Abstract
The United States is swiftly moving toward increased legalization of medical and recreational cannabis. Currently considered the most commonly used illicit psychoactive drug, recreational cannabis is legal in 11 states and Washington, DC, and male use is an important and understudied concern. Questions remain, however, about the potential long-term consequences of this exposure and how cannabis might impact the epigenetic integrity of sperm in such a way that could influence the health and development of offspring. This review summarizes cannabis use and potency in the USA, provides a brief overview of DNA methylation as an epigenetic mechanism that is vulnerable in sperm to environmental exposures including cannabis, and summarizes studies that have examined the effects of parental exposure to cannabis or delta-9 tetrahydrocannabinol (THC, the main psychoactive component of cannabis) on the epigenetic profile of the gametes and behavior of offspring. These studies have demonstrated significant changes to the sperm DNA methylome following cannabis use in humans, and THC exposure in rats. Furthermore, the use of rodent models has shown methylation and behavioral changes in rats born to fathers exposed to THC or synthetic cannabinoids, or to parents who were both exposed to THC. These data substantiate an urgent need for additional studies assessing the effects of cannabis exposure on childhood health and development. This is especially true given the current growing state of cannabis use in the USA.
Keywords: cannabis, marijuana, DNA methylation, sperm, paternal effects, preconception, heritability
Introduction
Cannabis sativa has been grown and used for thousands of years [1]. The versatile plant has served a variety of purposes including textile production, medicine and recreation [1]. Cannabis has been used in the United States since the 1800s, and today it is considered to be the most commonly used psychoactive drug [1]. Attitudes about cannabis safety and use have shifted over time, and more prominent legalization efforts have helped shape public opinion about medical and recreational cannabis consumption [1–3]. Along with changing attitudes toward cannabis use and acceptability, the composition of cannabis that is consumed has also changed dramatically in recent years, with more potent strains of cannabis being developed and used by consumers [1–3]. Surveys have gathered information about patterns and trends of cannabis use and to better understand the shifts in the public perception of the safety of cannabis. This is important and timely given that legislators and politicians are frequently discussing plans to increase access to some form of legal cannabis—either medical or recreational—at the federal level.
Largely absent from these conversations are the potential health consequences of cannabis consumption. This is especially troubling as studies are beginning to demonstrate that there are adverse health effects of cannabis consumption not only on the consumer, but also on their children [4, 5]. This is supported by research using animals exposed to delta-9-tetrahydrocannabinol (THC, the main psychoactive component of cannabis) [4, 5]. Epigenetic modifications influence the way a gene is expressed, for example through the addition or removal of molecular moieties to the DNA or to the tails of histone proteins around which the DNA is wrapped [6]. The pattern of the epigenetic modifications in a somatic cell is heritable—that is, transmitted during cellular division to the daughter cell [6]. Importantly, epigenetic modifications serve as an intermediary that transmits information between the environment and the DNA sequence of the genome [6]. Environmental exposures can elicit changes in the epigenome that result in altered gene activity without an alteration to the underlying sequence of DNA [6]. Epigenetics therefore provide one potential mechanism through which parental cannabis exposure can impact the health and development of their offspring.
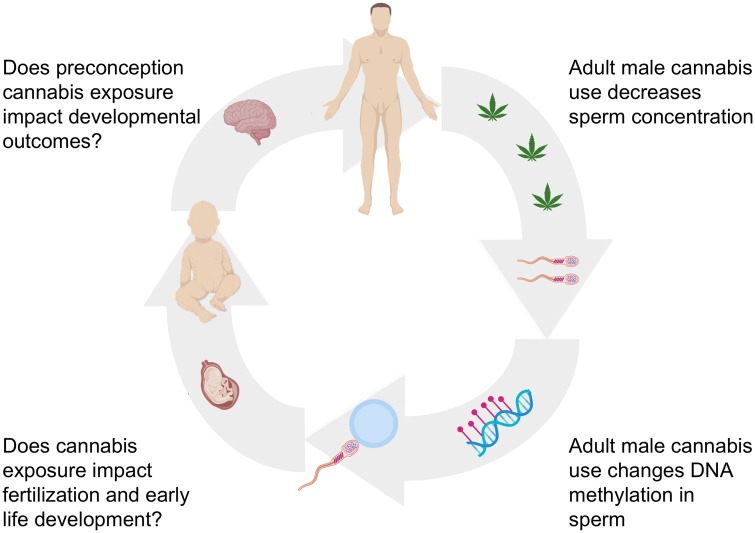
A schematic representation of this review is presented in Fig. 1. We will first highlight the trends in cannabis use and legalization in the USA and address the increasing potency of cannabis as well as the rationale for focusing on male cannabis use. We will next offer a brief description of epigenetic inheritance mechanisms, with a focus on DNA methylation. This will be followed by a summary of the current animal model and human studies that are beginning to reveal how THC exposure and cannabis use disrupt DNA methylation in sperm, and the potential impact of such preconception exposures on offspring development. Lastly, we will briefly discuss the studies that have focused on the effect of maternal exposure to cannabis in utero, highlight other potential epigenetic mechanisms that may also be affected by cannabis use, and outline important questions that remain unanswered. We will conclude by emphasizing the urgent need for more comprehensive studies to be conducted in this area, and the importance of conveying these research findings about the potential consequences of exposure to the general public and policymakers to help inform decisions about personal use and legalization.
Figure 1:
overview of cannabis exposure and potential consequences. Image made with graphics from ©BioRender (biorender.com)
Trends in Cannabis Use and Potency in the USA
Legalized cannabis is becoming increasingly available in the USA. As of 2020, there are 11 states and Washington, DC that have legalized the recreational sale and use of cannabis [7] (Table 1). Medicinal cannabis use has been made legal in 33 states and Washington, DC [7] (Table 1). Cannabidiol (CBD), a nonpsychoactive cannabinoid that can be derived from either the cannabis or hemp plant, has also achieved widespread acceptance and is federally legal as long as it is derived from the hemp plant and the dried product contains no more than 0.3% THC. Even in states without legalized cannabis use, CBD is available in enormous numbers of consumer products. Cannabis was first regulated at the federal level in the mid-1900s due to concerns about recreational use and adverse reactions to cannabis-containing products [1]. However, given that the federal regulation of cannabis has not been consistently enforced, cannabis has remained prevalent in the USA and is known to be the most commonly used illicit psychoactive drug. While legalization is most readily occurring at the state level in the USA, there is increasing discussion about changes to the federal regulation of cannabis in mainstream politics and news. Cannabis is currently illegal at the federal level, but individual states have autonomy to make decisions that are not generally subjected to federal jurisdiction.
Table 1:
list of states with legalized medical and/or recreational cannabis as of 2020
| State | Medical cannabis legalized | Recreational cannabis legalized |
|---|---|---|
| Alaska | Yes | Yes |
| Arizona | Yes | No |
| Arkansas | Yes | No |
| California | Yes | Yes |
| Colorado | Yes | Yes |
| Connecticut | Yes | No |
| Delaware | Yes | No |
| District of Columbia | Yes | Yes |
| Florida | Yes | No |
| Hawaii | Yes | No |
| Illinois | Yes | Yes |
| Louisiana | Yes | No |
| Maine | Yes | Yes |
| Maryland | Yes | No |
| Massachusetts | Yes | Yes |
| Michigan | Yes | Yes |
| Minnesota | Yes | No |
| Missouri | Yes | No |
| Montana | Yes | No |
| Nevada | Yes | Yes |
| New Hampshire | Yes | No |
| New Jersey | Yes | No |
| New Mexico | Yes | No |
| New York | Yes | No |
| North Dakota | Yes | No |
| Ohio | Yes | No |
| Oklahoma | Yes | No |
| Oregon | Yes | Yes |
| Pennsylvania | Yes | No |
| Rhode Island | Yes | No |
| Utah | Yes | No |
| Vermont | Yes | Yes |
| Washington | Yes | Yes |
| West Virginia | Yes | No |
The two most common cannabinoids present in the cannabis plant are THC and CBD [1, 8, 9]. THC is responsible for the psychoactive effects evoked by cannabis consumption, while CBD is not psychoactive and instead has purported relaxation and anti-anxiety properties [1]. The potency of cannabis, defined as the percentage by weight of THC, has been progressively increasing in the USA in recent years [5]. This is especially true in states with legalized recreational cannabis programs that are able to produce high-potency strains for hashish and hash oil products, that contain upwards of 65% THC [1, 3, 10, 11]. Several studies have analyzed changes in potency over time, from 1996 to 2014 as well as from 2008 to 2017 by measuring THC concentration present in samples that were confiscated by the Drug Enforcement Agency (DEA). This helps provide information about cannabis products that are being sold and consumed across that country that would otherwise not be reported, unlike in states with legal cannabis programs. These studies have consistently found increasing potency of THC in these samples over time [11]. Specifically, cannabis products that were confiscated in 1996 contained roughly 4% THC, while in 2017, the average amount of THC measured in confiscated samples was 17.1% [1, 11]. Additionally, the ratio of THC to CBD has increased nearly 10-fold, from 12 in 2008 to 104 in 2017 [1]. These increases in potency and the increased ratio of THC to CBD reflects the selective growth over time of plants with higher THC and lower CBD levels, as well as an increased desire for the consumption of these increasingly potent strains [1].
Higher potency strains of cannabis are increasingly associated with adverse health outcomes in users [12]. These adverse outcomes include an increased risk of developing a cannabis use disorder and increased risk of the development of, or relapse back into, cannabis-induced psychosis [13–15]. This is concerning considering that cannabis use has increased across multiple age groups in the USA as legalization has made cannabis more accessible [16]. A 2014 study of age differences in daily and nondaily cannabis consumers found that 26.3% of all adults who consumed cannabis were using it five or more days per week [17]. This is significant because the study reports that frequent cannabis use renders individuals more vulnerable to psychiatric symptoms and substance use disorders, similar to the harmful effects that are exacerbated by consuming more potent cannabis [17]. The study found that the adults who consumed cannabis most frequently were between the ages 18 and 35 [17]. Importantly, this age range includes peak reproductive years. Additionally, both daily and nondaily use were increased in these adults from 2002 to 2014, providing further evidence that more adults are using cannabis more frequently [17, 18].
Surveys of adolescents and adults in the USA during this same time period, 2002–2014, collected information about the perception of cannabis safety. Along with increased use, the perception of harm associated with cannabis use decreased, while the perception of its safety increased [2]. This trend was reported for both adolescents and adults. A ‘Monitoring the Future Study’ found that adolescents were using cannabis more and were perceiving it as less harmful. In 2016, about 30% of all 12th graders believed cannabis use is risky [2]. The National Survey of Drug Use and Health (NSDUH) found that between 2002 and 2014, the perception among adults of great risk associated with cannabis use declined from 50% to 33% [2, 12]. During this same period, adult perception of no risk increased from 6% to 15% [6, 12]. The 2018 NSDUH Substance Abuse and Mental Health Services (SAMHSA) report found that 43.5 million Americans ages 12 and older used cannabis in the past year [19]. This number represents about 15.9% of the population, and represents an increase in the number of Americans using cannabis compared to reports conducted between 2002 and 2017 [19]. These data support that cannabis use in the USA appears to still be on an upward trajectory.
Men use cannabis products more so than women, and men are more likely to develop cannabis use disorders [20, 21]. A cross-sectional anonymous online survey of 2374 cannabis users found not only that men use cannabis more frequently, but they consume higher quantities as compared to cannabis using women [20]. Furthermore, men are more likely than women are to become dependent on cannabis [8]. Sex differences were most pronounced for recreational versus medicinal use, with 73.4% of men reporting recreational cannabis use as compared to only 65.5% of women, a significant difference (P < 0.001) [1].
With expanding cannabis legalization, worldwide cannabis use and the perception that it is safe are increasing. These trends are observed among both adolescents and adults. However, while the normalization of acceptance and patterns of use expand, the associated health concerns remain understudied. We have little knowledge about the long-term health effects of this increasing level of cannabis exposure and how it might impact child development. Studies have focused on the potential harmful effects of maternal cannabis use during pregnancy, and the US Surgeon General and the American College of Obstetricians and Gynecologists have cautioned pregnant women about the potential hazards. However, little has been done to explore possible consequence of paternal cannabis use before conception. This is a critical gap in the literature given higher usage in men combined with emerging studies showing that exposure to other chemicals and drugs before conception has the potential to elicit harmful effects in children.
Epigenetics and Paternal Epigenetic Inheritance
How could cannabis use by males prior to conception influence the health of his children? One possible mechanism is through epigenetic modifications in the sperm that change gene expression after fertilization of an egg. Such changes are consistent with the concept of the Developmental Origins of Health and Disease (DOHaD), which posits that the environment encountered during specific critical developmental timepoints elicits a response in gene expression patterns that allow for optimizing survival under those conditions [22, 23]. However, those changes in gene expression, which are thought to be permanent, may be dysfunctional under different environmental conditions encountered at a later time, and could increase the propensity for adverse outcomes and development of disease [6, 23]. Epigenetic modifications, including the addition or removal of chemical moieties to histone tails, the actions of noncoding RNAs and the addition or removal of DNA methylation to cytosines in the DNA (usually in CpG context), serve as an arbitrator between the environment and the genome, are responsive to changes in the environment, and are one likely explanation for DOHaD [22]. The patterns of histone modifications and DNA methylation are transmissible during cell division, such that the epigenome in many ways serves as a compendium of an individual’s exposure history [22].
For the purposes of this review, we will be largely focused on DNA methylation, as this is the most extensively studied epigenetic modification, and the literature thus far has concentrated primarily on investigation of DNA methylation alterations and cannabis. There are two waves of DNA methylation reprogramming that occur throughout development that may provide routes for altered DNA methylation patterns to be inherited. Between gestational weeks 7 and 11 in humans, a wave of epigenetic reprogramming occurs in the primordial germ cells (PGCs) [24–37]. As PGCs migrate from the hindgut to colonize the genital ridge, they undergo a global demethylation to create a blank slate onto which an epigenetic profile reflective of the sex of the developing fetus is established [24, 25, 27, 32, 34, 35]. The second wave of reprogramming begins immediately post-fertilization, when DNA methylation marks are removed from the parental genomes. There is active removal of DNA methylation marks from the paternal genome, while the maternal genome undergoes a passive loss of methylation [31, 32, 34, 35, 38, 39]. Remethylation occurs in the epiblast during gastrulation, about 1-week post-fertilization [32, 34, 35].
Reprogramming provides an essential resetting of the epigenetic information in each generation. However, some epigenetic marks, including a small proportion of histones along with their post-translational modifications and some DNA methylation, are retained during these reprogramming events, providing a mechanism for potential transmission of altered epigenetic information from one generation to the next [23, 28, 29, 40, 41]. This means that it is possible that altered epigenetic information in the sperm could be delivered to the oocyte at fertilization and then be maintained in the developing embryo and fetus.
DNA methylation does indeed carry information that guides gene expression from the father to his children at regions of the genome subject to genomic imprinting [24]. Imprinted genes are characterized by expression of only one of the two alleles present in a given somatic cell, with the expressed allele dependent on the sex of the parent from whom it was inherited [24, 42]. This pattern of expression is established by DNA methylation that is set during formation of the gametes, and this methylation is subsequently resistant to post-fertilization reprogramming [42]. Any shifts in the establishment and/or maintenance of methylation at imprinted regions is carried forward into the next generation. Recently, thousands of other regions of the genome, aside from those that are imprinted or repetitive elements, have been shown to also resist post-fertilization DNA methylation reprogramming [40, 41]. These regions are therefore also able to carry gene regulatory information forward into the next generation and provide a mechanism for the intergenerational inheritance of altered epigenetic states from sperm in a manner that could impact the development and health of the child.
The majority of DOHaD-related studies have focused on the effects of in utero exposures on the health, development and epigenetic profile of the offspring, while the potential contribution of the father’s exposures to early life health has been long overlooked. Recent studies have begun to emphasize that the father’s exposure prior to conception may be playing an important role [43]. There are now emerging efforts to better understand the effects of paternal exposures prior to conception and how they impact the epigenetic integrity of the sperm [37, 44]. Studies have found that exposures such as obesity, nutrition, cigarette smoke and pesticides alter sperm DNA methylation, with obesity and cigarette smoke doing so in a heritable fashion in humans [37, 43–50]. The effects of cannabis on the sperm epigenome, a substance with increased access and use by men in particular, however, have been largely understudied, and the potential effects of paternal preconception cannabis exposure on offspring health are poorly understood.
Effects of THC and Synthetic Cannabinoids on DNA Methylation: Rodent Models
The effects of parental THC exposure on offspring behavior and epigenetic changes were analyzed by Szutorisz et al. [51], and Watson et al., using a rat model of exposure [52]. In their studies, both male and female rats were dosed with a moderate level of THC (1.5 mg/kg i.p.) or vehicle control (saline containing 0.3% Tween 80) during adolescence, one injection every third day during postnatal days 28 to 49. These rats were subsequently mated (THC-exposed male mated with THC-exposed female, and vehicle-exposed male mated with vehicle-exposed female) during adulthood and their offspring were analyzed for behavioral and molecular outcomes. There was a significant increased self-administration of heroin in the adult males born to THC-exposed parents compared to controls, as well as associated molecular changes in the brains of these offspring [51]. In particular, there were significant changes in expression of genes involved in synaptic plasticity as measured in the dorsal and ventral striatum of the adult offspring [51]. There were also changes in the expression of receptors involved in dopaminergic and glutamatergic signaling [51]. This is important and relevant for their observed phenotype, given the role of those pathways in addiction-related behaviors. There were significant changes in the expression of Cnr1, Grin2A and Grin1, Drd2, Gria1 and Gria2 [51]. Another endpoint that the authors analyzed was long-term depression (LTD), which plays an important role in synaptic plasticity, a neurological process that is critical for memory and learning [53]. They observed an increase in LTD in the dorsal striatum of the adult F1 offspring following parental THC exposure, demonstrating the ability for preconception parental THC exposure to have a physiological consequence [51].
Using the same parental THC exposure paradigm and focusing on epigenetic effects, Watson et al. demonstrated changes to DNA methylation in the F1 adults born to the THC-exposed parents as compared to the controls [52]. Methylation changes in the offspring were measured specifically in the nucleus accumbens, and were ultimately related to the behavioral changes identified by Szutorisz et al. as a result of the adolescent exposure to THC of the parents. DNA from these nucleus accumbens from these offspring underwent enhanced reduced representation bisulfite sequencing to measure DNA methylation changes, and quantitative reverse transcription PCR (qRT-PCR) was performed for a subset of genes to determine if the changes to methylation were associated with changes in expression in brain tissues.
The methylation data from this study demonstrated that there were 406 hypermethylated and 621 hypomethylated differentially methylated regions (DMRs) consisting of 5611 individual CpG sites [52]. There were 196 hypermethylated DMRs and 317 hypomethylated DMRs that mapped to the promoters or exons/introns of 492 RefSeq genes [52]. To understand the functional significance of the genes possessing differentially methylated regions, gene ontology terms were analyzed. There was enrichment for processes involved in synaptic plasticity and neurological behavior and function, all of which are in line with the behavioral and molecular findings reported by Szutorisz et al [51, 52]. However, the parental origin of the epigenetic changes observed in the offspring here cannot be attributed solely to one parent or the other, given that both parents were exposed to THC prior to mating, and no germline epigenetic analyses were conducted.
A rodent study that focused solely on paternal exposure was performed by Andaloussi et al. In this study, male rats were exposed to the synthetic cannabinoid, Win55,212-2, which acts as a CNR1 agonist. Male rats were then mated to drug-naïve females and offspring were examined for behavioral effects when they reached adolescence [54]. The offspring of exposed and control dads were first exposed to 1 week of unpredictable and variable stress [54]. One day after the cessation of the stress, behavioral tests were conducted in the adult animals to assess anxiety-like behaviors, locomotor behavior and episodic-like memory. There were no significant effects of the paternal exposure or unpredictable stress on locomotor behavior or episodic-like memory. However, there were significant effects of stress on anxiety-like behaviors as demonstrated with the open field test in the rats born to the Win55,212-2-exposed dads [54].
Global DNA methylation was analyzed in these animals through the use of a 5-mC ELISA assay [54]. These experiments showed that there was a significant interaction between stress and paternal Win55,212-2 exposure compared to control on global DNA methylation in offspring prefrontal cortex (PFC) [54]. Specifically, increased levels of genomic 5-mC was found in the PFC of these animals. Global DNA hypermethylation was accompanied by concurrent upregulation of Dnmt1 and Dnmt3a transcription [54].
Our group assessed the effects of paternal exposure to THC alone on DNA methylation in rat sperm. A group of adult male rats were exposed to 2 mg/kg THC (equivalent to moderate daily cannabis use) or vehicle control (10% ethanol, 1% Triton X-100 in saline) via oral gavage for 12 days to determine the effects of the exposure on rat sperm [55]. DNA from sperm of these animals underwent reduced representation bisulfite sequencing (RRBS) and demonstrated significant changes with the THC exposure. There were 621 genes associated with CpG sites significantly impacted by THC [55]. The majority of the affected CpG sites were located in intronic (44%) and exonic (33%) positions. Additionally, 25% were located in CpG islands, 24% were located in shores and 4% were located in shelves. The remaining 49% were located elsewhere throughout the genome outside of CpG islands [55]. If and how these sites might escape reprogramming remains unknown, but it is of interest that they are located primarily within the gene body and that a majority of them have some association with a CpG island, shore, or shelf. Analysis of the affected genes using the DAVID bioinformatics database revealed KEGG pathways enriched in differentially methylated genes including the hippo signaling pathway, pathways in cancer, MAPK signaling pathway and regulation of actin cytoskeleton [55]. THC-exposed and control male rats were bred to drug-naïve females and the adult offspring were examined for neurobehavioral outcomes. The F1 offspring exhibited long-lasting impairment of attention tasks as compared to controls, and they displayed a significant increase in the habituation of locomotor activity in the figure-8 maze [56].
We also examined the potential overlap between genes we identified as being significantly differentially methylated in sperm of THC-exposed rats and those affected in the nucleus accumbens of rats born to THC-exposed parents from the Watson et al. study. There was indeed a significant number of genes that were identified as differentially methylated in both datasets [52, 55].
These animal studies demonstrate the ability for paternal and parental exposure to THC or synthetic cannabinoids to significantly impact the epigenetic profile and behavior of offspring. These are important findings and may be relevant to humans since more adults of reproductive age are using increasingly potent strains of cannabis products. It is also important given that more than half of all US pregnancies are unplanned, meaning one or both of the parents may have been unknowingly exposed to cannabis prior to the time of conception. These findings stress the need for more research using rodent models and human studies to determine the potential breadth of intergenerational and potential for transgenerational effects of preconception cannabis use.
Effects of Male Cannabis Use on DNA Methylation: Human Studies
To date, only one study, published by our group, has reported on the effects of adult male cannabis use on the human sperm DNA methylome [55]. This study was done in tandem with our rat model, described above. Murphy et al. enrolled 12 men who used cannabis as well as 12 matched nonuser controls. All men provided urine samples to measure the concentration of THC metabolites present in urine, which was used to verify user status and to quantify exposure levels. Additionally, all men provided a semen sample which underwent semen analysis and from which DNA was extracted. The genomic DNA was then used to generate RRBS data, which provides quantitative DNA methylation data for millions of CpG sites across the genome.
The findings indicated a significant reduction in the sperm concentration of men who used cannabis compared to those who did not [55]. Furthermore, this was the first study to associate urinary THC concentrations with decreased sperm concentrations [55]. This finding is in line with the existing literature. Cannabis has been associated with decreased sperm quality and an increased risk of the development of testicular germ cell cancers in previous studies [57]. Others have attempted to determine the mechanism through which these effects occur, which led to the discovery that the endocannabinoid system (ECS) plays a critical role in the regulation of spermatogenesis [57]. There is stage and cell-type specific expression of ECS signaling components found in germ and somatic cells in the testes [57, 58]. While this helps to clarify the role of the ECS in spermatogenesis, the ability of cannabis to directly impair spermatogenesis through the ECS is still unresolved.
There were significant effects of cannabis use on sperm DNA methylation, with 3979 CpG sites that were differentially methylated in the sperm of cannabis users compared to the nonuser controls [55]. There were 46 genes for which there were 10 or more CpG sites affected by cannabis exposure [55]. About 78% of the altered CpGs exhibited decreased DNA methylation in the users compared to the controls [55]. For 183 individual CpG sites representing 177 genes, there was a significant correlation between the amount of THC measured in the urine and the level of methylation present, suggestive of a dose–response relationship [55]. We also assessed the genomic locations of these affected CpG sites and again found that the majority were located in introns (41%) and that 22% were in CpG islands, 16% were in shores and 4% were in shelves [55]. Intriguingly, both in our human and rat analyses, the majority of affected CpG sites are intronic. It is also noteworthy that there are substantial numbers of affected sites within CpG islands, shores and shelves.
Genes names associated with the affected CpG sites were entered into the DAVID bioinformatics database to determine if there was enrichment for genes with specific functions. Several KEGG pathways were identified, including ascorbate and aldarate metabolism, pathways in cancer, hippo signaling, MAPK signaling pathway and circadian entrainment [55]. These pathways are known to be involved in early life development, particularly hippo signaling and circadian entrainment, and may provide insights into the potential role that paternal cannabis use could have in affecting offspring health [59, 60]. Strikingly, there was a similarly in the pathways that were enriched for in both our rat and human studies. These include hippo signaling, pathways in cancer and MAPK signaling pathway. There was a small number of genes in common between the pathways in the two different species (hippo signaling pathway: APC2, GDF6, LLGL1, TCF7L1, BMP7, and BMP6; pathways in cancer: FGF12, PRKACA, GNG7, GNB2, APC2, TCF7L1; MAPK signaling pathway: CACNA2D1, CACNA1I, FGF12, PRKACA, CACNA1A) suggesting that there is potential for both gene-specific and pathway-specific effects of the exposure that are shared between humans and rodents [55]. The fact that there was minimal overlap in the genes in both species, yet similar pathways were significantly affected suggests that the function of the pathway, or sequence features that characterize the genes in these pathways, may be the target of the exposure. This finding also suggests that a significant portion of the effects of cannabis exposure in humans may be due to THC, since this was the common compound in both exposures. This is notable given that the amount of THC in cannabis has markedly and intentionally increased since the 1970s [11].
An unexpected finding relates to the aryl hydrocarbon receptor repressor (AHRR) gene. The RRBS data showed that there were 94 CpG sites within AHRR that were differentially methylated in the sperm of the cannabis users as compared to controls. While two of these CpG sites were found to be located very close to CpGs that are known to be hypomethylated in infants born to cigarette-smoking mothers, the other 92 are located in a 62-nucleotide repeat sequence of which there are 47.7 tandem copies [55]. This repetitive region is unique to the AHRR gene and is present in an intronic CpG island [55]. All 92 CpG sites were hypomethylated in the cannabis user’s sperm [55]. The consequences, if any, of this finding for expression of AHRR are unclear, but remain novel and appear to be specific to cannabis. Overall, the findings from this initial study demonstrated, for the first time, the association between cannabis use and altered DNA methylation in sperm.
Multi-Species Effects of Cannabis and THC on Methylation of Imprinted Autism Candidate Gene DLGAP2
In a follow up study, our group focused on the effects of cannabis exposure on DNA methylation of the gene Disks-large associated protein 2 (DLGAP2) [61]. This gene exhibited 17 differentially methylated CpG sites by RRBS in the sperm of cannabis-exposed men compared to controls [61]. DLGAP2 is implicated in autism spectrum disorders (ASD) [62, 63]. For this study, we used sperm DNA from the same cohort described in Murphy et al. to validate a significant loss of methylation present in the seventh intron of DLGAP2, a region that contained nine of the 17 initially identified differentially methylated CpG sites, all of which were hypomethylated. We validated with bisulfite pyrosequencing that there was a significant loss of methylation present across this intronic region in sperm of adult male cannabis users compared to controls, with methylation effect sizes ranging from 7% to 15% for the individually affected CpG sites in the sperm [61]. We also demonstrated that the methylation present at this region in human fetal brain tissues is correlated with gene expression levels, indicative of a functional methylation–expression relationship at this region [61]. Using human fetal testes tissues, a tissue type in which DLGAP2 is known to exhibit imprinted expression [64], we confirmed that the region of interest in DLGAP2 is unlikely to be the imprint control region for this gene given a very high level of methylation (average 72.5%) in these diploid cells.
To begin to address the question of intergenerational inheritance of an altered sperm DNA methylation pattern, we turned to a rat model of THC exposure. We first looked at the sperm of male rats exposed to 4 mg/kg THC (a dose reflecting daily consumption) or vehicle control (4% TWEEN-80 in saline) via injection for 28 days, and analyzed methylation changes in the first intron of this gene. From this we observed significant losses of methylation at Dlgap2 in the sperm of the THC-exposed rats compared to the controls [61]. To then address the question of intergenerational inheritance, DNA methylation of Dlgap2 was analyzed in the hippocampus and nucleus accumbens of rats born to THC-exposed fathers compared to controls, the regions of the brain where Dlgap2 function is most critical [61, 62]. A significant loss of methylation was detected at the same CpG sites in the nucleus accumbens as in the sperm of the exposed dads, beginning to suggest the potential for a paternal exposure that alters the epigenetic profile of sperm to contribute to the epigenetic profile of the offspring [61]. Interestingly, Watson et al. also found there to be significant changes in methylation and expression of Dlgap2 in the offspring of rats born to THC-exposed parents [52]. Given the small sample size of this pilot study, additional larger studies are necessary to confirm this finding.
Other Considerations: Maternal Exposure, Routes of Exposure and Alternative Epigenetic Mechanisms
Cannabis use among pregnant women is a serious growing concern [65], with 7.1% of pregnant women in 2016 reporting cannabis use, largely to alleviate morning sickness [66]. This percentage represents a 69% increase over the number of pregnant women reporting use in 2009 [66]. The health consequences of such in utero exposure are also understudied [67]. Some studies are beginning to suggest the possibility for changes in DNA methylation or gene expression in offspring born to mothers who use or are exposed to cannabis during pregnancy, but this too is an area that urgently needs further investigation [68]. Maternal use of cannabis during pregnancy has been associated with an increased likelihood for the newborn to require the neonatal intensive care unit, decreased infant birth weights, the potential of an impaired fetal immune system and neurodevelopmental delay and autistic-like deficits [69–72].
A study conducted by Reece and Hulse that analyzed the incidence of autism in states with legalized use of medicinal or recreational cannabis reported that the most common form of cannabis-associated clinical teratology in the USA is autism [69]. Their statistical models projected that there would ultimately be a 60% excess of autism cases in states with some form of legal cannabis compared to those without by the year 2030 [2]. In addition to autism and autism-like behaviors, Reece and Hulse also determined in the state of Colorado, where medicinal and recreational cannabis use has been legal since 2000 and 2014, respectively, that there was a correlation between cannabis use and congenital anomalies from 2000 to 2014, a time when cannabis was the only drug whose use increased over that period of time [73]. These associations and correlations were identified independent of epigenetics, but it is possible that such alterations may have a role in contributing to some of these phenotypes.
Another emerging issue to consider is the use of electronic cigarettes for both nicotine and cannabis products. The CDC reports that e-cigarettes are the most commonly used tobacco product among adolescents in the USA, though e-cigarettes are also frequently used as a marijuana delivery system [74]. In 2019, over five million teenagers in middle and high school reported use of an e-cigarette in the past 30 days [74]. Focusing specifically on THC in e-cigarettes, 14% of 12th graders and 12.6% of 10th graders reported THC vaping in the past 30 days, while 3.5% of 12th graders and 3% of 10th graders reported using THC e-cigarettes daily in 2019 [75]. In fact, the rates of teen vaping of cannabis almost doubled from 2018 to 2019, which raises serious concerns [76]. Little information is known about the potential effects of e-cigarette use on the health, development and epigenetic profile of the individual and the potential effects on their offspring. Studies are needed to delineate the effects of cannabis use via different routes of consumption.
It is important to recognize that no one epigenetic mechanism exerts its influence in isolation. In fact, different types of epigenetic modifications often work together to convey regulatory instructions to the cell in a meaningful way. Future studies are needed to assess other epigenetic mechanisms that may be impacted in sperm as a result of cannabis exposure. These include changes to chromatin structure, histone modifications, and the presence and activity of small noncoding RNAs [46, 77]. Integration of these findings will help to convey a more complete picture of how cannabis exposure can affect all parts of the sperm epigenome, how these epigenetic effects may work together to impact offspring health and potential strategies for amelioration of cannabis-induced changes.
What Research Is Needed?
There is a pressing need for additional fact-gathering studies in this field. Multiple questions remain about the health consequences of male cannabis use, but our policies are changing before we have all of the answers. One critical question that remains is whether or not abstinence from cannabis use will allow the methylation changes in sperm to be ‘washed out’ and resolved. If true, for family planning purposes, it needs to be determined how long of an abstinence period from cannabis use is necessary. This will provide answers as to whether the spermatogonial stem cells themselves are affected, or if the methylation changes are occurring in the differentiating sperm. A second important question is whether or not the cannabis-associated methylation changes present in sperm are retained following PGC and/or post-fertilization reprogramming in the offspring. Should they escape the reprogramming process and be retained, the role of the altered methylation state in early life development must be determined. A third area of need is to determine the long-term consequences of preconceptional cannabis use on offspring health and development. Given the preliminary studies exploring the statistical association between cannabis use and increased autism incidence, as well as our work focused on cannabis use and altered DNA methylation at an autism candidate gene, further studies exploring these associations are warranted. This is especially true as the incidence of autism continues to climb. Lastly, it will be critical to determine whether the methylation changes associated with cannabis use can be transmitted intergenerationally and transgenerationally through sperm of offspring born to cannabis using fathers.
Conclusions
Cannabis use is clearly on the rise across the USA, and the push toward legalization is moving rapidly. However, largely left out of the conversations surrounding increased legalization efforts are the potential health consequences resulting from the expansion of cannabis accessibility and use, and the long-term effects of this increased exposure. This is especially true for men of reproductive age, the predominant group of cannabis consumers. There is a large gap in knowledge about the associated risks of preconception cannabis exposure, and while the studies presented here begin to provide data about these effects, there is still more work to be done. Larger studies in both animals and humans are necessary to better understand the potential epigenetic consequences of cannabis use in order to allow individuals to make the best-informed decisions about their cannabis use habits, and to gain a comprehensive understanding of how their cannabis use may affect not only their health, but the health of their children.
Funding
This work was supported by grants 60564 and 60957 from the John Templeton Foundation.
Conflict of interest statement. None declared.
References
- 1. Chandra S, Radwan MM, Majumdar CG, Church JC, Freeman TP, ElSohly MA.. New trends in cannabis potency in USA and Europe during the last decade (2008–2017). Eur Arch Psychiatry Clin Neurosci 2019;269:5–15. [DOI] [PubMed] [Google Scholar]
- 2. Hasin DS. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology 2018;43:195–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smart R, Caulkins JP, Kilmer B, Davenport S, Midgette G.. Variation in cannabis potency and prices in a newly legal market: Evidence from 30 million cannabis sales in Washington state. Addiction 2017;112:2167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Szutorisz H, Hurd YL.. Epigenetic effects of cannabis exposure. Biol Psychiatry 2016;79:586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Szutorisz H, Hurd YL.. High times for cannabis: Epigenetic imprint and its legacy on brain and behavior. Neurosci Biobehav Rev 2018;85:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dolinoy DC, Jirtle RL.. Environmental epigenomics in human health and disease. Environ Mol Mutagen 2008;49:4–8. [DOI] [PubMed] [Google Scholar]
- 7. Blake SGJ. States where marijuana is legal. Business Insider, 2019.
- 8. Atakan Z. Cannabis, a complex plant: Different compounds and different effects on individuals. Ther Adv Psychopharmacol 2012;2:241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elsohly MA, Slade D.. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci 2005;78:539–48. [DOI] [PubMed] [Google Scholar]
- 10. Stuyt E. The problem with the current high potency THC marijuana from the perspective of an addiction psychiatrist. Mol Med 2018;115:482–6. [PMC free article] [PubMed] [Google Scholar]
- 11. ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC.. Changes in cannabis potency over the last 2 decades (1995–2014): Analysis of current data in the United States. Biol Psychiatry 2016;79:613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Compton WM, Han B, Jones CM, Blanco C, Hughes A.. Marijuana use and use disorders in adults in the USA, 2002–14: Analysis of annual cross-sectional surveys. Lancet Psychiatry 2016;3:954–64. [DOI] [PubMed] [Google Scholar]
- 13. Di Forti M, Marconi A, Carra E, Fraietta S, Trotta A, Bonomo M, Bianconi F, Gardner-Sood P, O'Connor J, Russo M, Stilo SA, Marques TR, Mondelli V, Dazzan P, Pariante C, David AS, Gaughran F, Atakan Z, Iyegbe C, Powell J, Morgan C, Lynskey M, Murray RM.. Proportion of patients in south London with first-episode psychosis attributable to use of high potency cannabis: A case-control study. Lancet Psychiatry 2015;2:233–8. [DOI] [PubMed] [Google Scholar]
- 14. Barkus E. High-potency cannabis increases the risk of psychosis. Evid Based Mental Health 2016;19:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blanco C, Hasin DS, Wall MM, Florez-Salamanca L, Hoertel N, Wang S, Kerridge BT, Olfson M.. Cannabis use and risk of psychiatric disorders: prospective evidence from a US national longitudinal study. JAMA Psychiatry 2016;73:388–95. [DOI] [PubMed] [Google Scholar]
- 16. Hall W, Stjepanovic D, Caulkins J, Lynskey M, Leung J, Campbell G, Degenhardt L.. Public health implications of legalising the production and sale of cannabis for medicinal and recreational use. Lancet 2019;394:1580–90. [DOI] [PubMed] [Google Scholar]
- 17. Mauro PM, Carliner H, Brown QL, Hasin DS, Shmulewitz D, Rahim-Juwel R, Sarvet AL, Wall MM, Martins SS.. Age differences in daily and nondaily cannabis use in the United States, 2002–2014. J Stud Alcohol Drugs 2018;79:423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, Jung J, Pickering RP, Ruan WJ, Smith SM, Huang B, Grant BF.. Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013. JAMA Psychiatry 2015;72:1235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US Department of Health and Human Services, SAMHSA, Center for Behavioral Health Statistics and Quality. National Survey on Drug Use and Health 2018 NSDUH-2018-DS0001, 2018. Rockville, MD. [Google Scholar]
- 20. Cuttler C, Mischley LK, Sexton M.. Sex differences in cannabis use and effects: A cross-sectional survey of cannabis users. Cannabis Cannabinoid Res 2016;1:166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Calakos KC, Bhatt S, Foster DW, Cosgrove KP.. Mechanisms underlying sex differences in cannabis use. Curr Addict Rep 2017;4:439–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heindel JJ, Vandenberg LN.. Developmental origins of health and disease: A paradigm for understanding disease cause and prevention. Curr Opin Pediatr 2015;27:248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Skinner MK. Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stability. Epigenetics 2011;6:838–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bartolomei MS, Tilghman SM.. Genomic imprinting in mammals. Annu Rev Genet 1997;31:493–525. [DOI] [PubMed] [Google Scholar]
- 25. De Felici M. The formation and migration of primordial germ cells in mouse and man. Results Probl Cell Differ 2016;58:23–46. [DOI] [PubMed] [Google Scholar]
- 26. Fernandez-Twinn DS, Constância M, Ozanne SE.. Intergenerational epigenetic inheritance in models of developmental programming of adult disease. Semin Cell Dev Biol 2015;43:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kota SK, Feil R.. Epigenetic transitions in germ cell development and meiosis. Dev Cell 2010;19:675–86. [DOI] [PubMed] [Google Scholar]
- 28. Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet 2002;3:662–73. [DOI] [PubMed] [Google Scholar]
- 29. Ly L, Chan D, Trasler JM.. Developmental windows of susceptibility for epigenetic inheritance through the male germline. Semin Cell Dev Biol 2015;43:96–105. [DOI] [PubMed] [Google Scholar]
- 30. Marques CJ, Joao Pinho M, Carvalho F, Bieche I, Barros A, Sousa M.. DNA methylation imprinting marks and DNA methyltransferase expression in human spermatogenic cell stages. Epigenetics 2011;6:1354–61. [DOI] [PubMed] [Google Scholar]
- 31. McSwiggin HM, O’Doherty AM.. Epigenetic reprogramming during spermatogenesis and male factor infertility. Reproduction 2018;156:R9–21. [DOI] [PubMed] [Google Scholar]
- 32. Messerschmidt DM, Knowles BB, Solter D.. DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Genes Dev 2014;28:812–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oakes CC, La Salle S, Smiraglia DJ, Robaire B, Trasler JM.. Developmental acquisition of genome-wide DNA methylation occurs prior to meiosis in male germ cells. Dev Biol 2007;307:368–79. [DOI] [PubMed] [Google Scholar]
- 34. Reik W, Dean W, Walter J.. Epigenetic reprogramming in mammalian development. Science 2001;293:1089–93. [DOI] [PubMed] [Google Scholar]
- 35. Stewart KR, Veselovska L, Kelsey G.. Establishment and functions of DNA methylation in the germline. Epigenomics 2016;8:1399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Trasler JM. Epigenetics in spermatogenesis. Mol Cell Endocrinol 2009;306:33–6. [DOI] [PubMed] [Google Scholar]
- 37. Wu H, Hauser R, Krawetz SA, Pilsner JR.. Environmental susceptibility of the sperm epigenome during windows of male germ cell development. Curr Environ Health Rep 2015;2:356–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vincent JJ, Huang Y, Chen P-Y, Feng S, Calvopiña JH, Nee K, Lee SA, Le T, Yoon AJ, Faull K, Fan G, Rao A, Jacobsen SE, Pellegrini M, Clark AT.. Stage-specific roles for Tet1 and Tet2 in DNA demethylation in primordial germ cells. Cell Stem Cell 2013;12:470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu X, Zhang Y.. TET-mediated active DNA demethylation: Mechanism, function and beyond. Nat Rev Genet 2017;18:517–34. [DOI] [PubMed] [Google Scholar]
- 40. Gkountela S, Zhang KX, Shafiq TA, Liao WW, Hargan-Calvopina J, Chen PY, Clark AT.. DNA demethylation dynamics in the human prenatal germline. Cell 2015;161:1425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tang WW, Dietmann S, Irie N, Leitch HG, Floros VI, Bradshaw CR, Hackett JA, Chinnery PF, Surani MA.. A unique gene regulatory network resets the human germline epigenome for development. Cell 2015;161:1453–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ferguson-Smith AC, Bourchis D.. The discovery and importance of genomic imprinting. eLife 2018;7:e42368. DOI: 10.7554/eLife.42368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Soubry A. POHaD: Why we should study future fathers. Environ Epigenet 2018;4:dvy007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Curley JP, Mashoodh R, Champagne FA.. Epigenetics and the origins of paternal effects. Horm Behav 2011;59:306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abbasi J. The paternal epigenome makes its mark. JAMA 2017;317:2049–51. [DOI] [PubMed] [Google Scholar]
- 46. Donkin I, Barres R.. Sperm epigenetics and influence of environmental factors. Mol Metab 2018;14:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Soubry A, Guo L, Huang Z, Hoyo C, Romanus S, Price T, Murphy SK.. Obesity-related DNA methylation at imprinted genes in human sperm: Results from the TIEGER study. Clin Epigenet 2016;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Soubry A, Murphy SK, Wang F, Huang Z, Vidal AC, Fuemmeler BF, Kurtzberg J, Murtha A, Jirtle RL, Schildkraut JM, Hoyo C.. Newborns of obese parents have altered DNA methylation patterns at imprinted genes. Int J Obes 2015;39:650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Soubry A, Schildkraut JM, Murtha A, Wang F, Huang Z, Bernal A, Kurtzberg J, Jirtle RL, Murphy SK, Hoyo C.. Paternal obesity is associated with IGF2 hypomethylation in newborns: Results from a Newborn Epigenetics Study (NEST) cohort. BMC Med 2013;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Morkve Knudsen GT, Rezwan FI, Johannessen A, Skulstad SM, Bertelsen RJ, Real FG, Krauss-Etschmann S, Patil V, Jarvis D, Arshad SH, Holloway JW, Scanes C.. Epigenome-wide association of father’s smoking with offspring DNA methylation: A hypothesis-generating study. Environ Epigenet 2019;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Szutorisz H, DiNieri JA, Sweet E, Egervari G, Michaelides M, Carter JM, Ren Y, Miller ML, Blitzer RD, Hurd YL.. Parental THC exposure leads to compulsive heroin-seeking and altered striatal synaptic plasticity in the subsequent generation. Neuropsychopharmacology 2014;39:1315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Watson CT, Szutorisz H, Garg P, Martin Q, Landry JA, Sharp AJ, Hurd YL.. Genome-wide DNA methylation profiling reveals epigenetic changes in the rat nucleus accumbens associated with cross-generational effects of adolescent THC exposure. Neuropsychopharmacology 2015;40:2993–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ramirez A, Arbuckle MR.. Synaptic plasticity: The role of learning and unlearning in addiction and beyond. Biol Psychiatry 2016;80:e73–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ibn Lahmar Andaloussi Z, Taghzouti K, Abboussi O.. Behavioural and epigenetic effects of paternal exposure to cannabinoids during adolescence on offspring vulnerability to stress. Int J Dev Neurosci 2019;72:48–54. [DOI] [PubMed] [Google Scholar]
- 55. Murphy SK, Itchon-Ramos N, Visco Z, Huang Z, Grenier C, Schrott R, Acharya K, Boudreau M-H, Price TM, Raburn DJ, Corcoran DL, Lucas JE, Mitchell JT, McClernon FJ, Cauley M, Hall BJ, Levin ED, Kollins SH.. Cannabinoid exposure and altered DNA methylation in rat and human sperm. Epigenetics 2018;13:1208–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Levin ED, Hawkey AB, Hall BJ, Cauley M, Slade S, Yazdani E, Kenou B, White H, Wells C, Rezvani AH, Murphy SK.. Paternal THC exposure in rats causes long-lasting neurobehavioral effects in the offspring. Neurotoxicol Teratol 2019;74:106806. [DOI] [PubMed] [Google Scholar]
- 57. Nielsen JE, Rolland AD, Rajpert-De Meyts E, Janfelt C, Jørgensen A, Winge SB, Kristensen DM, Juul A, Chalmel F, Jégou B, Skakkebaek NE.. Characterisation and localisation of the endocannabinoid system components in the adult human testis. Sci Rep 2019;9:12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Payne KS, Mazur DJ, Hotaling JM, Pastuszak AW.. Cannabis and male fertility: A systematic review. J Urol 2019; 202:674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mirmiran M, Kok JH, Boer K, Wolf H.. Perinatal development of human circadian rhythms: Role of the foetal biological clock. Neurosci Biobehav Rev 1992;16:371–8. [DOI] [PubMed] [Google Scholar]
- 60. Pan D. The hippo signaling pathway in development and cancer. Dev Cell 2010;19:491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schrott R, Acharya K, Itchon-Ramos N, Hawkey AB, Pippen E, Mitchell JT, Kollins SH, Levin ED, Murphy SK.. Cannabis use is associated with potentially heritable widespread changes in autism candidate gene DLGAP2 DNA methylation in sperm. Epigenetics 2019;15:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rasmussen AH, Rasmussen HB, Silahtaroglu A.. The DLGAP family: neuronal expression, function and role in brain disorders. Mol Brain 2017;10:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chien WH, Gau SS, Liao HM, Chiu YN, Wu YY, Huang YS, Tsai WC, Tsai HM, Chen CH.. Deep exon resequencing of DLGAP2 as a candidate gene of autism spectrum disorders. Mol Autism 2013;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Luedi PP, Dietrich FS, Weidman JR, Bosko JM, Jirtle RL, Hartemink AJ.. Computational and experimental identification of novel human imprinted genes. Genome Res 2007;17:1723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Young-Wolff KC, Adams SR, Wi S, Weisner C, Conway A.. Routes of cannabis administration among females in the year before and during pregnancy: Results from a pilot project. Addict Behav 2020;100:106125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Committee on Obstetric Practice. Committee opinion no. 722: marijuana use during pregnancy and lactation. Obstet Gynecol 2017;130:e205–9. [DOI] [PubMed] [Google Scholar]
- 67. Volkow ND, Han B, Compton WM, McCance-Katz EF.. Self-reported medical and nonmedical cannabis use among pregnant women in the United States. JAMA 2019;322:167–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. DiNieri JA, Wang X, Szutorisz H, Spano SM, Kaur J, Casaccia P, Dow-Edwards D, Hurd YL.. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol Psychiatry 2011;70:763–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Reece AS, Hulse GK.. Effect of cannabis legalization on US autism incidence and medium term projections. Clin Pediatr 2019;4:17. [Google Scholar]
- 70. Reece SA, Hulse GK.. Epidemiological associations of various substances and multiple cannabinoids with autism in USA. Clin Pediatr 2019;4. [Google Scholar]
- 71. Dong C, Chen J, Harrington A, Vinod KY, Hegde ML, Hegde VL.. Cannabinoid exposure during pregnancy and its impact on immune function. Cell Mol Life Sci 2019;76:729–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gunn JK, Rosales CB, Center KE, Nunez A, Gibson SJ, Christ C, Ehiri JE.. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open 2016;6:e009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Reece AS, Hulse GK.. Cannabis teratology explains current patterns of coloradan congenital defects: The contribution of increased cannabinoid exposure to rising teratological trends. Clin Pediatr 2019;58:1085–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cullen KA, Gentzke AS, Sawdey MD, Chang JT, Anic GM, Wang TW, Creamer MR, Jamal A, Ambrose BK, King BA.. e-Cigarette use among youth in the United States, 2019. JAMA 2019;322:2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.National Institute on Drug Abuse. Monitoring the Future 2019 Survey Results: Vaping, 2019. North Bethesda, MD.
- 76.Marijuana Factcheck. Marijuana Factcheck – Vaping, 2020. https://www.mjfactcheck.org/vaping. [Google Scholar]
- 77. Ben Maamar M, Sadler-Riggleman I, Beck D, McBirney M, Nilsson E, Klukovich R, Xie Y, Tang C, Yan W, Skinner MK.. Alterations in sperm DNA methylation, non-coding RNA expression, and histone retention mediate vinclozolin-induced epigenetic transgenerational inheritance of disease. Environ Epigenet 2018;4:dvy010. [DOI] [PMC free article] [PubMed] [Google Scholar]