Abstract
A previously developed and industrially feasible process for selective, Pd-mediated, liquid-phase heterogeneous catalytic hydrogenation of nitriles to primary amines was extended to the reduction of 3-phenylpropionitrile (PPN) to 3-phenylpropylamine (PPA). PPN, which belongs to the homologous series of benzonitrile (BN) and benzyl cyanide (BC), was hydrogenated under mild reaction conditions (30–80 °C, 6 bar), over Pd/C, in two immiscible solvents (dichloromethane/water) and using acidic additives (NaH2PO4 and H2SO4). Although relatively high conversion (76%) was achieved, the selectivity to PPA (26%) and its isolated yield (20%) were lesser than those in the case of the hydrogenation of BN or BC reported earlier. However, the purity of PPA was >99% without using any purification method. Quantum chemical calculations using a density functional theory (DFT) method were performed to compare the adsorption interactions of the different imine intermediates on palladium, as well as to clarify the differences observed in the primary amine selectivity. PPA is a valuable intermediate for the synthesis of carboxypeptidase B enzyme inhibitors, antimuscarinic drugs, or potential anticancer agents in the pharmaceutical industry.
Introduction
Our method1 developed previously allows the efficient, industrially feasible, and selective, Pd-catalyzed heterogeneous catalytic hydrogenation of nitriles to the corresponding primary amines in liquid phase. Full conversion, very high isolated yield (90%), and excellent selectivity (95%) can be achieved using this process. The reduction of benzonitrile (BN) to benzylamine (BA) was carried out over palladium on different supports, in two immiscible solvents (dichloromethane/water), applying a medium acidic additive (NaH2PO4), under mild conditions (30 °C, 6 bar), and a very pure product (>99%) was obtained without any purifying procedures.1 Later, this method was also applied in the hydrogenation of benzyl cyanide (BC) to 2-phenylethylamine (PEA), but the complete conversion of BC was accompanied by lower primary amine selectivity (45%) and isolated yield (40%).2
In this study, by adapting the aforementioned reduction process modified slightly, the liquid-phase catalytic hydrogenation of 3-phenylpropionitrile (PPN), which belongs to the homologous series of BN and BC, to 3-phenylpropylamine (PPA) was examined in detail. PPA is a valued product for using as a template molecule in the synthesis of vanadium oxide nanotubes3,4 or ion exchangers,5 as well as in that of Hofmann-type complexes containing cyanometallate groups.6 However, it plays the most significant role in the pharmaceutical industry because it can be applied to produce carboxypeptidase B (CpB) enzyme inhibitors,7 antimuscarinic drugs,8 or potential anticancer agents.9
Some recent reviews have provided overviews of the new developments in transition metal-catalyzed heterogeneous or homogeneous catalytic hydrogenation of nitriles.10−14 In addition, the general reduction methods of nitriles to primary amines were previously reported in detail.1,2 Recently, however, some very interesting multicomponent nanocatalyst systems have been fabricated for the transfer hydrogenation of nitrile compounds.15−17 These magnetite nanosphere-supported alloy catalysts contained mainly copper15−17 or iron,15 while precious metals (Pd15,16 or Rh17) were present only at a ppm level. Thus, for example, various nitriles were completely converted to primary amines with high selectivities (85–97%) over a Pd–Cu catalyst in the absence of iron, but secondary amines were obtained with good and excellent selectivities (76–98%) in the presence of Fe.15 Moreover, the catalyst could readily be separated by an external magnetic field and reused (5×) without significant activity loss.
Henceforth, we focus on the hydrogenation of PPN or its α,β-unsaturated derivative, cinnamonitrile (CN). Both heterogenous catalysts, such as nickel,18−23 cobalt,20,22,24 copper,22 ruthenium,22 or palladium,25,26 and homogeneous ones, such as Ir(I),27 Ru(II),28,29 Fe(II),30−32 Co(III),33,34 Mn(I),35,36 or Rh(I)37 complexes, were used in these reductions. Thus, CN was converted to cinnamylamine (CA) over Raney-type catalysts (Ni or Co), in methanol containing 14% NH3, at 100 °C and 80 bar with 60–80% selectivity and almost complete conversion (90–99%).20 Similarly, cinnamonitrile was hydrogenated to CA with 74% selectivity and complete conversion, over a highly dispersed 11% copper on silica catalyst, in toluene, at 130 °C and 40 bar, while over a 1.8% Ru/SiO2 catalyst, only 8% conversion of CN and 40% selectivity to CA were achieved also in toluene, at 100 °C and 13 bar.22 Using a 5% palladium on carbon catalyst at 60 °C and 4 bar, in methanol, the hydrogenation of PPN and CN, respectively, was also investigated.25 It was found that the reduction of CN resulted in exclusively PPN, that is, the saturation of C=C double bond took place fast and selectively, while no PPA was detected. When PPN was hydrogenated under the same conditions, no formation of PPA was also observed. Similar results were reported by Arai and co-workers when a two-phase medium (toluene and water) and supercritical carbon dioxide (sCO2, p = 10 bar) were used in the Pd-catalyzed hydrogenation of PPN and CN, respectively.26 With 5% palladium on alumina, only PPN was formed in the reduction of cinnamonitrile after 30 min, at 50 °C and 40 bar, while that of PPN resulted in PPA with 82% selectivity, after 60 min, but only in the presence of sCO2 and at a relatively low conversion (32%). These methods, however, have some disadvantages: using a large excess of ammonia, applying special reaction conditions, and no or low conversion of PPN achieved by palladium.
In this work, the influences of temperature, the acidic additives, reaction time, solvents, and the amount of the catalyst on the isolated yield and the selectivity to PPA, as well as on the conversion of PPN are discussed. Furthermore, a comparison of the observed differences in the selectivity in the hydrogenation of nitriles investigated previously and currently (BN, BC, PPN) was also made by quantum chemical calculations. The density functional theory (DFT) method was used for modeling the adsorption interactions between imine-type intermediates (benzaldimine, 2-phenylethylimine, 3-phenylpropylimine) and the palladium catalyst.
Results and Discussion
Reaction Pathways
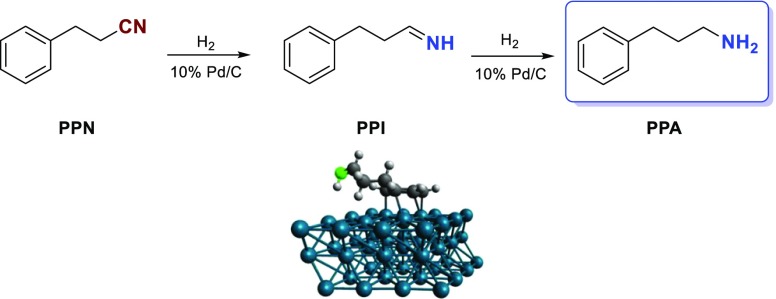
Scheme 1 shows the probable reaction pathways (intermediates, products) in the hydrogenation of PPN. As seen, the formation of the primary amine (PPA) takes place through the 3-phenylpropylimine (PPI) intermediate, but a secondary amine, bis(3-phenylpropyl)amine (BPPA), could also be formed in a condensation reaction of PPN and PPI, accompanied by the elimination of NH3. Further addition of PPI to BPPA followed by hydrogenation could provide a tertiary amine, tris(3-phenylpropyl)amine (TPPA). Whereas, other side reactions could arise because PPI could react with water to form 3-phenylpropanal (PPAL), which could also undergo hydrogenation to give 3-phenyl-1-propanol (PPOH).
Scheme 1. Possible Reaction Pathways in the Hydrogenation of PPN over Palladium.
Influence of Acidic Additives and Temperature
At first, similarly to our previous works,1,2 sodium dihydrogen phosphate (NaH2PO4) was used as an acidic additive in the hydrogenation of PPN. Its function is to form a salt with PPA and to hold that in the aqueous phase, decreasing the chance of byproduct formation.
As seen in Table 1, the use of 2.0 equimolar NaH2PO4 resulted in low conversion of PPN and selectivity to PPA (24 and 27%, respectively) in a dichloromethane/water solvent mixture, over 10% Pd/C (Selcat Q) catalyst (0.3 g·g–1 catalyst/substrate ratio), at 30 °C and 6 bar, after 7 h. Furthermore, the isolated yield of the product (7%) and its PPA-content (88.2%) were also much lower than those values obtained in the hydrogenation of benzonitrile1 or benzyl cyanide.2 To improve the conversion of PPN, the reaction temperature was increased to 50 and 80 °C, respectively (Table 1). As expected, higher conversions (36 and 53%) were achieved at these higher temperatures, but the selectivity to PPA decreased to 21–22%, and the purity of the product remained the same (88.8–90.0% PPA-content). On the basis of the gas chromatography–mass spectrometry (GC–MS) measurements, these low primary amine selectivities were due to the formation of the tertiary amine byproduct (TPPA) in a higher amount (14.8–45.0%), as shown in Table 2. Interestingly, no production of secondary amine (BPPA) was observed at any temperatures applied. Presumably, the more aliphatic PPI can easily react with BPPA to produce TPPA as well.
Table 1. Influence of Acidic Additives and Temperature in the Hydrogenation of PPNa.
| product |
|||||||
|---|---|---|---|---|---|---|---|
| no. | acidic additive/substrate ratio (mol mol–1) | temperature (°C) | conversion (%) | isolated yield (%) | PPA-content (%) | selectivity to PPA (%) | v0 (nL H2 gPd–1 h–1) |
| 1 | 2.0 NaH2PO4 | 30 | 24 | 7 | 88.2 | 27 | 0.95 |
| 50 | 36 | 9 | 88.5 | 22 | 1.23 | ||
| 80 | 53 | 12 | 90.0 | 21 | 2.10 | ||
| 2 | 1.0 NaH2PO4/1.0 H2SO4 | 30 | 46 | 22 | 99.5 | 48 | 1.98 |
| 50 | 61 | 22 | 99.1 | 36 | 2.63 | ||
| 80 | 76 | 20 | 99.3 | 26 | 3.19 | ||
| 3 | 2.0 NaH2PO4/2.0 H2SO4 | 80 | 75 | 20 | 99.2 | 26 | 3.20 |
Conditions: 5.0 g (38.2 mmol) PPN, 1.5 g 10% Pd/C (Selcat Q) catalyst, 250 mL water and 50 mL dichloromethane, 6 bar, reaction time: 7 h.
Table 2. Effect of Acidic Additives and Temperature on the Ratio of Byproducts in the Hydrogenation of PPNa.
| composition
of byproduct |
|||||||
|---|---|---|---|---|---|---|---|
| no. | acidic additive/substrate ratio (mol mol–1) | temperature (°C) | PPN (%) | TPPA (%) | BPPA (%) | PPAL (%) | PPOH (%) |
| 1 | 2.0 NaH2PO4 | 30 | 85.2 | 14.8 | 0 | 0 | 0 |
| 50 | 71.5 | 28.5 | 0 | 0 | 0 | ||
| 80 | 55.0 | 45.0 | 0 | 0 | 0 | ||
| 2 | 1.0 NaH2PO4/1.0 H2SO4 | 30 | 73.0 | 20.1 | 0 | 6.9 | 0 |
| 50 | 53.6 | 41.4 | 0 | 3.5 | 1.5 | ||
| 80 | 31.1 | 66.5 | 0 | 0.8 | 1.6 | ||
| 3 | 2.0 NaH2PO4/2.0 H2SO4 | 80 | 33.5 | 62.7 | 0 | 1.2 | 2.6 |
Conditions: 5.0 g (38.2 mmol) PPN, 1.5 g 10% Pd/C (Selcat Q) catalyst, 250 mL water and 50 mL dichloromethane, 6 bar, reaction time: 7 h.
These results suggest that NaH2PO4 with moderate acidity (pH = 3.5) was not able to form a stable salt with PPA, resulting in moderate conversion and low primary amine selectivity. Since PPA is a stronger organic base (pKb = 3.95) than 2-phenylethylamine (pKb = 4.16) or benzylamine (pKb = 4.57) obtained previously by applying this process,1,2 the reduction of PPN was also examined under more acidic conditions (pH < 2). Typically, mineral acids (e.g., hydrochloric acid, sulfuric acid, and ortho-phosphoric acid) can be the obvious choice for this purpose. Based on our previous experience in the hydrogenation of benzyl cyanide,2 where the use of HCl or H3PO4 proved to be less successful, H2SO4 was chosen as another acidic additive.
Accordingly, the half amount of sodium dihydrogen phosphate was replaced with sulfuric acid and the effects of these acidic additives on the conversion of PPN and the selectivity to PPA, under the same conditions (10% Pd/C (Selcat Q), dichloromethane/water, 30–80 °C, 6 bar), are also summarized in Table 1. As seen, the presence of 1.0 equimolar H2SO4 in addition to 1.0 equimolar NaH2PO4 resulted in better conversion (46%), isolated yield (22%), and primary amine selectivity (48%) at 30 °C compared to those values achieved with NaH2PO4 itself. Moreover, the product purity was also improved (>99% PPA-content). When the temperature was raised to 50 or 80 °C, the conversion of PPN was also increased (61 and 76%, respectively), but this was accompanied by diminishing the selectivity to PPA (35 and 26%), similarly to the use of NaH2PO4 itself. The observed low primary amine selectivities were also due to the formation of TPPA in a much higher amount (20.1–66.5%), and still no BPPA was detected (Table 2). However, the isolated yields (20–22%) and the PPA-content of the product (>99%) remained to be practically the same values. Further increase of the amount of H2SO4 and NaH2PO4 (2.0 equimolar separately) had no significant effect in the course of the hydrogenation of PPN, because the conversion (75%) and the selectivity to PPA remained unchanged (26%). Besides, the composition of the byproduct was also practically the same as found in the presence of a lesser amount of the acidic additives applied (Table 2). Thus, it is unnecessary to use them in a high proportion because this resulted in no further improvements, neither in the conversion nor in the primary amine selectivity.
According to our results, the combined application of these two acidic additives (H2SO4 and NaH2PO4) is a key parameter in terms of the conversion of PPN and the selectivity to PPA. Although the highest primary amine selectivity (48%) and isolated yield (22%) are obtained at 30 °C, the conversion (46%) is moderate. To achieve a relatively high conversion of PPN (76%), this hydrogenation has to be carried out at 80 °C, at most, but the selectivity to PPA is low (26%) due to the high amount of TPPA formed almost exclusively.
Effect of Reaction Time
Since the conversion of PPN was not complete after 7 h, even at 80 °C, the influence of reaction time on the primary amine selectivity, the conversion, and the isolated yield in this hydrogenation over 10% Pd/C (Selcat Q), in dichloromethane/water, in the presence of 1.0 equimolar NaH2PO4 and 1.0 equimolar H2SO4, at 80 °C and 6 bar is shown in Figure 1 and Table 3.
Figure 1.

Conversion and selectivity vs time in the hydrogenation of PPN over palladium. Conditions: 5.0 g (38.2 mmol) PPN, 1.5 g 10% Pd/C (Selcat Q) catalyst, 250 mL water and 50 mL dichloromethane, 5.27 g NaH2PO4·H2O (38.2 mmol) and 3.82 g conc. H2SO4 (38.2 mmol), 80 °C, 6 bar.
Table 3. Influence of Reaction Time in the Hydrogenation of PPNa.
| product |
composition of byproduct |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| no. | reaction time (h) | isolated yield (%) | PPA-content (%) | PPN (%) | TPPA (%) | BPPA (%) | PPAL (%) | PPOH (%) | v0 (nL H2 gPd–1 h–1) |
| 1 | 3.5 | 16 | 99.5 | 73.4 | 25.5 | 0 | 1.1 | 0 | 3.18 |
| 2 | 7 | 20 | 99.3 | 31.1 | 66.5 | 0 | 0.8 | 1.6 | 3.19 |
| 3 | 14 | 19 | 99.2 | 25.3 | 72.1 | 0 | 1.1 | 1.5 | 3.17 |
| 4 | 21 | 20 | 99.3 | 17.3 | 80.4 | 0 | 1.1 | 1.2 | 3.20 |
Conditions: 5.0 g (38.2 mmol) PPN, 1.5 g 10% Pd/C (Selcat Q) catalyst, 250 mL water and 50 mL dichloromethane, 5.27 g NaH2PO4·H2O (38.2 mmol) and 3.82 g conc. H2SO4 (38.2 mmol), 80 °C, 6 bar.
As seen, moderate conversion (41%) and selectivity to PPA (38%) were achieved after a relatively short reaction time (3.5 h), and a very pure product (99.5% PPA-content) was isolated in 16% yield. When the reaction time was increased to 7 h, the conversion of PPN (76%) and the isolated yield of PPA (20%) also became higher, but the selectivity to PPA significantly decreased to 26%. A further increase of the reaction time to 14 or 21 h resulted in higher conversions (81 and 86%, respectively) and a slight decrease in the selectivity values (23%), while the isolated yields remained unchanged (19–20%).
Although a very pure product (>99% PPA-content) was obtained in each hydrogenation of PPN with different reaction times, it was also observed that the poor selectivity to PPA after 14 or 21 h was due to a higher ratio of byproducts (Table 3), that is, the amount of tertiary amine (TPPA) was significantly increased (25.5 → 80.4%). The reaction profile for the hydrogenation of PPN (Figure 2) also shows that no formation of secondary amine (BPPA) was observed, at all, even after 21 h. The ratio of miscellaneous byproducts (PPAL and/or PPOH) was minimal (0.8–1.1 and 0–1.6%, respectively) and remained practically the same irrespectively of the reaction time. These results indicate that there are more opportunities for side reactions to take place, as shown in Scheme 1, after longer reaction times, presumably due to a weaker adsorption of the imine intermediate (PPI) on palladium. To confirm our hypothesis, quantum chemical calculations (DFT) were performed to model and compare the adsorption interactions between imines (benzaldimine, 2-phenylethylimine, PPI) and palladium, which will be discussed later.
Figure 2.

Reaction profile for the hydrogenation of PPN over palladium. Conditions: 5.0 g (38.2 mmol) PPN, 1.5 g 10% Pd/C (Selcat Q) catalyst, 250 mL water and 50 mL dichloromethane, 5.27 g NaH2PO4·H2O (38.2 mmol) and 3.82 g conc. H2SO4 (38.2 mmol), 80 °C, 6 bar.
On the basis of our results, it can be concluded that it is expedient to carry out this hydrogenation within 7 h to obtain a relatively high conversion value (76%), a reasonable isolated yield (20%) and primary amine selectivity (26%), namely, the further increase of the reaction time (up to 21 h) results in mainly the formation of TPPA and a slight improvement in the conversion of PPN.
Influence of Organic Solvents
As well-known,38 significant differences in the selectivity and the activity of the catalysts used in hydrogenation reactions can occur due to considerable solvent effects.
The results of the hydrogenation of PPN in the mixtures of water and different organic solvents, over 10% Pd/C (Selcat Q), using 1.0 equimolar NaH2PO4 and 1.0 equimolar H2SO4, at 80 °C and 6 bar, after 7 h are given in Table 4.
Table 4. Effect of Organic Solvents in the Hydrogenation of PPNa.
| product |
||||||
|---|---|---|---|---|---|---|
| no. | organic solvents | conversion (%) | isolated yield (%) | PPA-content (%) | selectivity to PPA (%) | v0 (nL H2 gPd–1 h–1) |
| 1 | dichloromethane | 76 | 20 | 99.3 | 26 | 3.19 |
| 2 | hexane | 52 | 1 | 87.5 | 2 | 2.28 |
| 3 | toluene | 56 | 5 | 88.3 | 7 | 2.74 |
| 4 | tert-butyl methyl ether | 78 | 7 | 84.7 | 8 | 3.65 |
| 5 | ethyl acetate | 76 | 9 | 95.9 | 11 | 3.42 |
Conditions: 5.0 g (38.2 mmol) PPN, 1.5 g 10% Pd/C (Selcat Q) catalyst, 250 mL water and 50 mL organic solvent, 5.27 g NaH2PO4·H2O (38.2 mmol) and 3.82 g conc. H2SO4 (38.2 mmol), 80 °C, 6 bar, reaction time: 7 h.
As seen, a dichloromethane/water solvent mixture provided the best isolated yield (20%) selectivity (26%) and product purity (99.3% PPA-content) at a relatively high conversion value (76%). When a hexane/water solvent mixture was applied, the conversion (52%), the isolated yield (1%), the selectivity (2%), and the purity of the product (87.5% PPA-content) decreased drastically. The main reason for these low values was the fact that the starting nitrile cannot be dissolved in hexane, and the catalyst was conglomerated by the substrate and it seceded onto the inner body of the autoclave. There was no solubility problem in toluene; however, both the conversion of PPN (56%) and the selectivity to PPA (7%) and the isolated yield (5%) and the purity of the product (88.3% PPA-content) became appreciably lower than those in the water/dichloromethane system. Applying tert-butyl methyl ether, a relatively high conversion value (78%) was obtained, but the other results (8% selectivity, 7% isolated yield, 84.7% PPA-content) were very similar to those achieved with toluene. In an ethyl acetate/water mixture, the hydrogenation of PPN was also fast, similarly to dichloromethane or tert-butyl methyl ether (Figure 3), and a slight increase in the conversion (76%), the selectivity (11%), the isolated yield (9%), and the purity of the product (95.9% PPA-content) was observed compared to toluene or tert-butyl methyl ether.
Figure 3.

Conversion of PPN vs time in the mixtures of water and different organic solvents. Conditions: 5.0 g (38.2 mmol) PPN, 1.5 g 10% Pd/C (Selcat Q) catalyst, 250 mL water and 50 mL organic solvent, 5.27 g NaH2PO4·H2O (38.2 mmol) and 3.82 g conc. H2SO4 (38.2 mmol), 80 °C, 6 bar.
As seen in Table 5, the use of different organic solvents instead of dichloromethane significantly altered the ratio of byproducts, resulting in very low selectivity to PPA. In all cases, similar to the hydrogenations carried out in the presence of dichloromethane, no formation of BPPA was observed, but in hexane or toluene, the amount of TPPA was appreciably lower (28.0 and 38.2%, respectively) than those detected in tert-butyl methyl ether, ethyl acetate, or dichloromethane (69.3, 68.6 or 66.5%). The lower tertiary amine contents were due to 3-phenylpropanal (PPAL) and 3-phenyl-1-propanol (PPOH) formed in higher ratios (7.5–18.5 and 1.9–4.7%, respectively).
Table 5. Influence of Organic Solvents on the Ratio of Byproducts in the Hydrogenation of PPNa.
| composition
of byproduct |
||||||
|---|---|---|---|---|---|---|
| no. | organic solvents | PPN (%) | TPPA (%) | BPPA (%) | PPAL (%) | PPOH (%) |
| 1 | dichloromethane | 31.1 | 66.5 | 0 | 0.8 | 1.6 |
| 2 | hexane | 51.6 | 28.0 | 0 | 18.5 | 1.9 |
| 3 | toluene | 49.6 | 38.2 | 0 | 7.5 | 4.7 |
| 4 | tert-butyl methyl ether | 25.4 | 69.3 | 0 | 0.9 | 4.4 |
| 5 | ethyl acetate | 22.8 | 68.6 | 0 | 0 | 8.6 |
Conditions: 5.0 g (38.2 mmol) PPN, 1.5 g 10% Pd/C (Selcat Q) catalyst, 250 mL water and 50 mL organic solvent, 5.27 g NaH2PO4·H2O (38.2 mmol) and 3.82 g conc. H2SO4 (38.2 mmol), 80 °C, 6 bar, reaction time: 7 h.
A significant amount of PPOH (8.6%) was also measured in ethyl acetate. Presumably, the different organic solvents significantly affected the adsorption strength of the imine type intermediate on palladium.
Accordingly, it is preferred to use a water-immiscible organic solvent as an accompanying solvent pair of water during this hydrogenation. Altogether, dichloromethane gave the best results related to conversion, selectivity, isolated yield, and purity among the solvents tested.
Influence of the Amount of the Catalyst
The effect of different amounts of 10% Pd/C (Selcat Q) catalyst on the selectivity to PPA, as well as on the conversion of PPN and the isolated yield in dichloromethane/water, using 1.0 equimolar NaH2PO4 and 1.0 equimolar H2SO4, at 80 °C and 6 bar is summarized in Table 6.
Table 6. Effect of the Amount of the Catalyst (10% Pd/C) in the Hydrogenation of PPNa.
| product |
||||||
|---|---|---|---|---|---|---|
| no. | catalyst/substrate ratio (g g–1) | conversion (%) | isolated yield (%) | PPA-content (%) | selectivity to PPA (%) | v0 (nL H2 gPd–1 h–1) |
| 1 | 0.30 | 74 | 20 | 99.3 | 26 | 3.19 |
| 2 | 0.25 | 74 | 19 | 99.4 | 25 | 3.83 |
| 3 | 0.20 | 61 | 15 | 99.8 | 24 | 3.94 |
| 4 | 0.15 | 57 | 14 | 99.6 | 24 | 4.91 |
| 5 | 0.10 | 51 | 11 | 99.0 | 21 | 6.59 |
Conditions: 5.0 g (38.2 mmol) PPN, 10% Pd/C (Selcat Q) catalyst, 250 mL water and 50 mL dichloromethane, 5.27 g NaH2PO4·H2O (38.2 mmol) and 3.82 g conc. H2SO4 (38.2 mmol), 80 °C, 6 bar, reaction time: 7 h.
As seen, the decrease of the catalyst/substrate ratio from 0.30 to 0.10 g·g–1 gradually by 0.05 g·g–1 was accompanied by diminishing the isolated yield (20 → 11%) and selectivity to PPA (26 → 21%) after a reaction time of 7 h. In addition, the conversion of PPN also became significantly lower (74 → 51%) by decreasing the amount of palladium on carbon. In all cases, however, the purity of the product was also very high (>99% PPA-content). Since the highest selectivity (25–26%) and isolated yield (19–20%) were obtained at 0.25–0.30 g·g–1 catalyst/substrate ratios, it is favorable to use these amounts of the catalyst for this hydrogenation.
As also noticeable in Table 6, the initial rates (v0) continuously increased from 3.19 to 6.59 nL H2 gPd–1 h–1 by decreasing the amount of 10% Pd/C (0.30 → 0.10 g·g–1). Obviously, in the case of kinetically controlled reaction, v0 (expressed as g–1 of Pd), should not change unless the reaction conditions are altered. Nevertheless, it was observed that the consistence of the reaction mixture had varied above 55 °C (Figure 4) at all catalyst/substrate ratios applied; namely, the normal suspension of the Pd/C catalyst (Figure 4a) had turned to a more viscous one (Figure 4b, red marker), resulting in a less effective stirring and difficulties in the contact of the different phases. Due to this alteration, at higher temperature (>55 °C), the mass transport became the dominant step in this hydrogenation.
Figure 4.

Consistence of the slurry reaction mixture at 30 °C (a) and above 55 °C (b) in the hydrogenation of PPN over palladium on carbon. Conditions: 5.0 g (38.2 mmol) PPN, 10% Pd/C (Selcat Q) catalyst, 250 mL water and 50 mL dichloromethane, 5.27 g NaH2PO4·H2O (38.2 mmol) and 3.82 g conc. H2SO4 (38.2 mmol), 6 bar, reaction time: 7 h.
Furthermore, it was found that there was no appreciable difference in the composition of byproducts by changing the amount of catalyst (Table 7). As seen, the ratio of the unreacted PPN increased from 31.1 to 55.5% by decreasing the catalyst/substrate ratio, but the main byproduct also remained to be TPPA (66.5–40.8%) accompanied by the minimal formation of PPAL (0.5–0.8%) or PPOH (0.9–3.7%). Similarly to the previous results, no secondary amine (BPPA) was detected as well. Accordingly, the different amounts of palladium on carbon had no effect on the ratio of the byproducts; namely, tertiary amine (TPPA) was almost solely formed.
Table 7. Influence of the Amount of Catalyst (10% Pd/C) on the Ratio of Byproducts in the Hydrogenation of PPNa.
| composition
of byproduct |
||||||
|---|---|---|---|---|---|---|
| no. | catalyst/substrate ratio (g g–1) | PPN (%) | TPPA (%) | BPPA (%) | PPAL (%) | PPOH (%) |
| 1 | 0.30 | 31.1 | 66.5 | 0 | 0.8 | 1.6 |
| 2 | 0.25 | 32.8 | 65.8 | 0 | 0.5 | 0.9 |
| 3 | 0.20 | 47.5 | 49.5 | 0 | 0 | 3.0 |
| 4 | 0.15 | 52.6 | 45.0 | 0 | 0 | 2.4 |
| 5 | 0.10 | 55.5 | 40.8 | 0 | 0 | 3.7 |
Conditions: 5.0 g (38.2 mmol) PPN, 1.5 g 10% Pd/C (Selcat Q) catalyst, 250 mL water and 50 mL dichloromethane, 5.27 g NaH2PO4·H2O (38.2 mmol), and 3.82 g conc. H2SO4 (38.2 mmol), 80 °C, 6 bar, reaction time: 7 h.
Although the applied catalyst/substrate ratios (0.10–0.30 g·g–1) are comparatively high with referring to those (0.01–0.10 g·g–1) commonly used in the industry,39 the 10% Pd/C catalyst is economically viable on an industrial scale, taking into account the high price of PPA.
Recycling the Used Catalyst or the Unreacted Starting Material
Reuse of the Pd/C Catalyst
The influence of the used 10% Pd/C (Selcat Q) on the primary amine selectivity, the conversion of PPN, and the isolated yield of PPA, in dichloromethane/water, in the presence of 1.0 equimolar NaH2PO4 and 1.0 equimolar H2SO4, at 80 °C and 6 bar, after 7 h is shown in Figure 5.
Figure 5.

Effect of the reused (a), as well as the prehydrogenated and reused (b) 10% Pd/C catalysts on conversion, isolated yield, and selectivity in the hydrogenation of PPN. Conditions: 5.0 g (38.2 mmol) PPN, 1.5 g 10% Pd/C (Selcat Q) catalyst, 250 mL water and 50 mL dichloromethane, 5.27 g NaH2PO4·H2O (38.2 mmol) and 3.82 g conc. H2SO4 (38.2 mmol), 80 °C, 6 bar, reaction time: 7 h.
As seen, the reused catalyst provided much worse results; namely, the conversion, the selectivity, and the isolated yield were significantly deteriorated (76 → 38, 26 → 12, and 21 → 7%, respectively) already in the second reaction (Figure 5a). This clearly indicates that the catalyst was partly deactivated during the second run, presumably due to the poisoning of the catalytically active centers or an intense effect of the strong mineral acid (H2SO4) on the highly dispersed Pd particles. The latter was evidenced by measuring the palladium content of the reused catalyst using an X-ray fluorescent (XRF) spectroscopy method; namely, the original value (10.0% Pd of the fresh catalyst) decreased to 8.7%. No Pd contamination, however, was detected in the final product (PPA) due to our very efficient work-up procedure,1,2 but its purity appreciably diminished from 99.0 to 69.6% (Table 8). The impurity was the starting material (PPN) due to its low conversion (38%). The byproduct, in addition to PPN (66.0%), contained mainly TPPA (26.3%) and a low amount of PPAL and PPOH (7.6 and 0.1%, respectively), that is, the components of the side product were the same as observed in the first reaction.
Table 8. Influence of the Reused 10% Pd/C Catalyst on the Ratio of Byproducts in the Hydrogenation of PPNa.
| composition
of byproduct |
|||||||
|---|---|---|---|---|---|---|---|
| no. | reusing the catalyst | purity of PPA (%) | PPN (%) | TPPA (%) | BPPA (%) | PPAL (%) | PPOH (%) |
| 1 | -(fresh) | 99.0 | 29.9 | 68.3 | 0 | 1.3 | 0.5 |
| 2 | 1st | 69.6 | 66.0 | 26.3 | 0 | 7.6 | 0.1 |
| 3 | -(fresh)b | 99.8 | 33.5 | 64.6 | 0 | 1.3 | 0.6 |
| 4 | 1stb | 99.7 | 32.5 | 65.8 | 0 | 1.2 | 0.5 |
Conditions: 5.0 g (38.2 mmol) PPN, 1.5 g 10% Pd/C (Selcat Q) catalyst, 250 mL of water and 50 mL dichloromethane, 5.27 g NaH2PO4·H2O (38.2 mmol), and 3.82 g conc. H2SO4 (38.2 mmol), 80 °C, 6 bar, reaction time: 7 h.
10% Pd/C catalyst was prehydrogenated in 200 mL water, at 30 °C and 6 bar, for 0.5 h.
To avoid Pd leaching, the catalyst was prehydrogenated (30 °C, 6 bar, 0.5 h) prior to the reaction, as well as before its reuse. As a result of this pretreatment, the Pd content of the catalyst also remained to be 10.0% even after the second run. As seen in Figure 5b, using the prehydrogenated Pd/C catalyst resulted in a slightly higher isolated yield (23%) and primary amine selectivity (30%) than those values obtained in the original reduction of PPN in the first run (Figure 5a). Furthermore, very similar conversion (73%), isolated yield (19%), and selectivity to PPA (27%) were achieved with the prehydrogenated Pd/C catalyst in the second run. These results indicate that the hydrogen-rich palladium is resistant to the strong acidic conditions.
In addition, the purity of the product (99.8 and 99.7%, respectively) and the composition of the side products were practically the same in each run using prehydrogenated palladium on carbon (Table 8). The side products contained mainly TPPA (64.6 and 65.8%. respectively), the unreacted PPN (33.5 and 32.5%, respectively), as well as a low amount of PPAL and PPOH (1.3–1.2 and 0.6–0.5%, respectively), that is, the ratios of the components in the side products were the same in these two reactions.
Accordingly, it is not expedient to reuse the 10% Pd/C spent catalyst in this hydrogenation because it showed lower activity and provided weaker conversion and primary amine selectivity already in the second catalytic run, whereas its application in a prehydrogenated form resulted in practically the same results after each reaction.
Recycling the Unreacted PPN
As it is mentioned above, the hydrogenation of PPN over 10% Pd/C (Selcat Q), in dichloromethane/water, using 1.0 equimolar NaH2PO4 and 1.0 equimolar H2SO4, at 30 °C and 6 bar, after 7 h resulted in a moderate selectivity to PPA (48%) and conversion of PPN (46%), but a relatively high ratio of PPN (73.0%) remained untouched (Table 2). Therefore, the reduction of PPN with recycling the unreacted starting material was also examined, and its result is depicted in Figure 6.
Figure 6.

Recycling the unreacted starting material and its influence on conversion, isolated yield, and selectivity in the hydrogenation of PPN. Conditions: (a) 5.0 g (38.2 mmol) PPN, 1.5 g 10% Pd/C (Selcat Q) catalyst, 250 mL water and 50 mL dichloromethane, 5.27 g NaH2PO4·H2O (38.2 mmol) and 3.82 g conc. H2SO4 (38.2 mmol), 30 °C, 6 bar, reaction time: 7 h; (b) 2.70 g (20.6 mmol) unreacted and 2.30 g (17.6 mmol) fresh PPN.
It was found that recycling the unreacted PPN could readily be realized; moreover, a moderate increase was achieved in the combined conversion (46 → 51%), selectivity (48 → 51%), and isolated yield (22 → 26%) values. Furthermore, as seen in Table 9, the purity of the product (99.8% PPA-content) and the composition of the byproduct were the same in both hydrogenations. Only the formation of TTPA (19.8–18.1%) and PPAL (7.2–8.2%) was also observed practically in unaltered proportions.
Table 9. Influence of the Unreacted Starting Material on the Ratio of Byproducts in the Hydrogenation of PPNa.
| composition of byproduct | ||||||
|---|---|---|---|---|---|---|
| no. | hydrogenation of PPN | purity of PPA (%) | PPN (%) | TPPA (%) | BPPA (%) | PPAL (%) |
| 1 | original reaction | 99.8 | 73.0 | 19.8 | 0 | 7.2 |
| 2b | recycling the unreacted PPN | 99.8 | 73.7 | 18.1 | 0 | 8.2 |
Conditions: 5.0 g (38.2 mmol) PPN, 1.5 g 10% Pd/C (Selcat Q) catalyst, 250 mL water and 50 mL dichloromethane, 5.27 g NaH2PO4·H2O (38.2 mmol) and 3.82 g conc. H2SO4 (38.2 mmol), 30 °C, 6 bar, reaction time: 7 h.
2.70 g (20.6 mmol) unreacted and 2.30 g (17.6 mmol) fresh PPN.
Based on these results, reusing the residual starting material could be an advantageous method from a technological aspect and could improve the efficiency of this hydrogenation process.
Theoretical Considerations
It was demonstrated in our previous work2 that there were no appreciable differences in the calculated energy profiles of the byproduct formation; thus, other interactions (e.g. diverse adsorption of the imine intermediate) could affect the primary amine selectivity during these reductions. Therefore, the adsorption of the imines, such as PPI, 2-phenylethylimine (PEI), and benzaldimine (BI), on the palladium catalyst was modeled by quantum chemical calculations (DFT method) to enable the explanation of the dissimilarities in the observed primary amine selectivity during the palladium-catalyzed hydrogenation of BN, BC, and PPN, respectively.
As shown in Figure 7, the computed most stable conformer of PPI, PEI, or BI was placed on an Pd48 cluster with a (111) surface, according to the energetically favored adsorption mode of benzene (the aromatic ring parallel to the surface) on Pd(111).40 To compare these interactions, the energy of adsorption (ΔEads) of these compounds on palladium was also calculated (Table 10).
Figure 7.

Computed adsorption modes of conformers with minimal energy of PPI (a), PEI (b), and BI (c) intermediates on a Pd48 three-layer slab with a (111) surface.
Table 10. Energy of Adsorption (ΔEads) of Imine-Type Intermediates on the Pd(111) Surface Applied in the Hydrogenation of the Corresponding Nitrilesa.
| no. | imine-type intermediates | ΔEads (kJ mol–1) |
|---|---|---|
| 1 | BI | –120.50 |
| 2 | PEI | –95.41 |
| 3 | PPI | –87.38 |
Calculated by using DFT method.
As seen in Figure 7c, the benzene ring and the imino group in the BI molecule are in the same plane, so they are simultaneously adsorbed on the catalyst surface. Accordingly, a stronger interrelationship forms between BI and Pd (ΔEads = −120.50 kJ mol–1), and this intermediate can remain on the catalyst surface for a longer period of time; therefore, there is a minimal chance of byproduct formation. On the contrary, the imino group is above the plane of the phenyl ring in the PEI or PPI molecule (Figure 7a,b), so these compounds can adsorb via their aromatic moiety only on palladium, resulting in weaker adsorption energies (ΔEads = −95.41 and −87.38 kJ mol–1, respectively). Thus, their imino segment can react more easily with another 2-phenylethylamine or PPA molecule to give secondary or tertiary amines in a higher amount. In addition, these intermediates can desorb easier from the surface of the catalyst, and they can react with the primary amines in the reaction mixture as well.
According to the results of these high-level DFT calculations, it can be stated that the observed low primary amine selectivity in the hydrogenation of PPN was due to weaker adsorption interaction between the imine intermediate (PPI) and palladium.
Conclusions
PPN was reduced to PPA over palladium on carbon, under mild reaction conditions (30–80 °C, 6 bar), in liquid phase by extending our selective, heterogeneous catalytic hydrogenation method for the conversion of nitriles to primary amines developed previously.1 PPN belongs to the homologous series of benzonitrile (BN) and benzyl cyanide (BC) whose hydrogenations were reported earlier.1,2 Despite the systematic variation of the reaction parameters, low primary amine selectivity (26%) and isolated yield (20%), at a relatively high conversion (76%), were achieved contrary to the results of the hydrogenation of BN or BC. The purity of the product, however, was over 99% without using any special purifying procedures. Although the unreacted starting material (PPN) could be recycled without any problems, the reused 10% Pd/C catalyst showed low activity and provided weaker conversion and primary amine selectivity values, respectively, whereas the prehydrogenation of the catalyst prior to its reusing improved its application in this hydrogenation.
In order to clarify the reasons of the differences in the primary amine selectivities, quantum chemical calculations using the DFT method were performed. The molecular modeling computations revealed that the diverse adsorption abilities of the imines could influence the selectivity of these hydrogenations. The calculated adsorption energies (ΔEads) of these intermediates on Pd(111) showed that their adsorption strength decreases in the following order
Due to these differences and the dissimilar structures of their conformers with minimal energy as well, the possibility of byproduct formation both on the surface of the catalyst and in the reaction mixture was increased with decreasing the adsorption strength of these imines, that is, there is a much higher chance for side reactions in the case of PPI.
Additional examinations to broaden this selective hydrogenation process to the reduction of other nitriles to primary amines are also proceeding.
Experimental Section
Materials
The 10% palladium on carbon catalyst (Selcat Q)41 was supplied by Szilor Ltd. (Budapest, Hungary). PPN was prepared from cinnamonitrile (98%) received from Merck (Darmstadt, Germany) by catalytic hydrogenation over 10% Pd/C, at 25 °C and 1 bar, in situ. Dichloromethane (p.a.), toluene (p.a.), ethyl acetate (p.a.), hexane (p.a.), tert-butyl methyl ether (p.a.), H2SO4 (98%), and NaH2PO4·H2O (p.a.) were purchased from Merck (Darmstadt, Germany).
Hydrogenation Reactions
The hydrogenation reactions were performed in a 0.5 dm3 BEP 280 (Büchi) glass autoclave (Büchi AG, Uster, Switzerland) equipped with a magnetically driven turbine stirrer (speed: 1800 rpm) and an automatic gas flow controlling and measuring unit (Büchi bpc 6010) at 6 bar and 30–80 °C. The initial rate values (v0) were calculated from the conversion curves.
The product (>99% PPA-content) was isolated with 20–23% yields in the same way as described in our previous works.1,2 The spectroscopic data of PPA are the following: 1H NMR (CDCl3, 300 MHz): δ ppm 1.56 (br s, 2H, NH2), 1.77 (dt, J = 14.5 and 7.2 Hz, 2H, CH2CH2CH2), 2.65 (t, J = 7.5 Hz, 2H, CH2Ph), 2.72 (t, J = 6.9 Hz, 2H, CH2NH2), 7.17–7.30 (m, 5H, Ar–H); 13C NMR (CDCl3, 75 MHz): δ ppm 33.39 (CH2CH2CH2), 35.43 (CH2Ph), 41.85 (CH2NH2), 125.91 (Ar–CH), 128.48 (2 × Ar–CH), 128.50 (2 × Ar–CH), 142.24 (Ar–C); MS m/z (rel %) 135 (3), 118 (100), 103 (7), 91 (30), 77 (9), 65 (8), 51 (6). The MS data of the starting material and the side products are the following: PPN m/z (rel %) 131 (30), 91 (100), 77 (4), 65 (10), 51 (6); BPPA m/z (rel %) 253 (18), 148 (100), 117 (16), 105 (7), 91 (99), 77 (10), 65 (12), 56 (11); TPPA m/z (rel %) 371 (4), 266 (90), 252 (2), 162 (30), 117 (10), 91 (100), 77 (5), 58 (80); PPAL m/z (rel %) 134 (71), 115 (10), 105 (35), 91 (100), 78 (44), 65 (16), 51 (21); PPOH m/z (rel %) 136 (21), 117 (100), 105 (13), 91 (88), 77 (18), 65 (17), 51 (13). These results are in conjunction with the literature analytical data.42
Catalyst Reusing
After filtering the spent 10% Pd/C catalyst, it was washed with distilled water (2 × 10 cm3) and collected carefully to store for the sequent reaction, in the wet form. No further regenerative treatments were applied prior to its reusing.
Recycling the Unreacted Starting Material
The second hydrogenation was carried out by recycling the byproduct obtained from the first reaction and complemented by fresh PPN to 5.0 g. The reactions were performed as described in section Hydrogenation Reactions.
Catalyst Characterization
The metal content of the fresh and the reused Pd/C catalysts was measured by energy-dispersive XRF spectroscopy using an isotope-excited XRF analyzer.43 A ring-shaped 125I source was applied for excitation, and a lead container with an aluminum collimator was used for shielding. The detector was a Canberra SSL 80160 Si(Li-drifted) semiconductor with a thickness of 5 mm and a surface area of 80 mm2 placed in a cooled space. Its resolution was 160 eV at an energy level of 5.9 keV.
Dispersion of the catalyst (D10%Pd/C = 0.50) was determined by O2-, H2-, and CO chemisorption measurements using an atmospheric flow system.44,45 Before the first adsorption of O2, the catalyst sample was treated with H2 in Ar at 90 °C for 2.5 h and then cooled to 25 °C in Ar only to avoid hydrogen absorption in the bulk phase of palladium. (Pd–H)s was titrated with O2 injections via a calibrated loop (0.1 cm3 each) at 25 °C. Then, (Pd–O)s was titrated with H2 in the same way. CO chemisorption was also measured after treating in hydrogen (90 °C, 2.5 h). The following stoichiometry was used for the calculations:
adsorption of O2
| 1 |
titration with H2
| 2 |
adsorption of CO
| 3 |
Analysis
The product and the byproducts were analyzed and identified by GC–MS, as well as 1H and 13C NMR measurements.
GC–MS analyses were performed using an Agilent 7890A GC-system (7683 autosampler and 7683B injector) connected to an Agilent 5975C mass spectrometer using a Restek Rxi-5Sil MS capillary column (15 m × 0.25 mm ID, 0.25 μm film). The temperature program was the following: 70 °C (1 min) to 300 °C at 33 °C/min, hold 2 min.
The NMR spectra were recorded in chloroform-D1 (CDCl3) on a Bruker AV-300 spectrometer operating at 300 and 75 MHz, respectively. The chemical shift values (δ) are given relatively to that of δTMS.
Computational Methods
DFT calculations of the adsorption geometries and energies were carried out using the Quantum ESPRESSO software package.46 The exchange–correlation functional utilized was the Perdew−Burke−Ernzerhof (PBE) of generalized gradient approximation (GGA) with the plane wave ultrasoft pseudopotential approach.47 The energy cut-off of the plane wave basis set was 50 Ry, and the convergence criterion of the self-consistent accuracy was adjusted to 1 × 10–6.
A three-layer periodic slab with upper atoms allowed to relax was used to model the Pd(111) surface. A (4 × 4) supercell including a 10 Å vacuum slab was applied to study the adsorption of the imine-type intermediates. The Monkhorst–Pack scheme k-point grid sampling of a (2 × 2 × 1) was used in all calculations over the entire Brillouin zone.
Adsorption energies (ΔEads) were calculated according to eq 4
| 4 |
where Eimine+Pd(111) is the total energy of the adsorbed imine intermediate on the Pd(111) surface, EPd(111) is the energy of the Pd(111) slab itself, and Eimine is the energy of the imine molecule in vacuum.
Acknowledgments
This work is connected to the scientific program of the “Talent Care and Cultivation in the Scientific Workshops at BME” project. The project is supported by the grant TÁMOP-4.2.2.B-10/1-2010-0009. The authors thank Dr. Antal Sárkány for determining catalyst dispersion, Dr. Iván Gresits for performing the XRF spectroscopy analysis, as well as Dr. Tibor Novák and Dr. Miklós Nyerges for providing GC–MS measurements.
The authors declare no competing financial interest.
Dedication
Dedicated to the memory of Dr. Tibor Máthé, an excellent researcher and inventor, who passed away 19 years ago.
References
- Hegedűs L.; Máthé T. Selective heterogeneous catalytic hydrogenation of nitriles to primary amines in liquid phase Part I. Hydrogenation of benzonitrile over palladium. Appl. Catal., A 2005, 296, 209–215. [Google Scholar]
- Hegedűs L.; Máthé T.; Kárpáti T. Selective heterogeneous catalytic hydrogenation of nitriles to primary amines in liquid phase Part II. Hydrogenation of benzyl cyanide over palladium. Appl. Catal., A 2008, 349, 40–45. [Google Scholar]
- Sediri F.; Touati F.; Gharbi N. A one-step hydrothermal way for the synthesis of vanadium oxide nanotubes containing the phenylpropylamine as template obtained via non-alkoxide route. Mater. Lett. 2007, 61, 1946–1950. 10.1016/j.matlet.2006.07.109. [DOI] [Google Scholar]
- Sediri F.; Gharbi N. From crystalline V2O5 to nanostructured vanadium oxides using aromatic amines as templates. J. Phys. Chem. Solids 2007, 68, 1821–1829. 10.1016/j.jpcs.2007.06.012. [DOI] [Google Scholar]
- Gerrard L. A.; Weller M. T. Characterisation of Four New Two-dimensional Lithium Beryllofluoro-layered Compounds. Chem.—Eur. J. 2003, 9, 4936–4942. 10.1002/chem.200304991. [DOI] [PubMed] [Google Scholar]
- Ünal A.; Şentürk Ş.; Şenyel M. Vibrational spectroscopic and thermal studies of some 3-phenylpropylamine complexes. Vib. Spectrosc. 2009, 51, 299–307. 10.1016/j.vibspec.2009.08.003. [DOI] [Google Scholar]
- Stanford D. J.; Fernandez R. A.; Zeller M.; Hunter A. D. N-(3-Phenylpropyl)guanidinium bromide. Acta Crystallogr. 2007, 63, o1934–o1936. 10.1107/s160053680701238x. [DOI] [Google Scholar]
- Košutić-Hulita N.; Žegarac M. Tolterodinium (+)-(2R,3R)-hydrogen tartrate. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 2005, 61, o171–o173. 10.1107/s0108270105001599. [DOI] [PubMed] [Google Scholar]
- Melchart M.; Habtemariam A.; Novakova O.; Moggach S. A.; Fabbiani F. P. A.; Parsons S.; Brabec V.; Sadler P. J. Bifunctional amine-tethered Ruthenium(II) Arene Complexes form Monofunctional Adducts on DNA. Inorg. Chem. 2007, 46, 8950–8962. 10.1021/ic700799w. [DOI] [PubMed] [Google Scholar]
- Werkmeister S.; Junge K.; Beller M. Catalytic Hydrogenation of Carboxylic Acid Esters, Amides, and Nitriles with Homogeneous Catalysts. Org. Process Res. Dev. 2014, 18, 289–302. 10.1021/op4003278. [DOI] [Google Scholar]
- Bagal D. B.; Bhanage B. M. Recent Advances in Transition Metal-catalyzed Hydrogenation of Nitriles. Adv. Synth. Catal. 2015, 357, 883–900. 10.1002/adsc.201400940. [DOI] [Google Scholar]
- Lévay K.; Hegedűs L. Selective Heterogeneous Catalytic Hydrogenation of Nitriles to Primary Amines. Period. Polytech., Chem. Eng. 2018, 62, 476–488. 10.3311/ppch.12787. [DOI] [Google Scholar]
- Allgeier A. M.; Sengupta S. K.. Nitrile hydrogenation. In Hydrogenation: Catalysts and Processes; Jackson S. D., Ed.; De Gruyter: Berlin, Boston, 2018; pp 107–154. [Google Scholar]
- Lévay K.; Hegedűs L. Recent Achievements in the Hydrogenation of Nitriles Catalyzed by Transitional Metals. Curr. Org. Chem. 2019, 23, 1881–1900. 10.2174/1385272823666191007160341. [DOI] [Google Scholar]
- Liu L.; Liu Y.; Ai Y.; Li J.; Zhou J.; Fan Z.; Bao H.; Jiang R.; Hu Z.; Wang J.; Jing K.; Wang Y.; Liang Q.; Sun H. Pd-CuFe Catalyst for Transfer Hydrogenation of Nitriles: Controllable Selectivity to Primary Amines and Secondary Amines. iScience 2018, 8, 61–73. 10.1016/j.isci.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Liu L.; Ai Y.; Hu Z.; Liu Z.; Guo R.; Zhang C.; Tian H.; Wu J.; Ruan M.; Sun H. Moderate Activity from Trace Palladium Alloyed with Copper for the Chemoselective Hydrogenation of −CN and −NO2 with HCOOH. ChemistrySelect 2019, 4, 7346–7350. 10.1002/slct.201902057. [DOI] [Google Scholar]
- Liu L.; Li J.; Ai Y.; Liu Y.; Xiong J.; Wang H.; Qiao Y.; Liu W.; Tan S.; Feng S.; Wang K.; Sun H.; Liang Q. A ppm level Rh-based composite as an ecofriendly catalyst for transfer hydrogenation of nitriles: triple guarantee of selectivity for primary amines. Green Chem. 2019, 21, 1390–1395. 10.1039/c8gc03595d. [DOI] [Google Scholar]
- Russell T. W.; Hoy R. C.; Cornelius J. E. Facile Reduction of Unsaturated Compounds Containing Nitrogen. J. Org. Chem. 1972, 37, 3552–3553. 10.1021/jo00795a042. [DOI] [Google Scholar]
- Yadav G. D.; Kharkara M. R. Liquid-phase hydrogenation of saturated and unsaturated nitriles: Activities and selectivities of bimetallic nickel–copper and nickel–iron catalysts supported on silica. Appl. Catal., A 1995, 126, 115–123. 10.1016/0926-860x(95)00039-9. [DOI] [Google Scholar]
- Kukula P.; Studer M.; Blaser H.-U. Chemoselective Hydrogenation of α,β-Unsaturated Nitriles. Adv. Synth. Catal. 2004, 346, 1487–1493. 10.1002/adsc.200404128. [DOI] [Google Scholar]
- Kukula P.; Koprivova K. Structure-selectivity relationship in the chemoselective hydrogenation of unsaturated nitriles. J. Catal. 2005, 234, 161–171. 10.1016/j.jcat.2005.06.011. [DOI] [Google Scholar]
- Segobia D. J.; Trasarti A. F.; Apesteguía C. R. Chemoselective hydrogenation of unsaturated nitriles to unsaturated primary amines: Conversion of cinnamonitrile on metal-supported catalysts. Appl. Catal., A 2015, 494, 41–47. 10.1016/j.apcata.2015.01.028. [DOI] [Google Scholar]
- Mokhov V. M.; Popov Y. V.; Shcherbakova K. V. Colloid and Nanosized Catalysts in Organic Synthesis: XII. Hydrogenation of Carbonitriles Catalyzed by Nickel Nanoparticles. Russ. J. Gen. Chem. 2016, 86, 273–280. 10.1134/s1070363216020110. [DOI] [Google Scholar]
- Kukula P.; Gabova V.; Koprivova K.; Trtik P. Selective hydrogenation of unsaturated nitriles to unsaturated amines over amorphous CoB and NiB alloys doped with chromium. Catal. Today 2007, 121, 27–38. 10.1016/j.cattod.2006.11.009. [DOI] [Google Scholar]
- McMillan L.; Gilpin L. F.; Baker J.; Brennan C.; Hall A.; Lundie D. T.; Lennon D. The application of a supported palladium catalyst for the hydrogenation of aromatic nitriles. J. Mol. Catal. A: Chem. 2016, 411, 239–246. 10.1016/j.molcata.2015.10.028. [DOI] [Google Scholar]
- Bhosale A.; Yoshida H.; Fujita S.-i.; Arai M. Carbon dioxide and water: An effective multiphase medium for selective hydrogenation of nitriles with a Pd/Al2O3 catalyst. J. CO2 Util. 2016, 16, 371–374. 10.1016/j.jcou.2016.09.006. [DOI] [Google Scholar]
- Chin C. S.; Lee B. Hydrogenation of nitriles with iridium-triphenylphosphine complexes. Catal. Lett. 1992, 14, 135–140. 10.1007/bf00764228. [DOI] [Google Scholar]
- Addis D.; Enthaler S.; Junge K.; Wendt B.; Beller M. Ruthenium N-heterocyclic carbene catalysts for selective reduction of nitriles to primary amines. Tetrahedron Lett. 2009, 50, 3654–3656. 10.1016/j.tetlet.2009.03.108. [DOI] [Google Scholar]
- Werkmeister S.; Junge K.; Wendt B.; Spannenberg A.; Jiao H.; Bornschein C.; Beller M. Ruthenium/imidazolylphosphine Catalysis: Hydrogenation of Aliphatic and Aromatic Nitriles to Form Amines. Chem.—Eur. J. 2014, 20, 4227–4231. 10.1002/chem.201303989. [DOI] [PubMed] [Google Scholar]
- Bornschein C.; Werkmeister S.; Wendt B.; Jiao H.; Alberico E.; Junge K.; Wendt B.; Beller M. Mild and selective hydrogenation of aromatic and aliphatic (di)nitriles with a well-defined iron pincer complex. Nat. Commun. 2014, 5, 4111. 10.1038/ncomms5111. [DOI] [PubMed] [Google Scholar]
- Lange S.; Elangovan S.; Cordes C.; Spannenberg A.; Jiao H.; Junge H.; Bachmann S.; Scalone M.; Topf C.; Junge K.; Beller M. Selective catalytic hydrogenation of nitriles to primary amines using iron pincer complexes. Catal. Sci. Technol. 2016, 6, 4768–4772. 10.1039/c6cy00834h. [DOI] [Google Scholar]
- Chakraborty S.; Leitus G.; Milstein D. Iron-Catalyzed Mild and Selective Hydrogenative Cross-Coupling of Nitriles and Amines to Form Secondary Aldimines. Angew. Chem., Int. Ed. 2017, 56, 2074–2078. 10.1002/anie.201608537. [DOI] [PubMed] [Google Scholar]
- Adam R.; Bheeter C. B.; Cabrero-Antonino J. R.; Junge K.; Jackstell R.; Beller M. Selective Hydrogenation of Nitriles to Primary Amines by Using a Cobalt Phosphine Catalyst. ChemSusChem 2017, 10, 842–846. 10.1002/cssc.201601843. [DOI] [PubMed] [Google Scholar]
- Schneekönig J.; Tannert B.; Hornke H.; Beller M.; Junge K. Cobalt pincer complexes for catalytic reduction of nitriles to primary amines. Catal. Sci. Technol. 2019, 9, 1779–1783. 10.1039/c9cy00225a. [DOI] [Google Scholar]
- Elangovan S.; Topf C.; Fischer S.; Jiao H.; Spannenberg A.; Baumann W.; Ludwig R.; Junge K.; Beller M. Selective Catalytic Hydrogenations of Nitriles, Ketones, and Aldehydes by Well-Defined Manganese Pincer Complexes. J. Am. Chem. Soc. 2016, 138, 8809–8814. 10.1021/jacs.6b03709. [DOI] [PubMed] [Google Scholar]
- Weber S.; Stöger B.; Kirchner K. Hydrogenation of Nitriles and Ketones Catalyzed by an Air-Stable Bisphosphine Mn(I) Complex. Org. Lett. 2018, 20, 7212–7215. 10.1021/acs.orglett.8b03132. [DOI] [PubMed] [Google Scholar]
- Nait Ajjou A.; Robichaud A. Chemoselective hydrogenation of nitriles to primary amines catalyzed by water-soluble transition metal catalysts. Appl. Organomet. Chem. 2018, 32, e4481 10.1002/aoc.4481. [DOI] [Google Scholar]
- Rylander P. N.Solvents in catalytic hydrogenation. In Catalysis in Organic Syntheses; Jones W. H., Ed.; Academic Press: New York, 1980; pp 155–171. [Google Scholar]
- Gunjal P. R.; Ranade V. V.. Catalytic Reaction Engineering. In Industrial Catalytic Processes for Fine and Specialty Chemicals; Joshi S. S., Ranade V. V., Eds.; Elsevier: Amsterdam, 2016; pp 263–314. [Google Scholar]
- Mittendorfer F.; Thomazeau C.; Raybaud P.; Toulhoat H. Adsorption of Unsaturated Hydrocarbons on Pd(111) and Pt(111): A DFT Study. J. Phys. Chem. B 2003, 107, 12287–12295. 10.1021/jp035660f. [DOI] [Google Scholar]
- Máthé T.; Tungler A.; Petró J.. Process for the preparation of supported metal catalysts. U.S. Patent 4,361,500A, 1982.
- Wallace W. E.Mass Spectra. In NIST Chemistry WebBook, NIST Standard Reference Database Number 69 [Online]; Linstrom P. J., Mallard W. G., Eds.; National Institute of Standards and Technology: Gaithersburg MD, 2020, 20899. (accessed Feb 14, 2020). [Google Scholar]
- Rácz L.; Solti Sz.; Gresits I.; Tölgyesi S.; Benedek D.; Valentínyi N.; Mizsey P. Measurement of Rarely Investigated Trace Elements As, P, Sr, Zr, Rb and Y in Waste Tires. Period. Polytech., Chem. Eng. 2016, 60, 78–84. 10.3311/ppch.8101. [DOI] [Google Scholar]
- Sárkány A.; Zsoldos Z.; Furlong B.; Hightower J. W.; Guczi L. Hydrogenation of 1-butene and 1,3-butadiene mixtures over Pd/ZnO catalysts. J. Catal. 1993, 141, 566–582. 10.1006/jcat.1993.1164. [DOI] [Google Scholar]
- Sárkány A.; Stefler G.; Hightower J. W. Participation of support sites in hydrogenation of 1,3-butadiene over Pt/Al2O3 catalysts. Appl. Catal., A 1995, 127, 77–92. 10.1016/0926-860x(95)00062-3. [DOI] [Google Scholar]
- Giannozzi P.; Baroni S.; Bonini N.; Calandra M.; Car R.; Cavazzoni C.; Ceresoli D.; Chiarotti G. L.; Cococcioni M.; Dabo I.; Dal Corso A.; de Gironcoli S.; Fabris S.; Fratesi G.; Gebauer R.; Gerstmann U.; Gougoussis C.; Kokalj A.; Lazzeri M.; Martin-Samos L.; Marzari N.; Mauri F.; Mazzarello R.; Paolini S.; Pasquarello A.; Paulatto L.; Sbraccia C.; Scandolo S.; Sclauzero G.; Seitsonen A. P.; Smogunov A.; Umari P.; Wentzcovitch R. M. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys.: Condens. Matter 2009, 21, 395502. 10.1088/0953-8984/21/39/395502. [DOI] [PubMed] [Google Scholar]
- Perdew J. P.; Burke K.; Ernzerhof M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. 10.1103/physrevlett.77.3865. [DOI] [PubMed] [Google Scholar]