Abstract
Background
Chlorpromazine is an aliphatic phenothiazine, which is one of the widely‐used typical antipsychotic drugs. Chlorpromazine is reliable for its efficacy and one of the most tested first generation antipsychotic drugs. It has been used as a ‘gold standard’ to compare the efficacy of older and newer antipsychotic drugs. Expensive new generation drugs are heavily marketed worldwide as a better treatment for schizophrenia, but this may not be the case and an unnecessary drain on very limited resources.
Objectives
To compare the effects of chlorpromazine with atypical or second generation antipsychotic drugs, for the treatment of people with schizophrenia.
Search methods
We searched the Cochrane Schizophrenia Group's Trials Register up to 23 September 2013.
Selection criteria
We included randomised controlled trials (RCTs) that compared chlorpromazine with any other atypical antipsychotic drugs for treating people with schizophrenia. Adults (as defined in each trial) diagnosed with schizophrenia, including schizophreniform, schizoaffective and delusional disorders were included in this review.
Data collection and analysis
At least two review authors independently screened the articles identified in the literature search against the inclusion criteria and extracted data from included trials. For homogeneous dichotomous data, we calculated the risk ratio (RR) and the 95% confidence intervals (CIs). For continuous data, we determined the mean difference (MD) values and 95% CIs. We assessed the risk of bias in included studies and rated the quality of the evidence using the GRADE approach.
Main results
This review includes 71 studies comparing chlorpromazine to olanzapine, risperidone or quetiapine. None of the included trials reported any data on economic costs.
1. Chlorpromazine versus olanzapine
In the short term, there appeared to be a significantly greater clinical response (as defined in each study) in people receiving olanzapine (3 RCTs, N = 204; RR 2.34, 95% CI 1.37 to 3.99, low quality evidence). There was no difference between drugs for relapse (1 RCT, N = 70; RR 1.5, 95% CI 0.46 to 4.86, very low quality evidence), nor in average endpoint score using the Brief Psychiatric Rating Scale (BPRS) for mental state (4 RCTs, N = 245; MD 3.21, 95% CI −0.62 to 7.05,very low quality evidence). There were significantly more extrapyramidal symptoms experienced amongst people receiving chlorpromazine (2 RCTs, N = 298; RR 34.47, 95% CI 4.79 to 248.30,very low quality evidence). Quality of life ratings using the general quality of life interview (GQOLI) ‐ physical health subscale were more favourable with people receiving olanzapine (1 RCT, N = 61; MD −10.10, 95% CI −13.93 to −6.27, very low quality evidence). There was no difference between groups for people leaving the studies early (3 RCTs, N = 139; RR 1.69, 95% CI 0.45 to 6.40, very low quality evidence).
2. Chlorpromazine versus risperidone
In the short term, there appeared to be no difference in clinical response (as defined in each study) between chlorpromazine or risperidone (7 RCTs, N = 475; RR 0.84, 95% CI 0.53 to 1.34, low quality of evidence), nor in average endpoint score using the BPRS for mental state 4 RCTs, N = 247; MD 0.90, 95% CI −3.49 to 5.28, very low quality evidence), or any observed extrapyramidal adverse effects (3 RCTs, N = 235; RR 1.7, 95% CI 0.85 to 3.40,very low quality evidence). Quality of life ratings using the QOL scale were significantly more favourable with people receiving risperidone (1 RCT, N = 100; MD −14.2, 95% CI −20.50 to −7.90, very low quality evidence). There was no difference between groups for people leaving the studies early (one RCT, N = 41; RR 0.21, 95% CI 0.01 to 4.11, very low quality evidence).
3. Chlorpromazine versus quetiapine
In the short term, there appeared to be no difference in clinical response (as defined in each study) between chlorpromazine or quetiapine (28 RCTs, N = 3241; RR 0.93, 95% CI 0.81 to 1.06, moderate quality evidence) nor in average endpoint score using the BPRS for mental state (6 RCTs, N = 548; MD −0.18, 95% CI −1.23 to 0.88, very low quality evidence). Quality of life ratings using the GQOL1‐74 scale were significantly more favourable with people receiving quetiapine (1 RCT, N = 59; MD −6.49, 95% CI −11.30 to −1.68, very low quality evidence). Significantly more people receiving chlorpromazine experienced extrapyramidal adverse effects (8 RCTs, N = 644; RR 8.03, 95% CI 4.78 to 13.51, low quality of evidence). There was no difference between groups for people leaving the studies early in the short term (12 RCTs, N = 1223; RR 1.04, 95% CI 0.77 to 1.41,moderate quality evidence).
Authors' conclusions
Most included trials included inpatients from hospitals in China. Therefore the results of this Cochrane review are more applicable to the Chinese population. Mostincluded trials were short term studies, therefore we cannot comment on the medium and long term use of chlorpromazine compared to atypical antipsychotics. Low qualityy evidence suggests chlorpromazine causes more extrapyramidal adverse effects. However, all studiesused varying dose ranges, and higher doses would be expected to be associated with more adverse events.
Plain language summary
Chlorpromazine compared with newer atypical antipsychotics
People with schizophrenia often hear voices or see things (hallucinations) and have strange beliefs (delusions). The main treatment for people with these symptoms of schizophrenia is antipsychotic drugs. Chlorpromazine was one of the first drugs discovered to be effective for treating people with schizophrenia. It remains one of the most commonly used and inexpensive treatments. However, being an older drug (typical or first generation) it also has serious side effects, including blurred vision, a dry mouth, tremors or uncontrollable shaking, depression, muscle stiffness and restlessness.
In this Cochrane review we examined the effects of chlorpromazine for treating people with schizophrenia compared with newer antipsychotic drugs.
We searched the literature for randomised controlled trials up to 23 September 2013, and included 71 trials. The included studies compared chlorpromazine with three newer antipsychotics: risperidone, quetiapine or olanzapine. Most included trials were short term studies and undertaken in China. Based on low quality evidence, we found that chlorpromazine is not much different to risperidone or quetiapine but is associated with more side effects. More favourable results were found for olanzapine with those receiving olanzapine experiencing fewer side effects and greater improvements in global state and quality of life than those receiving chlorpromazine, but again this is based on low quality evidence. Larger, longer, better conducted and reported trials should focus on important outcomes such as quality of life, levels of satisfaction with treatment or care, relapse, costs and hospital discharge or admission. Also, more international studies are needed. Outpatient treatment was under‐represented in the included studies, and future research should also include work with this group of people.
Due to the limitations of evidence in this Cochrane review, it is difficult to draw firm conclusions. Chlorpormazine is available widely, is comparable with the newer antipsychotics and is relatively cheap so despite its propensity to cause side effects, is likely to remain one of the benchmark antipsychotics.
The plain language summary has been written by a consumer. Ben Gray: Senior Peer Researcher, McPin Foundation. http://mcpin.org/.
Summary of findings
Summary of findings for the main comparison. CHLORPROMAZINE versus OLANZAPINE for schizophrenia.
| Chlorpromazine versus olanzapine for schizophrenia | ||||||
| Patient or population: people with schizophrenia Settings: inpatient and outpatient Intervention: chlorpromazine Comparison: olanzapine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Olanzapine | Chlorpromazine | |||||
| No significant clinical response: short term (up to 6 months) Follow‐up: 6 to 12 weeks | Low1 | RR 2.34 (1.37 to 3.99) | 204 (3 studies) | ⊕⊕⊝⊝ low2,3 | ‐ | |
| 700 per 1000 | 1000 per 1000 (959 to 1000) | |||||
| High1 | ||||||
| 190 per 1000 | 445 per 1000 (260 to 758) | |||||
| Relapse: long term (over 12 months) Follow‐up: mean 2 years | Study population | RR 1.5 (0.46 to 4.86) | 70 (1 study) | ⊕⊝⊝⊝ very low3,4 | ‐ | |
| 114 per 1000 | 171 per 1000 (53 to 555) | |||||
| Moderate | ||||||
| 114 per 1000 | 171 per 1000 (52 to 554) | |||||
| Mental state: short term (up to 6 months) BRPS average endpoint score (high = poor) Follow‐up: 6 to 12 weeks | The mean mental state: short term (up to 6 months) in the intervention groups was 3.21 higher (0.62 lower to 7.05 higher) | 245 (4 studies) | ⊕⊝⊝⊝ very low2,3,5,6,7 | ‐ | ||
| Adverse effects: any observed extrapyramidal symptoms ‐ short term (up to 6 months) Follow‐up: mean 8 weeks | Low | RR 34.47 (4.79 to 248.3) | 298 (2 studies) | ⊕⊝⊝⊝ very low2,3,8 | ‐ | |
| 10 per 1000 | 345 per 1000 (48 to 1000) | |||||
| Moderate | ||||||
| 100 per 1000 | 1000 per 1000 (479 to 1000) | |||||
| High | ||||||
| 250 per 1000 | 1000 per 1000 (1000 to 1000) | |||||
| Quality of life: short term (up to 6 months) GQOLI‐physical health subscale score (high = good) Follow‐up: mean 8 weeks | The mean quality of life: short term (up to 6 months) in the intervention groups was 10.10 lower (13.93 to 6.27 lower) | 61 (1 study) | ⊕⊝⊝⊝ very low2,3,7,9,10 | ‐ | ||
| Leaving the study early due to any reason: short term (up to 6 months) Follow‐up: mean 6 weeks | Study population11 | RR 1.69 (0.45 to 6.4) | 139 (3 studies) | ⊕⊝⊝⊝ very low3,12,13 | ‐ | |
| 159 per 1000 | 268 per 1000 (71 to 1000) | |||||
| Moderate11 | ||||||
| 229 per 1000 | 387 per 1000 (103 to 1000) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Control risk: the control risks are representative of the control group risks of the study population. 2Risk of bias: rated serious ‐ most included studies had unclear risk of bias in terms of allocation and blinding, hence selection and detection bias are likely to be present. Two studies also had high risk of reporting bias. 3Publication bias: strongly suspected ‐ we identified only small trial(s) from China for this outcome. Publication bias is highly suspected. 4Imprecision: serious ‐ only one small trial with unclear risk of selection and detection bias contributed data to this outcome. The result was imprecise with few event and a wide CI. 5Inconsistency: serious ‐ unexplained heterogeneity present, suggesting different magnitude of effect. 6Indirectness: serious ‐ binary outcome assessing mental state is unavailable. We, therefore, employed BPRS score as an alternative indicator. 7Imprecision: serious ‐ a relatively wide CI around the point of estimate of effect. Sample size is smaller than the optimal information size. 8Imprecision: serious ‐ although significant difference was observed between groups, the CI was wide (95% CI 4.97 to 248.3). 9Indirectness: serious ‐ binary outcome for quality of life is unavailable. We, thus, used outcome from QOL‐ physical health subscale as an alternative indicator. 10Imprecision: serious ‐ although significant difference was observed between group, the total sample size is smaller than the optimal information size. 11Control risk: the control risks in the study population are very close to the moderate risk observed here. Thus we adopted it to represent the control risk. 12Risk of bias: serious ‐ most included studies that contributed data to this outcome had high risk of bias with the selection, detection and reporting of the result. 13Imprecision: serious ‐ estimate of effect was not significant and the total sample size is smaller than the desired optimal information size.
Summary of findings 2. CHLORPROMAZINE versus RISPERIDONE for schizophrenia.
| Chlorpromazine versus risperidone for schizophrenia | ||||||
| Patient or population: people with schizophrenia Settings: inpatient and outpatient Intervention: chlorpromazine Comparison: risperidone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risperidone | Chlorpromazine | |||||
| No significant clinical response ‐ short term (up to 6 months) Follow‐up: median 8 to 12 weeks | Low1 | RR 0.84 (0.53 to 1.34) | 475 (7 studies) | ⊕⊕⊝⊝ low2,3 | ‐ | |
| 60 per 1000 | 50 per 1000 (32 to 80) | |||||
| Moderate1 | ||||||
| 114 per 1000 | 96 per 1000 (60 to 153) | |||||
| High1 | ||||||
| 240 per 1000 | 202 per 1000 (127 to 322) | |||||
| Mental state: short term (up to 6 months) BPRS endpoint scale score (high = poor) Follow‐up: 6 to 12 weeks | The mean mental state: short term (up to 6 months) in the intervention groups was 0.9 higher (3.49 lower to 5.28 higher) | 247 (4 studies) | ⊕⊝⊝⊝ very low2,4,5,6,7 | ‐ | ||
| Adverse effects: any observed extrapyramidal symptoms ‐ short term (up to 6 months) Follow‐up: 8 to 12 weeks | Low1 | RR 1.7 (0.85 to 3.4) | 235 (3 studies) | ⊕⊝⊝⊝ very low2,6,7 | ‐ | |
| 130 per 1000 | 221 per 1000 (111 to 442) | |||||
| High1 | ||||||
| 240 per 1000 | 408 per 1000 (204 to 816) | |||||
| Quality of life: short term (up to 6 months) QOL endpoint scale score (high = good) Follow‐up: mean 12 weeks | The mean quality of life: short term (up to 6 months) in the intervention groups was 14.2 lower (20.5 to 7.9 lower) | 100 (1 study) | ⊕⊝⊝⊝ very low7,8,9,10 | ‐ | ||
| Leaving the study early due to adverse effects ‐ short term (up to 6 months) Follow‐up: mean 8 weeks | Study population | RR 0.21 (0.01 to 4.11) | 41 (1 study) | ⊕⊝⊝⊝ very low7,10 | ‐ | |
| 95 per 1000 | 20 per 1000 (1 to 391) | |||||
| Moderate | ||||||
| 95 per 1000 | 20 per 1000 (1 to 390) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1Control risk: the control risks are representative of those observed in the study population. 2Risk of bias: serious ‐ most of the included studies had unclear risk of bias in terms of allocation and blinding, hence selection and detection bias are likely to be present. Some of the studies also had high risk of reporting bias. 3Publication bias: strongly suspected ‐ only Chinese studies with relatively small sample size were identified. Publication bias is highly likely. 4Inconsistency: serious ‐ unexplained heterogeneity present, suggesting different magnitude of effect. 5Indirectness: serious ‐ binary outcome assessing mental state is unavailable. We, therefore, employed BPRS score as an alternative indicator. 6Imprecision: serious ‐ although the CI around the estimate of effect relatively tight, the result was not significant and sample size was smaller than the optimal information size. 7Publication bias: strongly suspected ‐ only one study with unclear risk of selection and detection bias available for this outcome. 8Indirectness: serious ‐ binary outcome for quality of life was not available. Therefore, we adopted QOL score as an indicator. 9Imprecision: serious ‐ estimate of effect was significant with tight CI, but the study sample size is smaller than the optimal information size. 10Imprecision: serious ‐ estimate of effect was not significant and with relatively wide CI. Sample size was smaller than the optimal information size.
Summary of findings 3. CHLORPROMAZINE versus QUETIAPINE for schizophrenia.
| Chlorpromazine versus quetiapine for schizophrenia | ||||||
| Patient or population: people with schizophrenia Settings: inpatient and outpatient Intervention: chlorpromazine Comparison: quetiapine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Quetiapine | Chlorpromazine | |||||
| No significant clinical response: short term (up to 6 months) Follow‐up: 4 to 16 weeks | Low1 | RR 0.93 (0.81 to 1.06) | 3241 (28 studies) | ⊕⊕⊕⊝ moderate2 | ‐ | |
| 70 per 1000 | 65 per 1000 (57 to 74) | |||||
| Moderate1 | ||||||
| 128 per 1000 | 119 per 1000 (104 to 136) | |||||
| High1 | ||||||
| 360 per 1000 | 335 per 1000 (292 to 382) | |||||
| Mental state: short term (up to 6 months) BPRS endpoint scale score (high = poor) Follow‐up: 6 to 12 weeks | The mean mental state: short term (up to 6 months) in the intervention groups was 0.18 lower (1.23 lower to 0.88 higher) | 548 (6 studies) | ⊕⊝⊝⊝ very low2,3,4,5 | ‐ | ||
| Quality of life: short term (up to 6 months) GQOL1‐74 endpoint scale score (high = good) Follow‐up: mean 12 weeks | The mean quality of life: short term (up to 6 months) in the intervention groups was 6.49 lower (11.3 to 1.68 lower) | 59 (1 study) | ⊕⊝⊝⊝ very low6,7,8,9 | ‐ | ||
| Adverse effects: any observed extrapyramidal symptoms ‐ short term (up to 6 months) Follow‐up: 6 to 8 weeks | Study population | RR 8.03 (4.78 to 13.51) | 644 (8 studies) | ⊕⊕⊝⊝ low2,5 | ‐ | |
| 36 per 1000 | 293 per 1000 (174 to 493) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| High | ||||||
| 80 per 1000 | 642 per 1000 (382 to 1000) | |||||
| Leaving the study early due to any reason short term (up to 6 months) Follow‐up: 6 to 16 weeks | Moderate1 | RR 1.04 (0.77 to 1.41) | 1223 (12 studies) | ⊕⊕⊕⊝ moderate2 | ‐ | |
| 93 per 1000 | 97 per 1000 (72 to 131) | |||||
| High1 | ||||||
| 280 per 1000 | 291 per 1000 (216 to 395) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1Control risk: are representative of those observed in the study population. 2Risk of bias: serious ‐ most of the included studies had unclear risk of bias in terms of allocation and blinding, hence selection and detection bias are likely to be present. Some of the studies also had high risk of reporting bias. 3Indirectness: serious ‐ binary outcome on mental state is not available. Thus we employed BPRS rating as an indicator. 4Imprecision: serious ‐ although the estimate of effect was not significant, the CI was relatively tight around the point of estimate. The sample size is also relatively large and exceed the calculated optimal information size. 5Publication bias: strongly suspected ‐ only Chinese trials with small sample size were identified for this outcome. 6Risk of bias: serious ‐ the only study contributed data to this outcome had unclear risk of selection and detection bias. It also had attrition data that we excluded from the final analysis. 7Indirectness: serious ‐ there was no binary measurement available on quality of life. Therefore, we used GQOLI‐74 as an alternative indicator. 8Imprecision: serious ‐ sample size was smaller than the optimal information size. 9Publication bias: strongly suspected ‐ we only identified a Chinese trial with a small sample size with positive findings for this outcome.
Background
Description of the condition
Schizophrenia is a severe form of mental health disorder. It has a high lifetime prevalence rate, affecting (4 per 1000 people (Saha 2005), but low incident rate because of the chronic nature of the illness. The median incident rate of schizophrenia is 15.2 per 100,000 people (McGrath 2008).
The International Classification of Diseases (ICD) classifies the illness into categories F20 to F29 as ‘schizophrenia, schizotypal and delusional disorder’ (ICD‐10 1992), particularly ‘schizophrenia’ in F20. The ICD‐10 states that "schizophrenic disorders are characterized in general by fundamental and characteristic distortions of thinking and perception, and affects that are inappropriate or blunted". The Diagnostic and Statistical Manual of the American Psychiatric Association has also used the term ‘schizophrenia’ (DSM‐IV‐TR 2000).
The prognosis of schizophrenia is quite variable, and in the past psychiatrists were not very optimistic about its treatment (Kraeplin 1919). However, recent studies show that the outcome of schizophrenia treatment is better than previously thought. The use of phenothiazines may have contributed to this as well as other factors, such as improving community services (Bland 1978).
Description of the intervention
Psychiatrists have prescribed typical antipsychotic drugs since the 1950s, when the first antipsychotic medication, chlorpromazine, was synthesized. Chlorpromazine was first used as an antihistaminic agent to treat allergies. Later, surgeons used it as a pre‐surgical medication to sedate people before surgical procedures (Laborit 1951). In 1952, Paul Charpentier, from Laboratories Rhône‐Poulenc in France, and Delay and Deniker's team described the antipsychotic properties of chlorpromazine (Delay 1952). Chlorpromazine is considered a pivotal discovery in the field of psychosis treatment, with other antipsychotics often measured in 'chlorpromazine equivalents' (Turner 2007; Yorston 2000).
There are now many antipsychotic drugs available. They are broadly divided into two groups: ‘typical antipsychotic drugs’ and ‘atypical antipsychotic drugs’. Typical antipsychotic drugs are also known as ‘first generation’, ‘conventional’ or ‘classical’ antipsychotic drugs, e.g. chlorpromazine and haloperidol. Atypical antipsychotic drugs are also known as ‘second generation’ or ‘newer antipsychotic drugs’, e.g. clozapine, risperidone, quetiapine and olanzapine. Typical antipsychotic drugs have a good reputation regarding their efficacy in treating the 'positive' symptoms of schizophrenia (e.g. delusions and hallucinations) (Mathews 2007). They are also well known for their adverse effects, such as movement disorders (extrapyramidal symptoms (EPS) or extrapyramidal side effects (EPSE)), sedation, metabolic syndromes and sometimes potentially fatal conditions, such as neuroleptic malignant syndrome (Arana 2000). The second generation antipsychotic drugs arrived on the market, claiming notable differences. They had a reputed low side‐effect profile and, according to pharmaceutical companies, higher efficacy (Janssen 1988). However, research funded independently of pharmaceutical companies has suggested that there may be little difference between the older and newer drugs (Adams 2014). This has subsequently fuelled debate as to whether new atypical antipsychotics are more effective than older established first‐generation antipsychotics, and whether questioning the efficacy of the two classifications of drugs creates an improper generalisation of antipsychotics that do not form a homogenous class (Leucht 2009). Against this backdrop, chlorpromazine remains a benchmark drug in the treatment of schizophrenia. Although imperfect, it is relatively inexpensive and remains one of the most common drugs used for treating people with schizophrenia worldwide (Adams 2005).
How the intervention might work
Chlorpromazine is an aliphatic phenothiazine, which is one of the widely‐used typical antipsychotic drugs. Chlorpromazine is reliable for its efficacy and one of the most tested first generation antipsychotic drugs. It has been used as a ‘gold standard’ to compare the efficacy of older and newer antipsychotic drugs. It blocks alpha 1, 5HT2A, D2 and D1 receptors in the brain, and thus it works as an antipsychotic. It also has effect on muscarinic, serotonin and H1 receptors. By blocking D2 receptor it can also cause extrapyramidal side effects. Other adverse effects include dry mouth, blurred vision, restlessness, sedation, neuroleptic malignant syndrome (DSM‐IV 1994) etc. On the other hand, atypical antipsychotic drugs by definition may cause decreased or no extrapyramidal side effects (Kinon 1996). Different atypical antipsychotic drugs act in different ways; for example, clozapine blocks D2 and 5HT2 receptors (Meltzer 1989). Both clozapine and quetiapine blocks more 5HT2 receptors than D2 receptors. olanzapine blocks 5HT2A, 5HT6, D1, D2, D3 and muscarinic receptors (Zhang 1999).
Why it is important to do this review
This is one of a family of related Cochrane reviews on this important compound (Table 4).
1. Related Cochrane Reviews.
| Comparison | Reference |
| Chlorpromazine versus placebo | Adams 2014 |
| Chlorpromazine versus haloperidol | Leucht 2008 |
| Chlorpromazine doses | Liu 2009 |
| Chlorpromazine cessation | Almerie 2007 |
| Chlorpromazine for acute aggression | Ahmed 2010 |
Chlorpromazine is one of two oral antipsychotic drugs on the World Health Organization's Essential Drug list (WHO 2011). It is globally accessible and has been known for its effectiveness in schizophrenia treatment since the 1950s (Adams 2014), and it is also the most commonly used and inexpensive treatment for schizophrenia (Odejide 1982). Expensive new generation drugs are heavily marketed worldwide as a better treatment for schizophrenia. However, this may not be the case and may be an unnecessary drain on very limited resources (Adams 2006). Also, comparisons with new generation drugs, which are coming off‐patent and are therefore more accessible, are important to assist informed and independent choice of treatment for people with schizophrenia.
Objectives
To compare the effects of chlorpromazine with atypical or second generation antipsychotic drugs, for treatment of people with schizophrenia (seeDifferences between protocol and review).
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant randomised controlled trials (RCTs). If a trial was described as double‐blind but implied randomisation, we included such trials in a sensitivity analysis (see Sensitivity analysis). If their inclusion did not result in a substantive difference, we kept these trials in the analyses. If their inclusion resulted in statistically significant differences, we did not add the data from these lower quality studies to the results of the better trials, but presented such data within a subcategory. We excluded quasi‐RCTs (e.g. allocating by alternate days of the week). Where people were given additional treatments within the chlorpromazine and atypical antipsychotic groups, we only included data if the adjunct treatment was evenly distributed between groups and only the chlorpromazine and atypical antipsychotic groups were randomised.
Types of participants
Adults (as defined in each trial) diagnosed with schizophrenia, including schizophreniform, schizoaffective and delusional disorders (any means of diagnosis, including operational criteria (DSM‐IV 1994; ICD‐10 1992) or clinical opinion).
We are interested in making sure that information is as relevant to the current care of people with schizophrenia as possible so if the information was reported we clearly highlighted in the Characteristics of included studies and Description of studies, the current clinical state (acute, early post ‐ acute, partial remission, remission) as well as the stage (prodromal, first episode, early illness, persistent) and as to whether the studies primarily focused on people with particular problems (for example, negative symptoms, treatment‐resistant illnesses).
Types of interventions
1. Chlorpromazine
Any dose and any route of administration.
2. Any atypical antipsychotic
Atypical antipsychotic drugs including: amisulpride, aripiprazole, asenapine (Smith 2010), clozapine, clothiapine or clotiapine (Toren 1995), iloperidone (Caccia 2010), lurasidone (Risbood 2012), mosapramine (Takahashi 1999), olanzapine, paliperidone, perospirone (Bian 2008), quetiapine, remoxipride (Nadal 2001), risperidone, sertindole (Cincotta 2010), sulpiride (Rzewuska 1988), ziprasidone and zotepine (list non‐exhaustive).
Any dose and any route of administration.
Types of outcome measures
If possible, we divided outcomes into short term (up to six months), medium term (seven to 12 months) and long term (over one year).
Primary outcomes
1. Clinical response
Clinically significant improvement as defined by each included trial.
2. Relapse
As defined by each trial.
Secondary outcomes
1. Death: natural death or suicide
2. Global state
2.1 Any change in global state. 2.2 Deterioration. 2.3 Need for additional antipsychotic drugs. 2.4 Need for additional benzodiazepines. 2.5 Poor compliance.
3. Mental state
3.1 General symptoms
3.1.1 Any change in general symptoms. 3.1.2 Average endpoint general symptom score. 3.1.3 Average change in general symptom score.
3.2 Specific symptoms (positive and negative symptoms of schizophrenia, depression and mania/hypomania)
3.2.1 Any change of specific symptoms. 3.2.2 Average endpoint specific symptom score. 3.2.3 Average change specific symptom score.
4. Service involvement
4.1 Duration of hospital stay. 4.2 Re‐hospitalisation. 4.3 Engagement with community services. 4.4 Engagement with inpatient/outpatient services.
5. Functioning
5.1 General functioning
5.1.1 Any change in general functioning. 5.1.2 Average endpoint score in general functioning. 5.1.3 Average change score in general functioning.
5.2 Social functioning
5.1.1 Any change in social functioning. 5.1.2 Average endpoint score in social functioning. 5.1.3 Average change score in social functioning
5.3 Employment status
5.1.1 Any change in employment status. 5.1.2 Average endpoint score in employment functioning. 5.1.3 Average change score in employment functioning.
6. Behaviour
6.1 General behaviour. 6.2 Any improvement in behaviour, as defined in each trial. 6.3 Specific behaviour (e.g. agitation, aggression, violent incidents). 6.4 Average endpoint in behaviour scores. 6.5 Average change in behaviour scores.
7. Adverse effects
7.1 Anticholinergic. 7.2 Cardiovascular. 7.3 Central nervous system. 7.4 Gastrointestinal. 7.5 Endocrine (e.g. amenorrhoea, galactorrhoea, hyperlipidaemia, hyperglycaemia, hyperinsulinemia). 7.6 Haematology (e.g. haemogram, leukopenia, agranulocytosis/neutropenia). 7.7 Hepatitic (e.g. abnormal transaminase, abnormal liver function). 7.8 Metabolic. 7.9 Movement disorders. 7.10 Various other.
8. Satisfaction
8.1 Patient satisfaction. 8.2 Carer satisfaction. 8.3 Professional satisfaction (managers/doctors/nurses).
9. Economic outcomes
9.1 Direct costs, as defined in each study. 9.2 Indirect costs, as defined in each study. 9.3 Cost‐effectiveness, as defined in each study.
10. Quality of life
10.1 Average endpoint score in quality of life. 10.2 Average change score in quality of life. 10.3 Any improvement in quality of life.
11. Leaving the study early
'Summary of findings' tables
We used the GRADE approach to interpret findings (Schünemann 2008) and used GRADE profiler (GRADEPRO) to import data from RevMan 5 (Teview Manager) to create 'Summary of findings' tables. These tables provide outcome ‐ specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rated as important to patient care and decision making. We aimed to select the following main outcomes for inclusion in the 'Summary of findings' tables:
Clinical response: clinically significant improvement (as defined by each of the studies) by medium term.
Relapse (as defined by each of the studies) by medium term.
Mental state: average endpoint score (Brief Psychiatric Rating Scale (BPRS)) by medium term.
Adverse effects: extrapyramidal side effects: reported by the number participants by medium term.
Quality of life: improvement as defined by each of the study by medium term.
Participants leaving the study early by medium term.
Economic outcomes: cost effectiveness (as defined in each study) by long term.
Search methods for identification of studies
Electronic searches
1. Cochrane Schizophrenia Group Trials Register
We searched the Cochrane Schizophrenia Group's Trials Register up to 23 September 2013 using the phrase:
[((*chlorpromazine* AND (*amisulprid* or *aripiprazol* or *clozapin* or *olanzapin* or *quetiapin* or *risperidon* or *sertindol* or *ziprasidon* or *zotepin*or *sulpiride* or *remoxipride* or *paliperidone* or *perospirone* or asenapine or clothiapine or clotiapine or iloperidone or lurasidone or mosapramine or ((Atypical or (Second NEXT generation)) and antipsychotic*))) in title, abstract or index terms of REFERENCE or interventions of STUDY)]
The Cochrane Schizophrenia Group's Trials Register is compiled by systematic searches of major databases, handsearches and conference proceedings (see Group Module).
The seatch strategy was changed after protocol publication and we added new search terms to the strategy. The search strategy in protocol step is in Appendix 1.
Searching other resources
1. Reference searching
We inspected references of all included trials for further relevant studies published in any language.
2. Personal contact
If necessary we contacted the first author, relevant pharmaceutical companies, and drug approval agencies of trials for additional information.
Data collection and analysis
Selection of studies
Two review authors (BL and SZ) independently inspected citations from the searches and identified relevant abstracts. JX independently re‐inspected a random 20% sample to ensure reliability. Where disputes arose, the full report was acquired for more detailed scrutiny. BL obtained and inspected full reports of the abstracts meeting the review criteria and JX re‐inspected a random 20% of these reports in order to ensure reliable selection. Where it was not possible to resolve disagreement by discussion, we attempted to contact the authors of the study for clarification.
Data extraction and management
1. Extraction
Two review authors (BL and SZ) extracted data from all included trials. To ensure reliability of data extraction, JX independently extracted data from a random sample of these studies, comprising 10% of the total. We discussed and documented any disagreements. Another review author (SS) helped to clarify any remaining issues and we documented these final decisions. If possible, we extracted data presented only in graphs and figures, but only included this data if two review authors independently had the same result. If necessary, we attempted to contact the trial authors through an open‐ended request in order to obtain missing information or for clarification whenever necessary.
2. Management
2.1 Forms
We extracted data onto standard, pre‐designed simple forms.
2.2 Scale‐derived data
We included continuous data from rating scales only if:
The psychometric properties of the measuring instrument had been described in a peer‐reviewed journal (Marshall 2000); and
The measuring instrument had not been written or modified by one of the trialists for that particular trial.
Ideally, the measuring instrument should either be i. a self‐report or ii. completed by an independent rater or relative (not the therapist). We realise that this is not often reported clearly. We noted in the 'Description of studies' if this was the case or not.
2.3 Endpoint versus change data
There are advantages to both endpoint and change data. Change data can remove a component of between person variability from the analysis. However, calculation of change requires two assessments (baseline and endpoint), which can be difficult in unstable and difficult to measure conditions, such as schizophrenia. We decided to primarily use endpoint data, and only use change data if the former were unavailable. We combined endpoint and change data in the analysis as we preferred to use MD rather than standardised mean difference (SMD) values throughout (Higgins 2011).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data we applied the following standards to all data before inclusion:
For change data:
We entered change data, as when continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not. We presented and entered change data into statistical analyses.
For endpoint data:
a) When a scale started from the finite number 0, we subtracted the lowest possible value from the mean, and divided this by the SD. If this value was < 1, it strongly suggested a skew, and we excluded the study. If this ratio was higher than 1 but below 2, there was suggestion of skew. We entered the study and tested whether its inclusion or exclusion would change the results substantially. Finally, if the ratio was > 2, we included the study because skew was less likely (Altman 1996; Higgins 2011).
b) If a scale started from a positive value (such as the Positive and Negative Syndrome Scale (PANSS), which can have values from 30 to 210) (Kay 1986), we modified the calculation described above to take into account the scale starting point. In such cases skew was present if 2 SD > (S ‐ Smin), where S is the mean score and Smin is the minimum score.
(Please note, irrespective of the above rules, we entered endpoint data from studies of at least 200 participants in the analysis because skewed data pose less of a problem in large studies).
2.5 Common measure
To facilitate comparison between trials, we intended to convert variables that can be reported in different metrics, such as days in hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month). However, we did not identify such data.
2.6 Conversion of continuous to binary
Where possible, we made efforts to convert outcome measures to dichotomous data. This can be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that if there is a 50% reduction in a scale derived score, such as the BPRS (Overall 1962) or the PANSS (Kay 1986), this could be considered a clinically significant response (Leucht 2005a; Leucht 2005b).
2.7 Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicates a favourable outcome for chlorpromazine. Where keeping to this made it impossible to avoid outcome titles with clumsy double‐negatives (e.g. 'not un‐improved') we reported data where the left of the line indicates an unfavourable outcome. We have noted this in the relevant graphs.
Assessment of risk of bias in included studies
Two review authors (BL and SZ) independently assessed the risk of bias of included trials by using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This set of criteria is based on evidence of association between overestimate of effect and high risk of bias of the article, such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting.
If the two review authors disagreed, we made the final rating by consensus, with the involvement of another review author from the team. Where inadequate details of randomisation and other characteristics of trials were provided, we contacted trial authors for further information. We reported non‐concurrence in quality assessment, but if disputes arose as to which category a trial was to be allocated, again, we resolved this by discussion.
We described the results of the 'Risk of bias' assessments in both the review text and in the 'Summary of findings' tables.
Measures of treatment effect
1. Binary data−
For binary outcomes we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios (ORs) and that ORs tend to be interpreted as RRs by clinicians (Deeks 2000). The number needed to treat for an additional beneficial outcome (NNTB)/number needed to treat for an additional harmful outcome (NNTH) statistic with its CIs is intuitively attractive to clinicians but is problematic both in its accurate calculation in meta analyses and interpretation (Hutton 2009). For binary data presented in the 'Summary of findings' table(s), where possible, we calculated illustrative comparative risks.
2. Continuous data
For continuous outcomes, we estimated the MD between groups. We preferred not to calculate effect size measures (SMD). However, if scales of considerable similarity were used, we assumed there was a small difference in measurement, calculated effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, study authors often fail to account for intra‐class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, CIs unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
We did not identify any cluster‐randomised studies. However, if we identify such studies in future updates of this Cochrane review where clustering is not accounted for in primary studies, we will present data in a table, with an asterisk (*) to indicate the presence of a probable unit of analysis error. In updates of this review we will try to contact first authors of such studies to obtain intra‐class correlation coefficients (ICCs) for their clustered data and to adjust for this by using accepted methods (Gulliford 1999).
Where clustering was incorporated into the analysis of primary studies, we presented these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
We received statistical advice that binary data presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the ICC [Design effect = 1 + (m − 1)*ICC] (Donner 2002). If the ICC was not reported, we assumed it was 0.1 (Ukoumunne 1999).
Where cluster studies had been appropriately analysed taking into account ICCs and relevant data documented in the report, synthesis with other studies was possible using the generic inverse variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence on entry to the second phase, the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, we planned to only use data from the first phase of cross‐over studies. However, we did not identify any cross‐over trials for inclusion in this review.
3. Studies with multiple treatment groups
Where a study involves more than two treatment arms, if relevant, we presented the additional treatment arms in comparisons. If data were binary, we simply added these and combined within the two‐by‐two table. If data were continuous, we had planned to combine data following the formula in Section 7.7.3.8 (Combining groups) of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Where the additional treatment arms were irrelevant, we did not reproduce these data.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). We chose that, for any particular outcome, should more than 50% of data be unaccounted for, we would not reproduce these data or use them within analyses. If more than 50% of those in one arm of a study were lost but the total loss was less than 50%, we addressed this within the 'Summary of findings' table(s) by downgrading the quality of the evidence. We also downgraded the quality of the evidence within the 'Summary of findings' table(s) where loss was 25% to 50% in total.
2. Binary
In the case where attrition for a binary outcome was between 0% and 50% and where these data were not clearly described, we presented these data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat (ITT) analysis). We assumed all those leaving the study early to have the same rates of negative outcome as those who completed, with the exception of the outcome of death and adverse effects. For these outcomes, we used the rate of those who stayed in the study in that particular arm of the trial for those who did not. We undertook a sensitivity analysis to test how prone the primary outcomes were to change when 'completer' data only were compared to the ITT analysis using the above assumptions.
3. Continuous
3.1 Attrition
In cases where attrition for a continuous outcome was between 0% and 50% and completer‐only data was reported, we reproduced these.
3.2 Standard deviations
If SD values were not reported, we first tried to obtain the missing values from the trial authors. If unavailable, where there were missing measures of variance for continuous data, but an exact standard error (SE) and CIs available for group means, and either 'P' value or 't' value available for differences in mean, we calculated them according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011): when only the standard error (SE) is reported, SDs are calculated by the formula SD = SE * square root (n). Chapters 7.7.3 and 16.1.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a; Higgins 2011b) present detailed formulae for estimating SDs from P values, t or F values, CIs, ranges or other statistics. If these formula did not apply, we calculated the SDs according to a validated imputation method, which is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study's outcome and thus to lose information. We did not impute any continuous data in this review.
3.3 Last observation carried forward
We anticipated that in some studies would employ the method of last observation carried forward (LOCF) within the study report. As with all methods of imputation to deal with missing data, LOCF introduces uncertainty about the reliability of the results (Leucht 2007). Therefore, where LOCF data were used in the trial, if less than 50% of the data had been assumed, we reproduced these data and indicated that they were the product of LOCF assumptions.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for clearly outlying people or situations which we had not predicted would arise and discussed these.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods which we had not predicted would arise, and discussed.
3. Statistical heterogeneity
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I² statistic
We investigated heterogeneity between studies by considering the I² statistic alongside the Chi² test P value. The I² statistic provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed I² statistic value depends on: i. magnitude and direction of effects; and ii. strength of evidence for heterogeneity (e.g. P value from the Chi² test, or a CI for I² statistic). We interpreted an I² statistic estimate ≥ around 50% accompanied by a statistically significant Chi² test as evidence of substantial levels of heterogeneity (Section 9.5.2 ‐ Deeks 2011). When we found substantial levels of heterogeneity in the primary outcome, we explored reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
1. Protocol versus full study
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. These are described in Section 10.1 (Sterne 2011). We tried to locate protocols of included RCTs. If the protocol was available, we compared outcomes in the protocol and in the published report. If the protocol was unavailable, we compared outcomes listed in the methods section of the trial report with actually reported results.
2. Funnel plot
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are again described in Section 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We did not use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar sizes. In other cases, where funnel plots are possible, we sought statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. However, there is a disadvantage to the random‐effects model. It puts added weight onto small studies which often are the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. We used the random‐effects model for all analyses.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses
1.1 Primary outcomes
We did not anticipate any subgroup analyses.
1.2 Clinical state, stage or problem
We proposed to provide an overview of the effects of chlorpromazine versus atypical antipsychotic drugs for people with schizophrenia in general in this Cochrane review. In addition, we tried to report data on subgroups of people in the same clinical state, stage and with similar problems.
2. Investigation of heterogeneity
We reported if inconsistency in trial results was high. First, we investigated whether data had been entered correctly. Second, if data were correct, we visually inspected the graph and successively removed outlying studies to see if homogeneity was restored. For this Cochrane review, we decided that should this occur with data contributing to the summary finding of no more than around 10% of the total weighting, we presented these data. If not, we did not pool these data and discussed any issues. We know of no supporting research for this 10% cut‐off but are investigating use of prediction intervals as an alternative to this unsatisfactory state.
If we observed obvious unanticipated clinical or methodological heterogeneity, we simply stated hypotheses regarding these for future reviews or versions of this Cochrane review. We did not anticipate undertaking analyses relating to these.
Sensitivity analysis
1. Implication of randomisation
We aimed to include trials in a sensitivity analysis if they were described in some way as to imply randomisation. For the primary outcomes, we included these studies. If there was no substantive difference when we added the implied randomised studies to those with better description of randomisation, then we used relevant data from these studies.
2. Assumptions for lost binary data
Where assumptions have to be made regarding people lost to follow‐up (see Dealing with missing data), we compared the findings of the primary outcomes when we used our assumption compared with completer data only. If there was a substantial difference, we reported results and discussed them but continued to employ our assumption.
Where assumptions had to be made regarding missing SDs data (see Dealing with missing data), we compared the findings on primary outcomes when we used our assumption compared with completer data only. We performed a sensitivity analysis testing how prone results were to change when 'completer' data only were compared to the imputed data using the above assumption. If there was a substantial difference, we reported results and discussed them but continued to employ our assumption.
3. Risk of bias
We analysed the effects of excluding trials that we judged to be at 'high' risk of bias across one or more of the domains of randomisation (implied as randomised with no further detail available), allocation concealment, blinding and outcome reporting for the meta‐analysis of the primary outcome. If the exclusion of trials at 'high' risk of bias did not substantially alter the direction of effect or the precision of the effect estimates, then we included data from these trials in the analysis.
4. Imputed values
We had also planned to undertake a sensitivity analysis to assess the effects of including data from trials where we used imputed values for ICC in calculating the design effect in cluster‐RCTs. However, we did not impute any values in this Cochrane review.
5. Fixed‐effect and random‐effects
We synthesised all data using a random‐effects model. However, we also synthesised data for the primary outcome using a fixed‐effect model to evaluate whether this altered the significance of the results.
Results
Description of studies
Results of the search
We identified 444 references following our literature search, of which we excluded 298 after screening by title/abstract. For this Cochrane review with the three comparisons of olanzapine, risperidone and quetiapine, we obtained and scrutinised 146 full‐text articles. Of these, 42 articles did not meet the inclusion criteria (see Characteristics of excluded studies) and were excluded. We included 71 trials (Figure 1). In the same literature search, we identified 33 more studies comparing chlorpromazine to atypical antipsychotics, including aripiprazole, clotiapine, clozapine, iloperidone, remoxipride, sulpiride, ziprasidone and zotepine. We will include all of these comparisons in a future update of this Cochrane review.
1.
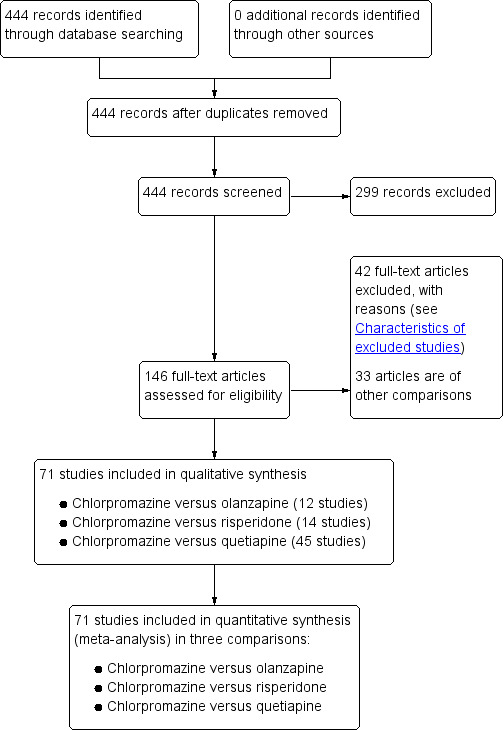
Study flow diagram.
Included studies
1. Chlorpromazine versus olanzapine
For this comparison, we included 12 studies (total N = 919; chlorpromazine N = 432 and olanzapine N = 487).
1. Trial length
Most included studies were eight weeks in duration (vs OLZ ‐ Chang 2003; vs OLZ ‐ Chen 2006; vs OLZ ‐ He 2003; vs OLZ ‐ Luo 2007; vs OLZ ‐ Wang 2002; vs OLZ ‐ Wang 2008; vs OLZ ‐ Zhao 2006), with three studies at six weeks in duration (HGCQ (Turkey) 2000; HGDV (Morocco) 1999; vs OLZ ‐ Loza 1999 (HGDT)). The final two studies were 12 weeks (vs OLZ ‐ Wu 2008) and two years (vs OLZ ‐ An 2006) in duration. Therefore most included studies provided data for our short term outcome, with only one study providing any data for the long term.
2. Design
All included studies were parallel arm RCTs. Four out of the 12 studies provided information as to randomisation methods, which included methods such as computer‐generated randomisation and random number tables (HGCQ (Turkey) 2000; HGDV (Morocco) 1999; vs OLZ ‐ Chang 2003; vs OLZ ‐ Luo 2007).
3. Participants
All included participants had a diagnosis of schizophrenia; diagnostic criteria used included DSM‐IV (HGCQ (Turkey) 2000; HGDV (Morocco) 1999; vs OLZ ‐ Loza 1999 (HGDT)) and CCMD (vs OLZ ‐ An 2006; vs OLZ ‐ Wang 2008; vs OLZ ‐ Wu 2008; vs OLZ ‐ Zhao 2006). Diagnostic criteria were unclear in the remaining studies, with participants described either as having 'schizophrenia' (vs OLZ ‐ Chang 2003; vs OLZ ‐ Chen 2006; vs OLZ ‐ He 2003) or 'first‐episode schizophrenia (vs OLZ ‐ Luo 2007; vs OLZ ‐ Wang 2002).
4. Setting
Nine of the included studies were undertaken in China, and included both inpatient and outpatient settings. Other studies were completed in Turkey (HGCQ (Turkey) 2000), Morocco (HGDV (Morocco) 1999) and Egypt (vs OLZ ‐ Loza 1999 (HGDT)).
5. Study size
Study sizes ranged from 30 (HGCQ (Turkey) 2000) to 100 participants (vs OLZ ‐ Wu 2008); and the mean sample size was 77 participants.
6. Interventions
6.1 Chlorpromazine
Chlorpromazine doses ranged from 25 to 600 mg/day (vs OLZ ‐ Wang 2002) to 200 to 800 mg/day (HGCQ (Turkey) 2000; HGDV (Morocco) 1999; vs OLZ ‐ Chen 2006; vs OLZ ‐ Loza 1999 (HGDT)). Doses tended to be fairy consistent between studies.
6.2 Olanzapine
Olanzapine doses between studies were largely uniform, with a range in 10 of the included studies of 5 mg/day to 20 mg/day, and in vs OLZ ‐ Luo 2007 the range was 5 mg/day to 25 mg/day, with vs OLZ ‐ Wang 2008 using a range of 5 mg/day to 15 mg/day.
7. Outcomes
7.1 General remarks
The included studies for this comparison provided generally well‐reported outcomes, including clinical response, global state outcomes, relapse rates and adverse events. Death was not reported in any of the included studies for any reason.
7.2 Acceptability and efficacy
Each included study provided data regarding mental and global state outcomes (widely accepted rating scales, including the BPRS, PANSS and CGI). However, some of these data were skewed and were presented in an additional table.
7.3 Adverse events
Adverse events, including extrapyramidal averse effects, anticholinergic effect, cardiovascular effects, gastrointestinal effects and 'others' were generally well‐reported in the included studies.
7.4 Outcome scales
7.4.1 Global state
i) Clinical Global Impression (CGI)
This is a rating instrument that enables clinicians to quantify severity of illness and overall clinical improvement during therapy (Guy 1976). A 7‐point scoring system is usually used, with low scores indicating decreased severity or greater recovery, or both.
7.4.2 Mental state
i) Brief Psychiatric Rating Scale (BPRS)
This scale is used to assess the severity of abnormal mental states (Overall 1962). The original scale has 16 items, but a revised 18‐item scale is commonly used. Each item is defined on a seven‐point scale varying from 'not present' to 'extremely severe', scoring from zero to six or one to seven. Scores can range from 0 to 108 or 18 to 126, respectively. High scores indicate more severe symptoms. The BPRS‐positive cluster comprises four items, which are conceptual disorganisation, suspiciousness, hallucinatory behaviour and unusual thought content. The BPRS‐negative cluster comprises only three items, which are emotional withdrawal, motor retardation and blunted affect.
ii) Hamilton Anxiety Scale (HAMA)
HAMA is a rating scale developed to quantify the severity of anxiety symptomatology and consists of 14 items, each defined by a series of symptoms (Maier 1988). Each item is rated on a 5‐point scale, ranging from zero (not present) to four (severe).
iii) Montgomery–Åsberg Depression Rating Scale (MADRS)
The MADRS is a 10‐ item clinician‐administered diagnostic questionnaire used to measure the severity of depressive episodes (Montgomery 1979). There are 10 items (including apparent sadness, reported sadness, inner tension, reduced sleep, reduced appetite, concentration difficulties, lassitude, inability to feel, pessimistic thoughts and suicidal thoughts), each rated zero (absent) to six (severe). Overall scores range from zero to 60.
iv) Nurses' Observation Scale for Inpatient Evaluation (NOSIE)
This scale assesses the behaviour of patients on an inpatient unit (Honigfeld 1965). The scale has 30 items, each rated from zero (not present) to four (markedly present), and includes such behaviours as 'gets angry or annoyed easily', 'cries' or 'is impatient'.
iv) Positive and Negative Syndrome Scale (PANSS)
The PANSS was originated as a method for evaluating positive, negative and other symptom dimensions in schizophrenia (Kay 1987). The scale has 30 items, and each item can be rated on a 7‐point scoring system varying from one (absent) to seven (extreme). This scale can be divided into three subscales for measuring the severity of general psychopathology, positive symptoms (PANSS‐P) and negative symptoms (PANSS‐N). A low score indicates low levels of symptoms.
v) Scale for Assessment of Positive Symptoms (SAPS)
This scale measures the positive symptoms of schizophrenia and is split into four items including hallucinations, delusions, bizarre behaviour and positive formal thought disorder, each rated on a scale of zero (absent) to five (severe) (Andreasen 1984).
7.4.3 Functioning
i) Wisconsin Card Sorting Test (WCST‐IQ/MQ)
The WCST is a neuropsychological test in which participants are expected to organise a set of specifically‐designed cards, without instruction (Monchi 2001). The test is a measure of executive functioning, assessing primarily strategic planning, organized searching, utilizing environmental feedback to shift cognitive sets, directing behavior toward achieving a goal, and modulating impulsive responding.
7.4.4 Adverse events
i) Extrapyramidal Symptom Rating Scale (ESRS)
The ESRS is a physical examination with 12 items that include both subjective and objective assessments (Chouinard 1980).
iii) Leeds Sleep Evaluation Questionnaire (LSEQ)
The LSEQ is a 10‐item, self‐rating measurement designed to assess changes in sleep quality over the course of psychopharmacological treatment (Parrott 1980). Four domains are rated, including 'ease of initiating sleep', 'quality of sleep', 'ease of waking' and 'behaviour following wakefulness'.
7.4.5 Quality of life
i) Gothenburg Quality of Life Instrument (GQOLI/ GQOL1 ‐ 74)
This scale assesses overall sleep quality; participants respond on a 7‐point Linkert‐type scale with one (excellent) and seven (very poor) (Sullivan 1993). Participants also rate other aspects of quality of life, including a self‐evaluation of their economic situation, social and friendship situation and home/family situation.
ii) Quality of life scale (QoL)
The QoL scale is a 16‐item instrument that measures six conceptual domains of quality of life: material and physical well‐being, relationships with other people, social, community and civic activities, personal development and fulfilment, recreation and independence (Flanagan 1982). Scores range from 16 to 112, with a higher score indicating a better outcome.
7.5 Missing outcomes
Studies did not provide vast amounts of data, nor even measured in many instances, outcomes including service involvement, functioning, behaviour, satisfaction with treatment, or economic outcomes.
2. Chlorpromazine versus risperidone
For this comparison, we included 14 studies, with a total of 991 participants (chlorpromazine N = 474; risperidone N = 517).
1. Trial length
Duration of included studies ranged from three weeks (vs RPD ‐ Liu 2005) to five months (vs RPD ‐ Chang 1998). The average length of studies was around eight weeks, therefore all included studies provided only short term data.
2. Design
All included studies were parallel‐arm RCTs. No included study adequately described randomisation methods.
3. Participants
All participants were diagnosed with schizophrenia according to Chinese Classification of Mental Disorders (CCMD) criteria (Chen 2002).
4. Setting
All included studies were undertaken in China with inpatient and outpatient settings both represented.
5. Study size
Sample sizes between studies ranged from 32 (vs RPD ‐ Liu 2000) to 107 participants (vs RPD ‐ Luo 2001), with a mean sample size of 70 participants.
6. Interventions
6.1 Chlorpromazine
All studies used different dosage ranges, and included 25 to 450 mg/day (vs RPD ‐ Wang 2005); 100 to 450 mg/day (vs RPD ‐ Lin 2005); 300/400 to 600 mg/day (vs RPD ‐ Wu 2002; vs RPD ‐ Wu 2004); and 100 to 700 mg/day (vs RPD ‐ Luo 2001). Not all studies reported mean doses, with only vs RPD ‐ Cui 2001 and vs RPD ‐ Feng 2003 using a mean dose of 426 mg/day and 355 mg/day respectively.
6.2 Risperidone
Risperidone doses also varied between studies, with the highest range of 1 mg/day to 9 mg/day used in vs RPD ‐ Ma 2004; most included studies used a range of risperidone up to 6 mg/day (vs RPD ‐ Chang 1998; vs RPD ‐ He 1999; vs RPD ‐ Wang 2005; vs RPD ‐ Wu 2002; vs RPD ‐ Wu 2004; vs RPD ‐ Zheng 2001). Again, only two studies described the mean doses used in the studies (vs RPD ‐ Cui 2001; vs RPD ‐ Feng 2003) of 4.19 mg/day and 3.62 mg/day respectively.
7. Outcomes
7.1 General remarks
The included studies for this comparison provided generally well‐reported outcomes, including clinical response, global state outcomes and adverse events. Death was not reported in any of the included studies for any reason.
7.2 Acceptability and efficacy
Data regarding mental and global state outcomes were measured using widely accepted rating scales, including the BPRS, PANSS and CGI. However some of these data were skewed and were presented in an additional table.
7.3 Adverse events
Adverse events, including extrapyramidal averse effects, anticholinergic effect, cardiovascular effects, gastrointestinal effects and 'others' were generally well reported in the included studies.
7.4 Outcome scales presenting useable data
7.4.1 Global state
i) Clinical Global Impression (CGI)
This is a rating instrument that enables clinicians to quantify severity of illness and overall clinical improvement during therapy (Guy 1976). A 7‐point scoring system is usually used with low scores indicating decreased severity or greater recovery, or both.
7.4.2 Mental state
i) Brief Psychiatric Rating Scale (BPRS)
This scale is used to assess the severity of abnormal mental states (Overall 1962). The original scale has 16 items, but a revised 18‐item scale is commonly used. Each item is defined on a 7‐point scale varying from 'not present' to 'extremely severe', scoring from zero to six or one to seven. Scores can range from zero to 108 or 18 to 126, respectively. High scores indicate more severe symptoms. The BPRS‐positive cluster comprises four items, which are conceptual disorganisation, suspiciousness, hallucinatory behaviour and unusual thought content. The BPRS‐negative cluster comprises only three items, which are emotional withdrawal, motor retardation and blunted affect.
ii) Positive and Negative Syndrome Scale (PANSS)
The PANSS was originated as a method for evaluating positive, negative and other symptom dimensions in schizophrenia (Kay 1987). The scale has 30 items, and each item can be rated on a 7‐point scoring system varying from one (absent) to seven (extreme). This scale can be divided into three subscales for measuring the severity of general psychopathology, positive symptoms (PANSS‐P) and negative symptoms (PANSS‐N). A low score indicates low levels of symptoms.
iii) Scale for Assessment of Negative Symptoms (SANS)
The SANS measures the incidence and severity of negative symptoms using a 25‐item scale, using a six‐point scoring system, of zero ( = better) to five ( = worse), where a higher score equals a more severe experience of negative symptoms (Andreasen 1982).
iv) Scale for Assessment of Positive Symptoms (SAPS)
This scale measures the positive symptoms of schizophrenia and is split into four items including hallucinations, delusions, bizarre behaviour and positive formal thought disorder, each rated on a scale of zero (absent) to five (severe) (Andreasen 1984).
7.4.3 Functioning
i) Wisconsin Card Sorting Test (WCST‐IQ/MQ)
The WCST is a neuropsychological test in which participants are expected to organise a set of specifically‐designed cards, without instruction (Monchi 2001). The test is a measure of executive functioning, assessing primarily strategic planning, organized searching, utilizing environmental feedback to shift cognitive sets, directing behavior toward achieving a goal, and modulating impulsive responding.
7.4.4 Adverse events
i) Treatment‐Emergent Signs and Symptoms (TESS)
The ICH E3 1995 guideline has stated that 'treatment‐emergent signs and symptoms' (TESS) are to be defined as "events not seen at baseline and events that worsened even if present at baseline". It can be difficult to document this accurately taking into account variables in time, dosage, adverse events and severity. Generally, TESS scores for particular adverse events are categorised as 'mild', 'moderate' or 'severe', with an appropriate action taken (e.g. none, discontinued, dose changed', hospitalisation and additional medication given). It is not often that studies publish continuous data from these measurements, with the more common presentation of dichotomised TESS ratings.
7.4.5 Quality of life
i) Quality of life scale (QoL)
The QoL scale is a 16‐item instrument that measures six conceptual domains of quality of life: material and physical well‐being; relationships with other people; social, community and civic activities; personal development and fulfilment; recreation and independence (Flanagan 1982). Scores range from 16 to 112, with a higher score indicating a better outcome.
7.5 Missing outcomes
Studies did not report any data for relapse rates, service use, behaviour, satisfaction with care or treatment, or economic outcomes.
3. Chlorpromazine versus quetiapine
For this comparison, we included 45 studies with a total of 4388 participants (chlorpromazine N = 2183; quetiapine N = 2205).
1. Trial length
Thirty‐seven of the 45 studies were under eight weeks in length. The shortest study was 42 days in length (vs QTP ‐ Peng 2006). The longest study was six months long (vs QTP ‐ Li 2003) and was the only study to provide data at the medium term. All other outcomes were reported at short term (< six months).
2. Design
All included studies were parallel‐arm RCTs. No included study adequately described randomisation methods.
3. Participants
All participants had a diagnosis of schizophrenia, with diagnosis using the Chinese Classification of Mental Disorders (CCMD) employed in 42 included studies. Two studies used DSM criteria to diagnose schizophrenia (vs QTP ‐ Hu 2003; vs QTP ‐ Peuskens 1997), and one study used a combination of CCMD and ICD‐10 criteria (vs QTP ‐ Zhou 2004).
4. Setting
All included studies were undertaken in China with both inpatient and outpatient settings represented.
5. Study size
Sample sizes between studies ranged from 30 (vs QTP ‐ Kong 2003) to 237 participants (vs QTP ‐ Zhang 2003).
6. Interventions
6.1 Chlorpromazine
The most common dose range between groups was between 300 to 600 mg/day. Only one study stated dosages used were 'less than 1000 mg/day' (vs QTP ‐ Guo 2008), with other maximum doses stated as 750 mg/day (vs QTP ‐ Peuskens 1997).
6.2 Quetiapine
Quetiapine doses were mostly between 300 and 700 mg/day. One study did not state the dosages used in either group (vs QTP ‐ Zhou 2003).
7. Outcomes
7.1 General remarks
The included studies for this comparison generally provided well‐reported outcomes, including clinical response, global state outcomes and adverse events. Death was not reported in any of the included studies for any reason. Most outcomes were reported using measurement scales, although we ensured they had been peer‐reviewed as the lack of dichotomous outcomes makes valuable interpretation of these results difficult.
7.2 Acceptability and efficacy
Data regarding mental and global state outcomes were reported using widely accepted rating scales, including the BPRS, PANSS and CGI. However some of these data were skewed and were presented in an additional table.
7.3 Adverse events
Adverse events, including extrapyramidal averse effects, anticholinergic effect, cardiovascular effects, gastrointestinal effects and 'others' were generally well reported in the included studies.
7.4 Outcome scales
7.4.1 Global state
i) Clinical Global Impression (CGI)
This is a rating instrument that enables clinicians to quantify severity of illness and overall clinical improvement during therapy (Guy 1976). A 7‐point scoring system is usually used with low scores indicating decreased severity or greater recovery, or both.
7.4.2 Mental state
i) Brief Psychiatric Rating Scale (BPRS)
This scale is used to assess the severity of abnormal mental states (Overall 1962). The original scale has 16 items, but a revised 18‐item scale is commonly used. Each item is defined on a 7‐point scale varying from 'not present' to 'extremely severe', scoring from zero to six or one to seven. Scores can range from zero to 108 or 18 to 126, respectively. High scores indicate more severe symptoms. The BPRS‐positive cluster comprises four items, which are conceptual disorganisation, suspiciousness, hallucinatory behaviour and unusual thought content. The BPRS‐negative cluster comprises only three items, which are emotional withdrawal, motor retardation and blunted affect.
ii) Hamilton Rating Scale for Depression (HAMD)
The HAMD rates severity of depression in adults including items of mood, feelings of guilt, suicide ideation, insomnia, agitation or retardation, anxiety, weight loss and somatic symptoms (Hamilton 1960). There are 20 items rated on a Linket‐type scale, where zero equals an absence of symptoms and a higher score indicates a worse outcome.
iii) Positive and Negative Syndrome Scale (PANSS)
The PANSS was originated as a method for evaluating positive, negative and other symptom dimensions in schizophrenia (Kay 1987). The scale has 30 items, and each item can be rated on a 7‐point scoring system varying from one (absent) to seven (extreme). This scale can be divided into three subscales for measuring the severity of general psychopathology, positive symptoms (PANSS‐P) and negative symptoms (PANSS‐N). A low score indicates low levels of symptoms.
7.4.3 Functioning
i) Wisconsin Card Sorting Test (WCST‐IQ/MQ)
The WCST is a neuropsychological test in which participants are expected to organise a set of specifically‐designed cards, without instruction (Monchi 2001). The test is a measure of executive functioning, assessing primarily strategic planning, organized searching, utilizing environmental feedback to shift cognitive sets, directing behaviour toward achieving a goal and modulating impulsive responding.
ii) Wechsler Memory Scale‐revised (WMS‐RC)
The WMS‐RC is a neuropsychological test that is used to measure a person's memory functions (WMS‐IV 2009). The scale consists of seven subtests: spatial addition, symbol span, design memory, general cognitive screener, logical memory, verbal paired associates and visual reproduction. A high score indicates a better outcome.
7.4.4 Adverse events
i) Treatment‐Emergent Signs and Symptoms (TESS)
The ICH E3 1995 guideline has stated that 'treatment‐emergent signs and symptoms' (TESS) are to be defined as "events not seen at baseline and events that worsened even if present at baseline". It can be difficult to document this accurately taking into account variables in time, dosage, adverse events and severity. Generally, TESS scores for particular adverse events are categorised as 'mild', 'moderate' or 'severe', with an appropriate action taken (e.g. none, discontinued, dose changed' hospitalisation, and additional medication given). It is not often that studies publish continuous data from these measurements, with the more common presentation of dichotomised TESS ratings.
7.4.5 Quality of life
i) Gothenburg Quality of Life Instrument (GQOLI/ GQOL1‐74)
This scale assesses overall sleep quality; participants respond on a 7‐point Linkert‐type scale ranging from one('excellent') and seven ('very poor') (Sullivan 1993). Participants also rate other aspects of quality of life, including a self‐evaluation of their economic situation, social and friendship situation and home/family situation.
7.5 Missing outcomes
Excluded studies
We excluded 42 studies; for details please consult the Characteristics of excluded studies section,
Risk of bias in included studies
For a graphical overview, please see Figure 2 and Figure 3.
2.
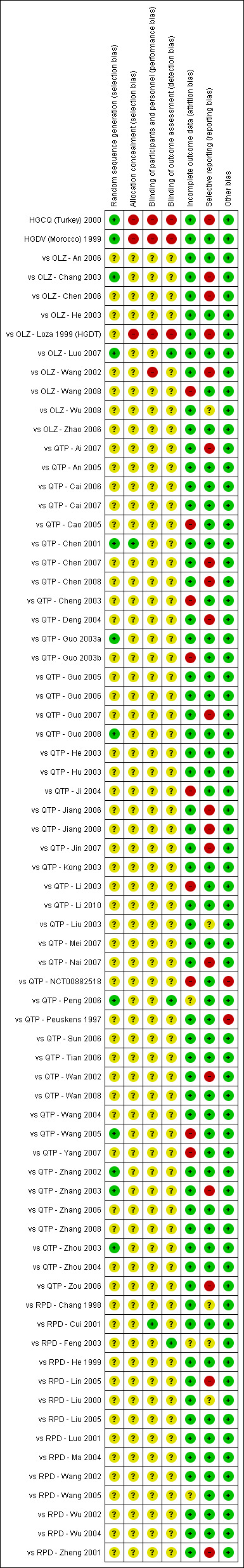
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
3.
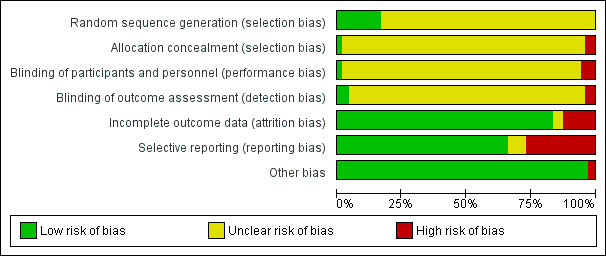
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
Allocation
1. Chlorpromazine versus olanzapine
Only four included studies provided adequate information regarding randomisation, which included methods such as a computer‐generated randomisation and random number tables (HGCQ (Turkey) 2000; HGDV (Morocco) 1999; vs OLZ ‐ Chang 2003; vs OLZ ‐ Luo 2007). We judged each of these studies as at 'low' risk of bias. The remaining studies in this comparison were rated as at 'unclear' risk of bias, as 'randomisation' or 'random allocated' was stated, however no description was provided. However, three studies were rated as a 'high' risk of bias due to the lack of allocation concealment (HGCQ (Turkey) 2000; HGDV (Morocco) 1999; vs OLZ ‐ Loza 1999 (HGDT)); each study stated that "the [study drug] bottles were labelled 'Olanzapine' or 'Chlorpromazine' in addition to the study number". We rated all other studies as 'unclear' for allocation bias.
2. Chlorpromazine versus risperidone
None of the included studies provided details as to randomisation methods and therefore we rated each as at 'unclear' risk of bias, since 'randomisation' or 'random allocated' was stated but no description was provided.
3. Chlorpromazine versus quetiapine
Only eight of the 45 included studies provided adequate data as to randomisation methods; five studies used a random number table (vs QTP ‐ Chen 2001; vs QTP ‐ Guo 2008; vs QTP ‐ Peng 2006; vs QTP ‐ Wang 2005; vs QTP ‐ Zhou 2003), two studies randomised by tossing a coin (vs QTP ‐ Guo 2003a; vs QTP ‐ Zhang 2002) and one study randomised through computer‐generated random numbers (vs QTP ‐ Zhang 2003). We rated these studies at 'low' risk of bias, with the remaining studies rated at 'unclear' risk.
Blinding
1. Chlorpromazine versus olanzapine
We rated four studies at 'high' risk of bias for blinding and detection bias (HGCQ (Turkey) 2000; HGDV (Morocco) 1999; vs OLZ ‐ Loza 1999 (HGDT); vs OLZ ‐ Wang 2002). Each of these were open label studies where both participant and study investigator knew what was being given and received. All other studies were rated at 'unclear' risk of bias.
2. Chlorpromazine versus risperidone
Only two studies were rated at 'low' risk of bias for performance and detection bias in blinding (vs RPD ‐ Cui 2001; vs RPD ‐ Feng 2003). Double blinding was used via, identical study drugs, dispensed by an independent pharmacist. All other studies were rated as at 'unclear' risk of bias.
3. Chlorpromazine versus quetiapine
We rated only one study as at 'low' risk of bias, since it specified assessor blinding (vs QTP ‐ Peng 2006). All other studies were rated at 'unclear' risk of bias.
Incomplete outcome data
1. Chlorpromazine versus olanzapine
All studies apart from one were rated at 'low' risk of attrition bias either because all participants were stated to have complete the study, or those that left the study early were included in the final analysis. We rated only one study as a 'high' risk of bias because 13 and 11 people dropped out of the olanzapine and chlorpromazine groups respectively (vs OLZ ‐ Wang 2008). These people were excluded from the final analysis and reasons for dropout were not given.
2. Chlorpromazine versus risperidone
We rated all but two studies as at 'low' risk of attrition bias either because all participants were stated to have completed the study, or those that left the study early were included in the final analysis. We rated two studies as at 'unclear' risk (vs RPD ‐ Feng 2003; vs RPD ‐ Wang 2005) as there were dropouts, however it was unclear whether these were included in the final analysis.
3. Chlorpromazine versus quetiapine
Thirty‐seven included studies were rated as at 'low' risk of attrition bias either because all participants were stated to have complete the study, or those that left the study early were included in the final analysis. We rated eight studies as at 'high' risk of attrition bias (vs QTP ‐ Cao 2005; vs QTP ‐ Cheng 2003; vs QTP ‐ Guo 2003b; vs QTP ‐ Ji 2004; vs QTP ‐ Li 2003; vs QTP ‐ NCT00882518; vs QTP ‐ Wang 2005; vs QTP ‐ Yang 2007) either because it was unclear if these were included in the final analysis, or dropouts were excluded from the analysis.
Selective reporting
1. Chlorpromazine versus olanzapine
We rated five studies at 'high' risk of bias for selective reporting (HGCQ (Turkey) 2000; vs OLZ ‐ Chang 2003; vs OLZ ‐ Chen 2006; vs OLZ ‐ Loza 1999 (HGDT); vs OLZ ‐ Wang 2002) due to pre‐specified outcomes in each study not being fully reported, or partially reported. One study was rated as at 'unclear' risk of bias (vs OLZ ‐ Wu 2008) as it was not explicit whether all outcomes were reported. We rated all remaining studies as at 'low' risk of bias.
2. Chlorpromazine versus risperidone
We rated two studies as at 'high' risk of bias for selective reporting due to pre‐specified outcomes in each study not being fully reported or partially reported (vs RPD ‐ Lin 2005; vs RPD ‐ Zheng 2001). Two studies were rated as at 'unclear' risk of bias as it was not explicit whether all outcomes were reported (vs RPD ‐ Feng 2003; vs RPD ‐ Liu 2000). We rated all other studies as at 'low' risk of bias.
3. Chlorpromazine versus quetiapine
Twelve studies were rated as at 'high' risk of bias for selective reporting due to pre‐specified outcomes in each study not being fully reported, or partially reported (vs QTP ‐ Ai 2007; vs QTP ‐ Chen 2007; vs QTP ‐ Chen 2008; vs QTP ‐ Deng 2004; vs QTP ‐ Guo 2007; vs QTP ‐ Jiang 2006; vs QTP ‐ Jiang 2008; vs QTP ‐ Jin 2007; vs QTP ‐ Nai 2007; vs QTP ‐ Wan 2002; vs QTP ‐ Zhang 2003; vs QTP ‐ Zou 2006). We rated one study as at 'unclear' risk of bias as it was not explicit whether all outcomes were reported (vs QTP ‐ Liu 2003). We rated all other studies as at 'low' risk of bias. See Figure 4.
4.
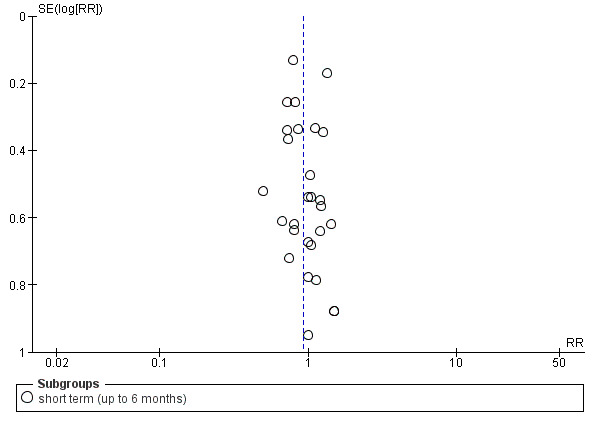
Funnel plot of comparison: 3 Chlorpromazine vs Quetiapine, outcome: 3.1 Clinical response: no significant clinical response.
Other potential sources of bias
1. Chlorpromazine versus olanzapine
All studies were rated at 'low' risk of other potential sources of bias, since we did not detect any obvious bias.
2. Chlorpromazine versus risperidone
We rated all studies at 'low' risk of other potential sources of bias, since we did not detect any obvious bias.
3. Chlorpromazine versus quetiapine
We rated two studies at 'high' risk of bias as they were both funded by the pharmaceutical company AstraZeneca, the manufacturers of quetiapine (vs QTP ‐ Peuskens 1997; vs QTP ‐ NCT00882518). All remaining studies were rated at 'low' risk of other potential sources of bias, since we did not detect any obvious bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Comparison 1: CHLORPROMAZINE versus OLANZAPINE
1.1 Clinical response: 1. No significant clinical response
1.1.1 short term ‐ up to 6 months
In this subgroup we found three relevant trials (N = 204). There was a statistically significant difference (P = 0.002) in favour of olanzapine (RR 2.34, 95% CI 1.37 to 3.99; Analysis 1.1).
1.1. Analysis.
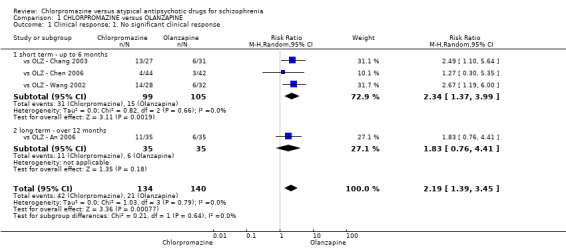
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 1 Clinical response: 1. No significant clinical response.
1.1.2 long term ‐ over 12 months
We only found one relevant trial in this subgroup (N = 70) (vs OLZ ‐ An 2006). There was no significant difference between chlorpromazine and olanzapine (RR 1.83, 95% CI 0.76 to 4.41; Analysis 1.1).
1.1.3 total
Overall there was a statistically significant difference in favour of olanzapine in both short and long term (4 RCTs, N = 274; RR 2.19, 95% CI 1.39 to 3.45; P = 0.0008; Analysis 1.1)
1.2 Clinical response: 2. Average endpoint score of CGI (high = poor) ‐ short term (up to 6 months)
In this subgroup we included three trials (N = 110). There was a statistically significant difference in favour of olanzapine (MD 0.93, 95% CI 0.36 to 1.51; P = 0.002; Analysis 1.2). This subgroup had moderate levels of heterogeneity (Chi² test = 3.9; df = 2; P = 0.142; I² statistic = 49%).
1.2. Analysis.
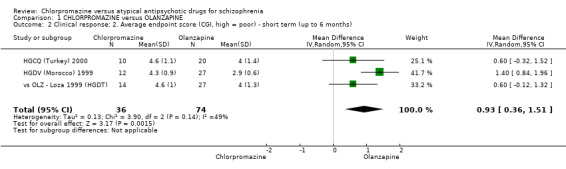
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 2 Clinical response: 2. Average endpoint score (CGI, high = poor) ‐ short term (up to 6 months).
1.3 Clinical response: 3. Relapse ‐ long term (over 12 months)
In this subgroup we only found one relevant trial (N = 70) (vs OLZ ‐ An 2006). There was no significant difference between chlorpromazine and olanzapine (RR 1.5, 95% CI 0.46 to 4.86; Analysis 1.3).
1.3. Analysis.
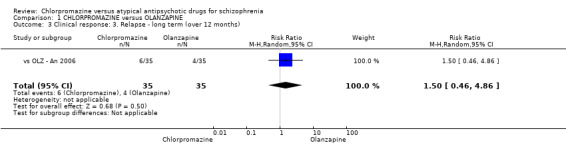
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 3 Clinical response: 3. Relapse ‐ long term (over 12 months).
1.4 Mental state: 1. Average endpoint score of various scales (high = poor) ‐ short term (up to 6 months)
1.4.1 BPRS total
In this subgroup we found four relevant trials (N = 245). There was no significant difference between chlorpromazine and olanzapine (MD 3.21, 95% CI −0.62 to 7.05; Analysis 1.4). This subgroup had important levels of heterogeneity (Chi² test = 23.38; df = 3; P = 0.0; I² statistic = 87%).
1.4. Analysis.
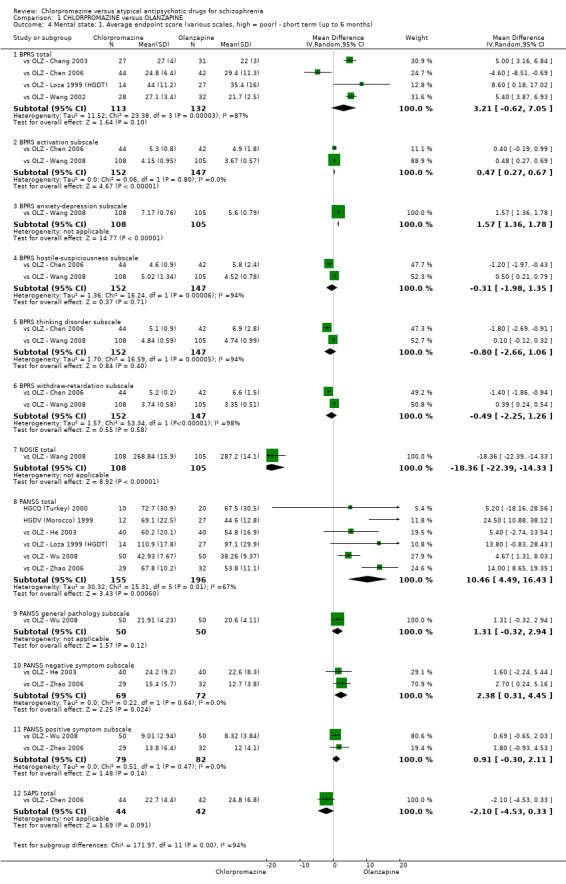
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 4 Mental state: 1. Average endpoint score (various scales, high = poor) ‐ short term (up to 6 months).
1.4.2 BPRS activation subscale
In this subgroup we found two relevant trials (N = 299). There was a statistically significant difference in favour of olanzapine (MD 0.47, 95% CI 0.27 to 0.67; P < 0.00001; Analysis 1.4).
1.4.3 BPRS anxiety‐depression subscale
In this subgroup we only included one trial (N = 213) (vs OLZ ‐ Wang 2008). There was a statistically significant difference in favour of olanzapine (MD 1.57, 95% CI 1.36 to 1.78; P < 0.00001; Analysis 1.4).
1.4.4 BPRS hostile‐suspiciousness subscale
In this subgroup we found two relevant trials (N = 299). There was no significant difference between chlorpromazine and olanzapine (MD −0.31, 95% CI −1.98 to 1.35; Analysis 1.4). This subgroup had important levels of heterogeneity (Chi² test = 16.24; df = 1; P = 0.0; I² statistic = 94%).
1.4.5 BPRS thinking disorder subscale
We found two relevant trials in this subgroup (N = 299). There was no significant difference between chlorpromazine and olanzapine (MD −0.8, 95% CI −2.66 to 1.06; Analysis 1.4). This subgroup had important levels of heterogeneity (Chi² test = 16.59; df = 1; P = 0.0; I² statistic = 94%).
1.4.6 BPRS withdraw‐retardation subscale
In this subgroup we found two relevant trials (N = 299). There was no significant difference between chlorpromazine and olanzapine (MD −0.49 95% CI −2.25 to 1.26; Analysis 1.4). This subgroup had important levels of heterogeneity (Chi² test = 53.34; df = 1; P = 0.0; I² statistic = 98%).
1.4.7 NOSIE total
We only included one trial in this subgroup (N = 213) (vs OLZ ‐ Wang 2008). There was a statistically significant difference in favour of chlorpromazine (MD −18.36, 95% CI −22.39 to −14.33; P < 0.00001; Analysis 1.4).
1.4.8 PANSS total
In this subgroup we included six trials (N = 351). There was a statistically significant difference in favour of olanzapine (MD 10.46, 95% CI 4.49 to 16.43; P = 0.006; Analysis 1.4). This subgroup had important levels of heterogeneity (Chi² test = 15.31; df = 5; P = 0.009; I² statistic = 67%).
1.4.9 PANSS general pathology subscale
In this subgroup we only found one relevant trial (N = 100) (vs OLZ ‐ Wu 2008). There was no significant difference between chlorpromazine and olanzapine (MD 1.31, 95% CI −0.32 to 2.94; Analysis 1.4).
1.4.10 PANSS negative symptom subscale
In this subgroup we found two relevant trials (N = 141). There was a statistically significant difference in favour of olanzapine (MD 2.38, 95% CI 0.31 to 4.45; P = 0.02; Analysis 1.4).
1.4.11 PANSS positive symptom subscale
We found two relevant trials in this subgroup (N = 161). There was no significant difference between chlorpromazine and olanzapine (MD 0.91, 95% CI −0.30 to 2.11; Analysis 1.4).
1.4.12 SAPS total
In this subgroup we only found one relevant trial (n = 86) (vs OLZ ‐ Chen 2006). There was no significant difference between chlorpromazine and olanzapine (MD −2.1, 95% CI −4.53 to 0.33; Analysis 1.4).
1.5 Mental state: 3. Average endpoint score (BPRS, high = poor) ‐ medium term (7 ‐ 12 months)
In this subgroup we only found one relevant trial (N = 60) (vs OLZ ‐ Wang 2002). There was a statistically significant difference in favour of olanzapine (MD 8.60, 95% CI 5.94 to 11.26; P < 0.00001; Analysis 1.5).
1.5. Analysis.
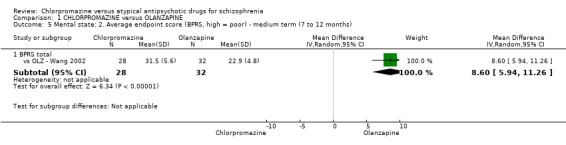
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 5 Mental state: 2. Average endpoint score (BPRS, high = poor) ‐ medium term (7 to 12 months).
1.6 Mental state: 2. Average endpoint score of various scales (high = poor) ‐ skewed data
Data using the various mental state scales, including the BPRS, PANSS, MADRS and HAMA, were skewed and are best inspected by viewing (Analysis 1.6).
1.6. Analysis.
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 6 Mental state: 3. Average endpoint score (various scales, high = poor) ‐ skewed data.
| Mental state: 3. Average endpoint score (various scales, high = poor) ‐ skewed data | ||||
|---|---|---|---|---|
| Study | Intervention | mean | SD | N |
| BPRS total | ||||
| HGCQ (Turkey) 2000 | Chlorpromazine | 24.7 | 18.4 | 10 |
| HGCQ (Turkey) 2000 | Olanzapine | 20.2 | 17.9 | 20 |
| HGDV (Morocco) 1999 | Chlorpromazine | 20.7 | 12.9 | 12 |
| HGDV (Morocco) 1999 | Olanzapine | 6.7 | 6.4 | 27 |
| vs OLZ ‐ Wang 2008 | Chlorpromazine | 30.16 | 27.4 | 108 |
| vs OLZ ‐ Wang 2008 | Olanzapine | 27.34 | 7.89 | 105 |
| HAMA (anxiety) | ||||
| HGDV (Morocco) 1999 | Chlorpromazine | 7.6 | 5.8 | 12 |
| HGDV (Morocco) 1999 | Olanzapine | 3.3 | 1.8 | 27 |
| MADRS (depression) | ||||
| HGDV (Morocco) 1999 | Chlorpromazine | 6.9 | 4.8 | 12 |
| HGDV (Morocco) 1999 | Olanzapine | 3.3 | 2.0 | 27 |
| PANSS negative symptom subscale | ||||
| vs OLZ ‐ Wu 2008 | Chlorpromazine | 11.62 | 3.93 | 50 |
| vs OLZ ‐ Wu 2008 | Olanzapine | 8.34 | 4.26 | 50 |
| PANSS positive symptom subscale | ||||
| vs OLZ ‐ He 2003 | Chlorpromazine | 11.7 | 6.2 | 40 |
| vs OLZ ‐ He 2003 | Olanzapine | 10.2 | 4.9 | 40 |
1.7 Service involvement: 1. Re‐hospitalisation
1.7.1 long term (over 12 months)
We found only one trial in this subgroup (N = 70) (vs OLZ ‐ An 2006). There was no significant difference between chlorpromazine and olanzapine (RR 1.50, 95% CI 0.46 to 4.86; Analysis 1.7).
1.7. Analysis.
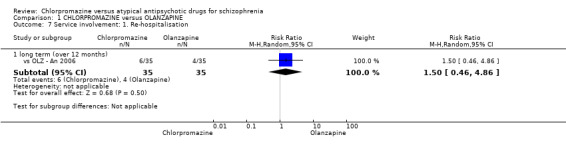
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 7 Service involvement: 1. Re‐hospitalisation.
1.8 Functioning: 1. Executive function ‐ average endpoint score (WCST, high = poor)
1.8.1 short term (up to 6 months)
In this subgroup we only found one relevant trial (N = 53) (vs OLZ ‐ An 2006). There was a statistically significant difference in favour of olanzapine (MD 10.96, 95% CI 1.01 to 20.91; P = 0.03; Analysis 1.8).
1.8. Analysis.
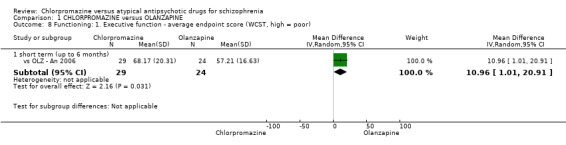
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 8 Functioning: 1. Executive function ‐ average endpoint score (WCST, high = poor).
1.9 Adverse effects: 1. Anticholinergic ‐ short term (up to 6 months)
1.9.1 blurred vision
In this subgroup we found three relevant trials (N = 241). There was no significant difference between chlorpromazine and olanzapine (RR 2.59, 95% CI 0.66 to 10.22; Analysis 1.9). This subgroup had moderate levels of heterogeneity (Chi² test = 3.4; df = 2; P = 0.183; I² statistic = 41%).
1.9. Analysis.
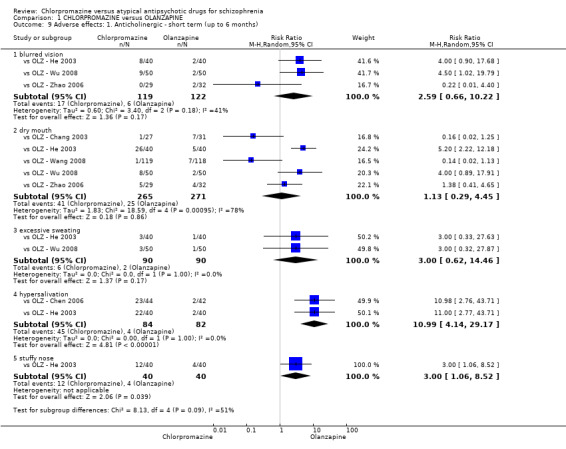
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 9 Adverse effects: 1. Anticholinergic ‐ short term (up to 6 months).
1.9.2 dry mouth
In this subgroup we found five relevant trials (N = 536). There was no significant difference between chlorpromazine and olanzapine (RR 1.13, 95% CI 0.29 to 4.45; Analysis 1.9). This subgroup had important levels of heterogeneity (Chi² test = 18.59; df = 4; P = 0.001; I² statistic = 78%).
1.9.3 excessive sweating
We included two relevant trials in this subgroup (N = 180). There was no significant difference between chlorpromazine and olanzapine (RR 3.00, 95% CI 0.62 to 14.46; Analysis 1.9).
1.9.4 hypersalivation
In this subgroup we found two relevant trials (N = 166). There was a statistically significant difference in favour of olanzapine (RR 10.99, 95% CI 4.14 to 29.17; P < 0.00001; Analysis 1.9).
1.9.5 stuffy nose
In this subgroup we only found one relevant trial (N = 80) (vs OLZ ‐ He 2003). There was a statistically significant difference in favour of olanzapine (RR 3.00, 95% CI 1.06 to 8.52; P = 0.04; Analysis 1.9).
1.10 Adverse effects: 2. Cardiovascular ‐ short term (up to 6 months)
1.10.1 abnormal ECG
In this subgroup we found two relevant trials (N = 180). There was no significant difference between chlorpromazine and olanzapine (RR 3.60, 95% CI 0.60 to 21.55; Analysis 1.10).
1.10. Analysis.
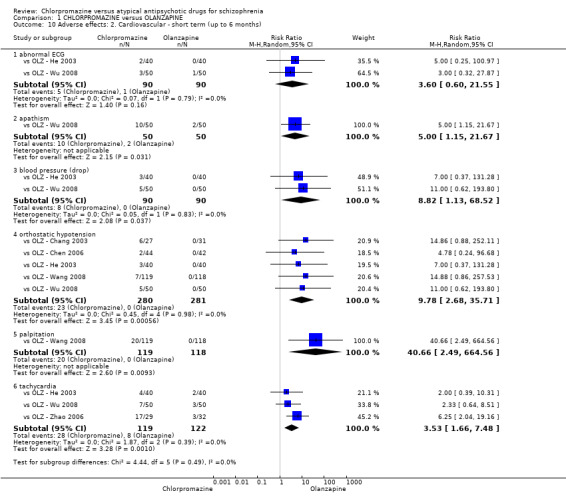
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 10 Adverse effects: 2. Cardiovascular ‐ short term (up to 6 months).
1.10.2 apathism ‐ short term (up to six months)
In this subgroup we included only one relevant trial (N = 100) (vs OLZ ‐ Wu 2008). There was a statistically significant difference in favour of olanzapine (RR 5.00, 95% CI 1.15 to 21.67; P = 0.03; Analysis 1.10).
1.10.3 blood pressure (drop)
We included two trials in this subgroup (N = 180). There was a statistically significant difference in favour of olanzapine (RR 8.82, 95% CI 1.13 to 68.52 Analysis 1.10.
1.10.4 orthostatic hypotension
In this subgroup we found five relevant trials (N = 561). There was a statistically significant difference in favour of olanzapine (RR 9.78, 95% CI 2.68 to 35.71; P = 0.006; Analysis 1.10).
1.10.5 palpitation
In this subgroup we only found one relevant trial (N = 237) (vs OLZ ‐ Wang 2008). There was a statistically significant difference in favour of olanzapine (RR 40.66, 95% CI 2.49 to 664.56; P = 0.009; Analysis 1.10).
1.10.6 tachycardia
In this subgroup we found three relevant trials (N = 241). There was a statistically significant difference in favour of olanzapine (RR 3.53, 95% CI 1.66 to 7.48; P = 0.001; Analysis 1.10).
1.11 Adverse effects: 3. Central nervous system ‐ short term (up to 6 months)
1.11.1 dizziness
We included two trials in this subgroup (N = 180). There was a statistically significant difference in favour of olanzapine (RR 3.85, 95% CI 1.11 to 13.32; P = 0.03; Analysis 1.11).
1.11. Analysis.
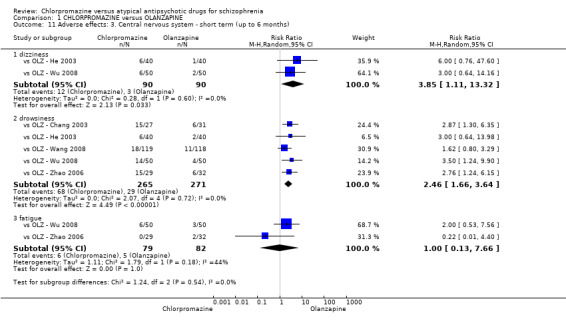
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 11 Adverse effects: 3. Central nervous system ‐ short term (up to 6 months).
1.11.2 drowsiness
In this subgroup we found five relevant trials (N = 536). There was a statistically significant difference in favour of olanzapine (RR 2.46, 95% CI 1.66 to 3.64; P < 0.00001; Analysis 1.11).
1.11.3 fatigue
In this subgroup we found two relevant trials (N = 161). There was no significant difference between chlorpromazine and olanzapine (RR 1.00, 95% CI 0.13 to 7.66; Analysis 1.11). This subgroup had moderate levels of heterogeneity (Chi² test = 1.79; df = 1; P = 0.181; I² statistic = 44%).
1.12 Adverse effects: 4. Gastrointestinal ‐ short term (up to 6 months)
1.12.1 appetite loss
In this subgroup we found two relevant trials (N = 180). There was a statistically significant difference in favour of olanzapine (RR 11.01, 95% CI 2.82 to 42.94; P = 0.0006; Analysis 1.12). This subgroup had moderate levels of heterogeneity (Chi² test = 1.78; df = 1; P = 0.182; I² statistic = 44%).
1.12. Analysis.
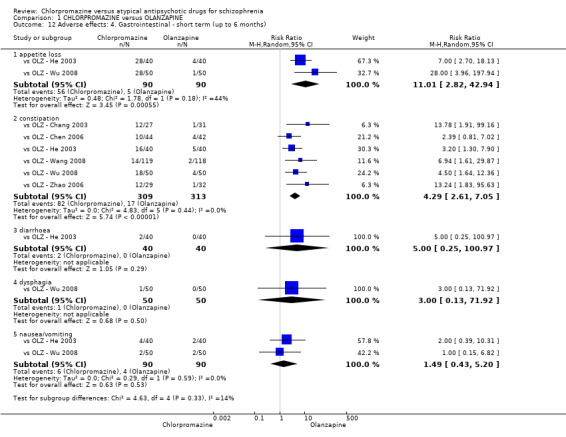
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 12 Adverse effects: 4. Gastrointestinal ‐ short term (up to 6 months).
1.12.2 constipation
In this subgroup we found six relevant trials (N = 622). There was a statistically significant difference in favour of olanzapine (RR 4.29, 95% CI 2.61 to 7.05; P < 0.00001; Analysis 1.12).
1.12.3 diarrhoea
We found only one trial in this subgroup (N = 80) (vs OLZ ‐ He 2003). There was no significant difference between chlorpromazine and olanzapine (RR 5.00, 95% CI 0.25 to 100.97; Analysis 1.12).
1.12.4 dysphagia
We found only one relevant trial in this subgroup (N = 100) (vs OLZ ‐ Wu 2008). There was no significant difference between chlorpromazine and olanzapine (RR 3.00, 95% CI 0.13 to 71.92; Analysis 1.12).
1.12.5 nausea/vomiting
We included two relevant trials in this subgroup (N = 180). There was no significant difference between chlorpromazine and olanzapine (RR 1.49, 95% CI 0.43 to 5.20; Analysis 1.12).
1.13 Adverse effects: 5. Haematology ‐ ‐ short term (up to 6 months)
1.13.1 abnormal haemogram
In this subgroup we found two relevant trials (N = 180). There was no significant difference between chlorpromazine and olanzapine (RR 4.00, 95% CI 0.87 to 18.31; Analysis 1.13).
1.13. Analysis.
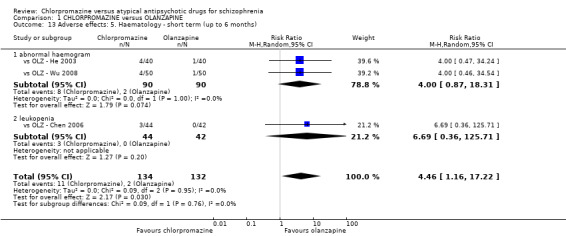
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 13 Adverse effects: 5. Haematology ‐ short term (up to 6 months).
1.13.2 leukopenia
In this subgroup we only found one relevant trial (N = 86) (vs OLZ ‐ Chen 2006). There was no significant difference between chlorpromazine and olanzapine (RR 6.69, 95% CI 0.36 to 125.71; Analysis 1.13).
1.14 Adverse effects: 6. Hepatitic ‐ short term (up to 6 months)
1.14.1 abnormal liver function
In this subgroup we only found one relevant trial (N = 100) (vs OLZ ‐ Wu 2008). There was no significant difference between chlorpromazine and olanzapine (RR 5.00, 95% CI 0.25 to 101.58; Analysis 1.14).
1.14. Analysis.
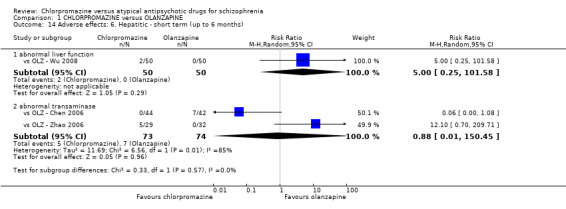
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 14 Adverse effects: 6. Hepatitic ‐ short term (up to 6 months).
1.14.2 abnormal transaminase
In this subgroup we found two relevant trials (N = 147). There was no significant difference between chlorpromazine and olanzapine (RR 0.88, 95% CI 0.01 to 150.45; Analysis 1.14). This subgroup had important levels of heterogeneity (Chi² test = 6.56; df = 1; P = 0.01; I² statistic = 85%).
1.15 Adverse effects: 7a Metabolic ‐ weight gain ‐ short term (up to 6 months)
In this subgroup we found five relevant trials (N = 536). There was no significant difference between chlorpromazine and olanzapine (RR 0.70, 95% CI 0.25 to 1.96; Analysis 1.15 ). This subgroup had important levels of heterogeneity (Chi² test = 12.17; df = 4; P = 0.016; I² statistic = 67%).
1.15. Analysis.
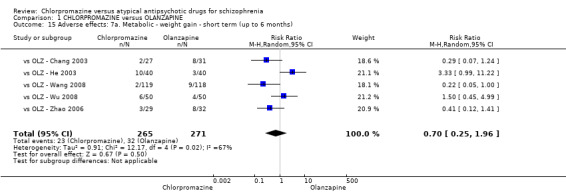
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 15 Adverse effects: 7a. Metabolic ‐ weight gain ‐ short term (up to 6 months).
1.16 Adverse effects: 7b. Metabolic ‐ weight gain ‐ continuous measures
1.16.1 short term (up to to 6 months)
In this subgroup we found four relevant trials (N = 160). There was a statistically significant difference in favour of chlorpromazine (MD −5.11, 95% CI −9.15 to −1.07; P = 0.01; Analysis 1.16).
1.16. Analysis.
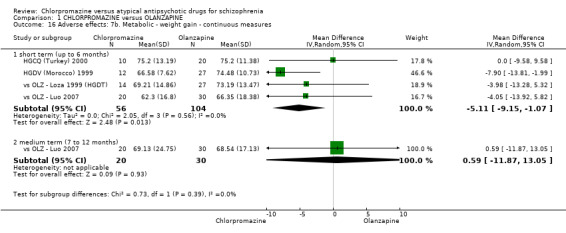
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 16 Adverse effects: 7b. Metabolic ‐ weight gain ‐ continuous measures.
1.16.1 medium term (7 to 12 months)
We included only one trial in this subgroup (N = 50) (vs OLZ ‐ Luo 2007). There was no significant difference between chlorpromazine and olanzapine (MD 0.59, 95% CI −11.87 to 13.05; Analysis 1.16).
1.17 Adverse effects: 7c. Metabolic ‐ other ‐ continuous measures
1.17.1 cholesterol (TC) ‐ short term (up to 6 months)
In this subgroup we included one relevant trial (N = 50) (vs OLZ ‐ Luo 2007). There was no significant difference between chlorpromazine and olanzapine (MD −0.40, 95% CI −1.02 to 0.22; Analysis 1.17).
1.17. Analysis.
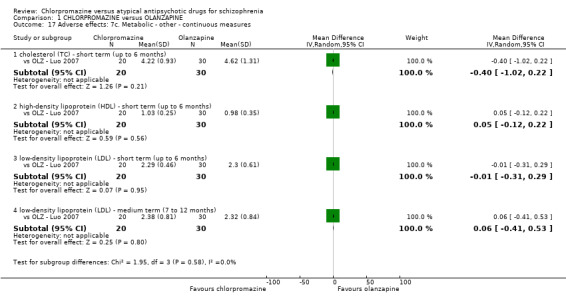
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 17 Adverse effects: 7c. Metabolic ‐ other ‐ continuous measures.
1.17.2 high‐density lipoprotein (HDL) ‐ short term (up to 6 months)
We only found one trial in this subgroup (N = 50) (vs OLZ ‐ Luo 2007). There was no significant difference between chlorpromazine and olanzapine (MD 0.05, 95% CI −0.12 to 0.22; Analysis 1.17).
1.17.3 low‐density lipoprotein (LDL) ‐ short term (up to 6 months)
We found only one relevant trial in this subgroup (N = 50) (vs OLZ ‐ Luo 2007). There was no significant difference between chlorpromazine and olanzapine (MD −0.01, 95% CI −0.31 to 0.29; Analysis 1.17).
1.17.4 low‐density lipoprotein (LDL) ‐ medium term (7 to 12 months)
In this subgroup we only found one relevant trial (N = 50) (vs OLZ ‐ Luo 2007). There was no significant difference between chlorpromazine and olanzapine (MD 0.06, 95% CI −0.41 to 0.53; Analysis 1.16).
1.18 Adverse effects: 7d. Metabolic ‐ other ‐ average endpoint scores ‐ skewed data
Data for endpoint scores in cholesterol, high‐density lipoprotein and triglyceride are skewed and are best inspected by viewing Analysis 1.18.
1.18. Analysis.
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 18 Adverse effects: 7d. Metabolic ‐ other ‐ average endpoint scores ‐ skewed data.
| Adverse effects: 7d. Metabolic ‐ other ‐ average endpoint scores ‐ skewed data | ||||
|---|---|---|---|---|
| Study | Intervention | mean | SD | N |
| cholesterol (TC) ‐ medium term (7 to 12 months) | ||||
| vs OLZ ‐ Luo 2007 | Chlorpromazine | 4.5 | 2.21 | 20 |
| vs OLZ ‐ Luo 2007 | Olanzapine | 4.52 | 2.73 | 30 |
| high‐density lipoprotein (HDL) ‐ short term (up to 6 months) | ||||
| vs OLZ ‐ Luo 2007 | Chlorpromazine | 0.89 | 0.33 | 20 |
| vs OLZ ‐ Luo 2007 | Olanzapine | 0.89 | 0.45 | 30 |
| triglyceride (TG) ‐ short term (up to 6 months) | ||||
| vs OLZ ‐ Luo 2007 | Chlorpromazine | 1.44 | 1.07 | 20 |
| vs OLZ ‐ Luo 2007 | Olanzapine | 2.13 | 2.07 | 30 |
| triglyceride (TG) ‐ medium term (7 to 12 months) | ||||
| vs OLZ ‐ Luo 2007 | Chlorpromazine | 2.26 | 2.04 | 20 |
| vs OLZ ‐ Luo 2007 | Olanzapine | 2.32 | 2.14 | 30 |
1.19 Adverse effects: 8a. Movement disorders ‐ extrapyramidal symptoms ‐ short term (up to 6 months)
1.19.1 akathisia
In this subgroup we found three relevant trials (N = 417). There was no significant difference between chlorpromazine and olanzapine (RR 1.86, 95% CI 0.29 to 11.84; Analysis 1.19). This subgroup had important levels of heterogeneity (Chi² test = 5.51; df = 2; P = 0.064; I² statistic = 64%).
1.19. Analysis.
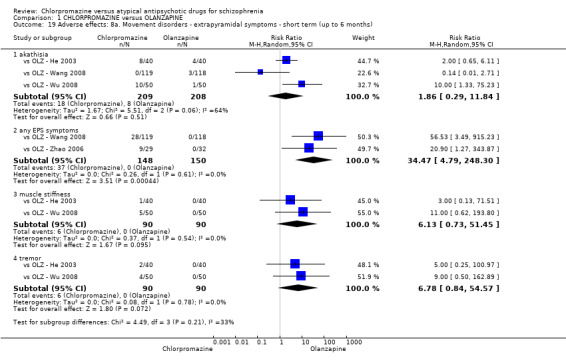
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 19 Adverse effects: 8a. Movement disorders ‐ extrapyramidal symptoms ‐ short term (up to 6 months).
1.19.2 any EPS symptoms
In this subgroup we found two relevant trials (N = 298). There was a statistically significant difference in favour of olanzapine (RR 34.47, 95% CI 4.79 to 248.3; P = 0.0004; Analysis 1.19).
1.19.3 muscle stiffness
In this subgroup we found two relevant trials (N = 180). There was no significant difference between chlorpromazine and olanzapine (RR 6.13, 95% CI 0.73 to 51.45; Analysis 1.19).
1.19.4 tremor
In this subgroup we found two relevant trials (N = 180). There was no significant difference between chlorpromazine and olanzapine (RR 6.78, 95% CI 0.84 to 54.57; Analysis 1.19).
1.20 Adverse effects: 8b. Movement disorders ‐ extrapyramidal symptoms ‐ average endpoint score (ESRS, high = poor)
1.20.1 short term (up to 6 months)
We included two relevant trials in this subgroup (N = 80). There was a statistically significant difference in favour of olanzapine (MD 0.9, 95% CI 0.14 to 1.66; P = 0.02; Analysis 1.20)
1.20. Analysis.
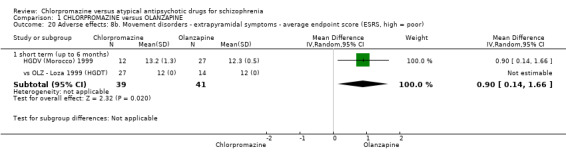
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 20 Adverse effects: 8b. Movement disorders ‐ extrapyramidal symptoms ‐ average endpoint score (ESRS, high = poor).
1.21 Adverse effects: 9a. Various other ‐ sleep ‐ average endpoint score (LSEQ, high = better) ‐ short term (up to 6 months)
1.21.1 awaking from sleep
We found only one relevant trial in this subgroup (N = 30) (HGCQ (Turkey) 2000). There was no significant difference between chlorpromazine and olanzapine (MD −5.30, 95% CI −21.91 to 11.31; Analysis 1.21).
1.21. Analysis.
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 21 Adverse effects: 9a. Various other ‐ sleep ‐ average endpoint score (LSEQ, high = better) ‐ short term (up to 6 months).
1.21.2 getting to sleep score
In this subgroup we only found one relevant trial (N = 30) (HGCQ (Turkey) 2000). There was no significant difference between chlorpromazine and olanzapine (MD −0.60, 95% CI −19.88 to 18.68; Analysis 1.21).
1.21.3 quality of sleep
In this subgroup we only found one relevant trial (N = 30) (HGCQ (Turkey) 2000). There was no significant difference between chlorpromazine and olanzapine (MD −15.00, 95% CI −33.07 to 3.07; Analysis 1.21).
1.22 Adverse effects: 9b. Various other ‐ sleep ‐ average length of sleep (hour/day)
1.22.1 short term (up to 6 months)
We found one trial in this subgroup (N = 50) (vs OLZ ‐ Luo 2007). There was a statistically significant difference in favour of olanzapine (MD 3.63, 95% CI 2.08 to 5.18; P < 0.00001; Analysis 1.22).
1.22. Analysis.
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 22 Adverse effects: 9b. Various other ‐ sleep ‐ average length of sleep (hour/day).
1.22.2 medium term (7 to 12 months)
In this subgroup we only found one relevant trial (N = 50) (vs OLZ ‐ Luo 2007). There was a statistically significant difference in favour of olanzapine (MD 4.41, 95% CI 2.82 to 6.00; P < 0.00001; Analysis 1.22).
1.23 Adverse effects: 9c. Various other ‐ sleep ‐ average score ‐ behaviour following waking ‐ skewed data
Data for this study are skewed and are best inspected by viewing Analysis 1.23.
1.23. Analysis.
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 23 Adverse effects: 9c. Various other ‐ sleep ‐ behaviour following waking (LSEQ) ‐ skewed data.
| Adverse effects: 9c. Various other ‐ sleep ‐ behaviour following waking (LSEQ) ‐ skewed data | ||||
|---|---|---|---|---|
| Study | Intervention | mean | SD | N |
| short term (up to 6 months) | ||||
| HGCQ (Turkey) 2000 | Chlorpromazine | 55.1 | 28.3 | 10 |
| HGCQ (Turkey) 2000 | Olanzapine | 55.9 | 18.8 | 20 |
1.24 Adverse effects: 9d. Various other ‐ rash
1.24.1 short term (up to 6 months)
We included one relevant trial in this subgroup (N = 30) (HGCQ (Turkey) 2000). There was no significant difference between chlorpromazine and olanzapine (RR 2.00, 95% CI 0.14 to 28.76; Analysis 1.24).
1.24. Analysis.
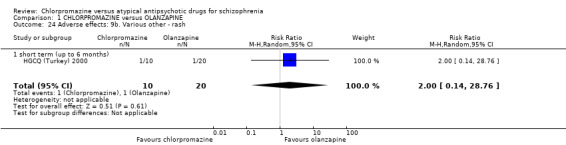
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 24 Adverse effects: 9b. Various other ‐ rash.
1.25 Quality of life: 1a. Average endpoint scores (various scales, high = better) ‐ short term (up to 6 months)
1.25.1 GQOLI ‐ living condition
In this subgroup we only found one relevant trial (N = 61) (vs OLZ ‐ Zhao 2006). There was no significant difference between chlorpromazine and olanzapine (MD −1.00, 95% CI −4.21 to 2.21; Analysis 1.25).
1.25. Analysis.
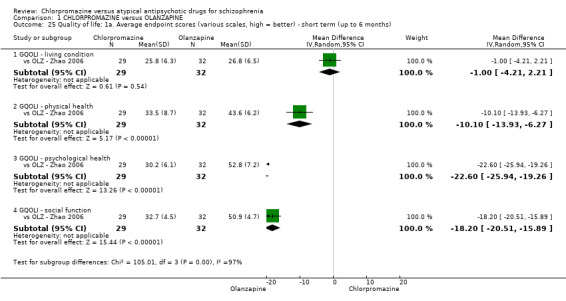
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 25 Quality of life: 1a. Average endpoint scores (various scales, high = better) ‐ short term (up to 6 months).
1.25.2 GQOLI ‐ physical health
In this subgroup we only found one relevant trial (N = 61) (vs OLZ ‐ Zhao 2006). There was a statistically significant difference in favour of olanzapine (MD −10.10, 95% CI −13.93 to −6.27; P < 0.00001; Analysis 1.25).
1.25.3 GQOLI ‐ psychological health
In this subgroup we only found one relevant trial (N = 61) (vs OLZ ‐ Zhao 2006). There was a statistically significant difference in favour of olanzapine (MD −22.60, 95% CI −25.94 to −19.26; P < 0.00001; Analysis 1.25).
1.25.4 GQOLI ‐ social function
In this subgroup we only found one relevant trial (N = 61) (vs OLZ ‐ Zhao 2006). There was a statistically significant difference in favour of olanzapine (MD −18.20, 95% CI −20.51 to −15.89; P < 0.00001; Analysis 1.25).
1.26 Quality of life: 1b. Average endpoint score (QoL, high = better) ‐ skewed data
Data for this outcome are skewed, and are best inspected by viewing Analysis 1.25.
1.27 Leaving the study early ‐ short term (up to 6 months)
1.27.1 due to any reason
In this subgroup we found three relevant trials (N = 139). There was no significant difference between chlorpromazine and olanzapine (RR 1.69, 95% CI 0.45 to 6.40; Analysis 1.27). This subgroup had moderate levels of heterogeneity (Chi² test = 3.64; df = 2; P = 0.162; I² statistic = 45%).
1.27. Analysis.
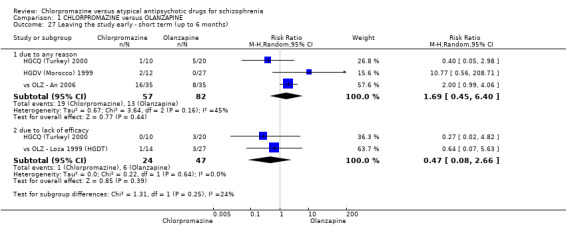
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 27 Leaving the study early ‐ short term (up to 6 months).
1.27.2 due to lack of efficacy
In this subgroup we found two relevant trials (N = 71). There was no significant difference between chlorpromazine and olanzapine (RR 0.47, 95% CI 0.08 to 2.66; Analysis 1.27).
Comparison 2: CHLORPROMAZINE versus RISPERIDONE
2.1 Clinical response: 1. No significant clinical response
2.1.1 short term (up to 6 months)
In this subgroup we found seven relevant trials (N = 475). There was no significant difference between chlorpromazine and risperidone (RR 0.84, 95% CI 0.53 to 1.34; Analysis 2.1).
2.1. Analysis.
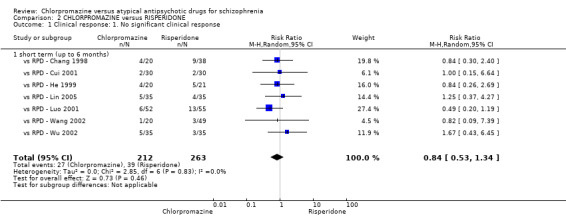
Comparison 2 CHLORPROMAZINE versus RISPERIDONE, Outcome 1 Clinical response: 1. No significant clinical response.
2.2 Global state: 1. Average endpoint score (CGI‐CI, high = poor) ‐ skewed data
Data for this outcome are skewed and are best inspected by viewing Analysis 2.2.
2.2. Analysis.
Comparison 2 CHLORPROMAZINE versus RISPERIDONE, Outcome 2 Global state: 1. Average endpoint score (CGI‐CI, high = poor) ‐ skewed data.
| Global state: 1. Average endpoint score (CGI‐CI, high = poor) ‐ skewed data | ||||
|---|---|---|---|---|
| Study | Intervention | mean | SD | N |
| short term (up to 6 months) | ||||
| vs RPD ‐ Ma 2004 | Chlorpromazine | 2.4 | 3.7 | 39 |
| vs RPD ‐ Ma 2004 | Risperidone | 1.8 | 6.6 | 39 |
2.3 Global state: 2. Need of additional benzhexol
2.3.1 short term (up to 6 months)
We included two trials in this subgroup (N = 137). There was no significant difference between chlorpromazine and risperidone (RR 1.20, 95% CI 0.26 to 5.53; Analysis 2.3). This subgroup had important levels of heterogeneity (Chi² test = 2.36; df = 1; P = 0.124; I² statistic = 58%).
2.3. Analysis.
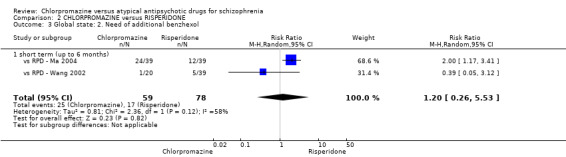
Comparison 2 CHLORPROMAZINE versus RISPERIDONE, Outcome 3 Global state: 2. Need of additional benzhexol.
2.4 Mental state: 1a. Average endpoint score (various scales, high = poor) short term (up to 6 months)
2.4.1 BPRS total
In this subgroup we found four relevant trials (N = 247). There was no significant difference between chlorpromazine and risperidone (MD 0.90, 95% CI −3.49 to 5.28; Analysis 2.4). This subgroup had important levels of heterogeneity (Chi² test = 14.61; df = 3; P = 0.002; I² statistic = 79%).
2.4. Analysis.
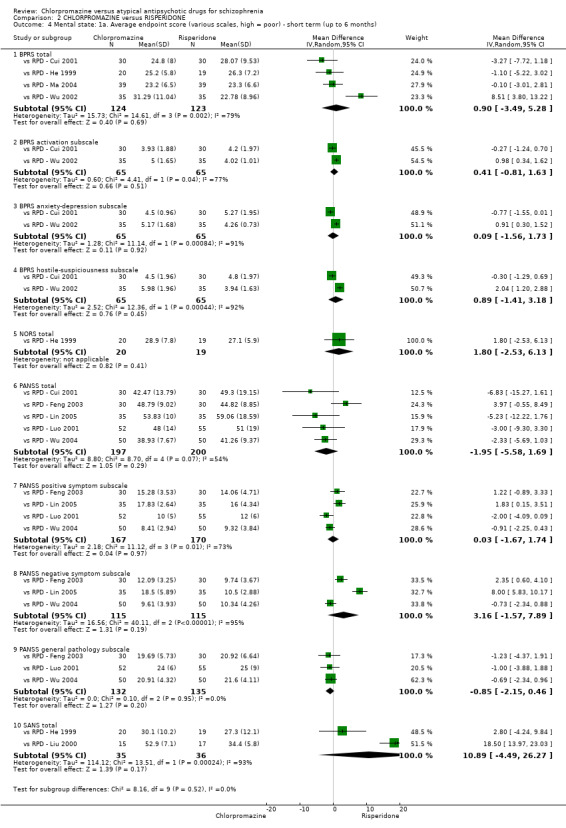
Comparison 2 CHLORPROMAZINE versus RISPERIDONE, Outcome 4 Mental state: 1a. Average endpoint score (various scales, high = poor) ‐ short term (up to 6 months).
2.4.2 BPRS activation subscale
In this subgroup we found two relevant trials (N = 130). There was no significant difference between chlorpromazine and risperidone (MD 0.41, 95% CI −0.81 to 1.63; Analysis 2.4). This subgroup had important levels of heterogeneity (Chi² test = 4.41; df = 1; P = 0.036; I² statistic = 77%).
2.4.3 BPRS anxiety‐depression subscale
In this subgroup we found two relevant trials (N = 130). There was no significant difference between chlorpromazine and risperidone (MD 0.09, 95% CI −1.56 to 1.73; Analysis 2.4). This subgroup had important levels of heterogeneity (Chi² test = 11.14; df = 1; P = 0.001; I² statistic = 91%).
2.4.4 BPRS hostile‐suspiciousness subscale
We found two relevant trials in this subgroup (N = 130). There was no significant difference between chlorpromazine and risperidone groups (MD 0.89, 95% CI −1.41 to 3.18; Analysis 2.4). This subgroup had important levels of heterogeneity (Chi² test = 12.36; df = 1; P = 0.0; I² statistic = 92%).
2.4.5 NORS total
In this subgroup we only found one relevant trial (N = 39) (vs RPD ‐ He 1999). There was no significant difference between chlorpromazine and risperidone (MD 1.80, 95% CI −2.53 to 6.13; Analysis 2.4).
2.4.6 PANSS total
In this subgroup we found five relevant trials (N = 397). There was no significant difference between chlorpromazine and risperidone (MD −1.95, 95% CI −5.58 to 1.69; Analysis 2.4). This subgroup had important levels of heterogeneity (Chi² test = 8.7; df = 4; P = 0.069; I² statistic = 54%).
2.4.7 PANSS positive symptom subscale
In this subgroup we found four relevant trials (N = 337). There was no significant difference between chlorpromazine and risperidone (MD 0.03, 95% CI −1.67 to 1.74; Analysis 2.4). This subgroup had important levels of heterogeneity (Chi² test = 11.12; df = 3; P = 0.011; I² statistic = 73%).
2.4.8 PANSS negative symptom subscale
We found three relevant trials in this subgroup (N = 230). There was no significant difference between chlorpromazine and risperidone (MD 3.16, 95% CI −1.57 to 7.89; Analysis 2.4). This subgroup had important levels of heterogeneity (Chi² test = 40.11; df = 2; P = 0.0; I² statistic = 95%).
2.4.9 PANSS general pathology subscale
In this subgroup we found three relevant trials (N = 267). There was no significant difference between chlorpromazine and risperidone (MD −0.85, 95% CI −2.15 to 0.46; Analysis 2.4).
2.4.10 SANS total
In this subgroup we found two relevant trials (N = 71). There was no significant difference between chlorpromazine and risperidone (MD 10.89, 95% CI −4.49 to 26.27; Analysis 2.4). This subgroup had important levels of heterogeneity (Chi² test = 13.51; df = 1; P = 0.0; I² statistic = 93%).
2.5 Mental state: 1b. Average endpoint score (various scales, high = poor) ‐ skewed data
Data for this outcome are skewed and are best inspected by viewing Analysis 2.5.
2.5. Analysis.
Comparison 2 CHLORPROMAZINE versus RISPERIDONE, Outcome 5 Mental state: 1b. Average endpoint score (various scales, high = poor) ‐ skewed data ‐ short term (up to 6 months).
| Mental state: 1b. Average endpoint score (various scales, high = poor) ‐ skewed data ‐ short term (up to 6 months) | ||||
|---|---|---|---|---|
| Study | Intervention | mean | SD | N |
| BPRS thinking disorder subscale | ||||
| vs RPD ‐ Wu 2002 | Chlorpromazine | 7.96 | 4.25 | 35 |
| vs RPD ‐ Wu 2002 | Risperidone | 5.13 | 1.89 | 35 |
| BPRS withdraw‐retardation subscale | ||||
| vs RPD ‐ Cui 2001 | Chlorpromazine | 5.73 | 2.21 | 30 |
| vs RPD ‐ Cui 2001 | Risperidone | 6.43 | 3.24 | 30 |
| vs RPD ‐ Wu 2002 | Chlorpromazine | 7.88 | 3.12 | 35 |
| vs RPD ‐ Wu 2002 | Risperidone | 5.02 | 2.87 | 35 |
| PANSS general pathology subscale | ||||
| vs RPD ‐ Cui 2001 | Chlorpromazine | 21.17 | 6.31 | 30 |
| vs RPD ‐ Cui 2001 | Risperidone | 24.47 | 9.51 | 30 |
| PANSS negative symptom subscale | ||||
| vs RPD ‐ Cui 2001 | Chlorpromazine | 9.5 | 3.53 | 30 |
| vs RPD ‐ Cui 2001 | Risperidone | 12.63 | 7.39 | 30 |
| vs RPD ‐ Luo 2001 | Chlorpromazine | 14 | 6 | 52 |
| vs RPD ‐ Luo 2001 | Risperidone | 14 | 8 | 55 |
| PANSS positive symptom subscale | ||||
| vs RPD ‐ Cui 2001 | Chlorpromazine | 11.67 | 13.6 | 30 |
| vs RPD ‐ Cui 2001 | Risperidone | 11.6 | 4.67 | 30 |
| SAPS total | ||||
| vs RPD ‐ He 1999 | Chlorpromazine | 12.7 | 5.9 | 20 |
| vs RPD ‐ He 1999 | Risperidone | 13.9 | 7.8 | 19 |
2.6 Mental state: 2. Average change score ‐ decreased rate (various scales, high = poor) ‐ short term (up to 6 months)
2.6.1 PANSS total
In this subgroup we only found one relevant trial (N = 57) (vs RPD ‐ Wang 2002). There was no significant difference between chlorpromazine and risperidone (MD −0.11, 95% CI −0.23 to 0.01; Analysis 2.6).
2.6. Analysis.
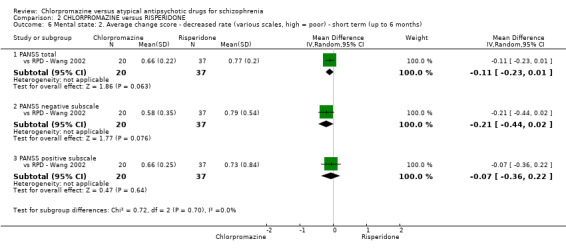
Comparison 2 CHLORPROMAZINE versus RISPERIDONE, Outcome 6 Mental state: 2. Average change score ‐ decreased rate (various scales, high = poor) ‐ short term (up to 6 months).
2.6.2 PANSS negative subscale
In this subgroup we only found one relevant trial (N = 57) (vs RPD ‐ Wang 2002). There was no significant difference between chlorpromazine and risperidone (MD −0.21, 95% CI −0.44 to 0.02; Analysis 2.6).
2.6.3 PANSS positive subscale
We only found one relevant trial in this subgroup (N = 57) (vs RPD ‐ Wang 2002). There was no significant difference between chlorpromazine and risperidone (MD −0.07, 95% CI −0.36 to 0.22; Analysis 2.6).
2.7 Functioning: average endpoint score (WCST subscales, high = good) ‐ short term (up to 6 months)
2.7.1 WCST‐IQ
In this subgroup we only found one relevant trial (N = 100) (vs RPD ‐ Wang 2005). There was a statistically significant difference in favour of risperidone (MD −11.30, 95% CI −18.34 to −4.26; P = 0.002; Analysis 2.7).
2.7. Analysis.
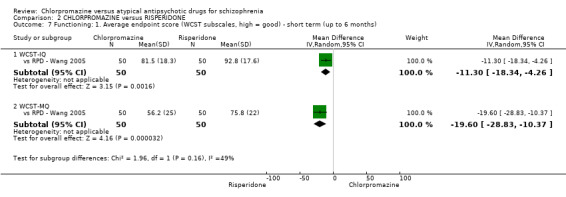
Comparison 2 CHLORPROMAZINE versus RISPERIDONE, Outcome 7 Functioning: 1. Average endpoint score (WCST subscales, high = good) ‐ short term (up to 6 months).
2.7.2 WCST‐MQ
We only found one relevant trial in this subgroup (N = 100) (vs RPD ‐ Wang 2005). There was a statistically significant difference in favour of risperidone (MD −19.6, 95% CI −28.83 to −10.37; P < 0.0001; Analysis 2.7).
2.8 Adverse effects: 1. Anticholinergic ‐ short term (up to 6 months)
2.8.1 blurred vision
In this subgroup we found five relevant trials (N = 387). There was a statistically significant difference in favour of risperidone (RR 2.44, 95% CI 1.32 to 4.50; P = 0.004; Analysis 2.8).
2.8. Analysis.
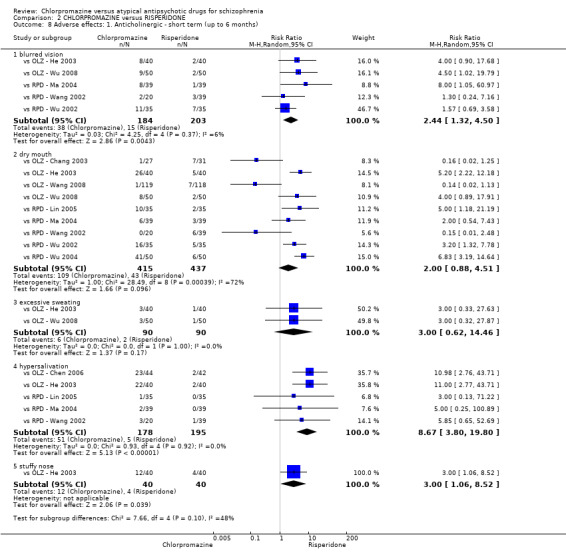
Comparison 2 CHLORPROMAZINE versus RISPERIDONE, Outcome 8 Adverse effects: 1. Anticholinergic ‐ short term (up to 6 months).
2.8.2 dry mouth
There were nine relevant trials in this subgroup (N = 852). There was no significant difference between chlorpromazine and risperidone (RR 2.00, 95% CI 0.88 to 4.51; Analysis 2.8). This subgroup had important levels of heterogeneity (Chi² test = 28.49; df = 8; P = 0.0; I² statistic = 72%).
2.8.3 excessive sweating
In this subgroup we found two relevant trials (N = 180). There was no significant difference between chlorpromazine and risperidone (RR 3.00, 95% CI 0.62 to 14.46; Analysis 2.8).
2.8.4 hypersalivation
In this subgroup we found five relevant trials (N = 373). There was a statistically significant difference in favour of risperidone (RR 8.67, 95% CI 3.80 to 19.8; P < 0.00001; Analysis 2.8).
2.8.5 stuffy nose
In this subgroup we only found one relevant trial (N = 80) (vs OLZ ‐ He 2003). There was a statistically significant difference in favour of risperidone (RR 3.00, 95% CI 1.06 to 8.52; P = 0.04; Analysis 2.8).
2.9 Adverse effects: 2a. Cardiovascular ‐ short term (up to 6 months)
2.9.1 abnormal ECG
In this subgroup we found three relevant trials (N = 229). There was no significant difference between chlorpromazine and risperidone (RR 2.41, 95% CI 0.96 to 6.06; Analysis 2.9).
2.9. Analysis.
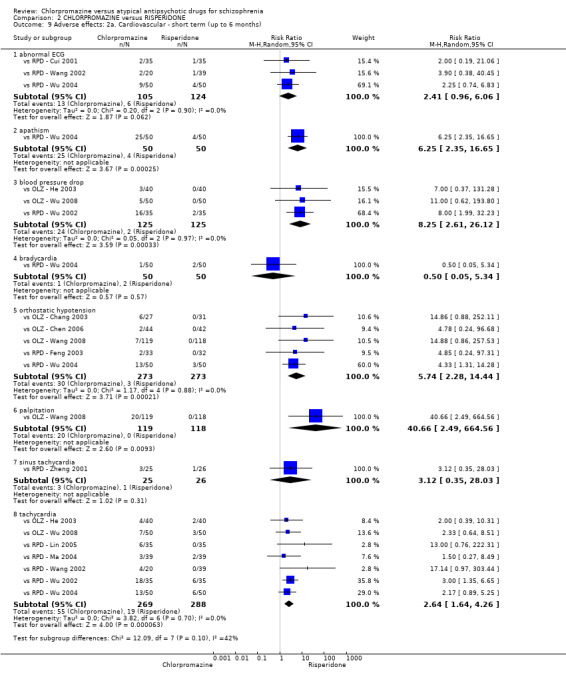
Comparison 2 CHLORPROMAZINE versus RISPERIDONE, Outcome 9 Adverse effects: 2a. Cardiovascular ‐ short term (up to 6 months).
2.9.2 apathism
In this subgroup we only found one relevant trial (N = 100) (vs RPD ‐ Wu 2004). There was a statistically significant difference in favour of risperidone (RR 6.25, 95% CI 2.35 to 16.65; P = 0.0002; Analysis 2.9).
2.9.3 bood pressure drop
In this subgroup we found three relevant trials (N = 250). There was a statistically significant difference in favour of risperidone (RR 8.25, 95% CI 2.61 to 26.12; P = 0.0003; Analysis 2.9).
2.9.4 bradycardia
In this subgroup we only found one relevant trial (N = 100) (vs RPD ‐ Wu 2004). There was no significant difference between chlorpromazine and risperidone (RR 0.50, 95% CI 0.05 to 5.34; Analysis 2.9).
2.9.5 orthostatic hypotension
In this subgroup we found five relevant trials (N = 546). There was a statistically significant difference in favour of risperidone (RR 5.74, 95% CI 2.28 to 14.44; P = 0.0002; Analysis 2.9).
2.9.6 palpitation
We only found one relevant trial in this subgroup (N = 237) (vs OLZ ‐ Wang 2008). There was a statistically significant difference in favour of risperidone (RR 40.66, 95% CI 2.49 to 664.56; P = 0.009; Analysis 2.9).
2.9.7 sinus tachycardia
In this subgroup we only found one relevant trial (N = 51) (vs RPD ‐ Zheng 2001). There was no significant difference between chlorpromazine and risperidone (RR 3.12, 95% CI 0.35 to 28.03; Analysis 2.9).
2.9.8 tachycardia
In this subgroup we found seven relevant trials (N = 557). There was a statistically significant difference in favour of risperidone (RR 2.64, 95% CI 1.64 to 4.26; P ≤ 0.0001; Analysis 2.9).
2.10 Adverse effects: 2b. Cardiovascular ‐ continuous measures ‐ short term (up to 6 months)
2.10.1 cardiac rate (upright position)
In this subgroup we only found one relevant trial (N = 100) (vs RPD ‐ Liu 2005). There was no significant difference between chlorpromazine and risperidone (MD 3.90, 95% CI −1.57 to 9.37; Analysis 2.10).
2.10. Analysis.
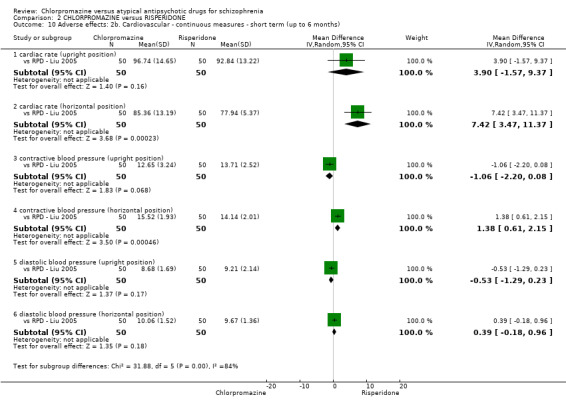
Comparison 2 CHLORPROMAZINE versus RISPERIDONE, Outcome 10 Adverse effects: 2b. Cardiovascular ‐ continuous measures ‐ short term (up to 6 months).
2.10.2 cardiac rate (horizontal position)
In this subgroup we only found one relevant trial (N = 100) (vs RPD ‐ Liu 2005). There was a statistically significant difference in favour of risperidone (MD 7.42, 95% CI 3.47 to 11.37; P = 0.0002; Analysis 2.10).
2.10.3 contractive blood pressure (upright position)
In this subgroup we only found one relevant trial (N = 100) (vs RPD ‐ Liu 2005). There was no significant difference between chlorpromazine and risperidone (MD −1.06, 95% CI −2.20 to 0.08; Analysis 2.10).
2.10.4 ontractive blood pressure (horizontal position)
In this subgroup we only found one relevant trial (N = 100) (vs RPD ‐ Liu 2005). There was a statistically significant difference in favour of risperidone (MD 1.38, 95% CI 0.61 to 2.15; P = 0.0005; Analysis 2.10).
2.10.5 diastolic blood pressure (upright position)
In this subgroup we only found one relevant trial (N = 100) (vs RPD ‐ Liu 2005). There was no significant difference between chlorpromazine and risperidone (MD −0.53, 95% CI −1.29 to 0.23; Analysis 2.10).
2.10.6 diastolic blood pressure (horizontal position)
We only found one relevant trial in this subgroup (N = 100) (vs RPD ‐ Liu 2005). There was no significant difference between chlorpromazine and risperidone (MD 0.39, 95% CI −0.18 to 0.96; Analysis 2.10).
2.11 Adverse effects: 3. Central nervous system ‐ short term (up to 6 months)
2.11.1 agitation
In this subgroup we only found one relevant trial (N = 65) (vs RPD ‐ Feng 2003). There was no significant difference between chlorpromazine and risperidone (RR 0.32, 95% CI 0.01 to 7.66; Analysis 2.11).
2.11. Analysis.
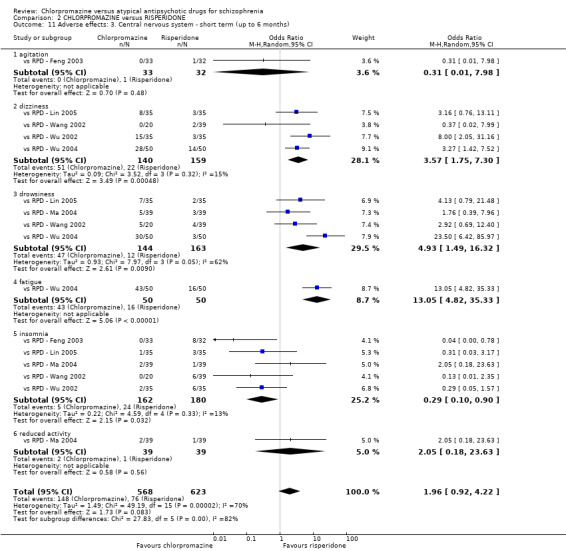
Comparison 2 CHLORPROMAZINE versus RISPERIDONE, Outcome 11 Adverse effects: 3. Central nervous system ‐ short term (up to 6 months).
2.11.2 dizziness
We found four relevant trials in this subgroup (N = 299). There was a statistically significant difference between chlorpromazine and risperidone (RR 2.37, 95% CI 1.38 to 4.07; Analysis 2.11).
2.11.3 drowsiness
In this subgroup we found four relevant trials (N = 307). There was a statistically significant difference in favour of risperidone (RR 3.62, 95% CI 1.56 to 8.39; P = 0.003; Analysis 2.11). This subgroup had moderate levels of heterogeneity (Chi² test = 5.14; df = 3; P = 0.162; I² statistic = 42%).
2.11.4 fatigue
We only found one relevant trial in this subgroup (N = 100) (vs RPD ‐ Wu 2004). There was a statistically significant difference in favour of risperidone (RR 2.69, 95% CI 1.77 to 4.09; P < 0.00001; Analysis 2.11).
2.11.4 insomnia
In this subgroup we found five relevant trials (N = 342). There was a statistically significant difference in favour of chlorpromazine (RR 0.33, 95% CI 0.12 to 0.91; P = 0.03; Analysis 2.11).
2.11.5 reduced activity
We only found one relevant trial in this subgroup (N = 78) (vs RPD ‐ Ma 2004). There was no significant difference between chlorpromazine and risperidone (RR 2.00, 95% CI 0.19 to 21.16; Analysis 2.11).
2.12 Adverse effects: 4. Gastrointestinal‐ short term (up to 6 months)
2.12.1 constipation
In this subgroup we found nine relevant trials (N = 868). There was a statistically significant difference in favour of risperidone (RR 3.00, 95% CI 2.05 to 4.39; P < 0.00001; Analysis 2.12).
2.12. Analysis.
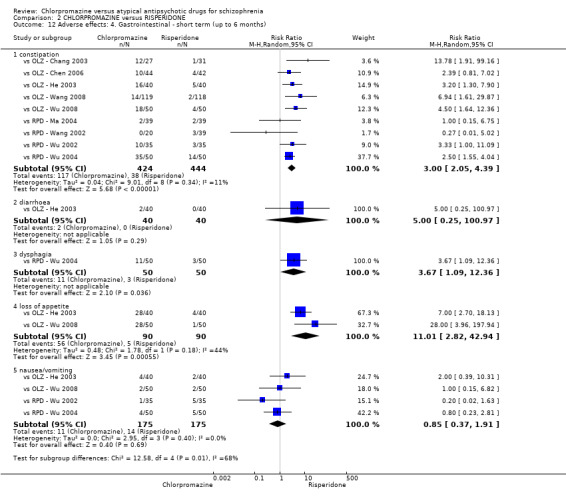
Comparison 2 CHLORPROMAZINE versus RISPERIDONE, Outcome 12 Adverse effects: 4. Gastrointestinal ‐ short term (up to 6 months).
2.12.2 diarrhoea
In this subgroup we only found one relevant trial (N = 80) (vs OLZ ‐ He 2003). There was no significant difference between chlorpromazine and risperidone (RR 5.00, 95% CI 0.25 to 100.97; Analysis 2.12).
2.12.3 dysphagia
In this subgroup we only found one relevant trial (N = 100) (vs RPD ‐ Wu 2004). There was a statistically significant difference (P = 0.04) in favour of risperidone (RR 3.67, 95% CI 1.09 to 12.36; Analysis 2.12).
2.12.4 loss of appetite
In this subgroup we found two relevant trials (N = 180). There was a statistically significant difference in favour of risperidone (RR 11.01, 95% CI 2.82 to 42.94; P = 0.0006; Analysis 2.12). This subgroup had moderate levels of heterogeneity (Chi² test = 1.78; df = 1; P = 0.182; I² statistic = 44%).
2.12.5 nausea/vomiting
We found four relevant trials in this subgroup (N = 350). There was no significant difference between chlorpromazine and risperidone (RR 0.85, 95% CI 0.37 to 1.91; Analysis 2.12).
2.13 Adverse effects: 5. Haematology ‐ short term (up to 6 months)
2.13.1 Abnormal haemogram
In this subgroup we found two relevant trials (N = 180). There was no significant difference between chlorpromazine and risperidone (RR 4.00, 95% CI 0.87 to 18.31; Analysis 2.13).
2.13. Analysis.
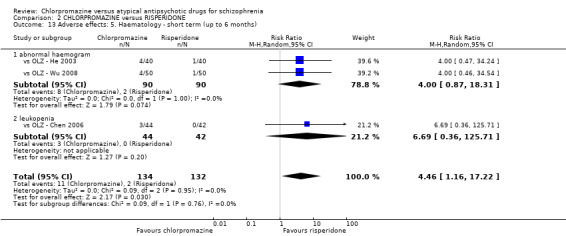
Comparison 2 CHLORPROMAZINE versus RISPERIDONE, Outcome 13 Adverse effects: 5. Haematology ‐ short term (up to 6 months).
2.13.2 leukopenia
We only found one relevant trial in this subgroup (N = 86) (vs OLZ ‐ Chen 2006). There was no significant difference between chlorpromazine and risperidone (RR 6.69, 95% CI 0.36 to 125.71; Analysis 2.13).
2.14 Adverse effects: 6. Hepatitic ‐ short term (up to 6 months)
2.14.1 abnormal liver function ‐ short term (up to six months)
In this subgroup we only found one relevant trial (N = 78) (vs RPD ‐ Ma 2004). There was no significant difference between chlorpromazine and risperidone (RR 5.00, 95% CI 0.25 to 100.89; Analysis 2.14).
2.14. Analysis.
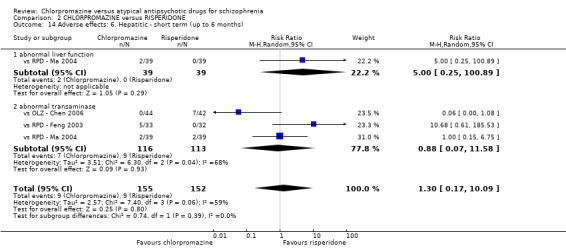
Comparison 2 CHLORPROMAZINE versus RISPERIDONE, Outcome 14 Adverse effects: 6. Hepatitic ‐ short term (up to 6 months).
2.14.2 abnormal transaminase ‐ short term (up to six months)
We found three relevant trials in this subgroup (N = 229). There was no significant difference between chlorpromazine and risperidone (RR 0.88, 95% CI 0.07 to 11.58; Analysis 2.14). This subgroup had important levels of heterogeneity (Chi² test = 6.3; df = 2; P = 0.043; I² statistic = 68%).
2.15 Adverse effects: 7. Metabolic ‐ weight gain
2.15.1 short term (up to six months)
In this subgroup we found four relevant trials (N = 302). There was no significant difference between chlorpromazine and risperidone (RR 1.36, 95% CI 0.52 to 3.59; Analysis 2.15). This subgroup had important levels of heterogeneity (Chi² test = 6.75; df = 3; P = 0.08; I² statistic = 56%).
2.15. Analysis.
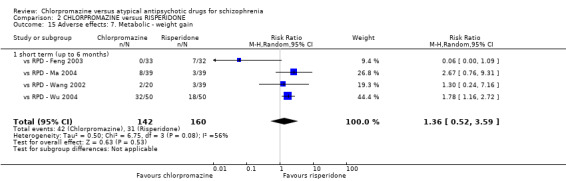
Comparison 2 CHLORPROMAZINE versus RISPERIDONE, Outcome 15 Adverse effects: 7. Metabolic ‐ weight gain.
2.16 Adverse effects: 8. Movement disorders ‐ short term (up to 6 months)
2.16.1 akathisia
In this subgroup we found six relevant trials (N = 435). There was a statistically significant difference in favour of risperidone (RR 2.37, 95% CI 1.46 to 3.85; P = 0.0005; Analysis 2.16).
2.16. Analysis.
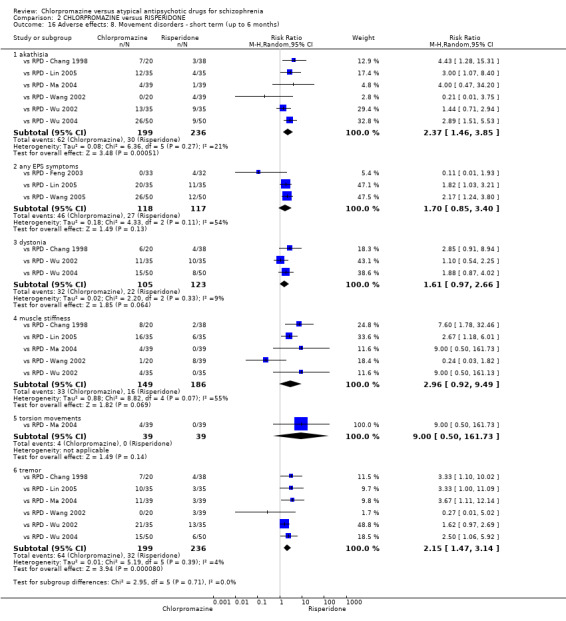
Comparison 2 CHLORPROMAZINE versus RISPERIDONE, Outcome 16 Adverse effects: 8. Movement disorders ‐ short term (up to 6 months).
2.16.2 any EPS symptoms
In this subgroup we found three relevant trials (N = 235). There was no significant difference between chlorpromazine and risperidone (RR 1.70, 95% CI 0.85 to 3.40; Analysis 2.16). This subgroup had important levels of heterogeneity (Chi² test = 4.33; df = 2; P = 0.115; I² statistic = 54%).
2.16.3 dystonia
In this subgroup we found three relevant trials (N = 228). There was no significant difference between chlorpromazine and risperidone (RR 1.61, 95% CI 0.97 to 2.66; Analysis 2.16).
2.16.4 muscle stiffness
In this subgroup we found five relevant trials (N = 335). There was no significant difference between chlorpromazine and risperidone (RR 2.96, 95% CI 0.92 to 9.49; Analysis 2.16). This subgroup had important levels of heterogeneity (Chi² test = 8.82; df = 4; P = 0.066; I² statistic = 55%).
2.16.5 torsion movements
We only found one relevant trial in this subgroup (N = 78) (vs RPD ‐ Ma 2004). There was no significant difference between chlorpromazine and risperidone (RR 9.00, 95% CI 0.50 to 161.73; Analysis 2.16).
2.16.6 tremor
In this subgroup we found six relevant trials (N = 435). There was a statistically significant difference in favour of risperidone (RR 2.15, 95% CI 1.47 to 3.14, P < 0.0001; Analysis 2.16).
2.17 Adverse effects: 9. Average endpoint score (TESS) ‐ skewed data
Data for this outcome are skewed and are best inspected by viewing Analysis 2.17.
2.17. Analysis.
Comparison 2 CHLORPROMAZINE versus RISPERIDONE, Outcome 17 Adverse events: 9. Average endpoint score (TESS) ‐ skewed data.
| Adverse events: 9. Average endpoint score (TESS) ‐ skewed data | ||||
|---|---|---|---|---|
| Study | Intervention | mean | SD | N |
| short term (up to 6 months) | ||||
| vs RPD ‐ Cui 2001 | Chlorpromazine | 3.23 | 2.87 | 30 |
| vs RPD ‐ Cui 2001 | Risperidone | 3.93 | 5.78 | 30 |
| vs RPD ‐ He 1999 | Chlorpromazine | 2.9 | 2.1 | 20 |
| vs RPD ‐ He 1999 | Risperidone | 2.3 | 2.4 | 19 |
| vs RPD ‐ Liu 2005 | Chlorpromazine | 2.58 | 2.93 | 50 |
| vs RPD ‐ Liu 2005 | Risperidone | 1.46 | 1.58 | 50 |
2.18 Adverse effects: 10. Various ‐ short term (up to 6 months)
2.18.1 concentration (poor)
In this subgroup we only found one relevant trial (N = 100) (vs RPD ‐ Wu 2004). There was a statistically significant difference in favour of risperidone (RR 1.95, 95% CI 1.35 to 2.82; P = 0.0003; Analysis 2.18).
2.18. Analysis.
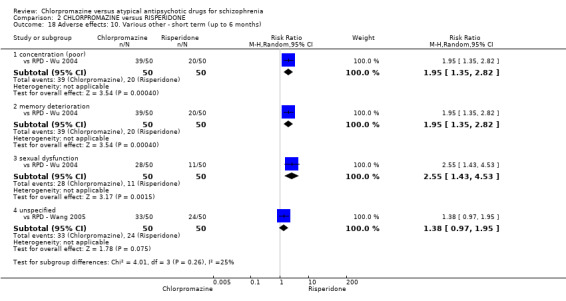
Comparison 2 CHLORPROMAZINE versus RISPERIDONE, Outcome 18 Adverse effects: 10. Various other ‐ short term (up to 6 months).
2.18.2 memory deterioration
In this subgroup we only found one relevant trial (N = 100) (vs RPD ‐ Wu 2004). There was a statistically significant difference in favour of risperidone (RR 1.95, 95% CI 1.35 to 2.82; P = 0.0004; Analysis 2.18).
2.18.3 sexual dysfunction
In this subgroup we only found one relevant trial (N = 100) (vs RPD ‐ Wu 2004). There was a statistically significant difference in favour of risperidone (RR 2.55, 95% CI 1.43 to 4.53; P = 0.002; Analysis 2.18).
2.18.4 unspecified
In this subgroup we only found one relevant trial (N = 100) (vs RPD ‐ Wang 2005). There was no significant difference between chlorpromazine and risperidone (RR 1.38, 95% CI 0.97 to 1.95; Analysis 2.18).
2.19 Quality of life: 1. Average endpoint scale score (QOL, high = good)
2.19.1 short term (up to 6 months)
In this subgroup we only found one relevant trial (N = 100) (vs RPD ‐ Wu 2004). There was a statistically significant difference in favour of risperidone (MD −14.20, 95% CI −20.50 to −7.90; P < 0.00001; Analysis 2.19).
2.19. Analysis.
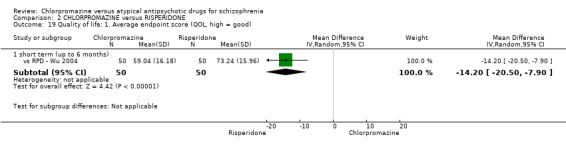
Comparison 2 CHLORPROMAZINE versus RISPERIDONE, Outcome 19 Quality of life: 1. Average endpoint score (QOL, high = good).
2.20 Leaving the study early ‐ short term (up to 6 months)
2.20.1 due to adverse events
In this subgroup we only found one relevant trial (N = 41) (vs RPD ‐ He 1999). There was no significant difference between chlorpromazine and risperidone (RR 0.21, 95% CI 0.01 to 4.11; Analysis 2.20).
2.20. Analysis.
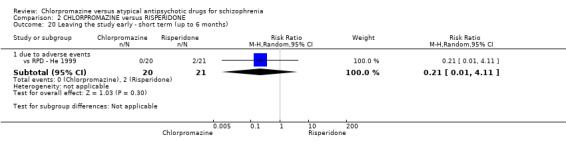
Comparison 2 CHLORPROMAZINE versus RISPERIDONE, Outcome 20 Leaving the study early ‐ short term (up to 6 months).
Comparison 3: CHLORPROMAZINE versus QUETIAPINE
3.1 Clinical response: No significant clinical response
3.1.1 short term (up to six months)
In this subgroup we found 28 relevant trials (N = 3241). There was no significant difference between chlorpromazine and quetiapine (RR 0.93, 95% CI 0.81 to 1.06; Analysis 3.1).
3.1. Analysis.
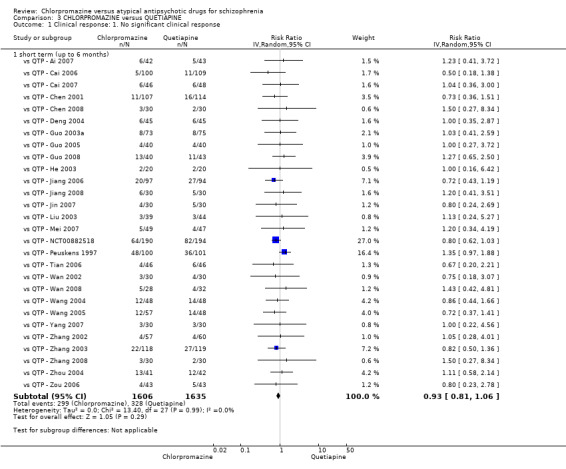
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 1 Clinical response: 1. No significant clinical response.
3.2 Global state: 1. Need of additional benzodiazepines/benzhexol
3.2.1 short term (up to 6 months)
In this subgroup we found two relevant trials (N = 290). There was a statistically significant difference in favour of quetiapine (RR 1.39, 95% CI 1.1 to 1.75; Analysis 3.2).
3.2. Analysis.
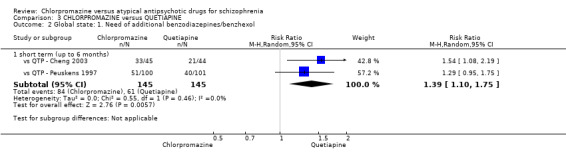
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 2 Global state: 1. Need of additional benzodiazepines/benzhexol.
3.3 Global state: 2a. Average endpoint scores (various scales, high = poor) ‐ short term (up to 6 months)
3.3.1 CGI‐SI
In this subgroup we found two relevant trials (N = 177). There was no significant difference between chlorpromazine and quetiapine (MD 0.01, 95% CI −0.82 to 0.84; Analysis 3.3. This subgroup had important levels of heterogeneity (Chi² test = 5.67; df = 1; P = 0.017; I² statistic = 82%).
3.3. Analysis.
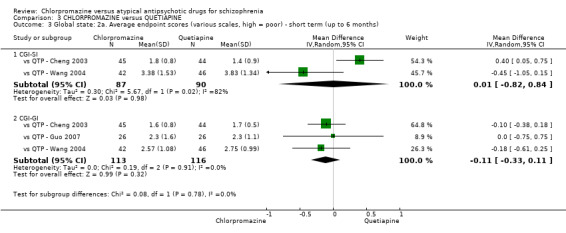
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 3 Global state: 2a. Average endpoint scores (various scales, high = poor) ‐ short term (up to 6 months).
3.3.2 CGI‐GI
In this subgroup we found three relevant trials (N = 229). There was no significant difference between chlorpromazine and quetiapine (MD −0.11, 95% CI −0.33 to 0.11; Analysis 3.3).
3.4 Global state: 2b. Average endpoint score (CGI‐SI, high = poor) ‐ skewed data
Data for this outcome are skewed and are best inspected by viewing Analysis 3.4.
3.4. Analysis.
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 4 Global state: 2b. Average endpoint score (CGI‐SI, high = poor) ‐ skewed data).
| Global state: 2b. Average endpoint score (CGI‐SI, high = poor) ‐ skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| short term (up to 6 months) | ||||
| vs QTP ‐ Guo 2007 | Chlorpromazine | 1.5 | 1.7 | 26 |
| vs QTP ‐ Guo 2007 | Quetiapine | 1.6 | 1.3 | 26 |
3.5 Global state: 3. Average change scores (CGI‐SI, high = poor)
3.5.1 short term (up to six months)
We only found one relevant trial in this subgroup (N = 384) (vs QTP ‐ NCT00882518). There was a statistically significant difference in favour of chlorpromazine (MD −0.30, 95% CI −0.32 to −0.28; P < 0.00001; Analysis 3.5).
3.5. Analysis.
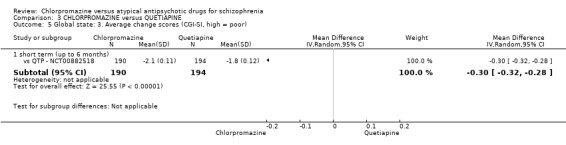
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 5 Global state: 3. Average change scores (CGI‐SI, high = poor).
3.6 Mental state: 1a. Average endpoint scores (various scale, high = poor) ‐ short term (up to 6 months)
3.6.1 BPRS total
In this subgroup we found six relevant trials (N = 548). There was no significant difference between chlorpromazine and quetiapine (MD −0.18, 95% CI −1.23 to 0.88; Analysis 3.6).
3.6. Analysis.
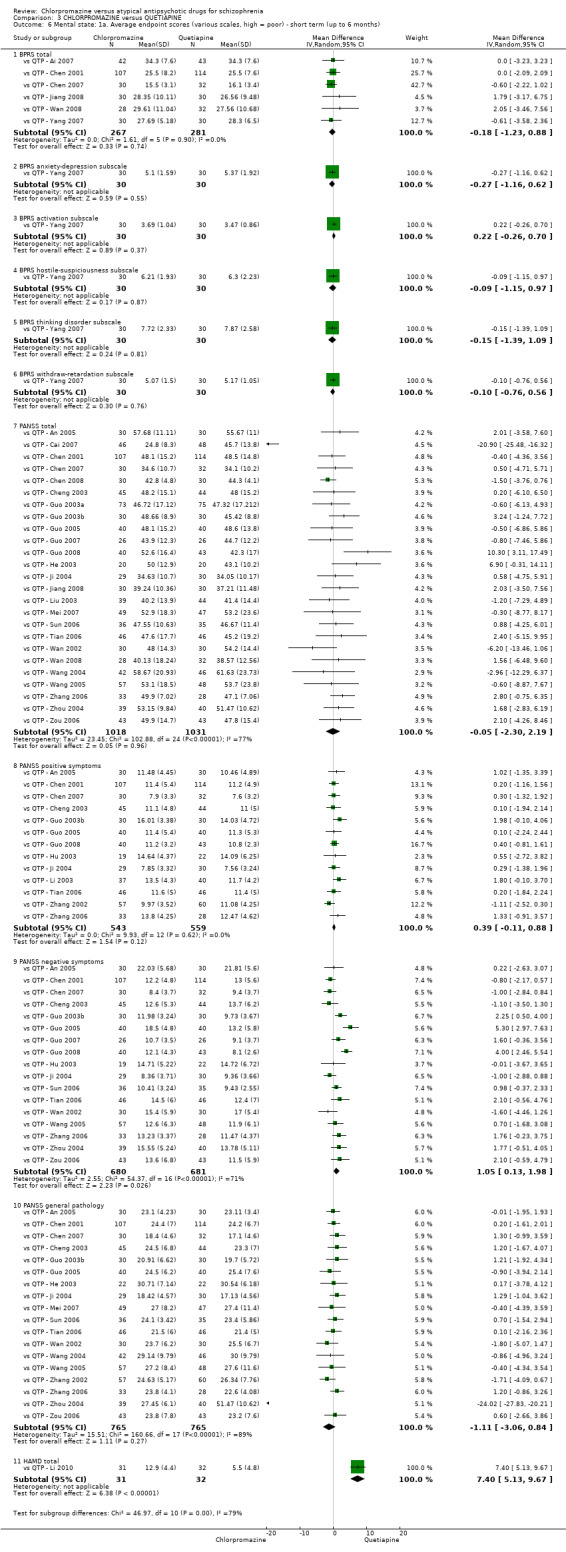
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 6 Mental state: 1a. Average endpoint scores (various scales, high = poor) ‐ short term (up to 6 months).
3.6.2 BPRS anxiety‐depression subscale
In this subgroup we only found one relevant trial (N = 60) (vs QTP ‐ Yang 2007). There was no significant difference between chlorpromazine and quetiapine (MD −0.27, 95% CI −1.16 to 0.62; Analysis 3.6).
3.6.3 BPRS activation subscale
In this subgroup we only found one relevant trial (N = 60) (vs QTP ‐ Yang 2007). There was no significant difference between chlorpromazine and quetiapine (MD 0.22, 95% CI −0.26 to 0.70; Analysis 3.6).
3.6.4 BPRS hostile‐suspiciousness subscale
In this subgroup we only found one relevant trial (N = 60) (vs QTP ‐ Yang 2007). There was no significant difference between chlorpromazine and quetiapine (MD −0.09, 95% CI −1.15 to 0.97; Analysis 3.6).
3.6.5 BPRS thinking disorder subscale
We only found one relevant trial in this subgroup (N = 60) (vs QTP ‐ Yang 2007). There was no significant difference between chlorpromazine and quetiapine (MD −0.15, 95% CI −1.39 to 1.09; Analysis 3.6).
3.6.6 BPRS withdraw‐retardation subscale
In this subgroup we only found one relevant trial (N = 60) (vs QTP ‐ Yang 2007). There was no significant difference between chlorpromazine and quetiapine (MD −0.10, 95% CI −0.76 to 0.56; Analysis 3.6).
3.6.7 PANSS total
In this subgroup we found 25 relevant trials (N = 2049). There was no significant difference between chlorpromazine and quetiapine (MD −0.05, 95% CI −2.3 to 2.19; Analysis 3.6). This subgroup had important levels of heterogeneity (Chi² test = 102.88; df = 24; P = 0.0; I² statistic = 77%).
3.6.8 PANSS positive symptom
In this subgroup we found 13 relevant trials (N = 1102). There was no significant difference between chlorpromazine and quetiapine (MD 0.39, 95% CI −0.11 to 0.88; Analysis 3.6).
3.6.9 PANSS negative symptoms
In this subgroup we found 17 relevant trials (N = 1361). There was a statistically significant difference in favour of quetiapine (MD 1.05, 95% CI 0.13 to 1.98; P = 0.03; Analysis 3.6). This subgroup had important levels of heterogeneity (Chi² test = 54.37; df = 16; P = 0.0; I² statistic = 71%).
3.6.10 PANSS general pathology
In this subgroup we found 18 relevant trials (N = 1530). There was no significant difference between chlorpromazine and quetiapine (MD −1.11, 95% CI −3.06 to 0.84; Analysis 3.6). This subgroup had important levels of heterogeneity (Chi² test = 160.66; df = 17; P = 0.0; I² statistic = 89%).
3.6.11 HAMD total
In this subgroup we only found one relevant trial (N = 63) (vs QTP ‐ Li 2010). There was a statistically significant difference in favour of quetiapine (MD 7.40, 95% CI 5.13 to 9.67; P < 0.00001; Analysis 3.6).
3.7 Mental state: 1b. Average endpoint scores (various scales, high = poor) ‐ medium term (6 to 12 months)
3.7.1 PANSS total
In this subgroup we only found one relevant trial (N = 41) (vs QTP ‐ Li 2003). There was a statistically significant difference in favour of quetiapine (MD 4.90, 95% CI 1.74 to 8.06; P = 0.002; Analysis 3.7).
3.7. Analysis.
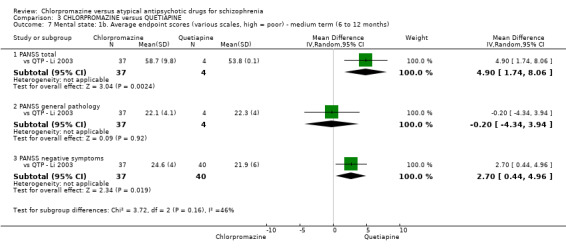
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 7 Mental state: 1b. Average endpoint scores (various scales, high = poor) ‐ medium term (6 to 12 months).
3.7.2 PANSS general pathology
We only found one relevant trial in this subgroup (N = 41) (vs QTP ‐ Li 2003). There was no significant difference between chlorpromazine and quetiapine (MD −0.20, 95% CI −4.34 to 3.94; Analysis 3.7).
3.7.3 PANSS negative symptoms
In this subgroup we only found one relevant trial (N = 77) (vs QTP ‐ Li 2003). There was a statistically significant difference in favour of quetiapine (MD 2.70, 95% CI 0.44 to 4.96; P = 0.02; Analysis 3.7).
3.8 Mental state: 1c. Average endpoint scores (various scales, high = poor) ‐ skewed data
Data for this outcome are skewed and are best inspected be viewing Analysis 3.8.
3.8. Analysis.
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 8 Mental state: 1c. Average endpoint scores (various scales, high = poor) ‐skewed data.
| Mental state: 1c. Average endpoint scores (various scales, high = poor) ‐skewed data | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| PANSS total ‐ short term (up to 6 months) | ||||
| vs QTP ‐ Zhang 2002 | Chlorpromazine | 28.39 | 16.57 | 57 |
| vs QTP ‐ Zhang 2002 | Quetiapine | 9.27 | 16.36 | 60 |
| PANSS positive symptoms ‐ short term (up to 6 months) | ||||
| vs QTP ‐ Guo 2003a | Chlorpromazine | 11.4 | 5.8 | 73 |
| vs QTP ‐ Guo 2003a | Quetiapine | 11.3 | 5.7 | 75 |
| vs QTP ‐ Guo 2007 | Chlorpromazine | 9.9 | 5.5 | 26 |
| vs QTP ‐ Guo 2007 | Quetiapine | 10.9 | 6.4 | 26 |
| vs QTP ‐ Mei 2007 | Chlorpromazine | 11.9 | 6.2 | 49 |
| vs QTP ‐ Mei 2007 | Quetiapine | 14.0 | 8.2 | 47 |
| vs QTP ‐ Sun 2006 | Chlorpromazine | 9.46 | 4.55 | 36 |
| vs QTP ‐ Sun 2006 | Quetiapine | 10.37 | 3.29 | 35 |
| vs QTP ‐ Wan 2002 | Chlorpromazine | 11.5 | 6.1 | 30 |
| vs QTP ‐ Wan 2002 | Quetiapine | 11.8 | 4.3 | 30 |
| vs QTP ‐ Wang 2004 | Chlorpromazine | 11.38 | 4.2 | 42 |
| vs QTP ‐ Wang 2004 | Quetiapine | 13.0 | 8.16 | 46 |
| vs QTP ‐ Wang 2005 | Chlorpromazine | 12.09 | 6.39 | 57 |
| vs QTP ‐ Wang 2005 | Quetiapine | 14.2 | 8.4 | 48 |
| vs QTP ‐ Zhang 2008 | Chlorpromazine | 11.47 | 5.71 | 30 |
| vs QTP ‐ Zhang 2008 | Quetiapine | 10.33 | 6.82 | 30 |
| vs QTP ‐ Zhou 2004 | Chlorpromazine | 17.12 | 7.82 | 39 |
| vs QTP ‐ Zhou 2004 | Quetiapine | 16.12 | 8.3 | 40 |
| vs QTP ‐ Zou 2006 | Chlorpromazine | 10.8 | 6.2 | 43 |
| vs QTP ‐ Zou 2006 | Quetiapine | 10.09 | 7.2 | 43 |
| PANSS negative symptoms ‐ short term (up to 6 months) | ||||
| vs QTP ‐ Guo 2003a | Chlorpromazine | 12.3 | 7.1 | 73 |
| vs QTP ‐ Guo 2003a | Quetiapine | 12.9 | 6.9 | 75 |
| vs QTP ‐ Mei 2007 | Chlorpromazine | 12.4 | 6.1 | 49 |
| vs QTP ‐ Mei 2007 | Quetiapine | 11.7 | 5.9 | 47 |
| vs QTP ‐ Wang 2004 | Chlorpromazine | 18.14 | 9.38 | 42 |
| vs QTP ‐ Wang 2004 | Quetiapine | 18.63 | 9.3 | 46 |
| vs QTP ‐ Zhang 2002 | Chlorpromazine | 15.88 | 4.11 | 57 |
| vs QTP ‐ Zhang 2002 | Quetiapine | 10.87 | 6.19 | 60 |
| PANSS general pathology ‐ short term (up to 6 months | ||||
| vs QTP ‐ Guo 2008 | Chlorpromazine | 20.1 | 10.9 | 40 |
| vs QTP ‐ Guo 2008 | Quetiapine | 19.2 | 11.4 | 43 |
3.9 Mental state: Average change score (various scales, high = poor) ‐ short term (up to 6 months)
3.9.1 PANSS total
In this subgroup we found two relevant trials (N = 426). There was a statistically significant difference in favour of chlorpromazine (MD −2.50, 95% CI −2.82 to −2.19; P < 0.00001; Analysis 3.9).
3.9. Analysis.
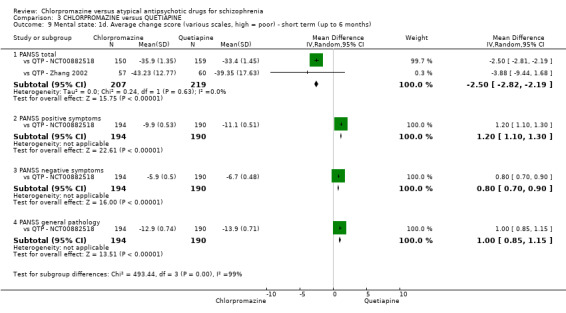
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 9 Mental state: 1d. Average change score (various scales, high = poor) ‐ short term (up to 6 months).
3.9.2 PANSS positive symptoms
In this subgroup we only found one relevant trial (N = 384) (vs QTP ‐ NCT00882518). There was a statistically significant difference in favour of quetiapine (MD 1.20, 95% CI 1.10 to 1.30, Analysis 3.9).
3.9.3 PANSS negative symptom
In this subgroup we only found one relevant trial (N = 384) (vs QTP ‐ NCT00882518). There was a statistically significant difference) in favour of quetiapine (MD 0.80, 95% CI 0.70 to 0.90; P < 0.00001; Analysis 3.9).
3.9.4 PANSS general pathology
In this subgroup we only found one relevant trial (N = 384) (vs QTP ‐ NCT00882518). There was a statistically significant differenc in favour of quetiapine (MD 1.00, 95% CI 0.85 to 1.15; P < 0.00001; Analysis 3.9).
3.10 Mental state: 1e. Average score decreased rate of BPRS/PANSS (%) ‐ short term (up to 6 months)
3.10.1 BPRS
In this subgroup we only found one relevant trial (N = 197) (vs QTP ‐ Cai 2006). There was no significant difference between chlorpromazine and quetiapine (MD −0.80, 95% CI −4.86 to 3.26; Analysis 3.10).
3.10. Analysis.
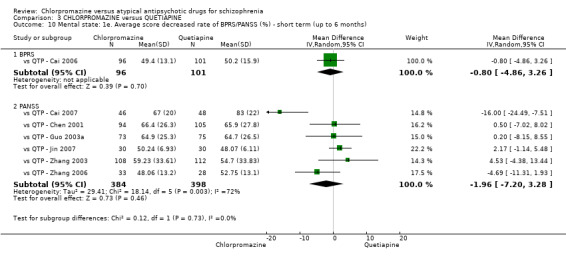
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 10 Mental state: 1e. Average score decreased rate of BPRS/PANSS (%) ‐ short term (up to 6 months).
3.10.2 PANSS
In this subgroup we found six relevant trials (N = 782). There was no significant difference between chlorpromazine and quetiapine (MD −1.96, 95% CI −7.20 to 3.28; Analysis 3.10). This subgroup had important levels of heterogeneity (Chi² test = 18.14; df = 5; P = 0.003; I² statistic = 72%).
3.11 Functioning: 1. Average endpoint score (various scales, high = better) ‐ short term (up to 6 months)
3.11.1 WCST‐IQ
In this subgroup we only found one relevant trial (N = 120) (vs QTP ‐ Nai 2007). There was a statistically significant difference in favour of quetiapine (MD −11.30, 95% CI −17.62 to −4.98; P = 0.0005; Analysis 3.11).
3.11. Analysis.
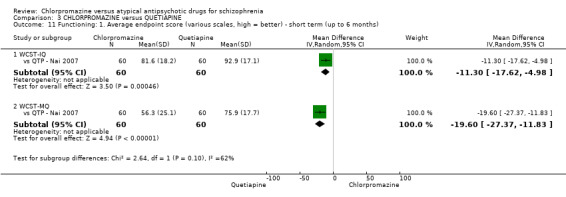
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 11 Functioning: 1. Average endpoint score (various scales, high = better) ‐ short term (up to 6 months).
3.11.2 WCST‐MQ
In this subgroup we only found one relevant trial (N = 120) (vs QTP ‐ Nai 2007). There was a statistically significant difference in favour of quetiapine (MD −19.6, 95% CI −27.37 to −11.83; P < 0.00001; Analysis 3.11).
3.12 Cognitive function: 1. Average endpoint scores (various scales, high = better) short term (up to 6 months)
3.12.1 WCST
In this subgroup we found two relevant trials (N = 123). There was a statistically significant difference in favour of quetiapine (MD 8.92, 95% CI 0.40 to 17.43; P = 0.04; Analysis 3.12).
3.12. Analysis.
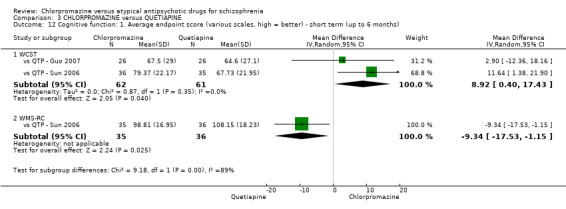
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 12 Cognitive function: 1. Average endpoint score (various scales, high = better) ‐ short term (up to 6 months).
3.12.2 WMS‐RC
In this subgroup we only found one relevant trial (N = 71) (vs QTP ‐ Sun 2006). There was a statistically significant difference in favour of chlorpromazine (MD −9.34, 95% CI −17.53 to −1.15; P = 0.03; Analysis 3.12).
3.13 Adverse effects: 1. Anticholinergic ‐ short term (up to 6 months)
3.13.1 blurred vision
In this subgroup we found 18 relevant trials (N = 1780). There was a statistically significant difference in favour of quetiapine (RR 5.00, 95% CI 3.46 to 7.22; P < 0.00001; Analysis 3.13).
3.13. Analysis.
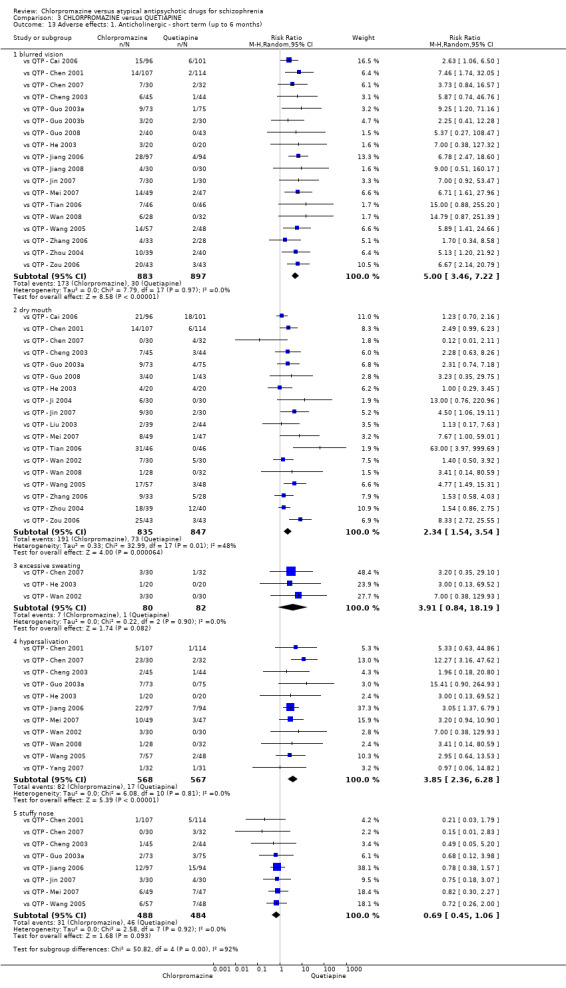
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 13 Adverse effects: 1. Anticholinergic ‐ short term (up to 6 months).
3.13.2 dry mouth
In this subgroup we found 18 relevant trials (N = 1682). There was a statistically significant difference (P < 0.0001) in favour of quetiapine (RR 2.34, 95% CI 1.54 to 3.54; Analysis 3.13). This subgroup had moderate levels of heterogeneity (Chi² test = 32.99; df = 17; P = 0.011; I² statistic = 48%).
3.13.2 excessive sweating
In this subgroup we found three relevant trials (N = 162). There was no significant difference between chlorpromazine and quetiapine (RR 3.91, 95% CI 0.84 to 18.19; Analysis 3.13).
3.13.3 hypersalivation
We found 11 relevant trials in this subgroup (N = 1135). There was a statistically significant difference in favour of quetiapine (RR 3.85, 95% CI 2.36 to 6.28; P < 0.0001; Analysis 3.13).
3.13.4 stuffy nose
In this subgroup we found eight relevant trials (N = 972). There was no significant difference between chlorpromazine and quetiapine (RR 0.69, 95% CI 0.45 to 1.06; Analysis 3.13).
3.14 Adverse effects: 2. Cardiovascular ‐ short term (up to 6 months)
3.14.1 abnormal ECG
In this subgroup we found seven relevant trials (N = 708). There was a statistically significant difference in favour of quetiapine (RR 1.82, 95% CI 1.11 to 2.98; P = 0.02; Analysis 3.14).
3.14. Analysis.
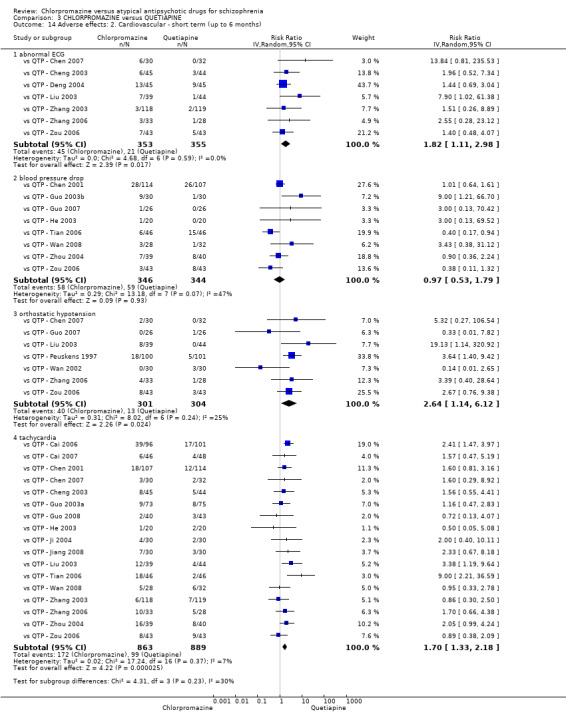
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 14 Adverse effects: 2. Cardiovascular ‐ short term (up to 6 months).
3.14.2 blood pressure drop
In this subgroup we found eight relevant trials (N = 690). There was no significant difference between chlorpromazine and quetiapine (RR 0.97, 95% CI 0.53 to 1.79; Analysis 3.14). This subgroup had moderate levels of heterogeneity (Chi² test = 13.18; df = 7; P = 0.068; I² statistic = 47%).
3.14.3 orthostatic hypotension
In this subgroup we found seven relevant trials (N = 605). There was a statistically significant difference in favour of quetiapine (RR 2.64, 95% CI 1.14 to 6.12; P = 0.02; Analysis 3.14).
3.14.4 tachycardia
In this subgroup we found 17 relevant trials (N = 1752). There was a statistically significant difference in favour of quetiapine (RR 1.70, 95% CI 1.33 to 2.18; P < 0.0001; Analysis 3.14).
3.15 Adverse effects: 3. Central nervous system ‐ short term (up to 6 months)
3.15.1 dizziness
In this subgroup we found 12 relevant trials (N = 1206). There was no significant difference between chlorpromazine and quetiapine (RR 1.40, 95% CI 0.83 to 2.35; Analysis 3.15). This subgroup had moderate levels of heterogeneity (Chi² test = 16.88; df = 11; P = 0.111; I² statistic = 35%).
3.15. Analysis.
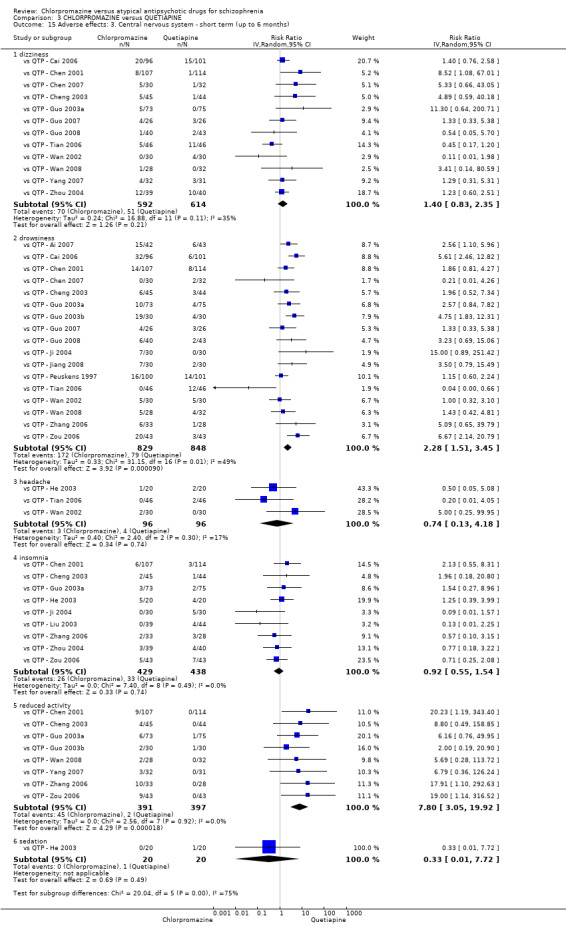
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 15 Adverse effects: 3. Central nervous system ‐ short term (up to 6 months).
3.15.2 drowsiness
In this subgroup we found 17 relevant trials (N = 1677). There was a statistically significant difference in favour of quetiapine (RR 2.28, 95% CI 1.51 to 3.45; P < 0.0001; Analysis 3.15). This subgroup had moderate levels of heterogeneity (Chi² test = 31.15; df = 16; P = 0.013; I² statistic = 49%).
3.15.3 headache
In this subgroup we found three relevant trials (N = 192). There was no significant difference between chlorpromazine and quetiapine (RR 0.74, 95% CI 0.13 to 4.18; Analysis 3.15).
3.15.4 insomnia
In this subgroup we found nine relevant trials (N = 867). There was no significant difference between chlorpromazine and quetiapine (RR 0.92, 95% CI 0.55 to 1.54; Analysis 3.15).
3.15.5 reduced activity
In this subgroup we found eight relevant trials (N = 788). There was a statistically significant difference in favour of quetiapine (RR 7.80, 95% CI 3.05 to 19.92; P < 0.0001; Analysis 3.15).
3.15.6 sedation
In this subgroup we only found one relevant trial (N = 40) (vs QTP ‐ He 2003). There was no significant difference between chlorpromazine and quetiapine (RR 0.33, 95% CI 0.01 to 7.72; Analysis 3.15).
3.16 Adverse effects: 4. Gastrointestinal ‐ short term (up to 6 months)
3.16.1 constipation
In this subgroup we found 22 relevant trials (N = 2048). There was a statistically significant difference in favour of quetiapine (RR 2.55, 95% CI 2.04 to 3.20; P < 0.00001; Analysis 3.16).
3.16. Analysis.
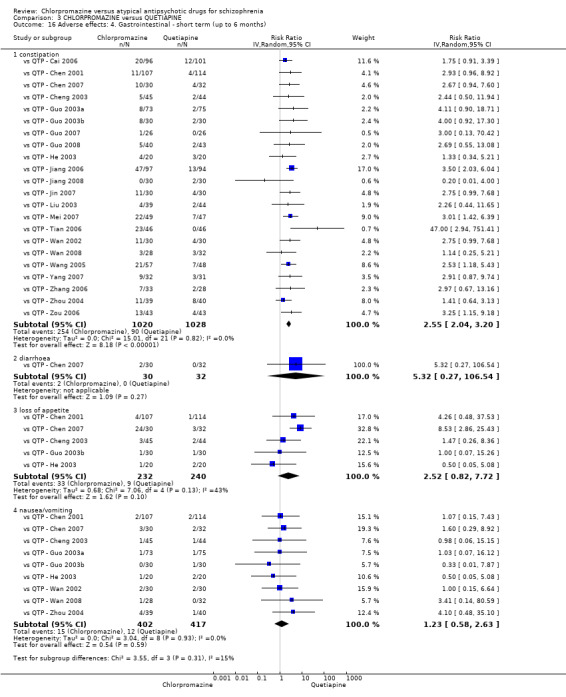
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 16 Adverse effects: 4. Gastrointestinal ‐ short term (up to 6 months).
3.16.2 diarrhoea
In this subgroup we only found one relevant trial (N = 62) (vs QTP ‐ Chen 2007). There was no significant difference between chlorpromazine and quetiapine (RR 5.32, 95% CI 0.27 to 106.54; Analysis 3.16).
3.16.3 loss of appetite
In this subgroup we found five relevant trials (N = 472). There was no significant difference between chlorpromazine and quetiapine (RR 2.52, 95% CI 0.82 to 7.72; Analysis 3.16). This subgroup had moderate levels of heterogeneity (Chi² test = 7.06; df = 4; P = 0.133; I² statistic = 43%).
3.16.4 nausea/vomiting
In this subgroup we found nine relevant trials (N = 819). There was no significant difference between chlorpromazine and quetiapine (RR 1.23, 95% CI 0.58 to 2.63; Analysis 3.16).
3.17 Adverse effects: 5a. Endocrine ‐ various ‐ short term (up to 6 months)
3.17.2 gynaecomastia, galactorrhoea
In this subgroup we only found one relevant trial (N = 83) (vs QTP ‐ Guo 2008). There was no significant difference between chlorpromazine and quetiapine (RR 5.37, 95% CI 0.27 to 108.47; Analysis 3.17).
3.17. Analysis.
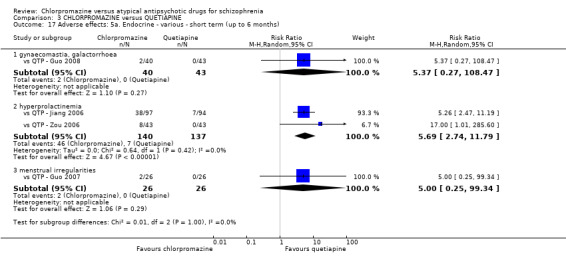
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 17 Adverse effects: 5a. Endocrine ‐ various ‐ short term (up to 6 months).
3.17.2 hyperprolactinemia
In this subgroup we found two relevant trials (N = 277). There was a statistically significant difference in favour of quetiapine (RR 5.69, 95% CI 2.74 to 11.79; P < 0.00001; Analysis 3.17).
3.17.3 menstrual irregularities
In this subgroup we only found one relevant trial (N = 52) (vs QTP ‐ Guo 2007). There was no significant difference between chlorpromazine and quetiapine (RR 5.00, 95% CI 0.25 to 99.34; Analysis 3.17).
3.18 Adverse effects: 5b. Endocrine ‐ average endpoint ‐ short term (up to 6 months)
3.1.18 prolactin level (ng/mL)
In this subgroup we only found one relevant trial (n = 30) (vs QTP ‐ Kong 2003). There was a statistically significant difference in favour of quetiapine (MD 24.62, 95% CI 17.76 to 31.48; P < 0.00001; Analysis 3.18).
3.18. Analysis.
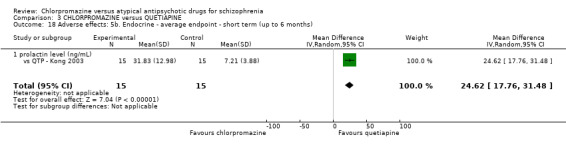
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 18 Adverse effects: 5b. Endocrine ‐ average endpoint ‐ short term (up to 6 months).
3.19 Adverse effect: 5c. Endocrine ‐ skewed data ‐ short term (up to 6 months)
3.19.1 average prolactin level (ng/mL)
Data for this outcome are skewed and are best inspected by viewing Analysis 3.19.
3.19. Analysis.
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 19 Adverse effects: 5c. Endocrine ‐ skewed data ‐ short term (up to 6 months).
| Adverse effects: 5c. Endocrine ‐ skewed data ‐ short term (up to 6 months) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| average prolactin level (ng/mL) | ||||
| vs QTP ‐ Jiang 2006 | Chlorpromazine | 680.2 | 688.0 | 97 |
| vs QTP ‐ Jiang 2006 | Quetiapine | 123.2 | 132.1 | 94 |
| vs QTP ‐ Wang 2005 | Chlorpromazine | 697.21 | 80.26 | 57 |
| vs QTP ‐ Wang 2005 | Quetiapine | 112.85 | 92.37 | 48 |
3.20 Adverse effects: 6a. Haematology ‐ short term (up to 6 months)
3.20.1 elevated ALT
In this subgroup we found eight relevant trials (N = 775). There was no significant difference between chlorpromazine and quetiapine (RR 1.62, 95% CI 0.92 to 2.87; Analysis 3.20).
3.20. Analysis.
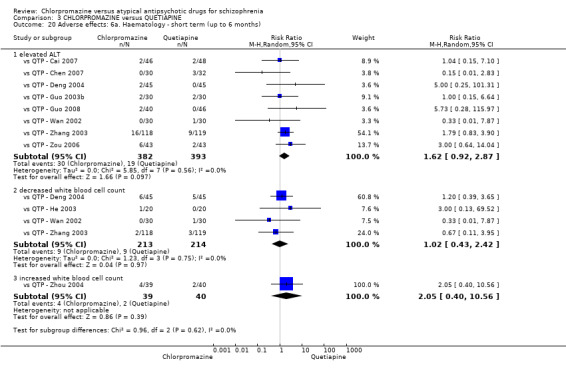
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 20 Adverse effects: 6a. Haematology ‐ short term (up to 6 months).
3.20.2 decreased white blood cell count
In this subgroup we found four relevant trials (N = 427). There was no significant difference between chlorpromazine and quetiapine (RR 1.02, 95% CI 0.43 to 2.42; Analysis 3.20).
3.20.3 increased white blood cell count
We only found one relevant trial in this subgroup (N = 79) (vs QTP ‐ Zhou 2004). There was no significant difference between chlorpromazine and quetiapine (RR 2.05, 95% CI 0.40 to 10.56; Analysis 3.20).
3.21 Adverse effects: 6b. Haematology ‐ average endpoint ‐ short term (up to 6 months)
3.21.1 blood glucose
In this subgroup we only found one relevant trial (N = 130) (vs QTP ‐ Guo 2006). There was no significant difference between chlorpromazine and quetiapine (MD 0.10, 95% CI −0.18 to 0.38; Analysis 3.21).
3.21. Analysis.
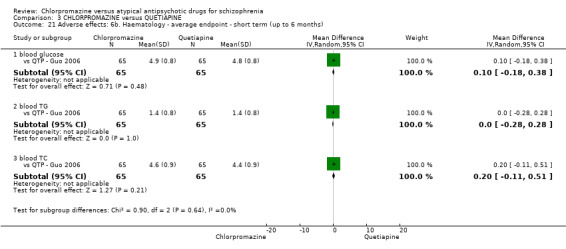
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 21 Adverse effects: 6b. Haematology ‐ average endpoint ‐ short term (up to 6 months).
3.21.2 blood TG
In this subgroup we only found one relevant trial (N = 130) (vs QTP ‐ Guo 2006). There was no significant difference between chlorpromazine and quetiapine (MD 0.00, 95% CI −0.28 to 0.28; Analysis 3.21).
3.21.3 blood TC
We only found one relevant trial (N = 130) in this subgroup (vs QTP ‐ Guo 2006). There was no significant difference between chlorpromazine and quetiapine (MD 0.20, 95% CI −0.11 to 0.51; Analysis 3.21).
3.22 Adverse effects: 7. Hepatitic ‐ short term (up to 6 months)
3.22.1 abnormal liver function
In this subgroup we found five relevant trials (N = 561). There was a statistically significant difference in favour of quetiapine (RR 2.10, 95% CI 1.32 to 3.33; P = 0.002; Analysis 3.22).
3.22. Analysis.
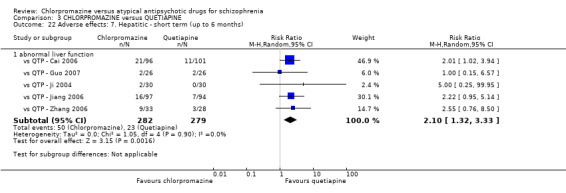
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 22 Adverse effects: 7. Hepatitic ‐ short term (up to 6 months).
3.23 Adverse effects: 8. Movement disorders ‐ short term (up to 6 months)
3.23.1 agitation
We found five relevant trials in this subgroup (N = 313). There was a statistically significant difference in favour of chlorpromazine (RR 0.36, 95% CI 0.14 to 0.95; P = 0.04; Analysis 3.23).
3.23. Analysis.
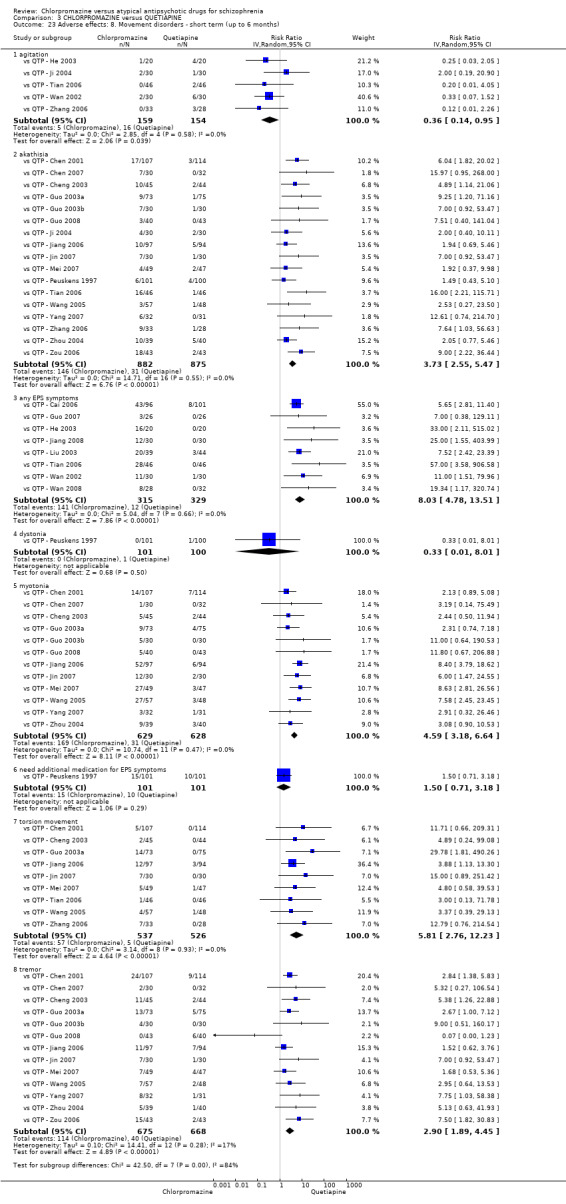
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 23 Adverse effects: 8. Movement disorders ‐ short term (up to 6 months).
3.23.2 akathisia
In this subgroup we found 17 relevant trials (N = 1757). There was a statistically significant difference in favour of quetiapine (RR 3.73, 95% CI 2.55 to 5.47; P < 0.00001; Analysis 3.23).
3.23.3 any EPS symptoms
In this subgroup we found eight relevant trials (N = 644). There was a statistically significant difference in favour of quetiapine (RR 8.03, 95% CI 4.78 to 13.51; P < 0.00001; Analysis 3.23).
3.23.4 dystonia
In this subgroup we only found one relevant trial (N = 201) (vs QTP ‐ Peuskens 1997). There was no significant difference between chlorpromazine and quetiapine (RR 0.33, 95% CI 0.01 to 8.01; Analysis 3.23).
3.23.5 myotonia
In this subgroup we found 12 relevant trials (N = 1257). There was a statistically significant difference in favour of quetiapine (RR 4.59, 95% CI 3.18 to 6.64; P < 0.00001; Analysis 3.23).
3.23.6 need additional medication for EPS symptoms
We only found one relevant trial in this subgroup (N = 202) (vs QTP ‐ Peuskens 1997). There was no significant difference between chlorpromazine and quetiapine (RR 1.50, 95% CI 0.71 to 3.18; Analysis 3.23).
3.23.7 torsion movement
In this subgroup we found nine relevant trials (N = 1063). There was a statistically significant difference in favour of quetiapine (RR 5.81, 95% CI 2.76 to 12.23; P < 0.00001; Analysis 3.23).
3.23.8 tremor
In this subgroup we found 13 relevant trials (N = 1343). There was a statistically significant difference in favour of quetiapine (RR 2.90, 95% CI 1.89 to 4.45; P < 0.00001; Analysis 3.23).
3.24 Adverse effects: 9a. Metabolic ‐ weight gain
3.24.1 short term (up to six months)
In this subgroup we found 15 relevant trials (N = 1259). There was a statistically significant difference in favour of quetiapine (RR 1.67, 95% CI 1.17 to 2.39; P = 0.005; Analysis 3.24).
3.24. Analysis.
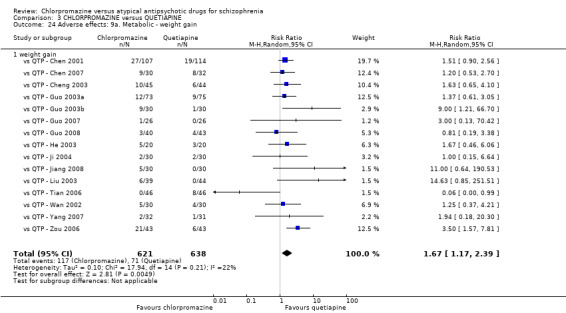
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 24 Adverse effects: 9a. Metabolic ‐ weight gain.
3.25 Adverse effects: 9b. Metabolic ‐ continuous ‐ short term (up to 6 months)
3.25.1 average BMI
In this subgroup we only found one relevant trial (N = 105) (vs QTP ‐ Wang 2005). There was no significant difference between chlorpromazine and quetiapine (MD 0.50, 95% CI −0.67 to 1.67; Analysis 3.25).
3.25. Analysis.
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 25 Adverse effects: 9b. Metabolic ‐ short term (up to 6 months).
3.25.2 average weight (KG)
In this subgroup we only found one relevant trial (N = 130) (vs QTP ‐ Guo 2006). There was no significant difference between chlorpromazine and quetiapine (MD −1.00, 95% CI −4.68 to 2.68; Analysis 3.25).
3.26 Adverse effects: 10. Various other ‐ short term (up to 6 months)
3.26.1 unspecified adverse effects
In this subgroup we found four relevant trials (N = 560). There was a statistically significant difference (P = 0.004) in favour of quetiapine (RR 1.73, 95% CI 1.20 to 2.51; Analysis 3.26). This subgroup had important levels of heterogeneity (Chi² test = 10.42; df = 3; P = 0.015; I² statistic = 71%).
3.26. Analysis.
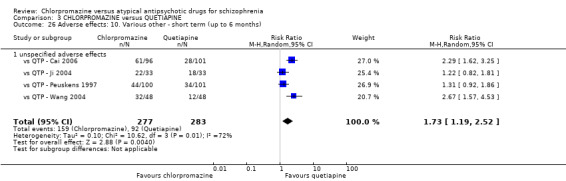
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 26 Adverse effects: 10. Various other ‐ short term (up to 6 months).
3.27 Adverse effects: 11. Average endpoint score (TESS, high = poor) ‐ skewed data
Data for this outcome are skewed and are best inspected by viewing Analysis 3.27.
3.27. Analysis.
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 27 Adverse effects: 11. Average endpoint score (TESS, high = poor) ‐ skewed data.
| Adverse effects: 11. Average endpoint score (TESS, high = poor) ‐ skewed data | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | SD | N |
| short term (up to 6 months) | ||||
| vs QTP ‐ Cai 2007 | Chlorpromazine | 3.0 | 2.2 | 46 |
| vs QTP ‐ Cai 2007 | Quetiapine | 2.4 | 2.3 | 48 |
| vs QTP ‐ Guo 2005 | Chlorpromazine | 4.2 | 3.0 | 40 |
| vs QTP ‐ Guo 2005 | Quetiapine | 2.0 | 2.4 | 40 |
| vs QTP ‐ Guo 2008 | Chlorpromazine | 2.8 | 2.2 | 40 |
| vs QTP ‐ Guo 2008 | Quetiapine | 1.9 | 1.6 | 43 |
| vs QTP ‐ Hu 2003 | Chlorpromazine | 5.33 | 3.94 | 22 |
| vs QTP ‐ Hu 2003 | Quetiapine | 6.92 | 4.35 | 19 |
| vs QTP ‐ Li 2003 | Chlorpromazine | 4.8 | 3.1 | 37 |
| vs QTP ‐ Li 2003 | Quetiapine | 2.9 | 2.8 | 40 |
| vs QTP ‐ Zhang 2002 | Chlorpromazine | 6.94 | 4.35 | 57 |
| vs QTP ‐ Zhang 2002 | Quetiapine | 6.07 | 5.04 | 60 |
| vs QTP ‐ Zhang 2008 | Chlorpromazine | 9.35 | 6.23 | 30 |
| vs QTP ‐ Zhang 2008 | Quetiapine | 6.63 | 5.2 | 30 |
| medium term (7 to 12 months) | ||||
| vs QTP ‐ Li 2003 | Chlorpromazine | 4.2 | 3.0 | 37 |
| vs QTP ‐ Li 2003 | Quetiapine | 2.1 | 2.3 | 40 |
3.28 Quality of life: 1. General ‐ average endpoint score (GQOL1 ‐ 74, high = better)
3.28.1 short term (up to 6 months)
In this subgroup we only found one relevant trial (N = 59) (vs QTP ‐ Ji 2004). There was a statistically significant difference in favour of quetiapine (MD −6.49, 95% CI −11.3 to −1.68; P = 0.008; Analysis 3.28).
3.28. Analysis.
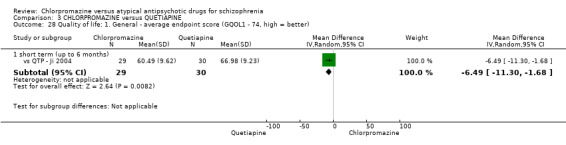
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 28 Quality of life: 1. General ‐ average endpoint score (GQOL1 ‐ 74, high = better).
3.29 Leaving the study early: 1a. Short term (up to six months)
3.29.1 due to adverse effect
In this subgroup we found ten relevant trials (N = 1680). There was a statistically significant difference in favour of quetiapine (RR 1.43, 95% CI 1.04 to 1.98; P = 0.03; Analysis 3.29).
3.29. Analysis.
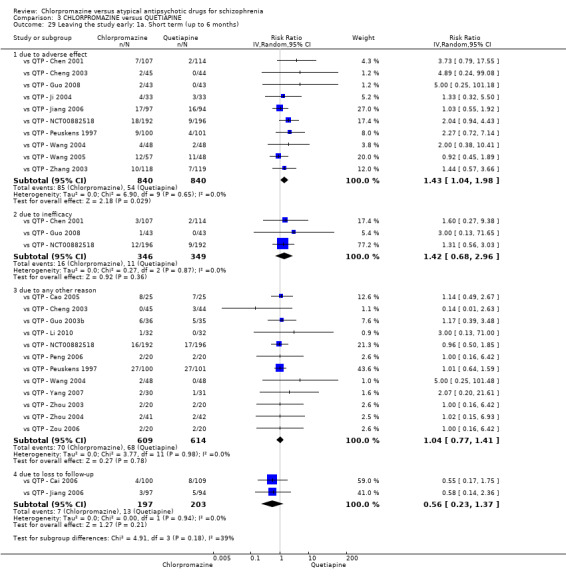
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 29 Leaving the study early: 1a. Short term (up to 6 months).
3.29.2 due to inefficacy
In this subgroup we found three relevant trials (N = 695). There was no significant difference between chlorpromazine and quetiapine (RR 1.42, 95% CI 0.68 to 2.96; Analysis 3.29).
3.29.3 due to any other reason
We found 12 relevant trials in this subgroup (N = 1223). There was no significant difference between chlorpromazine and quetiapine (RR 1.04, 95% CI 0.77 to 1.41; Analysis 3.29).
3.29.4 due to lost to follow‐up
In this subgroup we found two relevant trials (N = 400). There was no significant difference between chlorpromazine and quetiapine (RR 0.56, 95% CI 0.23 to 1.37; Analysis 3.29).
3.30 Leaving the study early: 1b. Medium term (seven to 12 months)
For this outcome we only found one relevant trial (N = 103) (vs QTP ‐ Li 2003). There was no significant difference between chlorpromazine and quetiapine (RR 1.19, 95% CI 0.61 to 2.32; Analysis 3.30).
3.30. Analysis.
Comparison 3 CHLORPROMAZINE versus QUETIAPINE, Outcome 30 Leaving the study early: 1b. Medium term (7 to 12 months).
4. Sensitivity analysis
1. Implication of randomisation
1.1 Chlorpromazine versus olanzapine ‐ clinical response: no significant clinical response
After removing studies that did not adequately explain randomisation methods, there was no substantial difference in the estimate of the effect, with results statistically significant in favour of olanzapine (1 RCT, N = 58; RR 2.49, 95% CI 1.10 to 5.64).
1.2 Chlorpromazine versus risperidone ‐ clinical response: no significant clinical response
After removing studies that did not adequately explain randomisation methods, there were no data left in the short term outcome to compare, since all included studies did not provide explanation.
1.3 Chlorpromazine versus quetiapine ‐ clinical response: no significant clinical response
After removing studies that did not adequately explain randomisation methods, there was no substantial difference in the estimate of the effect.
2. Assumptions for lost binary data
We did not assume any binary data from the included studies.
3. Risk of bias
3.1 Chlorpromazine versus olanzapine ‐ clinical response: no significant clinical response
After we removed studies that were rated as 'high' across one or more of the 'Risk of bias' domains, there were no data left in the short‐term outcome to compare, since all included studies were rated as 'high' (vs OLZ ‐ Chang 2003; vs OLZ ‐ Chen 2006; vs OLZ ‐ Wang 2002). However, there was no difference in long‐term outcome.
3.2 Chlorpromazine versus risperidone ‐ clinical response: no significant clinical response
After we removed studies that we had rated as 'high' across one or more of the 'Risk of bias' domains, there was no substantial difference in the estimate of the effect.
3.3 Chlorpromazine versus quetiapine ‐ clinical response: no significant clinical response
After removing studies that rated as 'high' across one or more of the 'Risk of bias' domains, there was no substantial difference in the estimate of the effect.
4. Imputed values
We had also planned to undertake a sensitivity analysis to assess the effects of including data from trials where we used imputed values for ICC in calculating the design effect in cluster‐RCTs. However, we did not impute any values in this Cochrane review.
5. Fixed‐effect and random‐effects
5.1 Chlorpromazine versus olanzapine ‐ clinical response: no significant clinical response
There was no difference in the estimate of effect if a fixed‐effect model was used in place of the random‐effects model.
5.2 Chlorpromazine versus risperidone ‐ clinical response: no significant clinical response
There was no difference in the estimate of effect if a fixed‐effect model was used in place of the random‐effects model.
5.3 Chlorpromazine versus quetiapine ‐ clinical response: no significant clinical response
There was no difference in the estimate of effect if a fixed‐effect model was used in place of the random‐effects model.
Discussion
Summary of main results
1. Chlorpromazine versus olanzapine
1.1 Clinical response
Data from included studies indicate that olanzapine is more effective than chlorpromazine when it comes to 'clinical response' outcomes, at least in the short term, since long‐term data are equivocal. Notably, each study providing data defined 'clinical response' differently; one study classified response as a decrease in BPRS score, while another measured the number of people who became stabilised on their study medication. The remaining studies did not explain how clinical response was measured. However, regarding average endpoint scores using the CGI, scores were significantly lower in the olanzapine groups. Disappointingly, only one small study (N = 70) provided data for relapse, which demonstrated no significant difference between groups; a result that will need to be confirmed by future large, high‐quality RCTs.
1.2 Mental state
These outcomes were difficult to interpret, with most included studies using a different scale of measurement to rate mental state in participants. Clearly there was no significant difference between the two compounds regarding total mean BPRS scores. However there was a significant level of heterogeneity in the studies. The source of this heterogeneity was vs OLZ ‐ Chen 2006 and removal of this study demonstrated a statistically significant difference between groups, in favour of olanzapine. The reasons for this heterogeneity have not been confirmed, since all studies shared a similar setting and used differing dose ranges of chlorpromazine (either 25 to 600 mg/day or 200 to 800 mg/day) and its olanzapine comparator. These findings need clarifying in future studies, as only a small sample provided data (N = 245). Changes in PANSS score were significant, with a greater decrease seen in people receiving olanzapine (N = 351).
1.3 Service involvement
Unfortunately, data for service usage/involvement was seriously lacking; the only study that provided data for re‐hospitalisation rates (N = 70) demonstrated an equivocal result between groups.
1.4 Functioning
Data from one study (N = 53) suggest that olanzapine is significantly better at improving functioning, using the WCST. A naturalistic study has suggested that people who received atypicals showed a better cognitive pattern in terms of WCST performance than those on typical antipsychotics (Rossi 2006). However, although this is a test that is generally accepted in schizophrenia research, it has not been widely used in the included studies, making it difficult to confirm this result.
1.5 Adverse effects
Extrapyramidal adverse effects were largely equivocal; only data for 'any observed EPS' event were significant, with more events in people receiving chlorpromazine; however, this finding is based on only two studies (N = 298). There is a strong dataset indicating that chlorpromazine causes significantly greater instances of drowsiness and constipation. Other significant findings, particularly associated with cardiovascular outcomes (palpitation, tachycardia and blood pressure decrease) are only based on the findings of two small RCTs.
1.6 Quality of life
Two different rating scales were used in the two studies that provided data, neither of which were meta‐analysed, with skewed data only available using the QoL scale. GQOLI scores show that ratings on specific areas of quality of life (including physical health, psychological health and social functioning) were significantly better in people receiving olanzapine. Again, this is a result that will need to be confirmed with future studies reporting data using these validated scales.
1.7 Leaving the study early
There was no difference between groups for people leaving the studies early for any reason.
2. Chlorpromazine versus risperidone
2.1 Clinical response
Data from the seven included RCTs (N = 475) indicate there is no significant difference between compounds in 'clinical response' (as defined in the different studies). Other global state outcomes are equivocal, with skewed data only available using the CGI, and data equivocal for amounts of participants requiring additional antiparkinsonian medication.
2.2 Mental state
Most included studies measured mental state outcomes using one of the widely‐accepted rating scales (BPRS, PANSS or SANS), with data for each subscale demonstrating no significant difference between groups.
2.3 Functioning
Data show significantly better scores for improvement in functioning using the WCST when receiving risperidone. Again, as only one small study reported data for two subscales of the WCST, it is impossible to make a generalisation with this result.
2.4 Adverse effects
Extrapyramidal adverse effects were largely equivocal; however, akathisia was significantly more prominent in people receiving chlorpromazine (31% versus 13%), as was tremor (32% versus 14%). vs RPD ‐ Wang 2002 was the source of heterogeneity in the two aforementioned significant findings, and this was the only study to use a fixed dose of chlorpromazine (400 mg/day) and two further treatment arms of risperidone (4 mg/day or 6 mg/day). Chlorpromazine was also associated significantly with more events of constipation (N = 868), loss of appetite (N = 180), tachycardia (N = 557), hypersalivation (N = 373), blurred vision (N = 387), drop of blood pressure (N = 250) and orthostatic hypotension (N = 546), drowsiness (N = 307) and dizziness (N = 299). However, significantly more people receiving risperidone experienced insomnia (N = 342).
2.5 Quality of life
With only one study providing data using the QoL scale demonstrating significantly greater improvement in people receiving risperidone (N = 100), it is difficult to generalise this finding to a real‐world setting.
2.6 Leaving the study early
There was no difference between groups for people leaving the studies early for any reason with roughly equal numbers of participants leaving their groups.
3. Chlorpromazine versus quetiapine
3.1 Clinical response
Data from the 28 included RCTs (N = 3241) indicate there is no significant difference in 'clinical response' (as defined in the different studies) between compounds; however, significantly more people receiving chlorpromazine required additional benzodiazepines or antiparkinsonian drugs. Furthermore, global state outcomes using the CGI are largely equivocal, making it difficult to weigh up any further benefits of either drug taking into account the fact that there was no difference in clinical response.
3.2 Mental state
Mental state outcomes using either the BPRS or PANSS were largely equivocal again, with no significant difference between groups. There were some results that suggested quetiapine was significantly more effective at reducing negative symptoms (using PANSS). However, there was a significant degree of heterogeneity in these results.
3.3 Functioning
Data showed significantly better scores for improvement in functioning using the WCST when receiving quetiapine. Again, with only one small study reporting data for two subscales of the WCST, it is impossible to make a generalisation with this result.
3.4 Adverse effects
Extrapryamidal symptoms were significantly more prominent in people receiving chlorpromazine, particularly with akathisia (N = 1757), 'any' EPS symptom (N = 644), myotonia (N = 1257), torsion movement (N = 1063) and tremor (N = 1343). However, levels of agitation tended to be higher in people receiving quetiapine. Other specific adverse effects, including drowsiness, reduced activity, weight gain, constipation, orthostatic hypotension, tachycardia, hypersalivation, blurred vision and dry mouth, were significantly greater in people receiving chlorpromazine.
3.5 Quality of life
GQOLI scores show that ratings on QoL were significantly better in people receiving quetiapine. Again, this needs to be confirmed with future studies reporting data using this validated scale.
3.6 Leaving the study early
More people left their study early due to adverse effects if they were receiving chlorpromazine; a finding that reflects the expected tolerability of this drug based on the adverse events recorded above. Leaving the studies early for other reasons were not significantly different between groups in the short or medium term.
Overall completeness and applicability of evidence
None of the included studies provided measured outcomes for economic consideration, nor behaviour or satisfaction with treatment or care. These outcomes are important for managers and policy makers' consideration, as well as for carers. Cearly data for 'satisfaction of treatment or care' would provide a basis for evidence of patient acceptability of either compound. No study reported the outcome of death. Reported outcomes were largely clinician oriented, and outcomes including service involvement was under‐reported, and future studies would need to address this patient important, policy important outcome. The majority of included studies provided data at the short term only (less than six months), making it impossible to assess any medium or long‐term effect either compound has on patients. Several studies were excluded because they did not provide any useable data.
Most included studies included inpatients from hospitals in China. Therefore the results of this Cochrane review are more applicable to the Chinese population and are not particularly suitable to apply to community‐treated patients. The dose range employed in most studies was as high as 1000 mg/day, which is far beyond the maximum BNF recommended dosage (BNF 2014). As most studies provided a range as opposed to a mean dosage, it is not possible to generalise these findings to other populations.
Quality of the evidence
Unfortunately, the overall quality of the evidence was poor (see Figure 2; Figure 3 for a graphical overview and summary). No included studies provided details as to randomisation methods and selective reporting was relatively prominent in most included studies. Furthermore, as most studies were undertaken in China (concerns have previously been raised about the quality of reporting in Chinese studies; Anon 2010; Wu 2006), this data requires interpreting with caution as biases may have inflated the estimate of effect in statistically significant outcomes. We identified duplicated data in two studies (vs OLZ ‐ Wu 2008; vs RPD ‐ Wu 2004) where these two studies compared chlorpromazine to olanzapine and risperidone, respectively. vs OLZ ‐ Wu 2008 measured quality of life using the SF‐36 scale, and vs RPD ‐ Wu 2004 measured QoL using the GQOLI scale; however the data for these are identical in both studies. The mean scores, SDs and sample size in each group were identical; and we therefore strongly suspect reporting biases, but need to clarify this with the trial authors. In the meantime, we have included the QoL data from vs RPD ‐ Wu 2004, and cross‐checked all other data between studies and found no further duplication.
Potential biases in the review process
Chlorpromazine is an old antipsychotic drug that has been extensively compared with other compounds. There exists the possibility that there are unpublished, unidentified studies that we have not included in this review. We strictly adhered to our protocol regarding data extraction and data management. However, we have presented comparisons with chlorpromazine with the specific name of the comparator drug, instead of the planned, generic 'chlorpromazine versus atypical antipsychotics'. We have only included data for three of the drug comparisons and plan in future updated of this Cochrane review to include all other comparator atypical antipsychotics. We also note that due to the size of the 2012 search results, the search date for this review is out‐of‐date. We intend to re‐run a search this year and republish as soon as possible.
Agreements and disagreements with other studies or reviews
This Cochrane review clarifies the evidence relating to the effects of chlorpromazine versus three comparator atypical antipsychotics, namely olanzapine, risperidone and quetiapine. It provides the evidence base for the disputed adverse effect profile, which has been claimed to be more severe in the older, atypical antipsychotics as compared to the newer compounds. However, 96% of the studies from which this data have been derived were undertaken in Chinese hospitals making it difficult to generalise the finding.
Authors' conclusions
Implications for practice.
1. For people with schizophrenia
Based on weak evidence, chlorpromazine is not much different to risperidone or quetiapine and slightly less effective than olanzapine for levels of clinical response. Chlorpromazine was associated with more extrapyramidal adverse effects, particularly akathisia and tremor compared to risperidone and quetiapine. Risperidone has a lower adverse event profile than any of the other atypical comparators. Generally, chlorpromazine was associated with more, and varied adverse effects. There is a lack of evidence relating to patient‐oriented outcomes, including satisfaction with treatment or care and well‐reported QoL outcomes.
2. For clinicians
It is surprising how few studies outside of China have investigated the effects of chlorpromazine versus other atypical antipsychotics on people with schizophrenia. Most data relate only to short‐term studies; therefore it is impossible to comment on the medium and long‐term use of chlorpromazine in the research setting. Chlorpromazine was generally associated with more adverse events. However all studies made use of varying dose ranges; and the use of higher doses would be expected to be associated with greater adverse events. As included studies did not provide mean doses, we could not perform a sensitivity analysis to assess such differences in the results.
3. For managers or policy makers
Included studies reported no data relating to service utilisation, functioning in the community or cost features. Chlorpromazine is a cheap drug compared to new 'atypical' antipsychotics. Therefore, investing some money in research on this drug could be cost saving in the long run.
Implications for research.
1. General
Future studies in this area need to be well reported, long term (> one year) and adhere to the Consolidated Standards of Reporting Trials (CONSORT) statement (CONSORT; Moher 2001). This states that all research needs to be clearly and transparently reported, and be accompanied by a flow diagram to simply display the study progress and process.
2. Specific
The trials that met the inclusion criteria of this Cochrane review were all undertaken in China, predominantly in an inpatient surrounding, and focused only on short‐term outcomes (average of eight weeks). Further research should be international in scope and fairly representative of other healthcare systems. Outpatient treatment was under‐represented in the included studies, and future research should work with this population. More participant focused outcomes (e.g. functioning in social, occupational, family life, satisfaction with care, etc.) and economic considerations could be addressed.
Acknowledgements
The editorial base of the Cochrane Schizophrenia Group (Nottingham, UK) produces and maintains standard text for use in the Methods section of their Cochrane reviews. We have used this text and adapted it as required.
We thank Khaled Turkmani for peer reviewing the Cochrane protocol, Martina Rojnic Kuzman for peer reviewing this review version. We would also like to thank Deirdre Walshe for copy editing.
“This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Schizophrenia Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.”
Appendices
Appendix 1. Previous searches
1.1 Search in 2013 (Protocol Step)
1.1.1 Electronic searches
1.1.1.1 Cochrane Schizophrenia Group Trials Register
The Trials Search Co‐ordinator will search the Cochrane Schizophrenia Group’s Trials Register using the phrase:
[((*chlorpromazine* AND (*amisulprid* or *aripiprazol* or *clozapin* or *olanzapin* or *quetiapin* or *risperidon* or *sertindol* or *ziprasidon* or *zotepin*or *sulpiride* or *remoxipride* or *paliperidone* or *perospirone*)) in title, abstract or index terms of REFERENCE or interventions of STUDY)]
The Cochrane Schizophrenia Group’s Trials Register is compiled by systematic searches of major databases, handsearches and conference proceedings (see Group Module). Incoming trials are assigned to existing or new review titles.
1.1.2 Searching other resources
1.1.2.1 Reference searching
We will inspect references of all included studies for further relevant studies published in any language.
1.1.2.2 Personal contact
We will contact the first author of each included study for information regarding unpublished trials.
Data and analyses
Comparison 1. CHLORPROMAZINE versus OLANZAPINE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical response: 1. No significant clinical response | 4 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [1.39, 3.45] |
| 1.1 short term ‐ up to 6 months | 3 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 2.34 [1.37, 3.99] |
| 1.2 long term ‐ over 12 months | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [0.76, 4.41] |
| 2 Clinical response: 2. Average endpoint score (CGI, high = poor) ‐ short term (up to 6 months) | 3 | 110 | Mean Difference (IV, Random, 95% CI) | 0.93 [0.36, 1.51] |
| 3 Clinical response: 3. Relapse ‐ long term (over 12 months) | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.46, 4.86] |
| 4 Mental state: 1. Average endpoint score (various scales, high = poor) ‐ short term (up to 6 months) | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 BPRS total | 4 | 245 | Mean Difference (IV, Random, 95% CI) | 3.21 [‐0.62, 7.05] |
| 4.2 BPRS activation subscale | 2 | 299 | Mean Difference (IV, Random, 95% CI) | 0.47 [0.27, 0.67] |
| 4.3 BPRS anxiety‐depression subscale | 1 | 213 | Mean Difference (IV, Random, 95% CI) | 1.57 [1.36, 1.78] |
| 4.4 BPRS hostile‐suspiciousness subscale | 2 | 299 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐1.98, 1.35] |
| 4.5 BPRS thinking disorder subscale | 2 | 299 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐2.66, 1.06] |
| 4.6 BPRS withdraw‐retardation subscale | 2 | 299 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐2.25, 1.26] |
| 4.7 NOSIE total | 1 | 213 | Mean Difference (IV, Random, 95% CI) | ‐18.36 [‐22.39, ‐14.33] |
| 4.8 PANSS total | 6 | 351 | Mean Difference (IV, Random, 95% CI) | 10.46 [4.49, 16.43] |
| 4.9 PANSS general pathology subscale | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 1.31 [‐0.32, 2.94] |
| 4.10 PANSS negative symptom subscale | 2 | 141 | Mean Difference (IV, Random, 95% CI) | 2.38 [0.31, 4.45] |
| 4.11 PANSS positive symptom subscale | 2 | 161 | Mean Difference (IV, Random, 95% CI) | 0.91 [‐0.30, 2.11] |
| 4.12 SAPS total | 1 | 86 | Mean Difference (IV, Random, 95% CI) | ‐2.10 [‐4.53, 0.33] |
| 5 Mental state: 2. Average endpoint score (BPRS, high = poor) ‐ medium term (7 to 12 months) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 BPRS total | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 8.60 [5.94, 11.26] |
| 6 Mental state: 3. Average endpoint score (various scales, high = poor) ‐ skewed data | Other data | No numeric data | ||
| 6.1 BPRS total | Other data | No numeric data | ||
| 6.2 HAMA (anxiety) | Other data | No numeric data | ||
| 6.3 MADRS (depression) | Other data | No numeric data | ||
| 6.4 PANSS negative symptom subscale | Other data | No numeric data | ||
| 6.5 PANSS positive symptom subscale | Other data | No numeric data | ||
| 7 Service involvement: 1. Re‐hospitalisation | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 long term (over 12 months) | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.46, 4.86] |
| 8 Functioning: 1. Executive function ‐ average endpoint score (WCST, high = poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 short term (up to 6 months) | 1 | 53 | Mean Difference (IV, Random, 95% CI) | 10.96 [1.01, 20.91] |
| 9 Adverse effects: 1. Anticholinergic ‐ short term (up to 6 months) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 blurred vision | 3 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 2.59 [0.66, 10.22] |
| 9.2 dry mouth | 5 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.29, 4.45] |
| 9.3 excessive sweating | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.62, 14.46] |
| 9.4 hypersalivation | 2 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 10.99 [4.14, 29.17] |
| 9.5 stuffy nose | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [1.06, 8.52] |
| 10 Adverse effects: 2. Cardiovascular ‐ short term (up to 6 months) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 abnormal ECG | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 3.60 [0.60, 21.55] |
| 10.2 apathism | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [1.15, 21.67] |
| 10.3 blood pressure (drop) | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 8.82 [1.13, 68.52] |
| 10.4 orthostatic hypotension | 5 | 561 | Risk Ratio (M‐H, Random, 95% CI) | 9.78 [2.68, 35.71] |
| 10.5 palpitation | 1 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 40.66 [2.49, 664.56] |
| 10.6 tachycardia | 3 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 3.53 [1.66, 7.48] |
| 11 Adverse effects: 3. Central nervous system ‐ short term (up to 6 months) | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 dizziness | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 3.85 [1.11, 13.32] |
| 11.2 drowsiness | 5 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 2.46 [1.66, 3.64] |
| 11.3 fatigue | 2 | 161 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.13, 7.66] |
| 12 Adverse effects: 4. Gastrointestinal ‐ short term (up to 6 months) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 appetite loss | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 11.01 [2.82, 42.94] |
| 12.2 constipation | 6 | 622 | Risk Ratio (M‐H, Random, 95% CI) | 4.29 [2.61, 7.05] |
| 12.3 diarrhoea | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.25, 100.97] |
| 12.4 dysphagia | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 71.92] |
| 12.5 nausea/vomiting | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.43, 5.20] |
| 13 Adverse effects: 5. Haematology ‐ short term (up to 6 months) | 3 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 4.46 [1.16, 17.22] |
| 13.1 abnormal haemogram | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 4.0 [0.87, 18.31] |
| 13.2 leukopenia | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 6.69 [0.36, 125.71] |
| 14 Adverse effects: 6. Hepatitic ‐ short term (up to 6 months) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 abnormal liver function | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [0.25, 101.58] |
| 14.2 abnormal transaminase | 2 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.01, 150.45] |
| 15 Adverse effects: 7a. Metabolic ‐ weight gain ‐ short term (up to 6 months) | 5 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.25, 1.96] |
| 16 Adverse effects: 7b. Metabolic ‐ weight gain ‐ continuous measures | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 short term (up to 6 months) | 4 | 160 | Mean Difference (IV, Random, 95% CI) | ‐5.11 [‐9.15, ‐1.07] |
| 16.2 medium term (7 to 12 months) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.59 [‐11.87, 13.05] |
| 17 Adverse effects: 7c. Metabolic ‐ other ‐ continuous measures | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 17.1 cholesterol (TC) ‐ short term (up to 6 months) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.02, 0.22] |
| 17.2 high‐density lipoprotein (HDL) ‐ short term (up to 6 months) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.12, 0.22] |
| 17.3 low‐density lipoprotein (LDL) ‐ short term (up to 6 months) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.31, 0.29] |
| 17.4 low‐density lipoprotein (LDL) ‐ medium term (7 to 12 months) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.41, 0.53] |
| 18 Adverse effects: 7d. Metabolic ‐ other ‐ average endpoint scores ‐ skewed data | Other data | No numeric data | ||
| 18.1 cholesterol (TC) ‐ medium term (7 to 12 months) | Other data | No numeric data | ||
| 18.2 high‐density lipoprotein (HDL) ‐ short term (up to 6 months) | Other data | No numeric data | ||
| 18.3 triglyceride (TG) ‐ short term (up to 6 months) | Other data | No numeric data | ||
| 18.4 triglyceride (TG) ‐ medium term (7 to 12 months) | Other data | No numeric data | ||
| 19 Adverse effects: 8a. Movement disorders ‐ extrapyramidal symptoms ‐ short term (up to 6 months) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19.1 akathisia | 3 | 417 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [0.29, 11.84] |
| 19.2 any EPS symptoms | 2 | 298 | Risk Ratio (M‐H, Random, 95% CI) | 34.47 [4.79, 248.30] |
| 19.3 muscle stiffness | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 6.13 [0.73, 51.45] |
| 19.4 tremor | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 6.78 [0.84, 54.57] |
| 20 Adverse effects: 8b. Movement disorders ‐ extrapyramidal symptoms ‐ average endpoint score (ESRS, high = poor) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20.1 short term (up to 6 months) | 2 | 80 | Mean Difference (IV, Random, 95% CI) | 0.90 [0.14, 1.66] |
| 21 Adverse effects: 9a. Various other ‐ sleep ‐ average endpoint score (LSEQ, high = better) ‐ short term (up to 6 months) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 21.1 awaking from sleep | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐5.30 [‐21.91, 11.31] |
| 21.2 getting to sleep score | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐19.88, 18.68] |
| 21.3 quality of sleep | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐15.0 [‐33.07, 3.07] |
| 22 Adverse effects: 9b. Various other ‐ sleep ‐ average length of sleep (hour/day) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 22.1 short term (up to 6 months) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 3.63 [2.08, 5.18] |
| 22.2 medium term (7 to 12 months) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 4.41 [2.82, 6.00] |
| 23 Adverse effects: 9c. Various other ‐ sleep ‐ behaviour following waking (LSEQ) ‐ skewed data | Other data | No numeric data | ||
| 23.1 short term (up to 6 months) | Other data | No numeric data | ||
| 24 Adverse effects: 9b. Various other ‐ rash | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.14, 28.76] |
| 24.1 short term (up to 6 months) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.14, 28.76] |
| 25 Quality of life: 1a. Average endpoint scores (various scales, high = better) ‐ short term (up to 6 months) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 25.1 GQOLI ‐ living condition | 1 | 61 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐4.21, 2.21] |
| 25.2 GQOLI ‐ physical health | 1 | 61 | Mean Difference (IV, Random, 95% CI) | ‐10.10 [‐13.93, ‐6.27] |
| 25.3 GQOLI ‐ psychological health | 1 | 61 | Mean Difference (IV, Random, 95% CI) | ‐22.60 [‐25.94, ‐19.26] |
| 25.4 GQOLI ‐ social function | 1 | 61 | Mean Difference (IV, Random, 95% CI) | ‐18.20 [‐20.51, ‐15.89] |
| 26 Quality of life: 1b. Average endpoint score (QoL, high = better) ‐ skewed data | Other data | No numeric data | ||
| 26.1 short term (up to 6 months) | Other data | No numeric data | ||
| 27 Leaving the study early ‐ short term (up to 6 months) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 27.1 due to any reason | 3 | 139 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.45, 6.40] |
| 27.2 due to lack of efficacy | 2 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.08, 2.66] |
1.26. Analysis.
Comparison 1 CHLORPROMAZINE versus OLANZAPINE, Outcome 26 Quality of life: 1b. Average endpoint score (QoL, high = better) ‐ skewed data.
| Quality of life: 1b. Average endpoint score (QoL, high = better) ‐ skewed data | ||||
|---|---|---|---|---|
| Study | Intervention | mean | SD | N |
| short term (up to 6 months) | ||||
| vs OLZ ‐ Loza 1999 (HGDT) | Chlorpromazine | 47.7 | 26.6 | 14 |
| vs OLZ ‐ Loza 1999 (HGDT) | Olanzapine | 56.6 | 31.3 | 27 |
Comparison 2. CHLORPROMAZINE versus RISPERIDONE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical response: 1. No significant clinical response | 7 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.53, 1.34] |
| 1.1 short term (up to 6 months) | 7 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.53, 1.34] |
| 2 Global state: 1. Average endpoint score (CGI‐CI, high = poor) ‐ skewed data | Other data | No numeric data | ||
| 2.1 short term (up to 6 months) | Other data | No numeric data | ||
| 3 Global state: 2. Need of additional benzhexol | 2 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.26, 5.53] |
| 3.1 short term (up to 6 months) | 2 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.26, 5.53] |
| 4 Mental state: 1a. Average endpoint score (various scales, high = poor) ‐ short term (up to 6 months) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 BPRS total | 4 | 247 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐3.49, 5.28] |
| 4.2 BPRS activation subscale | 2 | 130 | Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.81, 1.63] |
| 4.3 BPRS anxiety‐depression subscale | 2 | 130 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐1.56, 1.73] |
| 4.4 BPRS hostile‐suspiciousness subscale | 2 | 130 | Mean Difference (IV, Random, 95% CI) | 0.89 [‐1.41, 3.18] |
| 4.5 NORS total | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 1.80 [‐2.53, 6.13] |
| 4.6 PANSS total | 5 | 397 | Mean Difference (IV, Random, 95% CI) | ‐1.95 [‐5.58, 1.69] |
| 4.7 PANSS positive symptom subscale | 4 | 337 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐1.67, 1.74] |
| 4.8 PANSS negative symptom subscale | 3 | 230 | Mean Difference (IV, Random, 95% CI) | 3.16 [‐1.57, 7.89] |
| 4.9 PANSS general pathology subscale | 3 | 267 | Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐2.15, 0.46] |
| 4.10 SANS total | 2 | 71 | Mean Difference (IV, Random, 95% CI) | 10.89 [‐4.49, 26.27] |
| 5 Mental state: 1b. Average endpoint score (various scales, high = poor) ‐ skewed data ‐ short term (up to 6 months) | Other data | No numeric data | ||
| 5.1 BPRS thinking disorder subscale | Other data | No numeric data | ||
| 5.2 BPRS withdraw‐retardation subscale | Other data | No numeric data | ||
| 5.3 PANSS general pathology subscale | Other data | No numeric data | ||
| 5.4 PANSS negative symptom subscale | Other data | No numeric data | ||
| 5.5 PANSS positive symptom subscale | Other data | No numeric data | ||
| 5.6 SAPS total | Other data | No numeric data | ||
| 6 Mental state: 2. Average change score ‐ decreased rate (various scales, high = poor) ‐ short term (up to 6 months) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 PANSS total | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.23, 0.01] |
| 6.2 PANSS negative subscale | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.44, 0.02] |
| 6.3 PANSS positive subscale | 1 | 57 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.36, 0.22] |
| 7 Functioning: 1. Average endpoint score (WCST subscales, high = good) ‐ short term (up to 6 months) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 WCST‐IQ | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐11.30 [‐18.34, ‐4.26] |
| 7.2 WCST‐MQ | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐19.60 [‐28.83, ‐10.37] |
| 8 Adverse effects: 1. Anticholinergic ‐ short term (up to 6 months) | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 blurred vision | 5 | 387 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [1.32, 4.50] |
| 8.2 dry mouth | 9 | 852 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.88, 4.51] |
| 8.3 excessive sweating | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.62, 14.46] |
| 8.4 hypersalivation | 5 | 373 | Risk Ratio (M‐H, Random, 95% CI) | 8.67 [3.80, 19.80] |
| 8.5 stuffy nose | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [1.06, 8.52] |
| 9 Adverse effects: 2a. Cardiovascular ‐ short term (up to 6 months) | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 abnormal ECG | 3 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 2.41 [0.96, 6.06] |
| 9.2 apathism | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 6.25 [2.35, 16.65] |
| 9.3 blood pressure drop | 3 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 8.25 [2.61, 26.12] |
| 9.4 bradycardia | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 5.34] |
| 9.5 orthostatic hypotension | 5 | 546 | Risk Ratio (M‐H, Random, 95% CI) | 5.74 [2.28, 14.44] |
| 9.6 palpitation | 1 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 40.66 [2.49, 664.56] |
| 9.7 sinus tachycardia | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 3.12 [0.35, 28.03] |
| 9.8 tachycardia | 7 | 557 | Risk Ratio (M‐H, Random, 95% CI) | 2.64 [1.64, 4.26] |
| 10 Adverse effects: 2b. Cardiovascular ‐ continuous measures ‐ short term (up to 6 months) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 cardiac rate (upright position) | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 3.90 [‐1.57, 9.37] |
| 10.2 cardiac rate (horizontal position) | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 7.42 [3.47, 11.37] |
| 10.3 contractive blood pressure (upright position) | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐2.20, 0.08] |
| 10.4 contractive blood pressure (horizontal position) | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 1.38 [0.61, 2.15] |
| 10.5 diastolic blood pressure (upright position) | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.29, 0.23] |
| 10.6 diastolic blood pressure (horizontal position) | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.39 [‐0.18, 0.96] |
| 11 Adverse effects: 3. Central nervous system ‐ short term (up to 6 months) | 6 | 1191 | Odds Ratio (M‐H, Random, 95% CI) | 1.96 [0.92, 4.22] |
| 11.1 agitation | 1 | 65 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.98] |
| 11.2 dizziness | 4 | 299 | Odds Ratio (M‐H, Random, 95% CI) | 3.57 [1.75, 7.30] |
| 11.3 drowsiness | 4 | 307 | Odds Ratio (M‐H, Random, 95% CI) | 4.93 [1.49, 16.32] |
| 11.4 fatigue | 1 | 100 | Odds Ratio (M‐H, Random, 95% CI) | 13.05 [4.82, 35.33] |
| 11.5 insomnia | 5 | 342 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.10, 0.90] |
| 11.6 reduced activity | 1 | 78 | Odds Ratio (M‐H, Random, 95% CI) | 2.05 [0.18, 23.63] |
| 12 Adverse effects: 4. Gastrointestinal ‐ short term (up to 6 months) | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 constipation | 9 | 868 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [2.05, 4.39] |
| 12.2 diarrhoea | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.25, 100.97] |
| 12.3 dysphagia | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 3.67 [1.09, 12.36] |
| 12.4 loss of appetite | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 11.01 [2.82, 42.94] |
| 12.5 nausea/vomiting | 4 | 350 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.37, 1.91] |
| 13 Adverse effects: 5. Haematology ‐ short term (up to 6 months) | 3 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 4.46 [1.16, 17.22] |
| 13.1 abnormal haemogram | 2 | 180 | Risk Ratio (M‐H, Random, 95% CI) | 4.0 [0.87, 18.31] |
| 13.2 leukopenia | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 6.69 [0.36, 125.71] |
| 14 Adverse effects: 6. Hepatitic ‐ short term (up to 6 months) | 3 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.17, 10.09] |
| 14.1 abnormal liver function | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.25, 100.89] |
| 14.2 abnormal transaminase | 3 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.07, 11.58] |
| 15 Adverse effects: 7. Metabolic ‐ weight gain | 4 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.52, 3.59] |
| 15.1 short term (up to 6 months) | 4 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.52, 3.59] |
| 16 Adverse effects: 8. Movement disorders ‐ short term (up to 6 months) | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 akathisia | 6 | 435 | Risk Ratio (M‐H, Random, 95% CI) | 2.37 [1.46, 3.85] |
| 16.2 any EPS symptoms | 3 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.85, 3.40] |
| 16.3 dystonia | 3 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.97, 2.66] |
| 16.4 muscle stiffness | 5 | 335 | Risk Ratio (M‐H, Random, 95% CI) | 2.96 [0.92, 9.49] |
| 16.5 torsion movements | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [0.50, 161.73] |
| 16.6 tremor | 6 | 435 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [1.47, 3.14] |
| 17 Adverse events: 9. Average endpoint score (TESS) ‐ skewed data | Other data | No numeric data | ||
| 17.1 short term (up to 6 months) | Other data | No numeric data | ||
| 18 Adverse effects: 10. Various other ‐ short term (up to 6 months) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 concentration (poor) | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [1.35, 2.82] |
| 18.2 memory deterioration | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [1.35, 2.82] |
| 18.3 sexual dysfunction | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 2.55 [1.43, 4.53] |
| 18.4 unspecified | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.97, 1.95] |
| 19 Quality of life: 1. Average endpoint score (QOL, high = good) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 19.1 short term (up to 6 months) | 1 | 100 | Mean Difference (IV, Random, 95% CI) | ‐14.20 [‐20.50, ‐7.90] |
| 20 Leaving the study early ‐ short term (up to 6 months) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 20.1 due to adverse events | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.01, 4.11] |
Comparison 3. CHLORPROMAZINE versus QUETIAPINE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical response: 1. No significant clinical response | 28 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 short term (up to 6 months) | 28 | 3241 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.81, 1.06] |
| 2 Global state: 1. Need of additional benzodiazepines/benzhexol | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 short term (up to 6 months) | 2 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.10, 1.75] |
| 3 Global state: 2a. Average endpoint scores (various scales, high = poor) ‐ short term (up to 6 months) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 CGI‐SI | 2 | 177 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.82, 0.84] |
| 3.2 CGI‐GI | 3 | 229 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.33, 0.11] |
| 4 Global state: 2b. Average endpoint score (CGI‐SI, high = poor) ‐ skewed data) | Other data | No numeric data | ||
| 4.1 short term (up to 6 months) | Other data | No numeric data | ||
| 5 Global state: 3. Average change scores (CGI‐SI, high = poor) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 short term (up to 6 months) | 1 | 384 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.32, ‐0.28] |
| 6 Mental state: 1a. Average endpoint scores (various scales, high = poor) ‐ short term (up to 6 months) | 31 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 BPRS total | 6 | 548 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐1.23, 0.88] |
| 6.2 BPRS anxiety‐depression subscale | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐1.16, 0.62] |
| 6.3 BPRS activation subscale | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.26, 0.70] |
| 6.4 BPRS hostile‐suspiciousness subscale | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐1.15, 0.97] |
| 6.5 BPRS thinking disorder subscale | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐1.39, 1.09] |
| 6.6 BPRS withdraw‐retardation subscale | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.76, 0.56] |
| 6.7 PANSS total | 25 | 2049 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐2.30, 2.19] |
| 6.8 PANSS positive symptoms | 13 | 1102 | Mean Difference (IV, Random, 95% CI) | 0.39 [‐0.11, 0.88] |
| 6.9 PANSS negative symptoms | 17 | 1361 | Mean Difference (IV, Random, 95% CI) | 1.05 [0.13, 1.98] |
| 6.10 PANSS general pathology | 18 | 1530 | Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐3.06, 0.84] |
| 6.11 HAMD total | 1 | 63 | Mean Difference (IV, Random, 95% CI) | 7.4 [5.13, 9.67] |
| 7 Mental state: 1b. Average endpoint scores (various scales, high = poor) ‐ medium term (6 to 12 months) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 PANSS total | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 4.90 [1.74, 8.06] |
| 7.2 PANSS general pathology | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐4.34, 3.94] |
| 7.3 PANSS negative symptoms | 1 | 77 | Mean Difference (IV, Random, 95% CI) | 2.70 [0.44, 4.96] |
| 8 Mental state: 1c. Average endpoint scores (various scales, high = poor) ‐skewed data | Other data | No numeric data | ||
| 8.1 PANSS total ‐ short term (up to 6 months) | Other data | No numeric data | ||
| 8.2 PANSS positive symptoms ‐ short term (up to 6 months) | Other data | No numeric data | ||
| 8.3 PANSS negative symptoms ‐ short term (up to 6 months) | Other data | No numeric data | ||
| 8.4 PANSS general pathology ‐ short term (up to 6 months | Other data | No numeric data | ||
| 9 Mental state: 1d. Average change score (various scales, high = poor) ‐ short term (up to 6 months) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 PANSS total | 2 | 426 | Mean Difference (IV, Random, 95% CI) | ‐2.50 [‐2.82, ‐2.19] |
| 9.2 PANSS positive symptoms | 1 | 384 | Mean Difference (IV, Random, 95% CI) | 1.20 [1.10, 1.30] |
| 9.3 PANSS negative symptoms | 1 | 384 | Mean Difference (IV, Random, 95% CI) | 0.80 [0.70, 0.90] |
| 9.4 PANSS general pathology | 1 | 384 | Mean Difference (IV, Random, 95% CI) | 1.0 [0.85, 1.15] |
| 10 Mental state: 1e. Average score decreased rate of BPRS/PANSS (%) ‐ short term (up to 6 months) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 BPRS | 1 | 197 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐4.86, 3.26] |
| 10.2 PANSS | 6 | 782 | Mean Difference (IV, Random, 95% CI) | ‐1.96 [‐7.20, 3.28] |
| 11 Functioning: 1. Average endpoint score (various scales, high = better) ‐ short term (up to 6 months) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 WCST‐IQ | 1 | 120 | Mean Difference (IV, Random, 95% CI) | ‐11.30 [‐17.62, ‐4.98] |
| 11.2 WCST‐MQ | 1 | 120 | Mean Difference (IV, Random, 95% CI) | ‐19.60 [‐27.37, ‐11.83] |
| 12 Cognitive function: 1. Average endpoint score (various scales, high = better) ‐ short term (up to 6 months) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 WCST | 2 | 123 | Mean Difference (IV, Random, 95% CI) | 8.92 [0.40, 17.43] |
| 12.2 WMS‐RC | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐9.34 [‐17.53, ‐1.15] |
| 13 Adverse effects: 1. Anticholinergic ‐ short term (up to 6 months) | 22 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 blurred vision | 18 | 1780 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [3.46, 7.22] |
| 13.2 dry mouth | 18 | 1682 | Risk Ratio (M‐H, Random, 95% CI) | 2.34 [1.54, 3.54] |
| 13.3 excessive sweating | 3 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 3.91 [0.84, 18.19] |
| 13.4 hypersalivation | 11 | 1135 | Risk Ratio (M‐H, Random, 95% CI) | 3.85 [2.36, 6.28] |
| 13.5 stuffy nose | 8 | 972 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.45, 1.06] |
| 14 Adverse effects: 2. Cardiovascular ‐ short term (up to 6 months) | 22 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 14.1 abnormal ECG | 7 | 708 | Risk Ratio (IV, Random, 95% CI) | 1.82 [1.11, 2.98] |
| 14.2 blood pressure drop | 8 | 690 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.53, 1.79] |
| 14.3 orthostatic hypotension | 7 | 605 | Risk Ratio (IV, Random, 95% CI) | 2.64 [1.14, 6.12] |
| 14.4 tachycardia | 17 | 1752 | Risk Ratio (IV, Random, 95% CI) | 1.70 [1.33, 2.18] |
| 15 Adverse effects: 3. Central nervous system ‐ short term (up to 6 months) | 21 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 15.1 dizziness | 12 | 1206 | Risk Ratio (IV, Random, 95% CI) | 1.40 [0.83, 2.35] |
| 15.2 drowsiness | 17 | 1677 | Risk Ratio (IV, Random, 95% CI) | 2.28 [1.51, 3.45] |
| 15.3 headache | 3 | 192 | Risk Ratio (IV, Random, 95% CI) | 0.74 [0.13, 4.18] |
| 15.4 insomnia | 9 | 867 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.55, 1.54] |
| 15.5 reduced activity | 8 | 788 | Risk Ratio (IV, Random, 95% CI) | 7.80 [3.05, 19.92] |
| 15.6 sedation | 1 | 40 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.01, 7.72] |
| 16 Adverse effects: 4. Gastrointestinal ‐ short term (up to 6 months) | 22 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 16.1 constipation | 22 | 2048 | Risk Ratio (IV, Random, 95% CI) | 2.55 [2.04, 3.20] |
| 16.2 diarrhoea | 1 | 62 | Risk Ratio (IV, Random, 95% CI) | 5.32 [0.27, 106.54] |
| 16.3 loss of appetite | 5 | 472 | Risk Ratio (IV, Random, 95% CI) | 2.52 [0.82, 7.72] |
| 16.4 nausea/vomiting | 9 | 819 | Risk Ratio (IV, Random, 95% CI) | 1.23 [0.58, 2.63] |
| 17 Adverse effects: 5a. Endocrine ‐ various ‐ short term (up to 6 months) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.1 gynaecomastia, galactorrhoea | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 5.37 [0.27, 108.47] |
| 17.2 hyperprolactinemia | 2 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 5.69 [2.74, 11.79] |
| 17.3 menstrual irregularities | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.25, 99.34] |
| 18 Adverse effects: 5b. Endocrine ‐ average endpoint ‐ short term (up to 6 months) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 24.62 [17.76, 31.48] |
| 18.1 prolactin level (ng/mL) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 24.62 [17.76, 31.48] |
| 19 Adverse effects: 5c. Endocrine ‐ skewed data ‐ short term (up to 6 months) | Other data | No numeric data | ||
| 19.1 average prolactin level (ng/mL) | Other data | No numeric data | ||
| 20 Adverse effects: 6a. Haematology ‐ short term (up to 6 months) | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 20.1 elevated ALT | 8 | 775 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.92, 2.87] |
| 20.2 decreased white blood cell count | 4 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.43, 2.42] |
| 20.3 increased white blood cell count | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 2.05 [0.40, 10.56] |
| 21 Adverse effects: 6b. Haematology ‐ average endpoint ‐ short term (up to 6 months) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 21.1 blood glucose | 1 | 130 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.18, 0.38] |
| 21.2 blood TG | 1 | 130 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.28, 0.28] |
| 21.3 blood TC | 1 | 130 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.11, 0.51] |
| 22 Adverse effects: 7. Hepatitic ‐ short term (up to 6 months) | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 22.1 abnormal liver function | 5 | 561 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [1.32, 3.33] |
| 23 Adverse effects: 8. Movement disorders ‐ short term (up to 6 months) | 24 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 23.1 agitation | 5 | 313 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.14, 0.95] |
| 23.2 akathisia | 17 | 1757 | Risk Ratio (IV, Random, 95% CI) | 3.73 [2.55, 5.47] |
| 23.3 any EPS symptoms | 8 | 644 | Risk Ratio (IV, Random, 95% CI) | 8.03 [4.78, 13.51] |
| 23.4 dystonia | 1 | 201 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.01, 8.01] |
| 23.5 myotonia | 12 | 1257 | Risk Ratio (IV, Random, 95% CI) | 4.59 [3.18, 6.64] |
| 23.6 need additional medication for EPS symptoms | 1 | 202 | Risk Ratio (IV, Random, 95% CI) | 1.5 [0.71, 3.18] |
| 23.7 torsion movement | 9 | 1063 | Risk Ratio (IV, Random, 95% CI) | 5.81 [2.76, 12.23] |
| 23.8 tremor | 13 | 1343 | Risk Ratio (IV, Random, 95% CI) | 2.90 [1.89, 4.45] |
| 24 Adverse effects: 9a. Metabolic ‐ weight gain | 15 | 1259 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [1.17, 2.39] |
| 24.1 weight gain | 15 | 1259 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [1.17, 2.39] |
| 25 Adverse effects: 9b. Metabolic ‐ short term (up to 6 months) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 25.1 average BMI | 1 | 105 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐0.67, 1.67] |
| 25.2 average weight (KG) | 1 | 130 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐4.68, 2.68] |
| 26 Adverse effects: 10. Various other ‐ short term (up to 6 months) | 4 | 560 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.19, 2.52] |
| 26.1 unspecified adverse effects | 4 | 560 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.19, 2.52] |
| 27 Adverse effects: 11. Average endpoint score (TESS, high = poor) ‐ skewed data | Other data | No numeric data | ||
| 27.1 short term (up to 6 months) | Other data | No numeric data | ||
| 27.2 medium term (7 to 12 months) | Other data | No numeric data | ||
| 28 Quality of life: 1. General ‐ average endpoint score (GQOL1 ‐ 74, high = better) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 28.1 short term (up to 6 months) | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐6.49 [‐11.30, ‐1.68] |
| 29 Leaving the study early: 1a. Short term (up to 6 months) | 19 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 29.1 due to adverse effect | 10 | 1680 | Risk Ratio (IV, Random, 95% CI) | 1.43 [1.04, 1.98] |
| 29.2 due to inefficacy | 3 | 695 | Risk Ratio (IV, Random, 95% CI) | 1.42 [0.68, 2.96] |
| 29.3 due to any other reason | 12 | 1223 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.77, 1.41] |
| 29.4 due to loss to follow‐up | 2 | 400 | Risk Ratio (IV, Random, 95% CI) | 0.56 [0.23, 1.37] |
| 30 Leaving the study early: 1b. Medium term (7 to 12 months) | 1 | 103 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.61, 2.32] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
HGCQ (Turkey) 2000.
| Methods | Allocation: randomised, blocks, computer‐generated, 2:1 for each investigator, concealed from investigators.
Blindness: double, medication kits issued.
Duration: 6 weeks.
Design: parallel 2 centres, Turkey. Setting: inpatients and outpatients. |
|
| Participants | Diagnosis: schizophrenia (DSM‐IV). Length of illness: informed consent obtained. N = 30. Age: range 18 to 47 years, mean 32.8 years. Sex: male = 17, female = 13. Inclusion criteria: CGI severity at least 4. Excluded: pregnant, seriously unstable illness, including hepatic, renal, gastroenterologic, respiratory, cardiovascular, endocrinologic, neurologic or hematologic disease, glaucoma, uncontrolled thyroid disease, myasthenia gravis, urinary retention, seizures, leucopenia. | |
| Interventions |
|
|
| Outcomes | Leaving the study early.
Mental state: BPRS, PANSS.
Global state: CGI.
Adverse events: ESRS, COSTART list, weight change.
Quality of life: VPS, LSEQ. Unable to use: Adverse events: UKU, weight change (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation. |
| Allocation concealment (selection bias) | High risk | No concealment of allocation. Quote: "the bottles were labelled 'Olanzapine' or 'Chlorpromazine' in addition to the study number". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding, which may introduce detection bias, especially, for those subjective outcomes involving rating scales. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was included in the final analysis. |
| Selective reporting (reporting bias) | High risk | UKU adverse effect rating scale data and weight change data were not reported. |
| Other bias | Low risk | None obvious. |
HGDV (Morocco) 1999.
| Methods | Allocation: randomised, computer‐generated, blocks for each investigator, 2:1, olanzapine to chlorpromazine. Blindness: open‐label, medication kits issued. Duration: 6 weeks (preceded by washout phase; extension for responders). Design: single centre Morocco. | |
| Participants | Diagnosis: schizophrenia (DSM‐IV). Length of illness: previously hospitalised (mean ˜ 1.5 times), informed consent obtained. N = 40. Age: 18 to 47. Sex: 6 male, 33 female. Setting: inpatients and outpatients. Inclusion criteria: initial CGI severity score of 4. Excluded: pregnant, seriously unstable illness, including hepatic, renal, gastroenterologic, respiratory, cardiovascular, endocrinologic, neurologic or hematologic disease; glaucoma, uncontrolled thyroid disease, myasthenia gravis, urinary retention, seizures, leucopenia. | |
| Interventions |
|
|
| Outcomes | Leaving study early. Mental state: BPRS, PANSS, MADRS, HAMA. Global state: CGI. Adverse events: ESRS, COSTART list, weight gain. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation. |
| Allocation concealment (selection bias) | High risk | No concealment of allocation. Quote: "the bottles were labelled 'Olanzapine' or 'Chlorpromazine' in addition to the study number". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Quote: "This is an open label study...personnel at the study site, patients and personnel at Lilly were not blinded as to the treatment being administered." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding, which may introduce detection bias, especially, for those subjective outcomes involving rating scales. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants left chlorpromazine group early, but were included in the final analysis. |
| Selective reporting (reporting bias) | Low risk | It appears that all measured outcomes were reported. |
| Other bias | Low risk | None obvious. |
vs OLZ ‐ An 2006.
| Methods | Allocation: randomised, no further information.
Blinding: not stated.
Duration: 2 years. Design: parallel. Setting: inpatients, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3). N = 70. Age: mean ˜ 27 years, SD ˜ 6 years. Sex: male and female. Length of illness: mean ˜ 32 months, SD ˜ 13 months. Inclusion criteria: PANSS ≥ 60, length of illness less than 5 years, without organic diseases, without history of drug or alcohol dependence, clear of any antipsychotic medication for at least 1 month prior to hospital admission, able to complete cognitive test and consent to participation to the study. |
|
| Interventions |
|
|
| Outcomes | Clinical response: number of people stabilised on current medication. Relapse. Service involvement: re‐hospitalisation. Functioning: executive functioning as measured by WCST. Leaving the study early. Unable to use: Functioning: executive functioning WCST subscale score. The subscales are not validated. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but no detail provided. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Eight and 16 people dropped out of olanzapine and chlorpromazine group respectively. The drop out is unlikely to have had any impact on the outcomes measured, except for WCST (executive functioning) test. |
| Selective reporting (reporting bias) | Low risk | All measured outcomes were reported. |
| Other bias | Low risk | None obvious. |
vs OLZ ‐ Chang 2003.
| Methods | Allocation: randomised with random number table.
Blindness: not reported.
Duration: 8 weeks.
Design: parallel group. Setting: hospital and community, China. |
|
| Participants | Diagnosis: schizophrenia.
N = 62.
Age: 18 to 53 years.
Sex: male and female. Length of illness: 4 to 5 years. Inclusion criteria: not reported. |
|
| Interventions |
|
|
| Outcomes | Clinical response: no clinically significant improvement*.
Mental state: BPRS. Adverse effects. |
|
| Notes | *BPRS scale score decreased rate ≤ 29%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised with random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | TESS was measured, but not reported. |
| Other bias | Low risk | None obvious. |
vs OLZ ‐ Chen 2006.
| Methods | Allocation: randomised, no further detail.
Blindness: not reported.
Duration: 8 weeks.
Design: parallel group. Setting: inpatients, China. |
|
| Participants | Diagnosis: schizophrenia.
N = 86.
Age: 20 to 55 years.
Sex: male and female. Length of illness: 3 months to 5 years; median 2 years. Inclusion criteria: BPRS ≥ 30, SAPS ≥ 28, SANS ≤ 12. Exclusion criteria: severe physical impairment, history of drug or alcohol abuse, pregnant or lactating women. |
|
| Interventions |
|
|
| Outcomes | Clinical response: no clinically significant improvement*.
Mental state: BPRS and SAPS endpoint scale score. Adverse effects. Unable to use: Mental state: BPRS anxiety ‐ depression subscale data, as it was skewed. |
|
| Notes | *Unclear how this was assessed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further description. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | TESS was measured, but not reported. |
| Other bias | Low risk | None obvious. |
vs OLZ ‐ He 2003.
| Methods | Allocation: randomised, no further detail.
Blindness: not reported.
Duration: 8 weeks.
Design: parallel group. Setting: inpatients, China. |
|
| Participants | Diagnosis: schizophrenia.
N = 80.
Age: 20 to 55 years.
Sex: male and female. Length of illness: olanzapine group = 1.5 ± 3.2 years; chlorpromazine group = 2.2 ± 3.0 years. Inclusion criteria: PANSS ≥ 60 Exclusion criteria: severe liver, renal, heart, haematological diseases; people with glaucoma or history of drug or alcohol abuse, pregnant or lactating women. |
|
| Interventions |
|
|
| Outcomes | Mental state: PANSS endpoint scale score, subscale scores. Adverse events. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further description. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | All measured outcomes were reported. |
| Other bias | Low risk | None obvious. |
vs OLZ ‐ Loza 1999 (HGDT).
| Methods | Allocation: randomised, computer‐generated, blocks for each investigator, 2:1, olanzapine to chlorpromazine, concealed from investigators. Blindness: open label. Duration: 6 weeks (preceded by washout phase of 2 to 9 days; extension for responders). Setting: inpatients and outpatients, multi‐centre: two sites, Egypt. | |
| Participants | Diagnosis: schizophrenia (DSM‐IV). N = 41. Age: range 17 to 47 years, mean 32.3. Sex: male 33, female 8. Inclusion criteria: initial score of at least four on the CGI severity scale. Excluded: pregnant, seriously unstable illness, including hepatic, renal, gastroenterologic, respiratory, cardiovascular, endocrinologic, neurologic or hematologic disease; glaucoma, uncontrolled thyroid disease, myasthenia gravis, urinary retention, seizures, leucopenia. | |
| Interventions |
|
|
| Outcomes | Leaving study early.
Mental state: BPRS, PANSS.
Global state: CGI‐S.
Quality of Life Scale: QoL.
Adverse events: ESRS, COSTART list, weight change. Unable to use: Adverse events: UKU (no data). Hospital status: (no data). Laboratory tests & physiological measures: (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It's unclear if appropriate tools/methods were used to facility the random allocation. Quote: "qualified patients were assigned by random allocation at Visit 2 to one of the two treatment groups. Randomisation was performed at 2:1 ratio." |
| Allocation concealment (selection bias) | High risk | No concealment of allocation. Quote: "Medication was dispensed to the patients by the study site...the bottles were labelled 'Olanzapine' or 'Chlorpromazine' in addition to the study number". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This is an open label study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | LOCF was used to account for any missing values in the final analysis. |
| Selective reporting (reporting bias) | High risk | Laboratory tests, physiological measures and hospital status, as well as UKU Side Effect Rating Scale scores were not reported. |
| Other bias | Low risk | None obvious. |
vs OLZ ‐ Luo 2007.
| Methods | Allocation: randomised, using computer generated random numbers.
Blindness: not reported.
Duration: 8 weeks trial period, plus 52 weeks follow‐up.
Design: parallel group. Setting: inpatients, China. |
|
| Participants | Diagnosis: first episode schizophrenia.
N = 50.
Age: 18 to 60 years.
Sex: male and female. Length of illness: unclear, but stated as first episode schizophrenia. Inclusion criteria: CCMD‐3 diagnosed schizophrenia, not receiving any other antipsychotic medications in combination. Exclusion criteria: simple obesity, hypertension, hypothyroidism, severe heart, liver or renal disease. |
|
| Interventions |
|
|
| Outcomes | Cholesterol analysis: TC, TG, LDL, HDL. Adverse effects: weight, sleep time. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised with computer generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated, but most of the outcomes reported are objective lab test results, which is unlikely to introduce bias to the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | All measured outcomes are reported. |
| Other bias | Low risk | None obvious. |
vs OLZ ‐ Wang 2002.
| Methods | Allocation: randomised, using computer generated random numbers.
Blindness: not reported.
Duration: 8 weeks trial period, plus 52 weeks follow‐up.
Design: parallel group. Setting: inpatients, China. |
|
| Participants | Diagnosis: first episode schizophrenia.
N = 60.
Age: 18 to 60 years.
Sex: male and female. Length of illness: unclear, but stated as first episode schizophrenia. Inclusion criteria: CCMD‐3 diagnosed schizophrenia, BPRS > 30. Exclusion criteria: severe organic disease, heart, liver or renal disease, substance misuse induced mental disorders. |
|
| Interventions |
|
|
| Outcomes | Clinical response: no significant clinical response*. Mental state: BPRS Adverse effects. Unable to use: Mental state: 'BPRS reducing score', as it's unclear what the trial author meant by this. We are uncertain as to how the score is calculated or what it means. |
|
| Notes | *Paper did not report on how they measured significant clinical response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label. Quote: "test and control drugs were administrated openly." p2665. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated, but most of the outcomes reported are objective laboratory test results, which is unlikely to introduce bias to the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | TESS is measured, but not reported. |
| Other bias | Low risk | None obvious. |
vs OLZ ‐ Wang 2008.
| Methods | Allocation: randomised, no further information.
Blindness: not reported.
Duration: 1 week washout period, plus 8 weeks trial.
Design: parallel group. Setting: inpatients and community patients, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 237.
Age: mean age ˜ 27 years, SD ˜ 10 years.
Sex: male and female. Length of illness: mean ˜ 4 years, SD ˜ 5 years. Inclusion criteria: CCMD‐3 diagnosed schizophrenia, BPRS > 36, obtained informed consent, lab test results within normal range. Exclusion criteria: severe organic disease, heart, liver or renal disease and any other severe mental disorders. |
|
| Interventions |
|
|
| Outcomes | Mental state: BPRS subscale and endpoint scale score*. NOSIE endpoint scale score*. Adverse effects. Leaving the study early. Unable to use: Mental state: unvalidated NOSIE subscale scores. |
|
| Notes | *The published BPRS and NOSIE endpoint scale score were implied as 'mean ± SD', but we suspect they are 'mean ± SE' for the SDs are extremely tight around the mean. Therefore, we converted these reported SE to SD following SD = SEM X sq rt (N). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Thirteen and 11 people dropped out of Olazapine and chlorpromazine group respectively. These people were excluded from final analysis. Reason for drop out were not given. |
| Selective reporting (reporting bias) | Low risk | None obvious. All measured outcomes appear to be reported. |
| Other bias | Low risk | None obvious. |
vs OLZ ‐ Wu 2008.
| Methods | Allocation: randomised, no further information.
Blindness: not reported.
Duration: twelve weeks trial.
Design: parallel group. Setting: inpatients, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 100.
Age: 18 to 60 years.
Sex: male and female. Length of illness: mean ˜ 4 years, SD ˜ 5 years. Inclusion criteria: CCMD‐3 diagnosed schizophrenia, PANSS > 60, obtained informed consent. Exclusion criteria: severe organic disease, drug or alcohol dependence. |
|
| Interventions |
|
|
| Outcomes | Mental state: PANSS subscale and endpoint scale score. NOSIE endpoint scale score. Adverse effects. Leaving the study early. Unable to use: Mental state: invalidated SF‐36 subscale scores. Quality of life: SF‐36 total scale score*. |
|
| Notes | *Data derived from this scale overlapped with the QOL data reported in another study published by the same author. Therefore, we decided to report the QOL data that was published earlier (published in 2004) than this paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | None obvious. All measured outcomes appear to be reported. |
| Other bias | Low risk | None obvious. |
vs OLZ ‐ Zhao 2006.
| Methods | Allocation: randomised, no further information.
Blindness: not reported.
Duration: 8 weeks trial.
Design: parallel group. Setting: inpatients and community patients, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 61.
Age: mean ˜ 30 years, SD ˜ 9.6 years.
Sex: male and female. Length of illness: mean ˜ 1.5 years, SD ˜ 3 years. Inclusion criteria: CCMD‐3 diagnosed schizophrenia, PANSS > 60, obtained informed consent. Exclusion criteria: severe organic disease, drug or alcohol dependence, pregnant or lactating women, learning disability. |
|
| Interventions |
|
|
| Outcomes | Mental state: PANSS subscale and endpoint scale score. Quality of life: QGOLI score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | None obvious. All measured outcomes appear to be reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Ai 2007.
| Methods | Allocation: randomised, no further detail. Blinding: not reported. Duration: 12 weeks. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 85.
Sex: male = 44, female = 41.
Age: mean ˜ 30 years, SD ˜ 11 years.
Length of illness: mean ˜ 5.8 years, SD ˜ 3.3 years. Inclusion criteria: schizophrenia (CCMD‐3). Exclusion criteria: severe physical illness. |
|
| Interventions |
|
|
| Outcomes | Clinical response: no clinical improvement*. Mental state: BPRS. Adverse effects. |
|
| Notes | * BPRS decreased rate < 25%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | TESS measured, but not reported fully. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ An 2005.
| Methods | Allocation: randomised, no further information. Blindness: not stated. Duration: 8 weeks. Design: parallel. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R).
N = 60. Age: 23.7 ± 5.4 years. Sex*: male = 45, female = 12. Length of illness: 0.8 ± 0.6 years. Inclusion criteria: first episode schizophrenia (CCMD‐2‐R), did not receive systematic treatment prior to admission, PANSS ≥ 60, length of illness less than 2 years, laboratory tests normal. Exclusion criteria: patients with physical or organic diseases. |
|
| Interventions |
|
|
| Outcomes | Mental state: PANSS positive, negative, general pathology and total score. Function: general function as measured by WAIS ‐ RC endpoint subscale score, WMS endpoint subscale score. Uable to use unvalidated WMS subscale score. |
|
| Notes | *We contacted the trial author for clarification of the number of male and female participants but didn't receive any reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the trial. |
| Selective reporting (reporting bias) | Low risk | All measured outcomes were well reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Cai 2006.
| Methods | Allocation: randomised. Blindness: not stated. Duration: 1 week wash out, plus 8 weeks trial. Design: parallel. Setting: inpatients from 8 hospitals, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 197. Age: mean ˜ 31.1 years, SD ˜ 10.1 years. Sex: male = 109, female = 88. Length of illness: mean ˜ 5.6 years. Inclusion criteria: BRPS score ≥ 36. Exclusion criteria: patients with severe physical diseases, organic disease or pregnant women. |
|
| Interventions |
No ECT or any other antipsychotic medication were combined during the treatment, but benzodiazepine or other medications for adverse effects were allowed. |
|
| Outcomes | Mental state: decreased rate of BPRS score. Leave the study early**. Adverse effect. Unable to use. Clinical response: no clinical improvement*. |
|
| Notes | *The total N of this outcome exceeded the total number of people randomised, therefore, we are unable to determine the true proportion of cases without clinical improvement. **We adopted 96 and 101 as the number of participants in the chlorpromazine and quetiapine groups respectively, rather than using 100 and 109 as reported in the paper, as the total of the latter exceeds the number of people randomised. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details were reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Twelve cases left the study early with no reason reported, but ITT analysis was conducted. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Cai 2007.
| Methods | Allocation: randomised, no further detail were reported. Blindness: not reported. Duration: 1 week washout period, plus 8 week trial. Design: parallel. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 94. Age: Quetiapine group, range: 18 to 68 years, average: 37.6 ± 15.3 years; chlorpromazine group, range: 19 to 65 years, average: 34 ± 10.5 years. Sex: Quetiapine group, male 35, female 13; chlorpromazine group, male 32, female 14. Length of illness: quetiapine group, range: 2 months to 15 years, average: 3.3 ± 3.5 years; chlorpromazine group, range: 3 months to 16 years, average: 3.6 ± 4.3 years. Inclusion criteria: PANSS score ≥ 60. Exclusion criteria: patients with organic mental disorder, alcohol abuse or other drugs abuse, with learning disability, pregnant or breastfeeding women. Patients on slow release depot antipsychotic. |
|
| Interventions |
|
|
| Outcomes | Clinical response:no clinical improvement*, decreased rate of PANSS score. Mental state: PANSS endpoint scale score decreased rate, positive subscale score decreased rate, negative subscale score decreased rate. Adverse effect: TESS. Unable to use: Mental state: PANSS subscale scores on cognitive function, agitation and depression. These subscales are not validated. |
|
| Notes | *Decreased rate of PANSS score: < 25%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further detail were reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All the patients completed the study. |
| Selective reporting (reporting bias) | Low risk | All the outcomes were well reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Cao 2005.
| Methods | Allocation: randomised, no further detail. Blinding: open label. Duration: 16 weeks. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). N = 50. Age: 25 to 40 years. Sex: male. Length of illness: not stated. Exclusion: having other mental health problems, other physical illness, drug/alcohol dependent, abnormal lab test results, problematic marital relationships. | |
| Interventions |
|
|
| Outcomes | Leaving the study early. Unable to use: Adverse effects ‐ continuous data was reported, but the scale used was unclear. Sexual arousal, and other sexual related outcomes that were measured using an invalidated scale. |
|
| Notes | Only male participants were included in the study; therefore, gender bias is likely. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop outs were excluded from analysis. |
| Selective reporting (reporting bias) | Low risk | All measured outcomes reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Chen 2001.
| Methods | Allocation: randomised by using random number table. Blinding: double blind, no further detail. Duration: 1 week washout period, plus 8 weeks intervention. Setting: inpatients, multi‐centre, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R). N = 221. Sex: male = 113 and female = 108. Age: mean ˜ 35 years, SD ˜ 11 years. Length of illness: not stated. Inclusion criteria: CCMD‐3 diagnosed schizophrenia, BPRS ≥ 36. Exclusion criteria: severe heart, renal, liver illness, nerve system illness, hypertension, blood disease, pregnant/lactating women, received ECT within 2 weeks prior to current study, suicidal patients, participated in other clinical trials within 1 month prior to current study. |
|
| Interventions |
|
|
| Outcomes | Clinical response: no clinical improvement*, decreased rate of PANSS score. Global state: poor compliance, leave the study early. Mental state: BPRS, PANSS. Adverse events. |
|
| Notes | *Decreased rate of PANSS score < 25%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using random number table. |
| Allocation concealment (selection bias) | Low risk | Pharmacist produced identical pills, only they know which pill contains experimental drug. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT was used. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as measured. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Chen 2007.
| Methods | Allocation: randomised, no further detail. Blinding: not reported. Duration: 3 months. Setting: inpatients and outpatients, Xuzhou, Jiangsu province, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). N = 62. Sex: male = 33 and female = 29. Age: mean ˜ 20.1 years, SD ˜ 2.1 years. Length of illness: average, mean 1.6 years, SD 0.8 years. Inclusion criteria: length of illness between 6 months to 7 years. Exclusion criteria: severe physical illness, drug/alcohol dependent, pregnant/lactating women. |
|
| Interventions |
|
|
| Outcomes | Mental state: PANSS, BPRS. Adverse events. Unable to use: GQOLI‐74. Only subscale scores were reported. The subscales were not validated. |
|
| Notes | *Decreased rate of PANSS score < 25%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation, no further detail. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | Clinical response was measured, but not reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Chen 2008.
| Methods | Allocation: randomised, no further detail. Blindness: no further detail. Duration: 3 days wash out period, plus 8 weeks trial. Design: parallel. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 60. Age: 18 to 50 years. Sex: male = 33, female = 27. Length of illness: mean ˜ 21.25 months, SD ˜ 13.90 months; Inclusion criteria: PANSS score ≥ 60. Exclusion criteria: severe physical diseases or other mental diseases, pregnant or breastfeeding women, allergic to medication. |
|
| Interventions |
During 8 weeks treatment period, benzodiazepine or anticholinergics agents were used when necessary. |
|
| Outcomes | Clinical response: no clinical improvement* Mental health: PANSS scale score; PANSS negative. |
|
| Notes | *Decreased rate of PANSS score: < 25% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the trial. |
| Selective reporting (reporting bias) | High risk | TESS were not fully reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Cheng 2003.
| Methods | Allocation: Randomised, no further detail. Blindness: not reported. Duration: 8 weeks. Design: parallel. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R).
N = 89. Age: average, 33.1 ± 10.3 years; chlorpromazine group:34.8 ± 10.5 years. Sex: quetiapine group: male 20, female 24; chlorpromazine group: male 22, female 23. Length of illness: quetiapine group: 6.4 ± 5.8 years; chlorpromazine group: 6.4 ± 5.7 years. Inclusion criteria: PANSS score ≥ 60. Exclusion criteria: physical impairment. |
|
| Interventions | Chlorpromazine: titrated over a 1 week period from 50 mg/d to 200 mg/d, average dosage in the end of the 4th week post treatment was 400 ± 150 mg/d and 250 ± 100 mg/d at the end of 8th week. N = 45. Quetiapine: titrated over a 1 week period from 50 mg/d to 200 mg/d, average dosage in the end of the 4th week post treatment was 450 ± 250 mg/d and 260 ± 110 mg/d at the end of 8th week. N = 44. |
|
| Outcomes | Global state: CGI*, need of additional non‐antipsychotic drugs. Mental state: PANSS* Adverse effect: TESS. Leaving the study early. Uable to use: Clinical response, the total number of patients in each group doesn't match the number reported at baseline. We contacted the trial author, but did not receive any response. |
|
| Notes | *Numbers of participants in these two studies were not clearly stated in the paper. We assumed that each arm had the same number of participants as when randomised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Three cases left study early in the quetiapine group because of advanced condition; 2 cases left the study early in the chlorpromazine group because of adverse effects. Unclear if these were included in the final analysis |
| Selective reporting (reporting bias) | Low risk | All the outcomes were well reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Deng 2004.
| Methods | Allocation: Randomised, no further detail. Blindness: double blind, the medication was put into capsules. Duration: 3 to 7 days of wash out period, plus 12 weeks trial. Design: parallel. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2)
N = 90. Age: Quetiapine group, range: 18 to 70 years, average: 37.6 ± 15.5 years; chlorpromazine group, range: 20 to 65 years, average: 34.2 ± 10.6 years. Sex: quetiapine group: male 20, female 25; chlorpromazine group: male 26, female 19. Length of illness: quetiapine group, 3.3 ± 3.6 years; chlorpromazine group, 3.5 ± 4.2 years. Inclusion criteria:
Exclusion criteria: severe physical diseases, extremely agitated or restless. |
|
| Interventions | Chlorpromazine: dosage started from 50 mg/d and increased to 100 mg/d on the 3rd day of treatment, then 150 mg/d on the 5th day. Maintenance dosage: 200 to 800 mg/d. N = 45. Quetiapine: dosage started from 50 mg/d increased to 100 mg/d on the third day of treatment, then 150 mg/d on the fifth day. Maintenance dosage 200 to 800 mg/d. N = 45. Benzodiazepine or benzhexol were used when necessary. Without combination with other antipsychotic drugs. |
|
| Outcomes | Clinical response: no clinical improvement*. Effects on physiology: blood test result, liver function. |
|
| Notes | *Decreased rate of BPRS score: < 30%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included patients completed the trial. |
| Selective reporting (reporting bias) | High risk | Clinical response, BPRS, SANS, SAPS and TESS were not reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Guo 2003a.
| Methods | Allocation: randomised (by tossing a coin). Blinding: not reported. Duration: 8 weeks. Setting: outpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). N = 148. Sex: male (N = 74) and female (N = 74). Age: 18 to 55 years. Length of illness: range, 3 months to 1 year, mean 0.1 year, SD 0.5 year. Inclusion: PANSS ≥ 60, length of illness < 1 year, not receiving long‐acting antipsychotic drugs. Exclusion: severe physical illness, drug/alcohol dependent. |
|
| Interventions |
|
|
| Outcomes | Clinical response: no clinical improvement*, decreased rate of PANSS score. Mental state: PANSS (total, positive and negative score). Adverse events. Effects on physiology: laboratory findings. liver function. |
|
| Notes | *Decreased rate of PANSS score < 25%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by tossing a coin. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | Low risk | All the outcomes were well reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Guo 2003b.
| Methods | Allocation: randomised, no further detail. Blinding: single blind, assessor blind. Duration: 3 months. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 71. Sex*: male 28 and female 31. Age: 17 to 58 years, mean ˜ 26.22 years, SD ˜ 6.73 years. Length of illness: mean 5.72 months, SD 3.97 months. Inclusion: PANSS > 60, length of illness < 2 years. Exclusion: severe physical illness, pregnant or breast feeding women. |
|
| Interventions |
Antan was used when EPS appears, no other antipsychotic drugs were used during treatment. |
|
| Outcomes | Mental state: PANSS (total, positive and negative score). Leave the study early. Adverse events. Effects on physiology: laboratory findings. liver function. Unable to use: WHO‐QOL‐100, no scale scores reported and the subscales were not validated. |
|
| Notes | *The total number of male and female participants does not match to the total number randomised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further detail. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Five patients left the study early in the quetiapine group, 6 patients left the study early in the chlorpromazine group, without any reason reported. No ITT analysis was applied. |
| Selective reporting (reporting bias) | Low risk | All the outcomes were well reported |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Guo 2005.
| Methods | Allocation: randomised, no further detail reported. Blindness: not stated. Duration: 3 days wash out period, plus 8 weeks trial. Design: parallel. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 80.
Age: 16 to 57 years, mean ˜ 28.3 years, SD ˜ 10.2 years.
Sex: male = 42, female = 38.
Length of illness: 2 months to 10 years; mean ˜ 6.5 years, SD ˜ 5.9 years. Inclusion criteria: PANSS ≥ 60. Exclusion criteria: severe physical impairment or other mental disorders, pregnant or breastfeeding women, allergic to medication. |
|
| Interventions |
Antan was used for EPS; alprazolam was used for insomnia or anxiety; β‐blokers was used for tachycardia. Liver function, ECG and blood test were measured at baseline, 2 , 4 and 8 weeks post‐treatment. |
|
| Outcomes | Clinical response: no clinical improvement*. Globle state: need for non‐antipsychotic drugs. Mental state: PANSS scale score, positive score, negative score, general pathological score. Adverse effects: TESS. |
|
| Notes | *Decreased rate of PANSS score < 20%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All the patients completed the trial. |
| Selective reporting (reporting bias) | Low risk | All measured outcomes were reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Guo 2006.
| Methods | Allocation: randomised, no further details. Blindness: not stated. Duration: 8 weeks. Design: parallel. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 130.
Age: mean ˜ 33 years, SD ˜ 11 years.
Sex: male = 57, female = 73.
Length of illness: not stated. Inclusion criteria: FBG < 6.7 mmol/L, without use of antipsychotic, antidepressant or any other medication having an influence on the metabolites of blood glucose or blood lipid. Exclusion criteria: disturbance of carbohydrate or lipid metabolics, severe physical impairments. |
|
| Interventions |
Without combination of antipsychotic, antidepressant, or any other medication having a influence on the metabolites of blood glucose or blood lipid. |
|
| Outcomes | Adverse effects: weight gain/loss. Effects on physiology: blood glucose, cholesterol. |
|
| Notes | This trial was funded by the National Science and Technology Research Fund. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients completed this trial. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Guo 2007.
| Methods | Allocation: randomised, no further detail. Blinding: not reported. Duration: 8 weeks. Setting: inpatients and outpatients, Kangning hospital, Guangzhou city, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 52.
Age: 18 to 40 years, mean ˜ 26.8 years, SD ˜ 4.2 years.
Sex: male = 23, female = 29.
Length of illness: 0.5 to 2 years, mean ˜ 1.1 years, SD ˜ 0.6 years. Inclusion: PANSS ≥ 60, CGI‐SI ≥ 4. Exclusion: organic brain diseases, severe physical illness, drug/alcohol dependent, pregnant/lactating women. |
|
| Interventions |
Antan or propranolol were used when necessary. |
|
| Outcomes | Global state: CGI‐S, CGI‐I. Mental state: PANSS. Function: global function WCST. Adverse events. Unable to use: WCST subcale score, as it is not validated. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | TESS was measured, but not reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Guo 2008.
| Methods | Allocation: randomised, using random number table. Blindness: double‐blind, patients and assessor were blinded. Duration: 16 weeks. Design: parallel. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 86.
Age: mean ˜ 30 years, SD ˜ 10 years.
Sex: male = 39, female = 44.
Length of illness: less than 1 year: 57 cases; more than 1 year: 26 cases. Inclusion criteria: PANSS ≥ 60. Exclusion criteria: organic diseases or severe cardiac, liver, kidney diseases, patients receiving 2 or more antipsychotic drugs, patients taking antipsychotic drugs 1 week before randomisation. |
|
| Interventions |
|
|
| Outcomes | Clinical response: no clinical improvement* Mental state: PANSS. Quality of life: WHO‐QOL‐100 endpoint scale score. Adverse effect: TESS. Effects of physiology: laboratory findings,blood count, cholesterol, glucose, liver function. Leave the study early. Unable to use: WHO‐QOL‐100 subscale score. The subscales were not validated. |
|
| Notes | *Decreased rate of PANSS score < 50%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by using random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 3 patients in the quetiapine group left the study early: 1 case left due lack of efficacy, 2 cases due to adverse effects. ITT was employed in the final result analysis. |
| Selective reporting (reporting bias) | Low risk | All the outcomes were reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ He 2003.
| Methods | Allocation: randomised, no further details. Blinding: open label. Duration: six weeks. Setting: Jiangsu City, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R).
N = 40. Sex: male 24, female 16. Age: 18 to 50 years, mean ˜ 32.4 years, SD ˜ 8.1 years. Length of illness: one month to 10 years, mean 4.3 years, SD 3.4 years. Inclusion criteria: PANSS ≥ 60. Exclusion criteria: severe physical illness, alcohol/drug abuse, pregnant/lactating women. |
|
| Interventions |
|
|
| Outcomes | Clinical improvement: no clinical improvement*. Mental state: PANSS endpoint scale score, positive, negative, general pathology score. Adverse effects. |
|
| Notes | *Decreased rate of PANSS score < 40%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | Low risk | All the outcomes were well reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Hu 2003.
| Methods | Allocation: randomised, no further detail. Blinding: not reported. Duration: 4 weeks. Setting: inpatients, Mental health centre, Sichuan, China. | |
| Participants | Diagnosis: schizophrenia (DSM‐IV), first onset, inpatients. N = 41. Sex: male Age: mean ˜ 26.94 years, SD ˜ 8.82 years. Length of illness: not reported. Exclusion: severe physical illness, lactating/pregnant women. | |
| Interventions |
|
|
| Outcomes | Mental state: PANSS (positive, negative, general psychopathology subscale). Adverse effects: TESS, RSESE. Unable to use: GAF score ‐ some data missing. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | Low risk | Evidence of selective reporting was no found. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Ji 2004.
| Methods | Allocation: randomised, no further detail. Blinding: not reported. Duration: 3 months. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 66.
Age: 18 to 45 years, mean ˜ 25.6 years, SD ˜ 7 years.
Sex: male = 33 and female = 26.
Length of illness: mean ˜ 1.6 years, SD ˜ 0.5 years. Inclusion: PANSS ≥ 60. Exclusion: patients with severe physical illnesses, drug or alcohol dependency, pregnant or lactating women. |
|
| Interventions |
|
|
| Outcomes | Global state: poor compliance, leave the study early. Mental state: PANSS total, negative, positive, general psychopathology score. Quality of life: GQOLI ‐ 74 endpoint scale score. Adverse effects. Uable to use: Quality of life:GQOLI ‐ 74 subscale score. These subscales were not validated. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Four patients in the chlorpromazine group left the study early, 3 patients in the quetiapine group left the study early, all due to adverse effects. Dropouts were excluded from analysis. |
| Selective reporting (reporting bias) | Low risk | Evidence of selective reporting was not found. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Jiang 2006.
| Methods | Allocation: randomised, no further details. Blinding: not reported. Duration: 8 weeks. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 191.
Age: mean ˜ 34 years, SD ˜ 9.7 years.
Sex: not reported.
Length of illness: duration ill mean 58 months, SD 68 months. Inclusion: PANSS ≥ 60, or a score of at least 3 on two or more of the PANSS items 'delusion', 'hallucination', 'Incoherence', 'suspiciousness', 'persecution'. Exclusion: severe physical illness, drug/alcohol dependent, received experimental drugs within 4 weeks prior to the trial. |
|
| Interventions |
|
|
| Outcomes | Clinical response: no clinical improvement*. Adverse events: TESS. Effects on physiology: laboratory findings. Leave the study early. Unable to use: PANSS score, this data is not reported. |
|
| Notes | *No clinical improvement: decreased rate of PANSS score < 30%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Twenty‐one cases in the quetiapine group left the study early, 5 cases because of agitation, 10 because of abnormal liver function, 1 because of orthostatic hypotension, 5 cases lost to follow‐up. Twenty cases in the chlorpromazine group left the study early: 17 cases because of adverse effects and 3 were lost to follow‐up. The drop‐out cases were also reported. |
| Selective reporting (reporting bias) | High risk | PANSS measured, but not reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Jiang 2008.
| Methods | Allocation: randomised, no further detail. Blinding: not stated. Duration: 6 weeks. Setting: inpatient, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3).
Sex: male = 36, female = 24.
Age: 18 to 60 years, mean ˜ 33.54 years, SD ˜ 5.26 years
N = 60.
Length of illness: mean ˜ 8.24 years, SD ˜ 3.15 years. Inclusion criteria: PANSS ≥ 60, patients who weren't receiving any antipsychotic drugs before randomisation, or with a suspension of antipsychotic drugs at least 1 week before randomisation. Exclusion criteria: severe physical illness, organic brain disease, drug abuse, pregnant/lactating women, allergic to the medication. |
|
| Interventions |
Antan or benzodiazepine were used when necessary, no other antipsychotic medication were used during treatment course. |
|
| Outcomes | Clinical improvement: no clinical improvement*. Mental state: PANSS and BPRS endpoint total score. Measured at baseline, 2, 4 and 6 weeks post treatment. Adverse events: TESS. |
|
| Notes | *Decreased rate of PANSS score < 25%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included patients completed the trial. |
| Selective reporting (reporting bias) | High risk | TESS were not fully reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Jin 2007.
| Methods | Allocation: randomised, no further details. Blinding: not reported. Duration: 4 weeks. Setting: outpatients and inpatients, Hebei province, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). N = 60. Sex: male = 33 and female = 27. Age: 18 to 60 years. Length of illness: 1 to 24 months. Exclusion: severe physical illness, drug abuse, pregnant/lactating women, allergic to either of the intervention drugs. | |
| Interventions |
|
|
| Outcomes | Clinical response: no clinical improvement*, decreased rate of PANSS score. Adverse effects. |
|
| Notes | *Decreased rate of PANSS score < 25%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | TESS measured, but not reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Kong 2003.
| Methods | Allocation: randomised, no further details. Blinding: not reported. Duration: 6 weeks. Setting: outpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 30.
Sex: male 8, female 22.
Age: mean 26 to 38 years.
Length of illness: 0.5 to 5 years. Inclusion: patients did not take antipsychotic drugs 1 week before randomisation, without cardiac, liver, kidney or endocrine diseases. Exclusion: lactating/pregnant women. |
|
| Interventions |
|
|
| Outcomes | Effects on physiology: laboratory findings. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included patients completed the trial. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Li 2003.
| Methods | Allocation: randomised, no further details. Blinding: not reported. Duration: 6 months. Setting: inpatients and outpatients, mental health centre, Shandong, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R).
N = 103.
Sex: male 36, female 31.
Age: 18 to 56 years, mean ˜ 31.3 years, SD ˜ 9.5 years.
Length of illness: mean ˜ 1.3 years, SD ˜ 2.0 years. Inclusion: schizophrenia, CCMD‐3; PANSS ≥ 60. Exclusion: severe physical illness, drug/alcohol dependent, pregnant/lactating women. |
|
| Interventions |
|
|
| Outcomes | Mental state: PANSS scores. Adverse effects: TESS scores. Unable to use: Quality of life: WHO‐QOL‐100 subscale scores. The subscales were not validated. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Twelve cases left the study early in quetiapine group; 14 patients left the study early in chlorpromazine group. The reasons for dropout were either lack of efficacy or adverse effects. ITT was not used. |
| Selective reporting (reporting bias) | Low risk | We did not find any evidence of selective reporting. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Li 2010.
| Methods | Allocation: randomised, no further detail. Blinding: not reported. Duration: 6 weeks. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 64.
Sex: male = 37 and female = 27.
Age: mean ˜ 27.9 years, SD ˜ 7.22 years.
Length of illness: mean 32 months, SD 22 months. Inclusion: schizophrenia, CCMD‐3. Exclusion: not reported. |
|
| Interventions |
|
|
| Outcomes | Leave the study early. Mental state: HAMD scores. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One case in the chlorpromazine group left the study early. Reason not stated, neither was it included in the final analysis. |
| Selective reporting (reporting bias) | Low risk | All the outcomes were well reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Liu 2003.
| Methods | Allocation: randomised, no further detail. Blinding: not reported. Duration: 6 weeks. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R).
N = 83.
Sex: male = 45 and female = 38.
Age: mean ˜ 64.3 years, SD ˜ 2.8 years.
Length of illness: mean ˜ 3.98 months, SD ˜ 2.57 months. Inclusion: primary schizophrenia, CCMD‐2‐R; age ≥ 60 years, PANSS ≥ 60. Exclusion: not reported. |
|
| Interventions |
|
|
| Outcomes | Clinical response: no clinical improvement*. Mental state: PANSS scores. Adverse effects: TESS scores. Effects on physiology: laboratory findings, blood test and liver function. |
|
| Notes | *Decreased rate of PANSS score < 40%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included patients completed the trial. |
| Selective reporting (reporting bias) | Unclear risk | TESS were not well reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Mei 2007.
| Methods | Allocation: randomised, no further details. Blinding: not reported. Duration: 3 days wash out period, plus 8 weeks trial. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 96.
Sex: male = 43, female = 53.
Age: 17 to 60 years, mean ˜ 29.4 years, SD ˜ 11.5 years.
Length of illness: 3 months to 12 years, median = 6.1 years. Inclusion: PANSS ≥ 60, patients did not take antipsychotic drugs within 1 week before admission. patients did not accept long‐acting antipsychotic drugs within one month before admission. Exclusion: severe physical illness, organic brain diseases, drug/alcohol dependent, pregnant or breastfeeding women. |
|
| Interventions |
No other antipsychotic drugs were used during treatment, but some medications was used for EPS. |
|
| Outcomes | Clinical response: no clinical improvement*. Mental state: PANSS. Adverse events. Effects of physiology: Laboratory findings.(blood glucose, serum prolactin). |
|
| Notes | *PANSS decreased rate < 25%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Nai 2007.
| Methods | Allocation: randomised, no further details. Blinding: not reported. Duration: 3 days wash out period, plus 8 weeks trial. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 120.
Sex: male = 63, female = 57.
Age: 18 to 45 years, mean ˜ 28 years, SD ˜ 7.9 years.
Length of illness: 1 month to 24 months. Inclusion: primary schizophrenia, without taking any antipsychotic drugs, PANSS ≥ 60, did not take antipsychotic drugs within 1 week before admission, did not accept long‐acting antipsychotic drugs within one month before admission. Exclusion: severe physical illness, pregnant or breastfeeding women. |
|
| Interventions |
No other antipsychotic drugs will be used during treatment course, but Antan or benzodiazepine were used when necessary. |
|
| Outcomes | Function: global function, WCST‐IQ, WCST‐MQ scores. Unable to use: WCST subscale scores were not validated. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included patients completed the trial. |
| Selective reporting (reporting bias) | High risk | PANSS were not well reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ NCT00882518.
| Methods | Allocation: randomised, no further detail reported. Blindness: double‐blind, patients, caregiver and investigator were blinded. Duration: 6 weeks. Design: parallel. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia.
N = 388.
Age: mean ˜ 32.5 years, SD ˜ 10.52 years.
Sex: male = 202, female = 182.
Length of illness: not reported. Inclusion criteria: patients gave written informed consent. Exclusion criteria: AIDs or hepatitis, History of epilepsy, hospitalised for schizophrenic disorder 1 month prior to entering into study. |
|
| Interventions |
|
|
| Outcomes | Clinical response: no clinical improvement*. Global state: CGI, CGI severity of illness score, poor compliance, leave the study early**. Mental state: PANSS scale score, positive score, negative score, general pathological score. PANSS aggression score, depression clusters score. Adverse effects. Unable to use: PANSS aggression score, depression clusters score. The subscale is not validated. |
|
| Notes | *Decreased rate of PANSS score < 30%. **Patients who left the study early were categorised by the following reasons: "withdrawal by subject" , "severe non ‐ compliance to protocol" , "incorrect enrolment" , "central lab closure for national day" as "any reason" in our meta analysis. We added the number of these patients and imputed the total number into our meta analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details were reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Thirty‐eight patients in the quetiapine group and 44 patients in the chlorpromazine group left the study early and no ITT analysis were applied. Among them, 9 patients in the quetiapine group and 18 patients in the chlorpromazine group left because of adverse effects. Twelve patients in the quetiapine group and 9 patients in the chlorpromazine group left due to lack of efficacy. |
| Selective reporting (reporting bias) | Low risk | All the outcomes were well reported. |
| Other bias | High risk | The study was sponsored by AstraZenenca‐‐the producer of quetiapine fumarate extended‐release (SEROQUEL‐XR). |
vs QTP ‐ Peng 2006.
| Methods | Allocation: randomised using random number table. Blinding: double‐blind, patients, investigator and assessor were blinded. Duration: 42 days. Setting: inpatients, West China Hospital, Sichuan, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). N = 40. Age: mean ˜ 30.6 years, SD ˜ 11.26 years. Sex: male and female Length of illness: mean ˜ 28.5 years, SD ˜ 6.45years Exclusion: not stated. | |
| Interventions |
|
|
| Outcomes | Globle state: poor compliance, leaving the study early. Unable to use: PANSS, BPRS, TESS, RSESE scores ‐ no SD reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double blind, but untested. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blind. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether people dropped out are included in the analysis. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Peuskens 1997.
| Methods | Allocation: randomised, no further detail. Blinding: double blind, investigator‐blinded, no further details. Duration: six weeks. Design: parallel, multicentre. Setting: Belgium, UK, Spain, France, South Africa. | |
| Participants | Diagnosis: schizophrenia, schizophreniform disorder (DSM‐III‐R).
N = 201.
Sex: male = 129, female = 72.
Age: 18 to 65 years.
Length of illness: BPRS ≧ 27, at least 3 on 2 or more of the BPRS positive symptom items 'conceptual disorganisation', 'suspiciousness', hallucinatory behaviour' and 'unusual thought content'; CGI‐S ≧ 4. Inclusion:
Exclusion:
|
|
| Interventions |
|
|
| Outcomes | Clinical response: no clinical improvement*.
Global state: Need of additional antipsychotic drugs. Leave the study early. Adverse effects: extrapyramidal side effects (AIMS, BAS, SAS), haematology, liver function test, thyroid function tests, ECGs, need for medication to reduce EPS. Uable to use: CGI (no SD), PANSS (no raw data), BPRS (no SD). |
|
| Notes | *No clinical improvement: decreased rate of BPRS score ≤ 50%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for leaving study early were available. ITT method was not used but overall discontinuation rate was low (2.5%). |
| Selective reporting (reporting bias) | Low risk | Evidence of selective reporting was not found. |
| Other bias | High risk | Sponsored by manufacturer of quetiapine AstraZenenca. |
vs QTP ‐ Sun 2006.
| Methods | Allocation: randomised, no further details. Blinding: not reported. Duration: 8 weeks. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 71.
Sex: male = 46 and female = 25.
Age: mean ˜ 26.3 years, SD ˜ 5.9 years.
Length of illness: mean ˜ 1.2 years, SD ˜ 0.6 years. Inclusion: first episode schizophrenia and no history of taking antipsychotic drugs, PANSS ≥ 60. Exclusion: severe physical disease, pregnant or breastfeeding women, allergic to the related medication. |
|
| Interventions |
No combination with other antipsychotic drugs.Benzodiazepine, anticholinergic medication or propranolol was used for adverse effects. |
|
| Outcomes | Mental state: PANSS total score, positive score, negative score, general pathological score. Function: Global function‐WCST endpoint total score, WMS‐RC endpoint total score. Unable to use: WCST, WMS‐RS subscale score. The subscale is not validated. NCT total and subscale score. The NCT scale is not validated. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included patients completed this trial. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Tian 2006.
| Methods | Allocation: randomised, no further details reported. Blinding: not reported. Duration: 8 weeks. Setting: inpatients and outpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3).
Paticipant: N = 92.
Sex: male = 35 and female = 57.
Age: 18 to 61 years, mean ˜ 27.8 years, SD ˜ 8.4 years.
Length of illness: mean ˜ 6.2 years, SD ˜ 4.2 years. Inclusion: schizophrenia. Exclusion: severe physical illness, drug/alcohol dependent, pregnant/lactating women, aggressive and suicidal patients. |
|
| Interventions |
|
|
| Outcomes | Clinical response: no clinical improvement*. Mental state: PANSS score,positive score, negative score and general pathological score. Adverse effects. |
|
| Notes | *Decreased rate of PANSS score < 25%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | Low risk | We did not find any evidence of selective reporting. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Wan 2002.
| Methods | Allocation: randomised, no further information. Blinding: double‐blind. Duration: 6 weeks. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R). N = 60. Sex: not reported. Age: 18 to 60 years. Length of illness: not reported. Inclusion: first episode schizophrenia, PANSS ≥ 60. Exclusion: organic diseases, severe physical illness, alcohol or drug abuse, pregnant or breastfeeding women. |
|
| Interventions |
No other antipsychotic drugs were used during treatment course, but Antan or benzodiazepine were used when necessary. |
|
| Outcomes | Clinical response: no clinical improvement*. Mental state: PANSS scores (total, positive, negative and general pathological). Adverse effect: TESS. |
|
| Notes | *No clinical improvement: decreased rate of PANSS score ≤ 29% or symptoms deterioration. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included patients completed the trial. |
| Selective reporting (reporting bias) | High risk | SAS were not reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Wan 2008.
| Methods | Allocation: randomised, no further information. Blinding: not reported. Duration: 6 weeks. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). N = 60. Sex: male = 32, female = 28. Age: 18 to 60 years, mean ˜ 33.54 years, SD ˜ 5.26 years. Length of illness: mean ˜ 8.24 years, SD ˜ 3.15 years. Inclusion: PANSS ≥ 60, have no history of taking antipsychotic drugs or suspended antipsychotic drugs for 1 week before randomisation. Exclusion: organic brain diseases, severe physical illness, alcohol or drug abuse, pregnant or breastfeeding women. |
|
| Interventions |
No other antipsychotic drugs were used during treatment course, but Antan or benzodiazepine were used when necessary. |
|
| Outcomes | Clinical response: no clinical improvement*. Mental state: PANSS total score,BPRS total score. Adverse effect: TESS |
|
| Notes | *Decreased rate of PANSS score ≤ 29% or symptoms deterioration. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All the patients completed the trial. |
| Selective reporting (reporting bias) | Low risk | All the outcomes were well reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Wang 2004.
| Methods | Allocation: randomised, no further details provided. Blinding: no details. Duration: 6 weeks. Setting: inpatients, Guangzhou Mental Health Hospital, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). N = 96. Sex: male and female. Age: 19 to 58 years. Length of illness: 2 months to 14 years. Exclusion criteria: pregnant/lactating women, drug/alcohol dependent patients, severe physical illness, took quetiapine or chlorpromazine within 4 weeks prior to the study, suicidal, allergic to any of the intervention drugs, participated in other clinical trials within 30 days prior to the current study. | |
| Interventions |
|
|
| Outcomes | Leaving the study early. Mental state: PANSS (total, positive, negative,general psychopathology subscale). Global state: CGI endpoint total improvement score, illness severity score, no clinical improvement*. Adverse effects. |
|
| Notes | *PANSS decreased rate < 30%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT was used. |
| Selective reporting (reporting bias) | Low risk | We did not find any evidence of selective reporting. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Wang 2005.
| Methods | Allocation: randomised, randomised by the random number. Blinding: open label. Duration: 3 days washout period, plus 8 weeks trial. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 112.
Age: mean 33.3 years, SD 11.7 years.
Sex: not stated.
Length of illness: mean ˜ 65.1 days, SD ˜ 8.4 days. Inclusion: PANSS score ≥ 60, score of at least 4 on 2 or more of the PANSS items 'delusion', 'hallucination behavior' , 'Incoherence' , 'suspiciousness', 'persecution'. Exclusion: drug/alcohol dependent patients, severe physical illness, accept quetiapine or chlorpromazine within 4 weeks prior to the study and have no response to both of them. |
|
| Interventions |
|
|
| Outcomes | Clinical response:no clinical improvement*. Mental state: PANSS scores (total, positive, negative and general pathological). Adverse effect:TESS, weight gain/loss. Effects on physiology: laboratory findings, blood glucose and PRL. Unable to use: Leaving the study early: 7 cases left the study early because of poor compliance, use other medication, withdrew informed consent. We were unable to use this data, as it is not reported by groups and we were unable to separate them. |
|
| Notes | *decreased rate of PANSS score < 25%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation, randomised by the random number. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Seven patients left the study early because of poor compliance, or lost to follow‐up. They were not included in the final analysis. Twenty‐three patients left the study but were included in the adverse effects analysis: 12 cases from the chlorpromazine group and 11 cases from the quetiapine group. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Yang 2007.
| Methods | Allocation: randomised, no further detail. Blindness: not reported. Duration: 8 weeks. Design: parallel. Setting: outpatients and inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 63. Age: mean ˜ 33.1 years, SD ˜ 9.69 years Sex: male = 35, female = 28. Length of illness: mean ˜ 4.90 months, SD ˜ 3.60 months. Inclusion criteria: BPRS score > 35, did not take any antipsychotic drugs within 2 weeks before randomisation. Exclusion criteria: severe physical impairment or organic brain diseases, alcohol or drug abuse, pregnant or breastfeeding women. |
|
| Interventions |
|
|
| Outcomes | Clinical response: no clinical improvement*. Leave the study early. Mental state: BPRS Adverse effect: TESS |
|
| Notes | *Decreased rate of BPRS score < 25%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | One patient in the quetiapine group and 2 patients in the chlorpromazine group left the study early. No reason given and no ITT analysis was applied. |
| Selective reporting (reporting bias) | Low risk | All the patients completed the trial. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Zhang 2002.
| Methods | Allocation: randomised (by tossing a coin). Blinding: double‐blind, investigator was blinded. Duration: 8 weeks. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R). N = 117. Sex: male 89 and female 28. Age: average, mean 23.89 years, SD 5.63 years. Length of illness: mean 3.87 years, SD 1.03 years. Exclusion criteria: length of illness > 5 years, with severe physical or neurological illness, pregnant or lactating women. |
|
| Interventions |
|
|
| Outcomes | Clinical response: no clinical improvement*. Mental state: PANSS positive, negative, general psychopathology and endpoint score. Adverse event: TESS endpoint score. |
|
| Notes | *Decreased rate of PANSS score < 25%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by tossing a coin. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included patients completed the trial. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Zhang 2003.
| Methods | Allocation: randomised using computer generated random number. Blinding: double‐blinded, no further details provided. Duration: 1 week wash out period, plus 6 weeks of intervention, followed by 1 week of reduced dose of medication to help patients to withdraw from the interventions. Setting: inpatients, multicentre, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R).
N = 237.
Sex: male = 121 and female = 99.
Age: mean ˜ 32.27 years, SD ˜ 11.33 years.
Length of illness: mean ˜ 7.0 years, SD ˜ 7.3 years. Inclusion: BPRS score ≥ 36. Exclusion:
|
|
| Interventions |
|
|
| Outcomes | Clinical response:no clinical improvement*, decreased rate of PANSS score. Globel state: poor compliance, leave the study early. Mental state, PANSS endpoint score. Adverse effect: weight gain/loss, ECG, Tachycaridia. Effects on physiology: pulse, blood pressure, laboratory findings. |
|
| Notes | *Decreased rate of PANSS score < 30%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using computer‐generated random number. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Twenty‐eight patients left the study early with reasons reported in the study. ITT was used. |
| Selective reporting (reporting bias) | High risk | CGI and adverse events were measured, but data were not reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Zhang 2006.
| Methods | Allocation: randomised, no further details reported. Blindness: single blinded, assessor was blinded. Duration: 2 weeks wash out period, plus 8 weeks trial. Design: parallel. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 61.
Age: 18 to 59 years.
Sex: not reported.
Length of illness: < 2 years. Inclusion criteria: length of illness < 2 years, PANSS ≥ 60. Exclusion criteria: patients with severe physical impairment or abnormal laboratory findings, pregnant or breastfeeding women, allergic to quetiapine or chlorpromazine, patients with long‐acting antipsychotic drugs treatment at present. |
|
| Interventions |
|
|
| Outcomes | Clinical response: no clinical improvement*, decreased rate of PANSS score. Mental state: PANSS scale score, positive score, negative score, general pathological score. Adverse effects: TESS. |
|
| Notes | *Decreased rate of PANSS score < 30%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details were reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included patients completed the trial. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Zhang 2008.
| Methods | Allocation: randomised, no further details were reported. Blinding: not reported. Duration: 8 weeks. Setting: inpatients, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3).
Paticipan: N = 60.
Sex: male 45 and female 15.
Age: mean 35.6 years, SD 9.7 years.
Length of illness:4 to 17 years. Inclusion: PANSS score ≥ 60. Exclusion: patients with severe physical illnesses, pregnant or lactating women, alcohol or drug dependent patients, patients with allergy to quetiapine and chlorpromazine. |
|
| Interventions |
|
|
| Outcomes | Clinical response:no clinical improvement*.
Mental state, PANSS endpoint total score. Adverse effects: TESS. |
|
| Notes | *Decreased rate of PANSS score < 25%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details were reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included patients completed the trial. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Zhou 2003.
| Methods | Allocation: randomised, using a random number table. Blinding: assessors were blinded, unclear if the patients were. Duration: 6 weeks. Setting: inpatients, Mental health centre, Sichuan, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3), inpatients. N = 40. Sex: male and female. Age: mean ˜ 28, SD ˜ 10 years. Length of illness: mean ˜ 25 years, SD ˜ 6 years. Exclusion: not described. | |
| Interventions |
|
|
| Outcomes | Leave the study early. Unable to use: WCST subscale scores, as they are not validated. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using a random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One case of early discharge due to family emergency, 3 other patients withdrew for their family members were unsatisfied with the efficacy. The above drop out reasons are unlikely to have an impact on the outcome reported in this study. |
| Selective reporting (reporting bias) | Low risk | Evidence of selective reporting was not found. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Zhou 2004.
| Methods | Allocation: randomised, no further details provided. Blinding: not reported. Duration: 12 weeks. Setting: inpatients, Jinan psychiatric hospital, Shandong province, China. | |
| Participants | Diagnosis: schizophrenia (ICD‐10, CCMD‐3).
N = 83.
Age: 17 to 51 years old.
Sex: male and female.
Length of illness: 4 months to 21 years.
Inclusion: without receiving antipsychotic drugs, PANSS ≥ 60; patients without severe physical diseases and organic brain diseases, learning disability, allergic to quetiapine or chlorpormazine, abnormal laboratory findings; Exclusion: pregnant or breast feeding women. |
|
| Interventions |
|
|
| Outcomes | Clinical response: no clinical improvement* Leave the study early. Mental state: PANSS positive, negative, general psychopathology, total score. Adverse events: TESS score. Unable to use GQOLI subscale score. These subscales were not validated. |
|
| Notes | *PANSS decreased rate < 25%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four patients left the study early because of discharge or financial problems. They were not included in the efficacy and adverse effect analysis, but this should have minimal influence in the accuracy of results. |
| Selective reporting (reporting bias) | Low risk | Evidence of selective reporting was unclear. |
| Other bias | Low risk | None obvious. |
vs QTP ‐ Zou 2006.
| Methods | Allocation: randomised, no further details provided. Blinding: not reported. Duration: 8 weeks. Setting: Community and hospital, Zhejiang, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐3). N = 86. Age: 17 to 56 years. Sex: male and female. Length of illness: < 10 years. Exclusion: severe physical illness, drug/alcohol dependent, pregnant/lactating women, allergic to experimental drugs. | |
| Interventions |
|
|
| Outcomes | Clinical response: no clinical improvement*. Mental state: PANSS scores. Adverse events. |
|
| Notes | *Decreased rate of PANSS score < 25%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | TESS scores measured, but not reported. |
| Other bias | Low risk | None obvious. |
vs RPD ‐ Chang 1998.
| Methods | Allocation: randomised, no further information.
Blinding: not stated.
Duration: 21 weeks. Design: parallel. Setting: China. |
|
| Participants | Diagnosis: schizophrenia (CCMD). N = 58. Age: mean ˜ 35 years, SD ˜ 7 years. Sex: male and female. Length of illness: mean ˜ 12 years, SD ˜ 7 years. Inclusion criteria: without organic diseases. |
|
| Interventions |
|
|
| Outcomes | Clinical response: no significant clinical response. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but no detail provided. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Unclear what the study intended to measure, as we were unable to obtain its protocol. |
| Other bias | Low risk | None obvious. |
vs RPD ‐ Cui 2001.
| Methods | Allocation: randomised, no further information.
Blindness: not reported.
Duration: 1 week wash out period, plus 6 weeks trial.
Design: parallel group. Setting: inpatients, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R).
N = 60.
Age: 18 to 57 years.
Sex: male and female. Length of illness: in risperidone group mean ˜ 69 months, SD ˜ 82 months; in chlorpromazine group mean ˜ 59 months, SD ˜ 77 months. Inclusion criteria: BPRS > 35, without antipsychotic medication one week prior to trial, blood test normal, ECG and EEG normal. Exclusion criteria: nervous system and endocrine system disease, heart, liver, renal disease, pregnant and lactating women. |
|
| Interventions |
|
|
| Outcomes | Clinical response: no significant clinical response*. Mental state: BPRS. Adverse effects. |
|
| Notes | *BPRS score decreased rate <30% is regarded as no significant clinical response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further detail. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind (clinician and patients are blinded). Quote "risperidone and chlorpromazine were placed into capsules of equal size and same colour, dispensed by pharmacist. Neither patients or clinician are aware of the content of the capsule." p206. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | it appears that all measured outcomes were reported. |
| Other bias | Low risk | None obvious. |
vs RPD ‐ Feng 2003.
| Methods | Allocation: randomised, no further information.
Blindness: not reported.
Duration: three months.
Design: parallel group. Setting: inpatients, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R).
N = 65.
Age: mean ˜ 26 years, SD ˜ 8 years.
Sex: male and female. Length of illness: in chlorpromazine group mean ˜ 5.82 months, SD ˜ 3.97 months; in risperidone group mean ˜ 6.02 months, SD ˜ 4.38 months. Inclusion criteria: PANSS > 60, without systematic antipsychotic treatment, length of illness is within two years. Exclusion criteria: severe physical impairment, heart, liver, renal disease, drug or alcohol dependence, pregnant and lactating women. |
|
| Interventions |
|
|
| Outcomes | Mental state: PANSS. Adverse effects. Quality of life: QOL‐100. Leaving the study early. Unable to use: Adverse effects: we were unable to report on the following adverse effects for their data were pooled together ‐ fatigue, drowsiness, constipation, dry mouth, tachycardia, deteriorated memory, blurred vision. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further detail. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, but untested. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Three and 2 people dropped out of chlorpromazine and risperidone groups respectively. Trial authors did not report the reason for drop out. It's unclear if these participants were included in the final analysis. |
| Selective reporting (reporting bias) | Unclear risk | It appears that all measured outcomes were reported. |
| Other bias | Low risk | None obvious. |
vs RPD ‐ He 1999.
| Methods | Allocation: randomised, no further information.
Blindness: not reported.
Duration: 1 week wash out period, plus 8 weeks trial.
Design: parallel group. Setting: inpatients, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R).
N = 41.
Age: mean ˜ 45 years, SD ˜ 11 years.
Sex: male and female. Length of illness: mean ˜ 16 years, SD ˜ 10 years. Inclusion criteria: CCMD‐2‐R diagnosed schizophrenia. Exclusion criteria: severe organic or heart, liver, renal disease. |
|
| Interventions |
|
|
| Outcomes | Clinical response: no significant clinical response*. Mental state: BPRS, SAPS, SANS, NORS. Adverse effects: TESS. Leaving the study early. Unable to use: Mental state: unvalidated SAPS and SANS subscale scores. |
|
| Notes | *BPRS score decreased rate < 50% is regarded as no significant clinical response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further detail. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two people dropped out of risperidone group due to agitation and severe nausea. This is unlikely to have any serious impact on other outcomes assessed. |
| Selective reporting (reporting bias) | Low risk | It appears that all measured outcomes were reported. |
| Other bias | Low risk | None obvious. |
vs RPD ‐ Lin 2005.
| Methods | Allocation: randomised, no further information.
Blindness: not reported.
Duration: 8 weeks trial.
Design: parallel group. Setting: inpatients, China. |
|
| Participants | Diagnosis: first episode schizophrenia (CCMD‐3).
N = 70.
Age: mean ˜ 32 years, SD ˜ 14 years.
Sex: male and female. Length of illness: chlorpromazine group 25.19 ± 42.54 months; risperidone group 32.59 ± 45.80 months. Inclusion criteria: first episode schizophrenia, without receiving oral antipsychotic drugs one week prior to trial, or depot antipsychotic one month prior to trial, PANSS > 60. Exclusion criteria: severe organic or heart, liver, renal disease, pregnant or lactating women, or abnormal ECG. |
|
| Interventions |
|
|
| Outcomes | Clinical response: no significant clinical response*. Mental state: PANSS. Adverse effects. |
|
| Notes | *Paper did not describe the criteria of 'no significant clinical response'. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | High risk | CGI was measured, but not reported. |
| Other bias | Low risk | None obvious. |
vs RPD ‐ Liu 2000.
| Methods | Allocation: randomised, no further information.
Blindness: not reported.
Duration: 3 months.
Design: parallel group. Setting: inpatients, China. |
|
| Participants | Diagnosis: first episode schizophrenia (CCMD‐2).
N = 32.
Age: 18 to 45 years.
Sex: male and female. Length of illness: < 5 years. Inclusion criteria: no history of substance misuse prior to admission, without the use of antipsychotic drugs at least two weeks prior to admission, . Exclusion criteria: severe physical impairment or central nervous system disease or traumatic head injury. |
|
| Interventions |
|
|
| Outcomes | Mental state: SANS. Unable to use: Mental state: unvalidated SANS subscale scores. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | Unclear risk | Not enough information to make a judgement. |
| Other bias | Low risk | None obvious. |
vs RPD ‐ Liu 2005.
| Methods | Allocation: randomised, no further information.
Blindness: not reported.
Duration: 21 days.
Design: parallel group. Setting: inpatients, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R).
N = 100.
Age: 18 to 59 years.
Sex: male only. Length of illness: not stated. Inclusion criteria: no severe physical impairment or primary hypotension. Exclusion criteria: severe organic or heart, liver, renal disease. |
|
| Interventions |
|
|
| Outcomes | Adverse effects. Blood pressure. Unable to use: Adverse effects: TESS subscale score. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further detail. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | Low risk | All measured outcomes are reported. |
| Other bias | Low risk | None obvious. |
vs RPD ‐ Luo 2001.
| Methods | Allocation: randomised, no further information.
Blindness: double‐blind, but untested.
Duration: 8 weeks trial.
Design: parallel group. Setting: inpatients, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R).
N = 107.
Age: 15 to 46 years.
Sex: male and female. Length of illness: mean ˜ 3 years, SD ˜ 4 years. Inclusion criteria: not stated. Exclusion criteria: severe organic or heart, liver, renal disease, substance misuse, epilepsy, pregnant or lactating women. |
|
| Interventions |
|
|
| Outcomes | Clinical response: no significant clinical response*. Mental state: PANSS |
|
| Notes | *PANSS score decreased rate < 30%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further detail. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double blind, but untested. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | Low risk | All measured outcomes are reported. |
| Other bias | Low risk | None obvious. |
vs RPD ‐ Ma 2004.
| Methods | Allocation: randomised, no further information.
Blindness: not reported.
Duration: 1 week wash out period, plus 12 weeks trial.
Design: parallel group. Setting: inpatients, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 78.
Age: 19 to 65 years.
Sex: male and female. Length of illness: mean ˜ 6.6 years. Inclusion criteria: no severe physical impairment of chronic diseases, no epilepsy or history of organic disease; blood, urine test normal, liver function normal, ECG and EEG normal, BPRS > 35. |
|
| Interventions |
|
|
| Outcomes | Global state: CGI‐SI (skewed data), need of additional Benzhexol. Mental state: BPRS. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further detail. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data. |
| Selective reporting (reporting bias) | Low risk | All measured outcomes are reported. |
| Other bias | Low risk | None obvious. |
vs RPD ‐ Wang 2002.
| Methods | Allocation: randomised, no further information.
Blindness: not reported.
Duration: 8 weeks trial.
Design: parallel group. Setting: inpatients, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R).
N = 59.
Age: 18 to 58 years.
Sex: male and female. Length of illness: mean ˜ 100 months, SD ˜ 85 months. Inclusion criteria: without intake of antipsychotic medication one week prior to trial, or slow release depot antipsychotic one month prior to trial, PANSS > 60. Exclusion criteria: severe organic, brain or other disease. |
|
| Interventions |
|
|
| Outcomes | Clinical response: no significant clinical response*. Global state: need of additional Benzhexol. Mental state: PANSS change score. Adverse effects. Unable to use. Mental state: PANSS score at week 1. This is an eight weeks trial, but there was no endpoint PANSS score reported at week 8, instead the author reported improved PANSS score measured at week 1. |
|
| Notes | *Paper did not report on how this is measured. **We combined the 4 mg and 6 mg risperidone group, when reporting their outcomes. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One case was discharged early from risperidone (4 mg group), but it is unlikely to have had any significant impact on other outcome assessments such as PANSS score. |
| Selective reporting (reporting bias) | Low risk | All measured outcomes are reported. |
| Other bias | Low risk | None obvious. |
vs RPD ‐ Wang 2005.
| Methods | Allocation: randomised, no further information.
Blindness: not reported.
Duration: 8 weeks trial.
Design: parallel group. Setting: inpatients, China. |
|
| Participants | Diagnosis: first episode schizophrenia (CCMD‐3).
N = 100.
Age: 18 to 45 years.
Sex: male and female. Length of illness: 1 to 24 months. Inclusion criteria: not stated. Exclusion criteria: not stated. |
|
| Interventions |
|
|
| Outcomes | Functioning: WCST IQ and MQ score. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further detail. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | All measured outcomes are reported. |
| Other bias | Low risk | None obvious. |
vs RPD ‐ Wu 2002.
| Methods | Allocation: randomised, no further information.
Blindness: not reported.
Duration: 12 weeks trial.
Design: parallel group. Setting: inpatients, China. |
|
| Participants | Diagnosis: chronic schizophrenia (CCMD‐2‐R).
N = 70.
Age: 21 to 60 years.
Sex: male and female. Length of illness: 5 to 23 years. Inclusion criteria: BPRS > 38. Exclusion criteria: severe physical impairment. |
|
| Interventions |
|
|
| Outcomes | Clinical response: no significant clinical response*. Adverse effects. |
|
| Notes | *The paper did not report on how this is determined. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further detail. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | All measured outcomes are reported. |
| Other bias | Low risk | None obvious. |
vs RPD ‐ Wu 2004.
| Methods | Allocation: randomised, no further information.
Blindness: not reported.
Duration: 3 months.
Design: parallel group. Setting: inpatients, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 100.
Age: 18 to 43 years.
Sex: male and female. Length of illness: 3 months to 7.2 years. Inclusion criteria: PANSS > 60, give informed consent to participate. Exclusion criteria: severe organic or heart, liver, renal disease, drug or alcohol dependent. |
|
| Interventions |
|
|
| Outcomes | Mental state: PANSS. Quality of life: QOL. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further detail. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | All measured outcomes are reported. |
| Other bias | Low risk | None obvious. |
vs RPD ‐ Zheng 2001.
| Methods | Allocation: randomised, no further information.
Blindness: not reported.
Duration: 1 week washout period, plus 8 weeks trial.
Design: parallel group. Setting: inpatients, China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R).
N = 51.
Age: mean ˜ 45 years, SD ˜ 11 years.
Sex: male and female. Length of illness: mean ˜ 16 years, SD ˜ 10 years. Inclusion criteria: not stated. Exclusion criteria: severe organic or heart, liver, renal disease. |
|
| Interventions |
|
|
| Outcomes | Clinical response: no significant clinical response*. Adverse effects. |
|
| Notes | *BPRS score decreased rate < 50% is regarded as no significant clinical response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further information. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | BPRS, SAPS, SANS, NORS, TESS are all tested, but none were reported. |
| Other bias | Low risk | None obvious. |
Abbreviations: AIMS = Abnormal Involuntary Movement Scale BAS = Barne's Akathisa Scale BPRS = Brief Psychiatric Rating Scale CCMD = Chinese Classification of Mental Disorders CGI = Clinical Global Impression COSTART = Coding Symbols for a Thesaurus of Adverse Reaction Terms DSM = Diagnositc Statistics Manual ECG = Electrocardiogram EPS = Extrapyramidal Symptoms ESRS = Extrapyramidal Symptom Rating Scale FBG = fasting blood glucose GAF = Global Assessment of Functioning GQOL = General Quality of Life Scale HAMA = Hamilton Rating Scale for Anxiety HAMD = Hamilton Rating Scale for Depression HDL = High‐density lipoprotein LDL = Low‐density lipoprotein LSEQ = Leeds Sleep Evaluation Questionnaire MADRS = Montgomery–Åsberg Depression Rating Scale NORS = Nurse Observation Rating Scale NOSIE = Nurses' Observation Scale for Inpatient Evaluation PANSS = Positive and Negative Syndrome Scale PRL = prolactin QoL = Quality of Life RSESE = Rating Scale for Extrapyramidal Side Effects SANS = Scale for the Assessment of Negative Symptoms SAPS = Scale for the Assessment of Positive Symptoms SAS = Simpson‐Angus Extrapyramidal Side‐Effects scale SF‐36 = SF‐36 Health Survey TC = Total Cholesterol TESS = Treatment‐Emergent Signs and Symptoms TG = triglycerides UKU = Undersogelser side effect rating scale VPS = Vitality Plus Scale WAIS‐RC = Wechsler Adult Intelligence Scale‐Revised WCST = Wisconsin Card Sorting Test WHO‐QOL = World Health Organisation Quality of Life scale WMS = Wechsler Memory Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Appelberg 2004 | Allocation: randomised. Participants: schizophrenia. Intervention: olanzapine versus conventional neuroleptics (which may or may not include chlorpromazine). |
| Beuzen 1998 (HGCF) | Allocation: randomised. Participants: treatment‐resistant schizophrenia. |
| Bouchard 1998 | Allocation: randomised. Participants: treatment‐resistant schizophrenia. |
| Chen 2001 | Allocation: not randomised, summary of several trials. |
| Conley 1998 | Allocation: randomised. Participants: treatment‐resistant schizophrenia. |
| Czekalla 2001 | Allocation: randomised. Participants: schizophrenia. Intervention: olanzapine versus placebo versus haloperidol versus risperidone. |
| de Jesus Mari 2004 | Allocation: randomised. Participants: schizophrenia. Intervention: olanzapine versus conventional treatment (including chlorpromazine, as well as other drugs). Results cannot be separated. |
| Edgell 1998 | Allocation: randomised. Participants: schizophrenia. Intervention: olanzapine versus risperidone. |
| Feng 2001 | Allocation: randomised. Participants: treatment‐resistant schizophrenia. |
| Hu 2005 | Allocation: randomised. Participants: treatment‐resistant schizophrenia. |
| Huang 2000a | Allocation: quasi‐randomisation. Randomised according to admission order. |
| Huang 2000b | Allocation: randomised. Participants: schizophrenia. Intervention: risperidone versus chlorpromazine versus olanzapine Outcome: no usable data reported. |
| Kostakoglu 2001 | Allocation: unclear. Participants: not stated. Intervention: olanzapine versus chlorpromazine. Outcome: no usable data. |
| Li 2007 | Allocation: quasi‐randomisation. Randomised according the odd and even numbers of hospital admission order. |
| Pappas 1997 | Allocation: unclear. Participants: acute schizophrenia. Intervention: risperidone versus haloperidol versus chlorpromazine. Outcome: no usable data reported. |
| Qu 2006 | Allocation: quasi‐randomisation, according to odd and even admission numbers. |
| Shi 2007 | Allocation: randomised. Participants: schizophrenia. Intervention: chlorpromazine versus risperidone. Outcome: no usable data. Paper reported on tracked eye movement only. |
| Su 2002 | Allocation: quasi‐randomisation, according to hospital admission order. |
| Tian 2005 | Allocation: randomised. Participants: schizophrenia. Intervention: risperidone and chlorpromazine versus risperidone and benzodiazepines. |
| vs QTP ‐ Arvanitis 1996 | Allocation: double‐blind, randomised, multicentre.
Patients: schizophrenia. Intervention: five fixed dose of quetiapine versus a standard dose of haloperidol or placebo. |
| vs QTP ‐ AstraZeneca 2000 | Allocation: randomised multicentre, double‐blind, parallel‐group trial. Patients: treatment‐resistant schizophrenia. |
| vs QTP ‐ AstraZeneca 2005 | Allocation: randomised multicentre, double‐blind, parallel‐group trial. Patients: treatment‐resistant schizophrenia. |
| vs QTP ‐ Bai 2006 | Allocation: not randomised. The randomisation was based on the admission sequence. |
| vs QTP ‐ Cai 2008 | Allocation: not randomised. The randomisation was based on the admission sequence. |
| vs QTP ‐ Du 2004 | Allocation: not randomised. The randomisation was based on the admission sequence. |
| vs QTP ‐ Jiang 2004 | Allocation: randomised. Participants: schizophrenia. Intervention: chlorpromazine versus quetiapine. Outome: no usable outcome data reported. |
| vs QTP ‐ Li 2005 | Allocation: not randomised. The randomisation was based on the admission sequence. |
| vs QTP ‐ Ma 2004 | Allocation: not randomised. |
| vs QTP ‐ Ning 2008 | Allocation: randomised, no further information. Participants: schizophrenia, CCMD ‐ 3. Intervention: chlorpromazine versus quetaipine. The data is unable to use, as the number of each group is inconsistent in the result reporting and method statement. |
| vs QTP ‐ Tang 2004 | Allocation: not randomised. The randomisation was based on the admission sequence. |
| vs QTP ‐ Tang 2008 | Allocation: not randomised. The randomisation was based on the requirement (unclear from patients or physician) and patients financial capacity. |
| vs QTP ‐ Wang 2007 | Allocation: not randomised. The randomisation was based on the admission sequence. |
| vs QTP ‐ Zhang 2007 | Allocation: not randomised. The randomisation was based on the admission number. |
| vs QTP ‐ Zhong 2005 | Allocation: double‐blind, randomised, multicentre. Participants: schizophrenia, age range of 16 to 60 years but no average data reported. |
| Wang 1998 | Allocation: randomised. Participants: patients with schizophrenia aged between 16 and 62. The average age is approximately 24 years with a SD of 12.9 years. we consulted statistician and were unable to determine the proportion of people under 18 involved in this trial. |
| Wang 2004 | Allocation: randomised. Participants: treatment‐resistant schizophrenia. |
| Wang 2006 | Allocation: quasi‐randomisation, according to the odd and even numbers of hospital admission order. |
| Xiong 2004 | Allocation: randomised. Participants: children with schizophrenia (7 to 16 years). |
| Yu 2001 | Allocation: quasi‐randomisation, according to the odd and even numbers of hospital admission number. |
| Yuan 2006 | Allocation: quasi‐randomisation, according to the odd and even numbers of hospital admission number. |
| Zou 2005 | Allocation: quasi‐randomisation, according to the odd and even numbers of hospital admission number. |
Differences between protocol and review
We adhered to the Cochrane protocol for data extraction and data management. However, due to the large number of results we obtained from the literature searches, we have presented comparisons with chlorpromazine with the specific name of the comparator drug instead of the planned, generic 'chlorpromazine versus atypical antipsychotics'. We have only included data for three drug comparisons and will include all other comparator atypical antipsychotics in future updates of this Cochrane review. We clarified and amended the anticholinergic outcomes to include hypersalivation and leaving the study data.
The adverse effects outcomes have been arranged into new categories that are now used by the Cochrane Schizophrenia Group.
Contributions of authors
Kumar Saha developed the protocol. Rashid Zaman developed the protocol. Stephanie Sampson helped to develop the protocol, and wrote the review. Li Bo performed data screening and extraction, and contributed to writing the review. Sai Zhao performed data screening and data extraction. Jun Xia did reliability checks and extracted data.
Sources of support
Internal sources
Nottinghamshire Healthcare NHS Trust, UK.
External sources
-
National Institute for Health Research (NIHR), UK.
Cochrane Collaboration Programme Grant 2011; Reference number: 10/4001/15
-
National Natural Science Foundation of China, China.
NSFC project number: 81303151
-
Beijing Nova Program, China.
Project numbers: xxjh2015A093 and Z1511000003150125
Declarations of interest
Kumar Saha has no known conflicts of interest. Rashid Zaman has no known conflicts of interest. Stephanie Sampson has no known conflicts of interest. Li Bo has no known conflicts of interest. Sai Zhao has no known conflicts of interest. Jun Xia has no known conflicts of interest.
New
References
References to studies included in this review
HGCQ (Turkey) 2000 {unpublished data only}
- Dossenbach M. Sleep quality and early morning wakefulness of schizophrenic patients treated with olanzapine compared to chlorpromazine. European Neuropsychopharmacology 2000;10(Supplement 3):S328‐9. [Google Scholar]
- Eli Lilly, Company. Study F1D‐VI‐HGCQ olanzapine versus chlorpromazine in Turkey. Unpublished document internal to Eli‐Lilly 2000:1‐560.
- Kostakoglu E, Alptekin K, Kivicik BB, Martenyi F, Tunca Z, Gogus A, et al. Sleep quality and early morning wakefulness of schizophrenia patients treated with olanzapine compared to chlorpromazine [Errata]. European Neuropsychopharmacology 2001;11(Suppl 3):S369. [Google Scholar]
- Mraz K, Gogus A, Tunca Z, Martenyi F, Dossenbach M. Olanzapine versus chlorpromazine in Turkey. Schizophrenia Research (Abstracts of the Winter Workshop on Schizophrenia; February 5‐11, 2000; Davos, Switzerland). 2000.
HGDV (Morocco) 1999 {unpublished data only}
- Eli Lilly, Company. Study HGDV olanzapine (versus chlorpromazine in Morocco). Unpublished document internal to Eli‐Lilly and Company 2001:1‐512.
- HGDV/Morocco. Data supplied to the Cochrane Schizophrenia Group. Data on file 1999.
vs OLZ ‐ An 2006 {published data only}
- An BF, Li Y, Wang CH. Olanzapine on effect of executive dysfunction in schizophrenia. Journal of Clinical Psychiatry 2006;16(3):144‐5. [Google Scholar]
vs OLZ ‐ Chang 2003 {published data only}
- Chang FW, Wang CH, Zhao Z. Clinical effects of olanzapine vs chlorpromazine in treating positive symptoms of schizophrenia. Chinese Journal of New Drugs and Clinical Remedies 2003;22(6):357‐9. [Google Scholar]
vs OLZ ‐ Chen 2006 {published data only}
- Chen F. Olanzapine and chlorpromazine in the treatment of schizophrenia. Journal of Clinical Psychiatric Medicine 2006;16:303‐4. [Google Scholar]
vs OLZ ‐ He 2003 {published data only}
- He J, An Q. A comparative trial of olanzapine versus chlorpromazine in the treatment of schizophrenia. Shandong Mental Health Archive 2003;16:78‐80. [Google Scholar]
vs OLZ ‐ Loza 1999 (HGDT) {published and unpublished data}
- Eli Lilly, Company. HGDT olanzapine versus chlorpromazine in Egypt. Unpublished document internal to Eli Lilly and Company 2001:1‐513.
- Loza N, El‐Dosoky AM, Okasha TA, Khalil AH, Hasan NM, Dossenbach M, et al. Olanzapine compared to chlorpromazine in acute schizophrenia. European Neuropsychopharmacology 1999;9(Suppl 5):S291. [Google Scholar]
vs OLZ ‐ Luo 2007 {published data only}
- Luo C, Yang JW, Ren JP. Comparative study of the effect of olanzapine on serum lipid in schizophrenia. Journal of Clinical Psychiatric Medicine 2007;17:401‐2. [Google Scholar]
vs OLZ ‐ Wang 2002 {published data only}
- Wang CH, Zhao Z, Li Y, Pan M, Liu X. Evaluation of proximal therapeutic effect and distal social function restoration of olanzapine on schizophrenia patients. Zhongguo lin chuang kang fu [Chinese Journal of Clinical Rehabilitation] 2002;6(17):2664‐5. [Google Scholar]
vs OLZ ‐ Wang 2008 {published data only}
- Wang Q, Li L, Wang T. A comparative study of olanzapine in treatment of schizophrenia. Journal of Psychiatry 2008;21:373‐5. [Google Scholar]
vs OLZ ‐ Wu 2008 {published data only}
- Wu Y, Lu WH, Yi ZH, Wang J, Song BF, Shi DQ, et al. Controlled study of olanzapine versus chlorpromazine for the quality of life of patients with schizophrenia. Sichuan Mental Health Journal 2008;21(1):8‐11. [Google Scholar]
vs OLZ ‐ Zhao 2006 {published data only}
- Zhao Z, Yan F, Feng Y. A comparative study on olanzapine and chlorpromazine in the treatment of schizophrenia. China Journal of Health Psychology 2006;14:41‐2. [Google Scholar]
vs QTP ‐ Ai 2007 {published data only}
- Ai L. A comparative study of efficacy and safety of seroquel versus chlorpromazine in the treatment of schizophrenia. Heilongjiang Medical Journal 2007;31(4):285. [Google Scholar]
vs QTP ‐ An 2005 {published data only}
- An QH, Hu KY, Meng XL. A comparison of intelligence disorders and memory disorders in the first‐onset schizophrenia treated with quetiapine and chlorpromazine. Chinese Journal of Behavioral Medical Science 2005;14(1):62‐3. [Google Scholar]
vs QTP ‐ Cai 2006 {published data only}
- Cai DM. Clinical comparative study of quetiapine and chlorpromazine in the treatment of schizophrenia. Medical Journal of Chinese People's Health 2006;18(4):281‐2. [Google Scholar]
vs QTP ‐ Cai 2007 {published data only}
- Cai Q, Wang Z, Lin Y, Zhang X. Quetiapine and chlorpromazine in the treatment of schizophrenia: a random, controlled trial. Shanghai Archives of Psychiatry 2007;19(1):12‐4. [Google Scholar]
vs QTP ‐ Cao 2005 {published data only}
- Cao D, Xie SP, Chen QB, Yuan YG, Fang Q. Characteristics of the sexual disturbance caused by chlorpromazine, risperidone, quetiapine and olanzapine and their associations with the changes of blood glucose and blood lipids in male patients with schizophrenia. Chinese Journal of Clinical Rehabilitation [Zhongguo Lin Chuang Kang Fu] 2005;9(36):63‐8. [Google Scholar]
vs QTP ‐ Chen 2001 {published data only}
- Chen J, Zhao J, Li L. Multi‐center, double blind control study of domestic manufactured quetiapine on schizophrenia. Chinese Journal of Psychiatry 2001;34(4):193‐6. [Google Scholar]
- Zhao JP, Chen JD, Chen YG, Shu L, Ma C. A double‐blind and double‐dummy comparative study of quetiapine and chlorpromazine in the treatment of schizophrenia. Chinese New Drugs Journal 2002;11(2):149‐51. [Google Scholar]
vs QTP ‐ Chen 2007 {published data only}
- Chen F, Chen Y, Zhou B, Cheng F, Liu Y, Zho XW, et al. Quetiapine treatment in schizophrenia curative effect and quality of life. Journal of Clinical Psychiatry [Linchuang Jingshen Yixue Zazhi] 2007;17(5):319‐20. [Google Scholar]
vs QTP ‐ Chen 2008 {published data only}
- Chen S. Quetiapine and chlorpromazine in the treatment of schizophrenia. Medical Journal of Chinese People's Health 2008;20(7):660. [Google Scholar]
vs QTP ‐ Cheng 2003 {published data only}
- Cheng XF. Control observation of quetiapine vs chlorpromazine in schizophrenia. Journal of Clinical Psychosomatic Diseases 2003;9(2):78‐80. [Google Scholar]
vs QTP ‐ Deng 2004 {published data only}
- Deng YF, Liu CY, Liu ZR, Zhang W. 利培酮合用氯硝西泮治疗精神分裂症急性期兴奋状态疗效观察. 中国实用内科杂志 2004;24:496‐7. [Google Scholar]
vs QTP ‐ Guo 2003a {published data only}
- Guo BY, Wang YB. Control studies on efficacy of quetiapine vs chlorpromazine in first ‐ episode schizophrenics. Journal of Clinical Psychosomatic Diseases 2003;9(2):75‐7. [Google Scholar]
vs QTP ‐ Guo 2003b {published data only}
- Guo P, Guo H, Yang C. A comparative study on the effects of quetiapine and chlorpromazine upon the life quality of patients with schizophrenia. Nervous Diseases and Mental Hygiene 2003;3(6):454‐5. [Google Scholar]
vs QTP ‐ Guo 2005 {published data only}
- Guo HR, Song SY. Domestic quetiapine and chlorpromazine in the treatment of schizophrenia in controlled clinical studies. Modern Journal of Integrated Traditional Chinese and Western Medicine 2005;14:443‐4. [Google Scholar]
vs QTP ‐ Guo 2006 {published data only}
- Guo XF, Zhao JP, Chen JD, Zhang ZC. The effect of chlorpromazine and quetiapine on serum lipid and glucose. Journal of Clinical Psychiatry 2006;16(5):257‐9. [Google Scholar]
vs QTP ‐ Guo 2007 {published data only}
- Guo J, Cao C, Wu D. Effect of quetiapine and chlorpromazine on cognitive function in first‐episode schizophrenic. Chinese Journal of Health Psychology 2007;15(7):583‐4. [Google Scholar]
vs QTP ‐ Guo 2008 {published data only}
- Guo Z, Liu J, Yang J. Efficacy quetiapine in the treatment of schizophrenic patients and effect on the quality of life. Chinese Journal of Health Psychology 2008;16(5):544‐6. [Google Scholar]
vs QTP ‐ He 2003 {published data only}
- He YD, Zhao CM, Shao AL, Chen YN. A comparative study of quetiapine and chlorpromazine in the treatment of patients with schizophrenia. Herald of Medicine 2003;22(10):680‐2. [Google Scholar]
vs QTP ‐ Hu 2003 {published data only}
- Hu JM, Li Yi, Li Tao, Wang HM, Liu XH, Huo KJ. The effects of antipsychotics on serum prolactin in the first‐episode schizophrenia patients. West China Journal of Pharmaceutical Sciences 2003;18(6):467‐9. [Google Scholar]
vs QTP ‐ Ji 2004 {published data only}
- Ji J, Ou W. Comparative study between the quality of life and its curative effect in schizophrenic patients treated with quetiapine or chlorpromazine. Sichuan Mental Health 2004;17(2):73‐5. [Google Scholar]
vs QTP ‐ Jiang 2006 {published data only}
- Jiang KD, Bai YL, Peng DH, Tang MQ, Fan JX, Ma JS. A random and controlled study of quetiapine and chlorpromazine in patients with schizophrenia. Journal of Clinical Psychiatry [Linchuang Jingshen Yixue Zazhi] 2006;16(6):352‐3. [Google Scholar]
vs QTP ‐ Jiang 2008 {published data only}
- Jiang GQ, Luo J. A controlled study of quetiapine and chlorpromazine for schizophrenia. Chongqing Journal of Medicine 2008;37(16):1835‐6. [Google Scholar]
vs QTP ‐ Jin 2007 {published data only}
- Jin S, Liu S, Sang W, Zhao M. Seroquel and chlorpromazine in treatment of schizophrenia: a random, controlled trial. China Pharmaceuticals 2007;16(19):56‐7. [Google Scholar]
vs QTP ‐ Kong 2003 {published data only}
- Kong DL, Zhang SQ, Shu MQ. Effects of quetiapine and chlorpromazine on serum prolactin of schizophrenics. Journal of Clinical Psychosomatic Diseases 2003;9(4):200‐1. [Google Scholar]
vs QTP ‐ Li 2003 {published data only}
- Li M, Hu F, Wang S. A study of quetiapine and chlorpromazine on the effects and quality of life in the treatment of schizophrenia. Shandong Archives of Psychiatry 2003;16(3):135‐7. [Google Scholar]
vs QTP ‐ Li 2010 {published data only}
- Li YQ, Zhuang JH. Quetiapine and chlorpromazine in the treatment of schizophrenia with depression. Journal of Qiqi ha‐er Medical College 2010;31(3):395. [Google Scholar]
vs QTP ‐ Liu 2003 {published data only}
- Liu SF, Qin X. Quetiapine for late onset schizophrenia. Shandong Archive of Psychiatry 2003;16(3):164. [Google Scholar]
vs QTP ‐ Mei 2007 {published data only}
- Mei AC. Comparison of efficacy and safety of quetiapine and chlorpromazine in the treatment of schizophrenia. Medical Journal of Chinese People's Health 2007;19(17):723‐4. [Google Scholar]
vs QTP ‐ Nai 2007 {published data only}
- Nai X, Hu X, Qiu S. Effect of quetiapine on cognition function of patients with first episode schizophrenia. Journal of Medical Forum 2007;28(2):38‐9. [Google Scholar]
vs QTP ‐ NCT00882518 {published data only}
- NCT00882518. Efficacy and safety of quetiapine fumarate in the treatment of schizophrenic patients. http://www.clinicaltrials.gov (accessed 28 February 2012).
vs QTP ‐ Peng 2006 {published data only}
- Peng ZG, Zhou JX, Kuang WH, Li J, Huang MS. A randomized double ‐ blind controlled study on the efficacy of quetiapine and chlorpromazine in treatment of schizophrenia. West China Journal of Pharmaceutical Sciences 2006;21(6):606‐8. [Google Scholar]
vs QTP ‐ Peuskens 1997 {published data only}
- Peuskens J, Link CGG. A comparison of quetiapine and chlorpromazine in the treatment of schizophrenia. Acta Psychiatrica Scandinavica 1997;96(4):265‐73. [DOI] [PubMed] [Google Scholar]
vs QTP ‐ Sun 2006 {published data only}
- Sun XD, Zhou SB, Li ZM, Han ZF. Effect of cognitive function in first‐episode schizophrenia treated with quetiapine. Journal of Clinical Psychiatry 2006;16(2):94‐5. [Google Scholar]
vs QTP ‐ Tian 2006 {published data only}
- Tian H, He Q, Du L, Wu R, Hui S, Zheng Q, et al. Comparative study on the effect of seroquel and chlorpromazine on schizophrenia. China Pharmacy 2006;17(9):682‐3. [Google Scholar]
vs QTP ‐ Wan 2002 {published data only}
- Wan C, Lan SZ, Zhu XJ, Liao B, Wan J. A double blind controlled study of quetiapine and chlorpromazine for schizophrenia. Journal of Yichun University 2002;24(2):55‐6. [Google Scholar]
vs QTP ‐ Wan 2008 {published data only}
- Wan ZY, Mei HB. A controlled study of quetiapine and chlorpromazine for schizophrenia. Medical Journal of Chinese People's Health 2008;20(11):1151‐3. [Google Scholar]
vs QTP ‐ Wang 2004 {published data only}
- Wang XL, Jiang F, Li T. Comparison of efficacy and safety of quetiapine and larctigal in the treatment of schizophrenia. Chinese Journal of Behavioral Medical Science 2004;13(3):288‐90. [Google Scholar]
vs QTP ‐ Wang 2005 {published data only}
- Wang H, Peng D, Bai Y. Efficacy of quetiapine in the treatment of female patients with schizophrenia. Shanghai Archives of Psychiatry 2005;17(2):83‐6. [Google Scholar]
vs QTP ‐ Yang 2007 {published data only}
- Yang L, Yuan YG. Quetiapine and chlorpromazine in the treatment of people with schizophrenia. Medical Journal of Chinese People's Health 2007;19(12):1050‐1. [Google Scholar]
vs QTP ‐ Zhang 2002 {published data only}
- Zhang S, Li Y, Xu J. A double‐blind study of domestic quetiapine and chlorpromazine in the treatment of schizophrenia. Shandong Archives of Psychiatry 2002;15(3):149‐51. [Google Scholar]
vs QTP ‐ Zhang 2003 {published data only}
- Zhang HY, Wang X, Liu C, Shu L, Li H, Gu N, et al. A comparison study on efficacy and safety of quetiapine and chlorpromazine in the treatment of schizophrenia. Chinese Journal of Clinical Pharmacology 2003;19(3):163‐6. [Google Scholar]
vs QTP ‐ Zhang 2006 {published data only}
- Ma J. Quetiapine and chlorpromazine in the treatment of first‐episode schizophrenia controlled clinical studies. Chinese Clinical Practical Medicine 2007; Vol. 1, issue 2:51‐2.
- Ma J. Quetiapine and chlorpromazine in the treatment of first‐episode schizophrenia controlled clinical studies. Journal of Qiqihar Medical College [Qiqihar Yixueyuan Xuebao] 2007;28(9):1038‐9. [Google Scholar]
- Zhang W, Ma JG, Xu FL. Comparison study on treatment of primary schizophrenia with quetiapine and chlorpromazine. Heilongjiang Nursing Journal [Modern Nursing] 2006;30(2):81‐2. [Google Scholar]
vs QTP ‐ Zhang 2008 {published data only}
- Zhang J. A controlled study of quetiapine and chlorpromazine for schizophrenia. Neurological Disorders and Mental Health 2008;8(2):149‐50. [Google Scholar]
vs QTP ‐ Zhou 2003 {published data only}
- Zhou J, Li J, Kuang W. Comparison between quetiapine and chlorpromazine in cognitive function of schizophrenic patients. Chinese Mental Health Journal 2003;17(10):699‐701. [Google Scholar]
vs QTP ‐ Zhou 2004 {published data only}
- Zhou SB, Sun XD, Li YM. A comparative study on quetiapine and chlorpromazine in the treatment of schizophrenia. Medical Journal of Chinese People's Health 2004;16(11):657‐9. [Google Scholar]
vs QTP ‐ Zou 2006 {published data only}
- Zou X, Zhou Y, Zhang W. Study on quetiapine and chlorpromazine in treatment of schizophrenia. China Pharmaceuticals 2006;15(12):51‐2. [Google Scholar]
vs RPD ‐ Chang 1998 {published data only}
- Chang SL, Wang GH. Clinical observation of risperidone and chlorpromazine in the treatment of schizophrenia. Anhui Medical and Pharmaceutical Journal 1998; Vol. 2:25‐6.
vs RPD ‐ Cui 2001 {published data only}
- Cui L. Risperidone and chlorpromazine in the treatment of schizophrenia, double‐blind, controlled study, and serum prolactin efficacy. Hebei Archive of Psychiatry 2001;14:205‐8. [Google Scholar]
vs RPD ‐ Feng 2003 {published data only}
- Feng YX, Sun F. A comparative study on the effects of risperidone and chlorpromazine upon the life quality of patients with schizophrenia. Health Psychology Journal 2003;11:117‐8. [Google Scholar]
vs RPD ‐ He 1999 {published data only}
- He M, Feng Y, Chen R. A controlled comparative study on risperidone and chlorpromazine in treatment of schizophrenia. Chinese New Drugs Journal 1999;8:185‐7. [Google Scholar]
vs RPD ‐ Lin 2005 {published data only}
- Lin WX. Risperidone and chlorpromazine in the treatment of clinical controlled study of 70 patients with schizophrenia. Modern Chinese Practical Medicine Journal 2005;4:27‐8. [Google Scholar]
vs RPD ‐ Liu 2000 {published data only}
- Liu Y, Huang X, Xie L. A comparative study of risperidone and chlorpromazine in the treatment of the negative symptoms of schizophrenia. Journal of Medicine and Pharmacy 2000;19:323‐4. [Google Scholar]
vs RPD ‐ Liu 2005 {published data only}
- Liu GR, Zhang YH. Risperidone and chlorpromazine on schizophrenia blood pressure, heart rate. Occupation and Health 2005;21:443‐5. [Google Scholar]
vs RPD ‐ Luo 2001 {published data only}
- Luo X, Jiang K, Gu N, Yang X, Zhu S. Comparison of effects and factors between risperidone and chlorpromazine on schizophrenia. Chinese Journal of New Drugs and Clinical Remedies 2001;20:264‐6. [Google Scholar]
vs RPD ‐ Ma 2004 {published data only}
- Ma Z H, Liu A Y, Zhou C M, Zhang Z. A comparative study of risperidone and chlorpromazine in the treatment of schizophrenia. Medical Journal of Chinese People Health 2004;16:202‐4. [Google Scholar]
vs RPD ‐ Wang 2002 {published data only}
- Wang Q, Jia C. Controlled study of risperidone and chlorpromazine in the treatment of schizophrenia. Health Psychology Journal 2002;10:226‐8. [Google Scholar]
vs RPD ‐ Wang 2005 {published data only}
- Wang X, Hu X, Yang J. Effect of chlorpromazine and risperidone on cognitive function of the patients with first episode schizophrenia. Chinese Journal of Health Psychology 2005;13:342‐4. [Google Scholar]
vs RPD ‐ Wu 2002 {published data only}
- Wu S, Xing G. A comparative trial on the efficacy of risperidone vs chlorpromazine in chronic schizophrenia. Health Psychology Journal 2002;10:364‐5. [Google Scholar]
vs RPD ‐ Wu 2004 {published data only}
- Wu Y, Lu W, Yi Z. A comparative study of risperidone and chlorpromazine on quality of life in patients with schizophrenia. Shandong Archive of Psychiatry 2004;17:137‐40. [Google Scholar]
vs RPD ‐ Zheng 2001 {published data only}
- Zheng ZS, Huang JM, Yu XY. Efficacy of risperidone and chlorpromazine in the treatment of schizophrenia. Chinese Hospital Pharmacy Journal 2001;21:99‐100. [Google Scholar]
References to studies excluded from this review
Appelberg 2004 {published data only}
- Appelberg B, Sintonen H, Tuisku K, Joffe G. Is it worthwhile to change clinically stable schizophrenic outpatients with mild to moderate residual symptoms and/or side effects from conventional to atypical antipsychotics? – a randomised study with olanzapine. Proceedings of the 12th Biennial Winter Workshop on Schizophrenia; 2004 Feb 7‐13; Davos, Switzerland. 2004.
- Appelberg B, Tuisku K, Joffe G. Is it worth while changing clinically stable schizophrenic out‐patients with mild to moderate residual symptoms and/or side effects from conventional to atypical antipsychotics? A prospective, randomised study with olanzapine. European Psychiatry 2004;19(8):516‐8. [DOI] [PubMed] [Google Scholar]
- Appelberg B, Tuisku K, Joffe G. Is it worthwhile changing clinically stable schizophrenic out‐patients with mild to moderate residual symptoms and/or side effects from conventional to atypical antipsychotics?. Schizophrenia Research 2004;67(1):140‐1. [DOI] [PubMed] [Google Scholar]
- Joffe G, Sintonen H, Appelberg B. Shift from first generation antipsychotics to olanzapine may improve health‐related quality of life of stable but residually symptomatic schizophrenic outpatients: a prospective, randomized study. International Journal of Technology Assessment in Health Care 2008;24(4):399‐402. [DOI] [PubMed] [Google Scholar]
Beuzen 1998 (HGCF) {published and unpublished data}
- Beasley CM, Beuzen JN, Birkett MA. [Olanzapine versus clozapine: an international double‐blind study in the treatment of patients with treatment‐resistent schizophrenia]. New Clinical Drug Evaluation Unit (NCDEU) Meeting; 1999 June 12‐15; Boca Raton, FL, USA. 1999.
- Beasley CM, Beuzen JN, Birkett MA. Olanzapine versus clozapine: an international double‐blind study in the treatment of patients with treatment‐resistent schizophrenia. American College of Neuropsychopharmacology Annual Meeting. Hawaii, 1998.
- Beasley CM, Beuzen JN, Birkett MA. Olanzapine versus clozapine: an international double‐blind study in the treatment of patients with treatment‐resistent schizophrenia. American Psychiatric Association Meeting; 1999 January 31 ‐ February 2; Washington, DC, USA. 1999.
- Beasley CM, Beuzen JN, Birkett MA. Olanzapine versus clozapine: an international double‐blind study in the treatment of patients with treatment‐resistent schizophrenia. World Psychiatric Association; 1999 August 6‐11; Hamburg, Germany. 1999.
- Beuzen JN, Birkett M, Kiesler G, Wood A. Olanzapine versus clozapine in resistant schizophrenic patients ‐ results of an international double‐blind randomised clinical trial. Proceedings of XXIst Collegium Internationale Neuro‐psychopharmacologicum; 1998 July 12‐16; Glasgow, UK. 1998.
- Beuzen JN, Birkett MA, Kiesler GM. An investigation of subgroup effects in a study of olanzapine versus clozapine in the treatment of resistant schizophrenic patients. European Neuropsychopharmacology 1998;8(Suppl 2):S226‐7. [Google Scholar]
- David SR, Meehan KM, Sutton VK, Taylor CC. Treatment of negative symptoms with olanzapine in comparison with other novel antipsychotic agents. International Journal of Neuropsychopharmacology 2000;3(Suppl 1):S140. [Google Scholar]
- Dossenbach M, Bitter I, Slabber M, Pretorius J, Bartko GY, Banics Z, et al. Olanzapine versus clozapine in patients nonresponsive or intolerant to standard acceptable treatment for schizophrenia. 153rd Annual Meeting of the American Psychiatric Association; 2000 May 13‐18; Chicago, USA. 2000.
- Dossenbach M, Bitter I, Slabber M, Pretorius J, Bartko GY, Banics Z, et al. Olanzapine versus clozapine in patients nonresponsive or intolerant to standard acceptable treatment for schizophrenia. 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, PA, USA. 2002.
- Dossenbach M, Slabber M, Martenyi F, Bartko G, Bitter I. Olanzapine vs. clozapine in patients non responsive or intolerant to standard acceptable treatment of schizophrenia. 11th World Congress of Psychiatry; 1999 Aug 6‐11; Hamburg, Germany. 1999:148.
- Jean‐Noel B, Wood AJ, Kiesler GM, Birkett M, Tollefson GD. Olanzapine vs. clozapine: an international double blind study in the treatment of resistant schizophrenia. 11th World Congress of Psychiatry; 1999 Aug 6‐11; Hamburg, Germany. 1999:143.
- Tollefson GD, Birkett MA, Kiesler GM, Wood AJ, Lilly Resistant Schizophrenia Study Group. Double‐blind comparison of olanzapine versus clozapine in schizophrenic patients clinically eligible for treatment with clozapine. Biological Psychiatry 2001;49(1):52‐63. [DOI] [PubMed] [Google Scholar]
Bouchard 1998 {published data only}
- Bouchard RH. A comparative longitudinal study of risperidone versus classic neuroleptic drugs in the treatment of schizophrenia: 24 months observation. Journal of Clinical Psychopharmacology 2002;28:31‐2. [Google Scholar]
- Bouchard RH, Demers MF, Merette E, Pourcher E. Classical neuroleptics (CNLP) vs risperidone (RIS) interim 12 months efficacy analysis of a long‐term, naturalistic study in schizophrenia. 21st Congress of the Collegium Internationale Neuro‐Psychopharmacologicum (CINP); 1998 Jul 12‐16; Glassgow, UK. 1998.
- Bouchard RH, Merette C, Demers MF, Roy‐Gagnon MH. Risperidone versus classical neuroleptics: preliminary results of a prospective naturalistic one year study. 151st Annual Meeting of the American Psychiatric Association; 1998 May 30‐Jun 4; Toronto. Toronto, Canada, 1998:180.
- Bouchard RH, Mérette C, Pourcher E, Demers MF, Villeneuve J, Roy Gagnon MH, et al. Longitudinal comparative study of risperidone and conventional neuroleptics for treating patients with schizophrenia. The Quebec Schizophrenia Study Group. Journal of Clinical Psychopharmacology 2000;20(3):295‐304. [DOI] [PubMed] [Google Scholar]
- Bouchard RH, Pourcher E, Merette C, Demers MF, Villeneuve J, Roy Gagnon MH, et al. One‐year follow‐up of schizophrenic patients treated with risperidone or classical neuroleptics: a prospective, randomized, multicentred open‐study. 11th Congress of the European College of Neuropsychoharmacology; 1998 Oct 31‐ Nov 4; Paris, France. 1998.
- Bouchard RH, Pourcher E, Merette C, Demers MF, Villeneuve J, Roy MA, et al. Risperidone advantages in chronic schizophrenic patients: a 1‐year effectiveness study. Schizophrenia Research 1999;36(1‐3):271. [Google Scholar]
Chen 2001 {published data only}
- Chen GY, Li B, Li C. Clinical observation on schizophrenic patients treated with risperidone. Journal of Clinical Psychiatry 2001;11:325‐8. [Google Scholar]
Conley 1998 {published data only}
- Conley R, Gounaris C. Demographic differences in olanzapine responders with therapy‐refractory schizophrenia. Schizophrenia Research the VIth International Congress on Schizophrenia Research;1997 April 12‐16; Colorado Springs, CO. 1997; Vol. 24 Suppl:189.
- Conley RR, Tamminga CA, Bartko JJ, Richardson C, Peszke M, Lingle J, et al. Olanzapine compared with chlorpromazine in treatment‐resistant schizophrenia. American Journal of Psychiatry 1998;155(7):914‐20. [DOI] [PubMed] [Google Scholar]
- Conley RR, Tamminga CA, Beasley C. Olanzapine vs chlorpromazine in therapy‐refractory schizophrenia. Schizophrenia Research, The VIth International Congress on Schizophrenia Research; 1997 April 12‐16; Colorado Springs, CO. 1997; Vol. 24 Suppl:189.
- Conley RR, Tamminga CA, Beasley C. Olanzapine vs. chlorpromazine in treatment‐resistant schizophrenia. Biological Psychiatry 1997;41:1S‐120S. [Google Scholar]
- Conley RR, Tamminga CA, Beasley C, Maryland Study Group. Olanzapine vs. chlorpromazine in treatment‐resistant schizophrenia. Biological Psychiatry 1997;41:73S. [Google Scholar]
- Kelly DL, Conley RR, Tamminga CA. Differential olanzapine plasma concentrations by sex in a fixed‐dose study. Schizophrenia Research 1999;40(2):101‐4. [CSG NO. 4333] [DOI] [PubMed] [Google Scholar]
- Richardson C, Conley R. Olanzapine response in schizophrenic subjects with neuroleptic resistant disorganization. Schizophrenia Research, The VIth International Congress on Schizophrenia Research; 1997 April 12‐16; Colorado Springs, CO. 1997; Vol. 24 Suppl:191.
Czekalla 2001 {published data only}
- Czekalla J, Beasley CM Jr, Dellva MA, Berg PH, Grundy S. Analysis of the QTc interval during olanzapine treatment of patients with schizophrenia and related psychosis. Journal of Clinical Psychiatry 2001;62(3):191‐8. [DOI] [PubMed] [Google Scholar]
de Jesus Mari 2004 {published data only}
- Jesus Mari J, Lima MS, Costa AN, Alexandrino N, Rodrigues‐Filho S, Oliveira IR, et al. The prevalence of tardive dyskinesia after a nine month naturalistic randomized trial comparing olanzapine with conventional treatment for schizophrenia and related disorders. European Archives of Psychiatry and Clinical Neuroscience 2004;254(6):356‐61. [DOI] [PubMed] [Google Scholar]
Edgell 1998 {published data only}
- David SR, Meehan KM, Sutton VK, Taylor CC. Treatment of negative symptoms with olanzapine in comparison with other novel antipsychotic agents. International Journal of Neuropsychopharmacology 2000;3(Suppl 1):S140. [Google Scholar]
- Edgell ET, Andersen SW, Grainger D, Wang J. Resource use and quality of life of olanzapine compared with risperidone: results from an international randomized clinical trial. XXIst Collegium Internationale Neuro‐psychopharmacologicum; 1998, July 12‐16; Glasgow, UK. 1998.
Feng 2001 {published data only}
- Feng CX, Huang SX, Yang HZ. A comparative trial on the efficacy of risperidone vs chlorpromazine in treatment‐resistant schizophrenia. Shandong Archive of Psychiatry 2001;14:95‐6. [Google Scholar]
Hu 2005 {published data only}
- Hu TS. Comparative analysis of the positive symptoms of schizophrenia treated with risperidone. Chinese Journal of Behavioral Medical Science 2005;14:428. [Google Scholar]
Huang 2000a {published data only}
- Huang SX, Feng CX, Chen JY, Liu DQ. Risperidone vs chlorpromazine in treating schizophrenia: a randomized double‐blind study. Chinese Journal of New Drugs and Clinical Remedies 2000;19:396‐8. [Google Scholar]
Huang 2000b {published data only}
- Huang YH, Luo BM. Risperidone and chlorpromazine, clozapine drug‐control study. Chinese Journal of New Drugs and Clinical Remedies 2000;8:210‐1. [Google Scholar]
Kostakoglu 2001 {published data only}
- Kostakoglu E, Alptekin K, Kivircik BB, Dossenbach M, Tunca Z, Gogus A. Olanzapine vs. chlorpromazine‐ 6 weeks treatment of acute schizophrenia. Journal of European College of Neuropsychopharmacology 2001;11:S369. [Google Scholar]
Li 2007 {published data only}
- Li Z. Initial searching for the early influence and interfere about the regulating function of the blood‐glucose affected by chlorpromazine and risperidal. Journal of Health and Well Being 2007;4:18‐20. [Google Scholar]
Pappas 1997 {published data only}
- Pappas D, Konitsiotis S, Liakos A. Risperidone in the treatment of acute schizophrenic episodes. European Neuropsychopharmacology 1997;7:S206. [Google Scholar]
Qu 2006 {published data only}
- Qu ZW, Chen MD, Gu JQ, Fu WZ, Wang T, Gu LM, et al. Metabolism of the blood‐glucose that affected by chlorpromazine or risperidone treatment. Journal of Clinical Psychiatric Medicine [Linchuang Jingshen Yixue Zazhi] 2006;16:68‐70. [Google Scholar]
Shi 2007 {published data only}
- Shi Y, Wang Y, Li R. Effects of risperidone on NEF of eye movements in the first‐episode schizophrenia. Journal of Clinical Psychosomatic Diseases 2007;13:487‐9. [Google Scholar]
Su 2002 {published data only}
- Su YQ. Effects of low dosage risperdal to treat the first attack of schizophrenia. Youjiang Medical Journal 2002;30:380‐1. [Google Scholar]
Tian 2005 {published data only}
- Tian R. Risperidone combined with chlorpromazine in the treatment of insomnia clinical observation of patients with schizophrenia. Journal of Clinical Psychosomatic Diseases 2005;11:151‐2. [Google Scholar]
vs QTP ‐ Arvanitis 1996 {published data only}
- Arvanitis LA, Miller BG. An atypical antipsychotic: results from a multiple fixed dose, placebo‐controlled study. European Neuropsychopharmacology 1996;6:148. [Google Scholar]
vs QTP ‐ AstraZeneca 2000 {published data only}
- AstraZeneca. A multicentre, double‐blind, randomised trial to compare the effects of seroquel and chlorpromazine in patients with treatment‐resistant schizophrenia (5077IL/0054 TRESS). http://www.clinicalstudyresults.org/ (accessed 28 February 2012).
vs QTP ‐ AstraZeneca 2005 {published data only}
- AstraZeneca. A multicenter, double‐blind, randomized, comparison of quetiapine (seroquel®) and chlorpromazine in the treatment of subjects with treatment‐resistant schizophrenia. http://www.clinicalstudyresults.org/ (accessed 28 February 2012).
vs QTP ‐ Bai 2006 {published data only}
- Bai H. Quetiapine and chlorpromazine in the treatment of schizophrenia. Medical Journal of Chinese People's Health 2006;18:1028‐9. [Google Scholar]
vs QTP ‐ Cai 2008 {published data only}
- Cai J, Ji L, Liu X. Clinical effects comparison of quetiapine and chlorpromazine hydrochloride in treatment of female schizophrenia. China Pharmaceuticals 2008;17(21):52‐3. [Google Scholar]
vs QTP ‐ Du 2004 {published data only}
- Du QX, Yu CF, Zhong W. A clinical comparative analysis of quetiapine and chlorpromazine in treatment of schizophrenia. Medical Journal of Chinese People's Health 2004;16(7):398‐9. [Google Scholar]
vs QTP ‐ Jiang 2004 {published data only}
- Jiang K. Quetiapine vs chlorpromazine for schizophrenia – a multi‐centre, randomized controlled clinical trial. Proceedings of the 1st Chinese National Conference of Psycho‐Neuroscience; 2004 Unknown Dates; Changsha, China. Changsha, China, 2004:106.
vs QTP ‐ Li 2005 {published data only}
- Li YQ. Comparative analysis of side effects of quetiapine and chlorpromazine in the treatment of schizophrenia. Practical Clinical Medicine 2005;6:26‐7. [Google Scholar]
vs QTP ‐ Ma 2004 {published data only}
- Ma ZW, Li MX, Shi YZ, Wang LH. Quetiapine (35 patients) vs chlorpromazine (34 patients) in treatment of schizophrenia. Chinese Journal of New Drugs and Clinical Remedies 2004;23(5):273‐5. [Google Scholar]
vs QTP ‐ Ning 2008 {published data only}
- Ning Z, Liu S, Jin S. A comparison of cognitive function in first episode schizophrenia treated with seroquel or chlorpromazine. Chinese Journal of Health Psychology 2008;16(2):138‐40. [Google Scholar]
vs QTP ‐ Tang 2004 {published data only}
- Tang Y. Quetiapine and chlorpromazine in the treatment of schizophrenia observed. Chinese Modern Medicine 2004;3(11):14. [Google Scholar]
vs QTP ‐ Tang 2008 {published data only}
- Tang CG, Wang RC. Comparison of efficacy and safety of quetiapine and chlorpromazine in the treatment of schizophrenia. Medical Journal of Chinese People's Health 2008;20(9):917‐8. [Google Scholar]
vs QTP ‐ Wang 2007 {published data only}
- Wang Y, Li G, Xue JW, Li T. A comparative study of effects of quetiapine and chlorpromazine on the brain blood stream dynamics in the treatment of schizophrenia. Linchuang Jingshen Yixue Zazhi 2007;17(4):255‐6. [Google Scholar]
vs QTP ‐ Zhang 2007 {published data only}
- Zhang L. A comparative study of quetiapine and chlorpromazine in the treatment of schizophrenia. Chinese Modern Medicine and Pharmacy 2007;9(2):97‐8. [Google Scholar]
vs QTP ‐ Zhong 2005 {published data only}
- Zhong CL, Cui YH. Comparative study of influence of quetiapine on life quality in schizophrenic patients. Journal of Clinical Psychological Medicine 2005;15(2):103‐5. [Google Scholar]
Wang 1998 {published data only}
- Wang L, Song J, Su G. Random comparing research on the treatment of schizophrenia by risperidone and chlorpromazine. Tianjin Pharmacy 1998;10:57‐60. [Google Scholar]
Wang 2004 {published data only}
- Wang LG, Liu Y, Wan H. A comparative trial of the efficacy on olanzapine and chlorpromazine in treatment‐resistant schizophrenia. Heath Psychology Journal 2004;12:203‐4. [Google Scholar]
Wang 2006 {published data only}
- Wang T. Study on influence of the short ‐ term therapy by chlorpromazine or risperidal on 2hPG. Journal of Qiqihar Medical College [Qiqihar Yixueyuan Xuebao] 2006;27:1417‐8. [Google Scholar]
Xiong 2004 {published data only}
- Xiong Y. Comparison study of childhood schizophrenia treated with risperidone and chlorpromazine. Guizhou Medical Journal 2004;28:697‐8. [Google Scholar]
Yu 2001 {published data only}
- Yu J, Chai M, Chen J. A comparison of cognitive function in schizophrenia treated with risperidone and chlorpromazine. Journal of Clinical Psychological Medicine 2001;11:265‐6. [Google Scholar]
Yuan 2006 {published data only}
- Yuan J, Qu WZ. The first use of chlorpromazine and risperidone on glucose metabolism in patients with schizophrenia. Sichuan Mental Health 2006;19:197‐9. [Google Scholar]
Zou 2005 {published data only}
- Zou QL, Fan JH, Chen SQ. A comparative trial on the efficacy of risperidone vs chlorpromazine in the treatment of chronic schizophrenia. Medical Journal of Chinese People's Health 2005;17:153‐4. [Google Scholar]
Additional references
Adams 2005
- Adams CE, Rathbone J, Thornley B, Clarke M, Borrill J, Wahlbeck K, et al. Chlorpromazine for schizophrenia: a Cochrane systematic review of 50 years of randomised controlled trials. BMC Medicine 2005;3:15. [DOI: 10.1186/1741-7015-3-15] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adams 2006
- Adams CE, Tharyan P, Coutinho ES, Stroup TS. The schizophrenia drug‐treatment paradox: pharmacological treatment based on best possible evidence may be hardest to practise in high‐income countries. British Journal of Psychiatry 2006;189:391‐2. [PUBMED: 17077426] [DOI] [PubMed] [Google Scholar]
Adams 2014
- Adams CE, Awad G, Rathbone J, Thornley B. Chlorpromazine versus placebo for schizophrenia. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD000284.pub3] [DOI] [Google Scholar]
Ahmed 2010
- Ahmed U, Jones H, Adams CE. Chlorpromazine for psychosis induced aggression or agitation. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD007445.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Almerie 2007
- Almerie MQ, Alkhateeb H, Essali A, Matar HE, Rezk E. Cessation of medication for people with schizophrenia already stable on chlorpromazine. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD006329] [DOI] [PMC free article] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreasen 1982
- Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Archives of General Psychiatry 1982;39(7):784‐8. [DOI] [PubMed] [Google Scholar]
Andreasen 1984
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SANS). Iowa City: University of Iowa, 1984. [Google Scholar]
Anon 2010
- Anon. Scientific fraud: action needed in China. Lancet 2010;375(9709):94. [DOI] [PubMed] [Google Scholar]
Arana 2000
- Arana GW. An overview of side effects caused by typical antipsychotics. Journal of Clinical Psychiatry 2000;61(Suppl 8):5‐11. [PubMed] [Google Scholar]
Bian 2008
- Bian Q, Kato T, Monji A, Hashioka S, Mizoguchi Y, Horikawa H, et al. The effect of atypical antipsychotic drugs, perospirone, ziprasidone and quetiapine on microglial activation induced by interferon‐gamma. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 2008;32(1):42‐8. [DOI] [PubMed] [Google Scholar]
Bland 1978
- Bland RC, Parker JH, Orn H. Prognosis in schizophrenia. Prognostic predictors and outcome. Archives of General Psychiatry 1978;35(1):72‐7. [DOI: 10.1001/archpsyc.1978.01770250074007] [DOI] [PubMed] [Google Scholar]
Bland 1997
- Bland JM, Kerry SM. Statistics notes. Trials randomised in clusters. BMJ 1997;315(7108):600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [Apercu sur la problematique des indices d'efficacite therapeutique, 3: comparaison des indices et utilisation. Groupe d'etude des indices d'efficacite]. Therapie 1999;54(4):405‐11. [PUBMED: 10667106] [PubMed] [Google Scholar]
Caccia 2010
- Caccia S, Pasina L, Nobili A. New atypical antipsychotic drugs for schizophrenia: iloperidone. Drug Design, Development and Therapy 2010;4:33–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2002
- Chen YF. Chinese classification of mental disorders (CCMD‐3): towards integration in international classification. Psychopathology 2002;35(2‐3):171‐5. [DOI] [PubMed] [Google Scholar]
Chouinard 1980
- Chouinard G, Ross‐Chouinard A, Annable L, Jones B. Extrapyramidal Symptom Rating Scale (abstract). Canadian Journal of Neurogical Sciences 1980;7:233. [Google Scholar]
Cincotta 2010
- Cincotta SL, Rodefer JS. Emerging role of sertindole in the management of schizophrenia. Neuropsychiatric Disease and Treatment 2010;7:429‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; 2000 Oct 25‐28; Cape Town. Cape Town: The Cochrane Collaboration, 2000.
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org 2011.
Delay 1952
- Delay J, Deniker P. The treatment of psychosis in a derivative of the neuroleptic hibernotherapie method [Le traitement des psychoses par une méthode neuroleptique dérivée de l'hibernothérapie]. In: Ossa PC editor(s). CR Congrès des Médicins Aliénistes et Neurologistes de France et des Pays de Langue Française. Paris: Masson, 1952:479. [Google Scholar]
Divine 1992
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623‐9. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21(19):2971‐80. [DOI] [PubMed] [Google Scholar]
DSM‐IV 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington DC: American Psychiatric Association, 1994. [Google Scholar]
DSM‐IV‐TR 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington DC: American Psychiatric Association, 2000. [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne D, Altman DG, Higgins JPT, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Flanagan 1982
- Flanagan JC. Measurement of the quality of life: Current state of the art. Archives of Physical Medicine and Rehabilitation 1982;63(2):56‐9. [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7‐10. [DOI] [PubMed] [Google Scholar]
Gulliford 1999
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149(9):876‐83. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute of Mental Health. DHEW Publication NO (ADM), 1976:124‐585. [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011a
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org 2011.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org 2011.
Honigfeld 1965
- Honigfeld G, Klett CJ. The nurses' observation scale for inpatient evaluation: a new scale for measuring improvement in chronic schizophrenia. Journal of Clinical Psychology 1965;21:65‐71. [DOI] [PubMed] [Google Scholar]
Hutton 2009
- Hutton JL. Number needed to treat and number needed to harm are not the best way to report and assess the results of randomised clinical trials. British Journal of Haematology 2009;146(1):27‐30. [DOI] [PubMed] [Google Scholar]
ICD‐10 1992
- World Health Organization. F‐20. The ICD‐10 Classification of Mental and Behavioural Disorders. Geneva: Divison of Mental Health, WHO, 1992:86‐109. [Google Scholar]
ICH E3 1995
- ICH Expert Working Group. ICH harmonised tripartite guideline: structure and content of clinical study reports. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use 30 November 1995.
Janssen 1988
- Janssen PA, Niemegeers CJ, Awouters F, Schellekens KH, Megens AA, Meert TF. Pharmacology of risperidone (R 64 766), a new antipsychotic with serotonin‐S2 and dopamine‐D2 antagonistic properties. Journal of Pharmacology and Experimental Therapeutics 1988;244(2):685‐93. [PubMed] [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda, NY: Multi‐Health Systems, 1986. [Google Scholar]
Kay 1987
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin 1987;13(2):261‐76. [DOI] [PubMed] [Google Scholar]
Kinon 1996
- Kinon B J, Lieberman JA. Mechanisms of action of atypical antipsychotic drugs: a critical analysis. Psychopharmacology 1996;124(1‐2):2‐34. [DOI: 10.1007/BF02245602] [DOI] [PubMed] [Google Scholar]
Kraeplin 1919
- Kraeplin E. Dementia Praecox and Paraphrenia. Edinburgh: E&S Livingstone, 1919. [Google Scholar]
Laborit 1951
- Laborit H, Huguenard P. Artificial hibernation by physical and pharmacodynamic means [L'hibernation artificielle par moyens pharmacodynamiques et physiques]. Presse Médicale 1951;59:1329. [PubMed] [Google Scholar]
Leucht 2005a
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of Brief Psychiatric Rating Scale scores. British Journal of Psychiatry 2005;187:366‐71. [PUBMED: 16199797] [DOI] [PubMed] [Google Scholar]
Leucht 2005b
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophrenia Research 2005;79(2‐3):231‐8. [PUBMED: 15982856] [DOI] [PubMed] [Google Scholar]
Leucht 2007
- Leucht S, Engel RR, Bäuml J, Davis JM. Is the superior efficacy of new generation antipsychotic drugs an artifact of LOCF?. Schizophrenia Bulletin 2007;33(1):183‐91. [PUBMED: 16905632] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leucht 2008
- Leucht C, Kitzmantel M, Chua L, Kane J, Leucht S. Haloperidol versus chlorpromazine for schizophrenia. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD004278.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leucht 2009
- Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second‐generation versus first‐generation antipsychotic drugs for schizophrenia: a meta‐analysis. Lancet 2009;373(9657):31‐41. [DOI] [PubMed] [Google Scholar]
Liu 2009
- Liu X, Haan S. Chlorpromazine dose for people with schizophrenia. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007778] [DOI] [PubMed] [Google Scholar]
Maier 1988
- Maier W, Buller R, Philipp M, Heuser I. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. Journal of Affective Disorders 1988;14(1):61‐8. [DOI: 10.1016/0165-0327(88)90072-9] [DOI] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. [DOI] [PubMed] [Google Scholar]
Mathews 2007
- Mathews M, Muzina DJ. Atypical antipsychotics: new drugs, new challenges. Cleveland Clinic Journal of Medicine 2007;74(8):597‐606. [DOI] [PubMed] [Google Scholar]
McGrath 2008
- McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiologic Reviews 2008;30:67‐76. [DOI: 10.1093/epirev/mxn001] [DOI] [PubMed] [Google Scholar]
Meltzer 1989
- Meltzer HY. Clinical studies on the mechanism of action of clozapine: the dopamine‐serotonin hypothesis of schizophrenia. Psychopharmacology 1989;99 Suppl:S18‐27. [DOI: 10.1007/BF00442554] [DOI] [PubMed] [Google Scholar]
Monchi 2001
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event‐related functional magnetic resonance imaging. The Journal of Neuroscience 2001;21(19):7733‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. [DOI] [PubMed] [Google Scholar]
Nadal 2001
- Nadal R. Pharmacology of the atypical antipsychotic remoxipride, a dopamine D2 receptor antagonist. CNS Drug Reviews 2001;7(3):265‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Odejide 1982
- Odejide AO, Ban TA. Psychotropic drug prescription pattern in a developing country (Nigeria). The need for an essential psychotherapeutic drug list. International Pharmacopsychiatry 1982;17(3):163‐9. [DOI] [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Parrott 1980
- Parrott AC, Hindmarch I. The Leeds Sleep Evaluation Questionnaire in psychopharmacological Investigations ‐ a review. Psychopharmacology 1980;71(2):173‐9. [DOI] [PubMed] [Google Scholar]
Risbood 2012
- Risbood V, Lee JR, Roche‐Desilets J, Fuller MA. Lurasidone: an atypical antipsychotic for schizophrenia. Annals of Pharmacotherapy 2012;46(7‐8):1033‐46. [DOI] [PubMed] [Google Scholar]
Rossi 2006
- Rossi A, Daneluzzo E, Tomassini A, Struglia F, Cavallaro F, Smeraldi E, et al. The effect of verbalization strategy on wisconsin card sorting test performance in schizophrenic patients receiving classical or atypical antipsychotics. BMC Psychiatry 2006;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rzewuska 1988
- Rzewuska M. Sulpiride: the best known atypical, safe neuroleptic drug. Review of literature. Psychiatria Polska 1998;32(5):655‐66. [PUBMED: 9921002] [PubMed] [Google Scholar]
Saha 2005
- Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Medicine 2005;2(5):e141. [DOI: 10.1371/journal.pmed.0020141] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: The Cochrane Collaboration, 2008:359‐83. [Google Scholar]
Smith 2010
- Smith TLL, Carter CW. Asenapine: a novel atypical antipsychotic agent for schizophrenia and bipolar I disorder. Journal of Pharmacy Technology 2010;26:352‐61. [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D, on behalf of the Cochrane Bias Methods Group. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org 2011.
Sullivan 1993
- Sullivan M, Karlsson J, Bengtsson C, Furunes B, Lapidus L, Lissner L. The Göteborg Quality of Life Instrument – a psychometric evaluation of assessments of symptoms and well‐being among the women in a general population. Scandinavian Journal of Primary Health Care 1993;11(4):267‐75. [DOI] [PubMed] [Google Scholar]
Takahashi 1999
- Takahashi N, Terao T, Oga T, Okada M. Comparison of risperidone and mosapramine addition to neuroleptic treatment in chronic schizophrenia. Neuropsychobiology 1999;39(2):81‐5. [DOI] [PubMed] [Google Scholar]
Toren 1995
- Toren P, Samuel E, Weizman R, Golomb A, Eldar S, Laor N. Emergence of transient compulsive symptoms during treatment with clothiapine. Journal of the American Academy of Child & Adolescent Psychiatry 1995;34(11):1469‐72. [DOI: 10.1097/00004583-199511000-00013] [DOI] [PubMed] [Google Scholar]
Turner 2007
- Turner T. Chlorpromazine: unlocking psychosis. BMJ (Clinical research ed.) 2007;334 Suppl 1:s7. [PUBMED: 17204765] [DOI] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):iii‐92. [PubMed] [Google Scholar]
WHO 2011
- World Health Organization. WHO model list of essential medicines. 17th Edition. Geneva: World Health Organization, March 2011. [Google Scholar]
WMS‐IV 2009
- Wechsler D, Holdnack JA, Drozdick LW. Wechsler Memory Scale – Fourth Edition (WMS‐IV). San Antonio: Pearson, 2009. [Google Scholar]
Wu 2006
- Wu T, Li Y, Liu G, Bian Z, Li J, Zhang J, et al. Investigation of authenticity of 'claimed' randomized controlled trials (RCTs) and quality assessment of RCT reports published in China. Proceedings of the 14th Cochrane Colloquium; 2006 October 23‐26; Dublin. Dublin, 2006.
Xia 2009
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7. [Google Scholar]
Yorston 2000
- Yorston G, Pinney A. Chlorpromazine equivalents and percentage of British National Formulary maximum recommended dose in patients receiving high‐dose antipsychotics. Psychiatric Bulletin 2000;24(4):130‐2. [Google Scholar]
Zhang 1999
- Zhang W, Bymaster FPP. The in vivo effects of olanzapine and other antipsychotic agents on receptor occupancy and antagonism of dopamine D1, D2, D3, 5HT2A and muscarinic receptors. Psychopharmacology 1999;141(3):267‐78. [DOI: 10.1007/s002130050834] [DOI] [PubMed] [Google Scholar]


