Abstract
Meal ingestion provokes the release of hormones and transmitters, which in turn regulate energy homeostasis and feeding behavior. One such hormone, glucagon-like peptide-1 (GLP-1), has received significant attention in the treatment of obesity and diabetes due to its potent incretin effect. In addition to the peripheral actions of GLP-1, this hormone is able to alter behavior through the modulation of multiple neural circuits. Recent work that focused on elucidating the mechanisms and outcomes of GLP-1 neuromodulation led to the discovery of an impressive array of GLP-1 actions. Here, we summarize the many levels at which the GLP-1 signal adapts to different systems, with the goal being to provide a background against which to guide future research.
Keywords: Glucagon-like peptide-1, bariatric surgery, feeding behavior, reward
1. Introduction
Glucagon-like peptide-1 (GLP-1) has long been recognized as a potent stimulator of insulin secretion and key regulator of energy homeostasis. The importance and conservation of its role in physiology is reflected in its almost complete sequence homology across mammalian species(Ørskov, 1992). Over time, the list of physiological functions mediated by GLP-1 has expanded dramatically(Baggio and Drucker, 2007; Holst, 2007; Pabreja et al., 2014; Rowlands et al., 2018). First described as an incretin, GLP-1 protects against hyperglycemia by enhancing insulin secretion and inhibiting glucagon secretion. Within the gut, it also acts to inhibit gastric motility and secretion. More recently, GLP-1 in the brain has become increasingly appreciated to engage a range of neural circuits to regulate appetite and reward related behaviors. This widely expressed receptor system can participate in a diverse assortment of functions partly due to its highly tailorable architecture. From the production of the endogenous ligands, to the coupling of downstream signaling partners and cellular localization, the GLP-1 and GLP-1 receptor (GLP-1r) system exhibits complexity and diversity(Pabreja et al., 2014). This diversity allows for a customizable receptor system specifically attuned to the environment in which it is found. As we continue to uncover the nuances of the GLP-1 signal, it is important to remain aware of the currently known spectrum of its actions. This review will encompass the synthesis, secretion, and metabolism of GLP-1, the structure and expression of the GLP-1r, the intracellular signaling initiated by GLP-1r stimulation, and GLP-1 changes after bariatric surgery. Additionally, we end with a special focus on the role of GLP-1 signaling in feeding and drug reward. Each section will highlight the opportunity for diversity within the GLP-1 and GLP-1r system, from the level of a single amino acid to the impact of the hormone on feeding behavior and reward.
2. Synthesis and secretion of GLP-1
The regulation of GLP-1 synthesis is one mechanism by which the GLP-1 and GLP-1r system can be customized. GLP-1 is produced through the proteolytic cleavage of proglucagon, a protein expressed in the enteroendocrine cells, α cells of the pancreas, as well as in the nucleus of the solitary tract (NTS) in the brainstem. The amino acid sequence of this 18 kDa protein was first deduced from the translation of the nucleotide sequence of the glucagon gene(Bell et al., 1983a; Heinrich et al., 1984). The protein contains three separate hormonal sequences separated by two intervening peptides (IP), IP-1 and IP-2(Bell et al., 1983b). Proteolytic processing of proglucagon is performed by a number of prohormone convertase enzymes. Expression of prohormone convertase occurs in a tissue specific manner. Consequently, the products produced by cleavage of proglucagon are also tissue-specific(Ørskov et al., 1987).
In the pancreas, proglucagon undergoes posttranslational processing by prohormone convertase 2(Smeekens et al., 1991) to produce glucagon, GRPP, and the major proglucagon fragment (MPGF)(Holst et al., 1994). This MPGF contains both glucagon-like peptide (GLP)-1 and GLP-2, as well as the IP-2 sequence. A small amount (10–20%) of the MPGF is cleaved to produce GLP-1(Holst et al., 1994).
The main sources of GLP-1 in the periphery are the enteroendocrine GLP-1 producing cells (GLP-1 EECs) found in the small intestine and colon. GLP-1 EEC density varies along a proximal to distal axis, with a sparse number of cells localized in the duodenum, increasing number in the jejunum, and the greatest numbers in the ileum and colon(Baggio and Drucker, 2007). In GLP-1 EECs, proglucagon is cleaved by prohormone convertase 1/3 to produce glicentin, GLP-1 and GLP-2, liberating the IP-2 peptide. Glicentin can be further processed to produce GRPP and oxyntomodulin(Dhanvantari et al., 1996). Oxyntomodulin contains the glucagon peptide sequence in addition to the IP-1 sequence and acts as an agonist at both GLP-1r and the glucagon receptor(Jorgensen et al., 2007). The brain also synthesizes GLP-1, utilizing a strategy similar to that of the GLP-1 EECs. Prohormone convertase 1/3 is the main species expressed(Seidah et al., 1991), and it acts to produce GLP-1 in the NTS(Jin et al., 1988; Larsen et al., 1997; Rinaman, 1999a).
GLP-1 can be secreted in four different forms, produced by differential post-translational modification. These forms include GLP-1 (1–37), GLP-1 (1–36)NH2, GLP-1(7–37), and GLP-1(7–36)NH2. The truncated versions listed are produced through action of prohormone convertase 1/3(Dhanvantari et al., 1996). Terminal amination is then mediated by α-monooxygenase(Wettergren et al., 1998). Despite the variety of possible species, the vast majority (80%) of GLP-1 that reaches circulation in humans is the GLP-1(7–36)NH2 variety(Ørskov et al., 1994). The complex process leading to the production of these multiple species of GLP-1 affords multiple levels of control over what is ultimately secreted by the cell. Importantly, these different forms of GLP-1 have been shown to activate signaling pathways to different degrees(Furness et al., 2018). The ultimate form of GLP-1 secreted by the cell then has the potential to be directed to a specific signaling outcome, and this bias can be controlled by expression of enzymes controlling the post-translational modifications.
GLP-1 secretion from GLP-1 EECs is triggered the by carbohydrates(Baggio and Drucker, 2007), proteins(Belza et al., 2013), and fats(Hirasawa et al., 2005) present in a meal, an area that has been reviewed in great detail(Gribble and Reimann, 2017). Glucose induced GLP-1 secretion is mediated primarily by glucose uptake into GLP-1 EECs by the sodium coupled glucose transporters (SGLT), as shown by pharmacological and genetic interference of SGLT function or expression(Gorboulev et al., 2012; Parker et al., 2012). Co-transport of Na+ results in membrane depolarization, triggering opening of voltage-gated Ca2+ channels and exocytosis of GLP-1 vesicles(Reimann et al., 2008). Amino acids can influence GLP-1 secretion via a similar electrogenic mechanism mediated by Na+ cotransport(Reimann et al., 2004). Oligopeptides can act at G protein-coupled receptors (GPCRs), including CASR(Diakogiannaki et al., 2013) and GPR142(Lin et al., 2016). Fatty acids are also detected by GPCRs, with long chain fatty acids mainly signaling through GPR40 and GPR120(Gribble et al., 2016). Mono-oleoylglycerols signal through GPR119, a receptor that appears to be critical for GLP-1 secretion based on rodent knockout studies(Moss et al., 2016). However, human trials of a GPR119 agonist failed to significantly increase GLP-1 levels(Nunez et al., 2014), complicating the interpretation of previous results from model systems. In addition to direct sensing of fats, bile acids secreted upon fat ingestion are able to signal at GLP-1 EECs through the GPCR TGR5 and the nuclear receptor FXR(Albaugh et al., 2019). These above mechanisms are critical for GLP-1 secretion, and nutrients must pass through the gut to trigger GLP-1 secretion. This is best shown in studies where intravenous glucose administration produces no significant change in GLP-1 levels(Herrmann et al., 1995). However, early phase GLP-1 secretion that occurs before nutrients reach the distal intestine and the GLP-1 EECs relies on other mechanisms, likely mediated by vagus nerve transmission(Rocca and Brubaker, 1999). Enhancing the secretion of GLP-1 is a goal of diabetes and obesity pharmacological interventions, and thus, significant effort has been expended towards identifying the molecular mechanisms that trigger GLP-1 secretion The magnitude of GLP-1 secretion is strongly correlated to the rate of gastric emptying, which may explain some of the secretion-promoting effects of bariatric surgeries that reduce stomach volume and increase gastric pressure(Belza et al., 2013). Future work in this area could focus on specific signaling pathways that promote GLP-1 and other hormone secretion, promoting or augmenting natural satiety processes during a meal.
Central GLP-1 release is mediated by firing of preproglucagon expressing neurons in the NTS. These neurons show activation following gastric distension or systemic administration of lipopolysaccharide, lithium chloride, and cholecystokinin (CCK) (Rinaman, 1999a; Vrang et al., 2003). They also respond to local application of CCK and leptin(Hisadome et al., 2011, 2010). In addition to responding to systemic signals, preproglucagon neurons are also synaptically connected to inputs releasing fast neurotransmitters as well as others releasing neuromodulators. NTS preproglucagon neurons have been shown to express functional post-synaptic glutamate receptors(Hisadome et al., 2011). Additionally, they have been shown to be inhibited by exogenously applied 5-HT(Holt et al., 2017), and excited by epinephrine and norepinephrine(Hisadome et al., 2011), all of which likely endogenously originate from local modulatory inputs. An in depth analysis of the synaptic connectivity of the NTS preproglucagon neuron population has yet to be undertaken.
Peripheral GLP-1 secretion has the ability to trigger further GLP-1 secretion in the brain. GLP-1 released in the intestine is able to act through GLP-1 receptors expressed on the vagus nerve, leading to vagal excitation(Bucinskaite et al., 2009; Kakei et al., 2002). Vagal excitation then stimulates NTS preproglucagon neurons, resulting in GLP-1 release in the brain(Williams, 2009). In this model, peripheral GLP-1 secretion has a direct impact on central GLP-1 secretion, despite the rapid elimination of the circulating peptide. This connection has been shown to be functionally important for GLP-1 mediated anorexia through vagal deafferentation studies(Williams, 2009; Yeğen et al., 1997). Further, this route may play a significant role in GLP-1 enhancement of insulin response, as denervated mice show no response to low doses (0.1 nmol/kg) of GLP-1(Ahrén, 2004). Sensory denervated mice retain enhanced insulin response to glucose following high doses of GLP-1, and this has been interpreted as a preference for GLP-1 to signal via circuit mechanisms at physiological levels, but an ability to signal in an endocrine manner at higher doses(Ahrén, 2004).
Together, these results highlight clear differences in the stimuli that trigger GLP-1 release from the GLP-1 EECs and the preproglucagon expressing NTS neurons. While GLP-1 EECs primarily respond to nutrient signals, GLP-1 releasing neurons of the NTS are stimulated by hormonal signals and vagal firing. The combination of excitatory, inhibitory, and neuromodulatory influences on NTS GLP-1 neurons allows integration of different information prior to the propagation of the GLP-1 signal to other central nodes. While the majority of these signals are stimulated by nutrient signaling within the gut, the same nutrient signals that influence GLP-1 secretion from GLP-1 EECs, central GLP-1 signaling is gated behind the integration of these signals and thus is insulated from directly responding to nutrient availability in the gut. This allows for greater control over the central GLP-1 response, and may allow for longer term control over energy homeostasis by considering long term energy stores in addition to acute nutrient availability. It would be interesting to see if signal integration at the level of the NTS was influenced by long term metabolic manipulations, and how that signal integration altered central GLP-1 signaling. The dynamic responses of NTS GLP-1 releasing neurons also remains to be examined. By utilizing calcium reporter molecules driven by genetic identifiers, future researchers could characterize the responses of these neurons to food cues, nutrients, gastric distension, and other stimuli to build an understanding of how these neurons decide to release GLP-1. An interesting direction would be to examine whether that decision is subject to learning, similar to what has been found in other central integrators of energy state(Beutler et al., 2017; Su et al., 2017).
3. Metabolism of GLP-1 and the therapeutic actions of long-lasting agonists
GLP-1 has an extremely short half-life once released in the plasma, ranging from 2–11 minutes(Deacon et al., 1995; Ørskov et al., 1994, 1993; Wettergren et al., 1998). The enzyme responsible for the majority of GLP-1 metabolism is dipeptidyl-peptidase IV (DPP IV)(Hopsu-Havu and Glenner, 1966), a serine aminopeptidase that can exist in both a membrane bound as well as soluble form(Engel et al., 2003). It inactivates GLP-1 by cleaving a X-Pro or a X-Ala dipeptide, producing mainly GLP-1 (9–36) NH2(Kieffer et al., 1995). DPP IV is expressed in numerous organs, including the kidney(Kettmann et al., 1992), liver(Hanski et al., 1988), pancreas(Heymann et al., 1985), blood vessels(Lojda, 1979), gut(Mulvihill et al., 2017), and brain(Bernstein et al., 1987). In the periphery, the main functional burden of GLP-1 metabolism rests on the liver and endothelial populations of DPP IV(Mulvihill et al., 2017). In a recent study using a conditional DPP IV knockout mouse, it was shown that although enteric DPP IV contributes significantly to reducing GLP-1 in the intestine, knockout of enteric DPP IV did not significantly disrupt incretin hormone levels or glucose tolerance(Mulvihill et al., 2017). These results place increased importance on the endothelial population of DPP IV. Although present in neurons during development(Bernstein et al., 1987), in the mature brain the enzyme is found on the ependymal cells of the blood-brain barrier(Bernstein et al., 1987), and low concentrations of the soluble form of the enzyme can be found in the cerebrospinal fluid(Kato et al., 1979). GLP-1 readily crosses the blood-brain barrier by simple diffusion, although presumably the amount of peripheral endogenous GLP-1 that reaches the central nervous system is small based on its short half-life(Kastin et al., 2002). Indeed, one study showed that only 20% of GLP-1 released from GLP-1 EECs was able to pass through the liver and reach the pancreas through circulation(Hjøllund et al., 2011). These results underscore the emphasis on the vagal route by which peripheral GLP-1 spurs central GLP-1 release. DPP IV inhibition has been explored as a potential therapeutic route for type 2 diabetes with the goal of increasing circulating GLP-1 levels by reducing metabolism(Deacon and Lebovitz, 2016). There are now several DPP IV inhibitors on the market, including sitagliptin(Herman et al., 2006), and alogliptin(DeFronzo et al., 2008).
In order to improve the clinical efficacy of GLP-1 agonism for type II diabetes and obesity, long-lasting analogues for GLP-1 have been developed which delay metabolism by DPP IV and increase their half-life in the blood. One GLP-1 analogue, Exendin-4, was discovered in the venom of the Gila monster (Heloderma suspectum), a lizard native to the southwestern United States and Mexico(Eng et al., 1992). Exendin-4 is a 39 amino acid peptide with 53% sequence homology to GLP-1(Eng et al., 1992). The synthetic version of Exendin-4 is FDA approved and marketed as Exenatide. This analogue is metabolized much slower than endogenous GLP-1, resulting in a half-life of 3–4 hours(Pinkney et al., 2010). The increased half-life is due mainly to the substitution of a glycine for an alanine residue in the 8th position, making the peptide a poor substrate for DPP IV(Pinkney et al., 2010). Unlike Exenatide, the GLP-1 agonist Liraglutide shares 97% homology with endogenous GLP-1(Pinkney et al., 2010). This analogue features a arginine to lysine substitution at position 34, as well as the addition of a fatty acid moiety bonded to lysine 26(Pinkney et al., 2010).This fatty acid moiety facilitates binding to albumin, further increasing the half-life of the drug in circulation to 11–13 hours(Chia and Egan, 2008). Development of these analogues allowed GLP-1 agonism to become a widespread treatment strategy for type II diabetes and obesity, as well as expanding the potential for studies of the GLP-1 system in basic science. Notably, Exendin-4 has been shown to attenuate both the psychomotor stimulant action of amphetamine(Erreger et al., 2012) as well as the rewarding and addictive properties of cocaine(Graham et al., 2013; Reddy et al., 2016; Sørensen et al., 2015), topics covered later in this review..
Further progress has been made by identifying GLP-1 agonists that additionally target receptors for either the glucose-dependent insulinotropic polypeptide (GIP) receptor, the glucagon receptor, or both(Brandt et al., 2018). Polyagonists engage complementary signaling systems to enhance outcomes beyond additive effects while reducing adverse effects(Müller et al., 2018). This synergy has been shown with other gut hormones at the behavioral(Roth et al., 2007; Talsania et al., 2005) and neural level(Su et al., 2017). Unimolecular polyagonists represent a significant advancement over simple co-administration of multiple peptides by normalizing the absorption, distribution, and metabolism of the therapeutic. Since the first production in 2009(Day et al., 2009), substantial progress has been made in producing these polyagonists, utilizing previous successful strategies including lipidation and building off the exendin backbone(Evers et al., 2017; Finan et al., 2013). Notably, a dual GIP/GLP-1 receptor agonist has been shown in clinical trials to improve glucose control and reduce body weight in patients with type 2 diabetes(Frias et al., 2017).
These results show that combining the GLP-1 signal with other hormonal pathways produces a synergistic, or greater than additive, response. EECs expressing GLP-1 are known to produce other hormones(Egerod et al., 2012) and in some cases these products can even be found stored in the same vesicles(Billing et al., 2018; Fothergill et al., 2017). Similar to the GIP/GLP-1r polyagonists, co-administration of GLP-1 and neurotensin has been shown to produce a synergistic effect(Grunddal et al., 2016). This raises questions about whether there are mechanisms regulating co-secretion, and if that regulation can be manipulated for therapeutic value.
4. GLP-1r structural characteristics and expression
The GLP-1r was first discovered in 1987 through radioligand binding assays with GLP-1 in rat pancreatic islet cell cultures(Drucker et al., 1987). Soon after, it was localized to a wide variety of tissues, including the brain(Shimizu et al., 1987). Expression cloning from cDNA libraries allowed for the identification of the gene responsible for the production of the receptor(Dillon et al., 1993; Graziano et al., 1993; Thorens, 1992; Thorens et al., 1993; van Eyll et al., 1994). Many early studies of the receptor focused on the rat GLP-1r, which exhibits 90% sequence homology with the human form(Dillon et al., 1993), highlighting the conservation of this important physiological signaling system.
Based on its structure and similarity to the secretin receptor, GLP-1r belongs to the Class B family of GPCRs. This family is defined by its peptide ligands as well as its extensive extracellular N-terminal ligand binding domain(Lagerström and Schiöth, 2008). GLP-1r and other Class B GPCRs exhibit a characteristic tertiary structure, despite relatively low sequence homology across the class (40–60%)(Lagerström and Schiöth, 2008). The Class B GPCR common structure consists of a lengthy N-terminal extracellular binding domain, followed by seven transmembrane domains interspersed by intra and extra-cellular loops(Hollenstein et al., 2013; Liang et al., 2017; Neumann et al., 2008; ter Haar et al., 2010). Recent cryo-EM structures of GLP-1r show this characteristic structure with GLP-1 or modified exendin-4 bound between the N-terminal binding domain and the transmembrane domains(Liang et al., 2017; Zhang et al., 2017). Upon binding, the ligand extends into the transmembrane core of the receptor, allowing a plethora of interactions with almost every transmembrane helix(Liang et al., 2017; Zhang et al., 2017). While the interactions made with the receptor are largely similar between GLP-1 and modified exendin peptide, there are some subtle differences in the positioning of transmembrane residues(Liang et al., 2017). On the intracellular side, the C terminus is critical for the desensitization and internalization of the receptor due to three phosphorylation sites which regulate these processes(Vázquez et al., 2005; Widmann et al., 1997, 1996a, 1996b).
Polar residues within the transmembrane region, in addition to extracellular residues, have been shown to be important for producing differential signaling by different ligands, including the endogenously produced varieties of GLP-1 as well as agonists like exendin-4(Furness et al., 2018; Wootten et al., 2016, 2013). In pharmacological studies, alanine scanning mutagenesis shows that residues in the transmembrane region and extracellular loops are critical for the differential signaling response produced by different ligands(Furness et al., 2018; Wootten et al., 2016, 2013). These pharmacological data align with the differences in transmembrane residue orientation found between GLP-1r bound to GLP-1 or a modified exendin peptide(Liang et al., 2017). Thus the GLP-1 receptor is capable of diverse outcomes even when signaling though a common g protein. This occurs despite a relatively conserved intracellular face forming interactions with the g protein, even when compared to class A receptors(Zhang et al., 2017). These results point to an exquisitely tuned receptor able to differentiate between subtle differences in ligand structure to produce varied intracellular responses. The most well-known example of this in GLP-1r signaling was the failure of Taspoglutide in clinical trials. Taspoglutide failed due to the unwanted side-effects of severe nausea and vomiting, known consequences of intense GLP-1r stimulation, which occurred despite the agonist having a 93% sequence homology to endogenous GLP-1(Rosenstock et al., 2013). Thus, even small deviations in ligand structure can significantly alter physiological outcome.
GLP-1r is expressed in a wide variety of tissues, including the pancreas, lung, heart, kidneys, stomach, intestine, pituitary, vagus nerve, and multiple regions of the central nervous system(Baggio and Drucker, 2007). The highest densities of GLP-1r in the brain are found in the nucleus of the solitary tract, area postrema, interpeduncular nucleus, posterodorsal and ventral tegmental nucleus, hypothalamic nuclei, thalamic nuclei, nucleus accumbens, and lateral septum(Göke et al., 1995; Merchenthaler et al., 1999; Van Dijk et al., 1996). There are some discrepancies among studies examining GLP-1r expression by radioactive binding or mRNA expression in brain, likely as a result of presynaptic receptor populations. However, there may be alternative splicing of the receptor, differences in translation, or another receptor entirely producing this effect. Having such wide distribution of GLP-1r allows a ligand to exert a host of different effects on whole body physiology depending on where the receptor is found, as has been reviewed(Holst, 2007). This is especially important to consider when using a long lasting therapeutic agonist which will likely act at many different receptor populations, effecting resulting in an endocrinization of a signal which is normally restricted by specific spatial release (central synapses) and powerful metabolism constraints (degradation by DPP IV).
5. GLP-1r Intracellular Signaling
GLP-1 acts in the pancreas to decrease blood glucose concentrations. It achieves this by increasing the synthesis and release of insulin, in addition to increasing neogenesis, proliferation, and by decreasing apoptosis of β cells. GLP-1-induced insulin release from pancreatic β cells is the most well studied intracellular signaling mechanism by GLP-1r.
In this system, GLP-1r acts through Gαs to stimulate adenylate cyclase and increase cyclic AMP (cAMP) levels(Drucker et al., 1987). This increase in cAMP has been shown to result in both protein kinase A (PKA)-dependent intracellular signaling, as well as exchange protein directly activated by cAMP (EPAC) dependent processes. The activation of these pathways allows GLP-1 to initiate a wide variety of mechanisms within the cell to ultimately trigger insulin release and genetic alterations(Baggio and Drucker, 2007; Cho et al., 2014; Holst, 2007; MacDonald et al., 2002; Seino and Shibasaki, 2005). GLP-1 has been shown to act through PKA and EPAC (cAMP dependent mechanisms) to inhibit ATP-regulated potassium channels (Kang et al., 2006; Light et al., 2002; Nakazaki et al., 2002; Shiota et al., 2002), increase activity of L-type voltage gated calcium channels (VGCCs)(Britsch et al., 1995)(Yada et al., 1993), and trigger opening of non-specific cation channels (Figure 1)(Holz et al., 1995)(Leech and Habener, 1997). Together, these actions lead to increased calcium influx, thereby enhancing calcium-induced insulin secretion. Notably, inhibition of ATP-regulated potassium channels leads to increased glucose induced membrane depolarization, enhancing the sensitivity of that cell to glucose(Holz IV IV et al., 1993). There is limited evidence indicating that similar mechanisms may occur in hippocampal and hypothalamic GLP-1r expressing neurons(Beak et al., 1998; Gilman et al., 2003; Hayes et al., 2011).
Figure 1.
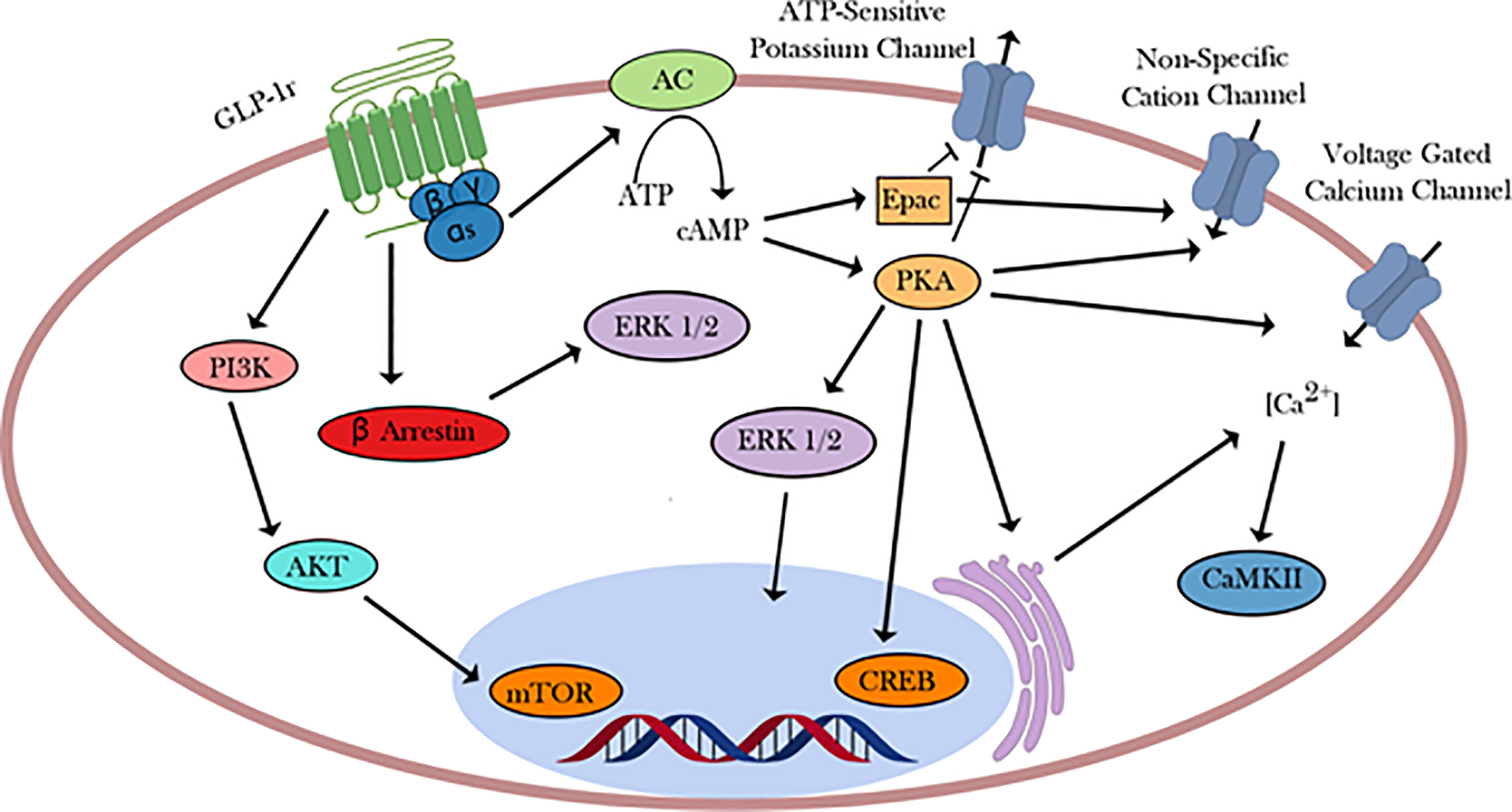
GLP-1r mediated intracellular signaling via Gαs. Although GLP-1r is known to associate with multiple different G proteins, Gαs is the most well studied coupling. Here, the diversity of downstream signaling is shown through the differential actions of ERK 1/2 depending on its activation. Activation by PKA results in transient translocation to the nucleus, while activation by β arrestin results in the targeting of cytoplasmic partners.
Other effects of GLP-1 in β cells may or may not result from Gαs signaling. GLP-1r has been shown to associate in cultured cells with not only Gαs, but also Gαq, Gαi, and Gαo(Montrose-Rafizadeh et al., 1999). There is also evidence supporting GLP-1 initiating a phospholipase C (PLC)-mediated mobilization of intracellular calcium, likely through Gαq (Gromada et al., 1995; Holz et al., 1999; Thompson and Kanamarlapudi, 2015). Additionally, limited evidence shows that GLP-1r signaling can initiate activation of CaMKII via calcium influx through L-type VGCCs, opening up further downstream pathways(Gomez et al., 2002). Further actions may be mediated through transactivation of other receptors. For example, GLP-1 acts to promote DNA synthesis and further inhibit potassium channels through transactivation of the epidermal growth factor receptor, leading to activation of phosphatidylinositol 3-kinase (PI3K)(Buteau et al., 2001). PI3K activation also leads to inhibition of apoptosis-related transcription factors. These actions also may result through Gβγ signaling. Coupling of GLP-1r to different G proteins opens up a wide variety of signaling pathways activated by the same ligand, underscoring the potential for diversity within the GLP-1r system.
The GLP-1r and other GPCRs inactivate following stimulation. GLP-1r signaling in β cells results in β-arrestin recruitment and signaling via extracellular signal-regulated kinases (ERK1/2)(Jorgensen et al., 2005; Quoyer et al., 2010; Sonoda et al., 2008). In pancreatic cells, GLP-1r internalizes following stimulation with GLP-1 or various agonists, whereby the receptor is ultimately recycled to the cell surface(Roed et al., 2014). The mechanism leading to this internalization is unclear. There is evidence in cultured cells for both a clathrin-dependent as well as a caveolin-dependent mechanism(Syme et al., 2006; Widmann et al., 1995). Moreover, additional evidence in HEK cells points to a Gαq dependent mechanism triggering activation of protein kinase C (PKC) and ERK 1/2, shown using specific chemical inhibitors of Gαq (Thompson and Kanamarlapudi, 2015). Under this mechanism, activation of Gαq triggers a PLC mediated increase in inositol triphosphate (IP3), raising intracellular calcium through mobilization of endoplasmic reticulum calcium stores. A rise in calcium concentrations activates PKC, which phosphorylates ERK 1/2, which in turn phosphorylates the C-terminus of GLP-1r. This C-terminal phosphorylation then leads to internalization via an unknown mechanism. It is possible that no one mechanism holds true across all contexts in which GLP-1r is expressed.
Although the observation that GLP-1 activates signaling in the brain has been known for over three decades(Hoosein and Gurd, 1984), much less is known about GLP-1r initiated intracellular signaling in the central nervous system. In the hindbrain, GLP-1r acts through Gαs and PKA to enhance calcium influx through VGCCs, and stimulate mitogen-activated protein kinase (MAPK) and AMP-activated protein kinase (AMPK)(Hayes et al., 2011). In the hypothalamus and hippocampus, GLP-1 acts to increase cAMP levels and activate L type VGCCs, indicating Gαs mediated signaling(Beak et al., 1998; Gilman et al., 2003; Hayes et al., 2011). In cultured neurons, GLP-1 activates PI3K, AKT, and mechanistic target of rapamycin (mTOR) to protect against apoptosis(Kimura et al., 2009). In the paraventricular nucleus of the hypothalamus, GLP-1 has been shown to enhance glutamatergic transmission via PKA mediated phosphorylation of the AMPA receptor GluR1 subunit(Liu et al., 2017). More work is needed to elucidate the intracellular actions enacted by GLP-1r in the CNS; it is likely there will be subtle differences between pathways activated depending on the brain region and cellular context.
Investigation of ligand bias in GLP-1r signaling has focused on the three main signaling pathways initiated by Gαs: cAMP production, ERK 1/2 activation, and increases in intracellular calcium(Furness et al., 2018; Wootten et al., 2016, 2013). As previously mentioned, these differences in signaling arise due to structural differences in the receptor conformations produced by binding of various ligands(Furness et al., 2018). This signaling bias has been meticulously studied mainly in conditions designed to mimic pancreatic GLP-1r signaling. However, it would be interesting to see if similar bias occurs at central GLP-1r populations and whether this alters the synaptic and behavioral outcomes of GLP-1r agonism.
The result of GLP-1r activation can differ depending on spatial and temporal factors. Compartmentalization can lead to a restriction of the action of downstream signaling partners(Calebiro and Maiellaro, 2014). This formation of microdomains has been shown to impact GLP-1r signaling. For example, PKA-induced activation of ERK 1/2 has been shown to be transient and result in its translocation to the nucleus(Khoo et al., 2003; Quoyer et al., 2010). In contrast, GLP-1r β-arrestin-induced ERK 1/2 activation does not result in translocation, and instead, preferentially targets cytoplasmic targets(Quoyer et al., 2010). The targeting of signaling partners to GLP-1rs is likely mediated by in some areas by A-kinase anchoring protein (AKAP) scaffolding proteins, which have been shown to target PKA to GLP-1r(Lester et al., 1997). Temporally, the action of GLP-1r is controlled through the timing of cAMP oscillations in β-cells, leading to only sustained elevations of cAMP triggering a nuclear translocation of activated PKA(Dyachok et al., 2006). Greater spatial and temporal resolution will likely provide a more complete picture of the factors influencing the environment specific outcomes of GLP-1r signaling.
6. Bariatric surgery, reduced food intake and the GLP-1-bile acid axis
The prevalence of obesity in the United States continues to increase. Bariatric procedures such as Roux-en Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG) are the most effective and durable treatments for obesity and coincident type 2 diabetes (T2D)(Vetter et al., 2009). These procedures rapidly normalize glucose metabolism and enhance insulin secretion both before(Isbell et al., 2010) and after significant weight loss (Salehi et al., 2011). Such discoveries have prompted intensive efforts into identifying the mechanisms by which GLP-1 and other proglucagon peptides contribute to metabolic improvements after these surgeries.
Enhanced incretin responsiveness is a major proposed mechanism by which bariatric procedures improve oral glucose tolerance. Our group(Hansen et al., 2011; Isbell et al., 2010) and others(Laferrère et al., 2007; le Roux et al., 2007; Morínigo et al., 2006) have described a large increase in postprandial GLP-1 immediately after RYGB that is sustained(Mousumi et al., 2010; Vidal et al., 2009). At one month, postoperative elevations in GLP-1 are concurrent with enhanced insulin responses to enteral glucose and other incretin effects(Laferrère et al., 2007). Hansen et al. investigated nutrient bypass of the foregut for its contributions to enhanced glucose tolerance, insulin sensitivity and incretin responses in humans after a mixed meal was delivered orally before RYGB (gastric), and both orally (jejunal) and by gastrostomy tube (gastric) postoperatively (Hansen et al., 2011). They observed GLP-1 (active form) responses were absent preoperatively and restored postoperatively by both jejnual and gastric feeding routes. Such findings are consistent with more responsive GLP-1 EECs, located primarily in the distal ileum and proximal colon, to the rapid delivery of nutrients to the distal small intestine with both administration routes. We assessed GLP-1 responses upon enteral glucose via nasogastric or nasojejunal tubes in non-surgical glucose-tolerant obese subjects(Breitman et al., 2013). In this crossover study, glucose excursions obtained with nasogastric and nasojejunal delivery were replicated using isoglycemic glucose infusions, enabling determination of the GI tract to postprandial glucose disposal. Such determinations were made by comparing the amount of intravenous glucose required to match plasma glucose curves obtained following delivery of the same glucose load via nasogastric and nasojejunal delivery. Comparison of glucose excursion curves upon nasogastric versus nasojejunal glucose delivery revealed there was a more rapid and six-fold greater GLP-1 response via nasojejunal delivery. Additionally, there was a twofold increase in the contribution of the GI tract to glucose disposal after nasojejunal compared to nasogastric delivery. These findings suggest that foregut bypass may alter GLP-1 responses by enhanced nutrient delivery to the hindgut.
We have also investigated bile acids for their role in regulating metabolic improvements, GLP-1 secretion, and weight loss after bariatric procedures. We(Albaugh et al., 2015), and others(Kohli et al., 2013; Patti et al., 2009; Pournaras et al., 2012; Steinert et al., 2013)have observed increases in serum bile acids after bariatric procedures. More specifically, we examined total and species-specific BA changes in Class III obese (BMI ≥ 40 kg/m2), pre-operatively and followed longitudinally at 1, 6, 12 and 24 months after RYGB. One month after surgery, bile acid levels increased nearly 3-fold relative to pre-operative levels, declined slightly at 6 months, then progressively increased up to 2-years after surgery. The bile acid increases we detected were concordant with improvements in both hepatic and muscle insulin sensitivity as we previously reported(Fabbrini et al., 2010; Tamboli et al., 2014). We hypothesized that the altered enterohepatic circulation of bile acids after RYGB mediates the metabolic improvements that underlie the resolution of T2D after the procedure. We utilized a novel surgical procedure in diet-induced obese (DIO)(Flynn et al., 2015) and lean(Albaugh et al., 2019) mice wherein bile is diverted from the gallbladder (GB) to the duodenum (GB-D, a sham procedure), the jejunum (GB-J), or ileum (GB-IL). This biliary diversion (BD) procedure permitted examination of altered bile flow, similar to that observed after RYGB, independent of alterations in gastrointestinal tract anatomy. Remarkably, the GB-IL procedure, but not GB-D or GB-J, recapitulated many of the beneficial metabolic outcomes observed after RYBG. In obese mice fed a high fat (60%) diet after GB-IL, dramatic reductions in food intake, body weight, hepatic steatosis and adiposity were concurrent with increases in serum bile acids, significantly improved oral glucose tolerance and changes in the gut microbiome. DIO GB-IL mice additionally exhibited significant fat malabsorption confounding the establishment of a direct cause and effect relationship between bile acids and improved glucose metabolism. To mitigate these confounding variables, we implemented GB-IL and sham (GB-D) surgeries in normal, low-fat (4.5%) chow-fed mice and again assessed their effects on nutrient metabolism and physiology. Remarkably, neither GB-IL of GB-D had effects on food intake, body weight or body composition. However, GB-IL mice maintained on a low-fat chow diet displayed significant elevations in serum bile acids as well as dramatic improvements in oral glucose tolerance. These improvements were not attributable to detectable differences in hepatic glycogen content, insulin sensitivity or intestinal glucose uptake, but were associated with enhanced insulin secretion. To investigate the potential contribution of GLP-1 to this phenomenon, we utilized a lymphatic fistula procedure and measured incretin concentrations in intestinal lymph. GLP-1 levels in the fasted state were significantly higher than controls. Postprandial GLP-1 excursions after a mixed meal, however, were similar. These observations contrasted with those of lymphatic GIP, which were similar to controls in both the fasted and fed states. Importantly, pre-treatment of GB-IL mice with exendin 9 (Ex-9; 50 μg intraperitoneally), a GLP-1r antagonist, or cholestyramine (500 mg/kg p.o., daily for 3 days), a bile acid resin, mitigated improvements in fasting glycemia after GB-IL. Improvements in oral glucose tolerance after GB-IL were also assessed in GLP-1r knockout mice maintained on a low-fat, normal chow diet. Body weight and adiposity after GB-IL in Glp-1r null mice were unaffected by GB-IL surgery and were similar to controls, suggesting the importance of the Glp-1r in mediating the responses of the GB-IL procedure.
Finally, recent findings additionally suggest that the GLP1r also modulates systemic metabolism by limiting the bioavailability of intestinal GLP-1(He et al., 2019). Deletion of integrin β7+ (Itβ7−/−) renders mice deficient of natural gut intraepithelial T lymphocytes (IELs) resident throughout the enterocyte layer of the small intestine. These mice exhibit metabolic hyperactivity when fed a high-fat and high sugar diet and are additionally resistant to obesity, hypercholesterolaemia, hypertension, diabetes, atherosclerosis and were protected from cardiovascular disease. These mice, on a pro-atherosclerotic Ldlr−/− background (chimeras) exhibited high basal GLP-1 tone with high gut gcg mRNA levels contrasting the relative GLP1r deficiency observed in Itβ7−/− mice containing fewer and mostly GLP1rlow T cells. in other words, loss of the GLP1r in natural IELs associated with increased plasma GLP-1. Chimeras lacking GLP1R (Glp1r−/− β7−/− Ldlr−/−) exhibited elevated GLP-1 tone were protected from glucose intolerance, hypercholesterolaemia and cardiovascular disease. These results indicate that β7+ IELS as critical gatekeepers of dietary metabolism in a GLP-1R manner.
7. GLP-1 in feeding and reward
GLP-1 is able to influence energy homeostasis through a multiplicity of actions. Although it is best known for its incretin effect, GLP-1 also exerts a potent effect on feeding behavior to control nutrient intake. Early studies utilized intracerebroventricular injections to show that exogenous modulation of GLP-1 action could dose dependently increase (through antagonism)(Meeran et al., 1999) or decrease (through agonism)(Donahey et al., 1998; Tang-Christensen et al., 1996; Turton et al., 1996) food intake in rodents. Intravenous infusions of synthetic GLP-1 also reduced food intake and subjective reporting of hunger in human participants(Gutzwiller et al., 1999). While the anorectic effect of GLP-1 was clear, it was more difficult to localize the effect to a specific circuit within the brain. Due to the wide distribution of GLP-1r, different groups attributed the anorectic effect to interactions with homeostatic(Goldstone et al., 1997; Gunn et al., 1996; Meeran et al., 1999; Turton et al., 1996) or aversion circuitry(Rinaman, 1999b; Seeley et al., 2000; Thiele et al., 1997). By directing GLP-1 infusions to specific brain regions, it was shown that specific populations of GLP-1r expressing cells mediated the visceral illness response associated with conditioned aversion, and that the anorectic effect could be separated from the aversion phenotype(Kinzig et al., 2002). Following these findings, there has been considerable focus on GLP-1’s role in brainstem and hypothalamic nuclei in the regulation of homeostatic feeding.
7.1. Hindbrain
In the hindbrain, the role of GLP-1 in feeding has been studied in the parabrachial nucleus (PBN). The PBN receives monosynaptic connections from GLP-1 producing neurons in the NTS(Alhadeff et al., 2014), and applying exendin-4 to this region results in a suppression in chow and palatable food intake(Alhadeff et al., 2014; Richard et al., 2014). Exendin-4 also elevates the firing rate of neurons within the PBN, and enhances cFos expression, consistent with a stimulatory effect on neuronal activity(Richard et al., 2014). Further, lesion of the PBN disrupts the ability of exendin-4 to alter feeding behavior(Swick et al., 2015). Calcitonin gene-related peptide (CGRP) expressing cells within the PBN are likely the cell type mediating the effect of GLP-1r signaling within the PBN, as shown by localizing Fos immunoreactivity to CGRP cells and by silencing these neurons during exendin-4 administration(Campos et al., 2016). The PBN is thought to regulate satiety processes within the feeding network(Sternson and Eiselt, 2017), and an increase in activity following intra-PBN exendin-4 administration is consistent with this role. Further work in this area could integrate these GLP-1 responsive PBN neurons into currently known feeding circuits and behavioral circuits(Alhadeff et al., 2018), as started by work integrating the role of the arcuate nucleus input to the PBN(Campos et al., 2016). GLP-1 is also capable of acting within the NTS at local receptors(Card et al., 2018; Richard et al., 2015). Knockdown studies of GLP-1r within the NTS has shown that endogenous GLP-1 signaling in this region suppresses chow intake and meal size as well as self-administration of palatable food on both a fixed and progressive ratio schedule(Alhadeff et al., 2017). Interestingly, GLP-1 within the NTS has been shown to be taken up by astrocytes and influence calcium dynamics of these astrocytes, indicating they may play a role in the function of GLP-1 within this nucleus(Reiner et al., 2016).
7.2. Hypothalamus
GLP-1 is able to act within several hypothalamic nuclei to alter feeding behavior. In the arcuate nucleus, studies of GLP-1 have yielded conflicting results. One group using intra arcuate nucleus microinjections of GLP-1 itself found no effect on 2 hour cumulative food intake(Beiroa et al., 2014), while another found a reduction of 24 hour food intake applying liraglutide(Sandoval et al., 2008). It is clear that GLP-1 is taken up by proopiomelanocortin (POMC) cells within the arcuate nucleus, and these neurons are depolarized by application of GLP-1(Secher et al., 2014). This same study found that intra-arcuate nucleus application of the GLP-1 antagonist exendin-9 attenuated liraglutide-induced weight loss, examined at 14 days following chronic administration(Secher et al., 2014). Further work has found that GLP-1 also acts at kisspeptin expressing neurons(Heppner et al., 2017), indicating that GLP-1 in the arcuate nucleus may be participating in nodes connected to the hypothalamic-pituitary-gonadal axis. Importantly, the arcuate nucleus receives GLP-1 positive terminals(Heppner et al., 2017), but is also able to take up peripherally administered GLP-1r agonists(Secher et al., 2014). While it is clear that GLP-1 is able to alter neuronal function within the arcuate nucleus, the specific circuits and behavioral phenotypes modulated by this signal are still being elucidated.
In the paraventricular nucleus of the hypothalamus (PVN), both GLP-1 and exendin-4 reduce food intake(Burmeister et al., 2017; Sandoval et al., 2008). Glp-1r neurons in the PVN show markers of activity following refeeding, and inactivation of these neurons results in increased food intake and increased breakpoint on a progressive ratio task(Li et al., 2018). Expectedly, inducible silencing of GLP-1r expressing neurons in the PVN does not fully ablate the anorectic effect of peripherally administered liraglutide, likely due to redundancy in the ability of GLP-1 to alter feeding behavior(Li et al., 2018). A study investigating the molecular mechanisms of GLP-1 acting within the PVN found that using a genetic strategy to block AMPA receptor trafficking following exendin-4 administration to the PVN resulted in a blunted reduction in food intake(Liu et al., 2017). Thus post synaptic modulation of excitatory transmission appears to be a key player in the effect of GLP-1r on food intake within the PVN. However, the circuits in which these GLP-1r expressing neurons are participating also remain unknown. Future work could investigate the role of these hypothalamic GLP-1r expressing neurons in homeostatic feeding following weight loss, or whether GLP-1 in this region alters feeding to different degrees for different nutrients.
7.3. Mesolimbic dopamine system
Nodes of the mesolimbic reward circuitry are also impacted by local GLP-1r signaling. Intra VTA injections of exendin-4 result in a reduction in palatable food intake(Alhadeff et al., 2012; Mietlicki-Baase et al., 2013), as well as a reduction in the willingness to work for a palatable food reward on a progressive ratio schedule(Dickson et al., 2012). Based on functional studies of excitatory transmission, the GLP-1r receptor population mediating the effect of exendin-4 in the VTA is thought to be presynaptic(Mietlicki-Baase et al., 2013). Circuit analysis revealed the medial NAc shell VTA population to be modulated by exendin-4(Wang et al., 2015). Intra NAc exendin-4 injections have shown that GLP-1r signaling in the NAc core, but not NAc shell, result in a reduction in food intake(Dossat et al., 2011). A more detailed analysis of licking microstructure found that the effect of GLP-1r in the NAc primarily effects the size of a “meal”, as defined by lick bursts(Dossat et al., 2013). Interestingly, this effect was specific to sucrose solution and did not occur with a saccharine solution, indicating a nutrient dependent effect. Similar to the VTA, analysis of excitatory transmission shows that GLP-1r stimulation primarily changes presynaptic measures of release probability, again indicating a presynaptic GLP-1r localization(Mietlicki-Baase et al., 2014). However, the specific input at which the GLP-1r dependent effect is produced remains unknown.
The GLP-1r exhibits a wide central distribution, with some populations outside of brain regions classically associated with modulation of feeding behavior. There is some evidence for that GLP-1 can act within the hippocampus to alter feeding behavior(Hsu et al., 2015). A more recent line of work has shown that GLP-1r agonism in the lateral septum is able to alter feeding behavior(Terrill et al., 2018, 2016).
7.4. Psychostimulants
Studies on the role of GLP-1 in regulating food intake led to the discovery that GLP-1 signaling also regulates reward and motivation associated with drugs of abuse(Mietlicki-Baase et al., 2013). This led to the discovery that administration of GLP-1 blunted the action of psychostimulants(Erreger et al., 2012; Graham et al., 2013). Notably, GLP-1r activation in the VTA and NAc has been linked to a reduction in cocaine seeking behavior(Hernandez et al., 2018, 2017). GLP-1 agonism has been shown to reduce locomotor activity stimulated by amphetamine(Erreger et al., 2012), a process that is known to depend on dopamine signaling in the NAc(Heusner et al., 2003). More recent work has shown that GLP-1 is able to dampen phasic dopamine transmission in the NAc core(Fortin and Roitman, 2017). This suppression of dopamine signaling may explain the GLP-1 induced alterations in psychostimulant reward-related behaviors, including conditioned place preference(Graham et al., 2013)(Reddy et al., 2016) and self-administration(Sørensen et al., 2015). Notably, in self-administration studies, researchers were able to determine that GLP-1 agonism had decreased the efficacy of cocaine as a reinforcer rather than simply reduced cocaine potency(Sørensen et al., 2015). However, it is likely that other brain regions also contribute to the ability of GLP-1 to dampen drug reward. A GLP-1r population in the lateral septum has been shown to be critical for the influence of GLP-1 agonism over cocaine conditioned place preference(Harasta et al., 2015; Reddy et al., 2016). This GLP-1r population occupies the dorsal portion of the lateral septum, a region which has been shown to constitute an important relay mediating the incorporation of contextual information from the hippocampus into reward processing in the VTA(Luo et al., 2011). Further studies have shown that GLP-1 agonism in the lateral septum is able to alter cocaine’s action and dopamine homeostasis through the modulation of endocannabinoid signaling and the function of the dopamine transporter(Reddy et al., 2016). Thus, the modulation of the dopamine transporter by GLP-1r signaling likely plays an important role in the impact of GLP-1 agonism on psychostimulant induced behaviors in the lateral septum. The antagonistic relationship between GLP-1 and cocaine is well supported by preclinical work, as shown above. However, a study finding reduced serum GLP-1 levels in human participants following intravenous cocaine administration shows that this relationship may be bidirectional(Bouhlal et al., 2017).
7.5. Alcohol and nicotine
Subsequent work has expanded the effect of GLP-1 to impact alcohol intake and reward(Egecioglu et al., 2013b; Shirazi et al., 2013). GLP-1 agonism is able to dampen alcohol self-administration as well as alcohol conditioned place preference, indicating that these effects may also be mediated by the same circuitry controlling response to psychostimulants(Shirazi et al., 2013). In humans, a genetic variant of the GLP-1r has been found to be associated with alcohol use disorder, enhancing the validity of the preclinical results in this area(Suchankova et al., 2015). In contrast to these preclinical data, there have been reports that bariatric surgeries in humans, that elevate gut hormones like GLP-1, may result in an increased propensity to abuse alcohol in a small proportion of the population(Ertelt et al., 2008). Follow up studies in animals have produced mixed results(Davis et al., 2012; Hajnal et al., 2012). A more detailed and controlled analysis of the clinical population is necessary to determine whether this represents a real consequence of bariatric surgery. Correlation to pre and post-operative variables would allow a better idea of what could be contributing to the phenomena. A more detailed discussion of the role of GLP-1 in preclinical studies of alcohol related behaviors can be found elsewhere(Jerlhag, 2018). GLP-1 has also been shown to influence nicotine intake (Egecioglu et al., 2013a; Tuesta et al., 2017). The influence of GLP-1 agonism on nicotine is likely mediated by classical reward circuitry in addition to aversion circuits.
Due to the widespread expression of GLP-1r, it is important to consider the context in which the peptide is signaling when understanding its effect. For example, GLP-1r populations in the VTA and nucleus accumbens likely allow GLP-1 to modulate salience and rewarding efficacy of drug rewards(Schmidt et al., 2016; Sørensen et al., 2015). On the other hand, the GLP-1r population in the lateral septum likely mediates associations of context and reward, measured through place preference and reinstatement paradigms(Luo et al., 2011; Reddy et al., 2016; Sartor and Aston-Jones, 2012). Thus, GLP-1 is able to alter distinct properties of the rewarding signal depending on the circuit context. Furthermore, the mechanism by which GLP-1 acts to alter the behavior of cells within the circuit can differ. While it has been shown that GLP-1 can act to blunt dopamine transmission in both the nucleus accumbens(Fortin and Roitman, 2017) and the lateral septum(Reddy et al., 2016), this has been shown to occur via a transporter-independent and a transporter-dependent manner, respectively. This may arise from differences in intracellular signaling initiated by the receptor, directed by receptor localization and grouping with intracellular signaling partners(Calebiro and Maiellaro, 2014; Lester et al., 1997; Quoyer et al., 2010). Future work is needed to identify the intracellular events that lead to these different signaling outcomes, and greater anatomical resolution will illuminate the precise localization of the receptor populations mediating these outcomes.
Concluding Remarks
The GLP-1 signal represents a propagating message which spreads throughout multiple tissues to modulate an astonishingly wide variety of processes. The broad reach of this signal and its evolutionary conservation underscore its critical role in normal physiological functioning. Thus, it should come as no surprise that GLP-1 is able to potently regulate systems ranging from glucose homeostasis to motivated behavior. It should be equally recognizable that this multiplicity of actions requires immense diversity of the GLP-1 signal. This diversity has been found to be expressed at many levels, including synthesis, receptor function, and downstream signaling partners. There is little doubt that there are additional complexities of the GLP-1 system that will be found to differentiate populations. Future work will rely on relatively recent technologies which allow for the conditional expression of genes and the delicate pharmacological manipulation of intracellular signaling, as has been used to produce the most recent results presented here. With these tools and the knowledge accrued from the previous three decades of GLP-1 research, we will hopefully see novel clinical applications of the already FDA approved GLP-1 receptor agonists.
Highlights:
GLP-1 synthesis, metabolism, and the expression of the receptor show diversity across tissues.
Bile acid signaling may represent a novel target for enhancing GLP-1 action.
The diversity and complexity of GLP-1 signaling in the brain points to the multiple regulatory roles of GLP-1 in the CNS.
Abbreviations:
- AMPK
AMP-activated protein kinase
- CCK
cholecystokinin
- CGRP
Calcitonin gene-related peptide
- DPP IV
dipeptidyl-peptidase IV
- EPAC
exchange protein directly activated by cAMP
- ERK1/2
extracellular signal-regulated kinases
- GIP
glucose-dependent insulinotropic polypeptide
- GLP-1
Glucagon-like peptide-1
- GLP-1r
Glucagon-like peptide-1 receptor
- GLP-1 EECs
enteroendocrine GLP-1 producing cells
- GPCRs
G protein-coupled receptors
- IP3
inositol triphosphate
- MAPK
mitogen-activated protein kinase
- mTOR
mechanistic target of rapamycin
- NTS
nucleus of the solitary tract
- PBN
parabrachial nucleus
- PI3K
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- PLC
phospholipase C
- POMC
proopiomelanocortin
- SGLT
sodium coupled glucose transporters
- VGCCs
voltage gated calcium channels
References
- Ahrén B, 2004. Sensory nerves contribute to insulin secretion by glucagon-like peptide-1 in mice. Am. J. Physiol. Integr. Comp. Physiol 286, R269–R272. [DOI] [PubMed] [Google Scholar]
- Albaugh VL, Banan B, Antoun J, Xiong Y, Guo Y, Ping J, Alikhan M, Clements BA, Abumrad NN, Flynn CR, 2019. Role of Bile Acids and GLP-1 in Mediating the Metabolic Improvements of Bariatric Surgery. Gastroenterology 156, 1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albaugh VL, Flynn CR, Cai S, Xiao Y, Tamboli RA, Abumrad NN, 2015. Early increases in bile acids post Roux-en-Y gastric bypass are driven by insulin-sensitizing, secondary bile acids. J. Clin. Endocrinol. Metab 100, E1225–E1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadeff AL, Baird J-P, Swick JC, Hayes MR, Grill HJ, 2014. Glucagon-like peptide-1 receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake and motivation to feed. Neuropsychopharmacology 39, 2233–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadeff AL, Mergler BD, Zimmer DJ, Turner CA, Reiner DJ, Schmidt HD, Grill HJ, Hayes MR, 2017. Endogenous glucagon-like peptide-1 receptor signaling in the nucleus tractus solitarius is required for food intake control. Neuropsychopharmacology 42, 1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadeff AL, Rupprecht LE, Hayes MR, 2012. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 153, 647–58. 10.1210/en.2011-1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadeff AL, Su Z, Hernandez E, Klima ML, Phillips SZ, Holland RA, Guo C, Hantman AW, De Jonghe BC, Betley JN, 2018. A neural circuit for the suppression of pain by a competing need state. Cell 173, 140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio LL, Drucker DJ, 2007. Biology of Incretins: GLP-1 and GIP. Gastroenterology 132, 2131–2157. 10.1053/j.gastro.2007.03.054 [DOI] [PubMed] [Google Scholar]
- Beak SA, Heath MM, Small CJ, Morgan DGA, Ghatei MA, Taylor AD, Buckingham JC, Bloom SR, Smith DM, 1998. Glucagon-like peptide-1 stimulates luteinizing hormone-releasing hormone secretion in a rodent hypothalamic neuronal cell line. J. Clin. Invest 101, 1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, Serrano M, Fernø J, Salvador J, Escalada J, 2014. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 63, 3346–3358. [DOI] [PubMed] [Google Scholar]
- Bell GI, Sanchez-Pescador R, Laybourn PJ, Najarian RC, 1983a. Exon duplication and divergence in the human preproglucagon gene. Nature 304, 368–371. [DOI] [PubMed] [Google Scholar]
- Bell GI, Santerre RF, Mullenbach GT, 1983b. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature 302, 716–718. [DOI] [PubMed] [Google Scholar]
- Belza A, Ritz C, Sørensen MQ, Holst JJ, Rehfeld JF, Astrup A, 2013. Contribution of gastroenteropancreatic appetite hormones to protein-induced satiety. Am. J. Clin. Nutr 97, 980–989. [DOI] [PubMed] [Google Scholar]
- Bernstein H-G, Schön E, Ansorge S, Röse I, Dorn A, 1987. Immunolocalization of dipeptidyl aminopeptidase (DAP IV) in the developing human brain. Int. J. Dev. Neurosci 5, 237–242. [DOI] [PubMed] [Google Scholar]
- Beutler LR, Chen Y, Ahn JS, Lin Y-C, Essner RA, Knight ZA, 2017. Dynamics of Gut-Brain Communication Underlying Hunger. Neuron 96, 461–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billing LJ, Smith CA, Larraufie P, Goldspink DA, Galvin S, Kay RG, Howe JD, Walker R, Pruna M, Glass L, 2018. Co-storage and release of insulin-like peptide-5, glucagon-like peptide-1 and peptideYY from murine and human colonic enteroendocrine cells. Mol. Metab 16, 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhlal S, Ellefsen KN, Sheskier MB, Singley E, Pirard S, Gorelick DA, Huestis MA, Leggio L, 2017. Acute effects of intravenous cocaine administration on serum concentrations of ghrelin, amylin, glucagon-like peptide-1, insulin, leptin and peptide YY and relationships with cardiorespiratory and subjective responses. Drug Alcohol Depend. 180, 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt SJ, Götz A, Tschöp MH, Müller TD, 2018. Gut hormone polyagonists for the treatment of type 2 diabetes. Peptides 100, 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitman I, Isbell JM, Saliba J, Jabbour K, Flynn CR, Marks-Shulman PA, Laferrère B, Abumrad NN, Tamboli RA, 2013. Effects of proximal gut bypass on glucose tolerance and insulin sensitivity in humans. Diabetes Care 36, e57–e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britsch S, Krippeitdrews P, Lang F, Gregor M, Drews G, 1995. Glucagon-like peptide-1 modulates Ca 2+ current but not K+ ATP current in intact mouse pancreatic B-cells. Biochem. Biophys. Res. Commun 207, 33–39. [DOI] [PubMed] [Google Scholar]
- Bucinskaite V, Tolessa T, Pedersen J, Rydqvist B, Zerihun L, Holst JJ, Hellström PM, 2009. Receptor-mediated activation of gastric vagal afferents by glucagon-like peptide-1 in the rat. Neurogastroenterol. Motil 21, 978. [DOI] [PubMed] [Google Scholar]
- Burmeister MA, Ayala JE, Smouse H, Landivar-Rocha A, Brown JD, Drucker DJ, Stoffers DA, Sandoval DA, Seeley RJ, Ayala JE, 2017. The hypothalamic glucagon-like peptide 1 receptor is sufficient but not necessary for the regulation of energy balance and glucose homeostasis in mice. Diabetes 66, 372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buteau J, Foisy S, Rhodes CJ, Carpenter L, Biden TJ, Prentki M, 2001. Protein kinase Cζ activation mediates glucagon-like peptide-1–induced pancreatic β-cell proliferation. Diabetes 50, 2237–2243. [DOI] [PubMed] [Google Scholar]
- Buteau J, Roduit R, Susini S, Prentki M, 1999. Glucagon-like peptide-1 promotes DNA synthesis, activates phosphatidylinositol 3-kinase and increases transcription factor pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding activity in beta (INS-1)-cells. Diabetologia 42, 856–864. [DOI] [PubMed] [Google Scholar]
- Calebiro D, Maiellaro I, 2014. cAMP signaling microdomains and their observation by optical methods. Front. Cell. Neurosci 8, 350 10.3389/fncel.2014.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos CA, Bowen AJ, Schwartz MW, Palmiter RD, 2016. Parabrachial CGRP neurons control meal termination. Cell Metab. 23, 811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Johnson AL, Llewellyn-Smith IJ, Zheng H, Anand R, Brierley DI, Trapp S, Rinaman L, 2018. GLP-1 neurons form a local synaptic circuit within the rodent nucleus of the solitary tract. J. Comp. Neurol 526, 2149–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia CW, Egan JM, 2008. Incretin-based therapies in type 2 diabetes mellitus. J. Clin. Endocrinol. Metab 93, 3703–3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YM, Fujita Y, Kieffer TJ, 2014. Glucagon-like peptide-1: glucose homeostasis and beyond. Annu. Rev. Physiol 76, 535–559. [DOI] [PubMed] [Google Scholar]
- Davis JF, Schurdak JD, Magrisso IJ, Mul JD, Grayson BE, Pfluger PT, Tschöp MH, Seeley RJ, Benoit SC, 2012. Gastric bypass surgery attenuates ethanol consumption in ethanol-preferring rats. Biol. Psychiatry 72, 354–360. [DOI] [PubMed] [Google Scholar]
- Day JW, Ottaway N, Patterson JT, Gelfanov V, Smiley D, Gidda J, Findeisen H, Bruemmer D, Drucker DJ, Chaudhary N, 2009. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat. Chem. Biol 5, 749. [DOI] [PubMed] [Google Scholar]
- Deacon CF, Lebovitz HE, 2016. Comparative review of dipeptidyl peptidase-4 inhibitors and sulphonylureas. Diabetes, Obes. Metab 18, 333–347. [DOI] [PubMed] [Google Scholar]
- Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ, 1995. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes 44, 1126–1131. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Fleck PR, Wilson CA, Mekki Q, 2008. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care 31, 2315–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanvantari S, Seidah NG, Brubaker PL, 1996. Role of prohormone convertases in the tissue-specific processing of proglucagon. Mol. Endocrinol 10, 342–355. [DOI] [PubMed] [Google Scholar]
- Diakogiannaki E, Pais R, Tolhurst G, Parker HE, Horscroft J, Rauscher B, Zietek T, Daniel H, Gribble FM, Reimann F, 2013. Oligopeptides stimulate glucagon-like peptide-1 secretion in mice through proton-coupled uptake and the calcium-sensing receptor. Diabetologia 56, 2688–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP, 2012. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J. Neurosci 32, 4812–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon JS, Tanizawa Y, Wheeler MB, Leng X-H, Ligon BB, Rabin DU, Yoo-Warren H, Permutt MA, Boyd AE 3rd, 1993. Cloning and functional expression of the human glucagon-like peptide-1 (GLP-1) receptor. Endocrinology 133, 1907–1910. [DOI] [PubMed] [Google Scholar]
- Donahey JCK, van Dijk G, Woods SC, Seeley RJ, 1998. Intraventricular GLP-1 reduces short-but not long-term food intake or body weight in lean and obese rats. Brain Res. 779, 75–83. [DOI] [PubMed] [Google Scholar]
- Dossat AM, Diaz R, Gallo L, Panagos A, Kay K, Williams DL, 2013. Nucleus accumbens GLP-1 receptors influence meal size and palatability. Am. J. Physiol. Metab 304, E1314–E1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossat AM, Lilly N, Kay K, Williams DL, 2011. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J. Neurosci 31, 14453–14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF, 1987. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc. Natl. Acad. Sci 84, 3434–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyachok O, Isakov Y, Sågetorp J, Tengholm A, 2006. Oscillations of cyclic AMP in hormone-stimulated insulin-secreting β-cells. Nature 439, 349–352. [DOI] [PubMed] [Google Scholar]
- Egecioglu E, Engel JA, Jerlhag E, 2013a. The Glucagon-Like Peptide 1 Analogue Exendin-4 Attenuates the Nicotine-Induced Locomotor Stimulation, Accumbal Dopamine Release, Conditioned Place Preference as well as the Expression of Locomotor Sensitization in Mice. PLoS One 8, e77284 10.1371/journal.pone.0077284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Steensland P, Fredriksson I, Feltmann K, Engel JA, Jerlhag E, 2013b. The glucagon-like peptide 1 analogue Exendin-4 attenuates alcohol mediated behaviors in rodents. Psychoneuroendocrinology 38, 1259–1270. 10.1016/j.psyneuen.2012.11.009 [DOI] [PubMed] [Google Scholar]
- Egerod KL, Engelstoft MS, Grunddal KV, Nøhr MK, Secher A, Sakata I, Pedersen J, Windeløv JA, Füchtbauer E-M, Olsen J, 2012. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology 153, 5782–5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng J, Kleinman WA, Singh L, Singh G, Raufman J-P, 1992. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J. Biol. Chem 267, 7402–7405. [PubMed] [Google Scholar]
- Engel M, Hoffmann T, Wagner L, Wermann M, Heiser U, Kiefersauer R, Huber R, Bode W, Demuth H-U, Brandstetter H, 2003. The crystal structure of dipeptidyl peptidase IV (CD26) reveals its functional regulation and enzymatic mechanism. Proc. Natl. Acad. Sci 100, 5063–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erreger K, Davis AR, Poe AM, Greig NH, Stanwood GD, Galli A, 2012. Exendin-4 decreases amphetamine-induced locomotor activity. Physiol. Behav 106, 574–8. 10.1016/j.physbeh.2012.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertelt TW, Mitchell JE, Lancaster K, Crosby RD, Steffen KJ, Marino JM, 2008. Alcohol abuse and dependence before and after bariatric surgery: a review of the literature and report of a new data set. Surg. Obes. Relat. Dis 4, 647–650. [DOI] [PubMed] [Google Scholar]
- Evers A, Haack T, Lorenz M, Bossart M, Elvert R, Henkel B, Stengelin S, Kurz M, Glien M, Dudda A, 2017. Design of novel exendin-based dual glucagon-like peptide 1 (GLP-1)/glucagon receptor agonists. J. Med. Chem 60, 4293–4303. [DOI] [PubMed] [Google Scholar]
- Fabbrini E, Tamboli RA, Magkos F, Marks–Shulman PA, Eckhauser AW, Richards WO, Klein S, Abumrad NN, 2010. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology 139, 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan B, Ma T, Ottaway N, Müller TD, Habegger KM, Heppner KM, Kirchner H, Holland J, Hembree J, Raver C, 2013. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci. Transl. Med 5, 209ra151–209ra151. [DOI] [PubMed] [Google Scholar]
- Flynn CR, Albaugh VL, Cai S, Cheung-Flynn J, Williams PE, Brucker RM, Bordenstein SR, Guo Y, Wasserman DH, Abumrad NN, 2015. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nat. Commun 6, 7715 10.1038/ncomms8715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin SM, Roitman MF, 2017. Central GLP-1 receptor activation modulates cocaine-evoked phasic dopamine signaling in the nucleus accumbens core. Physiol. Behav [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fothergill LJ, Callaghan B, Hunne B, Bravo DM, Furness JB, 2017. Costorage of enteroendocrine hormones evaluated at the cell and subcellular levels in male mice. Endocrinology 158, 2113–2123. [DOI] [PubMed] [Google Scholar]
- Frias JP, Bastyr III EJ, Vignati L, Tschöp MH, Schmitt C, Owen K, Christensen RH, DiMarchi RD, 2017. The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090–2746, in patients with type 2 diabetes. Cell Metab. 26, 343–352. [DOI] [PubMed] [Google Scholar]
- Furness SGB, Christopoulos A, Sexton PM, Wootten D, 2018. Differential engagement of polar networks in the glucagon-like peptide 1 receptor by endogenous variants of the glucagon-like peptide 1. Biochem. Pharmacol 156, 223–240. [DOI] [PubMed] [Google Scholar]
- Gilman CP, Perry T, Furukawa K, Grieg NH, Egan JM, Mattson MP, 2003. Glucagon-like peptide 1 modulates calcium responses to glutamate and membrane depolarization in hippocampal neurons. J. Neurochem 87, 1137–1144. [DOI] [PubMed] [Google Scholar]
- Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP, 1995. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur. J. Neurosci 7, 2294–2300. [DOI] [PubMed] [Google Scholar]
- Goldstone AP, Mercer JG, Gunn I, Moar KM, Edwards CMB, Rossi M, Howard JK, Rasheed S, Turton MD, Small C, 1997. Leptin interacts with glucagon-like peptide-1 neurons to reduce food intake and body weight in rodents. FEBS Lett 415, 134–138. [DOI] [PubMed] [Google Scholar]
- Gomez E, Pritchard C, Herbert TP, 2002. cAMP-dependent protein kinase and Ca2+ influx through L-type voltage-gated calcium channels mediate Raf-independent activation of extracellular regulated kinase in response to glucagon-like peptide-1 in pancreatic β-cells. J. Biol. Chem 277, 48146–48151. [DOI] [PubMed] [Google Scholar]
- Gorboulev V, Schürmann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard R, 2012. Na+-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 61, 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Erreger K, Galli A, Stanwood GD, 2013. GLP-1 analog attenuates cocaine reward. Mol. Psychiatry 18, 961–962. 10.1038/mp.2012.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano MP, Hey PJ, Borkowski D, Chicchi GG, Strader CD, 1993. Cloning and functional expression of a human glucagon-like peptide-1 receptor. Biochem. Biophys. Res. Commun 196, 141–146. [DOI] [PubMed] [Google Scholar]
- Gribble FM, Diakogiannaki E, Reimann F, 2016. Gut hormone regulation and secretion via FFA1 and FFA4, in: Free Fatty Acid Receptors. Springer, pp. 181–203. [DOI] [PubMed] [Google Scholar]
- Gribble FM, Reimann F, 2017. Signalling in the gut endocrine axis. Physiol. Behav 176, 183–188. [DOI] [PubMed] [Google Scholar]
- Gromada J, Dissing S, Bokvist K, Renström E, Frøkjær-Jensen J, Wulff BS, Rorsman P, 1995. Glucagon-like peptide I increases cytoplasmic calcium in insulin-secreting βTC3-cells by enhancement of intracellular calcium mobilization. Diabetes 44, 767–774. [DOI] [PubMed] [Google Scholar]
- Grunddal KV, Ratner CF, Svendsen B, Sommer F, Engelstoft MS, Madsen AN, Pedersen J, Nøhr MK, Egerod KL, Nawrocki AR, 2016. Neurotensin is coexpressed, coreleased, and acts together with GLP-1 and PYY in enteroendocrine control of metabolism. Endocrinology 157, 176–194. [DOI] [PubMed] [Google Scholar]
- Gunn I, O’Shea D, Turton MD, Beak SA, Bloom SR, 1996. Central glucagon-like peptide-I in the control of feeding 24, 581–584. [DOI] [PubMed] [Google Scholar]
- Gutzwiller JP, Göke B, Drewe J, Hildebrand P, Ketterer S, Handschin D, Winterhalder R, Conen D, Beglinger C, 1999. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut 44, 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnal A, Zharikov A, Polston JE, Fields MR, Tomasko J, Rogers AM, Volkow ND, Thanos PK, 2012. Alcohol reward is increased after Roux-en-Y gastric bypass in dietary obese rats with differential effects following ghrelin antagonism. PLoS One 7, e49121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen EN, Tamboli RA, Isbell JM, Saliba J, Dunn JP, Marks-Shulman PA, Abumrad NN, 2011. Role of the foregut in the early improvement in glucose tolerance and insulin sensitivity following Roux-en-Y gastric bypass surgery. Am. J. Physiol. Liver Physiol 300, G795–G802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski C, Huhle T, Gossrau R, Reutter W, 1988. Direct evidence for the binding of rat liver DPP IV to collagen in vitro. Exp. Cell Res 178, 64–72. [DOI] [PubMed] [Google Scholar]
- Harasta AE, Power JM, von Jonquieres G, Karl T, Drucker DJ, Housley GD, Schneider M, Klugmann M, 2015. Septal Glucagon-Like Peptide 1 Receptor Expression Determines Suppression of Cocaine-Induced Behavior. Neuropsychopharmacology 40, 1969–1978. 10.1038/npp.2015.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, De Jonghe BC, Kanoski SE, Grill HJ, Bence KK, 2011. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab. 13, 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Kahles F, Rattik S, Nairz M, McAlpine CS, Anzai A, Selgrade D, Fenn AM, Chan CT, Mindur JE, 2019. Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease. Nature 566, 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich G, Gros P, Habener JF, 1984. Glucagon gene sequence. Four of six exons encode separate functional domains of rat pre-proglucagon. J. Biol. Chem 259, 14082–14087. [PubMed] [Google Scholar]
- Heppner KM, Baquero AF, Bennett CM, Lindsley SR, Kirigiti MA, Bennett B, Bosch MA, Mercer AJ, Rønnekleiv OK, True C, 2017. GLP-1R signaling directly activates arcuate nucleus kisspeptin action in brain slices but does not rescue luteinizing hormone inhibition in ovariectomized mice during negative energy balance. eNeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman GA, Bergman A, Stevens C, Kotey P, Yi B, Zhao P, Dietrich B, Golor G, Schrodter A, Keymeulen B, 2006. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J. Clin. Endocrinol. Metab 91, 4612–4619. [DOI] [PubMed] [Google Scholar]
- Hernandez NS, Ige KY, Mietlicki-Baase EG, Molina-Castro GC, Turner CA, Hayes MR, Schmidt HD, 2018. Glucagon-like peptide-1 receptor activation in the ventral tegmental area attenuates cocaine seeking in rats. Neuropsychopharmacology 43, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez NS, O’donovan B, Ortinski PI, Schmidt HD, 2017. Activation of glucagon-like peptide-1 receptors in the nucleus accumbens attenuates cocaine seeking in rats. Addict. Biol 24, 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann C, Göke R, Richter G, Fehmann H-C, Arnold R, Göke B, 1995. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion 56, 117–126. [DOI] [PubMed] [Google Scholar]
- Heusner CL, Hnasko TS, Szczypka MS, Liu Y, During MJ, Palmiter RD, 2003. Viral restoration of dopamine to the nucleus accumbens is sufficient to induce a locomotor response to amphetamine. Brain Res. 980, 266–274. [DOI] [PubMed] [Google Scholar]
- Heymann E, Mentlein R, Nausch I, Erlanson-Albertsson C, Yoshimoto T, Feller AC, 1985. Processing of pro-colipase and trypsinogen by pancreatic dipeptidyl peptidase IV. Biomed. Biochim. Acta 45, 575–584. [PubMed] [Google Scholar]
- Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G, 2005. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med 11, 90–94. [DOI] [PubMed] [Google Scholar]
- Hisadome K, Reimann F, Gribble FM, Trapp S, 2011. CCK Stimulation of GLP-1 Neurons Involves α1-Adrenoceptor–Mediated Increase in Glutamatergic Synaptic Inputs. Diabetes 60, 2701–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisadome K, Reimann F, Gribble FM, Trapp S, 2010. Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: electrical properties of glucagon-like Peptide 1 neurons. Diabetes 59, 1890–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjøllund KR, Deacon CF, Holst JJ, 2011. Dipeptidyl peptidase-4 inhibition increases portal concentrations of intact glucagon-like peptide-1 (GLP-1) to a greater extent than peripheral concentrations in anaesthetised pigs. Diabetologia 54, 2206–2208. [DOI] [PubMed] [Google Scholar]
- Hollenstein K, Kean J, Bortolato A, Cheng RKY, Doré AS, Jazayeri A, Cooke RM, Weir M, Marshall FH, 2013. Structure of class B GPCR corticotropin-releasing factor receptor 1. Nature 499, 438–443. [DOI] [PubMed] [Google Scholar]
- Holst JJ, 2007. The physiology of glucagon-like peptide 1. Physiol. Rev 87, 1409–1439. [DOI] [PubMed] [Google Scholar]
- Holst JJ, Bersani M, Johnsen AH, Kofod H, Hartmann B, Orskov C, 1994. Proglucagon processing in porcine and human pancreas. J. Biol. Chem 269, 18827–18833. [PubMed] [Google Scholar]
- Holt MK, Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S, 2017. Serotonergic modulation of the activity of GLP-1 producing neurons in the nucleus of the solitary tract in mouse. Mol. Metab 6, 909–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Leech CA, Habener JF, 1995. Activation of a cAMP-regulated Ca-Signaling Pathway in Pancreatic β-Cells by the Insulinotropic Hormone Glucagon-like Peptide-1. J. Biol. Chem 270, 17749–17757. [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Leech CA, Heller RS, Castonguay M, Habener JF, 1999. cAMP-dependent Mobilization of Intracellular Ca2+ Stores by Activation of Ryanodine Receptors in Pancreatic β-Cells A Ca2+ signaling system stimulated by the insulinotropic hormone glucagon-like peptide-1-(7–37). J. Biol. Chem 274, 14147–14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz IV IV GG, Kiihtreiber WM, Habener JF, 1993. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1 (7–37). Nature 361, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoosein NM, Gurd RS, 1984. Human glucagon-like peptides 1 and 2 activate rat brain adenylate cyclase. FEBS Lett 178, 83–86. [DOI] [PubMed] [Google Scholar]
- Hopsu-Havu VK, Glenner GG, 1966. A new dipeptide naphthylamidase hydrolyzing glycyl-prolyl-β-naphthylamide. Histochem. Cell Biol 7, 197–201. [DOI] [PubMed] [Google Scholar]
- Hsu TM, Hahn JD, Konanur VR, Lam A, Kanoski SE, 2015. Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology 40, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, Marks-Shulman PA, Abumrad NN, 2010. The importance of caloric restriction in the early improvements in insulin sensitivity following Roux-en-Y gastric bypass surgery. Diabetes Care 33, 1438–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, 2018. GLP-1 signaling and alcohol-mediated behaviors; preclinical and clinical evidence. Neuropharmacology 136, 343–349. [DOI] [PubMed] [Google Scholar]
- Jin S-LL, Han VK, Simmons JG, Towle AC, Lauder JM, Lund PK, 1988. Distribution of glucagonlike peptide I (GLP-I), glucagon, and glicentin in the rat brain: an immunocytochemical study. J. Comp. Neurol 271, 519–532. 10.1002/cne.902710405 [DOI] [PubMed] [Google Scholar]
- Jorgensen R, Kubale V, Vrecl M, Schwartz TW, Elling CE, 2007. Oxyntomodulin differentially affects glucagon-like peptide-1 receptor β-arrestin recruitment and signaling through Gα. J. Pharmacol. Exp. Ther 322, 148–154. [DOI] [PubMed] [Google Scholar]
- Jorgensen R, Martini L, Schwartz TW, Elling CE, 2005. Characterization of glucagon-like peptide-1 receptor β-arrestin 2 interaction: a high-affinity receptor phenotype. Mol. Endocrinol 19, 812–823. [DOI] [PubMed] [Google Scholar]
- Kakei M, Yada T, Nakagawa A, Nakabayashi H, 2002. Glucagon-like peptide-1 evokes action potentials and increases cytosolic Ca 2+ in rat nodose ganglion neurons. Auton. Neurosci 102, 39–44. [DOI] [PubMed] [Google Scholar]
- Kang G, Chepurny OG, Malester B, Rindler MJ, Rehmann H, Bos JL, Schwede F, Coetzee WA, Holz GG, 2006. cAMP sensor Epac as a determinant of ATP-sensitive potassium channel activity in human pancreatic β cells and rat INS-1 cells. J. Physiol 573, 595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V, Pan W, 2002. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J. Mol. Neurosci 18, 7–14. [DOI] [PubMed] [Google Scholar]
- Kato T, Iwase K, Nagatsu T, Sakakibara S, Fujita K, 1979. Comparison of X-prolyl dipeptidyl-aminopeptidase activity in human cerebrospinal fluid with that in serum. Cell. Mol. Life Sci 35, 20–21. [DOI] [PubMed] [Google Scholar]
- Kettmann U, Humbel B, Holzhausen H-J, 1992. Ultrastructural localization of dipeptidylpeptidase IV in the glomerulum of the rat kidney. Acta Histochem. 92, 225–227. [DOI] [PubMed] [Google Scholar]
- Khoo S, Griffen SC, Xia Y, Baer RJ, German MS, Cobb MH, 2003. Regulation of insulin gene transcription by ERK1 and ERK2 in pancreatic β cells. J. Biol. Chem 278, 32969–32977. [DOI] [PubMed] [Google Scholar]
- Kieffer TJ, McIntosh CH, Pederson RA, 1995. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 136, 3585–3596. [DOI] [PubMed] [Google Scholar]
- Kimura R, Okouchi M, Fujioka H, Ichiyanagi A, Ryuge F, Mizuno T, Imaeda K, Okayama N, Kamiya Y, Asai K, 2009. Glucagon-like peptide-1 (GLP-1) protects against methylglyoxal-induced PC12 cell apoptosis through the PI3K/Akt/mTOR/GCLc/redox signaling pathway. Neuroscience 162, 1212–1219. [DOI] [PubMed] [Google Scholar]
- Kinzig KP, D’Alessio DA, Seeley RJ, 2002. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J. Neurosci 22, 10470–10476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli R, Setchell KDR, Kirby M, Myronovych A, Ryan KK, Ibrahim SH, Berger J, Smith K, Toure M, Woods SC, 2013. A surgical model in male obese rats uncovers protective effects of bile acids post-bariatric surgery. Endocrinology 154, 2341–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laferrère B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, Hart AB, Olivan B, 2007. Incretin levels and effect are markedly enhanced one month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 30, 1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerström MC, Schiöth HB, 2008. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov 7, 339–357. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Tang-Christensen M, Holst JJ, Ørskov C, 1997. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77, 257–270. [DOI] [PubMed] [Google Scholar]
- le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lönroth H, Fändriks L, Ghatei MA, Bloom SR, 2007. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann. Surg 246, 780–785. [DOI] [PubMed] [Google Scholar]
- Leech CA, Habener JF, 1997. Insulinotropic glucagon-like peptide-1-mediated activation of non-selective cation currents in insulinoma cells is mimicked by maitotoxin. J. Biol. Chem 272, 17987–17993. [DOI] [PubMed] [Google Scholar]
- Lester LB, Langeberg LK, Scott JD, 1997. Anchoring of protein kinase A facilitates hormone-mediated insulin secretion. Proc. Natl. Acad. Sci 94, 14942–14947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Navarrete J, Liang-Guallpa J, Lu C, Funderburk SC, Chang RB, Liberles SD, Olson DP, Krashes MJ, 2018. Defined Paraventricular Hypothalamic Populations Exhibit Differential Responses to Food Contingent on Caloric State. Cell Metab. 29, 681–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y-L, Khoshouei M, Radjainia M, Zhang Y, Glukhova A, Tarrasch J, Thal DM, Furness SGB, Christopoulos G, Coudrat T, 2017. Phase-plate cryo-EM structure of a class B GPCR–G-protein complex. Nature 546, 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light PE, Manning Fox JE, Riedel MJ, Wheeler MB, 2002. Glucagon-like peptide-1 inhibits pancreatic ATP-sensitive potassium channels via a protein kinase A-and ADP-dependent mechanism. Mol. Endocrinol 16, 2135–2144. [DOI] [PubMed] [Google Scholar]
- Lin HV, Efanov AM, Fang X, Beavers LS, Wang X, Wang J, Valcarcel ICG, Ma T, 2016. GPR142 controls tryptophan-induced insulin and incretin hormone secretion to improve glucose metabolism. PLoS One 11, e0157298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Conde K, Zhang P, Lilascharoen V, Xu Z, Lim BK, Seeley RJ, Zhu JJ, Scott MM, Pang ZP, 2017. Enhanced AMPA receptor trafficking mediates the anorexigenic effect of endogenous glucagon-like peptide-1 in the paraventricular hypothalamus. Neuron 96, 897–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lojda Z, 1979. Studies on dipeptidyl (amino) peptidase IV (glycyl-proline naphthylamidase). Histochem. Cell Biol 59, 153–166. [DOI] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G, 2011. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science (80-. ) 333, 353–7. 10.1126/science.1204622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald PE, El-kholy W, Riedel MJ, Salapatek AMF, Light PE, Wheeler MB, 2002. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes 51, S434–S442. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Wellman PJ, 1998. PVN infusion of GLP-1-(7—36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am. J. Physiol. Integr. Comp. Physiol 274, R23–R29. [DOI] [PubMed] [Google Scholar]
- Meeran K, O’shea D, Edwards CMB, Turton MD, Heath MM, Gunn I, Abusnana S, Rossi M, Small CJ, Goldstone AP, 1999. Repeated Intracerebroventricular Administration of Glucagon-Like Peptide-1-(7–36) Amide or Exendin-(9–39) Alters Body Weight in the Rat. Endocrinology 140, 244–250. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P, 1999. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J. Comp. Neurol 403, 261–280. [DOI] [PubMed] [Google Scholar]
- Mietlicki-Baase EG, Ortinski PI, Reiner DJ, Sinon CG, McCutcheon JE, Pierce RC, Roitman MF, Hayes MR, 2014. Glucagon-like peptide-1 receptor activation in the nucleus accumbens core suppresses feeding by increasing glutamatergic AMPA/kainate signaling. J. Neurosci 34, 6985–6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Pierce RC, Hayes MR, 2013. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am. J. Physiol. Endocrinol. Metab 305, E1367–74. 10.1152/ajpendo.00413.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montrose-Rafizadeh C, Avdonin P, Garant MJ, Rodgers BD, Kole S, Yang H, Levine MA, Schwindinger W, Bernier M, 1999. Pancreatic Glucagon-Like Peptide-1 Receptor Couples to Multiple G Proteins and Activates Mitogen-Activated Protein Kinase Pathways in Chinese Hamster Ovary Cells 1. Endocrinology 140, 1132–1140. [DOI] [PubMed] [Google Scholar]
- Morínigo R, Moizé V, Musri M, Lacy AM, Navarro S, Marín JL, Delgado S, Casamitjana R, Vidal J, 2006. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J. Clin. Endocrinol. Metab 91, 1735–1740. [DOI] [PubMed] [Google Scholar]
- Moss CE, Glass LL, Diakogiannaki E, Pais R, Lenaghan C, Smith DM, Wedin M, Bohlooly-y M, Gribble FM, Reimann F, 2016. Lipid derivatives activate GPR119 and trigger GLP-1 secretion in primary murine L-cells. Peptides 77, 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousumi B, Teixeira J, Olivan B, Baani B, Arias S, Machineni S, PI-SUNYER FX, Scherer PE, Laferrere B, 2010. Weight loss and incretin responsiveness improve glucose control independently after gastric bypass surgery. J. Diabetes 2, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller TD, Clemmensen C, Finan B, DiMarchi RD, Tschöp MH, 2018. Anti-obesity therapy: from rainbow pills to polyagonists. Pharmacol. Rev 70, 712–746. [DOI] [PubMed] [Google Scholar]
- Mulvihill EE, Varin EM, Gladanac B, Campbell JE, Ussher JR, Baggio LL, Yusta B, Ayala J, Burmeister MA, Matthews D, 2017. Cellular Sites and Mechanisms Linking Reduction of Dipeptidyl Peptidase-4 Activity to Control of Incretin Hormone Action and Glucose Homeostasis. Cell Metab. 25, 152–165. [DOI] [PubMed] [Google Scholar]
- Nakazaki M, Crane A, Hu M, Seghers V, Ullrich S, Aguilar-Bryan L, Bryan J, 2002. cAMP-activated protein kinase-independent potentiation of insulin secretion by cAMP is impaired in SUR1 null islets. Diabetes 51, 3440–3449. [DOI] [PubMed] [Google Scholar]
- Neumann J-M, Couvineau A, Murail S, Lacapere J-J, Jamin N, Laburthe M, 2008. Class-B GPCR activation: is ligand helix-capping the key? Trends Biochem. Sci 33, 314–319. [DOI] [PubMed] [Google Scholar]
- Nunez DJ, Bush MA, Collins DA, McMullen SL, Gillmor D, Apseloff G, Atiee G, Corsino L, Morrow L, Feldman PL, 2014. Gut hormone pharmacology of a novel GPR119 agonist (GSK1292263), metformin, and sitagliptin in type 2 diabetes mellitus: results from two randomized studies. PLoS One 9, e92494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørskov C, 1992. Glucagon-like peptide-1, a new hormone of the entero-insular axis. Diabetologia 35, 701–711. [PubMed] [Google Scholar]
- Ørskov C, Holst JJ, Poulsen SS, Kirkegaard P, 1987. Pancreatic and intestinal processing of proglucagon in man. Diabetologia 30, 874–881. 10.1007/BF00274797 [DOI] [PubMed] [Google Scholar]
- Ørskov C, Rabenhøj L, Wettergren A, Kofod H, Holst JJ, 1994. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes 43, 535–539. [DOI] [PubMed] [Google Scholar]
- Ørskov C, Wettergren A, Holst JJ, 1993. Biological effects and metabolic rates of glucagonlike peptide-1 7–36 amide and glucagonlike peptide-1 7–37 in healthy subjects are indistinguishable. Diabetes 42, 658–661. [DOI] [PubMed] [Google Scholar]
- Pabreja K, Mohd MA, Koole C, Wootten D, Furness SGB, 2014. Molecular mechanisms underlying physiological and receptor pleiotropic effects mediated by GLP-1R activation. Br. J. Pharmacol 171, 1114–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker HE, Adriaenssens A, Rogers G, Richards P, Koepsell H, Reimann F, Gribble FM, 2012. Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia 55, 2445–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti M, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, Badman MK, Maratos-Flier E, Mun EC, Pihlajamaki J, 2009. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity 17, 1671–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkney J, Fox T, Ranganath L, 2010. Selecting GLP-1 agonists in the management of type 2 diabetes: differential pharmacology and therapeutic benefits of liraglutide and exenatide. Ther. Clin. Risk Manag 6, 401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D, Bekker JHR, Ghatei MA, Bloom SR, Walters JRF, 2012. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology 153, 3613–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quoyer J, Longuet C, Broca C, Linck N, Costes S, Varin E, Bockaert J, Bertrand G, Dalle S, 2010. GLP-1 mediates antiapoptotic effect by phosphorylating Bad through a β-arrestin 1-mediated ERK1/2 activation in pancreatic β-cells. J. Biol. Chem 285, 1989–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy IA, Pino JA, Weikop P, Osses N, Sørensen G, Bering T, Valle C, Bluett RJ, Erreger K, Wortwein G, Reyes JG, Graham D, Stanwood GD, Hackett TA, Patel S, Fink-Jensen A, Torres GE, Galli A, 2016. Glucagon-like peptide 1 receptor activation regulates cocaine actions and dopamine homeostasis in the lateral septum by decreasing arachidonic acid levels. Transl. Psychiatry 6, e809 10.1038/tp.2016.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM, 2008. Glucose sensing in L cells: a primary cell study. Cell Metab. 8, 532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann F, Williams L, da Silva Xavier G, Rutter GA, Gribble FM, 2004. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia 47, 1592–1601. [DOI] [PubMed] [Google Scholar]
- Reiner DJ, Mietlicki-Baase EG, McGrath LE, Zimmer DJ, Bence KK, Sousa GL, Konanur VR, Krawczyk J, Burk DH, Kanoski SE, Hermann GE, Rogers RC, Hayes MR, 2016. Astrocytes Regulate GLP-1 Receptor-Mediated Effects on Energy Balance. J. Neurosci 36, 3531–3540. 10.1523/JNEUROSCI.3579-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JE, Anderberg RH, Göteson A, Gribble FM, Reimann F, Skibicka KP, 2015. Activation of the GLP-1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PLoS One 10, e0119034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JE, Farkas I, Anesten F, Anderberg RH, Dickson SL, Gribble FM, Reimann F, Jansson J-O, Liposits Z, Skibicka KP, 2014. GLP-1 receptor stimulation of the lateral parabrachial nucleus reduces food intake: neuroanatomical, electrophysiological, and behavioral evidence. Endocrinology 155, 4356–4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L, 1999a. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am. J. Physiol. Integr. Comp. Physiol 277, R582–R590. [DOI] [PubMed] [Google Scholar]
- Rinaman L, 1999b. A functional role for central glucagon-like peptide-1 receptors in lithium chloride-induced anorexia. Am. J. Physiol. Integr. Comp. Physiol 277, R1537–R1540. [DOI] [PubMed] [Google Scholar]
- Rocca AS, Brubaker PL, 1999. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion 1. Endocrinology 140, 1687–1694. [DOI] [PubMed] [Google Scholar]
- Roed SN, Wismann P, Underwood CR, Kulahin N, Iversen H, Cappelen KA, Schäffer L, Lehtonen J, Hecksher-Soerensen J, Secher A, 2014. Real-time trafficking and signaling of the glucagon-like peptide-1 receptor. Mol. Cell. Endocrinol 382, 938–949. [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Balas B, Charbonnel B, Bolli GB, Boldrin M, Ratner R, Balena R, Group, T. 2 S., 2013. The fate of taspoglutide, a weekly GLP-1 receptor agonist, versus twice-daily exenatide for type 2 diabetes. Diabetes Care 36, 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth JD, Coffey T, Jodka CM, Maier H, Athanacio JR, Mack CM, Weyer C, Parkes DG, 2007. Combination therapy with amylin and peptide YY [3–36] in obese rodents: anorexigenic synergy and weight loss additivity. Endocrinology 148, 6054–6061. [DOI] [PubMed] [Google Scholar]
- Rowlands J, Carlessi R, Newsholme P, Heng J, 2018. Pleiotropic Effects of GLP-1 and analogs on cell signaling, metabolism and function. Front. Endocrinol. (Lausanne) 9, 672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi M, Prigeon RL, D’Alessio DA, 2011. Gastric Bypass Surgery Enhances Glucagon-Like Peptide 1–Stimulated Postprandial Insulin Secretion in Humans. Diabetes 60, 2308–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval DA, Bagnol D, Woods SC, D’alessio DA, Seeley RJ, 2008. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes 57, 2046–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor GC, Aston-Jones GS, 2012. A Septal-Hypothalamic Pathway Drives Orexin Neurons, Which Is Necessary for Conditioned Cocaine Preference. J. Neurosci 32, 4623–4631. 10.1523/jneurosci.4561-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Mietlicki-Baase EG, Ige KY, Maurer JJ, Reiner DJ, Zimmer DJ, Van Nest DS, Guercio LA, Wimmer ME, Olivos DR, De Jonghe BC, Hayes MR, 2016. Glucagon-Like Peptide-1 Receptor Activation in the Ventral Tegmental Area Decreases the Reinforcing Efficacy of Cocaine. Neuropsychopharmacology 41, 1917–1928. 10.1038/npp.2015.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secher A, Jelsing J, Baquero AF, Hecksher-Sørensen J, Cowley MA, Dalbøge LS, Hansen G, Grove KL, Pyke C, Raun K, 2014. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J. Clin. Invest 124, 4473–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley RJ, Blake K, Rushing PA, Benoit S, Eng J, Woods SC, D’Alessio D, 2000. The role of CNS glucagon-like peptide-1 (7–36) amide receptors in mediating the visceral illness effects of lithium chloride. J. Neurosci 20, 1616–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah NG, Marcinkiewicz M, Benjannet S, Gaspar L, Beaubien G, Mattei MG, Lazure C, Mbikay M, Chrétien M, 1991. Cloning and primary sequence of a mouse candidate prohormone convertase PC1 homologous to PC2, Furin, and Kex2: distinct chromosomal localization and messenger RNA distribution in brain and pituitary compared to PC2. Mol. Endocrinol 5, 111–122. 10.1210/mend-5-1-111 [DOI] [PubMed] [Google Scholar]
- Seino S, Shibasaki T, 2005. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol. Rev 85, 1303–1342. [DOI] [PubMed] [Google Scholar]
- Shimizu I, Hirota M, Ohboshi C, SHIMA K, 1987. Identification and localization of glucagon-like peptide-1 and its receptor in rat brain. Endocrinology 121, 1076–1082. [DOI] [PubMed] [Google Scholar]
- Shiota C, Larsson O, Shelton KD, Shiota M, Efanov AM, Høy M, Lindner J, Kooptiwut S, Juntti-Berggren L, Gromada J, 2002. Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose. J. Biol. Chem 277, 37176–37183. [DOI] [PubMed] [Google Scholar]
- Shirazi RH, Dickson SL, Skibicka KP, 2013. Gut Peptide GLP-1 and Its Analogue, Exendin-4, Decrease Alcohol Intake and Reward. PLoS One 8, e61965 10.1371/journal.pone.0061965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens SP, Avruch AS, LaMendola J, Chan SJ, Steiner DF, 1991. Identification of a cDNA encoding a second putative prohormone convertase related to PC2 in AtT20 cells and islets of Langerhans. Proc. Natl. Acad. Sci. U. S. A 88, 340–344. 10.1073/pnas.88.2.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda N, Imamura T, Yoshizaki T, Babendure JL, Lu J-C, Olefsky JM, 2008. β-arrestin-1 mediates glucagon-like peptide-1 signaling to insulin secretion in cultured pancreatic β cells. Proc. Natl. Acad. Sci 105, 6614–6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen G, Reddy IA, Weikop P, Graham DL, Stanwood GD, Wortwein G, Galli A, Fink-Jensen A, 2015. The glucagon-like peptide 1 (GLP-1) receptor agonist exendin-4 reduces cocaine self-administration in mice. Physiol. Behav 149, 262–268. 10.1016/j.physbeh.2015.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert RE, Peterli R, Keller S, Meyer-Gerspach AC, Drewe J, Peters T, Beglinger C, 2013. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity 21, E660–E668. [DOI] [PubMed] [Google Scholar]
- Sternson SM, Eiselt A-K, 2017. Three pillars for the neural control of appetite. Annu. Rev. Physiol 79, 401–423. [DOI] [PubMed] [Google Scholar]
- Su Z, Alhadeff AL, Betley JN, 2017. Nutritive, post-ingestive signals are the primary regulators of AgRP neuron activity. Cell Rep. 21, 2724–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchankova P, Yan J, Schwandt ML, Stangl BL, Caparelli EC, Momenan R, Jerlhag E, Engel JA, Hodgkinson CA, Egli M, 2015. The glucagon-like peptide-1 receptor as a potential treatment target in alcohol use disorder: evidence from human genetic association studies and a mouse model of alcohol dependence. Transl. Psychiatry 5, e583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick JC, Alhadeff AL, Grill HJ, Urrea P, Lee SM, Roh H, Baird J-P, 2015. Parabrachial nucleus contributions to glucagon-like peptide-1 receptor agonist-induced hypophagia. Neuropsychopharmacology 40, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syme CA, Zhang L, Bisello A, 2006. Caveolin-1 regulates cellular trafficking and function of the glucagon-like peptide 1 receptor. Mol. Endocrinol 20, 3400–3411. [DOI] [PubMed] [Google Scholar]
- Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL, 2005. Peripheral exendin-4 and peptide YY3–36 synergistically reduce food intake through different mechanisms in mice. Endocrinology 146, 3748–3756. [DOI] [PubMed] [Google Scholar]
- Tamboli RA, Breitman I, Marks-Shulman PA, Jabbour K, Melvin W, Williams B, Clements RH, Feurer ID, Abumrad NN, 2014. Early weight regain after gastric bypass does not affect insulin sensitivity but is associated with elevated ghrelin. Obesity 22, 1617–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M, Sheikh SP, 1996. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am. J. Physiol. Integr. Comp. Physiol 271, R848–R856. [DOI] [PubMed] [Google Scholar]
- ter Haar E, Koth CM, Abdul-Manan N, Swenson L, Coll JT, Lippke JA, Lepre CA, Garcia-Guzman M, Moore JM, 2010. Crystal structure of the ectodomain complex of the CGRP receptor, a class-B GPCR, reveals the site of drug antagonism. Structure 18, 1083–1093. [DOI] [PubMed] [Google Scholar]
- Terrill SJ, Jackson CM, Greene HE, Lilly N, Maske CB, Vallejo S, Williams DL, 2016. Role of lateral septum glucagon-like peptide 1 receptors in food intake. Am. J. Physiol. Integr. Comp. Physiol 311, R124–R132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrill SJ, Maske CB, Williams DL, 2018. Endogenous GLP-1 in lateral septum contributes to stress-induced hypophagia. Physiol. Behav 192, 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Seeley RJ, D’Alessio D, Eng J, Bernstein IL, Woods SC, Van Dijk G, 1998. Central infusion of glucagon-like peptide-1-(7–36) amide (GLP-1) receptor antagonist attenuates lithium chloride-induced c-Fos induction in rat brainstem. Brain Res. 801, 164–170. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Van Dijk G, Campfield LA, Smith FJ, Burn P, Woods SC, Bernstein IL, Seeley RJ, 1997. Central infusion of GLP-1, but not leptin, produces conditioned taste aversions in rats. Am. J. Physiol. Integr. Comp. Physiol 272, R726–R730. [DOI] [PubMed] [Google Scholar]
- Thompson A, Kanamarlapudi V, 2015. Agonist-induced internalisation of the glucagon-like peptide-1 receptor is mediated by the Gα q pathway. Biochem. Pharmacol 93, 72–84. [DOI] [PubMed] [Google Scholar]
- Thorens B, 1992. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc. Natl. Acad. Sci 89, 8641–8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B, Porret A, Bühler L, Deng S-P, Morel P, Widmann C, 1993. Cloning and functional expression of the human islet GLP-1 receptor: demonstration that exendin-4 is an agonist and exendin-(9–39) an antagonist of the receptor. Diabetes 42, 1678–1682. [DOI] [PubMed] [Google Scholar]
- Tuesta LM, Chen Z, Duncan A, Fowler CD, Ishikawa M, Lee BR, Liu X-A, Lu Q, Cameron M, Hayes MR, 2017. GLP-1 acts on habenular avoidance circuits to control nicotine intake. Nat. Neurosci 20, 708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turton MD, Shea DO, Gunn I, Beak SA, 1996. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379, 69. [DOI] [PubMed] [Google Scholar]
- Van Dijk G, Thiele TE, 1999. Glucagon-like peptide-1 (7–36) amide: a central regulator of satiety and interoceptive stress. Neuropeptides 33, 406–414. [DOI] [PubMed] [Google Scholar]
- Van Dijk G, Thiele TE, Donahey JC, Campfield LA, Smith FJ, Burn P, Bernstein IL, Woods SC, Seeley RJ, 1996. Central infusions of leptin and GLP-1-(7–36) amide differentially stimulate c-FLI in the rat brain. Am. J. Physiol. Integr. Comp. Physiol 271, R1096–R1100. [DOI] [PubMed] [Google Scholar]
- van Eyll B, Lankat-Buttgereit B, Bode HP, Göke R, Göke B, 1994. Signal transduction of the GLP-1-receptor cloned from a human insulinoma. FEBS Lett. 348, 7–13. [DOI] [PubMed] [Google Scholar]
- Vázquez P, Roncero I, Blázquez E, Alvarez E, 2005. The cytoplasmic domain close to the transmembrane region of the glucagon-like peptide-1 receptor contains sequence elements that regulate agonist-dependent internalisation. J. Endocrinol 186, 221–231. [DOI] [PubMed] [Google Scholar]
- Vetter ML, Cardillo S, Rickels MR, Iqbal N, 2009. Narrative review: effect of bariatric surgery on type 2 diabetes mellitus. Ann. Intern. Med 150, 94–103. [DOI] [PubMed] [Google Scholar]
- Vidal J, Nicolau J, Romero F, Casamitjana R, Momblan D, Conget I, Morínigo R, Lacy AM, 2009. Long-term effects of Roux-en-Y gastric bypass surgery on plasma glucagon-like peptide-1 and islet function in morbidly obese subjects. J. Clin. Endocrinol. Metab 94, 884–891. [DOI] [PubMed] [Google Scholar]
- Vrang N, Phifer CB, Corkern MM, Berthoud H-R, 2003. Gastric distension induces c-Fos in medullary GLP1/2 containing neurons. Am. J. Physiol. Integr. Comp. Physiol 285, R470–478. [DOI] [PubMed] [Google Scholar]
- Wang X-F, Liu J-J, Xia J, Liu J, Mirabella V, Pang ZP, 2015. Endogenous glucagon-like peptide-1 suppresses high-fat food intake by reducing synaptic drive onto mesolimbic dopamine neurons. Cell Rep. 12, 726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettergren A, Pridal L, Wøjdemann M, Holst JJ, 1998. Amidated and non-amidated glucagon-like peptide-1 (GLP-1): non-pancreatic effects (cephalic phase acid secretion) and stability in plasma in humans. Regul. Pept 77, 83–87. [DOI] [PubMed] [Google Scholar]
- Widmann C, Dolci W, Thorens B, 1997. Internalization and homologous desensitization of the GLP-1 receptor depend on phosphorylation of the receptor carboxyl tail at the same three sites. Mol. Endocrinol 11, 1094–1102. [DOI] [PubMed] [Google Scholar]
- Widmann C, Dolci W, Thorens B, 1996a. Desensitization and phosphorylation of the glucagon-like peptide-1 (GLP-1) receptor by GLP-1 and 4-phorbol 12-myristate 13-acetate. Mol. Endocrinol 10, 62–75. [DOI] [PubMed] [Google Scholar]
- Widmann C, Dolci W, Thorens B, 1996b. Heterologous desensitization of the glucagon-like peptide-1 receptor by phorbol esters requires phosphorylation of the cytoplasmic tail at four different sites. J. Biol. Chem 271, 19957–19963. [DOI] [PubMed] [Google Scholar]
- Widmann C, Dolci W, Thorens B, 1995. Agonist-induced internalization and recycling of the glucagon-like peptide-1 receptor in transfected fibroblasts and in insulinomas. Biochem. J 310, 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, 2009. Minireview: finding the sweet spot: peripheral versus central glucagon-like peptide 1 action in feeding and glucose homeostasis. Endocrinology 150, 2997–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootten D, Reynolds CA, Smith KJ, Mobarec JC, Koole C, Savage EE, Pabreja K, Simms J, Sridhar R, Furness SGB, 2016. The extracellular surface of the GLP-1 receptor is a molecular trigger for biased agonism. Cell 165, 1632–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootten D, Simms J, Miller LJ, Christopoulos A, Sexton PM, 2013. Polar transmembrane interactions drive formation of ligand-specific and signal pathway-biased family BG protein-coupled receptor conformations. Proc. Natl. Acad. Sci 110, 5211–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yada T, Itoh K, Nakata M, 1993. Glucagon-like peptide-1-(7–36) amide and a rise in cyclic adenosine 3’, 5’-monophosphate increase cytosolic free Ca2+ in rat pancreatic beta-cells by enhancing Ca2+ channel activity. Endocrinology 133, 1685–1692. [DOI] [PubMed] [Google Scholar]
- Yeğen BÇ, Bozkurt A, Coşkun T, Villanueva-Peñacarrillo ML, Ulusoy NB, 1997. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am. J. Physiol. Liver Physiol 273, G920–G927. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Sun B, Feng D, Hu H, Chu M, Qu Q, Tarrasch JT, Li S, Kobilka TS, Kobilka BK, 2017. Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature 546, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]


