Abstract
Volume status assessment in left ventricular assist device (LVAD) patients remains challenging. Cardiac resynchronization therapy (CRT) devices are common in LVAD patients, and the impedance across the CRT leads may be associated with hemodynamics and serve as a tool for noninvasive estimation of volume status. Ninety-one sets of measurements including cardiac filling pressures and lead impedances were prospectively obtained during ramp tests from 11 LVAD patients (65.5 ± 9.7 years old; nine male) with CRT devices. Right atrial (RA), right ventricular (RV), and left ventricular (LV) lead impedances were all significantly associated with central venous pressure (CVP) (p < 0.05). We derived the following equation: estimated CVP = 47.90− (0.086 x RA lead impedance) + (0.013 x RV lead impedance)−(0.020 x LV lead impedance). The estimated CVP had a significant correlation (r = 0.795) and good agreement with the measured CVP (mean difference −0.14 ± 1.77 mmHg). Applying the above equation on the validation cohort of twenty-one patients also maintained a strong association with measured CVP (r = 0.705). In conclusion, we have derived a novel equation to estimate CVP using lead impedance measurements. This finding may allow noninvasive monitoring of volume status in LVAD patients.
Keywords: left ventricular assist device, hemodynamics, ramp, cardiac resynchronization therapy
Left ventricular assist devices (LVADs) have become a main-stay of therapy for patients with advanced heart failure (HF), both as destination therapy and as bridge to transplantation.1 However, the rate of right ventricular failure (RVF) remains high and readmissions for HF continue to impose a significant morbidity burden on LVAD patients.2,3
Not only for the diagnosis of RVF but also for the follow-up of the degree of RVF, accurate measurement of CVP is essential. However, repeating invasive hemodynamic measurements, which is a gold standard, may have a risk of complications for LVAD patients with enhanced coagulopathy.
Noninvasive evaluation of CVP for the assessment of RVF includes physical examination,4 echocardiographic assessment of inferior vena cava (IVC) size and collapsibility,5 and the recently introduced method of transient elastography.6 These procedures are semiquantitative, require expert technique, and may lack sufficient reproducibility. Noninvasive methodologies to overcome these demerit are desired thus far.
Cardiac resynchronization therapy (CRT) devices typically contain three intracardiac leads, and changes in the pacing impedances of one or more may be associated with changes in cardiac filling pressures. In this study, we sought to evaluate the usefulness of CRT lead impedance as a predictor of CVP in LVAD patients.
Methods
Patient Selection
We prospectively collected data from clinically stable out-patients with the HVAD LVAD (Medtronic, Minneapolis, MN) or the HeartMate II LVAD (Abbott, Abbott Park, IL) who also had a CRT device. CVP and lead impedances were evaluated during a hemodynamic ramp test according to our institutional protocol. Written informed consent was obtained from all participants before the ramp test. The study protocol was approved by the University of Chicago Institutional Review Board.
Ramp Test Protocol
Hemodynamic ramp tests were performed according to our previously described protocol.7 Impedances were measured for the right atrial (RA) lead, right ventricular (RV) lead, and left ventricular (LV) lead using each CRT device’s specific programmer. Measurements of CVP and impedances of each lead were performed after the devices were turned down to a minimum speed (8,000 rpm for HeartMate II and 2,300 rpm for HVAD); subsequently, measurements were taken at progressively increasing LVAD speeds to a maximal value (12,000 rpm for HeartMate II and 3,200 rpm for HVAD). After the change of LVAD speed, each measurement was performed after at least 2-minute rest. Measurements of lead impedances were performed three times via the baseline programmed pacing configuration and then averaged at each LVAD speed. There were no patients with suspicion of lead insulation failure, conductor fracture, device malfunction, sepsis, or myocarditis.
Variables Evaluated
Patients’ preoperative background characteristics before LVAD implantation were obtained. During the ramp test, CVP, and lead impedance data were collected. All data were reviewed by the attending cardiologists. Right atrial and RV lead impedances were measured in a bipolar pacing configuration. Left ventricular lead impedance measurements were taken using a narrow bipoles (one LV electrode to a nearby LV electrode) or a wider bipoles (an LV electrode to the RV lead). Impedance was measured as a single value in ohms. CVP and impedance were measured and recorded simultaneously during the ramp study. A single recording of impedance and CVP were obtained at each LVAD speed.
Validation and Longitudinal Cohorts
For our validation cohort, we collected data from LVAD patients with a CRT device who did not receive the afore-described ramp test but underwent measurements of both lead impedance by CRT interrogation8 and CVP level by right heart catheterization (RHC) at the same timing. For the longitudinal assessment, we collected data from patients who did not receive the ramp test but underwent repeated measurements of both CVP and lead impedance.
Statistical Analyses
Statistical analyses were performed using SPSS Statistics 22 (SPSS Inc, Chicago, IL). A two-tailed p value < 0.05 was considered statistically significant. All continuous variables were described as mean ± standard deviation unless otherwise specified. Relationships between CVP and lead impedances were assessed by Pearson’s correlation coefficient. The statistical impact of lead impedance on CVP was analyzed using linear regression analysis. Significant variables with p < 0.05 in the univariate analysis were enrolled into the multivariate analysis after confirmation that a variance inflation factor was under 5.0 for each.
Based on the results of the multivariate analysis, an equation to estimate CVP was derived. The agreement between measured CVP and the CVP estimated by the equation was analyzed by the Bland-Altman test. The accuracy of the equation’s estimate of CVP in the validation cohort was assessed using Pearson’s correlation coefficient and Bland-Altman test. A receiver operating characteristic analysis was used to calculate sensitivity and specificity to estimate CVP ≥ 12 mmHg by using the equation.
Results
Baseline Characteristics
Eleven LVAD patients with CRT devices (65.5 ± 9.7 years old; nine male) were enrolled. Baseline characteristics are shown in Table 1. The majority of patients (82%) were implanted as destination therapy, and seven patients (64%) had an ischemic etiology of their HF. CRT support duration was 1,219 (inter-quartile range [IQR], 939–1,633) days.
Table 1.
Baseline Characteristics
| Total (N = 11) | |
|---|---|
| Age (years), mean ± SD | 65.5 ± 9.7 |
| Gender (male), n (%) | 9 (82) |
| Race (Caucasian), n (%) | 8 (73) |
| Body mass index, mean ± SD | 27.8 ± 5.6 |
| Ischemic etiology, n (%) | 7 (64) |
| Destination therapy, n (%) | 9 (82) |
| LVAD duration before ramp test (days), median (IQR) | 859 (246–1,310) |
| CRT-D support duration (days), median (IQR) | 1,219 (939–1,633) |
| LVAD type, n (%) | |
| HeartMate II | 8 (73) |
| HVAD | 3 (27) |
| CRT-D type, n (%) | |
| Boston scientific | 3 (27) |
| Medtronics | 6 (55) |
| St. Jude | 2 (18) |
| Hypertension | 5 (45) |
| Diabetes mellitus | 4 (36) |
| History of stroke | 2 (18) |
| Atrial fibrillation | 4 (36) |
| History of ventricular arrhythmia | 3 (27) |
CRT-D, cardiac resynchronization therapy defibrillator; IQR, interquartile range; LVAD, left ventricular assist device; SD, standard deviation.
Correlation of CVP and Lead Impedances
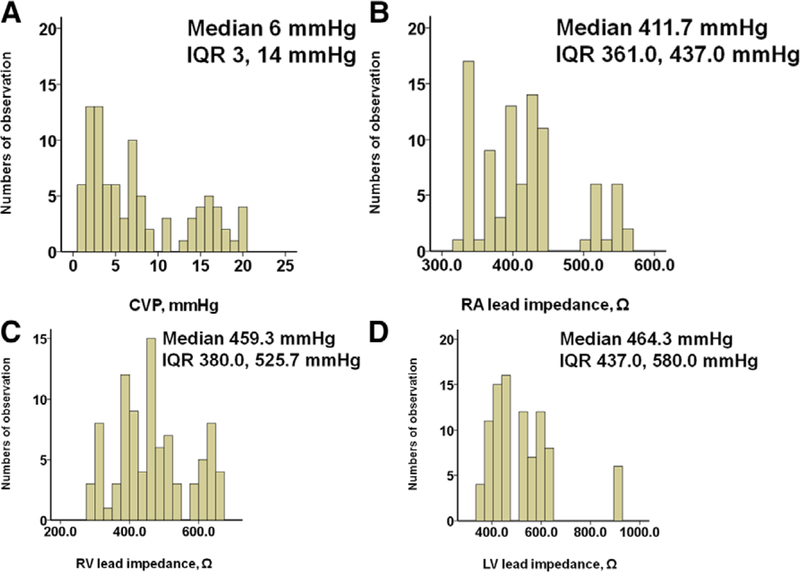
Wide distributions of CVP, RA, RV, and LV lead impedances are observed (Figure 1A-D). Variabilities of each three measurements were 0.998, 0.996, and 0.998, respectively. There was a moderate negative correlation between CVP and RA lead impedance (r = −0.668); whereas CVP had weak negative associations with both RV lead impedance (r = −0.374) and LV lead impedance (r = −0.222) (Table 2).
Figure 1.
Histograms of the distribution of lead impedances for CVP (A), RA (B), RV (C), and LV (D). CVP, central venous pressure; IQR, interquartile range; LV, left ventricular; RA, right atrial; RV, right ventricular.
Table 2.
Correlation Between CVP and Lead Impedances
| p Value | r Value | |
|---|---|---|
| RA lead impedance,Ω | < 0.00* | −0.668 |
| RV lead impedance, Ω | < 0.001* | −0.374 |
| LV lead impedance, Ω | 0.034* | −0.222 |
p < 0.05 by Pearson’s correlation coefficient.
CVP, central venous pressure; LV, left ventricular; RA, right atrial; RV, right ventricular.
Estimation of CVP From Measured Lead Impedances
Univariate and multivariate linear regression analyses showed that RA, RV, and LV lead impedances were independently associated with CVP (Table 3; p < 0.05 for all). An equation to estimate CVP was subsequently derived (see Appendix for more precise methodology [see Appendix, Supplemental Digital Content, http://links.lww.com/ASAIO/A394]): estimated CVP (mmHg) = 47.9− (0.086 × RA lead impedance [Ω]) + (0.013 × RV lead impedance [Ω])−(0.020 × LV lead impedance [Ω]).
Table 3.
Linear Regression Analyses for the Estimation of CVP
| Univariate Analysis |
Multivariate Analysis |
||||
|---|---|---|---|---|---|
| B Value | p Value | B Value | p Value | VIF | |
| RA lead impedance, Ω | −0.062 | < 0.001* | −0.086 | < 0.001* | 1.932 |
| RV lead impedance, Ω | −0.021 | < 0.001* | 0.013 | 0.009* | 1.825 |
| LV lead impedance, Ω | −0.01 | 0.034* | −0.020 | < 0.001* | 1.122 |
CVP, central venous pressure; LV, left ventricular; RA, right atrial; RV, right ventricular; VIF, variant inflation factor.
p < 0.05 by linear regression analyses.
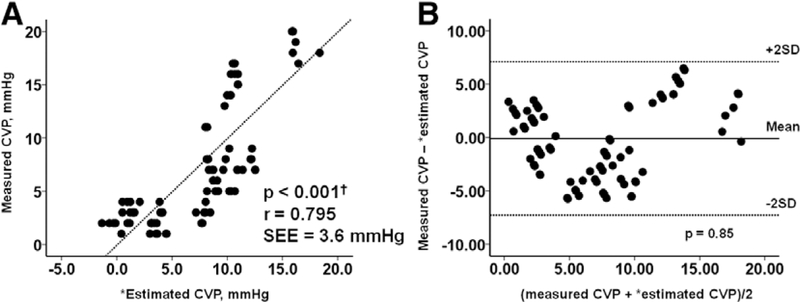
There was a strong linear relationship between the equation to estimate CVP and directly measured CVP, although there was significant scatter around the regression (Figure 2A; r = 0.795). The results are shown with individual patients identified by colored dots in Appendix Figure 1 (see Appendix, Supplemental Digital Content, http://links.lww.com/ASAIO/A394). Linearity seems to be preserved in each individual. Bland-Altman plots showed that the mean difference between estimated and measured CVPs was −0.14 ± 1.77 mmHg, with no tendency toward systematic bias (Figure 2B; p = 0.85).
Figure 2.
The correlation between measured CVP and estimated CVP in the derivation cohort are shown in part A. The Bland-Altman plot for the comparison of measured CVP and estimated CVP in the derivation cohort is shown in part B. There is a moderate positive correlation (r = 0.795) and good agreement (mean difference −0.14 ± 1.77 mmHg) between the measured CVP and estimated CVP. ‘Estimated CVP = 47.90–0.086 ×(RA lead impedance) + 0.013 × (RV lead impedance)− 0.020 × (LV lead impedance). CVP, central venous pressure; LV, left ventricular; RA, right atrial; RV, right ventricular; SD, standard deviation; SEE, standard error of estimate.
A receiver operating characteristic analysis showed 0.905 of area under curve, 100% of sensitivity, and 79.1% of specificity to estimate CVP ≥ 12 mmHg by using the equation (Appendix Figure 2 [see Appendix, Supplemental Digital Content, http://links.lww.com/ASAIO/A394]).
As subanalyses, there were still strong linear correlations between estimated and measured CVPs among HeartMate II population (r = 0.783, N = 68) and HVAD population (r = 0.768, N = 23). Similar trends were observed among Boston scientific device (r = 0.805, N = 26), Medtronics device (r = 0.777, N = 48), and St. Jude device (r = 0.980, N = 17). Strong correlations remained among those with CRT duration shorter than median (r = 0.659, N = 55) and those with longer duration (r = 0.902, N = 36).
Validation Study for the Derived Equation
Datasets of CVPs directly measured by RHC and lead impedances measured by interrogation were collected from another twenty-one LVAD patients who did not receive the ramp test (63.7 ± 10.6 years old, 15 male) for a validation study.
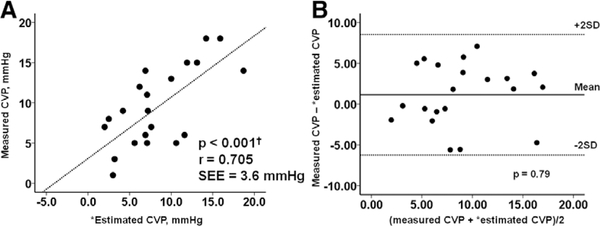
When the equation was applied to the validation group, estimated CVP still had a strong correlation with directly measured CVP (Figure 3A; r = 0.705). Bland-Altman plots showed that the mean difference between estimated and measured CVP averaged 1.15 ± 3.70 mmHg, with no tendency toward systematic bias (Figure 3B).
Figure 3.
The correlation between measured CVP and estimated CVP in the validation cohort are shown in part A. The Bland-Altman plot for the comparison of measured CVP and estimated CVP in the validation cohort is shown in part B. There is a moderate positive correlation (r = 0.705) and good agreement (mean difference 1.15 ± 3.70 mmHg) between the measured CVP and estimated CVP. ‘Estimated CVP = 47.90−0.086 × (RA lead impedance) + 0.013 × (RV lead impedance)−0.020 × (LV lead impedance). CVP, central venous pressure; LV, left ventricular; RA, right atrial; RV, right ventricular; SD, standard deviation; SEE, standard error of estimate.
Longitudinal Assessment of the Created Equation
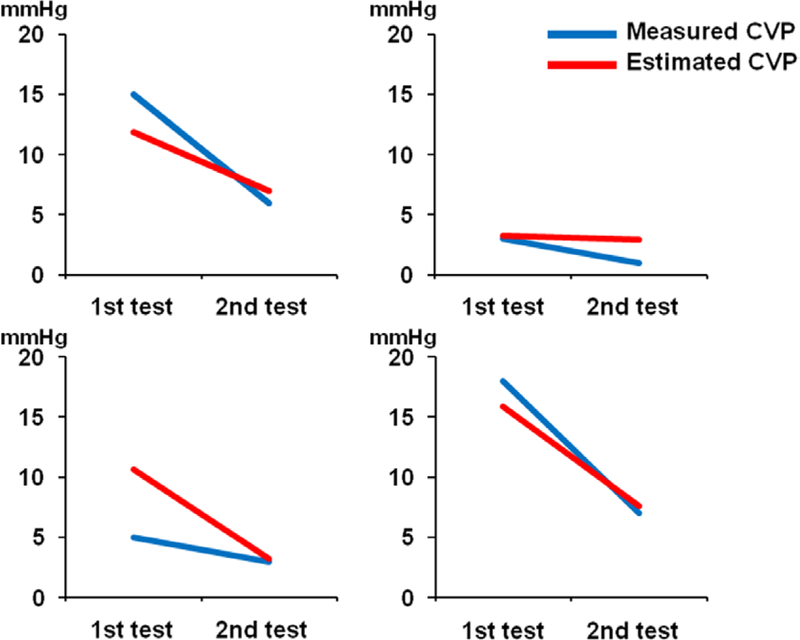
Four patients underwent repeated measurements of both CVP and lead impedance. Second tests were performed at 43, 119, 148, and 154 days, respectively after the first tests. The estimated CVPs changed in parallel with the actually measured CVP in all cases (Figure 4).
Figure 4.
The results of measured CVP and estimated CVP at two different time points are shown for four patients who underwent repeat testing. Decreases in CVP for all four patients were reflected by similar decreases in estimated CVP. CVP, central venous pressure.
Discussion
In this prospective study, we analyzed the relationship between invasively measured CVP and noninvasively measured lead impedances in LVAD patients with CRT. The main findings are as follows: 1) there was a moderate negative correlation between CVP and RA lead impedance, and a weak correlation between CVP and RV and LV lead impedances and 2) an equation to estimate CVP based on the results of linear regression analysis demonstrated a strong correlation between measured and estimated CVPs.
Conventional Noninvasive Estimation of CVP
The most traditional but essential method to estimate CVP remains the physical examination. Jugular venous pressure (JVP), measured at the highest point of oscillation of the internal jugular vein, can be used to estimate CVP.4 Although convenient and easily performed, JVP estimation may be inaccurate when the jugular vein is constricted or tortuous, and it may be difficult to assess in patients with short necks, with prior neck surgery, or with prior repeated catheter placement in the jugular vein, as is often the case in advanced HF patients. We have previously demonstrated that physical examination had low sensitivity to predict hemodynamics including CVP in the LVAD population.9
Another widely-used noninvasive method to predict CVP is transthoracic echocardiography. CVP is estimated using multiple echocardiographic factors, such as the size and respiratory variation of the IVC and RA.5 However, IVC measurement as a surrogate marker for CVP was never validated in LVAD patients. Echocardiography only offers a semiquantitative assessment of CVP and may lead to inaccurate estimates, particularly in the intermediate range (5–10 mmHg).10 Further-more, optimal estimation requires expert technique particularly in LVAD patients.11
Use of Impedance Measurements to Reflect Physiology
Impedance measurements derived from cardiac implantable electronic devices (CIEDs) have long been used to assess changes or trends in physiology. Studies have demonstrated correlations between thoracic impedance measurements, measured between the RV lead and pulse generator, and both degree of pulmonary congestion12–14 and minute ventilation.15 Such technology is incorporated into some ICD and cardiac resynchronization therapy defibrillator (CRT-D) models (OptiVol; Medtronic, Minneapolis, MN). Changes in LV volume achieved with CRT are also associated with impedance measurements taken between the LV and RV leads.16 Nevertheless, the accuracy and utility of CIED technology have been questioned in the recent clinical practice.
Localized impedance measurements at the tip of the RV lead have been used as a proxy for ventricular contractility and incorporated in some pacemakers as a closed-loop feedback system for determining heart rate-responsiveness. Given the increase in ventricular contractility with increased sympathetic tone, such a system can increase pacing rate proactively in response to factors like emotional stress, in addition to the more commonly used parameters for heart rate-response such as movement and minute ventilation.15,17
There is thus extensive experience with the use of impedance measurements across a variety of intrathoracic spaces to assess physiology and influence therapy. Our data adds the benefit of impedance analysis during the rapidly changing filling pressures seen with routine ramp studies, effectively isolating those changes from other confounding factors.
Clinical Implications of the Novel Equation Model
Remote measurements of lead impedances (patients are outside of the hospital setting) can be utilized for remote estimation of the CVP using the equation provided in this paper. This remote estimation of CVP may provide the LVAD team the ability to adjust diuretics without physically evaluating the patient and may address fluid accumulation earlier in the course of the disease. Better fluid status control may reduce the high rate of HF readmission associated with LVAD and improve patients’ quality of life and exercise performance.
With the recent improvement in device technology and significant reduction in complications such as pump thrombosis and stroke,3 the next generation of devices—smart pumps— should devote greater focus to improve patient quality of life through the dynamic adjustment of pump speeds in response to hemodynamic cues. These smart pumps will need to take into consideration multiple parameters including 1) patients activity level, 2) LV preload, 3) LV afterload, and 4) RV preload.
Although an easy solution is available only for the first parameters (activity level can be assessed using information derived from permanent pacing devices), the others remain more challenging. Although LV preload can be measured by implantable pulmonary artery monitors such as CardioMEMS (Abbott, Abbott Park, IL),18 up to 50% of the LVAD patients may experience decoupling between the pulmonary capillary wedge pressure (PCWP) and diastolic pulmonary artery pressure (i.e., diastolic pulmonary pressure overestimates the PCWP).19 This decoupling could lead to suction events if LVAD speed adjustment is just based on the level of pulmonary artery pressure alone. Recently our group reported a way to estimate PCWP from the HVAD LVAD waveform,20 and called for further evaluation of waveforms as a source for LV preload assessment.
Currently, we are lacking a way to assess both LV afterload and RV preload in LVAD patients. Measurement of systemic blood pressure in continuous-flow LVAD patients remains challenging. Our team has completed a pilot study of a non-invasive investigational device that can provide continuous blood pressure monitoring of LVAD patients with excellent accuracy in comparison to an invasive arterial catheter.21 On-going studies will further determine the efficacy of this device to provide an accurate measurement of LV afterload. Our current study suggests that lead impedances can provide a reliable estimate of RV preload. The combination of these technologies may provide a comprehensive view of biventricular loading conditions that can be incorporated into a smart pump design.
Although this is the first study describing the significant association between CVP and lead impedance, its precise mechanism remains uncertain. Ultimately, the most likely explanation for these findings are high atrial load resulting in structural and electrical change in RA myocardium, which may impact lead impedance.22,23 Our next priority is to the impact of atrial load on lead impedance. Other factors, including myocardial properties, fibrosis, and heart rate may also affect pacing lead impedances.
Study Limitations
Several potential limitations of this study should be considered. First, although dataset is moderate size, patient number is small. A prospective multicenter longitudinal study to demonstrate the utility of the lead impedance-guided therapy, in which diuretic dose is adjusted based on the equation, should be pursued. Also, the validity of the equation during longitudinal follow-up of individual patients should be studied further. Second, our equation cannot replicate the pressure waveform of right atrium, which is useful to interpret specific pathology such as constrictive pericarditis. A full invasive hemodynamic assessment is necessary for complete evaluation in such specific situations. Third, our equation was constructed from LVAD patients and cannot be adapted to patients without LVADs. Fourth, all measurements were made with the patients supine. It is uncertain whether the lead impedances may have differed significantly with posture and activity. Finally, we did not routinely perform echocardiographic IVC measurements during ramp test. We did not demonstrate superiority of our equation over IVC measurements in this study.
Conclusion
We derived a novel equation to estimate CVP levels using lead impedance measurements. This finding may help with the noninvasive monitoring and management of volume status in LVAD patients.
Supplementary Material
Footnotes
Disclosure: Dr. Imamura receives financial support from Postdoctoral Fellowship for Research Abroad of Japan Society for the Promotion of Science; Dr. Uriel receives grant support from Abbott and Medtronic; Dr. Sayer has received consulting fees from Medtronic; Dr. Jeevanandam receives consultant fee from Abbott. Dr. Burkhoff receives consultant fee from Medtronic, Corvia Medical, Sensible Medical, Impulse Dynamics, Cardiac Implants, and educational grant support from Abiomed. The other authors have no conflicts of interest to report.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML and PDF versions of this article on the journal’s Web site (www.asaiojournal.com).
References
- 1.Kirklin JK, Pagani FD, Kormos RL, et al. : Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant 36: 1080–1086, 2017. [DOI] [PubMed] [Google Scholar]
- 2.Rogers JG, Pagani FD, Tatooles AJ, et al. : Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med 376: 451–460, 2017. [DOI] [PubMed] [Google Scholar]
- 3.Mehra MR, Goldstein DJ, Uriel N, et al. ; MOMENTUM 3 Investigators: Two-year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med 378: 1386–1395, 2018. [DOI] [PubMed] [Google Scholar]
- 4.Drazner MH, Rame JE, Stevenson LW, Dries DL: Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. N Engl J Med 345: 574–581, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Rudski LG, Lai WW, Afilalo J, et al. : Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23: 685–713; quiz 786–788, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Taniguchi T, Sakata Y, Ohtani T, et al. : Usefulness of transient elastography for noninvasive and reliable estimation of right-sided filling pressure in heart failure. Am J Cardiol 113: 552–558, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Uriel N, Sayer G, Addetia K, et al. : Hemodynamic ramp tests in patients with left ventricular assist devices. JACC Heart Fail 4: 208–217, 2016. [DOI] [PubMed] [Google Scholar]
- 8.Winters SL, Packer DL, Marchlinski FE, et al. ; North American Society of Electrophysiology and Pacing: Consensus statement on indications, guidelines for use, and recommendations for follow-up of implantable cardioverter defibrillators. North American Society of Electrophysiology and Pacing. Pacing Clin Electrophysiol 24: 262–269, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Anyanwu E, Bhatia A, Tehrani DM, et al. : The accuracy of physical exam compared to RHC in LVAD patients. J Heart Lung Transplant 36(4 suppl): S341–S342, 2017. [Google Scholar]
- 10.Beigel R, Cercek B, Luo H, Siegel RJ: Noninvasive evaluation of right atrial pressure. J Am Soc Echocardiogr 26: 1033–1042, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Estep JD, Vivo RP, Krim SR, et al. : Echocardiographic evaluation of hemodynamics in patients with systolic heart failure supported by a continuous-flow LVAD. J Am Coll Cardiol 64: 1231–1241, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Yu CM, Wang L, Chau E, et al. : Intrathoracic impedance monitoring in patients with heart failure: Correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation 112: 841–848, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Wang L: Fundamentals of intrathoracic impedance monitoring in heart failure. Am J Cardiol 99(10A): 3G–10G, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Vollmann C, Nägele H, Schauerte P, et al. ; European InSync Sentry Observational Study Investigators: Clinical utility of intrathoracic impedance monitoring to alert patients with an implanted device of deteriorating chronic heart failure. Eur Heart J 28: 1835–1840, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Cole CR, Jensen DN, Cho Y, et al. : Correlation of impedance minute ventilation with measured minute ventilation in a rate responsive pacemaker. Pacing Clin Electrophysiol 24: 989–993, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Maines M, Landolina M, Lunati M, et al. ; Italian Clinical Service Optivol-CRT Group: Intrathoracic and ventricular impedances are associated with changes in ventricular volume in patients receiving defibrillators for CRT. Pacing Clin Electrophysiol 33: 64–73, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Osswald S, Cron T, Grädel C, et al. : Closed-loop stimulation using intracardiac impedance as a sensor principle: Correlation of right ventricular dP/dtmax and intracardiac impedance during dobutamine stress test. Pacing Clin Electrophysiol 23(10 pt 1): 1502–1508, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Abraham WT, Adamson PB, Bourge RC, et al. ; CHAMPION Trial Study Group: Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet 377: 658–666, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Imamura T, Chung B, Nguyen A, et al. : Decoupling between diastolic pulmonary artery pressure and pulmonary capillary wedge pressure as a prognostic factor after continuous flow ventricular assist device implantation. Circ Heart Fail 10: e003882, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grinstein J, Rodgers D, Kalantari S, et al. : HVAD waveform analysis as a noninvasive marker of pulmonary capillary wedge pressure: A first step toward the development of a smart left ventricular assist device pump. ASAIO J 64: 10–15, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sayer G, Kim G, Rodgers D, et al. : Highly accurate continuous blood pressure measurement in LVAD patients with a non-invasive, non-oscillometric wearable device: A pilot study. J Heart Lung Transplant 37(4S): S44, 2018. [Google Scholar]
- 22.Thijssen VL, Ausma J, Liu GS, Allessie MA, van Eys GJ, Borgers M: Structural changes of atrial myocardium during chronic atrial fibrillation. Cardiovasc Pathol 9: 17–28, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Allessie MA : Atrial fibrillation-induced electrical remodeling in humans: What is the next step? Cardiovasc Res 44: 10–12, 1999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.