Abstract
Good symptom management in oncology is associated with improved patient and family quality of life, greater treatment compliance, and may even offer survival advantages. With population growth and aging, the proportion of patients with multiple symptoms—both related and unrelated to their cancer—is anticipated to increase, supporting calls for a more routine and integrated approach to symptom management. This article presents a summary of the literature for the use of symptom assessment tools and reviews the management of four common and distressing symptoms commonly experienced by people with advanced cancer: pain, breathlessness, nausea and vomiting, and fatigue. We also discuss the role of palliative care in supporting a holistic approach to symptom management throughout the cancer trajectory.
INTRODUCTION
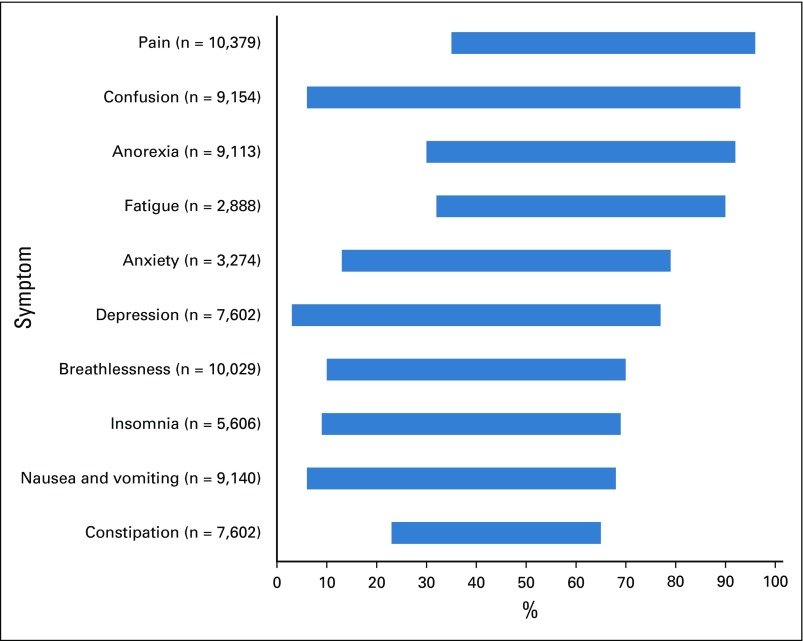
Most patients with cancer experience symptoms, the prevalence and severity of which vary according to cancer type, stage, treatment(s), and comorbidities.1-4 In advanced cancer, 35% to 96% of patients experience pain, 32% to 90% experience fatigue, and 10% to 70% experience breathlessness (Fig 1).2
FIG 1.
Minimum-maximum symptom prevalence (%) for patients with cancer (n = total number of patients involved in the studies for each symptom). Adapted from systematic review findings of Solano et al.1
Patients typically experience more than one symptom at any one time.5 Those with metastatic cancer and breathlessness (as a marker of advanced disease) have, on average, 14 symptoms.6 Grond et al5 found that 94% of those referred to a cancer pain clinic experienced additional symptoms, with 15% reporting at least five. Symptoms can be caused by the cancer itself, direct or indirect consequences of the cancer, early or late adverse effects of treatment, and/or comorbid conditions.7 The last two causes are becoming increasingly common as treatments advance and the population ages. Accurate symptom assessment and diagnosis are essential for effective treatment.8 Good symptom management is associated with improved patient and family quality of life,9-11 greater treatment compliance,12,13 and may even offer survival advantages.13-17 Yet, despite these benefits, pain and other symptoms remain poorly managed and/or undertreated in many cases, highlighting the need for additional improvements in care.18-20
This article provides a summary of symptom assessment tools and reviews the management of four common and distressing symptoms frequently experienced by patients with advanced cancer: pain, breathlessness, nausea and vomiting, and fatigue. We also discuss the role of palliative care in supporting a holistic approach to symptom management throughout the cancer trajectory.
SYMPTOM ASSESSMENT TOOLS
Many symptom assessment tools are available for patients with advanced cancer, as exemplified by a survey of palliative care professionals (n = 331), in which 99 tools for clinical practice and 94 for research were identified.21 These assessment tools differ in various aspects, such as symptom selection, the inclusion of global quality-of-life questions, measurement of function, type of assessment scales (ie, visual analog v numeric rating scale), and validation for research and/or clinical practice. Commonly cited tools for both clinical practice and research are the Edmonton Symptom Assessment System Revised (ESAS-r),22,23 the Palliative Care Outcome Scale (POS),24,25 and the Palliative Performance Scale.26
The incorporation of patient-reported outcome measures (PROMs) into routine clinical practice is supported by evidence that they improve symptom assessment and monitoring over time, help identify patients’ unmet needs or concerns, and assist clinicians with decision making and treatment planning.27-31 Examples of PROMs for patients with advanced cancer include the ESAS-r23 and the POS.25 The ESAS-r replaces the original ESAS that was first developed by Bruera et al22 in 1991. ESAS-r is a self-report tool, designed to capture multidimensional symptom profiles over time. It uses 11-point numeric rating scales to measure the intensity of nine symptoms (pain, tiredness, nausea, depression, anxiety, drowsiness, appetite, well-being, shortness of breath), and includes the option of measuring an additional patient-specific symptom. ESAS-r is validated for self- and proxy-reporting, has guidance on interpreting its numeric rating scale,23 and has transcultural adaptation for use in low- and middle-income countries.32
The POS was initially developed in 1999 as a tool to measure the palliative care needs of patients and their families. It has 10 items covering physical symptoms (n = 2), psychological symptoms (n = 2), spiritual considerations (n = 1), social needs (n = 4), and carer concerns (n = 1), with each item scored using a 0 to 4 (Likert) scale, with numeric and descriptive labels. The POS also includes a free text section, where patients are asked, “If any, what have been your main problems in the last three days?”33 A number of adapted POS versions now exist, including those specific to certain populations, for example, MyPOS for patients with myeloma.34 Transcultural adaptations are also available for more than 13 countries.35,36 All POS versions are validated for self- and proxy-reporting and have a clinical decision support tool to aid use.37
For older patients with advanced cancer, Van Lancker et al38 developed and validated the Assessment Symptoms Palliative Elderly, a 36-item instrument to assess symptom frequency and intensity. Although longer than ESAS-r and POS, the tool places a greater emphasis on assessing function, social symptoms, and psychological issues, all of which are prominent areas of concern for older patients with cancer.
Use of technology is likely to be an important and key component to implementing PROMs into routine clinical practice.39 Examples where integration has been successful include home asthma telemonitoring systems40 and Web-based support platforms for patients with insulin-dependent diabetes.41 In an oncology setting, Velikova et al42 found that regular repeated assessment of health-related quality of life of patients with cancer (using touch-screen computers in outpatient clinics) improved patients’ emotional well-being and resulted in more frequent discussions of chronic nonspecific symptoms. Similarly, in a randomized trial of 766 patients with metastatic cancer, Basch et al30,31 found that those receiving care incorporating routine patient-reported electronic monitoring of symptoms had greater improvements in health-related quality of life (34% v 18%), fewer emergency department visits or admissions to hospital (34% v 41% and 45% v 49%, respectively), and longer quality-adjusted survival (mean, 8.7 months v 8.0 months) compared with those receiving usual care. In a study of patients with lung cancer, Denis et al43-45 investigated the E-MOSAIC intervention, which used real-time electronic monitoring of PROMs, with measures completed weekly by participants on a palm-based device (ClinicalTrials.gov identifier: NCT00477919). They found that weekly reporting of symptoms resulted in improved survival compared with those receiving usual care (median survival, 22.4 months v 16.7 months). Compared with the control group, those in the intervention group also had greater improvements in symptoms, communication, and coping, but not overall quality of life.46
SYMPTOM MANAGEMENT
High-quality symptom assessment and management are fundamental to providing holistic, patient-centered care that results in positive outcomes for patients and their families. Despite their ubiquity, most symptoms experienced by patients with advanced cancer can be effectively managed using pharmacologic and/or nonpharmacologic approaches; symptom assessment and management is therefore an expected core skill of all clinicians involved in caring for patients with cancer. The following section reviews the latest evidence for the management of four symptoms commonly experienced by patients with advanced cancer: pain, breathlessness, nausea and vomiting, and fatigue.
Pain
Despite its ubiquity and the availability of management guidelines, more than 30% of patients with cancer receive inadequate analgesia for pain.47 Identifying the pain modality (nociceptive, neuropathic, or combined) helps direct effective therapy, with the WHO analgesic ladder providing a therapeutic framework.48
Nonpharmacologic treatments in the management of cancer pain include physical (massage, aromatherapy, transcutaneous electrical nerve stimulation, and acupuncture) and cognitive modalities (relaxation, distraction, and imagery exercises).49 Evidence to support the effectiveness of aromatherapy,50 transcutaneous electrical nerve stimulation,51 and acupuncture52 for reducing pain intensity in patients with cancer is lacking. For massage, study findings have been mixed, although most positive effects are not sustained beyond the intervention period or immediately after it.49 For cognitive modalities, evidence supports immediate reductions in pain intensity; however, similar to the finding for massage, evidence to support sustained reductions in pain are lacking.53,54
For the pharmacologic management of mild to moderate pain, nonopioid analgesics, such as acetaminophen and nonsteroidal anti-inflammatory drugs, remain widely used in clinical practice. Opioids for mild to moderate pain are added at step two of the WHO analgesic ladder. Codeine phosphate and/or tramadol are commonly used, although this is supported by limited evidence. Two recent Cochrane reviews found only weak evidence to support their use,55,56 and some authors have suggested bypassing step two of the WHO analgesic ladder and proceeding directly from step one to step three.57,58 Opioids, specifically morphine, remain the first-choice analgesic for moderate to severe cancer-related pain. A 2017 review of nine systematic reviews, (incorporating 152 individual studies) examining any opioid for cancer-related pain found that, on average, 19 of 20 patients with cancer with moderate or severe pain who receive opioids and can tolerate them will have their pain reduced to mild or no pain within 14 days.59 Opioids should be given orally where possible and titrated individually to the lowest effective, tolerable dose.58 Either immediate- or modified-release preparations can be prescribed regularly, with immediate-release preparations also available as required for breakthrough pain.60 No evidence of superiority across opioids for moderate to severe pain exists: morphine remains the first-line opioid of choice in international guidance because of its familiarity, availability, and cost.58 Fentanyl and buprenorphine are recommended in renal impairment (estimated glomerular filtration rate < 30)58 when morphine is contraindicated.61 Despite limited evidence, switching between opioid preparations is common practice when the first-line opioid chosen is ineffective or poorly tolerated.62 Adverse effects from opioid therapy are common and predictable.59 Constipation, nausea, and vomiting are most commonly reported, and guidelines recommend the use of laxatives with all opioid prescriptions.58
More than 20% of patients with cancer experience neuropathic pain.63 Although anticonvulsant or antidepressant medications are considered standard treatment of nonmalignant neuropathic pain, evidence for their effectiveness in cancer is mixed. A meta-analysis of four randomized controlled trials (RCTs) found no analgesic benefit from adding pregabalin or gabapentin to opioids in patients with cancer-related pain.64 By comparison, Jongen et al65 found antidepressants and anticonvulsants effective and well tolerated in patients with confirmed cancer-related neuropathic pain, and in a separate double-blind crossover RCT, an opioid-sparing effect was found with the addition of pregabalin to opioid therapy.66 As such, neuropathic pain agents remain recommended in international pain guidelines and should be considered an appropriate adjunct to opioids for patients with neuropathic pain.58 Ketamine has not been shown to be effective for patients with cancer-related neuropathic pain, although it may have a role in a specific subsection of patients with hyperalgesia.67,68 A Cochrane systematic review found a lack of evidence to support the use of methadone as an adjuvant for cancer-related neuropathic pain.69 Limited data suggest that it may have a role in patients with cancer-related pain unresponsive to morphine or other opioids.70 Its complex pharmacology necessitates use by specialist physicians.
There is mixed evidence to support bisphosphonates for cancer-related bone pain. One systematic review found that 22 of 28 RCTs did not identify any analgesic benefit.71 However, a meta-analysis of studies including only patients with multiple myeloma (n = 8) found a difference in amelioration of pain with use of bisphosphonates compared with placebo or no treatment (pooled risk ratio, 0.75; 95% CI, 0.60 to 0.95), although the overall quality of evidence was found to be low.72 By comparison, radiotherapy has been shown to be highly effective in the management of cancer-related bone pain. A meta-analysis found that almost a third of those treated with radiotherapy experienced total resolution of pain at 4 weeks, with a single fraction of 8 Gy being effective.73 Specialist pain interventions, for example, nerve blocks, should be considered in patients with moderate to severe pain refractory to standard pharmacologic treatments.58
Breathlessness
Breathlessness is a common symptom that becomes increasingly prevalent as disease progresses.74,75 Refractory breathlessness (that which persists despite optimal treatment of the underlying condition) is associated with a shortened life expectancy,76,77 can be especially frightening for patients and families,78,79 and often results in use of acute hospital services.80-83 Despite increased understanding of the mechanisms of breathlessness, new and effective treatment options remain elusive.84 Thus, clinicians also experience distress when faced with refractory breathlessness because of the limited availability of effective interventions.6,78,79
Management should start with optimizing the treatment of any underlying causes of breathlessness, especially bronchoconstriction. Nonpharmacologic treatments should then be considered, in particular, positioning and breathing techniques, mobility aids, and muscle strengthening.85 The importance and potential effectiveness of simple aids, such as a hand-held fan, should also not be overlooked.86 For pharmacologic treatments, the European Respiratory Society and American Thoracic Society have both concluded that beyond oxygen and opioids, there is no robust evidence for other pharmacologic agents.84,87 Oxygen has a clear and accepted role for patients with hypoxia. However, in patients with mild or nonhypoxemic breathlessness, the benefit derived from oxygen is similar to medical air, and there are limitations to its use (eg, safety, cost).88,89 Relevant systematic reviews of effectiveness and clinical trials are available for opioids, oxygen, and benzodiazepines.88,90-95 Although opioids by mouth and injection can reduce breathlessness, their effects are modest or small, and the optimal dosing, titration, and potential issues arising from long-term use (eg, safety, tolerance, dependence, misuse) remain to be determined.90,96 The evidence from Cochrane reviews does not support a role for benzodiazepines, except as second- or third-line treatment if opioids fail, because there is no overall evidence of benefit and some evidence of possible harms.94
Holistic breathlessness services combine tailored nonpharmacologic and pharmacologic breathlessness management,97 typically with input from multiple specialties and professions (eg, medicine, nursing, physiotherapy).98 Such services represent an evidence-based means for early integration of palliative care on the basis of need rather than prognosis.99 They are highly valued by patients and their carers and overall can lead to significant improvements in distress due to breathlessness and aspects of psychological health, including depression.97 In a single-blind randomized trial, Higginson et al98 assessed the effectiveness of a new breathlessness support service compared with usual care for patients with advanced disease and refractory breathlessness. The service comprised an initial outpatient clinic appointment with respiratory medicine and palliative care clinicians during which time patients were also provided with a breathlessness pack that included information, management, and pacing guidance, a hand-held fan or water spray, a poem (a short mantra to help breathing and relaxation during crises), and an individualized crisis management plan. Approximately 2 to 3 weeks later, patients received a home visit by a physiotherapist and/or occupational therapist to assess their need for aids and adaptations, as well as to reinforce self-management of breathlessness and provide additional guidance on pacing and exercises. A second and final outpatient appointment with a palliative care specialist followed and allowed any additional actions to be incorporated and a discharge plan to be developed.98 The study found significant improvements in breathlessness mastery in the breathlessness support service group compared with the control group (mean difference, 0.58; 95% CI, 0.01 to 1.15), as well as improvements in overall survival for patients with chronic obstructive pulmonary disease and interstitial lung disease but not cancer.98
Nausea and Vomiting
Nausea, defined as the unpleasant subjective feeling of wanting to vomit or retch, and/or vomiting,100 are experienced by as many as 68% of patients with cancer at some point during their illness; during the last 6 weeks of life, the prevalence of nausea and vomiting is 40% or more.101 Poorly controlled nausea and vomiting is associated with physical, cognitive, and psychosocial distress, and can contribute to patient and family fears of death from dehydration and/or starvation.102,103
Nausea and vomiting secondary to antineoplastic agents or radiation therapy should be anticipated and managed according to ASCO antiemetic, or equivalent, clinical practice guidelines.104 The latest ASCO antiemetic update includes evidence-based recommendations and information on the appropriate use of olanzapine, neurokinin 1 receptor antagonists, and use of subcutaneous 5-hydroxytryptamine-3 receptor antagonists.104
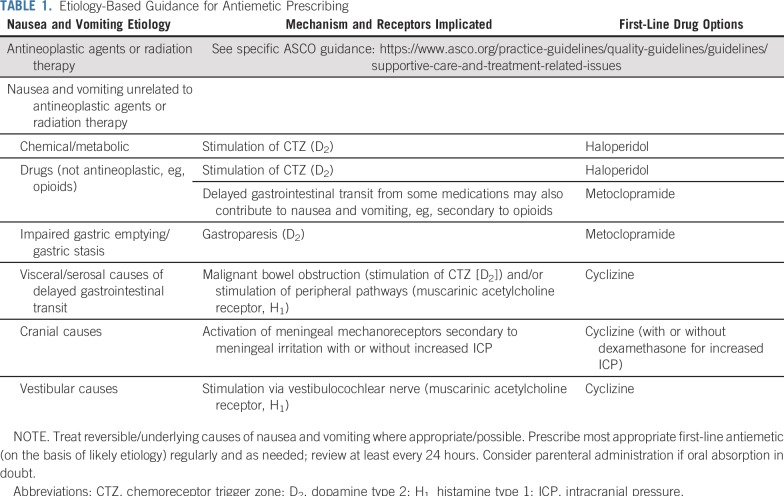
Much less trial evidence is available for the use of antiemetics in patients with advanced cancer and nausea and vomiting unrelated to antineoplastic agents or radiation therapy.105,106 Instead, an etiologic or mechanism-based approach to choosing an antiemetic is commonly recommended.107 This approach requires clinicians to take a detailed history and perform a focused examination to determine the most likely underlying cause(s) of the patient’s nausea and vomiting. In the advanced cancer population, the most common underlying causes of nausea and vomiting are chemical abnormalities (eg, renal or liver failure, hyponatremia, hypercalcemia); drugs (eg, opioids, antidepressants, antibiotics); infection; and impaired gastric emptying, as well as visceral and serosal causes of delayed gastrointestinal transit (bowel obstruction, gastric bleed, enteritis, constipation).108-111 Once the most likely underlying cause of the patient’s nausea and vomiting is determined, an appropriate antiemetic can then be selected based on the pathophysiology and receptors implicated (Table 1).108
TABLE 1.
Etiology-Based Guidance for Antiemetic Prescribing
Unless contraindicated, antiemetics should be prescribed regularly and with a low threshold for being administered parenterally. If, despite titration, treatment with a single agent remains ineffective, a second-line antiemetic should be commenced. The addition of a second-line antiemetic is preferred over switching, because cancer-related nausea and vomiting is often multifactorial, involving multiple neurotransmitters and receptor sites. Currently, only limited and low-quality evidence exists for the use of corticosteroids,112 olanzapine,113 and cannabinoids114,115 for nausea and vomiting that is not secondary to antineoplastic agents or radiation therapy. Nonpharmacologic measures, such as dietary advice,116 psychological services,117 and acupuncture/acupressure,118 may offer some benefit when used alongside standard pharmacologic approaches, although again, evidence of their effectiveness for nausea and vomiting unrelated to antineoplastic treatment is limited.
Fatigue
Fatigue is “a subjective, unpleasant symptom which incorporates total body feelings ranging from tiredness to exhaustion creating an unrelenting overall condition which interferes with individuals’ ability to function to their normal capacity.”119(p527) The severe and unrelenting nature of fatigue negatively affects patients and those close to them.120,121 It is highly prevalent, affecting three quarters of patients with advanced cancer, perhaps related to the proinflammatory state that plays a role in its pathogenesis.122 Other contributing factors include anemia, malnutrition, neuro-endocrine impairment, and muscle dysfunction.123 Assessment of fatigue can be via single-item tools (eg, 0 to 10 numeric rating scale), unidimensional (eg, Functional Assessment of Cancer Therapy: Fatigue), or multidimensional (eg, European Organisation for Research and Treatment of Cancer QLQ-FA13 and Chalder Fatigue Scale) scales (for a comprehensive review, see Minton and Stone124).
Both nonpharmacologic and pharmacologic treatments for fatigue are available. These should be considered once treatment of any underlying/reversible causes of fatigue have been optimized. Proactive monitoring and protocolized management of physical symptoms can improve general fatigue, as well as affect activity levels and motivation.125 The use of exercise for fatigue is supported both during and after anticancer treatment (standardized mean difference [SMD], −0.27; 95% CI, −0.37 to −0.17),126 with consistent secondary effects on depression and sleep quality,127 although most studies are limited to patients with primary breast cancer receiving adjuvant chemotherapy or patients with prostate cancer. The strongest evidence is for aerobic exercise (eg, walking, cycling). Resistance training may have an additional role in cancers where cachexia is highly prevalent, for example, lung and pancreatic, although studies for these groups are fewer in number and smaller.126
Evidence is relatively weaker and less consistent for other nonpharmacologic treatments, although arguably more applicable, being limited to those with advanced, incurable disease. There is restricted support for psychosocial interventions, including cognitive behavioral or expressive group therapies (SMD, −0.25; −0.50 to 0.00),128 although benefit has been found after cancer treatment.129 Educational interventions involving information giving with reinforcement or problem-solving led to small improvements in fatigue intensity (SMD, −0.28; 95% CI, −0.52 to −0.04), general fatigue, distress related to fatigue, and interference with daily life.130 Music interventions also led to a small-to-moderate effect (SMD, −0.38; 95% CI, −0.72 to −0.04), although some studies feature a high risk of bias, including inadequate randomization, and the anxiety-relieving effect is stronger.131 There is insufficient evidence for complementary and alternative medicines, including acupuncture and hypnosis.132,133
Once nonpharmacologic treatments have been used, the psychostimulant methylphenidate can be considered.134,135 In 2011, a meta-analysis of five psychostimulant trials, four of which related to methylphenidate, found an overall SMD for psychostimulant use of −0.28 (95% CI, −0.48 to −0.09).136 However, more recent and larger RCTs have found methylphenidate to be ineffective for the management of cancer-related fatigue, with evidence of benefit limited to patients with narcotic-induced fatigue and/or depression.137-142 Evidence for modafinil is mixed, with two trials concluding either no benefit143 or benefit only with severe fatigue.144 For patients with anemia, including during chemotherapy, the hemopoietic growth factor erythropoietin reduces fatigue (SMD, −0.36; 95% CI, −0.46 to −0.26), whereas evidence for darbepoetin is less consistent.145 Single trials support short-term use of dexamethasone, although efficacy and safety beyond 2 weeks are undetermined.146 There is currently no evidence of benefit from l-carnitine supplementation,147 progestational steroids, or paroxetine.145
In summary, most patients with advanced cancer experience symptoms throughout the disease trajectory, often with greater intensity as death approaches. If poorly managed, such symptoms can have a considerable impact on patients’ ability to function, quality of life, ability to comply with anticancer treatments, and use of health care resources. All clinicians involved in the care of patients with cancer should be competent in symptom assessment and management. For complex, multiple, and/or refractory symptoms, patients may benefit from additional support services, such as those provided by specialist palliative care. Multidisciplinary palliative care teams have been shown to improve patient outcomes,148-150 and current ASCO guidelines therefore recommend that patients with advanced cancer should receive dedicated palliative care services, early in the disease course, and concurrent with active treatment.151 At a service level, managers and policymakers should consider incorporating routine screening of symptoms into usual care structures, with evidence that symptom assessment tools can improve patient outcomes and possibly even survival.
AUTHOR CONTRIBUTIONS
Conception and design: Lesley A. Henson, Matthew Maddocks, Catherine Evans, Irene J. Higginson
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Palliative Care and the Management of Common Distressing Symptoms in Advanced Cancer: Pain, Breathlessness, Nausea and Vomiting, and Fatigue
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Matthew Maddocks
Consulting or Advisory Role: Helsinn Therapeutics
No other potential conflicts of interest were reported.
REFERENCES
- 1.J Pain Symptom Manage. 1996;12:3–10. doi: 10.1016/0885-3924(96)00042-5. Vainio A, Auvinen A: Prevalence of symptoms among patients with advanced cancer: An international collaborative study. Symptom Prevalence Group. [DOI] [PubMed] [Google Scholar]
- 2.Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage. 2006;31:58–69. doi: 10.1016/j.jpainsymman.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Al Qadire M, Al Khalaileh M. Prevalence of symptoms and quality of life among Jordanian cancer patients. Clin Nurs Res. 2016;25:174–191. doi: 10.1177/1054773814564212. [DOI] [PubMed] [Google Scholar]
- 4.Chiu TY, Hu WY, Chen CY. Prevalence and severity of symptoms in terminal cancer patients: A study in Taiwan. Support Care Cancer. 2000;8:311–313. doi: 10.1007/s005209900112. [DOI] [PubMed] [Google Scholar]
- 5.Grond S, Zech D, Diefenbach C, et al. Prevalence and pattern of symptoms in patients with cancer pain: A prospective evaluation of 1635 cancer patients referred to a pain clinic. J Pain Symptom Manage. 1994;9:372–382. doi: 10.1016/0885-3924(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 6.Bausewein C, Booth S, Gysels M, et al. Understanding breathlessness: Cross-sectional comparison of symptom burden and palliative care needs in chronic obstructive pulmonary disease and cancer. J Palliat Med. 2010;13:1109–1118. doi: 10.1089/jpm.2010.0068. [DOI] [PubMed] [Google Scholar]
- 7.Twycross R, Harcourt J, Bergl S. A survey of pain in patients with advanced cancer. J Pain Symptom Manage. 1996;12:273–282. doi: 10.1016/s0885-3924(96)00149-2. [DOI] [PubMed] [Google Scholar]
- 8.Higginson IJ, Costantini M. Dying with cancer, living well with advanced cancer. Eur J Cancer. 2008;44:1414–1424. doi: 10.1016/j.ejca.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 9.Kehl KA. Moving toward peace: An analysis of the concept of a good death. Am J Hosp Palliat Care. 2006;23:277–286. doi: 10.1177/1049909106290380. [DOI] [PubMed] [Google Scholar]
- 10.Portenoy RK, Thaler HT, Kornblith AB, et al. Symptom prevalence, characteristics and distress in a cancer population. Qual Life Res. 1994;3:183–189. doi: 10.1007/BF00435383. [DOI] [PubMed] [Google Scholar]
- 11.Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;291:88–93. doi: 10.1001/jama.291.1.88. [DOI] [PubMed] [Google Scholar]
- 12.Cheville AL, Alberts SR, Rummans TA, et al. Improving adherence to cancer treatment by addressing quality of life in patients with advanced gastrointestinal cancers. J Pain Symptom Manage. 2015;50:321–327. doi: 10.1016/j.jpainsymman.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruera E, Yennurajalingam S. Palliative care in advanced cancer patients: How and when? Oncologist. 2012;17:267–273. doi: 10.1634/theoncologist.2011-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faller H, Bülzebruck H, Drings P, et al. Coping, distress, and survival among patients with lung cancer. Arch Gen Psychiatry. 1999;56:756–762. doi: 10.1001/archpsyc.56.8.756. [DOI] [PubMed] [Google Scholar]
- 15.Irwin KE, Greer JA, Khatib J, et al. Early palliative care and metastatic non-small cell lung cancer: Potential mechanisms of prolonged survival. Chron Respir Dis. 2013;10:35–47. doi: 10.1177/1479972312471549. [DOI] [PubMed] [Google Scholar]
- 16.Pinquart M, Duberstein PR. Depression and cancer mortality: A meta-analysis. Psychol Med. 2010;40:1797–1810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 18.Addington-Hall J, McCarthy M. Dying from cancer: Results of a national population-based investigation. Palliat Med. 1995;9:295–305. doi: 10.1177/026921639500900404. [DOI] [PubMed] [Google Scholar]
- 19.Breuer B, Fleishman SB, Cruciani RA, et al. Medical oncologists’ attitudes and practice in cancer pain management: A national survey. J Clin Oncol. 2011;29:4769–4775. doi: 10.1200/JCO.2011.35.0561. [DOI] [PubMed] [Google Scholar]
- 20.Deandrea S, Montanari M, Moja L, et al. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19:1985–1991. doi: 10.1093/annonc/mdn419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harding R, Simon ST, Benalia H, et al. The PRISMA Symposium 1: Outcome tool use. Disharmony in European outcomes research for palliative and advanced disease care: Too many tools in practice. J Pain Symptom Manage. 2011;42:493–500. doi: 10.1016/j.jpainsymman.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 23.Watanabe SM, Nekolaichuk C, Beaumont C, et al. A multicenter study comparing two numerical versions of the Edmonton Symptom Assessment System in palliative care patients. J Pain Symptom Manage. 2011;41:456–468. doi: 10.1016/j.jpainsymman.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 24. Collins ES, Witt J, Bausewein C, et al: A systematic review of the use of the Palliative Care Outcome Scale and the Support Team Assessment Schedule in Palliative Care. J Pain Symptom Manage 50:842-53.e19, 2015. [DOI] [PubMed]
- 25. Pos-pal.org: Palliative Care Outcome Scale (POS). https://pos-pal.org/maix/
- 26.Anderson F, Downing GM, Hill J, et al. Palliative performance scale (PPS): A new tool. J Palliat Care. 1996;12:5–11. [PubMed] [Google Scholar]
- 27.Dawson J, Doll H, Fitzpatrick R, et al. The routine use of patient reported outcome measures in healthcare settings. BMJ. 2010;340:c186. doi: 10.1136/bmj.c186. [DOI] [PubMed] [Google Scholar]
- 28. doi: 10.1016/j.jpainsymman.2014.07.010. Etkind SN, Daveson BA, Kwok W, et al: Capture, transfer, and feedback of patient-centered outcomes data in palliative care populations: Does it make a difference? A systematic review. J Pain Symptom Manage 49:611-6242014. [DOI] [PubMed] [Google Scholar]
- 29. Evans CJ, Benalia H, Preston NJ, et al: The selection and use of outcome measures in palliative and end-of-life care research: The MORECare International Consensus Workshop. J Pain Symptom Manage 46:925-937,2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318:197–198. doi: 10.1001/jama.2017.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol. 2016;34:557–565. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chinda M, Jaturapatporn D, Kirshen AJ, et al. Reliability and validity of a Thai version of the Edmonton Symptom Assessment Scale (ESAS-Thai) J Pain Symptom Manage. 2011;42:954–960. doi: 10.1016/j.jpainsymman.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 33. doi: 10.1136/qshc.8.4.219. Hearn J, Higginson IJ: Development and validation of a core outcome measure for palliative care: The palliative care outcome scale. Palliative Care Core Audit Project Advisory Group. Qual Health Care 8:219-227, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osborne TR, Ramsenthaler C, Schey SA, et al. Improving the assessment of quality of life in the clinical care of myeloma patients: The development and validation of the Myeloma Patient Outcome Scale (MyPOS) BMC Cancer. 2015;15:280. doi: 10.1186/s12885-015-1261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harding R, Selman L, Agupio G, et al. Validation of a core outcome measure for palliative care in Africa: The APCA African Palliative Outcome Scale. Health Qual Life Outcomes. 2010;8:10. doi: 10.1186/1477-7525-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pukrittayakamee P, Sapinum L, Suwan P, et al. Validity, reliability and responsiveness of the Thai Palliative Care Outcome Scale staff and patient versions among cancer patients. J Pain Symptom Manage. 2018;56:414–420. doi: 10.1016/j.jpainsymman.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 37.van Vliet LM, Harding R, Bausewein C, et al. How should we manage information needs, family anxiety, depression, and breathlessness for those affected by advanced disease: Development of a clinical decision support tool using a Delphi design. BMC Med. 2015;13:263. doi: 10.1186/s12916-015-0449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Lancker A, Beeckman D, Verhaeghe S, et al. An instrument to collect data on frequency and intensity of symptoms in older palliative cancer patients: A development and validation study. Eur J Oncol Nurs. 2016;21:38–47. doi: 10.1016/j.ejon.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Selby JV, Beal AC, Frank L. The Patient-Centered Outcomes Research Institute (PCORI) national priorities for research and initial research agenda. JAMA. 2012;307:1583–1584. doi: 10.1001/jama.2012.500. [DOI] [PubMed] [Google Scholar]
- 40.Finkelstein J, O’Connor G, Friedmann RH. Development and implementation of the home asthma telemonitoring (HAT) system to facilitate asthma self-care. Stud Health Technol Inform. 2001;84:810–814. [PubMed] [Google Scholar]
- 41.Riva A, Bellazzi R, Stefanelli M. A Web-based system for the intelligent management of diabetic patients. MD Comput. 1997;14:360–364. [PubMed] [Google Scholar]
- 42.Velikova G, Booth L, Smith AB, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: A randomized controlled trial. J Clin Oncol. 2004;22:714–724. doi: 10.1200/JCO.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 43.Denis F, Viger L, Charron A, et al. Detection of lung cancer relapse using self-reported symptoms transmitted via an Internet web-application: Pilot study of the sentinel follow-up. Support Care Cancer. 2014;22:1467–1473. doi: 10.1007/s00520-013-2111-1. [DOI] [PubMed] [Google Scholar]
- 44.Denis F, Viger L, Charron A, et al. Detecting lung cancer relapse using self-evaluation forms weekly filled at home: The sentinel follow-up. Support Care Cancer. 2014;22:79–85. doi: 10.1007/s00520-013-1954-9. [DOI] [PubMed] [Google Scholar]
- 45.Denis F, Yossi S, Septans AL, et al. Improving survival in patients treated for a lung cancer using self-evaluated symptoms reported through a web application. Am J Clin Oncol. 2017;40:464–469. doi: 10.1097/COC.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 46.Strasser F, Blum D, von Moos R, et al. The effect of real-time electronic monitoring of patient-reported symptoms and clinical syndromes in outpatient workflow of medical oncologists: E-MOSAIC, a multicenter cluster-randomized phase III study (SAKK 95/06) Ann Oncol. 2016;27:324–332. doi: 10.1093/annonc/mdv576. [DOI] [PubMed] [Google Scholar]
- 47.Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: An update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32:4149–4154. doi: 10.1200/JCO.2014.56.0383. [DOI] [PubMed] [Google Scholar]
- 48. World Health Organization: Cancer pain relief: With a guide to opioid availability, 2nd ed. http://www.who.int/iris/handle/10665/37896.
- 49.Hökkä M, Kaakinen P, Pölkki T. A systematic review: Non-pharmacological interventions in treating pain in patients with advanced cancer. J Adv Nurs. 2014;70:1954–1969. doi: 10.1111/jan.12424. [DOI] [PubMed] [Google Scholar]
- 50.Soden K, Vincent K, Craske S, et al. A randomized controlled trial of aromatherapy massage in a hospice setting. Palliat Med. 2004;18:87–92. doi: 10.1191/0269216304pm874oa. [DOI] [PubMed] [Google Scholar]
- 51.Hurlow A, Bennett MI, Robb KA, et al. Transcutaneous electric nerve stimulation (TENS) for cancer pain in adults. Cochrane Database Syst Rev. 2012;(3):CD006276. doi: 10.1002/14651858.CD006276.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paley CA, Johnson MI, Tashani OA, et al. Acupuncture for cancer pain in adults. Cochrane Database Syst Rev. 2015;(10):CD007753. doi: 10.1002/14651858.CD007753.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwekkeboom KL, Abbott-Anderson K, Wanta B. Feasibility of a patient-controlled cognitive-behavioral intervention for pain, fatigue, and sleep disturbance in cancer. Oncol Nurs Forum. 2010;37:E151–E159. doi: 10.1188/10.ONF.E151-E159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsai PS, Chen PL, Lai YL, et al. Effects of electromyography biofeedback-assisted relaxation on pain in patients with advanced cancer in a palliative care unit. Cancer Nurs. 2007;30:347–353. doi: 10.1097/01.NCC.0000290805.38335.7b. [DOI] [PubMed] [Google Scholar]
- 55.Straube C, Derry S, Jackson KC, et al. Codeine, alone and with paracetamol (acetaminophen), for cancer pain. Cochrane Database Syst Rev. 2014;(9):CD006601. doi: 10.1002/14651858.CD006601.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wiffen PJ, Derry S, Moore RA. Tramadol with or without paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst Rev. 2017;5:CD012508. doi: 10.1002/14651858.CD012508.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bandieri E, Romero M, Ripamonti CI, et al. Randomized trial of low-dose morphine versus weak opioids in moderate cancer pain. J Clin Oncol. 2016;34:436–442. doi: 10.1200/JCO.2015.61.0733. [DOI] [PubMed] [Google Scholar]
- 58.Fallon M, Giusti R, Aielli F, et al. Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann Oncol. 2018;29(suppl 4):iv166–iv191. doi: 10.1093/annonc/mdy152. [DOI] [PubMed] [Google Scholar]
- 59.Wiffen PJ, Wee B, Derry S, et al. Opioids for cancer pain - An overview of Cochrane reviews. Cochrane Database Syst Rev. 2017;7:CD012592. doi: 10.1002/14651858.CD012592.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klepstad P, Kaasa S, Borchgrevink PC. Starting step III opioids for moderate to severe pain in cancer patients: Dose titration: A systematic review. Palliat Med. 2011;25:424–430. doi: 10.1177/0269216310386280. [DOI] [PubMed] [Google Scholar]
- 61.Sande TA, Laird BJA, Fallon MT. The use of opioids in cancer patients with renal impairment-a systematic review. Support Care Cancer. 2017;25:661–675. doi: 10.1007/s00520-016-3447-0. [DOI] [PubMed] [Google Scholar]
- 62.Riley J, Branford R, Droney J, et al. Morphine or oxycodone for cancer-related pain? A randomized, open-label, controlled trial. J Pain Symptom Manage. 2015;49:161–172. doi: 10.1016/j.jpainsymman.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 63.Rayment C, Hjermstad MJ, Aass N, et al. Neuropathic cancer pain: Prevalence, severity, analgesics and impact from the European Palliative Care Research Collaborative-Computerised Symptom Assessment study. Palliat Med. 2013;27:714–721. doi: 10.1177/0269216312464408. [DOI] [PubMed] [Google Scholar]
- 64.Kane CM, Mulvey MR, Wright S, et al. Opioids combined with antidepressants or antiepileptic drugs for cancer pain: Systematic review and meta-analysis. Palliat Med. 2018;32:276–286. doi: 10.1177/0269216317711826. [DOI] [PubMed] [Google Scholar]
- 65. Jongen JLM, Huijsman ML, Jessurun J, et al: The evidence for pharmacologic treatment of neuropathic cancer pain: Beneficial and adverse effects. J Pain Symptom Manage 46:581-590.e1, 2013. [DOI] [PubMed]
- 66.Dou Z, Jiang Z, Zhong J. Efficacy and safety of pregabalin in patients with neuropathic cancer pain undergoing morphine therapy. Asia Pac J Clin Oncol. 2017;13:e57–e64. doi: 10.1111/ajco.12311. [DOI] [PubMed] [Google Scholar]
- 67.Bell RF, Eccleston C, Kalso EA. Ketamine as an adjuvant to opioids for cancer pain. Cochrane Database Syst Rev. 2017;6:CD003351. doi: 10.1002/14651858.CD003351.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fallon MT, Wilcock A, Kelly CA, et al. Oral ketamine vs placebo in patients with cancer-related neuropathic pain: A randomized clinical trial. JAMA Oncol. 2018;4:870–872. doi: 10.1001/jamaoncol.2018.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McNicol ED, Ferguson MC, Schumann R. Methadone for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;5:CD012499. doi: 10.1002/14651858.CD012499.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Good P, Afsharimani B, Movva R, et al. Therapeutic challenges in cancer pain management: A systematic review of methadone. J Pain Palliat Care Pharmacother. 2014;28:197–205. doi: 10.3109/15360288.2014.938883. [DOI] [PubMed] [Google Scholar]
- 71.Porta-Sales J, Garzón-Rodríguez C, Llorens-Torromé S, et al. Evidence on the analgesic role of bisphosphonates and denosumab in the treatment of pain due to bone metastases: A systematic review within the European Association for Palliative Care guidelines project. Palliat Med. 2017;31:5–25. doi: 10.1177/0269216316639793. [DOI] [PubMed] [Google Scholar]
- 72.Mhaskar R, Kumar A, Miladinovic B, et al. Bisphosphonates in multiple myeloma: An updated network meta-analysis. Cochrane Database Syst Rev. 2017;12:CD003188. doi: 10.1002/14651858.CD003188.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chow R, Hoskin P, Hollenberg D, et al. Efficacy of single fraction conventional radiation therapy for painful uncomplicated bone metastases: A systematic review and meta-analysis. Ann Palliat Med. 2017;6:125–142. doi: 10.21037/apm.2016.12.04. [DOI] [PubMed] [Google Scholar]
- 74.Bausewein C, Booth S, Gysels M, et al. Individual breathlessness trajectories do not match summary trajectories in advanced cancer and chronic obstructive pulmonary disease: Results from a longitudinal study. Palliat Med. 2010;24:777–786. doi: 10.1177/0269216310378785. [DOI] [PubMed] [Google Scholar]
- 75.Seow H, Barbera L, Sutradhar R, et al. Trajectory of performance status and symptom scores for patients with cancer during the last six months of life. J Clin Oncol. 2011;29:1151–1158. doi: 10.1200/JCO.2010.30.7173. [DOI] [PubMed] [Google Scholar]
- 76.Frostad A, Soyseth V, Haldorsen T, et al. Respiratory symptoms and long-term cardiovascular mortality. Respir Med. 2007;101:2289–2296. doi: 10.1016/j.rmed.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 77.Frostad A, Søyseth V, Haldorsen T, et al. Impact of respiratory symptoms on lung cancer: 30-year follow-up of an urban population. Lung Cancer. 2008;60:22–30. doi: 10.1016/j.lungcan.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 78.Gysels MH, Higginson IJ. The lived experience of breathlessness and its implications for care: A qualitative comparison in cancer, COPD, heart failure and MND. BMC Palliat Care. 2011;10:15. doi: 10.1186/1472-684X-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Malik FA, Gysels M, Higginson IJ. Living with breathlessness: A survey of caregivers of breathless patients with lung cancer or heart failure. Palliat Med. 2013;27:647–656. doi: 10.1177/0269216313488812. [DOI] [PubMed] [Google Scholar]
- 80.Barbera L, Atzema C, Sutradhar R, et al. Do patient-reported symptoms predict emergency department visits in cancer patients? A population-based analysis. Ann Emerg Med. 2013;61:427–437.e5. doi: 10.1016/j.annemergmed.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 81.0. Barbera L, Taylor C, Dudgeon D: Why do patients with cancer visit the emergency department near the end of life? CMAJ 182:563-568, 201.
- 82.Currow DC, Abernethy AP. Breathlessness in an age of noncommunicable diseases. Curr Opin Support Palliat Care. 2012;6:127–128. doi: 10.1097/SPC.0b013e3283537d26. [DOI] [PubMed] [Google Scholar]
- 83. Dudgeon DJ: Managing dyspnea and cough. Hematol Oncol Clin North Am 16:557-77, viii, 2002. [DOI] [PubMed]
- 84.Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: Update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185:435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. doi: 10.1080/23809000.2018.1524708. Booth S, Chin C, Spathis A, et al: Non-pharmacological interventions for breathlessness in people with cancer. Expert Rev Qual Life Cancer Care . [epub ahead of print on October 19, 2018] [DOI] [Google Scholar]
- 86.Bausewein C, Booth S, Gysels M, et al. Non-pharmacological interventions for breathlessness in advanced stages of malignant and non-malignant diseases. Cochrane Database Syst Rev. 2008;(2):CD005623. doi: 10.1002/14651858.CD005623.pub2. [DOI] [PubMed] [Google Scholar]
- 87. Bausewein C, Currow D, Johnson M (eds): Management of Chronic Breathlessness, in Palliative Care in Respiratory Disease. ERS Monograph. ERS, Sheffield, United Kingdom, 2016, pp 153-171. [Google Scholar]
- 88.Abernethy AP, McDonald CF, Frith PA, et al. Effect of palliative oxygen versus room air in relief of breathlessness in patients with refractory dyspnoea: A double-blind, randomised controlled trial. Lancet. 2010;376:784–793. doi: 10.1016/S0140-6736(10)61115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rocker G. Harms of overoxygenation in patients with exacerbation of chronic obstructive pulmonary disease. CMAJ. 2017;189:E762–E763. doi: 10.1503/cmaj.170196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barnes H, McDonald J, Smallwood N, et al. Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness. Cochrane Database Syst Rev. 2016;3:CD011008. doi: 10.1002/14651858.CD011008.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ben-Aharon I, Gafter-Gvili A, Leibovici L, et al. Interventions for alleviating cancer-related dyspnea: A systematic review and meta-analysis. Acta Oncol. 2012;51:996–1008. doi: 10.3109/0284186X.2012.709638. [DOI] [PubMed] [Google Scholar]
- 92.Booth S, Moosavi SH, Higginson IJ. The etiology and management of intractable breathlessness in patients with advanced cancer: A systematic review of pharmacological therapy. Nat Clin Pract Oncol. 2008;5:90–100. doi: 10.1038/ncponc1034. [DOI] [PubMed] [Google Scholar]
- 93.Jennings AL, Davies AN, Higgins JP, et al. A systematic review of the use of opioids in the management of dyspnoea. Thorax. 2002;57:939–944. doi: 10.1136/thorax.57.11.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Simon ST, Higginson IJ, Booth S, et al. Benzodiazepines for the relief of breathlessness in advanced malignant and non-malignant diseases in adults. Cochrane Database Syst Rev. 2016;10:CD007354. doi: 10.1002/14651858.CD007354.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Simon ST, Köskeroglu P, Gaertner J, et al. Fentanyl for the relief of refractory breathlessness: A systematic review. J Pain Symptom Manage. 2013;46:874–886. doi: 10.1016/j.jpainsymman.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 96.Ekström M, Bajwah S, Bland JM, et al. One evidence base; three stories: Do opioids relieve chronic breathlessness? Thorax. 2018;73:88–90. doi: 10.1136/thoraxjnl-2016-209868. [DOI] [PubMed] [Google Scholar]
- 97. doi: 10.1136/thoraxjnl-2018-211589. Brighton L, Miller S, Farquhar M, et al: Holistic services for people with advanced disease and chronic breathlessness: A systematic review and meta-analysis. Thorax 74:270-281, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Higginson IJ, Bausewein C, Reilly CC, et al. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: A randomised controlled trial. Lancet Respir Med. 2014;2:979–987. doi: 10.1016/S2213-2600(14)70226-7. [DOI] [PubMed] [Google Scholar]
- 99.Maddocks M, Lovell N, Booth S, et al. Palliative care and management of troublesome symptoms for people with chronic obstructive pulmonary disease. Lancet. 2017;390:988–1002. doi: 10.1016/S0140-6736(17)32127-X. [DOI] [PubMed] [Google Scholar]
- 100.Hasler WL, Chey WD. Nausea and vomiting. Gastroenterology. 2003;125:1860–1867. doi: 10.1053/j.gastro.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 101.Reuben DB, Mor V. Nausea and vomiting in terminal cancer patients. Arch Intern Med. 1986;146:2021–2023. [PubMed] [Google Scholar]
- 102.Krishna L. Nasogastric feeding at the end of life: A virtue ethics approach. Nurs Ethics. 2011;18:485–494. doi: 10.1177/0969733011403557. [DOI] [PubMed] [Google Scholar]
- 103.Osoba D, Zee B, Warr D, et al. Effect of postchemotherapy nausea and vomiting on health-related quality of life. Support Care Cancer. 1997;5:307–313. doi: 10.1007/s005200050078. [DOI] [PubMed] [Google Scholar]
- 104.Hesketh PJ, Kris MG, Basch E, et al. Antiemetics. J Clin Oncol. 2017;35:3240–3261. doi: 10.1200/JCO.2017.74.4789. [DOI] [PubMed] [Google Scholar]
- 105.Cox L, Darvill E, Dorman S. Levomepromazine for nausea and vomiting in palliative care. Cochrane Database Syst Rev. 2015;(11):CD009420. doi: 10.1002/14651858.CD009420.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;(11):CD006271. doi: 10.1002/14651858.CD006271.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stephenson J, Davies A. An assessment of aetiology-based guidelines for the management of nausea and vomiting in patients with advanced cancer. Support Care Cancer. 2006;14:348–353. doi: 10.1007/s00520-005-0897-1. [DOI] [PubMed] [Google Scholar]
- 108.Gordon P, LeGrand SB, Walsh D. Nausea and vomiting in advanced cancer. Eur J Pharmacol. 2014;722:187–191. doi: 10.1016/j.ejphar.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 109.Rhodes VA, McDaniel RW. Nausea, vomiting, and retching: Complex problems in palliative care. CA Cancer J Clin. 2001;51:232–248, quiz 249-252. doi: 10.3322/canjclin.51.4.232. [DOI] [PubMed] [Google Scholar]
- 110.Webb A. Management of nausea and vomiting in patients with advanced cancer at the end of life. Nurs Stand. 2017;32:53–63. doi: 10.7748/ns.2017.e10993. [DOI] [PubMed] [Google Scholar]
- 111.Wood GJ, Shega JW, Lynch B, et al. Management of intractable nausea and vomiting in patients at the end of life: “I was feeling nauseous all of the time . . . nothing was working. JAMA. 2007;298:1196–1207. doi: 10.1001/jama.298.10.1196. [DOI] [PubMed] [Google Scholar]
- 112.Vayne-Bossert P, Haywood A, Good P, et al. Corticosteroids for adult patients with advanced cancer who have nausea and vomiting (not related to chemotherapy, radiotherapy, or surgery) Cochrane Database Syst Rev. 2017;7:CD012002. doi: 10.1002/14651858.CD012002.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Langley-DeGroot M, Ma JD, Hirst J, et al. Olanzapine in the treatment of refractory nausea and vomiting: A case report and review of the literature. J Pain Palliat Care Pharmacother. 2015;29:148–152. doi: 10.3109/15360288.2015.1035831. [DOI] [PubMed] [Google Scholar]
- 114.Ernst G, Kongsgaard UE. Use of cannabinoids in palliative medicine [in Norwegian] Tidsskr Nor Laegeforen. 2008;128:822–825. [PubMed] [Google Scholar]
- 115.Mücke M, Carter C, Cuhls H, et al. Cannabinoids in palliative care: Systematic review and meta-analysis of efficacy, tolerability and safety [in German] Schmerz. 2016;30:25–36. doi: 10.1007/s00482-015-0085-2. [DOI] [PubMed] [Google Scholar]
- 116.Strang P. Quality of life is the most important goal of nutritional support of the dying [in Swedish] Lakartidningen. 2000;97:1141–1144. [PubMed] [Google Scholar]
- 117.Oyama H, Kaneda M, Katsumata N, et al. Using the bedside wellness system during chemotherapy decreases fatigue and emesis in cancer patients. J Med Syst. 2000;24:173–182. doi: 10.1023/a:1005591626518. [DOI] [PubMed] [Google Scholar]
- 118.Nystrom E, Ridderstrom G, Leffler AS. Manual acupuncture as an adjunctive treatment of nausea in patients with cancer in palliative care--A prospective, observational pilot study. Acupunct Med. 2008;26:27–32. doi: 10.1136/aim.26.1.27. [DOI] [PubMed] [Google Scholar]
- 119.Ream E, Richardson A. Fatigue: A concept analysis. Int J Nurs Stud. 1996;33:519–529. doi: 10.1016/0020-7489(96)00004-1. [DOI] [PubMed] [Google Scholar]
- 120.Peters MEWJ, Goedendorp MM, Verhagen SAHHVM, et al. A prospective analysis on fatigue and experienced burden in informal caregivers of cancer patients during cancer treatment in the palliative phase. Acta Oncol. 2015;54:500–506. doi: 10.3109/0284186X.2014.953254. [DOI] [PubMed] [Google Scholar]
- 121.Stone P, Richards M, A’Hern R, et al. A study to investigate the prevalence, severity and correlates of fatigue among patients with cancer in comparison with a control group of volunteers without cancer. Ann Oncol. 2000;11:561–567. doi: 10.1023/a:1008331230608. [DOI] [PubMed] [Google Scholar]
- 122.Baracos VE, Martin L, Korc M, et al. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4:17105. doi: 10.1038/nrdp.2017.105. [DOI] [PubMed] [Google Scholar]
- 123.Yennurajalingam S, Bruera E. Palliative management of fatigue at the close of life: “It feels like my body is just worn out. JAMA. 2007;297:295–304. doi: 10.1001/jama.297.3.295. [DOI] [PubMed] [Google Scholar]
- 124.Minton O, Stone P. A systematic review of the scales used for the measurement of cancer-related fatigue (CRF) Ann Oncol. 2009;20:17–25. doi: 10.1093/annonc/mdn537. [DOI] [PubMed] [Google Scholar]
- 125.de Raaf PJ, de Klerk C, Timman R, et al. Systematic monitoring and treatment of physical symptoms to alleviate fatigue in patients with advanced cancer: A randomized controlled trial. J Clin Oncol. 2013;31:716–723. doi: 10.1200/JCO.2012.44.4216. [DOI] [PubMed] [Google Scholar]
- 126.Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2012;11:CD006145. doi: 10.1002/14651858.CD006145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tomlinson D, Diorio C, Beyene J, et al. Effect of exercise on cancer-related fatigue: A meta-analysis. Am J Phys Med Rehabil. 2014;93:675–686. doi: 10.1097/PHM.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 128.Poort H, Peters M, Bleijenberg G, et al. Psychosocial interventions for fatigue during cancer treatment with palliative intent. Cochrane Database Syst Rev. 2017;7:CD012030. doi: 10.1002/14651858.CD012030.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lengacher CA, Reich RR, Paterson CL, et al: Examination of broad symptom improvement resulting from mindfulness-based stress reduction in breast cancer survivors: A randomized controlled trial. J Clin Oncol 34:2827-2834, 2016. [DOI] [PMC free article] [PubMed]
- 130.Bennett S, Pigott A, Beller EM, et al. Educational interventions for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2016;11:CD008144. doi: 10.1002/14651858.CD008144.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bradt J, Dileo C, Magill L, et al. Music interventions for improving psychological and physical outcomes in cancer patients. Cochrane Database Syst Rev. 2016;(8):CD006911. doi: 10.1002/14651858.CD006911.pub3. [DOI] [PubMed] [Google Scholar]
- 132.Finnegan-John J, Molassiotis A, Richardson A, et al. A systematic review of complementary and alternative medicine interventions for the management of cancer-related fatigue. Integr Cancer Ther. 2013;12:276–290. doi: 10.1177/1534735413485816. [DOI] [PubMed] [Google Scholar]
- 133.Zeng Y, Luo T, Finnegan-John J, et al. Meta-analysis of randomized controlled trials of acupuncture for cancer-related fatigue. Integr Cancer Ther. 2014;13:193–200. doi: 10.1177/1534735413510024. [DOI] [PubMed] [Google Scholar]
- 134. doi: 10.1200/JCO.2014.55.8353. Ruddy KJ, Barton D, Loprinzi CL: Laying to rest psychostimulants for cancer-related fatigue. J Clin Oncol 32:1865-1867, 2014. [DOI] [PubMed] [Google Scholar]
- 135.Stone PC. Methylphenidate in the management of cancer-related fatigue. J Clin Oncol. 2013;31:2372–2373. doi: 10.1200/JCO.2013.50.0181. [DOI] [PubMed] [Google Scholar]
- 136.Minton O, Richardson A, Sharpe M, et al. Psychostimulants for the management of cancer-related fatigue: A systematic review and meta-analysis. J Pain Symptom Manage. 2011;41:761–767. doi: 10.1016/j.jpainsymman.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 137.Bruera E, Yennurajalingam S, Palmer JL, et al. Methylphenidate and/or a nursing telephone intervention for fatigue in patients with advanced cancer: A randomized, placebo-controlled, phase II trial. J Clin Oncol. 2013;31:2421–2427. doi: 10.1200/JCO.2012.45.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Escalante CP, Meyers C, Reuben JM, et al. A randomized, double-blind, 2-period, placebo-controlled crossover trial of a sustained-release methylphenidate in the treatment of fatigue in cancer patients. Cancer J. 2014;20:8–14. doi: 10.1097/PPO.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Homsi J, Nelson KA, Sarhill N, et al. A phase II study of methylphenidate for depression in advanced cancer. Am J Hosp Palliat Care. 2001;18:403–407. doi: 10.1177/104990910101800610. [DOI] [PubMed] [Google Scholar]
- 140.Moraska AR, Sood A, Dakhil SR, et al. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. J Clin Oncol. 2010;28:3673–3679. doi: 10.1200/JCO.2010.28.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rozans M, Dreisbach A, Lertora JJ, et al. Palliative uses of methylphenidate in patients with cancer: A review. J Clin Oncol. 2002;20:335–339. doi: 10.1200/JCO.2002.20.1.335. [DOI] [PubMed] [Google Scholar]
- 142.Wilwerding MB, Loprinzi CL, Mailliard JA, et al. A randomized, crossover evaluation of methylphenidate in cancer patients receiving strong narcotics. Support Care Cancer. 1995;3:135–138. doi: 10.1007/BF00365854. [DOI] [PubMed] [Google Scholar]
- 143.Spathis A, Fife K, Blackhall F, et al. Modafinil for the treatment of fatigue in lung cancer: Results of a placebo-controlled, double-blind, randomized trial. J Clin Oncol. 2014;32:1882–1888. doi: 10.1200/JCO.2013.54.4346. [DOI] [PubMed] [Google Scholar]
- 144.Jean-Pierre P, Morrow GR, Roscoe JA, et al. A phase 3 randomized, placebo-controlled, double-blind, clinical trial of the effect of modafinil on cancer-related fatigue among 631 patients receiving chemotherapy: A University of Rochester Cancer Center Community Clinical Oncology Program Research base study. Cancer. 2010;116:3513–3520. doi: 10.1002/cncr.25083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Minton O, Richardson A, Sharpe M, et al. Drug therapy for the management of cancer-related fatigue. Cochrane Database Syst Rev. 2010;(7):CD006704. doi: 10.1002/14651858.CD006704.pub2. [DOI] [PubMed] [Google Scholar]
- 146. Yennurajalingam S, Frisbee-Hume S, Palmer JL, et al: Reduction of cancer-related fatigue with dexamethasone: A double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol 31:3076-3082, 2013. [DOI] [PubMed]
- 147.Cruciani RA, Zhang JJ, Manola J, et al. L-carnitine supplementation for the management of fatigue in patients with cancer: An Eastern Cooperative Oncology Group phase III, randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2012;30:3864–3869. doi: 10.1200/JCO.2011.40.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Higginson IJ, Evans CJ. What is the evidence that palliative care teams improve outcomes for cancer patients and their families? Cancer J. 2010;16:423–435. doi: 10.1097/PPO.0b013e3181f684e5. [DOI] [PubMed] [Google Scholar]
- 149.Higginson IJ, Finlay I, Goodwin DM, et al. Do hospital-based palliative teams improve care for patients or families at the end of life? J Pain Symptom Manage. 2002;23:96–106. doi: 10.1016/s0885-3924(01)00406-7. [DOI] [PubMed] [Google Scholar]
- 150.Zimmermann C, Riechelmann R, Krzyzanowska M, et al. Effectiveness of specialized palliative care: A systematic review. JAMA. 2008;299:1698–1709. doi: 10.1001/jama.299.14.1698. [DOI] [PubMed] [Google Scholar]
- 151.Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35:96–112. doi: 10.1200/JCO.2016.70.1474. [DOI] [PubMed] [Google Scholar]