Key Points
Question
Is there an association between the V122I genetic variant of hereditary transthyretin amyloidosis with heart failure among individuals of African or Hispanic/Latino ancestry?
Findings
In this observational study that included 9694 participants from 2 biobank registries, there was a significant association of the transthyretin V122I genetic variant with heart failure (adjusted odds ratio, 1.7 in a cohort of African ancestry and 1.8 in a separate cohort of African or Hispanic/Latino ancestry).
Meaning
Among individuals of African or Hispanic/Latino ancestry, the transthyretin V122I genetic variant was significantly associated with heart failure.
Abstract
Importance
Hereditary transthyretin (TTR) amyloid cardiomyopathy (hATTR-CM) due to the TTR V122I variant is an autosomal-dominant disorder that causes heart failure in elderly individuals of African ancestry. The clinical associations of carrying the variant, its effect in other African ancestry populations including Hispanic/Latino individuals, and the rates of achieving a clinical diagnosis in carriers are unknown.
Objective
To assess the association between the TTR V122I variant and heart failure and identify rates of hATTR-CM diagnosis among carriers with heart failure.
Design, Setting, and Participants
Cross-sectional analysis of carriers and noncarriers of TTR V122I of African ancestry aged 50 years or older enrolled in the Penn Medicine Biobank between 2008 and 2017 using electronic health record data from 1996 to 2017. Case-control study in participants of African and Hispanic/Latino ancestry with and without heart failure in the Mount Sinai BioMe Biobank enrolled between 2007 and 2015 using electronic health record data from 2007 to 2018.
Exposures
TTR V122I carrier status.
Main Outcomes and Measures
The primary outcome was prevalent heart failure. The rate of diagnosis with hATTR-CM among TTR V122I carriers with heart failure was measured.
Results
The cross-sectional cohort included 3724 individuals of African ancestry with a median age of 64 years (interquartile range, 57-71); 1755 (47%) were male, 2896 (78%) had a diagnosis of hypertension, and 753 (20%) had a history of myocardial infarction or coronary revascularization. There were 116 TTR V122I carriers (3.1%); 1121 participants (30%) had heart failure. The case-control study consisted of 2307 individuals of African ancestry and 3663 Hispanic/Latino individuals; the median age was 73 years (interquartile range, 68-80), 2271 (38%) were male, 4709 (79%) had a diagnosis of hypertension, and 1008 (17%) had a history of myocardial infarction or coronary revascularization. There were 1376 cases of heart failure. TTR V122I was associated with higher rates of heart failure (cross-sectional cohort: n = 51/116 TTR V122I carriers [44%], n = 1070/3608 noncarriers [30%], adjusted odds ratio, 1.7 [95% CI, 1.2-2.4], P = .006; case-control study: n = 36/1376 heart failure cases [2.6%], n = 82/4594 controls [1.8%], adjusted odds ratio, 1.8 [95% CI, 1.2-2.7], P = .008). Ten of 92 TTR V122I carriers with heart failure (11%) were diagnosed as having hATTR-CM; the median time from onset of symptoms to clinical diagnosis was 3 years.
Conclusions and Relevance
Among individuals of African or Hispanic/Latino ancestry enrolled in 2 academic medical center–based biobanks, the TTR V122I genetic variant was significantly associated with heart failure.
This study assesses the association between the TTR V122I variant and prevalent heart failure in individuals with African and Hispanic/Latino ancestry and identifies rates of hereditary transthyretin amyloid cardiomyopathy (hATTR-CM) diagnosis among carriers with heart failure.
Introduction
Genetic variation in the gene TTR, encoding the protein transthyretin, can result in misfolding of the tetrameric transthyretin protein complex, leading to the accumulation of insoluble, extracellular amyloid fibrils that clinically result in hereditary transthyretin amyloidosis (hATTR).1 Although polyneuropathy is one prominent clinical presentation of hATTR, the deposition of amyloid fibrils in the myocardium can lead to cardiomyopathy (hATTR-CM), characterized by heart failure and arrhythmias.2,3 One of the most common genetic causes of hATTR-CM is a valine to isoleucine amino acid substitution at position 122 (V122I) in the TTR coding sequence that is primarily found in individuals of African ancestry.4,5,6,7,8,9
Although treatment of ATTR-CM has traditionally been limited to supportive care, targeted TTR therapies have recently been developed. The transthyretin-stabilizing small-molecule tafamidis significantly decreased cardiovascular-related hospitalizations, improved quality of life, and decreased all-cause mortality in patients with ATTR-CM10 and was approved by the US Food and Drug Administration in May 2019 for the treatment of heart failure due to ATTR-CM.
Given recent advances in treatment for ATTR-CM, it is important to distinguish these patients from those with other forms of cardiomyopathy (CM) and heart failure. Although hATTR-CM is likely underdiagnosed overall,7 individuals of African ancestry with hATTR-CM due to V122I may be especially overlooked.5,6,7,11 Previous studies have proposed using routine genetic testing in individuals of African ancestry presenting with heart failure8,9,11; however, the scope of underdiagnosis is not clear and this is not current practice.
In the current study, the association of the TTR V122I variant with the clinical diagnosis of heart failure was evaluated using longitudinal electronic health record (EHR)–linked genetic data from 2 large integrated academic health systems. Among TTR V122I variant carriers with heart failure, the rates of evaluation for and diagnosis with hATTR-CM were assessed.
Methods
The studies were approved by the institutional review boards of the University of Pennsylvania and the Icahn School of Medicine at Mount Sinai. All participants provided written informed consent.
Study Design and Outcomes
Because the TTR V122I variant predominantly occurs in individuals of African ancestry, we analyzed the association between TTR V122I variant carrier status and heart failure in individuals of African and Hispanic/Latino ancestry enrolled in either of 2 biobanks affiliated with large tertiary care academic medical centers, the Penn Medicine Biobank (PMBB) and the Icahn School of Medicine at Mount Sinai BioMe biobank. The exposure variable was the presence of the rare pathogenic allele for the genetic variant in TTR that encodes the V122I amino acid substitution. The primary outcome was prevalent heart failure at the time of data extraction. Using data from PMBB, a cross-sectional cohort analysis was performed, comparing the rate of heart failure between TTR V122I variant carriers and noncarriers among individuals of genetically inferred African ancestry aged 50 years or older. The analysis in BioMe used a case-control design among individuals of self-reported African or Hispanic/Latino ancestry, comparing the number of TTR V122I carriers and noncarriers between all participants with prevalent heart failure (cases) and individuals older than the age of 65 years without heart failure (controls). Additional outcomes included echocardiographic parameters in both studies.
Study Cohorts
The cross-sectional cohort is derived from a genomic and precision medicine research cohort enrolled from throughout the University of Pennsylvania Health System; participants in this analysis were recruited between November 21, 2008, and January 4, 2017. Participants actively consented to allow the linkage of biospecimens to their longitudinal EHR. Plasma, buffy coat, and DNA were isolated and stored for downstream analysis. The primary analysis included a subset of individuals of African genetic ancestry with whole-exome sequencing, performed through collaboration with the Regeneron Genetics Center (Tarrytown, New York) and additional individuals genotyped on the Global Screening Array (Illumina Inc) (Figure 1). Because hATTR-CM occurs predominantly in the elderly population,12 the primary analysis was limited to individuals aged 50 years or older at the time of analysis.
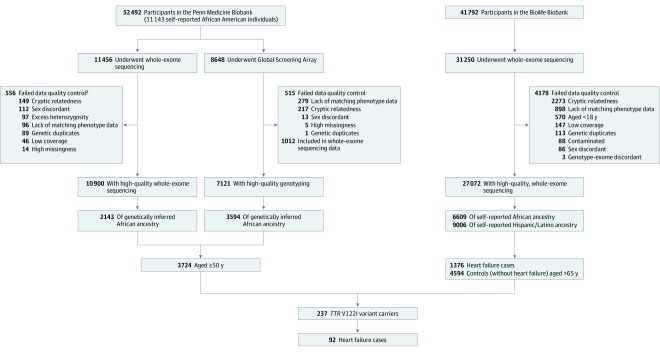
Figure 1. Evaluation and Diagnosis of Study Participants for Hereditary Transthyretin Amyloid Cardiomyopathy.
Across both studies, there were 9694 individuals of African or Hispanic/Latino ancestry entered into the primary analysis. Of these, there were 237 TTR V122I variant carriers, 92 of whom had confirmed heart failure based on clinical criteria after physician medical review. Only 10 of the 92 TTR V122I carriers with heart failure had been clinically evaluated for and diagnosed as having cardiac amyloidosis.
aCriteria for quality control were not mutually exclusive and therefore the numbers do not sum to the total number of unique individuals failing quality control.
The BioMe Biobank is an EHR-linked clinical care cohort comprised of participants from diverse ancestries (African, Hispanic/Latino, European, and other ancestries).13 Participants in this analysis were recruited between 2007 and 2015. Enrollment of participants was predominantly through ambulatory care practices. As a result, participants have a high median number of encounters per patient.14 Genetic data were linked to a wide array of longitudinal biomedical traits, including clinical outcomes, imaging results, and exposure data, originating from the systemwide EHR. This study was derived from a subset of participants of self-reported (based on fixed categories) African or Hispanic/Latino ancestry in whom whole-exome sequencing and genotyping on the Global Screening Array was generated by the Regeneron Genetics Center (Figure 1). For the primary analysis, cases were defined as individuals with prevalent heart failure at the time of analysis. Controls included individuals aged older than 65 years who did not have a clinical diagnosis of heart failure. This age cutoff for controls with heart failure was based on previous work showing that hATTR-CM mainly presents in the seventh decade of life12 and was designed to prevent the inclusion of individuals in the control group who were at genetic risk for, but had not yet developed, heart failure.
Genetic Sequencing, Variant Calling, and Genotype Assignment
In the cross-sectional cohort, genomic DNA underwent sample preparation and whole-exome sequencing via standard methodology as previously described.15 Genetic ancestry was inferred from genetic principal components using kernel density estimates based on HapMap3 ancestral super classes as described elsewhere.16 Following completion of cohort sequencing, samples showing disagreement between genetically determined and reported sex, low-quality sequencing data, and genetically identified sample duplicates were excluded; 1 individual from every pair of individuals with closer than third-degree relatedness was removed. Ancestry-specific principal components were calculated using PLINK version 1.9b.17 The TTR V122I variant is encoded by a nonsynonymous single-nucleotide polymorphism consisting of a guanine to adenosine substitution in the TTR gene located on chromosome 18 position 27178618 in the Genome Reference Consortium Human Build 37 (GRCh37/hg19).
Genomic DNA from an additional sample of individuals was genotyped on the Global Screening Array using standard protocols at the Children’s Hospital of Philadelphia Research Institute Center for Applied Genomics (Philadelphia, Pennsylvania). Data processing, quality control, and inference of genetic ancestry were performed as described in the eMethods in the Supplement. Among individuals of genetically inferred African ancestry, ancestry-specific principal components were calculated using PLINK version 1.9b. The TTR V122I protein coding variant was directly genotyped on the Global Screening Array platform and had a marker call rate of greater than 95%.
In the case-control study population, participants’ genomic DNA underwent sample preparation and exome sequencing and genotyping on the Global Screening Array as previously described.15 Ancestry was ascertained by self-report. Sample-level quality control was performed based on a number of steps including sex discordance, low coverage, contamination, duplicates, and discordance with genotyped data. One individual from every pair of individuals with closer than second-degree relatedness was removed. TTR V122I genotypes were obtained from the exome sequencing data and had a mean depth of coverage of 37.1x, a call rate of greater than 99.9%, and did not deviate from Hardy-Weinberg Equilibrium (P > .05 for Hardy-Weinberg test). The genome-wide genotype data were used to calculate principal components, which were used as covariates in association tests to adjust for genetic ancestry.
Phenotype Evaluation
For the cross-sectional cohort, data were directly queried from the Penn Data Store on January 18, 2017; data were extracted for the case-control study as of May 10, 2018. Age was calculated as the age on the date of data extraction for individuals who were alive at analysis and the age at death for deceased individuals in the cross-sectional cohort analysis and as age at enrollment for the case-control study. Clinical parameters were extracted based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Clinical Modification (ICD-10-CM) diagnostic codes, which have been shown to have more than 80% accuracy in heart failure diagnosis.18 Participants were considered to have heart failure if they had ICD-9-CM code 428 or 425 or ICD-10-CM code I50 or I42 listed as an encounter diagnosis on 2 or more distinct dates for the cross-sectional cohort or ICD-9-CM code 428 or ICD-10-CM code I50 listed as an encounter diagnosis or in the problem list on 1 or more distinct encounters for the case-control study.
Clinical covariates, including sex, body mass index (calculated as weight in kilograms divided by height in meters squared), systolic and diastolic blood pressure, hypertension, diabetes, smoking status, history of myocardial infarction or coronary revascularization, or use of antihypertensive or lipid-lowering medications, were extracted from structured EHR data elements. Binary categorical variables were encoded as present or absent, leading to complete ascertainment.
In the cross-sectional cohort, the quantitative echocardiographic parameters of left ventricular (LV) ejection fraction (%), left atrial volume index (mL/m3), interventricular septum wall thickness (mm) in diastole, LV posterior wall diastolic thickness (mm), and LV end-diastolic diameter (mm) were extracted from the outpatient reports for clinically obtained echocardiograms; relative wall thickness and LV mass (g) were calculated from the aforementioned parameters.19 For individuals with multiple echocardiograms, the median value for each parameter was used for analysis. In the case-control population, echocardiographic parameters were extracted from all clinically obtained echocardiograms for participants without heart failure and with available data; the median was used for continuous values and presence during first echocardiogram for categorical values. LV hypertrophy was said to be present if the physician entered “concentric/localized LV hypertrophy” into the comments field of the echocardiogram and absent if there was no physician ascertainment of LV hypertrophy. Continuous parameters included LV ejection fraction (%), left atrial volume index (mL/m3), interventricular septum wall thickness (mm) in diastole, LV posterior wall diastolic thickness (mm), LV end-diastolic diameter (mm), and LV mass (g).
To understand the detailed phenotypes of TTR V122I variant carriers with heart failure, we conducted physician medical record review. In addition, the medical records of participants in the cross-sectional cohort who carried the TTR V122I variant but did not have a diagnosis code for heart failure were also reviewed. Algorithmically determined heart failure was confirmed based on clinical data. Data relating to the underlying presence of heart failure risk factors (hypertension, coronary disease) and amyloid-related cardiac (arrhythmias, electrocardiographic abnormalities, low-voltage electrocardiogram) and systemic (carpal tunnel symptoms, neuropathy) features were extracted. Participants were assessed for clinical evaluation of hATTR-CM by medical record review (both studies), as well as by screening for structured data elements (case-control study only), comprised of diagnosis and procedure codes for cardiac biopsy, genetic testing, and nuclear imaging (eTable 1 in the Supplement).
Statistical Analysis
Counts and percentages were used to summarize categorical variables and medians and interquartile ranges (IQRs) were used to describe continuous variables. Demographic and clinical characteristics were compared by exposure or case status using the Fisher exact and χ2 tests for categorical variables and the Wilcoxon test for continuous variables. Confidence intervals for the absolute difference in the median values of continuous variables were calculated using bootstrapping. Data completeness is presented with summary statistics for measures with evidence of missingness; all other covariates were completely assessed. Complete case analysis was used for all analyses.
In the cross-sectional cohort analysis, we tested the association of TTR V122I variant carrier status with the primary outcome of prevalent heart failure in a cross-sectional cohort study design using all prevalent data available at the time of analysis. Logistic regression was used to model the exposure outcome relationship, controlling for age, sex, and population stratification (by incorporating the first 5 ancestry-specific genetic principal components to minimize confounding by ancestry).20 We additionally used sequential models to control for prevalent hypertension and a history of myocardial infarction or coronary revascularization. For logistic regression modeling, individuals who had their carrier status ascertained from sequencing were analyzed separately from those who underwent genotyping and the results combined with fixed-effects, inverse-variance weighted meta-analysis using the meta package in R.21
Because the V122I variant is thought to have the highest penetrance in elderly men, we performed additional sex-stratified analyses. These models sequentially controlled for age and principal components 1 through 5, hypertension, and myocardial infarction or coronary revascularization. Among men, we performed further age-stratified analyses using logistic regression controlling for principal components 1 through 5. Differences between subgroups were quantified by calculating the ratio of odds ratios (RORs).22
The association of echocardiographic parameters was tested using linear regression; parameters were logarithmically transformed using the ln(x +1) transformation. Building on an unadjusted model, we sequentially controlled for (1) age, sex, and principal components 1 through 5; (2) prevalent hypertension; and (3) a history of myocardial infarction or coronary revascularization.
In the case-control study, the association of TTR V122I carrier status with the primary outcome of prevalent heart failure was tested using logistic regression, controlling for age, sex, and principal components 1 through 10. We additionally controlled for prevalent hypertension and a history of myocardial infarction or coronary revascularization in sequential models. In instances when there was a zero count in one of the cells in the 2 × 2 contingency table of carrier status and case-control status, Firth’s logistic regression was used to account for sparsity of counts.
To examine whether TTR V122I carriers exhibit subclinical cardiac features before a diagnosis of heart failure is made, logistic regression was used to assess the association between TTR V122I carrier status and LV hypertrophy in participants from the case-control study population without heart failure. Linear regression was used to assess the association between TTR V122I carrier status and continuous echocardiographic traits after applying a natural logarithm transformation. Analyses were performed separately by self-reported ancestry group (participants with African and Hispanic/Latino ancestry) and combined with fixed-effects inverse-variance weighted meta-analysis using the meta package in R.21 Because LV hypertrophy can appear at any age in adult life, we examined the association stratified by age at enrollment (>65, >45-≤65, and ≤45 years old). Differences between subgroups were quantified by calculating the ROR or differences in regression coefficients (β).22,23
A predetermined 2-tailed P < .05 was considered statistically significant for both primary and secondary outcomes. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. For subgroup comparisons between multiple subgroups, P values were Bonferroni corrected for the number of pairwise subgroup comparisons. Statistical analyses were performed using R (version 3.4.3).24
Results
Cohorts and Carrier Rates
At the time of analysis, 52 492 participants were enrolled in PMBB, of whom 11 143 (21%) were of self-reported African ancestry as indicated in the EHR. Whole-exome sequencing was performed on 11 456 participants, with 10 900 passing sample-level quality control, and of whom 2143 were determined to be of genetically inferred African ancestry (Figure 1). An additional 3594 individuals of genetically inferred African ancestry were identified from among 8648 individuals who underwent genome-wide genotyping. Among all individuals of African ancestry with available genetic data (n = 5737), there were 190 carriers (3.3%) of the TTR V122I variant (eTable 2 in the Supplement). A total of 3724 participants were 50 years of age or older and were included in the analytic cohort for the primary analysis.
Among the 41 792 individuals in the population from which the case-control study was derived at the time of analysis, 31 250 had available genotype data; 27 072 passed genotype quality control and of these, 6609 participants were of self-identified African ancestry, 9006 of Hispanic/Latino ancestry, 8710 of European ancestry, 816 of East and Southeast Asian ancestry, 79 of Native American ancestry, and 1852 of other ancestries based on self-report (Figure 1). TTR V122I was polymorphic in individuals of self-reported African, Hispanic/Latino, and other ancestries, but not in those of European ancestry (eTable 2 in the Supplement), with most carriers being of self-reported African or Hispanic/Latino ancestry. In total, 211 individuals of African ancestry (3.2%) and 114 individuals of Hispanic/Latino ancestry (1.3%) carried at least 1 copy of the rare allele for the TTR V122I coding variant.
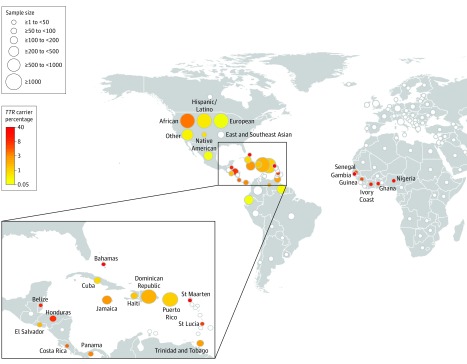
The TTR V122I variant was polymorphic in 30 of 154 total countries/regions (Figure 2). The highest rates of carriers were observed in those from West African countries, including Gambia (n = 2/5 individuals [40%] had at least 1 copy of the rare allele for the TTR V122I variant) and Ghana (n = 4/42 [9.5%]), as well as in those from Caribbean and Central American countries with known African ancestry, including St Maarten (n = 1/3 [33%]), St Croix (n = 1/3 [33%]), the Bahamas (n = 2/11 [18%]), Belize (n = 3/39 [7.7%]), and Honduras (n = 9/128 [7.0%]). The TTR V122I variant was monomorphic in European, East Asian, Middle Eastern, and all South American countries, except Venezuela (n = 1/47 [2.1%]), Guyana (n = 1/244 [0.4%]), and Ecuador (n = 1/378 [0.3%]) (eTable 3 in the Supplement).
Figure 2. Carrier Rate of TTR V122I in Self-reported Countries/Regions of Origin.
Carrier rate (percentage of individuals who have at least 1 copy of the rare allele) of the TTR V122I variant in self-reported countries/regions of origin. The size of the bubble is proportional to the sample size and the color coding is proportional to the carrier rate of the TTR V122I variant denoted in the key, with white bubbles representing a carrier rate of 0%. Participants with self-reported “USA” country of origin were categorized further into 6 different ancestry groups. Although countries from American and African continents are emphasized here, the complete country list is tabulated in eTable 3 in the Supplement.
Among the 15 615 individuals of self-reported African or Hispanic/Latino ancestry with high-quality whole-exome sequencing data in the population from which the case-control study was derived, there were 1376 cases (8.8%) of clinically diagnosed heart failure. There were 4594 individuals (29%) older than 65 years of age of self-reported African or Hispanic/Latino ancestry who had no clinical diagnosis of heart failure and comprised the control group for the primary analysis. These 2 groups of individuals comprised the analytic case-control cohort for the primary analysis.
V122I and Heart Failure in the Cross-Sectional Cohort
Among the 3724 participants of African ancestry aged 50 years or older, there were 116 carriers (3.1%) of the TTR V122I variant (Table 1). Participants had a median of 14 years (IQR, 8-17) of EHR data available for analysis and 1618 (43%) had at least 1 echocardiogram. There were no significant differences in these rates by carrier status. Proportionally, significantly more carriers than noncarriers were men (carriers: n = 70 [60%]; noncarriers: n = 1685 [47%]; difference, 13.6% [95% CI, 4.1%-23.1%]; P = .004) but the groups were otherwise similar with respect to age, body mass index, blood pressure, diabetes, hypertension, hypertensive medication usage, lipid-lowering agent use, current smoking status, or history of myocardial infarction or coronary revascularization. There was complete ascertainment of binary clinical covariates and missingness for the continuous measures of body mass index and blood pressure were less than 5% with no significant differences in completeness between carriers and noncarriers (Table 1).
Table 1. Analytic Cohort Characteristics.
| Characteristic | Cross-Sectional Cohort | Case-Control Study | ||||
|---|---|---|---|---|---|---|
| TTR V122I Carriers | TTR V122I Noncarriers | Difference, % (95% CI)a |
Heart Failure Cases | Heart Failure Controls | Difference, % (95% CI)a |
|
| No. of participants | 116 | 3608 | 1376 | 4594 | ||
| Years of electronic health record data, median (IQR) | 14 (9 to 17) | 14 (8 to 17) | −0.2 (−1.6 to 1.4) | 9 (6 to 12) | 8 (4 to 11) | 1.5 (1.1 to 1.9) |
| Echocardiogram, No. (%) | 56 (48) | 1562 (43) | 5.0 (−4.7 to 14.7) | 1220 (89) | 1866 (41) | 48.0 (46.0 to 50.0) |
| Age, median (IQR), y | 64 (58 to 70) | 64 (57 to 71) | 0 (−2.5 to 2.4) | 70 (61 to 79) | 74 (69 to 80) | −4.0 (−5.0 to −3.1) |
| Sex, No. (%) | ||||||
| Male | 70 (60) | 1685 (47) | 13.6 (4.1 to 23.1) | 601 (44) | 1670 (36) | 7.3 (4.3 to 10.0) |
| Female | 46 (40) | 1923 (53) | −13.6 (−23.1 to −4.1) | 775 (56) | 2924 (64) | −7.3 (−10.0 to −4.3) |
| Race/ethnicity, No. (%)b | ||||||
| African ancestry | 116 (100) | 3608 (100) | 0 | 570 (41) | 1737 (38) | 3.6 (0.61 to 6.6) |
| Hispanic/Latino | 806 (59) | 2857 (62) | −3.6 (−6.6 to 0.61) | |||
| Body mass indexc | ||||||
| No. (%) | 114 (98.2) | 3545 (98.3) | 0.02 (−2.4 to 2.5) | 1341 (97.5) | 4174 (90.9) | 6.6 (5.4 to 7.8) |
| Median (IQR) | 30 (26 to 25) | 30 (26 to 35) | 0 (−1.3 to 2.4) | 30 (26 to 36) | 29 (25 to 33) | 1.0 (−1.3 to 1.4) |
| Systolic blood pressure, mm Hg | ||||||
| No. (%) | 113 (97.4) | 3485 (96.6) | 0.8 (−2.6 to 4.2) | 1372 (99.7) | 4338 (94.4) | 5.3 (4.5 to 6.1) |
| Median (IQR) | 130 (120 to 138) | 130 (122 to 140) | 0 (−2.9 to 5.2) | 130 (118 to 145) | 132 (120 to 148) | −2.0 (−2.8 to 0.03) |
| Diastolic blood pressure, mm Hg | ||||||
| No. (%) | 113 (97.4) | 3485 (96.6) | 0.8 (−2.6 to 4.2) | 1372 (99.7) | 4338 (94.4) | 5.3 (4.5 to 6.1) |
| Median (IQR) | 77 (70 to 80) | 77 (71 to 81) | 0 (−2.9 to 2.5) | 75 (68 to 82) | 77 (70 to 82) | −2.0 (−4.1 to −0.65) |
| Blood pressure, No. (%) | ||||||
| Systolic ≥140 mm Hg | 26 (23) | 872 (25) | −1.8 (−9.9 to 6.4) | 492 (36) | 1766 (41) | −4.8 (−7.8 to −1.9) |
| Diastolic ≥90 mm Hg | 3 (2.7) | 187 (5.4) | −2.6 (−6.0 to 0.8) | 226 (16) | 616 (14) | 2.3 (0.003 to 4.5) |
| Hypertension diagnosis, No. (%) | 97 (84) | 2799 (78) | 6.0 (−1.2 to 13) | 1244 (90) | 3465 (75) | 15.0 (13.0 to 17.0) |
| Antihypertensive medication, No. (%) | 104 (89.6) | 3294 (91) | −1.6 (−7.7 to 4.4) | 1366 (99) | 4151 (90) | 8.9 (7.9 to 9.9) |
| Diabetes, No. (%) | 52 (45) | 1349 (38) | 7.4 (−2.1 to 17.1) | 761 (55) | 1936 (42) | 13.0 (10.0 to 16.0) |
| Lipid-lowering medication, No. (%) | 88 (76) | 2452 (68) | 7.9 (−0.5 to 16.3) | 930 (68) | 2574 (56) | 12.0 (8.7 to 14.0) |
| Current smoker, No. (%) | 12 (11) | 527 (15) | −4.3 (−10.3 to 1.8) | 252 (18) | 666 (14) | 3.8 (1.5 to 6.1) |
| Myocardial infarction or coronary revascularization, No. (%) | 31 (27) | 722 (20) | 6.7 (−1.9 to 15.3) | 412 (30) | 596 (13) | 17.0 (14.0 to 20.0) |
Abbreviation: IQR, interquartile range.
The absolute difference between groups was calculated as a difference in proportions (percentages) for count data and as the difference in medians for continuous data. The 95% CIs for the difference between medians was calculated by bootstrapping.
Genetically inferred ancestry in cross-sectional cohort data; self-reported ancestry in case-control study.
Calculated as weight in kilograms divided by height in meters squared.
Fifty-one of 116 TTR V122I variant carriers (44%) older than age 50 years had prevalent heart failure or CM compared with 1070 of 3608 noncarriers (30%) (difference, 14.3% [95% CI, 4.5%-23.9%]; P = .001). V122I variant carriers had significantly higher age- and sex-adjusted odds of prevalent heart failure or CM (OR, 1.7 [95% CI, 1.2-2.4]; P = .006) compared with noncarriers. Although the point estimate attenuated, this association was robust to sequential adjustment for the clinical diagnosis of hypertension and history of myocardial infarction or coronary revascularization (Table 2).
Table 2. Association of TTR V122I Carrier Status With Heart Failure.
| Model | Covariates | Cross-Sectional Cohort | Case-Control Study | ||
|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | ||
| 1 | V122I + age + sex + principal componentsa | 1.7 (1.2-2.4) | .006 | 1.8 (1.2-2.7) | .008 |
| 2 | Model 1 + hypertension | 1.6 (1.1-2.3) | .01 | 1.8 (1.2-2.7) | .009 |
| 3 | Model 2 + myocardial infarction/coronary revascularization | 1.6 (1.06-2.3) | .03 | 1.8 (1.1-2.8) | .01 |
Genetic principal components were calculated from genome-wide genotype data and included in the analysis to control for confounding by ancestry.
Sex-stratified analyses demonstrated stronger associations between TTR V122I variant and prevalent heart failure or CM in men (age- and genetic principal component–adjusted OR, 2.7 [95% CI, 1.6-4.5]; P < .001) than in women (age- and genetic principal component–adjusted OR, 0.92 [95% CI, 0.46-1.9]; P = .81) with a significant difference in the OR (ROR, 2.9 [95% CI, 1.2-7.0]; P = .02). This was robust to sequential adjustment for age, sex, principal components, hypertension, and myocardial infarction or coronary revascularization (eTable 4 in the Supplement).
Among elderly (age ≥70 years) male TTR V122I carriers, the rate of heart failure or CM was 70%, and was 100% among those older than 80 years of age (eFigures 1 and 2 in the Supplement). Sensitivity analyses of male participants stratified by age in decades demonstrated significant associations of the V122I variant with heart failure or CM in individuals 60 to 69 years of age (genetic principal component–adjusted OR, 3.4 [95% CI, 1.3-9.5]; P = .02) and in those 70 years of age and older (genetic principal component–adjusted OR, 3.1 [95% CI, 1.2-8.3]; P = .02), but not in individuals between 50 and 59 years of age (genetic principal component–adjusted OR, 1.6 [95% CI, 0.63-3.9]; P = .33) (eTable 5 in the Supplement). The pairwise differences in point estimates between the age groups aged 50 to 59 years and 60 to 69 years (ROR, 2.2 [95% CI, 0.57-8.7]; P = .25) or the 70 years and older group (ROR, 2.0 [95% CI, 0.52-7.6]; P = .31) were not significant.
V122I and Heart Failure in the Case-Control Study
There were 1376 individuals of self-reported African or Hispanic/Latino ancestry with prevalent heart failure and 4594 individuals of self-reported African or Hispanic/Latino ancestry older than 65 years without heart failure. Heart failure cases had a longer follow-up time (9 years [IQR, 6-12]) compared with controls with heart failure (8 years [IQR 4-11]) (difference, 1.5 years [95% CI, 1.1-1.9]; P < .001). Individuals with heart failure were significantly more likely to be younger (cases: 70 years [IQR, 61-79]; controls: 74 years [IQR, 69-80]; difference, −4.0 years [95% CI, −5.0 to −3.1]; P < .001) and have higher body mass index (cases: 30 [IQR 26-36]; controls: 29 [IQR 25-33]; difference, 1.0 [95% CI, −1.3 to 1.4]; P < .001) and lower systolic blood pressure (cases: 130 mm Hg [IQR, 118-145]; controls: 132 mm Hg [IQR, 120-148]; difference, −2.0 mm Hg [95% CI, −2.8 to 0.03]; P < .001) (Table 1).
Compared with controls, a significantly greater proportion of heart failure cases were men (cases: n = 601 [44%]; controls: n = 1670 [36%]; difference, 7.3% [95% CI, 4.3%-10%]; P < .001), with proportionally more hypertension diagnoses (cases: n = 1244 [90%]; controls: n = 3465 [75%]; difference, 15% [95% CI, 13%-17%]; P < .001), more participants with a diastolic blood pressure greater than or equal to 90 mm Hg (cases: n = 226 [16%]; controls: n = 616 [14%]; difference, 2.3% [95% CI, 0.003%-4.5%]; P < .001), more antihypertensive medication prescriptions (cases: n = 1366 [99%]; controls: n = 4151 [90%]; difference, 8.9% [95% CI, 7.9%-9.9%]; P < .001), more diabetes diagnoses (cases: n = 761 [55%]; controls: n = 1936 [42%]; difference, 13% [95% CI, 10%-16%]; P < .001), more prescriptions for lipid-lowering medications (cases: n = 930 [68%]; controls: n = 2574 [56%]; difference, 12.0% [95% CI, 8.7%-14.0%]; P < .001), more current smokers (cases: n = 252 [18%]; controls: n = 666 [14%]; difference, 3.8% [95% CI, 1.5%-6.1%]; P < .001), and more myocardial infarction or coronary revascularization diagnoses (cases: n = 412 [30%]; controls: n = 596 [13%]; difference, 17% [95% CI, 14%-20%]; P < .001) (Table 1).
Echocardiograms were obtained clinically in a significantly greater proportion of heart failure cases (n = 1220 [89%]) than controls (n = 1866 [41%]) (difference, 48% [95% CI, 46%-50%]; P < .001). There was complete ascertainment of binary clinical covariates, and missingness for the continuous measures of body mass index and blood pressure were less than 10% (Table 1). Significantly more heart failure cases had a recorded body mass index (n = 1341 [97.5%]) or blood pressure (n = 1372 [99.7%]) than controls (body mass index: n = 4147 [90.9%]; difference, 6.6% [95% CI, 5.4%-7.8%], P < .001; blood pressure: n = 4338 [94.4%]; difference, 5.3% [95% CI, 4.5%-6.1%], P < .001).
Among the combined group of individuals of self-reported African and Hispanic/Latino ancestry, 36 of 1376 individuals (2.6%) with heart failure and 82 of 4594 controls (1.8%) carried the TTR V122I variant (difference, 0.8% [95% CI, −0.1% to 1.8%]; P = .07). This translated into V122I carriers having significantly higher odds of heart failure (OR, 1.8 [95% CI, 1.2 to 2.7]; P = .008) than noncarriers among individuals of self-reported African and Hispanic/Latino ancestry, after adjusting for age, sex, and 10 genetic principal components. The associations remained statistically significant after adjusting for clinical covariates including hypertension and myocardial infarction or coronary revascularization (Table 2). There were no differential associations of carrier status by self-reported ancestry (ROR, 0.90 [95% CI, 0.38-2.1]; P = .81) (eFigure 3 in the Supplement) despite the observed differences in carrier rate seen between self-reported African and Hispanic/Latino ancestry groups (eTable 2 in the Supplement).
V122I and Cardiac Morphology
In the cross-sectional cohort, the availability of clinically obtained outpatient echocardiogram data were not significantly different between V122I variant carriers and noncarriers (carriers: n = 56 [48%]; noncarriers: n = 1562 [43%]; difference, 5.0% [95% CI, −4.7% to 15.0%]; P = .29) and although all parameters were not recorded for all patients with data, there were no significant differences by carrier status. Using these data, we compared the cardiac morphology of TTR V122I variant carriers with that of noncarriers. TTR V122I carrier status was associated with significantly thicker interventricular septal wall thickness (carriers: 12 mm [IQR, 11-14], noncarriers: 11 mm [IQR, 10-13], P = .01; adjusted β [log-transformed], 0.08 [95% CI, 0.03-0.14], P = .02), LV posterior wall thickness in diastole (carriers: 12 mm [IQR, 11-14], noncarriers: 11 mm [IQR, 10-12], P = .002; adjusted β [log-transformed], 0.1 [95% CI, 0.05-0.14], P < .001), relative wall thickness (carriers: 0.49 mm [IQR, 0.43-0.63], noncarriers: 0.48 mm [IQR, 0.40-0.56], P = .09; adjusted β [log-transformed], 0.04 [95% CI, 0.01-0.06], P = .003), and LV mass (carriers: 206 g [IQR, 165-292], noncarriers: 189 g [IQR, 147-242], P = .02; adjusted β [log-transformed], 0.11 [95% CI, 0.01-0.20], P = .03) as shown in eTable 6 in the Supplement. LV cavity size (end-diastolic diameter) was the same in carriers (46 mm [IQR, 41-52]) and noncarriers (46 mm [IQR, 41-52]; P = .70). These changes are consistent with concentric LV wall thickening and are robust to controlling for underlying hypertension and myocardial infarction or coronary revascularization (eTable 7 in the Supplement). Similar findings were seen when the analysis was restricted to participants with heart failure (eTables 8 and 9 in the Supplement). There were no significant differences in LV ejection fraction after accounting for age, sex, and principal components in any of the analyses.
We analyzed echocardiographic data from 4124 participants of self-reported African and Hispanic/Latino ancestry without a diagnosis of heart failure from the population from which the case-control study was derived stratified by age category. Of these, 4094 participants (99.3%) had available data relating to LV hypertrophy and 1045 (25.5%) had LV hypertrophy diagnosed by the echocardiographer reading the study (eTable 10 in the Supplement). We observed a significantly higher risk of LV hypertrophy in TTR V122I carriers (n = 5/10 [50%]) compared with noncarriers (n = 39/468 [8.3%]) with an age at enrollment of 45 years or younger (difference, 42.0% [95% CI, 5.5%-78.0%]; adjusted OR, 10 [95% CI, 2.2-47]; P < .01) (eTable 11 and eFigure 4 in the Supplement). Similarly, interventricular septal thickness during diastole was significantly thicker in TTR V122I variant carriers (11 mm [IQR, 10-12]) compared with noncarriers (9 mm [IQR, 8-10]) aged 45 years or younger (adjusted β [log-transformed], 0.12 [95% CI, 0.013-0.23], P = .02), as was LV posterior wall thickness in diastole (carriers: 11 mm [IQR 10-11], noncarriers: 9 mm [IQR 8-10]; adjusted β [log-transformed], 0.12 [95% CI, 0.008-0.14], P = .04) as shown in eTables 12 and 13 and eFigure 5 in the Supplement.
No significant associations between TTR V122I and LV hypertrophy or interventricular septal thickness were observed in participants older than age 65 years or between older than age 45 years and 65 years or younger. The differences in each of these groups was significantly different than that of the youngest age group for LV hypertrophy (group aged ≤45 years old compared with >65 years old: ROR, 9.5 [95% CI, 1.8-51.0], P = .009; group aged ≤45 years old compared with >45-≤65 years old: ROR, 8.9 [95% CI, 1.5-52.0], P = .02) but not LV posterior wall thickness (group aged ≤45 years old compared with >65 years old: difference in β, 0.12 [95% CI, 0.00-0.24], P = .05; group aged ≤45 years old compared with >45-≤65 years old: difference in β, 0.12 [95% CI, −0.01 to 0.25], P = .06) or interventricular septal thickness in diastole (group aged ≤45 years old compared with >65 years old: difference in β, 0.14 [95% CI, 0.01-0.26], P = .03; group aged ≤45 years old compared with >45-≤65 years old: difference in β, 0.11 [95% CI, −0.01 to 0.24], P = .08). There were no significant differences in echocardiographic parameter ascertainment by TTR Vq122I variant carrier status in these analyses (eTable 13 in the Supplement).
Diagnosis of hATTR-CM in TTR V122I Carriers
We examined in detail the EHRs of the 116 V122I carriers in the cross-sectional cohort by physician medical record review (Figure 1). All but 2 of the 51 carriers who had ICD codes for heart failure or CM were confirmed to have heart failure based on medical record review, demonstrating a positive predictive value of 96% in this cohort. Conversely, 4 of the carriers without an ICD code for heart failure or CM were identified as having clinical heart failure. Of the 53 V122I carriers with heart failure based on medical record review (eTable 14 in the Supplement), there were 19 cases (36%) of ischemic CM. Only 9 carriers had been evaluated for, and subsequently diagnosed as having, hATTR-CM (Figure 1). In 8 of the 9 cases, the diagnosis of amyloid was made via cardiac biopsy with subsequent confirmatory clinical genetic testing revealing the participants were carriers of the V122I variant. The remaining individual was identified by direct genetic testing, which was positive for the V122I variant.
We similarly reviewed the EHRs of the 39 TTR V122I carriers with heart failure in the case-control study (Figure 1; eTable 14 in the Supplement). All individuals with a heart failure code were clinically determined to have heart failure. There were 6 individuals (15%) with ischemic CM. Only 1 participant had an hATTR-CM diagnosis, which was based on cardiac biopsy.
Across both analyses, there were a total of 92 individuals who carried the V122I allele and had a clinical diagnosis of heart failure (Table 3). Even after excluding the 25 with evidence of ischemic heart disease, there were 67 individuals whose heart failure was likely specifically due to hATTR-CM caused by TTR V122I, of whom only 10 had been diagnosed. The median time from onset of symptoms to diagnosis of hATTR-CM was 3 years (IQR, 2-5).
Table 3. Characteristics of the TTR V122I Carriers With Heart Failure or Cardiomyopathy.
| No. (%) | ||
|---|---|---|
| Patients Diagnosed as Having hATTR-CM (n = 10) | Patients Not Evaluated for hATTR-CM (n = 82) | |
| Age, median (IQR), y | 70 (65-75) | 69 (61-78) |
| Sex | ||
| Male | 10 (100) | 46 (56) |
| Female | 0 | 36 (44) |
| Hypertension | 8 (80) | 71 (87) |
| Ischemic cardiomyopathy | 3 (30) | 22 (27) |
| Arrhythmias | 8 (80) | 29 (35) |
| Electrocardiogram abnormalities | 7 (70) | 26 (32) |
| Low-voltage electrocardiogram | 4 (40) | 12 (15) |
| Carpal tunnel syndrome | 5 (50) | 8 (10) |
| Neuropathy | 5 (50) | 19 (23) |
| Cardiac biopsy | 9 (90) | 0 |
| Genetic testing | 8 (80) | 0 |
| Delay in diagnosis, median (IQR), y | 3 (2-5) | NA |
Abbreviations: hATTR-CM, hereditary transthyretin amyloid cardiomyopathy; IQR, interquartile range; NA, not applicable.
Discussion
In this study of individuals of African or Hispanic/Latino ancestry undergoing care in integrated academic health care systems, TTR V122I carrier status was significantly associated with prevalent heart failure and cardiac morphology, consistent with concentric LV wall thickening and hypertrophy. This is consistent with findings from previous prospective cohort studies, including both the Cardiovascular Health Study and the Atherosclerosis Risk in Communities Study.6,12
Additionally, previous studies have attempted to highlight the relative underdiagnosis of hATTR-CM due as a cause of CM and heart failure in elderly individuals of African ancestry.8,9,25,26 The current study adds to this literature, providing data from routine clinical care indicating that despite having phenotypic characteristics of hATTR-CM, very few individuals of African ancestry with heart failure underwent evaluation for hATTR-CM or genetic testing for TTR V122I in standard clinical practice. Furthermore, for the minority that were diagnosed, there were lengthy delays in obtaining a molecular diagnosis of hATTR-CM. This suggests that even at tertiary referral centers, there is significant under-recognition, underdiagnosis, and delay in diagnosis of TTR cardiac amyloid as a cause of heart failure in older individuals of African ancestry.
Individuals of self-reported Hispanic/Latino ancestry from the New York City region are known to have significant levels of recent African ancestry due to admixture,13,14,27 and thus harbor an unrecognized risk of hATTR-CM due to the TTR V122I variant, which may be overlooked for testing and treatment. This is in counterdistinction to self-identifying Hispanic/Latino individuals from other geographic locations, such as the West Coast, where population migrations have resulted in higher rates of individuals from Mexico and Central and South America with predominantly admixed Amerindian and European genetic backgrounds.28 In these populations, the underlying rates of the TTR V122I variant would be expected to be extremely low, as reflected in the analysis of allele carrier rate by self-identified country of origin (Figure 2 and eTable 3 in the Supplement). The data also suggest that the highest TTR V122I carrier rates are in the subpopulation of individuals specifically with West African ancestry, similar to what has been reported by Jacobson and colleagues4 in their examination of the prevalence of the TTR V122I allele in Africa. This study emphasized the need to consider screening for hATTR-CM due to V122I in diverse populations, particularly in underserved ethnic minority populations, and incorporating detailed ancestry information into clinical care.
The recent development of efficacious targeted therapies for ATTR has increased the urgency of prompt diagnosis of hATTR-CM, including that due to the TTR V122I variant. The APOLLO trial with the small interfering RNA patisiran29 demonstrated significant improvement in symptoms of both neuropathy, as well as a subset of patients with CM, leading to approval of patisiran by the US Food and Drug Administration in August 2018 for the treatment of hATTR polyneuropathy. The NEURO-TTR trial with the antisense oligonucleotide inotersen30 demonstrated significant benefit and led to Food and Drug Administration approval of inotersen in October 2018, again with the indication of amyloid polyneuropathy. Both of these approaches are being studied for their benefit in ATTR-CM (ClinicalTrials.gov NCT03997383 and NCT03702829). Most relevant at this time for the treatment of ATTR-CM, the Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT),10 demonstrated that treatment with the small-molecule tafamidis resulted in reductions in all-cause mortality and lower rates of functional decline in patients with ATTR-CM and led to its approval in May 2019 for treating ATTR-CM. The availability of tafamidis, and these additional emerging therapies, emphasize the importance of early identification of hereditary TTR cardiac amyloidosis, of which TTR V122I is by far the most common cause, as a treatable cause of heart failure.
Higher rates of LV hypertrophy and greater interventricular septal thickness among younger TTR V122I carriers without overt heart failure were detected, suggesting a subtle cardiac phenotype may develop years prior to the onset of overt disease. The absolute differences in echocardiographic parameters between TTR V122I variant carriers and noncarriers are small and represent subtle differences between the 2 populations. These differences are unlikely to be of clinical significance, or even detectable, at the individual level. Nonetheless, this is in contrast to findings reported in baseline echocardiographic data from the Atherosclerosis Risk in Communities Study.12 The current analysis of echocardiographic parameters included approximately 3 times the number of participants as those analyzed in the Atherosclerosis Risk in Communities Study and 2 times more carriers of the V122I allele, providing more statistical power to detect subclinical differences. Alternatively, the differences in results could be due to differences in the baseline characteristics of the populations or bias introduced by our use of clinically obtained echocardiograms.
Limitations
This study has several limitations. First, the analyses were conducted in EHR-linked genetic biobanks comprised of patients seeking care at 1 of 2 integrated academic health care systems and relying on clinically obtained data. Because phenotype data were extracted from the EHR and assignment of heart failure or CM was based on diagnosis codes, phenotype misclassification may be greater than that which would be seen in an adjudicated cohort. The medical record review of TTR V122I carriers with heart failure or CM suggests that this is minimal.
Second, in keeping with this, the echocardiographic measures were extracted from participants who had clinically obtained studies, which could have produced a bias in the resulting distribution of echocardiographic parameters.
Third, we used complete case analysis for our statistical modeling, which has the potential to introduce bias. For the analysis of the primary outcomes, this should be minimal because there was complete ascertainment of all exposures, covariates, and outcomes.
Fourth, incomplete penetrance of the TTR V122I variant and other prevalent causes of heart failure, such as ischemic CM, result in the fact that some carriers of TTR V122I with heart failure likely do not have hATTR-CM, leading to possible overestimation of the under-recognized nature of hATTR-CM.
Fifth, despite both cohorts using recruitment strategies that were agnostic to participant phenotype, this approach undoubtedly introduced some bias into the cohort. The University of Pennsylvania has a robust advanced heart failure and transplant program, which likely leads to increased rates of individuals with heart failure and bias toward more advanced heart failure phenotypes overall among participants in the cohort study. As a result, these results may not be representative of the general elderly population of African ancestry. With respect to patients recruited from the primary care setting for the case-control study and associated analyses, these individuals may be more likely to seek and receive routine health care than the population at large. Because of these limitations, the point estimates from our association testing and assessment of the rates of hATTR-CM underdiagnosis must be interpreted with caution.
Conclusions
Among individuals of African or Hispanic/Latino ancestry enrolled in 2 academic medical center–based biobanks, the TTR V122I genetic variant was significantly associated with heart failure.
eMethods. Illumina Global Screening Array Genotyping and Quality Control
eTable 1. List of CPT (Current Procedural Terminology), Diagnosis and Procedure Codes to Identify Evaluation for Hereditary Transthyretin (TTR) Amyloid Cardiomyopathy (hATTR-CM) and Diagnosis of Amyloid
eTable 2. Carrier Rates of TTR V122I by Ancestry Group
eTable 3. Carrier Counts and Rates of TTR V122I by 154 Countries/Regions of Origin
eTable 4. Sex-Stratified Analysis of Prevalent Heart Failure or Cardiomyopathy in PMBB
eTable 5. Age-Stratified Analysis of Prevalent Heart Failure or Cardiomyopathy Among Male Participants in PMBB
eTable 6. Clinically Obtained Echocardiographic Characteristics of Participants by TTR V122I Carrier Status in PMBB
eTable 7. Association of TTR V122I Carrier Status With Clinically Obtained Transthoracic Echocardiographic Parameters in PMBB
eTable 8. Clinically Obtained Echocardiographic Characteristics of Participants With Heart Failure or Cardiomyopathy by TTR V122I Carrier Status in PMBB
eTable 9. Association of TTR V122I Carrier Status With Clinically Obtained Transthoracic Echocardiographic Parameters in Participants With Heart Failure or Cardiomyopathy in PMBB
eTable 10. Number and Percentage of Participants With Left Ventricular Hypertrophy Among BioMe Participants (Self-Reported African and Hispanic/Latino Ancestries) Without Heart Failure (total N=4,094)
eTable 11. Genetic Association of TTR V122I on Left Ventricular (LV) Hypertrophy Among Individuals Without Heart Failure in BioMe
eTable 12. Genetic Association of TTR V122I With Diastolic Intraventricular Septal Thickness Among Individuals Without Heart Failure in BioMe
eTable 13. Genetic Association of TTR V122I With Additional Echocardiographic Parameters Among Individuals Without Heart Failure in BioMe
eTable 14. Characteristics of TTR V122I Carriers With Heart Failure That Underwent Detailed Chart Review
eFigure 1. Prevalence of Clinically Diagnosed Heart Failure or Cardiomyopathy by Age and Sex in TTR V122I Carriers in PMBB
eFigure 2. Cumulative Prevalence of Clinically Diagnosed Heart Failure or Cardiomyopathy by Age in TTR V122I Carriers in PMBB
eFigure 3. Ancestry-Specific Association of TTR V122I Carrier Status With Prevalent Heart Failure in BioMe
eFigure 4. Genetic Association Results of Transthyretin (TTR) V122I on Left Ventricular Hypertrophy Among BioMe Participants Without Heart Failure
eFigure 5. Genetic Association Results of Transthyretin (TTR) V122I on Diastolic Intraventricular Septal Thickness Among BioMe Participants Without Heart Failure
References
- 1.Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126(10):1286-1300. doi: 10.1161/CIRCULATIONAHA.111.078915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rapezzi C, Quarta CC, Riva L, et al. Transthyretin-related amyloidoses and the heart: a clinical overview. Nat Rev Cardiol. 2010;7(7):398-408. doi: 10.1038/nrcardio.2010.67 [DOI] [PubMed] [Google Scholar]
- 3.Gorevic PD, Prelli FC, Wright J, Pras M, Frangione B. Systemic senile amyloidosis. Identification of a new prealbumin (transthyretin) variant in cardiac tissue: immunologic and biochemical similarity to one form of familial amyloidotic polyneuropathy. J Clin Invest. 1989;83(3):836-843. doi: 10.1172/JCI113966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobson DR, Alexander AA, Tagoe C, et al. The prevalence and distribution of the amyloidogenic transthyretin (TTR) V122I allele in Africa. Mol Genet Genomic Med. 2016;4(5):548-556. doi: 10.1002/mgg3.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobson DR, Alexander AA, Tagoe C, Buxbaum JN. Prevalence of the amyloidogenic transthyretin (TTR) V122I allele in 14 333 African-Americans. Amyloid. 2015;22(3):171-174. doi: 10.3109/13506129.2015.1051219 [DOI] [PubMed] [Google Scholar]
- 6.Quarta CC, Buxbaum JN, Shah AM, et al. The amyloidogenic V122I transthyretin variant in elderly black Americans. N Engl J Med. 2015;372(1):21-29. doi: 10.1056/NEJMoa1404852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maurer MS, Hanna M, Grogan M, et al. ; THAOS Investigators . Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J Am Coll Cardiol. 2016;68(2):161-172. doi: 10.1016/j.jacc.2016.03.596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobson DR, Reveille JD, Buxbaum JN. Frequency and genetic background of the position 122 (Val----Ile) variant transthyretin gene in the black population. Am J Hum Genet. 1991;49(1):192-198. [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobson D, Tagoe C, Schwartzbard A, Shah A, Koziol J, Buxbaum J. Relation of clinical, echocardiographic and electrocardiographic features of cardiac amyloidosis to the presence of the transthyretin V122I allele in older African-American men. Am J Cardiol. 2011;108(3):440-444. doi: 10.1016/j.amjcard.2011.03.069 [DOI] [PubMed] [Google Scholar]
- 10.Maurer MS, Schwartz JH, Gundapaneni B, et al. ; ATTR-ACT Study Investigators . Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016. doi: 10.1056/NEJMoa1805689 [DOI] [PubMed] [Google Scholar]
- 11.Buxbaum JN, Ruberg FL. Transthyretin V122I (pV142I)* cardiac amyloidosis: an age-dependent autosomal dominant cardiomyopathy too common to be overlooked as a cause of significant heart disease in elderly African Americans. Genet Med. 2017;19(7):733-742. doi: 10.1038/gim.2016.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buxbaum J, Alexander A, Koziol J, Tagoe C, Fox E, Kitzman D. Significance of the amyloidogenic transthyretin Val 122 Ile allele in African Americans in the Arteriosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Am Heart J. 2010;159(5):864-870. doi: 10.1016/j.ahj.2010.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.BioMeTM BioBank Program https://icahn.mssm.edu/research/ipm/programs/biome-biobank. Accessed May 10, 2018.
- 14.Tayo BO, Teil M, Tong L, et al. Genetic background of patients from a university medical center in Manhattan: implications for personalized medicine. PLoS One. 2011;6(5):e19166. doi: 10.1371/journal.pone.0019166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dewey FE, Gusarova V, Dunbar RL, et al. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med. 2017;377(3):211-221. doi: 10.1056/NEJMoa1612790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dewey FE, Murray MF, Overton JD, et al. Distribution and clinical impact of functional variants in 50,726 whole-exome sequences from the DiscovEHR study. Science. 2016;354(6319):aaf6814. doi: 10.1126/science.aaf6814 [DOI] [PubMed] [Google Scholar]
- 17.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4(1):7. doi: 10.1186/s13742-015-0047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of heart failure diagnoses in administrative databases: a systematic review and meta-analysis. PLoS One. 2014;9(8):e104519. doi: 10.1371/journal.pone.0104519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57(6):450-458. doi: 10.1016/0002-9149(86)90771-X [DOI] [PubMed] [Google Scholar]
- 20.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904-909. doi: 10.1038/ng1847 [DOI] [PubMed] [Google Scholar]
- 21.Schwarzer G. meta: {A}n {R} package for meta-analysis. R News. 2007;7(3):40-45. [Google Scholar]
- 22.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326(7382):219-219. doi: 10.1136/bmj.326.7382.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthews JN, Altman DG. Interaction 3: how to examine heterogeneity. BMJ. 1996;313(7061):862. doi: 10.1136/bmj.313.7061.862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.R Core Team R: A Language and Environment for Statistical Computing. 2017.
- 25.Damy T, Costes B, Hagège AA, et al. Prevalence and clinical phenotype of hereditary transthyretin amyloid cardiomyopathy in patients with increased left ventricular wall thickness. Eur Heart J. 2016;37(23):1826-1834. doi: 10.1093/eurheartj/ehv583 [DOI] [PubMed] [Google Scholar]
- 26.Buxbaum J, Jacobson DR, Tagoe C, et al. Transthyretin V122I in African Americans with congestive heart failure. J Am Coll Cardiol. 2006;47(8):1724-1725. doi: 10.1016/j.jacc.2006.01.042 [DOI] [PubMed] [Google Scholar]
- 27.Bryc K, Durand EY, Macpherson JM, Reich D, Mountain JL. The genetic ancestry of African Americans, Latinos, and European Americans across the United States. Am J Hum Genet. 2015;96(1):37-53. doi: 10.1016/j.ajhg.2014.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conomos MP, Laurie CA, Stilp AM, et al. Genetic diversity and association studies in US Hispanic/Latino populations: applications in the Hispanic Community Health Study/Study of Latinos. Am J Hum Genet. 2016;98(1):165-184. doi: 10.1016/j.ajhg.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams D, Gonzalez-Duarte A, O’Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11-21. doi: 10.1056/NEJMoa1716153 [DOI] [PubMed] [Google Scholar]
- 30.Benson MD, Waddington-Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):22-31. doi: 10.1056/NEJMoa1716793 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Illumina Global Screening Array Genotyping and Quality Control
eTable 1. List of CPT (Current Procedural Terminology), Diagnosis and Procedure Codes to Identify Evaluation for Hereditary Transthyretin (TTR) Amyloid Cardiomyopathy (hATTR-CM) and Diagnosis of Amyloid
eTable 2. Carrier Rates of TTR V122I by Ancestry Group
eTable 3. Carrier Counts and Rates of TTR V122I by 154 Countries/Regions of Origin
eTable 4. Sex-Stratified Analysis of Prevalent Heart Failure or Cardiomyopathy in PMBB
eTable 5. Age-Stratified Analysis of Prevalent Heart Failure or Cardiomyopathy Among Male Participants in PMBB
eTable 6. Clinically Obtained Echocardiographic Characteristics of Participants by TTR V122I Carrier Status in PMBB
eTable 7. Association of TTR V122I Carrier Status With Clinically Obtained Transthoracic Echocardiographic Parameters in PMBB
eTable 8. Clinically Obtained Echocardiographic Characteristics of Participants With Heart Failure or Cardiomyopathy by TTR V122I Carrier Status in PMBB
eTable 9. Association of TTR V122I Carrier Status With Clinically Obtained Transthoracic Echocardiographic Parameters in Participants With Heart Failure or Cardiomyopathy in PMBB
eTable 10. Number and Percentage of Participants With Left Ventricular Hypertrophy Among BioMe Participants (Self-Reported African and Hispanic/Latino Ancestries) Without Heart Failure (total N=4,094)
eTable 11. Genetic Association of TTR V122I on Left Ventricular (LV) Hypertrophy Among Individuals Without Heart Failure in BioMe
eTable 12. Genetic Association of TTR V122I With Diastolic Intraventricular Septal Thickness Among Individuals Without Heart Failure in BioMe
eTable 13. Genetic Association of TTR V122I With Additional Echocardiographic Parameters Among Individuals Without Heart Failure in BioMe
eTable 14. Characteristics of TTR V122I Carriers With Heart Failure That Underwent Detailed Chart Review
eFigure 1. Prevalence of Clinically Diagnosed Heart Failure or Cardiomyopathy by Age and Sex in TTR V122I Carriers in PMBB
eFigure 2. Cumulative Prevalence of Clinically Diagnosed Heart Failure or Cardiomyopathy by Age in TTR V122I Carriers in PMBB
eFigure 3. Ancestry-Specific Association of TTR V122I Carrier Status With Prevalent Heart Failure in BioMe
eFigure 4. Genetic Association Results of Transthyretin (TTR) V122I on Left Ventricular Hypertrophy Among BioMe Participants Without Heart Failure
eFigure 5. Genetic Association Results of Transthyretin (TTR) V122I on Diastolic Intraventricular Septal Thickness Among BioMe Participants Without Heart Failure