Abstract

Deep eutectic solvents (DESs), featured as promising green solvents, were applied to examine their effectiveness in pretreating raw ramie fibers (RFs) for cellulose nanofibril (CNF) production. The pretreatment performance of three DESs, i.e., choline chloride–urea (CU), choline chloride–oxalic acid dihydrate (CO), and choline chloride–glycerol (CG), was evaluated based on chemical composition analysis and structural and morphological changes. CO attained the most dramatic morphological changes of RFs, followed by CG and CU. Its high structural disruption of RFs during the pretreatment process, shown in the results from scanning electron microscopy and atomic force microscopy, could be due to an outstanding ability to remove amorphous cellulose and noncellulosic components from raw RFs, confirmed by the results of chemical composition analysis, Fourier transform infrared spectroscopy, and X-ray diffractometry. Overall, this study provided an innovative and effective pretreatment process for fractionating raw cellulosic fibers, so as to promote the subsequent preparation of CNFs.
Introduction
Cellulose particles measuring 100 nm or less in at least one dimension are referred to as nanocellulose. Due to its natural abundance, renewability, biodegradability, mechanical durability, and chemical tunability, nanocellulose is an ideal material to lay the foundation for a new biopolymer composites industry.1,2 Depending on the cellulosic sources and the production conditions, nanocellulose can be further divided into four main categories of cellulose nanocrystals (CNCs), cellulose nanofibrils (CNFs), bacterial cellulose (BC), and electrospun cellulose nanofibers (ECNFs).3,4 Among them, CNCs and CNFs are generated by the separation of cellulosic fibers into nanosize fragments (top-down process),5,6 while BC and ECNFs are produced by a buildup of nanofibers (bottom-up process) from low-molecular-weight carbohydrates by bacteria7 or from cellulose solution using an electrospinning technique,8 respectively. Here, we focus our attention on CNFs.
CNFs are finer cellulose fibrils extracted from wood, plant, algae, and bacterial source materials by mechanical processes, such as high-pressure homogenization.9 However, the mechanical separation of CNFs from cellulosic fibers especially consumes a large amount of energy because of the cell wall recalcitrance, which results from a rigid and crystalline structure of cellulose surrounded by noncellulosic materials like hemicelluloses, lignin, pectin, and protein, and which also makes cellulose resistant to liquid penetration.10,11 The isolation of CNFs generally requires two successive stages. The first stage is a pretreatment of the source material by various pulping and bleaching methods. The particular pretreatment involves the isolation of individual intact fibers and the partial or complete elimination of noncellulosic materials.12 The second stage is the mechanical treatment as well as biological and chemical pretreatments, which involves the disintegration of the above-mentioned “purified” cellulosic fibers into CNFs.13 However, when compared to the direct use of raw cellulosic fibers, the pulping and bleaching pretreatments in the first stage have many drawbacks, including the higher cost of chemicals, the increased environmental burden (the use of toxic chemicals), and the lower production yield of CNFs.14,15
In this study, the ramie fibers (RFs) in their native state were directly treated for CNF production without the pulping and bleaching pretreatments. Ramie, which belongs to the Urticaceae family, is a perennial herbaceous plant and an important natural fiber crop.16 Raw ramie consists of many individual fibers, which are linked to each other to form a fiber bundle structure; its main composition is cellulose and the noncellulosic components such as hemicelluloses, pectin, lignin, waxes, and fats bind the fiber bundles together.17,18
To facilitate the disintegration of cellulose microfibrils into thinner CNFs in the second stage, biological and chemical pretreatments are usually applied that markedly weaken their interfibrillar hydrogen bonding: enzymatic hydrolysis, acid hydrolysis, alkaline hydrolysis, TEMPO oxidation, carboxymethylation, organic solvents/ionic liquids, etc.19,20 Unfortunately, biological processes are expensive and time-consuming, and conventional chemical approaches also can be costly and toxic. Thus, green and inexpensive chemicals capable of loosening the cell wall structure are highly desired in the preparation of CNFs. Recently, a new class of green solvents called deep eutectic solvents (DESs) as media for biomass processing has been presented.21,22 A DES usually consists of two or three cheap and safe components that can associate with each other to form a eutectic mixture, often through hydrogen bonding.23 Generally, DESs are featured by a melting point below that of each individual component and being liquid at temperatures below 100 °C. In most cases, DESs are prepared by mixing a quaternary ammonium salt as a hydrogen bond acceptor (HBA) with a melt salt or hydrogen bond donor (HBD).24 Choline chloride, featured as a cheap, nontoxic, and biodegradable quaternary ammonium salt, is often used to form a DES in combination with HBDs, such as urea, oxalic acid dihydrate, or glycerol. DESs have been used as a pretreatment for the nanofibrillation of bleached birch kraft pulp,25,26 waste board,27 NaOH-treated grass,28 degummed silk fibers,29 bleached polar kraft pulp,30 and degummed RFs.31 However, to the best of our knowledge, the use of DESs for the pretreatment of raw cellulosic fibers prior to the isolation of CNFs has been rarely reported.
In this work, DES was applied to examine its effectiveness in pretreating raw RFs for CNF production. Three representative DESs, namely, choline chloride–urea (CU), choline chloride–oxalic acid dihydrate (CO), and choline chloride–glycerol (CG) were selected. Subsequently, their pretreatment performances were evaluated based on chemical composition analysis and structural and morphological changes.
Results and Discussion
Chemical Composition
In this study, three different DESs were evaluated comparatively for their efficiency in pretreating raw RFs, and the chemical compositions of un-pretreated and pretreated RFs are summarized in Table 1. Reaction time was one of the most important factors affecting the efficiency of the DES pretreatment. Table 1 reflects that the contents of cellulose, hemicelluloses, pectin, lignin, and water solubles in RFs changed sharply from 0 to 4 or 6 h with prolonged reaction time and then showed little changes when the reaction time exceeded 4 or 6 h. However, no significant changes in the content of waxes were observed with a further increase in reaction time. Consequently, 6, 4, and 6 h were determined as appropriate reaction times of CU, CO, and CG treatments, respectively. It needs to be pointed out here that the CO system could not be investigated at 8 and 10 h, as the pretreated RFs formed a highly viscous paste that could not be filtered. A similar observation was made by Procentese et al.32
Table 1. Chemical Compositions of Raw RFs and Pretreated RFs.
| composition (%) |
|||||||
|---|---|---|---|---|---|---|---|
| pretreatment | solid recovery (%) | cellulose | hemicelluloses | pectin | lignin | waxes | water solubles |
| untreated | 68.64 | 16.96 ± 0.51a | 5.48 ± 0.05 | 2.06 ± 0.11 | 0.42 ± 0.03 | 6.44 ± 0.11 | |
| CU 2 h | 80.62 ± 3.31 | 84.52 | 9.23 ± 0.37 | 1.29 ± 0.02 | 2.46 ± 0.09 | 0.43 ± 0.01 | 2.07 ± 0.10 |
| CU 4 h | 79.47 ± 2.78 | 86.33 | 8.96 ± 0.28 | 0.07 ± 0.02 | 2.17 ± 0.12 | 0.43 ± 0.01 | 2.04 ± 0.05 |
| CU 6 h | 76.41 ± 3.05 | 89.23 | 6.83 ± 0.16 | 0.08 ± 0.01 | 1.96 ± 0.07 | 0.39 ± 0.03 | 1.51 ± 0.04 |
| CU 8 h | 76.32 ± 2.97 | 89.80 | 6.42 ± 0.13 | 0.05 ± 0.02 | 1.83 ± 0.07 | 0.45 ± 0.03 | 1.45 ± 0.08 |
| CU 10 h | 75.86 ± 3.59 | 90.34 | 6.25 ± 0.26 | 0.03 ± 0.03 | 1.74 ± 0.13 | 0.37 ± 0.02 | 1.27 ± 0.07 |
| CO 2 h | 74.62 ± 2.10 | 68.13 | 26.21 ± 0.17 | 1.19 ± 0.05 | 1.59 ± 0.06 | 0.44 ± 0.04 | 2.44 ± 0.05 |
| CO 4 h | 73.26 ± 3.14 | 72.09 | 22.93 ± 0.07 | 0.85 ± 0.04 | 1.54 ± 0.12 | 0.50 ± 0.01 | 2.09 ± 0.03 |
| CO 6 h | 71.49 ± 1.88 | 72.85 | 22.67 ± 0.13 | 0.70 ± 0.01 | 1.24 ± 0.08 | 0.51 ± 0.02 | 1.96 ± 0.07 |
| CO 8 h | NDb | ND | ND | ND | ND | ND | ND |
| CO 10 h | ND | ND | ND | ND | ND | ND | ND |
| CG 2 h | 78.57 ± 1.01 | 86.94 | 8.06 ± 0.25 | 0.81 ± 0.08 | 2.48 ± 0.21 | 0.45 ± 0.04 | 1.26 ± 0.13 |
| CG 4 h | 75.09 ± 1.25 | 91.33 | 4.73 ± 0.21 | 0.00 ± 0.00 | 2.53 ± 0.11 | 0.49 ± 0.07 | 0.92 ± 0.12 |
| CG 6 h | 73.36 ± 2.18 | 93.28 | 3.03 ± 0.18 | 0.04 ± 0.01 | 2.56 ± 0.23 | 0.37 ± 0.01 | 0.76 ± 0.05 |
| CG 8 h | 73.15 ± 1.73 | 93.31 | 2.97 ± 0.15 | 0.06 ± 0.01 | 2.67 ± 0.04 | 0.38 ± 0.04 | 0.68 ± 0.04 |
| CG 10 h | 73.03 ± 1.11 | 93.89 | 2.44 ± 0.27 | 0.03 ± 0.02 | 2.60 ± 0.19 | 0.39 ± 0.05 | 0.69 ± 0.01 |
The data were presented as mean value ± standard deviation.
Not determined.
The solid recovery larger than 76% was achieved for CU pretreatment at 140 °C in this study, while approximately 73% was achieved with CO at 100 °C and CG at 180 °C (Table 1). The results of this study can be compared to the data reported by Hou et al.,33 who investigated rice straw pretreatment using the choline chloride sequences of DESs. The solid recovery as reported by Hou et al.33 was 78% of their biomass for choline chloride–urea pretreatment at 120 °C, being very similar to that obtained here; while 68 and 79.5%, which were slightly different from those obtained here, were reported for choline chloride–oxalic acid dihydrate at 80 °C and choline chloride–glycerol at 120 °C, respectively. As shown in Figure 1, both CU and CG pretreatments were quite effective in terms of fractionating RFs. CU removed 69.23% hemicelluloses, 98.88% pectin, 27.30% lignin, and 82.08% water solubles, while CG took away 86.89% hemicelluloses, 99.46% pectin, 8.83% lignin, and 91.34% water solubles. However, compared to CU and CG, CO showed the lowest hemicellulose removal (0.95%), slightly lower pectin removal (99.64%) and water soluble removal (76.22%), and slightly higher lignin removal (45.23%). In this study, the content of hemicelluloses in pretreated RFs was determined by the NaOH method, which could detect all oligosaccharides containing hemicelluloses and other oligosaccharides.34 Unlike CU and CG, CO could induce cellulose hydrolysis during the pretreatment of lignocellulosic biomass,35 which was typical for the sulfuric acid hydrolysis of wood pulp for CNC production and resulted in an increase in cellulose removal and a decrease in “hemicelluloses” removal (shown in Figure 1). Similar results were reported by Liu et al.34
Figure 1.
Removal of individual components in pretreated RFs.
FT-IR Analysis
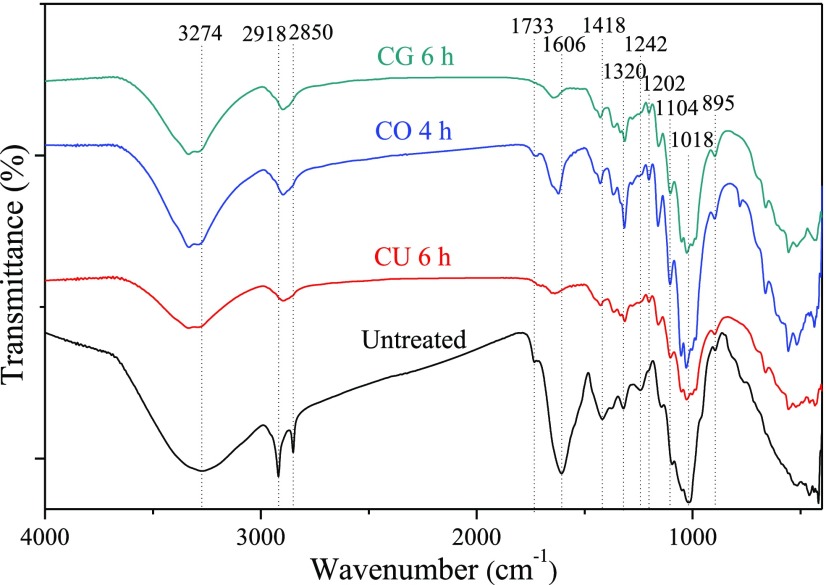
FT-IR analysis was carried out to understand the effect of DES pretreatment on the chemical structure of pretreated RFs, and the results obtained from the IR analysis are shown in Figure 2 and Table S1.
Figure 2.
FT-IR spectra of raw RFs and pretreated RFs.
The absorbance peaks near 3274 cm–1 correspond to the free O–H stretching and bending vibrations of the OH groups of cellulose and lignin.17 The peaks at 2918 and 2850 cm–1 are due to the stretching of C–H in cellulose and hemicelluloses.36 The sharp peak at 2918 cm–1 in raw RFs became a little shoulder in pretreated RFs and the peak at 2850 cm–1 disappeared completely in pretreated RFs. The removal of hemicelluloses during DES pretreatments, shown in Figure 1, caused these changes in the peak positions. The peak located at 1733 cm–1 in raw RFs and CO pretreated RFs is associated to the C=O stretching vibration in hemicelluloses and lignin,37 or the carbonyl group in carboxyl and esters.38 This peak disappeared in the CU and CG pretreated RFs, indicating the removal of hemicelluloses and lignin during the DES pretreatment, while this peak occurred in the CO pretreated RFs, signifying the esterification reaction between cellulose and oxalic acid during the DES pretreatment. The bands around 1606 cm–1 in the spectra of all samples represent the O–H bending of adsorbed water.39 The absorption bands at 1418 cm–1 (CH2 bending), 1320 cm–1 (C–H bending), 1104 cm–1 (C–O vibration), 1018 cm–1 (C–O–C stretching), and 895 cm–1 (C–H deformation) are the typical bonds of cellulose.31,40,41 These bands were present in all of the spectra; however, the CO-pretreated RFs exhibited stronger absorption intensities, which could be attributed to the degradation of amorphous cellulose during the DES pretreatment.34 This result is consistent with the chemical composition analysis from Figure 1. The peak at 1242 cm–1 in raw RFs is attributed to the C–O stretching in lignins,42 and this peak shifted to lower values at 1202 cm–1 in pretreated RFs, which was regarded as a reduction in the amount of lignin after the DES pretreatment.
Crystallinity
To determine the effect of DES pretreatment on the crystallinity of pretreated RFs, XRD analysis was carried out. Figure 3a presents the XRD patterns obtained for raw RFs and pretreated RFs, and the corresponding CrIs were calculated and are presented in Figure 3b.
Figure 3.
XRD patterns (a) and CrIs (b) of raw RFs and pretreated RFs.
For real biomass, the measurement of true cellulose crystallinity is very difficult because conventional X-ray methods determine the crystallinity of the entire material including amorphous cellulose, hemicelluloses, and lignin. However, chemical and physicochemical pretreatments can alter the crystal structure of the cellulose by destroying the inter- and intramolecular hydrogen bonds in cellulose chains, and X-ray measurements of CrI are still the best option for evaluating their effects on biomass crystallinity.43 As shown in Figure 3b, the CrI of RFs was increased from 79.17 to 81.75% after the CG pretreatment. This was unlikely to reflect an increase in cellulose crystallinity but revealed only an increase in the proportion of cellulose in RFs, shown in Table 1.32 However, the CrIs of CU and CO pretreated RFs were slightly reduced to 77.42 and 77.65%, respectively. According to previous reports,44,45 the CrI values of cellulose declined sharply after the same pretreatment process. Putting these two results together indicates that the relative contents of cellulose in RFs increased after CU or CO pretreatments, owing to the removal of hemicelluloses, pectin, and lignin. These results are in perfect accordance with the results obtained in Table 1.
Morphology
Finally, based on the results from the chemical composition analysis, FT-IR, and XRD, SEM and AFM were used to find out the effect of the DES pretreatment on the cell wall ultrastructure of RFs.
Figure 4A–L illustrates how the fiber morphology was changed from macro- to microscale by DES pretreatment. When untreated, the cross section of the harvested, chopped RFs was easily recognizable (Figure 4C), with single raw fibers composed of individual microfibrils linked by hemicelluloses and lignin.42 After DES pretreatments, the digital photographs (Figure 4A,D,G,J) displayed a general trend toward a greater abundance of long, thin, and individual fibers and a reduction in the particle size. Especially, the CO-pretreated RFs exhibited the maximum size reduction and their SEM image revealed the formation of some transverse cracks on the biomass surface (Figure 4I), which might be due to the dissolution of amorphous cellulose during the DES pretreatment. These photographs also showed a color change from light yellow for raw RFs to nearly white or light brown for pretreated RFs, which was similar to the result obtained previously with thermochemical alkaline pretreatments.46 In the CU- and CO-pretreated RFs (Figure 4F,I), the coarse surface became somewhat more loose with the attachment of some small particles, which might be residual hemicelluloses and lignin aggregates after DES pretreatment. The similar results explained by the relocalization of hemicelluloses and lignin were reported during dilute acid pretreatment.47,48 In contrast, Figure 4L shows the surface of the CG-pretreated RFs, which produced the smoothest and the cleanest surface. This result revealed the effective removal of a vast majority of noncellulosic components (shown in Table 1) and the efficient disintegration of bundle fibers into individual/discrete fibers.49 Overall, after DES pretreatments, a partial lesion of the fiber bundle and a typical weakening of the cell wall structure were observed in RFs.
Figure 4.
Digital photographs and SEM images of raw RFs and pretreated RFs.
AFM was used to get nanoscale morphological information on the surface of ramie cell walls. Figure 5A shows the morphology of raw RFs. It clearly appeared as aggregates of grains covering the surface. It was believed that the grains mainly consisted of noncellulosic components such as lignin, waxes, and hemicelluloses.50,51 It is well known that lignin usually forms aggregates on the surface of plant cell walls and exists in a grainy shape. Figure 5B–D shows the AFM images of pretreated RFs. These images clearly revealed the appearance of bundles of cellulose microfibers, all oriented in the same direction, indicating that most of the noncellulosic components in RFs were removed after DES pretreatment (shown in Figure 1). However, some links oriented perpendicular to the microfiber bundle were clearly deficient in the features of continuous microfibers (Figure 5B,D). It could be suggested that these links were chains of residual hemicelluloses in pretreated RFs.50,52 Particularly, the CO-pretreated RFs formed a network structure (Figure 5C), which corresponded to a degraded surface in this case.53 This observation is in agreement with the SEM analysis from Figure 4I.
Figure 5.
AFM images of raw RFs and pretreated RFs.
The findings obtained in this study by different analysis methods are summarized in Table 2, which included the comparisons of reaction time, physical changes after pretreatment, biomass crystallinity, and structural and morphological changes, along with viscosity of DES.54 Compared to other DESs, CO achieved a higher level of performance because it exhibited the highest structural disruption, resulting in the most dramatic changes in RF morphology. Moreover, it had the lowest reaction temperature with the lowest viscosity among the DESs investigated, implying less energy consumption and easy solvent handling during the pretreatment process. Bundles of RFs were not observed after CO pretreatment, which could be due to the fracturing behavior of long cellulose microfibrils during the pretreatment process (shown in Figure 4I).
Table 2. Summary of Analyses of DES on RF Pretreatmenta.
| pretreatment | reaction temperature (°C) | physical changes | CrI (%) | structural changes | morphological changes | viscosity |
|---|---|---|---|---|---|---|
| CU 6 h | 140 | bundling | 77.42 | C, H+ +, W+ +, P+ + +, L+ | + | > > > |
| CO 4 h | 100 | without bundling | 77.65 | C+, H+ +, W+ +, P+ + +, L+ + | + + + | > |
| CG 6 h | 180 | bundling | 81.75 | C, H+ + +, W+ + +, P+ + +, L+ | + | > > |
C = cellulose. H = hemicelluloses. W = water solubles. P = pectin. L = lignin. + = high level of structural disruption. > = high level of viscosity.
Potential Utilization Methods of Cellulose Nanofibrils
Due to its intrinsic properties, abundance, and renewability, nanocellulose is involved in the fabrication of a variety of nanomaterials and nanocomposites, including templating agents, hydrogels, aerogels, nanocellulose–nanocarbon composites, nanocellulose–organic polymer matrixes, and nanocellulose–inorganic nanoparticle composites.55 Its applications include mechanical reinforcement, packaging, medical treatment, sensing, electronics, energy storage, environmental remediation, catalysis, and fire prevention. However, how to make better use of CNFs has always been a concern.
According to Hietala et al.,56 cellulose nanofibers were prepared from sugar beet pulp, and the reinforcing capability of the obtained nanofibers in the poly(vinyl alcohol) matrix was studied. Interestingly, the sugar beet nanofibers containing higher amounts of pectin and hemicellulose resulted in better redispersion properties and a nanocomposite with better mechanical properties. Laitinen et al.57 isolated CNFs from recycled waste fibers by employing a Masuko grinder after pretreatment with DES. In the following step, a combination of two types of silylation agents and freeze-drying was used to fabricate nanostructured aerogels. The obtained CNF aerogels exhibited excellent absorption performances for cleaning oil and chemical spills. Kumar et al.58 produced cellulose nanocrystal-reinforced poly(vinyl alcohol)/silica glass hybrid scaffolds via a freeze-drying method. The product showed a highly porous structure with an improved mechanical performance by the addition of cellulose nanocrystals and silica-based bioactive glass, making it suitable for bone tissue regeneration. Moreover, recently, some new techniques have emerged to produce both nanoscale and microscale fibers. These techniques include electrohydrodynamic processing, centrifugal spinning, and pressurized gyration.59,60 With these techniques, polymer solutions produce micro/nanofibers in the form of yarns. Since CNFs can be uniformly dispersed in a polymer solution, they could be spun into fiber mats for preparing nanocomposites by centrifugal spinning.
Conclusions
In this study, CO showed the most dramatic morphological changes of RFs during DES pretreatment, followed by CG and CU. The high capability to remove amorphous cellulose and noncellulosic components such as hemicelluloses, water solubles, pectin, and lignin from raw RFs contributed to its high structural disruption of RFs during the pretreatment process. This study demonstrated a green pretreatment process with great potential for the preparation of CNFs from raw cellulosic fibers. A future goal is to use the raw RFs pretreated with CO for preparing CNFs.
Experimental Section
Materials
RFs were collected from Changsha Ramie Garden of Institute of Bast Fiber Crops, Chinese Academy of Agricultural Sciences. The raw material was air-dried, cut into short fibers (less than 3 cm in length), and stored in an airtight container at room temperature prior to use. Choline chloride (≥98%), urea (≥99.5%), oxalic acid dihydrate (≥98%), and glycerol (≥99.5%) were obtained from Shanghai Macklin Biochemical Co., Ltd. All other chemicals were of the highest purity commercially available.
DES Synthesis
Three different DESs were tested: CU, CO, and CG in molar ratios of 1:2, 1:1, and 1:2, respectively. The DESs were synthesized by mixing the two constituents (HBA and HBD), and then the mixture was heated and stirred in a closed flask at 100 °C in an oil bath to obtain a homogeneous liquid. The synthesized DESs were stored in a desiccator prior to use.
DES Pretreatment
Two grams of ramie short fibers was added into 200 g of DES, then the suspension was mixed for 2–10 h at a predetermined temperature (140 °C for CU; 100 °C for CO; 180 °C for CG). The detailed reaction times used are presented in Table 1 and the experimental results of different reaction temperatures are shown in Table S2. After the DES pretreatment, the mixture was taken away from the heating source (oil bath) and 200 mL of deionized water was added while mixing. Then, the suspension was filtrated under vacuum and washed with deionized water until the filtrate ran clear. Finally, the pretreated RFs were dried at 60 °C and stocked in sealed plastic bags.
Characterization
The chemical composition of RFs was tested according to the Chinese national standards (see the Supporting Information) of GB5883-8661 and GB5889-86,62 and expressed as wt % content of waxes, water-soluble components, pectin, hemicelluloses, lignin, and cellulose.17,49 Each sample was tested at least three times. Besides, some values were calculated as follows63
| 1 |
| 2 |
The functional groups of RFs were analyzed using a Nicolet IS10 Fourier transform infrared (FT-IR) spectrometer (Thermo Electron). The data were recorded over the range between 400 and 4000 cm–1 with 32 scans per spectrum and a 4 cm–1 resolution.
The X-ray diffraction (XRD) patterns were measured using a D8 Advance diffractometer (Bruker, Germany) with a Cu Kα radiation (λ = 0.154 nm) at 40 kV and 40 mA. The diffraction data of RFs were collected in the 2θ range of 5–40° in steps of 0.02° at a scanning speed of 4°/min. The crystallinity index (CrI) was measured by the Segal method as follows (without a baseline substrate)64
| 3 |
where I200 is the peak intensity of the main crystalline plane (200) diffraction and Iam is the minimum diffraction intensity at a 2θ angle close to 18°.
The surface morphology features of RFs were observed using a JSM-6490LV scanning electron microscope (JEOL, Japan). The samples were well scattered on the conducting resin and coated with Au. SEM images were recorded with a 20 kV acceleration voltage under standard conditions. Additionally, a Multimode 8 atomic force microscope (Bruker) was used to characterize slight changes in the surface morphology of the samples. The scanasyst mode automatically optimized the imaging parameters with a scan rate of 1 Hz. All images were acquired in air.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 21878326) and the Training Program for Excellent Young Innovators of Changsha (No. kq1802018).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00506.
Experimental procedure of the method of quantitative analysis of ramie chemical components; FT-IR analysis of raw ramie fibers and pretreated ramie fibers; and chemical compositions of raw ramie fibers and pretreated ramie fibers under different reaction temperatures (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Huang P.; Zhao Y.; Kuga S.; Wu M.; Huang Y. A versatile method for producing functionalized cellulose nanofibers and their application. Nanoscale 2016, 8, 3753–3759. 10.1039/C5NR08179C. [DOI] [PubMed] [Google Scholar]
- Moon R. J.; Martini A.; Nairn J.; Simonsen J.; Youngblood J. Cellulose nanomaterials review: structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. 10.1039/c0cs00108b. [DOI] [PubMed] [Google Scholar]
- Abdul Khalil H. P. S.; Davoudpour Y.; Islam M. N.; Mustapha A.; Sudesh K.; Dungani R.; Jawaid M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. 10.1016/j.carbpol.2013.08.069. [DOI] [PubMed] [Google Scholar]
- Nechyporchuk O.; Belgacem M. N.; Bras J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crops Prod. 2016, 93, 2–25. 10.1016/j.indcrop.2016.02.016. [DOI] [Google Scholar]
- Lu Z. X.; Fan L. W.; Zheng H. Y.; Lu Q. L.; Liao Y. Q.; Huang B. Preparation, characterization and optimization of nanocellulose whiskers by simultaneously ultrasonic wave and microwave assisted. Bioresour. Technol. 2013, 146, 82–88. 10.1016/j.biortech.2013.07.047. [DOI] [PubMed] [Google Scholar]
- Ferrer A.; Filpponen I.; Rodríguez A.; Laine J.; Rojas O. J. Valorization of residual Empty Palm Fruit Bunch Fibers (EPFBF) by microfluidization: Production of nanofibrillated cellulose and EPFBF nanopaper. Bioresour. Technol. 2012, 125, 249–255. 10.1016/j.biortech.2012.08.108. [DOI] [PubMed] [Google Scholar]
- Fan X.; Gao Y.; He W. Y.; Hu H.; Tian M.; Wang K. X.; Pan S. Y. Production of nano bacterial cellulose from beverage industrial waste of citrus peel and pomace using Komagataeibacter xylinus. Carbohydr. Polym. 2016, 151, 1068–1072. 10.1016/j.carbpol.2016.06.062. [DOI] [PubMed] [Google Scholar]
- Montaño-Leyva B.; Rodriguez-Felix F.; Torres-Chavez P.; Ramirez-Wong B.; Lopez-Cervantes J.; Sanchez-Machado D. Preparation and characterization of durum wheat (Triticum durum) straw cellulose nanofibers by electrospinning. J. Agric. Food Chem. 2011, 59, 870–875. 10.1021/jf103364a. [DOI] [PubMed] [Google Scholar]
- Kargarzadeh H.; Mariano M.; Gopakumar D.; Ahmad I.; Thomas S.; Dufresne A.; Huang J.; Lin N. Advances in cellulose nanomaterials. Cellulose 2018, 25, 2151–2189. 10.1007/s10570-018-1723-5. [DOI] [Google Scholar]
- Iiyama K.; Lam T.; Stone B. A. Covalent cross-links in the cell wall. Plant Physiol. 1994, 104, 315–320. 10.1104/pp.104.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmel M. E.; Ding S. Y.; Johnson D. K.; Adney W. S.; Nimlos M. R.; Brady J. W.; Foust T. D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- Rajinipriya M.; Nagalakshmaiah M.; Robert M.; Elkoun S. Importance of agricultural and industrial waste in the field of nanocellulose and recent industrial developments of wood based nanocellulose: A Review. ACS Sustainable Chem. Eng. 2018, 6, 2807–2828. 10.1021/acssuschemeng.7b03437. [DOI] [Google Scholar]
- Shak K. P. Y.; Pang Y. L.; Mah S. K. Nanocellulose: Recent advances and its prospects in environmental remediation. Beilstein J. Nanotechnol. 2018, 9, 2479–2498. 10.3762/bjnano.9.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirviö J. A.; Visanko M. Anionic wood nanofibers produced from unbleached mechanical pulp by highly efficient chemical modification. J. Mater. Chem. A 2017, 5, 21828–21835. 10.1039/C7TA05668K. [DOI] [Google Scholar]
- Visanko M.; Sirvio J. A.; Piltonen P.; Sliz R.; Liimatainen H.; Illikainen M. Mechanical fabrication of high-strength and redispersible wood nanofibers from unbleached groundwood pulp. Cellulose 2017, 24, 4173–4187. 10.1007/s10570-017-1406-7. [DOI] [Google Scholar]
- Angelini L. G.; Tavarini S. Ramie Boehmeria nivea (L.) Gaud. as a potential new fibre crop for the Mediterranean region: Growth, crop yield and fibre quality in a long-term field experiment in Central Italy. Ind. Crops Prod. 2013, 51, 138–144. 10.1016/j.indcrop.2013.09.009. [DOI] [Google Scholar]
- Li Z. L.; Yu C. W. Effect of peroxide and softness modification on properties of ramie fiber. Fibers Polym. 2014, 15, 2105–2111. 10.1007/s12221-014-2105-8. [DOI] [Google Scholar]
- Li Z. L.; Chen J.; Zhou J. J.; Zheng L.; Pradel K. C.; Fan X.; Guo H. Y.; Wen Z.; Yeh M. H.; Yu C. W.; Wang Z. L. High-efficiency ramie fiber degumming and self-powered degumming wastewater treatment using triboelectric nanogenerator. Nano Energy 2016, 22, 548–557. 10.1016/j.nanoen.2016.03.002. [DOI] [Google Scholar]
- Khalil H. P. S. A.; Davoudpour Y.; Saurabh C. K.; Hossain M. S.; Adnan A. S.; Dungani R.; Paridah M. T.; Sarker M. Z. I.; Fazita M. R. N.; Syakir M. I.; Haafiz M. K. M. A review on nanocellulosic fibres as new material for sustainable packaging: Process and applications. Renewable Sustainable Energy Rev. 2016, 64, 823–836. 10.1016/j.rser.2016.06.072. [DOI] [Google Scholar]
- Klemm D.; Cranston E. D.; Fischer D.; Gama M.; Kedzior S. A.; Kralisch D.; Kramer F.; Kondo T.; Lindstrom T.; Nietzsche S.; Petzold-Welcke K.; Rauchfuss F. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. 10.1016/j.mattod.2018.02.001. [DOI] [Google Scholar]
- Domínguez de María P.; Maugeri Z. Ionic liquids in biotransformations: from proof-of-concept to emerging deep-eutectic-solvents. Curr. Opin. Chem. Biol. 2011, 15, 220–225. 10.1016/j.cbpa.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Zdanowicz M.; Wilpiszewska K.; Spychaj T. Deep eutectic solvents for polysaccharides processing. A review. Carbohydr. Polym. 2018, 200, 361–380. 10.1016/j.carbpol.2018.07.078. [DOI] [PubMed] [Google Scholar]
- Zhang Q. H.; Vigier K. D. O.; Royer S.; Jerome F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. 10.1039/c2cs35178a. [DOI] [PubMed] [Google Scholar]
- Smith E. L.; Abbott A. P.; Ryder K. S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. 10.1021/cr300162p. [DOI] [PubMed] [Google Scholar]
- Sirviö J. A.; Visanko M.; Liimatainen H. Deep eutectic solvent system based on choline chloride-urea as a pre-treatment for nanofibrillation of wood cellulose. Green Chem. 2015, 17, 3401–3406. 10.1039/C5GC00398A. [DOI] [Google Scholar]
- Li P. P.; Sirvio J. A.; Haapala A.; Liimatainen H. Cellulose nanofibrils from nonderivatizing urea-based deep eutectic solvent pretreatments. ACS Appl. Mater. Interfaces 2017, 9, 2846–2855. 10.1021/acsami.6b13625. [DOI] [PubMed] [Google Scholar]
- Suopajärvi T.; Sirvio J. A.; Liimatainen H. Nanofibrillation of deep eutectic solvent-treated paper and board cellulose pulps. Carbohydr. Polym. 2017, 169, 167–175. 10.1016/j.carbpol.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Hosseinmardi A.; Annamalai P. K.; Wang L.; Martin D.; Amiralian N. Reinforcement of natural rubber latex using lignocellulosic nanofibers isolated from spinifex grass. Nanoscale 2017, 9, 9510–9519. 10.1039/C7NR02632C. [DOI] [PubMed] [Google Scholar]
- Tan X. X.; Zhao W. C.; Mu T. C. Controllable exfoliation of natural silk fibers into nanofibrils by protein denaturant deep eutectic solvent: nanofibrous strategy for multifunctional membranes. Green Chem. 2018, 20, 3625–3633. 10.1039/C8GC01609G. [DOI] [Google Scholar]
- Ma Y.; Xia Q. Q.; Liu Y. Z.; Chen W. S.; Liu S. X.; Wang Q. W.; Liu Y. X.; Li J.; Yu H. P. Production of nanocellulose using hydrated deep eutectic solvent combined with ultrasonic treatment. ACS Omega 2019, 4, 8539–8547. 10.1021/acsomega.9b00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W.; Wang C. Y.; Yi Y. J.; Zhou W. L.; Wang H. Y.; Yang Y. R.; Tan Z. J. Choline chloride-based deep eutectic solvent systems as a pretreatment for nanofibrillation of ramie fibers. Cellulose 2019, 26, 3069–3082. 10.1007/s10570-019-02290-7. [DOI] [Google Scholar]
- Procentese A.; Johnson E.; Orr V.; Garruto Campanile A.; Wood J. A.; Marzocchella A.; Rehmann L. Deep eutectic solvent pretreatment and subsequent saccharification of corncob. Bioresour. Technol. 2015, 192, 31–36. 10.1016/j.biortech.2015.05.053. [DOI] [PubMed] [Google Scholar]
- Hou X. D.; Li A. L.; Lin K. P.; Wang Y. Y.; Kuang Z. Y.; Cao S. L. Insight into the structure-function relationships of deep eutectic solvents during rice straw pretreatment. Bioresour. Technol. 2018, 249, 261–267. 10.1016/j.biortech.2017.10.019. [DOI] [PubMed] [Google Scholar]
- Liu Y. Z.; Guo B. T.; Xia Q. Q.; Meng J.; Chen W. S.; Liu S. X.; Wang Q. W.; Liu Y. X.; Li J.; Yu H. P. Efficient cleavage of strong hydrogen bonds in cotton by deep eutectic solvents and facile fabrication of cellulose nanocrystals in high yields. ACS Sustainable Chem. Eng. 2017, 5, 7623–7631. 10.1021/acssuschemeng.7b00954. [DOI] [Google Scholar]
- Sirviö J. A.; Visanko M.; Liimatainen H. Acidic deep eutectic solvents as hydrolytic media for cellulose nanocrystal production. Biomacromolecules 2016, 17, 3025–3032. 10.1021/acs.biomac.6b00910. [DOI] [PubMed] [Google Scholar]
- Ray D.; Sarkar B. K. Characterization of alkali-treated jute fibers for physical and mechanical properties. J. Appl. Polym. Sci. 2001, 80, 1013–1020. 10.1002/app.1184. [DOI] [Google Scholar]
- Karimi S.; Tahir P. M.; Karimi A.; Dufresne A.; Abdulkhani A. Kenaf bast cellulosic fibers hierarchy: A comprehensive approach from micro to nano. Carbohydr. Polym. 2014, 101, 878–885. 10.1016/j.carbpol.2013.09.106. [DOI] [PubMed] [Google Scholar]
- Li W. Y.; Jin A. X.; Liu C. F.; Sun R. C.; Zhang A. P.; Kennedy J. F. Homogeneous modification of cellulose with succinic anhydride in ionic liquid using 4-dimethylaminopyridine as a catalyst. Carbohydr. Polym. 2009, 78, 389–395. 10.1016/j.carbpol.2009.04.028. [DOI] [Google Scholar]
- Beltramino F.; Roncero M. B.; Torres A. L.; Vidal T.; Valls C. Optimization of sulfuric acid hydrolysis conditions for preparation of nanocrystalline cellulose from enzymatically pretreated fibers. Cellulose 2016, 23, 1777–1789. 10.1007/s10570-016-0897-y. [DOI] [Google Scholar]
- Chen Z.; Reznicek W. D.; Wan C. X. Deep eutectic solvent pretreatment enabling full utilization of switchgrass. Bioresour. Technol. 2018, 263, 40–48. 10.1016/j.biortech.2018.04.058. [DOI] [PubMed] [Google Scholar]
- Xu G. C.; Ding J. C.; Han R. Z.; Dong J. J.; Ni Y. Enhancing cellulose accessibility of corn stover by deep eutectic solvent pretreatment for butanol fermentation. Bioresour. Technol. 2016, 203, 364–369. 10.1016/j.biortech.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Kargarzadeh H.; Ahmad I.; Abdullah I.; Dufresne A.; Zainudin S. Y.; Sheltami R. M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. 10.1007/s10570-012-9684-6. [DOI] [Google Scholar]
- Kumar R.; Mago G.; Balan V.; Wyman C. E. Physical and chemical characterizations of corn stover and poplar solids resulting from leading pretreatment technologies. Bioresour. Technol. 2009, 100, 3948–3962. 10.1016/j.biortech.2009.01.075. [DOI] [PubMed] [Google Scholar]
- Zhang C. W.; Xia S. Q.; Ma P. S. Facile pretreatment of lignocellulosic biomass using deep eutectic solvents. Bioresour. Technol. 2016, 219, 1–5. 10.1016/j.biortech.2016.07.026. [DOI] [PubMed] [Google Scholar]
- Fang C.; Thomsen M. H.; Frankær C. G.; Brudecki G. P.; Schmidt J. E.; AlNashef I. M. Reviving pretreatment effectiveness of deep eutectic solvents on lignocellulosic date palm residues by prior recalcitrance reduction. Ind. Eng. Chem. Res. 2017, 56, 3167–3174. 10.1021/acs.iecr.6b04733. [DOI] [Google Scholar]
- Karp E. M.; Donohoe B. S.; O’Brien M. H.; Ciesielski P. N.; Mittal A.; Biddy M. J.; Beckham G. T. Alkaline pretreatment of corn stover: Bench-scale fractionation and stream characterization. ACS Sustainable Chem. Eng. 2014, 2, 1481–1491. 10.1021/sc500126u. [DOI] [Google Scholar]
- Donohoe B. S.; Decker S. R.; Tucker M. P.; Himmel M. E.; Vinzant T. B. Visualizing lignin coalescence and migration through maize cell walls following thermochemical pretreatment. Biotechnol. Bioeng. 2008, 101, 913–925. 10.1002/bit.21959. [DOI] [PubMed] [Google Scholar]
- Brunecky R.; Vinzant T. B.; Porter S. E.; Donohoe B. S.; Johnson D. K.; Himmel M. E. Redistribution of xylan in maize cell walls during dilute acid pretreatment. Biotechnol. Bioeng. 2009, 102, 1537–1543. 10.1002/bit.22211. [DOI] [PubMed] [Google Scholar]
- Li Z. F.; Li Z. L.; Ding R. Y.; Yu C. W. Composition of ramie hemicelluloses and effect of polysaccharides on fiber properties. Text. Res. J. 2016, 86, 451–460. 10.1177/0040517515592811. [DOI] [Google Scholar]
- Yan L. F.; Li W.; Yang J. L.; Zhu Q. S. Direct visualization of straw cell walls by AFM. Macromol. Biosci. 2004, 4, 112–118. 10.1002/mabi.200300032. [DOI] [PubMed] [Google Scholar]
- Kaparaju P.; Felby C. Characterization of lignin during oxidative and hydrothermal pre-treatment processes of wheat straw and corn stover. Bioresour. Technol. 2010, 101, 3175–3181. 10.1016/j.biortech.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Kristensen J. B.; Thygesen L. G.; Felby C.; Jørgensen H.; Elder T. Cell-wall structural changes in wheat straw pretreated for bioethanol production. Biotechnol. Biofuels 2008, 1, 5. 10.1186/1754-6834-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simola J.; Malkavaara P.; Alén R.; Peltonen J. Scanning probe microscopy of pine and birch kraft pulp fibres. Polymer 2000, 41, 2121–2126. 10.1016/S0032-3861(99)00379-1. [DOI] [Google Scholar]
- Thi S.; Lee K. M. Comparison of deep eutectic solvents (DES) on pretreatment of oil palm empty fruit bunch (OPEFB): Cellulose digestibility, structural and morphology changes. Bioresour. Technol. 2019, 282, 525–529. 10.1016/j.biortech.2019.03.065. [DOI] [PubMed] [Google Scholar]
- Thomas B.; Raj M. C.; B A. K.; H R. M.; Joy J.; Moores A.; Drisko G. L.; Sanchez C. Nanocellulose, a versatile green platform: From biosources to materials and their applications. Chem. Rev. 2018, 118, 11575–11625. 10.1021/acs.chemrev.7b00627. [DOI] [PubMed] [Google Scholar]
- Hietala M.; Sain S.; Oksman K. Highly redispersible sugar beet nanofibers as reinforcement in bionanocomposites. Cellulose 2017, 24, 2177–2189. 10.1007/s10570-017-1245-6. [DOI] [Google Scholar]
- Laitinen O.; Suopajarvi T.; Osterberg M.; Liimatainen H. Hydrophobic, superabsorbing aerogels from choline chloride-based deep eutectic solvent pretreated and silylated cellulose nanofibrils for selective oil removal. ACS Appl. Mater. Interfaces 2017, 9, 25029–25037. 10.1021/acsami.7b06304. [DOI] [PubMed] [Google Scholar]
- Kumar A.; Negi Y. S.; Choudhary V.; Bhardwaj N. K.; Han S. S. Morphological, mechanical, and in vitro cytocompatibility analysis of poly(vinyl alcohol)–silica glass hybrid scaffolds reinforced with cellulose nanocrystals. Int. J. Polym. Anal. Charact. 2017, 22, 139–151. 10.1080/1023666X.2016.1263909. [DOI] [Google Scholar]
- Crabbe-Mann M.; Tsaoulidis D.; Parhizkar M.; Edirisinghe M. Ethyl cellulose, cellulose acetate and carboxymethyl cellulose microstructures prepared using electrohydrodynamics and green solvents. Cellulose 2018, 25, 1687–1703. 10.1007/s10570-018-1673-y. [DOI] [Google Scholar]
- Alenezi H.; Cam M. E.; Edirisinghe M. Experimental and theoretical investigation of the fluid behavior during polymeric fiber formation with and without pressure. Appl. Phys. Rev. 2019, 6, 041401 10.1063/1.5110965. [DOI] [Google Scholar]
- Jiang F. C.; Shao K.. Testing Method of Moisture Content of Ramie Fiber, GB5883-86; Standardization Administration of China: Beijing, China, 1986. [Google Scholar]
- Jiang F. C.; Shao K.. Method of Quantitative Analysis of Ramie Chemical Components, GB5889-86; Standardization Administration of China: Beijing, China, 1986. [Google Scholar]
- Chen Z.; Wan C. X. Ultrafast fractionation of lignocellulosic biomass by microwave-assisted deep eutectic solvent pretreatment. Bioresour. Technol. 2018, 250, 532–537. 10.1016/j.biortech.2017.11.066. [DOI] [PubMed] [Google Scholar]
- Segal L.; Creely J. J.; Martin A. E. Jr.; Conrad C. M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. 10.1177/004051755902901003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.