EV-D68 is a serious threat to human health, and there are currently no effective treatments or vaccines. SGs play an important role in cellular innate immunity as a target with antiviral effects. This manuscript describes the formation of SGs induced by EV-D68 early infection but inhibited during the late stage of infection. Moreover, TIA1, HUR, and G3BP1 can chelate a specific site of the 3′ UTR of EV-D68 to inhibit viral replication, and this interaction is sequence and complex dependent. However, this inhibition can be antagonized by overexpression of the minireplicon. These findings increase our understanding of EV-D68 infection and may help identify new antiviral targets that can inhibit viral replication and limit the pathogenesis of EV-D68.
KEYWORDS: 3′ UTR, EV-D68, stress granule
ABSTRACT
Stress granules (SGs) are formed in the cytoplasm under environmental stress, including viral infection. Human enterovirus D68 (EV-D68) is a highly pathogenic virus which can cause serious respiratory and neurological diseases. At present, there is no effective drug or vaccine against EV-D68 infection, and the relationship between EV-D68 infection and SGs is poorly understood. This study revealed the biological function of SGs in EV-D68 infection. Our results suggest that EV-D68 infection induced the accumulation of SG marker proteins Ras GTPase-activated protein-binding protein 1 (G3BP1), T cell intracellular antigen 1 (TIA1), and human antigen R (HUR) in the cytoplasm of infected host cells during early infection but inhibited their accumulation during the late stage. Simultaneously, we revealed that EV-D68 infection induces HUR, TIA1, and G3BP1 colocalization, which marks the formation of typical SGs dependent on protein kinase R (PKR) and eIF2α phosphorylation. In addition, we found that TIA1, HUR, and G3BP1 were capable of targeting the 3′ untranslated regions (UTRs) of EV-D68 RNA to inhibit viral replication. However, the formation of SGs in response to arsenite (Ars) gradually decreased as the infection progressed, and G3BP1 was cleaved in the late stage as a strategy to antagonize SGs. Our findings have important implications in understanding the mechanism of interaction between EV-D68 and the host while providing a potential target for the development of antiviral drugs.
IMPORTANCE EV-D68 is a serious threat to human health, and there are currently no effective treatments or vaccines. SGs play an important role in cellular innate immunity as a target with antiviral effects. This manuscript describes the formation of SGs induced by EV-D68 early infection but inhibited during the late stage of infection. Moreover, TIA1, HUR, and G3BP1 can chelate a specific site of the 3′ UTR of EV-D68 to inhibit viral replication, and this interaction is sequence and complex dependent. However, this inhibition can be antagonized by overexpression of the minireplicon. These findings increase our understanding of EV-D68 infection and may help identify new antiviral targets that can inhibit viral replication and limit the pathogenesis of EV-D68.
INTRODUCTION
Human enterovirus D68 (EV-D68) was originally isolated from throat swab samples from four critically ill patients in California in 1962 (1, 2). Patients infected with EV-D68 typically present with severe respiratory illnesses (3). There are also reports indicating that this virus causes nervous system diseases (4). In 2014, a major epidemic of EV-D68 infection occurred in the United States, and 1,153 patients with severe respiratory symptoms were diagnosed with EV-D68 (1, 5, 6). Unfortunately, there are currently no effective drugs and vaccines to treat EV-D68 infection.
EV-D68 is a member of the enterovirus D group of the Picornavirus family, and its genome contains approximately 7.6 kb of single-stranded RNA (1, 7). EV-D68 contains an independent open reading frame (ORF) encoding a polyprotein, a 3′ poly(A) tail, and an untranslated region (UTR) at the 3′ and 5′ terminals. The noncoding region encodes a precursor polyprotein, which, upon autocatalytic cleavage, forms four structural proteins (VP1 to VP4) and seven nonstructural proteins (2A, 2B, 2C, 3A, 3B, 3C, and 3D) (7). VP1 has serotype specificity and can effectively distinguish EV-D family viruses and their newly discovered strains (8). It has been reported that 2A and 3C proteins of EV-D68 regulate the host innate immune response and are potential targets for potential antiviral drugs.
Stress granules (SGs), aggregates of stable, translationally silenced mRNAs formed in eukaryotic cells, are triggered by various environmental stresses, including viral infections (9). Nontranslated mRNA; eukaryotic translation initiation factor (eIF4E, eIF4G, eIF4A, eIF2); 40S ribosomal subunit; and RNA-binding proteins such as poly(A) binding protein (PABP), T cell intracellular antigen 1 (TIA1), TIA1-related protein (TIAR), Ras GTPase-activated protein-binding protein 1 (G3BP1), and human antigen R (HUR) (10) are all basic components of SGs (9, 11–13). It has been reported that viral infection regulates the formation of SGs as a manner in which to regulate translation of host proteins. Viral infection causes a cellular stress response, which activates several different signaling pathways within the cell—the most classical being the protein kinase R (PKR)-triggered pathway. PKR regulates the transcription and translation of cells through its phosphorylation initiation factor eIF2α, which plays an important role in cell growth (14). After viral infection, PKR binds to viral double-stranded RNA (dsRNA), which activates the autophosphorylation of the kinase. Activation of PKR phosphorylates eIF2α (15–18), inhibits the formation of the ternary complex tRNAiMet-GTP-eIF2, and initiates assembly of SGs, thereby preventing cell translation (9, 16, 17, 19). For example, RSV-induced SGs are mediated by PKR-dependent eIF2α phosphorylation (9, 20).
Previous reports have claimed that the formation of SGs regulates viral replication (21). For example, herpes simplex virus 1 (HSV-1) infection does not form obvious SGs, but the expression of TIA1, TIAR, and TTP in cells was upregulated, mainly because the viral RNA stem-loop structure captured TIA1/TIAR to assist in its own replication (22). Moreover, there is also a phenomenon in which SGs inhibit viral replication. It has been reported that the Chikungunya virus (CHIKV) nonstructural protein nsP3 localizes to SGs containing G3BP1/2 together with nsP1 and dsRNA, limiting viral replication (23). However, to antagonize the host autoimmune response, many viruses have developed strategies to disrupt the formation of SGs in order to release transcripts that are arrested in SGs and efficiently translate their own proteins (24, 25). For example, the C and V proteins of the paramyxovirus inhibit the formation of SGs by inhibiting the activity of PKR and its terminal RNA, which also interacts with TIAR (26–28). The 2A proteins of picornaviruses, including EV-A71, CV-B3, and EV-D68, inhibit the formation of SGs (29, 30). Reovirus particles induce the formation of eIF2α-dependent SGs early during infection (26, 31, 32). West Nile virus and dengue virus block SGs via specific interactions of TIA1/TIAR with the end of viral negative-strand RNA (33–35). Therefore, the noncoding region plays an important role in the interaction with the SG protein.
The aim of this study was to investigate the relationship between EV-D68 infection and SGs. In this study, we first demonstrated that EV-D68 infection induced the formation of typical SGs during the early stages of infection. However, as the infection progressed, SGs were gradually decomposed. In addition, we found that the overexpression of SGs proteins inhibited viral replication, indicating that SGs negatively regulated EV-D68 replication. Results further showed specific sites involved in the binding of SGs to EV-D68 and that SGs inhibited viral replication by chelation of viral RNA. These findings provide a new potential mechanism for the role of SGs in EV-D68 infection.
RESULTS
EV-D68 infection induces the formation of SGs during the early stage of infection and subsequently inhibits SGs.
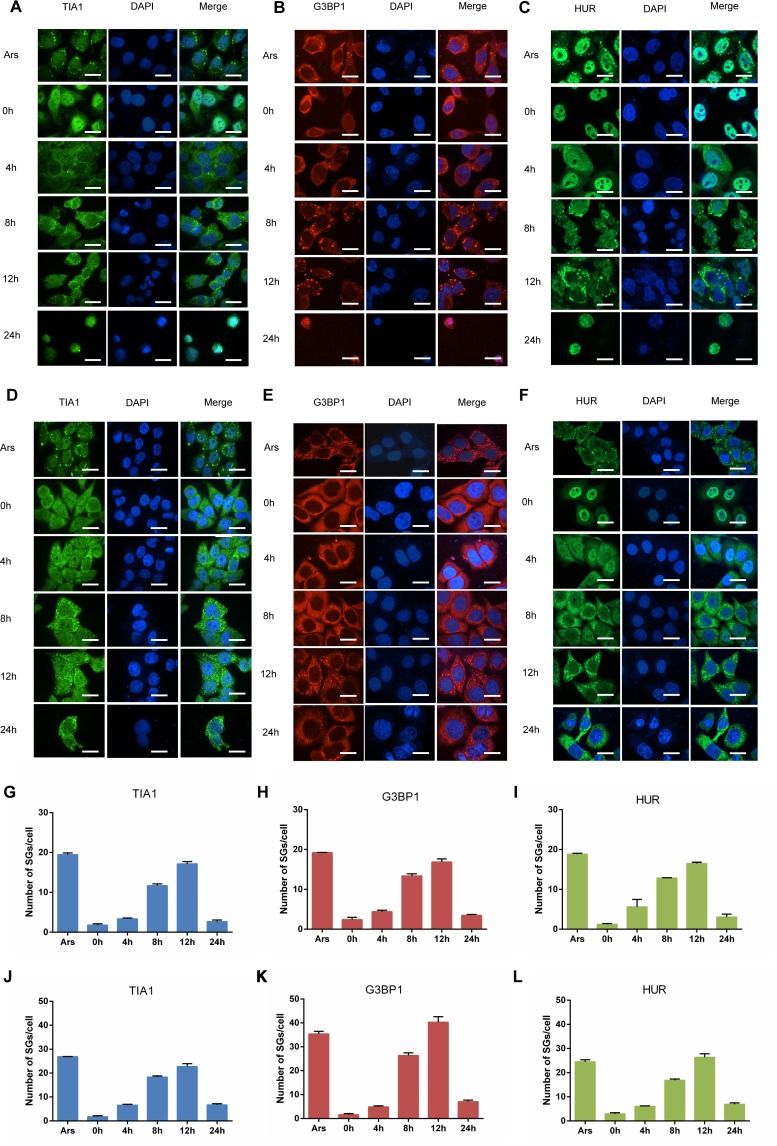
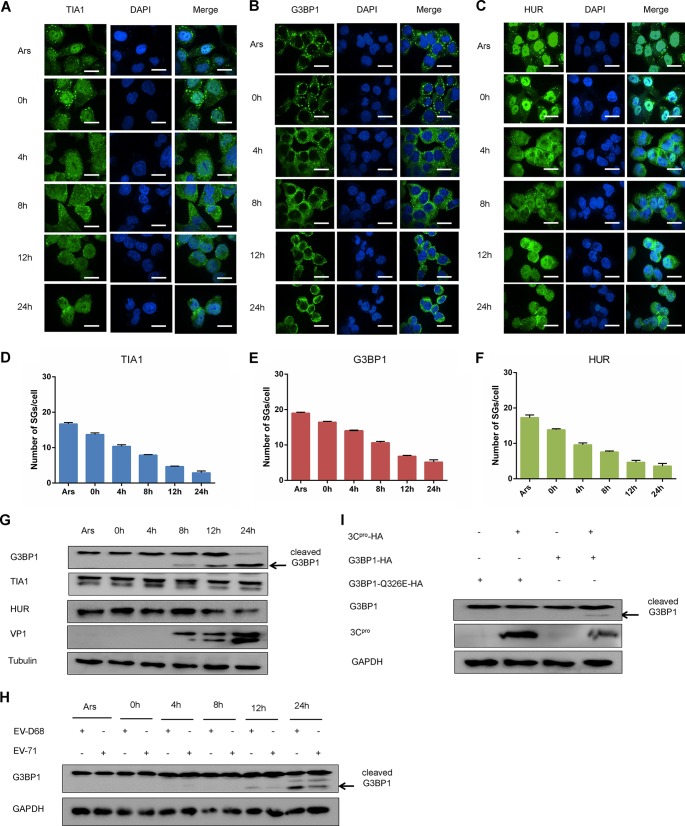
It has been reported that various picornaviruses, such as enterovirus 71 (EV-71) and coxsackievirus B3 (CVB3), induce the formation of SGs (36, 37), and the formed SGs are decomposed during the late stage of infection. However, the effect of EV-D68 infection on the formation of SGs is not fully understood. First, we verified the kinetics of SG formation in different cells infected with EV-D68. Rhabdomyosarcoma (RD) cells were infected with EV-D68 at a multiplicity of infection (MOI) of 1 and stained with antibodies against TIA1. The cells were treated with arsenite (Ars) as a positive control. It is well-known that HUR, TIA1, and G3BP1 are components of SGs and proteins that mark the formation of SGs. The formation of aggregated particles containing TIA1 began to appear in the cytoplasm at 4 h after virus infection. As the infection progressed, the number of SGs containing TIA1 gradually increased, reaching the maximum at 12 h postinfection. Interestingly, SGs were significantly reduced at 24 h postinfection, indicating that SGs were inhibited during the late stages of viral infection (Fig. 1A). At the same time, we stained RD cells with antibodies against G3BP1 and HUR and observed the formation of SGs by confocal microscopy in order to detect whether G3BP1 and HUR were involved in SGs formation during EV-D68 infection. As expected, similar results were observed with TIA1 (Fig. 1B and C). We performed a quantitative analysis to calculate the number of SGs in each cell (Fig. 1G, H, and I). To further verify the above results, we also obtained similar results by infecting HeLa cells with EV-D68 (Fig. 1D, E, F, J, K, and L). These results indicated that the formation of SGs is induced early during EV-D68 infection and is inhibited during the late stages of infection.
FIG 1.
EV-D68 infection induces the formation of SGs during the early stage and inhibits the formation of SGs during the late stage. (A, B, C, D, E, and F) RD/HeLa cells were infected with EV-D68 (MOI of 1), fixed at 0, 4, 8, 12, and 24 h, and then stained with a rabbit anti-TIA1 monoclonal antibody (A and D), a rabbit anti-G3BP1 monoclonal antibody (B and E), or a rabbit anti-HUR monoclonal antibody (C and F) for immunofluorescence. (G, H, I, J, K, and L) Quantitative analysis of the number of SGs in each cell.
EV-D68 infection induces PKR and eIF2α phosphorylation-dependent typical SGs.
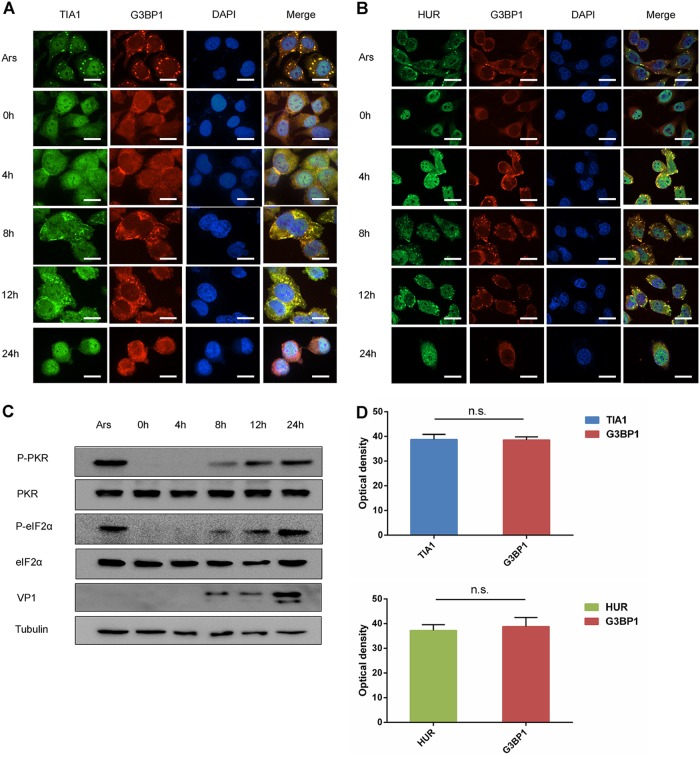
The above results indicated that EV-D68 infection induces HUR, TIA1, and G3BP1 proteins to form SGs in the cytoplasm. It has been reported that EV-71 infection induces the formation of atypical SG-like structures (38). We aimed to determine if the SGs induced by EV-D68 infection were different from the SGs formed by EV-71. We infected RD cells with EV-D68 at an MOI of 1 and fixed the cells at different times postinfection. Our results showed that TIA1 and G3BP1 were colocalized. At the same time, optical density analysis was performed on the colocalization of TIA1 and G3BP1 (Fig. 2A and D). As with the above results, EV-D68 infection induced the formation of cytoplasmic SGs of TIA1 and G3BP1 during the early stage of infection, while the formation of SGs was inhibited during the late stage of infection. It is well known that Ars treatment induces the formation of typical SGs in cells (38), which includes HUR, TIA1, and G3BP1. Our results showed that EV-D68 infection induces TIA1 and G3BP1 to colocalize, consistent with typical SGs in response to Ars stress. To further confirm the above results, we analyzed HUR and G3BP1 in EV-D68-infected cells by immunofluorescence and found that they colocalized together, and optical density analysis was performed on the colocalization of HUR and G3BP1 (Fig. 2B and D). As described above, we detected the formation of SGs in HeLa cells and obtained similar results (data not shown). These findings indicated that EV-D68 infection induces the formation of typical SGs.
FIG 2.
EV-D68 infection induces the formation of typical SGs. (A and B) RD cells were infected with EV-D68 (MOI of 1), fixed at 0, 4, 8, 12, and 24 h, and costained with a rabbit anti-TIA1 monoclonal antibody and a mouse anti-G3BP1 monoclonal antibody (A) or a mouse anti-G3BP1 monoclonal antibody and a rabbit anti-HUR monoclonal antibody (B) for immunofluorescence. (C) RD cells were infected with EV-D68, and cells were harvested after 0, 4, 8, 12, and 24 h of infection. Cells treated with 0.5 μm of Ars was used as a control. The expression of PKR, P-PKR, eIF2α, and P-eIF2α was detected by Western blot analysis. (D) Optical density analysis of two colocalized proteins by ImageJ software.
Phosphorylation of eIF2α is the main pathway leading to the induction of typical SGs by Ars. To explore the potential mechanisms by which EV-D68 regulates SGs formation, we first assessed the kinetics of eIF2α kinase activation and PKR activation. We evaluated the phosphorylation of PKR and eIF2α because they are usually associated with cellular stress resulting from viral infections. Western blot analysis showed that eIF2α and PKR had similar kinetics. There was no significant change as the infection progressed. At the same time, phosphorylation of eIF2α and PKR increased as the infection progressed (Fig. 2C). The formation of SGs in response to Ars is dependent upon the phosphorylation of PKR and eIF2α (39). As such, we used Ars treatment as a control. These findings indicated that EV-D68 induces the formation of PKR and eIF2α phosphorylation-dependent SGs.
Overexpression of SG proteins inhibits EV-D68 replication.
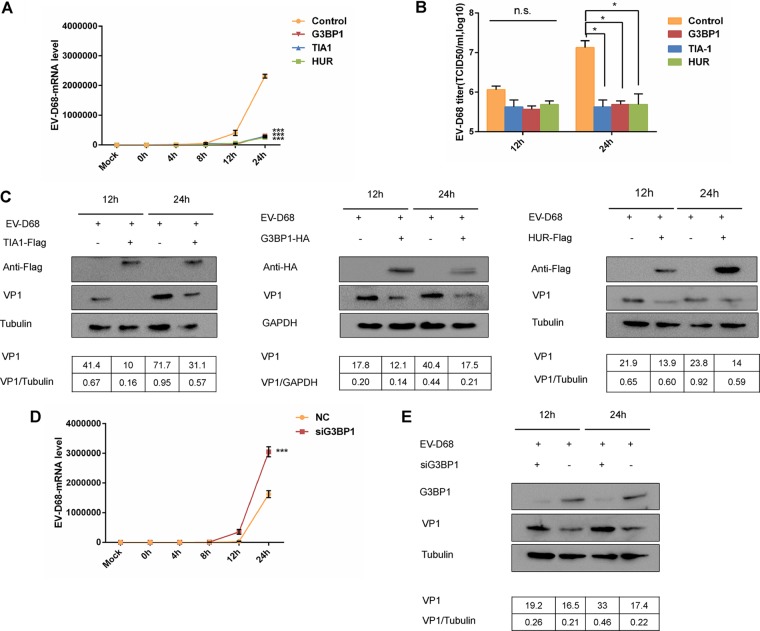
As a class of antiviral proteins, SGs have antiviral activity against certain viruses and mediate interactions with innate immunity (26). EV-D68 infection initially induces and subsequently reduces SGs, suggesting a key role for SGs in the innate immune response to viral infection. Overexpression of G3BP1 has been reported to induce the assembly of cytosolic SGs (40). To investigate the effect of SGs on EV-D68 replication, RD cells were infected with EV-D68 at an MOI of 0.1 after overexpressing HUR, TIA1, or G3BP1, and quantitative PCR (qPCR) was used to detect the abundance of viral RNA. Our results showed that the abundance of EV-D68 RNA was significantly higher than that of EV-D68 RNA transfected with SG proteins 24 h after virus infection (Fig. 3A). At the same time, Western blot analysis showed that SGs significantly inhibited the expression of viral protein (Fig. 3C). To determine if SGs would inhibit viral replication, SG proteins were transfected before viral infection, and virus titers were determined at 12 and 24 h. The virus titer was significantly reduced compared to cells mock transfected with SG proteins at 24 h (Fig. 3B). We also detected similar levels of mRNA levels, viral protein, and viral titers of EV-D68 in HeLa and 293T cells, which was consistent with our previous data (data not shown). In order to further confirm the negative regulation of SGs on EV-D68 replication, G3BP1 was knocked down in 293T cells. The cells were then infected with EV-D68 to detect the abundance of viral RNA of EV-D68. Our results showed that the abundance of EV-D68 RNA increased significantly after knocking down G3BP1 (Fig. 3D). Western blot analysis showed enhanced viral protein expression after G3BP1 knockdown (Fig. 3E). These data indicated that SGs can negatively regulate viral replication and have potential antiviral capabilities.
FIG 3.
Overexpression of SG proteins inhibits the replication of EV-D68. (A) HUR, TIA1, G3BP1, or an empty vector control were overexpressed in RD cells. RD cells were infected with EV-D68 (MOI of 0.1) 24 h after transfection, and cells were harvested after infection at 0, 4, 8, 12, and 24 h. RNA was extracted, and qPCR was used to detect viral mRNA in cells. (B) HUR, TIA1, G3BP1, and an empty vector control were overexpressed in RD cells. Cells were infected with EV-D68 (MOI of 0.1) 24 h after transfection. Cells were harvested 12 and 24 h after infection, and the cells were repeatedly thawed three times in order to assess the 50% tissue culture infective dose (TCID50) virus titer. (C) HUR, TIA1, G3BP1, or an empty vector control were overexpressed in RD cells. Cells were infected with EV-D68 (MOI of 0.1) 24 h after transfection, and cells were harvested after 12 and 24 h postinfection for SDSPAGE gel electrophoresis. Western blotting detected the expression of viral protein VP1 in the cell lysate. (D) 293T cells were transfected with siG3BP1 (100 nM) using Lipofectamine 2000. The cells were infected with EV-D68 (MOI of 0.1) 48 h after transfection, and the cells were harvested at 0, 4, 8, 12, and 24 h after infection. (E) Western blot analysis detected the expression of viral protein VP1 in the cell lysate. Knockdown of G3BP1 was assessed by Western blotting.
SG proteins interact with EV-D68 RNA to inhibit viral replication.
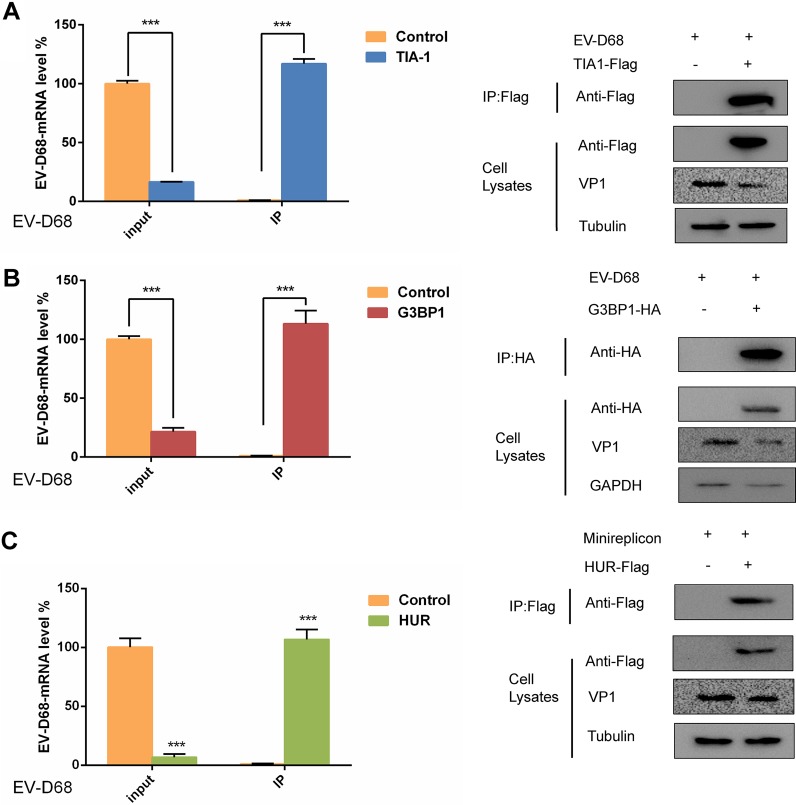
It has been reported that the West Nile virus and dengue virus infection inhibit the formation of SGs since minus-strand 3′-terminal stem-loop (SL) RNA was previously shown to bind specifically to cellular SG components TIA1 and the related protein TIAR (35, 41). This prompted us to explore whether SGs interact with EV-D68 RNA to regulate viral replication. To confirm this hypothesis, 293T cells were transfected with TIA1, G3BP1, and HUR, and then the cells were infected with EV-D68 at an MOI of 0.1 24 h after transfection. To accurately assess the presence or absence of the interaction of EV-D68 RNA with TIA1, G3BP1, and HUR, viral mRNA levels in cells were measured prior to coimmunoprecipitation. Consistent with the above results, the mRNA level of EV-D68 was reduced by about 5- to 10-fold after transfection with TIA1. However, the mRNA level of EV-D68 was more than 100 times that of the control group after coimmunoprecipitation. To ensure the reliability of the experimental system, we also examined the expression of TIA1 after coimmunoprecipitation and in the cell lysate. After transfection of TIA1, the expression of viral proteins was significantly reduced compared to the control (Fig. 4A), which further confirms that SGs have an inhibitory effect on viral replication. These data indicated that TIA1 inhibits EV-D68 replication by chelation of viral RNA. Previous results have confirmed that EV-D68 infection induces the formation of typical SGs, but it was not known if G3BP1 and HUR also interacted with viral RNA. In the same manner, coimmunoprecipitation was carried out after transfection of G3BP1 and HUR, and it was found that G3BP1 and HUR interacted with viral RNA, and similar results were obtained (Fig. 4B and C). These results confirmed that SGs inhibit viral replication by chelation of EV-D68 RNA.
FIG 4.
SG proteins interact with EV-D68 RNA to inhibit viral replication. (A) 293T cells infected with EV-D68 for 24 h were used for coimmunoprecipitation experiments 24 h after TIA1, G3BP1 (B), and HUR (C) transfection. qPCR was used to detect the viral mRNA levels before and after coimmunoprecipitation, and the expression of viral protein and TIA1, G3BP1, and HUR was detected by Western blot analysis.
The 3′ UTR of EV-D68 RNA interacts with SG proteins to inhibit viral replication.
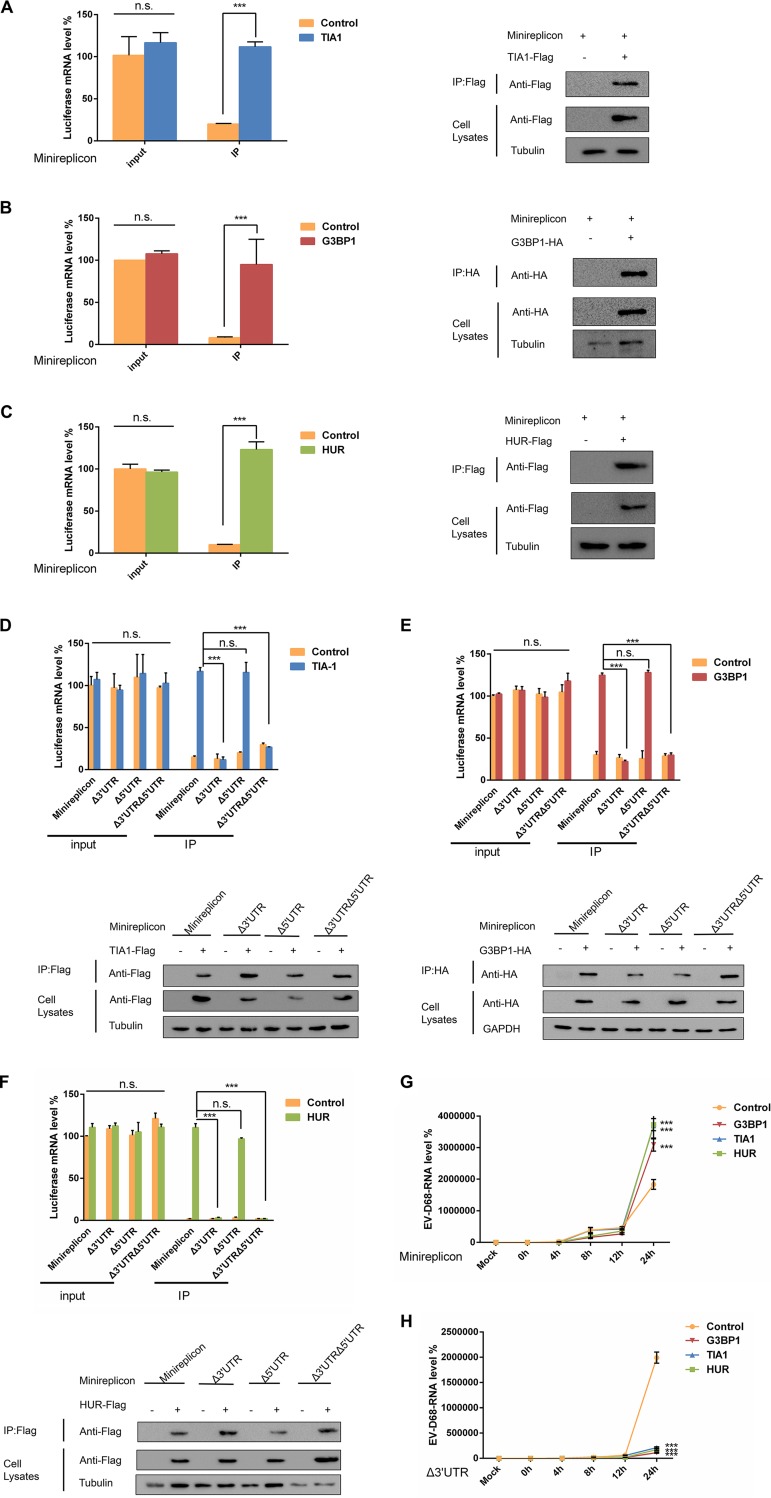
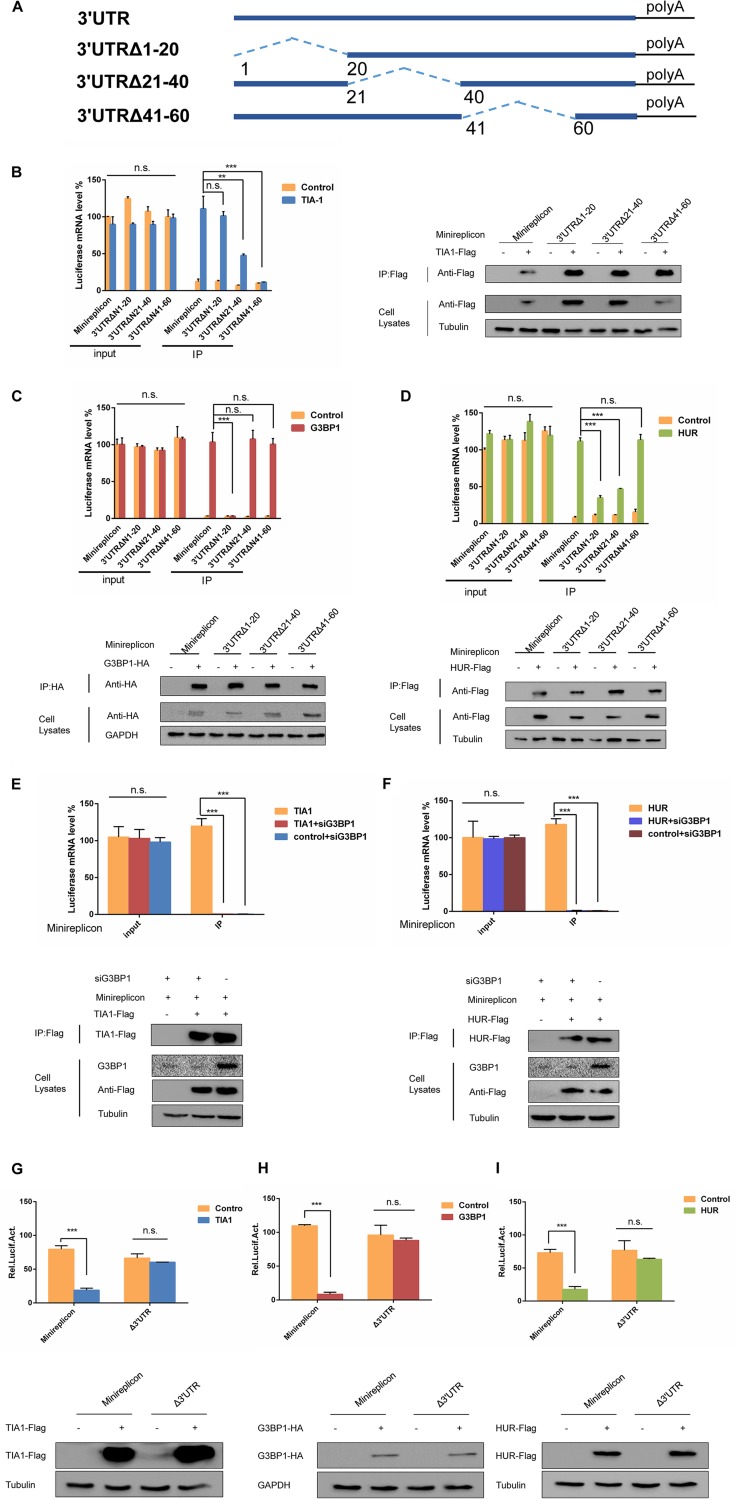
The EV-D68 minireplication subsystem plays an important role in the study of antiviral mechanisms as a key tool for studying the interaction between pathogens and hosts (41). Herein, we explored how EV-D68 minireplicon in SGs influence viral replication. Previous studies have found that EV-D68 RNA interacts with TIA1, G3BP1, and HUR. To further investigate the molecular mechanism by which SGs negatively regulate viral replication, the minireplicon was cotransfected in 293T cells with TIA1. Single transfection of minireplicon was used as a control for performing coimmunoprecipitation. The results showed that similar minireplicon mRNA levels were shown in the cells, but the mRNA levels of the minireplicon in the cells transfected with TIA1 after coimmunoprecipitation were about five times that of the control (Fig. 5A). To exclude the possibility of problems with the experimental system, we examined the expression of TIA1 after coimmunoprecipitation and in cell lysates by Western blotting. From this, we believe that TIA1 interacts with the EV-D68 minireplicon. To identify key regions where the minireplicon interacts with TIA1, we constructed deletion mutations in the minireplicon, namely, Δ3′ UTR, Δ5′ UTR, and Δ3′ UTR Δ5′ UTR. Their ability to bind to TIA1 was assessed individually, and it was found that when the Δ3′ UTR was deleted, the minireplicon substantially lost interaction with TIA1 (Fig. 5D). The expression of TIA1 was also detected by Western blotting. Based on the sequence of EV-D68 RNA, we predicted the secondary structure of its 3′ UTR and compared the structural model to that of the coxsackievirus (CVB) and poliovirus (PV) (42–44). The predicted structure of the 3′ UTR of EV-D68 RNA was comprised of a relatively long pairing region (18 bp) flanked by unpaired nucleotides (16 nucleotides at the 3′ terminal and 4 nucleotides at the 5′ terminal. In contrast, within the structures of the 3′ UTR of both CVB and PV (45, 46), the pairing regions (helices) were relatively short (5 to 10 bp) and interrupted by 1 or 2 bulges. Consistent with the secondary structure prediction, the tertiary structural model of the 3′ UTR of EV-D68 was different from that of the 3′ UTR of both CVB and PV. The structure of the 3′ UTR of EV-D68 largely resembled the double-helix model, with unpaired nucleotides mainly located at one end of the helix; however, the 3′ UTR of both CVB and PV featured large bulges (8 to 10 unpaired nucleotides) within the middle of their helices (data not shown). To find a specific binding site for the interaction of TIA1 with the 3′ UTR, we constructed deletion mutants of the 3′-UTR—3′ UTRΔN1-20, 3′ UTRΔN21-40, and 3′ UTRΔN41-60 (Fig. 6A)—and then cotransfected 293T cells with TIA1 for coimmunoprecipitation. The results showed that the 3′ UTRΔN21-40 and 3′ UTRΔN41-60 were necessary for the interaction between the TIA1 and 3′ UTR (Fig. 6B). These results indicated that the SGs formed by TIA1 were able to chelate EV-D68 RNA to inhibit viral replication. At the same time, we also examined the interaction G3BP1 and HUR with the minireplicon (Fig. 5B and C). It was found that when the 3′ UTR was deleted, the interaction between G3BP1 and the minireplicon was significantly reduced (Fig. 5E). At the same time, the interaction between HUR and the minireplicon was also significantly weakened (Fig. 5F). The 3′ UTR was also a binding site for the interaction between G3BP1 and HUR with EV-D68 RNA. Similarly, we examined the interaction between G3BP1 and the 3′-UTR deletion mutations and found that G3BP1 specifically interacted with the 3′ UTRΔN1-20 to inhibit viral replication (Fig. 6C). Interestingly, we found that HUR interacted with the 3′ UTRΔN1-20 and 3′ UTRΔN21-40 (Fig. 6D). At the same time, in order to detect whether the interaction between SG proteins and the 3′ UTR of EV-D68 RNA was dependent upon a complex formed by HUR, TIA1, and G3BP1, 293T cells were cotransfected with TIA1, minireplicon, and siG3BP1 for coimmunoprecipitation. The results showed that TIA1 does not interact with the minireplicon when G3BP1 was knocked down (Fig. 6E). We also detected that HUR no longer interacted with the minireplicon when G3BP1 was knocked down (Fig. 6F). These results confirmed that the interaction of EV-D68 RNA with HUR and TIA1 is complex dependent.
FIG 5.
SG proteins interact with the 3′ UTR of EV-D68 RNA to inhibit viral replication. TIA1 (A), G3BP1 (B), HUR (C), and the minireplicon were cotransfected into 293T cells, and 293T cells were cotransfected with empty vector and the minireplicon as a control. Coimmunoprecipitation was performed 48 h after transfection, and minireplicon mRNA levels were detected by qPCR before and after coimmunoprecipitation. Western blotting was performed to detect the expression of TIA1, G3BP1, and HUR. (D, E, and F) SG proteins and minireplicon deletion mutations were cotransfected into 293T cells and coimmunoprecipitated by the above method. RD cells were transfected with minireplicon (G) and mutants (H) and infected with EV-D68 24 h posttransfection, and the cells were then harvested at 0, 4, 8, 12, and 24 h postinfection. EV-D68 RNA was extracted, and qPCR was used to detect viral mRNA in the cells.
FIG 6.
The interaction of SG proteins with the 3′ UTR of EV-D68 RNA is sequence and complex dependent. (A) Schematic diagram showing the construction of the 3′ UTR mutant of EV-D68. (B, C, and D) SG proteins and 3′ UTR mutants were cotransfected into 293T cells. 293T cells were also cotransfected with empty vector and 3′ UTR mutants as a control. Coimmunoprecipitation was performed 48 h after transfection, and the mRNA levels of the 3′ UTR mutants were detected by qPCR before and after coimmunoprecipitation. SG protein expression was detected by Western blot analysis. (E and F) siG3BP1, HUR/TIA1, and the minireplicon were cotransfected into 293T cells. 293T cells were also cotransfected with an empty vector, siG3BP1, and minireplicon as a control. The cells were cotransfected with HUR/TIA1 and minireplicon as a positive control. Coimmunoprecipitation was performed 48 h after transfection, and minireplicon mRNA levels were detected by qPCR before and after coimmunoprecipitation. HUR and TIA1 expression were detected by Western blot analysis. (G, H, and I) 293T cells were cotransfected with SG proteins and minireplicon or SG proteins and Δ3′ UTR. Minireplicon and empty vectors were used as controls, and all the cells were transfected with pRL-SV40 as an intracellular reference. Luciferase expression levels were detected by a dual-report detection system after 48 h.
To establish the relationship between viral replication and the interaction between EV-D68 RNA and HUR, TIA1, and G3BP1, we cotransfected 293T cells with TIA1 and minireplicon and TIA1 and Δ3′ UTR. Minireplicon and empty vectors were used as controls, and all the cells were transfected with pRL-SV40 as an intracellular reference. The dual-reporter assay system detected luciferase expression and found that luciferase levels were inhibited after transfection with TIA1, but there was no significant difference compared to the control group after transfection with the Δ3′ UTR (Fig. 6G). These data showed that TIA1 can inhibit the expression of minireplicon. We also examined the relationship between G3BP1, HUR, and minireplicon and found that they all inhibited the expression of minireplicon (Fig. 6H and I). These data indicate that overexpression of TIA1, HUR, and G3BP1 can inhibit viral replication, which is dependent upon the interaction with the 3′ UTR of EV-D68 RNA.
In order to further determine the interaction between SG proteins and EV-D68 3′ UTR, RD cells were cotransfected with the minireplicon and SG proteins, respectively. The cells were then infected with EV-D68 at an MOI of 0.1 after 24 h. The results showed that SG proteins did not affect the abundance of EV-D68 RNA compared with the control group (Fig. 5G). In contrast, the abundance of EV-D68 RNA was inhibited after the cotransfection of the SG proteins with the 3′-UTR-deficient minireplicon (Fig. 5H). To further confirm the reliability of the data, we found that the inhibition of EV-D68 replication by SG proteins can be restored after transfection of the minireplicon in HeLa cells. The minireplicon was able to competitively bind to overexpressed SG proteins to reverse their inhibition of EV-D68 replication. In conclusion, SG proteins can inhibit viral replication by interacting with the EV-D68 3′ UTR.
EV-D68 infection blocks the formation of typical SGs during the late stage by cleaving G3BP1.
The above results indicated that SGs negatively regulate the replication of EV-D68. However, further research is needed to determine if the virus has antagonistic mechanisms to provoke the formation of SGs. RD cells were infected with EV-D68 at an MOI of 1, and the cells were treated with Ars as a positive control. We treated the cells with Ars for 1 h after different time points of infection and then fixed the cells for immunofluorescence analysis. Our results showed that cells treated with Ars showed a large amount of cytoplasmic G3BP1-containing SGs. However, cytoplasmic SGs decreased significantly after 12 h, and the ability of cytoplasmic SGs to form at 24 h decreased drastically, resulting eventually in no SGs (Fig. 7B). Next, we performed the same immunofluorescence analysis on HUR and TIA1. The results showed that SGs formed by HUR and TIA1 were significantly degraded after 12 h and were substantially eliminated at 24 h, which was significantly lower than that of cells treated with Ars alone (Fig. 7A and C). We performed quantitative analysis to calculate the number of SGs in each cell (Fig. 7D, E, and F). SGs induced in response to Ars were degraded during the late stage of EV-D68 infection, accompanied by disintegration of G3BP1-containing SGs. In addition, we performed a visual kinetic analysis of endogenous SGs in HeLa cells infected with EV-D68, and similar results were obtained by immunofluorescence (data not shown). In general, EV-D68 was able to inhibit the formation of SGs induced by Ars during the late stage of infection. Our above results further demonstrated that EV-D68 infection not only induces the formation of cytoplasmic SGs but also inhibits the response to Ars-induced SGs.
FIG 7.
EV-D68 infection inhibits the formation of SGs responding to Ars. (A, B, and C) RD cells were infected with EV-D68 (MOI of 1), treated with 0.5 μm Ars for 1 h after infection for 0, 4, 8, 12, and 24 h, fixed, and stained with a rabbit anti-TIA1 monoclonal antibody (A), rabbit anti-G3BP1 monoclonal antibody (B), or rabbit anti-HUR monoclonal antibody (C) for immunofluorescence. (D to F) Quantitative analysis of the number of SGs in each cell. (G) RD cells were infected with EV-D68 (MOI of 1). Cells were harvested at 0, 4, 8, 12, and 24 h after infection and treated with Ars for 1 h as a positive control. The influence of EV-D68 on G3BP1, TIA1, and HUR was detected by Western blotting. (H) RD cells were infected with EV-D68 and EV-71. Cells were harvested at 0, 4, 8, 12, and 24 h after infection. Western blotting was used to assess the expression of G3BP1. (I) RD cells were cotransfected with G3BP1 and 3C or G3BP1 Q326E, and 3C. The expression of G3BP1 and G3BP1 Q326E in the cell lysates was detected 48 h after transfection.
To determine whether the disintegration of SGs is associated with SG protein expression levels, Western blotting was used to analyze SG protein expression in RD cells during EV-D68 infection. It showed that the expression of HUR and TIA1 was consistent in EV-D68-infected cells. Interestingly, G3BP1 was cleaved during the middle and late stages of viral infection (Fig. 7G). Thus, the dispersion of SGs during the late stage of EV-D68 infection may be due to the fact that G3BP1 is cleaved during the middle and late stages of viral infection. It has been reported that the 3C protein of EV-71 and CVB3 cleaves G3BP1. This prompted us to explore the potential mechanism of EV-D68 3C protein cutting G3BP1 (36, 37). RD cells were infected with EV-D68 and EV-71, and the state of G3BP1 was detected at different time points. It was found that G3BP1 had a consistently cleaved band (Fig. 7H). Therefore, we hypothesized that the EV-D68 3C protein also has a shearing effect on G3BP1. We successfully constructed an EV-D68 3C plasmid and cotransfected RD cells with 3C and G3BP1. We then used Western blot analysis to show that G3BP1 had obvious cleavage. At the same time, a G3BP1 mutant was constructed to detect the specific site of 3C protein cutting G3BP1. We found that G3BP1Q326E is an important anti-cutting site. (Fig. 7I).
DISCUSSION
In the past few years, EV-D68 has become a global public health threat. Although there have been a large number of reports on the manipulation of SGs by viruses, little is known about the mechanism of action at the molecular level. Mammalian orthoreovirus (MRV) has been shown to induce the formation of SGs, which is dependent on elF2α phosphorylation during the early stage of infection, and also promotes viral replication (47). At the end of MRV infection, the phosphorylation level of elF2α was still high, but the formation of SGs was reduced (31). The poliovirus (PV) protease 2A was shown to induce SGs early during infection, and by late infection, the PV 3C protease cleaved G3BP1, which resulted in the depolymerization of the SGs (48). However, there it was reported that there is a cytoplasmic granule that is stable in the late stage of PV infection and is an atypical SG that contains viral RNA and TIA1 but not elF4G and PABP (49). Foot-and-mouth disease virus (FMDV) was shown to cleave G3BP1 and G3BP2 by the L protein in order to inhibit the formation of SGs (50). It was reported that EV-71 cuts elF4GI via protease 2A to induce atypical SGs, but inhibits the generation of typical SGs (40). Our study demonstrated that EV-D68 infection induces the formation of typical SGs during early infection (Fig. 2), including G3BP1, HUR, and TIA1, while the formation of SGs was inhibited during the late stages of viral infection (Fig. 1). It has previously been shown that SG formation can be induced by phosphorylation of eIF2α and interference with translation initiation factors (eIF4A, eIF4B, eIF4H, or PABP) (51). In our study, EV-D68-induced SGs were also dependent on PKR-eIF2α phosphorylation, suggesting that EV-D68-induced SGs are similar to typical SGs. Based on this similarity, we verified whether EV-D68 infection induces the formation of typical SGs. It is well-known that Ars and Newcastle disease virus treatment induces the formation of typical SGs. We treated the cells with Ars for 1 h after infecting with EV-D68. The results showed that the SG proteins HUR, TIA1, and G3BP1 were colocalized (Fig. 2). These data showed that EV-D68 infection induced the formation of typical SGs, unlike the “SG-like structure” induced by EV-71. Although both viruses belong to the Picornavirus family, the types and mechanisms of induction of SGs are different.
Studies have shown that various viruses regulate replication by regulating SGs. According to reports, the 3′ stem ring structure of the West Nile and dengue virus (DENV) interacts with TIA1/TIAR to inhibit the formation of SGs, which affects viral replication (33). A previous study demonstrated that DENV-infected A549 cells induced non-TIA1-dependent G3BP1 aggregation (52). Proteomic analysis revealed that G3BP1, G3BP2, Caprin1, and USP10 interact with DENV subgenomic flavivirus RNAs (sfRNAs) to promote DENV replication (52). Our study showed that TIA1, G3BP1, and HUR interacted with the 3′ UTR of EV-D68 RNA. The 3′ UTR region in picornaviruses is a highly conserved sequence with a heterogeneous spatial structure that regulates viral replication. We predicted the secondary structure and tertiary structure of the 3 UTR of EV-D68, PV, and CVB3 and found that the 3′ UTR of EV-D68 is significantly different from the 3′ UTRs of PV and CVB3. In this study, we made truncation mutations to the 3′ UTR of EV-D68, and a specific site for binding to the SG proteins was found by creating a deletion mutation on the 3′ UTR of EV-D68. Previous data have shown that SGs inhibited viral replication. However, TIA1, G3BP1, and HUR interact with the 3′ UTR. We hypothesize that SGs chelate viral RNA to inhibit viral mRNA transcription and affect viral protein translation to inhibit viral replication. Although we have demonstrated that the interaction between SGs and the 3′ UTR inhibits viral replication, SGs can affect viral replication through various pathways, and SGs may also have multiple roles in regulating the replication of various viruses. Therefore, further research is needed in order to determine whether SGs influence viral replication in other ways.
Our results indicated that EV-D68 inhibited SGs during the late stage of infection (Fig. 7). It has been confirmed in previous reports that the cleavage of G3BP1 serves as a mechanism by which PV inhibits the formation of SGs (48). At the same time, G3BP1 shearing occurred in the late stage of EV-71 infection (36). This study also confirmed the cleavage of G3BP1 during the late stage of EV-D68 infection (Fig. 7). This suggests that the inhibition of SGs may be due to the cleavage of G3BP1 in the late stage of EV-D68 infection. To further confirm this possibility, we constructed the 3C protein of EV-D68, which has been shown to disperse the formation of SGs by cleaving G3BP1 at the Q326E site. These results indicated the mechanism by which EV-D68 infection inhibits SGs in the late stage. In short, we found that Picornaviridae viruses, including CVB3, EV71, PV, and EV-D68, were able to cleave G3BP1 during the middle and late stages of infection. However, studies have shown that the late inhibition of SGs by picornaviruses is due to 2A protein. Thus, further research is needed in order to elucidate if 3C plays a role in inhibiting the formation of SGs.
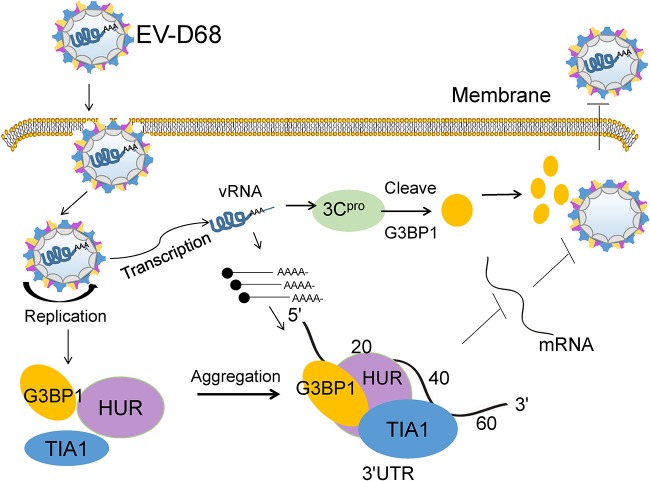
In summary, our research has shown that EV-D68 infection induced the formation of typical SGs early during infection but inhibited SGs during late infection. The interaction of the SGs with the EV-D68 3′ UTR inhibited viral replication, revealing that SGs may contribute to the innate immune response by negatively regulating EV-D68 replication. Based on these results, we developed a schematic diagram of SG formation and dynamic changes during EV-D68 infection (Fig. 8). EV-D68 infection induces the formation of typical SGs, and the SGs chelate the EV-D68 RNA and inhibit viral replication. However, EV-D68 3C protein eliminated the SGs by cleaving the G3BP1 protein as the infection progressed. Thus, SGs are dispersed during the late stage of viral infection. These findings increase our understanding of EV-D68 infection and may help identify new antiviral targets that can inhibit viral replication and limit the pathogenesis of EV-D68.
FIG 8.
Schematic diagram of SG formation and dynamic changes during EV-D68 infection. EV-D68 infection induces the formation of typical SGs in which TIA1, HUR, and G3BP1 colocalize early during infection, and the resulting SGs chelate EV-D68 RNA and inhibit viral replication. However, EV-D68 3C protein eliminated the SGs by cleaving the G3BP1 protein as the infection progressed. Thus, SGs are dispersed during the late stage of viral infection.
MATERIALS AND METHODS
Reagents and antibodies.
FastPfu DNA polymerase (lot no. M21105), First-Strand cDNA synthesis supermix kit (lot no. N10116), and Top Green qPCR supermix kit (lot no. M30321) were purchased from TransGen (Beijing, China). High-glucose Dulbecco’s modified Eagle’s medium (DMEM) (lot no. 8119102) and 0.25% trypsin-EDTA (lot no. 2061500) were purchased from Gibco (USA). Lipofectamine 2000 (lot no. 1952312) was purchased from Invitrogen (CA, USA). Fetal bovine serum (FBS) (catalog no. VS500T) was purchased from Ausbian.
Anti-HA affinity matrix (catalog no. 11815016001) was purchased from Roche. Mouse monoclonal anti-G3BP1 antibody (lot no. l0004925), rabbit monoclonal anti-G3BP1 antibody (lot no. 00047654), rabbit monoclonal anti-HUR antibody (lot no. 00018686), rabbit monoclonal anti-TIA1 antibody (lot no. 00055350), and rabbit anti-HA monoclonal antibody (lot no. 00060457) were purchased from Proteintech (Wuhan, China). Rabbit monoclonal anti-P-eIF2α (catalog no. 9721) and rabbit monoclonal anti-eIF2α (catalog no. 9722) were purchased from Cell Signaling Technology (MA, USA). Rabbit monoclonal anti-P-PKR (catalog no. ab32036) and rabbit monoclonal anti-PKR (catalog no. ab32506) were purchased from Abcam (Cambridge, MA, USA). Mouse anti-Flag monoclonal antibody was purchased from Tianjin Sungene Biotech Co., Ltd.
Cells and viruses.
Human embryonic kidney 293 cells/HEK293T (293T cells), rhabdomyosarcoma (RD), and HeLa cervical carcinoma cells (HeLa cells) were maintained in high-glucose DMEM supplemented with 10% FBS and 1% penicillin/streptomycin (HyClone, USA) at 37°C with 5% CO2. The original prototype EV-D68 strain Fermon (GenBank accession no. KU844179.1) was obtained from Xiaofang Yu (Johns Hopkins University, Baltimore, MD). The original prototype EV-71 was obtained from Jun Han (Chinese Center for Disease Control and Prevention, Beijing). All the cells were purchased from ATCC.
For cell infection, RD or HeLa cells were infected with EV-D68 with a multiplicity of infection (MOI) of 1 at 37°C in FBS-free medium. After 1 h incubation, the unadsorbed virus particles were washed away with phosphate-buffered saline (PBS), and a new medium containing 2% FBS was used. Further treatment was carried out after incubation at 37°C for the indicated time points.
For transfection, the plasmid was transfected with polyethylenimine (PEI), and the cells were further processed after 24 h. For drug treatments, cells were treated with 0.5 μM arsenite (Ars) for 1 h before being harvested for further analysis.
Construction of plasmid.
The EV-D68Fermon 3C plasmid was obtained by molecular cloning. PCR was carried out using the following primers: forward, 5′-CTGCAGACCATGGGACCAGGATTTGATTTTGCG-3′, and reverse, 5′-TAGCGGCCGCTTATACCCATACGACGTCCCAGACTACGCTAAAAACTTGATGGAAGACACTGG-3′. The HUR, TIA1, and G3BP1 plasmid was obtained by molecular cloning, and PCR was carried out using the following primers: HUR (forward, 5′-GCGTCGACACCATGTCTAATGGTTATGAAGACCAC-3′, and reverse, 5′-GCTCTAGATTACTTATCGTCGTCATCCTTGTAATCTTTGTGGGACTTGTTGGTTTTG-3′), TIA1 (forward, 5′-GCGTCGACACCA TGGAGGACGAGATGCCCAAGAC-3′, and reverse, 5′-GCTCTATCACTTAT CGTCGTCATCCTTGTAATCCTGGGTTTCATACCCTGCCAC-3′), and G3BP1 (forward, 5′-TCTGCAGTCACCGTCGTCGACACCATGGTGATGGAGAAGCCTAGTC-3′, and reverse, 5′-GTCTAGAGCGGCCGCGATATCAGCGTAGTCTG GGACGTCGTATGGGTACTGCCGTGGCGCAAGCCCCC-3′). Deletion mutations were made to the minireplicons of EV-D68 (preserved in our laboratory) by molecular cloning using the following primers for PCR amplification: Δ3′ UTR (forward, 5′-ATCGCGTCTCCTATTTTAAAACAGCTCTGGGGTT GTTCCCACCTC-3′, and reverse, 5′-CGATCGTCTCCGGGATTACACGGCG ATCTTTCCGCCCTT CTTGG-3′), Δ5′ UTR (forward, 5′-ATCGCGTCTCCTATT ATGGAAGACGCCAAAAACATA AAGAAAGGCCCG-3′, and reverse, 5′-CGAT CGTCTCCGGGAGGTCCCCAA GTGACCAAA ATTTACCTCTAAG-3′), Δ3′ UTR Δ5′ UTR (forward, 5′-ATCGCGT CTCCTATTATGGAAGACGCCAAAA ACATAAAGAAAGGCCCG-3′, and reverse, 5′-CGATCGTCTCCGGGATTACA CGGCGATCTTTCCGCCCTTCTTGG-3′), 3′ UTRΔN1-20 (forward, 5′-CGGAAAGATCGCCGTGTAAAGTTACAGTTACTTTC-3′, and reverse, 5′-CCTCTAAGTGAAAGTAACTGTAACTTTACACGGCG-3′), 3′ UTR ΔN21-40 (forward, 5′-GTAAATAACTCTAATTGAAACCCAAGAGGTAAATTTT GG-3′, and reverse, 5′-CAAGTGACCAAAATTTACCTCTTGGGTTT CAATTAGAG-3′), and 3′ UTR ΔN41-60 (forward, 5′-CCAAGTTACAGTTACTTTCACTTTG GGGACC-3′, and reverse, 5′-GCGGCCGCTTTTTTTTTTTTTTTTT TTTTTTTTGGTCCCCAAAGTGAAAGTAACTG-3′). The PCR product was digested with DpnI restriction enzyme, and then the mixture was transformed into DH5α competent bacteria (TransGen, Beijing, China) to screen for the correct mutant. The presence of a 3′ UTR mutation in the resulting plasmid was confirmed by DNA sequence analysis software (SeqMan).
RNA extraction and quantitative PCR.
Total intracellular RNA was extracted by TRIzol (Qiagen, Germany) extraction. RNA was reverse transcribed into cDNA using TransScript first-strand cDNA synthesis supermix. cDNA was subjected to qPCR to detect changes in mRNA levels using the TransStart Top Green qPCR supermix kit. mRNA levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. The following primers were used for EV-D68 mRNA: forward, 5′-GGCAGCCTATCAGGTGGAGAG-3′, and reverse, 5′-GAGTTTGTATGGCTTCTTCTGGT-3′. The following primers were used for the EV-D68 minireplicon: forward, 5′-TGGGCGCGTTATTTATCGGA-3′, and reverse, 5′-GCTGCGAAATGCCCATACTG-3′ (reverse). Results were analyzed as fold change using the 2−ΔΔCt method.
Western blotting.
RD and HeLa cells were infected with EV-D68 after transfection with an SG-associated protein (HUR/TIA1/G3BP1). Cells were harvested at different time points after infection and lysed with radioimmunoprecipitation assay (RIPA) lysis buffer. The samples were separated using reduced SDS-PAGE and transferred to a 0.2-μm nitrocellulose membrane using a semidry transfer membrane tester. The membrane was blocked in 5% skim milk for half an hour, and the corresponding primary antibody was diluted with 5% skim milk and incubated at 4°C for 12 h. The corresponding secondary antibody was then incubated with the membrane and finally scanned with Image Lab software for color development.
Immunofluorescence assay.
RD/HeLa cells were infected with EV-D68, fixed with 4% paraformaldehyde (Solarbio) at different time points, and permeabilized with 0.5% Triton X-100 solution for 10 min at 25°C. The cells were blocked with bovine serum albumin (BSA) (Solarbio) for 20 min at 37°C. The primary antibody was diluted in 1% BSA and incubated at 4°C for 12 h. The secondary antibody was incubated at 37°C for 40 min, stained with 1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) (Sigma, USA) for 10 min, mounted with 50% glycerol, and observed under a confocal microscope.
Immunoprecipitation.
The minireplicon and its mutant were cotransfected into 293T cells with SG proteins. After 48 h, the cells were harvested, and 1/10 of the total cells were used to detect the expression of luciferase in the cells. The remaining cells were lysed with lysis buffer supplemented with protease inhibitor (Roche)and RNase inhibitor for 30 min at 4°C. A portion of the cells was used to detect the expression of SG proteins in the cells, followed by incubation with the corresponding beads (Sigma, USA) at 4°C for 6 h. The beads were washed repeatedly with cold PBS, and a portion was eluted with a glycine elution buffer with a pH of 2.4. The remainder was used for RNA extraction, and the mRNA level of luciferase was detected by qPCR. Western blotting was used to detect protein expression.
siRNA knockdown.
siRNAg3bp1 (53) was purchased from GenePharma (Shanghai, China). Cells were seeded into 24-well plates 24 h before two sequential transfections with small interfering RNA (siRNA) oligonucleotide (100 nM) using Lipofectamine 2000 (Invitrogen). The knockdown efficiency of siRNA was examined by Western blotting.
RNA structure identification.
The secondary structures of the RNA from three viruses (3′ UTRs of EV-D68, CVB3, and PV) were predicted using the CentroidFold server with default parameters. Based on the predicted secondary structures, the tertiary structural models of this RNA were constructed using molecular dynamics simulations on the iFoldRNA server 2.3. iFoldRNA used replica exchange for the rapid search of RNA conformations. The simulation time was set as 200 ns. The default values of eight replica temperatures and the heat exchange coefficient were adopted. For each RNA, only one cluster of models was generated, indicating ready convergence of the simulation.
Dual-luciferase assays.
Minireplicons or Δ3′ UTR were transfected with TIA1 and pRL-SV40 in 293T cells in 24-well plates. Minireplicons or Δ3′ UTR were cotransfected with empty vector and pRL-SV40 as controls. Forty-eight hours after transfection, luciferase activity in cell lysates was determined by dual-luciferase reporter assay. Renilla luciferase activity was determined as a control for FLuc activity.
Statistical analysis.
All results were analyzed with GraphPad Prism and are presented as means ± standard deviations (SDs). Statistical significance was determined using two-tailed Student’s unpaired t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., not significant).
Data availability.
All of the data are fully available without restriction.
ACKNOWLEDGMENTS
We thank Xiaofang Yu and Jun Han for providing critical reagents, and Siqi Hu, Zhongwen Zhang, and Minglei Pan for advice and technical assistance. We thank LetPub for linguistic assistance during the preparation of the manuscript.
This work was supported by the National Key Research and Development Program of China (2017YFA0205102) and the National Science Foundation of Tianjin (16JCQNJC09800).
Author contributions are as follows. This study was conceptualized by T.W. and Z.W. Data was curated by J.C., S.L., C.Z., and J.L. Formal analysis was performed by J.C., S.G., and J.K. T.W. and J.K. handled funding acquisition. Investigations were done by J.C., S.L., and S.G. J.C., S.L., S.G., and C.Z. oversaw the methodology. T.W. and Z.W. handled project administration. T.W. managed the resources. T.W. and Z.W. supervised the study. J.C., S.G., and S.L. visualized the study. J.C. wrote the original draft. Review and editing were done by T.W. and Z.W.
All authors declare that they have no competing interests.
REFERENCES
- 1.Sun J, Hu X-Y, Yu X-F, Sun J, Hu X-Y, Yu X-F. 2019. Current understanding of human enterovirus D68. Viruses 11:490. doi: 10.3390/v11060490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schieble JH, Fox VL, Lennette EH. 1967. A probable new human picornavirus associated with respiratory diseases. Am J Epidemiol 85:297–310. doi: 10.1093/oxfordjournals.aje.a120693. [DOI] [PubMed] [Google Scholar]
- 3.Oberste MS, Maher K, Schnurr D, Flemister MR, Lovchik JC, Peters H, Sessions W, Kirk C, Chatterjee N, Fuller S, Hanauer JM, Pallansch MA. 2004. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J Gen Virol 85:2577–2584. doi: 10.1099/vir.0.79925-0. [DOI] [PubMed] [Google Scholar]
- 4.Greninger AL, Naccache SN, Messacar K, Clayton A, Yu G, Somasekar S, Federman S, Stryke D, Anderson C, Yagi S, Messenger S, Wadford D, Xia D, Watt JP, Van Haren K, Dominguez SR, Glaser C, Aldrovandi G, Chiu CY. 2015. A novel outbreak enterovirus D68 strain associated with acute flaccid myelitis cases in the USA (2012–14): a retrospective cohort study. Lancet Infect Dis 15:671–682. doi: 10.1016/S1473-3099(15)70093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Midgley CM, EV-D68 Working Group, Watson JT, Nix WA, Curns AT, Rogers SL, Brown BA, Conover C, Dominguez SR, Feikin DR, Gray S, Hassan F, Hoferka S, Jackson MA, Johnson D, Leshem E, Miller L, Nichols JB, Nyquist AC, Obringer E, Patel A, Patel M, Rha B, Schneider E, Schuster JE, Selvarangan R, Seward JF, Turabelidze G, Oberste MS, Pallansch MA, Gerber SI. 2015. Severe respiratory illness associated with a nationwide outbreak of enterovirus D68 in the USA (2014): a descriptive epidemiological investigation. Lancet Respir Med 3:879–887. doi: 10.1016/S2213-2600(15)00335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poelman R, ESCV-ECDC EV-D68 Study Group, Schuffenecker I, Van Leer-Buter C, Josset L, Niesters HG, Lina B. 2015. European surveillance for enterovirus D68 during the emerging North-American outbreak in 2014. J Clin Virol 71:1–9. doi: 10.1016/j.jcv.2015.07.296. [DOI] [PubMed] [Google Scholar]
- 7.Opanda SM, Wamunyokoli F, Khamadi S, Coldren R, Bulimo WD. 2014. Genetic diversity of human enterovirus 68 strains isolated in Kenya using the hypervariable 3′-end of VP1 gene. PLoS One 9:e102866. doi: 10.1371/journal.pone.0102866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tokarz R, Firth C, Madhi SA, Howie SR, Wu W, Sall AA, Haq S, Briese T, Lipkin WI. 2012. Worldwide emergence of multiple clades of enterovirus 68. J Gen Virol 93:1952–1958. doi: 10.1099/vir.0.043935-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Q, Sharma NR, Zheng ZM, Chen M. 2019. Viral regulation of RNA granules in infected cells. Virol Sin 34:175–191. doi: 10.1007/s12250-019-00122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baez MV, Boccaccio GL. 2005. Mammalian Smaug is a translational repressor that forms cytoplasmic foci similar to stress granules. J Biol Chem 280:43131–43140. doi: 10.1074/jbc.M508374200. [DOI] [PubMed] [Google Scholar]
- 11.Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, Anderson P. 2004. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell 15:5383–5398. doi: 10.1091/mbc.e04-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson P, Kedersha N. 2002. Visibly stressed: the role of eIF2, TIA-1, and stress granules in protein translation. Cell Stress Chaperones 7:213–221. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert SJ, Meakin LB, Bonnet CS, Nowell MA, Ladiges WC, Morton J, Duance VC, Mason DJ. 2014. Deletion of P58(IPK), the cellular inhibitor of the protein kinases PKR and PERK, causes bone changes and joint degeneration in mice. Front Endocrinol (Lausanne) 5:174. doi: 10.3389/fendo.2014.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakagawa K, Narayanan K, Wada M, Makino S. 2018. Inhibition of stress granule formation by Middle East respiratory syndrome coronavirus 4a accessory protein facilitates viral translation, leading to efficient virus replication. J Virol 92. doi: 10.1128/JVI.00902-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holcik M, Sonenberg N. 2005. Translational control in stress and apoptosis. Nat Rev Mol Cell Biol 6:318–327. doi: 10.1038/nrm1618. [DOI] [PubMed] [Google Scholar]
- 16.Garcia MA, Meurs EF, Esteban M. 2007. The dsRNA protein kinase PKR: virus and cell control. Biochimie 89:799–811. doi: 10.1016/j.biochi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Farrell PJ, Balkow K, Hunt T, Jackson RJ, Trachsel H. 1977. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell 11:187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- 18.Wek RC, Jiang HY, Anthony TG. 2006. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans 34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 19.Anderson P, Kedersha N. 2008. Stress granules: the Tao of RNA triage. Trends Biochem Sci 33:141–150. doi: 10.1016/j.tibs.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Lindquist ME, Lifland AW, Utley TJ, Santangelo PJ, Crowe JE. Jr. 2010. Respiratory syncytial virus induces host RNA stress granules to facilitate viral replication. J Virol 84:12274–12284. doi: 10.1128/JVI.00260-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McInerney GM, Kedersha NL, Kaufman RJ, Anderson P, Liljestrom P. 2005. Importance of eIF2alpha phosphorylation and stress granule assembly in alphavirus translation regulation. Mol Biol Cell 16:3753–3763. doi: 10.1091/mbc.e05-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esclatine A, Taddeo B, Roizman B. 2004. Herpes simplex virus 1 induces cytoplasmic accumulation of TIA-1/TIAR and both synthesis and cytoplasmic accumulation of tristetraprolin, two cellular proteins that bind and destabilize AU-rich RNAs. J Virol 78:8582–8592. doi: 10.1128/JVI.78.16.8582-8592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Utt A, Das PK, Varjak M, Lulla V, Lulla A, Merits A. 2015. Mutations conferring a noncytotoxic phenotype on chikungunya virus replicons compromise enzymatic properties of nonstructural protein 2. J Virol 89:3145–3162. doi: 10.1128/JVI.03213-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reineke LC, Lloyd RE. 2013. Diversion of stress granules and P-bodies during viral infection. Virology 436:255–267. doi: 10.1016/j.virol.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai WC, Lloyd RE. 2014. Cytoplasmic RNA granules and viral infection. Annu Rev Virol 1:147–170. doi: 10.1146/annurev-virology-031413-085505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onomoto K, Yoneyama M, Fung G, Kato H, Fujita T. 2014. Antiviral innate immunity and stress granule responses. Trends Immunol 35:420–428. doi: 10.1016/j.it.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeuchi K, Komatsu T, Kitagawa Y, Sada K, Gotoh B. 2008. Sendai virus C protein plays a role in restricting PKR activation by limiting the generation of intracellular double-stranded RNA. J Virol 82:10102–10110. doi: 10.1128/JVI.00599-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iseni F, Garcin D, Nishio M, Kedersha N, Anderson P, Kolakofsky D. 2002. Sendai virus trailer RNA binds TIAR, a cellular protein involved in virus-induced apoptosis. EMBO J 21:5141–5150. doi: 10.1093/emboj/cdf513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Visser LJ, Langereis MA, Rabouw HH, Wahedi M, Muntjewerff EM, de Groot RJ, van Kuppeveld FJM. 2019. Essential role of enterovirus 2A protease in counteracting stress granule formation and the induction of type I interferon. J Virol 93. doi: 10.1128/JVI.00222-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White JP, Cardenas AM, Marissen WE, Lloyd RE. 2007. Inhibition of cytoplasmic mRNA stress granule formation by a viral proteinase. Cell Host Microbe 2:295–305. doi: 10.1016/j.chom.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Qin Q, Carroll K, Hastings C, Miller CL. 2011. Mammalian orthoreovirus escape from host translational shutoff correlates with stress granule disruption and is independent of eIF2alpha phosphorylation and PKR. J Virol 85:8798–8810. doi: 10.1128/JVI.01831-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fritzlar S, Aktepe TE, Chao Y-W, Kenney ND, McAllaster MR, Wilen CB, White PA, Mackenzie JM. 2019. Mouse norovirus infection arrests host cell translation uncoupled from the stress granule-PKR-eIF2alpha axis. mBio 10. doi: 10.1128/mBio.00960-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emara MM, Brinton MA. 2007. Interaction of TIA-1/TIAR with West Nile and dengue virus products in infected cells interferes with stress granule formation and processing body assembly. Proc Natl Acad Sci U S A 104:9041–9046. doi: 10.1073/pnas.0703348104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuki H, Takahashi M, Higuchi M, Makokha GN, Oie M, Fujii M. 2013. Both G3BP1 and G3BP2 contribute to stress granule formation. Genes Cells 18:135–146. doi: 10.1111/gtc.12023. [DOI] [PubMed] [Google Scholar]
- 35.Yang X, Hu Z, Zhang Q, Fan S, Zhong Y, Guo D, Qin Y, Chen M. 2019. SG formation relies on eIF4GI-G3BP interaction which is targeted by picornavirus stress antagonists. Cell Discov 5:1. doi: 10.1038/s41421-018-0068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Yao L, Xu X, Han H, Li P, Zou D, Li X, Zheng L, Cheng L, Shen Y, Wang X, Wu X, Xu J, Song B, Xu S, Zhang H, Cao H. 2018. Enterovirus 71 inhibits cytoplasmic stress granule formation during the late stage of infection. Virus Res 255:55–67. doi: 10.1016/j.virusres.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Fung G, Ng CS, Zhang J, Shi J, Wong J, Piesik P, Han L, Chu F, Jagdeo J, Jan E, Fujita T, Luo H. 2013. Production of a dominant-negative fragment due to G3BP1 cleavage contributes to the disruption of mitochondria-associated protective stress granules during CVB3 infection. PLoS One 8:e79546. doi: 10.1371/journal.pone.0079546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Y, Wang B, Huang H, Zhao Z. 2016. Enterovirus 71 induces anti-viral stress granule-like structures in RD cells. Biochem Biophys Res Commun 476:212–217. doi: 10.1016/j.bbrc.2016.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu Z, Wang Y, Tang Q, Yang X, Qin Y, Chen M. 2018. Inclusion bodies of human parainfluenza virus type 3 inhibit antiviral stress granule formation by shielding viral RNAs. PLoS Pathog 14:e1006948. doi: 10.1371/journal.ppat.1006948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang X, Hu Z, Fan S, Zhang Q, Zhong Y, Guo D, Qin Y, Chen M. 2018. Picornavirus 2A protease regulates stress granule formation to facilitate viral translation. PLoS Pathog 14:e1006901. doi: 10.1371/journal.ppat.1006901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan M, Gao S, Zhou Z, Zhang K, Liu S, Wang Z, Wang T. 2018. A reverse genetics system for enterovirus D68 using human RNA polymerase I. Virus Genes 54:484–492. doi: 10.1007/s11262-018-1570-3. [DOI] [PubMed] [Google Scholar]
- 42.Sato K, Hamada M, Asai K, Mituyama T. 2009. CENTROIDFOLD: a web server for RNA secondary structure prediction. Nucleic Acids Res 37:W277–80. doi: 10.1093/nar/gkp367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krokhotin A, Houlihan K, Dokholyan NV. 2015. iFoldRNA v2: folding RNA with constraints. Bioinformatics 31:2891–2893. doi: 10.1093/bioinformatics/btv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma S, Ding F, Dokholyan NV. 2008. iFoldRNA: three-dimensional RNA structure prediction and folding. Bioinformatics 24:1951–1952. doi: 10.1093/bioinformatics/btn328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Bakkers JM, Galama JM, Bruins Slot HJ, Pilipenko EV, Agol VI, Melchers WJ. 1999. Structural requirements of the higher order RNA kissing element in the enteroviral 3′UTR. Nucleic Acids Res 27:485–490. doi: 10.1093/nar/27.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiang Z, Xie Z, Liu L, Ren L, Xiao Y, Paranhos-Baccalà G, Wang J. 2016. Genetic divergence of enterovirus D68 in China and the United States. Sci Rep 6:27800. doi: 10.1038/srep27800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qin Q, Hastings C, Miller CL. 2009. Mammalian orthoreovirus particles induce and are recruited into stress granules at early times postinfection. J Virol 83:11090–11101. doi: 10.1128/JVI.01239-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dougherty JD, Tsai WC, Lloyd RE. 2015. Multiple poliovirus proteins repress cytoplasmic RNA granules. Viruses 7:6127–6140. doi: 10.3390/v7122922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Piotrowska J, Hansen SJ, Park N, Jamka K, Sarnow P, Gustin KE. 2010. Stable formation of compositionally unique stress granules in virus-infected cells. J Virol 84:3654–3665. doi: 10.1128/JVI.01320-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Visser LJ, Medina GN, Rabouw HH, de Groot RJ, Langereis MA, de Los Santos T, van Kuppeveld F. 2019. Foot-and-mouth disease virus leader protease cleaves G3BP1 and G3BP2 and inhibits stress granule formation. J Virol 93:e00922-18. doi: 10.1128/JVI.00922-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lloyd RE. 2012. How do viruses interact with stress-associated RNA granules? PLoS Pathog 8:e1002741. doi: 10.1371/journal.ppat.1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bidet K, Dadlani D, Garcia-Blanco MA. 2017. Correction: G3BP1, G3BP2 and CAPRIN1 are required for translation of interferon stimulated mRNAs and are targeted by a dengue virus non-coding RNA. PLoS Pathog 13:e1006295. doi: 10.1371/journal.ppat.1006295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu S, Li J, Xu F, Mei S, Le Duff Y, Yin L, Pang X, Cen S, Jin Q, Liang C, Guo F. 2015. SAMHD1 inhibits LINE-1 retrotransposition by promoting stress granule formation. PLoS Genet 11:e1005367. doi: 10.1371/journal.pgen.1005367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the data are fully available without restriction.