Abstract
A total of 201 patients with major depressive disorder from four hospitals in Malaysia were followed up for 5 years to determine the prognostic factors of recurrent major depressive disorder that could potentially contribute to improving the management of MDD patients. For each individual patient, at the time of recruitment as part of a case-control study, information was collected on recent threatening life events, personality and social and occupational functioning, while blood samples were collected to genotype single nucleotide polymorphisms of vitamin D receptor (VDR), zinc transporter-3 (ZnT3), dopamine transporter-1 (DAT1), brain-derived neurotropic factor (BDNF), serotonin receptor 1A (HT1A) and 2A (HT2A) genes. Kaplan-Meier and Cox-regression were used to estimate hazard functions for recurrence of major depressive disorder. Individuals with severe MDD in previous major depressive episodes had five and a half times higher hazard of developing recurrence compared to mild and moderate MDD (HR = 5.565, 95% CI = 1.631–18.994, p = 0.006). Individuals who scored higher on social avoidance had three and a half times higher hazard of recurrence of MDD (HR = 3.525, 95% CI = 1.349–9.209; p = 0.010). There was significant interaction between ApaI +64978C>A single nucleotide polymorphism and severity. The hazard ratio increased by 6.4 times from mild and moderate to severe MDD for A/A genotype while that for C/A genotype increased by 11.3 times. Social avoidance and severity of depression at first episode were prognostic of recurrence. Screening for personality factors at first encounter with MDD patients needs to be considered as part of the clinical practice. For those at risk of recurrence in relation to social avoidance, the psychological intervention prescribed should be customized to focus on this modifiable factor. Prompt and appropriate management of severe MDD is recommended to reduce risk of recurrence.
Introduction
Major depressive disorder (MDD) is defined as “discrete episodes of at least 2 weeks’ duration with clear-cut changes in affect, cognition, and neuro-vegetative functions, and inter-episode remissions” and often is recurrent in nature [1]. Recurrence is the return of symptoms after at least 2 consecutive months between separate episodes during which time criteria are not met for a major depressive episode (MDE) and there must be the return of at least 5 out of 9 symptoms of depression [1]. Many patients are at substantial risk of later recurrence, with 60% lifetime risk of recurrence after the first major depressive episode. As many as 70% of those with 2 MDEs have recurrences throughout their life, and 90% of those with three or more episodes will experience further recurrent episodes [2]. In addition, one third to half of the patients had recurrence within one year of discontinuation of treatment [3]. Each recurrence also carries a 10–20% risk of becoming unremitting and chronic [4]. Recurrent MDD in turn increases risk of significant functional impairment, suicide and comorbid physical health problems [5–9], incurring heavy health and economic burdens [10].
Many studies showed that sociodemographic factors such as younger age, women, and those who have never been married were at higher risk of recurrence [7, 11–15]; however, results of other studies on these risk factors have been inconsistent. A systematic review by Hardeveld and colleagues (2009) did not find any association between sociodemographic factors such as age, gender, socioeconomic status, marital status and risk of recurrence [16, 17, 18]. However, clinical variables such as number of previous episodes [7, 11, 19–22], severity of last episode [21, 22] and family history of MDD [21– 23] were strong predictors of recurrence. In addition, patients who had residual depression symptoms despite responding to treatment, had higher risk of recurrence (Serafini et al., 2018; Nierenberg et al., 2010; Judd et al., 1998) [19, 24, 25]. Studies also showed that personality traits were related to risk of recurrence [26]; Nobbelin et al. (2018) reported that patients with premorbid nervous or tense personalities had greater likelihood of recurrence [27] (Nobbelin et al., 2018).
Burcasa and Iacono in their review suggested that vulnerability to recurrent MDD has a genetic component [28]. Mutations in the brain-derived neurotropic factor (BDNF) [29, 30] and serotonin receptor genes [31] were shown to be associated with recurrent MDD. In addition, findings by Kuningas et al. (2009) [32] and Lye et al. [33] that single nucleotide polymorphisms (SNPs) of vitamin D receptor (VDR) and zinc transporter-3 (SLC30A3) genes were respectively associated with MDD, and together with evidence that SNPs of dopamine transporter-1 gene increased risk of MDD[34, 35], led to renewed interest in determining if an association exists between VDR, ZnT3 SNPs and recurrence of MDD.
Although acute management of MDDs is generally effective, many patients experience recurrence following remission. Identifying prognostic factors in patients at high risk of recurrence provides a window of opportunity for specific secondary prevention as well as initiation of long-term maintenance treatment. The objective of the present study is to determine the prognostic factors of recurrence that could potentially contribute to improving the management of patients with MDD.
Materials and methods
Ethics statement
This study was approved by the Medical Research Ethics Committee of the Ministry of Health Malaysia (NMRR No.: NMRR-14-688-19696). Written informed consent was obtained from all the participants.
Data collection
The original study was a case-control study in which the methodology has been described elsewhere [33]. Inclusion criteria included male and female patients from 18 to 65 years of age, who were diagnosed with single or recurrent, non-psychotic episode of Major Depressive Disorder (using the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [1]) diagnosed less than 2 years prior to recruitment. Patients who had significant suicidal risk as assessed by the psychiatrist or diagnosed with dementia, schizophrenia or other psychotic disorder, bipolar I or II disorder, or anxiety disorders including panic disorder, generalized anxiety disorder, obsessive-compulsive disorder and post-traumatic stress disorder, were excluded from the study.
Baseline sociodemographic characteristics as well as comorbidities (such as hypertension, diabetes mellitus, heart disease, stroke, cancer and asthma) were captured. Standardized questionnaires such as Lists of Threatening Events (LTEs) [34] and Temperament and Personality (T&P) [36] were used. The English version of the 12-item LTE questionnaire was reported to have good internal consistency and test-retest reliability (Cronbach's alpha = 0.84; Cohen's kappa = 0.72), with sensitivity of 89% and specificity of 74% [37]; the Malay version by Ng et al. (2009) was shown to be reliable as well (Kappa ĸ = 0.7 to 0.9) [38].
The T&P questionnaire consisted of 109 questions to capture information on eight personality dimensions and two personality functioning dimensions with Cronbach's α coefficients ranging from 0.62 to 0.91 and intra-class correlations from 0.72 to 0.93 [36, 39]. Items measuring each dimension add up to a scaled score, in which different cut off values were used to determine the tendencies of the dimensions. Cut off points for the different dimensions were as follows: Anxious worrying (18 and above—indicates a greater tendency to become stressed, worried and anxious); Personal reserve (17 and above—is associated with a tendency to keep one’s inner feelings to oneself); Perfectionism (31 and above—is associated with a tendency to be very responsible, to have high standards for oneself and to be highly committed to tasks and duties); Irritability (21 and above—is associated with a tendency to be quick-tempered and to externalise stress by becoming snappy and irritated by little things); Social avoidance (17 and above—is associated with a tendency to be introverted); Interpersonal sensitivity (14 and above—is associated with a tendency to worry about rejection or abandonment); Self-criticism (10 and above—is associated with a tendency to be very tough on oneself); Self-focus (9 and over—indicating prioritizing one’s own needs over other peoples’); Cooperativeness (20 and above—is associated with a tendency to be generally helpful); and Effectiveness (18 and above—indicates an ability to cope well with different situations and to be confident in problem solving). Higher scores indicate a higher tendency for those dimensions. A clinical record form endorsed by a psychiatrist (NI) (S1 Appendix) was used to identify recurrence based on the definition mentioned above and the symptoms reported at each visit, time from first diagnosis to occurrence of first episode of recurrence of MDD, duration of first episode of recurrence, subsequent episodes of MDD following the first episode of recurrence, severity of first and subsequent MDDs, full or partial remission for each recurrent episode, and family history of MDE. Severity was classified as mild, moderate and severe, according to the number symptoms based on ICD-10 [40], taking into consideration the level of severity of those symptoms and the degree of functional disability using DSM-5 [1]. Mild MDD was defined as presence of two or three MDD symptoms, which were distressing but manageable with minor functional impairment; moderate MDD was characterized by four or more MDD symptoms and patients were functionally impaired; severe MDD was defined by several marked and distressing MDD symptoms which were seriously distressing and unmanageable, with severe functional impairment associated with loss of self-esteem and ideas of worthlessness or guilt, suicidal thoughts or acts [1]. Full remission is defined by DSM-5 as having no significant signs or symptoms of MDD in the past 2 months [1]. Partial remission is defined as presence of symptoms of the immediate previous MDE but they do not fulfil full criteria for MDD or “there is a period lasting less than 2 months without any significant symptoms of MDE following the end of such an episode” [1].
Blood collection
5 mL of blood was collected using evacuated ethylenediamine tetra acetic acid (EDTA) tubes (Vacutainer Tubes, Becton-Dickinson, NJ, USA) from each subject, was stored under 4°C and processed on the same day.
Extraction of genomic DNA
We used the QIAamp DNA Mini Kit (QIAgen, USA) to extract genomic DNA [41]. 25μL of proteinase K and 200μL of lysis buffer were added to the buffy coat and incubated at 56°C for 30 minutes to maximize the cell lysis. We then precipitated DNA in 200μL of absolute ethanol before spinning through a filtered spin column. The column was washed twice in AW1 and AW2 wash buffer, followed by a drying spin at maximum speed for 1 minute. DNA was eluted with 50μL of nuclease free water and DNA yield. We then purified and quantified the extracted genomic DNA using a NanoPhotometer®Classic (Implen, USA). DNA purity of all samples were assessed using the 260/280nm ratio within the range of 1.7–2.0.
Determination of Vitamin D Receptor, zinc transporter-3, Dopamine Transporter-1, Brain-derived neurotropic factor, serotonin receptor 1A and 2A Single Nucleotide Polymorphisms (SNPs)
Primers (both sense and antisense) were used to amplify the targeted single nucleotide polymorphisms (SNPs) namely, BsmI +63980 G>A (rs1544410), ApaI +64978C>A (rs7975232) and TaqI +65058 T>C (rs731236) in the Vitamin D Receptor (VDR) gene, SLC30A3 rs11126396 of ZnT3 gene, rs40184 C>T of Dopamine Transporter-1 (DAT1) gene, rs6265 G196A of Brain-derived neurotropic factor (BDNF) gene, rs6295 -1019C>G of serotonin receptor 1A(HT1A) gene, and rs6311 -1438A>G of serotonin receptor 2A (HT2A) gene. Details of the genotyping methods have been fully described elsewhere [33, 42] and in S2 Appendix and S3 Appendix.
Data analysis
Relative frequencies were used to describe variables studied including sociodemographic factors and distribution of single nucleotide polymorphisms of all the genes. Chi-square test or Fisher’s exact test were used to determine differences in sociodemographic variables between recurrent patients and non-recurrent patients. Kaplan-Meier analysis was used to perform univariate analysis for sociodemographic variables, family history of psychiatric illnesses, severity of first MDE, comorbidities, and SNPs of BsmI (rs1544410), TaqI (rs731236) and ApaI (rs7975232) of VDR gene, SLC30A3 rs11126396 of ZnT3 gene, rs40184 of DAT1 gene, rs6265 of BDNF gene, rs6295 of HT1A, rs6311 HT2A gene, LTE and T&P domains. Subject(s) who did not experience recurrent MDE at the end of the study period were censored. From the Kaplan-Meier results, variables with p values less than 0.25 were then selected for the Cox regression model which estimated hazard ratios [43, 44, 45].
The Cox-proportionate hazards model was used in regressing time to recurrence on severity of first MDE, social avoidance, irritability, anxious worrying, interpersonal sensitivity, self-criticism, effectiveness, age of first MDE, gender, educational level, family history of psychiatric illness, and comorbidities. Interactions between the significant predictor variables and genotypes of single nucleotide polymorphisms of VDR genes namely, BsmI +63980 G>A, ApaI +64978C>A and TaqI +65058 T>C, and SLC30A3 rs11126396 of ZnT3 gene, rs40184 of DAT1 gene, rs6265 of BDNF gene, rs6295 of HT1A and rs6311 HT2A gene were tested. Statistical significance was set at alpha of 0.05.
Results
Over the five-year period, of the 201 patients traced from the case histories, 145 patients were available for survival analysis. 20.9% of the MDD patients suffered first recurrence; the rest were censored. Table 1 shows that there was no difference between the patients with data available for survival analysis and those for whom records were not available. Table 2 depicts the baseline information among those with and without recurrence. The age distribution was similar among both groups. The proportion of patients who experienced recurrence was slightly higher in females compared to males, with a female to male ratio of 1.5:1. Recurrence of MDD was also higher in those with academic degrees and postgraduate qualifications. However, those differences were not statistically significant.
Table 1. Comparison of cases included for analysis versus missing cases.
| Variables | Followed up | Missing | Statistica | p- value | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Race | 1.354 | 0.508 | ||||
| Malay | 100 | 51.0 | 49 | 47.1 | ||
| Chinese | 60 | 30.6 | 30 | 28.8 | ||
| Indian and others | 36 | 18.4 | 25 | 24.0 | ||
| Gender | 0.765 | 0.382 | ||||
| Male | 60 | 30.6 | 37 | 35.6 | ||
| Female | 136 | 69.4 | 67 | 64.4 | ||
| Education level | 0.638 | 0.888 | ||||
| Primary or | 95 | 48.5 | 52 | 50.0 | ||
| Secondary | ||||||
| Certificate | 18 | 9.2 | 7 | 6.7 | ||
| Diploma | 30 | 15.3 | 15 | 14.4 | ||
| Degree/Postgraduate | 53 | 27.0 | 30 | 28.8 | ||
| Comorbidities | 0.004 | 0.949 | ||||
| Yes | 69 | 35.2 | 37 | 35.6 | ||
| No | 127 | 64.8 | 67 | 64.4 | ||
| Family history | 0.918 | 0.632 | ||||
| Yes | 54 | 27.6 | 26 | 25.0 | ||
| No | 124 | 63.2 | 71 | 68.3 | ||
| Unsure | 18 | 9.2 | 7 | 6.7 | ||
| TaqI | 0.693 | 0.707 | ||||
| T/T | 139 | 70.9 | 78 | 75.0 | ||
| T/C | 44 | 22.4 | 21 | 20.2 | ||
| C/C | 13 | 6.6 | 5 | 4.8 | ||
| ApaI | 0.123 | 0.940 | ||||
| A/A | 41 | 20.9 | 20 | 19.2 | ||
| A/C | 75 | 38.3 | 41 | 39.4 | ||
| C/C | 80 | 40.8 | 43 | 41.4 | ||
| Anxious worrying | 0.75 | 0.386 | ||||
| High tendency | 51 | 26.6 | 21 | 21.9 | ||
| Low tendency | 141 | 73.4 | 75 | 78.1 | ||
| Irritability | 2.963 | 0.085 | ||||
| High tendency | 25 | 13.0 | 20 | 20.8 | ||
| Low tendency | 167 | 87.0 | 76 | 79.2 | ||
| Social avoidance | 1.169 | 0.280 | ||||
| High tendency | 16 | 8.4 | 12 | 12.4 | ||
| Low tendency | 175 | 91.6 | 85 | 87.6 | ||
| Interpersonal | 0.097 | 0.755 | ||||
| sensitivity | 57 | 30.5 | 31 | 32.3 | ||
| High tendency | 130 | 69.5 | 65 | 67.7 | ||
| Low tendency | ||||||
| Self-criticism | 0.629 | 0.428 | ||||
| High tendency | 140 | 72.9 | 65 | 68.4 | ||
| Low tendency | 52 | 27.1 | 30 | 31.6 | ||
| Effectiveness | 0.129 | 0.719 | ||||
| High tendency | 60 | 33.3 | 29 | 31.2 | ||
| Low tendency | 120 | 66.7 | 64 | 68.8 | ||
| Mean Age, years (sd) | 196 | 38.9 (13.01) | 104 | 38.5 (11.93) | 0.228* | 0.820 |
a The statistic reported for all variables was the chi-square value, except the variable labelled * (t-statistic)
Table 2. Sociodemographic characteristics of study population by recurrence (n = 145).
| Variables | Recurrencen (%) | No Recurrence n (%) | χ 2 | p value | ||
|---|---|---|---|---|---|---|
| Age (years old) | 0.017 | 0.898 | ||||
| 18–39 | 24 | 21.2 | 89 | 78.8 | ||
| 40–65 | 17 | 20.5 | 66 | 79.5 | ||
| Race | 1.051 | 0.305 | ||||
| Malay | 18 | 18.0 | 82 | 82.0 | ||
| Others | 23 | 24.0 | 73 | 76.0 | ||
| Gender | 1.831 | 0.176 | ||||
| Male | 9 | 15.0 | 51 | 85.0 | ||
| Female | 32 | 23.5 | 104 | 76.5 | ||
| Education level | 1.672 | 0.196 | ||||
| Primary /Secondary/Certificate | 20 | 17.7 | 93 | 82.3 | ||
| Diploma/Degree/Postgraduate | 21 | 25.3 | 62 | 74.7 | ||
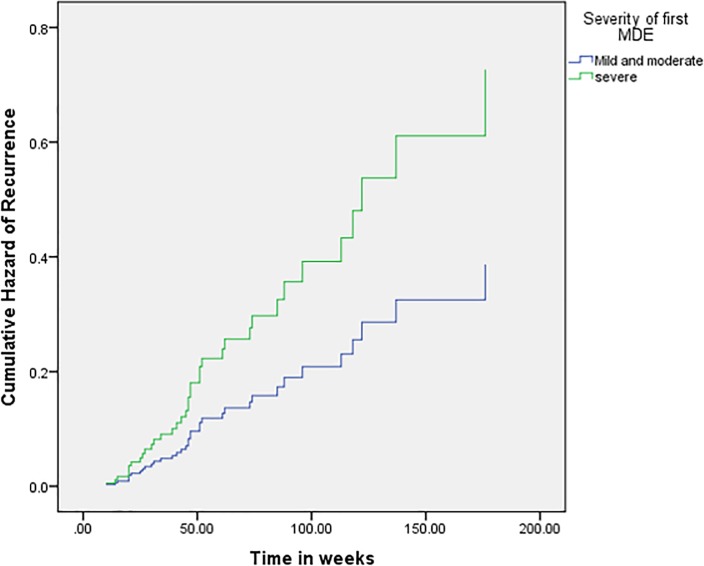
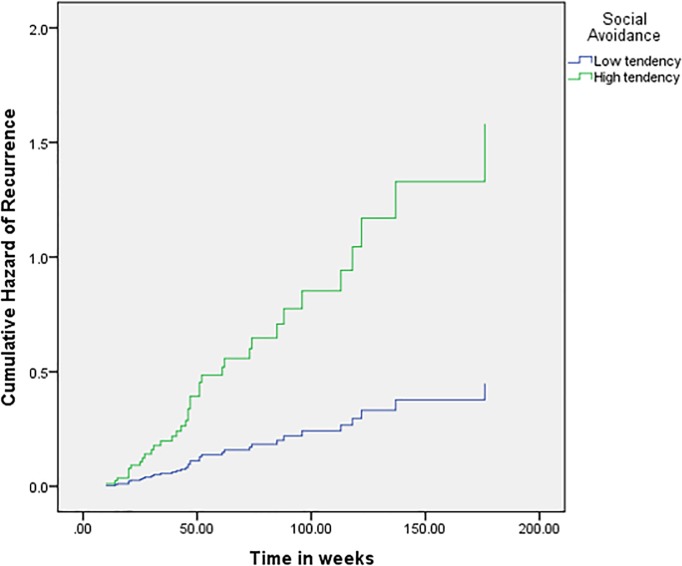
Severity, social avoidance and interaction between ApaI polymorphism and severity significantly prognosticated recurrence (Table 3). Individuals with severe MDD at the first MDE had five and a half times higher hazard of developing recurrence compared to mild and moderate MDD (HR = 5.565, 95% CI = 1.631–18.994, p = 0.006). Individuals with a higher score of social avoidance had three and a half times higher hazard of recurrence of MDD (HR = 3.525, 95% CI = 1.349–9.209; p = 0.010). The hazard functions for severity and social avoidance are depicted in Fig 1 and Fig 2 respectively.
Table 3. Cox proportionate hazard analysis for predictors of MDD recurrence (n = 145).
| Variables | Recurrencen (%) | No Recurrence n (%) | Adjusted HRa | 95% CI | p value | |||
|---|---|---|---|---|---|---|---|---|
| Age group | 18–39 | 24 | 21.2 | 89 | 78.8 | 1 | ||
| 40–65 | 17 | 20.5 | 66 | 79.5 | 0.778 | (0.323–1.874) | 0.575 | |
| Gender | Male | 9 | 15.0 | 51 | 85.0 | 1 | ||
| Female | 32 | 23.5 | 104 | 76.5 | 0.996 | (0.401–2.476) | 0.994 | |
| Educational level | Primary / Secondary/Certificate | 20 | 17.7 | 93 | 82.3 | 1 | ||
| Diploma/Degree/ Postgraduate | 21 | 25.3 | 62 | 74.7 | 1.252 | (0.544–2.883) | 0.597 | |
| Comorbidities | Yes | 10 | 14.3 | 60 | 85.7 | 1 | ||
| No | 31 | 23.7 | 100 | 76.3 | 0.936 | (0.376–2.334) | 0.888 | |
| Family History of psychiatric illness | Yes | 45 | 83.3 | 9 | 16.7 | 1 | ||
| No | 102 | 79.1 | 27 | 20.9 | 1.937 | (0.804–4.666) | 0.141 | |
| Unsure | 13 | 72.2 | 5 | 27.8 | 1.756 | (0.478–6.448) | 0.397 | |
| Severity | Mild to Moderate | 20 | 17.4 | 95 | 82.6 | 1 | ||
| Severe | 21 | 25.9 | 60 | 74.1 | 5.565 | (1.631–18.994) | 0.006* | |
| Anxious Worrying | Low | 27 | 19.0 | 115 | 81.0 | 1 | ||
| High | 13 | 26.0 | 37 | 74.0 | 0.769 | (0.285–2.071) | 0.603 | |
| Irritability | Low | 32 | 19.2 | 135 | 80.8 | 1 | ||
| High | 7 | 28.0 | 18 | 72.0 | 1.328 | (0.413–4.274) | 0.634 | |
| Social Avoidance | Low | 31 | 17.7 | 144 | 82.3 | 1 | ||
| High | 8 | 50.0 | 8 | 50.0 | 3.525 | (1.349–9.209) | 0.010* | |
| Interpersonal Sensitivity | Low | 27 | 20.6 | 104 | 79.4 | 1 | ||
| High | 12 | 21.4 | 44 | 78.6 | 0.942 | (0.371–2.394) | 0.900 | |
| Self-criticism | Low | 4 | 7.5 | 49 | 92.5 | 1 | ||
| High | 36 | 25.9 | 103 | 74.1 | 2.964 | (0.810–10.850) | 0.101 | |
| Effectiveness | Low | 27 | 22.5 | 93 | 77.5 | 1 | ||
| High | 10 | 16.7 | 50 | 83.3 | 1.048 | (0.435–2.523) | 0.917 | |
| TaqI | T/T | 33 | 23.9 | 105 | 76.1 | 1 | ||
| T/C | 7 | 15.6 | 38 | 84.4 | 0.700 | (0.258–1.900) | 0.483 | |
| C/C | 1 | 7.7 | 12 | 92.3 | 0.141 | (0.016–1.264) | 0.080 | |
| ApaI*Severity at first MDE | ||||||||
| ApaI*Severity | 1 | |||||||
| b ApaI (1)*Severity | 0.582 | (0.181–1.876) | 0.365 | |||||
| b ApaI (2)*Severity | 0.122 | (0.024–0.610) | 0.010* | |||||
aHazard ratio, controlling for age and gender
b Dummy variables: ApaI (1) = C/A; ApaI (2) = C/C Reference genotype = A/A
*p<0.05
Fig 1. Hazard function for recurrence of MDD by severity at first MDE.
Fig 2. Hazard function for recurrence of MDD by social avoidance.
ApaI +64978C>A was shown to significantly potentiate the effect of severity (Table 4). For A/A genotype, the hazard of recurrence increased by 6.4 times when the severity shifted from mild and moderate to severe; similarly, for the C/A genotype, the hazard ratio increased by a factor of 11.3.
Table 4. Hazard ratios for ApaI +64978C>A genotypes by severity at first MDE.
| Severity | ||
|---|---|---|
| ApaI | Mild and Moderate | Severe |
| A/A | 0.469 (p = 0.239; 95% CI = 0.133–1.656) * | 3.018 (p = 0.105, 95% CI = 0.794–14.468) * |
| C/A | 0.289 (p = 0.033, 95% CI = 0.092–0.908) * | 3.265 (p = 0.041, 95% CI = 1.047–10.182) * |
| C/C | 1 | 1 |
*Hazard ratio, controlling for age at first MDE
Discussion
Accurate prediction and identification of factors leading to recurrence of MDD is important to improve customized management to the individual patients. However, accurate ascertainment of the diagnosis is also important before identifying those factors. A number of reviews and research articles used definitions of remission, recovery, relapse and recurrence by Frank et al. [46] but the definitions in DSM-5 [1] are clinically more pragmatic and useful, hence these were used in the current study.
From our study, the proportion of first recurrence of MDD was 20.9%. Hardeveld and colleagues reported that the rate of recurrence in specialised mental health care centres was 26.8% [47]. In another article, Hardeveld et al. reported that the rate of recurrence over a 5-year period was 60% [16]. According to the American Psychiatric Association [1], there was at least a 60% lifetime risk of recurrence after the first major depressive episode. On the other hand, the 4.3% recurrence rate reported by ten Have et al. [48] was much lower than those from this and other studies.
Our study showed that depressed individuals with a higher tendency towards social avoidance were at greater risk of recurrence of MDD. Studies regarding the influence of personality functioning on recurrence of MDD is limited. To the best of our knowledge, no previous study has reported on social avoidance as a predictor of recurrence of MDD. Empirical evidence has been accumulated to show that personality functioning is not only a modifier or a sequela of depressive disorder, but also a predictor of its incidence [49], relapses and recurrences [50, 51]. Ferster (1973) suggested that avoidance plays a role in which frequent avoidance of perceived unpleasant conditions by depressed individuals results in limited exposure to positively-reinforced behaviors and social activities [52, 53] hence increasing the risk of recurrence. In addition, anticipation of previously experienced discrimination may further cause depressed individuals to avoid participation in certain life areas, leading to greater isolation and social marginalization [54]. This finding from our study could potentially be useful for application in clinical practice; by identifying MDD patients with a high tendency to social avoidance, targeted interventions could be undertaken by the psychiatrist or clinical psychologist to mitigate the effect of this modifiable risk factor.
Similar to the results of our study, Kudo et al. (2017) did not observe a significant association between the self-criticism personality domain and recurrence of MDD [55]. However, the tools of measurement of the personality dimension (such as anxious worrying, personal reserve and perfectionism) and personality functioning (such as cooperativeness and effectiveness) in both studies were slightly different (Black Dog Institute, 2017). Individuals with a higher tendency towards neuroticism, which was not measured in our study, were being consistently reported to be associated with a higher risk of recurrence [56, 57].
Our study found that severity at first MDE increased hazard of recurrence by five and a half times. However, population and clinical studies that have been conducted used different classification systems for severity of first MDE which involved the duration of the first major depressive episode and number of symptoms present, but a more severe first MDE has consistently been shown to be related to recurrence [22]. Barkow et al. (2003) used ICD-10 criteria for severity to study the risk factors of recurrent MDE in the primary care setting and showed that severe MDD at baseline tripled the risk for development of subsequent depressive episodes [58]. NEMESIS-2, a Dutch cohort study using the Sheehan Disability Scale (SDS) to classify severity of depression, showed that severity of the last depressive episode increased recurrence of MDD by almost two times [48]. The results consistently suggested that severity of previous MDE predicted recurrence and this was also demonstrated in our study.
In terms of age at onset and gender, the results of our study replicated results of several earlier studies showing that age at onset of first MDE [17, 18, 59–60] and gender [16, 18, 59–61] did not predict subsequent episode of MDE. This is contrary to what some other studies have found [7, 11–15]. The inconsistencies in findings regarding age and gender could possibly be due to the effect of socio-cultural differences in the populations studied. In a meta-analysis of the effect of gender and age on depressive symptoms, Salk et al. put forward Eagly and Wood’s social-structural theory [62] that “larger gender differences should be observed in nations with more gender inequality”. In addition, Salk et al. found quadratic trends for gender differences in its interaction with age and age-related factors such as “stressors in adolescence and the hormonal and neurodevelopmental changes that vary by sex and peaking at ages 13–15 years and declining in the 20s”. Depression may also manifest differently in different cultures [63]. We postulate that these differences could possibly apply to recurrence, and explain the inconsistent findings between studies conducted in different countries and with different age groups studied.
Results regarding family history and recurrence also have been inconsistent–a number of previous studies have reported that a family history of psychiatric illnesses was not associated with recurrence of MDD [11, 44, 64, 65], similar to what we have found in our study. Other studies [21–23] have reported that family history significantly predicted recurrence. In a recent systematic review and meta-analysis, Buckman et al. (2018) [66] reported that “there was very limited and inconsistent evidence that family history of depression is prognostic of recurrence”. In their meta-analysis, the pooled effect size of an odds ratio of 1.36 (95% CI = 0.92, 2.01) for 3 studies was not statistically significant. The authors reported that “many studies fail to discriminate patients in their first episode from those with a history of multiple previous depressive episodes” and that “differences in the case mix across studies can lead to spurious conclusions”. This could have partially accounted for some of the inconsistencies found in the published literature.
Although ApaI +64978C>A SNP by itself is not a predictor of recurrence in the present study, we found that the interaction of ApaI +64978C>A genotype with severity at first MDE to be significant in predicting its recurrence. The hazard of recurrence for carriers of the A/A and C/A genotypes of ApaI with a severe first episode of MDD is much higher than for a less severe episode, indicating that ApaI +64978C>A polymorphisms potentiated the effect of severity on recurrence of MDD (Table 4). Kuningas et al. (32) found that Dutch patients with the baT haplotype of the Vitamin D receptor (VDR) gene were less prone to depression compared with carriers of BAt. This propensity of the A allele to increase risk of depression could possibly play a role in the interaction between ApaI polymorphism and severity. The C>A transversion at the ApaI restriction site is located in the 3’ VDR gene transcription initiation site—a ligand binding site of the VDR gene [67]—and may affect the downstream effect of the ligand-binding properties of the Vitamin D receptor, in turn affecting the Vitamin D Response Element (VDRE) complex and activation of the transcription of tryptophan hydroxylase 2 (TPH2) gene, leading to an imbalance of serotonin levels in the brain [68–70]. Although several polymorphisms, including ApaI, of the VDR gene have been described, their effects on VDR function and interaction with severity of depression are still poorly understood.
The limitations of the study include those inherent in the design of a case-control study from which data for this study was derived. Biases inherent in a case-control study include recall and selection bias. We attempted to minimize recall bias by administering the questionnaires using enumerators trained in interviewing patients. Since the questionnaires were self-administered, enumerators were on standby to ensure that any questions the patients may have were adequately addressed, with intimations from non-verbal communication from interviewers kept to a minimum. Representativeness of the sample was ensured as far as possible with the recruitment of patients who came from the catchment area of the hospital representative of the cross-section of the community as shown by their socio-demographic profiles (Table 2). The sample size undoubtedly was affected by loss to follow-up; however, there was no significant difference in the baseline profiles between those who were followed up and those lost to follow-up, indicating that the bias could have been kept to a minimum.
This study adds new information to the body of knowledge on modifiable risk factors and predictors for recurrence of MDD; future studies could further examine the role of genetic biomarkers in prognosticating recurrence in assisting health care workers in early identification and preventive management of major depressive disorder. We believe that this new finding has the potential to inform the management of MDD in which clinicians can now be alerted to potential recurrence from a fore knowledge of social avoidance and severity of a previous episode of MDD, both of which are modifiable risk factors.
Conclusion
This study suggests that social avoidance and severity of previous MDD prognosticate recurrence, with ApaI +64978C>A genotypes acting in synergy with severity in potentiating the hazard of recurrence. We suggest screening for personality factors—in particular, social avoidance; this could potentially be performed at the first major depressive episode. Psychological intervention could then be customized to focus on this modifiable factor. In addition, prompt and appropriate management of severe MDD is recommended to reduce risk of recurrence.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank the Director General of Health Malaysia for approval to conduct the study (NMRR No.: NMRR-14-688-19696). We also thank Dr Azizul Awaluddin, Dr Sharifah Suziah Syed Mokhtar, Dr Mazni Mat Junus, Dr Elinda Tunan, Ms Siti Zubaidah Redzuan, Dr Vaidehi Ulanganathan, research assistants as well as staff of the hospitals involved.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The study was funded by Research Management Centre Universiti Putra Malaysia (GP-IPB/2013/9415700). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed Washington, DC: American Psychiatric Publishing; 2014. [Google Scholar]
- 2.Monroe SM, Harkness KL. Recurrence in major depression: A conceptual analysis. Psychol Rev. 2011; 118: 655–674. 10.1037/a0025190 [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119–38. 10.1146/annurev-publhealth-031912-114409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee AS. Better outcomes for depressive disorders? Psychol Med. 2003; 33: 769–774. 10.1017/s003329170300802x [DOI] [PubMed] [Google Scholar]
- 5.Judd LJ. The clinical course of unipolar major depressive disorders. Arch Gen Psychiatry. 1997; 54(11): 989–91. 10.1001/archpsyc.1997.01830230015002 [DOI] [PubMed] [Google Scholar]
- 6.Kessler RC, Wang PS. The epidemiology of depression In Gotlib I.H., & Hammen C.L., 3rd ed Handbook of Depression 2. New York: Guilford Press; 2009: pp. 5–22. [Google Scholar]
- 7.Mueller TI, Leon AC, Keller MB, Solomon DA, Endicott J, Coryell W, et al. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am J Psychiatry. 1999; 156(7): 1000–6. 10.1176/ajp.156.7.1000 [DOI] [PubMed] [Google Scholar]
- 8.Murray C, Lopez A. Evidence-based health policy–lessons from the Global Burden of Disease Study. Science. 1996; 274(5288): 740–3. 10.1126/science.274.5288.740 [DOI] [PubMed] [Google Scholar]
- 9.Roiser JP, Elliot E, Sahakian BJ. Cognitive Mechanisms of Treatment in Depression. Neuropsychopharmacology. 2012; 37(1): 117–36. 10.1038/npp.2011.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greden J. The burden of recurrent depression: causes, consequences, and future prospects. J Clin Psychiatr. 2001; 62 Suppl 22 5–9. [PubMed] [Google Scholar]
- 11.Winokur G, Coryell W, Keller M, Endicott J, Akiskal H. A prospective follow-up of patients with bipolar and primary unipolar affective disorder. Arch Gen Psychiatry. 1993; 50(6): 457–65. 10.1001/archpsyc.1993.01820180059006 [DOI] [PubMed] [Google Scholar]
- 12.Holma KM, Holma IA, Melartin TK, Rytsala HJ, Isometsa ET. Long-term outcome of major depressive disorder in psychiatric patients is variable. J Clin Psychiatry. 2008; 69(2):196–205. 10.4088/jcp.v69n0205 [DOI] [PubMed] [Google Scholar]
- 13.Eaton WW, Anthony JC, Gallo J, Cai G, Tien A, Romanoski A, et al. Natural history of diagnostic interview schedule/DSM-IV major depression. The Baltimore Epidemiologic Catchment Area follow-up. Arch Gen Psychiatry. 1997; 54(11): 993–9. 10.1001/archpsyc.1997.01830230023003 [DOI] [PubMed] [Google Scholar]
- 14.Gueorguieva R, Chekroud AM, Krystal JH. Trajectories of relapse in randomised, placebo-controlled trials of treatment discontinuation in major depressive disorder: an individual patient-level data meta-analysis. Lancet Psy. 2017; 4:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serafini G, Santi F, Gonda X, Aguglia A, Fiorillo A, Pompili M, et al. Predictors of recurrence in a sample of 508 outpatients with major depressive disorder. J Psy Research. 2019; 114: 80–87. [DOI] [PubMed] [Google Scholar]
- 16.Hardeveld F, Spijker J, De Graaf R, Nolen WA, Beekman AT. Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr Scand. 2010; 122(3): 184–191. 10.1111/j.1600-0447.2009.01519.x [DOI] [PubMed] [Google Scholar]
- 17.Lewinsohn PM, Zeiss AM, Duncan EM. Probability of relapse after recovery from an episode of depression. J Abnormal Psychol. 1989; 98(2): 107–116. [DOI] [PubMed] [Google Scholar]
- 18.Kovac M, Obrosky DS, Sherrill J. Developmental changes in the phenomenology of depression in girls compared to boys from childhood onward. J Affect Disord. 2003;74: 33–48. 10.1016/s0165-0327(02)00429-9 [DOI] [PubMed] [Google Scholar]
- 19.Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, et al. (1998). Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998; 50(2–3): 97–108. 10.1016/s0165-0327(98)00138-4 [DOI] [PubMed] [Google Scholar]
- 20.Maj M, Veltro F, Pirozzi R, Lobrace S, Magliano L. Pattern of recurrence of illness after recovery from an episode of major depressive episodes: A Prospective Study. Am J Psychiatry. 1992; 149: 795–800. 10.1176/ajp.149.6.795 [DOI] [PubMed] [Google Scholar]
- 21.Pintor L, Gasto C, Navarro V, Torres X, Fananas L. Relapse of major depression after complete and partial remission during a 2-year follow-up. J Affect Disord. 2003; 73(3): 237–44. 10.1016/s0165-0327(01)00480-3 [DOI] [PubMed] [Google Scholar]
- 22.Pintor L, Torres X, Navarro V, Matrai S, Gasto C. Is the type of remission after a major depressive episode an important risk factor to relapses in a 4-year follow up? J Affect Disord. 2004; 82: 291–6. 10.1016/j.jad.2003.11.008 [DOI] [PubMed] [Google Scholar]
- 23.Lewinsohn PM, Rohde P, Seeley JR, Klein DN, Gotlib IH. Natural course of adolescent major depressive disorder in a community sample: Predictors of recurrence in young adults. Am J Psychiatry. 2000; 157:1584–1591. 10.1176/appi.ajp.157.10.1584 [DOI] [PubMed] [Google Scholar]
- 24.Serafini G, Nebbia J, Cipriani N, Conigliaro C, Erbuto D, Pompili M, et al. Number of illness episodes as predictor of residual symptoms in major depressive disorder. Psychiatr Res. 2018; 262: 469–476. [DOI] [PubMed] [Google Scholar]
- 25.Nierenberg AA, Husain MM, Trivedi MH, Fava M, Warden D, Wisniewski SR, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010; 40: 41–50. 10.1017/S0033291709006011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nery FG, Hatch JP, Nicoletti MA, Monkul ES, Najt P, Matsuo K, et al. Temperament and character traits in major depressive disorder: influence of mood state and recurrence of episodes. Depress Anxiety. 2009;26(4):382–8 10.1002/da.20478 [DOI] [PubMed] [Google Scholar]
- 27.Nöbbelin L, Bogren M, Mattisson C, Brådvik L. Risk factors for recurrence in depression in the Lundby population, 1947–1997. J Affect Disord. 2008; 228: 125–131. [DOI] [PubMed] [Google Scholar]
- 28.Burcusa SL, Iacono WG. Risk for Recurrence in Depression. Clin Psychol Rev. 2007; 27(8): 959–985. 10.1016/j.cpr.2007.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Na KS, Won E, Kang J, Chang HS, Yoon HK, Tae WS, et al. Brain-derived neurotrophic factor promoter methylation and cortical thickness in recurrent major depressive disorder. Sci Rep. 2016; 6: 21089 10.1038/srep21089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao ZM, Liu WH, Gao K, Wan QR, Yang C, Wang HL, et al. Interaction between CRHR1 and BDNF genes increases the risk of recurrent major depressive disorder in Chinese population. PLoS ONE. 2011; 6(12): e28733 10.1371/journal.pone.0028733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drevets WC, Thase M, Moses E, Price J, Frank E, Kupfer DJ, et al. Serotonin-1A Receptor Imaging in Recurrent Depression: Replication and Literature Review. Nucl Med Biol. 2007; 34(7): 865–877. 10.1016/j.nucmedbio.2007.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuningas M, Mooijaart SP, Jolles J, Slagboom PE, Westendorp RGJ, van Heemst D. VDR gene variants associate with cognitive function and depressive symptoms in old age. Neurobiol Aging. 2009; 30:466–73. 10.1016/j.neurobiolaging.2007.07.001 [DOI] [PubMed] [Google Scholar]
- 33.Lye MS, Shahbudin AF, Tey YY, Tor YS, Ling KH, Loh SP, et al. Zinc transporter-3 [SLC30A3 (rs11126936)] polymorphism is associated with major depressive disorder in Asian Subjects. Neuroscience Research Notes. 2019; 2(3): 20–28. [Google Scholar]
- 34.Felten A, Montag C, Markett S, Walter NT, Reuter M. Genetically determined dopamine availability predicts disposition for depression. Brain Behav. 2011; 1(2): 109–118. 10.1002/brb3.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haeffel GJ, Getchell M, Koposov RA, Yrigollen CM. Association between polymorphisms in the dopamine transporter gene and depression: Evidence for a gene-environment interaction in a sample of juvenile detainees. Psychol Sci. 2008; 19(1): 62–9. 10.1111/j.1467-9280.2008.02047.x [DOI] [PubMed] [Google Scholar]
- 36.Black Dog Institute. https://www.blackdoginstitute.org.au/clinical-resources/health-professional-resources/temperament-and-personality(t-p)-questionnaire. Assessed on: 3 August 2018
- 37.Bhugra TS, Cragg D. The list of threatening experiences: the relialibility and validity of a brief Life Events Questionaire. Acta Psychiatr Scand. 1990; 82(1): 97–104. [DOI] [PubMed] [Google Scholar]
- 38.Ng CG, Amer Siddiq AN, Aida SA, Koh OH, Nor Zuraida Z. The Reliability Of Malay Version Of List Of Threatening Experiences Questionnaire: A Study On A Group Of Medical Students In Malaysia. Malaysian Journal of Psychiatry 2009; 18(2). [Google Scholar]
- 39.Spanemberg L, Parker G, Caldieraro MA, Vares EA, Costa F, Costa MM, et al. Translation and cross-cultural adaptation of the Temperament & Personality Questionnaire into Brazilian Portuguese. Trends Psychiatry Psychother. 2014; 36(4): 214–218. 10.1590/2237-6089-2014-1007 [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10). 2016. http://apps.who.int/classifications/icd10/browse/2016/en#/F32.2 Assessed on: 3 August 2018
- 41.Qiagen. QIAamp DNA Mini and Blood Mini Handbook. 5th ed. 2016.
- 42.Faris A, Md Yusof HH, Zainal Abidin S, Cheah PS. Development and validation of High Resolution Melting assays for high-throughput screening of BDNF rs6265 and DAT1 rs40184. Malaysian Journal of Medicine and Health Sciences. 2018; 14: 64–71. [Google Scholar]
- 43.Singh R, Tripathi V, Kalpana Singh MK, Dwivedi SN. Determinants of Birth Intervals in Tamil Nadu in India: Developing Cox Hazard Models with Validations and Predictions. Revista Colombiana de Estadística. 2012; 35: 289–307. [Google Scholar]
- 44.Sendek EM, Hebo SH. Modeling time-to-good control of hypertension using Cox proportional hazard and frailty models at Bahir-Dar Felege Hiwot Referral Hospital. Open Access Medical Statistics. 2017; 7:27–36. [Google Scholar]
- 45.Robinson DH, Wainer H. On the Past and Future of Null Hypothesis Significance Testing. (Research Report No. RR-01-24) Statistics & Research Division Princeton, NJ, USA. Educational Testing Service. 2001; 25.
- 46.Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991; 48(9):851–5. 10.1001/archpsyc.1991.01810330075011 [DOI] [PubMed] [Google Scholar]
- 47.Hardeveld F, Spijker J, De Graaf R, Hendriks SM, Licht CM, Nolen WA et al. Recurrence of major depressive disorder across different treatment settings: Results from the NESDA study. J Affect Disord. 2013; 147(1–3): 225–231. 10.1016/j.jad.2012.11.008 [DOI] [PubMed] [Google Scholar]
- 48.ten Have M, de Graaf R, van Dorsselaer S, Tuithof M, Kleinjan M, Penninx BWJH. Recurrence and chronicity of major depressive disorder and their risk indicators in a population cohort. Acta Psychiatri Scand. 2018; 137(6): 503–515. 10.1111/acps.12874 [DOI] [PubMed] [Google Scholar]
- 49.Klein DN, Kotov R, Bufferd SJ. Personality and depression: explanatory models and review of the evidence. Annu Rev Clin Psychol. 2011; 7: 269–295. 10.1146/annurev-clinpsy-032210-104540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fava GA, Ruini C, Belaise C. The concept of recovery in major depression. Psychol Med. 2007; 37(3): 307–317. 10.1017/S0033291706008981 [DOI] [PubMed] [Google Scholar]
- 51.Huber D, Zimmermann J, Klug G. Change in personality functioning during psychotherapy for depression predicts long-term outcome. Psychoanalytic Psychology, 2017; 34(4): 434–445. 10.1037/pap0000129 [DOI] [Google Scholar]
- 52.Ferster CB. A functional analysis of depression. American Psychologist. 1973; 28, 857–870. 10.1037/h0035605 [DOI] [PubMed] [Google Scholar]
- 53.Ottenbreit ND, Dobson KS, Quigley L. An examination of avoidance in major depression in comparison to social anxiety disorder. Behav Res Ther. 2014; 56: 82–90. 10.1016/j.brat.2014.03.005 [DOI] [PubMed] [Google Scholar]
- 54.Farrelly S, Clement S, Gabbidon J, Jeffery D, Dockery L, Lassman F, et al. Anticipated and experienced discrimination amongst people with schizophrenia, bipolar disorder and major depressive disorder: a cross-sectional study. BMC Psychiatry. 2014; 14(157). ISSN 1471-244X 10.1186/1471-244X-14-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kudo Y, Nakagawa A, Wake T, Ishikawa N, Kurata C, Nakahara M, et al. Temperament, personality, and treatment outcome in major depression: a 6-month preliminary prospective study. Neuropsychiatric Dis Treat. 2017; 13: 17–24. 10.2147/NDT.S123788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ormel J, Rosmalen J, Farmer A. Neuroticism: a non-informative marker of vulnerability to psychopathology. Soc Psychiatry Psychiatr Epidemiol. 2004; 39(11): 906–912. 10.1007/s00127-004-0873-y [DOI] [PubMed] [Google Scholar]
- 57.Klein DN, Lewinsohn PM, Rohde P, Seeley JR, Durbin CE. Clinical features of major depressive disorder in adolescents and their relatives: Impact on familial aggregation, implications for phenotype definition, and specificity of transmission. J Abnormal Psychol. 2002; 111(1): 98–106. [PubMed] [Google Scholar]
- 58.Barkow K, Maier W, Ustun TB, Gansicke M, Wittchen HU, Heun R. Risk factors for depression at 12-month follow-up in adult primary health care patients with major depression: An international prospective study. J Affect Disord. 2003; 76(1–3): 157–169. 10.1016/s0165-0327(02)00081-2 [DOI] [PubMed] [Google Scholar]
- 59.Wainwright NWJ, Surtees PG. Childhood adversity, gender and depression over the life-course. J Affect Disord. 2002; 72(1); 33–44. 10.1016/s0165-0327(01)00420-7 [DOI] [PubMed] [Google Scholar]
- 60.Kessler RC, Magee WJ. Childhood adversities and adult depression: Basic patterns of association in a US national survey. Psychol Med. 1993; 27(5): 679–690. [DOI] [PubMed] [Google Scholar]
- 61.Simpson HB, Nee JC, Endicott J. First-episode major depression: Few sex differences in course. Arch Gen Psychiatry. 1997; 54(7): 633–639. 10.1001/archpsyc.1997.01830190059006 [DOI] [PubMed] [Google Scholar]
- 62.Wood W, Eagly AH. Biosocial construction of sex differences and similarities in behavior. Advances in Experimental Social Psychology. 2012; 46: 55–123. [Google Scholar]
- 63.Tsai JL, Chentsova-Dutton Y. Understanding depression across cultures In Gotlib IH, Hammen CL, editors. Handbook of depression. New York: Guilford Press; 2002. pp. 467–491. [Google Scholar]
- 64.Lewinsohn PM, Rohde P, Seeley JR, Klein DN, Gotlib IH. Natural course of adolescent major depressive disorder in a community sample: Predictors of recurrence in young adults. Am J Psychiatry. 2000; 157(10): 1584–1591. 10.1176/appi.ajp.157.10.1584 [DOI] [PubMed] [Google Scholar]
- 65.Kennedy N, Abbott R, Paykel ES. Remission and recurrence of depression in the maintenance era: long-term outcome in a Cambridge cohort. Psychol Med. 2003; 33(5):827–38. 10.1017/s003329170300744x [DOI] [PubMed] [Google Scholar]
- 66.Buckman JEJ, Underwood A, Clarke K, Saunders R, Hollon SD, Fearon P, et al. Risk factors for relapse and recurrence of depression in adults and how they operate: A four-phase systematic review and meta-synthesis. Clin Psychol Rev. 2018; 64:13–38. 10.1016/j.cpr.2018.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Keyimu K, Zhou XH, Miao HJ, Zou T. Relationship between vitamin D receptor gene polymorphism and mild cognitive impairment in elderly Uygur people. Int J Clin Exp Med. 2014; 7:5282–8. [PMC free article] [PubMed] [Google Scholar]
- 68.Orme RP, Middleditch C, Waite L, Fricker RA. The Role of Vitamin D₃ in the Development and Neuroprotection of Midbrain Dopamine Neurons In: Gerald L. Vitamins and Hormones. London: Elsevier; 2016. pp. 273–297. 10.1016/bs.vh.2015.10.007 [DOI] [PubMed] [Google Scholar]
- 69.Hasegawa H, Nakamura K. Tryptophan Hydroxylase and Serotonin Synthesis Regulation In: Müller CP, Jacobs BL, editors. Handbook of Behavioral Neuroscience. London: Elsevier; 2010. pp. 183–202. [Google Scholar]
- 70.Côté F, Thévenot E, Fligny C, Fromes Y, Darmon M, Ripoche M-A, et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci USA. 2003; 100:13525–30. 10.1073/pnas.2233056100 [DOI] [PMC free article] [PubMed] [Google Scholar]