ABSTRACT
While the complement cascade is an important component of the innate immune system, uncontrolled activation can cause severe disease. This concept is illustrated by the prototypical complement-mediated renal disease atypical haemolytic uraemic syndrome (aHUS), which causes renal failure if untreated but when managed with the complement inhibitor eculizumab leaves the patient vulnerable to infection with encapsulated organisms. Complement activation is also implicated in the pathogenesis of many other renal and non-renal diseases, necessitating an understanding of complement biology and diagnostics. We review renal diseases in which complement over-activation is known to cause tissue injury; aHUS and C3 glomerulopathy. We also discuss the contribution of complement more widely to the pathophysiology of renal disease, and highlight the significance and side effects of anti-complement therapy relevant to the general physician.
KEYWORDS: Complement, atypical haemolytic uraemic syndrome, C3 glomerulopathy, thrombotic microangiopathy, glomerulonephritis n
Key points
The complement cascade is vital for maintaining health, but the kidney is particularly vulnerable to complement over-activation.
Complement-mediated atypical haemolytic uraemic syndrome (aHUS) is an important differential in the patient with thrombotic microangiopathy.
Emerging evidence implicates complement dysregulation in a wide range of kidney diseases, potentially identifying new therapeutic areas.
Measurement of serum C3 and C4 levels can provide important diagnostic information in the assessment of a patient with kidney disease.
Eculizumab is the only complement inhibitor currently in clinical use in the UK and is highly effective in treating aHUS, but puts the patient at risk of infection with encapsulated organisms.
Introduction
The complement cascade is part of the innate immune system and plays an important role in defence against foreign pathogens such as bacteria and viruses. There are over 40 proteins involved in the complement cascade, and complement accounts for approximately 10% of circulating protein.1 Complement activation is complex and is achieved through three separate pathways (Fig 1).
Classical pathway: linking the innate and adaptive immune systems, the classical pathway is activated when immunoglobulin (Ig) M or IgG, complexed with antigen, bind complement protein C1q.
Mannose-binding lectin (MBL) pathway: activated when MBL, collectins or ficolins bind to carbohydrate residues found on pathogen cell surfaces.
Alternative pathway: unlike the other pathways, the alternative pathway is constantly active at a low level, allowing rapid amplification of complement in response to pathogens.
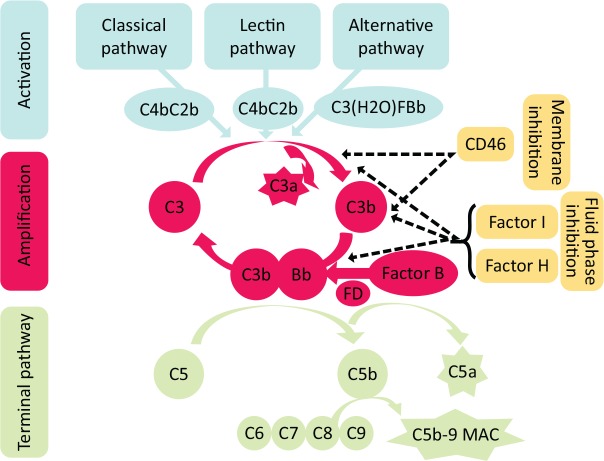
Fig 1.
Overview of complement activation pathways. Complement can be activated by three pathways: the classical pathway when antibody binds antigen, the lectin pathway activated by proteins that recognise carbohydrate residues on pathogens and the alternative pathway which is in a continuous state of low-level activation. All pathways converge to form a C3 convertase which converts C3 to its active split product C3b. Subsequent amplification is mainly driven by the alternative pathway when C3b binds to factor B which is then activated by the serine protease factor D. This results in formation of a C5 convertase complex and the activation of the terminal, effector pathway. Complement activation is controlled by cell surface and fluid phase inhibitory proteins that possess decay accelerating activity and cofactor activity to disrupt complexes and allow degradation of activated proteins by the protease factor I. FD = serine protease factor D; MAC = membrane attack complex.
If not regulated, activation of any pathway leads to generation of C3b, the activated form of C3 (Fig 1), and activation of the final common pathway that generates inflammatory mediators (C5a), and the membrane attack complex (MAC; C5b-9), which can cause cell lysis. To prevent excessive complement activation and injury to self, there are a series of soluble (fluid phase) and membrane bound inhibitory proteins. Failure of these mechanisms to limit complement activation is one of the main causes of complement-mediated renal disease.
Prototypical complement-mediated kidney diseases; atypical haemolytic uraemic syndrome and C3 glomerulopathy
The kidney is particularly susceptible to damage by complement.2 The role of complement is well established in the prototypical diseases; atypical haemolytic uraemic syndrome (aHUS) and C3 glomerulopathy. Complement-mediated aHUS is often a diagnosis of exclusion in a patient with a thrombotic microangiopathy (TMA), and therefore the differential diagnosis of TMA will be discussed.3
Atypical haemolytic uraemic syndrome
TMA is a process of microvascular thrombosis, commonly affecting small renal vessels but potentially affecting any organ. Microangiopathic haemolytic anaemia (MAHA) occurs as erythrocytes are destroyed when they pass through damaged vessels, leading to red cell fragmentation (shistocytes on a blood film, raised lactate dehydrogenase and bilirubin, and low haptoglobin). Platelet consumption leads to thrombocytopenia. The site of thrombus formation determines the clinical presentation, with renal involvement most frequently seen in haemolytic uraemic syndrome (HUS) or aHUS, and central nervous system involvement in thrombotic thrombocytopenic purpura (TTP). However, classification on clinical grounds alone is inaccurate as considerable overlap exists. Therefore, TMAs should be classified by aetiology, with TTP due to an inherited or functional deficiency in ADAMTS13 enzyme.
The most common cause of HUS is infection with Shiga toxin-producing Shigella or Escherichia coli (particularly E coli O157), accounting for 90% of cases. This is referred to as Shiga toxin-producing E coli (STEC) HUS. It usually presents with severe renal dysfunction days to weeks after a colitic illness, and management is supportive.
The remaining cases of HUS are collectively referred to as aHUS, but it is now clear that this term encompasses a range of diagnoses. Forty per cent of these cases are due to secondary causes such as malignant hypertension, infection, medications, cancer or autoimmune disease, and management is targeted at treating the underlying cause.3 The remaining 60% are primarily due to excessive activation of complement.
A clinical diagnosis of complement-mediated aHUS is made based on the exclusion of STEC infection, TTP (Table 1), and other secondary causes of TMA. This is important as treatment is different for these diseases, and early diagnosis and treatment improves patient outcome.
Table 1.
Contrasting features of different causes of thrombotic microangiopathy
| Feature | STEC-HUS | TTP | Complement-mediated aHUS |
|---|---|---|---|
| Presentation | 1–2 weeks after colitis-like illness | Prominent neurological symptoms | May have personal or family history of TMA |
| Laboratory tests | AKI predominates | Severe thrombocytopenia | AKI predominates |
| Diagnostic test | Stool culture STEC PCR | ADAMTS13 activity level | Genetic and autoantibody screening |
| Treatment | Supportive | Emergency plasma exchange, corticosteroids | Eculizumab |
aHUS = atypical haemolytic uraemic syndrome; AKI = acute kidney injury; HUS = haemolytic uraemic syndrome; PCR = polymerase chain reaction; STEC = Shiga toxin-producing Escherichia coli; TMA = thrombotic microangiopathy; TTP = thrombotic thrombocytopenic purpura.
Pathogenesis of complement-mediated aHUS
In approximately 60% of patients with aHUS, an inherited or acquired defect in complement regulation can be identified.4 The defect in complement control leads to excessive complement activation, causing endothelial dysfunction in small blood vessels and TMA. Heterozygotic loss of function mutations in inhibitory proteins (including factor H (20–30%), factor I (5–10%) and membrane cofactor protein (CD46; 10%)), or gain of function mutations in the activators, C3 (5%) or factor B (<5%), can predispose to disease. In addition, acquired autoantibodies that interfere with factor H function can cause disease (10%). The development of a TMA in patients who carry a disease-associated mutation is often preceded by a trigger, which can include infections or pregnancy.5
Age of onset varies from early childhood to adulthood, and presents with a TMA and features related to the organ system affected, such as acute kidney injury (AKI), myocardial infarction, stroke, or gastrointestinal bleeding, with 15% of patients having extra-renal disease. A family history of similar presentations makes the diagnosis of complement-mediated TMA more likely.
Once STEC-HUS and TTP have been excluded as the cause of TMA, genetic and autoantibody testing is essential but, given results will not be immediately available, they will not guide early treatment choices. C3 levels may be low, but normal levels do not exclude the diagnosis of complement-mediated aHUS.6
Management of complement-mediated aHUS
Previously, plasma exchange was the only available treatment but response was variable and 50% of patients died or required dialysis within a year of diagnosis. As the liver is the major site of complement protein synthesis, liver transplantation was used as a curative treatment.7 However, with the introduction of the anti-complement drug eculizumab, this option is now rarely used.8
C3 glomerulopathy
C3 glomerulopathy (C3G) is a rare, complement-mediated kidney disease and includes two over-lapping pathologies; dense deposit disease (DDD) and C3 glomerulonephritis.9 C3G at presentation is clinically indistinguishable from other glomerulonephritides, presenting with non-visible haematuria, proteinuria, hypertension, nephrotic syndrome and renal impairment. Diagnosis is therefore histopathological on renal biopsy, with sole or dominant C3 deposition in the glomeruli. The clinical and histological features of C3G can resemble post-infectious glomerulonephritis (PIGN) and a diagnosis of C3G should be considered in cases of PIGN that do not resolve. Median age of onset is 23 years, 50% of patients develop end-stage kidney disease by 10 years after diagnosis, and risk of recurrence in a renal transplant is high.
C3G is caused by excessive activation of the alternative complement pathway due to a genetic or acquired defect in complement regulation. Acquired disease may be caused by autoantibodies that stabilise the C3 or C5 convertases (nephritic factors) or block regulatory protein function. More recently the potential of a monoclonal immunoglobulin (or light chain) to deposit in the kidney and activate complement has been recognised. The monoclonal proteins may not be evident in the blood and require special techniques to detect their presence in the kidney. It is important to consider this diagnosis in older patients presenting with C3G as treatment of an underlying plasma cell dyscrasia can induce remission of the renal disease. The activated C3 fragments (including C3b, iC3b, C3dg and C3d) are deposited in the glomerular basement membrane, disrupting membrane function and causing an inflammatory response that leads to glomerular damage. Low serum levels of C3 are usually present due to C3 consumption.
The broader spectrum of complement-mediated kidney disease
Serum complement levels should be measured if there is a suspicion of an autoimmune renal disease in a patient with AKI or chronic kidney disease. Most hospital laboratories routinely give a result for C3 and C4 levels, and low concentrations imply complement consumption. However, in some diseases, serum complement concentrations are normal but complement deposition is seen in the kidney if a renal biopsy is performed. Table 2 summarises the renal diseases in which complement activation is implicated in their pathogenesis, either due to low circulating levels or deposition in the kidney. In the diseases typically associated with low circulating complement concentrations normal levels are also possible, and therefore this finding does not completely exclude a complement-mediated disease.
Table 2.
Evidence of complement activation in systemic and renal-limited kidney diseases, typical changes seen in renal disease
| Disease | Serum C3 | Serum C4 | Staining on renal biopsy |
|---|---|---|---|
| Complement-mediated aHUS | Low | Normal | Evidence of TMA, no specific staining |
| Dense deposit disease | Low | Normal | Dominant C3 in glomerulus (dense deposits in GBM on EM)a |
| C3 glomerulonephritis | Low | Normal | Dominant C3 in glomerulus (ill-defined deposits on EM)a |
| Lupus nephritis | Low | Low | ‘Full house’ of complement components (including C1q), as well as IgG, IgA and IgM |
| Cryoglobulinaemia | Normal | Low | C1q and C4 staining, immune complexes in capillary loops and subendothelial space on LM |
| IgA nephropathy | Normal | Normal | Predominant IgA deposition, C3 also commonly seena |
| Anti-GBM disease (Goodpasture's) | Normal | Normal | Linear GBM IgG staining, frequent linear C3 and C1q staining observed |
| ANCA-associated vasculitis | Normal | Normal | Majority have positive but weak staining for complement or immunoglobulin |
| Bacterial endocarditis | Low | Low/Normal | C3 deposition, crescentic or endocapillary proliferative glomerulonephritis on LMa |
| Post-infectious glomerulonephritis | Low | Normal | Predominant C3 staining, proliferative glomerular injurya |
| Antibody-mediated rejection of renal transplant | Normal | Normal | Peritubular capillary and glomerular C4d deposition |
| Membranous nephropathy | Normal | Normal | C3 and IgG deposition in sub-epithelial immune complexesa |
a = staining for C3 will detect mainly activated C3 (C3b) and the fragments produced by factor I-mediated proteolytic breakdown (iC3b, C3dg and C3d); aHUS = atypical haemolytic uraemic syndrome; EM = electron microscopy; GBM = glomerular basement membrane; Ig = immunoglobulin; LM = light microscopy; TMA = thrombotic microangiopathy.
Evidence from genetic, pharmacological, biomarker and animal model studies now implicates complement in renal diseases previously not thought to be complement-mediated. As an example, antineutrophil cytoplasmic antibodies associated vasculitis (AAV) is often referred to as ‘pauci-immune’ vasculitis due to the absence of immune deposits in the glomerulus. However, on biopsy complement and/or immunoglobulin fragments can be detected, and increased complement activation products, such as C3a, C5a and sC5b-9, can be found in patients’ plasma.10 Evidence is accumulating that complement dysregulation is pathogenic in AAV and, in keeping with this, the oral C5a receptor inhibitor avacopan has shown promise as a steroid-sparing agent for inducing remission in clinical trials.11
Complement activation in kidney transplantation
Complement activation can occur at several stages during kidney transplantation, and contributes to kidney injury on reperfusion.12 However, most vitally, examining renal transplant biopsies for evidence of peritubular and glomerular C4d deposition has become key in diagnosing acute and chronic antibody mediated rejection.13 C4d is a small fragment of C4 that remains bound to cell membranes after activated C4 is degraded by factor I. The presence of C4d staining suggests classical pathway activation by donor specific antibodies, and its presence is predictive of poorer graft outcomes.14,15
Therapeutic complement inhibition
Eculizumab is currently the only complement inhibitor in clinical use in the UK. It is a recombinant, humanised, monoclonal antibody which binds C5, preventing C5 cleavage and subsequent formation of C5a and C5b-9. Eculizumab was approved for use in aHUS by the National Institute for Health and Care Excellence (NICE) in 2015 and has replaced plasma exchange as the standard treatment for complement-mediated aHUS.8 Because of its high cost, one condition for NICE approval was coordination of treatment through an expert centre which is currently based in Newcastle. Unlike the UK, access to eculizumab remains limited in some countries by cost. There is a high rate of recurrence of aHUS after kidney transplantation, and eculizumab can be administered prophylactically to prevent recurrence.16 Although not a licenced indication for eculizumab, NHS England have commissioned its use to treat C3G recurring after transplantation, but with strict criteria for access and coordination by an expert group based in Newcastle and at the Hammersmith Hospital, London.
Given eculizumab inhibits the terminal pathway, the major side effect is infection with encapsulated organisms, and a 1,000-fold increase in life-threatening meningococcal infections is observed.17 Therefore all patients should be immunised to reduce risk and prophylactic antibiotics should be considered.
The success of eculizumab and studies highlighting the crucial role of complement in many diseases has led to resurgence in anti-complement drug development, and many novel compounds are now in late stages of clinical trials.18 However, development of complement inhibiting drugs is challenging due to the high concentration of complement proteins in the circulation, uncertainty whether abnormal complement in a disease-state is a response to or driver of pathology, and the risks of infection when the cascade is suppressed.
A second anti-C5 monoclonal antibody ravulizumab has now been approved by the US Food and Drug Administration, and has been shown to be non-inferior to eculizumab in paroxysmal nocturnal haemoglobinuria.19 The oral C5a receptor antagonist, avacopan, is in phase III trials for AAV, and phase II trials for IgA nephropathy and C3G.11 There are inhibitors of the lectin pathway, factor B, factor D and C3 at varying stages of clinical development, and novel approaches to treatment include the use of antisense oligonucleotide to suppress complement protein synthesis.20
Conclusion
The complement system is central in the pathogenesis of aHUS and C3G, and is also implicated in a wide range of other renal diseases. The general physician needs to know when complement investigations are indicated, and when to refer for advice when managing thrombotic microangiopathy. It is likely that patients on anti-complement therapies will increasingly be presenting to the acute medical take, and so knowledge of indications and side effects of these drugs is important.
Conflicts of interest
Neil Sheerin has given lectures on behalf of and served on advisory boards for Alexion Pharmaceuticals.
References
- 1.Walport MJ. Complement. First of two parts. N Engl J Med 2001;344:1058–66. [DOI] [PubMed] [Google Scholar]
- 2.De Vriese AS, Sethi S, Van Praet J, et al. Kidney disease caused by dysregulation of the complement alternative pathway: an etiologic approach. J Am Soc Nephrol 2015;26:2917–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brocklebank V, Wood KM, Kavanagh D. Thrombotic microangiopathy and the kidney. Clin J Am Soc Nephrol 2018;13:300–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kavanagh D, Goodship TH, Richards A. Atypical hemolytic uremic syndrome. Semin Nephrol 2013;33:508–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodship TH, Cook HT, Fakhouri F, et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2017;91:539–51. [DOI] [PubMed] [Google Scholar]
- 6.Kavanagh D, Richards A, Fremeaux-Bacchi V, et al. Screening for complement system abnormalities in patients with atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol 2007;2:591–6. [DOI] [PubMed] [Google Scholar]
- 7.Saland JM, Ruggenenti P, Remuzzi G. Liver-kidney transplantation to cure atypical hemolytic uremic syndrome. J Am Soc Nephrol 2009;20:940–9. [DOI] [PubMed] [Google Scholar]
- 8.Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 2013;368:2169–81. [DOI] [PubMed] [Google Scholar]
- 9.Smith RJH, Appel GB, Blom AM, et al. C3 glomerulopathy – understanding a rare complement-driven renal disease. Nat Rev Nephrol 2019;15:129–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gou SJ, Yuan J, Wang C, et al. Alternative complement pathway activation products in urine and kidneys of patients with ANCA-associated GN. Clin J Am Soc Nephrol 2013;8:1884–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayne DRW, Bruchfeld AN, Harper L, et al. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J Am Soc Nephrol 2017;28:2756–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou W, Farrar CA, Abe K, et al. Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest 2000;105:1363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria – an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant 2003;3:708–14. [DOI] [PubMed] [Google Scholar]
- 14.Collins AB, Schneeberger EE, Pascual MA, et al. Complement activation in acute humoral renal allograft rejection: diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol 1999;10:2208–14. [DOI] [PubMed] [Google Scholar]
- 15.Herzenberg AM, Gill JS, Djurdjev O, Magil AB. C4d deposition in acute rejection: an independent long-term prognostic factor. J Am Soc Nephrol 2002;13:234–41. [DOI] [PubMed] [Google Scholar]
- 16.Zuber J, Fakhouri F, Roumenina LT, et al. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol 2012;8:643–57. [DOI] [PubMed] [Google Scholar]
- 17.McNamara LA, Topaz N, Wang X, et al. High risk for invasive meningococcal disease among patients receiving eculizumab (soliris) despite receipt of meningococcal vaccine. Morb Mortal Wkly Rep 2017;66:734–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris CL, Pouw RB, Kavanagh D, et al. Developments in anti-complement therapy; from disease to clinical trial. Mol Immunol 2018;102:89–119. [DOI] [PubMed] [Google Scholar]
- 19.Kulasekararaj AG, Hill A, Rottinghaus ST, et al. Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor-experienced adult patients with PNH: the 302 study. Blood 2019;133:540–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zelek WM, Xie L, Morgan BP, Harris CL. Compendium of current complement therapeutics. Mol Immunol 2019;114:341–52. [DOI] [PubMed] [Google Scholar]