CONSPECTUS:
Carbon is central to the chemistry of life, and in addition to its fundamental roles as a static component of all major biomolecules spanning proteins, nucleic acids, sugars, and lipids, emerging evidence shows that small and transient carbon-based metabolites, termed reactive carbon species (RCS), are dynamic signaling/stress agents that can influence a variety of biological pathways. Recent examples include the identification of carbon monoxide (CO) as an ion channel blocker and endogenous formaldehyde (FA) as a one-carbon metabolic unit formed from the spontaneous degradation of dietary folate metabolites. These findings motivate the development of analytical tools for transient carbon species that can achieve high specificity and sensitivity to further investigate RCS signaling and stress pathways at the cell, tissue, and whole-organism levels. This Account summarizes work from our laboratory on the development of new chemical tools to monitor two important one-carbon RCS, CO and FA, through activity-based sensing (ABS), where we leverage the unique chemical reactivities of these small and transient analytes, rather than lock-and-key binding considerations, for selective detection. Classic inorganic/organometallic and organic transformations form the basis for this approach. For example, to distinguish CO from other biological diatomics of similar shape and size (e.g., nitric oxide and oxygen), we exploit palladium-mediated carbonylation as a synthetic method for CO sensing. The high selectivity of this carbonylation approach successfully enables imaging of dynamic changes in intracellular CO levels in live cells. Likewise, we apply the aza-Cope reaction for FA detection to provide high selectivity for this one-carbon unit over other larger biological aldehydes that are reactive electrophiles, such as acetaldehyde and methylglyoxal. By relying on an activity-based trigger as a design principle for small-molecule detection, this approach can be generalized to create a toolbox of selective FA imaging reagents, as illustrated by a broad range of FA probes spanning turn-on and ratiometric fluorescence imaging, positron emission tomography imaging, and chemiluminescence imaging modalities. Moreover, these chemical tools have revealed new one-carbon biology through the identification of folate as a dietary source of FA and alcohol dehydrogenase 5 as a target for FA metabolism. Indeed, these selective RCS detection methods have been expanded to a wider array of imaging platforms, such as metal-complex-based time-gated luminescence and materials-based imaging scaffolds (e.g., nanotubes, nanoparticles, and carbon dots), with modalities extending to Raman and Rayleigh scattering readouts. This pursuit of leveraging selective chemical reactivity to develop highly specific ABS probes for imaging of RCS provides not only practical tools for deciphering RCS-dependent biology but also a general design platform for developing ABS probes for a broader range of biological analytes encompassing elements across the periodic table.
Graphical Abstract
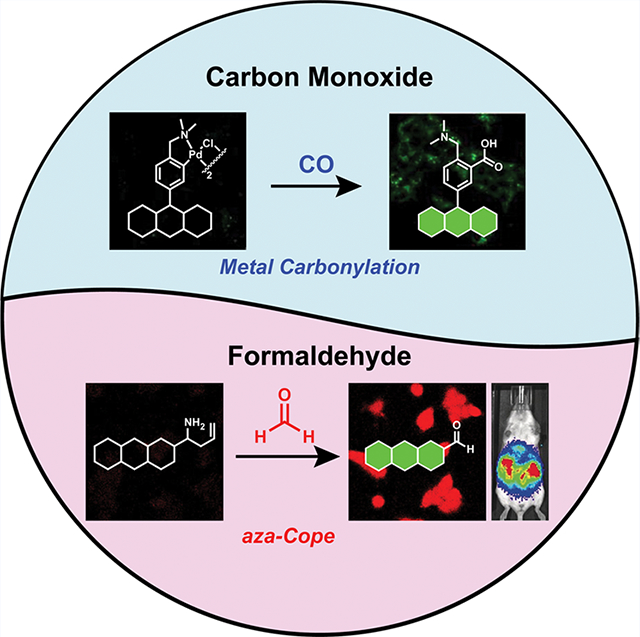
1. INTRODUCTION
The periodic table organizes the elements of life,1 with carbon occupying a central place in the formation of fundamental biomolecules such as proteins, nucleic acids, sugars, and lipids that sustain living organisms. Beyond these organic building blocks that regulate transcription, translation, and metabolism, emerging evidence shows that transient carbon metabolites, such as the simplest one-carbon species like carbon monoxide (CO) and formaldehyde (FA), can function as dynamic signaling and/or stress molecules for a diverse array of biological events. Indeed, the biological activity of CO ranges from regulating membrane ion channel function2,3 to intracellular signaling pathways,4–6 often through coordination to metalloprotein targets, including hemoglobin/myoglobin and cytochrome c oxidase,6 and FA contributes to one-carbon metabolism and epigenetic pathways.7–12 At the same time, aberrant environmental exposure to CO and FA and/or internal dysregulation of these reactive carbon species (RCS) is detrimental, providing motivation to develop new methods for monitoring CO and FA in biological settings to help decipher their native function. The dynamic and transient nature of these one-carbon units, including coordination/dissociation of CO to metal centers6 and catalytic FA production/consumption from pathways spanning protein and nucleic acid N-demethylation, glutathione redox cycling, and folate metabolism,13,14 presents a unique challenge for RCS detection, particularly with many competing analytes in the biological milieu that are similar in shape and size.
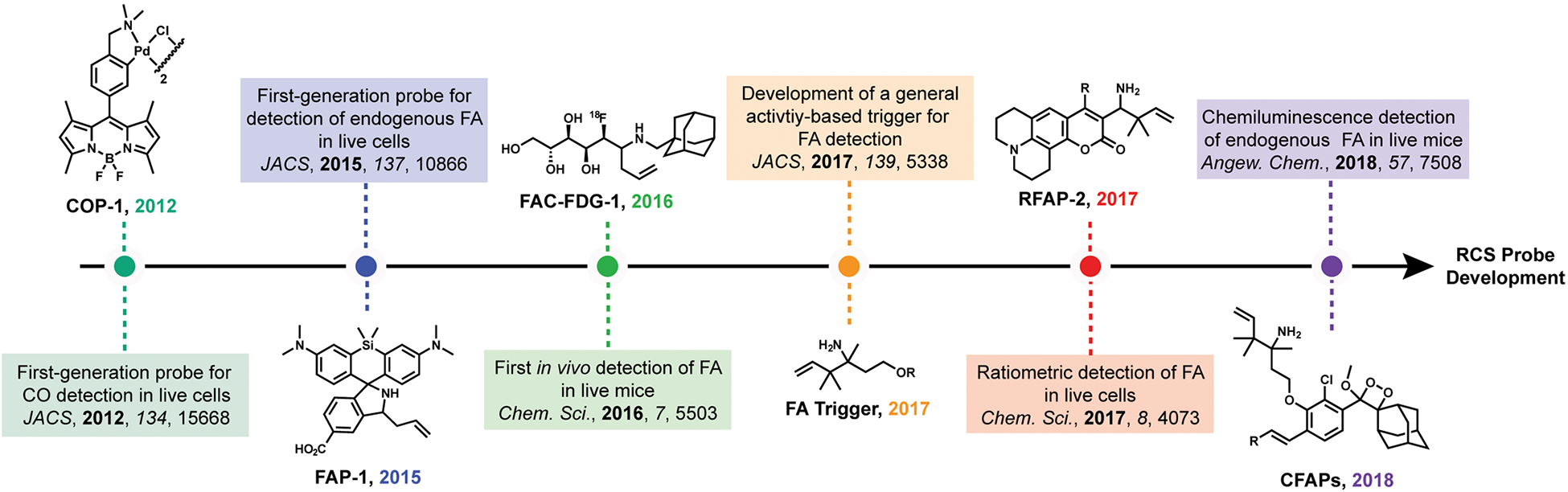
Against this backdrop, we and others have pursued an emerging approach for the design of chemical sensors for biological imaging, which we term activity-based sensing (ABS).15–21 In contrast to conventional sensors that operate by lock-and-key binding and molecular recognition, ABS leverages the intrinsic chemical reactivity of the analyte to enable its highly specific and sensitive detection with spatial and temporal resolution. Indeed, the power of ABS lies in the development of a robust synthetic reaction method, which can then be exploited to create a suite of useful reagents that can be tailored for a biological application at hand. In this Account, we describe research from our laboratory on the development of ABS probes for CO and FA detection and their application to discover new one-carbon biology. Figure 1 and Table 1 provide a summary of key chemical probes developed in this ongoing program. We also refer the reader to other leading work on activity-based detection of larger RCS like methylglyoxal22 and ethylene.23,24
Figure 1.
Development of activity-based sensing (ABS) probes for carbon monoxide (CO) and formaldehyde (FA).
Table 1.
Probes for the Detection of Carbon Monoxide (CO) and Formaldehyde (FA)
| probe name | probe properties | turn-ona | notable experiments |
|---|---|---|---|
| COP-1 | λex = 499 nm/λem = 507 nm | 10-fold (50 μM CORM-3, 60 min) | exogenous CORM-3 (5–50 μM) in live cells |
| FAP-1 | λex = 645 nm/λem = 655 nm | 12-fold (100 μM FA, 120 min) | endogenous FA imaging in a histone demethylation cell model |
| FAC-FDG-1 | 18F positron emission | 90% yield (1 mM FA, 180 min) | in vivo exogenous FA in a xenograft model |
| FAP385 | λex = 385 nm/λem = 498 nm | 4.5-fold (100 μM FA, 120 min) | exogenous FA in vitro (not cell-trappable) |
| FAP498 | λex = 498 nm/λem = 515 nm | 2.25-fold (100 μM FA, 120 min) | exogenous FA (200–1000 μM) in living cells |
| FAP555 | λex = 555 nm/λem = 572 nm | 11-fold (100 μM FA, 120 min) | exogenous FA (200–1000 μM) in living cells |
| FAP573 | λex = 573 nm/λem = 585 nm | 4.25-fold (100 μM FA, 120 min) | endogenous FA from an impaired metabolism cell model |
| RFAP-1 | ratiometric, λex = 420 nm to λex = 470 nm | 3-fold (100 μM FA, 120 min) | exogenous FA (50–200 μM) in living cells |
| RFAP-2 | ratiometric, λex = 420 nm to λex = 470 nm | 3-fold (100 μM FA, 120 min) | endogenous FA from an impaired metabolism cell model |
| CFAP540 | chemiluminescent, λem = 540 nm | 500-fold (10 mM FA, 60 min) | exogenous FA (200–1000 μM) in living cells |
| CFAP700 | chemiluminescent, λem = 700 nm | 33-fold (10 mM FA, 60 min) | endogenous FA from folate degradation in living mice |
Reported as the maximum in vitro turn-on to the indicated analyte concentration at the listed time point.
2. CARBON MONOXIDE DETECTION IN LIVE CELLS BY PALLADIUM-MEDIATED CARBONYLATION
Detection of CO is challenging in large part because of its low abundance and relative chemical inertness in biological settings. In eukaryotes, endogenous CO production occurs mainly from enzymatic degradation of the heme cofactor by heme oxygenase, releasing CO at a rate of ca. 16 μmol/h in humans.3,25 Despite its well-known toxicity via coordination to the iron center of heme, CO is a relatively stable molecule in biological environments compared with nitric oxide, an isostructural compound that has radical character and versatile chemical reactivity.26 Our laboratory developed Carbon Monoxide Probe 1 (COP-1), which exploits palladium-mediated carbonylation as a synthetic method for CO detection in an ABS mode (Figure 2).27 In the absence of CO, Pd serves as a heavy-atom fluorescence quencher. Binding of CO to the Pd center triggers a carbonylation reaction, in which C–C coupling with concomitant release of Pd leads to a fluorescence increase. COP-1 exhibits high selectivity for CO over other biologically relevant small molecules, including NO and hydrogen peroxide, with a 10-fold turn-on fluorescence response. This reagent is capable of detecting changes in CO levels in living cells, as shown by studies in human embryonic kidney (HEK) 293T cells treated with carbon monoxide releasing molecule-3 (CORM-3, [Ru(CO)3Cl(glycinate)]). This carbonylation approach has been generalized by others for CO detection,28–30 with other chemical probes expanding the biocompatible and bioorthogonal nature of palladium chemistry to operate in living environments.31–33
Figure 2.
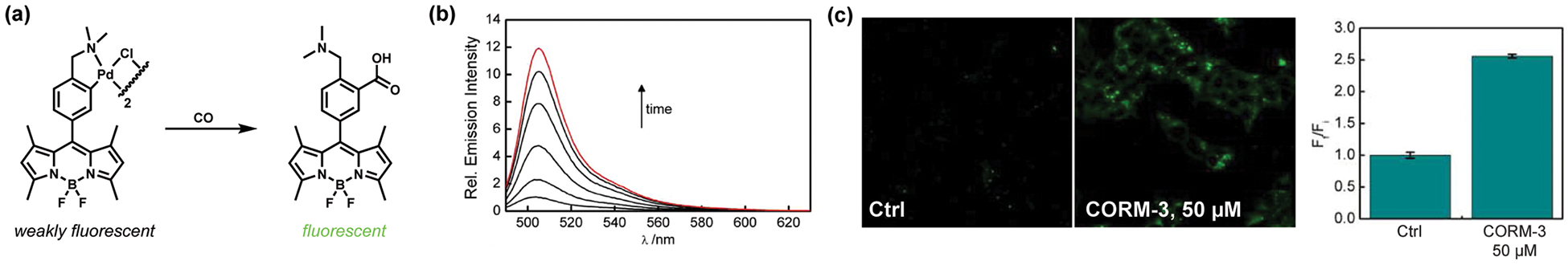
Activity-based sensing of CO through palladium-mediated carbonylation. (a) CO detection with the BODIPY-based palladium complex COP-1. (b) Fluorescence spectra of 1 μM COP-1 in the presence of 50 μM CORM-3 over 120 min (red trace). (c) Confocal images of HEK293T cells stained with 1 μM COP-1 at 37 °C for 30 min. The bar graph presents mean fluorescence intensities, with error bars representing the standard error of the mean (SEM) (n = 3). Adapted from ref 27. Copyright 2012 American Chemical Society.
3. FORMALDEHYDE DETECTION IN LIVE CELLS BY AZA-COPE CHEMISTRY
Formaldehyde is one of the most abundant aldehydes in living systems, with blood concentrations estimated to be in the 10–100 μM range.34,35 FA is produced endogenously through a diverse array of biochemical pathways spanning protein and nucleic acid N-demethylation, one-carbon metabolism, and folate metabolism.8 Our laboratory,36 along with Chan’s laboratory,37 reported a synthetic ABS method for FA detection using the aza-Cope rearrangement (Figure 3).38 The aza-Cope reaction offers a prime example showcasing the advantages of activity-based detection, as initial Schiff base formation can in principle occur with many electrophilic aldehydes,39,40 but when a homoallyl moiety is tethered onto the amine group, Schiff base formation with FA (but not other aldehydes because of steric effects) leads to a 2-aza Cope rearrangement. The first-generation FA probe FAP-1 shows a 12-fold turn-on response to FA with high selectivity over other similar carbon electrophiles such as acetaldehyde and methylglyoxal, establishing the preference of the sigmatropic rearrangement process for FA. FAP-1 is capable of FA detection in live cells, as shown in HEK293T and MCF-7 models, and can be used to monitor changes in basal FA levels through decreased lysine demethylation induced by small-molecule inhibition of lysine-specific demethylase 1 (LSD1) (Figure 3c). Patel and co-workers applied FAP-1 to help discover spontaneous generation of FA from folate metabolism and showed that oxidation-sensitive folate derivatives such as dihydrofolate (DHF), tetrahydrofolate (THF), and 5,10-dimethyltetrahydrofolate (5,10-me-THF) have differing FA-release capacities, connecting dietary folate and its regulation to endogenous FA production (Figure 3d).14
Figure 3.
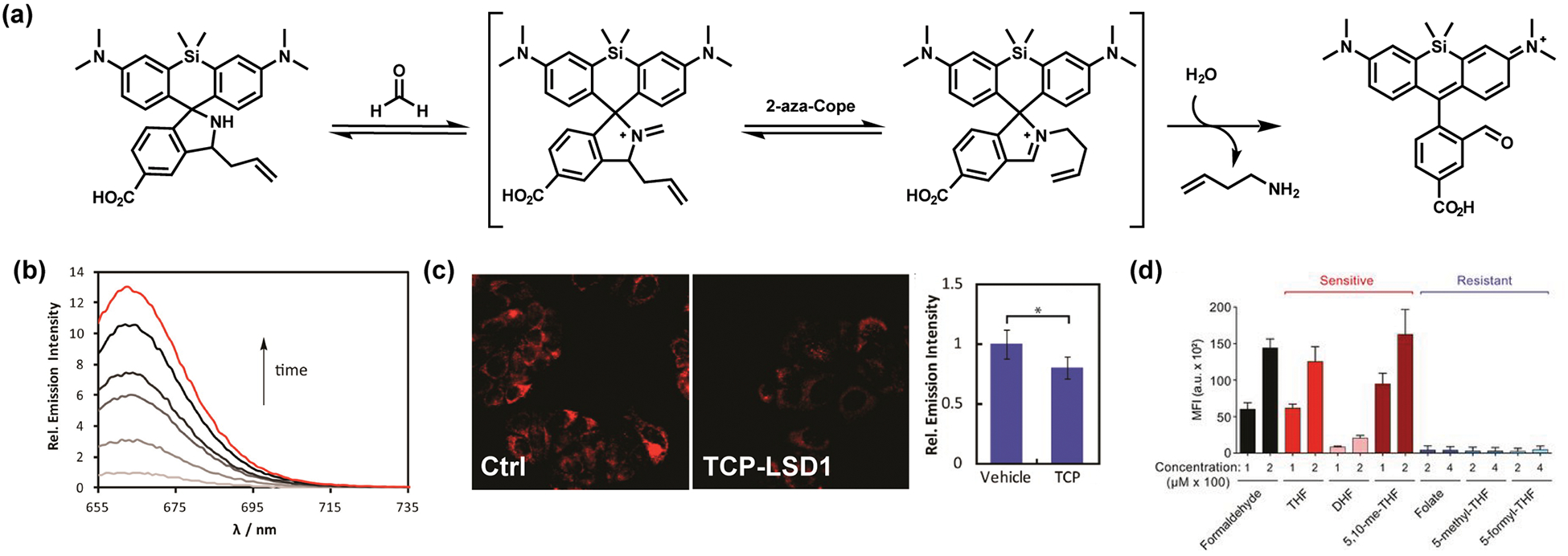
First-generation ABS fluorescence probes for FA based on the aza-Cope rearrangement reaction. (a) Reaction scheme for FA detection with the silicon rhodamine-based dye FAP-1. (b) Fluorescence response of 10 μM FAP-1 to 100 μM FA over 120 min (red trace). (c) Confocal microscopy images of MCF7 cells stained with 10 μM FAP-1 at 37 °C for 60 min: (left) vehicle (Ctrl) with no additive; (right) 20 μM tranylcypromine (TCP), an inhibitor of the demethylase enzyme lysine-specific demethylase 1 (LSD1). The bar graph presents fluorescence intensities (mean ± SEM, n = 6; *, P ≤ 0.005). (d) FA-dependent fluorescence responses of folate derivatives analyzed by FAP-1, showing that THF, DHF, and 5,10-me-THF derivatives are high folate producers. The bar graph presents fluorescence intensities (mean ± SEM, n = 3). Adapted from refs 14 and 36. Copyright 2015 American Chemical Society.
4. ACTIVITY-BASED DETECTION OF FORMALDEHYDE IN LIVE ANIMALS BY POSITRON EMISSION TOMOGRAPHY
We reported the first example of in vivo FA imaging in live animals through the development of Formaldehyde Caged Fluorodeoxyglucose 1 (FAC-FDG-1), a positron emission tomography (PET) tracer where the acyclic form of 18F-fluorodeoxyglucose (FDG) is masked with a FA-reactive homoallylamine moiety for the 2-aza-Cope reaction (Figure 4a).41 This strategy complements work from our laboratory on activity-based 18F PET probes for H2O2 and hypoxia,42,43 where the FA-triggered uncaging process generates the known18F PET tracer FDG.44 FAC-FDG-1 can be used to monitor changes in FA levels in PC3 prostate cancer cells as well as in living mice with PC3-derived tumor xenografts. FAC-FDG-1 detects uptake of FA exclusively in tumor over other healthy organs such as brain, heart, and muscle (Figure 4b) upon FA exposure.
Figure 4.
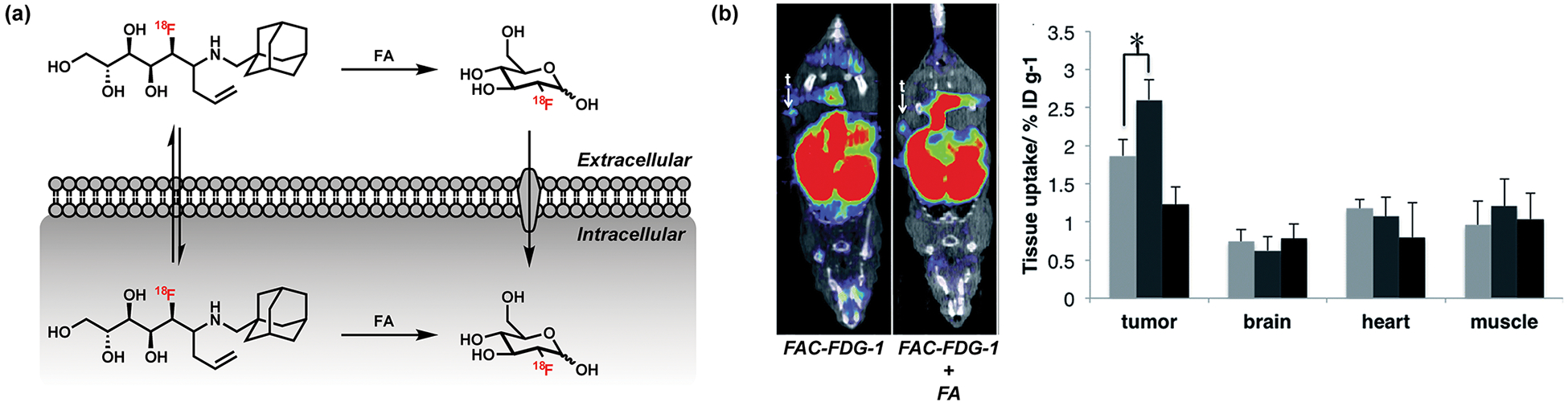
ABS probe for in vivo FA imaging using positron emission tomography (PET). (a) Mechanism for the detection of FA using the [18F]fluorodeoxyglucose probe FAC-FDG-1. (b) (left) 18F PET images of xenograft mice treated with FAC-FDG-1 and (right) bar graphs presenting quantification of 18F uptake in different organs (mean ± SEM, n = 3). Statistical analyses were performed with a two-tailed Student’s t test (*, P < 0.05). Panel (b) adapted from ref 41. Published by The Royal Society of Chemistry.
5. DESIGN OF A MODULAR ACTIVITY-BASED FORMALDEHYDE TRIGGER
Although the first-generation reagent FAP-1 was capable of selective and sensitive detection of endogenous FA levels in cells, its spirocyclization-dependent fluorescence modulation requires this motif to be directly incorporated into the fluorophore scaffold. To expand the utility of aza-Cope reactivity for FA detection, we sought to design a new FA-dependent reaction trigger that could be more generally applied to multiple types of platforms (Figure 5).45 To this end, we created a homoallylamine trigger with a self-immolative two-carbon linker to cage phenols, where FA would induce imine formation, aza-Cope rearrangement, imine hydrolysis, and β-elimination in a stepwise fashion. Further structural optimization of the trigger moiety by introduction of two methyl groups at the allylic position dramatically promoted the aza-Cope reaction as a result of the Thorpe–Ingold effect.46 Indeed, the development of this new activity-based trigger enables late-stage functionalization of phenol-containing fluorophores like coumarin, fluorescein, rhodol, and resorufin to furnish a panel of FA-reactive fluorophores covering the entire visible color region (Figure 5a). Green (FAP498), orange (FAP555), and red (FAP573) probes can be used to monitor changes in FA levels in live cells upon exogenous FA exposure. Moreover, FAP573 imaging can detect aberrant elevations in FA by genetic knockout of the FA-metabolizing enzyme alcohol dehydrogenase 5 (ADH5), identifying a role for this enzyme in regulating cellular FA pools (Figures 5b,c), akin to the enzymes superoxide dismutase47 and catalase48 that regulate and detoxify the reactive oxygen species superoxide and hydrogen peroxide, respectively.
Figure 5.
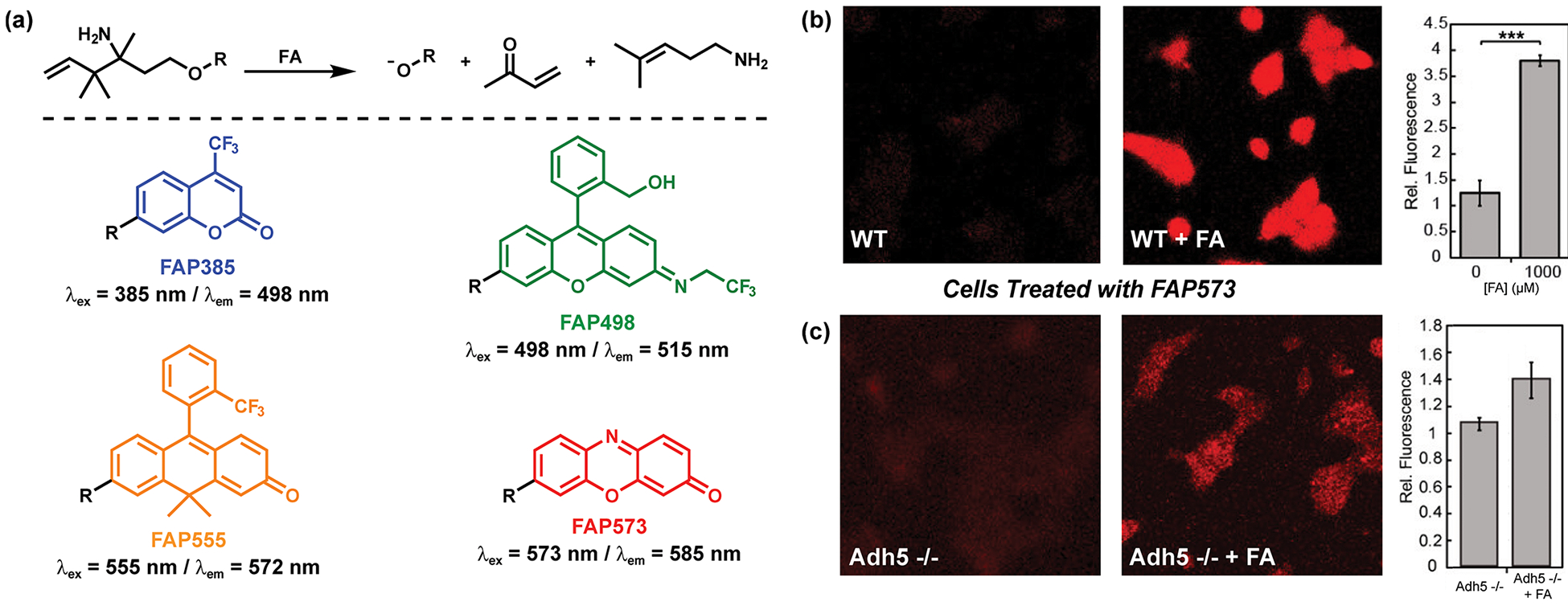
Next-generation ABS fluorescence probes for FA that share a general activity-based trigger operating through tandem aza-Cope rearrangement and β-elimination reactions. (a) (top) Reaction scheme for the detection of FA by the generalizable FA trigger and (bottom) structures of a color palette of FA probes. (b) Confocal microscopy images of wild-type (WT) HEK293T cells stained with FAP573 (10 μM) for 60 min in the presence (1000 μM) or absence of FA. (c) Confocal microscopy images of near-haploid (HAP-1) alcohol dehydrogenase knockout (Adh5 −/−) cells stained with FAP573 (10 μM) for 60 min in the presence (100 μM) or absence of FA. In (b) and (c), bar graphs present fluorescence intensities (mean ± SEM, n = 3; ***, P < 5 × 10−5). Adapted from ref 45. Copyright 2017 American Chemical Society.
6. RATIOMETRIC FORMALDEHYDE DETECTION BY AZA-COPE CHEMISTRY
Fluorescent probes that operate by an intensity-based turn-on response are of utility, but ratiometric probes that can leverage a ratio change of fluorescence excitation and/or emission wavelengths upon analyte detection can potentially provide even more quantitative information by minimizing potential experimental artifacts derived from variations in probe loading, light intensity, and sample thickness.18 To this end, we designed Ratiometric Formaldehyde Probes 1 and 2 (RFAP-1 and RFAP-2), a first-generation set of indicators based on a coumarin scaffold where the FA-mediated transformation of homoallylamine into an aldehyde through aza-Cope chemistry results in a change in the excitation wavelength from 420 to 470 nm (Figure 6a,b).49 Our design exploited the electronic change in the donor–acceptor properties of the coumarin scaffold when the electron-rich homoallylamine group is converted to the more electron-poor aldehyde moiety after the aza-Cope rearrangement and hydrolysis. RFAP-2, bearing a chloroalkylether chain, shows substantially enhanced FA-sensing ability in a variety of cell lines (HeLa, MCF-7, MCF-10A, RKO, SH-SY5Y, and U-2OS), presumably due to better cellular retention and staining. Finally, RFAP-2 can also be used to detect changes in basal FA levels with genetic disruption of ADH5 (Figure 6c).
Figure 6.
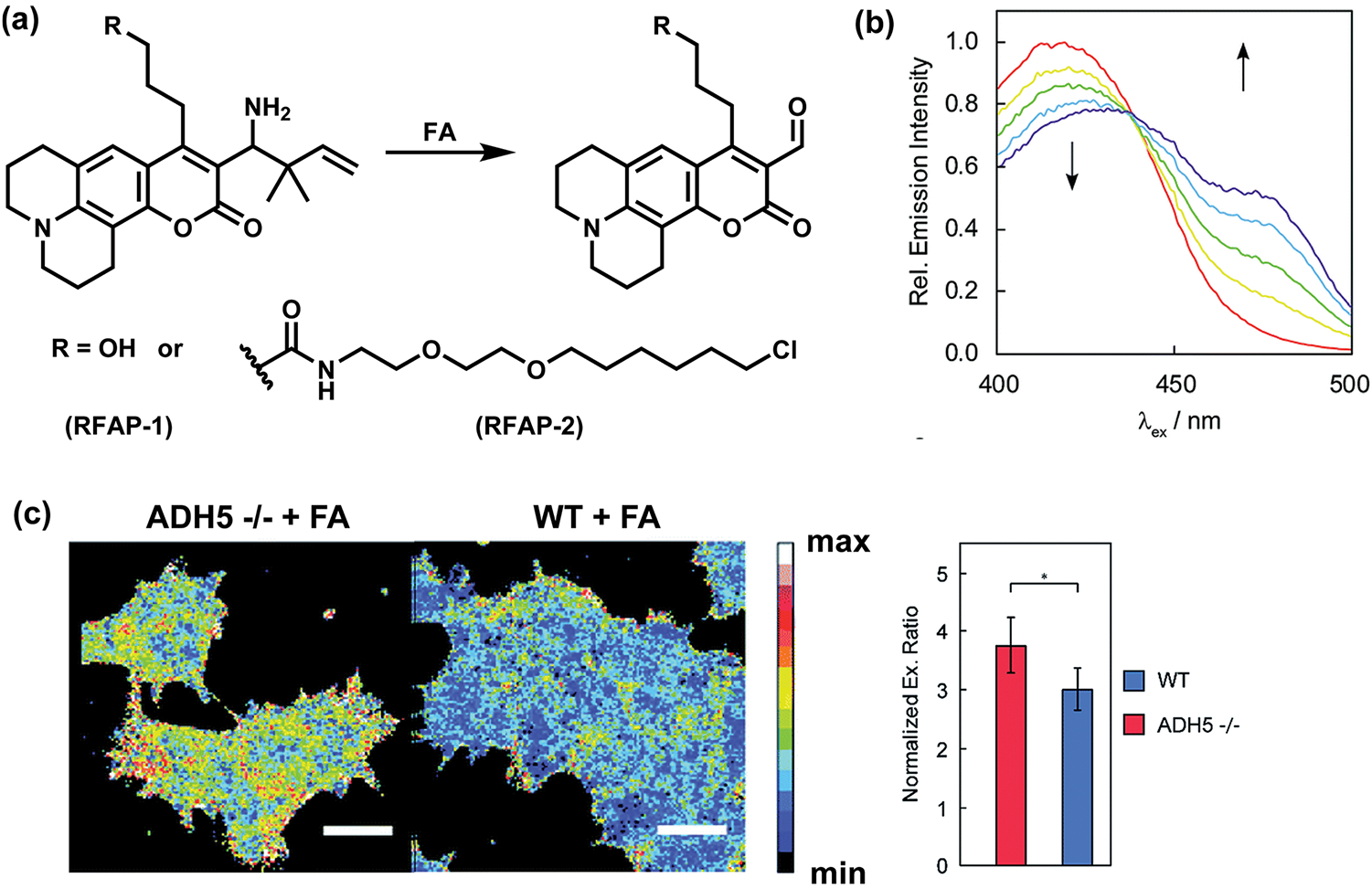
Ratiometric ABS probes for FA. (a) Reaction scheme for FA detection by ratiometric probes RFAP-1 and RFAP-2. (b) Excitation spectra for 10 μM RFAP-2 responding to 100 μM FA at 0, 30, 60, 90, and 120 min (red, yellow, green, blue, and purple curves, respectively). (c) Confocal microscopy images of near-haploid (HAP-1) cells stained with 0.5 μM RFAP-2 for 60 min. WT, wild type; ADH5 −/−, alcohol dehydrogenase 5 knockout cells. Bar graphs present normalized excitation ratios (mean ± SEM, n = 5; *, P < 0.05). Adapted from ref 49. Published by The Royal Society of Chemistry.
7. CHEMILUMINESCENT ACTIVITY-BASED SENSING OF FORMALDEHYDE IN LIVE CELLS AND ANIMALS
With a growing toolbox of chemical probes for FA detection in cells and animals, we initiated a collaboration with Shabat and co-workers to develop chemiluminescent reagents that could operate in both cells and animals.50 Specifically, we functionalized a Schaap’s dioxetane-based chemiluminescent platform bearing a phenol moiety51,52 with the same general FA-responsive trigger we utilized for fluorescence imaging (Figure 7). Through tandem aza-Cope rearrangement and β-elimination steps upon interaction with the FA analyte, the released phenol undergoes chemiexcitation through liberation of an emissive methyl ester compound from the dioxetane scaffold (Figure 7a). Modulation of the π-conjugation system of the dioxetane moiety facilitated creation of green (CFAP540) and red (CFAP700) chemiluminescence with high FA sensitivity and selectivity. CFAP540 and CFAP700 are effective probes for visualizing FA levels in live cells and mice, respectively. In particular, CFAP700 identified elevations in FA levels upon treatment of live mice with tetrahydrofolate (THF), establishing the susceptibility of folate derivatives to release FA in vivo (Figure 7b).
Figure 7.
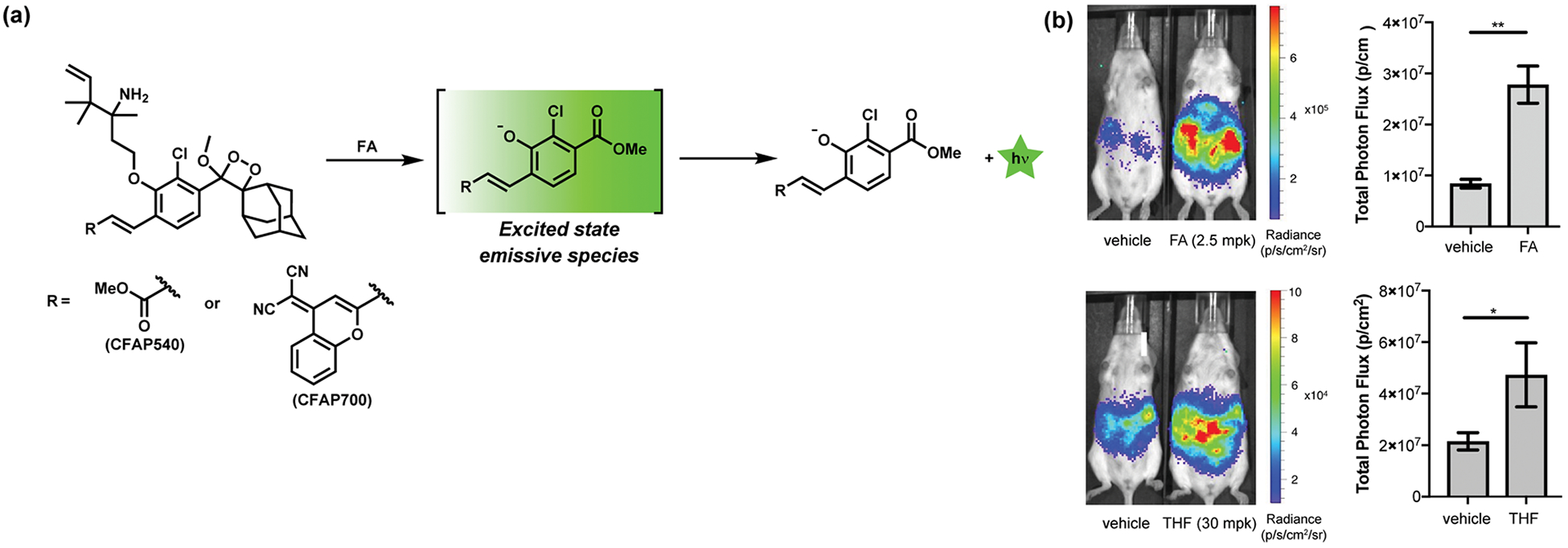
Chemiluminescent ABS probes for FA. (a) Reaction scheme for the detection of FA by chemiluminescent probes CFAP540 and CFAP700. (b) Chemiluminescence imaging of mice injected with 100 μM CFAP700 in the presence of FA or tetrahydrofolate (THF) at the indicated concentrations compared with vehicle. Bar graphs present total photon fluxes integrated from 0 to 25 min postinjection (mean ± SEM, n = 3–4). Statistical analyses were performed with a two-tailed Student’s t test (*, P ≤ 0.05; **, P ≤ 0.01). Adapted with permission from ref 50. Copyright 2018 Wiley-VCH.
8. CONCLUDING REMARKS
In this Account, we have described the development of activity-based sensing (ABS) methods for advancing studies of reactive carbon species (RCS), focusing on carbon monoxide (CO) and formaldehyde (FA) as emerging one-carbon metabolites in biology. Leveraging the unique structures and reactivities of these transient analytes provides opportunities to take a synthetic methods approach for biological discovery. Indeed, we have utilized classic palladium-mediated carbonylation and aza-Cope rearrangement reactions to form the basis for selective activity-based detection of CO and FA, respectively, and these versatile sensing mechanisms have been generalized and expanded into a variety of different imaging platforms,28–30,38,53,54 including multicolor and ratiometric fluorescence,49,55,56 positron emission tomography-based detection,41 and chemiluminescence.50 New biological principles of one-carbon biology learned include the identification of folate derivatives as a dietary FA source and specific folate metabolites that release FA during metabolism, as well as alcohol dehydrogenase 5 as a regulator of FA fluxes, akin to the enzymes superoxide dismutase and catalase that regulate superoxide and hydrogen peroxide, respectively. In addition to new synthetic opportunities to refine signal-to-noise responses and push the limits of spatial and temporal resolution to the subcellular organelle and single-molecule levels as well as expanding RCS imaging to a wider range of imaging modalities for translational studies, application of these imaging probes and related activity-based chemical tools can help identify the targets and underlying biochemical pathways of these transient one-carbon metabolites and larger RCS,57–59 which will certainly be a worthwhile field of study. Recent examples that expand this aza-Cope ABS method to other platforms and modalities include ruthenium(II) complex-based time-gated luminescence imaging60 and material-based sensors such as carbon dots,53,61 which offer promising emerging approaches for the study of FA in biological systems. In a broader sense, the expansion of ABS methods to study and utilize elements across the periodic table offers a powerful approach for biological discovery.20,16,21
ACKNOWLEDGMENTS
We thank the NIH (ES28096 and ES4705 to C.J.C.) for research support. J.O. thanks the Japan Society for the Promotion of Science for a postdoctoral fellowship, and K.J.B. thanks the National Science Foundation for a graduate fellowship. C.J.C. is an Investigator of the Howard Hughes Medical Institute and a CIFAR Senior Fellow.
Biographies
Jun Ohata was born and raised in Japan. He received his B.S. in 2011 and his M.S. in 2013 from Osaka Prefecture University, where he worked with Prof. Hiroyuki Matsuzaka studying the reactive C1 species on diruthenium complexes. He earned his Ph.D. in 2018 in Prof. Zach Ball’s group at Rice University, studying protein bioconjugation by transition-metal catalysis, and is currently a Japan Society for the Promotion of Science Postdoctoral Fellow in the Chang group at the University of California, Berkeley.
Kevin J. Bruemmer received his B.S. in Chemistry and Mathematics from Southern Methodist University in 2014, where he worked with Prof. Alex Lippert on the development of fluorescence and magnetic resonance probes for reactive nitrogen and sulfur species. He then moved to UC Berkeley in 2015 to continue studying reactive species with Prof. Chris Chang, where his work as an NSF Graduate Fellow has focused on creating and applying activity-based sensing methods to study the physiological roles of formaldehyde.
Christopher J. Chang is the Class of 1942 Chair Professor of Chemistry and Molecular and Cell Biology at the University of California, Berkeley, Investigator of the Howard Hughes Medical Institute, and Faculty Scientist at Lawrence Berkeley National Laboratory. He graduated with B.S. and M.S. degrees from Caltech in 1997, working with Prof. Harry Gray, spent a year as a Fulbright scholar with Dr. Jean-Pierre Sauvage, and earned his Ph.D. from MIT in 2002 with his thesis advisor Prof. Dan Nocera. After postdoctoral studies at MIT with Prof. Steve Lippard, he joined the faculty at UC Berkeley in 2004. Research in the Chang laboratory focuses on the study of metals and redox-active molecules in biology and energy, focusing on the development of activity-based sensing and proteomics probes and catalysts and applying them to questions in neuroscience, metabolism, and sustainable synthesis.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Chang CJ Searching for Harmony in Transition-Metal Signaling. Nat. Chem. Biol 2015, 11, 744–747. [DOI] [PubMed] [Google Scholar]
- (2).Peers C; Boyle JP; Scragg JL; Dallas ML; Al-Owais MM; Hettiarachichi NT; Elies J; Johnson E; Gamper N; Steele DS Diverse Mechanisms Underlying the Regulation of Ion Channels by Carbon Monoxide. Br. J. Pharmacol 2015, 172, 1546–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Heinemann SH; Hoshi T; Westerhausen M; Schiller A Carbon Monoxide - Physiology, Detection and Controlled Release. Chem. Commun 2014, 50, 3644–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Kim HP; Ryter SW; Choi AMK CO as a Cellular Signaling Molecule. Annu. Rev. Pharmacol. Toxicol 2006, 46, 411–449. [DOI] [PubMed] [Google Scholar]
- (5).Gullotta F; di Masi A; Coletta M; Ascenzi P CO Metabolism, Sensing, and Signaling. BioFactors 2012, 38, 1–13. [DOI] [PubMed] [Google Scholar]
- (6).Motterlini R; Foresti R Biological Signaling by Carbon Monoxide and Carbon Monoxide-Releasing Molecules. Am. J. Physiol. Cell Physiol 2017, 312, C302–C313. [DOI] [PubMed] [Google Scholar]
- (7).Ducker GS; Rabinowitz JD One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Reingruber H; Pontel LB Formaldehyde Metabolism and Its Impact on Human Health. Curr. Opin. Toxicol 2018, 9, 28–34. [Google Scholar]
- (9).Zheng Y; Cantley LC Toward a Better Understanding of Folate Metabolism in Health and Disease. J. Exp. Med 2018, 216, 253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Wang F; Chen D; Wu P; Klein C; Jin C Formaldehyde, Epigenetics, and Alzheimer’s Disease. Chem. Res. Toxicol 2019, 32, 820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Garaycoechea JI; Patel KJ Why Does the Bone Marrow Fail in Fanconi Anemia? Blood 2014, 123, 26–34. [DOI] [PubMed] [Google Scholar]
- (12).Pontel LB; Rosado IV; Burgos-Barragan G; Garaycoechea JI; Yu R; Arends MJ; Chandrasekaran G; Broecker V; Wei W; Liu L; Swenberg JA; Crossan GP; Patel KJ Endogenous Formaldehyde Is a Hematopoietic Stem Cell Genotoxin and Metabolic Carcinogen. Mol. Cell 2015, 60, 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Ducker GS; Chen L; Morscher RJ; Ghergurovich JM; Esposito M; Teng X; Kang Y; Rabinowitz JD Reversal of Cytosolic One-Carbon Flux Compensates for Loss of the Mitochondrial Folate Pathway. Cell Metab. 2016, 23, 1140–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Burgos-Barragan G; Wit N; Meiser J; Dingler FA; Pietzke M; Mulderrig L; Pontel LB; Rosado IV; Brewer TF; Cordell RL; Monks PS; Chang CJ; Vazquez A; Patel KJ Mammals Divert Endogenous Genotoxic Formaldehyde into One-Carbon Metabolism. Nature 2017, 548, 549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Lippert AR; Van de Bittner GC; Chang CJ Boronate Oxidation as a Bioorthogonal Reaction Approach for Studying the Chemistry of Hydrogen Peroxide in Living Systems. Acc. Chem. Res 2011, 44, 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Chan J; Dodani SC; Chang CJ Reaction-Based Small-Molecule Fluorescent Probes for Chemoselective Bioimaging. Nat. Chem 2012, 4, 973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Yang Y; Zhao Q; Feng W; Li F Luminescent Chemodosimeters for Bioimaging. Chem. Rev 2013, 113, 192–270. [DOI] [PubMed] [Google Scholar]
- (18).Lee MH; Kim JS; Sessler JL Small Molecule-Based Ratiometric Fluorescence Probes for Cations, Anions, and Biomolecules. Chem. Soc. Rev 2015, 44, 4185–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Lin VS; Chen W; Xian M; Chang CJ Chemical Probes for Molecular Imaging and Detection of Hydrogen Sulfide and Reactive Sulfur Species in Biological Systems. Chem. Soc. Rev 2015, 44, 4596–4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Aron AT; Ramos-Torres KM; Cotruvo JA; Chang CJ Recognition- and Reactivity-Based Fluorescent Probes for Studying Transition Metal Signaling in Living Systems. Acc. Chem. Res 2015, 48, 2434–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Iovan DA; Jia S; Chang CJ Inorganic Chemistry Approaches to Activity-Based Sensing: From Metal Sensors to Bioorthogonal Metal Chemistry. Inorg. Chem 2019, DOI: 10.1021/acs.inorgchem.9b01221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Wang T; Douglass EF; Fitzgerald KJ; Spiegel DAA “Turn-On” Fluorescent Sensor for Methylglyoxal. J. Am. Chem. Soc 2013, 135, 12429–12433. [DOI] [PubMed] [Google Scholar]
- (23).Toussaint SNW; Calkins RT; Lee S; Michel BW Olefin Metathesis-Based Fluorescent Probes for the Selective Detection of Ethylene in Live Cells. J. Am. Chem. Soc 2018, 140, 13151–13155. [DOI] [PubMed] [Google Scholar]
- (24).Tang Y; Ma Y; Yin J; Lin W Strategies for Designing Organic Fluorescent Probes for Biological Imaging of Reactive Carbonyl Species. Chem. Soc. Rev 2019, 48, 4036–4048. [DOI] [PubMed] [Google Scholar]
- (25).Coburn RF The Carbon Monoxide Body Stores. Ann. N. Y. Acad. Sci 1970, 174, 11–22. [DOI] [PubMed] [Google Scholar]
- (26).Heinrich TA; da Silva RS; Miranda KM; Switzer CH; Wink DA; Fukuto JM Biological Nitric Oxide Signalling: Chemistry and Terminology. Br. J. Pharmacol 2013, 169, 1417–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Michel BW; Lippert AR; Chang CJ A Reaction-Based Fluorescent Probe for Selective Imaging of Carbon Monoxide in Living Cells Using a Palladium-Mediated Carbonylation. J. Am. Chem. Soc 2012, 134, 15668–15671. [DOI] [PubMed] [Google Scholar]
- (28).Kumar N; Bhalla V; Kumar M Recent Developments of Fluorescent Probes for the Detection of Gasotransmitters (NO, CO and H2S). Coord. Chem. Rev 2013, 257, 2335–2347. [Google Scholar]
- (29).Zhou X; Lee S; Xu Z; Yoon J Recent Progress on the Development of Chemosensors for Gases. Chem. Rev 2015, 115, 7944–8000. [DOI] [PubMed] [Google Scholar]
- (30).Strianese M; Pellecchia C Metal Complexes as Fluorescent Probes for Sensing Biologically Relevant Gas Molecules. Coord. Chem. Rev 2016, 318, 16–28. [Google Scholar]
- (31).Yusop RM; Unciti-Broceta A; Johansson EMV; Sánchez-Martín RM; Bradley M Palladium-Mediated Intracellular Chemistry. Nat. Chem 2011, 3, 239–243. [DOI] [PubMed] [Google Scholar]
- (32).Li J; Yu J; Zhao J; Wang J; Zheng S; Lin S; Chen L; Yang M; Jia S; Zhang X; Chen PR Palladium-Triggered Deprotection Chemistry for Protein Activation in Living Cells. Nat. Chem 2014, 6, 352–361. [DOI] [PubMed] [Google Scholar]
- (33).Jbara M; Maity SK; Brik A Palladium in the Chemical Synthesis and Modification of Proteins. Angew. Chem., Int. Ed 2017, 56, 10644–10655. [DOI] [PubMed] [Google Scholar]
- (34).Luo W; Li H; Zhang Y; Ang CYW Determination of Formaldehyde in Blood Plasma by High-Performance Liquid Chromatography with Fluorescence Detection. J. Chromatogr., Biomed. Appl 2001, 753, 253–257. [DOI] [PubMed] [Google Scholar]
- (35).Nagy K; Pollreisz F; Takáts Z; Vékey K Atmospheric Pressure Chemical Ionization Mass Spectrometry of Aldehydes in Biological Matrices. Rapid Commun. Mass Spectrom 2004, 18, 2473–2478. [DOI] [PubMed] [Google Scholar]
- (36).Brewer TF; Chang CJ An Aza-Cope Reactivity-Based Fluorescent Probe for Imaging Formaldehyde in Living Cells. J. Am. Chem. Soc 2015, 137, 10886–10889. [DOI] [PubMed] [Google Scholar]
- (37).Roth A; Li H; Anorma C; Chan J A Reaction-Based Fluorescent Probe for Imaging of Formaldehyde in Living Cells. J. Am. Chem. Soc 2015, 137, 10890–10893. [DOI] [PubMed] [Google Scholar]
- (38).Bruemmer KJ; Brewer TF; Chang CJ Fluorescent Probes for Imaging Formaldehyde in Biological Systems. Curr. Opin. Chem. Biol 2017, 39, 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Song H; Rajendiran S; Kim N; Jeong SK; Koo E; Park G; Thangadurai TD; Yoon S A Tailor Designed Fluorescent ‘Turn-on’ Sensor of Formaldehyde Based on the BODIPY Motif. Tetrahedron Lett. 2012, 53, 4913–4916. [Google Scholar]
- (40).Zhou W; Dong H; Yan H; Shi C; Yu M; Wei L; Li Z HCHO-Reactive Molecule with Dual-Emission-Enhancement Property for Quantitatively Detecting HCHO in near 100% Water Solution. Sens. Actuators, B 2015, 209, 664–669. [Google Scholar]
- (41).Liu W; Truillet C; Flavell RR; Brewer TF; Evans MJ; Wilson DM; Chang CJ A Reactivity-Based [18F]FDG Probe for in Vivo Formaldehyde Imaging Using Positron Emission Tomography. Chem. Sci 2016, 7, 5503–5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Carroll V; Michel BW; Blecha J; VanBrocklin H; Keshari K; Wilson D; Chang CJ A Boronate-Caged [18F]FLT Probe for Hydrogen Peroxide Detection Using Positron Emission Tomography. J. Am. Chem. Soc 2014, 136, 14742–14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Flavell RR; Truillet C; Regan MK; Ganguly T; Blecha JE; Kurhanewicz J; VanBrocklin HF; Keshari KR; Chang CJ; Evans MJ; Wilson DM Caged [18F]FDG Glycosylamines for Imaging Acidic Tumor Microenvironments Using Positron Emission Tomography. Bioconjugate Chem. 2016, 27, 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Alauddin MM Positron Emission Tomography (PET) Imaging with 18F-Based Radiotracers. Am. J. Nucl. Med. Mol. Imaging 2011, 2, 55–76. [PMC free article] [PubMed] [Google Scholar]
- (45).Bruemmer KJ; Walvoord RR; Brewer TF; Burgos-Barragan G; Wit N; Pontel LB; Patel KJ; Chang CJ Development of a General Aza-Cope Reaction Trigger Applied to Fluorescence Imaging of Formaldehyde in Living Cells. J. Am. Chem. Soc 2017, 139, 5338–5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Jung ME; Piizzi G Gem-Disubstituent Effect: Theoretical Basis and Synthetic Applications. Chem. Rev 2005, 105, 1735–1766. [DOI] [PubMed] [Google Scholar]
- (47).Wang Y; Branicky R; Noë A; Hekimi S Superoxide Dismutases: Dual Roles in Controlling ROS Damage and Regulating ROS Signaling. J. Cell Biol 2018, 217, 1915–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Glorieux C; Calderon PB Catalase, a Remarkable Enzyme: Targeting the Oldest Antioxidant Enzyme to Find a New Cancer Treatment Approach. Biol. Chem 2017, 398, 1095–1108. [DOI] [PubMed] [Google Scholar]
- (49).Brewer TF; Burgos-Barragan G; Wit N; Patel KJ; Chang CJA 2-Aza-Cope Reactivity-Based Platform for Ratiometric Fluorescence Imaging of Formaldehyde in Living Cells. Chem. Sci 2017, 8, 4073–4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Bruemmer KJ; Green O; Su TA; Shabat D; Chang CJ Chemiluminescent Probes for Activity-Based Sensing of Formaldehyde Released from Folate Degradation in Living Mice. Angew. Chem., Int. Ed 2018, 57, 7508–7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Schaap AP; Chen T-S; Handley RS; DeSilva R; Giri BP Chemical and Enzymatic Triggering of 1,2-Dioxetanes. 2: Fluoride-Induced Chemiluminescence from Tert-Butyldimethylsilyloxy-Substituted Dioxetanes. Tetrahedron Lett. 1987, 28, 1155–1158. [Google Scholar]
- (52).Green O; Eilon T; Hananya N; Gutkin S; Bauer CR;Shabat D Opening a Gateway for Chemiluminescence Cell Imaging: Distinctive Methodology for Design of Bright Chemiluminescent Dioxetane Probes. ACS Cent. Sci 2017, 3, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Bi A; Yang S; Liu M; Wang X; Liao W; Zeng W Fluorescent Probes and Materials for Detecting Formaldehyde: From Laboratory to Indoor for Environmental and Health Monitoring. RSC Adv. 2017, 7, 36421–36432. [Google Scholar]
- (54).Xu Z; Chen J; Hu L-L; Tan Y; Liu S-H; Yin J Recent Advances in Formaldehyde-Responsive Fluorescent Probes. Chin. Chem. Lett 2017, 28, 1935–1942. [Google Scholar]
- (55).Feng W; Hong J; Feng G Colorimetric and Ratiometric Fluorescent Detection of Carbon Monoxide in Air, Aqueous Solution, and Living Cells by a Naphthalimide-Based Probe. Sens. Actuators, B 2017, 251, 389–395. [Google Scholar]
- (56).Xie X; Tang F; Shangguan X; Che S; Niu J; Xiao Y; Wang X; Tang B Two-Photon Imaging of Formaldehyde in Live Cells and Animals Utilizing a Lysosome-Targetable and Acidic PH-Activatable Fluorescent Probe. Chem. Commun 2017, 53, 6520–6523. [DOI] [PubMed] [Google Scholar]
- (57).Galligan JJ; Wepy JA; Streeter MD; Kingsley PJ; Mitchener MM; Wauchope OR; Beavers WN; Rose KL; Wang T; Spiegel DA; Marnett LJ Methylglyoxal-Derived Posttranslational Arginine Modifications Are Abundant Histone Marks. Proc. Natl. Acad. Sci. U. S. A 2018, 115, 9228–9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Bollong MJ; Lee G; Coukos JS; Yun H; Zambaldo C; Chang JW; Chin EN; Ahmad I; Chatterjee AK; Lairson LL; Schultz PG; Moellering RE A Metabolite-Derived Protein Modification Integrates Glycolysis with KEAP1-NRF2 Signalling. Nature 2018, 562, 600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Long MJC; Parvez S; Zhao Y; Surya SL; Wang Y; Zhang S; Aye Y Akt3 Is a Privileged First Responder in Isozyme-Specific Electrophile Response. Nat. Chem. Biol 2017, 13, 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Liu C; Zhang R; Zhang W; Liu J; Wang Y-L; Du Z; Song B; Xu ZP; Yuan J Dual-Key-and-Lock” Ruthenium Complex Probe for Lysosomal Formaldehyde in Cancer Cells and Tumors. J. Am. Chem. Soc 2019, 141, 8462–8472. [DOI] [PubMed] [Google Scholar]
- (61).Liu H; Sun Y; Li Z; Yang J; Aryee AA; Qu L; Du D; Lin Y Lysosome-Targeted Carbon Dots for Ratiometric Imaging of Formaldehyde in Living Cells. Nanoscale 2019, 11, 8458–8463. [DOI] [PubMed] [Google Scholar]


