Presentation of cases
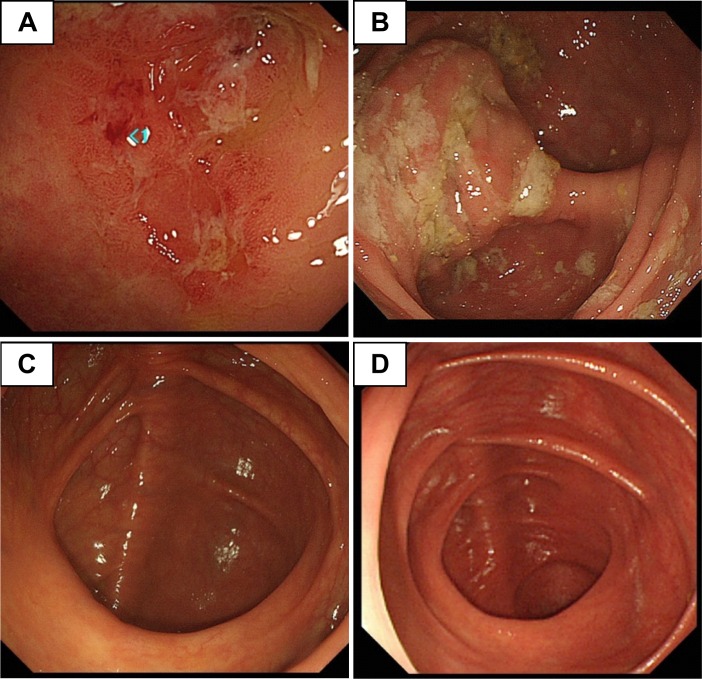
Patient 1 who was a 43-year-old male was first referred to our institute because of an allergic reaction to metronidazole with oral mucosal erosions during his sixth treatment for amebic colitis. He had a history of 5 recurrent episodes of amebic colitis (last treatment was 3 years earlier, using metronidazole followed by paromomycin) (S1 Table). Besides oral mucosal erosions, he complained of soft or loose stools 2 to 3 times daily without abdominal pain or fever. Although we proposed admission for close observation during his treatment, he selected outpatient treatment at a nearby hospital. Three months later, the patient returned to our hospital because his wife was also diagnosed with Entamoeba histolytica infection. The couple operated a Japanese inn in a suburban area of Tokyo. They had no travel history to developing countries within the past 10 years. He denied extramarital sexual intercourse and oral–anal sexual contact. He did not have any past histories, except for recurrent amebiasis. There were no reported outbreaks of gastrointestinal diseases for over 10 years in the couple’s residential area. Results of a blood examination showed no particular abnormalities (Table 1). Although a direct microscopic examination was negative for any protozoa, the patient’s stool tested positive for E. histolytica with polymerase chain reaction (PCR). Total colonoscopy showed white-coated ulcerative lesions at the cecum (Fig 1A). In a pathological examination, Entamoeba was identified on the surface mucosa in a biopsy sample (S1 Fig). We treated the patient with a lumen-active agent (paromomycin monotherapy) because (1) he had a past history of acute oral mucosal lesions owing to metronidazole, (2) tinidazole is not approved to treat amebiasis in Japan, and (3) his symptoms of E. histolytica were mild. Negative PCR results for E. histolytica were confirmed in stool samples taken at 1, 2, and 4 months after treatment. Follow-up colonoscopy showed that lesions of the cecum were completely resolved (Fig 1C).
Table 1. Laboratory and endoscopic findings in the 2 cases.
| Patient 1 | Patient 2 | |
|---|---|---|
| Blood tests | ||
| White blood cell count (per μL) | 5,830 | 6,050 |
| Eosinophils (per μL) | 140 | 563 |
| C-reactive protein (mg per dL) | 0.02 | 0.02 |
| Antibody titer against E. histolytica, immunofluorescence assay | Negative | Positive (1:800) |
| Stool testing | ||
| Fecal occult blood, immunoassay | Positive | Positive |
| Microscopy | Negative | Cystic form of Entamoeba |
| Antigen detectiona | Negative | Negative |
| PCR for E. histolytica (tRNA STR genotype) | Positive (genotype J8)b | Positive (genotype J13)b |
| Endoscopic findings | ||
| Multiple or sporadic | Sporadic | Multiple |
| Distribution | Cecum | Cecum ascending colon, transverse colon descending colon, sigmoid colon |
| Pathological findings | Entamoeba | Negative |
aTests were performed using frozen stocked samples by E. HISTOLYTICA QUIK CHEK (Techlab, Inc., Blacksburg VA, USA).
bSequences of each STR genotypes are shown in S2 Fig.
PCR, polymerase chain reaction; STR, short tandem repeat; tRNA, transfer RNA
Fig 1. Macroscopic findings during colonoscopy.
(A) Sporadic ulcerative lesions with edema were identified in the cecum of patient 1 before treatment. (B) Multiple ulcerative lesions with white moss were identified in patient 2 at the cecum before treatment. After completion of paromomycin monotherapy, all lesions were completely healed in (C) patient 1 and (D) patient 2.
Patient 2 was a 43-year-old female and the wife of patient 1. She was referred to our institute because of Entamoeba infection as confirmed on microscopic examination of a stool sample. One month before diagnosis, she had a positive fecal occult blood test result in an advanced health check. Dysentery, abdominal pain, and fever were not documented at referral. She denied extramarital sexual intercourse. She had a high anti–E. histolytica antibody titer (1:800). Direct microscopy of stool samples showed the cystic form of Entamoeba. E. histolytica was confirmed using PCR (Table 1). Total colonoscopy showed multiple, white-coated, ulcerative lesions from the cecum to the sigmoid colon (Fig 1B). The patient was treated with paromomycin monotherapy because her symptoms were mild, and she wished to avoid the potential adverse events of metronidazole experienced by her husband. PCR for E. histolytica in stool samples collected at 1, 2, and 4 months after treatment were negative. Follow-up colonoscopy showed that all lesions had completely resolved (Fig 1D).
We informed both patients about risk behavior for acquiring E. histolytica, such as oral–anal sexual contact or food and waterborne infections in poor sanitary settings. More than 2 years after treatment, neither patient has experienced recurrence of invasive E. histolytica infection.
Discussion
Epidemiology of sexually transmitted E. histolytica infection
Amebiasis is transmitted by oral ingestion of the transmissible cystic form of E. histolytica in human stool. This transmission occurs by ingestion of fecally contaminated food and water, mainly in developing countries. The pathogen can also be transmitted directly from human to human, with clustering of E. histolytica strains due to sexual contact. [1, 2] Over the past 2 decades, amebic infection has been increasingly reported as a sexually transmitted infection (STI) in developed countries of East Asia and in Australia. [3] E. histolytica has also been recently recognized as a comorbidity among HIV-infected men or as a domestic STI in developed European countries. [4, 5] Neither of our 2 patients had a history of travel to a developing country in their lifetime. Additionally, we performed genotyping of 6 short tandem repeat (STR) loci in transfer RNA lesions of E. histolytica in stool samples from both patients. [6] Interestingly, the STR genotypes showed different patterns at all 6 loci between the 2 patients. However, both genotypes were identical to previously reported genotypes of Japanese origin (S2 Fig; J8 in patient 1 and J13 in patient 2). Therefore, we could not identify the transmission route of the patients’ infection. Additionally, because samples were not available at referral, we did not know the reason (reinfection, treatment failure by poor adherence, or drug resistance) why patient 1 repeatedly developed E. histolytica infection before referral.
Treatment for endoscopically diagnosed amebic colitis
Noninvasive infections (described as asymptomatic intestinal colonization [7]) can be treated using a lumen-active agent, such as paromomycin [8], without a tissue-active agent. Tissue-active agents, such as metronidazole, should be administered before a lumen-active agent for invasive intestinal diseases (amebic colitis). However, in clinical settings similar to the 2 present patients, the difference between intestinal colonization and invasive colitis is often uncertain. [9–12] Our patients had minimal symptoms but showed macroscopically visible intestinal ulcers upon colonoscopy and microscopically identified inflammation caused by E. histolytica in biopsy samples. This difference is presumably because disease severity (colonization or colitis) in amebiasis is not currently determined using pathophysiological criteria but is based on patients’ symptoms. Furthermore, colonoscopy is not routinely recommended for asymptomatic cyst passers who may have ulcerative lesions in their large intestine, such as patient 2 (cystic form of Entamoeba in the stool and visible ulcers identified by colonoscopy). However, these cases are commonly treated by a lumen-active agent without a tissue-active agent. [7] These results raise the unresolved issue that monotherapy by a lumen-active agent can be inadequate treatment for asymptomatically infected patients with E. histolytica who are diagnosed by stool tests, such as microscopy or PCR. Additionally, as warranted in our cases, extra-intestinal lesions, such as liver abscess, should be ruled out before determining their treatment for all asymptomatically infected cases. However, imaging studies before treatment with a lumen-active agent are currently not recommended for these cases. [7] At a minimum, close follow-up is required when a tissue-active agent is not administered prior to a lumen-active agent. Additionally, nitroimidazole agents, especially metronidazole, often show toxicity in patients, such as drug hypersensitivity, neurological disorders, and sometimes Stevens–Johnson syndrome. [13, 14] Another problem in the clinical settings is that there are no available alternative regimens for invasive diseases for patients who are unable to tolerate a nitroimidazole agent. In our cases, we did not use tissue-active agents before paromomycin because of concern about adverse effects. Both patients achieved complete healing of ulcerative lesions after paromomycin monotherapy (Fig 1C and 1D). A study from Japan reported that 11 of 143 cases of amebiasis were treated with paromomycin monotherapy, although symptoms and outcomes of the treatment were unclear from the report. [15] Additionally, older reports published before the era of PCR differentiation of E. histolytica from nonpathogenic Entamoeba described the effectiveness of a single dose or 5 days of paromomycin, even for symptomatic amebic colitis. [16, 17] However, 6.3% to 44.0% of the cases experienced recurrence within 10 weeks of follow-up. Tissue-active agents are generally used for initial treatment, according to recent case reports of endoscopically diagnosed, asymptomatic amebic colitis. [9, 18] Furthermore, paromomycin is not systemically absorbed, and 100% is excreted in an unchanged form from stool. Taken together, these results suggest that the best treatment regimen for endoscopically diagnosed asymptomatic ulcers in the large intestine due to E. histolytica remains undetermined. Paromomycin monotherapy, which might be considered only for patients who are intolerant to nitroimidazole after excluding extraintestinal involvement, will not adequately treat all cases of intestinal amebiasis. Further investigations are required to determine appropriate treatment for asymptomatically infected patients with E. histolytica who are diagnosed via colonoscopy or stool tests.
Conclusion
Successful treatment with paromomycin monotherapy due to metronidazole intolerance was microbiologically and endoscopically confirmed in 2 patients with mild symptoms of E. histolytica infection and visible colonic ulcers. This finding suggests that this treatment is an option for amebic ulcers in the setting of metronidazole intolerance if there is no evidence of intestinal and extraintestinal invasive diseases. Additionally, a future proof-of-concept study is required for the appropriate treatment choice for asymptomatically infected individuals.
Key learning points
E. histolytica infection is a common STI in Japan, and it is a comorbidity among HIV-infected men or as a domestic STI in developed European countries.
E. histolytica can cause endoscopically visible ulcers in the large intestine, even if the patient does not have any abdominal symptoms.
Appropriate treatment for such cases (asymptomatic or mildly symptomatic patients with endoscopically visible colonic ulcers) is still undetermined.
Consent for publication
Written informed consent was obtained from the patients for publication of details of the clinical courses.
Supporting information
(DOCX)
(PPTX)
(PPTX)
Acknowledgments
We thank Dr. Kumiko Nakada-Tsukui (Department of Parasitic Diseases, National Institute of Infectious Diseases, Tokyo, Japan) for technical support with laboratory diagnosis and genotyping of E. histolytica, Dr. Eiko Okubo (Department of Gastroenterology, National Center for Global Health and Medicine, Tokyo, Japan) for supplying clinical images of patients’ colonoscopies, and all staff of the National Center for Global Health and Medicine for completion of the patients’ treatment. We also thank Analisa Avila, ELS, of Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Funding Statement
KW recieved the Emerging/Re-emerging Infectious Diseases Project of Japan from the Japan Agency for Medical Research and Development (AMED, https://www.amed.go.jp/) under Grant Number 18k0108046 and 20fk0108138s0301 and a grant from the National Center for Global Health and Medicine (https://www.ncgm.go.jp/) (29-2013 and 19A2016). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Salit IE, Khairnar K, Gough K, Pillai DR. A possible cluster of sexually transmitted Entamoeba histolytica: genetic analysis of a highly virulent strain. Clin Infect Dis. 2009;49(3): 346–53. 10.1086/600298 [DOI] [PubMed] [Google Scholar]
- 2.Escola-Verge L, Arando M, Vall M, Rovira R, Espasa M, Sulleiro E, et al. Outbreak of intestinal amoebiasis among men who have sex with men, Barcelona (Spain), October 2016 and January 2017. Euro Surveill. 2017;22(30). pii: 30581 10.2807/1560-7917.ES.2017.22.30.30581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hung CC, Chang SY, Ji DD. Entamoeba histolytica infection in men who have sex with men. Lancet Infect Dis. 2012;12(9): 729–36. 10.1016/S1473-3099(12)70147-0 [DOI] [PubMed] [Google Scholar]
- 4.Timsit BL, Deroux A, Lugosi M, Colombe B, Bouillet L. [Amoebosis: May sexual transmission be an underestimated way of contamination?]. Rev Med Interne. 2018;39(7): 586–8. 10.1016/j.revmed.2018.04.004 [DOI] [PubMed] [Google Scholar]
- 5.Roure S, Valerio L, Soldevila L, Salvador F, Fernandez-Rivas G, Sulleiro E, et al. Approach to amoebic colitis: Epidemiological, clinical and diagnostic considerations in a non-endemic context (Barcelona, 2007–2017). PLoS ONE. 2019;14(2): e0212791 10.1371/journal.pone.0212791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe K, Gatanaga H, Escueta-de Cadiz A, Tanuma J, Nozaki T, Oka S. Amebiasis in HIV-1-infected Japanese men: clinical features and response to therapy. PLoS Negl Trop Dis. 2011;5(9): e1318 10.1371/journal.pntd.0001318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petri WA Jr., Haque R, 2015. Entamoeba species, Including Amebic Colitis and Liver Abscess. In: Bennett JE, Dolin R, and Blaser MJ(ed): Mandell, Douglas, and Bennett's principles and practice of infectious diseases, 8th edition Philadelphia, PA: Elsevier/Saunders, 3047–3058. 2015. [Google Scholar]
- 8.Blessmann J, Tannich E. Treatment of asymptomatic intestinal Entamoeba histolytica infection. N Engl J Med. 2002;347:1384 10.1056/NEJM200210243471722 [DOI] [PubMed] [Google Scholar]
- 9.Watanabe K, Nagata N, Sekine K, Watanabe K, Igari T, Tanuma J, et al. Asymptomatic Intestinal Amebiasis in Japanese HIV-1-Infected Individuals. Am J Trop Med Hyg. 2014;91(4):816–20. 10.4269/ajtmh.14-0278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okamoto M, Kawabe T, Ohata K, Togo G, Hada T, Katamoto T, et al. Amebic colitis in asymptomatic subjects with positive fecal occult blood test results: clinical features different from symptomatic cases. Am J Trop Med Hyg. 2005;73(5): 934–5. [PubMed] [Google Scholar]
- 11.Ishikane M, Arima Y, Kanayama A, Takahashi T, Yamagishi T, Yahata Y, et al. Epidemiology of Domestically Acquired Amebiasis in Japan, 2000–2013. Am J Trop Med Hyg. 2016;94(5): 1008–14. 10.4269/ajtmh.15-0560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spinzi G, Pugliese D, Filippi E. An Unexpected Cause of Chronic Diarrhea. Gastroenterology 2016;150(1): e5–6. 10.1053/j.gastro.2015.05.054 [DOI] [PubMed] [Google Scholar]
- 13.Johnson M. Metronidazole: An overview Hooper DC ed. UpToDate. Waltham, MA: UpToDate Inc; https://www.uptodate.com. [cited 2019 Oct 1]. [Google Scholar]
- 14.Mazumdar G, Shome K. Stevens–Johnson syndrome following use of metronidazole in a dental patient. Indian J Pharmacol. 2014;46(1): 121–122. 10.4103/0253-7613.125193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kikuchi T, Koga M, Shimizu S, Miura T, Maruyama H, Kimura M. Efficacy and safety of paromomycin for treating amebiasis in Japan. Parasitol Int. 2013;62:497–501. 10.1016/j.parint.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 16.Chavarria AP, Hunter GW 3rd, Redmond DL, Lizono C, Loria AR. Single Dose of Paromomycin (Humatin) against Intestinal Amebiasis. Mil Med. 1964;129: 947–51. [PubMed] [Google Scholar]
- 17.Carter CH, Bayles A, Thompson PE. Effects of paromomycin sulfate in man against Entamoeba histolytica and other intestinal protozoa. Am J Trop Med Hyg. 1962;11: 448–51. 10.4269/ajtmh.1962.11.448 [DOI] [PubMed] [Google Scholar]
- 18.Wu N, Freiman JS. Caecal ulceration in an asymptomatic man. Gut. 2017;66(5): 886 10.1136/gutjnl-2016-312811 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(PPTX)
(PPTX)