Abstract
Background
Pelvic floor muscle training (PFMT) is a first‐line conservative treatment for urinary incontinence in women. Other active treatments include: physical therapies (e.g. vaginal cones); behavioural therapies (e.g. bladder training); electrical or magnetic stimulation; mechanical devices (e.g. continence pessaries); drug therapies (e.g. anticholinergics (solifenacin, oxybutynin, etc.) and duloxetine); and surgical interventions including sling procedures and colposuspension. This systematic review evaluated the effects of adding PFMT to any other active treatment for urinary incontinence in women
Objectives
To compare the effects of pelvic floor muscle training combined with another active treatment versus the same active treatment alone in the management of women with urinary incontinence.
Search methods
We searched the Cochrane Incontinence Group Specialised Register, which contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE in process, ClinicalTrials.gov, WHO ICTRP and handsearching of journals and conference proceedings (searched 5 May 2015), and CINAHL (January 1982 to 6 May 2015), and the reference lists of relevant articles.
Selection criteria
We included randomised or quasi‐randomised trials with two or more arms, of women with clinical or urodynamic evidence of stress urinary incontinence, urgency urinary incontinence or mixed urinary incontinence. One arm of the trial included PFMT added to another active treatment; the other arm included the same active treatment alone.
Data collection and analysis
Two review authors independently assessed trials for eligibility and methodological quality and resolved any disagreement by discussion or consultation with a third party. We extracted and processed data in accordance with the Cochrane Handbook for Systematic Reviews of Interventions. Other potential sources of bias we incorporated into the 'Risk of bias' tables were ethical approval, conflict of interest and funding source.
Main results
Thirteen trials met the inclusion criteria, comprising women with stress urinary incontinence (SUI), urgency urinary incontinence (UUI) or mixed urinary incontinence (MUI); they compared PFMT added to another active treatment (585 women) with the same active treatment alone (579 women). The pre‐specified comparisons were reported by single trials, except bladder training, which was reported by two trials, and electrical stimulation, which was reported by three trials. However, only two of the three trials reporting electrical stimulation could be pooled, as one of the trials did not report any relevant data. We considered the included trials to be at unclear risk of bias for most of the domains, predominantly due to the lack of adequate information in a number of trials. This affected our rating of the quality of evidence.
The majority of the trials did not report the primary outcomes specified in the review (cure or improvement, quality of life) or measured the outcomes in different ways. Effect estimates from small, single trials across a number of comparisons were indeterminate for key outcomes relating to symptoms, and we rated the quality of evidence, using the GRADE approach, as either low or very low. More women reported cure or improvement of incontinence in two trials comparing PFMT added to electrical stimulation to electrical stimulation alone, in women with SUI, but this was not statistically significant (9/26 (35%) versus 5/30 (17%); risk ratio (RR) 2.06, 95% confidence interval (CI) 0.79 to 5.38). We judged the quality of the evidence to be very low. There was moderate‐quality evidence from a single trial investigating women with SUI, UUI or MUI that a higher proportion of women who received a combination of PFMT and heat and steam generating sheet reported a cure compared to those who received the sheet alone: 19/37 (51%) versus 8/37 (22%) with a RR of 2.38, 95% CI 1.19 to 4.73). More women reported cure or improvement of incontinence in another trial comparing PFMT added to vaginal cones to vaginal cones alone, but this was not statistically significant (14/15 (93%) versus 14/19 (75%); RR 1.27, 95% CI 0.94 to 1.71). We judged the quality of the evidence to be very low. Only one trial evaluating PFMT when added to drug therapy provided information about adverse events (RR 0.84, 95% CI 0.45 to 1.60; very low‐quality evidence).
With regard to condition‐specific quality of life, there were no statistically significant differences between women (with SUI, UUI or MUI) who received PFMT added to bladder training and those who received bladder training alone at three months after treatment, on either the Incontinence Impact Questionnaire‐Revised scale (mean difference (MD) ‐5.90, 95% CI ‐35.53 to 23.73) or on the Urogenital Distress Inventory scale (MD ‐18.90, 95% CI ‐37.92 to 0.12). A similar pattern of results was observed between women with SUI who received PFMT plus either a continence pessary or duloxetine and those who received the continence pessary or duloxetine alone. In all these comparisons, the quality of the evidence for the reported critical outcomes ranged from moderate to very low.
Authors' conclusions
This systematic review found insufficient evidence to state whether or not there were additional effects by adding PFMT to other active treatments when compared with the same active treatment alone for urinary incontinence (SUI, UUI or MUI) in women. These results should be interpreted with caution as most of the comparisons were investigated in small, single trials. None of the trials in this review were large enough to provide reliable evidence. Also, none of the included trials reported data on adverse events associated with the PFMT regimen, thereby making it very difficult to evaluate the safety of PFMT.
Plain language summary
Pelvic floor muscle training added to another active treatment versus the same active treatment alone for urinary incontinence in women
Background
Involuntary leakage of urine (urinary incontinence) affects women of all ages, particularly older women who live in residential care, such as nursing homes. Some women leak urine during exercise or when they cough or sneeze (stress urinary incontinence). This may occur as a result of weakness of the pelvic floor muscles, which may be a result of factors such as damage during childbirth. Other women leak urine before going to the toilet when there is a sudden and compelling need to pass urine (urgency urinary incontinence). This may be caused by involuntary contraction of the bladder muscle. Mixed urinary incontinence is the combination of both stress and urgency urinary incontinence. Pelvic floor muscle training is a supervised treatment that involves muscle‐clenching exercises to strengthen the pelvic floor muscles. It is a common treatment used by women to stop urine leakage. Other treatments are also available, which can be used either alone, or in combination with pelvic floor muscle training.
The main findings of the review
In this review, we included 13 trials that compared a combination of pelvic floor muscle training and another active treatment in 585 women with the same active treatment alone in 579 women to treat all types of urine leakage. There was not enough evidence to say whether or not the addition of pelvic floor muscle training to another active treatment would result in more reports of a cure or improvement in urine leakage and better quality of life, when compared to the same active treatment alone.
Adverse effects
There was also insufficient evidence to evaluate the adverse events associated with the addition of PFMT to other active treatment as none of the included trials reported data on adverse events associated with the PFMT regimen.
Limitations of the review
Most of the comparisons were investigated by single trials, which were small. None of the trials included in this systematic review were large enough to answer the questions they were designed to answer. The quality of the evidence was rated as either low or very low for the outcomes of interest. The main limitations of the evidence were poor reporting of study methods, and lack of precision in the findings for the outcome measures.
Summary of findings
Background
Different treatment options are currently available for the management of urinary incontinence in women. Conservative interventions include:
physical therapies such as pelvic floor muscle training (PFMT) with or without biofeedback (Dumoulin 2014; Herderschee 2011);
electrical or magnetic stimulation;
vaginal cones (Herbison 2013);
behavioural therapies including bladder training (Wallace 2004);
timed voiding (Ostaszkiewicz 2004);
prompted voiding (Eustice 2000);
anti‐incontinence devices (Lipp 2011); and
lifestyle interventions such as weight reduction.
Drug therapies include anticholinergics (Madhuvrata 2012; Nabi 2006), duloxetine (Mariappan 2005), local vaginal oestrogens (Cody 2012) and intravesical botulinum toxin (Duthie 2011). Surgical interventions include sling procedures (Ogah 2009; Rehman 2011), colposuspension (Dean 2006; Lapitan 2012), and injection of peri‐urethral bulking agents (Kirchin 2012).
The focus of this review is to determine the benefits of adding PFMT to any of the treatments above for the management of urinary incontinence in women. There is a separate Cochrane review dealing with the conservative treatment of postprostatectomy urinary incontinence in men (Campbell 2012).
Description of the condition
Urinary incontinence or loss of bladder control, according to the International Continence Society (ICS), is defined as the complaint of any involuntary loss of urine (Abrams 2013). It is a common problem that may affect women of all ages with a wide range of severity and a variety of symptoms; however, it is more prevalent in older women, particularly amongst those in institutionalised care (Milsom 2009).
The prevalence of urinary incontinence varies, depending on the age of the study population, the study methods and settings and the definition of the problem (Culligan 2000). In the general population, the estimated prevalence of urinary incontinence in middle‐aged and older women ranges from 30% to 60% and increases with advancing age; the prevalence of daily urinary incontinence ranges from 5% to 15%, and is over 15% in institutionalised women who are over the age of 70 (Milsom 2009). Nonetheless, these figures may not actually reflect the true nature, size and scope of this problem, for it is usually under‐diagnosed and under‐reported due to its embarrassing nature and associated stigmatisation (Shaw 2001a).
Urinary incontinence has an impact on many aspects of a woman's life (Grimby 1993; Hunskaar 1991; Sinclair 2011). Women with urinary incontinence have a significant reduction in their quality of life (Shaw 2001b). It significantly affects couples' relationships (Nilsson 2009); it is reported that 25% to 50% of incontinent women experience sexual dysfunction (Barber 2002). Evidence has also shown that women with urinary incontinence have coexisting psychiatric illness. Melville, et al reported that major depression was three times more common in incontinent women compared to their continent counterparts (6.1% versus 2.2%; Melville 2002). The financial impact of urinary incontinence is enormous; the estimated annual direct cost of treating urinary incontinence in women in the USA was estimated at USD 12.4 billion in 2001 (Wilson 2001). In the UK, the annual NHS cost of treating clinically significant urinary storage symptoms in women was estimated to be GBP 233 million (Turner 2004). With an increasingly ageing population, these costs are likely to increase in the future.
Types of urinary incontinence
There are three main types of urinary incontinence.
Stress urinary incontinence (SUI)
This is defined by the ICS and the International Urogynecological Association (IUGA) as the complaint of involuntary leakage of urine with coughing, sneezing or physical exertion (Haylen 2010). The term urodynamic stress incontinence (USI) is used to describe involuntary leakage of urine with increased intra‐abdominal pressure in the absence of detrusor contraction during urodynamic evaluation (Abrams 2013). Stress urinary incontinence is the most common type of urinary incontinence, affecting an estimated 50% (half) of all incontinent women (Milsom 2009). It is more prevalent in young and middle‐aged women, particularly those who are white and non‐Hispanic (Milsom 2009). It is often associated with weakness of pelvic floor support (muscles and collagen‐dependent tissues (Long 2008), damage to the bladder sphincter mechanism, or both, resulting in bladder neck hypermobility and rotational descent of the proximal urethra with associated intrinsic sphincter deficiency (Schorge 2008). This results in reduction of urethral closure pressure and consequently urine leakage during exertion or physical exercise.
Risk factors for SUI in women include pregnancy, vaginal delivery, increasing parity, advancing age, post‐menopausal state, obesity (MacArthur 2006; MacLennan 2000), and gynaecological procedures such as hysterectomy (Allahdin 2008). The aim of treatment is to strengthen the pelvic floor support, restore the normal function of the sphincter mechanism, or both.
Urgency urinary incontinence (UUI)
This is defined by the IUGA and ICS as the complaint of involuntary leakage of urine associated with urgency (Haylen 2010). Urgency is a sudden and compelling desire to void urine which is difficult to defer (Abrams 2013). Overactive bladder (OAB) is the presence of urinary urgency, usually associated with frequency and nocturia, with (OAB‐wet) or without UUI (OAB‐dry), in the absence of urinary tract infection (UTI) or other pathology (Haylen 2010). Urinary frequency is defined as passing urine more than eight times in 24 hours (Fitzgerald 2003; Fitzgerald 2002), while nocturia is waking up from sleep more than once per night to urinate (van Kerrebroeck 2002). In patients with detrusor overactivity (DO), a spontaneous or induced detrusor contraction is observed during urodynamic testing (Abrams 2013). Urgency urinary incontinence is more prevalent in older women and accounts for a small proportion of women with urinary incontinence (Milsom 2009). In continent individuals, reflex (involuntary) contraction of the pelvic floor muscles and the striated muscle of the urethra occurs during the filling (storage) phase of the bladder (Morrison 1995). This in turn leads to increased intra‐urethral pressure and reflex inhibition of detrusor contraction, thereby preventing urine leakage and urgency. Thus, any abnormality of the pelvic floor muscles (structural or neural) which disrupts this reflex inhibition of detrusor during the filing phase may result in urgency urinary incontinence.
In some cases, the cause of urgency urinary incontinence is idiopathic (unknown cause). Other causes include neurogenic (multiple sclerosis, Alzheimer's or Parkinson's disease), stroke, tumour of the bladder and bladder pain syndrome (interstitial cystitis), defined by the ICS as "an unpleasant sensation (pain, pressure, discomfort) perceived to be related to the urinary bladder, associated with lower urinary tract symptom(s) of more than six weeks duration, in the absence of infection or other identifiable causes" (Abrams 2013). The aim of treatment is to reduce the symptoms of OAB or UUI.
Mixed urinary incontinence (MUI)
This is the complaint of involuntary leakage of urine associated with urgency, exertion, effort, sneezing and coughing (Abrams 2013). The prevalence of MUI increases with age. It has been suggested that mixed urinary incontinence should initially be managed conservatively, or with drugs, to reduce the need for surgical intervention (Karram 1989). However, if symptoms persist without significant evidence of detrusor overactivity on urodynamics, surgery may be performed.
Description of the intervention
Pelvic floor muscle training (PFMT)
Pelvic floor muscle training (PFMT) was popularised by Arnold Kegel for the management of urinary incontinence and has since remained a first‐line conservative measure (Kegel 1948). It is commonly recommended for the treatment of patients with stress or mixed urinary incontinence (Dumoulin 2014). Less commonly, it can be used for urgency urinary incontinence.
The main aim of PFMT is to improve the function of the pelvic floor muscles in terms of strength, endurance and co‐ordination, thereby providing maximum support to the pelvic organs (particularly, the bladder neck and the proximal urethra), before and during an increase in intra‐abdominal pressure, to prevent urine leakage. There are different ways through which PFMT appears to work (Bø 2004):
Patients can learn how to use conscious pelvic floor muscle pre‐contraction before and during exertion to prevent urine leakage (co‐ordination).
Pelvic floor muscle strength training increases long‐lasting muscle volume, thereby providing structural support to the pelvic organs (strengthening).
The reported cure rates of PFMT vary, depending on a number of factors (Bernstein 1997; Bø 1999; Kegel 1948). These factors include the type and severity of incontinence, type of instruction and follow‐up, patients' adherence and the outcome measures used. Structured, supervised and more intensive programmes have been associated with more success than simple verbal instructions (Bø 1990; Dumoulin 2014).
How the intervention might work
Strong, fast and well‐timed voluntary pelvic floor muscle contractions have the effect of pressing the urethra against the posterior aspect of the symphysis pubis, thereby producing a mechanical increase in intra‐urethral pressure (DeLancey 1988). Thus, a positive urethral closure pressure is maintained during an increase in intra‐abdominal pressure, resulting in correction of the negative closure pressure usually observed in patients with stress incontinence.
Pelvic floor muscle strength training also aims to provide more support to the bladder neck and proximal urethra, which are observed to be poorly supported in some patients with urinary incontinence, by raising the position of the levator ani muscle through increased muscle volume (hypertrophy) and muscle stiffness (Bø 2004). The overall effect of this is to raise urethral closure pressure at rest and during increased intra‐abdominal pressure.
In urgency urinary incontinence, there is an inability to inhibit detrusor contractions, leading to abnormally high detrusor pressures. Reflex inhibition of detrusor activity has been shown to follow electrical stimulation of pelvic floor muscles (Godec 1975), and may also accompany repeated and conscious pelvic floor muscle contraction, thereby controlling UUI (Polden 1990). However, the timing, number, intensity and duration of pelvic floor muscle contractions considered adequate to inhibit detrusor contraction are unknown (Dumoulin 2014).
It is possible that adding other active treatments to basic PFMT may enhance its effectiveness, particularly if those treatments are effective in their own right.
Why it is important to do this review
To date, there is no sufficient evidence‐based rationale indicating that PFMT, in combination with another active treatment, is a better treatment of choice than the active treatment alone for urinary incontinence in women. Adding a treatment such as PFMT might be time consuming, increase resource use and decrease adherence. Therefore, if adding PFMT does not improve outcome over and above the other treatment, then there is no point incurring extra cost (both direct and indirect) for no added benefit. Thus, a considerable doubt exists about the real and potential therapeutic effectiveness, cost‐effectiveness and risks of PFMT added to another active treatment, in comparison with the active treatment alone, for the treatment of women with urinary incontinence. Therefore, there is a compelling need for a systematic review of the existing trial‐based evidence. The outcome of this review will complement what is already known about the effectiveness of PFMT (Boyle 2012; Dumoulin 2014; Hay‐Smith 2011; Herderschee 2011).
Objectives
To compare the effects of pelvic floor muscle training combined with another active treatment versus the same active treatment alone, in the management of women with urinary incontinence.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials and quasi‐randomised trials (for example allocation by alternation) of pelvic floor muscle training added to an active treatment versus the active treatment alone, for urinary incontinence in women. We also included trials using more than two arms of interventions, providing one of the arms involved the use of PFMT plus an active treatment and another arm involved the same active treatment alone. We excluded other forms of clinical trials.
Types of participants
Adult women with stress urinary incontinence (SUI), urgency urinary incontinence (UUI) or mixed urinary incontinence (MUI).
Weincluded trials that used any mode of diagnosis of incontinence (symptoms, signs, urodynamic evaluation, or any combination). This is because many patients are referred for PFMT solely on the basis of symptoms, with or without clinical signs, as there is no consensus yet on the need for urodynamic testing before PFMT is performed (Glazener 2012; Thuroff 2011). Also, the outcome of a conservative management of urinary incontinence has been shown to be no different with respect to the mode of diagnosis (Elser 1999). We included trials that recruited men and women providing demographic and outcome data were reported separately for women.
We excluded studies of women with urinary incontinence whose symptoms were due to significant external factors, for example, cognitive impairment, neurological disorders or lack of independent mobility, which are considered to be outside the urinary tract. We also excluded studies that recruited women with nocturnal enuresis.
We excluded studies that specifically investigated antenatal or postnatal women (up to three months after delivery). The effect of PFMT might differ in this group of women, given the physiological changes that occur during pregnancy and the postpartum period. These women have been considered in another Cochrane review (Boyle 2012).
We also excluded studies that recruited women in long‐term care facilities. Urinary incontinence in this category of women is often associated with other co‐morbid conditions such as dementia, depression, lack of independent mobility, etc., which might influence the outcome of PFMT or their ability to comply with treatment (Milsom 2009).
Types of interventions
One arm of the trial used pelvic floor muscle training (PFMT) added to another active treatment. The comparison was the same active treatment alone.
In this review, we counted PFMT as a programme of repeated voluntary pelvic floor muscle contractions taught or supervised (or both) by healthcare professionals. All types of PFMT programmes were considered for inclusion, for example, variations in timing and purpose of PFMT (such as PFMT for strengthening or urge suppression), ways of teaching PFMT, and types and number of contractions. If biofeedback was used at least once in the teaching or delivery of PFMT, we called this a PFMT intervention, and clearly labelled any trial that used biofeedback as a 'PFMT plus biofeedback' trial to recognise the potential additional effect of biofeedback. We considered trials in which PFMT was combined with advice on frequency or urgency strategies, or both (but without a scheduled voiding regimen characteristic of bladder training), or other lifestyle advice (such as weight reduction), with leaflets or verbal instructions only to be 'pure PFMT'.
The comparisons were:
A Physical
1. PFMT added to vaginal cones versus vaginal cones alone
B Behavioural
2. PFMT added to lifestyle intervention (e.g. weight reduction) versus lifestyle intervention alone (lifestyle intervention must be structured or supervised)
3. PFMT added to bladder training versus bladder training alone (bladder training must include scheduled voiding regimen)
C Electrical or magnetic
4. PFMT added to electrical stimulation versus electrical stimulation alone (excluding implanted electrodes)
5. PFMT added to magnetic stimulation versus magnetic stimulation alone
D Mechanical
6. PFMT added to continence pessaries versus continence pessaries alone
E Drugs
7. PFMT added to drug therapy (e.g. tolterodine, duloxetine) versus drug therapy alone
F Surgery
8. PFMT prior to surgical intervention (e.g. tension‐free vaginal tape (TVT)) versus surgical intervention alone
G Other
9. PFMT added to any other stand‐alone active treatment versus the same stand‐alone active treatment.
Types of outcome measures
The Standardisation Committee of the International Continence Society recommended that research looking into the effects of therapeutic interventions for women with urinary incontinence should take into consideration the following five outcome domains: patient's observations with respect to the symptoms of urinary incontinence, quantification of patient's symptoms, clinician's observations (functional and anatomical), patient's quality of life and socioeconomic implication of treatment (Lose 1998). For this review, one or more outcomes of interest were considered from each domain.
Primary outcomes
Women's observations
Number of women cured of symptoms of urinary incontinence (within first year, as reported by the participants, not the clinicians)
Number of women cured or improved (as reported by the participants, not the clinicians)
Symptom‐ and condition‐specific quality of life assessed by various measures, such as the Urinary Incontinence Quality of Life (I‐QoL) scale, King's Health Questionnaire, the Incontinence Impact Questionnaire (IIQ), the Social Activity Index, the Leicester Impact Scale, etc.
Number of women improved on patient global impression of improvement in the first three months after the end of treatment
Secondary outcomes
1. Quantification of symptoms
Number of women reporting incontinence at one year or more after treatment (subjective)
Number of micturitions during the day
Number of micturitions during the night
Urine loss (measured on pad or paper towel weight tests)
Other quantification of symptoms reported by individual trials
2. Clinician's observations
Objective measurement of incontinence, such as observation of urine leakage during cough test
Measurement of pelvic floor muscle function, such as electromyography, vaginal squeeze pressure, pelvic floor muscle force and morphological measurements (dynamometry, ultrasound)
3. Generic quality of life
General health status evaluation e.g. Short Form (SF)‐36, Norwegian version of the Quality of Life Scale (QoLS‐N), etc.
Other quality of life measures as reported by individual trials
4. Economic analysis
Costs of intervention, resource implications of differences in outcomes and overall cost utility and cost‐effectiveness
5. Adverse effects
Number of women reporting adverse events
Pain or discomfort
Other adverse outcomes as reported by individual trials
6. Other outcomes
Sexual function
Pelvic organ prolapse
Number of women requiring further treatment, such as surgery, drugs, mechanical devices (relapse)
Treatment adherence evaluation using, for example, a self administered treatment adherence questionnaire
Patient satisfaction with treatment assessed using, for example, the validated Patient Satisfaction Questionnaire
Other outcomes not pre‐specified but considered to be important during the review, e.g. long‐term follow‐up
Quality of evidence
We assessed the quality of evidence by adopting the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach. The following factors were considered for assessing the quality of evidence:
Limitations in the study design
Inconsistency of results
Indirectness of evidence
Imprecision
Publication bias
The review authors classified primary and secondary outcomes, as defined above, as 'critical', 'important' or 'not important' for decision making from the woman's perspective. The GRADE working group strongly recommends including up to seven critical outcomes in a systematic review (Guyatt 2011a; Guyatt 2011b).
In this systematic review, the seven critical outcomes for assessing the quality of evidence were as follows:
Number of women cured or improved (subjective)
Condition‐specific quality of life assessed by patient questionnaire such as Incontinence Impact Questionnaire (IIQ), King's Health Questionnaire (KHQ)
Number of women reporting incontinence at one year or more after treatment (subjective)
Objective measure of urine leakage (e.g. pad test)
Number of women reporting adverse events
General health status evaluation e.g. Short Form (SF‐36)
Number of women requiring further treatment such as surgery, drugs, mechanical devices
Search methods for identification of studies
We did not impose any restrictions, for example language or publication status, on the searches described below.
Electronic searches
This review drew on the search strategy developed for the Cochrane Incontinence Group. We identified relevant trials from the Cochrane Incontinence Group Specialised Register of trials. For more details of the search methods used to build the Specialised Register please see the Group's module in the Cochrane Library. The register contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE in Process, ClinicalTrials.gov and WHO ICTRP and handsearching of journals and conference proceedings. Most of the trials in the Cochrane Incontinence Group Specialised Register are also contained in CENTRAL. The date of the last search of the Specialised Register was 5 May 2015.
The terms used to search the Incontinence Group Specialised Register are given in Appendix 1:
For this review, we also specifically searched CINAHL on EBSCO Host from January 1982 to May 2015. The last search was performed on 6 May 2015; the search strategy is given in Appendix 1:
For details of the specific searches performed for the first version of this review, please see Appendix 2 (Ayeleke 2013).
Searching other resources
We searched the reference lists of relevant articles, and the included and excluded studies in other relevant Cochrane reviews.
Data collection and analysis
Selection of studies
Only randomised and quasi‐randomised controlled trials were included. Two review authors independently screened the list of titles and abstracts generated by the search. We retrieved full‐text articles of potentially relevant studies. Two review authors independently assessed the full‐text articles for eligibility. Any differences of opinion were resolved through discussion or by involving a third party. We listed studies formally considered for the review but excluded, with reasons given for their exclusion.
Data extraction and management
Two review authors independently extracted the data from the included studies using a standardised form. Any disagreement was resolved by discussion or by consulting a third party. Where there was insufficient information regarding the outcomes or other relevant aspects of the published reports, we contacted study authors. For data entry, we used Review Manager software (RevMan 2012). We processed the data from the included trials according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of risk of bias in included studies
We assessed the risk of bias in the included studies using the Cochrane 'Risk of bias' assessment tool (Higgins 2011). This included:
sequence generation;
allocation concealment;
blinding of participants or therapists;
blinding of outcome assessors;
completeness of outcome data;
selective outcome reporting;
Other potential sources of bias we incorporated into the 'Risk of bias' tables were ethical approval, conflict of interest and funding source. Some of these additional domains are also used in another systematic review (Omar 2014). Two review authors independently assessed the above mentioned domains. Any differences of opinion were resolved through consensus or by consulting a third party.
Measures of treatment effect
Analyses were based on available data from all included trials relevant to the comparisons and outcomes of interest. For trials with multiple publications, only the most up‐to‐date of the trials or those with complete data for each outcome were included. We had planned to undertake a meta‐analysis, but this could not be done for most of the outcome measures because each of the pre‐specified comparisons (except bladder training and electrical stimulation) was addressed by single trials. For categorical outcomes, we related the numbers reporting an outcome to the numbers at risk in each group to calculate a risk ratio (RR) with 95% confidence intervals (CI). For continuous variables, we used means and standard deviations to calculate a mean difference (MD) with 95% CI. Where data we required to calculate RRs or MDs were not given, we utilised the most detailed numerical data available (e.g. test statistics, P values) to calculate the actual numbers or means and standard deviations.
Unit of analysis issues
The primary analysis was per woman randomised. Initially, we had planned to analyse two‐period, two‐intervention cross‐over trials with continuous outcomes by determining the mean person difference between the two treatment periods and the standard error of this mean to obtain the effect estimates for inclusion in a meta‐analysis, by using the generic inverse variance method (Higgins 2011). However, cross‐over trials were not identified for inclusion in this review. Similarly, we had intended to analyse cluster‐randomised trials by reducing them to their effective sample size (that is, original sample size divided by design effect; design effect = 1 + (M ‐ 1) x ICC, where M is the average cluster size and ICC is the intra‐cluster correlation coefficient), and then combine the data obtained (dichotomous or continuous) in a meta‐analysis (Higgins 2011). In the end, no cluster‐randomised trial was included in this review.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis as far as possible. By intention‐to‐treat analysis, we mean that: 1. outcome data must be measured on all participants; 2. all randomised participants must be included in the analysis; and 3. participants must be retained in the intervention groups to which they were assigned (Higgins 2011). However, for this review, the criterion set for intention‐to‐treat analyses was that participants be retained and analysed in the intervention groups to which they were assigned. Where this was not the case, we considered whether the trial should be excluded. We made attempts to obtain missing data from the original trialists. However, where this was not possible, data were reported as given in the trial reports, except where there was evidence of differential loss to follow‐up between the intervention groups. In that case, the use of imputation of missing data was considered.
Assessment of heterogeneity
We assessed heterogeneity between studies by visual inspection of plots of the data, the Chi² test for heterogeneity and the I² statistic (Higgins 2003). We also used the thresholds for interpretation of the I² statistic as defined by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). An I² measurement greater than 50% was taken to indicate substantial heterogeneity..
Assessment of reporting biases
In view of the difficulty in detecting and correcting for publication bias and other reporting biases, we minimised their potential impact by ensuring a comprehensive search for eligible studies, and by watching out for duplication of data.
Data synthesis
We combined trials with similar interventions in a meta‐analysis, using a fixed‐effect model approach, as there was no evidence of significant heterogeneity across studies.
Subgroup analysis and investigation of heterogeneity
We had intended to do subgroup data analyses by the type of underlying urinary incontinence or lower urinary tract symptoms:
stress urinary incontinence;
urgency urinary incontinence;
mixed urinary incontinence (both stress and urgency urinary incontinence);
'unclear' if there was no clear cut diagnosis with respect to the type of urinary incontinence.
Ultimately, we could not perform subgroup analysis because there were few trials, with most addressing different interventions.
Where heterogeneity between trials was found to be substantive, we had planned to conduct an investigation to identify its cause(s). The investigation of heterogeneity was meant to address populations and interventions in the individual trials. The investigation could also include subgroup analyses, meta‐regression and sensitivity analyses. Where heterogeneity persisted after appropriate investigation and possible removal of outlying trials, a random‐effects model could have be used in the meta‐analysis. In the end, there was no need to investigate heterogeneity as most of the included trials tested different comparisons.
Sensitivity analysis
We had planned to perform sensitivity analyses by including or excluding trials at high risk of bias. However, this was not applicable as meta‐analyses could not be performed for most of the comparisons.
Results
Description of studies
Results of the search
In the first version of this review, the search produced a total of 641 titles and abstracts, out of which we considered 132 full‐text articles for further assessment. Eleven trials in 29 reports met the eligibility criteria for inclusion in the review, while 84 studies in 103 reports were excluded (reasons for exclusion are stated in the Characteristics of excluded studies table). In the current updated version of this review, the updated searches produced 173 records to assess, from which an additional two new studies in four reports were identified (Bezerra 2009; Kaya 2015). Thus, we included a total of 13 trials in 33 reports in the current updated version. The PRISMA flow chart in Figure 1 illustrates the flow of literature through the search and assessment process.
1.
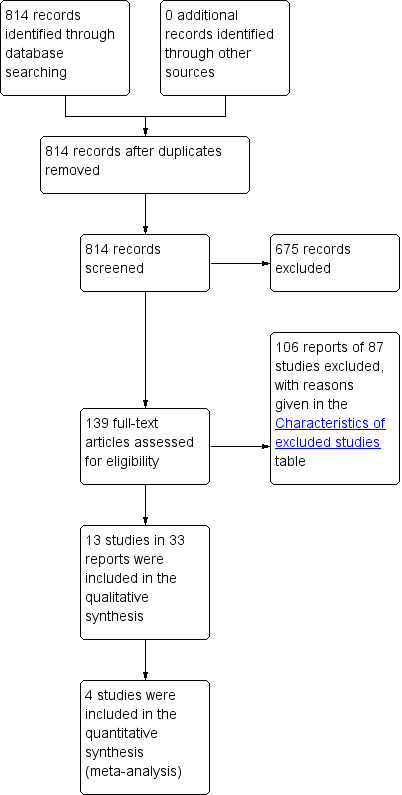
PRISMA study flow diagram.
Included studies
Nine of the included trials (Ghoniem 2005; Hofbauer 1990; Ishiko 2000; Jeyaseelan 2002; Jin 2012; Kim 2011; Richter 2010; Wise 1993; Wyman 1998) contained more than two intervention arms; descriptions and data were provided for all the arms in this review. The trials included a total of 1164 women, 585 of whom received some form of PFMT added to another active treatment, while 579 received comparator treatments, which were the other active treatment alone.
In terms of number of participants per comparison group:
the largest trial had more than 100 per comparison group (Richter 2010);
three trials had more than 50 but fewer than 100 per comparison group (Jin 2012; Kaya 2015; Wyman 1998);
one trial had between 47 and 52 per comparison group (Ghoniem 2005);
three trials had more than 20 but fewer than 50 per comparison group (Bezerra 2009; Burgio 2010a; Kim 2011);
one trial had between 20 and 21 per comparison group (Wise 1993);
one trial had between 18 and 20 per comparison group (Ishiko 2000); and
three trials had fewer than 20 per comparison group (Chen 2008; Hofbauer 1990; Jeyaseelan 2002).
Three trials reported an a priori power calculation (Kaya 2015; Richter 2010; Wyman 1998); another one used an a priori power calculation at an early stage of the trial, but later decided to use a conditional power calculation (based on available participants) due to slow accrual of participants (Burgio 2010a).
Sample characteristics
Mode of diagnosis of urinary incontinence
The trials based the diagnosis of urinary incontinence on:
symptoms, signs, or both: five trials (Chen 2008; Ishiko 2000; Kaya 2015; Kim 2011; Richter 2010);
urodynamics: three trials (Hofbauer 1990; Jin 2012; Wise 1993);
symptoms and urodynamics: four trials (Bezerra 2009; Burgio 2010a; Ghoniem 2005; Wyman 1998);
unspecified mode: one trial (Jeyaseelan 2002).
Types of urinary incontinence
The trials recruited women with:
SUI only: five trials (Bezerra 2009; Hofbauer 1990; Ishiko 2000; Jeyaseelan 2002; Wise 1993);
SUI or SUI predominant MUI: two trials (Ghoniem 2005; Richter 2010);
UUI or UUI predominant MUI: one trial (Burgio 2010a);
SUI, UUI or MUI: three trials (Kaya 2015; Kim 2011; Wyman 1998).
Age
The included trials recruited women aged:
18 to 75 years (Ghoniem 2005);
18 years or older (Kaya 2015; Richter 2010);
30 to 75 years or older (Ishiko 2000);
45 years or older Bezerra 2009; (Wyman 1998);
70 years or older (Kim 2011).
Four trials did not set age limits (either a lower or an upper limit; Burgio 2010a; Chen 2008; Hofbauer 1990; Jin 2012), while two trials did not present any data on the age of the included women (Jeyaseelan 2002; Wise 1993).
Frequency of urinary incontinence episodes
Five trials used frequency of incontinence episodes as one of the inclusion criteria:
more than once a month (Kim 2011);
at least once per week (Wyman 1998);
at least twice per week (Burgio 2010a);
twice or more per day (Ghoniem 2005); or
at least two episodes on seven‐day bladder diary (Richter 2010).
Duration of urinary incontinence symptoms
In six trials, duration of UI was reported as one of the baseline characteristics, with none using this as an inclusion criterion (Burgio 2010a; Ishiko 2000; Jin 2012; Kaya 2015; Kim 2011; Wyman 1998). The reported mean or median duration of symptoms varied between 2.1 and 8.6 years.
Other characteristics
Exclusion criteria were reported by eight of the included trials (Ghoniem 2005; Hofbauer 1990; Ishiko 2000; Jin 2012; Kaya 2015; Kim 2011; Richter 2010; Wyman 1998). Common reasons for excluding participants across trials were: presence of uncontrolled diabetes mellitus, persistent urinary tract infection, disease of the nervous system, impaired mental state, advanced pelvic organ prolapse, antenatal or postnatal women (up to three months after delivery), and post‐void residual volume more than a specified amount.
Interventions
Pelvic floor muscle training (PFMT)
Detailed descriptions of the PFMT programmes of the included trials are given in the Characteristics of included studies table. The purpose of this review was to examine the additional effects of adding PFMT to another active treatment. Therefore, the review authors were particularly interested in the effectiveness of PFMT with respect to the confirmation of a correct voluntary pelvic floor muscle contraction, duration of PFMT, and PFMT 'dose'. Additionally, we were interested in whether the 'experimental group' received any additional intervention to enhance the effectiveness of PFMT.
Confirmation of a correct pelvic floor muscle contraction
Only two trials reported that the correct type of voluntary pelvic floor muscle contraction was confirmed, but full details about the mode of confirmation were not reported (Ghoniem 2005; Wise 1993).
Duration of PFMT
One trial did not specify the duration of PFMT in weeks; it stated that participants underwent 24 sessions of PFMT training (Bezerra 2009). The duration of PFMT in the remaining trials varied between four and 12 weeks among the trials:
four weeks (Jin 2012);
six weeks (Hofbauer 1990; Kaya 2015);
eight weeks (Burgio 2010a; Chen 2008; Jeyaseelan 2002; Richter 2010); and
12 weeks (Ghoniem 2005; Ishiko 2000; Kim 2011; Wise 1993; Wyman 1998).
'Dose' of PFMT
A PFMT programme may be prescribed to:
increase strength (i.e. the maximum force generated in a single contraction by a muscle), characterised by low numbers of repetitions with high 'loads'('loads' can be increased by increasing the amount of voluntary efforts with each contraction);
increase endurance (i.e. the ability to contract repetitively or sustain a single contraction over time), characterised by high numbers of repetitions or prolonged contractions with low to moderate 'loads';
co‐ordinate muscle activity by using voluntary pelvic floor muscle contraction to either minimise urine leakage (with increased intra‐abdominal pressure) or suppress urge (suppression of detrusor contraction; i.e. behavioural training); or
-
a combination of these. In this review, the trials included the following programmes:
one trial targeted endurance training (Chen 2008), two trials targeted a combination of strength and endurance training (Kaya 2015; Kim 2011), while one trial used a combination of endurance and co‐ordination training (Burgio 2010a);
two trials used a combination of strength, endurance and co‐ordination training programmes (Ghoniem 2005; Wyman 1998).
In seven trials, it was difficult to characterise the PFMT programme (contraction effort, frequency, number and duration), because full details were not provided about the key training parameters, such as amount and duration of voluntary contractions (Bezerra 2009; Hofbauer 1990; Ishiko 2000; Jeyaseelan 2002; Jin 2012; Richter 2010; Wise 1993).
Additional intervention to enhance PFMT effectiveness
Some trials added extra interventions to the PFMT regimen in order to increase its effects:
biofeedback in the form of perineal surface electromyography (Chen 2008; Jeyaseelan 2002), or a strip‐chart recorder from vaginal balloon (Wyman 1998);
feedback by means of manual palpation (Ghoniem 2005); and
abdominal muscle exercises (Hofbauer 1990; Kim 2011).
Comparators
The active and concomitant comparators were:
vaginal cones (Wise 1993);
bladder training (Kaya 2015; Wyman 1998);
electrical stimulation (Bezerra 2009; Hofbauer 1990; Jeyaseelan 2002);
continence pessary (Richter 2010);
drug therapy: duloxetine (Ghoniem 2005); oxybutynin (Burgio 2010a); solifenacin (Jin 2012); clenbuterol (Ishiko 2000), and unspecified drugs (but assumed to be an anticholinergic as it was for participants with overactive bladder, (Chen 2008);
other active treatment: heat and steam generating sheet (HSGS, Kim 2011).
Further details about the participants, interventions and comparators are provided in the Characteristics of included studies table.
Outcome measures
The choice of outcome measures varied considerably among trials and this made it impossible to combine results from the majority of individual trials. Only the outcomes reported at endpoints (at the end of, or shortly after the end of the interventions) were used in the analysis based on the assumption that the maximum benefits could be expected to have been gained at that time. One trial reported all its outcomes in medians and ranges and was therefore not included in the analysis of data (Jeyaseelan 2002).
Excluded studies
We excluded 87 trials (106 reports); reasons for their exclusion are given in the Characteristics of excluded studies table. Most trials were excluded because either the interventions or the comparators were not relevant. For example, Millard and colleagues administered PFMT via a two‐page written instruction sheet (Millard 2004); another trial added bladder training, another active treatment, to PFMT as a component of lower urinary tract exercise (i.e. the exercise did not contain 'pure' PFMT and this combination was added to another active treatment, (Berghmans 2000); while Fitzgerald and colleagues used behavioural therapy, which included PFMT and timed voiding (BE‐DRI 2008); the latter is an active treatment on its own (Ostaszkiewicz 2004).
Risk of bias in included studies
Figure 2 and Figure 3 summarise the risk of bias of the included trials. Five trials were published as conference abstracts and it was therefore difficult to assess the risk of bias, with most domains being assessed as 'unclear' risk (Bezerra 2009; Chen 2008; Jeyaseelan 2002; Jin 2012; Wise 1993).
2.
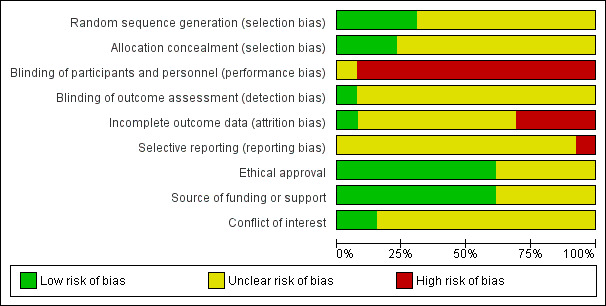
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
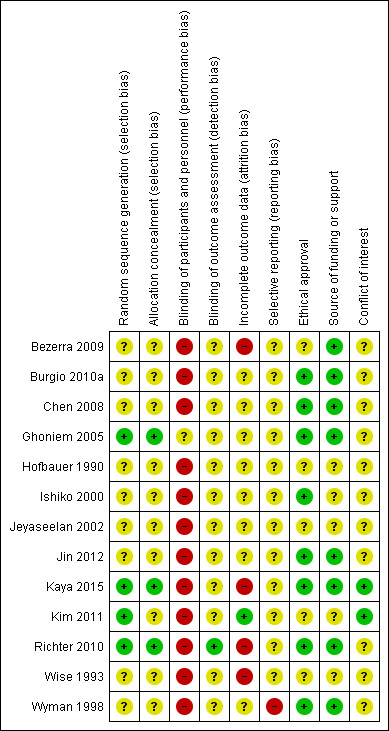
'Risk of bias' summary: review authors' judgements about each risk of bias domain for each included study.
Allocation
Sequence generation
Four trials provided sufficient details about the methods used in random sequence generation to be sure this was genuine and adequate (Ghoniem 2005; Kaya 2015; Kim 2011; Richter 2010). Therefore, we considered these trials to be at low risk. For the remaining trials, the risk of bias was unclear because they did not provide enough details about the methods used in sequence generation.
Allocation concealment
Three trials gave enough details to be sure there was adequate allocation concealment and we thus considered them to be at low risk (Ghoniem 2005; Kaya 2015; Richter 2010). Other trials did not give clear and sufficient information about allocation concealment and thus the risk of bias was unclear.
Blinding
It was decided that, given the nature of an intervention such as PFMT, blinding of women as well as therapists was not practical. Though one trial attempted this by blinding the participants (gave 'sham' PFMT to one of the treatment groups), the adequacy and genuineness of such a blinding process was unclear, thus, we categorised this trial as unclear with regard to performance bias (Ghoniem 2005). We categorised the remaining trials as being at high risk with respect to performance bias.
In the domain of detection bias, only one trial clearly stated that outcome assessors were blinded, therefore, we categorised it as being at low risk (Richter 2010). The remaining trials did not provide sufficient, or any, information about outcome assessment and we thus categorised them as unclear.
Incomplete outcome data
Description of dropout and withdrawal
Four trials did not clearly state whether or not there was loss to follow‐up (Chen 2008; Hofbauer 1990; Jeyaseelan 2002; Jin 2012), though in three of these trials, it appeared there were no dropouts (Hofbauer 1990; Jeyaseelan 2002; Jin 2012). In the remaining trials, the proportion of losses to follow‐up for all the treatment groups was as follows:
less than 10% (Burgio 2010a; Kim 2011; Wyman 1998);
between 10% and 20% (Ishiko 2000; Kaya 2015; Richter 2010; Wise 1993);
more than 20% (Bezerra 2009; Ghoniem 2005).
One trial did not report the number of withdrawals by treatment group (Wyman 1998). In another trial, there were no dropouts in either the experimental or the control groups (Kim 2011). In three trials, the proportion of losses to follow‐up was higher in the PFMT plus active treatment group than the active treatment group (Bezerra 2009; Burgio 2010a; Wise 1993), while in another three trials, more women dropped out of the active treatment group than the PFMT plus the active treatment group (Ishiko 2000; Kaya 2015; Richter 2010). In the remaining trial, the proportion of dropouts did not differ significantly between the experimental and the control groups (Ghoniem 2005).
Analysis by full intention‐to‐treat (ITT) principle
Trials were required to retain and analyse participants in the group to which they were randomly assigned. Only three trials clearly reported that the primary analysis was by intention‐to‐treat (Burgio 2010a; Ghoniem 2005; Richter 2010). However, it was difficult to ascertain if any of these trials actually met the above criterion for intention‐to‐treat analysis.
Therefore, we categorised trials as being at low risk of bias if the proportion of loss to follow‐up was 10% or less, and there was no evidence of differential loss to follow‐up between the comparison groups of interest. In this regard, we rated one trial as being at low risk (Kim 2011); we categorised four trials as being at high risk (Bezerra 2009; Kaya 2015; Richter 2010; Wise 1993), while the remaining trials were unclear.
Selective reporting
It was difficult to assess whether the included trials selectively reported their outcomes or not, as the protocols for these trials were not available for review. In some of the trials, there was incomplete data reporting with data not made available for one or more of the outcomes specified in the methods section. Therefore, we rated all the trials as unclear for this domain of risk of bias.
Other potential sources of bias
Ethical approval
In five trials, it was neither stated that ethical approval was obtained, nor that informed consent was sought from participants (Bezerra 2009; Hofbauer 1990; Jeyaseelan 2002; Kim 2011; Wise 1993).
Source of funding or financial assistance
Three trials received funding or support from public sources (Burgio 2010a; Kaya 2015; Wyman 1998); another two were funded either by pharmaceutical companies (Ghoniem 2005), or a private organisation (Richter 2010). Three trials stated that no funding or financial assistance was received (Bezerra 2009; Chen 2008; Jin 2012). The remaining trials did not give any report on their source of funding or financial support (Hofbauer 1990; Ishiko 2000; Jeyaseelan 2002; Kim 2011; Wise 1993).
Conflict of interest
Three trials clearly made conflict of interest statements in which some of the authors had financial or other relationships with some pharmaceutical companies (Burgio 2010a; Ghoniem 2005; Richter 2010); in Ghoniem 2005, some of the authors had financial interests or other relationships with one of the organisations that supported the trial. Two trials stated that the authors had no conflict of interest (Kaya 2015; Kim 2011). The remaining trials did not make any statement with respect to their conflict of interest (Bezerra 2009; Chen 2008; Hofbauer 1990; Ishiko 2000; Jeyaseelan 2002; Jin 2012; Wise 1993; Wyman 1998).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9
Summary of findings for the main comparison. PFMT added to vaginal cones versus vaginal cones alone for urinary incontinence in women.
| PFMT added to vaginal cones versus vaginal cones alone for urinary incontinence in women | ||||||
| Patient or population: women with urinary incontinence Settings: Secondary care Intervention: PFMT added to vaginal cones versus vaginal cones alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | PFMT added to vaginal cones versus vaginal cones alone | |||||
| Number of women cured or improved (subjective) ‐ not reported | Not estimable | Not reported | ||||
| Number of women reporting incontinence at 1 year or more after treatment (subjective) ‐ not reported | Not estimable | Not reported | ||||
| Objective measure of urine leakage (pad test) | Study population | RR 1.27 (0.94 to 1.71) | 34 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 737 per 1000 | 936 per 1000 (693 to 1000) | |||||
| Number of women experiencing pain ‐ not reported | Not estimable | Not reported | ||||
| Condition‐specific quality of life assessed by patient questionnaire such as Incontinence Impact Questionnaire (IIQ), King's Health Questionnaire (KHQ) ‐ not reported | Not estimable | Not reported | ||||
| General health status evaluation e.g. Short Form (SF)‐36 ‐ not reported | Not estimable | Not reported | ||||
| Number of women requiring further treatment such as surgery, drugs, mechanical devices (relapse) ‐ not reported | Not estimable | Not reported | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PFMT: pelvic floor muscle training; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Random sequence generation and allocation concealment unclear. 2Confidence interval is very wide (0.94 to 1.71).
Summary of findings 2. PFMT added to lifestyle intervention versus lifestyle intervention alone for urinary incontinence in women.
| PFMT added to lifestyle intervention versus lifestyle intervention alone for urinary incontinence in women | ||||||
| Patient or population: women with urinary incontinence Settings: Secondary care Intervention: PFMT added to lifestyle intervention versus lifestyle intervention alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | PFMT added to lifestyle intervention versus lifestyle intervention alone | |||||
| Number of women cured or improved (subjective) ‐ not reported | Not estimable | Not reported | ||||
| Number of women reporting incontinence at 1 year or more after treatment (subjective) ‐ not reported | Not estimable | Not reported | ||||
| Objective measure of urine leakage (e.g. pad test) ‐ not reported | Not estimable | Not reported | ||||
| Number of women reporting adverse events ‐ not reported | Not estimable | Not reported | ||||
| Condition‐specific quality of life ‐ not reported | Not estimable | Not reported | ||||
| General health status evaluation e.g. Short Form (SF)‐36 ‐ not reported | Not estimable | Not reported | ||||
| Number of women requiring further treatment such as surgery, drugs, mechanical devices (relapse) ‐ not reported | Not estimable | Not reported | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PFMT: pelvic floor muscle training | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 3. PFMT added to bladder training versus bladder training alone for urinary incontinence in women.
| PFMT added to bladder training versus bladder training alone for urinary incontinence in women | ||||||
| Patient or population: women with urinary incontinence Settings: Secondary care Intervention: PFMT added to bladder training versus bladder training alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | PFMT added to bladder training versus bladder training alone | |||||
| Number of women cured ‐ 3 months after treatment | Study population | RR 1.71 (0.84 to 3.46) | 122 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 159 per 1000 | 271 per 1000 (133 to 549) | |||||
| Number of women reporting incontinence at 1 year or more after treatment (subjective) ‐ not reported | Not estimable | Not reported | ||||
| Objective measure of urine leakage (e.g. pad test) ‐ not reported | Not estimable | Not reported | ||||
| Number of women experiencing pain ‐ not reported | Not estimable | Not reported | ||||
| Condition‐specific quality of life ‐ 3 months after treatment Incontinence Impact Questionnaire‐ Revised (IIQ‐R) | The mean condition‐specific quality of life ‐ 3 months after treatment in the intervention groups was 5.9 lower (35.53 lower to 23.73 higher) | 118 (1 study) | ⊕⊝⊝⊝ very low1,3 | lower scores imply lower impact of incontinence on quality of life | ||
| General health status evaluation e.g. Short Form (SF)‐36 ‐ not reported | Not estimable | Not reported | ||||
| Number of women requiring further treatment such as surgery, drugs, mechanical devices (relapse) | 396 per 1000 | 376 per 1000 (226 to 621) | RR 0.95 (0.57 to 1.57) | 96 (1 study) | ⊕⊝⊝⊝ very low1,4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PFMT: pelvic floor muscle training; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Random sequence generation and allocation concealment is unclear. 2Confidence interval is very wide (0.84 to 3.46). 3Confidence interval is very wide (‐35.53 to 23.73). 4Confidence interval is very wide (0.57 to 1.57).
Summary of findings 4. PFMT added to electrical stimulation versus electrical stimulation alone (excluding implanted electrodes) for urinary incontinence in women.
| PFMT added to electrical stimulation versus electrical stimulation alone (excluding implanted electrodes) for urinary incontinence in women | ||||||
| Patient or population: women with urinary incontinence Settings: Secondary care Intervention: PFMT added to electrical stimulation versus electrical stimulation alone (excluding implanted electrodes) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | PFMT added to electrical stimulation versus electrical stimulation alone (excluding implanted electrodes) | |||||
| Number of women cured | Study population | RR 2.06 (0.79 to 5.38) | 56 (2 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 167 per 1000 | 343 per 1000 (132 to 897) | |||||
| Number of women reporting incontinence at 1 year or more after treatment (subjective) ‐ not reported | Not estimable | Not reported | ||||
| Objective measure of urine leakage (e.g. pad test) ‐ not reported | Not estimable | Not reported | ||||
| Number of women experiencing pain ‐ not reported | Not estimable | Not reported | ||||
| Condition‐specific quality of life assessed by patient questionnaire such as Incontinence Impact Questionnaire (IIQ), King's Health Questionnaire (KHQ) ‐ not reported | Not estimable | Not reported | ||||
| General health status evaluation e.g. Short Form (SF)‐36 ‐ not reported | Not estimable | Not reported | ||||
| Number of women requiring further treatment such as surgery, drugs, mechanical devices (relapse) ‐ not reported | Not estimable | Not reported | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PFMT: pelvic floor muscle training; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Random sequence generation and allocation concealment unclear. 2Confidence interval very wide (0.79 to 5.38).
Summary of findings 5. PFMT added to magnetic stimulation versus magnetic stimulation alone for urinary incontinence in women.
| PFMT added to magnetic stimulation versus magnetic stimulation alone for urinary incontinence in women | ||||||
| Patient or population: women with urinary incontinence Settings: Secondary care Intervention: PFMT added to magnetic stimulation versus magnetic stimulation alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | PFMT added to magnetic stimulation versus magnetic stimulation alone | |||||
| Number of women cured or improved (subjective) ‐ not reported | Not estimable | Not reported | ||||
| Number of women reporting incontinence at 1 year or more after treatment (subjective) ‐ not reported | Not estimable | Not reported | ||||
| Objective measure of urine leakage (e.g. pad test) ‐ not reported | Not estimable | Not reported | ||||
| Number of women reporting adverse events ‐ not reported | Not estimable | Not reported | ||||
| Condition‐specific quality of life assessed by patient questionnaire such as Incontinence Impact Questionnaire (IIQ), King's Health Questionnaire (KHQ) ‐ not reported | Not estimable | Not reported | ||||
| General health status evaluation e.g. Short Form (SF)‐36 ‐ not reported | Not estimable | Not reported | ||||
| Number of women requiring further treatment such as surgery, drugs, mechanical devices (relapse) ‐ not reported | Not estimable | Not reported | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PFMT: pelvic floor muscle training | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 6. PFMT added to continence pessary versus continence pessary alone for urinary incontinence in women.
| PFMT added to continence pessary versus continence pessary alone for urinary incontinence in women | ||||||
| Patient or population: women with urinary incontinence Settings: Secondary care Intervention: PFMT added to continence pessary versus continence pessary alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | PFMT added to continence pessary versus continence pessary alone | |||||
| Number of women cured or improved (subjective) at 12 months | Study population | RR 0.88 (0.67 to 1.16) | 207 (1 study) | ⊕⊕⊕⊝ moderate1 | ||
| 531 per 1000 | 468 per 1000 (356 to 616) | |||||
| Number of women reporting incontinence at 1 year or more after treatment (subjective) ‐ not reported | Not estimable | Not reported | ||||
| Objective measure of urine leakage (e.g. pad test) ‐ not reported | Not estimable | Not reported | ||||
| Number of women reporting adverse events ‐ not reported | Not estimable | Not reported | ||||
| Condition‐specific quality of life at 12 months Urogenital Distress Inventory (UDI) | Study population | RR 0.81 (0.62 to 1.08) | 207 (1 study) | ⊕⊕⊕⊝ moderate2 | ||
| 542 per 1000 | 439 per 1000 (336 to 585) | |||||
| General health status evaluation e.g. Short Form (SF)‐36 ‐ not reported | Not estimable | Not reported | ||||
| Number of women requiring further treatment such as surgery, drugs, mechanical devices (relapse) ‐ not reported | Not estimable | Not reported | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PFMT: pelvic floor muscle training; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Wide confidence interval (0.67 to 1.16). 2Confidence interval is wide (0.62 to 1.08).
Summary of findings 7. PFMT added to drug therapy versus drug therapy alone for urinary incontinence in women.
| PFMT added to drug therapy versus drug therapy alone for urinary incontinence in women | ||||||
| Patient or population: women with urinary incontinence Settings: Secondary care Intervention: PFMT added to drug therapy versus drug therapy alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | PFMT added to drug therapy versus drug therapy alone | |||||
| Number of women cured ‐ PFMT + clenbuterol versus clenbuterol | Study population | RR 1.16 (0.83 to 1.63) | 32 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 769 per 1000 | 892 per 1000 (638 to 1000) | |||||
| Number of women reporting incontinence at 1 year or more after treatment (subjective) ‐ not reported | Not estimable | Not reported | ||||
| Objective measure of urine leakage (e.g. pad test) ‐ not reported | Not estimable | Not reported | ||||
| Number of women reporting adverse events | 207 per 1000 | 174 per 1000 (1000 to 332) | RR 0.84 (45 to 1.60) | 162 (1 study) | ⊕⊝⊝⊝ very low1,3 | |
| Condition‐specific quality of life on I‐QoL Questionnaire ‐ PFMT + duloxetine versus duloxetine Incontinence Quality of Life questionnaire | The mean condition‐specific quality of life on I‐QoL questionnaire ‐ PFMT + duloxetine versus duloxetine in the intervention groups was 5.84 higher (2.08 lower to 13.76 higher) | 101 (1 study) | ⊕⊕⊝⊝ low4 | Higher scores mean less symptom impact on the quality of life (better) | ||
| General health status evaluation e.g. Short Form (SF)‐36 ‐ not reported | Not estimable | Not reported | ||||
| Number of women requiring further treatment such as surgery, drugs, mechanical devices (relapse) ‐ not reported | Not estimable | Not reported | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PFMT: pelvic floor muscle training; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Random sequence generation and allocation concealment is unclear. 2Confidence interval is very wide (0.83 to 1.63). 3Confidence interval is very wide (0.45 to 1.50). 4Confidence interval is very wide (‐2.08 to 13.76).
Summary of findings 8. PFMT prior to surgical intervention versus surgical intervention alone for urinary incontinence in women.
| PFMT prior to surgical intervention versus surgical intervention alone for urinary incontinence in women | ||||||
| Patient or population: women with urinary incontinence Settings: Secondary care Intervention: PFMT prior to surgical intervention versus surgical intervention alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | PFMT prior to surgical intervention versus surgical intervention alone | |||||
| Number of women cured or improved (subjective) ‐ not reported | Not estimable | Not reported | ||||
| Number of women reporting incontinence at 1 year or more after treatment (subjective) ‐ not reported | Not estimable | Not reported | ||||
| Objective measure of urine leakage (e.g. pad test) ‐ not reported | Not estimable | Not reported | ||||
| Number of women reporting adverse events ‐ not reported | Not estimable | Not reported | ||||
| Condition‐specific quality of life assessed by patient questionnaire such as Incontinence Impact Questionnaire (IIQ), King's Health Questionnaire (KHQ) ‐ not reported | Not estimable | Not reported | ||||
| General health status evaluation e.g. Short Form (SF)‐36 ‐ not reported | Not estimable | Not reported | ||||
| Number of women requiring further treatment such as surgery, drugs, mechanical devices (relapse) ‐ not reported | Not estimable | Not reported | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PFMT: pelvic floor muscle training | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 9. PFMT added to HSGS versus HSGS alone for urinary incontinence in women.
| PFMT added to HSGS versus HSGS alone for urinary incontinence in women | ||||||
| Patient or population: women with urinary incontinence Settings: Secondary care Intervention: PFMT added to other versus other treatment alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | PFMT added to other versus other treatment alone | |||||
| Number of women cured | Study population | RR 2.38 (1.19 to 4.73) | 74 (1 study) | ⊕⊕⊕⊝ moderate1 | ||
| 216 per 1000 | 515 per 1000 (257 to 1000) | |||||
| Number of women reporting incontinence at 1 year or more after treatment (subjective) ‐ not reported | Not estimable | Not reported | ||||
| Objective measure of urine leakage (e.g. pad test) ‐ not reported | Not estimable | Not reported | ||||
| Number of women experiencing pain ‐ not reported | Not estimable | Not reported | ||||
| Condition‐specific quality of life assessed by patient questionnaire such as Incontinence Impact Questionnaire (IIQ), King's Health Questionnaire (KHQ) ‐ not reported | Not estimable | Not reported | ||||
| General health status evaluation e.g. Short Form (SF)‐36 ‐ not reported | Not estimable | Not reported | ||||
| Number of women requiring further treatment such as surgery, drugs, mechanical devices (relapse) ‐ not reported | Not estimable | Not reported | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HSGS: heat and steam generating sheet; PFMT: pelvic floor muscle training; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Allocation concealment unclear.
The 13 included trials compared PFMT added to another active treatment (585 women) with the same active treatment alone (579 women). Nine trials reported data on at least one or more of the pre‐specified primary outcomes, while nine trials contained data on at least one or more of the pre‐specified secondary outcomes. None of the trials reported any data on socioeconomic outcomes.
The following comparisons were addressed:
A Physical interventions
1. PFMT added to vaginal cones versus vaginal cones alone
One small trial (Wise 1993) compared the effects of a combined PFMT and vaginal cones treatment with vaginal cones treatment alone for women with SUI.
Secondary outcome measures
Number of women cured or improved (objective assessment)
A number of outcomes were reported, but only one contained usable data; the number of women cured or improved on pad testing (objective assessment of cure or improvement). There were no statistically significant differences in the estimated size of treatment effect between the two intervention groups at endpoint (RR 1.27, 95% CI 0.94 to1.71, Analysis 1.1).
1.1. Analysis.
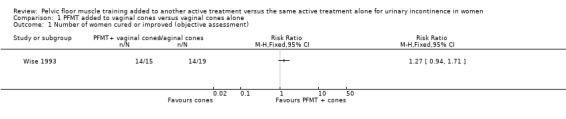
Comparison 1 PFMT added to vaginal cones versus vaginal cones alone, Outcome 1 Number of women cured or improved (objective assessment).
B Behavioural interventions
2. PFMT added to lifestyle intervention (e.g. weight reduction) versus lifestyle intervention alone (lifestyle intervention must be structured or supervised)
None of the trials addressed this comparison. We have added Table 2 to highlight lack of evidence.
3. PFMT added to bladder training versus bladder training alone (bladder training must include scheduled voiding regimen)
For this comparison, two trials with 336 participants contributed data (Kaya 2015; Wyman 1998). However, Kaya 2015 contributed data to only one outcome (patient global impression of improvement). The trials compared the effects of interventions in women with SUI, UUI or MUI.
Primary outcome measures
Number of women 'cured' or 'improved' (as reported by the women)
Cure rate was assessed immediately after and at three months after treatment, using a standardised diary; cure was defined as complete cessation (100% reduction) of incontinence. Immediately after treatment, women who received combined PFMT and bladder training were more likely to be cured than those who received bladder training alone, but this difference was not statistically significant (19/61 versus 12/67; RR 1.74, 95% CI 0.92 to 3.28; Analysis 3.1). At three months after treatment, there was also no statistically significant difference in the estimated size of treatment effect between the two intervention groups (16/59 versus 10/63; RR 1.71, 95% CI 0.84 to 3.46; Analysis 3.1).
3.1. Analysis.
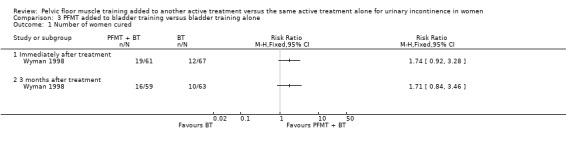
Comparison 3 PFMT added to bladder training versus bladder training alone, Outcome 1 Number of women cured.
Improvement was defined as the proportion of women who had 50% or greater reduction in incontinence episodes in a standardised diary. More women who received a combination of PFMT and bladder training reported cure or improvement immediately after treatment compared to those who were treated with bladder training alone (43/61 versus 35/67; RR 1.35, 95% CI 1.02 to 1.79; Analysis 3.2.1), but there was no statistically significant difference between the two intervention groups at three months after intervention (35/59 versus 28/61; RR 1.29, 95% CI 0.92 to 1.82; Analysis 3.2.2).
3.2. Analysis.
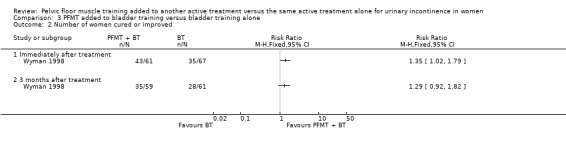
Comparison 3 PFMT added to bladder training versus bladder training alone, Outcome 2 Number of women cured or improved.
Symptom‐ and condition‐specific quality of life
The impact of urinary incontinence on quality of life was assessed by two validated scales: the Incontinence Impact Questionnaire‐Revised (IIQ‐R) and the Urogenital Distress Inventory (UDI) scale. Both instruments have established validity and reliability for assessing the impact of urinary incontinence on the quality of life of women (Shumaker 1994). On these scales, lower scores imply lower impact of incontinence on quality of life and vice versa. Assessment was carried out immediately and at three months after treatment.
Data analysis indicated that immediately after treatment, the addition of PFMT to bladder training resulted in statistically significantly lower impact on the quality of life (better) than with bladder training alone on both scales (IIQ‐R: MD ‐25.50, 95% CI ‐49.95 to ‐1.05; Analysis 3.3.1; UDI: MD ‐31.10, 95% CI ‐48.94 to ‐13.26; Analysis 3.4.1). However, this difference did not persist at three months after treatment on either scale (IIQ‐R: MD ‐5.90, 95% CI ‐35.53 to 23.73; Analysis 3.3.2; UDI: MD ‐18.90, 95% CI ‐37.92 to 0.12; Analysis 3.4.2).
3.3. Analysis.
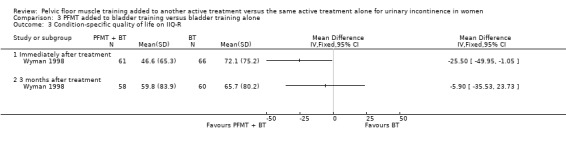
Comparison 3 PFMT added to bladder training versus bladder training alone, Outcome 3 Condition‐specific quality of life on IIQ‐R.
3.4. Analysis.
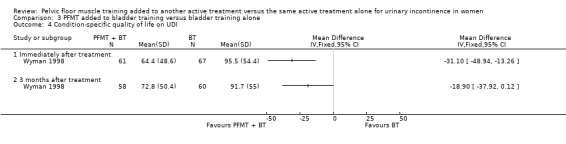
Comparison 3 PFMT added to bladder training versus bladder training alone, Outcome 4 Condition‐specific quality of life on UDI.
Patient global impression of improvement
Data analysis from two trials showed that immediately after treatment, the addition of PFMT to bladder training resulted in statistically significantly higher proportion of women being cured or improved than with bladder training alone (111/117 versus 86/118; RR 1.29, 95% CI 1.15 to 1.45; I² = 35%; Analysis 3.5). However, this difference did not persist at three months after treatment as reported in Wyman 1998 (RR 1.21, 95% CI 0.94 to 1.55; Analysis 3.5).
3.5. Analysis.
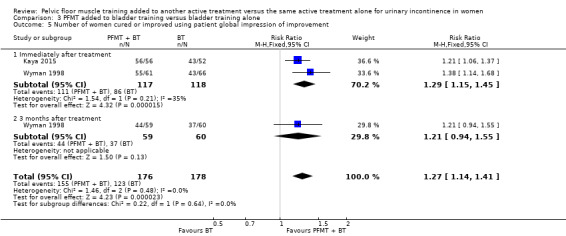
Comparison 3 PFMT added to bladder training versus bladder training alone, Outcome 5 Number of women cured or improved using patient global impression of improvement.
Secondary outcome measures
Frequency of incontinence episodes per week was assessed from the records in a standardised diary immediately after the intervention (Analysis 3.6). While women had fewer episodes of incontinence in the combined treatment group compared with bladder training alone group, this result was not statistically significant (6.8 versus 10.6; MD ‐3.80, 95% CI ‐8.51 to 0.91; Analysis 3.6).
3.6. Analysis.
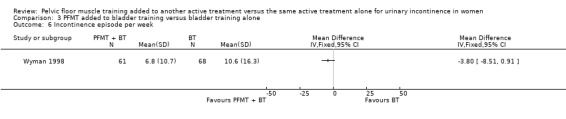
Comparison 3 PFMT added to bladder training versus bladder training alone, Outcome 6 Incontinence episode per week.
Other outcome measures
Patient satisfaction with treatment outcome
The instrument used to assess the level of satisfaction of the women with their treatment outcome was not reported. However, the pattern of more women improved immediately after treatment, but not three months later, was repeated; details are available in Analysis 3.7).
3.7. Analysis.
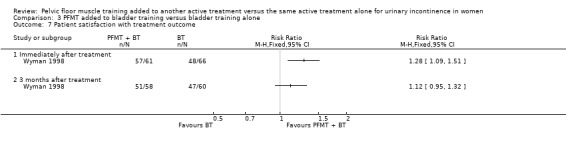
Comparison 3 PFMT added to bladder training versus bladder training alone, Outcome 7 Patient satisfaction with treatment outcome.
Number of women requiring further treatment (relapse)
After completion of the 12‐week treatment, women were followed for approximately three years. A similar number of women had sought further treatment such as surgical intervention or drug therapy among those who received PFMT in combination with bladder training and the control (bladder training alone).. No statistically significant difference was found in the estimated size of treatment effect (18/48 (38%) versus 19/48 (40%); RR 0.95, 95% CI 0.57 to 1.57; Analysis 3.8)
3.8. Analysis.
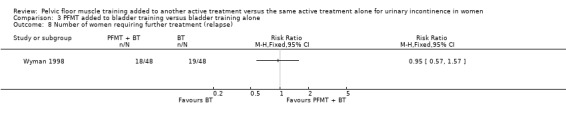
Comparison 3 PFMT added to bladder training versus bladder training alone, Outcome 8 Number of women requiring further treatment (relapse).
C Electrical or magnetic interventions
4. PFMT added to electrical stimulation versus electrical stimulation alone (excluding implanted electrodes)
Three small trials investigated the effects of this comparison in women with SUI (Bezerra 2009 (N = 48); Hofbauer 1990 (N = 43); Jeyaseelan 2002 (N = 19)) . However, Jeyaseelan 2002 provided no useable data.
Bezerra 2009 and Hofbauer 1990 reported the following outcomes of interest:
Primary outcome measures
Number of women 'cured' or 'improved' (as reported by the women)
The number of women 'cured' was reported by Hofbauer 1990 only. Cure was self reported by the women and was defined as the proportion of women who became continent (free of symptoms of urinary incontinence) at a specified point after treatment onset. The trial was too small to detect statistically significant differences in cure rates between women who received PFMT added to electrical stimulation and those who were given electrical stimulation alone (3/11 versus 1/11; RR 3.00, 95% CI 0.37 to 24.58; Analysis 4.1).
4.1. Analysis.
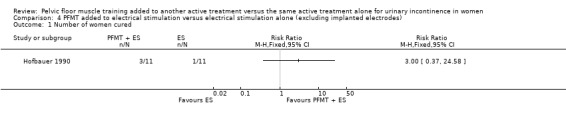
Comparison 4 PFMT added to electrical stimulation versus electrical stimulation alone (excluding implanted electrodes), Outcome 1 Number of women cured.
Both Bezerra 2009 and Hofbauer 1990 reported the number of women 'improved'. Improvement was also self reported, but the success threshold was not defined. Again, there was no statistically significant difference in the estimated size of the treatment effect between the two intervention groups (9/26 versus 5/30; RR 2.06, 95% CI 0.79 to 5.38; ; Analysis 4.2).
4.2. Analysis.
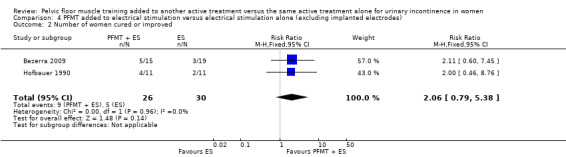
Comparison 4 PFMT added to electrical stimulation versus electrical stimulation alone (excluding implanted electrodes), Outcome 2 Number of women cured or improved.
Other outcome measure
Patient satisfaction with treatment outcome
This outcome was reported by Bezerra 2009 only. There was no statistically significant difference in the proportion of women satisfied with their treatment outcome between the two treatment groups either immediately after treatment (RR 0.84, 95% CI 0.47 to 1.52; Analysis 4.3), or 12 months after treatment (RR 0.99, 95% CI 0.48 to 2.02; Analysis 4.3)
4.3. Analysis.
Comparison 4 PFMT added to electrical stimulation versus electrical stimulation alone (excluding implanted electrodes), Outcome 3 Patient satisfaction with treatment outcome.
Jeyaseelan 2002 reported the following outcomes of interest:
Primary outcome measures
Condition‐specific quality of life
Condition‐specific quality of life was assessed at endpoint using two scales: Incontinence Impact Questionnaire (IIQ) and Urogenital Distress Inventory (UDI). Further details about these tools or the interpretation of scores were not given, and data were reported in medians and ranges. Women who received PFMT added to electrical stimulation had lower median scores (better) than those who received electrical stimulation alone on both instruments: IIQ: ‐27 (‐63 to 0) versus 7 (‐50 to 150); UDI: ‐32 (‐50 to 18) versus ‐28 (‐86 to 22).
Secondary outcome measures
Objective assessment of improvement on pad test
Women who received PFMT added to electrical stimulation had lower median pad weights compared to those who received electrical stimulation alone: ‐53 (‐77 to ‐23) versus 39 (‐39 to 29), implying less urine loss.
Frequency of incontinence episodes
Details about how this outcome was measured were not reported. However, women who received a combination of PFMT and electrical stimulation had fewer median episodes of urine leakage compared to those who were treated with electrical stimulation alone: ‐58 (‐100 to ‐50) versus ‐36 (‐58 to 166).
5. PFMT added to magnetic stimulation versus magnetic stimulation alone
This comparison was not investigated by any of the included trials. We have added Table 5 to highlight lack of evidence.
D Mechanical interventions
6. PFMT added to pessaries versus pessaries alone
Only one trial with 446 participants reported a number of outcomes on the effects of adding PFMT to continence pessary versus continence pessary alone, for women with SUI (Richter 2010).
Primary outcome measures
Number of women cured or improved (as reported by the women)
Cure or improvement rate was assessed using the seven‐day bladder diary and success (improvement) was defined as the proportion of women who had 75% or greater reduction in frequency of incontinence episodes per week. Assessment was carried out at three, six and 12 months after the start of treatment (but data were only available at three and 12 months). The result indicated that there were no statistically significant differences in cure or improvement rates between women who received PFMT added to continence pessaries and those who were treated with pessaries alone either at six months (80/132 versus 69/110; RR 0.97, 95% CI 0.79 to 1.18; Analysis 6.1.1), or at 12 months (52/111 versus 51/96; RR 0.88, 95% CI 0.67 to 1.16; Analysis 6.1.2) after the start of treatment.
6.1. Analysis.
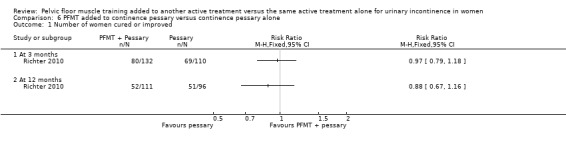
Comparison 6 PFMT added to continence pessary versus continence pessary alone, Outcome 1 Number of women cured or improved.
Symptom‐ and condition‐specific quality of life
This outcome was assessed at three and 12 months after the onset of intervention. The instrument used was the Urogenital Distress Inventory stress incontinence sub‐scale of the Pelvic Floor Distress Inventory, a validated tool that measures the impact of pelvic floor disorders on the quality of life of women (Barber 2001). On this scale, success was defined as the proportion of women without 'bothersome' stress incontinence symptoms. For more details, see the Characteristics of included studies table. There were no statistically significant differences in the estimated size of treatment effect between the two intervention groups either at three months (RR 1.12, 95% CI 0.86 to 1.47; Analysis 6.2.1), or at 12 months (RR 0.81, 95% CI 0.62 to 1.08; Analysis 6.2.2) post‐randomisation.
6.2. Analysis.
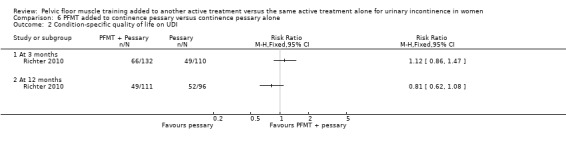
Comparison 6 PFMT added to continence pessary versus continence pessary alone, Outcome 2 Condition‐specific quality of life on UDI.
Patient global impression of improvement
This outcome was assessed at three, six and 12 months post‐randomisation, using the validated Patient Global Impression of Improvement (PGI‐I) Questionnaire. The validity and reliability of this instrument have been established by Yalcin and colleague (Yalcin 2003). Success was defined as the proportion of women with a response of 'much better' or 'very much better' on this scale. There were no statistically significant differences in the women's global impression of improvement between the two intervention groups at any of the endpoints: three months (RR 1.13, 95% CI 0.91 to 1.41; Analysis 6.3.1), six months (RR 1.00, 95% CI 0.78 to 1.30; Analysis 6.3.2) or 12 months (RR 0.90, 95% CI 0.67 to 1.21; Analysis 6.3.3).
6.3. Analysis.
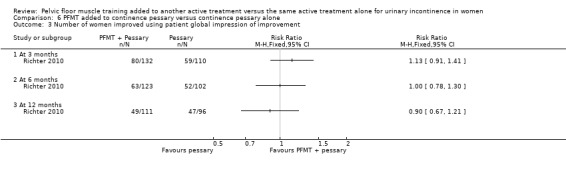
Comparison 6 PFMT added to continence pessary versus continence pessary alone, Outcome 3 Number of women improved using patient global impression of improvement.
Other outcome measure
Patient satisfaction with treatment outcome
This was assessed at three, six and 12 months after the start of treatment using the Patient Satisfaction Question, which has been found to be valid and reliable in assessing the extent to which women were satisfied with treatment (Burgio 2006). Success criteria were not reported. Analysis of data showed that there were no statistically significant differences in satisfaction between women who were treated with PFMT added to continence pessary and those who received continence pessary alone with approximately equal proportions of women in each treatment group reporting the same level of satisfaction at each time point: three months (118/132 versus 94/110; RR 1.05, 95% CI 0.95 to 1.15; Analysis 6.4.1); six months (104/123 versus 87/102; RR 0.99, 95% CI 0.89 to 1.11; Analysis 6.4.2); and 12 months (81/111 versus 75/96; RR 0.93, 95% CI 0.80 to 1.09; Analysis 6.4.3).
6.4. Analysis.
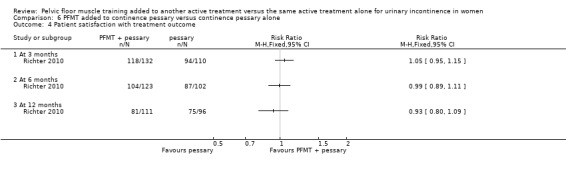
Comparison 6 PFMT added to continence pessary versus continence pessary alone, Outcome 4 Patient satisfaction with treatment outcome.
E Drug interventions
Each drug was tested only in single trials.
7. Duloxetine
One trial with 201 participants reported a number of outcomes on the benefits of adding PFMT to duloxetine therapy for women with SUI (Ghoniem 2005). The trial was too small to assess differences in outcomes reliably, and the confidence intervals were wide.
Primary outcome measures
Number of women cured or improved (as reported by the women)
Cure or improvement was assessed from the paper diaries completed by the women at the endpoint of treatment. Success was defined as the proportion of women who had 50% or greater reduction in the frequency of incontinence episodes per week. There were no statistically significant differences in the estimated size of treatment effect between women who were treated with PFMT added to duloxetine and those who received duloxetine alone (RR 1.09, 95% CI 0.77 to 1.53; Analysis 7.2.1).
7.2. Analysis.
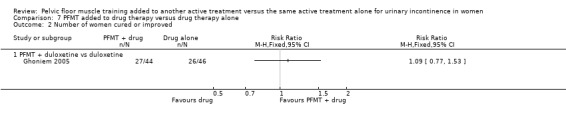
Comparison 7 PFMT added to drug therapy versus drug therapy alone, Outcome 2 Number of women cured or improved.
Symptom‐ and condition‐specific quality of life
This outcome was assessed at the endpoint of treatment, using the Incontinence Quality of Life (I‐QoL) Questionnaire. The validity of this instrument has been established by Patrick and colleagues (Patrick 1999). Scores were assigned to different domains of the questionnaire and mean (SD) scores calculated. Higher scores mean less symptom impact on the quality of life (better). The results indicated that there were no statistically significant differences in this outcome between the two intervention groups (MD 5.84, 95% CI ‐2.08 to 13.76; Analysis 7.3.1).
7.3. Analysis.
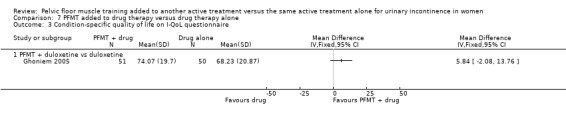
Comparison 7 PFMT added to drug therapy versus drug therapy alone, Outcome 3 Condition‐specific quality of life on I‐QoL questionnaire.
Patient global impression of improvement
Patient global impression of improvement was determined within the first three months after randomisation, using the validated Patient Global Impression of Improvement (PGI‐I) Questionnaire (Yalcin 2003). Success was defined as the number of women with a PGI‐I score in one of the three 'better' categories, that is, 'very much better', 'much better' or 'a little better'. The estimated size of treatment effect was not statistically significant between the women who received a combined PFMT and duloxetine and those who received duloxetine alone (RR 1.31, 95% CI 0.96 to 1.78; Analysis 7.4.1).
7.4. Analysis.
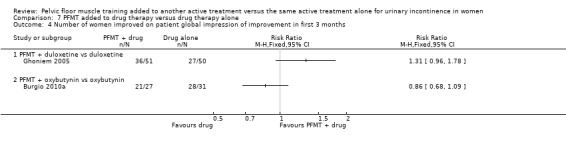
Comparison 7 PFMT added to drug therapy versus drug therapy alone, Outcome 4 Number of women improved on patient global impression of improvement in first 3 months.
Secondary outcome measures
Frequency of incontinence episodes per week
This outcome was determined three months after randomisation using paper diaries completed by the women. No statistically significant differences in outcome were detected between the two intervention groups (MD 0.31, 95% CI ‐3.55 to 4.17; Analysis 7.5.1).
7.5. Analysis.
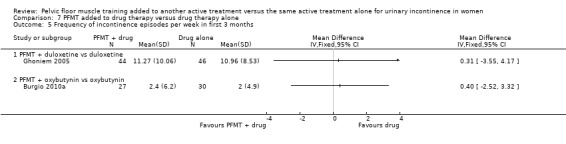
Comparison 7 PFMT added to drug therapy versus drug therapy alone, Outcome 5 Frequency of incontinence episodes per week in first 3 months.
Number of continence pads used per week
This outcome was computed for each intervention group at the endpoint of treatment. There were no statistically significant differences in number of continence pads used between women who received a combination of PFMT and duloxetine and those who were given duloxetine alone (MD 0.61, 95% CI ‐2.18 to 3.40; Analysis 7.9).
7.9. Analysis.
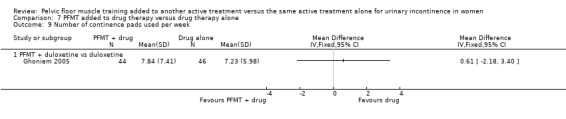
Comparison 7 PFMT added to drug therapy versus drug therapy alone, Outcome 9 Number of continence pads used per week.
8. Oxybutynin
One small trial investigated the effects of adding PFMT to oxybutynin treatment for women with urgency predominant urinary incontinence (Burgio 2010a). The following outcomes were reported and contributed data to the comparison.
Primary outcome measures
Patient global impression of improvement
One trial addressed this outcome three months after randomisation, using the validated Patient Global Impression of Improvement (PGI‐I) Questionnaire (Burgio 2006; Yalcin 2003). Success was defined as the proportion of women who felt 'much better' at endpoint. Analysis of data showed that there was no statistically significant difference in the estimated size of treatment effect between the two intervention groups (RR 0.86, 95% CI 0.68 to 1.09; Analysis 7.4.2).
Secondary outcome measures
Frequency of incontinence episodes per week
This outcome was measured at three months and at 12 months post‐randomisation, using the seven‐day bladder diary. Women were more likely to be incontinent with PFMT in combination with oxybutynin versus those who were treated with oxybutynin alone, but this did not reach statistical significance within either the first three months (MD 0.40, 95% CI ‐2.52 to 3.32; Analysis 7.5.2) or at 12 months (MD 2.80, 95% CI ‐2.19 to 7.79; Analysis 7.6.1) post‐randomisation.
7.6. Analysis.
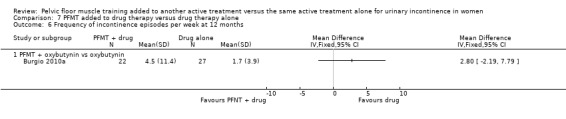
Comparison 7 PFMT added to drug therapy versus drug therapy alone, Outcome 6 Frequency of incontinence episodes per week at 12 months.
Frequency of micturitions per 24 hours
Although women emptied their bladders more often in the combined treatment group, this difference did not reach statistical significance at the end of treatment (MD 0.20, 95% CI ‐1.11 to 1.51; Analysis 7.7.1).
7.7. Analysis.
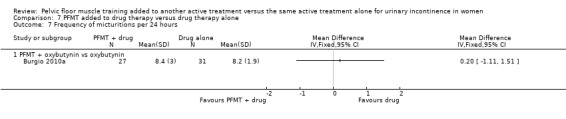
Comparison 7 PFMT added to drug therapy versus drug therapy alone, Outcome 7 Frequency of micturitions per 24 hours.
Volume of urine per micturition
For this outcome, higher volumes of urine per void means better treatment effect. Women tended to have higher volumes on the drug alone, but this did not differ significantly between the two intervention groups when subjected to statistical analysis and the difference was only 16 ml (MD ‐16.30, 95% CI ‐73.77 to 41.17; Analysis 7.8.1).
7.8. Analysis.
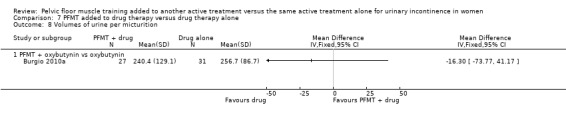
Comparison 7 PFMT added to drug therapy versus drug therapy alone, Outcome 8 Volumes of urine per micturition.
Other outcome measures
Patient satisfaction with treatment outcome
The validated Patient Satisfaction Questionnaire was used to assess each woman's level of satisfaction with her treatment outcome at endpoint (Burgio 2006). The number of women who were 'completely satisfied' with their treatment outcome was determined. Analysis of data showed that although more women were satisfied with the drug alone, there were no statistically significant differences in the estimated size of treatment effect between the two intervention groups (RR 0.89, 95% CI 0.70 to 1.14; Analysis 7.11.1).
7.11. Analysis.
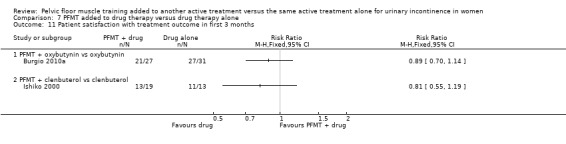
Comparison 7 PFMT added to drug therapy versus drug therapy alone, Outcome 11 Patient satisfaction with treatment outcome in first 3 months.
9. Solifenacin
One trial contributed data towards the analysis of the effects of adding PFMT to solifenacin treatment for women with over‐active bladder (Jin 2012). Only one of the reported outcomes had usable data, that is, treatment adverse effects, a secondary outcome measure (Analysis 7.10). Adverse effects were assessed with respect to the side effects of solifenacin, a treatment taken by both intervention groups. No statistically significant differences were found in adverse effects due to treatment between women who were treated with combined PFMT and solifenacin treatment and those who received solifenacin treatment alone (RR 0.84, 95% CI 0.45 to 1.60; Analysis 7.10.1).
7.10. Analysis.
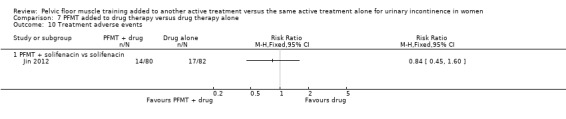
Comparison 7 PFMT added to drug therapy versus drug therapy alone, Outcome 10 Treatment adverse events.
10. Clenbuterol
One small trial compared the effects of combined PFMT and clenbuterol treatment with clenbuterol treatment alone for women with SUI (Ishiko 2000) .
Primary outcome measure
Number of women cured (as reported by the women)
Cure rate was defined as the proportion of women who reported 100% reduction in symptoms of urinary incontinence at the end of treatment. There were no statistically significant differences in reports of self reported cure between the two intervention groups (RR 1.16, 95% CI 0.83 to 1.63; Analysis 7.1.1).
7.1. Analysis.
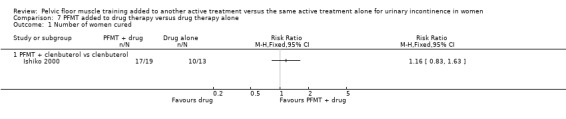
Comparison 7 PFMT added to drug therapy versus drug therapy alone, Outcome 1 Number of women cured.
Other outcome measures
Women's satisfaction with treatment outcome
The scale used in measuring this outcome was not specified. The trial was too small to identify significant differences in the number of women who were satisfied with either treatment (Analysis 7.11.2).
11. Other drugs (unspecified)
One very small trial tested the effects of adding PFMT to an unspecified drug therapy for women with overactive bladder (Chen 2008).
Other outcome measures
Treatment benefits
Treatment benefits were assessed using the Benefit Questionnaire (further detail was not reported). More women reported that they benefited from combined treatment in the intervention group compared to the control group treated with the drug alone: 11/15, 73% versus 4/14, 29%. This result was statistically significant (RR 2.57, 95% CI 1.06 to 6.20; Analysis 7.12.1).
7.12. Analysis.
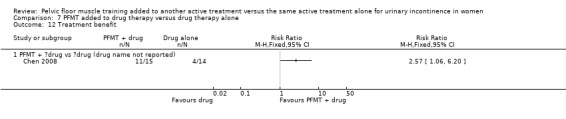
Comparison 7 PFMT added to drug therapy versus drug therapy alone, Outcome 12 Treatment benefit.
F Surgical interventions
12. PFMT prior to surgical intervention (e.g. tension‐free vaginal tape (TVT)) versus surgical intervention alone
This comparison was not tested by any of the included trials. We have added Table 8 to highlight lack of evidence.
G Other interventions
13. PFMT + heat and steam generating sheet versus heat and steam generating sheet alone
This comparison was tested by one trial in women with UUI or MUI (Kim 2011). The trialists hypothesised that the heat and steam generating sheet (HSGS) would reduce incontinent episodes by heating the abdominal and lower back, which in turn might result in positive effects on renal function, such as, suppression of the activity of renal sympathetic nerves and promotion of bladder emptying. Details about the heat and steam generating sheet are available in the Characteristics of included studies table.
Primary outcome measures
Number of women cured (as reported by the women)
Cure was assessed by interview and success was defined as the proportion of women with complete cessation of urine loss episodes at the end of treatment. Analysis of data indicated that more women were cured in the intervention group (PFMT added to HSGS) compared to the comparison group (HSGS alone): 19/37, 51% versus 8/37, 22%. This result was statistically significant (RR 2.38, 95% CI 1.19 to 4.73; Analysis 9.1.1).
9.1. Analysis.
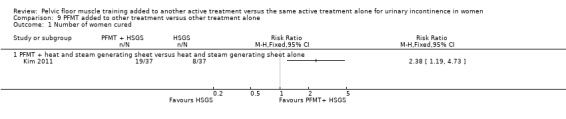
Comparison 9 PFMT added to other treatment versus other treatment alone, Outcome 1 Number of women cured.
Discussion
This is an updated version of a Cochrane review on the effects of adding pelvic floor muscle training (PFMT) to another active treatment versus the same active treatment alone, for urinary incontinence in women (Ayeleke 2013), and should be considered in the context of the other Cochrane reviews on pelvic floor muscle training (Boyle 2012; Dumoulin 2014; Hay‐Smith 2011; Herbison 2013; Herderschee 2011). The review examines whether the addition of PFMT to another active treatment is more beneficial than the same active treatment alone, for the treatment of women with urinary incontinence.
Summary of main results
Is PFMT added to another active treatment more effective than the same active treatment alone?
This question was addressed by 13 trials (Bezerra 2009; Burgio 2010a; Chen 2008; Ghoniem 2005; Hofbauer 1990; Ishiko 2000; Jeyaseelan 2002; Jin 2012; Kaya 2015; Kim 2011; Richter 2010; Wise 1993; Wyman 1998). We classified the primary and secondary outcomes, as defined earlier, as 'critical', 'important' or 'not important' for decision making from the woman's perspective. Seven outcomes from the comparisons were considered to be 'critical'; we applied the GRADE approach to determine the quality of evidence associated with these outcomes. The results are presented in the 'Summary of findings' tables.
Vaginal cones
The additional effects of adding PFMT to vaginal cones was examined by Wise and colleagues in women with stress urinary incontinence (SUI; Wise 1993). Although more women were either cured or improved with combined PFMT and vaginal cones than the control on objective assessment of urine leakage, this difference was not statistically significant (Analysis 1.1). The 12‐week PFMT was potentially too short and not adequately described to decide whether the exercise dose was theoretically sufficient. Adherence was not reported. We considered the quality of the evidence to be very low for the objective measure of urine leakage (pad test), when adopting the GRADE approach;none of the other outcomes which we considered critical for decision‐making were reported (Table 1).
Bladder training
The addition of PFMT to bladder training in women with SUI, urgency urinary incontinence (UUI) or mixed urinary incontinence (MUI) did not result in a statistically significant difference in the number of women cured either immediately after treatment (three months after randomisation; Analysis 3.1.1) or at three months after intervention (six months after randomisation; Analysis 3.1.2; Kaya 2015; Wyman 1998). There was no statistically significant benefit from adding PFMT to bladder training on the quality of life at three months after intervention (Analysis 3.4.2). A similar number of women (around 40%) who received PFMT added to bladder training versus bladder training alone required further treatments at approximately three years after treatment.. The description of the 12‐week PFMT programme in Wyman 1998 suggested it could theoretically increase strength, endurance and co‐ordination, although it was probably of insufficient duration for muscle strengthening. Training adherence was not reported. We judged the quality of evidence to be very low for the three reported critical outcomes, when adopting the GRADE approach; none of the other four outcomes which we considered critical for decision‐making from the patient's perspective were reported (Table 3).
Electrical stimulation
Adding PFMT to electrical stimulation in women with SUI did not result in a statistically significant difference in the cure or improvement rates (Bezerra 2009; Hofbauer 1990). The content of PFMT was not described, and at six weeks duration (Hofbauer 1990), or 24 sessions (Bezerra 2009), it was probably insufficient to maximise any possible training effect. Adherence was not reported. We considered the quality of the evidence to be very low for the number of women either cured or improved, when adopting the GRADE approach; none of the other outcomes which we considered critical for decision‐making were reported (Table 4).
Continence pessary
The addition of PFMT to a continence pessary did not result in statistically significant benefits in women with SUI in terms of the number of women cured or improved (Analysis 6.1.2) or the impact of urinary incontinence on the quality of life (Analysis 6.2.2) at 12 months after treatment (Richter 2010). At eight weeks duration, the incompletely described PFMT programme (that included stress and urgency strategies) was probably insufficient to maximise any possible training effect, and adherence was not reported. We considered the quality of the evidence to be moderate for the number of women cured or improved (subjective) at 12 months and condition‐specific quality of life at 12 months, when adopting the GRADE approach; none of the other outcomes which we considered critical for decision‐making were reported (Table 6).
Drug treatment
The benefits of adding PFMT to drug treatment did not show any statistically significant difference between the experimental (PFMT plus drug) and the control (drug alone) groups. There was no statistically significant difference in the number of women cured when adding PFMT to clenbuterol (Ishiko 2000). The 12‐week PFMT programme was potentially too short and not adequately described to decide whether the exercise dose was theoretically sufficient, and adherence was not reported. Similarly, the addition of PFMT to duloxetine did not result in a statistically significant better outcome in the domain of symptom‐ and condition‐specific quality of life (Ghoniem 2005). The description of the 12‐week PFMT programme suggested it could theoretically increase strength, endurance and co‐ordination, although it was probably of insufficient duration for muscle strengthening. In addition, training adherence was not reported. The quality of the evidence was low for condition‐specific quality of life on the I‐QoL Questionnaire, and very low for the number of women cured and the number of women reporting adverse events,when adopting the GRADE methodology; none of the other outcomes which we considered critical for decision‐making were reported (Table 7).
Other active treatments
The additional benefits of PFMT over and above a heat and steam generating sheet (HSGS) were investigated by Kim and colleagues (Kim 2011). More women were cured in the combined PFMT and HSGS group compared to the HSGS alone group and this result was statistically significant (Analysis 9.1.1). The description of the 12‐week PFMT programme suggested it could theoretically increase strength and endurance, but this duration was probably insufficient for muscle strengthening. Training adherence was not reported. We considered the effect estimate to be of moderate quality when adopting the GRADE approach (Table 9).
Problems with pelvic floor muscle training regimens
In most of the included trials, the absence of additional effects of PFMT over and above the active treatment alone might have been due to a number of factors. Of particular concern was the difficulty in evaluating the potential effectiveness of the PFMT intervention, the obvious inadequacy of the exercise dose offered to women, or both. All but three of the trials gave insufficient detail of the PFMT programme, or the PFMT programme was too short for muscle strengthening, suggesting that the exercise dose was not sufficient for treatment effect. Wyman, Kim and Ghoniem described PFMT programmes that might theoretically strengthen pelvic floor muscles (although in all three trials the treatment duration of 12 weeks was probably too short to establish muscle hypertrophy). It is also worth considering whether participants might have regarded the addition of PFMT as an additional treatment burden and thus either carried it out suboptimally or abandoned it altogether. None of the trials reported training adherence, which further compromises the ability to appraise the potential effectiveness of the PFMT intervention.
However, a likely explanation is that all the trials were too small (and hence underpowered) to detect statistically significant differences between the interventions.
Overall completeness and applicability of evidence
Three of the pre‐specified objectives (interventions) were not investigated by any of the included trials (PFMT with lifestyle intervention versus lifestyle intervention alone, PFMT with magnetic stimulation versus magnetic stimulation alone and PFMT prior to surgical intervention versus surgical intervention alone). The remaining pre‐specified comparisons were each addressed by single trials, except PFMT added to bladder training versus bladder training alone, which was reported by two trials (Kaya 2015; Wyman 1998) and PFMT plus electrical stimulation versus electrical stimulation alone, which was investigated by three trials (Bezerra 2009; Hofbauer 1990; Jeyaseelan 2002).
Jeyaseelan 2002 reported results using median and range, so the results were not included in the meta‐analysis. Therefore, it was not possible to improve the power for most of the comparisons. Some of the trialists used combinations of interventions with no regard to the types of urinary incontinence, and this might have influenced the results of the reported outcomes, e.g. combining PFMT and anticholinergics (without the appropriate 'dose' or 'doses') for women with UUI or urgency predominant urinary incontinence, when PFMT has been shown to work better for women with SUI or MUI (Dumoulin 2014).
None of the included trials reported any data on socioeconomic implications of the intervention, while only one trial reported data in a usable form on long‐term follow‐up (Wyman 1998). Also, only one trial, evaluating PFMT added to drug therapy, provided information about treatment adverse events, with respect to the side effects of the drug therapy (Jin 2012); none of the included trials reported data on adverse events associated with the PFMT regimen, thereby making it very difficult for us to evaluate the safety of PFMT. Treatment adherence, which might impact other outcome measures, was not reported in a usable form by any of the included trials and therefore, not analysed.
Quality of the evidence
Trial quality and methodological assessment
Methodological assessment plays a crucial role in determining the quality of the evidence supporting the estimated size of treatment effects of any intervention. In this review, we assessed the methodological flaws of the included trials, using the reports of the trials. Therefore, our judgement of methodological quality and hence the quality of effect estimates was influenced by the quality of reporting.
Five trials were published as conference abstracts, with few details on study designs, methods or data, thereby, making it difficult to assess their methodological quality (Bezerra 2009; Chen 2008; Jeyaseelan 2002; Jin 2012; Wise 1993). Of the 13 included trials, only three gave detailed descriptions of the randomisation process for the review authors to be sure there was adequate sequence generation and allocation concealment (Ghoniem 2005; Kaya 2015; Richter 2010). Thus, we judged them to be at low risk with respect to selection bias. A key challenge of the intervention in this review is that given the nature of PFMT, it was difficult to blind the participants or treatment providers to group allocation (performance bias). With regard to detection bias, outcome assessors were adequately blinded in only one of the included trials (Richter 2010).
In the domain of attrition bias, the rates of withdrawals and losses to follow‐up were high in some of the included trials, but with small differences in rates within treatment groups. In terms of size, most of the included trials were small, meaning that a high attrition rate would result in under‐powering of the trials and hence the occurrence of type II error (false negative results). A common problem with most of the included trials was incomplete reporting, particularly with respect to the trial methods and data. Thus, we assessed some domains of the risk of bias as 'unclear' due to incomplete reporting of methods.
In this review, the estimated sizes of treatment effects were generally small and therefore the quality of the evidence was not upgraded for any of the outcomes. However, the quality of the body of evidence was downgraded if we considered the randomisation process (sequence generation and allocation concealment) of the trial to be inadequate, if the effect estimate crossed the line of 'no effect' by 25% or 50% on either side (that is, effect estimate with a wide confidence interval), or both.
Potential biases in the review process
We searched all the important databases and imposed no language restriction in the course of the search. However, we were mindful of the fact that these databases might not have contained all the potentially eligible trials.
Agreements and disagreements with other studies or reviews
We identified one systematic review in the 5th edition of the International Consultation on Incontinence (ICI), which addressed the effects of adding PFMT to other active treatment versus the same active treatment alone (Moore 2013). Moore and colleagues included eight trials (Burgio 2008; Burgio 2010a; Ghoniem 2005; Hofbauer 1990; Ishiko 2000; Wilson 1998; Wise 1993; Wyman 1998), six of which were also included in this review (Burgio 2010a; Ghoniem 2005; Hofbauer 1990; Ishiko 2000; Wise 1993; Wyman 1998). One trial (Wilson 1998) was not included in this review as the participants were postpartum women; whereas the other trial was excluded because the behavioural intervention consisted of PFMT in addition to delayed voiding to increase voiding intervals, and individualised fluid management, which we considered as active treatments on their own (Burgio 2008). Moore's review did not include seven trials which were included in this review (Bezerra 2009; Chen 2008; Jeyaseelan 2002; Jin 2012; Kaya 2015; Kim 2011; Richter 2010). Overall, the findings of the review conducted by Moore and colleagues are in agreement with those of this review (Moore 2013).
Authors' conclusions
Implications for practice.
This review did not find sufficient evidence to support or refute that pelvic floor muscle training (PFMT), added to other active treatments results in better results than the same active treatment alone, for urinary incontinence in women. The identified trials randomised between six and 150 participants per treatment arm and thus, were not powered to detect any significant difference in the primary outcomes of interest in the review (Jeyaseelan 2002; Richter 2010 respectively). In addition, most of the trials addressed different participants and interventions with disparate and few outcome data. This limited our ability to combine data in a pooled analysis for most of the comparisons. Therefore, our confidence in the estimated size of treatment effects for most of the comparisons is uncertain.
These results should be interpreted with caution as most of the comparisons were investigated by single, small trials, and none of the trials in this review were large enough to answer the questions they were designed to answer.
Implications for research.
This review has demonstrated that there is insufficient evidence to conclude whether or not adding PFMT to another active treatment is more beneficial either in the short or the long term than the same active treatment alone. This was partly due to either scanty or no trials on the various interventions postulated. Additionally, the methodological quality of some of the included trials fell short of the recommendations and principles set out in the CONSORT statement as illustrated in Figure 2 and Figure 3. Moreover, a majority of the trials did not report the required information for making decisions, and we judged them as 'unclear'.
Therefore, there is a need for more research on the effect of PFMT when added to other treatments. For example, this review could not identify any trial which investigated the additional effects of PFMT over and above common active treatments such as surgical intervention (PFMT prior to surgical intervention versus surgical intervention alone) or structured lifestyle intervention (PFMT added to structured lifestyle intervention versus strictured lifestyle intervention alone).
Future research should also take into consideration the synergistic effects of combined PFMT and another active treatment in relation to the types of urinary incontinence before combination: for example, PFMT plus duloxetine versus duloxetine for stress urinary incontinence; or PFMT plus anticholinergic versus anticholinergic for urgency urinary incontinence or overactive bladder.
In addition, future research should equally focus on quality of life, socioeconomic implications, long‐term effects and adverse events associated with combining PFMT with other active treatments. Above all, future research should be conducted in accordance with the recommendations and principles outlined in the CONSORT statement for improving the reporting of trials.
What's new
| Date | Event | Description |
|---|---|---|
| 27 October 2015 | New citation required but conclusions have not changed | In this update the review authors have added the following two trials: Bezerra 2009; Kaya 2015 |
| 27 October 2015 | New search has been performed | In this update the review authors have added the following two trials: Bezerra 2009; Kaya 2015. |
Acknowledgements
The review authors would like to acknowledge the team of the Cochrane Incontinence Group for their help.
Appendices
Appendix 1. Search strategies for the first update of this review
Cochrane Incontinence Group Specialised Register
The terms used to search the Incontinence Group Specialised Register are given below. The date of the last search of the Specialised Register was: 5 May 2015.
(({DESIGN.CCT*} OR {DESIGN.RCT*}) AND
{TOPIC.URINE.INCON*} AND
({INTVENT.PHYS.PFMT*} OR {INTVENT.PHYS.BIOFEED*}) AND
({INTVENT.SURG*} OR {INTVENT.CHEM.DRUG*} OR {INTVENT.PSYCH*} OR {INTVENT.LIFESTYLE*} OR {INTVENT.MECH*} OR {INTVENT.ELECTSTIM*} OR {INTVENT.CONES*})
(All searches were of the keyword field of Reference Manager 2012).
CINAHL
CINAHL on EBSCOhost covering January 1982 to 1 May 2015 was searched using the search strategy given below. Date of last search: 6 May 2015.
| # | Query |
| S41 | S31 AND S40 |
| S40 | S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 |
| S39 | AB (exerci* or train*) N5 muscle* |
| S38 | TI (exerci* or train*) N5 muscle* |
| S37 | TI ( PFMT OR PFE ) OR AB ( PFMT OR PFE ) |
| S36 | TI pelvi* N5 floor* OR AB pelvi* N5 floor* |
| S35 | TI pelvi* N5 muscle* OR AB pelvi* N5 muscle* |
| S34 | TI kegel* OR AB kegel* |
| S33 | (MM "Pelvic Floor Muscles") |
| S32 | (MH "Kegel Exercises") OR (MH "Therapeutic Exercise+") OR (MH "Muscle Strengthening+") |
| S31 | S23 AND S30 |
| S30 | S24 OR S25 OR S26 OR S27 OR S28 OR S29 |
| S29 | TI overactiv* N3 bladder* OR AB overactiv* N3 bladder* |
| S28 | TI urin* N3 leak* OR AB urin* N3 leak* |
| S27 | TI ( incontinen* OR continen* ) OR AB ( incontinen* OR continen* ) |
| S26 | MH incontinence |
| S25 | MH overactive bladder |
| S24 | MH Urinary incontinence+ |
| S23 | S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 |
| S22 | TI ( singl* N25 blind* OR singl* N25 mask* OR doubl* N25 blind* or doubl* N25 mask* OR trebl* N25 blind* OR trebl* N25 mask*OR tripl* N25 blind* OR tripl* N25 mask* ) or AB ( singl* N25 blind* OR singl* N25 mask* OR doubl* N25 blind* or doubl* N25 mask* OR trebl* N25 blind* OR trebl* N25 mask*OR tripl* N25 blind* OR tripl* N25 mask* ) |
| S21 | (MH "Comparative Studies") |
| S20 | (MH "Clinical Research+") |
| S19 | (MH "Static Group Comparison") |
| S18 | (MH "Quantitative Studies") |
| S17 | (MH "Crossover Design") or (MH "Solomon Four‐Group Design") |
| S16 | (MH "Factorial Design") |
| S15 | (MH "Community Trials") |
| S14 | (MH "Random Sample") |
| S13 | TI balance* N2 block* or AB balance* N2 block* |
| S12 | TI "latin square" or AB "latin square" |
| S11 | TI factorial or AB factorial |
| S10 | TI clin* N25 trial* or AB clin* N25 trial* |
| S9 | (MH "Study Design") |
| S8 | (AB random*) OR (TI random*) |
| S7 | (AB placebo*) OR (TI placebo*) |
| S6 | (MH "Placebos") |
| S5 | PT Clinical Trial OR (PT "randomized controlled trial") |
| S4 | (MH "Clinical Trials+") |
| S3 | MH (random assignment) OR (crossover design) |
| S2 | cross‐over |
| S1 | crossover |
Appendix 2. Search strategies for the first version of this review
Other electronic searches performed specifically for this version of the review (Ayeleke 2013) are detailed below.
EMBASE Classic and EMBASE (on OVID SP) covering 1947 to 2013 Week 9. Date of last search: 7 March 2013. The search strategy is given below.
1. Randomized Controlled Trial/
2. crossover procedure/ or double blind procedure/ or parallel design/ or single blind procedure/
3. Placebo/
4. placebo$.tw,ot.
5. random$.tw,ot.
6. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw,ot.
7. crossover.tw,ot.
8. cross over$.tw,ot.
9. allocat$.tw,ot.
10. trial.ti.
11. parallel design/
12. triple blind procedure/
13. or/1‐12
14. exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/
15. exp human/ or exp "human tissue, cells or cell components"/
16. 14 and 15
17. 14 not 16
18. 13 not 17
19. pelvic floor muscle training/
20. exp feedback system/
21. kegel*.tw.
22. (pelvi* adj4 (exercis* or train* or muscle*)).tw.
23. PFMT.tw.
24. 19 or 20 or 21 or 22 or 23
25. incontinence/ or mixed incontinence/ or stress incontinence/ or urge incontinence/ or urine incontinence/
26. continence/
27. overactive bladder/
28. micturition disorder/ or lower urinary tract symptom/ or pollakisuria/
29. urinary dysfunction/ or bladder instability/ or detrusor dyssynergia/ or neurogenic bladder/ or urinary urgency/ or urine extravasation/
30. (incontinen$ or continen$).tw.
31. ((bladder or detrusor or vesic$) adj5 (instab$ or stab$ or unstab* or irritab$ or hyperreflexi$ or dys?ynerg$ or dyskinesi$ or irritat$)).tw.
32. (urin$ adj2 leak$).tw.
33. ((bladder or detrusor or vesic$) adj2 (hyper$ or overactiv$)).tw.
34. (bladder$ adj2 (neuropath$ or neurogen* or neurolog$)).tw.
35. (nervous adj pollakisur$).tw.
36. or/25‐35
37. 18 and 24 and 36
38. (2011* or 2012* or 2013*).em.
39. 37 and 38
The EMBASE search was limited by entry month to 2011, 2012 and 2013 to cover those years that are not currently included in the EMBASE search that is searched by The Cochrane Collaboration and incorporated into the CENTRAL database.
Key: / = EMTREE term; .tw. = text word search; .ot. = original title (for non‐English titles); $ = truncation; adjn = within n words of other word in any word order.
CINAHL on EBSCO Host (covering January 1982 to 5 March 2013). Date of last search: 5 March 2013. The search strategy is given below.
| # | Query |
| S39 | S31 AND S38 |
| S38 | S32 OR S33 OR S34 OR S35 OR S36 OR S37 |
| S37 | TI ( PFMT OR PFE ) OR AB ( PFMT OR PFE ) |
| S36 | TI pelvi* N5 floor* OR AB pelvi* N5 floor* |
| S35 | TI pelvi* N5 muscle* OR AB pelvi* N5 muscle* |
| S34 | TI kegel* OR AB kegel* |
| S33 | (MM "Pelvic Floor Muscles") |
| S32 | (MM "Kegel Exercises") OR (MH "Therapeutic Exercise+") OR (MH "Muscle Strengthening+") |
| S31 | S23 AND S30 |
| S30 | S24 OR S25 OR S26 OR S27 OR S28 OR S29 |
| S29 | TI overactiv* N3 bladder* OR AB overactiv* N3 bladder* |
| S28 | TI urin* N3 leak* OR AB urin* N3 leak* |
| S27 | TI ( incontinen* OR continen* ) OR AB ( incontinen* OR continen* ) |
| S26 | MH incontinence |
| S25 | MH overactive bladder |
| S24 | MH Urinary incontinence+ |
| S23 | S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 |
| S22 | TI ( singl* N25 blind* OR singl* N25 mask* OR doubl* N25 blind* or doubl* N25 mask* OR trebl* N25 blind* OR trebl* N25 mask*OR tripl* N25 blind* OR tripl* N25 mask* ) or AB ( singl* N25 blind* OR singl* N25 mask* OR doubl* N25 blind* or doubl* N25 mask* OR trebl* N25 blind* OR trebl* N25 mask*OR tripl* N25 blind* OR tripl* N25 mask* ) |
| S21 | (MH "Comparative Studies") |
| S20 | (MH "Clinical Research+") |
| S19 | (MH "Static Group Comparison") |
| S18 | (MH "Quantitative Studies") |
| S17 | (MH "Crossover Design") or (MH "Solomon Four‐Group Design") |
| S16 | (MH "Factorial Design") |
| S15 | (MH "Community Trials") |
| S14 | (MH "Random Sample") |
| S13 | TI balance* N2 block* or AB balance* N2 block* |
| S12 | TI "latin square" or AB "latin square" |
| S11 | TI factorial or AB factorial |
| S10 | TI clin* N25 trial* or AB clin* N25 trial* |
| S9 | (MH "Study Design") |
| S8 | (AB random*) OR (TI random*) |
| S7 | (AB placebo*) OR (TI placebo*) |
| S6 | (MH "Placebos") |
| S5 | PT Clinical Trial |
| S4 | (MH "Clinical Trials+") |
| S3 | MH (random assignment) OR (crossover design) |
| S2 | cross‐over |
| S1 | crossover |
Key: MH = exact CINAHL subject heading; + = exploded CINAHL heading; MM = exact major CINAHL subject heading; N = within n words of the other word, in any order; PT = publication type; AB = abstract; TI = title.
Searching for ongoing trials
ClinicalTrials.gov (date of last search: 30 May 2013). The search terms used are given below.
Pelvic training
Pelvic exercise
Pelvic exercises
WHO ICTRP (date of last search: 3 June 2013). The search terms used are given below.
Pelvic floor muscle training
Pelvic floor muscle exercise*
Pelvic floor exercise*
Pelvic exercise*
Pelvic training
Key: * indicates truncation
Data and analyses
Comparison 1. PFMT added to vaginal cones versus vaginal cones alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of women cured or improved (objective assessment) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. PFMT added to bladder training versus bladder training alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of women cured | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Immediately after treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 3 months after treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of women cured or improved | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Immediately after treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 3 months after treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Condition‐specific quality of life on IIQ‐R | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Immediately after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 3 months after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Condition‐specific quality of life on UDI | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Immediately after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 3 months after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of women cured or improved using patient global impression of improvement | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.14, 1.41] |
| 5.1 Immediately after treatment | 2 | 235 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.15, 1.45] |
| 5.2 3 months after treatment | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.94, 1.55] |
| 6 Incontinence episode per week | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Patient satisfaction with treatment outcome | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Immediately after treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 3 months after treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Number of women requiring further treatment (relapse) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 4. PFMT added to electrical stimulation versus electrical stimulation alone (excluding implanted electrodes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of women cured | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Number of women cured or improved | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.79, 5.38] |
| 3 Patient satisfaction with treatment outcome | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.57, 1.43] |
| 3.1 Immediately after treatment | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.47, 1.52] |
| 3.2 After 12 months | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.48, 2.02] |
Comparison 6. PFMT added to continence pessary versus continence pessary alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of women cured or improved | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Condition‐specific quality of life on UDI | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of women improved using patient global impression of improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 At 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 At 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 At 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Patient satisfaction with treatment outcome | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 At 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 At 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 At 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. PFMT added to drug therapy versus drug therapy alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of women cured | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 PFMT + clenbuterol vs clenbuterol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of women cured or improved | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 PFMT + duloxetine vs duloxetine | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Condition‐specific quality of life on I‐QoL questionnaire | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 PFMT + duloxetine vs duloxetine | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of women improved on patient global impression of improvement in first 3 months | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 PFMT + duloxetine vs duloxetine | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 PFMT + oxybutynin vs oxybutynin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Frequency of incontinence episodes per week in first 3 months | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 PFMT + duloxetine vs duloxetine | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 PFMT + oxybutynin vs oxybutynin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Frequency of incontinence episodes per week at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 PFMT + oxybutynin vs oxybutynin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Frequency of micturitions per 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 PFMT + oxybutynin vs oxybutynin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Volumes of urine per micturition | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 PFMT + oxybutynin vs oxybutynin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Number of continence pads used per week | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 PFMT + duloxetine vs duloxetine | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Treatment adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1 PFMT + solifenacin vs solifenacin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Patient satisfaction with treatment outcome in first 3 months | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.1 PFMT + oxybutynin vs oxybutynin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 PFMT + clenbuterol vs clenbuterol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Treatment benefit | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.1 PFMT + ?drug vs ?drug (drug name not reported) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. PFMT added to other treatment versus other treatment alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of women cured | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 PFMT + heat and steam generating sheet versus heat and steam generating sheet alone | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bezerra 2009.
| Methods | 2‐arm parallel RCT | |
| Participants | 48 women with SUI | |
| Interventions |
A. PFMT + electrical stimulation (ES) (N = 24) PFMT was individually designed by physiotherapist. No other details were provided about PFMT, including duration of treatment. ES was delivered with vaginal electrodes, with 50 Hz of frequency, 1 ms pulse and fixed 20 mA B. Electrical stimulation (ES) (N = 24) ES was delivered with vaginal electrodes, with 50 Hz of frequency, 1 ms pulse and fixed 20 mA |
|
| Outcomes |
1. Improvement in urinary symptoms This outcome was assessed using voiding diary, no other details were reported A. 5/15; B. 3/19 2. Satisfaction with treatment No detail was reported on how this outcome was measured Immediately after treatment A. 8/15; B. 12/19 After 12 months A. 7/15; B. 9/19 |
|
| Notes | This study is a conference abstract with little detail reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not reported |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind participants and personnel to PFMT |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | proportions of withdrawals differ between the 2 treatment groups; reasons for withdrawals were not reported and it was not clear whether or not analysis was based on ITT |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Ethical approval | Unclear risk | Details not reported |
| Source of funding or support | Low risk | It was stated that there was no source of funding |
| Conflict of interest | Unclear risk | Details not reported |
Burgio 2010a.
| Methods | 2‐arm randomised controlled trial, parallel design | |
| Participants | 64 women with urgency predominant incontinence | |
| Interventions |
A: Drug therapy alone group (N = 32). Individuals in this group received oxybutynin 5 mg daily with dose gradually increased during visits to the maximum level the individual could tolerate (dose range: 5 to 30 mg) B: Behavioural therapy + drug therapy (N = 32): participants in this group received drug therapy as described above and behavioural therapy. Behavioural therapy included PFMT and urge suppression strategies. PFMT consisted of 3 sessions of 15 exercises daily (total of 45 exercises). During each session, participants were instructed to contract for 10 seconds and relax for another 10 seconds (maximum duration of 10 seconds was achieved on a gradual basis). They were also taught the skills on urge suppression strategies |
|
| Outcomes |
1. Patient global perception of improvement: this was measured using the Global Perception of Improvement rating. Success was defined as the proportion of participants who felt 'much better' at the end of the treatment At 8 weeks: A: 28/31; B: 21/27 2. Condition‐specific quality of life: assessed using the Incontinence Impact Questionnaire and Urogenital Distress Inventory (reported as mean score and SD; details of data not reported) 3. Patient satisfaction with treatment outcome: success was defined as the proportion of participants who were completely satisfied with the treatment outcome. It was assessed using the Patient Satisfaction Questionnaire At 8 weeks: A: 27/31; B: 21/27 4. Frequency of incontinence episodes per week: mean (SD) of incontinence episodes frequency was assessed at endpoint using the 7‐day bladder diary At 8 weeks: A: 2.0 (4.9), N = 30: B: 2.4 (6.2), N = 27 At 12 months: A: 1.7 (3.9), N = 27; B: 4.5 (11.4), N = 22 5. Frequency of micturition per 24 hours (in mean and SD) At 8 weeks: A: 8.2 (1.9), N = 31; B: 8.4 (3.0), N = 27 6. Volumes of urine voided per 24 hours (in mean and SD) At 8 weeks: A: 256.7 (86.7), N = 31; B: 240.4 (129.1), N = 27 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as "stratified block randomisation". Exact process not specified |
| Allocation concealment (selection bias) | Unclear risk | It was not stated whether or not the allocations were concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Completed questionnaires were submitted in sealed envelopes and given to the nurses who administered the intervention. However, it is not specified whether the same or different nurses assessed the outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5/64 dropped out of the trial: A 1/32; B 4/32. Reasons not specified |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Ethical approval | Low risk | Approved by the institutional review board |
| Source of funding or support | Low risk | Stated (received grants from public institutions) |
| Conflict of interest | Unclear risk | Some of the authors had financial and other relationships with some pharmaceutical companies |
Chen 2008.
| Methods | 2‐arm randomised controlled trial, parallel design | |
| Participants | 29 women with over‐active bladder | |
| Interventions |
A: Drug alone (N = 14): details of drug including name and dose not stated B: PFMT + drug (N = 15). PFMT was assisted by perineal surface electromyography and was taught inially. Participants were then instructed to perform 3 sets of PFMT per day, 15 contractions per set, continuously at home for 8 weeks. Drug regimen: as stated above |
|
| Outcomes |
1. Urgency episodes per 24 hours: this was determined at baseline and endpoint using the 3‐day voiding diary and mean percentage change was calculated for the 2 groups (no useable data) 2. Daytime frequency per 24 hours: this was obtained before and after treatment using the 3‐day voiding diary and mean percentage change calculated (no useable data) 3. Treatment benefit: this was determined 4 weeks post‐treatment using the 'Benefit Questionnaire' and proportion of participants with perceived benefits calculated for each group A: 4/14; B: 11/15 4. Symptom bothersome: scores were obtained before and after treatment and mean percentage change in bothersome scores was obtained for the 2 groups (no useable data) 5. Quality of life: total scores were calculated for different domains of the quality of life (such as sleeping, concern and coping) before and after treatment. Mean percentage increase was calculated for the 2 groups (no useable data) |
|
| Notes | Dropouts: not reported, only the number of participants who completed the trial was stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Process involved in randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Process involved in allocation concealment was not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible (assumed not done) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported, only the number of participants who completed the trial was stated |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Ethical approval | Low risk | Approved by the Ethics committee |
| Source of funding or support | Low risk | Stated "none" according to the authors |
| Conflict of interest | Unclear risk | Not declared |
Ghoniem 2005.
| Methods | 4‐arm randomised controlled trial, parallel design | |
| Participants | 201 women with predominant symptoms of stress urinary incontinence (SUI) | |
| Interventions |
A: No active treatment (N = 47). Received placebo plus imitation (sham) PFMT for 12 weeks. Imitation PFMT consisted of initial therapist‐supervised instructions on how to train the hip abductors. Participants were then given written instructions and a training log with the recommendation of 3 sets of 10 long and 2 sets of 10 rapid contractions 4 days weekly. However, no instructions were given to the participants to contract the pelvic floor muscles with physical activities associated with urine leakage (skill training) B. PFMT only (N = 50). Received placebo plus PFMT for 12 weeks. PFMT comprised 30 minutes of initial therapist supervised instructions on how to contract the pelvic floor muscles. The correct type of contraction was confirmed by pelvic examination. Then participants received instructions to perform 3 sets of 10 long (6 to 8 seconds) and 2 sets of 10 rapid (1 to 2 seconds) contractions 4 days weekly (total of 200 contractions per week). At 4 and 8 weeks, participants received 15 minutes of re‐instruction and manual feedback and a training log was completed. Finally, skill training was giving by instructing participants to contract the pelvic floor muscles with physical events usually associated with urine loss C: Duloxetine + sham PFMT (N = 52). This group received duloxetine and sham PFMT. Duloxetine was given at a dose of 40 mg twice daily for 12 weeks. Sham PFMT (as described above) D: PFMT + duloxetine (N = 52). This is the combined group. Participants in this group received PFMT and duloxetine as described above For this review comparison D versus C is relevant |
|
| Outcomes |
1. Incontinence episode frequency (IEF) per week. This was computed from participant completed paper diaries at each visit. Mean (SD) weekly IEF at the endpoint was calculated for each treatment group A: 18.50 (17.10), N = 44; B: 20.93 (16.26), N = 46; C: 10.96 (8.53), N = 46; D: 11.27 (10.06), N = 44 2. Improvement (IEF responder rate): this was defined as the proportion of participants who had a 50% or greater decrease in IEF with treatment as computed from the paper diaries A: 11/44; B: 12/46; C: 26/46; D: 27/44 3. Number of continence pads used. Mean (SD) pads per week was calculated for each treatment group at endpoint A: 10.22 (7.56), N = 44; B: 11.48 (8.36), N = 46; C: 7.23 (5.98), N = 46; D: 7.84 (7.41), N = 44 4. Condition‐specific quality of life: this was assessed at endpoint using the Incontinence Quality of Life (I‐QoL) score questionnaire and mean (SD) score was obtained for each group A: 69.34 (20.69), N = 45; B: 68.76 (22.70), N = 49; C: 68.23 (20.87), N = 50; D: 74.07 (19.70), N = 51 5. Patient Global Impression of Improvement (PGI‐I): this was defined as the proportion of participants with a PGI‐I score in one of the following 3 categories: 1. 'very much better', 2. 'much better' or 3. 'a little better'. This was obtained using the validated PGI‐I questionnaire A: 19/45; B: 32/49; C: 27/50; D: 36/51 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...treatments were assigned using a centralised computer voice response" |
| Allocation concealment (selection bias) | Low risk | "...treatments were assigned using a centralised computer voice response" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Duloxetine and placebo were given in double‐blind fashion. However, it is not specified who exactly was blinded. Participants were blinded to PFMT or sham PFMT |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated whether or not outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts: all: 56/201; A: 10/47; B: 10/50; C: 19/52; D: 17/52 No differential loss to follow‐up between group C and D. However, there is excessive loss to follow‐up as 56/201 participants were dropped‐out. |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol not available |
| Ethical approval | Low risk | Approved by the ethics committee |
| Source of funding or support | Low risk | Stated, supported by private organisations |
| Conflict of interest | Unclear risk | Stated but some of the authors had financial and other relationships with one of the organisations which supported the trial |
Hofbauer 1990.
| Methods | 4‐arm randomised controlled trial, parallel design | |
| Participants | 43 women with urodynamic evidence of stress urinary incontinence (SUI) | |
| Interventions |
A. PFMT + electrical stimulation (ES) (N = 11): participants in this group received both PFMT and ES. PFMT was part of an exercise programme which also included abdominal and hip exercise and was administered twice weekly for 20 minutes by a therapist in addition to a daily home exercise programme. Electrical stimulation consisted of vaginal and lumbar electrodes which were administered for 10 minutes, 3 times weekly for a total of 6 weeks. Output was increased until noticeable contraction was achieved and participant then added voluntary effort B. PFMT alone (N = 11): as described above C. Electrical stimulation (ES) alone (N = 11): as described above D. Sham electrical stimulation (N = 10): as for ES above but current was so low that no effect (contraction) was possible For this review comparison A versus C is relevant |
|
| Outcomes |
1. Cure: this is the proportion of participants who became continent (free of symptoms of incontinence) at the end of the treatment as reported by the participants At 10 to 12 weeks from the onset of treatment: A: 3/11; B: 6/11; C: 1/11; D: 0/11 2. Improvement: proportion of participants who reported improvement in the symptoms of incontinence; success threshold not defined At 10 to 12 weeks from the onset of treatment: A: 4/11; B: 1/11; C: 2/11; D: 0/11 3. Success rate: this is the proportion of participants who reported cure of or significant improvement in the symptoms of incontinence At 10 to 12 weeks from the onset of treatment: A: 7/11; B:7/11; C: 3/11; D: 0/11 |
|
| Notes | Dropouts: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as "prospektiv randomisierten". No additional information provided. |
| Allocation concealment (selection bias) | Unclear risk | Reported as "prospektiv randomisierten". No additional information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants undergoing PFMT not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts: not reported |
| Selective reporting (reporting bias) | Unclear risk | Not specified |
| Ethical approval | Unclear risk | Not specified |
| Source of funding or support | Unclear risk | Not specified |
| Conflict of interest | Unclear risk | Not specified |
Ishiko 2000.
| Methods | 3‐arm randomised controlled trial, parallel design | |
| Participants | 61 women with symptoms of stress, urinary incontinence | |
| Interventions |
A. Drug therapy (DT) group (N = 18). Participants in this group received clenbuterol tablets 20 µg twice daily B. PFMT group (N = 20). Participants in this group received instructions on PFMT from gynaecologic specialists until they understood the technique. They were then instructed to perform the exercise for 10 minutes daily (other details not reported). Video tapes that demonstrated the proper method of performing PFMT were also given to the participants C. PFMT + DT group (N = 23): participants in this group received both clenbuterol and PFMT as described above For this review comparison C versus A is relevant |
|
| Outcomes |
1. Cure: as reported by participants and is the proportion of participants who reported 100% reduction in symptoms of incontinence (i.e. no incontinence at all) A: 10/13; B: 10/19; C: 17/19 2. Patient satisfaction with treatment outcome: defined as the proportion of participants who were completely satisfied with treatment outcome. Scale used for the assessment not stated A: 11/13; B: 6/19; C: 13/19 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The envelope method was used to randomise participants to treatment groups; not stated whether envelopes were sequentially numbered, opaque and sealed |
| Allocation concealment (selection bias) | Unclear risk | The envelope method was used to randomise participants to treatment groups; not stated whether envelopes were sequentially numbered, opaque and sealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants undergoing PFMT not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts: all: 10/61; A: 5/18, B: 1/20; C: 4/23 Differential loss to follow‐up: not fully reported (2 and 3 participants withdrew from groups A and C respectively due to adverse drug effects; other reasons for withdrawal not reported) |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol not available |
| Ethical approval | Low risk | Approved by the ethics committee |
| Source of funding or support | Unclear risk | Not stated |
| Conflict of interest | Unclear risk | Not stated |
Jeyaseelan 2002.
| Methods | 3‐arm randomised controlled trial, parallel design | |
| Participants | 16 women with stress incontinence | |
| Interventions |
A. Electrical stimulation (ES) group (N = 6): participants in this group used electrical stimulator for 1 hour a day every day (except when menstruating) B. Pelvic floor muscle training (PFMT) alone (N = 7): PFMT consisted of individualised exercise regimen with instruction to the participants to carry out a minimum of 3 exercises per day with progression over the treatment period. Biofeedback was provided by means of a Periform probe. Other details not given C. PFMT + ES (combined) (N = 6). Participants in this group received both PFMT and ES as described above For this review comparison C versus A is relevant |
|
| Outcomes |
1. Severity of incontinence assessed using 24‐hour pad test and 3‐day voiding diary 2. Condition‐specific quality of life assessed using Incontinence Impact Questionnaire (IIQ) and Urogenital Distress Inventory (UDI). |
|
| Notes | No useable data were reported in the trial (data reported in median and range) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible. Assumed not done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not explicitly reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Ethical approval | Unclear risk | Not reported |
| Source of funding or support | Unclear risk | Not reported |
| Conflict of interest | Unclear risk | Not reported |
Jin 2012.
| Methods | 3‐arm randomised controlled trial, parallel design | |
| Participants | 242 women with urodynamic evidence of over‐active bladder | |
| Interventions |
A. Drug alone (N = 82). Participants in this group received oral solifenacin 5 mg once daily B. PFMT alone (N = 80). Participants in this group performed PFMT once daily; other details were not given C. PFMT + drug (N = 80). Participants in this group received both PFMT and drug as stated above For this review comparison C versus A is relevant |
|
| Outcomes |
1. Frequency of micturition per 24 hours (no useable data) 2. Number of episodes of over‐active bladder in 24 hours (no useable data) 3. Volume of urine voided per micturition in 24 hour (no useable data) 4. Adverse events: proportion (%) of participants who reported adverse events (mainly dry mouth) with solifenacin A: 17/82; B: 0/80; C: 14/80 |
|
| Notes | Dropouts: not reported All participants randomised at baseline included in analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible for PFMT |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol not available |
| Ethical approval | Low risk | "ethics not required" according to the authors Trial was conducted in accordance with Helsinki declaration Informed consent was obtained from participants |
| Source of funding or support | Low risk | No funding source according to the authors |
| Conflict of interest | Unclear risk | Not stated |
Kaya 2015.
| Methods | 2‐arm parallel RCT | |
| Participants | 132 women with SUI, UUI and MUI from 2 centres in Turkey | |
| Interventions |
A. PFMT + bladder training (N = 67): Participants in this group completed a progressive home‐based exercise program consisting of strength and endurance training. They were taught both fast (2‐s) and slow voluntary PFM contractions (VPFMCs). One slow contraction took 15 s (5‐s contraction, 5‐s hold, 5‐s relaxation). One set of exercises involved ten fast and ten slow VPFMCs. During week 1, participants were instructed to perform five sets of exercises per day (5×10 fast and 10 slow = 50 fast and 50 slow VPFMCs daily), which was progressively increased by five sets/week: ten sets per day at week 2; 15 at week 3; 20 at week 4; 25 at week 5, and 30 at week 6 [600 VPFMCs daily (300 fast and 300 slow)]. Patients were advised to exercise while in the supine, seated, and upright positions and to integrate these exercises into their daily activities, e.g., while watching television, waiting for something, travelling. B. Bladder training (N = 65) Based on the three frequency‐volume charts obtained at baseline, the longest voiding interval achieved several times was deemed the initial voiding interval. During week 1, participants were encouraged to hold urine for 30 min beyond the initial voiding interval. Then, the schedule was increased by 15 min per week depending on the patient’s tolerance to the schedule. Urgency suppression strategies, including distraction, relaxation, and PFM contraction, were explained to each participant. Techniques to control urgency were: (1) Deep and slow breathing (2) Contracting PFMs while relaxing other body parts (3) Using mental imagery or self‐motivational statements, such as “I can wait” and “I can take control” (4) Incorporating mental distractions, such as mathematical calculation |
|
| Outcomes |
1. Global rating of improvement A four‐point scale (worse, unchanged, improved, cured) was used to determine participants’ global perception of UI improvement at the end of the intervention period compared with baseline. Improvement was defined as the proportion of women 'cured' or 'improved 'At 6 weeks A. 56/56; B. 43/52 2. Frequency and volume of incontinence (no usable data) 3. Symptom distress and quality of life (no usable data) 4. Incontinent episodes (No../day) (no usable data) 5. Micturition frequency (No./day) (no usable data) |
|
| Notes | 6‐week treatment protocol was implemented for both groups by an experienced physical therapist over four visits (baseline and weeks 2, 4, and 6 of the program) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A stratified block randomization procedure was used to assign blocks of four participants to either treatment arm using opaque and sealed envelopes that contained a group allocation number from a computer generated random‐number table.” |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed using sealed and opaque envelope |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind participants and personnel to PFMT |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportions of withdrawals and reasons for withdrawals differ between the 2 groups and data analysis was not based on ITT |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Ethical approval | Low risk | Ethics approval was said to be obtained |
| Source of funding or support | Low risk | Source of funding was declared |
| Conflict of interest | Low risk | It was reported that there was no known conflict of interest |
Kim 2011.
| Methods | 4‐arm randomised controlled trial | |
| Participants | 147 women with stress, urge or mixed UI from a single centre in Tokyo | |
| Interventions |
A. General education (GE) group (N = 36): general education classes were held (topics including cognitive function, osteoporosis and oral hygiene) once a month, a total of 3 times B. Heat and steam generating sheet (HSGS) group (N = 37): participants in this group received HSGS, a thin, flexible, filmed sheet that generated heat and steam. When placed on the skin surface. It raises the temperature to 38 to 40°C by generating heat and steam continuously for up to 5 hours. Participants were asked to place the HSGS on their lower back once daily immediately after waking period, taking note of the time they started and ended C. Exercise (Ex) group (N = 37): this group received stretching exercise, fitness exercise and PFM exercise. Participants were initially instructed to perform 10 fast contractions (3 seconds) with a 5‐second rest and 10 sustained contractions (8 to 10 seconds) with a 10‐second rest between the contractions D. Ex + HSGS group (N = 37): participants in this group received both exercise and HSGS as described above For this review comparison D versus B is relevant |
|
| Outcomes | Cure of urine loss episodes (assessed by interview, with cure defined as the proportion of participants with complete cessation of urine loss episodes) At 3 months: A: 1/34; B: 8/37; C: 12/35; D: 19/37 |
|
| Notes | Changes in frequency of urine loss episodes: assessed based on changes on a 5‐point scale obtained in the interviews conducted at baseline (before treatment) and at 3 months after treatment. Data not available, only graphical presentation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used "computer‐generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Used "computer generated random numbers", however, no further information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of the participants not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/147 dropped out of the trial. However, there were no dropouts in the comparison of interest and there was no differential loss to follow‐up in the other group |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Ethical approval | Unclear risk | Not stated |
| Source of funding or support | Unclear risk | Not stated |
| Conflict of interest | Low risk | Declared (no conflict of interest) |
Richter 2010.
| Methods | 3‐arm randomised controlled trial, parallel design | |
| Participants | 446 women with symptoms of stress urinary incontinence | |
| Interventions |
A. Continence pessary alone group (N = 149). Individuals in this group were fitted with a continence ring or dish either by a physician or a nurse. Most participants were fitted successfully in 1 clinic visit while up to 3 visits at 1 to 2‐week intervals were allowed for others to achieve optimal fitting. At the end of the 8‐week treatment period, participants were encouraged to continue to use the pessary B. Behavioural therapy (PFMT + continence strategies) (N = 146). Intervention in this group consisted of pelvic floor muscle training (PFMT) and exercise and additional skills and strategies on the use of muscles to prevent urgency and stress incontinence. Treatment was administered by registered nurses, nurse practitioners and physical therapists and was implemented in 4 visits at 2‐week intervals. During each visit, participants received instructions on PFMT and exercise and also acquired additional skills and strategies on stress urge incontinence prevention. They were then given individualised prescriptions for daily PFM exercise and practice. At the end of the 8‐week treatment period, participants received an individualised home maintenance programme to enable them sustain their skills and muscle strength C. Continence pessary + behavioural therapy (combined) (N = 150). Treatment regimen was as described for both pessary and behavioural therapy groups. In addition, participants in this group could continue in the trial with only 1 of the therapies at the end of the 8‐week treatment period For this review comparison C versus A is relevant |
|
| Outcomes |
1. The patient global impression of improvement (PGI‐I) was assessed for the 3 groups using a validated PGI‐I questionnaire with success defined as the proportion of participants with a response of 'much better' or 'very much better' At 3 months: A: 59/110; B: 72/124; C: 80/132 At 6 months: A: 52/102; B: 59/116; C: 63/123 At 12 months: A: 47/96; B: 48/99; C: 49/111 2. Condition‐specific quality of life (in form of the Pelvic Floor Distress inventory): this was assessed using the Urogenital Distress Inventory ‐ stress incontinence sub‐scale with success defined as the proportion of participants with absence of bothersome stress incontinence symptoms (indicated by an answer of 'no' to all 6 items on the sub‐scale or a response of 'yes' but with a bother of 'not at all' or 'somewhat' At 3 months: A: 49/110; B: 71/124; C: 66/132 At 6 months: data not reported At 12 months: A: 52/96; B: 59/99; C: 49/111 3. Frequency of incontinence episodes per week (self reported improvement) assessed by using the 7‐day diary with success defined as the proportion of women with 75% or more reduction in frequency of incontinence episodes At 3 months: A: 69/110; B: 68/124; C: 80/132 At 6 months: data not reported At 12 months: A: 51/96; B: 54/99; C: 52/111 4. Patient satisfaction with treatment: this was assessed using the validated Patient Satisfaction Questionnaire At 3 months: A: 94/110; B: 110/124; C: 118/132 At 6 months: A: 87/102; B: 95/116; C: 104/123 At 12 months: A: 75/96; B: 79/99; C: 81/111 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block randomisation schedule was used |
| Allocation concealment (selection bias) | Low risk | Allocation contained in sealed envelopes, opened by the interventionist only after the participants met all the inclusion/exclusion criteria |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible especially for PFMT |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcome assessors were blinded to the treatment group assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts: at 3 months: all 79/445, C: 18/150, B: 22/146, A: 39/149; at 6 months: all 104/445, C: 27/150, B: 30/146, A: 47/149; at 12 months: all: 139/445; C: 39/150; B: 47/146; A: 53/149 "After randomization, dropout patterns differed among the three treatment groups (P = 0.015) with the pessary only group having the highest attrition rate ..." |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol not available |
| Ethical approval | Low risk | Approved by the ethics committee |
| Source of funding or support | Low risk | Disclosed, funded by "Eunice Kennedy Shriver" |
| Conflict of interest | Unclear risk | Declared some of the authors were associated with a major pharmaceutical company |
Wise 1993.
| Methods | 3‐arm randomised controlled trial, parallel design | |
| Participants | 62 women with urodynamically proven genuine stress, urinary incontinence (GSI) | |
| Interventions |
A. Maximal electrical stimulation alone (N = 20). Participants in this group received a battery‐powered vaginal stimulator (impulse frequency: 20 Hz; duration: 0.75 ms; current intensity: 0 to 90 mA) at home daily for 20 minutes B. Vaginal cones alone (N = 21). Participants in this group were instructed to use cones twice daily for 15 minutes and to increase the weight of the cones when successful on 2 occasions. They did not undergo vaginal examination. It was not reported whether participants were instructed to contract PFMs in order to hold the cones C. Kegel exercise + vaginal cones (N = 21). Participants in this group received vaginal cones as stated above. In addition, they were taught by vaginal examination to voluntarily contract their pelvic floor muscles and carried out 10 sessions of 10 contractions daily. No further details were reported For this review comparison C versus B is relevant |
|
| Outcomes |
1. Improvement: threshold not defined, unclear whether self reported, detailed data not reported, only the level of significance was given for each treatment group 2. Reduction in urine leakage: this was assessed objectively (using pad testing); success threshold was not defined, details of data not reported, only P values were given 3. Decrease in pad weight: only the P values were reported, other details not given 4. Improvement on pad testing: objective assessment of improvement using pad testing; only proportions of participants were reported, success threshold was not defined A: 12/16; B: 14/19; C: 14/15 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible, especially for PFMT |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts: all 12/62; C: 6/21, B: 2/21; A: 4/20 There is differential loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol not available |
| Ethical approval | Unclear risk | Not stated |
| Source of funding or support | Unclear risk | Not disclosed |
| Conflict of interest | Unclear risk | Not disclosed |
Wyman 1998.
| Methods | 3‐arm randomised controlled trial, parallel design | |
| Participants | 204 women with urodynamic evidence of stress urinary incontinence (GSI), detrusor instability (DI) or both (mixed incontinence). | |
| Interventions |
A. Bladder training (BT) group (N = 68): involved a progressive voiding schedule that was altered every week for the first 6 weeks of the programme but remained unchanged for the last 6 weeks. The voiding interval was initially set at 30 or 60 minutes, depending on the baseline voiding diary and increased by 30 minutes each week if there was reduction in episodes of incontinence B. Pelvic floor muscle training (PFMT) alone (N = 69). PFMT was also structured and it consisted of an initial teaching session (which also included instructions on continence strategies) followed by a graded home exercise with audio cassette practice tapes and 4 office biofeedback sessions. In all, 10 fast (3‐second) contractions and 40 sustained (10‐second) contractions (a total of 50 contractions) with 10‐second rest periods between contractions were performed daily by the third week. Patients received 4 weekly 30‐minute sessions of visual and verbal biofeedback. Visual biofeedback was provided via a strip‐chart recorder demonstrating vaginal and abdominal pressures as measured by vaginal balloons C. PFMT + BT (combined) (N = 67). Treatment regimen was as described for the BT and PFMT groups. BT was implemented initially while PFMT was added during the third week, including instructions on continence strategies (urge inhibition and preventive contractions) For this review comparison C versus A is relevant |
|
| Outcomes |
1. Incontinence episodes per week (mean (SD)): this was assessed at endpoint using the records in a standardised diary Immediately after treatment: A: 10.6 (16.3), N = 68; B: 9.6 (10.8), N = 64; C: 6.8 (10.7), N = 61 3 months after treatment: data not reported 2. Cure rates: cure was defined as the proportion of participants who had 100% reduction in incontinence episodes, assessed using the standardised diary Immediately after treatment: A: 12/67; B: 8/62; C: 19/61 3 months after treatment: A: 10/63; B: 13/65; C: 16/59 3. Improvement rates: improvement was defined as the proportion of participants who had 50% or greater reduction in incontinence episodes, assessed using the standardised diary Immediately after treatment: A: 35/67; B: 36/63; C: 43/61 3 months after treatment: A: 28/61; B: 36/64; C: 35/59 4. Patient perceived improvement: instrument used in assessment not stated, success threshold was not defined but will be taken as the proportion of participants who were 'much better' or 'somewhat better' for the purpose of this review Immediately after treatment: A: 43/66; B: 48/63; C: 55/61 3 months after treatment: A: 37/60; B: 45/64; C: 44/59 5. Patient satisfaction with treatment outcome: instrument used in assessment not stated, success threshold was not defined but will be taken as the proportion of participants who were 'very satisfied' or 'slightly satisfied' with treatment outcome for the purpose of this review Immediately after treatment: A: 48/66; B: 56/63; C: 57/61 3 months after treatment: A: 47/60; B: 53/64; C: 51/58 6. Condition‐specific quality of life assessed at endpoint using: i. Urogenital Distress Inventory (UDI); reported as mean (SD): Immediately after treatment: A: 95.5 (54.4), N = 67; B: 90.8 (52.0), N = 63; C: 64.4 (48.6), N = 61 3 months after treatment: A: 91.7 (55.0), N = 60; B: 85.0 (52.4), N = 64; C: 72.8 (50.4), N = 58 ii. Incontinence Impact Questionnaire‐Revised (IIQ‐R); reported as mean (SD): Immediately after treatment: A: 72.1 (75.2), N = 66; B: 56.8 (61.4), N = 63; C: 46.6 (65.3), N = 61 3 months after treatment: A: 65.7 (80.2), N = 60; B: 59.3 (67.7), N = 64; C: 59.8 (83.9), N = 58 7. Treatment adherence: this was defined as the proportion of participants adhering to the voiding schedule; assessed using treatment logs or standardised questionnaire (no useable data were: only percentages, without the actual proportions, were reported) 8. Number of women requiring further treatment (relapse): women were followed up for a mean time of 3.2 years and the overall number of women requiring additional treatment such as surgery, drug, etc. was determined for each treatment group A: 19/48; B: 29/52: C: 18/48 |
|
| Notes | Dropouts in each treatment group were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible especially to PFMT |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts: immediately after treatment 9/204; 3 months after treatment 16/204 Differential loss to follow‐up: not reported |
| Selective reporting (reporting bias) | High risk | According to the authors, one pre‐specified outcome (pad weight) was eventually not reported due to large number of missing data |
| Ethical approval | Low risk | Approved by the ethics committee |
| Source of funding or support | Low risk | Disclosed (public institutions) |
| Conflict of interest | Unclear risk | Not stated |
BT: bladder training DT: drug therapy ES: electrical stimulation Ex: exercise GE: general education HSGS: heat and steam generating sheet IEF: incontinence episode frequency PFM: pelvic floor muscle PFMT: pelvic floor muscle training SD: standard deviation UI: urinary incontinence
SUI˜: Stress Urinary Incontinence
UUI: Urgency Urinary Incontinence
MUI: Mixed Urinary Incontinence
Hz: Hertz
mA: milliampere
µg: microgram
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alewijnse 2003 | Intervention not relevant |
| Aslan 2008 | Intervention not relevant |
| Barber 2008 | Participants and intervention not relevant |
| Bawden 1992 | Intervention not relevant |
| BE‐DRI 2008 | Intervention not relevant |
| Berghmans 2000 | Intervention not relevant |
| Berghmans 2000a | Intervention not relevant |
| Berghmans 2001a | Intervention not relevant |
| Berghmans 2002 | Intervention not relevant |
| Beuttenmuller 2010 | Intervention not relevant |
| Bidmead 2002 | Intervention not relevant |
| Bo 2002 | Intervention not relevant |
| Bo 2012 | Systematic review |
| Burgio 1998 | Intervention not relevant |
| Burgio 2001a | Intervention not relevant |
| Burgio 2007 | Intervention not relevant |
| Cammu 1996 | Intervention not relevant |
| Capobianco 2012 | Participants not relevant |
| Chancellor 2008 | Intervention not relevant |
| Crothers 2003 | Intervention not relevant |
| de Jong 2006 | Intervention not relevant |
| Dowell 1997 | Design not relevant |
| Driusso 2008 | Intervention not relevant |
| Dumoulin 2011 | Intervention not relevant |
| Firra 2013 | Intervention not relevant |
| Fonda 1995 | Intervention not relevant |
| Goode 2003 | Intervention not relevant |
| Goode 2011a | Post‐prostatectomy patients |
| Greer 2012 | Systematic review |
| Gronwald 2010 | Intervention not relevant |
| Gunthorpe 1994 | Intervention not relevant |
| Ha 2008 | Intervention not relevant |
| Hahn 1991 | Intervention not relevant |
| Haken 1991 | Intervention not relevant |
| Henalla 1989 | Intervention not relevant |
| Herschorn 2004 | Intervention not relevant |
| Huang 2006 | Design not relevant |
| Huang 2012 | Intervention not relevant |
| Kafri 2007 | Intervention not relevant |
| Kangchai 2002 | The study is about the efficacy of a self management promotion programme and the participants were not relevant |
| Kaya 2011 | Intervention not relevant |
| Kim 2001 | Intervention not relevant |
| Kim 2006 | Design not relevant |
| Kim 2007 | Intervention not relevant |
| Kim 2009 | Intervention not relevant |
| Kincade 2007 | Intervention not relevant |
| Kirschner‐Hermanns 1995 | Intervention not relevant |
| Kobayashi 2009 | Intervention not relevant |
| Lagro‐Janssen 1991 | Intervention not relevant |
| Laycock 1988 | Intervention not relevant |
| Laycock 1993 | Intervention not relevant |
| Laycock 1995 | Intervention not relevant |
| Laycock 2001 | Intervention not relevant |
| Lee 2005 | Intervention not relevant |
| Madersbacher 2003 | Intervention not relevant |
| Madersbacher 2004 | Intervention not relevant; recruited both men and women with no separate data for women |
| Maher 2009 | Intervention not relevant |
| McCormack 2004 | Design not relevant |
| Millard 2003 | Participants were provided with a leaflet and were not under a structured PFMT programme and included both men and women (no separate data for women) |
| Millard 2003a | Participants were provided with a leaflet and were not under a structured PFMT programme and included both men and women (no separate data for women) |
| Millard 2003b | Participants were provided with a leaflet; were not under a structured PFMT programme; included both men and women (no separate data for women) |
| Millard 2004 | Participants were provided with a leaflet and were not under a structured PFMT programme; included both men and women (no separate data for women) |
| Mørkved 2002 | Intervention not relevant |
| O'Brien 1996 | Intervention not relevant |
| Oldham 2010 | Intervention not relevant |
| PRIDE 2004 | Intervention not relevant |
| Rutledge 2012 | Intervention not relevant |
| Sampselle 2003 | Design not relevant |
| Sanchez 2008 | Intervention not relevant |
| Savage 2005 | Design not relevant |
| Scott 1979 | Randomisation was not done for intervention (incontinence versus no incontinence) |
| Smith 1994 | Intervention not relevant |
| Sran 2011 | Intervention not relevant |
| Sultana 2014 | Intervention not relevant |
| Suzuki 2003 | Intervention not relevant |
| Tapp 1987 | Intervention not relevant |
| Terry 1996 | Intervention not relevant |
| Van Hespen 2006 | Participants and intervention not relevant |
| Viereck 2011 | Intervention not relevant |
| Voigt 1996 | Intervention not relevant |
| von der Heide 2003 | Intervention not relevant |
| Waterfield 2007 | Participant and intervention not relevant |
| Wells 1999 | Intervention not relevant |
| Wilson 1984 | Intervention not relevant |
| Yamanishi 2006 | Intervention not relevant |
| Yoon 1999 | Intervention not relevant |
| Zhao 2000 | Intervention not relevant |
PFMT: pelvic floor muscle training
Differences between protocol and review
For this first update of the review (2015) ClinicalTrials.gov and WHO ICTRP were not searched separately as searches of these two databases are now incorporated into the search for the Cochrane Incontinence Group Specialised Register. Embase and Embase Classic were not searched specifically for this version of the review as the Cochrane Collaboration has now run centralised searches of these databases for randomised controlled trials and incorporated them into CENTRAL which is searched for the Cochrane Incontinence Group Specialised Register.
Contributions of authors
For 2015 update of the review Reuben Olugbenga Ayeleke (ROA), and Muhammad Imran Omar (MIO) performed abstract screening, full‐text screening, data extraction, 'Risk of bias' assessment and quality of evidence assessment. All review authors contributed in the analysis of data and writing the manuscript of the review.
Reuben Olugbenga Ayeleke (ROA), E. Jean C Hay‐Smith (JHS) and Muhammad Imran Omar (MIO) were responsible for the conception and writing of the protocol . ROA and MIO performed abstract screening, full‐text screening, data extraction, 'Risk of bias' assessment and quality of evidence assessment. All review authors contributed in the analysis of data and writing the manuscript of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
NIHR, UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to the Incontinence Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Reuben Olugbenga Ayeleke: None known
E. Jean C Hay‐Smith (JHS): None known
Muhammad Imran Omar: None known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bezerra 2009 {published data only}
- Bezerra CA, Wroclawski ER, Rodrigues AO, Tristao R, Borrelli M. Randomized clinical trial do not show that PFMT plus vaginal electrical stimulation is better than electrical stimulation alone for SUI in women (Abstract number 1561). Journal of Urology 2009;181(4 Suppl):561. [sr‐incont62404] [Google Scholar]
- Furst MC, Mendonca RR, Rodrigues AO, Matos LL, Pompeo AC, Bezerra CA. Long‐term results of a clinical trial comparing isolated vaginal stimulation with combined treatment for women with stress incontinence. Einstein 2014;12(2):168‐74. [sr‐incont62563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burgio 2010a {published data only}
- Burgio KL, Goode PS, Richter HE, Markland AD, Johnson TM, Redden DT. Combined behavioral and individualized drug therapy versus individualized drug therapy alone for urge urinary incontinence in women. Journal of Urology 2010;184(2):598‐603. [DOI] [PubMed] [Google Scholar]
Chen 2008 {published data only}
- Chen S, Tsai C, Tong Y, Chen C. Does pelvic floor muscle exercises adding on effects on overactive bladder patients with unsatisfactory drug responses? (Abstract number 183). Proceedings of the 38th Annual Meeting of the International Continence Society (ICS), 2008 Oct 20‐24, Cairo, Egypt. 2008.
Ghoniem 2005 {published data only}
- Eli Lilly, Company. Efficacy and safety of duloxetine compared with placebo, pelvic floor muscle training, and combined duloxetine/pelvic floor muscle training in subjects with moderate to severe stress urinary incontinence. CT Registry ID#2615. Study F1J‐MC‐SBAF. Eli Lilly Clinical Trial Registry 2008 (accessed 4 June 2008).
- Freeman R. Initial management of stress urinary incontinence: pelvic floor muscle training and duloxetine. BJOG: an International Journal of Obstetrics & Gynaecology 2006;113:10‐6. [DOI] [PubMed] [Google Scholar]
- Ghoniem GM, Leeuwen JS, Elser DM, Freeman RM, Zhao YD, Yalcin I, et al. A randomized controlled trial of duloxetine alone, pelvic floor muscle training alone, combined treatment and no active treatment in women with stress urinary incontinence. Journal of Urology 2005;173(5):1647‐53. [DOI] [PubMed] [Google Scholar]
- Schagen van Leeuwen JH, Elser D, Freeman R, Ghoniem G, Zhao Y, Yalcin I, et al. Controlled trial of duloxetine alone, pelvic floor muscle training alone, combined treatment, and no treatment in women with stress urinary incontinence (SUI) (Abstract). European Urology Supplements 2004;3(2):52. [DOI] [PubMed] [Google Scholar]
Hofbauer 1990 {published data only}
- Hofbauer J, Preisinger F, Nurnberger N. The value of physical therapy in genuine female stress incontinence. [German]. Zeitschrift Fur Urologie Und Nephrologie 1990;83(5):249‐54. [PubMed] [Google Scholar]
- Preisinger E, Hofbauer J, Nurnberger N, Sadil S, Schneider B. Possibilities of physiotherapy for urinary stress incontinence. Zeitschrift Physische Medizin, Balneologische Medizin Klimatologie 1990;19(2):75‐9. [Google Scholar]
Ishiko 2000 {published data only}
- Ishiko O, Ushiroyama T, Saji F, Mitsuhashi Y, Tamura T, Yamamoto K, et al. Beta(2)‐adrenergic agonists and pelvic floor exercises for female stress incontinence. International Journal of Gynaecology & Obstetrics 2000;71(1):39‐44. [DOI] [PubMed] [Google Scholar]
Jeyaseelan 2002 {published data only}
- Jeyaseelan S, Haslam J, Oldham J. Can the effects of pelvic floor muscle exercises be enhanced with a new pattern of electrical stimulation in women with stress incontinence? Pilot data (Abstract number 135). Proceedings of the International Continence Society (ICS), 32nd Annual Meeting, 2002 Aug 28‐30, Heidelberg, Germany. 2002:66‐7.
- Jeyaseelan S, Oldham JA. Can the effects of pelvic floor muscle exercises be enhanced with a new pattern of electrical stimulation in women with stress incontinence (Abstract). Proceedings of the World Confederation for Physical Therapy (WCPT), 14th International Congress, 2003 June 7‐12 , Barcelona. 2003.
Jin 2012 {published data only}
- Jin Y, Song E, Lv L, Li D. The therapeutic effects of orally solifenacin combined with pelvic floor muscle exercises in treatment of female overactive bladder (Abstract number 67). Neurourology and Urodynamics 2012;31(6):813. [Google Scholar]
Kaya 2015 {published data only}
- Kaya S, Akbayrak T, Gursen C, Beksac S. Pelvic floor muscle training added to bladder training versus bladder training alone for female urinary incontinence: A randomized controlled trial (Abstract number 401). Neurourology and Urodynamics 2014;33(6):864‐6. [sr‐incont64341] [Google Scholar]
- Kaya S, Akbayrak T, Gursen C, Beksac S. Short‐term effect of adding pelvic floor muscle training to bladder training for female urinary incontinence: a randomized controlled trial. International Urogynecology Journal 2015;26(2):285‐93. [sr‐incont66433] [DOI] [PubMed] [Google Scholar]
Kim 2011 {published data only}
- Kim H, Yoshida H, Suzuki T. Effects of exercise treatment with or without heat and steam generating sheet on urine loss in community‐dwelling Japanese elderly women with urinary incontinence. Geriatrics & Gerontology International 2011;11(4):452‐9. [DOI] [PubMed] [Google Scholar]
Richter 2010 {published data only}
- Barber MD, Spino C, Janz NK, Brubaker L, Nygaard I, Nager CW, et al. The minimum important differences for the urinary scales of the Pelvic Floor Distress Inventory and Pelvic Floor Impact Questionnaire. American Journal of Obstetrics & Gynecology 2009;200(5):580.e1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley CS, Rahn DD, Nygaard IE, Barber MD, Nager CW, Kenton KS, et al. The questionnaire for urinary incontinence diagnosis (QUID): validity and responsiveness to change in women undergoing non‐surgical therapies for treatment of stress predominant urinary incontinence. Neurourology & Urodynamics 2010;29(5):727‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenton K, Barber M, Wang L, Hsu Y, Rahn D, Whitcomb E, et al. Pelvic floor symptoms improve similarly after pessary and behavioral treatment for stress incontinence. Female Pelvic Medicine & Reconstructive Surgery 2012;18(2):118‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nager CW, Richter HE, Nygaard I, Paraiso MF, Wu JM, Kenton K, et al. Incontinence pessaries: size, POPQ measures, and successful fitting. International Urogynecology Journal 2009;20(9):1023‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter HE. A randomized trial of pessary vs. behavioral therapy vs. combined therapy for treatment of stress urinary incontinence (Abstract number 195). Neurourology & Urodynamics 2009;28(7):816‐7. [Google Scholar]
- Richter HE, Burgio KL, Brubaker L, Nygaard IE, Ye W, Weidner A, et al. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstetrics & Gynecology 2010;115(3):609‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter HE, Burgio KL, Goode PS, Borello‐France D, Bradley CS, Brubaker L, et al. Non‐surgical management of stress urinary incontinence: ambulatory treatments for leakage associated with stress (ATLAS) trial. Clinical Trials 2007;4:92‐101. [DOI] [PubMed] [Google Scholar]
- Schaffer J, Nager CW, Xiang F, Borello‐France D, Bradley CS, Wu JM, et al. Predictors of success and satisfaction of nonsurgical therapy for stress urinary incontinence. Obstetrics & Gynecology 2012;120(1):91‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wise 1993 {published data only}
- Wise BG, Haken J, Cardozo LD, Plevnik S. A comparative study of vaginal cone therapy, cones + Kegel exercises, and maximal electrical stimulation in the treatment of female genuine stress incontinence (Abstract number 76). Neurourology & Urodynamics 1993;12(4):436‐7. [Google Scholar]
Wyman 1998 {published data only}
- Barber MD, Visco AG, Wyman JF, Fantl JA, Bump RC, Continence Program for Women Research Group. Sexual function in women with urinary incontinence and pelvic organ prolapse. Obstetrics & Gynecology 2002;99(2):281‐9. [DOI] [PubMed] [Google Scholar]
- Elser DM, Fantl JA, McClish DK. Comparison of "subjective" and "objective" measures of severity of urinary incontinence in women. Program for Women Research Group. Neurourology & Urodynamics 1995;14(4):311‐6. [DOI] [PubMed] [Google Scholar]
- Elser DM, Wyman JF, McClish DK, Robinson D, Fantl JA, Bump RC. The effect of bladder training, pelvic floor muscle training, or combination training on urodynamic parameters in women with urinary incontinence. Continence Program for Women Research Group. Neurourology & Urodynamics 1999;18(5):427‐36. [DOI] [PubMed] [Google Scholar]
- Sale PG, Wyman JF. Achievement of goals associated with bladder training by older incontinent women. Applied Nursing Research 1994;7(2):93‐6. [DOI] [PubMed] [Google Scholar]
- Theofrastous JP, Wyman JF, Bump RC, McClish DK, Elser DM, Bland DR, et al. Effects of pelvic floor muscle training on strength and predictors of response in the treatment of urinary incontinence. Neurourology & Urodynamics 2002;21(5):486‐90. [DOI] [PubMed] [Google Scholar]
- Wyman JF, Fantl JA, McClish DK, Bump RC. Comparative efficacy of behavioral interventions in the management of female urinary incontinence. American Journal of Obstetrics & Gynecology 1998;179(4):999‐1007. [DOI] [PubMed] [Google Scholar]
- Wyman JF, McClish DK, Sale P, Earle B, Camp J. Long‐term follow‐up of behavioral interventions in incontinent women (Abstract). International Urogynecology Journal and Pelvic Floor Dysfunction 1999;10(Suppl 1):S33. [Google Scholar]
References to studies excluded from this review
Alewijnse 2003 {published data only}
- Alewijnse D, Metsemakers JF, Mesters IE, Borne B. Effectiveness of pelvic floor muscle exercise therapy supplemented with a health education program to promote long‐term adherence among women with urinary incontinence. Neurourology & Urodynamics 2003;22(4):284‐95. [DOI] [PubMed] [Google Scholar]
Aslan 2008 {published data only}
- Aslan E, Komurcu N, Beji NK, Yalcin O. Bladder training and Kegel exercises for women with urinary complaints living in a rest home. Gerontology 2008;54(4):224‐31. [DOI] [PubMed] [Google Scholar]
Barber 2008 {published data only}
- Barber M, Richter H, Menefee SA, Nager CW, Brubaker L, Visco A, et al. Operations and pelvic muscle training in the management of apical support loss: The OPTIMAL Trial. ControlledTrials.com (www.controlled‐trials.com) 2008 (accessed 22 May 2008).
Bawden 1992 {published data only}
- Bawden ME, Kartha AS, Borrie MJ, Kerr PS, Durko NA, Haslam IF, et al. Treating women with stress incontinence in a multidisciplinary clinic: a randomized study (Abstract number 276). Proceedings of the International Continence Society (ICS), 22nd Annual Meeting, 1992 Sep 1‐4, Halifax, UK. 1992.
BE‐DRI 2008 {published data only}
- Borello‐France D, Burgio KL, Goode PS, Markland AD, Kenton K, Balasubramanyam A, et al. Adherence to behavioral interventions for urge incontinence when combined with drug therapy: adherence rates, barriers, and predictors. Physical Therapy 2010;90(10):1493‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgio KL, Kraus SR, Borello‐France D, Chai TC, Kenton K, Goode PS, et al. The effects of drug and behavior therapy on urgency and voiding frequency. International Urogynecology Journal 2010;21(6):711‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgio KL, Kraus SR, Menefee S, Borello‐France D, Corton M, Johnson HW, et al. Behavioral therapy to enable women with urge incontinence to discontinue drug treatment: a randomized trial. Annals of Internal Medicine 2008;149(3):161‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald MP, Lemack G, Wheeler T, Litman HJ, for the Urinary Incontinence Treatment Network (UITN). Nocturia, nocturnal incontinence prevalence, and response to anticholinergic and behavioral therapy. International Urogynecology Journal 2008;19(11):1545‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode PS, Burgio KL, Kraus SR, Kenton K, Litman HJ, Richter HE, et al. Correlates and predictors of patient satisfaction with drug therapy and combined drug therapy and behavioral training for urgency urinary incontinence in women. International Urogynecology Journal 2011;22(3):327‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus SR, for the Urinary Incontinence Treatment Network (UITN). Combining behavior and drug therapy to improve drug withdrawal in the treatment of urge incontinence: a randomized controlled trial (Abstract number 8 Oral). Journal of Pelvic Medicine & Surgery 2007;13(5):233‐4. [Google Scholar]
- Steers WD, Urinary Incontinence Treatment Network (UITN). Behavior enhances drug reduction of incontinence (BE‐DRI). www.ClinicalTrials.gov 2004.
Berghmans 2000 {published data only}
- Berghmans LC, Waalwijk van Doorn ES, Nieman FH, Bie RA, Kerrebroeck PE, Brandt P. Efficacy of physiotherapy in women with proven overactive bladder (Abstract number FDP34). International Urogynecology Journal 2000;11(Suppl 1):S45. [Google Scholar]
Berghmans 2000a {published data only}
- Berghmans LCM, Nieman FHM, Waalwijk van Doorn ESC, Smeets LWH, Haaf WMM, Bie RA, et al. Effects of physiotherapy, using the adapted Dutch 1‐QOL in women with urge incontinence (Abstract). International Urogynecology Journal 2001;12(Suppl 3):S40. [Google Scholar]
- Berghmans LCM, Waalwijk van Doorn ESC, Nieman FHM, Bie RA, Smeets LWH, Haaf H, et al. Efficacy of extramural physical therapy modalities in women with proven bladder overactivity: a randomized clinical trial (Abstract number 92). Neurourology & Urodynamics 2000;19(4):496‐7. [Google Scholar]
Berghmans 2001a {published data only}
- Berghmans LCM, Nieman FHM, Waalwijk van Doorn ESC, Smeets LWH, Haaf WMM, Bie RA, et al. Effects of physiotherapy, using the adapted Dutch 1‐QOL in women with urge incontinence (Abstract). Neurourology & Urodynamics 2001;20(4):509‐10. [Google Scholar]
Berghmans 2002 {published data only}
- Berghmans B, Waalwijk van Doorn ES, Nieman F, Bie R, Brandt P, Kerrebroeck P. Efficacy of physical therapeutic modalities in women with proven bladder overactivity. European Urology 2002;41(6):581‐7. [DOI] [PubMed] [Google Scholar]
Beuttenmuller 2010 {published data only}
- Beuttenmuller L, Cader SA, Macena RHM, Araujo NDS, Nunes EFC, Dantas EHM. Muscle contraction of the pelvic floor and quality of life of women with stress urinary incontinence who underwent kinesitherapy. Fizjoterapia 2010;18(1):35‐41. [Google Scholar]
Bidmead 2002 {published data only}
- Bidmead J, Mantle J, Cardozo L, Hextall A, Boos K. Home electrical stimulation in addition to conventional pelvic floor exercises: A useful adjunct or expensive distraction? (Abstract number 68). Neurourology & Urodynamics 2002;21(4):372‐3. [Google Scholar]
- Parsons M, Mantle J, Cardozo L, Hextall A, Boos K, Bidmead J. A single blind, randomised, controlled trial of pelvic floor muscle training with home electrical stimulation in the treatment of urodynamic stress incontinence [abstract number 296]. Proceedings of the Joint Meeting of the International Continence Society (ICS) (34th Annual Meeting) and the International UroGynecological Association (IUGA), 2004 Aug 23‐27, Paris, France. 2004.
Bo 2002 {published data only}
- Bo K, Talseth T, Holme I. Single blind, randomised controlled trial of pelvic floor exercises, electrical stimulation, vaginal cones, and no treatment in management of genuine stress incontinence in women... reprinted with permission from BMJ Publishing Group. British Medical Journal. London: 1999; 318:487‐493. Copyright 1999, BMJ Publishing Group. Journal of the Section on Women's Health 2002;26(1):17‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bo 2012 {published data only}
- Bo K. Pelvic floor muscle training in treatment of female stress urinary incontinence, pelvic organ prolapse and sexual dysfunction. World Journal of Urology 2012;30(4):437‐43. [DOI] [PubMed] [Google Scholar]
Burgio 1998 {published data only}
- Burgio KL, Locher JL, Goode PS, Hardin JM, McDowell BJ, Candib D. Behavior vs drug therapy for urge incontinence in older women (Abstract 26). Proceedings of the American Urogynecology Society (AUGS), 15th Annual Meeting, 1994 Sep 21‐24, Toronto, Canada. 1994:48.
- Burgio KL, Locher JL, Goode PS, Hardin JM, McDowell BJ, Dombrowski M, et al. Behavioral vs drug treatment for urge urinary incontinence in older women. JAMA 1998;280(23):1995‐2000. [DOI] [PubMed] [Google Scholar]
- Burgio KL, Locher JL, Roth DL, Goode PS. Psychological improvements associated with behavioral and drug treatment of urge incontinence in older women. Journals of Gerontology Series B‐Psychological Sciences & Social Sciences 2001;56(1):46‐51. [DOI] [PubMed] [Google Scholar]
Burgio 2001a {published data only}
- Burgio KL, Goode PS, Locher JL, Umlauf MG, Varner RE, Lloyd LK, et al. The role of biofeedback in the treatment of urge incontinence in older women (Abstract number 19). Proceedings of the American Urogynecology Society (AUGS), 22nd Annual Meeting, 2001 Oct 25‐27, Chicago (IL) 2001.
Burgio 2007 {published data only}
- Burgio K, Urinary Incontinence Treatment Network. Combining behavior and drug therapy to improve drug withdrawal in the treatment of urge incontinence: a randomized trial (Abstract number 21). Neurourology & Urodynamics 2007;26(5):616‐7. [Google Scholar]
Cammu 1996 {published data only}
- Cammu H, Nylen M. Pelvic floor exercises (PFE) versus vaginal cones (VC) in the treatment of genuine stress incontinence (abstract number 225). Proceedings of the International Continence Society (ICS), 26th Annual Meeting, 1996 Aug 27‐30, Athens, Greece. 1996:223.
- Cammu H, Nylen M. Pelvic floor exercises versus vaginal weight cones in genuine stress incontinence. European Journal of Obstetrics, Gynecology, & Reproductive Biology 1998;77(1):89‐93. [DOI] [PubMed] [Google Scholar]
Capobianco 2012 {published data only}
- Capobianco G, Donolo E, Borghero G, Dessole F, Cherchi PL, Dessole S. Effects of intravaginal estriol and pelvic floor rehabilitation on urogenital aging in postmenopausal women. Archives of Gynecology & Obstetrics 2012;285(2):397‐403. [DOI] [PubMed] [Google Scholar]
Chancellor 2008 {published data only}
- Chancellor MB, Kianifard F, Beamer E, Mongay L, Ebinger U, Hicks G, et al. A comparison of the efficacy of darifenacin alone vs. darifenacin plus a Behavioural Modification Programme upon the symptoms of overactive bladder. International Journal of Clinical Practice 2008;62(4):606‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crothers 2003 {published data only}
- Crothers E. A randomised controlled clinical trial of a cueing device to enhance patient compliance to a programme of pelvic floor exercises. National Research Register 2003, Issue 1.
de Jong 2006 {published data only}
- Jong JH, Kampen M, Biemans B. The effect of whole body vibration training on women with stress urinary incontinence (Abstract number 416). Proceedings of the International Continence Society (ICS), 36th Annual Meeting, 2006 Nov 27‐Dec 1, Christchurch, New Zealand. 2006.
Dowell 1997 {published data only}
- Dowell CJ, Bryant CM, Moore KH, Prashar S. The efficacy and user friendliness of the urethral occlusive device (Abstract). Proceedings of the International Continence Society (ICS), 27th Annual Meeting, 1997 Sep 23‐26, Yokohama, Japan. 1997:295‐6.
Driusso 2008 {published data only}
- Driusso P, Correia GN, Pereira VS, Aveiro MC. Physiotherapy for women with stress urinary incontinence: effects of kinesiotherapy, vaginal cones and electrical stimulation. www.anzctr.org 2008 (accessed 19 April 2011).
Dumoulin 2011 {published data only}
- Dumoulin C, Sran M, Lieblich P, Wilson P. Physiotherapy significantly reduces leakage in postmenopausal women with osteoporosis and urinary incontinence: result of a parallel randomised controlled trial (Abstract number 130). Neurourology and Urodynamics 2011;30(6):985‐6. [Google Scholar]
Firra 2013 {published data only}
- Firra J, Thompson M, Smith SS. Paradoxical Findings in the Treatment of Predominant Stress and Urge Incontinence: A Pilot Study With Exercise and Electrical Stimulation. Journal of Women's Health Physical Therapy 2013;37(3):113‐23. [sr‐incont61836] [Google Scholar]
Fonda 1995 {published data only}
- Fonda D, Woodward M, D'Astoli M, Chin WF. Sustained improvement of subjective quality of life in older community‐dwelling people after treatment of urinary incontinence. Age and Ageing 1995;24(4):283‐6. [DOI] [PubMed] [Google Scholar]
- Fonda D, Woodward M, D'Astoli M, Chin WF. The continued success of conservative management for established urinary incontinence in older people. Australian Journal on Ageing 1994;13:12‐6. [Google Scholar]
Goode 2003 {published data only}
- Goode PS, Burgio KL, Locher JL, Roth DL, Umlauf MG, Richter HE, et al. Effect of behavioral training with or without pelvic floor electrical stimulation on stress incontinence in women: a randomized controlled trial. JAMA 2003;290(3):345‐52. [DOI] [PubMed] [Google Scholar]
Goode 2011a {published data only}
- Goode PS, Burgio KL, Johnson TM, Clay OJ, Roth DL, Markland AD, et al. Behavioral therapy with or without biofeedback and pelvic floor electrical stimulation for persistent postprostatectomy incontinence: a randomized controlled trial. JAMA 2011;305(2):151‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greer 2012 {published data only}
- Greer JA, Smith AL, Arya LA. Pelvic floor muscle training for urgency urinary incontinence in women: a systematic review. International Urogynecology Journal 2012;23(6):687‐97. [DOI] [PubMed] [Google Scholar]
Gronwald 2010 {published data only}
- Gronwald C, Pantel M. Osteopathic treatment in women suffering from urinary incontinence. A controlled clinical trial. http://www.therapiebedarf.at/record/7283?ln=en 2010. [sr‐incont49183]
Gunthorpe 1994 {published data only}
- Gunthorpe W, Redman S, Millard R, Brown W. A randomised trial to assess the general practice‐based treatment of female incontinence (Abstract). Proceedings of the International Continence Society (ICS), 24th Annual Meeting, 1994 Aug 30‐Sep 2, Prague, Czech Republic. 1994:371‐2.
Ha 2008 {published data only}
- Ha Y, Yun SJ, Kim Y, Lee S, Jung W, Kim W, et al. The development and effect of Ubiquitous program for female patients with overactive bladder: prospective, randomized 8‐weeks follow up with questionnaire and voiding diary based assessment (Abstract number 611). Proceedings of the 38th Annual Meeting of the International Continence Society (ICS), 2008 20‐24 Oct, Cairo, Egypt. 2008.
Hahn 1991 {published data only}
- Hahn I, Sommar S, Fall M. A comparative study of pelvic floor training and electrical stimulation for the treatment of genuine female stress urinary incontinence. Neurourology & Urodynamics 1991;10(6):545‐54. [Google Scholar]
Haken 1991 {published data only}
- Haken J, Benness C, Cardozo L, Cutner A. A randomised trial of vaginal cones and pelvic floor exercises in the management of genuine stress incontinence (Abstract). Neurourology & Urodynamics 1991;10(4):393‐4. [Google Scholar]
Henalla 1989 {published data only}
- Henalla SM, Hutchins CJ, Robinson P, MacVicar J. Non‐operative methods in the treatment of female genuine stress incontinence of urine. Journal of Obstetrics and Gynaecology 1989;9(3):222‐5. [Google Scholar]
Herschorn 2004 {published data only}
- Herschorn S, Becker D, Miller B, Thompson M, B, Forte L. The impact of a simple health education intervention in overactive bladder patients (Abstract). Proceedings of the International Continence Society (ICS), 33rd Annual Meeting, 2003 Oct 5‐9, Florence Italy. 2003:352‐3.
- Herschorn S, Becker D, Miller E, Thompson M, Forte L. Impact of a health education intervention in overactive bladder patients. Canadian Journal of Urology 2004;11(6):2430‐7. [PubMed] [Google Scholar]
Huang 2006 {published data only}
- Huang AJ, Thom DH, Kanaya AM, Wassel‐Fyr CL, Eeden SK, Ragins AI, et al. Urinary incontinence and pelvic floor dysfunction in Asian‐American women. American Journal of Obstetrics & Gynecology 2006;195(5):1331‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2012 {published data only}
- Huang X, Xu T, Lv B, Xie L. Effects of medical interfered‐pelvic floor muscle training in behavioral therapy and/or tolterodine in female patients with primary overactive bladder syndrome: a single‐blind randomized study (Abstract number 351). Proceedings of the 42nd Annual Meeting of the International Continence (ICS), 2012 Oct 15‐19, Beijing, China. 2012.
Kafri 2007 {published data only}
- Kafri R, Langer R, Dvir Z, Katz‐Leurer M. Rehabilitation vs drug therapy for urge urinary incontinence: short‐term outcome. International Urogynecology Journal and Pelvic Floor Dysfunction 2007;18(4):407‐11. [DOI] [PubMed] [Google Scholar]
Kangchai 2002 {published data only}
- Kangchai W, Srisuphun W, Kompayak J, Charoenyooth C, Jitapunkul S. Efficacy of self‐management promotion program for elderly women with urinary incontinence. Thai Journal of Nursing Research 2002;6(3):101‐14. [Google Scholar]
Kaya 2011 {published data only}
- Kaya S, Akbayrak T, Beksac S. Comparison of different treatment protocols in the treatment of idiopathic detrusor overactivity: a randomized controlled trial. Clinical Rehabilitation 2011;25(4):327‐38. [DOI] [PubMed] [Google Scholar]
Kim 2001 {published data only}
- Kim J. Continence efficacy intervention program for community residing women with stress urinary incontinence in Japan. Public Health Nursing 2001;18(1):64‐72. [DOI] [PubMed] [Google Scholar]
Kim 2006 {published data only}
- Kim JS, Yoon H, Chung WS, Shim BS. The long term effect of extracorporeal magnetic innervation therapy with pelvic floor muscle exercise for stress urinary incontinence. [Korean]. Korean Journal of Urology 2006;47(12):1334‐8. [Google Scholar]
Kim 2007 {published data only}
- Kim H, Suzuki T, Yoshida Y, Yoshida H. Effectiveness of multidimensional exercises for the treatment of stress urinary incontinence in elderly community‐dwelling Japanese women: A randomized, controlled, crossover trial. Journal of the American Geriatrics Society 2007;55(12):1932‐9. [DOI] [PubMed] [Google Scholar]
Kim 2009 {published data only}
- Kim H, Yoshida H, Suzuki T. Exercises treatment to reduce the urine leakage in elderly community‐dwelling Japanese women with stress, urge, and mixed urinary incontinence (Abstract number 772). Proceedings of the 39th Annual Meeting of the International Continence Society (ICS), 2009 Sep 29‐Oct 3, San Francisco, CA. 2009.
Kincade 2007 {published data only}
- Kincade JE, Dougherty MC, Carlson JR, Wells EC, Hunter GS, Busby‐Whitehead J. Factors related to urinary incontinence in community‐dwelling women. Urologic Nursing 2007;27(4):307‐17. [PubMed] [Google Scholar]
Kirschner‐Hermanns 1995 {published data only}
- Kirschner‐Hermanns R, Niehaus S, Schafer W, Jakse G. Pelvic floor re‐education in female stress‐incontinence I. and II. follow‐up results (mean 43 months) (Abstract number 216). Proceedings of the International Continence Society (ICS), 25th Annual Meeting, 1995 Oct 17‐20, Sydney, Australia. 1995:230‐1.
Kobayashi 2009 {published data only}
- Kobayashi H, Sawada N, Zakohji H, Yoshiyama M, Araki I, Takeda M. Researches on the improvement of QOL in both patients with overactive bladder syndrome and their caregivers. Comparison between pharmacotherapy alone and combination of pharmacotherapy, physio‐therapy, and education of both patients and caregiver (Abstract number 516). Proceedings of the 39th Annual Meeting of the International Continence Society (ICS), 2009 Sep 29‐Oct 3, San Francisco, CA. 2009.
Lagro‐Janssen 1991 {published data only}
- Lagro‐Janssen TL, Debruyne FM, Smits AJ, Weel C. Controlled trial of pelvic floor exercises in the treatment of urinary stress incontinence in general practice. British Journal of General Practice 1991;41(352):445‐9. [PMC free article] [PubMed] [Google Scholar]
Laycock 1988 {published data only}
- Laycock J. Interferential therapy in the treatment of genuine stress incontinence (Abstract). Neurourology & Urodynamics 1988;7(3):268‐9. [Google Scholar]
Laycock 1993 {published data only}
- Laycock J, Jerwood D. Does pre‐modulated interferential therapy cure genuine stress incontinence?. Physiotherapy 1993;79(8):553‐60. [Google Scholar]
Laycock 1995 {published data only}
- Laycock J, Knight S, Naylor D. Prospective, randomised, controlled clinical trial to compare acute and chronic electrical stimulation in combination therapy for GSI (Abstract). Neurourology & Urodynamics 1995;14(5):425‐6. [Google Scholar]
Laycock 2001 {published data only}
- Laycock J, Brown J, Cusack C, Green S, Jerwood D, Mann K, et al. A multi‐centre, prospective, randomised, controlled, group comparative study of the efficacy of vaginal cones and PFX (Abstract number 47). Neurourology & Urodynamics 1999;18(4):301‐2. [Google Scholar]
- Laycock J, Brown J, Cusack C, Green S, Jerwood D, Mann K, et al. A multi‐centre, prospective, randomised, controlled, group comparative study of the efficacy of vaginal cones and PFX (Abstract). International Urogynecology Journal and Pelvic Floor Dysfunction 1999;10(Suppl 1):S49. [Google Scholar]
- Laycock J, Brown J, Cusack C, Green S, Jerwood D, Mann K, et al. Pelvic floor reeducation for stress incontinence: comparing three methods. British Journal of Community Nursing 2001;6(5):230‐44. [DOI] [PubMed] [Google Scholar]
Lee 2005 {published data only}
- Lee C, Johnson C, Chiarelli P. Women's waterworks: evaluating an early intervention for incontinence among adult women. Australian and New Zealand Continence Journal 2005;11(1):11‐6. [Google Scholar]
Madersbacher 2003 {published data only}
- Madersbacher H, Pilloni S. Efficacy of extracorporeal magnetic innervation therapy (EXMI) in comparison to standard therapy for stress, urge and mixed incontinence: a randomised prospective trial (Abstract). Proceedings of the International Continence Society (ICS), 33rd Annual Meeting, 2003 Oct 5‐9, Florence Italy. 2003:296‐7.
Madersbacher 2004 {published data only}
- Madersbacher H, Pilloni S. Efficacy of extracorporeal magnetic innervation therapy (ExMI) in comparison to standard therapy for stress, urge and mixed incontinence: A randomised prospective trial (Abstract number 185). European Urology Supplements 2004;3(2):49. [Google Scholar]
Maher 2009 {published data only}
- Maher RM, Crowe L, Caulfield B. Comparison of two methods of electrical muscle stimulation training of pelvic floor musculature in the treatment of stress urinary incontinence. Journal of Women's Health Physical Therapy 2009;33(1):24. [Google Scholar]
McCormack 2004 {published data only}
- McCormack PL, Keating GM. Duloxetine in stress urinary incontinence. Drugs 2004;64(22):2567‐73. [DOI] [PubMed] [Google Scholar]
Millard 2003 {published data only}
- Millard RJ. Clinical efficacy of tolterodine with or without a simplified pelvic floor exercise regimen (Abstract). Australian and New Zealand Continence Journal 2003;9(4):81. [DOI] [PubMed] [Google Scholar]
Millard 2003a {published data only}
- Millard RJ. A simplified pelvic floor exercise regimen adds no additional benefit to drug treatment alone (Abstract). Proceedings of the International Continence Society (ICS), 33rd Annual Meeting, 2003 Oct 5‐9, Florence Italy. 2003:215‐6.
Millard 2003b {published data only}
- Millard RJ. Clinical efficacy of tolterodine with or without a simplified pelvic floor exercise regimen (Abstract). Australia and New Zealand Journal of Surgery 2003;73:A337. [DOI] [PubMed] [Google Scholar]
Millard 2004 {published data only}
- Millard RJ, Asia Pacific Tolterodine Study Group. Clinical efficacy of tolterodine with or without a simplified pelvic floor exercise regimen. Neurourology & Urodynamics 2004;23(1):48‐53. [DOI] [PubMed] [Google Scholar]
Mørkved 2002 {published data only}
- Mørkved S, Bø K, Fjortoft T. Effect of adding biofeedback to pelvic floor muscle training to treat urodynamic stress incontinence. Obstetrics & Gynecology 2002;100(4):730‐9. [DOI] [PubMed] [Google Scholar]
O'Brien 1996 {published data only}
- O'Brien J. Evaluating primary care interventions for incontinence. Nursing Standard 1996;10(23):40‐3. [PubMed] [Google Scholar]
- O'Brien J, Long H. Urinary incontinence: long term effectiveness of nursing intervention in primary care. BMJ 1995;311(7014):1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oldham 2010 {published data only}
- Oldham J. An evaluation of pelvic floor muscle exercises and electrical muscle stimulation in patients with stress incontinence: a randomised, double‐blind, controlled trial. http://isrctn.org/ISRCTN56654882 2000. [ISRCTN56654882]
- Oldham J, McBride K, Herbert J. Evaluation of a new electrostim technology for the treatment of urinary incontinence in women: a randomized controlled trial (Abstract number 182). Neurourology and Urodynamics 2010;29(6):1067. [DOI] [PubMed] [Google Scholar]
PRIDE 2004 {published data only}
- Grady D, Subak L, Kusek J, Nyberg L. PRIDE ‐ program to reduce incontinence by diet and exercise. www.controlled‐trials.com 2004.
Rutledge 2012 {published data only}
- Rutledge T, Lee S, Rogers R, Muller C. A pilot randomized controlled trial to evaluate pelvic floor muscle training for urinary incontinence among gynecologic cancer survivors (Abstract number 282). Gynecologic Oncology 2012;125(Suppl 1):S117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sampselle 2003 {published data only}
- Sampselle CM. Behavioral interventions in young and middle‐age women: simple interventions to combat a complex problem. American Journal of Nursing 2003;103(3 Suppl):9‐14, 16. [DOI] [PubMed] [Google Scholar]
Sanchez 2008 {published data only}
- Sanchez MTM, Aparicio MD, Hermoso VE, Jalil LGK, Carro JII, Martin MM. Effectiveness of a protocolized nursing intervention on the treatment of the urinary incontinence in women from the providence of Avila [Spanish]. Nure Investigacion 2008, issue 34.
Savage 2005 {published data only}
- Savage AM. Is lumbopelvic stability training (using the Pilates model) an effective treatment strategy for women with stress urinary incontinence? A review of the literature and report of a pilot study. Journal of the Association of Chartered Physiotherapists in Women's Health 2005;97:33‐48. [Google Scholar]
Scott 1979 {published data only}
- Scott RS, Hsueh GSC. A clinical study of the effects of galvanic vaginal muscle stimulation in urinary stress incontinence and sexual dysfunction. American Journal of Obstetrics & Gynecology 1979;135:663‐5. [DOI] [PubMed] [Google Scholar]
Smith 1994 {published data only}
- Smith JJ, Loehner D, Bingham W. Intravaginal electrical stimulation in the treatment of GSUI and DI: a controlled study (Abstract number 25). Proceedings of the American Urogynecology Society (AUGS), 15th Annual Meeting, 1994 Sep 21‐24, Toronto, Canada. 1994.
Sran 2011 {published data only}
- Sran M, Wilson P, Lieblich P, Dumoulin C. Physiotherapy significantly reduces leakage in postmenopausal women with osteoporosis and urinary incontinence: results of a randomised controlled trial (Abstract number RR‐PO‐311‐14‐Wed). Physiotherapy 2011; Vol. 97, issue Suppl S1.
Sultana 2014 {published data only}
- Sultana A. Clinical evaluation of stress urinary incontinence in women of reproductive age and its management with combination of two Unani drugs. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=6577 2014. [CTRI/2014/09/004997; sr‐incont64507]
Suzuki 2003 {published data only}
- Suzuki T, Yasuda K, Suda S, Shibuya H, Yamanishi T, Kitahara S, et al. Therapeutic outcomes of functional continuous magnetic stimulation (FCMS) combined with pelvic floor muscle exercise (PFME) in urinary incontinence (Abstract). Proceedings of the International Continence Society (ICS), 33rd Annual Meeting, 2003 Oct 5‐9, Florence, Italy. 2003:297‐9.
Tapp 1987 {published data only}
- Tapp AJS, Williams S, Hills B, Cardozo LD. The role of physiotherapy in the treatment of genuine stress incontinence (abstract). Proceedings of the International Continence Society (ICS), 17th Annual Meeting, 1987 Sep 2‐5, Bristol, UK. 1987:204‐5.
Terry 1996 {published data only}
- Terry PB, Whyte SM. Randomised trial comparing Enhance with physiotherapy for the treatment of GSI [abstract]. Proceedings of the International Continence Society (ICS), 26th Annual Meeting, 1996 Aug 27‐30, Athens, Greece. 1996:248‐9.
Van Hespen 2006 {published data only}
- Tak E, Hespen A, Dommelen P, Hopman‐Rock M. Incondition; a multi‐level randomized controlled trial of a programme to reduce and prevent urinary incontinence in women in homes for the elderly (Abstract). Proceedings of the Joint Meeting of the International Continence Society (ICS) (34th Annual Meeting) and the International UroGynecological Association (IUGA), 2004 Aug 23‐27, Paris, France. 2004:Abstract number 190.
- Hespen ATH, Tak ECPM, Dommelen P, Hopman‐Rock M. Evaluation of the urinary incontinence training programme, INCondition, for women living in homes for the elderly. Nederlands Tijdschrift voor Fysiotherapie 2006;116(6):136‐42. [Google Scholar]
Viereck 2011 {published data only}
- Viereck V, Rautenberge O. Comparison of solifenacin combined with pelvic floor muscle and whole body vibration training with solifenacin alone in patients with overactive bladder syndrome ‐ a prospective randomized parallel group trial. http://clinicaltrials.gov/show/NCT01314781 2011.
Voigt 1996 {published data only}
- Voigt R, Halaska M, Martan A, Wilke I, Michels W, Voigt P. Follow‐up after pelvic floor exercises and electro‐stimulation in stress incontinent women. Proceedings of the International Continence Society (ICS), 26th Annual Meeting, 1996 Aug 27‐30, Athens, Greece. 1996:435‐6.
von der Heide 2003 {published data only}
- Heide S, Emons G, Hilgers R, Viereck V. Effect on muscles of mechanical vibrations produced by the Galileo 2000 in combination with physical therapy in treating female stress urinary incontinence (Abstract). Proceedings of the International Continence Society (ICS), 33rd Annual Meeting, 2003 Oct 5‐9, Florence Italy. 2003:192‐3.
Waterfield 2007 {published data only}
- Waterfield A, Freeman R, Waterfield M, Campbell J. A community intervention study of female pelvic floor condition and knowledge of pelvic floor exercises: Part 2 randomised controlled trial of pelvic floor muscle training in women with a weak pelvic floor (Abstract number 238). Proceedings of the 37th Annual Meeting of the International Continence Society (ICS), 2007 Aug 20‐24, Rotterdam, the Netherlands. 2007.
Wells 1999 {published data only}
- Krause C, Wells T, Hughes S, Brink C, Mayer R. Incontinence in women: effect of expectancy to regain control and severity of symptoms on treatment outcomes. Urologic Nursing 2003;23(1):54‐61. [PubMed] [Google Scholar]
- Wells T, Mayer R, Brink C, Brown R. Pelvic muscle exercise: A controlled clinical trial. [unpublished manuscript] 1999.
- Wells TJ. Curiouser and curiouser. Journal of Wound, Ostomy, & Continence Nursing 2003;30(6):300‐4. [DOI] [PubMed] [Google Scholar]
Wilson 1984 {published data only}
- Wilson PD, Al Samarrai T, Deakin M, Kolbe E, Brown ADG. The value of physiotherapy in female genuine stress incontinence (abstract). Proceedings of the International Continence Society (ICS), 14th Annual Meeting, 1984 Sep 13‐15, Innsbruck, Austria. 1984:156‐8.
Yamanishi 2006 {published data only}
- Yamanishi T, Suzuki T, Yasuda K, Kitahara S, Yoshida KI. Randomised sham‐controlled evaluation of functional continuous magnetic stimulation with pelvic floor muscle training in patients with urinary incontinence. European Urology Supplements 2006;5(2):156. [Google Scholar]
Yoon 1999 {published data only}
- Yoon HN, Hong JY, Choi YH, Back SH. The effect of pelvic floor muscle exercises on genuine stress incontinence among Korean women: focusing on it's effects on the quality of life (Abstract number 516). Proceedings of the International Continence Society (ICS), 29th Annual Meeting, 1999 Aug 23‐26, Denver, Colorado. 1999:296.
Zhao 2000 {published data only}
- Zhao D, Li H. Microwave combined with pelvic floor muscle exercise treatment for the middle and elderly patients with urinary incontinence. Chinese Journal of Physical Therapy 2000;23(3):141‐3. [Google Scholar]
Additional references
Abrams 2013
- Abrams P, Andersson KE, Artibani W, Birder I, Bliss D, Brubaker L, et al. Recommendations of the International Scientific Committee: Evaluation and treatment of urinary Incontinence, Pelvic organ Prolapse and Faecal Incontinence. 5th International Consultation on Incontinence. ICUD‐EAU 2013, 2013. [Google Scholar]
Allahdin 2008
- Allahdin S, Harrild K, Warraich QA, Bain C. Comparison of the long‐term effects of simple total abdominal hysterectomy with transcervical endometrial resection on urinary incontinence. BJOG: an International Journal of Obstetrics and Gynaecology 2008;115(2):199‐204. [DOI] [PubMed] [Google Scholar]
Barber 2001
- Barber MD, Kuchibhatla MN, Pieper CF, Bump RC. Psychometric evaluation of 2 comprehensive condition‐specific quality of life instruments for women with pelvic floor disorders. American Journal of Obstetrics and Gynecology 2001;185:1388‐95. [DOI] [PubMed] [Google Scholar]
Barber 2002
- Barber MD, Visco AG, Wyman J, Fantl JA, Bump RC. Sexual function in women with urinary incontinence and pelvic organ prolapse. Obstetrics and Gynecology 2002;99(2):281‐9. [DOI] [PubMed] [Google Scholar]
Bernstein 1997
- Bernstein IT. The pelvic floor muscles: muscle thickness in healthy and urinary incontinent women measured by perineal ultrasonography with reference to the effect of pelvic floor training. Oestrogen receptor studies. Neurourology and Urodynamics 1997;16:237‐75. [DOI] [PubMed] [Google Scholar]
Boyle 2012
- Boyle R, Hay‐Smith EJC, Cody JD, Mørkved S. Pelvic floor muscle training for prevention and treatment of urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD007471.pub2] [DOI] [PubMed] [Google Scholar]
Burgio 2006
- Burgio KL, Goode PS, Richter HE, Locher JL, Roth DE. Global ratings of patient satisfaction and perception of improvement with treatment for urinary incontinence: validation of three global patient ratings. Neurourology and Urodynamics 2006;25:411‐7. [DOI] [PubMed] [Google Scholar]
Burgio 2008
- Burgio KL, Kraus SR, Menefee S, Borello‐France D, Corton M, Johnson HW, et al. Behavioural therapy to enable women with urge incontinence to discontinue drug treatment. Annals of Internal Medicine 2008;149:161‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bø 1990
- Bø K, Hagen RH, Kvarstein B, Jorgensen J, Larsen S. Pelvic floor muscle exercise for the treatment of female stress urinary incontinence. III: Effects of two different degrees of pelvic floor muscle exercise. Neurourology and Urodynamics 1990;9:489‐502. [Google Scholar]
Bø 1999
- Bø K, Talseth T, Holme I. Single blind, randomized controlled trial of pelvic floor muscles exercises, electrical stimulation, vaginal cones and no treatment in management of genuine stress incontinence in women. BMJ 1999;318:487‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bø 2004
- Bø K. Pelvic floor muscle training is effective in treatment of female stress urinary incontinence, but how does it work?. International Urogynecology Journal and Pelvic Floor Dysfunction 2004;15:76‐84. [DOI] [PubMed] [Google Scholar]
Campbell 2012
- Campbell SE, Glazener CMA, Hunter KF, Cody JD, Moore KN. Conservative management for postprostatectomy urinary incontinence. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD001843.pub4] [DOI] [PubMed] [Google Scholar]
Cody 2012
- Cody JD, Jacobs ML, Richardson K, Moehrer B, Hextall A. Oestrogen therapy for urinary incontinence in post‐menopausal women. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD001405.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Culligan 2000
- Culligan PJ, Heit M. Urinary incontinence in women: evaluation and management. American Family Physician 2000;62(11):2433‐44. [PubMed] [Google Scholar]
Dean 2006
- Dean NM, Ellis G, Herbison GP, Wilson PD. Laparoscopic colposuspension for urinary incontinence in women. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD002239.pub2] [DOI] [PubMed] [Google Scholar]
DeLancey 1988
- DeLancey JOL. Anatomy and mechanics of structures around the vesical neck: how vesical position may affect its closure. Neurourology and Urodynamics 1988;7:161‐2. [Google Scholar]
Dumoulin 2014
- Dumoulin C, Hay‐Smith J, Mac Habée‐Séguin G. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD005654.pub3] [DOI] [PubMed] [Google Scholar]
Duthie 2011
- Duthie JB, Vincent M, Herbison GP, Wilson DI, Wilson D. Botulinum toxin injections for adults with overactive bladder syndrome. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD005493.pub3] [DOI] [PubMed] [Google Scholar]
Elser 1999
- Elser DM, Wyman JF, McClish DK, Robinson D, Fantl JA, Bump RC. The effect of bladder training, pelvic floor muscle training or combination training on urodynamic parameters in women with urinary incontinence. Continence Programme for Women Research Group. Neurology & Urodynamics 1999;18:427‐36. [DOI] [PubMed] [Google Scholar]
Eustice 2000
- Eustice S, Roe B, Paterson J. Prompted voiding for the management of urinary incontinence in adults. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002113] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fitzgerald 2002
- Fitzgerald MP, Stablein U, Brubaker L. Urinary habits among asymptomatic women. American Journal of Obstetrics and Gynecology 2002;187(5):1384‐8. [DOI] [PubMed] [Google Scholar]
Fitzgerald 2003
- Fitzgerald MP, Brubaker L. Variability of 24‐hour voiding diary variables among asymptomatic women. Journal of Urology 2003;169(1):207‐9. [DOI] [PubMed] [Google Scholar]
Glazener 2012
- Glazener CMA, Lapitan MCM. Urodynamic studies for management of urinary incontinence in children and adults. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD003195.pub2] [DOI] [PubMed] [Google Scholar]
Godec 1975
- Godec C, Cass AS, Ayala GF. Bladder inhibition with functional electrical stimulation. Urology 1975;6(6):663‐6. [DOI] [PubMed] [Google Scholar]
Grimby 1993
- Grimby A, Milson I, Molander U, Wiklund I, Ekelund P. The influence of urinary incontinence on the quality of life of elderly women. Age and Aging 1993;22:82‐9. [DOI] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [DOI] [PubMed] [Google Scholar]
Hay‐Smith 2011
- Hay‐Smith EJC, Herderschee R, Dumoulin C, Herbison GP. Comparisons of approaches to pelvic floor muscle training for urinary incontinence in women. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD009508] [DOI] [PubMed] [Google Scholar]
Haylen 2010
- Haylen BT, Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourology and Urodynamics 2010;29:4‐20. [DOI] [PubMed] [Google Scholar]
Herbison 2013
- Herbison GP, Dean N. Weighted vaginal cones for urinary incontinence. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD002114] [DOI] [PMC free article] [PubMed] [Google Scholar]
Herderschee 2011
- Herderschee R, Hay‐Smith EJC, Herbison GP, Roovers JP, Heinemann MJ. Feedback or biofeedback to augment pelvic floor muscle training for urinary incontinence in women. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD009252] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Hunskaar 1991
- Hunskaar S, Vinsnes A. The quality of life in women with urinary incontinence as measured by the sickness impact profile. Journal of the American Geriatrics Society 1991;39:378‐82. [DOI] [PubMed] [Google Scholar]
Karram 1989
- Karram MM, Bhatia NN. Management of co‐existent stress and urge incontinence. British Journal of Urology 1989;57:641‐6. [Google Scholar]
Kegel 1948
- Kegel AH. Progressive resistance exercise in the functional restoration of the perineal muscles. American Journal of Obstetrics and Gynecology 1948;56:238‐49. [DOI] [PubMed] [Google Scholar]
Kirchin 2012
- Kirchin V, Page T, Keegan PE, Atiemo K, Cody JD, McClinton S. Urethral injection therapy for urinary incontinence in women. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD003881.pub3] [DOI] [PubMed] [Google Scholar]
Lapitan 2012
- Lapitan MCM, Cody JD. Open retropubic colposuspension for urinary incontinence in women. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD002912.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lipp 2011
- Lipp A, Shaw C, Glavind K. Mechanical devices for urinary incontinence in women. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD001756.pub5] [DOI] [PubMed] [Google Scholar]
Long 2008
- Long RM, Giri SK, Flood HD. Current concepts in female stress urinary incontinence. The Surgeon: Journal of the Royal Colleges of Surgeons of Edinburgh and Ireland 2008;6(6):366‐72. [DOI] [PubMed] [Google Scholar]
Lose 1998
- Lose G, Fantl JA, Victor A, Walter S, Wells TL, Wyman J, et al. Outcome measures for research in adult women with symptoms of lower urinary tract dysfunction. Neurourology & Urodynamics 1998;17(3):255‐62. [DOI] [PubMed] [Google Scholar]
MacArthur 2006
- MacArthur C, Glazener CM, Wilson PD, Lancashire RJ, Herbison GP, Grant AM. Persistent urinary incontinence and delivery mode history: a six‐year longitudinal study. BJOG: an international Journal of Obstetrics and Gynaecology 2006;113(2):218‐24. [DOI] [PubMed] [Google Scholar]
MacLennan 2000
- MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. BJOG: an international Journal of Obstetrics and Gynaecology 2000;107(12):1460‐70. [DOI] [PubMed] [Google Scholar]
Madhuvrata 2012
- Madhuvrata P, Cody JD, Ellis G, Herbison GP, Hay‐Smith EJ. Which anticholinergic drug for overactive bladder symptoms in adults. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD005429.pub2] [DOI] [PubMed] [Google Scholar]
Mariappan 2005
- Mariappan P, Alhasso AA, Grant A, N'Dow JMO. Serotonin and noradrenaline reuptake inhibitors (SNRI) for stress urinary incontinence in adults. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD004742.pub2] [DOI] [PubMed] [Google Scholar]
Melville 2002
- Melville J, Walker E, Katon W, Lentz G, Miller J, Fenner D. Prevalence of comorbid psychiatric illness and its impact on symptom perception, quality of life, and functional status in women with urinary incontinence. American Journal of Obstetrics and Gynecology 2002;187:80‐7. [DOI: 10.1067/mob.2002.124839] [DOI] [PubMed] [Google Scholar]
Milsom 2009
- Milsom I, Altman D, Lapitan MC, Nelson R, Sillén U, Thom D. Epidemiology of urinary (UI) and faecal (FI) incontinence and pelvic organ prolapse (POP). In: Abrams P, Cardozo L, Khoury S, Wein A editor(s). Incontinence. 4th Edition. Health Publication Ltd., 2009. [Google Scholar]
Moore 2013
- Moore K, Dumoulin C, Bradley C, Burgio K, Chambers T, Hagen S, et al. Adult conservative management. In: Abrams P, Cardozo L, Khoury S, Wein A editor(s). Incontinence. 5th Edition. ICUD‐EAU, 2013:1101‐227. [Google Scholar]
Morrison 1995
- Morrison JFB. The excitability of the micturition reflex. Scandinavian Journal of Urology and Nephrology 1995;29(Suppl 175):21‐5. [PubMed] [Google Scholar]
Nabi 2006
- Nabi G, Cody JD, Ellis G, Hay‐Smith J, Herbison GP. Anticholinergic drugs versus placebo for overactive bladder syndrome in adults. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003781.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nilsson 2009
- Nilsson M, Lalos A, Lalos O. The impact of female urinary incontinence and urgency on quality of life and partner relationship. Neurourology and Urodynamics 2009;28:976‐81. [DOI: 10.1002/nau.20709] [DOI] [PubMed] [Google Scholar]
Ogah 2009
- Ogah J, Cody JD, Rogerson L. Minimally invasive synthetic suburethral sling operations for stress urinary incontinence in women. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD006375.pub2] [DOI] [PubMed] [Google Scholar]
Omar 2014
- Omar MI, Lam TB, Alexander CE, Graham J, Mamoulakis C, Imamura M, et al. Systematic review and meta‐analysis of the clinical effectiveness of bipolar compared with monopolar transurethral resection of the prostate (TURP). British Journal of Urology International 2013 Oct 24 [Epub ahead of print];113(1):24‐35. [DOI: 10.1111/bju.12281] [DOI] [PubMed] [Google Scholar]
Ostaszkiewicz 2004
- Ostaszkiewicz J, Johnston L, Roe B. Timed voiding for the management of urinary incontinence in adults. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD002802.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patrick 1999
- Patrick DL, Martin ML, Bushnell DM, Yalcin I, Wagne TH, Buesching DP. Quality of life of women with urinary incontinence: further development of the incontinence quality of life instrument (I‐QOL). Urology 1999;53:71. [DOI] [PubMed] [Google Scholar]
Polden 1990
- Polden M, Mantle J. Physiotherapy in Obstetrics & Gynaecology. Oxford: Butterworth Heinemann, 1990. [Google Scholar]
Reference Manager 2012 [Computer program]
- Thomson Reuters. Reference Manager Professional Edition Version 12. New York: Thomson Reuters, 2012.
Rehman 2011
- Rehman H, Bezerra CCB, Bruschini H, Cody, JD. Traditional suburethral sling operations for urinary incontinence in women. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD001754.pub3] [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Schorge 2008
- Schorge JO, Schaffer JI, Halvorson LM, Hoffman BL, Bradshaw KD, Cunningham FG. Williams Gynaecology. First Edition. New York: McGraw Hill, 2008. [Google Scholar]
Shaw 2001a
- Shaw C, Tansey R, Jackson C, Hyde C, Allan R. Barriers to help seeking in people with urinary symptoms. Family Practice 2001;18(1):48‐52. [DOI] [PubMed] [Google Scholar]
Shaw 2001b
- Shaw C. A review of the psychosocial predictors of help seeking behaviour and impact on quality of life in people with urinary incontinence. Journal of Clinical Nursing 2001;10:15‐24. [DOI: 10.1046/j.1365-2702.2001.00443] [DOI] [PubMed] [Google Scholar]
Shumaker 1994
- Shumaker SA, Wyman JF, Uebersax JS, McClish D, Fantl JA. Health‐related quality of life measures for women with urinary incontinence‐ the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program in Women (CPW) Research Group. Quality of Life Research 1994;3:291‐306. [DOI] [PubMed] [Google Scholar]
Sinclair 2011
- Sinclair AJ, Ramsay IN. The psychosocial impact of urinary incontinence in women. The Obstetrician and Gynaecologist 2011;13(3):143‐8. [Google Scholar]
Thuroff 2011
- Thuroff JW, Abrams P, Andersson KE, Artibani W, Chapple CR, Drake MJ, et al. EAU Guidelines on urinary incontinence. European Urology 2011;59:387‐400. [DOI] [PubMed] [Google Scholar]
Turner 2004
- Turner DA, Shaw C, McGrother CW, Dallosso HM, Cooper NJ, MRC Incontinence Team. The cost of clinically significant urinary storage symptoms for community dwelling adults in the UK. British Journal of Urology International 2004;93:1246‐52. [DOI] [PubMed] [Google Scholar]
van Kerrebroeck 2002
- Kerrebroeck P, Abrams P, Chaikin D, Donovan J, Fonda D, Jackson S, et al. The standardisation of terminology in nocturia: report from the standardisation sub‐committee of the International Continence Society. Neurourology and Urodynamics 2002;21:179‐83. [DOI] [PubMed] [Google Scholar]
Wallace 2004
- Wallace SA, Roe B, Williams K, Palmer M. Bladder training for urinary incontinence in adults. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD001308.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 1998
- Wilson PD, Herbison GP. A randomized controlled trial of pelvic floor muscle exercises to treat postnatal urinary incontinence. International Urogynecology Journal and Pelvic Floor Dysfunction 1998;9(5):257‐64. [DOI] [PubMed] [Google Scholar]
Wilson 2001
- Wilson L, Brown J, Shin G, Luc KO, Subak LL. Annual direct cost of urinary incontinence. Obstetrics and Gynaecology 2001;98:398‐406. [DOI] [PubMed] [Google Scholar]
Yalcin 2003
- Yalcin I, Bump RC. Validation of two global impression questionnaires for incontinence. American Journal of Obstetrics and Gynecology 2003;189:98. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ayeleke 2013
- Ayeleke RO, Hay‐Smith EJC, Omar MI. Pelvic floor muscle training added to another active treatment versus the same active treatment alone for urinary incontinence in women. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD010551.pub2] [DOI] [PubMed] [Google Scholar]


