Abstract
Neocortical inhibitory neurons exhibit remarkably diverse morphology, physiological properties and connectivity. Genetic access to molecularly-defined subtypes of inhibitory neurons has aided their functional characterization in recent years. These studies have established that, instead of simply balancing excitatory neuron activity, inhibitory neurons actively shape excitatory circuits in a subtype-specific manner. We review the emerging view that inhibitory neuron subtypes perform context-dependent modulation of excitatory activity as well as regulate experience-dependent plasticity of excitatory circuits. We then review the roles of neuromodulators in regulating the subtype-specific functions of inhibitory neurons. Finally, we discuss the notion that dysfunctions of inhibitory neuron subtypes may be responsible for various aspects of neurological disorders.
Introduction
In adult neocortical circuits, inhibition is thought to be ‘balanced’ with excitation. Usually this refers to the observation that the level of cortical inhibition generally scales with the amount of excitation. However, compelling evidence suggests that, instead of passively reflecting excitatory activity, inhibitory networks can also actively modulate excitatory activity in a context-dependent manner and shape the excitatory circuits during experience-dependent plasticity and learning.
Active shaping of cortical networks by inhibitory neurons (INs) is facilitated by their heterogeneity. Distinct subtypes of INs carry out different functions based on their unique morphological, electrophysiological, connectivity and molecular properties. In particular, non-overlapping parvalbumin (PV), somatostatin (SOM) and vasoactive intestinal peptide (VIP)-expressing INs have been extensively studied in recent years1-3. IN subtypes usually share inputs4-7 and are electrically coupled4 (Fig. 1a). PV-INs and SOM-INs mostly target perisomatic and distal dendritic regions of postsynaptic excitatory neurons, respectively, and this unique subcellular targeting underlies their distinct inhibitory impact on excitatory neurons3,8. In contrast, VIP-INs mostly disinhibit excitatory neurons through inhibition of other IN subtypes3. Although IN groupings based on single molecular marker expression simplifies the complexity of cortical networks, these subtypes provide opportunities to use genetic tools to dissect cell type-specific functions. In this review, we mainly discuss the roles of three IN subtypes in context-dependent cortical activity modulation, regulation of experience-dependent plasticity, and how their abnormality might lead to pathological conditions. We will not focus on control of passive sensory responses1,3,8 or further subdivisions of IN types3,9.
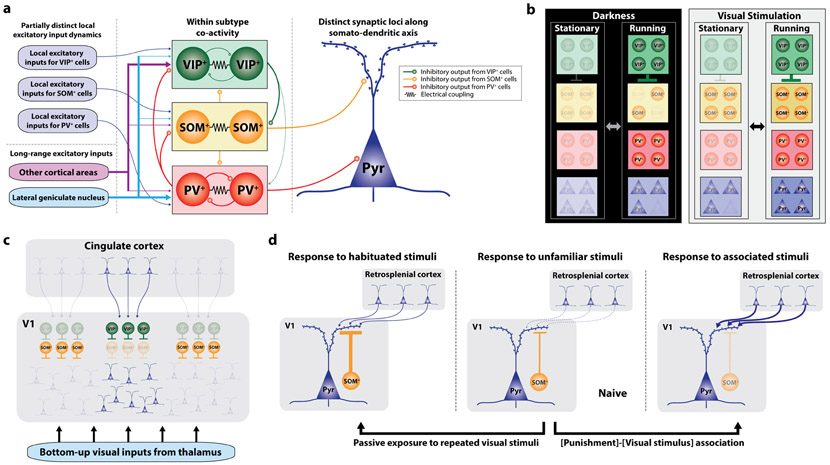
Figure 1: Context-dependent modulation of V1 by IN subtypes.
(a) A wiring diagram illustrating differential excitatory inputs, local inhibitory connectivity and inhibitory outputs in V14-7,23. (b) Locomotion-dependent modulation of IN activity in the presence or absence of concurrent visual inputs16,18. In the darkness, locomotion activates both PV-INs and VIP-INs, but SOM-INs and pyramidal neurons are heterogeneously modulated. With visual stimuli, locomotion activates all IN subtypes and pyramidal neurons. Color of each cell type reflects the activity level relative to its level during stationary state in darkness. (c) Top-down projections from cingulate cortex to specific retinotopic site in V1 induce local disinhibition by preferentially recruiting VIP-INs. In contrast, SOM-INs increase their activity at surrounding areas and cause surround suppression. To our knowledge, it has not been determined whether the cingulate neurons projecting to different V1 locations are intermingled or topographically organized as in the frontal eye field that mediates attentional modulation in primate visual cortex28. (d) Context-dependent gating of top-down inputs from retrosplenial cortex by SOM-INs37. Visual responses in SOM-INs increase after passive exposure to repeated visual stimuli. In contrast, visual responses in SOM-INs decrease when the mouse learns association between the visual stimulus and tail shock, permitting strong top-down modulation of visual response in excitatory neurons by retrosplenial cortex. Line thickness and opacity of cell reflect the connectivity strength and the activity, respectively.
Context-dependent shaping of excitatory activity by inhibitory subtypes
Cortical sensory responses do not solely depend on bottom-up sensory inputs; the behavioral context in which sensory inputs are received profoundly modulates cortical activity and perception. We propose that IN subtypes can alter the operation modes of cortical circuits depending on the context1-3,9-14, flexibly adjusting the way by which a given sensory stimulus is processed.
Behavioral contexts such as locomotion, task engagement and attention alter excitatory neuron response gain. For instance, locomotion enhances visual response of excitatory neurons in the primary visual cortex (V1) while maintaining their tuning properties15. This gain increase may be partly mediated by differential activation of local IN subtypes. Locomotion activates VIP-INs in V1 in an acetylcholine-dependent manner while heterogeneously modulating SOM-INs, leading to a proposal that VIP activation during locomotion causes disinhibition of excitatory neurons by inhibiting SOM-INs16. However, these recordings were performed in the darkness. In the presence of visual stimuli, these IN subtypes increase visual responsiveness during locomotion17,18 (Fig.1b), challenging the simple disinhibitory model. To determine how V1 IN subtypes mediate effects of locomotion, selective suppression of each IN subtype during locomotion and visual stimulation will be required.
In contrast to V1, the net effect of locomotion in the primary auditory cortex (A1) is suppression of excitatory neuron activity19-21. At the beginning of locomotion bouts, long-range projections from premotor cortex activate PV-INs20. This initial increase in PV-IN activity likely reduces recurrent excitation in A1, after which excitatory and inhibitory inputs to excitatory neurons are both reduced in a balanced manner21, leading to decreased activity of excitatory neurons. However, long-range inputs to A1 also recruit VIP-INs which could have disinhibitory effects22. The neural mechanisms underlying the difference in locomotion-related modulation between V1 (amplification) and A1 (suppression) have yet to be resolved. The balance of the strengths between inhibitory and disinhibitory pathways may be different across brain areas, resulting in variable locomotion effects. These examples point to a larger challenge for the coming years: to determine how feedback projections, microcircuit connectivity and synaptic weight structure differ between cortical regions.
The effect of locomotion is relatively homogeneous within each brain area. However, there is growing evidence of bidirectional modulation of excitatory neuron activity, such that some neurons are suppressed while others are activated, during task engagement or attention across sensory modalities23-27. In visual attention tasks, top-down modulation by higher brain areas such as the prefrontal cortex (PFC) likely plays a critical role28-30. Long-range excitatory projections from cingulate cortex in PFC to V1 exert top-down influence on sensory perception in mice23,31. Optogenetic activation of this long-range projection amplifies visual responses in V1 and improves behavioral performance in a visual discrimination task23. These long-range projections preferentially recruit local VIP-INs23 within the relevant retinotopic region of V1 which then disinhibits excitatory neurons via SOM-INs23,32. At the same time, SOM-INs in the surrounding areas increase their activity23, likely due to increased drive from excitatory neurons in the disinhibited area33-36, and suppress the activity of excitatory neurons within surrounding areas (Fig.1c). Thus, cingulate cortex can generate center-disinhibition/surround-inhibition of specific retinotopic sites in V1, providing a potential basis for spatially-selective visual attention.
Bidirectional modulation also extends to other sensory regions including auditory25,26 and somatosensory27 cortices. In A1, task engagement co-activates PV, SOM and VIP-INs in parallel, triggering broad suppression and selective facilitation of excitatory neurons25. Direct recording of subthreshold inputs to excitatory neurons showed that task engagement alters inhibition more than excitation. IN subtypes were critical for the bidirectional modulation, such that PV and SOM-INs directly suppressed some excitatory neurons while VIP-INs disinhibited others25. The potential benefits of bidirectional modulation in A1 remain unknown. One possibility is that inhibitory networks serve as a gateway for top-down and neuromodulatory signals that suppress task-irrelevant neurons while amplifying task-relevant neurons. In this model, suppression is a global response that may share mechanisms associated with locomotion. In parallel, sounds that have behavioral salience recruit a sub-network of task-relevant neurons via disinhibition. Such a model necessitates specific connectivity of a subset of INs onto task-relevant excitatory neurons and specific inputs to those INs, which remains to be demonstrated.
These studies have investigated general suppression or disinhibition at the level of individual excitatory neurons. In addition, recent studies indicate that certain operational modes of IN networks can selectively alter the effective weights of different inputs to excitatory neurons depending on context. For example, when mice are passively and repeatedly exposed to visual stimuli, the responses of excitatory neurons, PV-INs and VIP-INs in V1 gradually decreased in parallel with a specific enhancement of SOM-IN responses37,38. In contrast, when mice learned to associate the visual stimulus with subjectively important context such as punishment, SOM-INs gradually decreased their visual response, disinhibiting excitatory neurons at apical dendrites37. The dendritic disinhibition by SOM-INs occurred concurrently with increased activity of top-down projections from retrosplenial cortex arriving at the apical dendrites37, allowing top-down inputs to strongly modulate excitatory neuron activity (Fig. 1d). Importantly, these IN changes were stimulus specific, indicating that the circuit operation modes controlled by IN subtypes can switch rapidly and reversibly. Similar pathway-specific gating mechanisms have been also found in hippocampus and amygdala during fear learning, where SOM-INs disinhibit specific inputs in amygdala39 but inhibit specific inputs in hippocampus40. The context-dependent gating of specific input pathways in V1 is consistent with the notion that response to familiar or behaviorally salient stimuli is under strong influence of internally generated information. Also supporting this notion, V1 inherits internal representations of visual scenes through top-down projections from other areas such as anterior cingulate cortex31.
The examples above suggest an emerging view that IN subtypes can reversibly switch the operation modes of sensory cortex, such that sensory cortex processes an identical sensory stimulus differently depending on behavioral context. Context impinges on cortical IN subtypes in multiple and overlapping ways; IN subtypes express receptors for a wide-range of neuromodulators while also receiving long-range excitatory inputs from frontal regions. How do IN networks integrate these various signals and negotiate between potentially competing inputs? Future modeling studies will instruct targeted manipulations to test mechanistic hypotheses. Furthermore, the notion that each subtype has unique and dedicated functions is overly simplistic. For example, SOM-INs in barrel cortex are heterogeneously modulated during whisking behavior due to different levels of inhibition from VIP-INs in different lamina41. Moreover, VIP-INs can disinhibit other INs, but they also inhibit excitatory neurons22,42,43 and the relative contributions of disinhibitory and inhibitory effects by VIP-INs may not be fixed.
Regulation of excitatory neuron plasticity by inhibitory subtypes
In addition to contextual modulation of excitatory neuron activity, INs can actively control the synaptic plasticity of excitatory circuits. This mechanism was first described in studies of critical periods, developmental windows during which plasticity is enhanced. The maturation of PV-INs is thought to determine critical period onsets and durations44,45. Classically, occluding one eye during the critical period for ocular dominance leads to expansion of the spared eye representation. Recently, it has been shown that this plasticity is mediated by transient disinhibition due to a decrease in the excitatory inputs to PV-INs46,47. This disinhibition is followed by recovery of PV-IN responses46,47 and potentiation of PV-IN outputs48,49 within a few days. Manipulations of PV-IN activity demonstrated that the transient perisomatic disinhibition by PV-INs is necessary for the critical period plasticity of excitatory circuits46 (Fig. 2a-1).
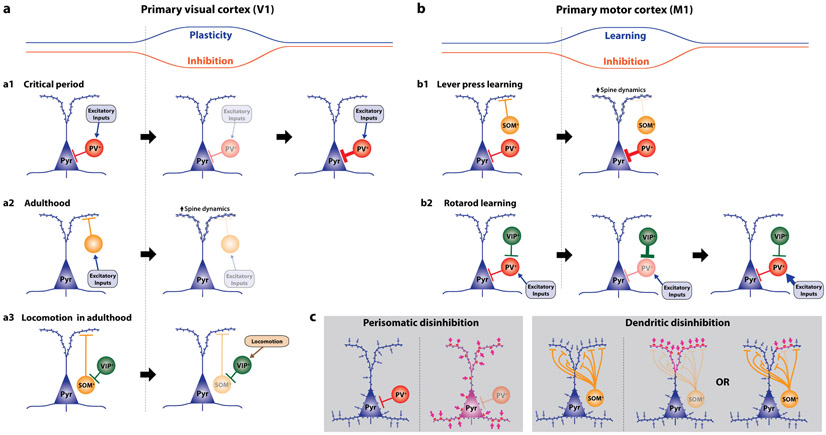
Figure 2: Relationship between disinhibition and excitatory neuron plasticity in sensory and motor cortex.
(a) Monocular deprivation reduces PV-IN-mediated perisomatic inhibition in V1 during the critical period46,47 (a1) whereas only reduction in dendritic inhibition is induced in adulthood46,51-56 (a2). In both cases, the plasticity of excitatory circuits is permitted by the transient disinhibition. The plasticity in adulthood can be enhanced by locomotion at least partially through activation of VIP-INs57,58 (a3). (b) Lever press motor learning (b1) reduces SOM-IN-mediated dendritic inhibition in M1, which permits structural plasticity of dendritic spines on excitatory neurons60. In contrast, PV-IN-mediated perisomatic inhibitory synapses increase in density to maintain the net excitatory/inhibitory input balance in pyramidal neurons60. Another study with rotarod learning reported transient increase of VIP-IN-mediated inhibitory synapses on PV-INs during learning62 (b2). As the learning performance saturates, the putative perisomatic disinhibition is terminated by decreased VIP-IN-mediated inhibitory synapses and increased excitatory synapses on PV-INs. (c) Hypothetical consequences of subtype-specific disinhibition. Perisomatic disinhibition by PV-INs may lower the threshold of plasticity induction all over the neuron. On the other hand, dendritic disinhibition by SOM-INs would lower the threshold locally at dendrites. Each SOM-IN may equally control plasticity of all targeted dendritic branches or selectively gate plasticity at narrow dendritic branch region.
Disinhibition opens a window of enhanced plasticity even in adulthood50. In V1 during passive experience, deprivation-induced plasticity in adulthood primarily involves dendritic disinhibition46,51-56, in contrast to the predominant role of PV-INs in critical period (Fig. 2a-2). The degree of experience-dependent plasticity is limited in adult V1 compared to during critical period, but certain behavioral contexts in adulthood can enhance plasticity. For example, locomotion enhances speed and degree of recovery from amblyopia in adult V157,58. It is suggested that suppression of SOM-INs by VIP-INs is responsible for this locomotion-dependent enhancement of V1 plasticity58 (Fig. 2a-3). Suppression of SOM-INs, in turn, results in disinhibition of excitatory neurons at their dendrites, which correlates with enhanced spine dynamics in V152,54,55. The dendritic disinhibition by SOM-INs can be recruited in a number of other contexts such as attention23 and associative learning2,37, and therefore SOM-INs may play a pivotal role in controlling excitatory neuron plasticity based on the context in which animals receive sensory inputs.
Another line of evidence supporting the role of INs in controlling excitatory neuron plasticity came from studies on primary motor cortex (M1) during motor learning. In a forelimb lever-press task, spine formation increases specifically on distal dendrites of excitatory neurons during the initial learning phase, followed by elimination of some spines that existed on the distal dendrites before learning59,60. The spine dynamics on pyramidal neurons occurs in parallel with elimination of SOM-IN synapses onto dendrites of excitatory neurons60 (Fig. 2b-1). Activation and suppression of SOM-INs increases and decreases spine elimination on apical dendrites and impairs learning60, supporting their critical role in controlling spine dynamics. In contrast, PV-IN synapses on the perisomatic region transiently increase during learning60, which might act to equalize the excitatory-inhibitory input ratio onto pyramidal neurons to prevent hyperactivity61.
Similar disinhibition has been suggested for PV-INs in M1 during rotarod motor learning, which may be subserved by increase in VIP-IN synapses on PV-INs62 (Fig.2b-2). The increased inhibition of PV-INs likely results in perisomatic disinhibition of excitatory neurons and promotes excitatory neuron plasticity. Interestingly, after the initial learning, VIP-IN synapses decrease and excitatory synapses increase on PV-INs62. As a result, PV-IN activity at the post-learning stage may be even higher than the pre-training period. Similar dynamics of disinhibition followed by hyperinhibition has been reported in human visual cortex during learning of orientation detection task63. Such hyperinhibition at the post-learning stage is consistent across species63-65 and may prevent excessive circuit reorganization and protect learned skills.
These studies on sensory and motor cortices have established a critical role of disinhibition in excitatory neuron plasticity. Subtype-specific disinhibition can potentially control the spatiotemporal specificity of cortical critical periods or other epochs of heightened plasticity. Perisomatic disinhibition would increase the global excitability of target excitatory neurons and lower the threshold for excitatory inputs to evoke sufficient postsynaptic activity for Hebbian or spike-timing-dependent plasticity. Dendritic disinhibition, on the other hand, may lower the threshold for Ca2+ spikes locally on dendrites and can selectively potentiate specific excitatory input pathways (Fig. 2c). Each dendrite-targeting IN may indiscriminately disinhibit all dendritic branches or may be able to selectively disinhibit a specific dendritic branch or even a subregion within a branch. In M1, for example, excitatory inputs for different motor skills cluster on different dendritic branches64,66. Elimination of SOM-IN synapses on selective dendritic regions may allow formation of new synaptic inputs specifically on the disinhibited branch. Future studies should test whether disinhibition is specific to task-related excitatory neurons and/or dendritic branches.
An important question is how plasticity can be controlled by disinhibition without compromising the ongoing circuit operation. Disinhibition would render the postsynaptic excitatory neuron hyperactive, so some type of homeostatic processes is necessary to ensure proper circuit functions. Excitatory neurons alone have homeostatic mechanisms to prevent hyperactivity67, but other IN subtypes can also compensate for disinhibition to maintain the general activity level of the circuit, as shown in the case of motor learning60. Another remaining issue is the mechanisms behind subtype-specific disinhibition. Top-down signals and neuromodulatory inputs may modulate each IN subtype and excitatory neurons differentially during learning to instruct subtype-specific disinhibition. However, for disinhibition to achieve cellular and subcellular specificity (e.g., disinhibition only on task-related neurons and/or dendritic branches), finer-scale mechanisms must be in place. One potential mechanism for inhibitory synapse plasticity with synapse specificity is spike-timing-dependent plasticity68. Furthermore, postsynaptic excitatory neurons may have intrinsic mechanisms to instruct subcellular specificity of disinhibition. For example, environmental enrichment triggers the expression of Npas4, which cell-autonomously redistributes inhibitory synapses from dendrites to soma in hippocampal excitatory neurons69.
Neuromodulatory control of inhibitory networks
The circuit mechanisms that enable inhibitory plasticity during context-dependent activity and associative learning are starting to be understood, with advances in imaging, molecular genetics, and intracellular recordings in behaving animals. Neuromodulation seems to be critical for induction of inhibitory and excitatory synaptic modifications during development and adulthood. Although the effects of a given neuromodulator can be quite complex70,71 and include changes to pre- and postsynaptic excitability, synaptic transmission, glial responses, and blood flow, one common theme that has emerged is that many neuromodulators directly alter GABA release50,72,73. In long-term plasticity, for example, neuromodulators such as acetylcholine and oxytocin initiate disinhibition to enable excitatory neuron plasticity. Here we discuss recent results that help connect modulation, inhibition, disinhibition, plasticity, and behavior for four different neuromodulators: acetylcholine from the basal forebrain25,74-77, norepinephrine from the locus coeruleus78-80, serotonin from the dorsal raphe nuclei81, and dopamine from the ventral tegmental area82. In addition to these canonical modulators, we also note that other neurochemicals such as adenosine72, estradiol83, and oxytocin73,84 have been reported to directly decrease cortical inhibition, potentially gating behaviorally-relevant plasticity in target circuits. We note that there is a large literature for each of these neurochemicals, particularly in regards to effects on spiking activity or sensory perception and cognition, beyond the scope of this review. Here we will focus on newer studies that examine how each modulator affects inhibitory networks in vivo in a behavioral context.
Acetylcholine
Cholinergic signaling has been postulated to play a role in enabling plasticity and in controlling brain state by directly working through cortical IN networks. Cholinergic neurons in basal forebrain are rapidly recruited by reward and punishment, and the magnitude of their activation is scaled by the unexpectedness of the reinforcement signals74. These cholinergic neurons innervate diverse cortical areas, including sensory cortex where reinforcement signals are paired with concurrent sensory inputs and provide opportunities for association learning. For example, pairing nucleus basalis stimulation with auditory input can enhance responses to the paired auditory stimuli in A1. If this pairing is repeated for several minutes, a long-lasting enhancement of the cortical representation of the paired sound is induced85,86. This plasticity seems to act through disinhibition87. Nucleus basalis stimulation leads to cortical muscarinic receptor activation, which reduces inhibitory inputs onto excitatory neurons elicited by the paired sound. Acetylcholine also reduces GABA release and disinhibits excitatory cells in mouse barrel cortex72. Reduction of inhibition is followed by a shift in excitatory synaptic tuning due to long-term potentiation of paired inputs, and then finally a fine-scale re-balancing of inhibition to match excitation. The shift in excitatory synaptic tuning also involves a reduction in excitatory and inhibitory synaptic responses to the unpaired, original best frequency. Together, these adjustments of synaptic strength lead to ‘x-axis’ translations in spiking responses and tuning curve peak, while conserving total synaptic strength87.
This plasticity of cortical representations has also been linked to changes in auditory perception. In associative fear conditioning experiments, recent work has begun to reveal circuit elements that implement disinhibitory computations88. During acquisition of auditory fear conditioning, foot-shocks recruit cholinergic inputs that activate layer 1 INs and possibly deeper layer VIP-INs which inhibit their target INs, including SOM-INs and PV-INs22,88. This disinhibition is required for the fear learning, suggesting the cholinergic control of cortical plasticity and learning via disinhibition. Nucleus basalis pairing can also improve performance on a target detection or recognition task, with rats showing transient or lasting enhancements in response rates to paired stimuli depending on how many days of pairing occurred87,89. Interestingly, changes at the level of tonotopic maps seem to recover, with the original map structure returning weeks after pairing although behavioral performance remains high89. This suggests that tonotopic map plasticity may not be the most relevant feature linking neural changes to behavior. Alternatively or in addition, improved behavioral performance after prolonged training periods might be supported by the auditory striatum90, as associative learning induces potentiation of field potentials in the auditory striatum91.
Acetylcholine, however, also acutely modulates cortical activity and is implicated in controlling brain state and context-dependent activity. Locomotion and arousal modulate the responses of cortical neurons; in V1, for example, neurons show increased stimulus-evoked responses when animals are running15. It has been shown that these effects are mediated by inhibitory networks. In V1, cholinergic activity excites VIP-INs, which increases pyramidal spiking via disinhibition16. In A1, engaging in a stimulus recognition go/no-go task leads to significant modulation of excitatory output25. Most excitatory neurons are suppressed but a select group are enhanced by task engagement. A recent study dissected this phenomenon and showed that cholinergic activity is elevated during task engagement. In A1, acetylcholine activates all major IN subtypes, including PV-INs, SOM-INs and VIP-INs, to create a balance of inhibition and disinhibition in the network which enables both suppression and facilitation of excitatory output25,92. It should be noted, however, that IN subtypes exhibit different sensitivity to acetylcholine, with VIP- and SOM-INs being most sensitive25,93,94 and PV-INs showing complex and potentially region-specific sensitivity25,72. A theoretical model could only recapitulate this outcome if neuromodulation activated all three inhibitory subtypes in parallel, ruling out inhibition or disinhibition as the sole relevant computation25. Going forward, it will be useful to incorporate insights gleaned from experimental data and network models into integrated behavioral models that account for the effects of different neuromodulators on decision-making processes95.
Overall, cholinergic activity in the cortex supports behavior both by promoting plasticity in a phasic manner through disinhibition, and by providing contextual signals by acting on both inhibitory and disinhibitory circuit elements. How can these two functions of the cholinergic system—reduction of inhibition for plasticity and co-activation of cortical INs during behavior—be reconciled? We speculate that there may be different operation modes that might vary as a function of cholinergic neuron firing rate, duration of activation, connectivity and muscarinic versus nicotinic sensitivity. For example, during task engagement, cholinergic neurons may fire at a low to moderate rate, leading to general recruitment of INs. But during initial learning phases or episodes of heightened reward, cholinergic neurons might fire at higher rates similar to what is seen during classic pairing experiments, producing a disinhibition permissive for long-term synaptic modifications. These hypotheses remain to be tested.
Noradrenaline
There is also a long literature supporting a role for noradrenergic signaling in attention, arousal, behavioral performance, and synaptic modulation or plasticity79,80. Norepinephrine is released from neurons in a number of brainstem nuclei, including the locus coeruleus. Locus coeruleus has extensive projections throughout the brain78,96,97, and locus coeruleus stimulation or norepinephrine iontophoresis can have complex effects on sensory cortical neurons. In S1, noradrenergic activation can enhance evoked activity while decreasing spontaneous activity98. In rat A1, noradrenaline and locus coeruleus stimulation can bidirectionally modulate evoked responses via alpha-adrenergic receptors99,100. In V1, noradrenergic projections have been shown to connect to cortical interneurons including SOM, NPY, and VIP interneurons101.
Noradrenaline can also act as a disinhibitory neuromodulator in rat A1, but in a different manner than acetylcholine. When a pure tone is repetitively paired with electrical or optogenetic locus coeruleus stimulation, responses to all tones are dramatically increased for minutes, up to ten-fold in strength. This is due to a reduction in tonic (spontaneous) inhibition, in contrast to the reduction in phasic (tone-evoked) inhibition. Gradually, responses recover in amplitude, leaving tuning curves shifted to the paired input as with nucleus basalis pairing. Thus noradrenergic modulation first produces transient ‘y-axis’ changes in overall response gain to any incoming stimulus, before leaving enduring changes to specific paired tones. These changes have an important impact on behavior: locus coeruleus stimulation enhances detection of auditory cues and accelerates reversal learning99. While the physiological and behavioral effects of a few minutes of nucleus basalis pairing last only for several hours, the effects of brief locus coeruleus pairing can last for days to weeks99. This long-lasting response enhancement is due to plasticity within the locus coeruleus itself. Locus coeruleus neurons can start directly responding to conditioned stimuli99,102, potentially enabling this system to come on-line during task engagement for state- or context-dependent modulation103.
Dopamine
There are two major sources of dopamine in the mammalian brain, the ventral tegmental area (VTA) and the substantia nigra. Axons from dopaminergic neurons in these areas densely innervate striatum and PFC but also have more sparse connectivity to other neocortical regions. Dopamine can bidirectionally regulate synaptic events; D1-type receptors enhance excitatory and inhibitory events while action through D2-type receptors reduces these events104. Pairing VTA stimulation with pure tones can bi-directionally adjust cortical representations, depending on the relative timing between sensory input and VTA activation105. The synaptic mechanisms by which dopamine controls this reorganization remain unknown.
The strongest evidence supporting a link between dopamine and inhibitory networks is from studies of the PFC. Inhibition is thought to shape task-related activity in the PFC106-109, a major site of dopaminergic innervation. Experiments in reduced preparations strongly support dopaminergic modulation of inhibitory networks110,111. It has been shown that dopamine enhances the excitability of fast-spiking INs (putatively PV-INs)112 while still depressing GABA release through direct inactivation of presynaptic terminals104. In contrast, dopamine enhances inhibitory transmission from non-fast spiking INs likely via postsynaptic action104. These cell-type specific effects of dopamine suggest a complex role for dopaminergic modulation of inhibitory networks during behavior. A recent study in the hippocampus, for example, implicates dopaminergic modulation of PV-INs as a proximal mechanism for long-term consolidation of memories113.
Serotonin
Serotonin (5-hydroxytriptamine; 5-HT) is released from neurons located in the brainstem dorsal raphe nuclei. Serotonergic signaling is thought to be involved in regulating mood, appetite, and reward-related behaviors but also modulates cortical activity114 (particularly in PFC) and potentially through inhibitory networks. The PFC is highly enriched in 5-HT receptors, including suppressive 5-HT1aRs and excitatory 5-HT2aRs. Both receptors reside on fast-spiking INs; most are inhibited via the 5-HT1aR115 but some are enhanced by the 5-HT2aR114. Similarly, serotonin reduces GABA release from fast-spiking INs onto excitatory cells in S1, consistent with a role in disinhibition72. One major class of cortical INs includes those that express the ionotropic serotonin 5-HT3A receptor (5-HT3aR), including VIP-INs. Whether and how serotonin interacts with cortical circuits during behavior via INs remains to be determined.
Inhibitory network contribution to neurological disorders
Inhibitory networks play a key role in many neurological disorders, from schizophrenia116 to Alzheimer’s disease117,118. What are the circuit mechanisms by which INs may mediate disease pathogenesis? Here, we outline three general ways that INs contribute to pathological conditions: hyperexcitability, network oscillations, and structural degradation of GABAergic synapses.
Loss of inhibitory control leading to hyperexcitability
INs provide cortical networks with the ability to balance spontaneous and evoked excitatory drive. This balance helps to prevent runaway excitation. A large body of work suggests that insufficient levels of inhibition promote epileptiform activity in a cell-type specific manner. PV–INs provide strong feedforward inhibition in the thalamocortical relay; disruptions to this feedforward inhibition can lead to runaway excitation and is implicated in generalized absence epilepsy in multiple mouse models119-121. The precise mechanisms that govern changes in feedforward inhibition via PV-INs are a potent target for therapeutic intervention. The activity of PV-INs can be modulated by voltage-gated calcium channels122 and sodium channels123. Moreover, direct activation of PV-INs has been used in mouse models to ameliorate epileptic activity121. SOM-INs stand out in their role in providing local feedback, as opposed to feedforward, inhibition to excitatory neurons. Partial deletion of SOM-INs results in epileptic behavior in mice121 suggesting that feedback inhibition is also critical for maintaining normal levels of excitation. VIP-INs contribute to excitatory activity mainly via disinhibition. Genetic removal of VIP-INs or blockade of VIP-IN activity leads to a marked reduction in seizure activity121,124.
In epilepsy, the therapeutic challenge is no longer to generically increase total amounts of inhibition, but rather, to selectively scale the appropriate type of inhibition for the particular form of epilepsy being treated. This strategy may apply to other diseases where abnormal inhibition is implicated such as Alzheimer’s disease, schizophrenia, autism and Rett syndrome. For example, Rett syndrome is a developmental disorder caused by loss of MeCP2 functions, and rodent studies suggested that loss of MeCP2 functions in INs is responsible for many of the symptoms including seizures125-127. Interestingly, loss of MeCP2 in either PV-INs or SOM-INs causes non-overlapping Rett-syndrome-like phenotypes in mice127. Selectively targeting therapeutic agents at specific IN subtypes may therefore have a rational basis. Gene therapy using new cell-type specific viruses may provide one therapeutic approach for particularly intractable conditions128.
Disruptions of gamma activity
Cortical and hippocampal brain regions exhibit striking rhythmic activity resulting from synchronicity of neuronal populations129. These oscillations occur in specific frequency bands, including theta (4-8 Hz), alpha (8-13 Hz), and gamma (30-80 Hz). Gamma “power” in the cortex and hippocampus has been shown to increase during attention, memory and demanding behavioral tasks130 and has been causally associated with cognition131,132. Synaptic inhibition plays a fundamental role in the generation of these oscillations129,133,134; for example, manipulating the firing rate of PV-INs fundamentally changes gamma band synchrony131. Now, evidence suggests that disruptions to oscillations may be a proximal cause of a wide range of neuropsychiatric and neurological disorders135.
In schizophrenia, patients exhibit reductions of gamma power during performance of cognitive tasks135. This has been linked to deficits in PV-IN activity. Schizophrenia-like behavioral symptoms and disruptions to gamma band oscillations have been observed in numerous mouse models in which aspects of PV-IN function have been impaired132,136-138, and stimulation of INs in PFC at gamma frequency can restore certain aspects of cognitive flexibility in a mouse model138. IN-related changes to gamma band synchrony have also been observed in Alzheimer’s disease. Transgenic mice with expression of human amyloid precursor protein (hAPP) exhibit abnormalities in gamma power that result from changes to the intrinsic properties of fast-spiking PV-INs118. These mice showed reduced expression of a specific voltage-gated sodium channel; deficits in gamma band oscillations and behavior were restored in hAPP transgenic mice by increasing Nav1.1 levels118. Gamma power is also affected by changes in apolipoprotein (apo) E4, the main genetic risk factor for Alzheimer’s disease. ApoE4 knock-in mice exhibit reduction in slow gamma activity that could be partially restored by elimination of ApoE4 in INs139. Furthermore, stimulation of PV-INs at gamma frequency is sufficient to reduce amyloid-β in a mouse model of Alzheimer’s disease140. Across diseases, the potential role of IN-mediated disruptions in gamma power suggests that this may be a convergent mechanism ripe for therapeutic intervention.
Structural degradation of INs
Many neurological diseases impair neural circuitry by introducing toxicity to a local region. In stroke, the loss of oxygenation leads to cell death. In Alzheimer’s disease, extracellular amyloid-beta and intracellular tau deposits are thought to initiate cellular degeneration pathways. In Parkinson’s, alpha synuclein aggregates and environmental toxins drive selective vulnerability of dopaminergic neurons. While most studies have focused on how excitatory neurons respond to these stressors, recent work is beginning to elucidate how these various insults may also impact INs.
In Alzheimer’s disease mouse models, axonal segments of SOM-INs in the hippocampus have recently been shown to be particularly sensitive to amyloidosis117. Axonal atrophy surprisingly did not depend on distance to amyloid plaques suggesting that INs, unlike their excitatory counterparts, may propagate dysfunctional synaptic activity well beyond the plaque periphery. In stroke models, transient global ischemia triggers rapid changes in both excitatory and inhibitory dendritic structure141. Reperfusion can rapidly recover dendritic structure of both excitatory and PV-INs, but surprisingly, GABAergic synaptic network activity remained impaired much longer. This suggests that PV-IN function may be particularly sensitive to stroke. In rat models of Parkinson’s disease, transplantation of embryonic MGE-derived neural precursors (which would normally mature into GABAergic INs) into the adult striatum ameliorated motor symptoms142.
Conclusions and outlook
Inhibitory control of cortical circuits remains an area of active exploration. As outlined above, significant progress has been made in uncovering the broad activity patterns of these IN subtypes in context-dependent control of activity and plasticity, and in neurological disorders. This builds on a strong foundation of characterizing the functional profile of INs in reduced preparations. Going forward, we see four major areas of exploration that will become more plausible with the advent of new behavioral, molecular, physiological and optogenetic tools.
Precise role of IN subtypes during well-defined behaviors
Thus far, the functional dissection of IN subtypes has been focused on relatively simple behaviors, largely in stimulus detection, recognition and discrimination tasks. We believe that understanding how GABAergic activity shapes behavior will require a more nuanced approach to behavioral manipulations. A first step will be to obtain parametric control of contextual influences. Currently, inhibitory control of context-dependent behavior appears to be highly dependent on task design and parameters. The continuum from passive sensation to active attentional control needs to be carefully dissected behaviorally and implications drawn about the cell-type specific role of different INs need to be considered with this in mind. For instance, specifying the role of disinhibition in selective versus global attention remains a mystery. A second step will be to consider more ethological behaviors. In auditory cortex, significant work has identified the possibility of motor influences on auditory cortical output. While locomotion or experimenter-defined motor-auditory tasks is a reasonable starting point, rodent vocalizations provide an ethological entry point to understand the fundamental circuit architecture of motor-auditory interactions. A third step will be to understand how INs mediate social behaviors. For example, maternal behavior and responses to infant distress calls appear to be gated by inhibitory plasticity in mouse auditory cortex73,143. Future studies should dissect the cell-type specific role of different INs “in the act” of performing parental behaviors. Overall, a fundamental goal of neuroscience is to link neural activity to behavior; to do so not only requires molecular and genetic access to the constituent elements of neural networks but also a deeper understanding of behavioral output.
Heterogeneity and interactions between cell types
Cell-type specific calcium imaging and electrophysiological recordings make it clear that INs, even within the same defined molecular class, exhibit heterogeneity in activity patterns. Explaining this heterogeneity will be a critical undertaking over the next years. One likely possibility is that molecular markers such as PV or SOM do not sufficiently define a neuronal subtype. In this view, greater molecular, functional or structural specificity will add in reducing the heterogeneity3,144. Another possibility that is not mutually exclusive from the first is that INs within a given subtype exhibit experience-dependent differences in activity. For example, not all INs in a given class may be recruited for a particular behavior or task. Heterogeneity, in this case, would derive from recruitment of INs into task-dependent ensembles. New tools, including activity-dependent optogenetics and cell-type specific imaging, should allow investigators to explore this heterogeneity in more detail. This includes examining interactions between cells of different types and identification of lower-level subtypes within existing classes. New transgenic approaches and intersectional genetic strategies will make it possible to examine these more elaborate molecular specifications. Simultaneous observation and/or manipulation of multiple inhibitory pools will be required to understand how inhibition sculpts cortical activity. Moreover, theoretical modeling can help provide testable hypotheses as to whether and how heterogeneity may emerge from interacting sub-ensembles.
Functional connectomics
INs exhibit broad output innervation and diverse input connectivity. How do synaptic inputs onto INs control their output activity pattern? How flexible and dynamic are these connections based on behavioral context? To answer these types of questions requires dissecting the synaptic partners of INs and the corresponding functional activity pattern of these neurons in distinct behavioral epochs and contexts. The methodological development of single-cell rabies virus tracing coupled with calcium imaging will soon make it possible to perform these types of experiments145. Moreover, functional characterization of neuronal activity during behavior followed by detailed structural analysis with electron and light microscopy can further help understand how structural connectivity patterns enable functional outputs.
Convergent inhibitory mechanisms in neurological disorders
Given the ability to target molecularly defined inhibitory subtypes with gene editing tools, inhibitory networks may provide powerful entry points for therapeutic intervention. Accumulating evidence suggests that inhibitory computations are complicated and generic increases or decreases to inhibition may not be an effective strategy. The challenge for the field will be to identify ways to operationalize changes to IN activity in a way that improves cognitive symptoms. The answer may reside in both the generality of the effects (e.g., multiple diseases exhibit disruptions to gamma oscillations) as well as the specific determinants (e.g., ApoE4-mediated disruptions to gamma in Alzheimer’s disease). To effectively intervene, we will have to titrate the cell-types being targeted (“who”), the intensity of the change (“how much”), and at what stage the intervention makes sense (“when”).
Acknowledgements
This work was supported by grants from NIH (R01 NS091010A, R01 EY025349, R01 DC014690 and U01 NS094342), Pew Charitable Trusts, David & Lucile Packard Foundation, McKnight Foundation, and New York Stem Cell Foundation to T.K. and from NIH (DC009635 and DC012557), Pew Charitable Trusts, McKnight Foundation, and the HHMI Faculty Scholars Program to R.C.F. and from NIH (DC05014) to K.V.K.
References
- 1.Hangya B, Pi H-J, Kvitsiani D, Ranade SP & Kepecs A From circuit motifs to computations: mapping the behavioral repertoire of cortical interneurons. Curr. Opin. Neurobiol 26, 117–124 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Letzkus JJ, Wolff SBE & Lüthi A Disinhibition, a Circuit Mechanism for Associative Learning and Memory. Neuron 88, 264–276 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Tremblay R, Lee S & Rudy B GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron 91, 260–292 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karnani MM et al. Cooperative Subnetworks of Molecularly Similar Interneurons in Mouse Neocortex. Neuron 90, 86–100 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wall NR et al. Brain-Wide Maps of Synaptic Input to Cortical Interneurons. J. Neurosci. Off. J. Soc. Neurosci 36, 4000–4009 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji X-Y et al. Thalamocortical Innervation Pattern in Mouse Auditory and Visual Cortex: Laminar and Cell-Type Specificity. Cereb. Cortex N. Y. N 1991 26, 2612–2625 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfeffer CK, Xue M, He M, Huang ZJ & Scanziani M Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat. Neurosci 16, 1068–1076 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naka A & Adesnik H Inhibitory Circuits in Cortical Layer 5. Front. Neural Circuits 10, 35 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kepecs A & Fishell G Interneuron cell types are fit to function. Nature 505, 318–326 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caroni P Inhibitory microcircuit modules in hippocampal learning. Curr. Opin. Neurobiol 35, 66–73 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Froemke RC Plasticity of cortical excitatory-inhibitory balance. Annu. Rev. Neurosci 38, 195–219 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roux L & Buzsáki G Tasks for inhibitory interneurons in intact brain circuits. Neuropharmacology 88, 10–23 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stryker MP A Neural Circuit That Controls Cortical State, Plasticity, and the Gain of Sensory Responses in Mouse. Cold Spring Harb. Symp. Quant. Biol 79, 1–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urban-Ciecko J & Barth AL Somatostatin-expressing neurons in cortical networks. Nat. Rev. Neurosci 17, 401–409 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niell CM & Stryker MP Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu Y et al. A cortical circuit for gain control by behavioral state. Cell 156, 1139–1152 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polack P-O, Friedman J & Golshani P Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat. Neurosci 16, 1331–1339 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pakan JM et al. Behavioral-state modulation of inhibition is context-dependent and cell type specific in mouse visual cortex. eLife 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson A et al. A circuit for motor cortical modulation of auditory cortical activity. J. Neurosci. Off. J. Soc. Neurosci 33, 14342–14353 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider DM, Nelson A & Mooney R A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature 513, 189–194 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou M et al. Scaling down of balanced excitation and inhibition by active behavioral states in auditory cortex. Nat. Neurosci 17, 841–850 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pi H-J et al. Cortical interneurons that specialize in disinhibitory control. Nature 503, 521–524 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang S et al. Selective attention. Long-range and local circuits for top-down modulation of visual cortex processing. Science 345, 660–665 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goard MJ, Pho GN, Woodson J & Sur M Distinct roles of visual, parietal, and frontal motor cortices in memory-guided sensorimotor decisions. eLife 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuchibhotla KV et al. Parallel processing by cortical inhibition enables context-dependent behavior. Nat. Neurosci 20, 62–71 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carcea I, Insanally MN & Froemke RC Dynamics of auditory cortical activity during behavioural engagement and auditory perception. Nat. Commun 8, 14412 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krupa DJ, Wiest MC, Shuler MG, Laubach M & Nicolelis MAL Layer-specific somatosensory cortical activation during active tactile discrimination. Science 304, 1989–1992 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Moore T & Armstrong KM Selective gating of visual signals by microstimulation of frontal cortex. Nature 421, 370–373 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Squire RF, Noudoost B, Schafer RJ & Moore T Prefrontal contributions to visual selective attention. Annu. Rev. Neurosci 36, 451–466 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Miller EK & Buschman TJ Cortical circuits for the control of attention. Curr. Opin. Neurobiol 23, 216–222 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiser A et al. Experience-dependent spatial expectations in mouse visual cortex. Nat. Neurosci 19, 1658–1664 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Karnani MM et al. Opening Holes in the Blanket of Inhibition: Localized Lateral Disinhibition by VIP Interneurons. J. Neurosci. Off. J. Soc. Neurosci 36, 3471–3480 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silberberg G & Markram H Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron 53, 735–746 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Kapfer C, Glickfeld LL, Atallah BV & Scanziani M Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat. Neurosci 10, 743–753 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fino E & Yuste R Dense inhibitory connectivity in neocortex. Neuron 69, 1188–1203 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adesnik H, Bruns W, Taniguchi H, Huang ZJ & Scanziani M A neural circuit for spatial summation in visual cortex. Nature 490, 226–231 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makino H & Komiyama T Learning enhances the relative impact of top-down processing in the visual cortex. Nat. Neurosci 18, 1116–1122 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamm JP & Yuste R Somatostatin Interneurons Control a Key Component of Mismatch Negativity in Mouse Visual Cortex. Cell Rep. 16, 597–604 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolff SBE et al. Amygdala interneuron subtypes control fear learning through disinhibition. Nature 509, 453–458 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Lovett-Barron M et al. Dendritic inhibition in the hippocampus supports fear learning. Science 343, 857–863 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muñoz W, Tremblay R, Levenstein D & Rudy B Layer-specific modulation of neocortical dendritic inhibition during active wakefulness. Science 355, 954–959 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Lee S, Kruglikov I, Huang ZJ, Fishell G & Rudy B A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat. Neurosci 16, 1662–1670 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-Junco-Clemente P et al. An inhibitory pull-push circuit in frontal cortex. Nat. Neurosci 20, 389–392 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Espinosa JS & Stryker MP Development and plasticity of the primary visual cortex. Neuron 75, 230–249 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lazarus MS & Huang ZJ Distinct maturation profiles of perisomatic and dendritic targeting GABAergic interneurons in the mouse primary visual cortex during the critical period of ocular dominance plasticity. J. Neurophysiol 106, 775–787 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuhlman SJ et al. A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature 501, 543–546 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hengen KB, Lambo ME, Van Hooser SD, Katz DB & Turrigiano GG Firing rate homeostasis in visual cortex of freely behaving rodents. Neuron 80, 335–342 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maffei A, Lambo ME & Turrigiano GG Critical period for inhibitory plasticity in rodent binocular V1. J. Neurosci. Off. J. Soc. Neurosci 30, 3304–3309 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kannan M, Gross GG, Arnold DB & Higley MJ Visual Deprivation During the Critical Period Enhances Layer 2/3 GABAergic Inhibition in Mouse V1. J. Neurosci. Off. J. Soc. Neurosci 36, 5914–5919 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Froemke RC, Merzenich MM & Schreiner CE A synaptic memory trace for cortical receptive field plasticity. Nature 450, 425–429 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Keck T et al. Massive restructuring of neuronal circuits during functional reorganization of adult visual cortex. Nat. Neurosci 11, 1162–1167 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Hofer SB, Mrsic-Flogel TD, Bonhoeffer T & Hübener M Experience leaves a lasting structural trace in cortical circuits. Nature 457, 313–317 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keck T et al. Loss of sensory input causes rapid structural changes of inhibitory neurons in adult mouse visual cortex. Neuron 71, 869–882 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Chen JL et al. Structural basis for the role of inhibition in facilitating adult brain plasticity. Nat. Neurosci 14, 587–594 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Versendaal D et al. Elimination of inhibitory synapses is a major component of adult ocular dominance plasticity. Neuron 74, 374–383 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Villa KL et al. Inhibitory Synapses Are Repeatedly Assembled and Removed at Persistent Sites In Vivo. Neuron 89, 756–769 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaneko M & Stryker MP Sensory experience during locomotion promotes recovery of function in adult visual cortex. eLife 3, e02798 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fu Y, Kaneko M, Tang Y, Alvarez-Buylla A & Stryker MP A cortical disinhibitory circuit for enhancing adult plasticity. eLife 4, e05558 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peters AJ, Chen SX & Komiyama T Emergence of reproducible spatiotemporal activity during motor learning. Nature 510, 263–267 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Chen SX, Kim AN, Peters AJ & Komiyama T Subtype-specific plasticity of inhibitory circuits in motor cortex during motor learning. Nat. Neurosci 18, 1109–1115 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xue M, Atallah BV & Scanziani M Equalizing excitation-inhibition ratios across visual cortical neurons. Nature 511, 596–600 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Donato F, Rompani SB & Caroni P Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature 504, 272–276 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Shibata K et al. Overlearning hyperstabilizes a skill by rapidly making neurochemical processing inhibitory-dominant. Nat. Neurosci 20, 470–475 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cichon J & Gan W-B Branch-specific dendritic Ca(2+) spikes cause persistent synaptic plasticity. Nature 520, 180–185 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vallentin D, Kosche G, Lipkind D & Long MA Neural circuits. Inhibition protects acquired song segments during vocal learning in zebra finches. Science 351, 267–271 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang G et al. Sleep promotes branch-specific formation of dendritic spines after learning. Science 344, 1173–1178 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turrigiano G Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu. Rev. Neurosci 34, 89–103 (2011). [DOI] [PubMed] [Google Scholar]
- 68.D’amour JA & Froemke RC Inhibitory and excitatory spike-timing-dependent plasticity in the auditory cortex. Neuron 86, 514–528 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bloodgood BL, Sharma N, Browne HA, Trepman AZ & Greenberg ME The activity-dependent transcription factor NPAS4 regulates domain-specific inhibition. Nature 503, 121–125 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marder E, O’Leary T & Shruti S Neuromodulation of circuits with variable parameters: single neurons and small circuits reveal principles of state-dependent and robust neuromodulation. Annu. Rev. Neurosci 37, 329–346 (2014). [DOI] [PubMed] [Google Scholar]
- 71.Bargmann CI & Marder E From the connectome to brain function. Nat. Methods 10, 483–490 (2013). [DOI] [PubMed] [Google Scholar]
- 72.Kruglikov I & Rudy B Perisomatic GABA release and thalamocortical integration onto neocortical excitatory cells are regulated by neuromodulators. Neuron 58, 911–924 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marlin BJ, Mitre M, D’amour JA, Chao MV & Froemke RC Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520, 499–504 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hangya B, Ranade SP, Lorenc M & Kepecs A Central Cholinergic Neurons Are Rapidly Recruited by Reinforcement Feedback. Cell 162, 1155–1168 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pinto L et al. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat. Neurosci 16, 1857–1863 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chubykin AA, Roach EB, Bear MF & Shuler MGH A cholinergic mechanism for reward timing within primary visual cortex. Neuron 77, 723–735 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goard M & Dan Y Basal forebrain activation enhances cortical coding of natural scenes. Nat. Neurosci 12, 1444–1449 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sara SJ The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci 10, 211–223 (2009). [DOI] [PubMed] [Google Scholar]
- 79.Bouret S & Sara SJ Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 28, 574–582 (2005). [DOI] [PubMed] [Google Scholar]
- 80.Aston-Jones G & Cohen JD An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci 28, 403–450 (2005). [DOI] [PubMed] [Google Scholar]
- 81.Dayan P & Huys QJM Serotonin in affective control. Annu. Rev. Neurosci 32, 95–126 (2009). [DOI] [PubMed] [Google Scholar]
- 82.Seamans JK & Yang CR The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog. Neurobiol 74, 1–58 (2004). [DOI] [PubMed] [Google Scholar]
- 83.Huang GZ & Woolley CS Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron 74, 801–808 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mitre M et al. A Distributed Network for Social Cognition Enriched for Oxytocin Receptors. J. Neurosci. Off. J. Soc. Neurosci 36, 2517–2535 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bakin JS & Weinberger NM Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc. Natl. Acad. Sci. U. S. A 93, 11219–11224 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kilgard MP & Merzenich MM Cortical map reorganization enabled by nucleus basalis activity. Science 279, 1714–1718 (1998). [DOI] [PubMed] [Google Scholar]
- 87.Froemke RC et al. Long-term modification of cortical synapses improves sensory perception. Nat. Neurosci 16, 79–88 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Letzkus JJ et al. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480, 331–335 (2011). [DOI] [PubMed] [Google Scholar]
- 89.Reed A et al. Cortical map plasticity improves learning but is not necessary for improved performance. Neuron 70, 121–131 (2011). [DOI] [PubMed] [Google Scholar]
- 90.Znamenskiy P & Zador AM Corticostriatal neurons in auditory cortex drive decisions during auditory discrimination. Nature 497, 482–485 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xiong Q, Znamenskiy P & Zador AM Selective corticostriatal plasticity during acquisition of an auditory discrimination task. Nature 521, 348–351 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nelson A & Mooney R The Basal Forebrain and Motor Cortex Provide Convergent yet Distinct Movement-Related Inputs to the Auditory Cortex. Neuron 90, 635–648 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xiang Z, Huguenard JR & Prince DA Cholinergic switching within neocortical inhibitory networks. Science 281, 985–988 (1998). [DOI] [PubMed] [Google Scholar]
- 94.Kawaguchi Y Selective cholinergic modulation of cortical GABAergic cell subtypes. J. Neurophysiol 78, 1743–1747 (1997). [DOI] [PubMed] [Google Scholar]
- 95.Dayan P Twenty-five lessons from computational neuromodulation. Neuron 76, 240–256 (2012). [DOI] [PubMed] [Google Scholar]
- 96.Schwarz LA et al. Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature 524, 88–92 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kebschull JM et al. High-Throughput Mapping of Single-Neuron Projections by Sequencing of Barcoded RNA. Neuron 91, 975–987 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Woodward DJ, Moises HC, Waterhouse BD, Hoffer BJ & Freedman R Modulatory actions of norepinephrine in the central nervous system. Fed. Proc 38, 2109–2116 (1979). [PubMed] [Google Scholar]
- 99.Martins ARO & Froemke RC Coordinated forms of noradrenergic plasticity in the locus coeruleus and primary auditory cortex. Nat. Neurosci 18, 1483–1492 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Manunta Y & Edeline J-M Noradrenergic induction of selective plasticity in the frequency tuning of auditory cortex neurons. J. Neurophysiol 92, 1445–1463 (2004). [DOI] [PubMed] [Google Scholar]
- 101.Paspalas CD & Papadopoulos GC Noradrenergic innervation of peptidergic interneurons in the rat visual cortex. Cereb. Cortex N. Y. N 1991 9, 844–853 (1999). [DOI] [PubMed] [Google Scholar]
- 102.Sara SJ & Segal M Plasticity of sensory responses of locus coeruleus neurons in the behaving rat: implications for cognition. Prog. Brain Res 88, 571–585 (1991). [DOI] [PubMed] [Google Scholar]
- 103.McGinley MJ, David SV & McCormick DA Cortical Membrane Potential Signature of Optimal States for Sensory Signal Detection. Neuron 87, 179–192 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tritsch NX & Sabatini BL Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron 76, 33–50 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bao S, Chan VT & Merzenich MM Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature 412, 79–83 (2001). [DOI] [PubMed] [Google Scholar]
- 106.Constantinidis C, Williams GV & Goldman-Rakic PS A role for inhibition in shaping the temporal flow of information in prefrontal cortex. Nat. Neurosci. 5, 175–180 (2002). [DOI] [PubMed] [Google Scholar]
- 107.Kvitsiani D et al. Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature 498, 363–366 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pinto L & Dan Y Cell-Type-Specific Activity in Prefrontal Cortex during Goal-Directed Behavior. Neuron 87, 437–450 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim D et al. Distinct Roles of Parvalbumin- and Somatostatin-Expressing Interneurons in Working Memory. Neuron 92, 902–915 (2016). [DOI] [PubMed] [Google Scholar]
- 110.Gao W-J, Wang Y & Goldman-Rakic PS Dopamine modulation of perisomatic and peridendritic inhibition in prefrontal cortex. J. Neurosci. Off. J. Soc. Neurosci 23, 1622–1630 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gao W-J & Goldman-Rakic PS Selective modulation of excitatory and inhibitory microcircuits by dopamine. Proc. Natl. Acad. Sci. U. S. A 100, 2836–2841 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gorelova N, Seamans JK & Yang CR Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J. Neurophysiol 88, 3150–3166 (2002). [DOI] [PubMed] [Google Scholar]
- 113.Karunakaran S et al. PV plasticity sustained through D1/5 dopamine signaling required for long-term memory consolidation. Nat. Neurosci 19, 454–464 (2016). [DOI] [PubMed] [Google Scholar]
- 114.Puig MV & Gulledge AT Serotonin and prefrontal cortex function: neurons, networks, and circuits. Mol. Neurobiol 44, 449–464 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lladó-Pelfort L, Santana N, Ghisi V, Artigas F & Celada P 5-HT1A receptor agonists enhance pyramidal cell firing in prefrontal cortex through a preferential action on GABA interneurons. Cereb. Cortex N. Y. N 1991 22, 1487–1497 (2012). [DOI] [PubMed] [Google Scholar]
- 116.Lewis DA Inhibitory neurons in human cortical circuits: substrate for cognitive dysfunction in schizophrenia. Curr. Opin. Neurobiol 26, 22–26 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schmid LC et al. Dysfunction of Somatostatin-Positive Interneurons Associated with Memory Deficits in an Alzheimer’s Disease Model. Neuron 92, 114–125 (2016). [DOI] [PubMed] [Google Scholar]
- 118.Verret L et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149, 708–721 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sasaki S, Huda K, Inoue T, Miyata M & Imoto K Impaired feedforward inhibition of the thalamocortical projection in epileptic Ca2+ channel mutant mice, tottering. J. Neurosci. Off. J. Soc. Neurosci 26, 3056–3065 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Paz JT et al. A new mode of corticothalamic transmission revealed in the Gria4(−/−) model of absence epilepsy. Nat. Neurosci 14, 1167–1173 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Paz JT & Huguenard JR Microcircuits and their interactions in epilepsy: is the focus out of focus? Nat. Neurosci 18, 351–359 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rossignol E, Kruglikov I, van den Maagdenberg AMJM, Rudy B & Fishell G CaV 2.1 ablation in cortical interneurons selectively impairs fast-spiking basket cells and causes generalized seizures. Ann. Neurol 74, 209–222 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tai C, Abe Y, Westenbroek RE, Scheuer T & Catterall WA Impaired excitability of somatostatin- and parvalbumin-expressing cortical interneurons in a mouse model of Dravet syndrome. Proc. Natl. Acad. Sci. U. S. A 111, E3139–3148 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Khoshkhoo S, Vogt D & Sohal VS Dynamic, Cell-Type-Specific Roles for GABAergic Interneurons in a Mouse Model of Optogenetically Inducible Seizures. Neuron 93, 291–298 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chao H-T et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 468, 263–269 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ure K et al. Restoration of Mecp2 expression in GABAergic neurons is sufficient to rescue multiple disease features in a mouse model of Rett syndrome. eLife 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ito-Ishida A, Ure K, Chen H, Swann JW & Zoghbi HY Loss of MeCP2 in Parvalbumin-and Somatostatin-Expressing Neurons in Mice Leads to Distinct Rett Syndrome-like Phenotypes. Neuron 88, 651–658 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dimidschstein J et al. A viral strategy for targeting and manipulating interneurons across vertebrate species. Nat. Neurosci 19, 1743–1749 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Buzsáki G & Wang X-J Mechanisms of gamma oscillations. Annu. Rev. Neurosci 35, 203–225 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fries P Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu. Rev. Neurosci 32, 209–224 (2009). [DOI] [PubMed] [Google Scholar]
- 131.Sohal VS, Zhang F, Yizhar O & Deisseroth K Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yizhar O et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, 171–178 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cardin JA et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Veit J, Hakim R, Jadi MP, Sejnowski TJ & Adesnik H Cortical gamma band synchronization through somatostatin interneurons. Nat. Neurosci 20, 951–959 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Uhlhaas PJ & Singer W Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci 11, 100–113 (2010). [DOI] [PubMed] [Google Scholar]
- 136.Belforte JE et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat. Neurosci 13, 76–83 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Carlén M et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol. Psychiatry 17, 537–548 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cho KKA et al. Gamma rhythms link prefrontal interneuron dysfunction with cognitive inflexibility in Dlx5/6(+/−) mice. Neuron 85, 1332–1343 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gillespie AK et al. Apolipoprotein E4 Causes Age-Dependent Disruption of Slow Gamma Oscillations during Hippocampal Sharp-Wave Ripples. Neuron 90, 740–751 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Iaccarino HF et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 540, 230–235 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xie Y, Chen S, Wu Y & Murphy TH Prolonged deficits in parvalbumin neuron stimulation-evoked network activity despite recovery of dendritic structure and excitability in the somatosensory cortex following global ischemia in mice. J. Neurosci. Off. J. Soc. Neurosci 34, 14890–14900 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Martínez-Cerdeño V et al. Embryonic MGE precursor cells grafted into adult rat striatum integrate and ameliorate motor symptoms in 6-OHDA-lesioned rats. Cell Stem Cell 6, 238–250 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lin FG, Galindo-Leon EE, Ivanova TN, Mappus RC & Liu RC A role for maternal physiological state in preserving auditory cortical plasticity for salient infant calls. Neuroscience 247, 102–116 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.He M et al. Strategies and Tools for Combinatorial Targeting of GABAergic Neurons in Mouse Cerebral Cortex. Neuron 92, 555 (2016). [DOI] [PubMed] [Google Scholar]
- 145.Wertz A et al. PRESYNAPTIC NETWORKS. Single-cell-initiated monosynaptic tracing reveals layer-specific cortical network modules. Science 349, 70–74 (2015). [DOI] [PubMed] [Google Scholar]