Abstract
Patient: Male, 22-year-old
Final Diagnosis: Anaplastic plasma cell myeloma
Symptoms: Bone pain
Medication:—
Clinical Procedure: BM examination
Specialty: Hematology
Objective:
Unusual clinical course
Background:
Plasma cell myeloma is a neoplastic plasma cell disorder that usually presents after the fifth decade of life; it is rarely described in younger population especially under 30 years of age. However, there are conflicting reports in the literature about the clinical behavior and overall survival in younger age groups. In approximately 2% of plasma cell myeloma, the morphology of the neoplastic cells is highly pleomorphic, quite anaplastic, and may resemble metastatic tumor cells. While this poses a challenge for morphological interpretation during diagnosis, it has been demonstrated that bone marrow morphologic features (including diffuse sheet growth pattern, immature cell morphology and high mitotic index) significantly correlates with high risk disease. Moreover, there is limited description available about the morphology of the neoplastic cells when correlating the age at presentation with the clinical outcome/biological behavior; hence, the need to report and collect such cases.
Case Report:
We report a case of plasma cell myeloma in a 22-year-old male who presented with non-specific clinical features and posed a diagnostic challenge during clinical, radiological, and laboratory examination. The pathology specimens showed anaplastic morphology. Unfortunately, after diagnosis, despite treatment with brotezomib, his disease had an aggressive clinical course and he passed away 4 months after diagnosis.
Conclusions:
Although plasma cell myeloma is rare in patients younger than 30 years, it must be considered in the differential diagnosis and investigated properly especially in patients with clinical suspicion of a metastatic non-hematological tumor. The anaplastic variant in a young patient is a diagnostic challenge and is associated with bizarre morphology, aggressive presentation, adverse cytogenetics, resistance to chemotherapy, and poor, short-term, survival.
MeSH Keywords: Bone Marrow Neoplasms; Multiple Myeloma; Neoplasms, Plasma Cell
Background
Plasma cell myeloma (PCM), a malignant, clonal plasma cell disorder that accounts for 1.6% of all malignancies and 10% of all hematologic neoplasms [1] is most commonly seen after the fifth decade of life [2] and is more common in males. The most common presenting clinical features are bone pain, anemia, impaired renal function, and hypercalcemia. The frequency of PCM in younger patients (less than 30 years of age) is extremely rare and account for 0.26% [3] of cases. In the majority of published series/case reports presentation at an earlier age is not associated with adverse clinical outcomes when compared to patients falling within the ‘usual’ age range of presentation. Rare case reports have mentioned an aggressive clinical course.
Case Report
Clinical presentation was of a 22-year-old male with low back pain and significant weight loss of 12 kg for 2 months with no fever, trauma, or neurological symptoms. Physical examination revealed localized tenderness in the anterior chest wall and lumbosacral spine but was otherwise unremarkable.
Laboratory workup revealed an increase in creatinine level 179 µmol/L (normal range 62–124 µmol/L) and hypercalcemia with a calcium level of 3.39 mmol/L (normal range 2.1–2.6 mmol/L) and high lactate dehydrogenase (LDH) 308 U/L (normal range 135–225). The complete blood count was normal with a hemoglobin of 13.6 g/dL (normal range 13–17 g/DL). Albumin was 44 g/L (normal range 35–50 g/L).
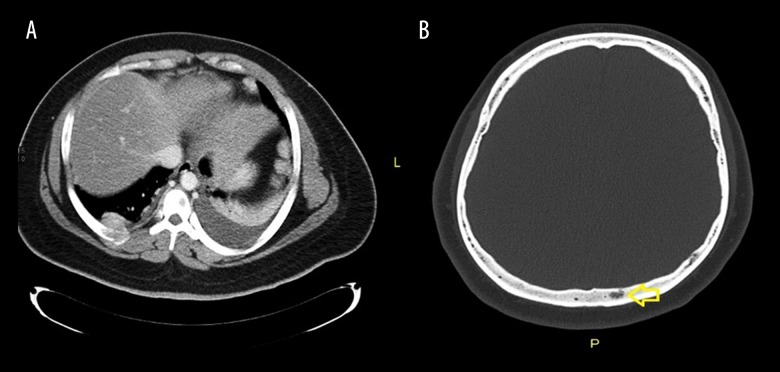
Computed tomography (CT) scan was done to rule out malignancy and it revealed the presence of a soft tissue mass at the anterior end of the 7th rib (6.5×4×7 cm) (Figure 1A), and extensive lytic lesions involving the skull base (Figure 1B), sternum, and clavicle.
Figure 1.
Computed tomography (CT) scan. (A) Selective axial CT image showing expanding soft tissue density mass lesion with underlying right rib osseous destruction. (B) A small lytic lesion is noted within the diploic space of the left posterior bony calvarium.
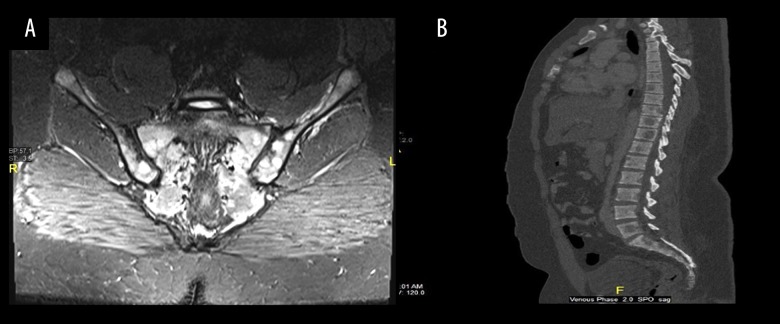
Radiologically, the features were suspicious of metastatic infiltration. Magnetic resonance imaging (MRI) spine showed disseminated bony lesions involving the whole spine and iliac bone (Figure 2A, 2B).
Figure 2.
Magnetic resonance imaging (MRI). (A) Coronal SITR of the sacrum and sacroiliac joints showing multiple bright foci of infiltration are noted bilaterally involving the sacrum and iliac side of both sacroiliac joints. (B) Sagittal reconstruction of the dorso-lumbosacral spine (bone window setting) showing multiple hypodense lytic lesions notably at L5, D11, D10, D9 as well as the proximal sacral pieces.
The clinical and radiological findings somewhat favored a metastatic tumor over PCM, though myeloma remained in the working differential diagnoses given the laboratory results.
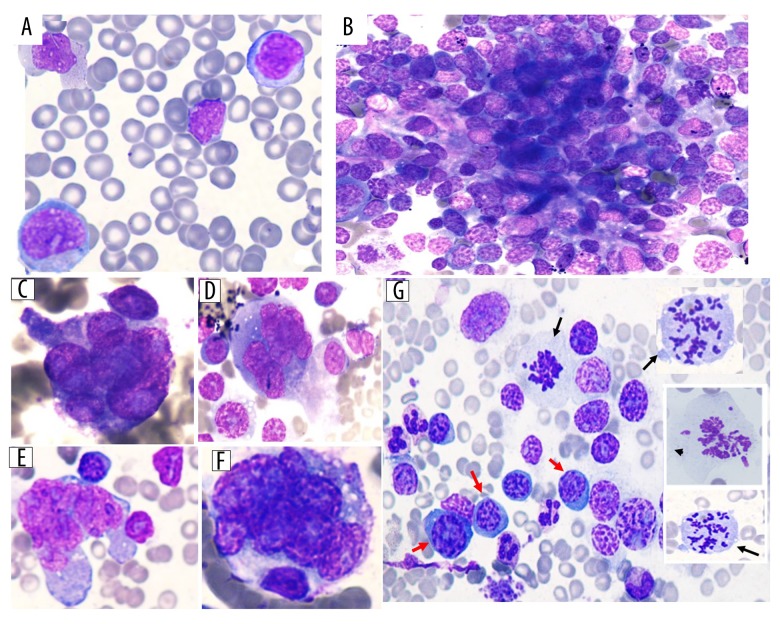
Further workup, including a peripheral blood smear which showed increased rouleaux formation with few circulating neoplastic cells with “blastoid” morphology (6%) (Figure 3A).
Figure 3.
(A) Peripheral blood smear (50×) showing few circulating neoplastic cells of blastoid morphology. (B) Bone marrow aspirate smear showing cohesive clusters/sheets of neoplastic cells mimicking metastatic tumors. (C–F) The malignant cells are large to giant in size with irregular cytoplasmic boundaries, bizarre shaped nuclei with markedly irregular contours (multilobated/abnormally lobated or multinucleated) with hyperchromatic nuclei and very prominent nucleoli (100×).(G) Some scattered plasma cells with hyperchromatic nuclei (red arrows) with frequent mitotic figures noted (black arrows).
The bone marrow aspirate smear was heavily infiltrated with cohesive clusters/sheets of highly pleomorphic neoplastic cells, many of which showed anaplastic morphology and mimicked the morphological changes seen in metastatic tumors (Figure 3B). The cells with anaplastic morphology were very large with abundant cytoplasm and markedly irregular contours. The nuclei were multi-lobated/abnormally lobated or multi-nucleated, and hyperchromatic and had very prominent nucleoli (Figure 3C–3F). Few cells show blast-like morphology. There were very few scattered plasma cells. Some cells with hyperchromatic nuclei and mitotic figures were also noted (Figure 3G).
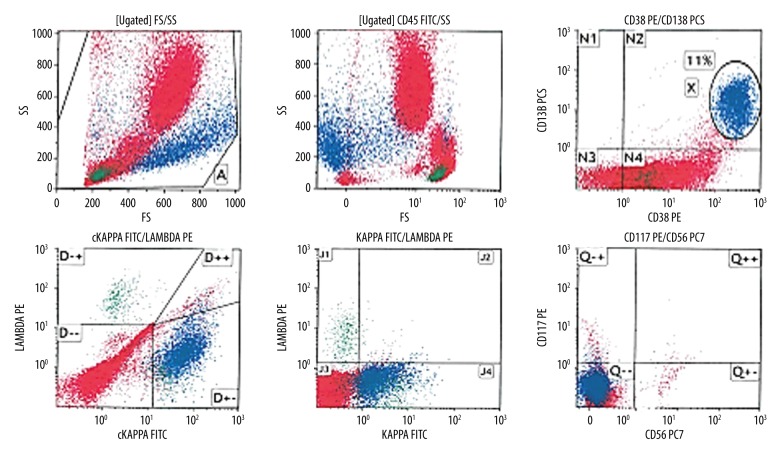
Flow cytometry analysis on bone marrow aspirate revealed an abnormal population of CD45-negative cells (11%), showing moderate side scatter and high heterogeneous forward light scatter. The population were positive for CD38 and CD138, indicative of plasma cells and showing cytoplasmic kappa light chain restriction (monotypic) with aberrant surface kappa light chain expression and negative for CD56 and CD117 (Figure 4).
Figure 4.
Flow cytometry analysis revealed a population comprising approximately 11% of the total showing moderate side light scatter and high heterogeneous forward light scatter and negative for CD45. The population was positive for CD38 and CD138 indicative of plasma cell showing cytoplasmic kappa light chain restriction (monotypic) with aberrant surface kappa light chain expression and negative for CD56 and CD117.
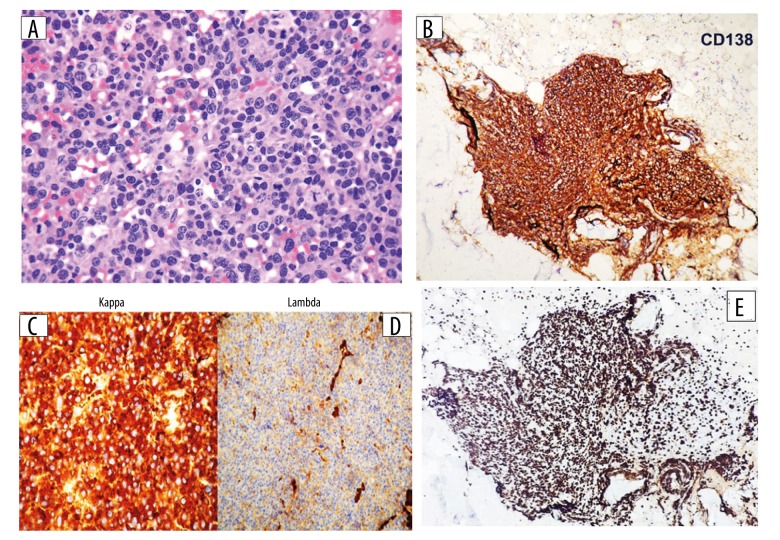
The bone marrow biopsy showed effacement of normal architecture due to a prominent interstitial infiltrate of neoplastic plasma cells that are arranged in large aggregates and a diffuse sheet-like distribution (Figure 5A) with focal areas of necrosis. The infiltrating cells exhibited anaplastic morphology with marked nuclear irregularities, prominent nucleoli, and multi-nucleation. The infiltrating plasma cells were positive for CD138 with kappa light chain restriction and high proliferation fraction (over 90% nuclear expression of Ki67 in plasma cells, Figure 5B–5E). The infiltrate was negative for CD20, CD56, IgG, IgM, IgD, and IgE.
Figure 5.
(A) Bone marrow biopsy specimen with hematoxylin and eosin stain (20×) shows core biopsy is diffusely infiltrated by sheets of neoplastic plasma cells. The cells are markedly pleomorphic, with marked nuclear irregularities, prominent nucleoli and some mitotic figures (black arrow). (B–E) Immunohistochemistry on core biopsy specimen (10×) shows (B) CD138 positive, (C) kappa: positive, (D) lambda: negative, and (E) Ki-67 (>90%).
Serum protein electrophoresis showed no monoclonal band. However, light chain analysis revealed free kappa light chain 329.2 mg/L (range 3.3–19.4) and free light chain lambda 1.24 mg/L (range 0.26–1.65), with kappa/lambda ratio at 265.
Bence-Jones protein was detected at 0.1 g/L and beta-2 microglobulin was 2.8 mg/L (range 0.8–2.2).
Cytogenetic studies performed on G-banded metaphase cells isolated from unstimulated short-term culture and Interleukin 4 stimulated long-term culture revealed a complex karyotype with 2 abnormal clones containing multiple numeric and structural abnormalities. A normal clone was also present, 4 cells.
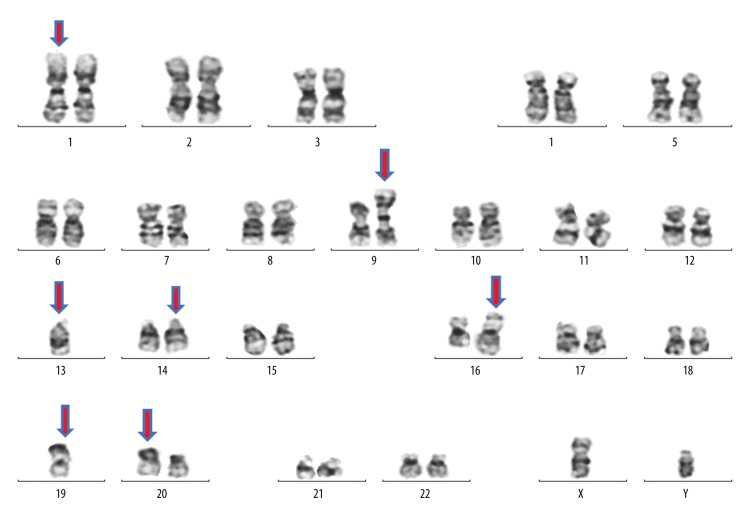
The main clone represented by 20 cells contained derivative chromosomes 16, 19, 20, and iso-chromosome 9q10, additional material on chromosome 14, and monosomy chromosome 13. A subclone represented by 6 cells contained derivative chromosome 16, insertional duplication in chromosome 1 at cyto-genetic bands 1q21q32, additional material on chromosome 14, monosomy of chromosomes 13 and 19. Furthermore, FISH analysis after cytogenetics studies confirmed a translocation between long arms of chromosomes 14 and 16 at cytogenetics bands 14q32.3 and 16q23, which results in fusion of IGHMAF genes (Figure 6).
Figure 6.
Representative karyotype of a metaphase cell with multiple numeric and structural abnormalities. ISCN nomenclature: 44,XY,i(9)(q10),-13,add(14)(q32),der(16)add(16)(p13q24),der(19)add(19)(p13.3),der(20)t(20;?)(p11;?)[20]/44,XY,dup(1) (q21q32),-13,add(14)(q32), der(16)add(16)(p13,q24),-19[6]/46,XY[4].
In view of these findings, the final diagnosis was (light chain) plasma cell myeloma, anaplastic morphological variant.
Concurrent with the bone marrow diagnosis; a CT guided biopsy (of the lower rib) confirmed infiltration by CD138-positive atypical cell infiltrate; compatible with plasma cell neoplasm.
Management and outcome
The patient was started on bortezomib-based triple therapy (bortezomib (1.3 mg/m2), cyclophosphamide (300 mg/m2), dexamethasone (40 mg), (D1, D4, D8, D11 every 21 days). Being a very young patient who presented with an advanced disease that demonstrated an aggressive clinical behavior, we planned an autologous stem cell transplantation for the patient. However, the patient travelled abroad and passed away in less than 4 months after his initial diagnosis.
Discussion
PCM is primarily and predominantly a disease of the bone marrow characterized by neoplastic plasma cell proliferation associated with a monoclonal immunoglobulin (M protein) in serum or urine. The diagnosis is based on clinical, radiological, laboratory, and cyto-histological findings.
Bone marrow examination is essential for the diagnosis of PCM, even in the presence of clinical, radiological and laboratory evidence of the disease. The bone marrow is used for both qualitative and quantitative assessment using immunophenotyping. Further cytogenetic studies, a key component of the diagnostic workup, provide prognostic information.
Based on the cytomorphology, myeloma cells are classified into 4 types: mature, immature, pleomorphic, and plasmablastic [4]
The well-differentiated or “mature” plasma cell (so called Marshalko-type) shows the characteristic round eccentric nuclei with “clock face” chromatin without nucleoli and abundant dense basophilic cytoplasm with clear perinuclear hof corresponding to the Golgi zone. In contrast, plasmablasts have vesicular nuclei with dispersed chromatin, a prominent nucleolus or nucleoli with loss of the perinuclear hof and some of the dense cytoplasmic texture and a high nucleocytoplasmic ratio [5]. Patients with plasmablastic myeloma have a median survival significantly shorter than for the other cytologic categories. It has been recently demonstrated by Hao et al. that bone marrow morphologic features, including diffuse sheet growth pattern, immature cell morphology, and high mitotic index, significantly correlates with high risk disease [6].
In approximately 2% of PCM cases, the morphology of the neo-plastic cells is highly pleomorphic, and may resemble that of metastatic epithelial tumor cells.
Anaplastic cells, by definition, show a marked degree of cytological atypia and pleomorphism with loss of features that can identify any relationship with a normal counterpart/cell of origin. Anaplastic pleomorphic plasma cells mimicking dysplastic megakaryocytes and osteoclast giant cells (similar to our case) have been recently reported in the literature [7]. The plasmablastic and anaplastic morphological variants that do not bear any resemblance to plasma cells can be very difficult to recognize on morphology alone, which may complicate the diagnosis without the aid of immunophenotyping by flow cytometry or immunohistochemistry [8].
Anaplastic myeloma variant has been reported to be more common in younger patients with a predisposition for the extramedullary site and poor prognosis. It may present initially at diagnosis [9,10] or as a feature of disease progression [11,12]. It is commonly associated with 1q21 amplification [13], 17p (p53) deletion, t(4;14), and/or chromosome 13 anomalies [14].
Most cases of PCM typically have low proliferation rate with Ki67 reported to be less than 10% [15]. In contrast, morphologically aggressive PCM with extramedullary involvement has been found to show very high Ki67 proliferative index.
There has been much interest in proliferation fraction-based prediction of clinical behavior in myeloma since the work done by Drach et al. [16].
In 2015, Juskevicius et al. [17] reported a Ki67 index up to 55% to 96% in 5 patients with morphologically aggressive myeloma and/or extramedullary involvement. All showed 13q deletion and +1q and 80% of patients were relatively young (in their 50s) and had kappa light chain restriction with serum M band of less than 3 g/dL. There was one report of a rising Ki67 in serial samples that correlated with adverse clinical outcome [18] and a proposal that the plasma cell proliferation index in light chain myeloma should be treated by stem cell transplantation [19].
PCM is a disease of the elderly and the incidence in patients younger than 30 years old is a very rare event. In 1976, Hewell et al. [20] described the first 3 well-documented cases of young patients with PCM, their ages ranged from 17 to 22 years with frequency of 1%.
Blade et al. [21] reviewed the record of 3278 patient treated with PCM at the Mayo Clinic between 1956 and 1992; the incidence of PCM in patients less than 30 years of age accounted for 0.3%. An analysis of a large case series including 10 549 patients by Ludwig et al. [3] found that only 27 patients were reported to be less than 30 years old, with a frequency of 0.26% [3]. Sophia et al. recently described the clinicopathologic features of PCM in children and young adolescent [22].
Most publications suggest that patients at a younger age present with more desirable features such as low International Staging System (ISS) and Durie-Salmon stage, good performance status, and absence of poor prognostic features as high C-reactive protein (CRP), anemia, and severe renal impairment and they have a better chance of survival [3,21,23] with rare exceptions reporting poor outcomes [24]. Hypercalcemia, thrombocytopenia, bone marrow plasmacytosis, and elevated serum LDH were associated with impaired survival among younger patients with myeloma.
As in the older age group, it appears that PCM in patients aged 30 years or less is a heterogeneous disease with some patients progressing rapidly despite therapy, and others remaining stable for years without treatment.
The median duration of survival for myeloma patients with standard cytogenetic risk is 8 to 10 years and 2 to 3 years for high-risk patients [25]. The survival of young patients with PCM was reported to be considerably longer than that of patients of all ages with PCM [21].
In contrast, young patients with anaplastic myeloma, as reported in this case, usually run an aggressive course with poor outcome even with novel therapy [26].
The poor outcome in our case could be attributed to several poor clinical and pathologic features including renal impairment at diagnosis, extensive bony lesions with extramedullary involvement, poor cytogenetics, and anaplastic morphology with very high proliferative index (Ki67 >90%).
Conclusions
In conclusion, our case demonstrates that although PCM is rare in patients younger than 30 years old, it must be considered in the differential diagnosis and investigated properly especially in patients with clinical suspicion of a metastatic non-hematological tumor. The anaplastic variant in a young patient is associated with the aggressive presentation, atypical morphology, adverse cytogenetics, resistance to chemo-therapy, and poor short-term survival. This case emphasizes the crucial role of the multidisciplinary approach in diagnosing hematological neoplasms. Prompt radiological and histo-pathological evaluation may help guide effective therapeutic interventions for aggressive cases.
Acknowledgments
Dr. Hasan Rivsi at University of Cambridge is acknowledged for his significant contribution to manuscript review.
Abbreviations
- PCM
plasma cell myeloma;
- CT
computed tomography;
- MRI
magnetic resonance imaging
Footnotes
Conflict of interests
None.
References:
- 1.Michels TC, Petersen KE. Multiple myeloma: Diagnosis and treatment. Am Fam Physician. 2017;95(6):373–83. [PubMed] [Google Scholar]
- 2.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 3.Ludwig H, Durie BG, Bolejack V, et al. Myeloma in patients younger than age 50 years presents with more favorable features and shows better survival: An analysis of 10 549 patients from the International Myeloma Working Group. Blood. 2008;111(8):4039–47. doi: 10.1182/blood-2007-03-081018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masahiko F. The histopathology of myeloma in the bone marrow. J Clin Exp Hematop. 2018;58(2):61–67. doi: 10.3960/jslrt.18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–48. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 6.Hao Y, Khaykin D, Machado L, et al. Bone marrow morphologic features, MyPRS, and gene mutation correlations in plasma cell myeloma. Mod Pathol. :2019. doi: 10.1038/s41379-019-0333-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Xie W, Tang G, Li S, et al. Anaplastic multiple myeloma resembling dysplastic megakaryocytes. Clin Case Rep. 2019;00:1–2. doi: 10.1002/ccr3.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aasen G, McKenna R. Plasma cell neoplasms: Morphology and Immunohistochemistry. In: Linden M, McKenna R, editors. Plasma cell neoplasms. Springer; Cham, Switzerland: 2016. pp. 43–64. [Google Scholar]
- 9.Rao S, Kar S, Pati H. Anaplastic myeloma: A morphologic diagnostic dilemma. Indian J Hematol Blood Transfus. 2008;24(4):188–89. doi: 10.1007/s12288-008-0046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subitha K, Renu T, Lillykutty P, Letha V. Anaplastic myeloma presenting as mandibular swelling: Diagnosis by cytology. J Cytol. 2014;31(2):114–16. doi: 10.4103/0970-9371.138691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foucar K, Raber M, Foucar E, et al. Anaplastic myeloma with massive extramedullar involvement: report of two cases. Cancer. 1983;51(1):166–74. doi: 10.1002/1097-0142(19830101)51:1<166::aid-cncr2820510132>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 12.Akihito F, Yasuhiro N, Naofumi Y, Yuji K. Morphological transformation of myeloma cells into multilobated plasma cell nuclei within 7 Days in a case of secondary plasma cell leukemia that finally transformed as anaplastic myeloma. Case Rep Hematol. 2017;2017:5758368. doi: 10.1155/2017/5758368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahmanyar M, Qi X, Chang H. Genomic aberrations in anaplastic multiple myeloma: High frequency of 1q21 (CKS1B) amplifications. Leuk Res. 2013;37(12):1726–28. doi: 10.1016/j.leukres.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 14.Sethi S, Miller I. Plasma cell myeloma with anaplastic transformation. Blood. 2016;128(16):2106. doi: 10.1182/blood-2016-08-731844. [DOI] [PubMed] [Google Scholar]
- 15.Marković O, Marisavljević D, Cemerikić V, et al. Proliferative activity of myeloma cells determined by Ki-67 antibody: Biological and clinical significance. Vojnosanit Pregl. 2005;62(1):33–38. doi: 10.2298/vsp0501033m. [DOI] [PubMed] [Google Scholar]
- 16.Drach J, Gattringer C, Glassl H, et al. The biological and clinical significance of the Ki-67 growth fraction in multiple myeloma. Hematol Oncol. 1992;10(2):125–34. doi: 10.1002/hon.2900100209. [DOI] [PubMed] [Google Scholar]
- 17.Juskevicius R, Murthy H, Dangott B. Plasma cell myeloma with very high Ki67 proliferation rate: comparison of visual estimation and computational image analysis with description of clinical and pathologic features. Am J Clin Pathol. 2015;144(2):A132. [Google Scholar]
- 18.Forsberg PA, Mark TM, Yadlapati S, et al. Rising plasma cell proliferation by Ki67/CD138 ratio at relapse is a marker of high-risk disease in multiple myeloma. Blood. 2015;126(23):2991. [Google Scholar]
- 19.Sidiqi H, Aljama MA, Jevremovic D, et al. Plasma cell proliferative index predicts outcome in immunoglobulin light chain amyloidosis treated with stem cell transplantation. Haematologica. 2018;103:1229–34. doi: 10.3324/haematol.2018.189985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hewell G, Alexanian R. Multiple myeloma in young persons. Ann Intern Med. 1976;84(4):441–43. doi: 10.7326/0003-4819-84-4-441. [DOI] [PubMed] [Google Scholar]
- 21.Bladé J, Kyle R, Greipp P. Presenting features and prognosis in 72 patients with multiple myeloma who were younger than 40 years. Br J Haematol. 1996;93(2):345–51. doi: 10.1046/j.1365-2141.1996.5191061.x. [DOI] [PubMed] [Google Scholar]
- 22.Sophia Y, Mark L, Troy L, et al. Plasma cell myeloma in children and young adults: A report of 4 cases from a single institution and a review of the literature. J Pediatr Hematol Oncol. 2017;39:452–57. doi: 10.1097/MPH.0000000000000907. [DOI] [PubMed] [Google Scholar]
- 23.Corso A, Klersy C, Lazzarino M, Bernasconi C. Multiple myeloma in younger patients: The role of age as prognostic factor. Ann Hematol. 1998;76(2):67–72. doi: 10.1007/s002770050365. [DOI] [PubMed] [Google Scholar]
- 24.Costello CL. Multiple myeloma in patients under 40 years old is associated with high-risk features and worse outcomes. Blood. 2013;122:5359. [Google Scholar]
- 25.Chng WJ, Dispenzieri A, Chim CS, et al. IMWG consensus on risk stratification in multiple myeloma. Leukemia. 2014;28(2):269–77. doi: 10.1038/leu.2013.247. [DOI] [PubMed] [Google Scholar]
- 26.Ammannagari N, Celotto K, Neppalli V, et al. Anaplastic multiple myeloma: An aggressive variant with a poor response to novel therapies. Clin Lymphoma Myeloma Leuk. 2016;16(9):e129–31. doi: 10.1016/j.clml.2016.06.008. [DOI] [PubMed] [Google Scholar]