Summary
Basal tumor propagating cells (TPCs) control squamous cell carcinoma (SCC) growth by self-renewing and differentiating into supra-basal SCC cells, which lack proliferative potential. While transcription factors such as Sox2 and Klf4 can drive these behaviors, their molecular roles and regulatory interactions with each other have remained elusive. Here we show PITX1 is specifically expressed in TPCs, where it co-localizes with SOX2 and TRP63, and determines cell fate in mouse and human SCC. Combining gene targeting with ChIP-seq and transcriptomic analyses reveals PITX1 cooperates with SOX2 and TRP63 to sustain a SCC-specific transcriptional feed-forward circuit that maintains TPC-renewal, while inhibiting KLF4 expression and preventing KLF4-dependent differentiation. Conversely, KLF4 represses PITX1, SOX2, and TRP63 expression to prevent TPC expansion. This bi-stable, multi-input network reveals a molecular framework that explains self-renewal, aberrant differentiation, and SCC growth in mice and humans, providing clues for developing differentiation-inducing therapeutic strategies.
Introduction
Balanced stem cell renewal and differentiation maintains tissue homeostasis, while their de-regulation enables tumor formation (Meacham and Morrison, 2013). Skin epithelium emerged as a powerful model in which lineage relationships between normal and malignant progenitor cells as well as their differentiated progeny have been determined (Blanpain and Fuchs, 2014). Still, the mechanisms governing self-renewal and squamous differentiation in homeostasis and carcinogenesis remain elusive.
Skin epithelium is comprised of epidermal, hair follicle and sebaceous lineages, which are specified during embryonic development and sustained by stem- or progenitor cells throughout life (Benitah and Frye, 2012; Jensen et al., 2009). While all skin epithelial cells express the squamous epithelial fate determinant TRP63 (Botchkarev and Flores, 2014; Truong and Khavari, 2014), each lineage requires additional transcription factors, which govern cell type specific programs (Frye and Benitah, 2012). Although epidermal and hair follicle lineages do not interconvert in homeostasis (Levy et al., 2007), epigenetic constraints erode upon wounding and tumor formation (Ge et al., 2017) as cells cross lineage boundaries to aide tissue regeneration (Ito et al., 2005; Tumbar et al., 2004).
Squamous cell carcinomas (SCCs) are hierarchically organized tumors, which originate in epidermal and hair follicle lineages (Sánchez-Danés and Blanpain, 2018). They are sustained by tumor propagating progenitor cell’s (TPCs) located within the basal layer where they express α6β4 and β1 integrin (Schober and Fuchs, 2011) and EPCAM (Lapouge et al., 2012). TPCs can self-renew and differentiate into supra-basal SCC cells without proliferative potential (Driessens et al., 2012; Pierce and Wallace, 1971) by mechanisms that are still unknown. However, transcriptomic analyses identified characteristic gene expression signatures, chromatin accessibility profiles and Histone H3-K27 acetylation (H3K27Ac) patterns which distinguish TPCs from normal epidermal progenitor cells (EPCs) and hair follicle stem cells (HFSCs) (Adam et al., 2015; Ge et al., 2017; Latil et al., 2017; Schober and Fuchs, 2011; Siegle et al., 2014; Yang et al., 2015). These differences suggest squamous carcinogenesis might select for stereotypical transcriptional programs, which compromise lineage commitment and thereby cause cell fate transformation, hyper-proliferation and cell survival. Still, the transcriptional network that governs TPC-renewal and squamous differentiation remains elusive.
Transcriptomic analyses uncovered de novo SRY-box 2 (SOX2) and Paired like homeodomain 1 (PITX1) expression in TPCs (Boumahdi et al., 2014; Schober and Fuchs, 2011; Siegle et al., 2014). Although Sox2 is epigenetically repressed in normal skin epithelium and dispensable for epidermal development and homeostasis (Arnold et al., 2011), it is critical for squamous carcinogenesis in mice and humans (Boumahdi et al., 2014; Siegle et al., 2014). Still, how SOX2 becomes expressed in SCCs and restricted to TPCs where it supports cell survival and clonal expansion remains unknown.
Here, we identify PITX1 as regulator of squamous carcinogenesis in mice and humans. We detect PITX1 in nuclei of TPCs, but not their differentiated progeny. PITX1 binds to and cooperates with SOX2 and TRP63 as they establish a tumor specific feed-forward circuit to sustain their own transcription and TPC renewal, as they repress Klf4 transcription to blunt squamous differentiation. Conversely, KLF4 competes with PITX1 on bi-stable transcriptional network motifs to inhibit Pitx1, Sox2 and Trp63 transcription, restricting TPC-renewal to the basal SCC layer. This bi-stable transcriptional network supports clonal expansion and aberrant differentiation characteristic of SCCs. Our research uncovers a transcriptional network, which is logically similar, but molecularly distinct from embryonic stem cells (Young, 2011). The identification of this cancer specific mechanism defines SCCs as ectopic growths rather than simply hyper-proliferative tissue, and it suggests that tumors could be effectively targeted with differentiation therapies which minimally affect skin epithelial homeostasis.
Results
PITX1 expression marks tumor propagating SCC cells
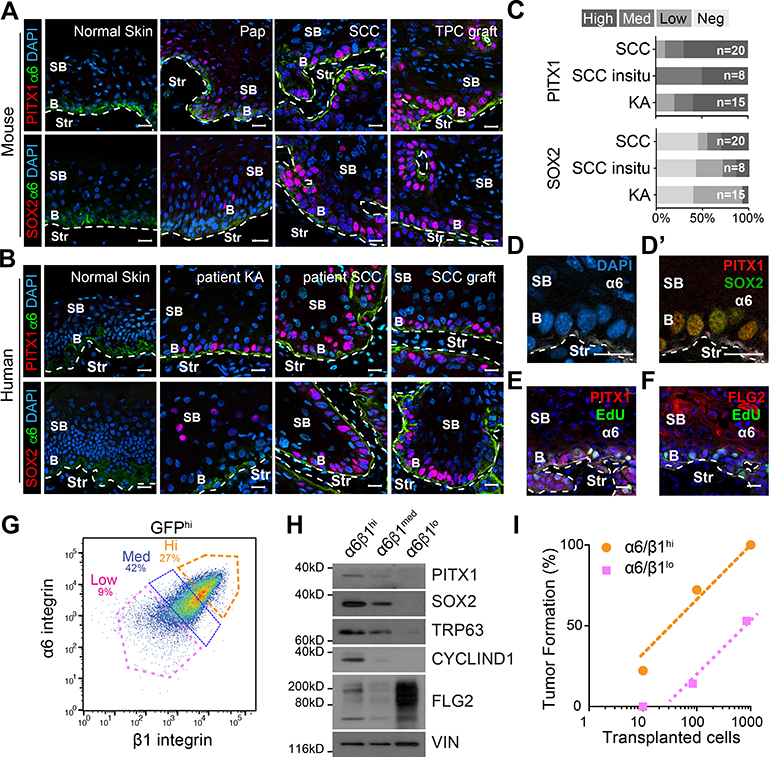
To determine the PITX1 and SOX2 expression pattern in cutaneous SCCs, we stained fresh frozen mouse and human skins and carcinomas with anti-PITX1 and anti-SOX2 antibodies (Figure 1A–C). PITX1 and SOX2 were not detectable in normal skin epithelia, but were spotted in DMBA initiated benign papillomas (Pap), well- and poorly- differentiated SCCs, and orthotopic SCC grafts (Figure 1A). A strikingly similar pattern was seen in patient specimens (Figure 1B, S1A), where nuclear PITX1 staining was commonly identified in basal Keratoacanthomas (KA), SCC in-situ, and malignant SCCs, as well as secondary SCC xenografts. Direct comparisons suggest PITX1 is consistently expressed at all stages of squamous tumorigenesis, preceding SOX2 in benign papillomas and early SCCs, before both proteins become broadly expressed in basal cells of advanced SCCs (Figure 1C). Western blotting (WB) also detected PITX1 and SOX2 in total protein lysates of mouse and human cutaneous (Figure S1B,C) and head and neck (HN) SCCs as well as their cell lines (Figure S1D–F), but not whole skins. Closer inspection showed PITX1 co-localizes with SOX2 in α6β4 expressing basal SCC cells (Figure 1D), which incorporated EdU in a short pulse (Figure 1E), unlike supra-basal, post-mitotic and Filaggrin (FLG2) positive SCC cells (Figure 1F).
Figure 1: PITX1 is de novo expressed in basal tumor propagating SCC cells.
(A,B) Confocal micrographs of mouse (A) and human (B) tissue sections. Basal (b), supra-basal (sb), and stromal (Str) cells in papillomas (Pap), primary SCCs (SCC) from mice and patients, patient keratoacanthoma (KA), and TPC grafts. Scale bars = 50μm. (C) Stacked bar graphs illustrate the prevalence of PITX1 and SOX2 expression in n patient specimens. (D-F) Confocal microscopy shows PITX1 colocalizes with SOX2 (D’) and EdU (E,F) in basal (b) but not Filaggrin (F) expressing supra-basal (sb) SCC cells. Scale bars = 50μm. (G) FACS separation of α6- and β1-integrin (CD49f and CD29) high (Hi), medium (Med) and low (lo) fractions of GFP marked SCC parenchyma. (H) Western blotting of parenchymal fractions with SOX2, PITX1, TRP63, and CYCLIN D1, and FLG2 antibodies. (I) Limited-dilution transplantation experiments of SCC fractions. (n=18 transplants; 3 independent SCCs). See also Figure S1.
To directly compare the tumor forming potential of basal and supra-basal SCC cells, we permanently labeled the parenchymal lineage with a fluorescent tracer (H2BGFP), dissociated SCCs into single cell suspensions, gated on GFP, and separated the tumor parenchyma into three fractions dependent on their α6 and β1 integrin, as well as Transferrin receptor (CD71) expression (Figure 1G, S1G). CD71 labels proliferative cells in epidermis and SCCs and helps to distinguish proliferative progenitor cells from post-mitotic and terminally differentiated SCC cells (Brown et al., 2017; Tani et al., 2000). Western blotting confirmed SOX2, PITX1, TRP63 and CyclinD1 expression in the GFPhiα6hiβ1hi basal cell fraction, while their loss along with elevated FLG2 expression validated the GFPhiα6loβ1lo fraction as supra-basal, post-mitotic and differentiated SCC cells (Figure 1H). Limited dilution and orthotopic transplantation of these two fractions indicate a higher tumor initiating potential of PITX1 and SOX2 expressing basal, compared to supra-basal SCC cells (Figure 1I), as previously suggested (Boumahdi et al., 2014; Driessens et al., 2012; Pierce and Wallace, 1971).
PITX1 is critical for SCC initiation and growth
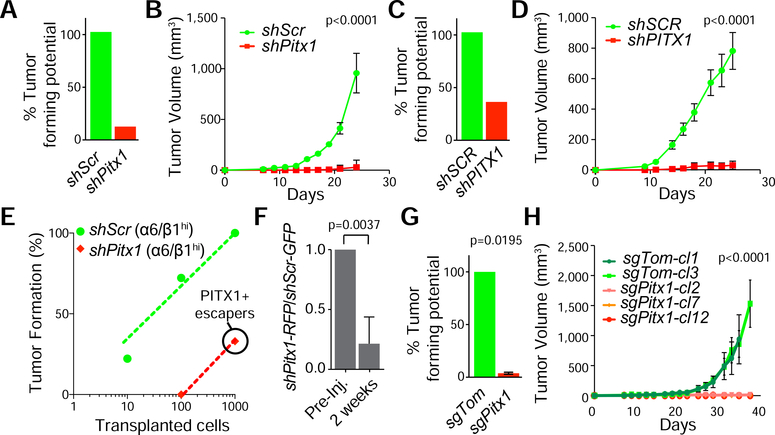
To determine if PITX1 is critical for squamous carcinogenesis, we isolated α6hiβ1hi cells from murine SCCs, expanded them briefly on 3T3 feeder layers, and transduced them with lentiviruses (LV) expressing short hairpin RNAs directed against Pitx1 (shPitx1) or scrambled (shScr) control sequences along with a PGK driven H2BRFP lineage tracer (Beronja et al., 2010; Siegle et al., 2014). We also transduced human SCC lines with LV-shSCR-H2BRFP or LV-shPITX1-H2BRFP. We validated Pitx1 knock-down (KD) by qPCR and Western blotting (Figure S1H,I) before transplanting these cells into the dermis of Nude mice. Pitx1KD impaired SCC formation in mouse and human (Figure 2 A,C). Pitx1KD tumors were unable to expand over time (Figure 2 B,D) and they failed to initiate secondary SCCs from α6hiβ1hi basal cells after serial transplantation (Figure 2E). Clonal lineage trace and competition experiments also revealed that shPitx1-RFP clones are outcompeted by shScr-GFP clones within two weeks (Figure 2F, S1J) and tumor initiation and growth rates diminished even further when we deleted the Pitx1 DNA binding domain (Figure 2G,H; S1K,L).
Figure 2: PITX1 controls the tumor propagating potential of basal SCC cells.
(A-D) Tumor initiation (A,C) and growth (B,D) rates after intradermal injection of 15,000 shScr (green) and shPitx1 (red) transduced mouse (A,B) and human A431 (C,D) TPCs (mean ±s.e.m., n=6 transplants). (E) Limited-dilution and orthotopic transplantation of shScr and shPitx1 α6hiβ1hi cells (n=6 transplants). Note: all shPitx1 derived SCCs expressed PITX1 and are escapers. (F) Competition analysis of shPitx1;H2B-RFP and shScr;H2B-GFP clones in 2-weeks old SCC grafts (n=3 transplants, mean +/−s.e.m., Student’s t-test). (G,H) Tumor formation (G) and growth (H) analyses of Pitx1WT and Pitx1KO clones in Nude mice (mean ±s.e.m., 2 Fcas9 control clones, n=3 transplants each; 3 sgPitx1 clones, n=3 transplants each, Student’s t-test). See also Figure S1.
PITX1 enhances proliferation and represses squamous differentiation gene transcription
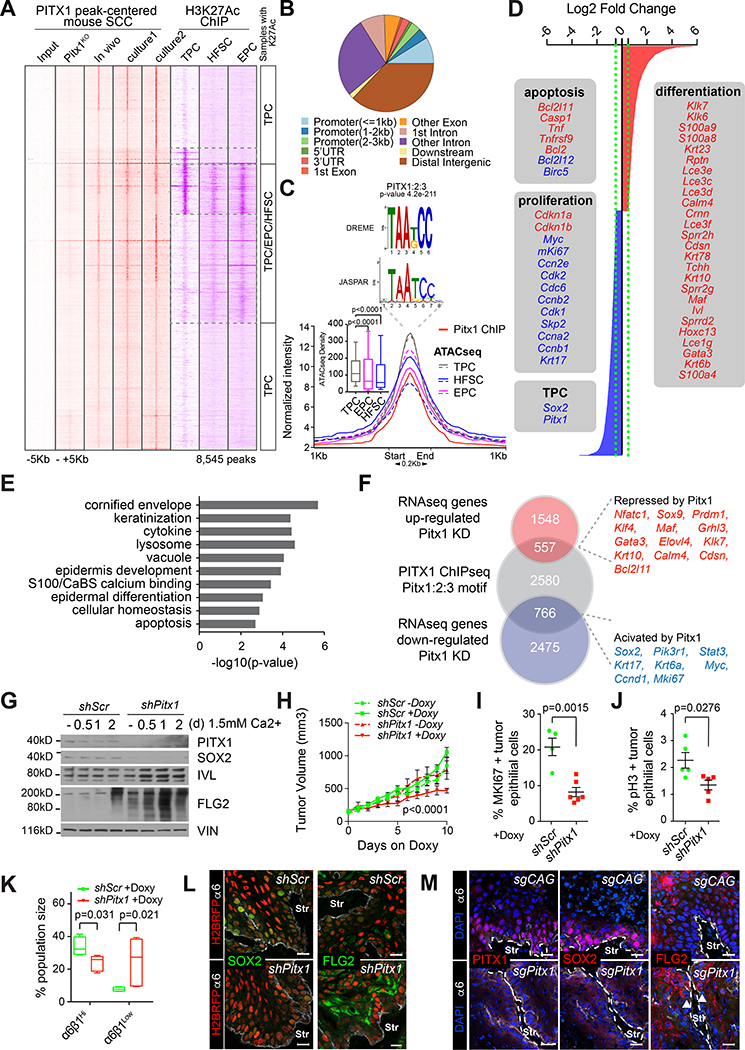
To determine how PITX1 promotes squamous carcinogenesis, we FACS isolated α6hiβ1hi TPCs from SCC grafts to identify PITX1 bound gene regulatory elements by Chromatin Immunoprecipitation and sequencing (ChIP-seq). We also mapped PITX1 bound chromatin in independent, primary TPC cultures before and after Pitx1 deletion. Alignment of short sequence reads against the mm10 genome revealed a highly concordant peak pattern across all PITX1 expressing data sets, comprising 8,545 significantly (p<0.01) enriched peaks (Figure 3A), located primarily at intergenic and intronic sequences (Figure 3B) with a centrally located PITX family consensus motif (p=4.2e-211, Figure 3C).
Figure 3: PITX1 promotes proliferation and survival and inhibits squamous differentiation in tumor propagating SCC cells.
(A) Heatmap representing PITX1 (red) and H3K27Ac (purple) ChIP-seq data centered around PITX1 peak summits on tumor propagating cells (TPCs), epidermal progenitor cells (EPCs) and hair follicle stem cells (HFSCs). (B) PITX1 peak distribution across the mouse genome. (C) Histogram shows mean PITX1 ChIP-seq (red) intensity along with ATAC-seq signals in HFSC (blue), TPC (gray) and EPCs (pink). Boxplots depict ATAC-seq signals around Pitx1 bound regions in TPCs, HFSCs, and EPCs (n=2, *P<0.0001, Mann-Whitney test). De novo motif discovery with MEME-ChIP identifies PITX1–3 motifs at the peak center. (D) Differentially expressed transcripts between shScr and shPitx1 transduced TPCs. (E) Gene ontology enrichment analysis of Pitx1 dependent transcripts. (F) Venn diagram shows intersection of genes in close proximity to PITX1 bound regulatory elements with Pitx1 dependent transcripts. (G) Western blotting confirms loss of PITX1 and SOX2 along with increasing Filaggrin (FLG2) and Involucrin (IVL) expression in shPitx1 compared to shScr control cells after Ca2+ induced differentiation in culture. (H) Growth analyses of Doxycycline (Dox)-inducible shPitx1 and shScr SCCs in established tumors (mean ±s.e.m. n=6 transplants, Sidak’s multiple comparisons test). (I,J) Quantification of the proliferation markers MKI67 (I) and mitotic phospho-Histone H3-ser10 (pH3, J) in Dox induced, murine shScr (n=5 tumors) and shPitx1 (n=6 tumors) SCCs. (K) Box plots depicting α6hiβ1hi and α6loβ1lo SCC fractions in shScr (n=4 tumors) and shPitx1 (n=5 tumors) SCCs (Mann-Whitney t-test). (L-M) Confocal micrographs of reveals decreased SOX2 and increased FLG2 expression after (L) constitutive and (M) Dox-induced Pitx1 CRISPRi mediated Pitx1 depletion. H2BRFP marks the tumor parenchyma. α6 integrin staining (white) demarcates the boundary between tumor epithelial cells and tumor stroma (Str). sgRNA targeting the CAG promoter was used as a control. Scale bars, 50μm. See also Figure S2 and S3 and Table S1–S2.
These data verify the specificity of our ChIP-experiments and they suggest that PITX1 binds preferentially to transcriptional enhancers. Because enhancers can be identified by chromatin accessibility and H3K27Ac (Heintzman et al., 2007), we first performed Assays for Transposase-Accessible Chromatin with Sequencing (ATAC-seq, (Buenrostro et al., 2013)) on freshly isolated TPCs and integrated our data with previously established ATAC-seq data sets from hair follicle stem cells (HFSCs), epidermal progenitor cells (EPCs), wound epithelial cells and TPCs (Ge et al., 2017; Latil et al., 2017). Global comparisons indicated that chromatin surrounding PITX1 peaks is significantly more accessible in TPCs, compared to EPCs and HFSCs (Figure 3C). Additional cluster analyses revealed similar peak patterns in TPCs of independent SCC models, which were distinct from EPCs and HFSCs (Figure S2A). For example, Clusters 2–4 comprise chromatin that is accessible in TPCs and wound epithelial cells, but not HFSCs and EPCs. These gene regulatory elements map distal to their closest transcriptional start sites, consistent with their putative function as transcriptional enhancers (Figure S2B). Further analysis with the Genomic Regions Enrichment of Annotations Tool (GREAT, (McLean et al., 2010)), revealed genes associated with clusters 2–3 belong to GO categories of increased skin tumor incidence and squamous carcinogenesis (Figure S3C). Cluster 4 gene sets are affiliated with fatty acid metabolism and reactive oxygen species, which are defining SCC characteristics (Brown et al., 2017; Pascual et al., 2017; Rinaldi et al., 2017). These analyses also suggest TPCs (clusters 2–4) are functionally distinct from EPCs (clusters 5–7) and HFSCs (clusters 7–8). Although TPCs express some embryonic stem cell (ESC) markers including SOX2 (Wong et al., 2008), we found gene regulatory elements in TPCs are fundamentally distinct from ESCs, as well as mouse embryonic fibroblasts (Maza et al., 2015) (Figure S2D), and ectodermal and skin epithelial progenitor cells at embryonic days 9 and 13 (Figure S3E) (Fan et al., 2018). Therefore, skin carcinogenesis may not simply present a developmental de-differentiation process, but it appears to acquire tumor specific gene regulatory programs de novo.
The significant enrichment of PITX and SOX motifs in ATAC cluster 2 (Figure S2F) suggests PITX1 and SOX2 may function to govern SCC specific enhancers that promote SCC initiation and growth. To test this hypothesis, we intersected PITX1 motif containing ChIP-seq peaks with H3K27Ac profiles of TPCs (Yang et al., 2015), HFSCs (Adam et al., 2015) and EPCs (Rinaldi et al., 2017)(Figure S2G). Most PITX1 bound chromatin was flanked by H3K27Ac marks in TPCs, while only a subset of PITX1 bound loci were also active in HFSCs and EPCs (Figure 3A). Indeed, Pitx1KD reduced chromatin accessibility (Figure S2H) and H3K27Ac (Figure S2I) preferentially in SCC specific ATAC-clusters, causing a significant (p<0.05) reduction in H3K27Ac at 3188 enhancers, including 1541 PITX1 bound sites (Figure S3A,A’). However, we also uncovered a significant increase in H3K27Ac at 1993 enhancers, including 300 PITX1 bound elements (Figure S3B,B’). PITX1 may therefore function as either transcriptional enhancer or repressor.
To define direct transcriptional PITX1 targets in TPCs, we first used GREAT to identify 3,894 genes with a transcriptional start site in close proximity to PITX1 bound, motif containing genomic loci. Next, we compared the transcriptomes of shPitx1 and shScr transduced murine TPCs to identify 3,708 transcripts significantly up- (red) or down-regulated (blue) upon Pitx1KD (Figure 3D, Table S1). Amongst these transcripts are well known regulators of cellular homeostasis (Cdkn1a, Cdkn1b, Mki67, Cdk2, Cdc6), cornified envelope formation (Lce, Flg2), keratinization (Krt1, Krt10, Krt6a, Krt17), epidermal differentiation (Klf4, Ivl, Maf, Grhl3, Gata3, Prdm1, Nfatc1), and apoptosis (Bcl2, Bcl2l11, Bcl2l12) (Figure 3E). By intersecting our PITX1 ChIP-seq results with our differential gene expression data, we uncovered 766 directly activated and 557 directly repressed PITX1 targets (Figure 3F, Table S2). Western blotting confirmed reduced SOX2 and elevated IVL and FLG2 expression in Pitx1KD cultures (Figure 3G) and qPCRs validated significantly reduced Pitx1, Sox2, Myc, and Mki67, along with elevated Klf4, Krt1, Krt10, Lor, and Flg2 expression in shPitx1 compared to shScr TPCs (Figure S3C).
Doxycycline (Dox) inducible shPitx1 KD (Figure S3D,E) also inhibited the expansion of already established SCCs (Figure 3H), suggesting PITX1 function is not just critical for SCC formation, but maintenance as well. Consistent with our differential expression data (Figure 3D,F), we detected significantly fewer proliferative, MKi67 (Figure 3I, S3F) and phospho-Ser10-Histone H3 (Figure 3J, S3G) positive cells in shPitx1 compared to shScr SCCs. We also measured reduced growth (Figure S3H–J) and EdU incorporation rates (Figure S3K–M) along with increased Caspase 3 activities (Figure S3N–Q) in cutaneous and HN-SCC cultures after Pitx1 KD.
To test if Pitx1KD simply affects TPC proliferation, or rather self-renewal due to confounding changes in squamous differentiation, we directly compared the relative abundance of basal and supra-basal cell fractions in LV-tetO-shScr and LV-tetO-shPitx1 transduced SCCs, where the parenchymal lineage was marked with H2BGFP as described above (Figure 1G,H). We found Pitx1KD depleted tumor propagating α6hiβ1hi SCC cells, while the α6loβ1lo SCC cell fraction expanded (Figure 3K). Consistent with these findings, we also noticed diminished SOX2 and emboldened FLG2 staining in Dox induced LV-tetO-Pitx1 compared to LV-tetO-Scr control SCCs (Figure 3L) and inducible Pitx1 depletion with CRISPR interference (Figure 3M, S3R–T). Collectively, these data suggest PITX1 promotes cell proliferation and its inhibition leads to cell cycle withdrawal and squamous differentiation. Because cell cycle withdrawal and differentiation are tightly linked in SCCs, we wondered whether Pitx1 is only detected in the proliferative, but not the quiescent TPC fraction (Brown et al., 2017). Interestingly, both TPC cohorts express Pitx1 and Sox2 at similar levels (Figure S3U), suggesting that they prevent TPC differentiation independent of cell proliferation.
Pitx1 cooperates with Trp63, Sox2 and Klf4 to govern a SCC specific transcriptional network
To determine if PITX1 controls gene transcription on its own, or together with other TFs, we used Cistrome to search for TF binding sites within 100 nucleotides of PITX1 peak summits. Interestingly, PITX1 motifs were highly enriched along with TRP63, KLF4, and SOX motifs (Figure 4A). Intrigued by this ensemble of TF motifs and the co-localization of PITX1 with SOX2 in TPCs (Figure 1D), we immuno-precipitated PITX1 and probed the precipitate with anti-SOX2, TRP63, PITX1 and KLF4 antibodies. SOX2 and TRP63 precipitated along with PITX1, whereas KLF4 was not enriched (Figure 4B). These data suggest PITX1 may physically interact with SOX2 and TRP63 in basal SCC cells, but not KLF4, which we detect in the first supra-basal SCC layers (Figure 4C). Encouraged by these interactions, we used ChIP-seq to search for bona fide SOX2, TRP63, and KLF4 bound regulatory elements in TPCs (Figure S4A–C).
Figure 4: PITX1 co-operates with SOX2 and TRP63 to control tumor propagating SCC cell defining gene regulatory elements.
(A) Transcription factor motifs surrounding PITX1 bound cis-regulatory elements. (B) Endogenous PITX1 immuno-precipitates TRP63 and SOX2, but not KLF4 or Vinculin (VIN). (C) Confocal micrographs identify PITX1 in basal and KLF4 in supra-basal SCC cells. (D) K-Means cluster analyses of ChIP-seq peaks centered on the union of PITX1, SOX2, TRP63, and KLF4 motif containing ChIP-seq peaks (left panel). Boxes right to the heat maps indicate the number of peaks present in each cluster. GO and PANTHER enrichment analyses by GREAT. (E) Venn diagram illustrates PITX1-SOX2-TRP63 bound genes in mouse and human TPCs. Genes repressed by PITX1 are red and activated ones are blue. (F) Working model for the regulation of TPC renewal and differentiation by a bi-stable transcriptional network. See also Figure S4 and S5 and Table S3–S5.
K-means cluster analyses of consensus motif containing ChIP-seq peaks revealed a complex pattern of TF interactions at SCC specific enhancers, where each cluster specifies well defined gene sets with functional links to epidermal homeostasis and squamous carcinogenesis (Figure 4D, S2J). At the center of these clusters we identified gene sets that are bound by PITX1, SOX2, and TRP63 in self-renewing TPCs and are increasingly occupied by KLF4 as soon as TPCs begin to differentiate in culture (Figure 4D), as well as tumors (Figure S5A–A”). Furthermore, PITX1, SOX2 and TRP63 bound to similar target gene sets in mouse and human SCCs, suggesting that SCC defining gene regulatory elements are evolutionarily conserved and governed by PITX1, SOX2 and TRP63 (Figure 4E, Figure S5BD, Table S3,4). Importantly, Pitx1, Sox2, Trp63, and Klf4 are themselves contained within this cluster (Figure 4D,E). Collectively, our RNA-seq and ChIP-seq data suggest PITX1, SOX2 and TRP63 promote TPC self-renewal and clonal expansion cooperatively by opposing KLF4 dependent squamous differentiation (Figure S5E, Table S5). Conversely, KLF4 function inhibits Pitx1, Sox2 and Trp63 transcription in supra-basal SCC cells to ensure their permanent cell cycle exit and differentiation into keratinized pearls, characteristic for SCCs (Figure 4F). This regulatory relationship is typical for bi-stable, multi-input network motifs, which allow cells to switch between two functionally distinct states of differentiation (Lee et al., 2002; Milo et al., 2002).
Pitx1, Trp63 and Sox2 enhance one another’s transcription
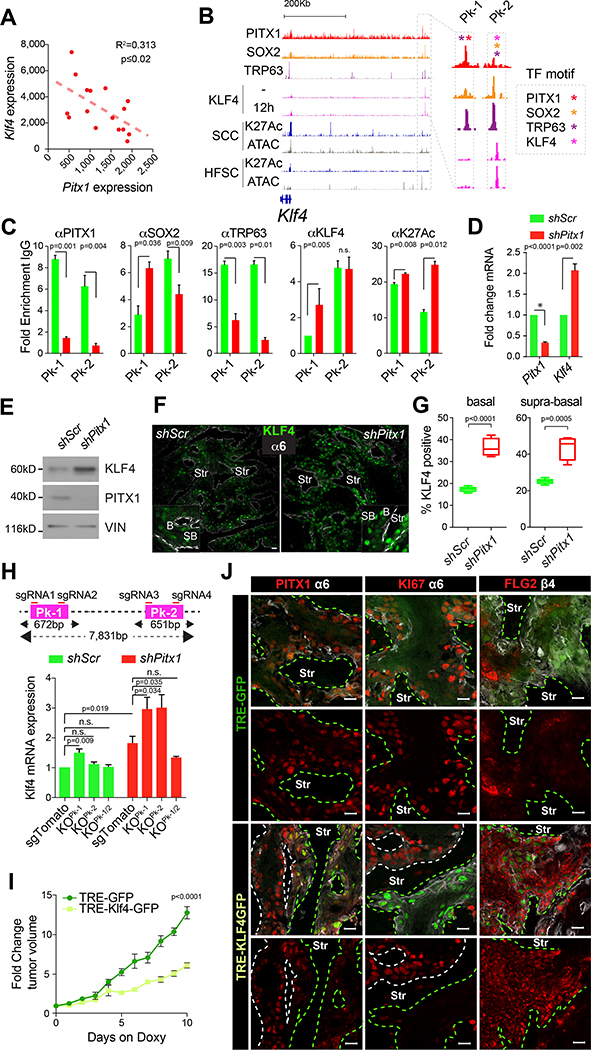
To test this hypothesis, we first quantified the association of these four transcription factors with their respective binding sites on the Sox2 (Figure 5A–A’, S6A–A’), Pitx1 (Figure 5B–B’, S6B–B‘) and Trp63 (Figure 5C–C’, S6C–C‘) gene regulatory elements in shScr control and shPitx1 TPCs. Focusing on a novel, SCC specific enhancer located ~300 kb downstream of the Sox2 transcriptional start site (TSS), which is active (H3K27Ac) and accessible (ATAC) in SCCs, but not HFSCs or embryonic stem cells (ESCs) (Figure S5F) we identified three overlapping, consensus motif (color coded asterisks) containing peaks (Pks, Figure 5A). Pk-2 and Pk-3 were highly enriched for PITX1, SOX2, and TRP63. Conversely, KLF4 binding increased at these gene regulatory elements as soon as TPCs began to differentiate, showing the strongest enrichment at Pk-1. ChIP-PCR results confirmed these findings in shScr TPCs and they revealed a significant decline in PITX1 and TRP63 binding to Pks-2&3 along with reduced SOX2 enrichment at Pks-1&3 in shPitx1 TPCs (Figure 5A’). Conversely, KLF4 binding increased significantly at all three sites once Pitx1 had been depleted. These data indicate PITX1 and SOX2 bind this enhancer cooperatively, but competitively with KLF4. Consistent with this notion, ChIP and re-ChIP experiments validate the interaction between PITX1 and SOX2, but not KLF4 (Figure S5G). Indeed, PITX1 depletion enables KLF4 binding to significantly diminish H3K27Ac at this enhancer (Figure 5A, A’). A similar behavior was seen at SCC specific Pitx1 (Figure 5B,B’) and Trp63 (Figure 5C,C’) enhancers, suggestive of a feed forward circuit that sustains transcription of these three TPC-defining transcription factors until intercepted by increased KLF4 activity.
Figure 5: PITX1, SOX2 and TRP63 promote one another’s transcription in TPCs.
(A-C) PITX1 (light red), SOX2 (orange) and TRP63 (purple) ChIP-seq tracks at the Sox2 (A), Pitx1 (B) and Trp63 (C) genomic loci. ATAC-seq and H3K27Ac ChIP-seq indicate open and active chromatin respectively in shScr (green) and shPitx1 (red) TPCs. Black arrowheads indicate statistically significant changes in H3K27Ac (p<0.001, n=2 ChIP-seq). ATAC-seq and H3K27Ac tracks of HFSCs (blue). Boxes highlight putative enhancers. Asterisks mark PITX1 (light red), SOX2 (orange) TRP63 (purple) or KLF4 (pink) transcription factor motifs. (A’-C’) ChIP-PCR data depict enrichment of PITX1, SOX2, TRP63, and KLF4 on nuclear chromatin in shScr (green) and shPitx1 (red) SCC cells. H3K27Ac indicates enhancer activity (mean ±s.e.m., n=3 ChIP-PCRs, Student’s t-test) (D) qPCR and (E) Western blot analyses show changes in PITX1, SOX2 and TRP63 expression between shScr, shPitx1, shTrp63, and shSox2 mouse TPCs. (mean ±s.e.m., n=4 experiments, Student’s t-test). See also Figure S5 and S6.
Consistent with this hypothesis, we measured significantly reduced Pitx1, Sox2 and Trp63 expression (Figure 5D,E), after Pitx1, Sox2, or Trp63 KD in TPCs. Although Pitx1 KD reduced Sox2 expression, it affected Trp63, an essential regulator of SCC growth (Figure S5H), more modestly. These variations could be due to differences in KD efficiency, or the complex regulation of Trp63 (Vanbokhoven et al., 2011). Regardless, our data suggest PITX1, SOX2 and TRP63 sustain one another’s association with their respective enhancers in mouse (Figure 5A–C) and human (Figure S5I) TPCs to boost their own transcription. Conversely, the dissociation or reduction of any of these three factors from their enhancers enables KLF4 chromatin binding, which in turn represses Pitx1, Sox2, and Trp63 transcription allowing TPCs to differentiate into SCC cells without proliferative potential.
Pitx1 represses Klf4 to block squamous differentiation in skin carcinogenesis
Consistent with this model, Klf4 and Pitx1 expression levels are inversely correlated in SCCs (Figure 6A) (Schober and Fuchs, 2011) and KLF4 expression increases once TPCs begin to differentiate, becoming transiently visible in nuclei of the first supra-basal SCC cell layers (Figure 4C) before their enucleation and keratinization. Klf4 expression increased significantly upon Pitx1 KD (Figure 3F). PITX1 might thus repress Klf4 transcription and squamous differentiation to support SCC expansion.
Figure 6: PITX1 represses KLF4 dependent differentiation in SCCs.
(A) Scatter plot illustrating inversely correlated Pitx1 and Klf4 expression in mouse TPCs. (B) PITX1 (red), SOX2 (orange), TRP63 (purple) and KLF4 (pink) ChIP-seq tracks at the Klf4 locus. ATAC-seq (grey) and H3K27Ac ChIP-seq (dark blue) indicate active and open chromatin in TPCs and HFSC. Box highlights a putative PITX1, SOX2 and KLF4 bound Klf4 enhancer. Asterisks indicate TF motifs. (C) ChIP-PCRs depict PITX1, SOX2, TRP63 and KLF4 enrichment on Klf4 enhancer and H3K27-acetylation in shScr (green) and shPitx1 (red) SCC cells (mean ±s.e.m., n=3 ChIP-PCRs, Student’s t-test). (D) qPCRs (n=4, mean ±s.e.m., Student’s t-test) and (E) Western blotting shows elevated KLF4 expression upon Pitx1 knockdown. (F) Confocal micrographs show KLF4 expression in shScr and shPitx1 SCCs. α6 integrin staining (white) demarcates the boundary between tumor epithelial cells and stroma (Str). Scale bars = 50μm. (G) KLF4 expression in basal and supra-basal layers of shScr and shPitx1 SCCs. (n=5, Mann-Whitney test). (H) Strategy to delete Klf4 enhancer Pk-1, Pk-2, or both with CRISPR/Cas9. qPCRs show changes in Klf4 expression upon Pk-1 and/or Pk-2 deletion (mean ±s.e.m., n=4 experiments, Student’s t-test). (I) Growth rate of fully established murine SCCs after doxycycline (Dox)-induced KLF4-GFP and GFP expression (mean ±s.e.m., n=6, Sidak’s multiple comparisons test). (J) Confocal images show PITX1, MKI67 and FLG2 expression in Dox induced GFP and KLF4-GFP clones in mouse SCCs. Green dotted lines mark GFP or KLF4-GFP expressing clones in SCCs. Scale bars = 50μm. See also Figure S7.
Consistent with this notion, we identified two overlapping, PITX1, SOX2 and TRP63 motif containing gene regulatory elements distal to the Klf4 TSS (Figure 6B, S6D–D’). Pk-1 was predominantly PITX1 and TRP63 bound, while Pk-2 was highly enriched for SOX2, TRP63, and KLF4 itself. ChIP-PCR experiments confirmed PITX1 and SOX2 enrichment on Pks-1&2 in shScr TPCs (Figure 6C), while PITX1 declined along with TRP63 from Pks-1&2 in shPitx1 SCCs. Although SOX2 binding waned on Pk-1, it amassed on Pk-2, a site bound by KLF4 irrespective of PITX1 function. These dynamic transcription factor interactions link PITX1 function to H3K27 acetylation, Klf4 transcription, and KLF4 expression (Figure 6C–E), along with more KLF4 expressing basal and supra-basal cells in shPitx1 SCCs (Figure 6F,G).
To test if this putative enhancer affects Klf4 transcription, we deleted Pk-1, Pk-2, or the entire 8kb locus with CRISPR/Cas9 (Figure S7A–B). Pk-1 deletion increased Klf4 transcription and this was further augmented by shPitx1 (Figure 6H), consistent with increased H3K27Ac upon PITX1, SOX2 and TRP63 loss from Pk2 (Figure 6B,C). Pk-2 deletion had no effect on Klf4 transcription by itself, but Klf4 transcript levels increased significantly as soon as Pitx1 depletion allowed KLF4 binding at Pk-1 (Figure 6C). In contrast, deletion of the entire enhancer rendered Klf4 transcription insensitive to its regulation by PITX1 and KLF4.
Our data suggest a model where competitive PITX1 and KLF4 interactions govern Klf4 transcription and squamous differentiation in TPCs. To test this hypothesis, we ectopically expressed Dox inducible tetON::KLF4-GFP, or tetON::GFP in TPCs. Although ectopic KLF4-GFP expression blunted tumor expansion (Figure 6I), reduced PITX1 and MKi67 along with elevated FLG2 staining were restricted to KLF4-GFP expressing clones within mosaic tumors (Figure 6J).
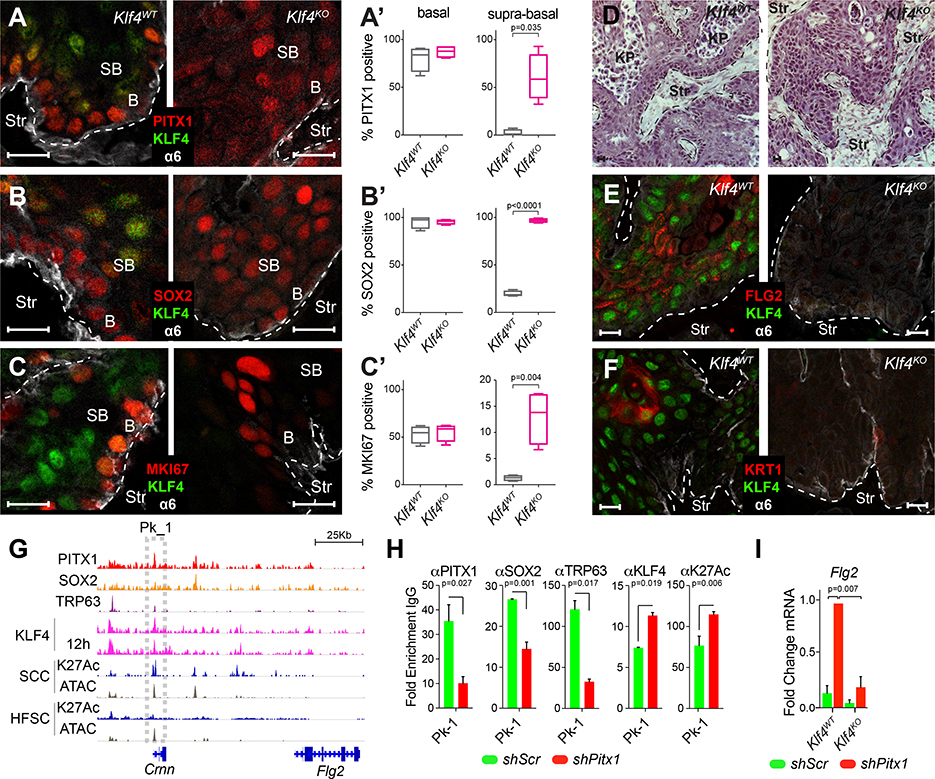
Consistent with this antagonistic relationship between PITX1 and KLF4 in TPCs, we also observed >75% of the 1805 PITX1 regulated transcripts became KLF4 bound once TPCs begin to differentiate and 955 direct KLF4 target genes were indirectly affected by PITX1 function (Figure S7C, Table S6). To further investigate the interaction between Pitx1 and Klf4 in SCCs, we deleted Klf4 in the same TPC lines, confirmed Klf4 deletion (Figure S7D), transplanted them into mouse dermis to measure tumor formation and SCC growth over time. Although tumor initiation and growth rates were hardly affected by Klf4-loss (Figure S7E), we noticed cells were tighter packed (Figure S7F) and Klf4KO SCCs had lost their hierarchical organization, as PITX1 and SOX2 expanded throughout the tumor parenchyma (Figure 7A–B, S7G–H), MKi67 staining increased in supra-basal SCC cells (Figure 7C, Figure S7I), while fewer keratinized pearls (Figure 7D) have been detected and FLG2 (Figure 7E) and KRT1 (Figure 7F) staining was lost.
Figure 7: KLF4 function restricts PITX1 and SOX2 expression to basal SCC cells.
(A-C) Confocal images of PITX1 (A), SOX2 (B), and MKI67 (C) expression in Klf4WT and Klf4KO SCCs. Scale bars = 50μm. (A’, B’ and C’) Box-plots illustrate PITX1 (A’), SOX2 (B’), and MKI67 (C’) expression in basal and supra-basal cells of murine Klf4wt (n=4) and Klf4KO (n=4, Mann-Whitney test) SCCs. (D) SCC sections stained with Hematoxylin and Eosin show loss of keratin pearls (KP) in Klf4KO SCCs. (E,F) FLG2 (E) and KRT1 (F) (Red) staining in Klf4WT and Klf4KO SCCs. (G) PITX1 (red), SOX2 (orange), TRP63 (purple) and KLF4 (pink) ChIP-seq tracks at the Crnn and Flg2 locus. ATAC-seq (grey) and H3K27Ac ChIP-seq (dark blue) indicate active and open chromatin in TPCs and HFSC. Boxes highlight putative Crnn and Flg2 enhancer. (H) ChIP-PCRs of PITX1, SOX2, TRP63, KLF4, and H3K27Ac in shScr (green) and shPitx1 (red) SCC cells (mean ±s.e.m., n=3 ChIP-PCRs, Student’s t-test). (I) qPCRs show significantly increased Flg2 expression after Pitx1 depletion in Klf4WT but not Klf4KO TPCs (mean ±s.e.m., n=3 experiments, Student’s t-test). See also Figure S7 and Table S6.
This reduction in keratinized pearls and Flg2 expression can be explained with persistent PITX1-SOX2-TRP63 binding of the Flg2 enhancer in Klf4KO tumors. Predominant PITX1-SOX2-TRP63 activity may thus prevent KLF4 binding and thereby inhibit Flg2 transcription (Figure 3G,L,M), whereas Pitx1KD releases SOX2 and TRP63 from chromatin to provide access for KLF4, which increases H3K27Ac, Flg2 transcription, and squamous differentiation in these tumors (Figure 7G–H). This model is consistent with epistasis experiments, which showed increased Flg2 transcription and squamous differentiation upon Pitx1KD in Klf4WT, but not Klf4KO TPCs (Figure 7I, Figure S7J).
Discussion
Our studies discovered PITX1, a TF that becomes de novo expressed at SCC initiation, is a pivotal regulator of squamous carcinogenesis and functional TPC marker in mouse and human SCCs. Although PITX1 is not expressed in normal skin epithelium, it is robustly detected in TPCs and lost as soon as they commit to differentiate into SCC cells without proliferative potential. PITX1 function is critical for TPC proliferation and survival, while its decline enables squamous differentiation. Increased PITX1 expression has also been observed in KRAS mutant colorectal cancers (Watanabe et al., 2011) and acute lymphoblastic leukemia (Nagel et al., 2011), while its increased expression correlates with good prognosis in melanoma (Osaki et al., 2013), osteosarcoma (Kong et al., 2015), prostate, lung, liver and gastric cancers (Calvisi et al., 2011; Chen et al., 2007), indicative of context dependent functions.
Indeed, we found PITX1 cooperates with SOX2 and TRP63 to govern a SCC specific transcriptional network. The core of this network is built around three network motifs that are commonly used in lower eukaryotes (Lee et al., 2002; Milo et al., 2002). We found that the most central circuit comprises multi–input motifs in tumor specific Pitx1, Sox2, Trp63, and Klf4 enhancers, which are collectively governed by their own gene products in auto-regulatory circuits. PITX1, SOX2 and TRP63 associate with their own enhancers to establish a self-sustaining, transcriptional feed forward circuit, while they inhibit Klf4 transcription and squamous differentiation. Conversely, reduced PITX1, SOX2 or TRP63 activity enables increased Klf4 transcription, thereby allowing KLF4 to occupy Pitx1, Sox2 and Trp63 gene regulatory elements to stall their transcription. This logic establishes a bi-stable system, which allows TPCs to self-renew and terminally differentiate. The SCC specific activity of this transcriptional circuit could be exploited by therapeutic strategies, which limit clonal tumor cell expansion by restricting TPC-renewal and stimulating their differentiation into post-mitotic SCC cells.
Although a feed forward circuit is also responsible for embryonic stem cell (ESC) self-renewal (Boyer et al., 2005; Ying et al., 2008; Young, 2011) we found the enhancers regulating Sox2 transcription in SCCs are different from those in ESCs (Figure S9A). Indeed, SOX2 partners with PITX1 and TRP63 to govern gene transcription in SCCs, where neither Oct4 nor Nanog are detected (Schober and Fuchs, 2011; Watanabe et al., 2014). We also find PITX1, SOX2, and TRP63 oppose KLF4 expression and function in SCCs, whereas SOX2 cooperates with KLF4 to enhance ESC-renewal. Consistent with these differences in gene transcription, our chromatin accessibility and enhancer maps suggest only minor regulatory similarities between TPCs and ESCs (Figure S2D). Surprisingly, SCC defining enhancers are also distinct from embryonic epidermal progenitor cells (Figure S2E)(Fan et al., 2018), suggesting that squamous carcinogenesis may not simply be due to de-differentiation as reported for melanoma (Kaufman et al., 2016).
Although our current data are able to mechanistically explain how the SCC specific transcriptional network can be sustained in order to enable long-term tumor growth, we are still unable to explain how this PITX1 and SOX2 dependent network is initially established. One possibility is that wound or oncogene induced stress signaling enables chromatin remodelers to open chromatin and thereby endow cells with opportunities to cope with demanding situations and changes in their cellular environment (Ge et al., 2017). Interestingly, the SWI/SNF2 complex components Hells and Smarca5 are significantly overexpressed in TPCs (Schober and Fuchs, 2011), ACTL6a is co-amplified with TP63 in HNSCCs (Saladi et al., 2016), and translocations altering SWI/SNF2 activity are linked to de novo SOX2 expression in Synovial sarcomas (Kadoch and Crabtree, 2013). Indeed, SWI/SNF2 complex activity is already known to govern epidermal self-renewal and differentiation (Bao et al., 2015; 2013). In addition, SOX2 transcription is repressed due to enhancer methylation in human astrocytoma (Modrek et al., 2017), raising the possibility that DNA methylation could repress Sox2 transcription in skin epithelial progenitor cells. Actually, loss of Dnmt3a and Dnmt3b accelerates SCC initiation and progression in a chemical carcinogenesis model, although Sox2 and Pitx1 were unaffected (Rinaldi et al., 2017; 2016). However, Polycomb represses Sox2 and Pitx1 transcription in EPCs to prevent their differentiation into Merkel cells (Bardot et al., 2013; Ezhkova et al., 2011). How these epigenetic mechanisms contribute to SCC initiation is still elusive. However, changes in chromatin accessibility provide an opportunity for Pitx1 and Sox2 transcription and the formation of a feed forward circuit, which drives long-term clonal expansion.
Alternatively, PITX1 could be a pioneering transcription factor that enables the establishment of TPC specific gene regulatory circuits. Although cellular identity is generally defined by a small set of fate-determining transcription factors during embryonic development and cellular fates remain invariable once they are established due to highly specialized epigenetic programs, genetic mutation or ectopic expression of master-regulatory homeobox proteins can trigger dramatic fate transformations (Lewis, 1992). For example, ectopic Antennapedia expression can transform fly antenna into legs (Schneuwly et al., 1987) and Paired box protein PAX6 can trigger ectopic eye formation (Halder et al., 1995). The most drastic fate change is caused by the ectopic expression of Pou5f1 (Oct4), Sox2 and Nanog, which induces terminally differentiated cells to revert to pluripotent ESCs (Takahashi and Yamanaka, 2006). It is intriguing to speculate that Paired like homeodomain box 1 (PITX1) might also have transformative, master regulatory functions, which are responsible for the transcriptional and epigenetic changes in squamous carcinogenesis. Irrespective of an active or permissive function, PITX1 cooperates with TRP63 and SOX2 to establish a SCC specific master regulatory circuit (Califano and Alvarez, 2017) in mice and man. Our data define a transcriptional framework for squamous carcinogenesis and provide opportunities towards the development of novel therapies that target stemness (Kreso and Dick, 2014) in these cancers.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Markus Schober (markus.schober@nyumc.org).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell lines
Primary murine SCC-TPC lines were established from malignant tumors induced with DMBA following the complete carcinogenesis protocol as previously described (Guasch et al., 2007; Schober and Fuchs, 2011; Siegle et al., 2014). Human A431, Cal-27, FaDu, and Detroit-562 (ATCC) and Cal33 (DSMZ) cell lines were grown in DMEM supplemented with 10% fetal bovine serum (FBS). Human SCC-25 and SCC-15 cells were grown in P-media (DMEM-F12 3:1 media (Gibco), sodium bicarbonate (Sigma), L-glutamine (Invitrogen) and Pen/Strep solution (Invitrogen)) supplemented with 10% FBS). Primary cells were induced to differentiate by raising Ca2+ from 0.05 to 1.5mM for the times indicated.
Mice
6-week-old, female Nude (NU/NU [088] Charles River) mice were used for orthotopic transplantations and xenograft studies. Tumors were detected by palpation, measured with digital calipers to calculate tumor volumes (VTumor. π/6xlxw2, where l=length in mm and w=width in mm). Mice were injected with 50 mg/g EdU (Invitrogen, A10044) intraperitoneally 6hrs before sacrifice. For inducible shRNA or sgRNA expression in tumors, mice were placed on doxycycline-containing (200 mg) chow once tumors were established. Tumor volume was measured every day for 10 days. All animal experiments were performed in accordance with the guidelines and approval of the Institutional Animal Care and Use Committee at New York University Langone Medical Center.
Clinical Specimens
Fresh frozen human SCC specimens have been collected by Dr. John Carucci, Director of Moh’s micrographic surgery, before they were provided de-identified by NYU-School of Medicine’s Center for Biospecimen Research & Development. Specimens were sectioned and stained with anti-SOX2 and anti-PITX1 antibodies as well as Hematoxillin and Eosin for single-blinded histopathological analyses.
METHOD DETAILS
Cell culture
All shRNAs were generated according to the sequences used in the Mission TRC-1 mouse and human library (Sigma, Broad Institute), sub-cloned into pLKO.1 (Addgene 8453) and subsequently transferred into pLKO Histone H2B-mRFP1 (Addgene 26001) or pLKO Histone H2BGFP (Addgene 25999) (Beronja et al., 2010), or cloned directly into pLKO Tet-on puro (Addgene 21915). The following shRNAs were used: shScramble (SHC002), shSox2 (TRCN0000424718), shPitx1 (TRCN0000020460 and TRCN0000421329) and shTrp63 (TRCN0000423330 and TRCN0000416977). For full hairpin sequences, see Table S7.
For stable cell line generation, VSV-G pseudotyped lentivirus was produced by Lipofectamine 2000 transfection of 293FT cells (Invitrogen) with pLKO shRNA or sgRNA-carrying vector or over-expression constructs and helper plasmids pMD2-VSVg and pPAX2 (Addgene plasmid 12259 and 12260, respectively). 293FT cells were maintained in DMEM (Gibco) supplemented with 10% FBS and Pen/Strep solution. Viral supernatant was collected 48 and 72 hours after transfection and filtered through 0.45-μm polyvinylidene difluoride filters. For infections, 3 × 105 cells were plated in a single well of a 6-well plate, incubated with a 1:3 dilution of viral supernatant containing 30 μg/mL Polybrene and spun at 1,100g for 30 min at 37°C. Forty-eight hours after infection, puromycin-resistant cells were selected with 1 μg/mL puromycin (Sigma-Aldrich). Infected cells were FACS-sorted 60 hrs post infection and used for in vivo tumor growth (whole tumor and clonal competition). For KLF4 over-expression analysis, cells were infected with rtTA-Hygromicin (Addgene 66810) and LV-tetO-GFP or LV-tetO-GFP-KLF4 (generous gift from E. Apostolou, Weill-Cornell), induced for 24 hours with Doxycycline, and selected by flow cytometer using GFP.
For tumor growth assays, cells were sorted on a BD FACSAria II equipped with 488, 633, 405, 562 and 355 nm lasers. Cells were gated on live, single cells, and sorted for either GFP or RFP (shRNA-encoding) cell populations. Sorted cells were suspended in 50% Matrigel (356237; BD) diluted with F medium at a concentration of 15,000 (murine) or 20,000 (human) cells per injection and injected intradermally into Nude (NU/NU [088] Charles River) recipient mice. For clonal competition assays, both viruses (shScr-GFP and shPitx1-RFP) were produced in the same well by mixing equimolar amounts of plasmid DNA. Infected cells were analyzed on a BD LSR II flow cytometer before injection to determine RFP/GFP ratios (Siegle et al., 2014).
Tumor isolation and flow cytometry
Tumors from murine allografts were isolated as previously described (Schober and Fuchs, 2011; Siegle et al., 2014). Briefly, after separating tumors from normal skin, blood vessels and connective tissue, tumors were minced and incubated with 0.25% collagenase (C2670, Sigma) in HBSS (Gibco) for 45min at 37°C shaking. During the last 10min of collagenase digestion, 62.5 U/ml DNaseI (LS002138, Worthington) was added. The cell suspensions were filtered through 70 and 40μm cell strainers. Tissue that was retained on filters was then digested for 10min with 0.25% trypsin (Invitrogen) at 37°C on a rocker before it was stra ined and combined with prior isolates. Cell suspensions were diluted in wash buffer (2% chelexed FBS in DPBS) and pelleted at 300g for 10min. Isolated cells were plated on 3T3 feeder cell layer in E medium with 15% Ca2+ free FBS, 300mM calcium and Antibiotic-Antimycotic (15240112, Invitrogen) (Nowak and Fuchs, 2009; Rheinwald and Green, 1975). For flow cytometry, cell suspensions were stained for 30min on ice and DAPI (40,6-diamidino-2-phenylindole; D1306, Invitrogen) was used for live/dead cell exclusion. FACS analyses were performed on a BD LSRII equipped with 488, 642, 407, 355 and 562nm lasers. EdU incorporation was determined by flow cytometry using the Click-iT EdU PacBlue Cell Proliferation Assay Kit for flow cytometry (C35002; Invitrogen) following the manufacturer’s instructions. Active Caspase was determined by flow cytometry after fixed cells (BD 554714) were stained with active caspase antibodies V405 (BD 560627) or AF-647 (BD 560626). Flow cytometry data were analyzed with FlowJo.
For limited dilution and serial orthotopic transplantation, tumors were processed as described above, sorted on a BD FACS Aria II following the strategy described in Figure S1G, and serially diluted to obtain 1,000, 100, 10 cells, mixed with 50% matrigel for intradermal injection into Nude (NU/NU [088] Charles River) recipient mice (Brown et al., 2017a, Schober and Fuchs, 2011). shPitx1 tumors were stained with anti-PITX1 antibodies and examined by immuno-fluorescence microscopy. If PITX1 was still detectable, these tumors were considered escapers and are marked as such on figures and figure legends.
CRISPR/Cas9 Knockouts in SCC-TPC Cells
The Pitx1 binding domain was deleted with, two sgRNAs targeting the 5ánd 3ŕegion of the sequence. For Klf4 CRISPR knock-outs, we designed single sgRNAs targeting its first exon. Klf4 enhancer deletion was performed with two sgRNAs flanking Pitx1 and Klf4 bound regions (Figure S9A). The most efficient guide RNAs were predicted using the CRISPR design tool MIT (Zhang Lab, MIT 2015). sgRNA against dTomato was used as a control. sgRNA sequences were cloned into pLKO U6-puro or GFP-modified vector (Addgene 52963). After sgRNA transduction, cells subsequently infected with lentiCas9-Blast (Addgene 52962), and selected with Blasticidin for 3 days (5μg/ml). For Pitx1 gene knockout, single clones were selected and screened by PCR on cDNA libraries. Clones were further validated by Western blot analysis with anti-PITX1 antibodies. Klf4 gene editing was performed in a pooled format and tumor cells were tested by Western blotting with anti-KLF4 antibodies. For Klf4 enhancer deletions, gDNA was extracted and screened by PCR. sgRNA sequences are listed in Table S7.
CRISPR interference
Pitx1 expression was interfered by using sgRNAs targeting Pitx1 TSS region to bring a doxycycline inducible deactivated Cas9 fused with the KRAB repressor (TRE-KRAB-dCas9-HA). sgRNAs were designed using the Cas9-Activators with SAM design tool (http://sam.genome-engineering.org/) and clone into the sgOpti (Gift from Eric Lander & David Sabatini, Addgene plasmid 85681). An sgRNA targeting the synthetic CAG promoter was use as a control. Primary TPCs were infected with the rtTA-N144 (gift from Andrew Yoo, Addgene plasmid # 66810) and a TRE-KRAB-dCas9-HA-IRES-GFP (gift from Eric Lander, Addgene plasmid # 85556), selected with hygromycin, induced for 24 hours with Dox, and isolated by flow cytometry using GFP. Purified cells were infected with the different sgRNAs and selected with puromycin. The efficiency of the sgRNAs was tested by Western blotting of cells that had been induced with Dox for 48 hours, using anti-PITX1 antibodies. KRAB-dCas9-HA expression was confirmed using anti-HA antibodies.
Immuno-fluorescence and imaging
Unfixed tumors were embedded in OCT (Tissue Tek). Frozen sections were cut to a thickness of 10μm on a Leica cryostat and mounted on SuperFrost Plus slides (Fisher). Slides were air-dried for 10min, then fixed for 10min with 4% formaldehyde, rinsed with PBS, permeabilized with 0.5% Triton X-100 in PBS for 15min, then blocked for 1h (5% normal donkey serum, 1% BSA, 0.3% Triton X-100 in PBS) and incubated in primary antibody diluted in blocking buffer at 4°C overnight. After washing with PBS, secondary antibodies, conjugated to Alexa 488, Alexa FITC, Alexa 568, DyLight 649, and Hoechst 33342 (83218, AnaSpec) were diluted in blocking buffer and incubated with the slides for 1hr at room temperature (RT). After washing, slides were mounted in ProLong Gold (Invitrogen). Imaging was performed on a Nikon Eclipse TiE Microscope or a Zeiss LSM780 Confocal Microscope. Images have been analyzed in NIS-Elements, Zen software or ImageJ. Antibodies for immunofluorescence were CD49f (1:200; Biolegend, 313618), SOX2 (1:1000; Abcam, Ab92494), pKi67 (1:1000; Novocastra, NCL-Ki67p), PITX1 (1:500; Novus, NBP1–88644), KLF4 (1:1000; R&D, AF3158), phospho Histone H3 (ser19) (1:1000; Millipore, 06–570), Filaggrin (1:1000; BioLegend, 905801) and Nidogen (1:1000; Santa Cruz, sc-33706).
Quantitative reverse-transcription PCR
mRNA was isolated using Qiazol (Qiagen) and Direct-zol RNA Mini Prep Kits (R2052, Zymo Research). Samples were quantified using a Nanodrop spectrophotometer (Thermo Scientific). Complementary DNA was synthesized from 1.5μg of total RNA using SuperScript VILO with random primers (Invitrogen). qRT–PCR was performed with FastStart Universal SYBR Green Master (Rox) (10802200, Roche) on a StepOnePlus™ Real-Time PCR System (Applied Biosystems). Measurements were recorded in triplicate. Differences between samples and controls were calculated based on the 2−ΔΔCT method and normalized to Rplp0. For detailed list of primer sequences, see Table S7.
RNA-seq library preparation
Total RNA was extracted from 2×105 TPCs using the Absolutely RNA Microprep Kit (400805, Agilent Technologies) according to manufacturer’s instructions. RNA quality was defined on an Agilent 2100 Bioanalyzer before we prepared ribo-depleted, multiplexed, paired end libraries with the Illumina TruSeq Stranded Ribozero Gold kit. Multiplexed libraries have been sequenced on an Illumina HiSeq 2500 Genome Analyzer using the 50-base pair paired end read method.
Western blotting
Cell lysates from culture or FACS isolated cells were prepared using RIPA buffer (150mM sodium chloride, 0.1% Triton-X 100, 0.5% SDS and 50mM Tris pH 8 in ddH2O) with complete Mini EDTA-free protease inhibitor tablets (04693159001, Roche). Protein concentrations were determined following the instructions of Pierce BCA Protein Assay Kit (23225, Pierce). Lysates were boiled with 5x Laemmli buffer (6% SDS, 15% β-mercaptoethanol, 30% glycerol, 0.006% bromophenol blue, 0.188M Tris–HCl) for 10 min at 95°C. Protein ladder used was Full-Range Rainbow Mo lecular Weight Markers (RPN800, GE Healthcare). 30μg of protein was loaded per lane. Gel electrophoresis was performed using a 10 or 12% Bis-Tris gel run for 75–150min at 120V, gel was transferred for 1.5h at 4°C at 100V to a 0.45μm nitrocellulose membrane (Whatmann) and transfer was assessed by Ponceau S staining (0.1% (w/v) Ponceau S in 5% (v/v) acetic acid). Membranes were blocked with 5% non-fat dry milk in TBST, then incubated with primary antibodies diluted in blocking overnight at 4°C with gentle agitation. Membranes were rinsed with TBST before incubating with horseradish peroxidase-conjugated secondary antibodies diluted in blocking buffer for 1h at RT. Membranes were washed with TBST before incubating with Supersignal West Pico or Femto Chemiluminscent substrate (Life Technologies, #34080 add #30095) and exposed to X-ray film (F-9024–8_10, GeneMate) using a Kodak X-Omat 2000A Processor. Antibodies used for western blotting were SOX2 (1:1,000; Abcam, ab92494), KLF4 (1:1,000; R&D, AF3158), TP63 (1:1,000; Cell Signaling, 13109), PITX1, (1:500; Santa Cruz, 18922), Tubulin (1:10,000; Sigma, T5201), Vinculin (1:10000; Sigma, V9131), Cyclin D1 (1:1000; Cell Signaling, 2978S), CASPASE-3 (1:1000; Cell Signaling, 9664S), Filaggrin (1:1000; BioLegend, 905801), Involucrin (1:1000; BioLegend, PRB-140C), HRP donkey anti-rabbit IgG (H+L) (1:3000; Jackson, 711–035-152), HRP donkey anti-mouse IgG (H+L) (1:3000; Jackson, 711–035-151), HRP donkey anti-goat IgG (H+L)(1:3,000; Jackson, 705–035-147).
Co-Immunoprecipitation
PITX1 or IgG antibody (1 μg) was bound to Dyna-beads (Invitrogen) and incubated with 1 mg of total protein extracts from primary TPCs cultured in 0.05 mM Ca2+ keratinocyte culture medium. Antibody bound beads were incubated with protein lysates overnight at 4°C in 1 ml of immunop recipitation buffer (20 mM HEPES [pH 8], 10 mM KCl, 0.15 mM EGTA, 0.15 mM EDTA, 150 mM NaCl, and 0.1% NP-40) containing a cocktail of protease inhibitors. After washing 4 times with immunoprecipitation buffer, proteins were eluted in 40 μl of Laemmli sample buffer by incubating them 10 minutes at 70C. The immune-complexes were separated by SDS-PAGE and then immune-blotted using anti-PITX1, anti-SOX2, anti-TRP63, anti-KLF4 and anti-VINCULIN antibodies.
ChIP-seq and ATAC-seq sample preparation
ChIP-seq
For ChIP-seq analyses 1×107 SCC cells were fixed in PBS containing 1% of formaldehyde for 8 min at room temperature in rotation. The reaction was quenched with 0.125M glycine 5 minutes at room temperature. After washing twice with cold PBS, cells were collected and the chromatin was prepared according to the ChIP-IT High Sensitivity protocol (Cat #53040). Cell pellets were re-suspended in 10ml of chromatin prep buffer supplemented with proteinase inhibitor cocktail (PIC) and PMSF 100 μM and nuclei were extracted using a chilled dounce homogenizer. Nuclei were re-suspended in ChIP buffer supplemented with PIC and PMSF 1mM and sonicated using a Diagenode Bioruptor 300 at maximum intensity and pulsed for 30 seconds on and 30 seconds off for 30 cycles. Previous to ChIP, chromatin size was confirmed by reverse-crosslinking of 2.5% of the input by incubation with 10μg of RNAse for 30min at 37°C followed by addition of 20μg proteinaseK and incubation for 30 min at 55°C and 2 hours at 80°C, making sure we had achieved an average length between 200–1200. Immunoprecipitation was performed overnight at 4 °C on 30μg of sheared chromatin using 4μg of PITX1 (Santa Cruz, 18922), SOX2 (Abcam, Ab92494), TP63 (Cell Signaling, 13109) and KLF4 (R&D, AF3758) antibodies previously blocked. Chromatin samples were washed five times before crosslink reversal. DNA was then extracted using in a DNA purification column (AM #103928) and eluted in 37μl of DNA Purification Elution Buffer (AM #103498) for ChIP-seq analysis. Sample quality and DNA concentration was assessed by Qubit. ChIP-Seq libraries were made using the ThruPLEX DNA-seq kit (Rubicon Genomics, R400427) using 200pg of ChIP DNA or KAPA Hyper Prep kit (KAPA, KK8505) using 1ng of ChIP DNA. Libraries were size selected prior to PCR amplification using AMPure XP beads (Beckman Coulter, A63880). Multiplexed libraries were run on an Illumina HiSeq 2500 instrument, Genome Analyzer using the 50-base pair single read method.
ChIP-PCR
For ChIP-PCR analysis, immunoprecipitated chromatin was eluted in 200ul of DNA purification and elution buffer. Input and IgG, PITX1, SOX2, TP63 and H3K27Ac-immunoprecipitated samples were analyzed by RT-PCRs using specific primers for the analyzed regions (See Table S7). Promoter region of Afm (Afamin or alpha-albumin) was used as negative control (Ruiz-Llorente et al., 2012). qRT–PCR was performed with FastStart Universal SYBR Green Master (Roche). Standard curves were done to validate the efficiency of the primers and calculate the ng of DNA in each ChIP-RT-PCR reaction. The enrichment of target sequences in ChIP material was calculated relative to the Afm negative control, and normalized to their relative amplification in the IgG sample.
ChIP Re-ChIP
For ChIP Re-ChIP analysis, fixed chromatin was prepared as described above and probed consecutively with anti-PITX1/KLF4 and KLF4/PITX1 antibodies according to Re-ChIP-IT® kit manufacturer protocol instructions (Active Motif 53016). Briefly, 30 μg of sheared chromatin was incubated with 4μg of anti-PITX1 or anti-KLF4 antibodies overnight at 4C. The next day, chromatin was washed, eluted, de-salted, and re-incubated with anti-KLF4 and anti-PITX1 antibodies. We also included anti-SOX2 antibodies as a positive control to test its binding to KLF4 and PITX1 bound elements, or a no-antibody control, to check for contaminations coming from our first immuno-precipitation step. After washing the immune-complexes, chromatin was eluted and crosslinks were reverted. Enrichment of chromatin was analyzed by RT-PCRs as described above, using specific primers for the analyzed regions (See Table S7).
ChIPmentation
ChIPmentation was performed as previously described (Schmidl et al., 2015). For KLF4 and PITX1 ChIP-seq experiments, 1×106 TPCs or supra-basal SCC cells were isolated by flow cytometry as described in Figure 1G and S1G. H3K27Ac ChIP-seq experiments were conducted with 2×106 shScr or shPitx1 TPCs in culture. Cells were fixed, resuspended in 200 μl of sonication buffer (10mM Tris pH8, 0.25% SDS, 2mM EDTA) supplemented with PIC, and sonicated as described above. Chromatin was then diluted 1:1.5 with equilibration buffer (10mM Tris, 233mM NaCl, 1.66 TritonX-100. 0.166 DOC, 1mM EDTA, PIC) and incubated over night with 4 μg of anti-PITX1, KLF4 or H3K27Ac antibodies, followed with a 3-hour incubation with 10 μl protein G Dynadeabs (previously blocked in 1%BSA). Immunocomplexes were washed twice with RIPA-LS, RIPA-HS, RIPA-LiCl and 10mM Tris pH8 supplemented with PIC (LS: 10mM Tris-HCl pH8, 140mM NaCl, 1mM pH8 EDTA, 0.1% SDS, 0.1% NA.Deoxycolate, 1% Triton X-100; HS: 10mM Tris-HCL pH8, 1mM EDTA, 500mM NaCl, 1% Triton X-100, 0.1% SDS, 0.1% DOC; LiCl: 10mM Tris-HCl pH8, 1mM EDTA, 250mM, 0,5% NP-40, 0.5% DOC), before they were incubated with the tagmentation reaction (1μl Tn5, 1x Tagmentation buffer (50mM Tris pH8, 25mM MgCl2, 50% v/v dimethylformamide)) for 1 minute at 37C. Tagmented DNA was washed twice with RIPA-LS and TE, eluted, and crosslinks were reversed over night in elution buffer (10mM Tris-HCl pH8, 05mM EDTA pH8, 300mM NaCl, 0,4% SDS) with proteinase K. DNA was then purified using Qiagen MinElute PCR purification kit (28004) in a final volume of 10 μl. To reduce GC and size bias during the PCR amplification step, the number of PCR cycles were optimized by qPCR and amplification was stopped before saturation, at a cycle number corresponding to a quarter of the maximum fluorescent intensity. Libraries were cleaned and size selected using SPRI beads (AMPureXP beads, Beckman). Multiplexed libraries were sequenced on an Illumina HiSeq 4000 Genome Analyzer using the 50-base single-end read method.
ATAC-seq
Assay for transposase accessible chromatin followed by sequencing (ATACseq) was perform as previously described (Buenrostro et al., 2013), with the following modifications (Wang et al., 2016). 1×105 FACS-purified TPCs were collected, washed once with cold PBS and pelleted by centrifugation for 5min at 4°C. Cell pellets were re-suspended in 50μl of lysis buffer per 50,000 cells processed (10mM Tris-HCl pH 7.4, 10mM NaCl, 3mM MgCl2, 0.1% Igepal CA-630) and nuclei were pelleted by centrifugation for 25min at 500g, 4°C using a swing rotor with low acceleration and brake settings. Nuclei were re-suspended in 50μl reaction buffers containing 2μl of Tn5 transposase and 22.5μl of TD buffer (Nextera DNA Sample Preparation Kit, Illumina). The reaction was incubated at 37°C for 30min. DNA was then purified using Qiagen MinElute PCR purification kit (28004) in a final volume of 10 μl. PCR amplification step was performed as described on the ChIPmentation section. Multiplexed libraries were sequenced on an Illumina HiSeq 2500 Genome Analyzer using the 50-base paired end read method.
QUANTITATION AND STATISTICAL ANALYSIS
Measurements, quantification, graphing and statistics
All experiments were carried out single blinded. All shRNA-mediated knockdown experiments in vivo and in vitro were repeated at least three independent times with biological replicates (3 SCC cell lines). All quantitative data were collected from experiments performed in at least triplicate, and expressed as mean±s.d., 95% confidence interval, min/max or s.e.m. Differences between groups were assayed using unpaired or paired two-tailed Student’s t-test, or Mann–Whitney test (proliferation and apoptosis quantifications, clonal size distributions and division axis) using Prism 7 (GraphPad Software). Box-and-whisker plots are used to describe the entire population without assumptions about the statistical distribution. Significant differences were considered when P<0.05 as indicated by asterisks in supplemental figures, or p-values in main figures. For clonal competition assays, population sizes were determined by counting RFP or GFP cells. For population analysis of the tumors, integrin gating was defined according with CD49f/α6 and CD29/β1 expression and their CD71 expression levels. For proliferation and apoptosis quantifications on the tumors, pictures were acquired at x20 magnification and deconvolved, before Ki67 or pH3 expression was automatically detected in cells expressing H2B-GFP in shScr or shPitx1 Doxycycline treated tumors using NIS elements software. The percentage of MKI67 or pH3 positive cells within the lineage was measured in more than 100 fields per condition in at least 3 independent tumors and plotted in Prism 7. For the analysis of expression patterns of KLF4, SOX2, PITX1 and MKI67, tumor parenchymal cells were consider basal, if they were positive for CD49f/α6, and supra-basal if the were not. The percentage of KLF4, SOX2, PITX1 and MKI67 basal or supra-basal cells was measured in at least 4 fields per condition in at least 3 different tumors and plotted in Prism7. Tumor cell density was measure counting the number of nuclei (DAPI) per area of tumor parenchyma in at least 4 fields per condition in at least 3 different tumors and plotted in Prism7. Statistical and graphical data analyses were performed in R and Prism 7. Images in Figures 1 A, B, C; 2 L, M; 3C; 4F,J, 6 B, C; Supp Figures 1A, D; 3 C, D, E; 10 G, H, I]; were acquired as composite images and cropped using ImageJ. Figures were prepared using Adobe Photoshop and Illustrator CC6.
PITX1 and SOX2 expression in human specimens
The percentage of PITX1 and SOX2 positive cell staining (Negative: 0%, Low: 1–5%, Medium: 5–20%, High: >20%) was assessed blinded prior to the assessment of histopathological characteristics. H&E stains were performed by the Histopathology core at NYULMC. Blinded slides were scored by a pathologist for differentiation status before pathology results have been combined with SOX2 and PITX1 staining results.
RNA-seq Analysis
Mouse assembly version mm10/NCBIm38 build and ENSEMBL annotations release 69 were used for the RNA-sequencing alignment, transcriptome quantification and differential expression analysis. More specifically, Bowtie (Langmead et al., 2009) version 2.2.6 was used for align sequenced reads. Transcriptome quantification and differential expression analysis was performed using the Cufflinks protocol (Trapnell et al., 2012) tophat version 2.0.9 (with parameters --no-coverage-search and --no-novel-juncs) and cufflinks version 2.2.0 (default parameters). Differential gene expression analyses were performed between shScr and shPitx1 populations isolated from two independent TPC cultures. Area proportional Venn diagrams were generated with BioVenn (Hulsen et al., 2008) and VENNY2.1 (Oliveros, J.C., 2005–2017). Gene ontology analyses were performed with the Database for Annotation, Visualization and Integrated Discovery (DAVID), version 6.7 (Huang et al., 2007).
ChIP-seq and ATAC-seq analysis
Mouse PITX1, SOX2, TRP63 and KLF4 ChIP-seq were done at least in biological replicates (two SCC cell lines). The effect of Pitx1 depletion on chromatin accessibility and H3K27Ac was evaluated performing ATACseq and H3K27Ac ChIP-seq in technical replicates. Mouse assembly version mm10/NCBI m38 or human version hg19/NCBI GRCh37 build were used for sequence alignments with bowtie (Langmead et al., 2009) version 2.1.0. Sequences with MAPQ scores <30 were removed with samtools (Li et al., 2009) and duplicates were removed with picard-tools version 1.88 (http://broadinstitute.github.io/picard). IGV viewer files were generated with igvtools count -z 5 -w 25 -e 250. ChIP-seq peaks were called with MACS (Zhang et al., 2008) version 2.1.0 in comparison to input controls (or Pitx1KO ChIP for mouse Pitx1 ChIP-seq) with the parameters q 0.001 --nomodel --extsize 400. ChIP peaks distribution was determined using the Rpackage ChIPseeker (Yu et al., 2015). Motif discovery was performed with MEME-ChIP (Bailey et al., 2009; Machanick and Bailey, 2011), CISTROME project (Liu et al., 2011) or HOMER (Heinz et al., 2010) using 200bp sequence surrounding peak summits. Motif containing ChIP-seq peaks were analyzed with the GREAT: Genomic Regions Enrichment of Annotations Tool (McLean et al., 2010), using default parameters. Integrative Genome Viewer (IGV) (Robinson et al., 2011) was used for track visualization and SEQminer (Ye et al., 2011) was used for visualization of ChIP-Seq heat maps and Kmean clustering. DeepTools (Ramírez et al., 2014) was used to generate histograms and heatmaps to visualize the average enrichment of ATAC signal over the indicated bound or clusters regions normalized to the effective genome size (mm10). Annotated TSS on mm10 was used to validate the correct normalization of the datasets. To quantify the density of the ATAC-seq or ChIP-seq on the different datasets, we used normalized bigwig files, defined the regions of interest with our reference data sets in bed format, and calculated signal intensities in each region by generating Matrices with Deeptools, using a 50bp bin size. For statistical analyses, we generated a sorted list of ChIP-seq or ATAC-seq peaks encompassing all experimental conditions, before we defined read counts for each peak in each condition with htseq, and analyzed these data sets with DEseq2 (Love et al., 2014) using p<0.05 as significance cutoff. RNA-seq and ChIP-seq data were integrated by extracting the closed up-stream and downstream gene associated to each ChIP peak using GREAT at default settings (McLean et al., 2010).
DATA AND SOFTWARE AVAILABILITY
The accession number for the RNA-seq, ATAC-seq and ChIP-seq data reported in this paper is NCBI GEO: GSE104145
Supplementary Material
Table S1, related to Figure 3. Differentially expressed genes in shPitx1 SCCs.
Table S2, related to Figure 3. PITX1 direct targets.
Table S5, related to Figure 4. Direct and indirect targets of PITX1 and its interaction with SOX2 and TRP63 in mouse SCC.
Table S6, related to Figure 7. Analysis of KLF4 and PITX1 interaction.
Table S7, related to STAR Methods. List of oligonucleotides.
Acknowledgments
We thank E. Fuchs for generous support and reagents, Eva Hernando-Monge, Slobodan Beronja, and Aris Tsirigos for discussions and comments on the manuscript. We are grateful to the NYU Medical Center Flow cytometry core facility, the Division of Comparative Medicine for expert handling and care of mice, the NYU genome technology center for next generation sequencing, and the High-Performance Computing Facility for cluster access and data storage. This research was supported by American Cancer Society grant RSG-16–033-01-DDC, the Orbuch-Brand Pilot Grant Program for Cancers of the Skin, and NIH grants R00AR057260 and R01CA181111 to M.S., T32CA009161 and T32AR064184 to A.S-P. and T32 CA009161 to S.H-P. Core funding was partially supported by NIH grant P30CA016087 to the Perlmutter Cancer Center. M-C.L. was supported by a Scholarship from the Austrian Marshall Plan Foundation.
Footnotes
Supplementary Information
Supplementary information includes 7 figures and 7 tables.
Declaration of Interests
The authors declare no competing interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam RC, Yang H, Rockowitz S, Larsen SB, Nikolova M, Oristian DS, Polak L, Kadaja M, Asare A, Zheng D, et al. (2015). Pioneer factors govern super-enhancer dynamics in stem cell plasticity and lineage choice. Nature 521, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold K, Sarkar A, Yram MA, Polo JM, Bronson R, Sengupta S, Seandel M, Geijsen N, and Hochedlinger K (2011). Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 9, 317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, and Noble WS (2009). MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 37, W202–W208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Rubin AJ, Qu K, Zhang J, Giresi PG, Chang HY, and Khavari PA (2015). A novel ATAC-seq approach reveals lineage-specific reinforcement of the open chromatin landscape via cooperation between BAF and p63. Genome Biol 16, 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Tang J, Lopez-Pajares V, Tao S, Qu K, Crabtree GR, and Khavari PA (2013). ACTL6a enforces the epidermal progenitor state by suppressing SWI/SNF-dependent induction of KLF4. Cell Stem Cell 12, 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardot ES, Valdes VJ, Zhang J, Perdigoto CN, Nicolis S, Hearn SA, Silva JM, and Ezhkova E (2013). Polycomb subunits Ezh1 and Ezh2 regulate the Merkel cell differentiation program in skin stem cells. Embo J 32, 1990–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beronja S, Livshits G, Williams S, and Fuchs E (2010). Rapid functional dissection of genetic networks via tissue-specific transduction and RNAi in mouse embryos. Nat Med 16, 821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, and Fuchs E (2014). Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science 344, 1242281–1242281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkarev VA, and Flores ER (2014). p53/p63/p73 in the epidermis in health and disease. Cold Spring Harb Perspect Med 4, a015248–a015248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Le Mercier M, Delatte B, Caauwe A, Lenglez S, Nkusi E, et al. (2014). SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature 511, 246–250. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, et al. (2005). Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122, 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Yonekubo Y, Hanson N, Sastre-Perona A, Basin A, Rytlewski JA, Dolgalev I, Meehan S, Tsirigos A, Beronja S, et al. (2017). TGF-beta-Induced Quiescence Mediates Chemoresistance of Tumor-Propagating Cells in Squamous Cell Carcinoma. Stem Cell 21, 650–664.e658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro JD, Giresi PG, Zaba LC, Chang HY, and Greenleaf WJ (2013). Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Califano A, and Alvarez MJ (2017). The recurrent architecture of tumour initiation, progression and drug sensitivity. Nat Rev Cancer 17, 116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvisi DF, Ladu S, Conner EA, Seo D, Hsieh J-T, Factor VM, and Thorgeirsson SS (2011). Inactivation of Ras GTPase-activating proteins promotes unrestrained activity of wild-type Ras in human liver cancer. J. Hepatol. 54, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Knösel T, Ye F, Pacyna-Gengelbach M, Deutschmann N, and Petersen I (2007). Decreased PITX1 homeobox gene expression in human lung cancer. Lung Cancer 55, 287–294. [DOI] [PubMed] [Google Scholar]
- Driessens G, Beck B, Caauwe A, Simons BD, and Blanpain C (2012). Defining the mode of tumour growth by clonal analysis. Nature 488, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezhkova E, Lien W-H, Stokes N, Pasolli HA, Silva JM, and Fuchs E (2011). EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes Dev 25, 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Wang D, Burgmaier JE, Teng Y, Romano R-A, Sinha S, and Yi R (2018). Single Cell and Open Chromatin Analysis Reveals Molecular Origin of Epidermal Cells of the Skin. Dev. Cell 1–23. [DOI] [PubMed] [Google Scholar]
- Frye M, and Benitah SA (2012). Chromatin regulators in mammalian epidermis. Semin Cell Dev Biol 23, 897–905. [DOI] [PubMed] [Google Scholar]
- Ge Y, Gomez NC, Adam RC, Nikolova M, Yang H, Verma A, Lu CP-J, Polak L, Yuan S, Elemento O, et al. (2017). Stem Cell Lineage Infidelity Drives Wound Repair and Cancer. Cell 169, 636–642.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasch G, SCHOBER M, Pasolli HA, Conn EB, Polak L, and Fuchs E (2007). Loss of TGFbeta signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell 12, 313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder G, Callaerts P, and Gehring WJ (1995). Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science 267, 1788–1792. [DOI] [PubMed] [Google Scholar]
- Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, Barrera LO, Van Calcar S, Qu C, Ching KA, et al. (2007). Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat. Genet. 39, 311–318. [DOI] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, and Glass CK (2010). Simple Combinations of Lineage-Determining Transcription Factors Prime cis-Regulatory Elements Required for Macrophage and B Cell Identities. Molecular Cell 38, 576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Tan Q, Kir J, Liu D, Bryant D, Guo Y, Stephens R, Baseler MW, Lane HC, et al. (2007). DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res 35, W169–W175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsen T, de Vlieg J, and Alkema W (2008). BioVenn - a web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genomics 9, 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, and Cotsarelis G (2005). Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 11, 1351–1354. [DOI] [PubMed] [Google Scholar]
- Kadoch C, and Crabtree GR (2013). Reversible disruption of mSWI/SNF (BAF) complexes by the SS18-SSX oncogenic fusion in synovial sarcoma. Cell 153, 71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman CK, Mosimann C, Fan ZP, Yang S, Thomas AJ, Ablain J, Tan JL, Fogley RD, van Rooijen E, Hagedorn EJ, et al. (2016). A zebrafish melanoma model reveals emergence of neural crest identity during melanoma initiation. Science 351, aad2197–aad2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong G, Liu Z, Wu K, Zhang Y, Deng Z, Feng W, Chen S, and Wang H (2015). Strong expression of paired-like homeodomain transcription factor 1 (PITX1) is associated with a favorable outcome in human osteosarcoma. Tumour Biol. 36, 7735–7741. [DOI] [PubMed] [Google Scholar]
- Kreso A, and Dick JE (2014). Evolution of the Cancer Stem Cell Model. Cell Stem Cell 14, 275–291. [DOI] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, and Salzberg SL (2009). Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10, R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapouge G, Beck B, Nassar D, Dubois C, Dekoninck S, and Blanpain C (2012). Skin squamous cell carcinoma propagating cells increase with tumour progression and invasiveness. Embo J 31, 4563–4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latil M, Nassar D, Beck B, Boumahdi S, Wang L, Brisebarre A, Dubois C, Nkusi E, Lenglez S, Checinska A, et al. (2017). Cell-Type-Specific Chromatin States Differentially Prime Squamous Cell Carcinoma Tumor-Initiating Cells for Epithelial to Mesenchymal Transition. Cell Stem Cell 20, 191–204.e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TI, Rinaldi NJ, Robert F, Odom DT, Bar-Joseph Z, Gerber GK, Hannett NM, Harbison CT, Thompson CM, Simon I, et al. (2002). Transcriptional regulatory networks in Saccharomyces cerevisiae. Science 298, 799–804. [DOI] [PubMed] [Google Scholar]
- Levy V, Lindon C, Zheng Y, Harfe BD, and Morgan BA (2007). Epidermal stem cells arise from the hair follicle after wounding. Faseb J. 21, 1358–1366. [DOI] [PubMed] [Google Scholar]
- Lewis EB (1992). The 1991 Albert Lasker Medical Awards. Clusters of master control genes regulate the development of higher organisms. Jama 267, 1524–1531. [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup (2009). The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Ortiz JA, Taing L, Meyer CA, Lee B, Zhang Y, Shin H, Wong SS, Ma J, Lei Y, et al. (2011). Cistrome: an integrative platform for transcriptional regulation studies. Genome Biol 12, R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, and Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machanick P, and Bailey TL (2011). MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics 27, 1696–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maza I, Caspi I, Zviran A, Chomsky E, Rais Y, Viukov S, Geula S, Buenrostro JD, Weinberger L, Krupalnik V, et al. (2015). Transient acquisition of pluripotency during somatic cell transdifferentiation with iPSC reprogramming factors. Nat Biotechnol 33, 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CY, Bristor D, Hiller M, Clarke SL, Schaar BT, Lowe CB, Wenger AM, and Bejerano G (2010). GREAT improves functional interpretation of cis-regulatory regions. Nat Biotechnol 28, 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham CE, and Morrison SJ (2013). Tumour heterogeneity and cancer cell plasticity. Nature 501, 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milo R, Shen-Orr S, Itzkovitz S, Kashtan N, Chklovskii D, and Alon U (2002). Network motifs: simple building blocks of complex networks. Science 298, 824–827. [DOI] [PubMed] [Google Scholar]
- Modrek AS, Golub D, Khan T, Bready D, Prado J, Bowman C, Deng J, Zhang G, Rocha PP, Raviram R, et al. (2017). Low-Grade Astrocytoma Mutations in IDH1, P53, and ATRX Cooperate to Block Differentiation of Human Neural Stem Cells via Repression of SOX2. Cell Reports 21, 1267–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel S, Venturini L, Przybylski GK, Grabarczyk P, Schneider B, Meyer C, Kaufmann M, Schmidt CA, Scherr M, Drexler HG, et al. (2011). Activation of Paired-homeobox gene PITX1 by del(5)(q31) in T-cell acute lymphoblastic leukemia. Leuk Lymphoma 52, 1348–1359. [DOI] [PubMed] [Google Scholar]
- Nowak JA, and Fuchs E (2009). Isolation and culture of epithelial stem cells. Methods Mol. Biol. 482, 215–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaki M, Chinen H, Yoshida Y, Ohhira T, Sunamura N, Yamamoto O, Ito H, Oshimura M, and Kugoh H (2013). Decreased PITX1 gene expression in human cutaneous malignant melanoma and its clinicopathological significance. Eur J Dermatol 23, 344–349. [DOI] [PubMed] [Google Scholar]
- Pascual G, Avgustinova A, Mejetta S, Martín M, Castellanos A, Attolini CS-O, Berenguer A, Prats N, Toll A, Hueto JA, et al. (2017). Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 541, 41–45. [DOI] [PubMed] [Google Scholar]
- Pierce GB, and Wallace C (1971). Differentiation of malignant to benign cells. Cancer Res 31, 127–134. [PubMed] [Google Scholar]
- Ramírez F, Dündar F, Diehl S, Grüning BA, and Manke T (2014). deepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Res 42, W187–W191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald JG, and Green H (1975). Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6, 331–343. [DOI] [PubMed] [Google Scholar]
- Rinaldi L, Avgustinova A, Martín M, Datta D, Solanas G, Prats N, and Benitah SA (2017). Loss of Dnmt3a and Dnmt3b does not affect epidermal homeostasis but promotes squamous transformation through PPAR-γ. Elife 6, 1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi L, Datta D, Serrat J, Morey L, Solanas G, Avgustinova A, Blanco E, Pons JI, Matallanas D, Kriegsheim, Von A, et al. (2016). Dnmt3a and Dnmt3b Associate with Enhancers to Regulate Human Epidermal Stem Cell Homeostasis. Stem Cell 19, 491–501. [DOI] [PubMed] [Google Scholar]
- Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, and Mesirov JP (2011). Integrative genomics viewer. Nat Biotechnol 29, 24–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Llorente S, Carrillo Santa de Pau E, Sastre-Perona A, Montero-Conde C, Gómez-López G, Fagin JA, Valencia A, Pisano DG, and Santisteban P (2012). Genome-wide analysis of Pax8 binding provides new insights into thyroid functions. BMC Genomics 13, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladi SV, Ross K, Karaayvaz M, Tata PR, Mou H, Rajagopal J, Ramaswamy S, and Ellisen LW (2016). ACTL6A Is Co-Amplified with p63 in Squamous Cell Carcinoma to Drive YAP Activation, Regenerative Proliferation, and Poor Prognosis. Cancer Cell 1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Danés A, and Blanpain C (2018). Deciphering the cells of origin of squamous cell carcinomas. Nat Rev Cancer 18, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidl C, Rendeiro AF, Sheffield NC, and Bock C (2015). ChIPmentation: fast, robust, low-input ChIP-seq for histones and transcription factors. Nat Meth 12, 963–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneuwly S, Klemenz R, and Gehring WJ (1987). Redesigning the body plan of Drosophila by ectopic expression of the homoeotic gene Antennapedia. Nature 325, 816–818. [DOI] [PubMed] [Google Scholar]
- Schober M, and Fuchs E (2011). Tumor-initiating stem cells of squamous cell carcinomas and their control by TGF-{beta} and integrin/focal adhesion kinase (FAK) signaling. Proc. Natl. Acad. Sci. U.S.a. 108, 10544–10549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle JM, Basin A, Sastre-Perona A, Yonekubo Y, Brown J, Sennett R, Rendl M, Tsirigos A, Carucci JA, and SCHOBER M (2014). SOX2 is a cancer-specific regulator of tumour initiating potential in cutaneous squamous cell carcinoma. Nature Communications 5, 4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, and Yamanaka S (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. [DOI] [PubMed] [Google Scholar]
- Tani H, Morris RJ, and Kaur P (2000). Enrichment for murine keratinocyte stem cells based on cell surface phenotype. Proc. Natl. Acad. Sci. U.S.a. 97, 10960–10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, and Pachter L (2012). Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7, 562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong AB, and Khavari PA (2014). Control of Keratinocyte Proliferation and Differentiation by p63. Cell Cycle 6, 295–299. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, and Fuchs E (2004). Defining the epithelial stem cell niche in skin. Science 303, 359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanbokhoven H, Melino G, Candi E, and Declercq W (2011). p63, a story of mice and men. J Invest Dermatol 131, 1196–1207. [DOI] [PubMed] [Google Scholar]
- Wang L, Siegenthaler JA, Dowell RD, and Yi R (2016). Foxc1 reinforces quiescence in self-renewing hair follicle stem cells. Science 351, 613–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Ma Q, Peng S, Adelmant G, Swain D, Song W, Fox C, Francis JM, Pedamallu CS, Deluca DS, et al. (2014). SOX2 and p63 colocalize at genetic loci in squamous cell carcinomas. J. Clin. Invest 124, 1636–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Kobunai T, Yamamoto Y, Matsuda K, Ishihara S, Nozawa K, Iinuma H, Ikeuchi H, and Eshima K (2011). Differential gene expression signatures between colorectal cancers with and without KRAS mutations: crosstalk between the KRAS pathway and other signalling pathways. Eur J Cancer 47, 1946–1954. [DOI] [PubMed] [Google Scholar]
- Wong DJ, Liu H, Ridky TW, Cassarino D, Segal E, and Chang HY (2008). Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell 2, 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Schramek D, Adam RC, Keyes BE, Wang P, Zheng D, and Fuchs E (2015). ETS family transcriptional regulators drive chromatin dynamics and malignancy in squamous cell carcinomas. Elife 4, e10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye T, Krebs AR, Choukrallah M-A, Keime C, Plewniak F, Davidson I, and Tora L (2011). seqMINER: an integrated ChIP-seq data interpretation platform. Nucleic Acids Res 39, e35–e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Q-L, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, and Smith A (2008). The ground state of embryonic stem cell self-renewal. Nature 453, 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RA (2011). Control of the embryonic stem cell state. Cell 144, 940–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Wang L-G, and He Q-Y (2015). ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 31, 2382–2383. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, et al. (2008). Model-based analysis of ChIP-Seq (MACS). Genome Biol 9, R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1, related to Figure 3. Differentially expressed genes in shPitx1 SCCs.
Table S2, related to Figure 3. PITX1 direct targets.
Table S5, related to Figure 4. Direct and indirect targets of PITX1 and its interaction with SOX2 and TRP63 in mouse SCC.
Table S6, related to Figure 7. Analysis of KLF4 and PITX1 interaction.
Table S7, related to STAR Methods. List of oligonucleotides.
Data Availability Statement
The accession number for the RNA-seq, ATAC-seq and ChIP-seq data reported in this paper is NCBI GEO: GSE104145