Abstract
Rationale & Objective
The renin angiotensin aldosterone system (RAAS) is associated with renal and cardiovascular disease in diabetes. Unfortunately, early RAAS blockade in patients with type 1 diabetes (T1D) does not prevent development of complications. We sought to examine the role of hyperfiltration and RAAS activation across a wide range of T1D duration to better understand the renal hemodynamic changes overtime.
Study Design
Post-hoc analysis of blood samples.
Setting & Participants
148 Canadian patients with T1D: 28 adolescents (16.2±2.0yrs), 54 young adults (25.4±5.6yrs), and 66 older adults (65.7±7.5yrs) studied in a clinical investigation unit.
Exposure
Angiotensin II infusion (ANGII, 1 ng∙kg−1∙min−1 – a measure of RAAS activation) during a euglycemic clamp.
Outcomes
Glomerular filtration rate (GFRINULIN), effective renal plasma flow (ERPFPAH), afferent (RA) and efferent (RE) arteriolar resistances, and glomerular hydrostatic pressure (PGLO) estimated using Gomez’s equations.
Results
In a step-wise fashion, GFRINULIN, ERPFPAH and PGLO were higher while renal vascular resistance (RVR) and RA were lower in adolescents vs. young adults vs. older adults. RE was similar in adolescents vs. young adults but was higher in older adults. ANGII resulted in blunted renal hemodynamic responses in older adults (RVR increase of 3.3±1.6% vs. 4.9±1.9% in adolescents, p < 0.001), suggesting a state of enhanced RAAS activation.
Limitations
Homogeneous study participants limit generalizability of findings to other populations. Studying older adult T1D participants may be associated with a survivorship bias.
Conclusions
A state of relatively low RAAS activity and predominant afferent dilation rather than efferent constriction characterize early adolescent and young adults with T1D. Given this state of endogenous RAAS inactivity in early T1D, may explain why pharmacological blockade of this neurohormonal system is often ineffective in reducing kidney disease progression in this setting. Older adults with longstanding T1D who have predominant afferent constriction and RAAS activation may experience renoprotection from therapies that target the afferent arteriole. Further work is required to understand the potential role of non-RAAS pharmacologic agents that target RA in patients with early and longstanding T1D.
Keywords: Type 1 Diabetes, Renal Hemodynamic Function, Diabetes Duration, Renin Angiotensin Aldosterone System
Nontechnical Summary
Renin angiotensin aldosterone system (RAAS) is associated with kidney and heart complications in type 1 diabetes (T1D), which are not completely prevented by RAAS blockers. We examined kidney function across a wide range of T1D durations by studying adolescents, young adults and older adults. The results of our analyses demonstrated that adolescents with T1D have low RAAS activity and have dilated arterioles flowing into the kidney. This may cause an increased glomerular filtration rate that is thought to mediate kidney injury. Older adults with T1D had increased RAAS activity and constricted arterioles flowing into the kidney. Our results suggest that medications that alter the tone of kidney inflow arterioles may protect or delay kidney complications in both early and longstanding T1D.
INTRODUCTION
In 1993, the Diabetes Control and Complications Trial (DCCT) demonstrated that intensive glycemic control reduces the risk of complications in patients with type 1 diabetes (T1D). Since that time, significant technological advances have been made leading to improved glycemic control and decreased rates of micro- and macrovascular complications in T1D 1,2. Despite the declining rates of advanced chronic kidney disease and end-stage renal disease (ESRD) in T1D, diabetic nephropathy (DN) remains relatively common. In the absence of DN the 20 year mortality risk for T1D is comparable to the general population 3. The presence of DN, however carries a substantial cardiovascular risk, which is the leading cause of mortality in T1D 4. Changes in renal hemodynamic function, such as intraglomerular hypertension or hyperfiltration, are linked with the initiation and progression of DN via increased glomerular wall tension. Although most studies suggest that hyperfiltration is an early marker of renal disease, some studies fail to detect such an association 5. One of the key factors in DN pathogenesis is the chronic renal and systemic activation of the renin angiotensin aldosterone system (RAAS), which initiates tissue injury via fibrosis, production of reactive oxygen species, proinflammatory pathways, and increases in intraglomerular pressure 4.
Although RAAS inhibitors are the current standard of care for nephroprotection in DN, they do not completely protect patients with T1D. In the Renin–Angiotensin System Study (RASS) in adults with uncomplicated T1D, angiotensin converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) did not prevent clinical or histological evidence of nephropathy over five years 6. RAAS blockade was also recently studied at even earlier stages of the disease in adolescents with T1D, since pathogenic processes leading to future complications may accelerate during puberty 7,8. Unfortunately, in the Adolescent Cardiorenal Intervention Trial (AdDIT), ACE inhibition was shown not to be effective as a primary renal disease prevention strategy 9.
Improving our understanding of changes in renal physiology and hemodynamic function from the earliest stages of T1D over time, including the role of hyperfiltration, may allow us to better characterise the mechanisms underlying DN pathogenesis. We have previously compared renal hemodynamic function in young T1D adults with and without hyperfiltration 10 cross-sectionally in a homogenous patient cohort. Yet, little is known about changes in renal hemodynamic function over time and in response to RAAS activation over a much broader range of age and T1D duration. Accordingly, our aim was to perform an exploratory analysis of kidney function, hemodynamic changes and RAAS activation over the natural history of T1D in patients across a wide range diabetes duration, including adolescents, young adults and older adults with T1D.
METHODS
Subject Inclusion Criteria and Experimental Design
We conducted a post-hoc analysis to compare renal hemodynamic function in patients with T1D: adolescents (n=28), young adults (n=54) and older adults (n=66) using archived plasma samples from our earlier studies where ANGII infusions were performed and primary study results were previously reported 4,11–17. Detailed baseline demographic characteristics were previously reported. All patients were studied under clamped euglycemic conditions (4–6 mmol/L). All participants from the older adult T1D cohort underwent RAAS inhibitor (ACE inhibitors, ARBs, direct renin inhibitors, aldosterone antagonists) washout 30 days prior to the study measurements. All studies were performed after a 7 day diet consisting of ≥150 mmol/day sodium and ≤1.5 g/kg/day protein. The sodium-replete diet was used to avoid circulating RAAS activation, volume contraction, between-subject heterogeneity and in an attempt to keep study conditions similar to typical North American dietary patterns. Pre-study protein intake was monitored to avoid the hyperfiltration effect of high protein diets. All study participants were instructed to avoid caffeine- containing products and to have the same light breakfast on the morning of each study visit. Studies were carried out in accordance with the Declaration of Helsinki, all study participants gave their informed consent and the study was approved by the University Health Network research ethics board.
Assessment of Renal Hemodynamic Function
Renal hemodynamic function (glomerular filtration rate [GFR] and ERPF) was measured using inulin and PAH clearance according to the plasma disappearance technique 15,18. The mean of the final 2 clearance periods represented baseline GFR and ERPF, expressed per 1.73 m2.
The following parameters were calculated:
Due to the inability to differentiate between afferent (RA) and efferent arteriolar resistances (RE) in humans, studies of renal haemodynamic function have relied primarily on assessments of GFR. In 1951, Gomez et al derived equations for indirect estimates of RA and RE, filtration pressure (PF) and glomerular hydrostatic pressure (PGLO) 19.
Indirect intraglomerular hemodynamic parameters were estimated using Gomez formulae based on data from animal studies (Figure 1). These equations were used in a similar manner to evaluate patients with conditions such as hypertension, endocrine disorders and T1D 20–22. Assumptions imposed by Gomez’s equations were the following: i) intrarenal vascular resistances are divided into afferent, post-glomerular and efferent; ii) hydrostatic pressures within the renal tubules, venules, Bowman’s space and interstitium (PBow) are in equilibrium of 10 mmHg; iii) glomerulus is in filtration disequilibrium; iv) the gross filtration coefficient (KFG) = 0.1012 ml/s per mmHg (PGLO = 56.4 mmHg) to reflect the PGLO values in diabetic conditions observed in previous micropuncture studies in Munich-Wistar rats 23. MAP (mmHg), ERPF (ml/s), GFR (mL/s) and total protein (g/dL) were used to calculate RE (dyne•sec•cm−5) and RA (dyne•sec•cm−5), PGLO (mmHg), ΔPF (mmHg) and πG (mmHg).
Figure 1.
Summary of Gomez equations and assumptions used to calculate intrarenal hemodynamic parameters.10
The filtration pressure across glomerular capillaries (ΔPF):
The glomerular oncotic pressure (πG) from the plasma protein mean concentration (CM) within the capillaries:
Glomerular hydrostatic pressure (PGLO):
RA and RE were estimated using principles of Ohm’s law, where 1328 is the conversion factor to dyne•sec•cm−5 19:
Assessment of Angiotensin II Infusion Response
After a stable euglycemic clamp for at least 3 hours, plasma renin and aldosterone levels were measured. In the adolescents with T1D group, such measurements were performed in 11 patients only. After baseline renal clearance measurements were complete, ANGII (Clinalpha, Laüfelfingen, Switzerland) was administered at 1 ng/kg/min over 30 minutes, followed by a 30-minute recovery phase 24. Oscillometric brachial artery blood pressure measurements were obtained in duplicates in a reclining position every 5 minutes during the ANGII infusion (Critikon, Tampa, Florida, USA). Blood was collected during the ANGII infusion period and after a 30-minute recovery for HCT, inulin and PAH to assess renal hemodynamic parameters. Renal and systemic hemodynamic responses to ANGII infusion were used to measure RAAS activation. Upon infusion, ANGII binds angiotensin 2 type 1 receptors and causes vasoconstriction, leading to decreased GFR, ERPF, RBF, and increased RVR and blood pressure. Blunted responses to the ANGII infusion, therefore, indicate saturation of the angiotensin 2 type 1 receptor due to high levels of endogenous RAAS activation.
Statistical Analyses
Data are presented as mean ± standard deviation (SD), unless otherwise specified. To assess for between-group differences, analysis of variance with post-hoc Tukey’s test was used. The difference between renal hemodynamic parameters at baseline euglycemic clamp and 30 minutes after the 3ng/kg/min ANGII infusion were used to compare the ANGII response between the patient groups. Sensitivity analysis was performed to compare renal, intraglomerular and systemic hemodynamic parameters between groups when adjusted for sex, HbA1c and BMI. All variables presented were normally distributed except for plasma renin and aldosterone levels. Non-parametric Kruskal-Wallis test was used to compare plasma renin and aldosterone levels. Statistical significance was defined as p<0.05. All statistical analyses were performed using SAS v9.1.3 and GraphPad Prism software (version 5.0).
RESULTS
Baseline Characteristics
At baseline, BMI was greater in older patients with T1D compared to adolescents and young adults. There was a stepwise decrease in HbA1c from adolescents to adults to older adults and an increase in plasma renin levels. Plasma aldosterone levels were increased in older patients with T1D compared to young adults.
Baseline Renal Hemodynamic Function
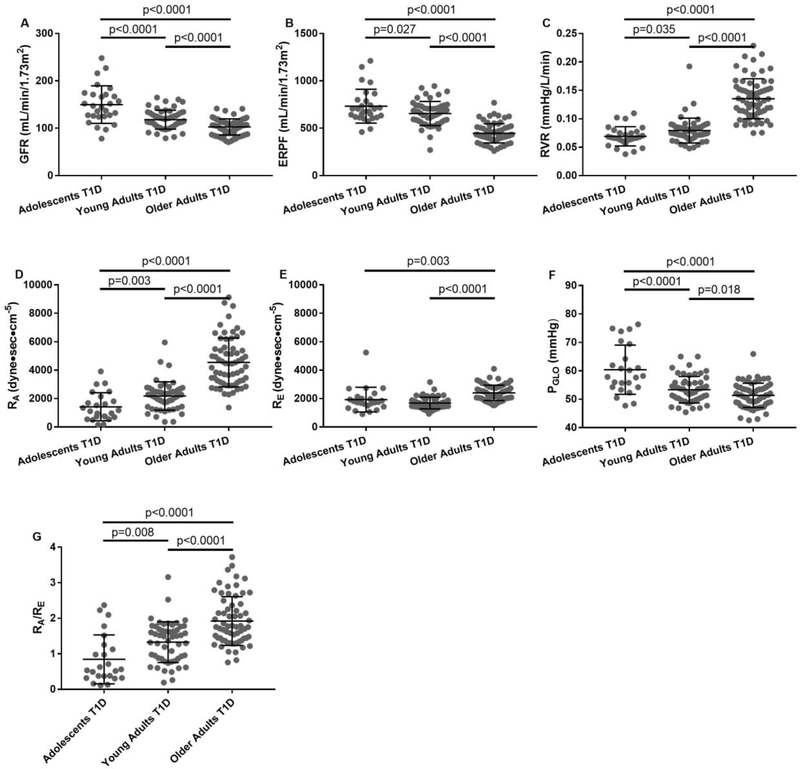
In a step-wise fashion, GFRinulin, ERPFPAH, RBF, and PGLO decreased, while FF, RVR and RA increased in adolescents vs. young adults vs. older adults with T1D (Table 1, Figure 2). Blood pressure, heart rate and RE were similar in adolescents vs. young adults, but significantly higher in older patients with T1D. Similar results were obtained in the sensitivity analysis, where renal, intraglomerular and systemic hemodynamic parameters adjusted for sex, HbA1c and BMI were compared between groups (Table 2).
Table 1.
Baseline Demographic Characteristics of Adolescent, Young Adult and Older Adult Patients with T1D.
| Parameter | Adolescents T1D (n=28) | Young Adults T1D (n=54) | Older Adults T1D (n=66) | p-value |
|---|---|---|---|---|
| Sex (% male) | 13 (46%) | 30 (56%) | 30 (45%) | |
| Age (years) | 16.2±2.0#& | 25.4±5.6*& | 65.7±7.5*# | <0.001 |
| Diabetes duration (years) | 11.8±2.6& | 14.1±6.7& | 54.6±5.7*# | <0.001 |
| Body mass index (kg/m2) | 24.3±3.5& | 25.1±3.3& | 26.6±3.9*# | 0.009 |
| Hemoglobin A1c – mmol/mol (%) | 69.1±13.5#& (8.5±1.2) #& | 62.3±13.7*& (7.8±1.2) *& | 57.0±9.3*# (7.4±0.8)*# | <0.001 |
| Hematocrit | 0.397±0.037& | 0.383±0.033& | 0.349±0.034*# | <0.001 |
| Plasma Protein | 71.4±23.8#& | 61.7±6.3* | 60.4±5.9* | <0.001 |
| Renal Hemodynamic Function | ||||
| ERPF (mL/min/1.73m2) | 733±179#& | 656±128*& | 447±101*# | <0.001 |
| GFR (mL/min/1.73m2) | 150±40#& | 118±20*& | 103±17*# | <0.001 |
| Filtration fraction | 0.214±0.072# | 0.187±0.039*& | 0.235±0.041# | <0.001 |
| RBF (mL/min/1.73m2) | 1214±280#& | 1065±212*& | 689±166*# | <0.001 |
| RVR (mmHg/L/min) | 0.069±0.017#& | 0.079±0.022*& | 0.135±0.035*# | <0.001 |
| Intraglomerular Hemodynamic Parameters | ||||
| PGLO (mmHg) | 60.4±8.7#& | 53.3±4.7*& | 51.4±4.26*# | <0.001 |
| RA (dyne•sec•cm−5) | 1424±990#& | 2183±995*& | 4546±1714*# | <0.001 |
| (RE (dyne•sec•cm−5) | 1928±867& | 1690±407& | 2403±544*# | <0.001 |
| RA/RE | 0.84±0.69#& | 1.33±0.57*& | 1.92±0.69*# | <0.001 |
| Systemic Hemodynamic Function | ||||
| HR (bpm) | 68±11 | 67±11 | 66±10 | 0.7 |
| SBP (mmHg) | 115±12& | 112±9& | 131±16*# | <0.001 |
| DBP (mmHg) | 63±8& | 66±7 | 68±5* | 0.008 |
| MAP (mmHg) | 80±9& | 81±8& | 88±7*# | <0.001 |
| Plasma RAAS Markers | ||||
| Renin (ng/L)*** | 0.16 (0.10–0.33)#&^ | 5.20 (3.20–8.90)*& | 11.50 (5.90–17.70)*# | <0.001 |
| Aldosterone (ng/dL)*** | 130 (86–185)^ | 73 (37–122)& | 165 (104–299)# | <0.001 |
| Therapy | ||||
| RAAS inhibitor use prior to study** (% of patients) | 0 (0%) | 0 (0%) | 54 (82%) | |
n, number of participants. Data are presented as mean ± SD.
p<0.05 vs. Adolescents with T1D
p<0.05 vs. Young Adults with T1D
p<0.05 vs. Older Adult with T1D
All the older adult T1D participants underwent RAAS inhibitor (ACE inhibitors, angiotensin receptor blockers, direct renin inhibitors, aldosterone antagonists) washout 30 days prior to the study measurements
Data are presented as a median (interquartile range)
Data available for 11 patients only.
ERPF: effective renal plasma flow; GFR: glomerular filtration rate; RBF: renal blood flow; RVR: renal vascular resistance; PGLO: glomerular hydrostatic pressure; RA: afferent arteriolar resistance; RE: efferent arteriolar resistance; HR: heart rate; SBP: systolic blood pressure; DBP: diastolic blood pressure; MAP: mean arterial pressure.
Figure 2. Baseline GFRINULIN (A), ERPFPAH (B), RVR (C), RA (D), RE (E), PGLO (F), and RA/RE ratio (J) in adolescents, young adults and older adult patients with T1D.
Adolescents T1D n=28, Young Adults T1D n=54, Older Adults T1D n=66; GFRINULIN: glomerular filtration rate measured by inulin clearance; ERPF: effective renal plasma flow measured by paraaminohippurate (PAH) clearance; RVR: renal vascular resistance; RA: afferent arteriolar resistance; RE: efferent arteriolar resistance; PGLO: glomerular hydrostatic pressure. Values are mean ± standard deviation. The bars in each figure represent significance levels.
Table 2.
Standardized Baseline Characteristics of Adolescent, Young Adult and Older Adult Patients with T1D adjusted for sex, HbA1c and BMI.
| Parameter | Adolescents T1D (n=27^) | Young Adults T1D (n=54) | Older Adults T1D (n=66) | p-value |
|---|---|---|---|---|
| Renal Hemodynamic Function | ||||
| (ERPF (mL/min/1.73m2) | 741±26#& | 655±18*& | 449±17*# | <0.001 |
| (GFR (mL/min/1.73m2) | 145±5#& | 118±3*& | 104±3*# | <0.001 |
| Filtration fraction | 0.203±0.009& | 0.187±0.006& | 0.236±0.006*# | <0.001 |
| RBF (mL/min/1.73m2) | 1234±41#& | 1061±28*& | 692±27*# | <0.001 |
| RVR (mmHg/L/min) | 0.066±0.006#& | 0.079±0.004*& | 0.136±0.004*# | <0.001 |
| Intraglomerular Hemodynamic Parameters | ||||
| PGLO (mmHg) | 59.2±1.1#& | 53.2±0.7* | 51.6±0.7* | <0.001 |
| RA (dyne•sec•cm−5) | 1394±301#& | 2181±192*& | 4573±178*# | <0.001 |
| RE (dyne•sec•cm−5) | 1728±102& | 1687±65& | 2414±60*# | <0.001 |
| RA/RE | 0.88±0.14#& | 1.32±0.09*& | 1.93±0.08*# | <0.001 |
| Systemic Hemodynamic Function | ||||
| HR (bpm) | 66±2 | 67±1 | 67±1 | 0.7 |
| SBP (mmHg) | 115±3& | 112±2& | 131±2*# | <0.001 |
| DBP (mmHg) | 63±1& | 66±1 | 68±1* | 0.009 |
| MAP (mmHg) | 79±2& | 80±1& | 89±1*# | <0.001 |
Data are presented as mean (adjusted for sex, HbA1c and BMI) ± SE. The numbers were calculated assuming 49% of male, HbA1c of 6.8% and BMI of 25.9 kg/m2 in all three groups.
p<0.05 vs. Adolescents with T1D
p<0.05 vs. Young Adults with T1D
p<0.05 vs. Older Adult with T1D
All the older adult T1D participants underwent RAAS inhibitor (ACE inhibitors, angiotensin receptor blockers, direct renin inhibitors, aldosterone antagonists) washout 30 days prior to the study measurements.
Data from 27 out of 28 adolescent T1D patients were used for the sensitivity analysis as 1 patient was missing HbA1c data and was excluded from this analysis.
ERPF: effective renal plasma flow; GFR: glomerular filtration rate; RBF: renal blood flow; RVR: renal vascular resistance; PGLO: glomerular hydrostatic pressure; RA: afferent arteriolar resistance; RE: efferent arteriolar resistance; HR: heart rate; SBP: systolic blood pressure; DBP: diastolic blood pressure; MAP: mean arterial pressure.
Renal Hemodynamic Function in Response to an ANGII Infusion
In response to infused ANGII, ERPF and RBF were most attenuated in the older adult T1D cohort compared to the other two cohorts (p<0.0001 for both comparisons), and intermediate in the young adult cohort compared to the adolescents (p=0.03 for ERPF and p=0.02 for RBF) (Figure 3, Table S1). RVR response to the ANGII infusion was also blunted the most in older patients with T1D (RVR increase of 0.033±0.016 vs. 0.049±0.019mmHg/L/min in adolescents, p=0.0006). RE and diastolic blood pressure responses were suppressed in the older adult T1D cohort compared to both other groups, whereas the systolic blood pressure response was lowest in the older adult T1D cohort vs. the young adult cohort only; the difference in the response vs. the adolescent cohort did not reach statistical significance.
Figure 3. Changes in GFRINULIN (A), ERPFPAH (B), RBF (C), RVR (D), PGLO (E) RA (F), RE (G), SBP (H), DBP (I), Heart Rate (J), and RA/RE ratio (K) in response to infused angiotensin II in adolescents, young adults and older adult patients with T1D.
Adolescents T1D n=28 (n=24 for PGLO, RA, and RE), Young Adults T1D n=53 (n=54 for GFR, SBP, DBP and HR), Older Adults T1D n=57; GFRINULIN: glomerular filtration rate measured by inulin clearance; ERPF: effective renal plasma flow measured by paraaminohippurate (PAH) clearance; RBF: renal blood flow; RVR: renal vascular resistance; PGLO: glomerular hydrostatic pressure; RA: afferent arteriolar resistance; RE: efferent arteriolar resistance; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate. Values are mean ± SD. The bars in each figure represent significance levels.
DISCUSSION
Animal models have provided crucial insights into the role of renal hemodynamic mechanisms that contribute to the development of DN. Data from animal models may not accurately reflect human physiology in health or disease, and observations from experimental models may not lead to the development of novel therapies 25,26, as demonstrated in several clinical trial development programs. Renal hemodynamic function is controlled by a number of complex autoregulatory mechanisms including tubuloglomerular feedback, neurohormonal mediators and myogenic responses, and each of these mechanisms may ultimately translate into a better understanding of human disease and/or therapy. Whatever new physiological insights and/or therapies are elucidated in present or future work, it is critical to determine the role of these therapies in the context of RAAS activation characteristic of diabetes. It is therefore of significant importance to better understand the role of the RAAS throughout the spectrum of diabetes duration. In this analysis, we used the Gomez equations to better define renal hemodynamic function changes over a wide range of age and T1D duration, and to examine the relationship between RAAS activation and segmental resistances in the kidney.
RAAS activation is physiologically important for the regulation of blood pressure and effective circulating volume. In addition, RAAS activation on the basis of chronic hyperglycemia in the setting of diabetes increases intraglomerular pressure and the initiation and progression of DN 4,27–30. From a hemodynamic perspective, RAAS inhibitors are therefore of use due to their ability to reduce efferent arteriolar resistance and hence intraglomerular pressure 31. Observations from our analyses suggest that the increase in efferent arteriolar resistance is present only in patients with long-standing T1D, but not in adolescents or young adults with shorter T1D duration. In addition to these baseline hemodynamic differences, patients with long-standing T1D exhibited evidence of RAAS overactivation compared to those with shorter duration of T1D, as was evident through the blunted RVR, RE and blood pressure responses to an ANGII infusion and by significantly greater plasma aldosterone and renin levels.
The high levels of efferent resistance due to RAAS overactivation with longer duration of T1D may be the reason why RAAS inhibitors are effectively used for nephroprotection in patients with DN, but were shown to be ineffective as primary prevention agents in young adult and even adolescent patients with shorter duration of T1D without complications 6,9. Moreover, our data suggest that the increased levels of afferent resistance are also predominant in the older longstanding T1D cohort, suggesting that pharmacologic targeting of the afferent arteriole could further improve nephroprotection already observed with RAAS inhibitors.
It is currently not known how to identify who is at risk for the development of DN prior to the onset of elevated albumin excretion or renal function decline. Elevated albumin excretion is one of the earliest manifestations to emerge clinically in patients with T1D and is evident in 12–16% of adolescents 32,33. However, albuminuria is not an ideal biomarker due to significant variability and lack of predictive value. Furthermore, primary prevention strategies with RAAS inhibitors have proven to be ineffective in people with T1D. It is therefore important to identify early subclinical renal hemodynamic changes in otherwise healthy adolescents and young adults with T1D who may preferentially benefit from the early intervention strategies. It is of further interest to define renal hemodynamic profiles in younger patients with T1D to help determine where to target new therapies in the renal microcirculation. For example, previous animal work directly measuring hydraulic pressure in diabetic rat afferent and efferent arteries and human work in adults with T1D estimating segmental resistances showed that hyperfiltration (GFR ≥135 ml/min/1.73m2, an early marker of DN) is a result of a decrease in afferent arteriolar resistance without changes to the resistance at the efferent arteriole during eu- and hyperglycemia 20,34. In the current analysis, we demonstrated that the afferent resistance is lower than the efferent resistance only in adolescents, the early T1D cohort that had significantly greater GFR with a large proportion of patients with hyperfiltration. Moreover, the increase in afferent arteriolar resistance is observed earlier than changes at the efferent arteriole, since the RA significantly increased and glomerular pressure decreased in a stepwise fashion from adolescents to young adults to older patients with T1D.
Resistance at the afferent renal arteriole is in part mediated by tubuloglomerular feedback mechanisms whereby hyperglycemia augments proximal renal tubular sodium reabsorption via sodium-glucose cotransporters (SGLT) including SGLT2. The resulting decrease in sodium delivery to the macula densa causes afferent renal arteriole vasodilatation and hyperfiltration. Accordingly, SGLT2 inhibition in young adult patients with T1D and hyperfiltration leads to an attenuation of GFR via an increase in RA 20,35. Given that hyperfiltration is a promising early marker of progressive diabetic kidney disease and the predominant afferent vasodilatation in the younger cohorts in the current analysis, our results suggest that pharmacologic agents that target RA in patients with shorter duration of T1D may be an important step towards early therapy for the prevention of hyperfiltration and DN. Although long-term renal effects of SGLT2 inhibition in T1D are not known, clinical trials have explored the use of SGLT2 inhibition in T1D for glycemic control 36. The use of SGLT2 inhibitors in patients with T1D is of particular importance given the cardiovascular and renal benefits that have been observed with SGLT2 inhibitors in cohorts of patients with T2D in the EMPA-REG OUTCOME and CANVAS Program trials 37. Background RAAS inhibition therapies were taken by 80% of these patients, suggesting that simultaneous targeting of RA and RE may provide synergistic nephroprotective effects in patients with longstanding diabetes. Another class of agent that acts to increase RA are the glucagon-like peptide-1 agonists (GLP-1RA), which are indicated for glucose control in patients with type 2 diabetes 38. Similarly to SGLT2 inhibitors, 4 year treatment of patients with type 2 diabetes with GLP-1RA in addition to standard care, which largely include RAAS inhibitors, resulted in lower rate of development and progression of diabetic kidney disease and lower rates of new onset of severely increased albuminuria compared to placebo in the LEADER trial 39. For patients with longstanding T1D with or without background CKD, although hyperfiltration at the whole-kidney level is exceedingly rare, hyperfiltration at the single-nephron level is likely to be present in a significant proportion of patients 40 – which should also be ameliorated with SGLT2 inhibition or GLP-1RA treatment. Therefore, based on this physiological rationale, patients with longstanding duration of T1D may also benefit from agents targeting RA – a possibility that merits exploration in future clinical trials.
Our study does have limitations. The relatively homogenous study groups limits the generalizability of our findings to other populations, such as those with proteinuria and overt nephropathy. We also acknowledge that in our older adult T1D group there may be a survivorship bias, where study participants had to survive 50 years with T1D to be eligible for the study. Moreover, improved glycemic control in the last 25 years has been linked to decreasing rate of developing complications in T1D. Therefore, due to the lack of longitudinal data for our study groups, we cannot determine what proportion of study participants, especially in the adolescent and young adult cohort, will eventually develop renal complications. Additionally, the lack of data for healthy control participants without diabetes in our cohort does not allow us to conclusively determine if our observations are a result of diabetes duration or age. Data on RA and RE in healthy controls compared to T1D are scarce. We previously observed that in a group similar to the young adult cohort there were no differences between RA and between RE when comparing T1D and age and sex-matched healthy controls 21. In another young adult cohort, we established that T1D with normofiltration have greater RA and T1D with hyperfiltration had lower RA, while no differences in RE were observed when compared to healthy controls. The data for RA and RE comparisons of our older T1D cohort compared to healthy controls were previously published and showed that RE is greater, but RA does not appear to differ when comparing healthy controls with participants with T1D with resistance to diabetic kidney disease 4. While these previous observations suggest there may be RA differences in the young adult T1D cohort and RE differences in the older T1D cohort compared to healthy controls, a direct comparison of temporal changes in renal hemodynamic function in healthy controls to the changes in T1D would allow us to more accurately tease out the diabetic versus age specific changes. Moreover, our previous findings in healthy controls suggested that age-related exaggerated renal and systemic hemodynamic responses to acute clamped hyperglycemia and ANGII infusions are only observed in T1D, but not in healthy controls 24. Although renal segmental resistances were not estimated in the aforementioned studies, observations suggest that the changes we observe in our cohorts may relate to duration of diabetes. To minimize the effects of small sample size, we used careful pre-study preparation and gold standard methods to quantify renal hemodynamic function. All study measurements were performed under clamped euglycemic conditions, which minimized the effects of our small sample size and concurrent glycemia on measured parameters. It should be noted that we previously showed an increase in GFR under clamped hyperglycemia in young adults with T1D, however our previous studies could not determine whether this observation is related to RA or RE 22. Therefore, the potential impact of hyperglycemia on our observations should be further examined. We also acknowledge that there are baseline differences between groups in glycemic control (HbA1c), blood pressure, heart rate and medication intake, however we believe these differences represent the natural progression of T1D that contribute to the outcomes presented in our analyses. To further limit intra-subject variability, future studies should be conducted that follow kidney function over time in each patient from diagnosis to long-standing T1D.
Additionally, we recognize that Gomez formulae are indirect estimates of intraglomerular hemodynamic parameters and are based on certain physiologic assumptions listed in the methods section and shown in Figure 1. These formulae have been developed in a canine model and are based on assumptions that could be generalized to human physiology. These formulae do not account for changes in intratubular pressure, such as tubular reabsorption of electrolytes or fluid, which may affect the pressure gradient across glomerular capillaries. While original formulae assume a KFG based on normal kidney physiology (Winston’s indirect estimates in a canine model that PGLO is roughly two-thirds of MAP and normal glomerular πG is 25 mmHg), we modified the formulae in an attempt to better reflect the physiology of a diabetic kidney by using the KFG obtained from diabetic Munich-Wistar rat models 23. We do recognize, however, that πG and KFG vary across different stages of kidney disease and albuminuria as the glomerulus increases in permeability with kidney disease progression 41 suggesting that our analysis is limited by using static πG and KFG. Nevertheless, these formulate were applied in a number of healthy and disease human populations in the last decade, including in patients with T1D and appear to reflect hemodynamic changes in renal function 21,22.
Our study uniquely characterises renal hemodynamic changes across a wide range of T1D duration without DN and in response to an ANGII infusion. T1D appears to first impact resistance at the afferent arteriole, which increases with longer duration of T1D. In contrast, the efferent arteriole resistance increases significantly only with longstanding T1D duration. Longer duration of T1D diabetes is associated with lower GFR, ERPF, elevated RVR, and evidence of RAAS activation as assessed by an infusion of ANGII and plasma renin and aldosterone levels. Further work is required to understand the potential role of non-RAAS, pharmacological agents that target RA as a primary prevention strategy in patients with short-standing T1D and as an additive nephroprotective strategy in patients with longstanding T1D when used with classic medications.
Supplementary Material
Acknowledgments
FUNDING: The Canadian Study of Longevity in Diabetes was funded by the JDRF (Operating Grant No. 17-2013-312). Y.L. is supported by a Canadian Diabetes Association (Diabetes Canada) Fellowship. J.A.L. was supported by a CaRE (Cardio-Renal-Endocrine) post-doctoral fellowship by the Division of Nephrology, Department of Medicine, University Health Network, University of Toronto, Toronto, Ontario, Canada. A.A. is a recipient of a Diabetes Investigator Award from Diabetes Canada. We acknowledge the contributions of the Steven and Ofra Menkes Fund for supporting aspects of this research. The authors acknowledge unrestricted financial support from the Boehringer Ingelheim Beta-Cell Preservation, Function and Regeneration project at Mount Sinai Hospital. The authors acknowledge unrestricted financial support from Bank of Montreal for the diabetes complications assessment unit. The funders of the study did not have a role in the design, collection, analysis and interpretation of the data presented in this manuscript nor did they have the decision to submit this report for publication.
DISCLOSURES: P.B. receives salary and research support by NIH/NIDDK (T32-DK063687, K23 DK116720-01), in addition to research support by Thrasher Foundation, Juvenile Diabetes Research Foundation (JDRF), International Society of Pediatric and Adolescent Diabetes (ISPAD), Colorado Clinical & Translational Sciences Institute (CCTSI) and Center for Women’s Health Research at University of Colorado. J.A.L. has received either consulting fees or speaking honorarium or both from Novo Nordisk, Eli Lilly & Co, Merck Sharp & Dohme, and AstraZeneca. G.B. has received speaker honoraria from Johnson & Johnson. A.A. has received research support from Boehringer Ingelheim and AstraZeneca, is listed as an inventor on a patent application (WO 2015/128453) submitted by Boehringer Ingelheim and has participated in advisory boards for Eli Lilly/Boehringer Ingelheim, Novo Nordisk, Abbott and Dexcom. B.A.P. has received speaker honoraria from Medtronic, Johnson & Johnson, Roche, GlaxoSmithKline Canada, Novo Nordisk, and Sanofi; has received research grant support from Medtronic and Boehringer Ingelheim; and serves as a consultant for NeuroMetrix. D.Z.I.C. has received consulting fees or speaking honorarium or both from Janssen, Boehringer Ingelheim-Eli, Lilly, AstraZeneca, Merck, and Sanofi, and has received operating funds from Janssen, Boehringer Ingelheim-Eli, Lilly, AstraZeneca and Merck.
REFERENCES
- 1.DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391(10138):2449–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathan DM, Zinman B, Cleary PA, et al. Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983–2005). Archives of internal medicine. 2009;169(14):1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2010;53(11):2312–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovshin JA, Boulet G, Lytvyn Y, et al. Renin-angiotensin-aldosterone system activation in long-standing type 1 diabetes. JCI Insight. 2018;3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia. 2009;52(4):691–697. [DOI] [PubMed] [Google Scholar]
- 6.Mauer M, Zinman B, Gardiner R, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361(1):40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kostraba JN, Dorman JS, Orchard TJ, et al. Contribution of diabetes duration before puberty to development of microvascular complications in IDDM subjects. Diabetes care. 1989;12(10):686–693. [DOI] [PubMed] [Google Scholar]
- 8.Lawson ML, Sochett EB, Chait PG, Balfe JW, Daneman D. Effect of puberty on markers of glomerular hypertrophy and hypertension in IDDM. Diabetes. 1996;45(1):51–55. [DOI] [PubMed] [Google Scholar]
- 9.Marcovecchio ML, Chiesa ST, Bond S, et al. ACE Inhibitors and Statins in Adolescents with Type 1 Diabetes. N Engl J Med. 2017;377(18):1733–1745. [DOI] [PubMed] [Google Scholar]
- 10.Bjornstad P, Skrtic M, Lytvyn Y, Maahs DM, Johnson RJ, Cherney DZ. The Gomez’ equations and renal hemodynamic function in kidney disease research. American journal of physiology Renal physiology. 2016;311(5):F967–F975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sochett EB, Cherney DZ, Curtis JR, Dekker MG, Scholey JW, Miller JA. Impact of renin angiotensin system modulation on the hyperfiltration state in type 1 diabetes. J Am Soc Nephrol. 2006;17(6):1703–1709. [DOI] [PubMed] [Google Scholar]
- 12.Lytvyn Y, Har R, Locke A, et al. Renal and Vascular Effects of Uric Acid Lowering in Normouricemic Patients With Uncomplicated Type 1 Diabetes. Diabetes. 2017;66(7):1939–1949. [DOI] [PubMed] [Google Scholar]
- 13.Cherney DZ, Sochett EB, Miller JA. Gender differences in renal responses to hyperglycemia and angiotensin-converting enzyme inhibition in diabetes. Kidney Int. 2005;68(4):1722–1728. [DOI] [PubMed] [Google Scholar]
- 14.Miller JA, Curtis JR, Sochett EB. Relationship between diurnal blood pressure, renal hemodynamic function, and the renin-angiotensin system in type 1 diabetes. Diabetes. 2003;52(7):1806–1811. [DOI] [PubMed] [Google Scholar]
- 15.Cherney DZ, Miller JA, Scholey JW, et al. The effect of cyclooxygenase-2 inhibition on renal hemodynamic function in humans with type 1 diabetes. Diabetes. 2008;57(3):688–695. [DOI] [PubMed] [Google Scholar]
- 16.Cherney DZ, Scholey JW, Nasrallah R, et al. Renal hemodynamic effect of cyclooxygenase 2 inhibition in young men and women with uncomplicated type 1 diabetes mellitus. American journal of physiology Renal physiology. 2008;294(6):F1336–1341. [DOI] [PubMed] [Google Scholar]
- 17.Cherney DZ, Miller JA, Scholey JW, et al. Renal hyperfiltration is a determinant of endothelial function responses to cyclooxygenase 2 inhibition in type 1 diabetes. Diabetes Care. 2010;33(6):1344–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherney DZ, Reich HN, Jiang S, et al. Hyperfiltration and effect of nitric oxide inhibition on renal and endothelial function in humans with uncomplicated type 1 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol. 2012;303(7):R710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez DM. Evaluation of renal resistances, with special reference to changes in essential hypertension. The Journal of clinical investigation. 1951;30(10):1143–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skrtic M, Yang GK, Perkins BA, et al. Characterisation of glomerular haemodynamic responses to SGLT2 inhibition in patients with type 1 diabetes and renal hyperfiltration. Diabetologia. 2014;57(12):2599–2602. [DOI] [PubMed] [Google Scholar]
- 21.Lytvyn Y, Skrtic M, Yang GK, et al. Plasma uric acid effects on glomerular haemodynamic profile of patients with uncomplicated Type 1 diabetes mellitus. Diabet Med. 2016;33(8):1102–1111. [DOI] [PubMed] [Google Scholar]
- 22.Skrtic M, Lytvyn Y, Yang GK, et al. Glomerular haemodynamic profile of patients with Type 1 diabetes compared with healthy control subjects. Diabet Med. 2015;32(7):972–979. [DOI] [PubMed] [Google Scholar]
- 23.Hostetter TH, Troy JL, Brenner BM. Glomerular hemodynamics in experimental diabetes mellitus. Kidney international. 1981;19(3):410–415. [DOI] [PubMed] [Google Scholar]
- 24.Cherney DZ, Reich HN, Miller JA, et al. Age is a determinant of acute hemodynamic responses to hyperglycemia and angiotensin II in humans with uncomplicated type 1 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol. 2010;299(1):R206–214. [DOI] [PubMed] [Google Scholar]
- 25.de Zeeuw D, Akizawa T, Audhya P, et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. The New England journal of medicine. 2013;369(26):2492–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuttle KR, McGill JB, Haney DJ, Lin TE, Anderson PW. Kidney outcomes in long-term studies of ruboxistaurin for diabetic eye disease. Clinical journal of the American Society of Nephrology : CJASN. 2007;2(4):631–636. [DOI] [PubMed] [Google Scholar]
- 27.Miller JA. Impact of hyperglycemia on the renin angiotensin system in early human type 1 diabetes mellitus. J Am Soc Nephrol. 1999;10(8):1778–1785. [DOI] [PubMed] [Google Scholar]
- 28.Cherney DZ, Reich HN, Scholey JW, et al. The effect of aliskiren on urinary cytokine/chemokine responses to clamped hyperglycaemia in type 1 diabetes. Diabetologia. 2013;56(10):2308–2317. [DOI] [PubMed] [Google Scholar]
- 29.Cherney DZ, Scholey JW, Miller JA. Insights into the regulation of renal hemodynamic function in diabetic mellitus. Curr Diabetes Rev. 2008;4(4):280–290. [DOI] [PubMed] [Google Scholar]
- 30.Cherney DZ, Scholey JW, Sochett E, Bradley TJ, Reich HN. The acute effect of clamped hyperglycemia on the urinary excretion of inflammatory cytokines/chemokines in uncomplicated type 1 diabetes: a pilot study. Diabetes Care. 2011;34(1):177–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollenberg NK, Price DA, Fisher ND, et al. Glomerular hemodynamics and the renin-angiotensin system in patients with type 1 diabetes mellitus. Kidney Int. 2003;63(1):172–178. [DOI] [PubMed] [Google Scholar]
- 32.Schultz CJ, Konopelska-Bahu T, Dalton RN, et al. Microalbuminuria prevalence varies with age, sex, and puberty in children with type 1 diabetes followed from diagnosis in a longitudinal study. Oxford Regional Prospective Study Group. Diabetes care. 1999;22(3):495–502. [DOI] [PubMed] [Google Scholar]
- 33.Quattrin T, Waz WR, Duffy LC, et al. Microalbuminuria in an adolescent cohort with insulin-dependent diabetes mellitus. Clinical pediatrics. 1995;34(1):12–17. [DOI] [PubMed] [Google Scholar]
- 34.Zatz R, Dunn BR, Meyer TW, Anderson S, Rennke HG, Brenner BM. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J Clin Invest. 1986;77(6):1925–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129(5):587–597. [DOI] [PubMed] [Google Scholar]
- 36.Garg SK, Henry RR, Banks P, et al. Effects of Sotagliflozin Added to Insulin in Patients with Type 1 Diabetes. The New England journal of medicine. 2017;377(24):2337–2348. [DOI] [PubMed] [Google Scholar]
- 37.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375(4):323–334. [DOI] [PubMed] [Google Scholar]
- 38.Tonneijck L, Smits MM, Muskiet MHA, et al. Acute renal effects of the GLP-1 receptor agonist exenatide in overweight type 2 diabetes patients: a randomised, double-blind, placebo-controlled trial. Diabetologia. 2016;59(7):1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mann JFE, Orsted DD, Brown-Frandsen K, et al. Liraglutide and Renal Outcomes in Type 2 Diabetes. N Engl J Med. 2017;377(9):839–848. [DOI] [PubMed] [Google Scholar]
- 40.Denic A, Mathew J, Lerman LO, et al. Single-Nephron Glomerular Filtration Rate in Healthy Adults. N Engl J Med. 2017;376(24):2349–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomlanovich S, Deen WM, Jones HW, 3rd, Schwartz HC, Myers BD. Functional nature of glomerular injury in progressive diabetic glomerulopathy. Diabetes. 1987;36(5):556–565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.