. [DOI] [Google Scholar]
- [135].Kim BS, Leong J, Yu SJ, Cho Y, Park CG, Kim D-H, Ko E, Im SG, Lee J, Kim YJ, Kong H, Stimulus-Responsive Anti-Oxidizing Drug Crystals and Its Ecological Implication, Small. (2019). doi: 10.1002/smll.201900765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Wilson DS, Dalmasso G, Wang L, Sitaraman SV, Merlin D, Murthy N, Orally delivered thioketal nanoparticles loaded with TNF-α–siRNA target inflammation and inhibit gene expression in the intestines, Nat. Mater 9 (2010) 923–928. doi: 10.1038/nmat2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Xu X, Saw PE, Tao W, Li Y, Ji X, Bhasin S, Liu Y, Ayyash D, Rasmussen J, Huo M, Shi J, Farokhzad OC, ROS-Responsive Polyprodrug Nanoparticles for Triggered Drug Delivery and Effective Cancer Therapy, Adv. Mater 29 (2017) 1700141. doi: 10.1002/adma.201700141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Sinananwanich W, Ueda M, Synthesis of a hyperbranched polythioketal with 100% degree of branching, J. Polym. Sci. Part A Polym. Chem 46 (2008) 2689–2700. doi: 10.1002/pola.22602. [DOI] [Google Scholar]
- [139].Chen D, Zhang G, Li R, Guan M, Wang X, Zou T, Zhang Y, Wang C, Shu C, Hong H, Wan L-J, Biodegradable, Hydrogen Peroxide, and Glutathione Dual Responsive Nanoparticles for Potential Programmable Paclitaxel Release, J. Am. Chem. Soc 140 (2018) 7373–7376. doi: 10.1021/jacs.7b12025. [DOI] [PubMed] [Google Scholar]
- [140].Sun C, Liang Y, Hao N, Xu L, Cheng F, Su T, Cao J, Gao W, Pu Y, He B, A ROS-responsive polymeric micelle with a π-conjugated thioketal moiety for enhanced drug loading and efficient drug delivery, Org. Biomol. Chem 15 (2017) 9176–9185. doi: 10.1039/C7OB01975K. [DOI] [PubMed] [Google Scholar]
- [141].Yue C, Yang Y, Zhang C, Alfranca G, Cheng S, Ma L, Liu Y, Zhi X, Ni J, Jiang W, Song J, de la Fuente JM, Cui D, ROS-responsive mitochondria-targeting blended nanoparticles: Chemo- and photodynamic synergistic therapy for lung cancer with on-demand drug release upon irradiation with a single light source, Theranostics. 6 (2016) 2352–2366. doi: 10.7150/thno.15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Sun C-Y, Cao Z, Zhang X-J, Sun R, Yu C-S, Yang X, Cascade-amplifying synergistic effects of chemo-photodynamic therapy using ROS-responsive polymeric nanocarriers, Theranostics. 8 (2018) 2939–2953. doi: 10.7150/thno.24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Zhang Q, Zhang F, Chen Y, Dou Y, Tao H, Zhang D, Wang R, Li X, Zhang J, Structure–Property Correlations of Reactive Oxygen Species-Responsive and Hydrogen Peroxide-Eliminating Materials with Anti-Oxidant and Anti-Inflammatory Activities, Chem. Mater 29 (2017) 8221–8238. doi: 10.1021/acs.chemmater.7b02412. [DOI] [Google Scholar]
- [144].Qiao C, Yang J, Shen Q, Liu R, Li Y, Shi Y, Chen J, Shen Y, Xiao Z, Weng J, Zhang X, Traceable Nanoparticles with Dual Targeting and ROS Response for RNAi-Based Immunochemotherapy of Intracranial Glioblastoma Treatment, Adv. Mater 30 (2018) 1705054. doi: 10.1002/adma.201705054. [DOI] [PubMed] [Google Scholar]
- [145].de Gracia Lux C, Joshi-Barr S, Nguyen T, Mahmoud E, Schopf E, Fomina N, Almutairi A, Biocompatible Polymeric Nanoparticles Degrade and Release Cargo in Response to Biologically Relevant Levels of Hydrogen Peroxide, J. Am. Chem. Soc 134 (2012) 15758–15764. doi: 10.1021/ja303372u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Feng S, Hu Y, Peng S, Han S, Tao H, Zhang Q, Xu X, Zhang J, Hu H, Nanoparticles responsive to the inflammatory microenvironment for targeted treatment of arterial restenosis, Biomaterials. 105 (2016) 167–184. doi: 10.1016/J.BIOMATERIALS.2016.08.003. [DOI] [PubMed] [Google Scholar]
- [147].Deng Z, Qian Y, Yu Y, Liu G, Hu J, Zhang G, Liu S, Engineering Intracellular Delivery Nanocarriers and Nanoreactors from Oxidation-Responsive Polymersomes via Synchronized Bilayer Cross-Linking and Permeabilizing Inside Live Cells, J. Am. Chem. Soc 138 (2016) 10452–10466. doi: 10.1021/jacs.6b04115. [DOI] [PubMed] [Google Scholar]
- [148].Bandyopadhyay A, Gao J, Iminoboronate-Based Peptide Cyclization That Responds to pH, Oxidation, and Small Molecule Modulators, J. Am. Chem. Soc 138 (2016) 2098–2101. doi: 10.1021/jacs.5b12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Hutin M, Bernardinelli G, Nitschke JR, An Iminoboronate Construction Set for Subcomponent Self-Assembly, Chem. - A Eur. J 14 (2008) 4585–4593. doi: 10.1002/chem.200800074. [DOI] [PubMed] [Google Scholar]
- [150].Lv W, Xu J, Wang X, Li X, Xu Q, Xin H, Bioengineered Boronic Ester Modified Dextran Polymer Nanoparticles as Reactive Oxygen Species Responsive Nanocarrier for Ischemic Stroke Treatment, ACS Nano. 12 (2018) 5417–5426. doi: 10.1021/acsnano.8b00477. [DOI] [PubMed] [Google Scholar]
- [151].Xiang J, Liu X, Zhou Z, Zhu D, Zhou Q, Piao Y, Jiang L, Tang J, Liu X, Shen Y, Reactive Oxygen Species (ROS)-Responsive Charge-Switchable Nanocarriers for Gene Therapy of Metastatic Cancer, ACS Appl. Mater. Interfaces 10 (2018) 43352–43362. doi: 10.1021/acsami.8b13291. [DOI] [PubMed] [Google Scholar]
- [152].Yang L, Yin T, Liu Y, Sun J, Zhou Y, Liu J, Gold nanoparticle-capped mesoporous silica-based H 2 O 2 -responsive controlled release system for Alzheimer’s disease treatment, Acta Biomater. 46 (2016) 177–190. doi: 10.1016/j.actbio.2016.09.010. [DOI] [PubMed] [Google Scholar]
- [153].Liao Y-H, Chang Y-J, Yoshiike Y, Chang Y-C, Chen Y-R, Negatively Charged Gold Nanoparticles Inhibit Alzheimer’s Amyloid-β Fibrillization, Induce Fibril Dissociation, and Mitigate Neurotoxicity, Small. 8 (2012) 3631–3639. doi: 10.1002/smll.201201068. [DOI] [PubMed] [Google Scholar]
- [154].Zhang T, Chen X, Xiao C, Zhuang X, Chen X, Synthesis of a phenylboronic ester-linked PEG-lipid conjugate for ROS-responsive drug delivery, Polym. Chem 8 (2017) 6209–6216. doi: 10.1039/C7PY00915A. [DOI] [Google Scholar]
- [155].Kim S, Seong K, Kim O, Kim S, Seo H, Lee M, Khang G, Lee D, Polyoxalate Nanoparticles as a Biodegradable and Biocompatible Drug Delivery Vehicle, Biomacromolecules. 11 (2010) 555–560. doi: 10.1021/bm901409k. [DOI] [PubMed] [Google Scholar]
- [156].Lee D, Bae S, Ke Q, Lee J, Song B, Karumanchi SA, Khang G, Choi HS, Kang PM, Hydrogen peroxide-responsive copolyoxalate nanoparticles for detection and therapy of ischemia–reperfusion injury, J. Control. Release 172 (2013) 1102–1110. doi: 10.1016/J.JCONREL.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Li J, Ke W, Wang L, Huang M, Yin W, Zhang P, Chen Q, Ge Z, Self-sufficing H2O2-responsive nanocarriers through tumor-specific H2O2 production for synergistic oxidation-chemotherapy, J. Control. Release 225 (2016) 64–74. doi: 10.1016/J.JCONREL.2016.01.029. [DOI] [PubMed] [Google Scholar]
- [158].Berwin Singh SV, Park H, Khang G, Lee D, Hydrogen peroxide-responsive engineered polyoxalate nanoparticles for enhanced wound healing, Macromol. Res 26 (2018) 40–47. doi: 10.1007/s13233-018-6003-6. [DOI] [Google Scholar]
- [159].Bae S, Park M, Kang C, Dilmen S, Kang TH, Kang DG, Ke Q, Lee SU, Lee D, Kang PM, Hydrogen Peroxide Responsive Nanoparticle Reduces Myocardial Ischemia/Reperfusion Injury, J. Am. Heart Assoc 5 (2016). doi: 10.1161/JAHA.116.003697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Lee JY, Jang YW, Kang HS, Moon H, Sim SS, Kim CJ, Anti-inflammatory action of phenolic compounds fromGastrodia elata root, Arch. Pharm. Res 29 (2006) 849–858. doi: 10.1007/BF02973905. [DOI] [PubMed] [Google Scholar]
- [161].Lee D, Bae S, Hong D, Lim H, Yoon JH, Hwang O, Park S, Ke Q, Khang G, Kang PM, H2O2-responsive molecularly engineered polymer nanoparticles as ischemia/reperfusion-targeted nanotherapeutic agents, Sci. Rep 3 (2013) 2233. doi: 10.1038/srep02233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Chen Q, Feng L, Liu J, Zhu W, Dong Z, Wu Y, Liu Z, Intelligent Albumin-MnO 2 Nanoparticles as pH-/H 2 O 2 -Responsive Dissociable Nanocarriers to Modulate Tumor Hypoxia for Effective Combination Therapy, Adv. Mater 28 (2016) 7129–7136. doi: 10.1002/adma.201601902. [DOI] [PubMed] [Google Scholar]
- [163].Alhadlaq HA, Akhtar MJ, Ahamed M, Different cytotoxic and apoptotic responses of MCF-7 and HT1080 cells to MnO2 nanoparticles are based on similar mode of action, Toxicology. 411 (2019) 71–80. doi: 10.1016/j.tox.2018.10.023. [DOI] [PubMed] [Google Scholar]
- [164].Pereira DR, Tapeinos C, Rebelo AL, Oliveira JM, Reis RL, Pandit A, Scavenging Nanoreactors that Modulate Inflammation, Adv. Biosyst 2 (2018) 1800086. doi: 10.1002/adbi.201800086. [DOI] [Google Scholar]
- [165].Li T, Shi T, Li X, Zeng S, Yin L, Pu Y, Li T, Shi T, Li X, Zeng S, Yin L, Pu Y, Effects of Nano-MnO2 on Dopaminergic Neurons and the Spatial Learning Capability of Rats, Int. J. Environ. Res. Public Health 11 (2014) 7918–7930. doi: 10.3390/ijerph110807918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Nosrati N, Hassanpour-Ezzati M, Zahra Mousavi S, Safi Rahmanifar M, Rezagholiyan S, Comparison of MnO 2 nanoparticles and microparticles distribution in CNS and muscle and effect on acute pain threshold in rats Size dependent effect of MnO 2 on acute pain threshold in rat, 2014. [Google Scholar]
- [167].Wu H, Li F, Wang S, Lu J, Li J, Du Y, Sun X, Chen X, Gao J, Ling D, Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing, Biomaterials. 151 (2018) 66–77. doi: 10.1016/J.BIOMATERIALS.2017.10.018. [DOI] [PubMed] [Google Scholar]
- [168].Selvaraj V, Nepal N, Rogers S, Manne NDPK, Arvapalli R, Rice KM, Asano S, Fankhanel E, Ma JJ, Shokuhfar T, Maheshwari M, Blough ER, Inhibition of MAP kinase/NF-kB mediated signaling and attenuation of lipopolysaccharide induced severe sepsis by cerium oxide nanoparticles, Biomaterials. 59 (2015) 160–171. doi: 10.1016/J.BIOMATERIALS.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Soh M, Kang D-W, Jeong H-G, Kim D, Kim DY, Yang W, Song C, Baik S, Choi I-Y, Ki S-K, Kwon HJ, Kim T, Kim CK, Lee S-H, Hyeon T, Ceria-Zirconia Nanoparticles as an Enhanced Multi-Antioxidant for Sepsis Treatment, Angew. Chemie 129 (2017) 11557–11561. doi: 10.1002/ange.201704904. [DOI] [PubMed] [Google Scholar]
- [170].Jin Kwon H, Cha M-Y, Kim D, Kyu Kim D, Soh M, Shin K, Hyeon T, Mook-Jung I, Mitochondria-Targeting Ceria Nanoparticles as Antioxidants for Alzheimer’s Disease, ACS Nano. 10 (2016). doi: 10.1021/acsnano.5b08045. [DOI] [PubMed] [Google Scholar]
- [171].Heckman KL, Decoteau W, Estevez A, Reed KJ, Costanzo W, Sanford D, Leiter JC, Clauss J, Knapp K, Gomez C, Mullen P, Rathbun E, Prime K, Marini J, Patchefsky J, Patchefsky AS, Hailstone RK, Erlichman JS, Custom Cerium Oxide Nanoparticles Protect against a Free Radical Mediated Autoimmune Degenerative Disease in the Brain, (2013). doi: 10.1021/nn403743b. [DOI] [PubMed] [Google Scholar]
- [172].Cimini A, D’Angelo B, Das S, Gentile R, Benedetti E, Singh V, Monaco AM, Santucci S, Seal S, Antibody-conjugated PEGylated cerium oxide nanoparticles for specific targeting of Aβ aggregates modulate neuronal survival pathways, Acta Biomater. 8 (2012) 2056–2067. doi: 10.1016/J.ACTBIO.2012.01.035. [DOI] [PubMed] [Google Scholar]
- [173].Ma Y, Dong W-F, Hempenius MA, Möhwald H, Julius Vancso G, Redox-controlled molecular permeability of composite-wall microcapsules, Nat. Mater 5 (2006) 724–729. doi: 10.1038/nmat1716. [DOI] [PubMed] [Google Scholar]
- [174].Staff RH, Gallei M, Mazurowski M, Rehahn M, Berger R, Landfester K, Crespy D, Patchy Nanocapsules of Poly(vinylferrocene)-Based Block Copolymers for Redox-Responsive Release, ACS Nano. 6 (2012) 9042–9049. doi: 10.1021/nn3031589. [DOI] [PubMed] [Google Scholar]
- [175].Nakahata M, Takashima Y, Yamaguchi H, Harada A, Redox-responsive self-healing materials formed from host–guest polymers, Nat. Commun 2 (2011) 511. doi: 10.1038/ncomms1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].N VR, Han HS, Lee H, Nguyen VQ, Jeon S, Jung D-W, Lee J, Yi G-R, Park JH, ROS-responsive mesoporous silica nanoparticles for MR imaging-guided photodynamically maneuvered chemotherapy, Nanoscale. 10 (2018) 9616–9627. doi: 10.1039/C8NR00888D. [DOI] [PubMed] [Google Scholar]
- [177].Yuan Y, Zhang C-J, Liu B, A Photoactivatable AIE Polymer for Light-Controlled Gene Delivery: Concurrent Endo/Lysosomal Escape and DNA Unpacking, Angew. Chemie Int. Ed 54 (2015) 11419–11423. doi: 10.1002/anie.201503640. [DOI] [PubMed] [Google Scholar]
- [178].Bio M, Nkepang G, You Y, Click and photo-unclick chemistry of aminoacrylate for visible light-triggered drug release, Chem. Commun 48 (2012) 6517. doi: 10.1039/c2cc32373g. [DOI] [PubMed] [Google Scholar]
- [179].Shen Y, Cao B, Snyder NR, Woeppel KM, Eles JR, Cui XT, ROS responsive resveratrol delivery from LDLR peptide conjugated PLA-coated mesoporous silica nanoparticles across the blood–brain barrier, J. Nanobiotechnology 16 (2018) 13. doi: 10.1186/s12951-018-0340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Lee H, Lee K, Kim IK, Park TG, Synthesis, characterization, and in vivo diagnostic applications of hyaluronic acid immobilized gold nanoprobes, Biomaterials. 29 (2008) 4709–4718. doi: 10.1016/J.BIOMATERIALS.2008.08.038. [DOI] [PubMed] [Google Scholar]
- [181].Lee H, Lee K, Kim I-K, Park TG, Fluorescent Gold Nanoprobe Sensitive to Intracellular Reactive Oxygen Species, Adv. Funct. Mater 19 (2009) 1884–1890. doi: 10.1002/adfm.200801838. [DOI] [Google Scholar]
- [182].Ramos S, Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways, Mol. Nutr. Food Res 52 (2008) 507–526. doi: 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]
- [183].Ashafaq M, Raza SS, Khan MM, Ahmad A, Javed H, Ahmad ME, Tabassum R, Islam F, Siddiqui MS, Safhi MM, Islam F, Catechin hydrate ameliorates redox imbalance and limits inflammatory response in focal cerebral ischemia, Neurochem. Res 37 (2012) 1747–1760. doi: 10.1007/s11064-012-0786-1. [DOI] [PubMed] [Google Scholar]
- [184].Wang C, Wang J, Zhang X, Yu S, Wen D, Hu Q, Ye Y, Bomba H, Hu X, Liu Z, Dotti G, Gu Z, In situ formed reactive oxygen species-responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy., Sci. Transl. Med 10 (2018) eaan3682. doi: 10.1126/scitranslmed.aan3682. [DOI] [PubMed] [Google Scholar]
- [185].Hong H-Y, Choi JS, Kim YJ, Lee HY, Kwak W, Yoo J, Lee J-T, Kwon T-H, Kim I-S, Han H-S, Lee B-H, Detection of apoptosis in a rat model of focal cerebral ischemia using a homing peptide selected from in vivo phage display., J. Control. Release 131 (2008) 167–72. doi: 10.1016/j.jconrel.2008.07.020. [DOI] [PubMed] [Google Scholar]
- [186].Gautam DK, Misro MM, Chaki SP, Sehgal N, H2O2 at physiological concentrations modulates Leydig cell function inducing oxidative stress and apoptosis, Apoptosis. 11 (2006) 39–46. doi: 10.1007/s10495-005-3087-1. [DOI] [PubMed] [Google Scholar]
- [187].Kim DK, Cho ES, Um H-D, Caspase-Dependent and -Independent Events in Apoptosis Induced by Hydrogen Peroxide, Exp. Cell Res 257 (2000) 82–88. doi: 10.1006/EXCR.2000.4868. [DOI] [PubMed] [Google Scholar]
- [188].Coakley RJ, Taggart C, Mcelvaney NG, O’neill SJ, Cytosolic pH and the inflammatory microenvironment modulate cell death in human neutrophils after phagocytosis, 2002. [DOI] [PubMed] [Google Scholar]
- [189].Takahashi K, Relationship between Acidity and Swelling in the Brain, 1966. [DOI] [PubMed] [Google Scholar]
- [190].Tohill M, Mantovani C, Wiberg M, Terenghi G, Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration, Neurosci. Lett 362 (2004) 200–203. doi: 10.1016/J.NEULET.2004.03.077. [DOI] [PubMed] [Google Scholar]
- [191].Crigler L, Robey RC, Asawachaicharn A, Gaupp D, Phinney DG, Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis, Exp. Neurol 198 (2006) 54–64. doi: 10.1016/J.EXPNEUROL.2005.10.029. [DOI] [PubMed] [Google Scholar]
- [192].Cooney DS, Wimmers EG, Ibrahim Z, Grahammer J, Christensen JM, Brat GA, Wu LW, Sarhane KA, Lopez J, Wallner C, Furtmüller GJ, Yuan N, Pang J, Sarkar K, Lee WPA, Brandacher G, Mesenchymal Stem Cells Enhance Nerve Regeneration in a Rat Sciatic Nerve Repair and Hindlimb Transplant Model, Sci. Rep 6 (2016) 31306. doi: 10.1038/srep31306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Liu Y, Ai K, Liu J, Deng M, He Y, Lu L, Dopamine-Melanin Colloidal Nanospheres: An Efficient Near-Infrared Photothermal Therapeutic Agent for In Vivo Cancer Therapy, Adv. Mater 25 (2013) 1353–1359. doi: 10.1002/adma.201204683. [DOI] [PubMed] [Google Scholar]
- [194].Liu Y, Ai K, Ji X, Askhatova D, Du R, Lu L, Shi J, Comprehensive Insights into the Multi-Antioxidative Mechanisms of Melanin Nanoparticles and Their Application To Protect Brain from Injury in Ischemic Stroke, J. Am. Chem. Soc 139 (2017) 856–862. doi: 10.1021/jacs.6b11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Yang F, Hu S, Zhang Y, Cai X, Huang Y, Wang F, Wen S, Teng G, Gu N, A Hydrogen Peroxide-Responsive O 2 Nanogenerator for Ultrasound and Magnetic-Resonance Dual Modality Imaging, Adv. Mater 24 (2012) 5205–5211. doi: 10.1002/adma.201202367. [DOI] [PubMed] [Google Scholar]
- [196].Ohno Y, Basics concepts and clinical applications of oxygen-enhanced MR imaging, Eur. J. Radiol 64 (2007) 320–328. doi: 10.1016/J.EJRAD.2007.08.006. [DOI] [PubMed] [Google Scholar]
- [197].Lee KH, Won CY, Chu CC, Gitsov I, Hydrolysis of biodegradable polymers by superoxide ions, J. Polym. Sci. Part A Polym. Chem 37 (1999) 3558–3567. doi:. [DOI] [Google Scholar]
- [198].Jahn M, Baynes JW, Spiteller G, The reaction of hyaluronic acid and its monomers, glucuronic acid and N-acetylglucosamine, with reactive oxygen species, Carbohydr. Res 321 (1999) 228–234. doi: 10.1016/S0008-6215(99)00186-X. [DOI] [PubMed] [Google Scholar]
- [199].Shaw LM, Korecka M, Clark CM, Lee VM-Y, Trojanowski JQ, Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics, Nat. Rev. Drug Discov 6 (2007) 295–303. doi: 10.1038/nrd2176. [DOI] [PubMed] [Google Scholar]
- [200].Mathew A, Fukuda T, Nagaoka Y, Hasumura T, Morimoto H, Yoshida Y, Maekawa T, Venugopal K, Kumar DS, Curcumin Loaded-PLGA Nanoparticles Conjugated with Tet-1 Peptide for Potential Use in Alzheimer’s Disease, PLoS One. 7 (2012) e32616. doi: 10.1371/journal.pone.0032616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Sun D, Li N, Zhang W, Zhao Z, Mou Z, Huang D, Liu J, Wang W, Design of PLGA-functionalized quercetin nanoparticles for potential use in Alzheimer’s disease, Colloids Surfaces B Biointerfaces. 148 (2016) 116–129. doi: 10.1016/J.COLSURFB.2016.08.052. [DOI] [PubMed] [Google Scholar]
- [202].Liu Y, Zhou H, Yin T, Gong Y, Yuan G, Chen L, Liu J, Quercetin-modified gold-palladium nanoparticles as a potential autophagy inducer for the treatment of Alzheimer’s disease, J. Colloid Interface Sci 552 (2019) 388–400. doi: 10.1016/J.JCIS.2019.05.066. [DOI] [PubMed] [Google Scholar]
- [203].Joshi SA, Chavhan SS, Sawant KK, Rivastigmine-loaded PLGA and PBCA nanoparticles: Preparation, optimization, characterization, in vitro and pharmacodynamic studies, Eur. J. Pharm. Biopharm 76 (2010) 189–199. doi: 10.1016/J.EJPB.2010.07.007. [DOI] [PubMed] [Google Scholar]
- [204].Wilson B, Samanta MK, Santhi K, Kumar KPS, Paramakrishnan N, Suresh B, Poly(n-butylcyanoacrylate) nanoparticles coated with polysorbate 80 for the targeted delivery of rivastigmine into the brain to treat Alzheimer’s disease, Brain Res. 1200 (2008) 159–168. doi: 10.1016/J.BRAINRES.2008.01.039. [DOI] [PubMed] [Google Scholar]
- [205].Tsai M-J, Huang Y-B, Wu P-C, Fu Y-S, Kao Y-R, Fang J-Y, Tsai Y-H, Oral Apomorphine Delivery from Solid Lipid Nanoparticles with Different Monostearate Emulsifiers: Pharmacokinetic and Behavioral Evaluations, J. Pharm. Sci 100 (2011) 547–557. doi: 10.1002/JPS.22285. [DOI] [PubMed] [Google Scholar]
- [206].Md S, Khan RA, Mustafa G, Chuttani K, Baboota S, Sahni JK, Ali J, Bromocriptine loaded chitosan nanoparticles intended for direct nose to brain delivery: Pharmacodynamic, Pharmacokinetic and Scintigraphy study in mice model, Eur. J. Pharm. Sci 48 (2013) 393–405. doi: 10.1016/J.EJPS.2012.12.007. [DOI] [PubMed] [Google Scholar]
- [207].Gambaryan PY, Kondrasheva IG, Severin ES, Guseva AA, Kamensky AA, Increasing the Efficiency of Parkinson’s Disease Treatment Using a poly(lactic-coglycolic acid) (PLGA) Based L-DOPA Delivery System., Exp. Neurobiol 23 (2014) 246–52. doi: 10.5607/en.2014.23.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Saraiva C, Praça C, Ferreira R, Santos T, Ferreira L, Bernardino L, Nanoparticlemediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases, J. Control. Release 235 (2016) 34–47. doi: 10.1016/J.JCONREL.2016.05.044. [DOI] [PubMed] [Google Scholar]
- [209].Yemisci M, Caban S, Gursoy-Ozdemir Y, Lule S, Novoa-Carballal R, Riguera R, Fernandez-Megia E, Andrieux K, Couvreur P, Capan Y, Dalkara T, Systemically administered brain-targeted nanoparticles transport peptides across the blood-brain barrier and provide neuroprotection, J. Cereb. Blood Flow Metab 35 (2015) 469–475. doi: 10.1038/jcbfm.2014.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Koffie RM, Farrar CT, Saidi L-J, William CM, Hyman BT, Spires-Jones TL, Nanoparticles enhance brain delivery of blood-brain barrier-impermeable probes for in vivo optical and magnetic resonance imaging, Proc. Natl. Acad. Sci 108 (2011) 18837–18842. doi: 10.1073/pnas.1111405108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Chaumeil MM, Valette J, Baligand C, Brouillet E, Hantraye P, Bloch G, Gaura V, Rialland A, Krystkowiak P, Verny C, Damier P, Remy P, Bachoud-Levi A-C, Carlier P, Lebon V, pH as a Biomarker of Neurodegeneration in Huntington’s Disease: A Translational Rodent-Human MRS Study, J. Cereb. Blood Flow Metab 32 (2012) 771–779. doi: 10.1038/jcbfm.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]