Abstract
Although robust evidence demonstrates that specialty palliative care integrated into oncology care improves patient and health system outcomes, few clinicians are familiar with the standards, guidelines, and quality measures related to integration. These types of guidance outline principles of best practice and provide a framework for assessing the fidelity of their implementation. Significant advances in the understanding of effective methods and procedures to guide integration of specialty palliative care into oncology have led to a proliferation of guidance documents around the world, with several areas of commonality but also some key differences. Commonalities originate from a shared vision for integration; differences arise from diverse roles of palliative care specialists within cancer care globally. In this review we discuss three of the most cited standards/guidelines, as well as quality measures related to integrated palliative and oncology care. We also recommend changes to the quality measurement framework for palliative care and a new way to match palliative care services to patients with advanced cancer on the basis of care complexity and patient needs, irrespective of prognosis.
INTRODUCTION
Until recently, the integration of specialty palliative care into oncology occurred near the end of life, when patients had few or no options for disease-modifying treatment. As a growing body of evidence has highlighted the benefits of adding palliative care services earlier in the course of illness for patients with cancer, oncologists’ perspectives have shifted. With that sense of opportunity, researchers have conducted several rigorous prospective trials to examine the efficacy of specialty palliative care integration into routine oncology care.1-3 Studies have consistently demonstrated improvement in patient, caregiver, and health system outcomes of importance, and this wave of evidence has necessitated translating evidence-based processes and expert consensus into guidance for busy clinical teams.4 Furthermore, challenges in achieving integration reported by oncology clinicians and administrators,5 including inconsistent access to palliative care services6,7 and variations in palliative care quality,8 have necessitated the development of guidance to direct practice.
In meeting clinicians’ and administrators’ needs during the transition from evidence development to clinical implementation, the field has focused on methods to ensure the fidelity of palliative care delivery. Ensuring fidelity involves providing guidance to clinicians on the best practice components (eg, timing, frequency, duration, patient populations) that improve outcomes of interest. Such efforts to ensure fidelity of specialty palliative care integration into oncology comprise two forms: standards/guidelines and quality measures. Standards and guidelines synthesize and prioritize best practices. Quality measures provide methods for measuring the frequency of achieving ideal practice, benchmarking across practices, and identifying gaps in health care. Here we describe the various methods to guide best practices in oncology and palliative care integration, presented in an increasing order of granularity from high-level standards and guidelines to quality measures evaluating practice. For the purposes of this review, we have limited our scope to those documents focusing on integrated specialty palliative care in patients with cancer, recognizing that several others exist for more disease-agnostic realms. We highlight commonalities and differences among these documents and suggest three ideas to make efforts more patient centered and useful to clinical teams.
STANDARDS AND GUIDELINES FOR PALLIATIVE CARE INTEGRATION INTO ONCOLOGY
Standards and guidelines provide general frameworks for how palliative care should be operationalized in the oncology context. Because standards and guidelines provide general guidance, clinicians and administrators often first reference them to perform service planning and resource allocation and discuss broadly the current performance of an organization. Standards and guidelines typically include high-level descriptions of best practices, with lesser attention given to how concordance with best practices is measured. This latter construct is better described by quality measures. Quality measures generally include details on how to define whether a practice is followed, which patients or visits are eligible for that best practice, and how results should be interpreted.
On the basis of considerable evidence for feasibility and efficacy, several national and international oncology organizations have produced recommendations regarding consistent integration of palliative care into oncology. From the United States, these include ASCO,9 which recently published its clinical practice guideline as follow-up to a provisional clinical opinion in 2013,10 the National Comprehensive Cancer Network,11 the Oncology Nursing Society,12 and the Commission on Cancer.13 Around the globe, several oncology organizations have led nation- and health ministry–level strategies to further palliative care integration. These include the European Society of Medical Oncology (ESMO),14 European Association for Palliative Care,15 Italian Association of Medical Oncology,16 German Guideline Program in Oncology,17 and German Comprehensive Cancer Centre Network.18 Although some countries seem to lack their own recommendations, many19-21 actually defer to the WHO22 or ASCO global recommendations.23 Collectively, these guidelines emphasize the role of concurrent palliative and oncology care for all patients, regardless of treatment intent or prognosis.
Recommendations come from varying organizations and geographies; however, they all share four common themes. These themes include: the need to guarantee access to specialty palliative care services for all patients with cancer, use of stratification (triage) methods to determine referral to specialty palliative care services, focus on informal caregivers alongside patients, and interdisciplinary composition of specialty palliative care teams (not simply limited to medical and nursing members).
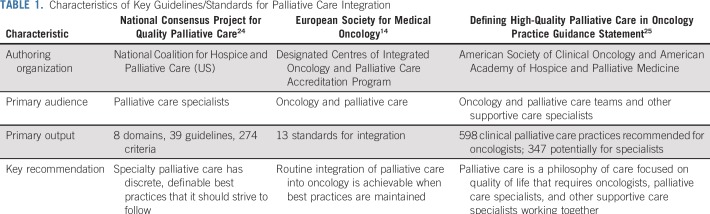
Our group focused on three key sets of palliative care integration standards and guidelines commonly cited by experts. These were chosen because of their attention to integration methods, focus on specialist palliative care teams, and development by multidisciplinary clinical organizations. These are listed in Table 1.
TABLE 1.
Characteristics of Key Guidelines/Standards for Palliative Care Integration
Fourth Edition Guidelines From the National Consensus Project for Quality Palliative Care in the United States
The National Consensus Panel (NCP) guidelines are the leader in guidelines and quality domains for specialty palliative care in the United States.26,27 The 2018 update to the guidelines,24 led by the National Coalition for Hospice and Palliative Care and funded by private foundations, represents one of the largest, most comprehensive and rigorous undertakings to define best practices in specialty palliative care. The update was accompanied by a systematic review,28 examining both the breadth and quality of the science supporting each best practice. The update was motivated by the need to broadly update the standards from 2013 and develop additional guidelines for community-based palliative care, as US care models further embrace care outside of hospital settings. Furthermore, the expert panel focused on informal caregivers because of an increased recognition of the need for specialty teams to care for caregivers and embrace caregivers as partners in improving patient quality of life, given that most time in the last year of life is spent in the community, and family and friends provide the majority of day-to-day care.29 The guidelines present an eight-domain framework to assess quality in palliative care, ranging from Structures and Process of Care to Care Near the End of Life. In total, 39 guidelines with 274 total criteria for meeting the guidelines from the NCP were published.
We highlight a few of the NCP guidelines presented. In the Structure and Process domain, the guidelines recommend that specialty palliative care teams include certified (or those with additional education) interdisciplinary team members and that patients and family members have access to teams at all times of the day and night. In addition, the guidelines also recommend that all palliative care clinicians are educated in safe prescribing of opioids and that their standard practice includes completing accessible care plans and comprehensively assessing both patient and caregiver distress early in the course of illness. The Psychological and Psychiatric Aspects of Care domain of the guidelines states that all teams have access to a social worker and support coping and emotional growth while assessing decisional regret. Overall, the guidelines emphasize the need for and composition of interdisciplinary teams to care for patients with cancer and also highlight the importance of addressing caregiver needs across all domains.
ESMO Designated Centres of Integrated Oncology and Palliative Care Accreditation Program
The ESMO Designated Centres of Integrated Oncology and Palliative Care Accreditation Program recognizes cancer centers that provide comprehensive services in supportive and palliative care as part of their routine cancer care. A similar program in the United States from the Joint Commission also exists.30 The program, initiated in 2003, has recognized nearly 200 cancer programs in 41 countries. A modified Delphi process using expert consensus produced 13 standards for integration, within topics of: integration issues, credentialing, service provision, research, and education. An example of a standard includes oncology centers demonstrating that they incorporate expert care in pain, symptom management, and psychosocial distress. Furthermore, the guidelines require that oncology centers ensure access to expert care for inpatient symptom stabilization, incorporate programmatic support of family members, and maintain physical facilities and clinical expertise to provide available and responsive inpatient end-of-life care. The standards focus on the integration and codelivery of care of the oncology and palliative care teams, in both outpatient and inpatient settings.
Defining High-Quality Palliative Care in Oncology Practice: An ASCO/American Academy of Hospice and Palliative Medicine Guidance Statement
In 2016, Bickel et al25 explored essential elements of palliative care within the professional purview of the oncology team at any time from diagnosis onward, thus highlighting particular patients and clinical situations where specialty-level integration of palliative care should occur. Compared with the other sets of standards, this work focused on standardizing clinical processes of care and identifying clinicians who may be responsible for the delivery of that care, which could include oncology teams, specialist palliative care teams, or other supportive care professionals. The Delphi process using a multidisciplinary team of oncology and palliative care professionals together with lay persons produced a consensus statement on components of care related to high-quality palliative care delivery in US medical oncology practices. Notable standards include monthly, systematic symptom assessment and psychosocial well-being of patients, spiritual issues of patients, and caregivers’ distress deployed by oncology teams as part of routine care. Regular prognostic disclosure and communication to the patient and other clinical team members, assessment of prognostic understanding, and initiation of advance care planning at the time of recognition of advanced cancer are also considered clinical standards for oncology teams. Patients with complex or uncontrolled care needs, including pain or other symptoms requiring significant adjuvant medications or interventions, or those with complex psychosocial or spiritual needs should be referred to specialist palliative care services.
These sets of guidelines/standards address care within both types of palliative care delivery: primary (oncologist delivered) and specialist (palliative care specialist delivered). Such equal focus recognizes that oncologists are the primary deliverers of palliative care for a majority of patients with cancer; however, specialists play an important role in complex patient cases. Furthermore, the research by Bickel et al25 highlights areas in between, where care delivery could be delivered by either oncologists or palliative care specialists, on the basis of access, resources, and time. This work also recognizes that certain domains of care could be delivered by other supportive care experts outside the palliative care team.
Although we could not summarize all guidelines or standards related to palliative care integration, readers are invited to evaluate other standards focused on pediatric populations31 or nutritional support.32 Another review by Kaasa et al33 and the Lancet Commission summarizes several bodies of evidence related to care pathways and models guiding integration and includes sections on standards related to education of the workforce and referral to specialists. Such reviews complement work by Waller et al34,35 on needs-based assessments to trigger referral to palliative care.
Despite the breadth of published standards or guidelines for palliative care integration, as presented previously, there remain no agreed-on national or international guidelines regarding criteria for referral to palliative care (despite the fact that palliative care is a referral-based specialty). Certainly, not every person with advanced cancer needs, wants, or will benefit from referral to palliative care. Most patients do benefit, but the timing and reasons for referral remain ill defined.
Rapid evolutions in cancer treatments and the associated dynamic nature of patient outcomes dictate a need to transition away from prognosis- or disease severity–based criteria for specialist palliative care integration. At the heart of the palliative care philosophy is meeting the needs of patients and their caregivers, regardless of diagnosis, severity of condition, or consideration of disease-modifying treatments. Data now demonstrate the ability of specialist palliative care to improve patient outcomes when integration is agnostic to disease prognosis, as shown by integration efforts in patients with hematologic malignancies undergoing stem-cell transplantation with curative intent.36 Furthermore, the proliferation of novel and highly effective anticancer treatments are producing scenarios where many cancers resemble chronic illnesses rather than acute causes of decline or death. This transition warrants evolution in how we identify parameters to guide integration of specialist palliative care and oncology.37-39
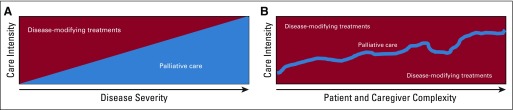
Although a few recommendations frame referrals to specialist palliative care around patient and caregiver distress and needs,40,41 many begin with discussions regarding disease severity, prognosis, or treatability/curability.10,11,42 We propose in Figure 1 an evolution from current referral criteria, often on the basis of disease severity, avoidance of health care expenditures, or use patterns, to those focusing on patient and caregiver complexity and needs.43 In Figure 1, palliative care intensity mirrors the complexity of the needs of the patient and caregiver, rather than reflecting few treatment options or short prognosis. For example, severe symptoms such as breathlessness, which often presents early in the disease course long before death, may alone be triggers for consultation.44 This approach requires clinicians to perform regular and systematic screening and assessments of multiple domains of quality of life (eg, symptoms, emotional concerns, independence, and function), starting from the time of diagnosis. Such comprehensive assessments, when inclusive of patient and caregiver needs, allow clinicians to determine overall care complexity within palliative care and supportive care realms. Then, when thresholds for care complexity and high patient needs are met, referrals to palliative care specialists are made. Fundamentally, we propose a shift away from disease severity and prognosis toward the needs of the patient, which should guide the type and intensity of palliative care he or she receives.
FIG 1.
(A) Current model and (B) proposed complexity-based integration framework for specialty palliative care in oncology. Disease-modifying treatments (background) are provided throughout the continuum of care, because they make sense in the clinical situation and in alignment with patient preferences. Palliative care intensity increases to mirror patient and caregiver complexity, not necessarily because treatment options are fewer or the disease is more advanced.
DOMAINS OF QUALITY MEASURES FOR PALLIATIVE CARE INTEGRATION IN ONCOLOGY
The Centers for Medicare and Medicaid Services in the United States defines health care quality measures as “tools that help measure or quantify healthcare processes, outcomes, patient perceptions, and organizational structure and/or systems that are associated with the ability to provide high-quality health care and/or that relate to one or more quality goals for health care.”45 Health care quality measures typically include specificity around measurement settings (ie, when and where the measure is applicable), numerators (how adherence to the measure is concluded), denominators (who is eligible for the measure), exclusion (under what circumstances the measure does not apply), reporting requirements (how is adherence to measures reported), and measurement philosophy (whether measurement was performed for internal improvement or external reporting).
The frequently cited Donabedian Framework for Quality Measurement46 classifies quality measures into a structure, process, or outcome category. Quality measures, which focus on the people, resources, and assets related to care, are deemed structural. An example includes a measure regarding teams incorporating educational initiatives and programs to prevent clinician burnout and increase resilience. Process measures relate to how care is delivered. For example, a measure that assesses what percentage of patients had an advance care planning discussion would fall into this category. Last, outcome measures are most commonly identified as those that change a patient’s health state. There are now many well-validated outcome measures available for palliative care. Examples include the Palliative care Outcome Scale and the Edmonton Symptom Assessment System. Guidance on how to choose and use these measures in practice has been developed by the European Association for Palliative Care.47 Other constructs that are related to improvements in patient outcomes, but that do not directly change a health state (eg, access, service use), are not classically considered outcome measures in the Donabedian framework; however, they are included in other frameworks as intermediate outcomes.48 Overwhelmingly, palliative care quality measures evaluate processes of care. Less commonly, structure and outcome measures are used.49,50
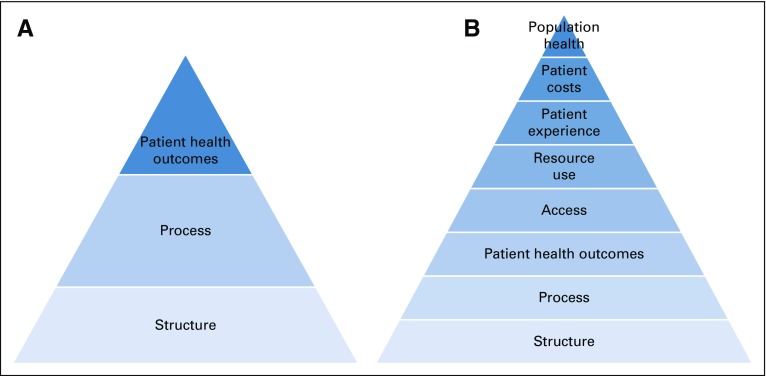
Knowing that palliative care specialists drive improvements in several individual and health system outcomes, we propose an expansion of the Donabedian framework for quality measurement in palliative care integration (Fig 2). Figure 2 depicts a hierarchic relationship within palliative care quality measurement, where structure and processes are established first, with the goal of improving several patient and system outcomes. Such an expanded framework allows for assessment of all the outcomes improved by palliative care at the individual, system, and population levels, while identifying areas where additional quality measures require development. For example, despite robust evidence demonstrating decrease in hospitalizations and emergency room visits for patients receiving specialty palliative care,51,52 no defined quality measures related to this use outcome exist, although they are critical to benchmarking, making comparisons, and standardizing measurement techniques. Without an expanded framework for quality measurement, we miss opportunities to develop measures in domains important to palliative care delivery (eg, access, population health) and standardize the definitions and procedures for measuring those impacts.
FIG 2.
(A) Current framework and (B) proposed updated quality measurement framework for palliative care patients and caregivers. Pyramids depict a hierarchic approach to quality measurement in palliative care, with structures of care established first, followed by processes of care, leading to improvement in several patient and system outcomes.
Busy clinicians and administrators are challenged by the increasing number of available palliative care quality measures, with ranges of approximately 50 measures in 200653 increasing to 142 in 200954 and approximately 300 measures more recently.55,56 With a shift toward value-based reimbursement that requires regular measurement of quality, alongside recent reports highlighting the staggering burden of quality measurement responsibilities placed on clinicians,57-59 there is an ongoing imperative to prioritize among the large list of measures. One such prioritization initiative comes from the American Academy of Hospice and Palliative Medicine and Hospice and Palliative Nurses Association: the Measuring What Matters initiative.60 In this initiative, a technical expert panel and clinical user panel sorted through the expansive list of existing quality measures and prioritized 10 of the most technically impactful and clinically feasible to measure. The successful identification of a top 10 measures list served three goals: guiding measurement efforts by oncologists in providing primary palliative care, assisting busy palliative care clinicians in prioritizing among many measures from which to choose, and, because of peer review by methodologists and clinicians as part of the panel review, providing feasibility for measurement. Often, much attention is paid to developing and testing new quality measures. The Measuring What Matters experience reminds us that emphasizing implementation, by using feasibility and usefulness criteria to guide prioritization of a streamlined list, is also a necessary endeavor for the field.
In addition to the large number of measures, another challenge we face is the lack of quality measures to evaluate the fidelity of specialty palliative care integration into oncology. Cancer-specific palliative and supportive care quality measures must remain focused on the processes of primary and specialist palliative care delivery themselves, such as addressing mucositis and nausea during chemotherapy treatment.61 However, there exists much potential in evaluating the nature of the integration itself, defining measurement characteristics (eg, numerator, denominator, exclusion criteria) more specifically than the existing ESMO guidelines discussed earlier. For example, a proposed quality measure could assess the percentage of patients referred to specialty palliative care for whom an easily accessible collaborative oncology/palliative care comprehensive care plan (process measure), accessible at all times to both teams, has been developed by the third visit. Such a plan could serve as a centralized and accessible repository of the patient’s wishes and preferences and understanding of his or her disease and prognosis, as well as caregiver needs. Another example could be assessment of whether clearly defined triggers or thresholds for consultation are established between oncologists and palliative care specialists (structure measure) and then adhered to in 80% of cases (process measure). Measures regarding coordinated palliative care plans and availability of standardized triggers with associated compliance assessments are not yet standard. Such measures to guide the integration itself would assess the degree to which care at the intersection of the two specialties meets expectations and, if gaps are uncovered, spur improvement efforts to further coordinate care.
BUILDING ON A STRONG FOUNDATION
Standards/guidelines and quality measures addressing palliative care integration into oncology have proliferated around the world, demonstrating remarkable growth since the early 2000s. Reports share a cohesive message: oncology teams should provide dedicated attention to the experience of patients with cancer and regularly use palliative care specialists when criteria-based thresholds are met. We recommend evolutions in a few key areas, including clinicians looking beyond disease severity and prognosis to the distress experienced by patients and their caregivers, their symptom burden, and any impairment in physical function that has or is likely to occur. Furthermore, we recommend advancing the nature of the quality measurement framework for specialty palliative care, including the addition of five categories of outcomes beyond the patient health outcomes in the classic Donabedian framework. Inherently, specialist palliative care aims to improve care across all components of a health system, and therefore, measurement of its outcomes should expand beyond individual patients. Last, we recommend development and testing of new quality measures (and associated measurement specifications) focused on the sharing of palliative care service delivery responsibilities between oncology teams and palliative care specialist teams. Integration efforts have come a long way, and future efforts like those recommended will build upon and strengthen the impressive work to date.
Footnotes
Supported by the Agency for Healthcare Research and Quality (Grant No. K08-HS023681-04) (A.H.K.).
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Administrative support: Arif H. Kamal
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Standards, Guidelines, and Quality Measures for Successful Specialty Palliative Care Integration Into Oncology: Current Approaches and Future Directions
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Arif H. Kamal
Leadership: Prepared Health, Acclivity Health
Stock and Other Ownership Interests: Acclivity Health
Consulting or Advisory Role: Insys Therapeutics, Medtronic, Huron Therapeutics
Research Funding: Cambia Health Foundation
Travel, Accommodations, Expenses: Janssen Oncology
David J. Casarett
Employment: Curio Wellness, Melix, Zelda Therapeutics, Curaleaf, Bristol Hospice
Stock and Other Ownership Interests: Northern Swan, Melix, Curio Wellness
Consulting or Advisory Role: Resolve Digital, Shoppers Drug Mart
Patents, Royalties, Other Intellectual Property: Sole authorship of book Stoned: A Doctor’s Case for Medical Marijuana, Penguin Random House, 2015.
David C. Currow
Consulting or Advisory Role: Mayne Pharma, Specialised Therapeutics, Helsinn Therapeutics
Patents, Royalties, Other Intellectual Property: Intellectual property, Mayne Pharma
Travel, Accommodations, Expenses: Helsinn Therapeutics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes: A systematic review and meta-analysis. JAMA. 2016;316:2104–2114. doi: 10.1001/jama.2016.16840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis MP, Temel JS, Balboni T, et al. A review of the trials which examine early integration of outpatient and home palliative care for patients with serious illnesses. Ann Palliat Med. 2015;4:99–121. doi: 10.3978/j.issn.2224-5820.2015.04.04. [DOI] [PubMed] [Google Scholar]
- 3.El-Jawahri A, Greer JA, Temel JS. Does palliative care improve outcomes for patients with incurable illness? A review of the evidence. J Support Oncol. 2011;9:87–94. doi: 10.1016/j.suponc.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Kamal AH. Time to define high-quality palliative care in oncology. J Clin Oncol. 2013;31:3047. doi: 10.1200/JCO.2013.50.2484. [DOI] [PubMed] [Google Scholar]
- 5.Davis MP, Strasser F, Cherny N. How well is palliative care integrated into cancer care? A MASCC, ESMO, and EAPC project. Support Care Cancer. 2015;23:2677–2685. doi: 10.1007/s00520-015-2630-z. [DOI] [PubMed] [Google Scholar]
- 6.Hui D, Elsayem A, De la Cruz M, et al. Availability and integration of palliative care at US cancer centers. JAMA. 2010;303:1054–1061. doi: 10.1001/jama.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calton BA, Alvarez-Perez A, Portman DG, et al. The current state of palliative care for patients cared for at leading US cancer centers: The 2015 NCCN palliative care survey. J Natl Compr Canc Netw. 2016;14:859–866. doi: 10.6004/jnccn.2016.0090. [DOI] [PubMed] [Google Scholar]
- 8.Kamal AH, Hanson LC, Casarett DJ, et al. The quality imperative for palliative care. J Pain Symptom Manage. 2015;49:243–253. doi: 10.1016/j.jpainsymman.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: ASCO clinical practice guideline update summary. J Oncol Pract. 2017;13:119–121. doi: 10.1200/JOP.2016.017897. [DOI] [PubMed] [Google Scholar]
- 10.Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: The integration of palliative care into standard oncology care. J Clin Oncol. 2012;30:880–887. doi: 10.1200/JCO.2011.38.5161. [DOI] [PubMed] [Google Scholar]
- 11.Dans M, Smith T, Back A, et al. NCCN guidelines insights: Palliative care, version 2.2017. J Natl Compr Canc Netw. 2017;15:989–997. doi: 10.6004/jnccn.2017.0132. [DOI] [PubMed] [Google Scholar]
- 12.Oncology Nursing Society Oncology Nursing Society position: Position statement on palliative care. Oncol Nurs Forum. 2015;42:11–12. [PubMed] [Google Scholar]
- 13.Sheldon LK. Implementing the new commission on cancer standard on palliative care services. Clin J Oncol Nurs. 2014;18(suppl):37–38. doi: 10.1188/14.CJON.S1.37-38. [DOI] [PubMed] [Google Scholar]
- 14.Cherny N, Catane R, Schrijvers D, et al. European Society for Medical Oncology (ESMO) program for the integration of oncology and palliative care: A 5-year review of the designated centers’ incentive program. Ann Oncol. 2010;21:362–369. doi: 10.1093/annonc/mdp318. [DOI] [PubMed] [Google Scholar]
- 15.Radbruch L, de Lima L, Lohmann D, et al. The Prague Charter: Urging governments to relieve suffering and ensure the right to palliative care. Palliat Med. 2013;27:101–102. doi: 10.1177/0269216312473058. [DOI] [PubMed] [Google Scholar]
- 16.Zagonel V, Franciosi V, Brunello A, et al. Position paper of the Italian Association of Medical Oncology on early palliative care in oncology practice (simultaneous care) Tumori. 2017;103:9–14. doi: 10.5301/tj.5000593. [DOI] [PubMed] [Google Scholar]
- 17. German Guideline Program in Oncology: Evidence-based guideline: Palliative care for patients with incurable cancer. https://www.leitlinienprogramm-onkologie.de/fileadmin/_migrated/content_uploads/Guideline_Palliative_Care_Short_Version_01.pdf.
- 18.Berendt J, Stiel S, Simon ST, et al. Integrating palliative care into comprehensive cancer centers: Consensus-based development of best practice recommendations. Oncologist. 2016;21:1241–1249. doi: 10.1634/theoncologist.2016-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malloy P, Boit J, Tarus A, et al. Providing palliative care to patients with cancer: Addressing the needs in Kenya. Asia Pac J Oncol Nurs. 2017;4:45–49. doi: 10.4103/2347-5625.199073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shamieh O, Jazieh AR, MENA Cancer Palliative Care Regional Guidelines Committee Modification and implementation of NCCN guidelines on palliative care in the Middle East and North Africa region. J Natl Compr Canc Netw. 2010;8(suppl 3):S41–S47. doi: 10.6004/jnccn.2010.0124. [DOI] [PubMed] [Google Scholar]
- 21.Jeba J, Atreya S, Chakraborty S, et al. Joint position statement Indian Association of Palliative Care and Academy of Family Physicians of India: The way forward for developing community-based palliative care program throughout India—Policy, education, and service delivery considerations. J Family Med Prim Care. 2018;7:291–302. doi: 10.4103/jfmpc.jfmpc_99_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vitillo R, Puchalski C. World Health Organization authorities promote greater attention and action on palliative care. J Palliat Med. 2014;17:988–989. doi: 10.1089/jpm.2014.9411. [DOI] [PubMed] [Google Scholar]
- 23.Osman H, Shrestha S, Temin S, et al. Palliative care in the global setting: ASCO resource-stratified practice guideline. J Glob Oncol. 2018;4:1–24. doi: 10.1200/JGO.18.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferrell B: National Consensus Project guidelines, fourth edition. J Hosp Palliat Nurs 20:507, 2018.
- 25.Bickel KE, McNiff K, Buss MK, et al. Defining high-quality palliative care in oncology practice: An American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine guidance statement. J Oncol Pract. 2016;12:e828–e838. doi: 10.1200/JOP.2016.010686. [DOI] [PubMed] [Google Scholar]
- 26.Ferrell B, Connor SR, Cordes A, et al. The national agenda for quality palliative care: The National Consensus Project and the National Quality Forum. J Pain Symptom Manage. 2007;33:737–744. doi: 10.1016/j.jpainsymman.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 27.American Academy of Hospice and Palliative Medicine. Center to Advance Palliative Care. Hospice and Palliative Nurses Association. et al. National Consensus Project for Quality Palliative Care: Clinical practice guidelines for quality palliative care, executive summary. J Palliat Med. 2004;7:611–627. doi: 10.1089/jpm.2004.7.611. [DOI] [PubMed] [Google Scholar]
- 28. Ahluwalia SC, Chen C, Raaen L, et al: A systematic review in support of the National Consensus Project clinical practice guidelines for quality palliative care, fourth edition. J Pain Symptom Manage 56:831-870, 2018. [DOI] [PubMed] [Google Scholar]
- 29.Rosenwax LK, McNamara BA, Murray K, et al. Hospital and emergency department use in the last year of life: a baseline for future modifications to end-of-life care. Med J Aust. 2011;194:570–573. doi: 10.5694/j.1326-5377.2011.tb03106.x. [DOI] [PubMed] [Google Scholar]
- 30. The Joint Commission: Certification for palliative care programs. https://www.jointcommission.org/certification/palliative_care.aspx.
- 31.Weaver MS, Heinze KE, Bell CJ, et al. Establishing psychosocial palliative care standards for children and adolescents with cancer and their families: An integrative review. Palliat Med. 2016;30:212–223. doi: 10.1177/0269216315583446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bachmann P, Marti-Massoud C, Blanc-Vincent MP, et al. Standards, options and recommendations: nutritional support in palliative or terminal care of adult patients with progressive cancer [in French] Bull Cancer. 2001;88:985–1006. [PubMed] [Google Scholar]
- 33.Kaasa S, Loge JH, Aapro M, et al. Integration of oncology and palliative care: A Lancet Oncology Commission. Lancet Oncol. 2018;19:e588–e653. doi: 10.1016/S1470-2045(18)30415-7. [DOI] [PubMed] [Google Scholar]
- 34.Waller A, Girgis A, Currow D, et al. Development of the palliative care needs assessment tool (PC-NAT) for use by multi-disciplinary health professionals. Palliat Med. 2008;22:956–964. doi: 10.1177/0269216308098797. [DOI] [PubMed] [Google Scholar]
- 35.Waller A, Girgis A, Lecathelinais C, et al. Validity, reliability and clinical feasibility of a needs assessment tool for people with progressive cancer. Psychooncology. 2010;19:726–733. doi: 10.1002/pon.1624. [DOI] [PubMed] [Google Scholar]
- 36.El-Jawahri A, LeBlanc T, VanDusen H, et al. Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: A randomized clinical trial. JAMA. 2016;316:2094–2103. doi: 10.1001/jama.2016.16786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaufmann TL, Kamal AH. Oncology and palliative care integration: Cocreating quality and value in the era of health care reform. J Oncol Pract. 2017;13:580–588. doi: 10.1200/JOP.2017.023762. [DOI] [PubMed] [Google Scholar]
- 38.Sanderson CR, Currow DC. Palliative care meets immunotherapy: What happens as cancer paradigms change? BMJ Support Palliat Care. 2018;8:431–432. doi: 10.1136/bmjspcare-2018-001598. [DOI] [PubMed] [Google Scholar]
- 39.Temel JS, Shaw AT, Greer JA. Challenge of prognostic uncertainty in the modern era of cancer therapeutics. J Clin Oncol. 2016;34:3605–3608. doi: 10.1200/JCO.2016.67.8573. [DOI] [PubMed] [Google Scholar]
- 40.Adelson K, Paris J, Horton JR, et al. Standardized criteria for palliative care consultation on a solid tumor oncology service reduces downstream health care use. J Oncol Pract. 2017;13:e431–e440. doi: 10.1200/JOP.2016.016808. [DOI] [PubMed] [Google Scholar]
- 41.Swetz KM, Kamal AH. Palliative care. Ann Intern Med. 2018;168:ITC33–ITC48. doi: 10.7326/AITC201803060. [DOI] [PubMed] [Google Scholar]
- 42.Smith CB, Phillips T, Smith TJ. Using the new ASCO clinical practice guideline for palliative care concurrent with oncology care using the TEAM approach. Am Soc Clin Oncol Educ Book. 2017;37:714–723. doi: 10.1200/EDBK_175474. [DOI] [PubMed] [Google Scholar]
- 43.Waller A, Girgis A, Johnson C, et al. Improving outcomes for people with progressive cancer: Interrupted time series trial of a needs assessment intervention. J Pain Symptom Manage. 2012;43:569–581. doi: 10.1016/j.jpainsymman.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 44.Maddocks M, Lovell N, Booth S, et al. Palliative care and management of troublesome symptoms for people with chronic obstructive pulmonary disease. Lancet. 2017;390:988–1002. doi: 10.1016/S0140-6736(17)32127-X. [DOI] [PubMed] [Google Scholar]
- 45. Centers for Medicare and Medicaid Services: Quality measures. https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/qualitymeasures/index.html.
- 46.Donabedian A. Evaluating the quality of medical care. Milbank Mem Fund Q. 1966;44(3) Suppl:166–206. [PubMed] [Google Scholar]
- 47.Bausewein C, Daveson BA, Currow DC, et al. EAPC white paper on outcome measurement in palliative care: Improving practice, attaining outcomes and delivering quality services—Recommendations from the European Association for Palliative Care (EAPC) Task Force on Outcome Measurement. Palliat Med. 2016;30:6–22. doi: 10.1177/0269216315589898. [DOI] [PubMed] [Google Scholar]
- 48.Antunes B, Rodrigues PP, Higginson IJ, et al. Outcome measurement: A scoping review of the literature and future developments in palliative care clinical practice. Ann Palliat Med. 2018;7(suppl 3):S196–S206. doi: 10.21037/apm.2018.07.03. [DOI] [PubMed] [Google Scholar]
- 49.Currow DC, Allingham S, Yates P, et al. Improving national hospice/palliative care service symptom outcomes systematically through point-of-care data collection, structured feedback and benchmarking. Support Care Cancer. 2015;23:307–315. doi: 10.1007/s00520-014-2351-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pidgeon T, Johnson CE, Currow D, et al. A survey of patients’ experience of pain and other symptoms while receiving care from palliative care services. BMJ Support Palliat Care. 2016;6:315–322. doi: 10.1136/bmjspcare-2014-000748. [DOI] [PubMed] [Google Scholar]
- 51.Morrison RS, Dietrich J, Ladwig S, et al. Palliative care consultation teams cut hospital costs for Medicaid beneficiaries. Health Aff (Millwood) 2011;30:454–463. doi: 10.1377/hlthaff.2010.0929. [DOI] [PubMed] [Google Scholar]
- 52.Siderow S, Silvers A, Meier DE. Palliative care improves quality of care, lowers costs. Manag Care. 2016;25:40–41. [PubMed] [Google Scholar]
- 53.Lorenz KA, Lynn J, Dy S, et al. Quality measures for symptoms and advance care planning in cancer: A systematic review. J Clin Oncol. 2006;24:4933–4938. doi: 10.1200/JCO.2006.06.8650. [DOI] [PubMed] [Google Scholar]
- 54.Pasman HR, Brandt HE, Deliens L, et al. Quality indicators for palliative care: A systematic review. J Pain Symptom Manage. 2009;38:145–156. doi: 10.1016/j.jpainsymman.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 55.Kamal AH, Gradison M, Maguire JM, et al. Quality measures for palliative care in patients with cancer: A systematic review. J Oncol Pract. 2014;10:281–287. doi: 10.1200/JOP.2013.001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Roo ML, Leemans K, Claessen SJ, et al. Quality indicators for palliative care: Update of a systematic review. J Pain Symptom Manage. 2013;46:556–572. doi: 10.1016/j.jpainsymman.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 57.Casalino LP, Gans D, Weber R, et al. US physician practices spend more than $15.4 billion annually to report quality measures. Health Aff (Millwood) 2016;35:401–406. doi: 10.1377/hlthaff.2015.1258. [DOI] [PubMed] [Google Scholar]
- 58.Sheldon TA. The healthcare quality measurement industry: Time to slow the juggernaut? Qual Saf Health Care. 2005;14:3–4. doi: 10.1136/qshc.2004.013185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilensky G. The need to simplify measuring quality in health care. JAMA. 2018;319:2369–2370. doi: 10.1001/jama.2018.6858. [DOI] [PubMed] [Google Scholar]
- 60.Dy SM, Kiley KB, Ast K, et al. Measuring what matters: Top-ranked quality indicators for hospice and palliative care from the American Academy of Hospice and Palliative Medicine and Hospice and Palliative Nurses Association. J Pain Symptom Manage. 2015;49:773–781. doi: 10.1016/j.jpainsymman.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 61.Lorenz KA, Dy SM, Naeim A, et al. Quality measures for supportive cancer care: The Cancer Quality-ASSIST Project. J Pain Symptom Manage. 2009;37:943–964. doi: 10.1016/j.jpainsymman.2008.05.018. [DOI] [PubMed] [Google Scholar]