Abstract
Background:
Repeat antinuclear antibody (ANA) testing may be unnecessary, potentially harmful and costly. Our aim was to assess the frequency and correlates of repeat ANA testing in Ontario.
Methods:
We performed a retrospective descriptive study identifying ANA tests performed over 2008–2015 among adults within the Ontario Laboratories Information System. Our primary outcome was any ANA test performed within 1 year of a previous ANA test. Our secondary outcome was any repeat test after a previous positive result. Repeat testing overall (regardless of who performed the previous test) and repeat testing by the same provider who performed the previous test were determined separately. We assessed correlates of repeat testing (e.g., patient and physician characteristics) and of repeat testing after a positive result using separate logistic regression models by means of generalized estimating equations to account for clustering of repeat testing within patients and within physician practices.
Results:
In total, 587 357 ANA tests were performed in 437 966 patients over the study period, of which 126 322 (21.5%) gave a positive result and 164 913 (28.1%) were repeat tests. Family physicians ordered 358 422 tests (61.0%), and rheumatologists ordered 65 071 tests (11.1%). Of the repeat tests, 82 332 (49.9%) were ordered within 12 months of the previous test. Among the 73 961 repeat tests ordered by the same practitioner within 12 months, the previous test result was positive for 22 657 (30.6%). A higher proportion of rheumatologists than other physicians ordered repeat tests within 12 months (36.1% v. 11.3%). The most significant correlate of potentially redundant testing was testing among patients with suspected or confirmed connective tissue disease.
Interpretation:
Over a quarter of ANA tests in Ontario were repeat tests; rheumatologists were most likely to order repeat testing. Our findings may be useful to inform quality-improvement initiatives related to the appropriateness of ANA testing.
Laboratory testing is the highest-volume medical procedure, 1 and volumes are increasing annually.2,3 It has been estimated that 20% of tests are ordered unnecessarily.4,5 Misuse of laboratory tests is a major challenge affecting the sustainability of health care.6,7 Improving the appropriateness of rheumatology laboratory testing is a priority of Choosing Wisely campaigns.8,9
Concerns have been raised about the inappropriate use of antinuclear antibody (ANA) testing. Testing for ANA is indicated only if a patient’s clinical history and physical examination show symptoms or signs suggestive of systemic lupus erythematosus, scleroderma, Sjögren syndrome, polymyositis or dermatomyositis.10,11 The test has high sensitivity, and, thus, a positive test result can contribute to a diagnosis of these conditions. 12 However, it has low specificity, and ANA and can be seen in other conditions and in more than 20% of healthy people, 13 which makes interpretation of test results challenging.12
Choosing Wisely Canada recommends that “ANA testing should not be used to screen subjects without specific symptoms or without a clinical evaluation that may lead to a diagnosis of systemic lupus or other connective tissue disease.”8 International recommendations strongly advise that “ANA testing is primarily intended for diagnostic purposes, and not for monitoring disease progression” owing to its limited value in monitoring disease activity.14–17 Thus, it is not appropriate to repeat ANA following a positive test result.7–9,16,18
Inappropriate testing may cause patients confusion and anxiety, and lead to overdiagnosis, overtreatment, and unnecessary consultations and costs.19–23 Moreover, given the rare incidence of systemic autoimmune rheumatic diseases24–26 and previous research suggesting that ANA tests are often ordered serially or in settings of low pretest probability,19,27,28 understanding patterns of ANA testing is useful to inform quality-improvement initiatives assessing the appropriateness of ANA testing.
Therefore, our aim in the present study was to assess the frequency and correlates of repeat ANA testing.
Methods
We performed a retrospective study over 2008–2015 using health administrative databases in Ontario.
Sources of data
We identified ANA tests (including dates, test results and ordering physician) using Logical Observation Identifiers Names and Codes from the Ontario Laboratories Information System, a nearly population-wide database of laboratory test results in Ontario. The Ontario Laboratories Information System captures both community and hospital laboratory tests. At the time of analysis, the period of laboratory data spanned from Jan. 1, 2007, to Sept. 30, 2015, and the provincial coverage increased from 41% in 2008 to 71% in 2009, 86% in 2010 and 99% in 2014.
We linked patients with ANA tests performed between 2008 and 2015 to the Ontario Health Insurance Plan Claims Database to identify diagnoses (according to a modification of the 8th revision of the International Classification of Diseases), and to the Canadian Institute for Health Information Discharge Abstract Database to identify hospital admissions. We identified patient demographic characteristics from the Registered Persons Database and ordering physician specialty by linking with the ICES Physician Database.
These data sets are linked by means of unique encoded patient and physician identifiers and are held securely and analyzed at ICES, a prescribed entity under section 45 of Ontario’s Personal Health Information Protection Act.
Participant eligibility
Patients were excluded if they were less than 18 years of age, had missing patient or physician identifiers, lived out of province or died on the date of their first ANA test.
Outcome measures
Our primary outcome was any ANA test within 1 year of a previous ANA test. Our secondary outcome was any repeat ANA test after a previous positive test result.
Covariates
Patient-level covariates included age, sex, income quintile (as a proxy for socioeconomic status, based on patients’ postal code and census neighbourhood income quintile), rural versus urban residence (defined with the Rurality Index for Ontario29 based on participants’ postal code), year of testing, hospital admission in the 6 months before testing and Charlson Comorbidity Index score (computed with a 2-yr look-back period from the index test). As a proxy for testing or confirmation of connective tissue disease, we determined whether patients had at least 1 Ontario Health Insurance Plan diagnosis code for connective tissue disease (710 or 695) within a 1-year period before or 6 months after the test date.
Physician-level covariates included specialty (rheumatologist, internist, family medicine or other), age, sex, whether they were international medical graduates, and whether they practised in an academic or community setting (determined by physician primary practice location based on postal codes linked to an academic hospital location).
Statistical analysis
We assessed the frequency of ANA testing at the health system level, patient level and provider level, and repeat testing for individual patients within 12 months of a previous ANA test. The frequency of total ANA tests performed, positive test results and repeat tests performed within 12 months of a previous test was determined overall and by ordering physician type. Repeat testing overall (regardless of who performed the previous test) and repeat testing by the same provider who ordered the previous test were determined separately.
For results at the level of ANA testing, we expressed percentages using the denominator for the total number of ANA tests. For patient-level results, the denominator included the total number of patients with at least 1 ANA test. We assessed intervals between testing in relation to preceding negative or positive test results. We compared characteristics between patients with 2 or more ANA tests and those with 1 test.
To assess correlates of repeat testing within 12 months of a previous test, and any repeat test in which the previous test result was positive, we fit 2 separate logistic regression models by means of generalized estimating equations to account for clustering of testing within patients and within physician practices in order to assess the unique contribution of the variables to each outcome. Models accounted for both physician-level and patient-level characteristics (the aforementioned covariates). The results of the regression analysis were expressed as odds ratios (ORs) and their 95% confidence intervals (CIs), representing the population-averaged effects of covariates on each outcome of interest.
The primary analysis focused on all repeat testing (within 12 mo) irrespective of the provider who ordered the previous test. Finally, we performed a sensitivity analysis to assess correlates of repeat ANA testing confined to the same provider ordering the previous test.
Ethics approval
This study was authorized under section 45 of the Personal Health Information Protection Act, which does not require review by a research ethics board.
Results
We identified 456 726 patients with ANA tests between 2008 and 2015, of whom 18 760 were excluded because they were less than 18 years old (n = 18 170) or lived out of province (n = 562). The remaining 28 patients were excluded owing to invalid health card numbers and death occurring on the date of the index test.
Testing-level results
In total, 587 357 ANA tests were performed over the study period, of which 164 913 (28.1%) were repeat tests during the study period and 82 332 (14.0%) were repeat tests within 12 months of a previous test (Table 1). Of the 587 357, 126 322 (21.5%) gave a positive result.
Table 1:
Frequency of total and repeat antinuclear antibody tests overall and by ordering physician type
| Variable | No. (%) of tests | ||||
|---|---|---|---|---|---|
| Total n = 7136 |
Family physicians n = 4643 |
Rheumatologists n = 188 |
Internal medicine n = 313 |
Other n = 1992 |
|
| No. of tests (% of total tests) | 587 357 (100.0) | 358 422 (61.0) | 65 071 (11.1) | 26 409 (4.5) | 137 455 (23.4) |
| Positive result | 126 322 (21.5) | 64 262 (17.9) | 28 393 (43.6) | 5884 (22.3) | 27 783 (20.2) |
| Repeat test within 12 mo of previous test (regardless of who ordered previous test) | 82 332 (14.0) | 32 994 (9.2) | 23 507 (36.1) | 4707 (17.8) | 21 124 (15.4) |
| Repeat test within 12 mo of previous test ordered by same practitioner type who ordered previous test* | 51 411 (8.8) | 25 213 (7.0) | 13 093 (20.1) | 1656 (6.3) | 11 071 (8.0) |
Individual categories do not total 51 411 as practitioner specialty was unknown for 378 tests.
We identified 7084 physicians who ordered ANA testing, of whom 188 were rheumatologists and 4643 were family physicians (Table 1). Family physicians ordered 358 422 tests (61.0%), rheumatologists ordered 65 071 tests (11.1%), and internists ordered 26 409 tests (4.5%). The top specialties for the remaining physicians were gastroenterology (22 239 tests [3.8%]), neurology (20 120 [3.4%]), dermatology (16 331 [2.8%]) and nephrology (14 484 [2.5%]). Rheumatologists had the highest frequency of positive results, and more rheumatologists ordered repeat tests within 12 months of a previous test than other practitioners (Table 1).
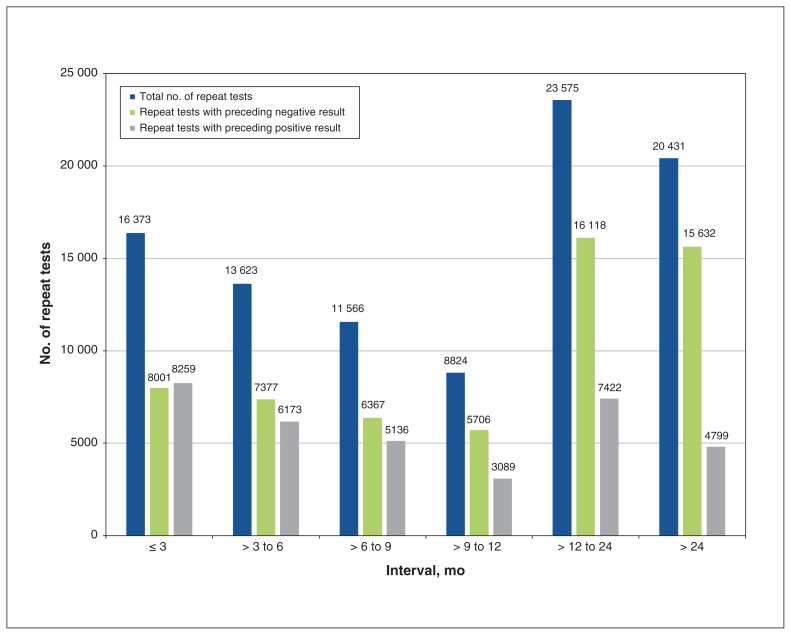
In our sensitivity analysis, of the 164 913 repeat tests, 94 392 (57.2%) were ordered by the same practitioner: 16 373 (17.3%) were ordered within 3 months of the previous test, and 73 961 (78.4%) were ordered within 12 months of the previous test (Figure 1). Among the repeat tests ordered within 12 months, the previous test result was positive for 22 657 (30.6%).
Figure 1:
Number of repeat antinuclear antibody (ANA) tests ordered by the same practitioner, according to result and by time interval. A small proportion (0.005%–0.8%) of tests had unknown results, and, therefore, the number of positive and negative results within each time interval may not add up to the cumulative total.
Patient-level results
The 587 357 ANA tests were performed for 437 966 patients (294 130 women [67.2%]; mean age 52.4 [standard deviation 16.3] yr). Of the 437 966 patients, 346 282 (79.1%) had 1 test, and 91 684 (20.9%) had more than 1 test: 63 084 (14.4%) had 2 tests, 17 000 (3.9%) had 3 tests, 5857 (1.3%) had 4 tests, and 5743 (1.3%) had 5 or more tests.
Of the 437 966 patients, 74 849 (17.1%) had a positive result of their first test recorded in the data source. Of the 91 684 patients who had more than 1 test, 61 684 (67.3%) had their first test result reported as negative; of the 61 684, 4641 (7.5%) had a subsequent positive test result.
Compared to the patients with a single ANA test, there was a higher proportion of female patients (65.4% v. 73.9%) and presence of suspected or confirmed diagnosis of connective tissue disease (3.9% v. 11.4%) among patients with multiple tests (Table 2).
Table 2:
Characteristics of patients with antinuclear antibody tests, overall and by number of tests
| Characteristic | No. (%) of patients* | ||
|---|---|---|---|
| Total n = 437 966 |
1 test n = 346 282 |
≥ 2 tests n = 91 684 |
|
| Age, yr, mean ± SD | 52.4 ± 16.3 | 51.9 ± 16.5 | 54.5 ± 15.3 |
| Female sex | 294 130 (67.2) | 226 363 (65.4) | 67 767 (73.9) |
| Connective tissue disease | 24 037 (5.5) | 13 610 (3.9) | 10 427 (11.4) |
| Hospital admission in 2 yr preceding index test | 22 600 (5.2) | 17 701 (5.1) | 4899 (5.3) |
| Urban residence | 378 822 (86.5) | 299 480 (86.5) | 79 342 (86.5) |
Note: SD = standard deviation.
Except where noted otherwise.
Among the 63 084 patients with 2 tests, we identified 43 706 patients for whom a family physician ordered the initial test. Of the 43 706, 5461 (12.5%) had their repeat test ordered by a rheumatologist, and 30 168 (69.0%) had their repeat test ordered by a family physician.
Correlates of repeat testing
Family physicians, internists and all other care practitioners were significantly less likely than rheumatologists to order repeat testing within 1 year or repeat testing after a positive test result (Table 3). When we confined our analyses to repeat testing ordered by the same physician, the ORs remained significant. Physician demographic characteristics did not appear to be significantly associated with repeat testing, with the exception that internationally trained medical graduates were less likely than Canadian medical graduates to order repeat tests (adjusted OR 0.81, 95% CI 0.70–0.93) and to order repeat tests after a previous positive result (OR 0.75, 95% CI 0.63–0.88).
Table 3:
Provider and patient characteristics associated with repeat antinuclear antibody testing within 12 months of previous test and repeat testing after a positive test result
| Characteristic | Any physician, adjusted OR (95% CI)* | Same physician, adjusted OR (95% CI)* | ||
|---|---|---|---|---|
|
|
|
|||
| Repeat testing within 12 mo of previous test | Repeat testing after prior positive test result | Repeat testing within 12 mo of previous test | Repeat testing after prior positive test result | |
| Physicians | ||||
|
| ||||
| Family physicians (reference = rheumatologists) | 0.26 (0.22–0.31) | 0.23 (0.20–0.28) | 0.80 (0.64–1.00) | 0.55 (0.44–0.69) |
|
| ||||
| Internists (reference = rheumatologists) | 0.59 (0.44–0.79) | 0.58 (0.44–0.76) | 0.63 (0.47–0.85) | 0.66 (0.50–0.87) |
|
| ||||
| All other practitioners (reference = rheumatologists) | 0.39 (0.32–0.48) | 0.33 (0.26–0.42) | 0.63 (0.47–0.84) | 0.56 (0.42–0.73) |
|
| ||||
| Physician age ≤ 50 year (reference = > 50 yr) | 0.98 (0.88–1.10) | 0.90 (0.79–1.03) | 1.29 (1.15–1.46) | 1.12 (0.97–1.29) |
|
| ||||
| Female (reference = male) | 0.93 (0.84–1.03) | 1.05 (0.93–1.19) | 0.95 (0.85–1.07) | 1.10 (0.96–1.27) |
|
| ||||
| Academic centre (reference = community practice) | 1.53 (0.87–2.69) | 1.32 (0.83–2.12) | 1.53 (0.94–2.48) | 1.33 (0.89–1.98) |
|
| ||||
| International medical school graduate (reference = Canadian medical school graduate) | 0.96 (0.87–1.07) | 0.91 (0.80–1.05) | 0.81 (0.70–0.93) | 0.75 (0.63–0.88) |
|
| ||||
| Patients | ||||
|
| ||||
| Age | 1.01 (1.00–1.01) | 1.00 (1.00–1.01) | 1.01 (1.01–1.01) | 1.01 (1.00–1.01) |
|
| ||||
| Female (reference = male) | 1.29 (1.25–1.34) | 1.82 (1.73–1.91) | 1.29 (1.23–1.36) | 1.83 (1.72–1.94) |
|
| ||||
| Income quintile† (reference = 1 lowest) | ||||
| 2 | 1.03 (0.99–1.07) | 1.06 (1.00–1.12) | 1.05 (1.00–1.10) | 1.06 (0.99–1.15) |
|
| ||||
| 3 | 1.05 (1.01–1.10) | 1.12 (1.06–1.19) | 1.04 (0.99–1.09) | 1.10 (1.02–1.19) |
|
| ||||
| 4 | 1.07 (1.03–1.12) | 1.19 (1.12–1.26) | 1.04 (0.99–1.10) | 1.16 (1.07–1.25) |
|
| ||||
| 5 (highest) | 1.08 (1.03–1.12) | 1.17 (1.10–1.25) | 1.02 (0.97–1.07) | 1.10 (1.01–1.19) |
|
| ||||
| Urban residence (reference = rural) | 0.93 (0.86–0.99) | 0.96 (0.89–1.05) | 1.01 (0.93–1.09) | 1.03 (0.94–1.13) |
|
| ||||
| Connective tissue disease | 2.20 (2.01–2.41) | 4.18 (3.70–4.73) | 3.08 (2.70–3.51) | 5.37 (4.69–6.14) |
|
| ||||
| Hospital admission in previous 6 mo | 0.95 (0.89–1.00) | 0.92 (0.83–1.02) | 0.92 (0.84–1.01) | 0.94 (0.80–1.10) |
|
| ||||
| Charlson Comorbidity Index score‡ (reference = 0) | ||||
|
| ||||
| 1 | 1.17 (1.10–1.25) | 1.14 (1.03–1.26) | 1.20 (1.10–1.30) | 1.18 (1.04–1.34) |
|
| ||||
| ≥ 2 | 1.11 (1.02–1.21) | 0.97 (0.86–1.09) | 1.16 (1.05–1.29) | 0.98 (0.85–1.13) |
|
| ||||
| Year of index test (reference = 2010) | ||||
|
| ||||
| 2008 | 0.24 (0.21–0.28) | 0.19 (0.16–0.24) | 0.27 (0.22–0.32) | 0.23 (0.17–0.30) |
|
| ||||
| 2009 | 0.75 (0.71–0.80) | 0.74 (0.67–0.81) | 0.76 (0.70–0.83) | 0.74 (0.65–0.83) |
|
| ||||
| 2011 | 1.09 (1.03–1.16) | 1.16 (1.07–1.25) | 1.06 (0.99–1.13) | 1.08 (0.97–1.20) |
|
| ||||
| 2012 | 1.17 (1.10–1.26) | 1.31 (1.19–1.44) | 1.12 (1.04–1.21) | 1.20 (1.07–1.34) |
|
| ||||
| 2013 | 1.30 (1.22–1.39) | 1.32 (1.21–1.46) | 1.26 (1.16–1.37) | 1.33 (1.17–1.50) |
|
| ||||
| 2014 | 1.45 (1.36–1.56) | 1.44 (1.32–1.58) | 1.39 (1.27–1.52) | 1.36 (1.21–1.51) |
|
| ||||
| 2015 | 1.70 (1.56–1.86) | 1.68 (1.50–1.88) | 1.79 (1.59–2.01) | 1.80 (1.54–2.09) |
Note: CI = confidence interval, OR = odds ratio.
Adjusted for patient covariates (age, sex, income quintile, urban residence, year of testing, prior hospital admissions, Charlson Comorbidity Index score, testing or diagnosis for connective tissue disease) and physician covariates (specialty, age, sex, international medical graduate, academic setting).
Based on patients’ postal code and census neighbourhood income quintile.
With a look-back period of 2 years before the index test.
Female patients, those with a higher socioeconomic status and those with more comorbidities were more likely to undergo repeat testing within 12 months. Patients with suspected or confirmed connective tissue disease were significantly more likely than those without such a diagnosis to undergo repeat testing within 12 months (OR 2.20, 95% CI 2.01–2.41 for any physician; OR 3.08, 95% CI 2.70–3.51 for the same physician). Patients with suspected or confirmed connective tissue disease were also 4–5 times (OR 4.18, 95% CI 3.70–4.73 for any physician, OR 5.37, 95% CI 4.69–6.14 for the same physician) more likely to undergo repeat testing after a previous positive result (Table 3).
Interpretation
In our sample, over a quarter of all ANA tests were repeat tests, with a substantial number of potentially redundant tests. Half of repeat tests were performed within 12 months of the previous test. Among the tests repeated within 12 months, the result of the previous test was positive for 31%. Family physicians ordered the most ANA tests; however, rheumatologists were more likely to order repeat tests and repeat testing after a positive result than other practitioners. The most significant correlate of potentially redundant testing involved testing in patients with suspected or confirmed connective tissue disease. Moreover, the volume of ANA testing far exceeded the number of expected new cases of connective tissue disease,24–26 which raises concerns of potential overuse of ANA testing.
Our findings are consistent with previous studies showing that ANA testing is pervasive in rheumatology practice. 22,23,27,28,30–32 We observed similar frequencies of ANA positivity in our sample and similarly identified that family physicians order the majority of ANA tests.27,31 Although there are far more family physicians than other specialists, it has been suggested that family physicians have limited expertise in interpreting ANA tests and may ignore results.27
Our findings show that rheumatologists were most likely to order repeat testing, which is consistent with a recent Canadian study.27 However, the proportion of potentially redundant repeat tests in our sample is higher than in previous studies.27,31,33 Potential explanations for this may be our ability to capture most tests performed across settings in Ontario and the publicly funded health care system in which this study was performed (i.e., patients not required to pay out of pocket for testing).
There are several potential reasons why rheumatologists order repeat tests, including issues surrounding access to and perceived accuracy of previous results, testing for research participants and the introduction of biologic therapy for immune-mediated diseases, which may drive ANA testing for drug-induced systemic lupus. Yet a recent Canadian survey showed that many rheumatologists feel they are ordering ANA tests correctly and that Choosing Wisely recommendations do not apply to them since only family physicians order ANA tests inappropriately.34 In one Canadian city, rheumatologists were found to be the third-highest laboratory spenders per physician by specialty,35 which raises concerns over the volume of laboratory testing in their patients.
Unnecessary test repetition is readily modifiable both through increasing education and awareness of overuse, and by enhancing access to outside health records and sharing results.5 Targeted strategies are effective in improving the appropriateness of testing.33 Multiple linked interventions coupled with computerized order set modifications can effect lasting change in ordering behaviours.36
It is difficult to extrapolate costs associated with repeat testing. In Ontario, an ANA test costs $6.85,37 which equates to about $1.1 million for the 164 913 repeat tests performed during the study period. In addition to the direct costs related to the test itself, there are direct labour costs (e.g., phlebotomists), indirect labour costs (e.g., administrative), direct material costs (e.g., collection needles, tubes) and indirect material costs (e.g., facilities, analyzer). Potential downstream costs, such as unnecessary specialist consultations, may also be incurred.
Limitations
We did not have clinical data to inform reasons for repeat testing. As it is difficult to standardize ANA tests between laboratories, we used each laboratory’s reported interpretation (positive or negative) and thus were unable to assess ANA titres, which could potentially influence physicians’ ordering of repeat testing. We did not assess ANA subserology.
We were also unable to assess testing that was ordered but not carried out by the patient. We did not study underscreening in targeted populations, which is another form of poor quality of care. A limitation of the laboratory data used in this study is the incompleteness of data coverage in the earliest years of our study period. Thus, we may have underestimated the frequency of repeat testing if patients had tests conducted at laboratories not captured in our source databases. Moreover, our primary outcome was a repeat test within 12 months of the previous test, and the first ANA test appearing in our data source may not have been a patient’s first test.
Conclusion
We identified a substantial number of potentially redundant ANA tests. Our findings have implications for quality-improvement initiatives related to the appropriateness of ANA testing. These may include developing strategies to support both primary care physicians (to ensure that appropriate patients undergo testing) and rheumatologists (to limit repeat testing). Health policy changes could eliminate duplicate orders for tests within specified time frames. In Canada, certain rheumatologic tests can be ordered only by specialists, to reduce unnecessary testing. Further research is also required to understand why physicians order ANA tests and repeat tests, and to explore ANA subserology testing patterns.
Supplementary Material
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Funding: This study was supported by the University of Toronto Pfizer Chair in Rheumatology Research Award and by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care.
Disclaimer: This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information. The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
Contributors: Jessica Widdifield, Shirley Lake, Amanda Steiman and Natasha Gakhal conceived the study. Gillian Hawker, Jessica Widdifield and Zhan Yao acquired the data. Zhan Yao analyzed the data. Shirley Lake and Jessica Widdifield drafted the manuscript. All of the authors contributed to the study design and to data interpretation, revised the manuscript critically for important intellectual contact, approved the final version to be published and agreed to be accountable for all aspects of the work.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/8/1/E184/suppl/DC1.
References
- 1.McGregor MJ, Martin D. Testing 1, 2, 3: Is overtesting undermining patient and system health? Can Fam Physician. 2012;58:1191–3. e615–7. [PMC free article] [PubMed] [Google Scholar]
- 2.McGrail KM, Evans RG, Barer ML, et al. Diagnosing senescence: contributions to physician expenditure increases in British Columbia, 1996/97 to 2005/06. Healthc Policy. 2011;7:41–54. [PMC free article] [PubMed] [Google Scholar]
- 3.Sivananthan SN, Peterson S, Lavergne R, et al. Designation, diligence and drift: understanding laboratory expenditure increases in British Columbia, 1996/97 to 2005/06. BMC Health Serv Res. 2012;12:472. doi: 10.1186/1472-6963-12-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhi M, Ding EL, Theisen-Toupal J, et al. The landscape of inappropriate laboratory testing: a 15-year meta-analysis. PLoS One. 2013;8:e78962. doi: 10.1371/journal.pone.0078962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyu H, Xu T, Brotman D, et al. Overtreatment in the United States. PLoS One. 2017;12:e0181970. doi: 10.1371/journal.pone.0181970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization. The true consequences of false-positive results. JAMA. 1991;265:365–9. [PubMed] [Google Scholar]
- 7.Fritzler MJ. Choosing Wisely: review and commentary on anti-nuclear antibody (ANA) testing. Autoimmun Rev. 2016;15:272–80. doi: 10.1016/j.autrev.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Chow SL, Carter Thorne J, Bell MJ, et al. Choosing Wisely: the Canadian Rheumatology Association’s list of 5 items physicians and patients should question. J Rheumatol. 2015;42:682–9. doi: 10.3899/jrheum.141140. [DOI] [PubMed] [Google Scholar]
- 9.Yazdany J, Schmajuk G, Robbins M, et al. Choosing Wisely: the American College of Rheumatology’s Top 5 list of things physicians and patients should question. Arthritis Care Res (Hoboken) 2013;65:329–39. doi: 10.1002/acr.21930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas C, Robinson JA. The antinuclear antibody test. When is a positive result clinically relevant? Postgrad Med. 1993;94:55–8. 63, 66. [PubMed] [Google Scholar]
- 11.Shojania K. Rheumatology: 2. What laboratory tests are needed? CMAJ. 2000;162:1157–63. [PMC free article] [PubMed] [Google Scholar]
- 12.Castro C, Gourley M. Diagnostic testing and interpretation of tests for autoimmunity. J Allergy Clin Immunol. 2010;125(Suppl 2):S238–47. doi: 10.1016/j.jaci.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wandstrat AE, Carr-Johnson F, Branch V, et al. Autoantibody profiling to identify individuals at risk for systemic lupus erythematosus. J Autoimmun. 2006;27:153–60. doi: 10.1016/j.jaut.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Solomon DH, Kavanaugh AJ, Schur PH, et al. Evidence-based guidelines for the use of immunologic tests: antinuclear antibody testing. Arthritis Rheum. 2002;47:434–44. doi: 10.1002/art.10561. [DOI] [PubMed] [Google Scholar]
- 15.Raissi TC, Hewson C, Pope JE. Repeat testing of antibodies and complements in systemic lupus erythematosus: When is it enough? J Rheumatol. 2018;45:827–34. doi: 10.3899/jrheum.161365. [DOI] [PubMed] [Google Scholar]
- 16.Agmon-Levin N, Damoiseaux J, Kallenberg C, et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann Rheum Dis. 2014;73:17–23. doi: 10.1136/annrheumdis-2013-203863. [DOI] [PubMed] [Google Scholar]
- 17.Five things physicians and patients should question [Choosing Wisely] Philadelphia: American College of Rheumatology; 2013. [accessed 2019 July 9]. Available: www.choosingwisely.org/societies/american-college-of-rheumatology-pediatric-rheumatology/ [Google Scholar]
- 18.Kavanaugh A, Tomar R, Reveille J, et al. Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens. American College of Pathologists. Arch Pathol Lab Med. 2000;124:71–81. doi: 10.5858/2000-124-0071-GFCUOT. [DOI] [PubMed] [Google Scholar]
- 19.Fitch-Rogalsky C, Steber W, Mahler M, et al. Clinical and serological features of patients referred through a rheumatology triage system because of positive antinuclear antibodies. PLoS One. 2014;9:e93812. doi: 10.1371/journal.pone.0093812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narain S, Richards HB, Satoh M, et al. Diagnostic accuracy for lupus and other systemic autoimmune diseases in the community setting. Arch Intern Med. 2004;164:2435–41. doi: 10.1001/archinte.164.22.2435. [DOI] [PubMed] [Google Scholar]
- 21.Rolfe A, Burton C. Reassurance after diagnostic testing with a low pretest probability of serious disease: systematic review and meta-analysis. JAMA Intern Med. 2013;173:407–16. doi: 10.1001/jamainternmed.2013.2762. [DOI] [PubMed] [Google Scholar]
- 22.Mohammed AS, Boddu P, Mael D, et al. Inappropriate use of commercial antinuclear antibody testing in a community-based US hospital: a retrospective study. J Community Hosp Intern Med Perspect. 2016;6:32031. doi: 10.3402/jchimp.v6.32031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohammadi S, Shaik I, Chevli P, et al. Improper use of antinuclear antibody (ANA) test can result in misdiagnosis, increased patient anxiety, and wasted health care resources [abstract]. Arthritis Rheumatol; Proceedings from the 2014 ACR/ARHP Annual Meeting; 2014 Nov 14–19; Boston. 2014. [Google Scholar]
- 24.Ungprasert P, Sagar V, Crowson CS, et al. Incidence of systemic lupus erythematosus in a population-based cohort using revised 1997 American College of Rheumatology and the 2012 Systemic Lupus International Collaborating Clinics classification criteria. Lupus. 2017;26:240–7. doi: 10.1177/0961203316657434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fatoye F, Gebrye T, Svenson LW. Real-world incidence and prevalence of systemic lupus erythematosus in Alberta, Canada. Rheumatol Int. 2018;38:1721–6. doi: 10.1007/s00296-018-4091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayes MD. Scleroderma epidemiology. Rheum Dis Clin North Am. 2003;29:239–54. doi: 10.1016/s0889-857x(03)00022-x. [DOI] [PubMed] [Google Scholar]
- 27.Man A, Shojania K, Phoon C, et al. An evaluation of autoimmune antibody testing patterns in a Canadian health region and an evaluation of a laboratory algorithm aimed at reducing unnecessary testing. Clin Rheumatol. 2013;32:601–8. doi: 10.1007/s10067-012-2141-y. [DOI] [PubMed] [Google Scholar]
- 28.Abeles AM, Abeles M. The clinical utility of a positive antinuclear antibody test result. Am J Med. 2013;126:342–8. doi: 10.1016/j.amjmed.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Kralj B. Measuring ‘rurality’ for purposes of health-care planning: an empirical measure for Ontario. Ont Med Rev. 2000;67:33–52. [Google Scholar]
- 30.Lake S, Gakhal N, Steiman A, et al. The frequency and cost of repeat ANA testing at two University of Toronto-affiliated teaching hospitals. J Rheumatol. 2018;45:1036–7. [Google Scholar]
- 31.Davis LA, Goldstein B, Tran V, et al. Applying Choosing Wisely: antinuclear antibody (ANA) and sub-serology testing in a safety net hospital system. Open Rheumatol J. 2015;9:82–7. doi: 10.2174/1874312901409010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bulbin D, Meadows A, Denio A, et al. Abstracts of the 2013 Annual Meeting of the American College of Rheumatology. October 25–30, 2013. San Diego, California, USA. Do rheumatologists (and other specialists) practice what we preach? A study of serology ordering patterns with attention to subserologies when the antinuclear antibody by enzyme linked immunosorbent assay is negative; and the clinical significance of these positive subserology results [abstract] Arthritis Rheum. 2013;65:S1–1331. [Google Scholar]
- 33.Lesuis N, Hulscher ME, Piek E, et al. Choosing Wisely in daily practice: an intervention study on antinuclear antibody testing by rheumatologists. Arthritis Care Res (Hoboken) 2016;68:562–9. doi: 10.1002/acr.22725. [DOI] [PubMed] [Google Scholar]
- 34.Zeiadin N, Averns H. CRA survey results: Choosing Wisely. Can Rheumatol Assoc J. 2018;28:26–8. [Google Scholar]
- 35.Naugler C, Thomas R, Turin TC, et al. Yearly clinical laboratory test expenditures for different medical specialties in a major Canadian city. Am J Clin Pathol. 2015;144:97–102. doi: 10.1309/AJCP80REPIUGVXPH. [DOI] [PubMed] [Google Scholar]
- 36.Sadowski BW, Lane AB, Wood SM, et al. High-value, cost-conscious care: iterative systems-based interventions to reduce unnecessary laboratory testing. Am J Med. 2017;130:1112.e1–1112.e7. doi: 10.1016/j.amjmed.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 37.Ontario Health Insurance Plan: OHIP Schedule of Benefits and Fees. Toronto: Ministry of Health and Long-Term Care; 2019. [accessed 2019 Aug 14]. Available: http://health.gov.on.ca/en/pro/programs/ohip/sob/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.