Abstract
Objectives:
To compare patterns of care and overall survival (OS) between stereotactic body radiotherapy (SBRT) and percutaneous local tumor ablation (LTA) for non-surgically managed early-stage non-small-cell lung cancer (NSCLC).
Materials and methods:
The National Cancer Database (NCDB) was queried from 2004 to 2014 for adults with non-metastatic, node-negative invasive adenocarcinoma or squamous cell carcinoma of the lung with primary tumor size ≤5.0 cm who did not undergo surgery or chemotherapy and received SBRT or LTA. Patterns of care were assessed with multivariate logistic regression. After propensity-score weighting with generalized boosted regression, OS was assessed with univariate and doubly-robust multivariate Cox regression.
Results:
Of 15,792 patients, 14,651 (93%) received SBRT and 1141 (7%) received LTA. Increasing age (OR 1.01, p = .035), treatment at an academic institution (OR 2.94, p < .001), increasing tumor size (OR 1.05, p < .001), and more recent year of diagnosis (OR 1.43, p < .001) were predictive of treatment with SBRT, whereas comorbidities (OR 0.74, p = .003) and treatment at a high-volume facility (OR 0.05, p < .001) were predictive for LTA. At a median follow-up of 26.2 months, SBRT was associated with improved OS relative to LTA within a propensity-score weighted doubly-robust multivariate analysis (HR 0.71, p < .001). On weighted subgroup analyses, improved OS was observed with SBRT for tumor sizes > 2.0 cm (HR 0.72, p < .001) and for those treated at high-volume facilities (HR 0.71, p < .001). No OS difference was found with SBRT or LTA in tumor sizes ≤2.0 cm (HR 0.90, p = .227).
Conclusion:
Within the NCDB, SBRT was more commonly utilized and was associated with improved OS when compared to percutaneous LTA for patients with non-surgically managed early-stage NSCLC. Patients with small tumor volumes likely represent an appropriate population for future prospective randomized comparisons between SBRT and LTA.
Keywords: NSCLC, Stereotactic body radiotherapy, Percutaneous ablation, Radiofrequency ablation, Microwave ablation
1. Introduction
Lung cancer represents the leading cause of cancer mortality in the United States, with 234,030 new diagnoses and 154,050 deaths estimated for 2018 [1]. Non-small cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancer diagnoses with the majority (~80%) presenting with locally advanced or metastatic disease. However, with the recent implementation of low-dose computed tomography [2] screening protocols, rising rates of detection of early-stage disease have contributed to a reduction in lung cancer mortality [3].
Surgical resection remains the historical gold standard for definitive management of medically operable early-stage NSCLC with prospective randomized evidence suggesting superiority of lobectomy over sublobar resection [4,5]. However, a substantial proportion of patients with early-stage NSCLC are deemed medically inoperable at diagnosis, most commonly due to pre-existing pulmonary or cardiovascular comorbidities. In those with medically inoperable early-stage NSCLC, stereotactic body radiotherapy (SBRT) has emerged as a safe and efficacious standard-of-care [6-17]. As an alternative to SBRT, percutaneous image-guided local tumor ablation (LTA) achieved most commonly via interventional radiological procedures utilizing thermal ablation (radiofrequency ablation [RFA], microwave ablation, laser ablation, or cryosurgery) has become an increasingly recognized treatment option [18-33]. Despite this trend, comparative effectiveness data for SBRT and LTA in early-stage NSCLC are limited. A pooled analysis of published experiences suggested possible improved local control with SBRT, but this local control benefit was not found to result in improved rates of overall survival (OS) [34]. As a result, we sought to compare OS and patterns of care for patients receiving definitive SBRT or LTA in non-surgically managed early-stage NSCLC.
2. Methods
2.1. Data source and study population
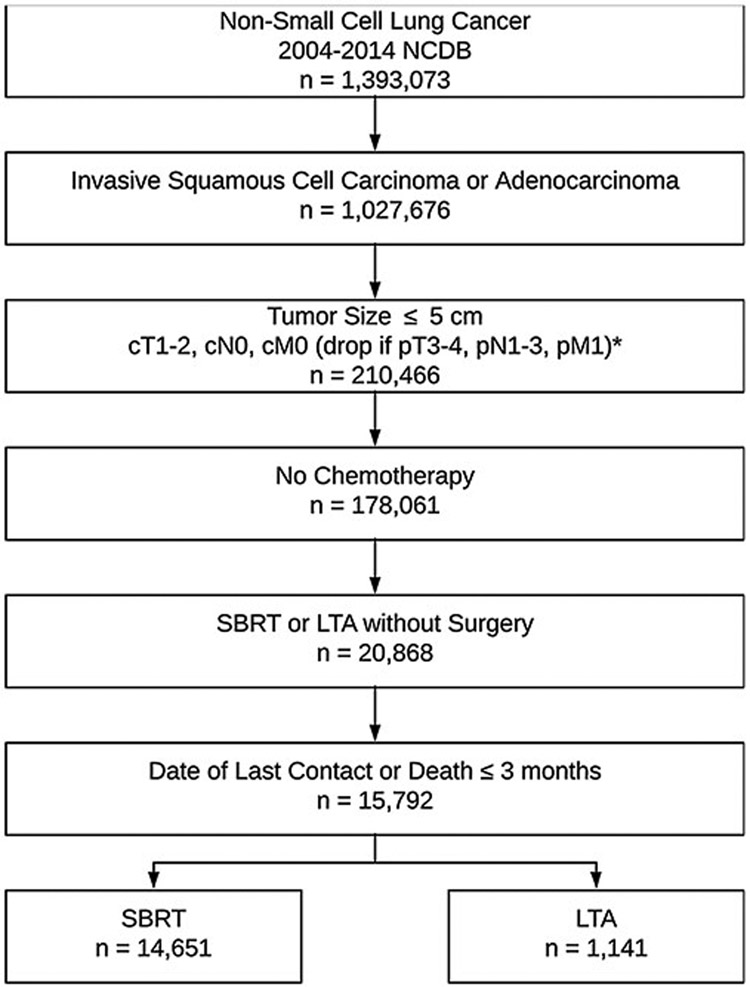
Patient data were obtained from the National Cancer Database (NCDB), which includes data from approximately 1500 accredited cancer facilities and captures nearly 70% of new cancer diagnoses made in the United States [35]. The NCDB was queried from 2004 through 2014 for adult patients aged 18 or older diagnosed with NSCLC (International Classification of Diseases in Oncology [ICD-O]-3 C340-C349 primary site codes) (Fig. 1). Included tumor histologies were invasive squamous cell carcinoma (ICD-O-3 8052, 8070, 8071, 8072, 8073, 8074, 8075, 8076, 8078, 8083, 8084) or invasive adenocarcinoma (IDC-O-3 8140, 8141, 8143, 8144, 8255, 8260, 8310, 8323, 8480, 8481, 8490, 8570, 8571, 8572, 8573, 8574). Included patients had primary tumor sizes ≤ 5.0 cm with American Joint Committee on Cancer (AJCC) 6th or 7th Edition clinical tumor (T) stage 1–2 disease and no clinical or pathologic evidence of metastasis to regional lymph nodes or distant sites. Patients receiving chemotherapy or surgery other than SBRT or LTA were excluded. The LTA cohort included those receiving laser ablation or cryosurgery, electrocautery/fulguration, and local tumor destruction not otherwise specified. Several percutaneous ablation techniques, notably radiofrequency and microwave ablation, were not explicitly recorded by the NCDB. Patients were required to have undergone first-line treatment with either SBRT or LTA but were excluded if they received both treatments. The SBRT cohort was defined as any of the following dose fractionations: 34 Gray (Gy) in 1 fraction, 54 Gy in 3 fractions, 48 Gy in 3 fractions, 45 Gy in 3 fractions, 50 Gy in 4 fractions, 48 Gy in 4 fractions, 55 Gy in 5 fractions, or 50 Gy in 5 fractions. Patients with a recorded date of last contact or death within three months of diagnosis were excluded.
Fig. 1.
Consort diagram of study population.
NOTE. Clinical and pathologic tumor staging as per the American Joint Committee on Cancer 6th Edition for patients diagnosed from 2004 – 2009. Patients diagnosed from 2010 – 2014 were staged according to the 7th Edition.
Abbreviations: NCDB, National Cancer Database; cm, centimeter; cT, clinical tumor stage; cN, clinical nodal stage; cM, clinical metastasis stage; pT, pathologic tumor stage; pN, pathologic nodal stage; pM, pathologic metastasis stage; SBRT, stereotactic body radiotherapy; LTA, local tumor ablation.
2.2. Covariates
Demographic and clinical characteristics included for analysis were age, sex, race, Charlson-Deyo comorbidity score, facility type (academic versus other), facility treatment volume (composite of facilities treating the highest 10th percentile versus lowest 90th percentile of patient volume with LTA or SBRT, calculated separately), tumor size, histology, tumor grade, clinical T stage (AJCC 6th or 7th edition), SBRT dose fractionation, LTA type, and year of diagnosis.
2.3. Objectives
The primary objective of this study was to compare rates of OS for non-surgically managed patients with early-stage, node-negative, non-metastatic NSCLC with primary tumor size ≤ 5.0 cm treated with either SBRT or LTA. OS was measured in months from the date of diagnosis to the date of death from any cause. A secondary objective was to compare patterns of care by determining factors associated with receipt of SBRT or LTA. Other meaningful endpoints such as local or regional recurrence, progression-free survival, disease-specific survival, and rates of toxicity were unable to be evaluated as these data were not recorded within the NCDB.
2.4. Statistical analysis
Differences in the distribution of demographic and clinical characteristics among groups were assessed using Pearson’s chi-squared analysis for categorical variables and two-sided t tests for continuous variables. Multivariate logistic regression was performed to quantify the predictive value of clinicodemographic characteristics in treatment allocation. Generalized boosted regression modeling was employed with the propensity-score in an attempt to equally weight clinicodemographic factors between treatment groups. All factors common to both treatment groups were included in the propensity-score weighted model, including age, sex, race, Charlson-Deyo comorbidity score, facility type, facility treatment volume, tumor size, histology, tumor grade, clinical T stage, and year of diagnosis.
The primary endpoint of OS was assessed using Kaplan-Meier analyses, log-rank regression, and Cox proportional hazards modeling. Doubly-robust estimation was performed with multivariate Cox proportional hazards modeling on the propensity-score weighted cohort. Significance was defined as any two-sided P value < .05. All analyses were performed using the STATA 14.2 statistical package (Stata Corporation, College Station, TX).
3. Results
3.1. Factors associated with use of SBRT
Of the 15,792 patients meeting criteria, 14,651 (93%) received SBRT and 1141 (7%) received LTA. The distribution of demographic and clinical characteristics by treatment group is shown in Table 1. On multivariate logistic regression, increasing age, treatment at an academic institution, increasing tumor size, and more recent year of diagnosis were associated with receipt of SBRT over LTA, whereas patients with comorbidities (Charlson-Deyo score ≥ 1) and those receiving treatment at a high-volume center for SBRT or LTA were more likely to receive LTA (Table 2). After propensity-score weighting, demographic and clinical characteristics were well-balanced between treatment groups (Supplemental Table A1).
Table 1.
Distribution of demographic and clinical characteristics among treatment groups.
| Characteristic | SBRT | LTA | P |
|---|---|---|---|
| No. of patients | 14,651 (93) | 1141 (7) | – |
| Follow-up, months | < .001 | ||
| Median | 26.1 | 28.0 | |
| Range | 3.0–148.7 | 3.0–144.4 | |
| Age, years Median |
75 | 75 | .488 |
| Range | 26–90 | 32–90 | |
| Sex Male |
6,758 (46) | 515 (45) | .518 |
| Female | 7,893 (54) | 626 (55) | |
| Race | < .001 | ||
| Caucasian | 12,905 (89) | 1045 (92) | |
| Other | 1,651 (11) | 85 (8) | |
| Charlson comorbitity score | < .001 | ||
| 0 | 8,256 (56) | 542 (48) | |
| 1 | 4,003 (27) | 381 (33) | |
| 2 | 1,671 (11) | 169 (15) | |
| 3+ | 721 (5) | 49 (4) | |
| Facility type | .019 | ||
| Academic | 6,400 (44) | 539 (47) | |
| Other | 8,248 (56) | 601 (53) | |
| Facility treatment volume | < .001 | ||
| Lowest 90% | 13,321 (91) | 595 (52) | |
| Highest 10% | 1330 (9) | 546 (48) | |
| Tumor size, cm | < .001 | ||
| Median | 2.1 | 1.8 | |
| Range | 0.1–5.0 | 0.1–5.0 | |
| Tumor size, cm | < .001 | ||
| ≤ 2.0 | 6,924 (47) | 695 (61) | |
| 2.1–3.0 | 4,781 (33) | 342 (30) | |
| 3.1–5.0 | 2,946 (20) | 104 (9) | |
| Histology | .127 | ||
| Squamous cell carcinoma | 6,324 (43) | 466 (41) | |
| Adenocarcinoma | 8,327 (57) | 675 (59) | |
| Grade | .360 | ||
| Well-differentiated | 1,279 (18) | 99 (18) | |
| Moderately-differentiated | 2,929 (42) | 254 (45) | |
| Poorly-differentiated | 2,722 (39) | 207 (37) | |
| Clinical T stage | < .001 | ||
| cT1 | 11,439 (78) | 1017 (89) | |
| cT2 | 3,212 (22) | 124 (11) | |
| SBRT dose | – | ||
| 34 Gy in 1 fx | 60 (< 1) | – | |
| 48 Gy in 3 fx | 165 (1) | – | |
| 45 Gy in 3 fx | 151 (1) | – | |
| 54 Gy in 3 fx | 2,887 (20) | – | |
| 50 Gy in 4 fx | 1,278 (9) | – | |
| 48 Gy in 4 fx | 3,797 (26) | – | |
| 55 Gy in 5 fx | 720 (5) | – | |
| 50 Gy in 5 fx | 5,593 (38) | – | |
| LTA type | – | ||
| Laser/Cryotherapy | – | 564 (49) | |
| Electrocautery/Fulguration | – | 97 (9) | |
| LTA NOS | – | 480 (42) | |
| Year of diagnosis | < .001 | ||
|
2004 |
12 (< 1) | 25 (2) | |
| 2005 | 32 (< 1) | 32 (3) | |
| 2006 | 114 (< 1) | 54 (5) | |
| 2007 | 196 (1) | 87 (8) | |
| 2008 | 439 (3) | 122 (11) | |
| 2009 | 757 (5) | 143 (13) | |
| 2010 | 1,435 (10) | 133 (12) | |
| 2011 | 1,906 (13) | 162 (14) | |
| 2012 | 2,578 (18) | 123 (11) | |
| 2013 | 3,299 (23) | 135 (12) | |
| 2014 | 3,883 (27) | 125 (11) |
Note: Data presented as No. (%) unless otherwise noted. P values given by Pearson’s chi-squared and two-sided t tests for categorical and continuous variables, respectively.
Abbreviations: SBRT, stereotactic body radiotherapy; LTA, local tumor ablation; No., number; cm, centimeter; Clinical T Stage, clinical tumor stage as per American Joint Committee on Cancer 6th or 7th Edition; Gy, gray; fx, fraction; NOS, not otherwise specified.
Table 2.
Multivariate logistic regression model of factors predictive of SBRT.
| OR | 95% CI | P | |
|---|---|---|---|
| Age | 1.01 | 1.00–1.02 | .035 |
| Sex | |||
| Male | Reference | ||
| Female | 1.05 | 0.86–1.28 | .655 |
| Race | |||
| Caucasian | Reference | ||
| Other | 1.18 | 0.83–1.67 | .362 |
| Charlson comorbidity score | |||
| 0 | Reference | ||
| ≥ 1 | 0.74 | 0.61–0.90 | .003 |
| Facility Type | |||
| Other | Reference | ||
| Academic | 2.94 | 2.30–3.75 | < .001 |
| Facility treatment volume | |||
| Lowest 90% | Reference | ||
| Highest 10% | 0.05 | 0.04–0.07 | < .001 |
| Tumor size | 1.05 | 1.03–1.07 | < .001 |
| Histology | |||
| Squamous cell carcinoma | Reference | ||
| Adenocarcinoma | 1.04 | 0.84–1.29 | .712 |
| Grade | |||
| Well-differentiated | Reference | ||
| Moderately-differentiated | 0.84 | 0.63–1.13 | .247 |
| Poorly-differentiated | 1.07 | 0.79–1.44 | .675 |
| Clinical T stage | |||
| cT1 | Reference | ||
| cT2 | 1.12 | 0.76–1.64 | .564 |
| Year of diagnosis | 1.43 | 1.37–1.50 | < .001 |
Note: Age (per year), tumor size (per centimeter), and year of diagnosis (per year) were treated as continuous variables.
Abbreviations: SBRT, stereotactic body radiotherapy; OR, odds ratio; CI, confidence interval; Clinical T Stage, clinical tumor stage as per American Joint Committee on Cancer 6th or 7th Edition.
3.2. Survival analysis
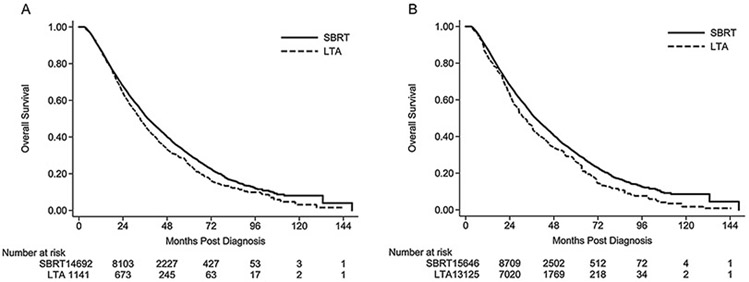
At a median follow-up of 26.2 months (range 3.0–148.7 months), SBRT resulted in a lower risk of death when compared to LTA for both the unadjusted cohort (hazard ratio [HR] 0.86, 95% confidence interval [CI] 0.80–0.93, p < .001; log-rank p < .001) and propensity-score weighted cohort (HR 0.83, 95% CI 0.73–0.94, p = .002) (Fig. 2). Adjusted rates of OS at 1, 2, 3, and 5 years were 87.5% vs. 83.5%, 68.0% vs. 63.0%, 52.2% vs. 45.9%, and 31.0% vs. 26.2% for SBRT and LTA, respectively. The superiority of SBRT over LTA with respect to OS was confirmed with doubly-robust multivariate analysis of the weighted cohort (Table 3). In this doubly-robust multivariate model, female sex, non-Caucasian race, and treatment at a high-volume facility were associated with a lower risk of death; whereas increasing age, increasing tumor size, and higher tumor grades portended worse OS.
Fig. 2.
Kaplan-Meier analysis of overall survival by treatment modality for A) unadjusted and B) propensity-score weighted cohorts.
NOTE. Number at risk in panel A represent actual numbers of patients at risk, whereas the number at risk in panel B represent a pseudo-population generated after propensity-score weighting the unadjusted population.
Abbreviations: SBRT, stereotactic body radiotherapy; LTA, local tumor ablation.
Table 3.
Doubly-robust estimation of overall survival with multivariate Cox proportional hazards modeling of the propensity-score weighted cohort.
| HR | 95% CI | P | |
|---|---|---|---|
| Local therapy | |||
| LTA | Reference | ||
| SBRT | 0.71 | 0.59–0.85 | < .001 |
| Age | 1.01 | 1.00–1.02 | .003 |
| Sex | |||
| Male | Reference | ||
| Female | 0.79 | 0.67–0.93 | .005 |
| Race | |||
| Caucasian | Reference | ||
| Other | 0.61 | 0.48–0.76 | < .001 |
| Charlson comorbidity score | |||
| 0 | Reference | ||
| ≥ 1 | 1.07 | 0.91–1.26 | .391 |
| Facility Type | |||
| Other | Reference | ||
| Academic | 1.08 | 0.90–1.31 | .412 |
| Facility treatment volume | |||
| Lowest 90% | Reference | ||
| Highest 10% | 0.77 | 0.63–0.94 | .010 |
| Tumor size, cm | |||
| ≤ 2.0 | Reference | ||
| 2.1-3.0 | 1.21 | 1.00–1.46 | .051 |
| 3.1-5.0 | 1.76 | 1.15–2.71 | .010 |
| Histology | |||
| Squamous cell carcinoma | Reference | ||
| Adenocarcinoma | 0.83 | 0.70–1.00 | .050 |
| Grade | |||
| Well-differentiated | Reference | ||
| Moderately-differentiated | 1.39 | 1.02–1.89 | .035 |
| Poorly-differentiated | 1.48 | 1.08–2.03 | .015 |
| Clinical T stage | |||
| cT1 | Reference | ||
| cT2 | 1.02 | 0.68–1.54 | .922 |
| Year of diagnosis | 1.03 | 0.99–1.08 | .108 |
Note: Age (per year) and year of diagnosis (per year) were treated as continuous variables.
Abbreviations: HR, hazard ratio; CI, confidence interval; LTA, local tumor ablation; SBRT, stereotactic body radiotherapy; cm, centimeter; Clinical T Stage, clinical tumor stage as per American Joint Committee on Cancer 6th or 7th Edition.
3.3. Subgroup analysis
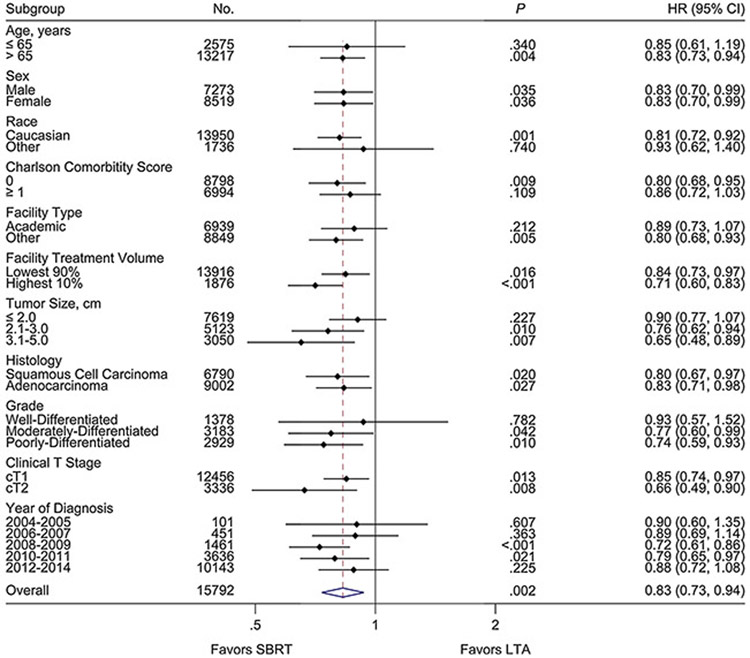
In an exploratory analysis of the propensity-score weighted cohort, the OS benefit for SBRT appeared to be consistent across multiple subgroups (Fig. 3). Within this propensity-score weighted population, lower risks of death were observed with SBRT for tumor sizes > 2.0 cm (HR 0.72, 95% CI 0.61–0.86, p < .001). No differences in rates of OS were found between SBRT and LTA for tumor sizes ≤ 2.0 cm. In the patient subset treated at high-volume facilities, a persistent lower risk of death was seen with SBRT versus LTA. No patient subgroups were identified that experienced improved OS with LTA relative to SBRT.
Fig. 3.
Forest plot of propensity-score weighted subgroup analyses of overall survival by treatment modality.
NOTE. Hazard ratios, 95% confidence intervals, and P values determined by univariate Cox proportional hazards modeling of the propensity-score weighted cohort.
Abbreviations: No., number; HR, hazard ratio; CI, confidence interval; cm, centimeter; Clinical T Stage, clinical tumor stage as per American Joint Committee on Cancer 6th or 7th Edition; SBRT, stereotactic body radiotherapy; LTA, local tumor ablation.
4. Discussion
Emerging evidence has suggested that percutaneous image-guided LTA may be a feasible treatment option for medically inoperable early-stage NSCLC [18-33]. However, no randomized prospective trials have been performed to validate LTA alongside SBRT, which prospective studies have firmly established as a safe and highly-effective standard-of-care [6-16]. In a 2016 pooled analysis of published experiences, SBRT was found to have superior local control at 1 (97% vs. 77%), 2 (92% vs. 48%), 3 (88% vs. 55%), and 5 years (86% vs. 42%) when compared to percutaneous LTA performed with RFA [34]. In this report, incidence of grade ≥ 3 toxicities also appeared to numerically favor SBRT (radiation pneumonitis, 2%; rib fracture, 2%) over RFA (pneumothorax requiring intervention, 13%). A single-institutional study evaluating the efficacy of microwave ablation also demonstrated a high rate of pneumothorax (32%) while the tumor control rates were only 78%, 64%, and 56% at 1, 2, and 3 years, respectively [36]. Others have suggested that SBRT may be more cost-effective than RFA, with an incremental cost-effectiveness ratio of $14,100 per quality-adjusted-life-year [37]. Although these findings appear to favor treatment with SBRT, limited published experiences suggest rates of OS are similar between SBRT and RFA [34,38].
Our analysis of a propensity-score weighted cohort from the NCDB demonstrated superior rates of OS with SBRT when compared to LTA for patients with non-surgically managed early-stage NSCLC. The survival advantage with SBRT persisted after a propensity-score weighted doubly-robust multivariate adjustment for age, sex, race, comorbidities, facility type, facility treatment volume, tumor size, histology, tumor grade, clinical T stage, and year of diagnosis. In an exploratory analysis, rates of OS appeared to favor SBRT across multiple subgroups, including those with larger tumor sizes (> 2.0 cm) and for those treated at high-volume facilities. Notably, no propensity-score weighted subgroups were identified that exhibited improved OS with LTA relative to SBRT.
These findings are in contrast to a similar 2004–2014 NCDB analysis that found no statistical differences in rates of OS between SBRT and LTA within a propensity-score matched patient cohort treated for tumor sizes ≤ 3.0 cm at high-volume facilities [38]. However, several factors may have contributed to the observed superiority of SBRT within the current study. Attempts were made in both studies to account for the quality of treatments by controlling for facility treatment volume, but this surrogate measure may not have been sufficient. In contrast to the referenced study, our analysis utilized a strict definition of SBRT, including only clinically-relevant prospectively-studied SBRT dose fractionation schemes achieving biologically effective doses ≥ 100 Gy (α/β = 10), a dose threshold which is known to be prognostic [11]. Conversely, no analogous treatment metrics were available within the NCDB to ensure high-quality percutaneous ablation techniques were performed. The NCDB registry does not include explicit codes for treatment with RFA or microwave ablation for cancers of the lung (ICD-O-3 C340-9). As a result, it is unclear how the RFA treatment cohort was defined in the referenced study. As defined by the NCDB, our percutaneous LTA cohort consisted of patients coded to have received laser ablation or cryosurgery, electrocautery/fulguration, or local tumor destruction not otherwise specified, with the assumption that patients receiving RFA or microwave ablation may also have been included. The limitations of the dataset in selecting for delivery of high-quality LTA certainly could have contributed to the observed superiority of SBRT in the current study.
Our results may also be partially explained by the inclusion of larger tumor sizes up to 5.0 cm. Historically, LTA has often been clinically reserved for smaller tumors measuring ≤ 3.0 cm due to declining rates of local control observed with increasing tumor size [23,31]. Indeed, we found no significant difference in rates of OS between SBRT and LTA within a propensity-score weighted subset of patients with tumor sizes ≤ 2.0 cm. However, improved OS was seen with SBRT in the weighted subgroup comprised of patients with tumor sizes of 2.1–3.0 cm. These findings highlight the need for future randomized prospective trials that can assist in further defining optimal patient selection and comparative-effectiveness between SBRT and LTA.
Our study was strengthened by a large sample size, achieved via a nationwide hospital-based population and relatively broad inclusion criteria, allowing for higher statistical power and potentially more generalizable findings. Within this population, treatment with SBRT was much more common than LTA (93% vs. 7%, respectively). SBRT was also relatively more common than LTA in patients with increasing age, in patients with fewer comorbidities, in those treated at academic facilities, in those with larger tumor sizes, and in patients treated more recently. Relative to SBRT, LTA was more commonly performed at high-volume facilities. These observed patterns of care suggest that SBRT remains the prevailing standard-of-care for the majority of medically inoperable early-stage NSCLC.
This study has several limitations, including potential effects from occult biases. To minimize potential confounders and biases, we utilized stout statistical methodology with propensity-score weighting via generalized boosted regression modeling and doubly-robust estimation with multivariate analysis of the weighted sample [39-41]. However, it remains difficult to control for all unknown confounders outside of a prospective randomized trial. In our analysis, relevant covariates were selected for propensity-score weighting a priori; contributions from potential unidentified confounders were otherwise unaccounted for. In addition, our analysis was restricted only to available data within the NCDB. Examples of potentially meaningful unavailable data included patient-level toxicity data, quality-of-life data, tumor location (e.g., central versus peripheral), local and regional tumor control, salvage treatments, progression-free survival, and disease-specific survival.
5. Conclusion
Within a propensity-score weighted NCDB hospital-based population, higher rates of OS were observed with SBRT versus percutaneous LTA in patients treated definitively for non-surgically managed early-stage NSCLC. This finding persisted across multiple subgroups, including those with tumor sizes > 2.0 cm and for those treated at high-volume facilities. In the subset of patients with small tumor sizes ≤ 2.0 cm, rates of OS were not significantly different between SBRT and LTA. These findings are hypothesis-generating and warrant further prospective evaluation of the comparative effectiveness of SBRT and LTA in early-stage NSCLC.
Supplementary Material
Acknowledgments
The authors would like to thank Michelle Denney for her contributions in editing and formatting this publication.
Funding
Greg Stoddard’s salary is supported by the University of Utah Population Health Research Foundation, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 5UL1TR001067-05 (formerly 8UL1TR000105 and UL1RR025764).
Abbreviations:
- NSCLC
non-small cell lung cancer
- SBRT
stereotactic body radiotherapy
- LTA
local tumor ablation
- RFA
radiofrequency ablation
- OS
overall survivor
- NCDB
National Cancer Database
- ICD-O
International Classification of Diseases in Oncology
- AJCC
American Joint Committee on Cancer
- T
tumor
- Gy
gray
Footnotes
Declaration of Competing Interest
Dr. Kokeny owns stock in Merit Medical and Becton Dickinson outside of the submitted work. The other authors declare no conflicts of interest.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.Org/10.1016/j.lungcan.2019.09.009.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2018, CA Cancer J. Clin 68 (1) (2018) 7–30. [DOI] [PubMed] [Google Scholar]
- [2].McMeekin DS, Filiaci VL, Aghajanian C, Cho J, Kim JW, DiSilvestro PA, O’Malley D, Rutherford TJ, Le LV, Randall ME, 1A randomized phase III trial of pelvic radiation therapy (PXRT) versus vaginal cuff brachytherapy followed by paclitaxel/carboplatin chemotherapy (VCB/C) in patients with high risk (HR), early stage endometrial cancer (EC): a Gynecologic Oncology Group trial, Gynecol. Oncol 134 (2) (2014) 438. [Google Scholar]
- [3].T. National Lung Screening Trial Research, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD, Reduced lung-cancer mortality with low-dose computed tomographic screening, N. Engl. J. Med 365 (5) (2011) 395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ginsberg RJ, Rubinstein LV, Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group, Ann. Thorac. Surg 60 (3) (1995) 615–622 discussion 622-3. [DOI] [PubMed] [Google Scholar]
- [5].Fernando HC, Landreneau RJ, Mandrekar SJ, Nichols FC, Hillman SL, Heron DE, Meyers BF, DiPetrillo TA, Jones DR, Starnes SL, Tan AD, Daly BD, Putnam JB Jr, Impact of brachytherapy on local recurrence rates after sublobar resection: results from ACOSOG Z4032 (Alliance), a phase III randomized trial for high-risk operable non-small-cell lung cancer, J. Clin. Oncol 32 (23) (2014) 2456–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Timmerman R, Papiez L, McGarry R, Likes L, DesRosiers C, Frost S, Williams M, Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer, Chest 124 (5) (2003) 1946–1955. [DOI] [PubMed] [Google Scholar]
- [7].Fakiris AJ, McGarry RC, Yiannoutsos CT, Papiez L, Williams M, Henderson MA, Timmerman R, Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study, Int. J. Radiat. Oncol. Biol. Phys 75 (3) (2009) 677–682. [DOI] [PubMed] [Google Scholar]
- [8].Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G, Johnstone D, Fowler J, Gore E, Choy H, Stereotactic body radiation therapy for inoperable early stage lung cancer, JAMA 303 (11) (2010) 1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Videtic GM, Hu C, Singh AK, Chang JY, Parker W, Olivier KR, Schild SE, Komaki R, Urbanic JJ, Timmerman RD, Choy H , A randomized phase 2 study comparing 2 stereotactic body radiation therapy schedules for medically inoperable patients with stage I peripheral non-small cell lung cancer: NRG oncology RTOG 0915 (NCCTG N0927), Int. J. Radiat. Oncol. Biol. Phys 93 (4) (2015) 757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nyman J, Hallqvist A, Lund JA, Brustugun OT, Bergman B, Bergstrom P, Friesland S, Lewensohn R, Holmberg E, Lax I, SPACE - a randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC, Radiother. Oncol 121 (1) (2016) 1–8. [DOI] [PubMed] [Google Scholar]
- [11].Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, Niibe Y, Karasawa K, Hayakawa K, Takai Y, Kimura T, Takeda A, Ouchi A, Hareyama M, Kokubo M, Hara R, Itami J, Yamada K, Araki T, Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study, J. Thorac. Oncol 2 (7 Suppl 3) (2007) S94–100. [DOI] [PubMed] [Google Scholar]
- [12].Videtic GM, Paulus R, Singh AK, Chang JY, Parker W, Olivier KR, Timmerman RD, Komaki RR, Urbanic JJ, Stephans KL, Yom SS, Robinson CG, Belani CP, Iyengar P, Ajlouni MI, Gopaul DD, Lele SB, McGarry RC, Choy H, Bradley JD, Long term follow-up on NRG Oncology RTOG 0915 (NCCTG N0927): a randomized phase II study comparing 2 stereotactic body radiation therapy schedules for medically inoperable patients with stage I peripheral non-small cell lung cancer, Int. J. Radiat. Oncol. Biol. Phys (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Baumann P, Nyman J, Hoyer M, Wennberg B, Gagliardi G, Lax I, Drugge N, Ekberg L, Friesland S, Johansson KA, Lund JA, Morhed E, Nilsson K, Levin N, Paludan M, Sederholm C, Traberg A, Wittgren L, Lewensohn R, Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy, J. Clin. Oncol 27 (20) (2009) 3290–3296. [DOI] [PubMed] [Google Scholar]
- [14].Koto M, Takai Y, Ogawa Y, Matsushita H, Takeda K, Takahashi C, Britton KR, Jingu K, Takai K, Mitsuya M, Nemoto K, Yamada S, A phase II study on stereotactic body radiotherapy for stage I non-small cell lung cancer, Radiother. Oncol 85 (3) (2007) 429–434. [DOI] [PubMed] [Google Scholar]
- [15].De Ruysscher D, Faivre-Finn C, Nestle U, Hurkmans CW, Le Pechoux C, Price A, Senan S, European Organisation for Research and Treatment of Cancer recommendations for planning and delivery of high-dose, high-precision radiotherapy for lung cancer, J. Clin. Oncol 28 (36) (2010) 5301–5310. [DOI] [PubMed] [Google Scholar]
- [16].Schneider BJ, Daly ME, Kennedy EB, Antonoff MB, Broderick S, Feldman J, Jolly S, Meyers B, Rocco G, Rusthoven C, Slotman BJ, Sterman DH, Stiles BM, Stereotactic body radiotherapy for early-stage non-small-cell lung cancer: american society of clinical oncology endorsement of the American society for radiation oncology evidence-based guideline, J. Clin. Oncol 36 (7) (2018) 710–719. [DOI] [PubMed] [Google Scholar]
- [17].Ball D, Mai GT, Vinod S, Babington S, Ruben J, Kron T, Chesson B, Herschtal A, Vanevski M, Rezo A, Elder C, Skala M, Wirth A, Wheeler G, Lim A, Shaw M, Schofield P, Irving L, Solomon B, Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial, Lancet Oncol. 20 (4) (2019) 494–503. [DOI] [PubMed] [Google Scholar]
- [18].Dupuy DE, Fernando HC, Hillman S, Ng T, Tan AD, Sharma A, Rilling WS, Hong K, Putnam JB, Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial, Cancer 121 (19) (2015) 3491–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ahmed M, Brace CL, Lee FT Jr., Goldberg SN, Principles of and advances in percutaneous ablation, Radiology 258 (2) (2011) 351–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zemlyak A, Moore WH, Bilfinger TV, Comparison of survival after sublobar resections and ablative therapies for stage I non-small cell lung cancer, J. Am. Coll. Surg 211 (1) (2010) 68–72. [DOI] [PubMed] [Google Scholar]
- [21].Ambrogi MC, Fanucchi O, Cioni R, Dini P, De Liperi A, Cappelli C, Davini F, Bartolozzi C, Mussi A, Long-term results of radiofrequency ablation treatment of stage I non-small cell lung cancer: a prospective intention-to-treat study, J. Thorac. Oncol 6 (12) (2011) 2044–2051. [DOI] [PubMed] [Google Scholar]
- [22].Dupuy DE, Image-guided thermal ablation of lung malignancies, Radiology 260 (3) (2011) 633–655. [DOI] [PubMed] [Google Scholar]
- [23].Simon CJ, Dupuy DE, DiPetrillo TA, Safran HP, Grieco CA, Ng T, Mayo-Smith WW, Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients, Radiology 243 (1) (2007) 268–275. [DOI] [PubMed] [Google Scholar]
- [24].Lencioni R, Crocetti L, Cioni R, Suh R, Glenn D, Regge D, Helmberger T, Gillams AR, Frilling A, Ambrogi M, Bartolozzi C, Mussi A, Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study), Lancet Oncol. 9 (7) (2008) 621–628. [DOI] [PubMed] [Google Scholar]
- [25].Hess A, Palussiere J, Goyers JF, Guth A, Auperin A, de Baere T, Pulmonary radiofrequency ablation in patients with a single lung: feasibility, efficacy, and tolerance, Radiology 258 (2) (2011) 635–642. [DOI] [PubMed] [Google Scholar]
- [26].Hiraki T, Gobara H, Mimura H, Matsui Y, Toyooka S, Kanazawa S, Percutaneous radiofrequency ablation of clinical stage I non-small cell lung cancer, J. Thorac. Cardiovasc. Surg 142 (1) (2011) 24–30. [DOI] [PubMed] [Google Scholar]
- [27].Okuma T, Matsuoka T, Yamamoto A, Oyama Y, Hamamoto S, Toyoshima M, Nakamura K, Miki Y, Determinants of local progression after computed tomography-guided percutaneous radiofrequency ablation for unresectable lung tumors: 9-year experience in a single institution, Cardiovasc. Intervent. Radiol 33 (4) (2010) 787–793. [DOI] [PubMed] [Google Scholar]
- [28].Pennathur A, Luketich JD, Abbas G, Chen M, Fernando HC, Gooding WE, Schuchert MJ, Gilbert S, Christie NA, Landreneau RJ, Radiofrequency ablation for the treatment of stage I non-small cell lung cancer in high-risk patients, J. Thorac. Cardiovasc. Surg 134 (4) (2007) 857–864. [DOI] [PubMed] [Google Scholar]
- [29].Belfiore G, Moggio G, Tedeschi E, Greco M, Cioffi R, Cincotti F, Rossi R, CT-guided radiofrequency ablation: a potential complementary therapy for patients with unresectable primary lung cancer–a preliminary report of 33 patients, AJR Am. J. Roentgenol 183 (4) (2004) 1003–1011. [DOI] [PubMed] [Google Scholar]
- [30].Kim SR, Han HJ, Park SJ, Min KH, Lee MH, Chung CR, Kim MH, Jin GY, Lee YC, Comparison between surgery and radiofrequency ablation for stage I non-small cell lung cancer, Eur. J. Radiol 81 (2) (2012) 395–399. [DOI] [PubMed] [Google Scholar]
- [31].Lanuti M, Sharma A, Willers H, Digumarthy SR, Mathisen DJ, Shepard JA, Radiofrequency ablation for stage I non-small cell lung cancer: management of locoregional recurrence, Ann. Thorac. Surg 93 (3) (2012) 921–927 discussion 927-88. [DOI] [PubMed] [Google Scholar]
- [32].Lee H, Jin GY, Han YM, Chung GH, Lee YC, Kwon KS, Lynch D, Comparison of survival rate in primary non-small-cell lung cancer among elderly patients treated with radiofrequency ablation, surgery, or chemotherapy, Cardiovasc. Intervent. Radiol 35 (2) (2012) 343–350. [DOI] [PubMed] [Google Scholar]
- [33].Sofocleous CT, May B, Petre EN, Gonen M, Thornton RH, Alago W, Rizk NP, Dupuy DE, Solomon SB, Pulmonary thermal ablation in patients with prior pneumonectomy, AJR Am. J. Roentgenol 196 (5) (2011) W606–12. [DOI] [PubMed] [Google Scholar]
- [34].Bi N, Shedden K, Zheng X, Kong FS, Comparison of the effectiveness of radiofrequency ablation with stereotactic body radiation therapy in inoperable stage I non-small cell lung cancer: a systemic review and pooled analysis, Int. J. Radiat. Oncol. Biol. Phys 95 (5) (2016) 1378–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bilimoria KY, Stewart AK, Winchester DP, Ko CY, The national cancer data base: a powerful initiative to improve cancer care in the United States, Ann. Surg. Oncol 15 (3) (2008) 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Healey TT, March BT, Baird G, Dupuy DE, Microwave ablation for lung neoplasms: a retrospective analysis of long-term results, J. Vasc. Interv. Radiol 28 (2) (2017) 206–211. [DOI] [PubMed] [Google Scholar]
- [37].Sher DJ, Wee JO, Punglia RS, Cost-effectiveness analysis of stereotactic body radiotherapy and radiofrequency ablation for medically inoperable, early-stage non-small cell lung cancer, Int. J. Radiat. Oncol. Biol. Phys 81 (5) (2011) e767–74. [DOI] [PubMed] [Google Scholar]
- [38].Lam A, Yoshida EJ, Bui K, Fernando D, Nelson K, Abi-Jaoudeh N, A national cancer database analysis of radiofrequency ablation versus stereotactic body radiotherapy in early-stage non-small cell lung cancer, J. Vasc. Interv. Radiol 29 (9) (2018) 1211–1217 e1. [DOI] [PubMed] [Google Scholar]
- [39].Austin PC, An introduction to propensity score methods for reducing the effects of confounding in observational studies, Multivariate Behav. Res 46 (3) (2011) 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].McCaffrey DF, Ridgeway G, Morral AR, Propensity score estimation with boosted regression for evaluating causal effects in observational studies, Psychol. Methods 9 (4) (2004) 403–425. [DOI] [PubMed] [Google Scholar]
- [41].Bang H, Robins JM, Doubly robust estimation in missing data and causal inference models, Biometrics 61 (4) (2005) 962–973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.