Abstract
Stem cell-based therapies hold great promise as alternative therapeutic strategies for various chronic diseases, including ischemic cardiomyopathy. Tracking the engraftment of transplanted stem cells is critical to the assessment of donor cell survival in the host environment. Fluorescent proteins, such as green fluorescent protein (GFP), have been widely used to track the fate of donor cells; however, GFP labeling has limitations with regard to noninvasively measuring cell engraftment in vivo. Our research indicates that near-infrared fluorescent protein (iRFP) labeling offers advantages for noninvasive in vivo imaging and histological assessment. Here, we present a protocol for using the lentiviral vector-mediated iRFP vector system to label and track donor stem cells in ischemic mouse hearts.
Keywords: Stem cells, iRFP, Green fluorescent protein (GFP), Lentivirus, Myocardial infarction
1. Introduction
Ischemic heart disease caused mainly by occlusive coronary atherosclerosis remains the leading causes of morbidity and mortality in western world [1]. Although appropriate application of currently available therapies is highly beneficial, the prognosis of ischemic cardiomyopathy caused by myocardial dysfunction post-myocardial infarction (MI) remains poor. Stem cell-based therapies represent an alternative, promising therapeutic strategy to improve heart function in ischemic cardiomyopathy [2-5]. To optimize stem cell-mediated heart repair, an efficient method is needed to image the engrafted donor cells and track their fate, including survival, death, proliferation, migration, and differentiation in the ischemic microenvironment. Fluorescent proteins have been widely used to label donor cells in recent decades. Traditional green fluorescent protein (GFP)-like fluorescent proteins, such as eGFP, DsRed, and mCherry, can be used to label and identify stem cells in vitro [6-8]. However, due to tissue scattering, limited penetration depth, and spatial resolution caused by absorbance and autofluorescence, GFP labeling has limited applicability for in vivo imaging [9]. Near-infrared fluorescent protein (iRFP), a fluorescent protein with near-infrared wavelength emission and excitation spectra, is a fluorescent protein with less scattering and absorption than visible light [10-13]. In this chapter, we present a detailed protocol for labeling cardiac mesenchymal stem cells (CMSCs) with lentiviral iRFP vector and demonstrate the advantage of iRFP labeling over conventional GFP for in vivo imaging to quantify and track donor cells in the recipient ischemic myocardium.
2. Materials
2.1. Lentivirus Packaging
pRRLSIN.cPPT.PGK-iRFP-IRES-Puro.WPRE (pPGK-iRFP-Puro) plasmid
pRRLSIN.cPPT.PGK-GFP.WPRE (pPGK-GFP) (a gift from Didier Trono, Addgene_12252)
pMD2.G envelope plasmid (a gift from Didier Trono, Addgene_12259)
psPAX2 packing plasmid (a gift from Didier Trono, Addgene_12260)
Lipofectamine™ 3000 (Thermo Fisher)
293FT cells (Thermo Fisher)
10 cm tissue culture dishes (Thermo Fisher)
DMEM with L-glutamine, 4.5 g/L glucose and sodium pyruvate (Corning)
Fetal bovine serum (FBS) (Thermo Fisher)
OPTI-MEM® Reduced Serum Medium (Thermo Fisher)
ViralBoost Reagent (500 ×) (Alstem)
50 mL polypropylene conical centrifuge tube (Thermo Fisher)
0.45 μm syringe filters
50% PEG 6000 solution (Calbiochem)
4 M NaCl solution
Phosphate buffered saline (PBS, 1×, pH 7.4)
2.2. Stem Cell Labeling
Cardiac mesenchymal stem cells (CMSC) from wild-type C57BL/6 mice (JAX)
3.5 cm tissue culture dish
Polybrene infection/transfection reagent (Sigma)
EVOS cell imaging systems (Thermo Fisher)
2.3. Myocardial Infarction Model and Intramyocardial Cell Transplantation
Accutase solution (Sigma)
Ketamine
Xylazine
9-0 suture (Ethicon)
24-gauge catheter
BD Ultra Fine U-100 Insulin Syringes 31G
Rodent ventilator (Harvard Apparatus)
2.4. Imaging and Tracking Stem Cells in Infarct Hearts
Pearl® Trilogy Small Animal Imaging System (LI-COR)
Odyssey Infrared imaging system (LI-COR)
2.5. Immunohistochemistry Identification of Engrafted Stem Cells in Ischemic Myocardium
Optimal cutting temperature compound (OCT compound)
4% paraformaldehyde
1% Triton™ X-100
Goat serum (Sigma)
Streptavidin/Biotin-Blocking Kit (Vectorlabs)
Biotinylated anti-green fluorescent protein (GFP) (Vectorlabs)
Rabbit anti-iRFP antibody (custom-synthesized antibody by GenScript)
Streptavidin, Alexa Fluor™ 488 conjugate (Thermo Fisher)
Goat anti-rabbit IgG, Alexa Fluor™ 647 conjugate (Thermo Fisher)
Vector® TrueVIEW™ Autofluorescence Quenching Kit (Vectorlabs)
VECTASHIELD® Antifade Mounting Media (Vectorlabs)
Confocal microscope (Zeiss 780)
3. Methods
3.1. Lentivirus Packaging
Day 1
Seed 2.2 × 106 293FT cells in a 10 cm cell culture dish with 10 mL DMEM containing 10% FBS. Incubate at 37 °C in 5% CO2 overnight (see Notes 1 and 2).
Day 2
In tube 1, dilute 25 μL Lipofectamine™ 3000 regent in 300 μL Opti-MEM medium and mix thoroughly.
In tube 2, add 4 μg pPGK-iRFP-Puro or pPGK-GFP plus helper plasmids including 1 μg pMD2.G and 3 μg psPAX2 plasmids to 300 μL Opti-MEM medium, then add 25 μL P3000™ Reagent and mix thoroughly (see Note 3).
Add diluted plasmids to diluted Lipofectamine™ 3000 Regent. Mix by gently flicking the tube. Incubate for 10–15 min at room temperature.
Replace the culture medium of 293FT cells with 6 mL Opti-MEM.
Gently and evenly add plasmid-lipid complex to 293FT cells. Incubate at 37 °C in 5% CO2 overnight (see Note 4).
Day 3
In the morning, add 4 mL DMEM with 10% FBS to 293FT cells. Add 20 μL ViralBoost Reagent to 10 mL culture medium. Incubate at 37 °C in 5% CO2 for 24 h.
Day 4
Collect the supernatant containing lentivirus from the culture dish and transfer to 50 mL polypropylene tube. Add fresh 10 mL DMEM with 10% FBS to 293FT cells (see Note 5). Incubate at 37 °C in 5% CO2.
Day 5
Collect the 2nd supernatant, centrifuge with the 1st supernatant at 220 × g for 3 min to remove the cell pellet. Then filter the supernatant through a 0.45 μm syringe filter to remove cell debris.
Add PEG 6000 (8.5% final concentration) and NaCl (0.4 M final concentration) to the filtered supernatant.
Vortex thoroughly, then incubate at 4 °C overnight.
Day 6
Centrifuge at 600 × g for 30 min at 4 °C. Discard the supernatant, and resuspend the pellet in 1 mL PBS. After mixing, aliquot and store at −80 °C until use (see Note 6)
3.2. Stem Cell Labeling
Seed 3 × 105 CMSCs into a 3.5 cm cell culture dish with 3 mL DMEM containing 10% FBS. Incubate at 37 °C in 5% CO2.
Replace with fresh culture medium containing 8 μg/mL polybrene when monolayer cells reach 80% confluence (see Note 7). Add 100 μL Lenti-GFP vector to one dish and 100 μL lenti-GFP vector plus 100 μL lenti-iRFP-Puro vector to another dish, and incubate at 37 °C and 5% CO2 for 3 days.
Use fluorescence-activated cell sorting (FACS) to purify GFP-positive cells and GFP and iRFP double-positive cells.
Use fluorescent microscopy to perform cell imaging (see Note 8) (Fig. 1).
Fig. 1.
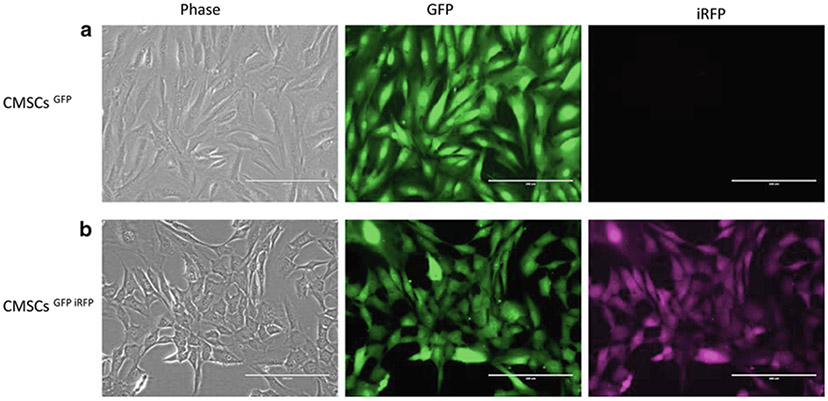
CMSC modified with lentivirus and visualized under fluorescent microscope: (a) CMSCs infected with lentiviral-GFP vector (CMSCsGFP). (b) CMSCs infected with both lentiviral-GFP and lentiviral-iRFP vectors (CMSCsGFP iRFP). Scale bar = 200 μm
3.3. Myocardial Infarction Model and Intramyocardial Cell Transplantation
Harvest the GFP-labeled and GFP-iRFP-labeled stem cells using Accutase. Prepare the cell suspension at a concentration of 5 × 105 cells in 30 μL PBS for cell transplantation. Keep cells on ice before injection.
Anesthetize C57/BL6 mice with intraperitoneal (i.p.) injection of ketamine/xylazine (100 mg/kg BW of ketamine with 10 mg/kg BW of xylazine) (see Note 9).
Use tape to secure mice in supine position on a surgical platform and a 3-0 suture to hold the upper jaw in place.
Apply the depilatory cream to remove the hair of the left side of the mouse chest.
Orally intubate mice with a 24 gauge IV catheter, and ventilate with room air at a rate of 195 breaths per minute by a rodent ventilator.
Disinfect the skin with 75% alcohol and 10% povidone iodine.
Make a lateral incision on left chest, and cut through the pectoralis major and pectoralis minor. Make a left thoracotomy through the fourth intercostal space, and then incise the pericardium. Identify the left anterior descending coronary artery (LAD), and permanently occlude the LAD by ligation with a 9-0 suture (see Note 10).
Identify the border zone of the infarcted myocardium; intra-myocardially inject with 30 μL cell suspension using a 31-gauge syringe (see Notes 11 and 12).
Use 6-0 sutures to close the thoracic cavity, pectoralis muscles, and skin in sequence.
Recover mice. Observe and record their condition after surgery.
3.4. Imaging and Tracking Stem Cells in Infarct Hearts
After 2 days following cell transplantation, perform noninvasive near-infrared fluorescence imaging to evaluate cell engraftment in hearts via Pearl® Trilogy Small Animal Imaging System (Fig. 2a) (see Note 13).
Euthanize mice, harvest hearts, and cut into three pieces; scan at 700 nm using an Odyssey Infrared imaging system (Fig. 2b).
Fig. 2.
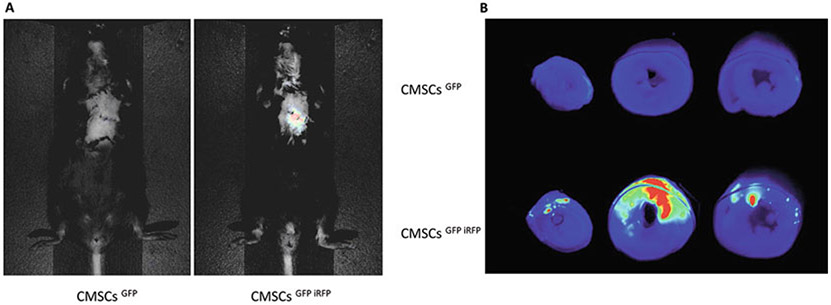
Comparison of iRFP and GFP for imaging and tracking transplanted CMSCs in infarcted hearts. (a) In vivo noninvasive near-infrared fluorescence imaging in mice. Two days after MI and cell transplantation, near-infrared fluorescence imaging of whole animals demonstrates that only CMSCsGFP iRFP-treated mice have detectable signals in the chest. (b) Infrared imaging of heart sections using 700 nm wavelength. Infrared signals can only be detected in the hearts of CMSCsGFP iRFP-treated mice
3.5. Identifying Engrafted Stem Cells in Ischemic Myocardium by Confocal Fluorescent Microscopy
Embed hearts into OCT compound, and cut into 5-μm-thick sections.
Remove OCT with PBS, and fix with 4% paraformaldehyde for 8 min at room temperature.
Permeabilize sections for 1 h at room temperature with 1% Triton™ X-100.
Block sections with 5% goat serum in PBS for 1 h at room temperature.
Block sections with Streptavidin/Biotin-Blocking Kit.
Incubate sections with diluted primary antibody (Biotin anti-GFP antibody, 1:500 in PBS; rabbit anti-iRFP antibody, 1:200 in PBS) overnight at 4 °C (see Note 14).
Incubate sections with diluted secondary antibody (Streptavidin Alexa Fluor 488 conjugated; goat anti-rabbit Alexa Fluor 647—conjugated; 1:400 in PBS) for 45 min at room temperature.
Control autofluorescence using an autofluorescence quenching kit.
Mount slices with mounting medium with DAPI.
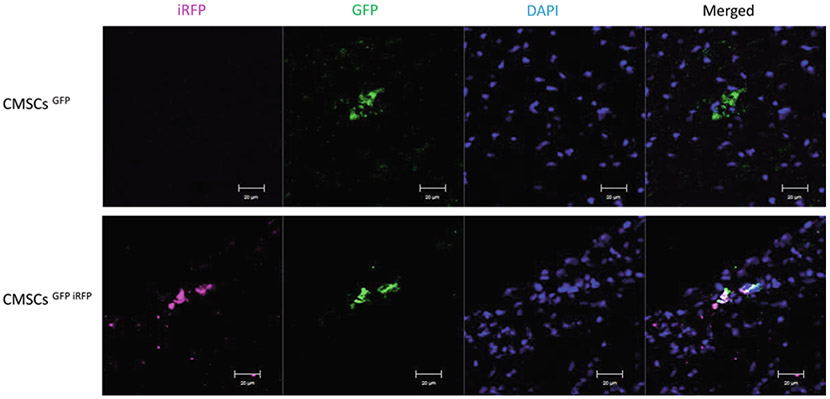
Examine sections under a confocal microscope (Fig. 3).
Fig. 3.
Confocal immunofluorescent imaging of transplanted cells in ischemic myocardium. Immunofluorescence staining demonstrates that both GFP and iRFP can be identified by immunofluorescent staining. Scale bar = 20 μm
Acknowledgments
Weintraub and Y. Tang were partially supported by the American Heart Association:GRNT31430008,NIH-AR070029, NIH-HL086555, and NIH-HL134354. All authors declare that he/she has no conflict of interest. This article does not contain any studies with human participants performed by any of the authors.
Footnotes
Institutional Animal Care and Use Committee (IACUC) regulations must be adhered to. Lentivirus is a modified HIV virus that must be handled in a BL2+ designated hood, although it cannot replicate in the host.
293FT cells should be cultured without antibiotics.
Add the plasmids and transfection reagents directly to the Opti-MEM medium to avoid contact with the polypropylene tube wall prior to dilution.
293FT cells should be approximately 70% confluence upon transfection.
Avoid disturbing the transfected cells when collecting the supernatant.
The virus can be aliquoted into several tubes and stored at −80 °C to avoid repeated freeze/thaw cycles and reduce the loss of viral efficiency.
The use of polybrene significantly increases the transfection efficacy. The concentration of polybrene depends on the cell type and the amount of cells, as it may be toxic to cells.
Transfected cells can be selected by antibiotics or sorted by flow cytometry.
The depth of anesthesia can be confirmed by toe pinch, respiratory rate, and/or other appropriate methods.
The induction of myocardial infarction can be confirmed visually (i.e., development of hypokinesis and pallor in the distal myocardium).
Bending the tip of 31-gauge needle to about 20° can help keep the injected cells in myocardium while avoiding direct injection into left ventricular cavity.
Make sure no air bubbles are injected to myocardium. Successful transplantation can be confirmed by appearance of translucent edema area after cell injection.
Mice should be fed with a nonfluorescent diet at least 3 days in advance of the procedure to reduce interference with fluorescence detection.
The dilution ratio of primary or secondary antibodies can be adjusted according to the type of antibody and titer.
References
- 1.Laslett LJ, Alagona P Jr, Clark BA 3rd, Drozda JP Jr, Saldivar F, Wilson SR, Poe C, Hart M (2012) The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol 60: S1–S49 [DOI] [PubMed] [Google Scholar]
- 2.Ju C, Li Y, Shen Y, Liu Y, Cai J, Liu N, Ma G, Tang Y (2018) Transplantation of cardiac mesenchymal stem cell-derived exosomes for angiogenesis. J Cardiovasc Transl Res 11:429–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ju C, Shen Y, Ma G, Liu Y, Cai J, Kim IM, Weintraub NL, Liu N, Tang Y (2018) Transplantation of cardiac mesenchymal stem cell-derived exosomes promotes repair in ischemic myocardium. J Cardiovasc Transl Res 11:420–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su X, Jin Y, Shen Y, Ju C, Cai J, Liu Y, Kim IM, Wang Y, Yu H, Weintraub NL, Jiang M, Tang Y (2018) Exosome-derived dystrophin from allograft myogenic progenitors improves cardiac function in duchenne muscular dystrophic mice. J Cardiovasc Transl Res 11:412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Su X, Ashraf M, Kim IM, Weintraub NL, Jiang M, Tang Y (2018) Regenerative therapy for cardiomyopathies. J Cardiovasc Transl Res 11:357–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC (1994) Green fluorescent protein as a marker for gene expression. Science 263:802–805 [DOI] [PubMed] [Google Scholar]
- 7.Frangioni JV (2003) In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol 7:626–634 [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Zhang L, Pan Y, Chen L, Weintraub N, Tang Y (2013) Identification of stem cells after transplantation. Methods Mol Biol 1036:89–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaner NC, Steinbach PA, Tsien RY (2005) A guide to choosing fluorescent proteins. Nat Methods 2:905–909 [DOI] [PubMed] [Google Scholar]
- 10.Lecoq J, Schnitzer MJ (2011) An infrared fluorescent protein for deeper imaging. Nat Biotechnol 29:715–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen G, Zhang Y, Li C, Huang D, Wang Q, Wang Q (2018) Recent advances in tracking the transplanted stem cells using near-infrared fluorescent nanoprobes: turning from the first to the second near-infrared window. Adv Healthc Mater 7:e1800497. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Zhou M, Wang X, Qin G, Weintraub NL, Tang Y (2014) Assessing in vitro stem-cell function and tracking engraftment of stem cells in ischaemic hearts by using novel iRFP gene labelling. J Cell Mol Med 18:1889–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Phillips MI, Miao HL, Zeng R, Qin G, Kim IM, Weintraub NL, Tang Y (2014) Infrared fluorescent protein 1.4 genetic labeling tracks engrafted cardiac progenitor cells in mouse ischemic hearts. PLoS One 9:e107841. [DOI] [PMC free article] [PubMed] [Google Scholar]