Abstract
Background
Prostate cancer changes the phenotype of cells within the stromal microenvironment, including fibroblasts, which in turn promote tumour progression. Functional changes in prostate cancer-associated fibroblasts (CAFs) coincide with alterations in DNA methylation levels at loci-specific regulatory regions. Yet, it is not clear how these methylation changes compare across CAFs from different patients. Therefore, we examined the consistency and prognostic significance of genome-wide DNA methylation profiles between CAFs from patients with different grades of primary prostate cancer.
Results
We used Infinium MethylationEPIC BeadChips to evaluate genome-wide DNA methylation profiles from 18 matched CAFs and non-malignant prostate tissue fibroblasts (NPFs) from men with moderate to high grade prostate cancer, as well as five unmatched benign prostate tissue fibroblasts (BPFs) from men with benign prostatic hyperplasia. We identified two sets of differentially methylated regions (DMRs) in patient CAFs. One set of DMRs reproducibly differed between CAFs and fibroblasts from non-malignant tissue (NPFs and BPFs). Indeed, more than 1200 DMRs consistently changed in CAFs from every patient, regardless of tumour grade. The second set of DMRs varied between CAFs according to the severity of the tumour. Notably, hypomethylation of the EDARADD promoter occurred specifically in CAFs from high-grade tumours and correlated with increased transcript abundance and increased EDARADD staining in patient tissue. Across multiple cohorts, tumours with low EDARADD DNA methylation and high EDARADD mRNA expression were consistently associated with adverse clinical features and shorter recurrence free survival.
Conclusions
We identified a large set of DMRs that are commonly shared across CAFs regardless of tumour grade and outcome, demonstrating highly consistent epigenome changes in the prostate tumour microenvironment. Additionally, we found that CAFs from aggressive prostate cancers have discrete methylation differences compared to CAFs from moderate risk prostate cancer. Together, our data demonstrates that the methylome of the tumour microenvironment reflects both the presence and the severity of the prostate cancer and, therefore, may provide diagnostic and prognostic potential.
Keywords: Prostate cancer, Tumour microenvironment, Cancer-associated fibroblast, Stroma, Methylation, Field effect, EPIC microarray
Background
In solid cancers, tumour formation changes the composition and phenotype of surrounding tissue. This creates the complex tumour microenvironment where different cell types, including cancer-associated fibroblasts (CAFs), interact with cancer epithelial cells [1]. CAFs are a heterogeneous population of cells that regulate the phenotype of prostate epithelial cells, including their tumourigenicity, proliferation, migration, invasion, differentiation and responsiveness to therapeutics [2–10]. CAFs also shape the tumour microenvironment by depositing extracellular matrix, promoting the infiltration of immune cells and stimulating angiogenesis [9, 11–13].
The functions of CAFs also evolve with cancer progression [14–17]. Indeed, changes in the histopathological features, gene expression profile and length of telomeres in the stroma have all been associated with poorer relapse-free or overall survival of men with prostate cancer [14, 15, 17–21]. The phenotype of CAFs is also enduring and does not rely on continuous interactions with epithelial cancer cells. This is demonstrated by primary cultures of patient-derived CAFs, which retain distinctive transcriptomic and proteomic profiles and ability to promote tumour progression, even when cultured without tumour epithelium [8, 10, 22, 23]. This stable phenotype is not due to genomic aberrations [24, 25]. Rather, we recently showed that CAFs harbour DNA methylation alterations compared to non-malignant prostate tissue fibroblasts (NPFs), particularly enriched at regulatory regions of the genome [24]. Other studies have also identified differential methylation of candidate genes and an altered repertoire of transcription factor binding sites in CAFs [6, 26, 27].
Although many epigenetic changes in CAFs have now been identified, their conservation between patients and association with prostate cancer aggressiveness is not clear. Therefore, in this study we compared the genome-wide methylation profiles of CAFs and NPFs from a larger cohort of men with primary prostate cancer, some of whom later developed advanced disease. Our results reveal two main sets of differentially methylated regions (DMRs) in CAFs. One set is CAF-specific, with methylation alterations that are remarkably consistent between CAFs and NPFs across all prostate cancers irrespective of grade. The second group of discrete methylation alterations was associated with tumour grade and patient outcome and may provide a potential source of prognostic biomarkers for prostate cancer.
Results
Profiling DNA methylation of prostate fibroblasts using EPIC arrays
We used Infinium MethylationEPIC BeadChips (EPIC arrays) to examine the genome-wide DNA methylation profile of early passage CAFs and NPFs from eighteen men (n = 36 samples) with either moderate or high-grade prostate cancer (Fig. 1a). The fibroblasts were patient-matched, with CAFs from tumour tissue and NPFs from distant benign tissue from a contralateral region and/or different anatomical zone of the same prostate, most often the transition zone (Additional File 1: Table S1). A pathologist verified the histology of all patient tissues. Nine patients had moderate-grade prostate cancer, defined as grade group ≤ 3 (GG ≤ 3; Gleason score 6–7), and nine patients had high-grade disease, defined as grade group ≥ 4 (GG ≥ 4; Gleason 8–10). The GG ≥ 4 patients had significantly higher primary tumour volume, shorter relapse-free survival and greater incidence of distant metastases (Table 1 and Additional File 1: Table S1). For cross-platform validation, we included three cases with published whole genome bisulfite sequencing (WGBS) data (Additional File 1: Table S1) [24]. To enable more thorough comparison of the methylation profiles of fibroblasts from benign and malignant tissue, we also included unmatched benign prostate tissue fibroblasts (BPFs) from patients undergoing transurethral resection of the prostate for benign prostatic hyperplasia (n = 5 men). These patients had no evidence of prostate cancer after at least 5 years follow-up.
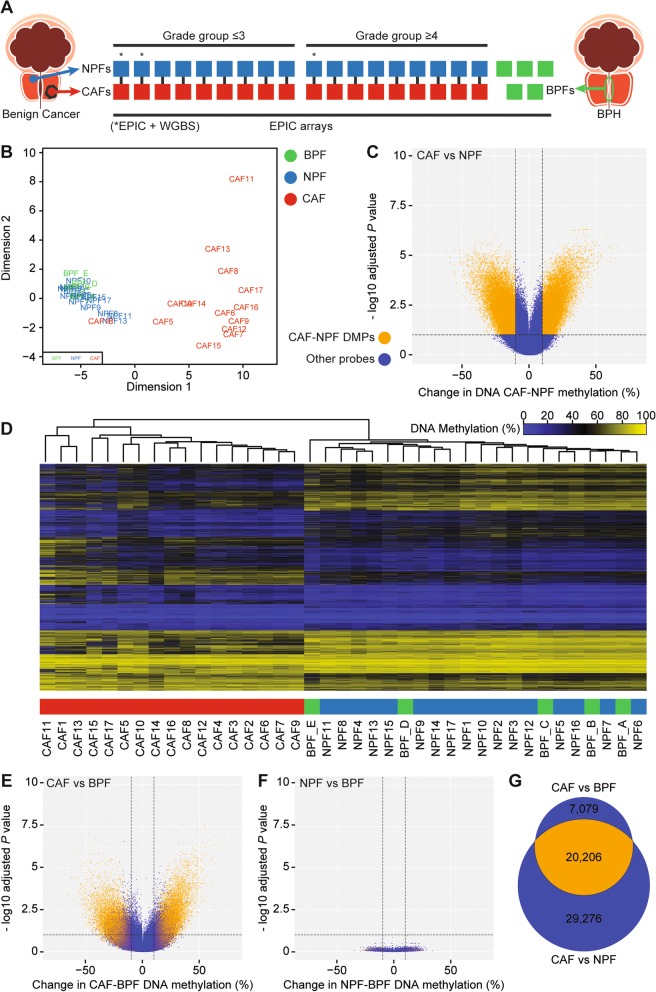
Fig. 1.
Prostate cancer-associated fibroblasts have distinctive changes in DNA methylation. a Schematic of the cohort of patient-derived fibroblasts analysed with EPIC arrays. Asterisks denote that WGBS data was available for three pairs of CAFs and NPFs. b MDS plot of the 1000 most variably methylated CpGs in EPIC array data showing clear separation of CAFs from NPFs and BPFs in patients 4–17; however, CAF18 clustered with NPFs and BPFs. c Volcano plot of differentially methylated positions (DMPs) in CAFs versus NPFs (patients 4–17). DMPs are shown in orange, while other probes are in blue. For all volcano plots, dotted lines indicate > 10% change in methylation and −log10 adjusted P value > 1 (adjusted p value > 0.1). d Dendrogram and heat map from unsupervised hierarchical clustering of the EPIC CAF-DMRs showing clear separation of CAFs from NPFs and BPFs. e and f Volcano plots of DMPs in CAFs versus BPFs and NPFs versus BPFs. DMPs from CAFs versus NPFs (panel c) are shown in orange. g Venn diagram showing the overlap between DMPs in CAFs versus NPFs compared to CAFs versus BPFs
Table 1.
Clinical features and follow-up of patients with ≤ GG3 and ≥ GG4 prostate cancer
| ≤ GG3 | ≥ GG4 | Pvalue | |
|---|---|---|---|
| Patients, no. | 9 | 9 | |
| Age, median (range) | 68 (53–72.4) | 65 (60–74) | 0.8249a |
| Gleason Grade Group, no. (%) | |||
| GG2 | 5 (56%) | 0 | |
| GG3 | 4 (44%) | 0 | |
| GG4 | 0 | 1 (11%) | |
| GG5 | 0 | 8 (89%) | |
| Clinical features, median (range) | |||
| PSA ng/mL | 6.7 (4-11) | 7 (4.3–22.6) | 0.3072a |
| Tumour volume | 4 (0.7-7.1) | 19.7 (0.7–30.2) | 0.0103a |
| Clinical features, no. (%) | |||
| Pathologic T stage 2 | 2 (22%) | 2 (22%) | 1.0b |
| Pathologic T stage 3 | 7 (78%) | 7 (78%) | |
| Positive margins | 6 (67%) | 3 (33%) | 0.6372c |
| Extra-prostatic extension | 7 (78%) | 7 (78%) | 1.0c |
| Seminal vesicle invasion | 3 (33%) | 7 (78%) | 0.1534c |
| Lymph node metastases at diagnosis | 0 | 4 (44%) | 0.0824c |
| Patient follow-upd, no. (%) | |||
| Disease relapsee | 2 (25%) | 8 (89%) | 0.0030e |
| Metastasis | 1 (13%) | 7 (78%) | 0.0152c |
| Castration-resistant prostate cancer | 0 (0%) | 3 (33%) | 0.2059c |
| Death from prostate cancer | 0 (0%) | 1 (11%) | 1.0c |
aUnpaired T test with Welch’s correction
bThe Fisher exact test comparing the proportion of patients with T2 versus T3 disease
cThe Fisher exact test comparing the proportion of patients with or without each clinical feature
dFollow-up information was unavailable for one GG ≤ 3 patient, so n = 8
eDisease relapse includes biochemical or clinical recurrence, HR = 6.937 (1.738–27.68), log rank test
The EPIC array methylation data showed technical reproducibility with WGBS. DNA methylation levels were highly correlated across the 796,222 CpG sites common to both platforms for three patient-matched pairs of CAFs and NPFs (patients 1–3, Pearson correlation 0.84–0.87) (Additional File 2: Figure S1a) [24]. As we previously reported, there was no evidence of global hypomethylation in CAFs versus NPFs (Additional File 2: Figure S1a) [24]. Nevertheless, CAFs clearly separated from NPFs in the first dimension of multidimensional scaling (MDS) plots of both datasets (Additional File 2: Figure S1b-c). Furthermore, there was excellent concordance in previously identified DMRs in CAFs versus NPFs (CAF-DMRs) between the two platforms (Additional File 2: Figure S1d), based on 3384 regions with probes on the EPIC array. For example, hypermethylated DMRs in the TBX3 gene were consistently detected in each patient by both EPIC arrays and WGBS (Additional File 2: Figure S1e). Altogether, this demonstrates the accuracy of the EPIC platform for measuring DNA methylation values and differential methylation in this study.
CAFs have distinct methylation profiles from NPFs and BPFs
To examine the methylation profile of BPFs, NPFs and CAFs, we generated an MDS plot of the EPIC methylation data, excluding the three patients used for technical validation (i.e., patients 4–18 only) (Fig. 1b). CAFs formed a separate group from NPFs and BPFs in the first dimension of the analysis, confirming their distinct DNA methylation profiles across patients. CAFs were also more dispersed than NPFs and BPFs in the second dimension of the plot. The plot suggests some patient-to-patient epigenetic variation in CAFs, but minimal differences in methylation among NPFs and BPFs.
Unexpectedly, CAF18 clustered with NPFs and BPFs rather than CAFs (Fig. 1b). To further analyse CAF18, we used an in vitro co-culture assay that measures the ability of fibroblasts to induce morphological changes in prostate epithelial cells [8, 28]. Unlike other CAFs, CAF18 did not induce significant phenotypic changes in prostate epithelial cells compared to its patient-matched NPF (Additional File 1: Table S1 and Additional File 2: Figure S2a & b). Given that CAF18 was atypical in both DNA methylation and the functional assay, we excluded this patient from further analyses. We speculate that CAF18 was originally misclassified as a CAF possibly due to poor sampling of the patient’s tumour tissue.
Identifying novel differentially methylated regions
Since the remaining CAFs formed a separate cluster in the MDS plot (Fig. 1b), we performed a new genome-wide analysis to identify specific regions of differential methylation. We excluded the three patients (patients 1–3) previously analysed with WGBS [21]. This revealed ~ 50,000 significantly differentially methylated positions (DMPs) between CAFs and NPFs (Fig. 1c, adjusted P value < 0.1 and absolute methylation difference > 10%). These DMPs could be further grouped into DMRs: 2369 hypermethylated and 3038 hypomethylated with more than 10% difference in methylation in CAFs versus NPFs (Additional File 1: Table S2 & S3). These regions are herein referred to as EPIC CAF-DMRs. Unsupervised clustering of samples using the methylation of probes within the EPIC CAF-DMRs separated all CAFs from both NPFs and BPFs (Fig. 1d). Notably, 2059 of the hypermethylated regions (87%) and 2501 of the hypomethylated regions (82%) were not previously reported with WGBS.
NPFs and BPFs have negligible differences in DNA methylation
Based on the MDS plot and EPIC CAF-DMRs (Fig. 1b, d), we found that NPFs are more similar to unmatched BPFs than they are to their patient-matched CAFs. To further compare each set of prostatic fibroblasts, we performed genome-wide analysis of differential methylation using limma. Strikingly, we identified 27,285 DMPs in CAFs versus BPFs (Fig. 1e), but no significant DMPs in NPFs versus BPFs (Fig. 1f; adjusted P value < 0.1 and absolute methylation difference > 10%). Therefore, we conclude that NPFs and BPFs share very similar methylomes, despite being from different patients with different prostatic diseases. Furthermore, the DMPs in CAFs versus BPFs were largely the same as those between CAFs versus NPFs (Fig. 1e, g).
CAF-DMRs are consistent across patients
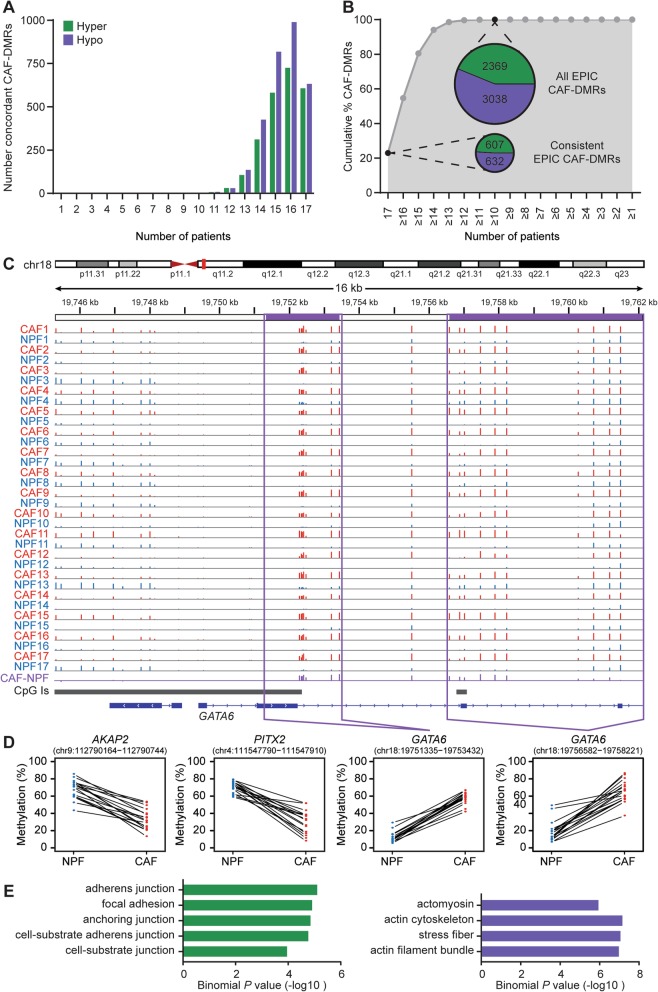
To further examine the EPIC CAF-DMRs, we determined how consistent they were across patients. The majority of hypermethylated and hypomethylated EPIC CAF-DMRs were present in most patients (Fig. 2a). Indeed, 80% of EPIC CAF-DMRs showed concordant methylation differences in at least 15 of the 17 patients, and all were shared by at least 10 of the patients (Fig. 2b). Furthermore, 1239 ‘consistent EPIC CAF-DMRs’ had concordant methylation differences in all 17 patients (607/2369 (26%) of hypermethylated and 632/3038 (21%) of hypomethylated EPIC CAF-DMRs; Fig. 2a, b and Additional File 1: Tables S2 and S3). These consistent EPIC CAF-DMRs encompassed 1.6% of the CpG sites assayed by the EPIC array (hypermethylated DMRs span 2161 probes, hypomethylated DMRs span 10,744 probes). The differentially methylated genes included GATA6, with two hypermethylated DMRs in all 17 CAFs compared to their matched NPFs (Fig. 2c, d). Conversely, PITX2 and AKAP2 had hypomethylated DMRs in all patient’s CAFs (Fig. 2d). We also confirmed that there were significant correlations between DNA methylation and mRNA abundance for candidate EPIC CAF-DMRs (Additional File 2: Figure S3A-B).
Fig. 2.
Consistently differentially methylated regions across patients in CAFs versus NPFs. a Graph showing the number of EPIC CAF-DMRs that are concordantly differentially methylated in the expected direction in each number of patients. b Graph showing the cumulative percentage of EPIC CAF-DMRs that are concordantly differentially methylated in the expected direction in each number of patients. Inset pie charts show the number of concordant EPIC CAF-DMRs in 17/17 patients (22.0% of DMRs) and 10/17 patients (100% of DMRs). c EPIC data for the GATA6 gene for each NPF (blue) and CAF (red). The average difference in DNA methylation in CAFs compared to NPFs is shown in purple. The height of each vertical line represents the percentage of DNA methylation at each CpG site. Purple boxes show the site of two EPIC CAF-DMRs. d Graphs showing DNA methylation levels in each NPF and CAF for representative hypomethylated (AKAP2 and PITX2) and hypermethylated (GATA6) consistent EPIC CAF-DMRs. Lines connect each patient-matched pair of fibroblasts. For each sample, the percentage of DNA methylation is averaged across CpG sites within each DMR. e Plots showing −log10 binomial P values of pathways within the cellular content category that were enriched in GREAT analysis of hypermethylated (green) and hypomethylated (purple) consistent EPIC CAF-DMRs
To investigate the possible functional importance of the 1239 consistent EPIC CAF-DMRs, we used the Genomic Regions Enrichment of Annotation Tool (GREAT) [29]. We observed enrichment of terms related to cell adhesion (focal adhesion, cell-substrate adherens junction, stress fibre, actin filament bundle), as well as ligand activated cell signalling, including TGFβ, insulin and PDGF signalling pathways (Fig. 2e and Additional File 1: Table S4). The remarkable concordance of the methylation changes at this large set of consistent EPIC CAF-DMRs across patients, coupled with their association with genes in biologically relevant pathways, suggests their likely importance in defining the identity and functions of CAFs in prostate cancer.
EDARADD is hypomethylated in grade group ≥ 4 CAFs
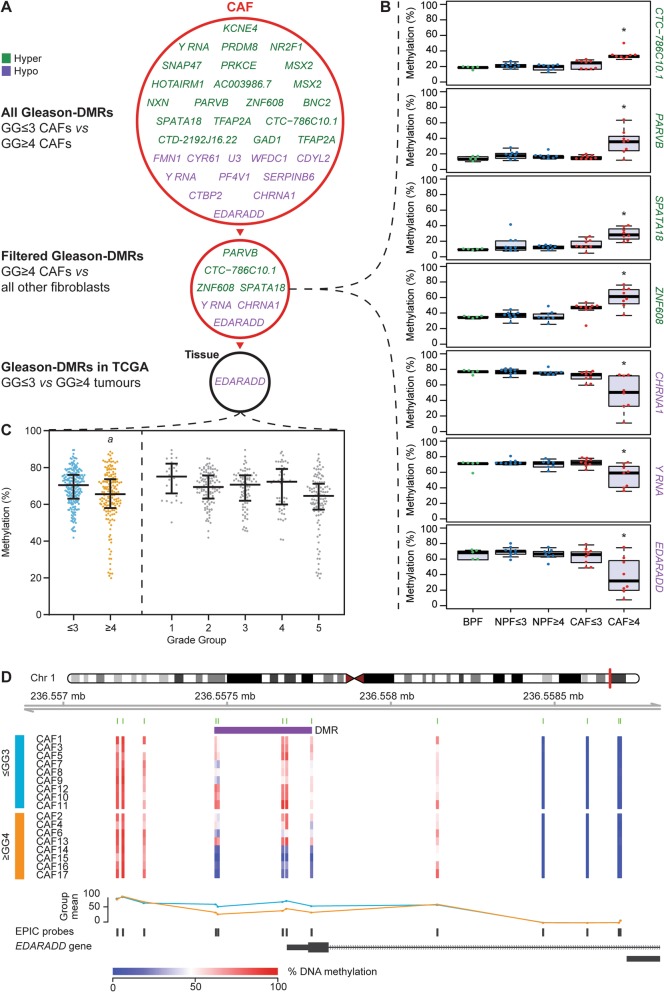
In addition to the set of DMRs that distinguish CAFs from NPFs, we next examined whether there is a set of DMRs that identify patients with more aggressive tumours. We compared the DNA methylation profiles of CAFs from GG ≤ 3 (n = 9) versus GG ≥ 4 tumours (n = 8) and used DMRcate to identify regions with absolute methylation differences greater than 10%, which we termed Gleason-DMRs. We found 31 Gleason-DMRs; only four of which were previously identified as CAF-DMRs [24] (Fig. 3a and Additional File 1: Table S5). To verify the cell-type specificity of the Gleason-DMRs, we used ANOVA models to compare methylation levels at Gleason-DMRs between the five sets of fibroblasts (BPFs, GG ≤ 3 NPFs, GG ≥ 4 NPFs, GG ≤ 3 CAFs and GG ≥ 4 CAFs). Seven of the Gleason-DMRs were significantly different between GG ≥ 4 CAFs and all other fibroblast groups (P < 0.05, Fig. 3a, b). Of these, the EDARADD Gleason-DMR had the greatest methylation difference in GG ≥ 4 CAFs versus other fibroblasts (mean methylation difference of 26%; Additional File 1: Table S5).
Fig. 3.
EDARADD is hypomethylated in CAFs from high-grade group prostate cancer. a Schematic of genes proximal to Gleason-DMRs in CAFs from GG ≤ 3 versus GG ≥ 4 prostate cancer. Gleason-DMRs that are hypermethylated in GG ≥ 4 CAFs are shown in green, while Gleason-DMRs that are hypomethylated in GG ≥ 4 CAFs are shown in purple. Seven of these Gleason-DMRs were also differentially methylated in GG ≥ 4 CAFs versus all other groups of fibroblasts (see panel b). Of these Gleason-DMRs, EDARADD was also significantly differentially methylated in GG ≥ 4 versus GG ≤ 3 tissues from TCGA (see panel c). b Boxplots showing DNA methylation of Gleason-DMRs in different groups of fibroblasts. Each dot represents a different fibroblast sample (*P < 0.05 One-way ANOVA GG ≥ 4 CAF vs all other groups). c Plot of EDARADD DNA methylation levels in patient tissue samples from TCGA. Samples are arranged as GG ≤ 3 versus GG ≥ 4 prostate cancer (aP = 8.3 × 10−5, diff = − 5.2%, Mann-Whitney test) and as individual grade groups. Each dot represents a different patient, with lines indicating median and ± IQR. d Schematic of the EDARADD Gleason-DMR showing the levels of DNA methylation at each CpG site in each CAF (blue = low methylation; red = high methylation). The trend lines show the average methylation status of GG ≤ 3 CAFs (light blue) versus GG ≥ 4 CAFs (orange). The location of the Gleason-DMR is shown in purple
Next, to assess their potential application as prognostic biomarkers we examined whether the seven Gleason-DMRs in CAFs could also be detected in whole patient tumour tissue, which contains heterogeneous cell types. We analysed the Infinium Methylation450 BeadChip (450 K array) data from 392 prostate cancer samples in TCGA. We found that only the EDARADD Gleason-DMR was significantly differentially methylated between GG ≤ 3 (n = 226) versus GG ≥ 4 tumours (n = 166) (Fig. 3a, c). A sequential decrease in DNA methylation of the EDARADD Gleason-DMR was also apparent from low to high-grade group samples (Fig. 3c). EDARADD is an adaptor in the EDAR pathway, which regulates the development of ectodermal tissues [30]. EDARADD is also differentially expressed in prostate and lung cancer [31, 32], which is notable since the Gleason-DMR lies in the potential gene promoter (Fig. 3d). Based on these observations, we examined the association between EDARADD methylation and gene expression levels and high risk prostate cancer in more detail.
EDARADD is differentially expressed in high-grade prostate cancer
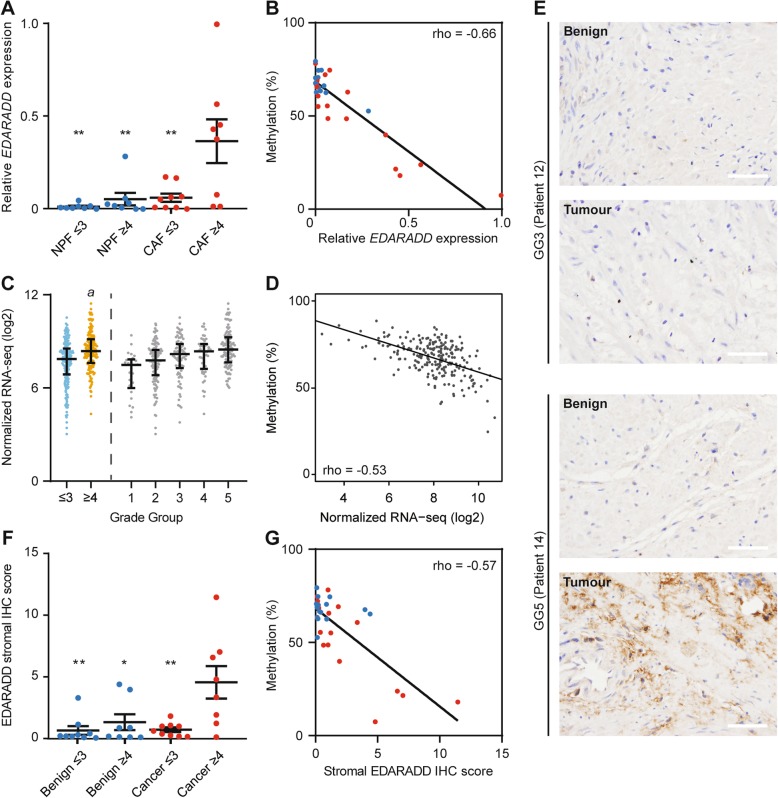
Since the EDARADD Gleason-DMR lies in a potential promoter for this gene, we assessed EDARADD mRNA and protein levels and their correlation with methylation. EDARADD mRNA levels were significantly higher in CAFs from grade group ≥ 4 tumours compared to all other groups of fibroblasts (BPFs, GG ≤ 3 NPFs, GG ≥ 4 NPFs and GG ≤ 3 CAFs), as measured using qPCR (Fig. 4a). Accordingly, there was a significant negative correlation between EDARADD mRNA abundance and DNA methylation at the Gleason-DMR across CAFs and NPFs (Fig. 4b). We observed the same pattern of EDARADD mRNA levels in patient tissues from TCGA, with significantly higher expression in high-grade group tumours (Fig. 4c) and a significant negative correlation with DNA methylation of probes in the Gleason-DMR (Fig. 4d).
Fig. 4.
EDARADD expression is increased in high-grade prostate cancer and correlated with DNA methylation. a Plot showing the average expression of EDARADD (± SEM) in each group of NPFs (blue) and CAFs (red). There was significantly higher mRNA abundance in ≥ GG4 CAFs versus each other fibroblast group (**P < 0.01 One-way ANOVA with Tukey post hoc analysis). b Scatter plot showing the significant negative correlation between EPIC data for EDARADD DNA methylation and qRT-PCR data for EDARADD mRNA abundance (Spearman correlation, P < 0.0001). Each dot represents a different fibroblast sample. c Plot of RNA-seq data showing higher EDARADD expression in ≥ GG4 versus ≤ GG3 prostate cancer specimens from TCGA (b logFC between GG1-3 vs GG4-5 = 1.57, genome-wide adjusted P = 6.9 × 10−07, generalized linear model using edgeR). d Scatter plot of matching EDARADD 450K DNA methylation data and RNA-seq data from TCGA showing a significant negative correlation (Spearman correlation, P = 3.2 × 1017). e Representative images of immunohistochemistry (IHC) for EDARADD in matched benign and tumour tissues. Scale bars equal 50 μm. f Plot of the average EDARADD stromal IHC score (± SEM) in each group of patient tissues. There was significantly higher EDARADD staining in ≥ GG4 tumours versus ≤ GG3 tumours and benign samples (*P < 0.05, **P < 0.01 One-way ANOVA with Tukey post hoc analysis). g Scatter plot showing the significant negative correlation between EDARADD DNA methylation in fibroblasts and the stromal EDARADD IHC score in matching patient tissues (Spearman correlation, P = 0.0006)
We also used immunohistochemistry, with appropriate controls, to examine EDARADD protein levels in the original patient samples from which the CAFs and NPFs were established (Fig. 4e and Additional File 2: Figure S4a-c). Stromal EDARADD staining was significantly higher in grade group ≥ 4 tumours compared to other patient samples (Fig. 4f) and negatively correlated with EDARADD methylation in the matching fibroblasts (Fig. 4 g). EDARADD was also expressed in the epithelium, but with no significant difference in staining between grade groups (Additional File 2: Figure S4d). We noted that a subset of patients have particularly pronounced changes in EDARADD methylation, expression and stromal staining compared to other patients. This is evident in the frequency histograms showing wider ranges of values for CAFs compared to NPFs, and longer tails of values for tumour compared to benign tissue from the TCGA cohort (Additional File 2: Figure S5a-e).
EDARADD methylation is associated with age in non-malignant prostate samples
Intriguingly, the precise region of the EDARADD Gleason-DMR, specifically EPIC probe cg09809672 (chr1:236,557,682, hg19), is known to be gradually hypomethylated with age in human blood and saliva samples [33, 34]. Indeed, we observed a significant negative correlation between cg09809672 methylation and patient age in NPFs and normal prostate tissues from TCGA (Additional File 2: Figure S6a-b), but this trend was much more subtle in CAFs and tumour tissues from TCGA (Additional File 2: Figure S6c-d). This concurs with a previous study showing a weaker association between DNA methylation and chronological age in cancer tissues compared with healthy tissues [35].
We also investigated whether the hypomethylation of EDARADD in the high-grade group samples might signify an accelerated aging phenotype, based on an established DNA methylation signature frequently observed in cancer [35]. However, we found no difference in the DNA methylation aging signature between NPFs and CAFs or grade group ≤ 3 CAFs and grade group ≥ 4 CAFs (Additional File 2: Figure S6e). Nor was there a relationship between the aging signature and EDARADD methylation across all fibroblasts (Additional File 2: Figure S6f). Thus, EDARADD is gradually hypomethylated with age in non-malignant prostate samples, but the significant decrease in EDARADD methylation in high-grade CAF samples is not linked to a more general aging phenotype in these cells. This is consistent with the known weakness of the relationship between an accelerated aging signature and tumour grade [35].
EDARADD methylation and expression are associated with poor clinical features and patient outcomes
Since EDARADD methylation and expression are associated with grade group in CAFs and tumour tissue, we examined whether there was any association with other clinical features or patient outcomes in several published prostate cancer cohorts. In each cohort, to capture the subset of patients with epigenetic changes in EDARADD, we compared patients in the bottom quartile of EDARADD methylation or top quartile of EDARADD expression to the rest of the cohort. In TCGA, patients in the lowest quartile of EDARADD methylation or highest quartile of expression had significantly higher grade group (Table 2). They also had significantly higher pathologic tumour stage and incidence of positive lymph nodes but no difference in age at diagnosis (Table 2).
Table 2.
Clinical features of TCGA patients based on EDARADD expression and methylation
| TCGA DNA methylation | Bottom 0.25 | Top 0.75 | Pvalue |
| Patients, no. | 97 | 290 | |
| Age, median (range) | 62 (47–75) | 62 (44–78) | 0.5339a |
| Gleason Grade Group, no. (%) | |||
| GG1 | 1 (1%) | 26 (9%) | < 0.0001b |
| GG2 | 18 (19%) | 96 (33%) | |
| GG3 | 18 (19%) | 63 (21%) | |
| GG4 | 16 (16%) | 36 (12%) | |
| GG5 | 44 (45%) | 69 (24%) | |
| Clinical features, no. (%) | |||
| Pathologic T stage 2 | 24 of 96 (25%) | 115 of 286 (40%) | 0.0072c |
| Pathologic T stage 3+ | 72 (75%) | 171 (60%) | |
| Lymph node involvement | 33 of 91 (36%) | 36 of 240 (13%) | < 0.0001d |
| Patient follow-up | |||
| Relapse, no of events | 44 | 26 | |
| Log rank teste | 0.0095 | ||
| Cox modele | 0.0167 | ||
| TCGA RNA levels | Top 0.25 | Bottom 0.75 | Pvalue |
| Patients, no. | 95 | 285 | |
| Age, median (range) | 62 (46–78) | 62 (44–77) | 0.7623a |
| Gleason Grade Group, no. (%) | |||
| GG1 | 1 (1%) | 26 (9%) | < 0.0001b |
| GG2 | 20 (24%) | 94 (33%) | |
| GG3 | 20 (24%) | 61 (21%) | |
| GG4 | 13 (15%) | 36 (13%) | |
| GG5 | 41 (48%) | 68 (24%) | |
| Clinical features, no. (%) | |||
| Pathologic T stage 2 | 16 of 94 (17%) | 120 of 281 (43%) | < 0.0001c |
| Pathologic T stage 3+ | 78 (83%) | 161 (57%) | |
| Lymph node involvement | 28 of 90 (31%) | 34 of 223 (15%) | 0.0026d |
| Patient follow up | |||
| Relapse, no of events | 42 | 27 | |
| Log rank testf | 0.0054 | ||
| Cox modelf | 0.0005 | ||
Sample numbers are based on the availability of clinical, methylation (387 samples) and expression (380 samples) data. Numbers in italics denote sample numbers where data was not available for some cases
aUnpaired T test with Welch’s correction
bChi-squared test for trend
cThe Fisher exact test comparing the proportion of patients with T2 versus T3 disease
dChi-squared test
eLog rank HR = 0.48 (0.28–0.84), Cox model HR = 0.10 (0.012–0.66)
fLog rank HR 1.96 (1.13–3.39), Cox model HR = 1.41 (1.16–1.72)
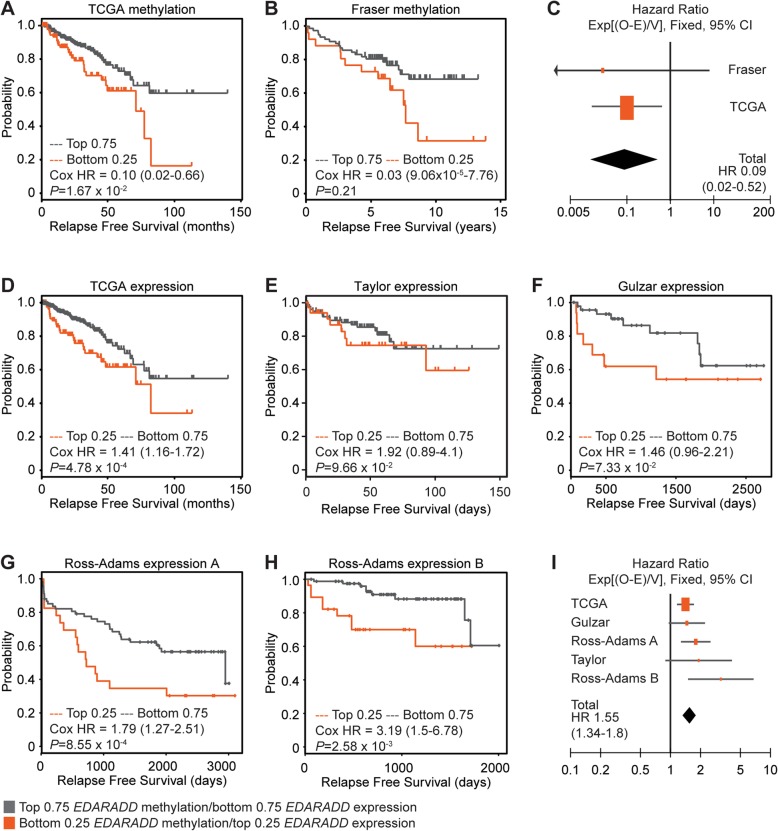
We also examined differences in relapse-free survival using Kaplan Meier curves and Cox models of EDARADD methylation or expression as continuous variables. Low EDARADD methylation was significantly associated with shorter relapse-free survival in TCGA patients (Fig. 5a and Table 2). We observed the same trend in methylation in the Fraser cohort, even though it is restricted to low-moderate risk prostate cancer (Fig. 5b) [36]. A meta-analysis confirmed that the association between EDARADD hypomethylation and poor relapse-free survival was significant across both datasets (Fixed effect model, Z = 3.14, P = 0.002, Fig. 5c).
Fig. 5.
EDARADD methylation and expression are associated with poor relapse-free survival in prostate cancer cohorts. a, b Kaplan Meier plots of relapse free survival for patients in the lowest quartile of EDARADD methylation (bottom 0.25, orange) versus the rest of each cohort (top 0.75, grey). c Forest plot showing the Cox hazard ratios (± 95% CI) for relapse free survival based on EDARADD methylation and a meta-analysis of both methylation datasets (Heterogeneity: Chi2 = 0.09, df = 1 (P = 0.76); I2 = 0%; Test for overall effect: Z = 3.14 (P = 0.002)). d–h Kaplan Meier plots of relapse free survival for patients in the highest quartile of EDARADD expression (top 0.25, orange) versus the rest of each cohort (bottom 0.75, grey) for the TCGA and Fraser datasets. i Forest plot showing the Cox hazard ratios (± 95% CI) for relapse-free survival based on EDARADD expression and a meta-analysis of all methylation datasets (Heterogeneity: Chi2 = 5.45, df = 4 (P = 0.24); I2 = 27%; Test for overall effect: Z = 5.74 (P < 0.00001))
Consistent with the inverse correlation between EDARADD methylation and expression, high EDARADD expression was significantly associated with poor relapse-free survival among patients in TCGA (Fig. 5d). There was the same trend in four other patient cohorts (Fig. 5e–h), reaching significance in both datasets from Ross-Adams and colleagues [37–39]. A meta-analysis of all five cohorts confirmed that higher EDARADD expression is significantly associated with shorter relapse-free survival (Fixed effect model, Z = 5.74, P < 0.00001, Fig. 5i). Finally, the levels of EDARADD methylation and expression in CAFs, and stromal staining in matched tumour tissues, were all significantly associated with poor relapse-free survival in our cohort (Additional File 2: Figure S7), consistent with the overall difference in survival between the grade group ≤ 3 and grade group ≥ 4 patients (Table 1). Therefore, epigenetic changes in EDARADD are consistently associated with adverse clinical features and poor relapse-free survival in men with localised prostate cancer.
Discussion
Tumourigenesis is associated with genome-wide DNA methylation alterations of cells within the tumour microenvironment, including CAFs [24]. Yet, how consistent these changes are across individual patients and whether they vary based on clinical features is unknown. Therefore, we assessed the DNA methylation profiles of prostatic fibroblasts from carefully validated patient samples spanning moderate- to high-grade prostate cancer. We found that a set of differently methylated regions accurately distinguished CAFs from patient-matched NPFs and non-matched BPFs, including a newly identified set of CAF-NPF DMRs with remarkable consistency across patients. We also identified DMRs associated with higher grade group disease, including at the promoter of EDARADD, which was associated with shorter relapse-free survival of patients.
Differences in the methylomes of CAFs or tumour stroma have been observed in primary cell cultures, patient tissue and mouse models of prostate cancer, although often in small numbers of samples [6, 24, 26, 40, 41]. To further examine the technical and biological reproducibility of DMRs, we used EPIC arrays to analyse genome-wide DNA methylation in a larger cohort of patients. There was strong cross-platform validation between EPIC arrays and WGBS, which both separated CAFs from NPFs. Furthermore, we identified numerous DMRs in CAFs versus NPFs, including 1239 CAF-DMRs that were detected in CAFs from every patient. This is remarkable given the heterogeneity of CAFs and the diversity of our cohort, with CAFs isolated from primary cancers with different grade groups, tumour stages, treatment outcomes and presumably genomic aberrations. These DMRs may be useful to validate primary cultures of CAFs and NPFs in future studies. Pathway analyses with the DMRs highlighted the importance of cell adhesion, cell morphology and the PDGF and TGFβ pathways, in concordance with previous studies [2, 42]. Thus, the common functional features of CAFs may include the mechanisms for attaching to the surrounding microenvironment and signalling to other cell types. Overall, the consistent CAF-DMRs imply that key molecular aspects of the identity of CAFs are preserved across prostate cancer samples.
In addition to comparing matched CAFs and NPFs, we examined unmatched BPFs to investigate whether NPFs bear traces of a cancer field effect [43]. The concept of field cancerisation is of particular interest in prostate cancer, because many patients have multifocal disease. Histologically normal tissues adjacent to tumour foci can also exhibit cytomorphological, transcriptional, genomic and epigenomic alterations close to tumour foci [16, 17, 44, 45]. Although some DNA methylation alterations have been reported in more distant regions of non-malignant prostate tissue [41, 46–50], we found negligible differences in our study between the DNA methylation profiles of NPFs and BPFs. Further analysis of CAF-DMRs in samples at varying distances from the tumour may define how far the cancer field effect extends and whether it is variable with tumour grade.
Previous studies have reported progressive changes in the histopathology and transcriptome of tumour stroma from low- to high-grade prostate cancer, so we hypothesised that there may also be changes in DNA methylation [17, 19]. Indeed, we identified a limited number of DNA methylation alterations associated with tumour grade. Notably EDARADD showed the greatest difference in methylation in grade group ≥ 4 CAFs. Loss of methylation at the EDARADD promoter is particularly interesting from a clinical perspective, since it is correlated with increased mRNA expression and stromal staining. EDARADD is an intracellular adaptor protein in the ectodysplasin pathway, activating downstream NFκB signalling when the EDA ligand binds to the EDAR receptor [30]. The ectodysplasin pathway fine-tunes the development of ectodermal tissues such as hair, teeth, sweat glands and mammary glands [51]. Patients with mutations in this pathway, including of EDARADD, have ectodermal dysplasias with malformations of ectodermal tissues. EDARADD is also associated with aging, through hypomethylation of cg09809672 [33, 34]. We observed that this CpG site is indeed hypomethylated with aging in non-malignant prostate tissue; however, the pronounced hypomethylation of EDARADD in high-grade tumour samples was not associated with a more widespread aging signature in this tissue. Although the function of EDARADD and the ectodysplasin pathway in the prostate is unknown, it is possible that it influences the paracrine interactions between stroma and epithelium, since it is expressed in both cell types and regulates the expression of Wnt, FGF and chemokines in other tissues [30, 51].
Further investigation of the role of EDARADD in tumour progression is warranted, given its association with poor patient outcomes across multiple cohorts. In this study, we observed that DNA methylation and gene expression levels of EDARADD are associated with tumour grade, stage, lymph node metastasis and relapse-free survival. EDARADD has also been linked to tumour severity in other studies. Shahabi and colleagues showed that EDARADD expression is upregulated in prostate cancer tissue from patients who develop clinical recurrence [31]. In addition hypomethylation and increased expression of EDARADD in CAFs and patient tissue is associated with poor overall survival in non-small cell lung cancer [32].
Conclusions
This study identified a set of DNA methylation alterations that are specific to CAFs and shared across patients regardless of tumour grade. These shared epigenetic changes potentially encode the phenotypic differences between CAFs and NPFs. We also demonstrated that EDARADD methylation and expression correlate with clinical features and patient outcomes, indicating that specific epigenome changes in CAFs reflect the features of the adjacent tumour. Importantly, EDARADD represents a potential prognostic biomarker to detect the severity of the tumour based on the molecular features of the surrounding microenvironment.
Methods
Patient tissue
Samples of fresh prostate tissue (n = 41, Additional File 1: Table S1) were collected from 23 patients undergoing radical prostatectomy (Table 1) or transurethral resection of the prostate (TURP) with written informed consent according to human ethics approval from Monash University (2004/145), Cabrini Hospital (03-14-04-08) and Epworth Hospital (53611). To isolate CAFs from radical prostatectomy specimens, the location of the tumour was determined using biopsy reports and palpation. The prostate was cut to expose the tumour, and frozen sections were examined by a board-certified pathologist to confirm that the area contained approximately 80% prostate cancer. Approximately, 200–1000 mg of tissue was then dissected from this site. To isolate patient-matched NPFs, benign tissue was obtained from a distant region of the same radical prostatectomy specimen, typically from a different side and zone of the prostate. Frozen sections were used to confirm the lack of tumour cells in the benign tissue. The prostate gland was then reassembled and processed for routine histopathology. BPFs were isolated from TURP chips from men with benign prostatic hyperplasia, but no diagnosis of prostate cancer for at least 5 years after the specimens were collected. Patient clinical features and follow-up were collected by the Melbourne Urological Research Alliance (MURAL). Gleason scores were converted to grade groups (GG) as described [52]. Relapse-free survival was calculated as the time from radical prostatectomy to biochemical relapse (two consecutive and rising serum PSA measurements > 0.2 ng/mL) [53] or clinical relapse [54], whichever occurred first.
Cell culture
Primary cultures of prostatic fibroblasts were established and validated as previously described [8, 43]. Briefly, fresh patient tissue was chopped into small pieces, approximately 2 mm3, and two or three pieces were retained for histology. Fibroblasts were only included in this study if a pathologist confirmed that these pieces of fixed tissue had the correct pathology: benign for BPF and NPF tissues and tumour for CAF tissues. The rest of each tissue was digested overnight at 37 °C in RPMI containing 10% fetal calf serum (FCS), 25 mM HEPES, 100 U/mL penicillin, 100 mg/mL streptomycin, 0.5 mg/mL Amphotericin B, 100 mg/mL gentamicin, 225 U/mL Collagenase Type I and 125 U/mL Hyaluronidase Type II (Sigma-Aldrich) as previously described [43]. Cells were then seeded in RPMI containing 5% FCS, penicillin/streptomycin, 1 nM testosterone (Sigma-Aldrich) and 10 ng/mL bFGF (Millipore), which selects for the growth of fibroblasts versus other prostatic cell types. Cells were grown at 37 °C in a humidified incubator with 5% O2 and 5% CO2. Early passage cultures of fibroblasts (median P4, range P2–7) were used for subsequent experiments.
Microarray genome-wide DNA methylation analysis
DNA was extracted from fibroblast samples with the DNeasy kit (Qiagen) with on-column RNase A digestion. DNA (500 ng) from 18 patient-matched CAF-NPF pairs and 5 BPF samples was treated with sodium bisulphite using the EZ-96 DNA methylation kit (Zymo Research, CA, USA). DNA methylation was quantified using the Illumina Infinium HumanMethylationEPIC (EPIC) BeadChip (Illumina, CA, USA) run on an Illumina HiScan System (Illumina, CA, USA) using the manufacturer’s standard protocol.
Raw intensity data (IDAT) files were imported into the R environment (version 3.1.1) [55] using the minfi package (version 1.20.2) [56]. Each sample passed all quality control steps. The data correctly predicted all patients to be male and control single nucleotide polymorphism (SNP) probes correctly paired the patient-matched CAFs and NPFs. Data was then normalised with background correction. Poor quality probes with a detection P value > 0.01 in at least 10% of the samples were removed. At least 99% of probes passed this step. Poor quality probes with a detection P value > 0.01 in less than 10% samples were considered undetected. Next, to reduce the risk of false discoveries, we removed probes that mapped to multiple locations or overlapped SNPs, as previously described [40]. The resulting dataset comprised 808,100 CpG sites. β values were calculated from unmethylated (U) and methylated (M) signal [M/(U + M + 100)] and ranged from 0 to 1 (0 to 100% methylation). The co-ordinates of all CpG sites were defined using the hg19 human genome assembly.
WGBS data extraction
To compare EPIC and WGBS data, we used in-house CAF and NPF WGBS sequencing data that was generated and processed as previously described [24]. All raw and processed WGBS data is publically available at NCBI Gene Expression Omnibus (GEO) (www.ncbi.nlm.nih.gov/geo) under accession number GSE86833. We used the getMeth function in R package bsseq [57] to extract CAF-NPF WGBS data for patients 1, 2 and 4 at the 796,222/808,100 CpG sites overlapping the EPIC probes in our dataset. To compare EPIC and extracted WGBS methylation data, we used base package functions in R to produce scatterplots, perform Pearson correlation analysis, and output bedGraph files of the data for visualisation in the IGV genome browser [58].
EPIC array statistical analysis
For initial visualisation of the EPIC data, multidimensional scaling plots were generated using the ‘mdsPlot’ function in the minfi Bioconductor package (version 1.20.2) [56]. We then performed differential methylation analysis between novel CAF versus NPF (n = 14 vs n = 14), CAF versus BPF (n = 17 vs n = 5), NPF versus BPF (n = 17 vs n = 5) and between Gleason grade groups (n = 8 GG ≤ 3 CAFs vs n = 7 GG ≥ 4 CAFs). In each case, β values were transformed using logit transformation: M = log2(β/(1−β)). We used the limma Bioconductor package [59] to identify DMPs between sample groups with adjusted p value cut-off of < 0.1. DMPs were visualised as volcano plots using the ggplot2 R package [60]. The R package DMRcate [61] was used to identify DMRs, with DMP p value cut-offs of FDR < 0.05 for CAF versus NPF and p< 0.0001 for GG ≤ 3 versus GG ≥ 4. DMRs were defined as regions with a maximum of 1000 nucleotides between consecutive probes and a minimum of 2 CpG sites, a methylation change > 10% and we applied Benjamini-Hochberg correction for multiple testing. DMRs were annotated for proximity with genetic features using the ‘annotateRegions’ function implemented in the R package aaRon (https://github.com/astatham/aaRon). DMRs were visualised as heat maps with dendrograms using the heatmap.2 function in the gplots R package [62], and bedGraph files of the data were generated for visualisation in the IGV genome browser [58]. ‘Consistent EPIC CAF-DMRs’ were identified by subtraction of methylation differences between each patient-matched CAF and NPF, and GREAT was used to analyse the functional significance of these DMRs [29].
To establish the cell-type specificity of the Gleason-DMRs, we used base package functions in R to perform one-way ANOVA and Tukey post hoc tests to compare Gleason-DMRs in GG ≥ 4 CAFs to all other fibroblast groups. This analysis was performed on a single methylation value for each Gleason-DMR per sample, obtained by calculating the mean methylation across all probes in the region and plotted using the beeswarm package in R [63].
To determine the DNA methylation age of each fibroblast sample, we uploaded β values from EPIC array data to the DNA Methylation Age Calculator (https://dnamage.genetics.ucla.edu/) [35].
Cellularised matrix co-culture model
A cellularised matrix co-culture model was used as previously described with some modifications [8, 28]. Briefly, CAFs and NPFs were seeded in 24 well plates at 1.5 × 104 cells/well and cultured for 5–8 days to yield a dense monolayer with extensive extracellular matrix deposition. RWPE-1 cells (American Type Culture Collection) [64] were maintained in keratinocyte serum-free medium supplemented with 5 ng/mL epidermal growth factor (Gibco), 50 μg/mL bovine pituitary extract (Gibco), 100 U/mL penicillin and 100 mg/mL streptomycin at 37 °C, 5% CO2. For co-cultures, RWPE-1 cells were pre-stained with CellTracker Green CMFDA (Invitrogen), seeded on top of the fibroblasts at 1.5 × 104 cells per well, and cultured at 37 °C, 5% CO2, 5% O2. After 24 h, cellularised matrix co-cultures were fixed with 4% paraformaldehyde for 12 min and then washed with phosphate-buffered saline.
Cellularised matrix co-cultures were imaged at 488 nm and with brightfield microscopy using a Nikon C1 Inverted Eclipse 90i confocal microscope with a × 20 objective lens. 2D quantitative analysis of RWPE-1 cell morphology was performed using ImageJ (NIH) as previously described [8]. Briefly, a maximum intensity projection was obtained of the green-labelled RWPE-1 cells, then a Gaussian Blur filter (σ: 2) was applied, followed by thresholding, and the watershed step to obtain the outlines of the cells. The shape factor, cell area, cell length and standard deviation of orientation of these outlines were then calculated in 8 random fields per co-culture with an average of 58 cells/field (range 20–140).
Quantitative RT-PCR
Total RNA was isolated from prostatic fibroblasts using the RNeasy Kit (QIAGEN) with an on-column DNaseI treatment. A mixture of RNA from human prostatic fibroblasts, epithelial cells and immune cells was pooled and used as a universal prostate control. Each sample (500 ng) was reverse transcribed into cDNA using the Superscript III First Strand Synthesis System (Invitrogen) according to the manufacturer’s instructions. Primer sequences are listed in Additional File 1: Table S6. Gene expression was quantified using Power SYBRTM Green Master Mix (ThermoFisher Scientific) and a Mx3000P qPCR System with MxPro Software (Stratagene). The relative mRNA abundance of the candidate genes compared to the universal prostate cancer control was calculated using the ΔΔCt method and the geometric mean of the three reference genes (GAPDH, HPRT1, RPLPO).
Immunohistochemistry
Small pieces of tissue were retained from the specimens used to establish fibroblast cultures. These samples were formalin-fixed and paraffin embedded. Sections were stained with a rabbit anti-EDARADD antibody (1 μg/mL, HPA018836, Sigma) or rabbit IgG control (1 μg/mL, Dako) using a Leica BOND-MAX-TM autostainer with BondTM epitope retrieval 1 and the Bond Refine Detection Kit (Leica). Slides were imaged using a ScanScope AT Turbo slide scanner (Aperio). Regions of stroma and epithelium in each tissue were circled separately using the ImageScope analysis software (Aperio), and staining was quantified with the positive pixel count v9 algorithm. Positive staining was defined as the percentage of strong positive pixels (intensity limit 0–100) versus the total number of pixels analysed per sample.
Analysis of 450K methylation datasets
Prostate adenocarcinoma (PRAD) 450K methylation data was downloaded from The Cancer Genome Atlas (TCGA) Data Portal website (http://tcga-data.nci.nih.gov/tcgafiles) and processed as described in [24], giving 414,133 CpG sites from 437 samples (of which 392 were tumour tissue). Gleason scores were converted to grade groups as described [52]. We identified probes that overlapped the seven CAF GG ≥ 4 Gleason-DMRs and calculated the mean methylation of probes within each region for each sample. The difference in methylation β values between tissues from n = 226 GG ≤ 3 versus n = 166 GG ≥ 4 prostate cancers was determined with a t test.
The 450K methylation data from the Fraser cohort was downloaded from the NCBI GEO database with accession GSE84043 [36, 65]. IDAT files were imported into the R environment (version 3.1.1) [55] using the minfi package (version 1.20.2) [56]. Data quality was checked with plots derived from control probes on the array. Data was then normalised with background correction. Poor quality probes with a detection P value > 0.01 in at least 10% samples were removed. Next, to reduce the risk of false discoveries we removed probes that mapped to multiple locations or overlapped SNPs [66]. The resulting dataset comprised 444,775 CpG sites. β values for the 160 tumour samples were calculated from unmethylated (U) and methylated (M) signal [M/(U + M + 100)] and ranged from 0 to 1 (0 to 100% methylation). β values were averaged across technical replicates, leaving 104 unique patient samples for analysis. Corresponding patient clinical data was obtained from Supplementary Table 1 in [36].
Analysis of TCGA RNAseq data
TCGA PRAD processed RNA-seq V2 data (level 3) was downloaded from the TCGA Data Portal website (http://tcga-data.nci.nih.gov/tcgafiles) on 19th April 2016. We extracted the samples matching the earlier TCGA PRAD 450K methylation data (described above—tumour n = 385/392). Spearman correlation analysis was used to assess the relationship between DNA methylation at probes within the EDARADD Gleason-DMR and EDARADD gene expression. Additionally, differential gene expression between Gleason grade groups (n = 226 GG ≤ 3 versus n = 159 GG ≥ 4) was calculated genome-wide using edgeR [67] and log fold change and Bonferroni adjusted P value extracted for EDARADD.
Analysis of patient clinical features in public datasets
Clinical data for the TCGA PRAD samples (corresponding to the methylation and expression data above) were downloaded from cBioPortal [68, 69] (TCGA—Provisional) on 13th August 2018. Samples with known disease-free status were included for further analyses (n = 387 for 450K methylation, n = 380 for RNAseq). Clinical 450K methylation data for the Fraser cohort [36] (GSE84043) and Affymetrix array expression data from the Taylor cohort [39] (GSE21032) were downloaded from the NCBI GEO database [65]. The Taylor dataset was processed as previously described [24]. Samples in the bottom quartile of EDARADD DNA methylation or top quartile of EDARADD expression were compared to the rest of the samples in each dataset. Relapse-free survival was visualised using Kaplan-Meier plots and was defined as biochemical relapse or disease-free survival as reported for each dataset. Cox proportional hazards models were used to calculate hazard ratios and P values using the R survival 2.39 package [70]. Additional survival analyses were performed with PROGgene2 [71] using data from the Gulzar cohort [37] (GSE40272) and two cohorts from Ross-Adams and colleagues (GSE70768 and GSE70769) [38].
Meta-analyses
Data from Cox proportional hazard models were used for meta-analyses of the association between relapse-free survival and EDARADD methylation and expression. The O-E and V values for each dataset were calculated as previously described [72]. The Review Manager version 5.3 software was used for meta-analyses [73]. The statistical model was Exp[(O-E)/Var], the statistical method was fixed effect and the effect measure was hazard ratios. The resulting Forest plots were ordered by the effect size of each dataset.
Supplementary information
Additional File 1. Supplementary Tables S1, S2, S3, S4, S5, S6.
Additional File 2. Figures S1, S2, S3, S4, S5, S6, S7.
Acknowledgements
We thank the Epigenetics Research and Prostate Cancer Groups (Garvan Institute of Medical Research) and Prostate Cancer Research Group (Monash University and Peter MacCallum Cancer Centre) for the helpful discussions, Wallace Crellin and Tony Maxwell for their advice and support, Drs Jeremy Grummet, Shomik Sengupta and Ross Snow for patient recruitment, Lucy Busija for guidance on statistics, the Monash Micro Imaging (MMI) facility and Monash Histology Platform for scientific and technical assistance, Tissupath Pathology for pathology analysis, the Australian Prostate Cancer BioResource for specimen collection, Melbourne Urological Research Alliance for providing primary cells, and the patients who donated their tissue. The results are in part based upon data generated by the TCGA Research Network (http://cancergenome.nih.gov).
Abbreviations
- 450K array
Infinium Methylation450 BeadChip
- BPF
Benign prostate tissue fibroblast
- CAF
Cancer-associated fibroblast
- DMR
Differentially methylated region
- EDARADD
EDAR associated death domain
- EPIC array
Illumina Infinium HumanMethylationEPIC BeadChip
- FCS
Fetal calf serum
- GEO
Gene Expression Omnibus
- GG
Grade group
- MDS
Multidimensional scaling
- MURAL
Melbourne Urological Research Alliance
- NPF
Non-malignant prostate tissue fibroblast
- RP
Radical prostatectomy
- RT-PCR
Reverse transcription polymerase chain reaction
- TCGA
The Cancer Genome Atlas
- TURP
Transurethral resection of the prostate
- WGBS
Whole genome bisulfite sequencing
Authors’ contributions
SJC, MGL, RP, GPR, CS and RAT conceived and designed the study and supervised the research. MGL and RP analysed and interpreted the data. MF and MP consented patients and collected follow-up. SN and AR analysed pathology. MGL, RP, BN, BAP, LT and MR performed experiments and analysed data. SJC, MGL, RP, GPR wrote and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was funded by Cancer Australia (1044458), the National Health and Medical Research Council (Project grant 1106870; Fellowships to SJC 1063559; GPR 1002648 & 1102752 and MGL 1035721), Cancer Institute of New South Wales (Fellowship to RP 14/ECF/1-23), Prostate Cancer Foundation of Australia (Movember Young Investigator Grant to MGL YI0911), Cancer Council New South Wales (RG 18-09), the Victorian Government through the Victorian Cancer Agency (Fellowships RAT MCRF15023 and MGL MCRF18017), the Australian Government Research Training Program (RTP) (Scholarship to BAP), RT Hall Trust and TissuPath Pathology.
Availability of data and materials
The data generated as part of this study are available from NCBI Gene Expression Omnibus (GEO) (www.ncbi.nlm.nih.gov/geo) under accession number GSE115413.
The datasets analysed during the current study are available in the following open access repositories:
GEO, https://www.ncbi.nlm.nih.gov/geo/ (GEO accession number: GSE21032, GSE84043, GSE86833)
TCGA, https://cancergenome.nih.gov/
PROGgene, http://watson.compbio.iupui.edu/chirayu/proggene/database/ (Datasets GSE40272, GSE70768, GSE70769).
Ethics approval and consent to participate
Patient samples were obtained with written, informed consent with human ethics approval from Monash University (2004/145), Cabrini Hospital (03-14-04-08) and Epworth Hospital (53611).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mitchell G. Lawrence, Ruth Pidsley, Gail P. Risbridger and Susan J. Clark contributed equally to this work.
Contributor Information
Gail P. Risbridger, Email: gail.risbridger@monash.edu
Susan J. Clark, Email: s.clark@garvan.org.au
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13148-020-00836-2.
References
- 1.Franco OE, Hayward SW. Targeting the tumor stroma as a novel therapeutic approach for prostate cancer. Adv Pharmacol. 2012;65:267–313. doi: 10.1016/B978-0-12-397927-8.00009-9. [DOI] [PubMed] [Google Scholar]
- 2.Ao M, Franco OE, Park D, Raman D, Williams K, Hayward SW. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 2007;67(9):4244–4253. doi: 10.1158/0008-5472.CAN-06-3946. [DOI] [PubMed] [Google Scholar]
- 3.Akerfelt M, Bayramoglu N, Robinson S, Toriseva M, Schukov HP, Harma V, Virtanen J, Sormunen R, Kaakinen M, Kannala J, et al. Automated tracking of tumor-stroma morphology in microtissues identifies functional targets within the tumor microenvironment for therapeutic intervention. Oncotarget. 2015;6(30):30035–30056. doi: 10.18632/oncotarget.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheteh EH, Augsten M, Rundqvist H, Bianchi J, Sarne V, Egevad L, Bykov VJ, Ostman A, Wiman KG. Human cancer-associated fibroblasts enhance glutathione levels and antagonize drug-induced prostate cancer cell death. Cell Death Dis. 2017;8(6):e2848. doi: 10.1038/cddis.2017.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato M, Placencio-Hickok VR, Madhav A, Haldar S, Tripathi M, Billet S, Mishra R, Smith B, Rohena-Rivera K, Agarwal P, et al. Heterogeneous cancer-associated fibroblast population potentiates neuroendocrine differentiation and castrate resistance in a CD105-dependent manner. Oncogene. 2018. [DOI] [PMC free article] [PubMed]
- 6.Mishra R, Haldar S, Placencio V, Madhav A, Rohena-Rivera K, Agarwal P, Duong F, Angara B, Tripathi M, Liu Z, et al. Stromal epigenetic alterations drive metabolic and neuroendocrine prostate cancer reprogramming. J Clin Invest. 2018;128(10):4472–4484. doi: 10.1172/JCI99397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pistore C, Giannoni E, Colangelo T, Rizzo F, Magnani E, Muccillo L, Giurato G, Mancini M, Rizzo S, Riccardi M, et al. DNA methylation variations are required for epithelial-to-mesenchymal transition induced by cancer-associated fibroblasts in prostate cancer cells. Oncogene. 2017;36(40):5551–5566. doi: 10.1038/onc.2017.159. [DOI] [PubMed] [Google Scholar]
- 8.Clark AK, Taubenberger AV, Taylor RA, Niranjan B, Chea ZY, Zotenko E, Sieh S, Pedersen JS, Norden S, Frydenberg M, et al. A bioengineered microenvironment to quantitatively measure the tumorigenic properties of cancer-associated fibroblasts in human prostate cancer. Biomaterials. 2013;34(20):4777–4785. doi: 10.1016/j.biomaterials.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Erdogan B, Ao M, White LM, Means AL, Brewer BM, Yang L, Washington MK, Shi C, Franco OE, Weaver AM, et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J Cell Biol. 2017;216(11):3799–3816. doi: 10.1083/jcb.201704053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59(19):5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8(9):2912–2923. [PubMed] [Google Scholar]
- 12.Ellem SJ, Taylor RA, Furic L, Larsson O, Frydenberg M, Pook D, Pedersen J, Cawsey B, Bioresource APC, Trotta A, et al. A pro-tumorigenic loop at the human prostate tumor interface orchestrated by estrogen, CXCL12 and mast cell recruitment. Journal of Pathology. 2014; (In Press). [DOI] [PubMed]
- 13.Tuxhorn JA, McAlhany SJ, Dang TD, Ayala GE, Rowley DR. Stromal cells promote angiogenesis and growth of human prostate tumors in a differential reactive stroma (DRS) xenograft model. Cancer Res. 2002;62(11):3298–3307. [PubMed] [Google Scholar]
- 14.Ayala G, Tuxhorn JA, Wheeler TM, Frolov A, Scardino PT, Ohori M, Wheeler M, Spitler J, Rowley DR. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin Cancer Res. 2003;9(13):4792–4801. [PubMed] [Google Scholar]
- 15.Heaphy CM, Yoon GS, Peskoe SB, Joshu CE, Lee TK, Giovannucci E, Mucci LA, Kenfield SA, Stampfer MJ, Hicks JL, et al. Prostate cancer cell telomere length variability and stromal cell telomere length as prognostic markers for metastasis and death. Cancer Discov. 2013;3(10):1130–1141. doi: 10.1158/2159-8290.CD-13-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia Z, Wang Y, Sawyers A, Yao H, Rahmatpanah F, Xia XQ, Xu Q, Pio R, Turan T, Koziol JA, et al. Diagnosis of prostate cancer using differentially expressed genes in stroma. Cancer Res. 2011;71(7):2476–2487. doi: 10.1158/0008-5472.CAN-10-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tyekucheva S, Bowden M, Bango C, Giunchi F, Huang Y, Zhou C, Bondi A, Lis R, Van Hemelrijck M, Andren O, et al. Stromal and epithelial transcriptional map of initiation progression and metastatic potential of human prostate cancer. Nat Commun. 2017;8(1):420. doi: 10.1038/s41467-017-00460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mo F, Lin D, Takhar M, Ramnarine VR, Dong X, Bell RH, Volik SV, Wang K, Xue H, Wang Y, et al. Stromal Gene Expression is Predictive for Metastatic Primary Prostate Cancer. Eur Urol. 2018;73(4):524–532. doi: 10.1016/j.eururo.2017.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayala GE, Muezzinoglu B, Hammerich KH, Frolov A, Liu H, Scardino PT, Li R, Sayeeduddin M, Ittmann MM, Kadmon D, et al. Determining prostate cancer-specific death through quantification of stromogenic carcinoma area in prostatectomy specimens. Am J Pathol. 2011;178(1):79–87. doi: 10.1016/j.ajpath.2010.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rochette A, Boufaied N, Scarlata E, Hamel L, Brimo F, Whitaker HC, Ramos-Montoya A, Neal DE, Dragomir A, Aprikian A, et al. Asporin is a stromally expressed marker associated with prostate cancer progression. Br J Cancer. 2017;116(6):775–784. doi: 10.1038/bjc.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blom S, Erickson A, Ostman A, Rannikko A, Mirtti T, Kallioniemi O, Pellinen T. Fibroblast as a critical stromal cell type determining prognosis in prostate cancer. Prostate. 2019;79(13):1505–1513. doi: 10.1002/pros.23867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen EV, Pereira BA, Lawrence MG, Ma X, Rebello RJ, Chan H, Niranjan B, Wu Y, Ellem S, Guan X, et al. Proteomic profiling of human prostate cancer-associated fibroblasts (CAF) reveals LOXL2-dependent regulation of the tumor microenvironment. Mol Cell Proteomics. 2019;18(7):1410–1427. doi: 10.1074/mcp.RA119.001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orr B, Riddick AC, Stewart GD, Anderson RA, Franco OE, Hayward SW, Thomson AA. Identification of stromally expressed molecules in the prostate by tag-profiling of cancer-associated fibroblasts, normal fibroblasts and fetal prostate. Oncogene. 2012;31(9):1130–1142. doi: 10.1038/onc.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pidsley R, Lawrence MG, Zotenko E, Niranjan B, Statham A, Song J, Chabanon RM, Qu W, Wang H, Richards M, et al. Enduring epigenetic landmarks define the cancer microenvironment. Genome Res. 2018;28(5):625–638. doi: 10.1101/gr.229070.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bianchi-Frias D, Basom R, Delrow JJ, Coleman IM, Dakhova O, Qu X, Fang M, Franco OE, Ericson NG, Bielas JH, et al. Cells comprising the prostate cancer microenvironment lack recurrent clonal somatic genomic aberrations. Mol Cancer Res. 2016;14(4):374–384. doi: 10.1158/1541-7786.MCR-15-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banerjee J, Mishra R, Li X, Jackson RS, 2nd, Sharma A, Bhowmick NA. A reciprocal role of prostate cancer on stromal DNA damage. Oncogene. 2014;33(41):4924–4931. doi: 10.1038/onc.2013.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nash C, Boufaied N, Mills IG, Franco OE, Hayward SW, Thomson AA. Genome-wide analysis of AR binding and comparison with transcript expression in primary human fetal prostate fibroblasts and cancer associated fibroblasts. Mol Cell Endocrinol. 2018;471:1–14. doi: 10.1016/j.mce.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Pereira BA, Lister NL, Hashimoto K, Teng L, Flandes-Iparraguirre M, Eder A, Sanchez-Herrero A, Niranjan B. Melbourne Urological Research A: Tissue engineered human prostate microtissues reveal key role of mast cell-derived tryptase in potentiating cancer-associated fibroblast (CAF)-induced morphometric transition in vitro. Biomaterials. 2019;197:72–85. doi: 10.1016/j.biomaterials.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 29.McLean CY, Bristor D, Hiller M, Clarke SL, Schaar BT, Lowe CB, Wenger AM, Bejerano G. GREAT improves functional interpretation of cis-regulatory regions. Nat Biotechnol. 2010;28(5):495–501. doi: 10.1038/nbt.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lefebvre S, Mikkola ML. Ectodysplasin research--where to next? Semin Immunol. 2014;26(3):220–228. doi: 10.1016/j.smim.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Shahabi A, Lewinger JP, Ren J, April C, Sherrod AE, Hacia JG, Daneshmand S, Gill I, Pinski JK, Fan JB, et al. Novel gene expression signature predictive of clinical recurrence after radical prostatectomy in early stage prostate cancer patients. Prostate. 2016;76(14):1239–1256. doi: 10.1002/pros.23211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vizoso M, Puig M, Carmona FJ, Maqueda M, Velasquez A, Gomez A, Labernadie A, Lugo R, Gabasa M, Rigat-Brugarolas LG, et al. Aberrant DNA methylation in non-small cell lung cancer-associated fibroblasts. Carcinogenesis. 2015;36(12):1453–1463. doi: 10.1093/carcin/bgv146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bocklandt S, Lin W, Sehl ME, Sanchez FJ, Sinsheimer JS, Horvath S, Vilain E. Epigenetic predictor of age. PLoS One. 2011;6(6):e14821. doi: 10.1371/journal.pone.0014821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bekaert B, Kamalandua A, Zapico SC, Van de Voorde W, Decorte R. Improved age determination of blood and teeth samples using a selected set of DNA methylation markers. Epigenetics. 2015;10(10):922–930. doi: 10.1080/15592294.2015.1080413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fraser M, Sabelnykova VY, Yamaguchi TN, Heisler LE, Livingstone J, Huang V, Shiah YJ, Yousif F, Lin X, Masella AP, et al. Genomic hallmarks of localized, non-indolent prostate cancer. Nature. 2017;541(7637):359–364. doi: 10.1038/nature20788. [DOI] [PubMed] [Google Scholar]
- 37.Gulzar ZG, McKenney JK, Brooks JD. Increased expression of NuSAP in recurrent prostate cancer is mediated by E2F1. Oncogene. 2013;32(1):70–77. doi: 10.1038/onc.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross-Adams H, Lamb AD, Dunning MJ, Halim S, Lindberg J, Massie CM, Egevad LA, Russell R, Ramos-Montoya A, Vowler SL, et al. Integration of copy number and transcriptomics provides risk stratification in prostate cancer: a discovery and validation cohort study. EBioMedicine. 2015;2(9):1133–1144. doi: 10.1016/j.ebiom.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18(1):11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pidsley R, Zotenko E, Peters TJ, Lawrence MG, Risbridger GP, Molloy P, Van Djik S, Muhlhausler B, Stirzaker C, Clark SJ. Critical evaluation of the Illumina MethylationEPIC BeadChip microarray for whole-genome DNA methylation profiling. Genome Biol. 2016;17(1):208. doi: 10.1186/s13059-016-1066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanson JA, Gillespie JW, Grover A, Tangrea MA, Chuaqui RF, Emmert-Buck MR, Tangrea JA, Libutti SK, Linehan WM, Woodson KG. Gene promoter methylation in prostate tumor-associated stromal cells. J Natl Cancer Inst. 2006;98(4):255–261. doi: 10.1093/jnci/djj051. [DOI] [PubMed] [Google Scholar]
- 42.Aoto K, Ito K, Aoki S. Complex formation between platelet-derived growth factor receptor beta and transforming growth factor beta receptor regulates the differentiation of mesenchymal stem cells into cancer-associated fibroblasts. Oncotarget. 2018;9(75):34090–34102. doi: 10.18632/oncotarget.26124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lawrence MG, Taylor RA, Toivanen R, Pedersen J, Norden S, Pook DW, Frydenberg M. Australian Prostate Cancer B, Papargiris MM, Niranjan B et al: A preclinical xenograft model of prostate cancer using human tumors. Nat Protoc. 2013;8(5):836–848. doi: 10.1038/nprot.2013.043. [DOI] [PubMed] [Google Scholar]
- 44.Nonn L, Ananthanarayanan V, Gann PH. Evidence for field cancerization of the prostate. Prostate. 2009;69(13):1470–1479. doi: 10.1002/pros.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooper CS, Eeles R, Wedge DC, Van Loo P, Gundem G, Alexandrov LB, Kremeyer B, Butler A, Lynch AG, Camacho N, et al. Analysis of the genetic phylogeny of multifocal prostate cancer identifies multiple independent clonal expansions in neoplastic and morphologically normal prostate tissue. Nat Genet. 2015;47(4):367–372. doi: 10.1038/ng.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Truong M, Yang B, Livermore A, Wagner J, Weeratunga P, Huang W, Dhir R, Nelson J, Lin DW, Jarrard DF. Using the epigenetic field defect to detect prostate cancer in biopsy negative patients. J Urol. 2013;189(6):2335–2341. doi: 10.1016/j.juro.2012.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang B, Bhusari S, Kueck J, Weeratunga P, Wagner J, Leverson G, Huang W, Jarrard DF. Methylation profiling defines an extensive field defect in histologically normal prostate tissues associated with prostate cancer. Neoplasia. 2013;15(4):399–408. doi: 10.1593/neo.13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moller M, Strand SH, Mundbjerg K, Liang G, Gill I, Haldrup C, Borre M, Hoyer S, Orntoft TF, Sorensen KD. Heterogeneous patterns of DNA methylation-based field effects in histologically normal prostate tissue from cancer patients. Sci Rep. 2017;7:40636. doi: 10.1038/srep40636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez-Canales J, Hanson JC, Tangrea MA, Erickson HS, Albert PS, Wallis BS, Richardson AM, Pinto PA, Linehan WM, Gillespie JW, et al. Identification of a unique epigenetic sub-microenvironment in prostate cancer. J Pathol. 2007;211(4):410–419. doi: 10.1002/path.2133. [DOI] [PubMed] [Google Scholar]
- 50.Richiardi L, Fiano V, Grasso C, Zugna D, Delsedime L, Gillio-Tos A, Merletti F. Methylation of APC and GSTP1 in non-neoplastic tissue adjacent to prostate tumour and mortality from prostate cancer. PLoS One. 2013;8(7):e68162. doi: 10.1371/journal.pone.0068162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadier A, Viriot L, Pantalacci S, Laudet V. The ectodysplasin pathway: from diseases to adaptations. Trends Genet. 2014;30(1):24–31. doi: 10.1016/j.tig.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Pierorazio PM, Walsh PC, Partin AW, Epstein JI. Prognostic Gleason grade grouping: data based on the modified Gleason scoring system. BJU Int. 2013;111(5):753–760. doi: 10.1111/j.1464-410X.2012.11611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mottet N, Bellmunt J, Briers E, van den Bergh RCN, Bolla M, van Casteren NJ, Cornford P, Culine S, Joniau S, Lam T, et al. EAU - ESTRO - ESUR - SIOG Guidelines on Prostate Cancer 2015. In: European Association of Urology Guidelines 2015 Edition. edn. European Association of Urology Guidelines Office: Arnhem, The Netherlands; 2015. [Google Scholar]
- 54.Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, Davis M, Peters TJ, Turner EL, Martin RM, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415–1424. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 55.Team RDC: R: A language and environment for statistical computing. In.: R Foundation for Statistical Computing; 2010.
- 56.Aryee MJ, Jaffe AE, Corrada-Bravo H, Ladd-Acosta C, Feinberg AP, Hansen KD, Irizarry RA. Minfi: a flexible and comprehensive bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics. 2014;30(10):1363–1369. doi: 10.1093/bioinformatics/btu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hansen KD, Langmead B, Irizarry RA. BSmooth: from whole genome bisulfite sequencing reads to differentially methylated regions. Genome Biol. 2012;13(10):R83. doi: 10.1186/gb-2012-13-10-r83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. Integrative genomics viewer. Nat Biotechnol. 2011;29(1):24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W. Smyth GK: limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wickham H: ggplot2: Elegant graphics for data analysis: Springer-Verlag New York; 2016.
- 61.Peters TJ, Buckley MJ, Statham AL, Pidsley R, Samaras K. R VL, Clark SJ. Molloy PL: De novo identification of differentially methylated regions in the human genome. Epigenetics Chromatin. 2015;8:6. doi: 10.1016/j.molp.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Warnes GR, Bolker B, Bonebakker L, Gentleman R, Liaw W, Lumley T, Maechler M, Magnusson A, Moeller S, Schwartz M et al: gplots: various R programming tools for plotting data. https://cranr-projectorg/package=gplots 2016.
- 63.Eklund A: beeswarm: The bee swarm plot, an alternative to stripchart. In.; 2016.
- 64.Bello D, Webber MM, Kleinman HK, Wartinger DD, Rhim JS. Androgen responsive adult human prostatic epithelial cell lines immortalized by human papillomavirus 18. Carcinogenesis. 1997;18(6):1215–1223. doi: 10.1093/carcin/18.6.1215. [DOI] [PubMed] [Google Scholar]
- 65.Edgar R, Domrachev M, Lash AE. Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30(1):207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen YA, Lemire M, Choufani S, Butcher DT, Grafodatskaya D, Zanke BW, Gallinger S, Hudson TJ, Weksberg R. Discovery of cross-reactive probes and polymorphic CpGs in the Illumina Infinium HumanMethylation450 microarray. Epigenetics. 2013;8(2):203–209. doi: 10.4161/epi.23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robinson MD, McCarthy DJ. Smyth GK: edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao J., Aksoy B. A., Dogrusoz U., Dresdner G., Gross B., Sumer S. O., Sun Y., Jacobsen A., Sinha R., Larsson E., Cerami E., Sander C., Schultz N. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Science Signaling. 2013;6(269):pl1–pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Therneau T: A package for survival analysis in S. Version 2.39. https://CRANR-projectorg/package=survival 2015.
- 71.Goswami CP, Nakshatri H. PROGgeneV2: enhancements on the existing database. BMC Cancer. 2014;14:970. doi: 10.1186/1471-2407-14-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Review Manager (RevMan). In., 5.3 edn. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional File 1. Supplementary Tables S1, S2, S3, S4, S5, S6.
Additional File 2. Figures S1, S2, S3, S4, S5, S6, S7.
Data Availability Statement
The data generated as part of this study are available from NCBI Gene Expression Omnibus (GEO) (www.ncbi.nlm.nih.gov/geo) under accession number GSE115413.
The datasets analysed during the current study are available in the following open access repositories:
GEO, https://www.ncbi.nlm.nih.gov/geo/ (GEO accession number: GSE21032, GSE84043, GSE86833)
TCGA, https://cancergenome.nih.gov/
PROGgene, http://watson.compbio.iupui.edu/chirayu/proggene/database/ (Datasets GSE40272, GSE70768, GSE70769).