Abstract
Introduction:
Febrile infection-related epilepsy syndrome (FIRES) is a syndrome of new-onset status epilepticus, preceded by fever, and highly refractory to treatment, thus resulting in high mortality and severe neurologic morbidity in surviving patients. Anakinra is an IL-1 receptor antagonist which has previously demonstrated efficacy in treating children with FIRES.
Case presentation:
A 21-year old previously-healthy woman presented with new onset super-refractory status epilepticus following a febrile illness. This was subsequently diagnosed as FIRES after an extensive evaluation failed to identify an alternative etiology. The patient’s seizures were refractory to numerous anti-epileptic drugs and immunomodulatory therapy. She was maintained under pharmacologic sedation for 31 days.
Management and Outcome:
Anakinra was initiated on day 32 of her hospital stay, with swift and complete remission of her status epilepticus. Seizures ceased within 24 hours. The patient remains in remission with minimal side effects from the medication and no known long-term morbidity.
Discussion:
Here we report a second case of super-refractory status epilepticus due to FIRES responding to anakinra, and the first such case in an adult patient. Anakinra was well-tolerated with few side effects. Our results are further evidence for the autoinflammatory nature of FIRES, and support the use of anakinra early in the treatment to prevent long-term sequelae.
Keywords: status epilepticus, anakinra, autoinflammatory epilepsy, FIRES, adult neurology
Introduction
Status epilepticus is a neurological disorder familiar to most physicians working in emergency or intensive care settings. Most cases of status epilepticus are attributable to an identifiable neurologic insult and are amenable to commonly available anticonvulsants and supportive measures1. When seizures are unresponsive to standard, adequately dosed benzodiazepines and a second line anti-seizure medication (e.g., valproic acid, levetiracetam, or fosphenytoin), the term refractory status epilepticus is used. When seizures continue for more than 24 hours despite the use of a continuous anesthetic infusion (e.g., midazolam), a small subset of patients achieve super-refractory status epilepticus. This condition is exceptionally challenging to manage and confers an ominous prognosis.
New-onset refractory status epilepticus (NORSE) defines a syndrome of refractory status epilepticus occurring in an individual without active epilepsy and without a clear structural, toxic, or metabolic cause2. A subcategory of NORSE where seizure onset is preceded by a febrile prodrome is termed febrile infection-related epilepsy syndrome (FIRES)*. Both the etiology and the optimal treatment of these disease entities remain unknown. Magnetic resonance is typically normal, though some frontal and temporal atrophy may occur over time3. Evidence suggests an immune-mediated process4,5, and thus, the mainstay has been immunomodulatory therapy including high-dose steroids, intravenous immunoglobulin (IVIG), and plasmapheresis6. Evidence for these therapies remains sparse, however, and a plurality of patients do not achieve meaningful remission6. Other treatments have been tried, including tacrolimus, cyclophosphamide, rituximab, hypothermia, and the ketogenic diet2. One particularly promising agent is anakinra, a recombinant form of the endogenously expressed IL-1 receptor antagonist (IL-1ra). There are now a handful of case reports of children with FIRES or other autoimmune status epilepticus syndromes being treated successfully with anakinra4,7,8, indicating that IL-1 blockade can be effective for refractory seizures associated with neuroinflammation.
In this report, we present a case of a young woman with super-refractory status epilepticus, diagnosed as FIRES, which responded rapidly to anakinra despite initiation several weeks into the acute presentation. This is the first reported case of FIRES successfully treated with anakinra in an adult. As in prior case studies, anakinra was rapidly effective and well-tolerated. This report provides additional evidence that anakinra may have benefit beyond traditional immunomodulatory therapies in new-onset super-refractory status epilepticus.
Case Description
Patient information
A 21-year-old woman developed generalized tonic-clonic seizures after one week of intermittent subjective fevers. Her preceding illness was nonspecific, with headaches as the only localizing symptom. Her past medical history was significant only for migraine headaches, and family history was unknown to the patient. At the time of her illness she was a college student also working as a beautician. She was unmarried but in a long-term, stable relationship.
Clinical findings
At the local hospital, her seizures did not respond to appropriately-dosed benzodiazepines, levetiracetam and lacosamide. She was intubated for airway protection, placed in pharmacologically-induced coma, and transferred to an academic hospital after three days. On admission she was febrile to 100.5F. She remained in pharmacologically-induced coma for the next 31 days.
Diagnostic assessment
On admission, continuous EEG monitoring revealed frequent electrographic seizures with onset over the right frontal and parietal regions (see Figure 1 for a representative example). Brain MRI revealed incidental developmental venous anomalies in the right frontal and parietal regions. C-reactive protein was elevated to 16.5 mg/dL (normal 0 – 1 mg/dL). Transaminases were initially elevated (ALT 155, AST 219 U/L) which resolved over the next three weeks. In an attempt to identify a surgical amenable seizure focus, an 18F-FDG PET scan was conducted on hospital day 22, revealing no hyper- or hypometabolic areas.
Figure 1.
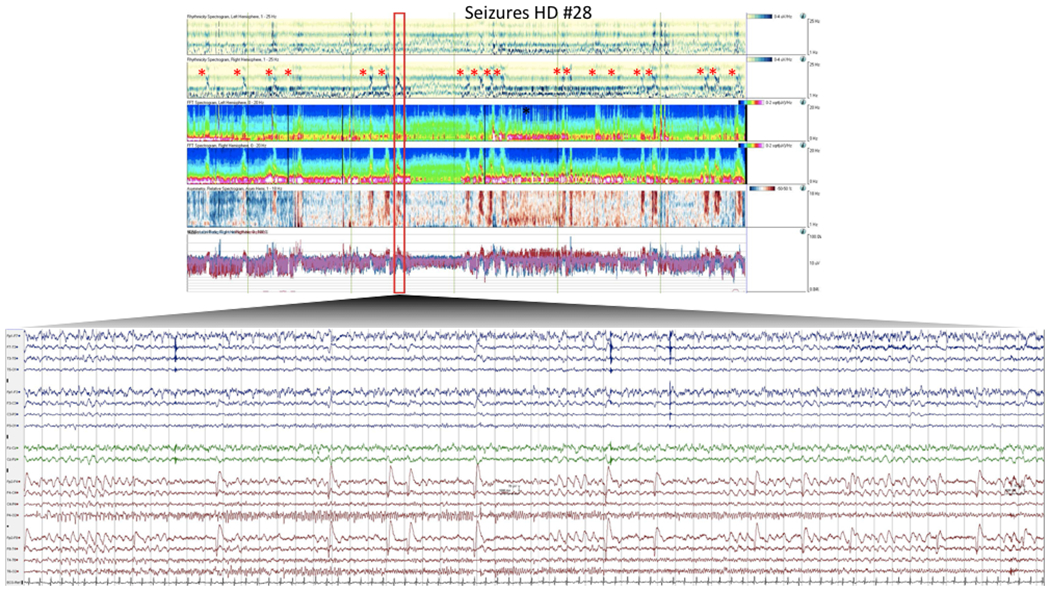
A representative seizure captured in this patient prior to initiation of anakinra.
Quantitative EEG (qEEG) and raw EEG demonstrating a typical right posterior seizure. qEEG encompasses a 2 hour epoch. Trends include (in order, top down) rhythmicity spectrogram (left and right hemispheres), compressed spectral array (left and right hemispheres), asymmetry spectrogram, and amplitude integrated EEG. Individual seizures are marked with *. Raw EEG is shown from one of the seizures. EEG is time compressed to allow visualization of entire seizure. Seizure is characterized by rhythmic, sharply contoured, 4 Hz evolving to 8 Hz frequency, right posterior discharge.
An extensive infectious workup was negative (see Table 1). CSF, blood, and urine cultures were negative at admission. A catheter-associated urinary tract infection developed later in her hospital stay.
Table 1.
Infectious workup for the patient.
| Viruses | Bacteria & other organisms |
|---|---|
| • HSV 1 & 2 | • Tuberculosis |
| • Epstein-Barr virus | • Syphilis |
| • Cytomegalovirus | • Cryptococcus |
| • Varicella zoster virus | • Lyme disease |
| • HIV 1 & 2 | • Ehrlichiosis |
| • Hepatitis A (prior immunity) | • Anaplasma |
| • Hepatitis B | • Coccidioides |
| • Hepatitis C | • Histoplasma |
| • Arbovirus | • Blastomyces |
| • West Nile virus | |
| • Mumps (prior immunity) | |
| • Rubeola | |
| • Respiratory virus panel | |
| ○ Influenza A Subtypes H1 and H3 | |
| ○ Influenza B | |
| ○ Parainfluenza 1, 2 & 3 | |
| ○ Rhinovirus | |
| ○ Enterovirus | |
| ○ Metapneumovirus | |
| ○ RSV subtypes A & B | |
| ○ Lymphocytic choriomeningitis virus |
Workup included CTs of the head, chest, abdomen, and pelvis, and transvaginal ultrasound. These studies were negative for neoplastic foci that might be associated with autoimmune encephalopathy. Repeated TSH remained within normal limits. CSF studies showed glucose 46 mg/dL, total protein 49 mg/dL, 39 nucleated cells (94% lymphocytes), and 4 oligoclonal bands. Tests for cytokines were not collected and no sample was available to perform them after the fact. A CSF autoimmune encephalitis panel was negative (see Table 2). A serum autoimmune encephalopathy panel drawn later (on hospital day 15) was positive for contactin-associated protein-like 2 (CASPR2) antibodies and weakly positive for anti-glutamic acid decarboxylase (GAD; 0.08 nmol/L). This panel was drawn after 5 days of IVIG administration, which complicates interpretation. Notably, repeat serum studies 3 months after hospital discharge were negative for all tested antibodies. An EMG/NCS performed 11 days after initiation of anakinra demonstrated findings of mild critical illness myopathy but was otherwise normal. C-reactive protein normalized to <1 mg/dL within 3 weeks, suggesting resolution of systemic inflammation.
Table 2.
Paraneoplastic antibodies tested in this patient.
| CSF | Serum |
|---|---|
| • NMDA-R Ab | • NMDA-R Ab |
| • Neuronal (V-G) K+ Channel Ab | • Neuronal (V-G) K+ Channel Ab |
| • LGI1-IgG | • LGI1-IgG |
| • CASPR2 IgG | • CASPR2 IgG (positive) |
| • GAD65 Ab | • GAD65 Ab (positive) |
| • GABA-B-R Ab | • GABA-B-R Ab |
| • AMPA-R Ab | • AMPA-R Ab |
| • ANNA type 1 | • ANNA type 1 |
| • ANNA type 2 | • ANNA type 2 |
| • ANNA type 3 | • ANNA type 3 |
| • AGNA type 1 | • AGNA type 1 |
| • PCA type 1 | • PCA type 1 |
| • PCA type 2 | • PCA type 2 |
| • PCA-Tr | • PCA-Tr |
| • Amphiphysin Ab | • Amphiphysin Ab |
| • CRMP-5-IgG | • N-type calcium channel Ab |
| • PQ-type calcium channel | |
| • Acetylcholine receptor (muscle) binding Ab | |
| • AChR ganglionic neuronal Ab | |
| • CRMP-5-IgG |
Consideration was given to a diagnosis of macrophage activation syndrome (MAS)/hemophagocytic lymphohistiocytosis (HLH), which is a primary autoinflammatory disorder that can cause CNS disturbance and seizure9. Ferritin (29 ng/mL) and soluble IL-2 receptor α (800 units/mL) were both normal, which is inconsistent with MAS/HLH, although these were not tested until 2 weeks after anakinra was started.
Therapy
The patient was initiated on anti-epileptic drugs (AEDs) and anesthetic medications which were adjusted to maintain her EEG in a burst-suppression pattern. This was accomplished with combinations of pentobarbital, propofol, and midazolam for approximately 3 weeks, after which time pentobarbital was cross-titrated to ketamine for another 1 week. Repeated attempts to wean anesthesia intermittently precipitated an EEG pattern of generalized periodic discharges with increasing frequency concerning for seizure and later more defined focal electrographic seizures. AEDs were gradually added until her final regimen consisted of 2,500 mg levetiracetam twice daily, 200 mg lacosamide twice daily, 20 mg clobazam twice daily, 130 mg phenobarbital three times daily, and perampanel 8mg daily. On this regimen she still had EEG-confirmed subclinical focal seizures when anesthesia was weaned.
In addition to her sedation and AED regimen, the patient received early immunomodulatory therapy. Specifically, she received IV methylprednisolone for five days starting on hospital day 8, followed by a prednisone taper; IVIG for five days starting on hospital day 11; and plasmapheresis treatments on hospital days 18, 20, 22, 24 and 26. She did not demonstrate appreciable clinical response throughout this time.
Following apparent therapeutic failure of first-line immunomodulatory therapies, the patient was initiated on anakinra on hospital day 32 at 100 mg three times daily. Total cessation of seizures was achieved within 24 hours of initiating anakinra and pharmacologic coma was weaned off within another 24 hours. Ketogenic therapy was also initiated on hospital day 34, but she did not achieve ketosis and this therapy was discontinued on hospital day 45. Her mental status began to improve quickly; she began following commands and moving her limbs within three days of cessation of sedation, and was fully oriented by 10 days (hospital day 42). She showed swift motor recovery, ambulating independently by hospital day 67. Anakinra was reduced to 100 mg twice daily on hospital day 48 and then to 100 mg once daily on hospital day 64. She progressed through physical therapy and rehabilitation and was discharged within six weeks (hospital day 73) on a scheduled taper of phenobarbital, levetiracetam, clobazam and lacosamide.
At 6 month outpatient follow-up she described regaining baseline physical and cognitive abilities, and returning to work and college courses. She has not experienced further seizures. She does not report side effects from the medication and has not been neutropenic. Tapering of antiseizure medications is expected to be complete within 1 year. Anakinra will be continued for 1 year, at which time discontinuation will be discussed. Repeat imaging and EEG are planned once she has discontinued her AEDs. (The full timeline of the patient’s treatment can be found in Figure 2.)
Figure 2.
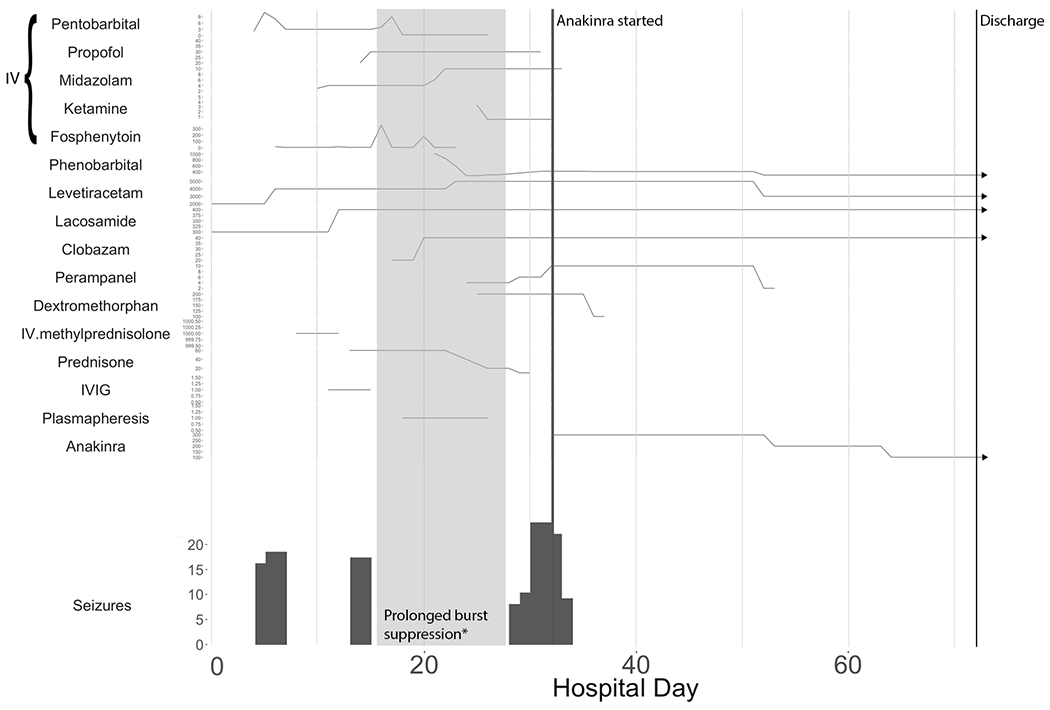
Timing of anti-seizure and anti-inflammatory medications relative to EEG-confirmed seizure burden. Hospital day is counted from the day of onset of SE. “Seizures” on the y-axis refers to number of EEG-confirmed seizures per day. Medications are reported as total daily doses except in the case of IV medications, which are reported as mg/kg/hr. Some IV medication doses were estimated based on the ordered rate.
Discussion
In this report we have described a previously-healthy 21-year-old woman who developed new-onset super-refractory status epilepticus consistent with febrile infection-related epilepsy syndrome (FIRES). Despite multiple treatment failures, she responded swiftly to the recombinant IL-1 receptor antagonist anakinra and appears to have achieved complete remission with minimal residual effects. Although it is possible that her clinical response was attributable to the delayed effects of another immunomodulatory therapy, the sudden and dramatic nature of her recovery would be unusual, and the timeline is more compelling for a response to anakinra. This is the second published report of FIRES responding to anakinra, and the first in an adult patient, which is notable due to the substantial morbidity and mortality of this syndrome in adult patients10. In addition to its utility in treating this challenging disorder, anakinra is generally a well-tolerated drug, with its more common side effects including local injection site reaction, leukopenia and transaminase elevation. There is an increased risk of infection, but serious infections are rare11.
There were several unusual aspects to this patient’s clinical presentation which bear discussion. First, her serum tested positive for CASPR2 and weakly positive for GAD antibodies, which raise the question of an autoimmune encephalitis rather than FIRES as her primary diagnosis. Regarding the CASPR2 antibodies, we are doubtful that they contributed to her presentation because (a) they were not present in CSF, (b) they were absent on follow-up testing, and (c) the initial studies were drawn at the end of a 5-day course of IVIG. In addition, her clinical presentation is inconsistent with a CASPR2-associated encephalitis, which is typified by subacute encephalitis and peripheral nervous dysfunction,12 but not status epilepticus. Anti-GAD encephalitis, likewise, is associated with encephalomyelitis and stiff-person syndrome13. Thus, we suspect that the positive serum CASPR2 and GAD results may have been due to circulating antibodies from an IVIG donor, and not from our patient.
Second, this patient also demonstrated elevated oligoclonal bands in CSF. This is an uncommon finding in NORSE/FIRES but does not preclude its diagnosis, as immunoglobulin production could certainly occur in the context of dysregulation of the innate immune system14. Cases of FIRES with oligoclonal bands have been previously reported6.
The success of anakinra in this patient’s treatment is valuable not only for future management of this disorder, but also in suggesting an autoinflammatory rather than autoimmune mechanism for the status epilepticus syndromes NORSE and FIRES. The majority of cases are not associated with known autoantibodies6, which argues against a classic autoimmune disorder in which antigen-specific T or B cells mediate neuroinflammation and neuronal dysfunction. The previously-reported association with elevations in pro-inflammatory cytokines that resolved with administration of anakinra4,5 instead support the assertion that FIRES represents an autoinflammatory disorder15. Autoinflammatory disorders are more recently described conditions in which the innate immune system is directly activated without the need for a specific antigen. From a clinical standpoint, autoinflammatory disorders respond to therapies targeting innate immune system cytokines such as IL-1, and may not respond to more familiar autoimmune therapies such as IVIG, tacrolimus or even steroids15. Thus, the responsiveness of FIRES to anakinra is highly suggestive of an IL-1-driven inflammatory cascade as a key pathogenic mechanism.
Conclusion
This is the first case report of an adult patient with acute onset super-refractory status epilepticus, presenting immediately after a febrile illness (i.e., FIRES), whose seizures did not respond to conventional immunotherapy but were abolished shortly after initiating anakinra. She recovered to her premorbid baseline. To put this case into broader perspective, the super-refractory status epilepticus syndromes NORSE and FIRES are rare but devastating conditions which are challenging to treat. Standard immunomodulatory modalities are the mainstays of therapy, but these are frequently unsuccessful. This case report and others provide increasing evidence for the benefit of novel immunomodulatory therapies targeting the innate immune system, particularly anakinra, in NORSE and FIRES. Because of its safety and the high risk of permanent neurologic sequelae from these disorders, we recommend considering initiating anakinra early in the course of treatment for adults as well as children presenting with new-onset super-refractory status epilepticus.
Footnotes
Other idiopathic new-onset SE and epilepsy syndromes have been described. A recent multinational consortium opted for subsuming all of these disorders under the NORSE/FIRES terminology2.
References
- 1.Trinka E & Kälviäinen R 25 years of advances in the definition, classification and treatment of status epilepticus. Seizure 44, 65–73 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Gaspard N et al. New-onset refractory status epilepticus (NORSE) and febrile infection–related epilepsy syndrome (FIRES): State of the art and perspectives. Epilepsia 59, 745–752 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Kramer U et al. Febrile infection–related epilepsy syndrome (FIRES): Pathogenesis, treatment, and outcome. Epilepsia 52, 1956–1965 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Kenney-Jung DL et al. Super-refractory status epilepticus and febrile infection-related epilepsy syndrome treated with anakinra. Ann. Neurol 80, 939–945 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakuma H et al. Intrathecal overproduction of proinflammatory cytokines and chemokines in febrile infection-related refractory status epilepticus. J. Neurol. Neurosurg. Psychiatry 86, 820–822 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Khawaja AM, DeWolfe JL, Miller DW & Szaflarski JP New-onset refractory status epilepticus (NORSE)--The potential role for immunotherapy. Epilepsy Behav. EB 47, 17–23 (2015). [DOI] [PubMed] [Google Scholar]
- 7.DeSena AD, Do T & Schulert GS Systemic autoinflammation with intractable epilepsy managed with interleukin-1 blockade. J. Neuroinflammation 15, 38 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shukla N et al. Anakinra(IL-1 blockade) use in children with suspected FIRES: A single institution experience (P4.346). Neurology 90, P4346 (2018). [Google Scholar]
- 9.Co DO, Bordini BJ, Meyers AB & Inglese C Immune-Mediated Diseases of the Central Nervous System: A Specificity-Focused Diagnostic Paradigm. Pediatr. Clin. North Am 64, 57–90 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Kramer U et al. Febrile infection–related epilepsy syndrome (FIRES): Does duration of anesthesia affect outcome? Epilepsia 52, 28–30 [DOI] [PubMed] [Google Scholar]
- 11.Kineret (anakinra) [package insert]. (Swedish Orphan Biovitrum AB, 2012).
- 12.Irani SR et al. Morvan syndrome: Clinical and serological observations in 29 cases. Ann. Neurol 72, 241–255 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Hernandez E et al. Clinical and immunologic investigations in patients with stiff-person spectrum disorder. JAMA Neurol 73, 714–720 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinarello CA Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev 281, 8–27 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doria A et al. Autoinflammation and autoimmunity: Bridging the divide. Autoimmun. Rev 12, 22–30 (2012). [DOI] [PubMed] [Google Scholar]


