Abstract
Background
Adolescent females living in agricultural areas where crops are routinely sprayed by pesticides are expected to be environmentally exposed to pesticidesˊ health hazards partially as those occupationally exposed.
Objective
to assess menstrual and neurobehavioral disorders among adolescent females environmentally exposed to pesticides.
Methods
This was a cross-sectional study conducted on 100 pesticide exposed adolescent females who had one or more of family members are pesticidesˊ seasonal applicators and 50 non- exposed adolescent females matched for age and education, served as controls at Menoufia governorate, Egypt during the period of pesticide application season of cotton crop from the first days of May to the end of September 2017. A self-administered and a series of neurobehavioral tests were administered and serum Acetylcholinesterase (AChE) activity was assessed.
Results
A significant lower AChE activity levels were found in the exposed group than controls (Mean±SD=238.49± 23.83 vs 303.35±78.54 IU/L; respectively). There were significant higher mean scores of trail making test (parts 1 and 2) and significant lower mean scores of (similarities test, Benton visual retention test, block design test, Santa Ana dexterity test (dominant and non-dominant hands) and Beery visuo-motor imitation test in the exposed group than the controls (P<0.05). Also, the exposed group reported more prevalent irregular menstrual cycle (26.8%) and intermenstrual bleeding (28.2%) compared to the control participants (8.1% and 8.1%; respectively).
Conclusion
Adolescent females living in agricultural areas and from families whose one or more members are pesticidesˊ applicators have significantly lower neurobehavioral performance, report more prevalent menstrual irregularities and have lower levels of serum AChE compared to a control group. The neurobehavioral deficits demonstrated a dose–response relationship AChE levels in the exposed participants. This necessitates the need for implementation of health education programs to prevent or reduce health effects associated with pesticide exposure to adolescent females.
Keywords: Environmental Pesticides’ Exposure, Adolescent females, Menstrual and Neurobehavioral Disorders, AChE
Introduction
Pesticides are toxic chemicals that are widely used throughout the world in agriculture on crops as well as for domestic purposes for mosquito and cockroach control operations (Rani et al., 2017). Organophosphate pesticides (OPs) represent a class of pesticides with high toxicity and are used in both farmlands and households (Zhu et al., 2015). The pesticide exposure of farmers’ families, especially children, can be potentially significant as there is a combination of para-occupational, environmental and domestic exposures (Silvério et al., 2017). Residential exposure depends on proximity of the house to areas treated with pesticides, the persistence of pesticides used in or around the home and domestic uses at home on pets (flies and ticks) and also on humans (lice and scabies) (Bouvier et al., 2005; Mamane et al., 2015). Direct exposure to pesticides can occur during the application whereas indirect exposure of farm spouses and children to pesticides can occur through spray drift, transfer of contaminated dust and soil from treated fields to farm vehicles and buildings, handling of contaminated clothing and personal contact with exposed individuals as they may track pesticides indoors on their clothes, shoes, skin and hair (El-Sebae, 1993; Lee et al., 2015). Also indirect exposures can occur through non-target species as air, water and soil and they represent routes of long-term generally low-level exposures. Pesticide use raises a number of environmental concerns. Over 98% of sprayed insecticides and 95% of herbicides reach a destination other than their target species (Miller, 2004). According to the Egyptian culture no females are recruited in pesticidesʹ application, hence their exposure to pesticides could be exclusively environmental either by living nearby agriculture fields, domestic use of home pesticides, or to pesticidesˊ residues spoiling clothes or skin and/or hair of their family members (brothers or fathers) working as pesticidesˊ applicators. Pesticide exposure can cause a variety of adverse health effects ranging from simple irritation of the skin and eyes to more severe effects such as endocrine disruption, blood and neurobehavioral disorders as well as the possibility of an increased risk of cancer especially, childhood cancers such as Non-Hodgkin lymphoma and leukemia (Clayton et al., 2003; Mamane et al., 2015). Organophosphate pesticides exposure can result in some neurobehavioral disorders such as memory loss, loss of coordination, reduced speed of response to stimuli, reduced visual ability, altered or uncontrollable mood; general behavior and reduced motor skills (Farahat et al., 2003; Silvério et al., 2017). Menstrual disorders like longer, irregular and missed menstrual cycles in addition to intermenstrual bleeding were detected among females exposed to carbamates and ∕ or OPs compared to women who never used pesticides (Farr et al. 2004). Pesticides are thought to pose a considerably higher risk to children than to adults as they are more vulnerable due to the significant anatomical and maturational physiological changes occurring in the brain during developmental periods including adolescence (Abdel-Rasoul et al., 2008). Most evidence for health effects of pesticides in adults comes from studies of occupationally exposed males. Relatively less is known about pesticide-related health effects in females, and there may be sex-specific risk differences with respect to reproductive toxicity. In addition, comparatively little is known about non-occupational pesticide exposure pathways (Rohlman et al., 2007). The aim of this study was to assess menstrual and neurobehavioral health disorders in the studied adolescent females that might arise due to environmental exposure of adolescent females to pesticides.
Subjects and Methods
Study design
This was a cross-sectional study.
Place and duration of the study
The study was conducted at three randomly chosen districts (Shebin El-Kom, El-Bagour and Menouf) out of the ten districts in Menoufia governorate. The pesticide exposed female adolescents’ homes lie within less than 1000 meters from the agricultural fields.
Study sample
The exposed group was chosen randomly from adolescent females ( aged from 9–18 years) whose one or more of their family members were pesticidesˊ seasonal applicators at cotton fields and / or working privately with their own backpack sprayers for other crops all over the year. Their homes lie within less than 1000 meters from the agricultural fields.
The control group included 50 adolescent females that were matched with the exposed group regarding age, residence, socioeconomic standard, educational level and their families were never involved in field pesticidesˊ applications. Their homes lied by more than 1000 meters away from the agricultural fields. All the chosen female adolescents in both groups were single.
Exclusion criteria included history of chronic medical disorders (diabetes, hypertension, asthma, thyroid disease, liver or kidney disease, peripheral neuropathy, vitamin deficiency) and illiteracy.
Study methods
each participant was subjected to the following:
- I: A predesigned questionnaire: included:
- Personal data as age, residence, level of education, distance of house from fields and past medical history of diseases.
- Menstrual history as age of menarche, regularity of cycles, intermenstrual bleeding.
- Pesticide exposure history as applying pes ticides at home, use of empty pesticide container, handling contaminated clothes of their relatives, existing in fields within 3 days after pesticide spraying.
- Occupational history of their pesticide applicators’ relatives: working days and duration of work.
- Neurological symptoms as headache, dizziness, blurred vision, impaired memory and concentration.
-
II: Neurobehavioral test batteries: included:
Age appropriate versions of the Wechsler Adult Intelligence Scale (WAIS) that were validated in an Arabic speaking population were used to assess neurobehavioral function for the studied participants. Better performance is evaluated by higher scores obtained on tests of Information, Similarities, Arithmetic, Digit Symbol, Block Design, Digit Span, and the Benton Visual Retention Test. In contrast, lower latencies or time to complete Trail Making A and B indicate better performance. Examiners were blind to the status of the participant as exposed or control (Abdel-Rasoul et al., 2008).
-
III: Serum acetylecholine esterase (AChE) assessment
Five milliliters of blood were drawn from all participants and serum AChE was determined according to Weber (1966) using standard kits (Test-combination Boehringer Mannheim GmbH Diagnostica). Serum AChE was selected because it is a better short-term indicator of cholinesterase inhibition than red blood corpuscles (RBCs) AChE due to its more rapid response to exposure; it is used as an indicator of recent, acute exposure to cholinesterase inhibiting pesticides. Also, because the primary pesticide being applied is chlorpyrifos, which has a preferentially inhibiting effect on serum AChE rather than RBC AChE (Abdel-Rasoul et al., 2008).
Consent
Written informed consents were signed by all participants before being enrolled into the study.
Ethical approval
Medical Ethics Committee at the Menoufia Faculty of Medicine approved the study protocol before starting.
Data management
Data were analyzed using IBM SPSS version 22 (SPSS Inc., Chicago, Illinois, USA). Chi- squared test (χ2) was used to examine the relation between qualitative variables. Z test is a significance test for testing proportions. For quantitative data, comparison between two groups was done using student t-test. Pearson correlation coefficient test was used to study the correlation between two quantitative variables. P values less than 0.05 were considered statistically significant.
Results
There was non-significant difference between exposed and control groups regarding socio-demographic data (age, income and education level), BMI, past clinical history (P>0.05) (data weren’t illustrated in tables).
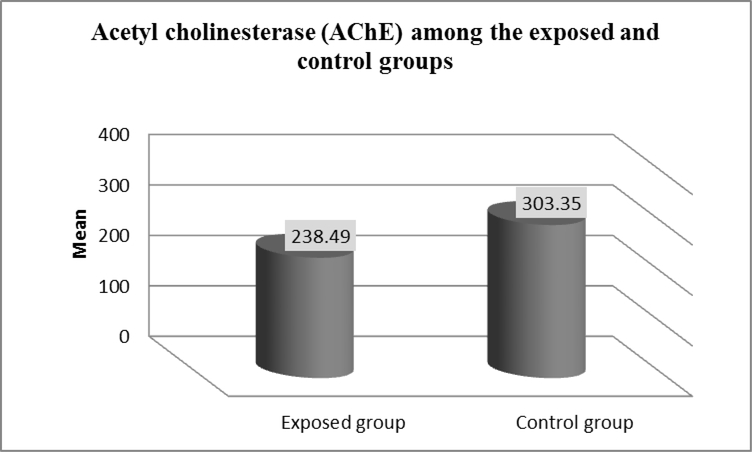
A significant lower activity levels of AChE was found in the exposed compared to the control group (Mean± SD =238.49± 23.83 versus 303.35 ±78.54 IU/L; P < 0.001) as shown in figure (1).
Figure 1:
Mean levels of serum AChE among exposed and control groups
Table 1 shows that there was a significantly higher prevalence of dizziness, blurred vision, difficulty in concentration, impaired memory, feelings of depression, fatigue and involuntary movements in limbs among exposed group than controls.
Table (1):
Prevalence of neurological manifestations in exposed and control groups
| Neurological manifestations | Studied groups | Z test | P value | |||
|---|---|---|---|---|---|---|
| Exposed (n=100) | Controls (n=50) | |||||
| No. | % | No. | % | |||
| Dizziness | 30 | 30.0 | 6 | 12.0 | 2.23 | 0.02* |
| Headache | 18 | 18.0 | 4 | 8.0 | 1.39 | 0.16 |
| Blurred vision | 27 | 27.0 | 4 | 8.0 | 2.50 | 0.01* |
| Difficulty in concentration | 24 | 24.0 | 5 | 10.0 | 1.83 | 0.03* |
| Impaired memory | 27 | 27.0 | 3 | 6.0 | 2.81 | 0.004* |
| Feeling depressed | 26 | 26.0 | 1 | 2.0 | 3.38 | 0.003* |
| Feeling anxious | 17 | 17.0 | 4 | 8.0 | 1.25 | 0.21 |
| Insomnia | 11 | 11.0 | 2 | 4.0 | 1.13 | 0.25 |
| Fatigue | 31 | 31.0 | 3 | 6.0 | 3.24 | 0.001* |
| Numbness | 13 | 13.0 | 5 | 10.0 | 0.27 | 0.78 |
| Twitches, or involuntary movements in limbs | 7 | 7.0 | 0 | 0.0 | 1.51 | 0.04* |
Significant
Table 2 shows that there were significantly higher mean scores of trail making test (parts 1 and 2) among the exposed group than the controls and significant lower mean scores of (similarities test, Benton visual retention test, block design test, Santa Ana dexterity test (dominant and non-dominant hands) and Beery visuo-motor imitation test (P<0.05).
Table (2):
Mean ± SD of neurobehavioral performance for the exposed and control groups
| Neurobehavioral tests | Studied groups |
t-test | P value | |
|---|---|---|---|---|
| Exposed (n=100) | Controls (n=50) | |||
| Attention: | ||||
| Trail making test part 1 | 89.60±16.44 | 81.70±18.11 | 2.58 | 0.008* |
| Trail making test part 2 | 176.35±39.27 | 158.55±39.52 | 2.61 | 0.009* |
| Concentration: | ||||
| Similarities test | 10.31±2.59 | 13.91±3.11 | 7.50 | <0.001* |
| Intellectual function: | ||||
| Block design test | 28.94±3.06 | 31.55±2.37 | 5.29 | 0.004* |
| Visual perception and memory: | ||||
| Benton visual retention test | 5.70±1.66 | 6.87±2.07 | 3.39 | 0.007* |
| Hand dexterity: | ||||
| Santa Ana dexterity test (dominant hand) | 40.49±2.58 | 46.31±2.91 | 12.47 | <0.001* |
| Santa Ana dexterity test (non-dominant hand) | 37.78±3.57 | 39.82±3.12 | 3.44 | 0.007* |
| Visuo-motor integration: | ||||
| Beery visuo-motor imitation test | 12.96±2.17 | 15.13±1.09 | 6.66 | 0.002* |
Significant
This table shows that there was a significantly positive correlation between AChE activity levels and duration of exposure with tests of Similarities, Santa Ana dexterity test dominant and non- dominant hand and Beery visuo-motor imitation. On the other hand, there was a significantly negative correlation between AChE activity levels with Trail making tests (part 1 and part 2).
DISCUSSION
This study revealed significantly higher prevalence of health effects in adolescent females environmentally exposed to pesticides compared to control group. Exposed participants had lower. AChE activity levels, impaired neurobehavioral performance, more prevalent neurological manifestations and menstrual irregularities.
The significant association between the exposure to pesticides and decrement in the different functions of neurobehavioral performance (P<0.05, Table 2) as exposure to OP pesticides has been associated with irreversible inhibition of the enzyme cholinesterase thus there is a massive accumulation of the neurotransmitter acetylcholine within the synaptic cleft, different nerves and receptors in the body including neuromuscular (skeletal, smooth and cardiac) and glandular tissues because acetylcholinesterase is blocked leading to overstimulation of nicotinic and muscarinic receptors in the central and peripheral nervous system and muscle overstimulation. This decrement was also revealed by the presence of the significantly negative correlation between the number of days worked by pesticide applicator family members at the current season and years worked as pesticide applicators with Similarities, BVRT, Santa Ana dexterity test dominant hand and Beery visuo-motor imitation subtests and by significant positive correlation with Trail Making B. Also, the significant correlation between AChE activity level and the same tests (P<0.05, Table 4). These findings were consistent with other research results (e.g. Roldan-Tapia et al., 2005), that also reported a significant relationship between the period of exposure and working in applying OP pesticides and performance deficits and increased prevalence of neurological manifestations.
Table (4):
Correlations between neurobehavioral tests’ performance with AChE activity levels, duration of work of pesticide applicator family member among the exposed group
| Neurobehavioral tests | Exposed group (n=100) |
||
|---|---|---|---|
| Days worked in the current season | Worked years | AChE (IU/l) | |
| r | r | r | |
| Attention: | |||
| Trail making test part 1 | 0.19* | 0.21* | −0.31* |
| Trail making test part 2 | 0.35* | 0.63* | −0.22* |
| Concentration: | |||
| Similarities test | −0.28* | −0.39* | 0.33* |
| Intellectual function: | |||
| Block design test | −0.25 | −0.14 | 0.03 |
| Visual perception and memory: | |||
| Benton visual retention test | −0.04 | −0.12 | 0.12 |
| Hand dexterity: | |||
| Santa Ana dexterity test (dominant hand) | −0.44* | −0.41* | 0.61* |
| Santa Ana dexterity test (non-dominant hand) | −0.39* | −0.42* | 0.43* |
| Visuo-motor integration: | |||
| Beery visuo-motor imitation test | −0.33* | −0.47* | 0.55 * |
r=Pearson correlation coefficient
P<0.05
This decrement of neurobehavioral performance and reporting more prevalent neurological manifestations in the exposed participants (P<0.05, Table 1) are consistent with a previous study that examined adult male pesticide workers in Egypt (Farahat et al., 2003) which found significant deficits in complex visual-motor processing and executive function, attention and short-term memory and memory/ perception. The exposure of the adults males was similar to the current study and some of the measurementss were the same across studies. Other studies in Egyptian populations comparing adult male pesticide applicators to controls have also reported increased prevalence of neurological, neuromuscular, and psychological symptoms and psychiatric disorders (Amr, 1997; Abdel-Rasoul et al., 2008).
The current results also showed that there was a significantly lower activity level of AChE in the exposed adolescent females compared to the control ones (P<0.05, Figure 1). AChE is used as a biomarker for exposure to OP pesticides (Abdel-Rasoul et al., 2008). In spite of this recommendation, there are several studies that reported cholinesterase level showing a consistent significant association between exposure to pesticides and cholinesterase inhibition in farm workers. All studies found that AChE was significantly lower in the exposed participants than the controls (Amr, 1997; Farahat et al., 2003; Abdel-Rasoul et al., 2008).
Exposed adolescent females who reported neurological manifestations had their relatives worked significantly more days than exposed participants who do not report these manifestations. These results are similar to studies with adults which found that increased time spent working is associated with increased symptom reporting (Farahat et al., 2003; Rohlman et al., 2007). The association between the level of AChE activity and neurobehavioral performance, was confirmed by presence of significant correlations between the AChE activity level and the performance in 6 out of 8 neurobeahvioral tests (Trail Making, Similarities, Santa Ana dexterity test dominant and non- dominant hand and Beery visuo-motor imitation subtests). These findings address the concerns raised by Roldan-Tapia et al. (2005), about the relationship between neurobehavioral performance and inhibition of AChE.
Exposed adolescent females showed a significantly higher prevalence of menstrual disturbances than controls from the same communities (P<0.05, Table 3). These findings confirm previous reports indicating that menstrual disorders like longer, irregular and missed menstrual cycles in addition to intermenstrual bleeding were detected among adult females exposed to carbamates and ∕ or OPs compared to adult females who never used pesticides (Farr et al., 2004). Exposure to pesticides has been associated with menstrual cycle disturbances most probably due to potential effects of endocrine disrupting pesticides on the female reproductive system as modulation of hormone concentrations, ovarian cycle irregularities and impaired fertility.
Table (3):
Menstrual history of the exposed and control groups
| Menstrual history | Studied groups |
χ2 test | P value | |||
|---|---|---|---|---|---|---|
| Exposed (n=100) |
Controls (n=50) |
|||||
| No. | % | No. | % | |||
| Menarche: | ||||||
| Yes | 71 | 71.0 | 37 | 74.0 | 0.15 | 0.69 |
| No | 29 | 29.0 | 13 | 26.0 | ||
| Menarche age |
n=71 |
n=37 |
t-test = 1.64 | 0.11 | ||
| 12.97±1.88 | 12.35±1.82 | |||||
| Menstrual cycle: | ||||||
| Regular | 52 | 73.2 | 34 | 91.9 | 5.22 | 0.02* |
| Irregular | 19 | 26.8 | 3 | 8.1 | ||
| Intermenstrual bleeding: | ||||||
| Present | 20 | 28.2 | 3 | 8.1 | 4.70 | 0.03* |
| Absent | 51 | 71.8 | 34 | 91.9 | ||
Significant
Table 3 shows that the exposed group had a significantly higher prevalence of irregular menstruation and intermenstrual bleeding compared to the control group.
Limitations of the study
The main limitation of our study is the scanty number of studies and cohorts.on the adolescent females environmentally exposed to pesticides Furthermore, not all neurobehavioral tests were used in all studies and cohorts. Greater precision would be achieved by the inclusion of additional studies. The second important limitation is the inconsistencies in exposure classification among the cohorts, ranging from purely categorical classification (agricultural workers/applicators vs controls) to more quantitative indices of exposure level including measurement of exposure across time.
Conclusions and Recommendations
Pesticides have been linked to numerous adverse health effects that are different in females than in males. Adolescent females living in agricultural areas and from families whose one or more members are pesticidesˊ sprayers have significantly lower neurobehavioral performance, report more prevalent manifestations of menstrual disturbances and have lower activity levels of serum AChE compared to a control group. The neurobehavioral deficits demonstrated a dose–response relationship between days and years of exposure and neurobehavioral performance and prevalence of manifestations reported. Since adolescents around the world are exposed to OP pesticides, these studies suggest an urgent need to evaluate this potential problem.
Future research should focus on the type of health educational training considering the perceived benefits and disadvantages while developing plans to decrease health disorders among adolescent females.
Acknowledgements
The authors would like to thank the participants who generously agreed to participate in this work for their time to provide valuable information. In addition, we appreciate the expert team who shared in application of the neurobehavioral test batteries for the participants recruited for this study.
Footnotes
Conflicts of Interest: None declared
REFERENCES
- 1.Abdel-Rasoul G, Abou Salem M, Mechael A, Hendy O, Rohlman D and Ismail A (2008): Effects of occupational pesticide exposure on children applying pesticides. Neuro; 29(5): 833–38. [DOI] [PubMed] [Google Scholar]
- 2.Amr M, Halim Z and Moussa S (1997): Psychiatric disorders among Egyptian pesticide applicators and formulators. Environ Res; 73:193–9. [DOI] [PubMed] [Google Scholar]
- 3.Bouvier G, Seta N and Vigouroux–Villard A (2005): Insecticide urinary metabolites in nonoccupationally exposed populations. J Toxicol Environ Health B Crit Rev; 8: 485–12. [DOI] [PubMed] [Google Scholar]
- 4.Clayton C, Pellizzari E, Whitmore R, Quackenboss J and Sefton K (2003): Distributions, associations, and partial aggregate exposure of pesticides and polynuclear aromatic hydrocarbons in the Minnesota Children’s Pesticide Exposure Study (MNCPES). J Exp Analys and Environl Epidemio; 13: 100–11. [DOI] [PubMed] [Google Scholar]
- 5.El-Sebae AH (1993): Special problems experienced with pesticide use in developing countries. Regul Toxicol Pharmacol; 17 (3): 287–91. [DOI] [PubMed] [Google Scholar]
- 6.Farahat T, Abdel Rasoul G, Amr M, Shebl M, Farahat F and Anger W (2003): Neurobehavioral effects among workers occupationally exposed to organophosphorus pesticides. Occup and Environ Med; 60: 279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farr S, Cooper G, Cai J, Savitz D and Sandler DP (2004): Pesticide use and menstrual cycle characteristics among premenopausal women in the Agricultural Health Study. Am J Epidemiol; 160(12):1194–204. [DOI] [PubMed] [Google Scholar]
- 8.Lee F, Chen W, Lin C, Lai C, Wu W, Lin I and Kao C (2015): Organophosphate Poisoning and Subsequent Acute Kidney Injury Risk. Medicine J; 94(47):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mamane A, Raherison C, Tessier J, Baldi I and Bouvier G (2015): Environmental exposure to pesticides and respiratory health. Eur Respir Rev; 24: 462–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller GT (2004): Sustaining the Earth, 6th edition. Thompson Learning, Inc. Pacific Grove, California; 9: 211–16. [Google Scholar]
- 11.Rani M, Shanker U and Jassal V (2017): Recent strategies for removal and degradation of persistent & toxic organochlorine pesticides using nanoparticles: A review. Journal of Environmental Management; 190: 208–22. [DOI] [PubMed] [Google Scholar]
- 12.Rohlman D, Lasarev M, Anger W, Scherer J, Stupfel J and McCauley L (2007): Neurobehavioral performance of adult and adolescent agricultural workers. Neurotoxicolgy; 28:374–80. [DOI] [PubMed] [Google Scholar]
- 13.Roldan-Tapia L, Parron T and Sanchez F (2005). Neuropsychological effects of long-term exposure to organophosphate pesticides. Neurotoxicol Teratol; 259–66. [DOI] [PubMed] [Google Scholar]
- 14.Silvério A, Machado S, Azevedo L, Nogueira D, de Castro Graciano M, Simões J, Viana A and Martins I (2017): Assessment of exposure to pesticides in rural workers in southern of Minas Gerais, Brazil. Environ Toxicol Pharmacol.; 55: 99–106. [DOI] [PubMed] [Google Scholar]
- 15.Weber H (1966): Quick and simple ultra-micro-method for the determination of cholinesterase. Dtsch Med Weschr; 91:1927–32. [DOI] [PubMed] [Google Scholar]
- 16.Zhu J, Dubois A, Ge Y, Olson JA and Ren X (2015): Application of human haploid cell genetic screening model in identifying the genes required for resistance to environmental toxicants: Chlorpyrifos as a case study. J Pharmacol Toxicol Methods; 76 (154): 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]