Abstract
Objective The main purpose of this article is to examine the prevalence, incidence, sociodemographic, and clinical characteristics of mental health disorders (MHDs) among patients with skull base malignancies.
Design Retrospective cohort study.
Settings/Participants Six-thousand seven-hundred sixty sinonasal/skull base cancer patients in the MarketScan database between 2005 and 2014.
Main Outcome Measures Frequency of MHDs pre- and post-diagnosis in patients harboring sinonasal/skull base malignancies.
Results A significant increase in MHDs was noted from pre- to post-cancer diagnosis (22 vs 31%, p < 0.0001). Despite an increase in the prevalence rate, the demographic profile of patients with MHDs post-diagnosis remained similar to pre-diagnosis. Those patients harboring MHDs were, however, more likely to be women (62.7 vs 47.4%), and carry a history of smoking (40.9 vs 26.3%) than those without MHDs. These comparisons were statistically significant ( p < 0.0001).
Conclusion The prevalence of MHDs increases following a diagnosis of a sinonasal/skull base malignancy. Patients with MHDs were more likely to be women and smokers.
Keywords: sinonasal cancer, skull base cancer, mental health disorders, survivorship
Introduction
The diagnosis of cancer is a known psychological stressor, manifesting in mental health disorders (MHDs) such as depression to anxiety. 1 In particular, patients with sinonasal/skull base malignancies experience unique challenges associated with visual changes, anosmia, the social stigma of facial disfiguration, and pain associated with nasal obstruction. They may also experience neuropsychiatric deficits from targeted radiation therapy and resection involving anatomical regions regulating mood and affect.
Many studies have investigated the effects of cancer diagnosis on MHDs, but it remains unclear how sinonasal/skull base malignancies in particular are associated with MHDs. 2 It has been suggested that quality of life is lowered in this patient population due to the significant psychological stress, but the level of MHDs has not been measured. 3 Measuring the mental health status unique to skull base malignancies may lead to measures, which improve quality of life of these patients and reduce healthcare costs. 4
While the link between MHDs and cancer has been reported for decades, the relationship between skull base malignancies and MHDs has not been fully elucidated. We suspect that the diagnosis and treatment of these specific cancers are a significant stressor and lead to clinical MHDs, namely anxiety and depression. We hypothesize that the prevalence of MHDs is higher in the sinonasal/skull base malignancy patient population. We perform a large, retrospective cohort study seek to address whether there is a temporal relationship between sinonasal/skull base cancer and MHD diagnosis, which demographic variables confer an increased risk, and the prevalence of MHDs in this population.
Methods
Study Design and Data Source
We performed a retrospective cohort study of skull base cancer patients from the MarketScan database. The Truven Health MarketScan database contains claims-based information of nearly 150 million patients in the United States from its three core databases—Commercial, Medicare Supplemental, and Medicaid ( https://truvenhealth.com ). MarketScan's claim-based data enables large-scale, comprehensive analysis by providing clinical information about the longitudinal care of patients, allowing this study to compare mental health prevalence temporally, between pre-diagnosis and post-diagnosis period. This study was approved by the Institutional Review Board of the Penn State College of Medicine, Pennsylvania, United States.
The diagnosis of skull base neoplasms was identified by the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for nasopharyngeal (147.0–147.9) and nasal cavity/sinus cancer (160.0, 160.2–160.9). The ICD-9-CM codes for psychiatric disorders and dispensing records of antianxiety and antidepression medications served as sources for tracking the presence of MHDs. The ICD-9-CM codes used to capture mental health diagnoses in the study population include the following diagnosis: episodic mood disorders (296.0–296.9), including depression, anxiety (300.0–300.9), drug dependence (304.0–304.9), adjustment disorder (309.0–309.1), and major depressive disorder, not otherwise specified (311.0–311.9). In addition, patients who were prescribed the antidepressants listed in ( Supplementary Table S1 , online only) were identified as having MHDs. “Pre-diagnosis” was defined as 6 months before the index date for ICD-9-CM diagnosis of cancer, and “post-diagnosis” was defined as from the index date for cancer diagnosis to 1 year after this date. Patient characteristics such as smoking and alcohol use are not directly captured by the claims database. Smoking history was coded for using the ICD-9-CM codes for chronic lung disease as an analogue, and history of alcohol 9 use was coded using the ICD-9-CM codes for alcohol dependence.
Statistical Analysis
A total of 6760 subjects meeting the inclusion criteria served as the study sample. The inclusion criteria of the cohort consisted of nasal cavity sinus and nasopharynx cancer patients aged 18 years or older who had continuous enrollment in claims. There were two major time points analyzed in this study: 6 months before index date diagnosis versus 3 months to 1 year after the index date. We defined the date of skull base cancer diagnosis as the index date. Treatment modalities were identified by Current Procedure Terminology-4 and ICD-9-CM codes for radiation, chemotherapy, and surgery.
We compared the frequency of MHDs 6 months before the date of skull base cancer diagnosis and compared it to the frequency of MHDs between 3 months to 1 year after the date of cancer diagnosis. Another point of comparison involved comparing those who only developed MHDs within 1 year after the cancer diagnosis to patients who never developed MHDs throughout the illness continuum. Pearson chi-square tests for independence were used for the above categorical comparisons.
Multivariable analysis was performed using binary logistic regression to assess the nature and strength of the association between dependent and independent variable by controlling the effect of potential confounders such as age, gender, history of smoking, and US regions. The descriptive results are reported as proportions and the multivariable logistic regression results are presented as adjusted odds ratios (OR) with 95% confidence intervals. The statistical tests were reported as significant if the level of significance is less than α equal to 0.05. The descriptive statistics and multivariable analysis were conducted using SAS version 9.4 (SAS Institute, Cary, North Carolina, United States).
Results
Table 1 delineates the sociodemographic characteristics of the patient cohort with MHDs and without MHDs. The majority of the patients (74%) were between 45 and 64 years of age. Fifty-eight percent of the cohort had cancer of the nasopharynx ( n = 3897). A slight female predominance was observed (female to male ratio = 1.12). The geographic distribution of the cohort was concentrated in the southern states, which comprise 37% of the cohort. Only 2% of the sinonasal cancer patients had a positive history of alcohol use, whereas 32% of the patients had a positive smoking history. A 9% absolute increase in MHDs was reported from the pre- to post-cancer diagnosis period (22 vs 31%, p < 0.0001).
Table 1. Comparison of characteristics between patients with and without MHDs.
| Characteristics | No MHDs n = 4336 (%) |
MHDs n = 2424 (%) |
|---|---|---|
| Mean age ± SD | 49.32 ± 10.69 | 50.54 ± 9.43 |
| Age | ||
| 18–34 | 463 (10.7) | 163 (6.7) |
| 35–44 | 721 (16.6) | 375 (15.5) |
| 45–54 | 1461 (33.7) | 908 (37.5) |
| 55–64 | 1691 (39.0) | 978 (40.4) |
| Sex | ||
| Male | 2282 (52.6) | 905 (37.3) |
| Female | 2054 (47.4) | 1519 (62.7) |
| US region | ||
| Northeast | 816 (18.8) | 371 (15.3) |
| Midwest | 864 (19.9) | 576 (23.8) |
| South | 1576 (36.4) | 945 (39.0) |
| West | 1030 (23.8) | 511 (21.1) |
| Unknown | 50 (1.2) | 21 (0.9) |
| History of smoking | ||
| No | 3196 (73.7) | 1432 (59.1) |
| Yes | 1140 (26.3) | 992 (40.9) |
| History of alcohol dependence | ||
| No | 4280 (98.7) | 2345 (96.7) |
| Yes | 56 (1.3) | 79 (3.3) |
| Type of cancer | ||
| Sinonasal | 1895 (43.7) | 968 (39.9) |
| Nasopharynx | 2441 (56.3) | 1456 (60.1) |
| Treatment modality | ||
| Chemotherapy | ||
| No | 3405 (78.5) | 1528 (63.0) |
| Yes | 931 (21.5) | 896 (37.0) |
| Radiation | ||
| No | 3161 (72.9) | 1347 (55.6) |
| Yes | 1175 (27.1) | 1077 (44.4) |
| Surgery | ||
| No | 615 (14.2) | 174 (7.2) |
| Yes | 3721 (85.8) | 2250 (92.8) |
Abbreviations: MHDs, mental health disorders; SD, standard deviation.
All comparisons between the MHD and no MHD populations were significant with p < 0.0001.
Table 2 delineates the adjusted multivariable OR for the association of sociodemographic factors with MHDs in patients with sinonasal/skull base malignancies. The presence of cancer, sex, smoking, and alcohol use had significant associations with MHDs. Our results showed that patients with MHDs were more likely to be women (63 vs 47%), and carry a history of smoking (41 vs 26%) than those without MHDs. A history of alcohol use was more prominent in patients with MHDs than those without (3 vs 1%). 23.4% of the patients with sinonasal/skull base malignancies had the diagnosis of depression, whereas 28.1% of patients had the diagnosis of anxiety. These comparisons were statistically significant ( p < 0.0001).
Table 2. Multivariate odds ratios for associations between sociodemographic factors and MHDs.
| Sociodemographic factors | Odds ratio, adjusted (95% CI) |
|---|---|
| Presence of cancer | |
| Yes | 2.58 (2.42–2.74) |
| No | 1 (Reference) |
| Sex | |
| Female | 2.18 (2.12–2.24) |
| Male | 1 (Reference) |
| US Region | |
| Northeast | 0.87 (0.83–0.91) |
| South | 1.02 (0.99–1.05) |
| West | 0.97 (0.94–1.01) |
| Midwest | 1 (Reference) |
| Age of diagnosis | 1.01 (1.01–1.01) |
| Type of residence | |
| Rural | 1.06 (1.02–1.10) |
| Urban | 1 (Reference) |
| Year of diagnosis | 0.90 (0.90–0.91) |
| History of smoking | |
| Yes | 1.82 (1.76–1.87) |
| No | 1 (Reference) |
| History of alcohol use | |
| Yes | 2.90 (2.68–3.14) |
| No | 1 (Reference) |
Abbreviations: CI, confidence interval; MHDs, mental health disorders.
All odds ratios were statistically significant with p < 0.0001.
Fig. 1 shows comparisons of patients without prior mental health history who developed MHDs after the cancer diagnosis and those who never developed MHDs. Female gender was associated with an OR of 1.38 (95% CI: 1.19–1.60) in developing MHDs compared with male patients with skull base cancer. Receiving radiation therapy was associated with 2.14 (95% CI: 1.78–2.56) times the odds of developing MHDs in the skull base cancer patient population, followed by surgery (OR 1.82, 95% CI: 1.34–2.45) and chemotherapy (OR 1.63, 95% CI: 1.35–1.96). History of smoking (OR 1.52, 95% CI: 1.30–1.78) and alcohol dependence (OR 3.18, 95% CI: 2.03–4.96) was associated with an increased risk of developing MHDs. There were no significant differences in age of diagnosis, year of diagnosis, US regions, and type of residence (urban vs rural).
Fig. 1.
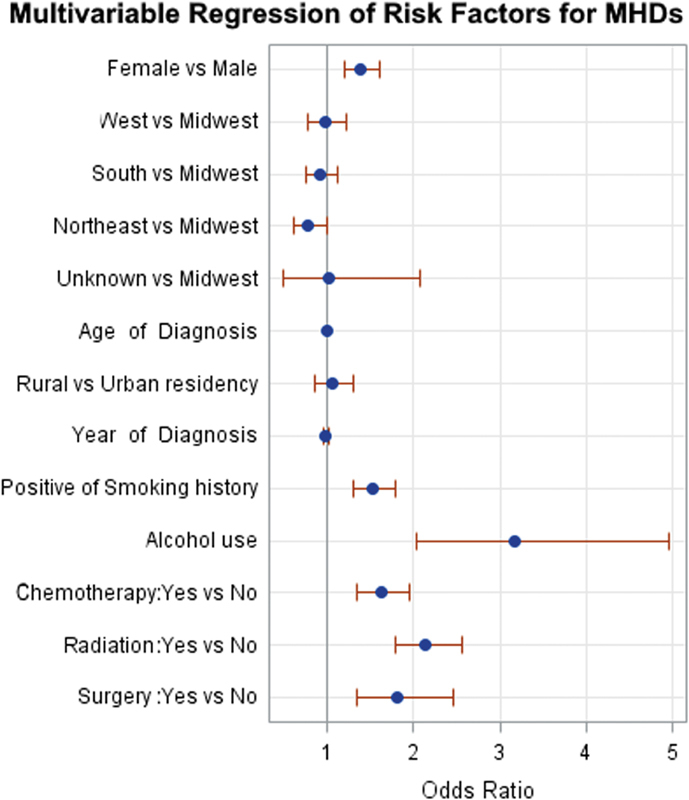
Logistic regression plot of odds ratios and 95% confidence intervals comparing patients who developed mental health disorders (MHDs) after skull base cancer diagnosis to those who never MHDs ( p < 0.0001).
Discussion
In this study, we measured the prevalence of MHDs in patients before the diagnosis of skull base malignancies and 1 year after the cancer diagnosis. A higher prevalence of MHDs was observed in skull base cancer patients compared with the general US population. Moreover, there was a significant increase in MHD prevalence after the diagnosis of cancer. Factors associated with increased psychiatric morbidity in skull base cancer patients include female gender, smoking, alcohol history, and residence in the southern states. The strongest association was shown between alcohol exposure and development of MHDs. Although smokers were more likely to have MHDs, it had a lower strength of association in comparison with alcohol use.
Our data supports that the diagnosis of cancer is a risk factor for MHDs. Possible explanations for the association between skull base cancer and MHD include the major craniofacial disfigurements associated with this particular type of cancer as well as treatment modalities. 5 Facial disfigurement is a common adverse effect of local extension and/or surgical treatment that imparts major social consequences, in the form of employment discrimination and interpersonal relationships. 6 It can also affect the senses resulting in a severe decline in quality of life. Vision changes from surgery and radiation can lead to blindness. 7 Anosmia often presents as an initial symptom of sinonasal cancer and is associated with a lower quality of life. 8 Quality of life has been shown to predict depression both in terms of diagnosis and severity. 9
The MHDs included in this study are depression, anxiety, and adjustment disorder. In particular, 24.5% of the skull base cancer patients received the diagnosis of depression, while 29.2% had anxiety disorder. A multicenter study that delineated the rates of psychiatric disorders making up the MHD diagnosis had 69% adjustment disorder, 13% major depressive disorder, and 4% anxiety disorder. Unlike this study, we observed a higher proportion of patients with diagnosis of anxiety than depression. Higher prevalence of anxiety may reflect the initial trauma associated with diagnosis since we tracked patients up to 1 year after the cancer diagnosis.
Our second aim was to identify risk factors particularly associated with skull base malignancies. We identified three major risk factors—female gender, history of smoking, and alcohol use. The strongest risk factor was alcohol use, which was associated with 2.90 times the likelihood of developing MHDs. The link between alcohol use and MHDs is well established, and this value is similar to the reported value in a study that investigated psychiatric comorbidities in patients with alcohol and other drug use disorders (OR = 2.3). 10 A plausible explanation for the higher OR may indicate the heightened use of maladaptive chemical coping mechanisms such as alcohol use after the acute stressor of cancer diagnosis, further exacerbating their mental health.
After the initial evaluation of patients, it may be helpful to identify this high-risk cohort with the routine use of CAGE or Alcohol Use Disorders Identification Test (AUDIT) questionnaire. 11 CAGE is an acronym for “cut-annoyed-guilty-eye opener” which represents the four questions that can be asked in an office setting to quickly screen for alcohol dependence. For males, two “yes” responses are considered a positive result, whereas for females, one “yes” would be a positive result. 12 The AUDIT is a reliable and validated 10-item screening questionnaire that was developed by the World Health Organization. These screening questionnaires can be used in an outpatient setting during initial consultations or preoperative visits to capture those suffering from MHDs and address the psychosocial issues that may exacerbate their treatment outcome. 13 14
Our study showed that women with skull base neoplasms are 2.18 times more likely to develop MHDs. There are two possible explanations for this risk factor. Many studies illustrated that females utilize mental health services more than males, which make it easier to capture MHDs. Males are also less likely to report mood symptoms and are less readily captured in behavioral health studies examining MHD prevalence. 15 16 17 18 Second, women are more likely to suffer from mood disorders, which we measured as the proxy for MHDs. On average, women are twice as more likely to be diagnosed with a mood disorder than men, and our finding parallels reported values in the literature. 19 20 In terms of symptoms, depressed women are more likely to experience somatic symptoms than men, who are more likely to report depressed mood not associated with fatigue and anxiety. 21 The gender differences in symptomatology of mood disorders may facilitate identification of those at risk.
History of smoking was the third significant risk factor associated with 1.82 times the likelihood of developing MHDs in the skull base cancer patients. Our finding corresponds to the reported value in literature that smokers are twice as likely to have mental illnesses in the United States. 22 Additionally, geographic differences, age of diagnosis, type of residence, and year of diagnosis were not significantly associated with MHDs in skull base cancer patients.
Finally, our study revealed that psychological outcomes differed slightly based on treatment modality. Highest prevalence of MHDs was observed in those who received radiation therapy compared with other modalities. Radiation could be a risk factor for MHDs because patients who underwent this treatment modality may have higher staged tumors than those who had definitive surgery. In reality, most of the patients in this cohort received two or more treatment modalities. Presence of MHDs has been found to be a significant factor influencing patients' quality of life undergoing radiotherapy. 24 Assessing the level of mental health comorbidity and need for psychosocial support will be critical in patients receiving treatment, especially with radiation.
Limitations
Retrospective studies may be subject to selection bias. Our cohort was matched to a noncancer patient group by age, gender, and exposure to cigarettes and alcohol to reduce such bias. Moreover, this study may underestimate the prevalence of MHDs as the selection criteria were based on the documentation of psychiatric diagnoses per ICD-9 codes. Patients who developed an MHD may not seek care. Accounting for the missed patient population will increase the number of patients suffering from MHDs in this cancer population. By excluding antipsychotics, mood stabilizers, and antiepileptics used off-label in psychiatric settings, we may have missed some skull base cancer patients with MHDs. However, because cancer patients suffer from significant emotional stress, cancer diagnosis is much more commonly associated with mood disorders such as depression and anxiety. 25 Health access is another important factor that may contribute to a missed MHD diagnosis. Those who had suffered MHDs for many years prior may not have accessed the healthcare system until the cancer diagnosis for reasons that may involve the stigma associated with seeking mental healthcare. 26
According to the 2015 National Survey on Drug Use and Health (NSDUH), 6.2% of the US population aged 18 or older had alcohol use disorder, which is higher than the rate of alcohol dependence we found in our study, 27 demonstrating a potential limitation of a claims-based database. NSDUH, however, incorporates both alcohol abuse and dependence in calculating the incidence, which would result in a higher reported rate than our study results. For our study, we classified alcohol dependence as the marker of alcoholism, given that this would have a higher likelihood of being captured by a claims-based database. However, this is dependent on an accurate capture and billing of the ICD-9 codes for alcohol dependence within the claims database.
Another potential limitation is that the extent of surgery and stage of tumor were not included in this study. The stage and extent of surgery may have direct correlation to post-operative morbidities and possible MHD incidence. 28 Information on the extent of surgery may elucidate the role of surgery in skull base cancer patients with MHDs. In addition, quality of life metrics were not captured in this database. MHDs are strongly associated with decreasing quality of life, and data on the quality of life of skull base cancer patients may yield insight into specific domains affected by the cancer diagnosis and treatment. 29
Future Directions
To capture the patient population not utilizing mental health services, we recommend a prospective study that identifies MHD patients by using both rating scales and a large database of MHD diagnoses. This would help identify the uncaptured population and allow for analysis of the differences between those utilizing mental health services and those who do not. However, given the rarity of this condition, a prospective study will involve multi-institutional collaboration for a reportable sample size. Future studies in elucidating the significance of MHD prevalence will help improve the quality of life and survivorship of the patients with skull base malignancies.
More research should be done on targeted interventions to help prevent or manage mood disorders after the cancer diagnosis to better meet the psychosocial needs of the vulnerable patient population. As low socioeconomic status is a well-known risk factor for mental illnesses, it will be helpful to investigate the link between socioeconomic status and MHDs in the skull base cohort. 30 In-office screening of MHDs with a questionnaire for all patients with skull base malignancies would greatly improve identification of patients suffering from these debilitating diseases and improve the quality of care. Future research may evaluate the efficacy of global screener of distress such as the distress thermometer in this unique population.
Conclusion
MHDs remain poorly understood in the sinonasal and skull base malignancy population. In a large-scale administrative database, we found a baseline level of 22% MHD, which increased significantly to 31% following the diagnosis. Patients with MHDs were more likely to be women and smokers. The levels of MHDs were higher than the general population according to NSDUH. Psychosocial needs of patients with skull base malignancies should be addressed in treatment discussions routinely to facilitate recovery and improve quality of life.
Funding Statement
Funding No external funding was received.
Footnotes
Conflict of Interest None.
Supplementary Material
References
- 1.Derogatis L R, Morrow G R, Fetting J et al. The prevalence of psychiatric disorders among cancer patients. JAMA. 1983;249(06):751–757. doi: 10.1001/jama.249.6.751. [DOI] [PubMed] [Google Scholar]
- 2.Ojo B, Genden E M, Teng M S, Milbury K, Misiukiewicz K J, Badr H. A systematic review of head and neck cancer quality of life assessment instruments. Oral Oncol. 2012;48(10):923–937. doi: 10.1016/j.oraloncology.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fasunla A J, Omokanye H. Psychological manifestation of patients with sinonasal tumors: an obscured and commonly neglected aspect of the disease. Otolaryngol (Sunnyvale) 2011;1(02):107. [Google Scholar]
- 4.Greenberg P E, Stiglin L E, Finkelstein S N, Berndt E R. The economic burden of depression in 1990. J Clin Psychiatry. 1993;54(11):405–418. [PubMed] [Google Scholar]
- 5.Palme C E, Irish J C, Gullane P J, Katz M R, Devins G M, Bachar G. Quality of life analysis in patients with anterior skull base neoplasms. Head Neck. 2009;31(10):1326–1334. doi: 10.1002/hed.21102. [DOI] [PubMed] [Google Scholar]
- 6.Stone A, Wright T. When your face doesn't fit: employment discrimination against people with facial disfigurements: when your face doesn't fit. J Appl Soc Psychol. 2013;43(03):515–526. [Google Scholar]
- 7.Hoppe B S, Wolden S L, Zelefsky M J et al. Postoperative intensity-modulated radiation therapy for cancers of the paranasal sinuses, nasal cavity, and lacrimal glands: technique, early outcomes, and toxicity. Head Neck. 2008;30(07):925–932. doi: 10.1002/hed.20800. [DOI] [PubMed] [Google Scholar]
- 8.Neuland C, Bitter T, Marschner H, Gudziol H, Guntinas-Lichius O. Health-related and specific olfaction-related quality of life in patients with chronic functional anosmia or severe hyposmia. Laryngoscope. 2011;121(04):867–872. doi: 10.1002/lary.21387. [DOI] [PubMed] [Google Scholar]
- 9.Özabacı N. Quality of life as a predictor of depression. Procedia Soc Behav Sci. 2010;2(02):2458–2463. [Google Scholar]
- 10.Regier D A, Farmer M E, Rae D S et al. Comorbidity of mental disorders with alcohol and other drug abuse. results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511–2518. [PubMed] [Google Scholar]
- 11.Del Fabbro E. Assessment and management of chemical coping in patients with cancer. J Clin Oncol. 2014;32(16):1734–1738. doi: 10.1200/JCO.2013.52.5170. [DOI] [PubMed] [Google Scholar]
- 12.Mayfield D, McLeod G, Hall P. The CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry. 1974;131(10):1121–1123. doi: 10.1176/ajp.131.10.1121. [DOI] [PubMed] [Google Scholar]
- 13.Saunders J B, Aasland O G, Amundsen A, Grant M. Alcohol consumption and related problems among primary health care patients: WHO collaborative project on early detection of persons with harmful alcohol consumption--I. Addiction. 1993;88(03):349–362. doi: 10.1111/j.1360-0443.1993.tb00822.x. [DOI] [PubMed] [Google Scholar]
- 14.Saunders J B, Aasland O G, Babor T F, de la Fuente J R, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on early detection of persons with harmful alcohol consumption–II. Addiction. 1993;88(06):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 15.Hibbard J H, Pope C R. Gender roles, illness orientation and use of medical services. Soc Sci Med. 1983;17(03):129–137. doi: 10.1016/0277-9536(83)90246-0. [DOI] [PubMed] [Google Scholar]
- 16.Cleary P D, Mechanic D, Greenley J R. Sex differences in medical care utilization: an empirical investigation. J Health Soc Behav. 1982;23(02):106–119. [PubMed] [Google Scholar]
- 17.Waldron I. Sex differences in illness incidence, prognosis and mortality: issues and evidence. Soc Sci Med. 1983;17(16):1107–1123. doi: 10.1016/0277-9536(83)90004-7. [DOI] [PubMed] [Google Scholar]
- 18.Verbrugge L M, Wingard D L. Sex differentials in health and mortality. Women Health. 1987;12(02):103–145. doi: 10.1300/J013v12n02_07. [DOI] [PubMed] [Google Scholar]
- 19.Kornstein S G, Schatzberg A F, Thase M E et al. Gender differences in chronic major and double depression. J Affect Disord. 2000;60(01):1–11. doi: 10.1016/s0165-0327(99)00158-5. [DOI] [PubMed] [Google Scholar]
- 20.Angst J, Dobler-Mikola A.Do the diagnostic criteria determine the sex ratio in depression? J Affect Disord 19847(3-4):189–198. [DOI] [PubMed] [Google Scholar]
- 21.Silverstein B. Gender difference in the prevalence of clinical depression: the role played by depression associated with somatic symptoms. Am J Psychiatry. 1999;156(03):480–482. doi: 10.1176/ajp.156.3.480. [DOI] [PubMed] [Google Scholar]
- 22.Lasser K, Boyd J W, Woolhandler S, Himmelstein D U, McCormick D, Bor D H. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284(20):2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 23.Frick E, Tyroller M, Panzer M. Anxiety, depression and quality of life of cancer patients undergoing radiation therapy: a cross-sectional study in a community hospital outpatient centre. Eur J Cancer Care (Engl) 2007;16(02):130–136. doi: 10.1111/j.1365-2354.2006.00720.x. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell A J, Chan M, Bhatti H et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. 2011;12(02):160–174. doi: 10.1016/S1470-2045(11)70002-X. [DOI] [PubMed] [Google Scholar]
- 25.Clement S, Schauman O, Graham T et al. What is the impact of mental health-related stigma on help-seeking? A systematic review of quantitative and qualitative studies. Psychol Med. 2015;45(01):11–27. doi: 10.1017/S0033291714000129. [DOI] [PubMed] [Google Scholar]
- 26.Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality.Key substance use and mental health indicators in the united states: results from the 2015 national survey on drug use and healthRockville, MD: U.S. Department of Health and Human Serviceshttps://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2015/NSDUH-FFR1-2015/NSDUH-FFR1-2015.pdf
- 27.Wu V, Cusimano M D, Lee J M. Extent of surgery in endoscopic transsphenoidal skull base approaches and the effects on sinonasal morbidity. Am J Rhinol Allergy. 2018;32(01):52–56. doi: 10.2500/ajra.2018.32.4499. [DOI] [PubMed] [Google Scholar]
- 28.Sharpe H, Patalay P, Fink E, Vostanis P, Deighton J, Wolpert M. Exploring the relationship between quality of life and mental health problems in children: implications for measurement and practice. Eur Child Adolesc Psychiatry. 2016;25(06):659–667. doi: 10.1007/s00787-015-0774-5. [DOI] [PubMed] [Google Scholar]
- 29.Weinberger A H, Gbedemah M, Martinez A M, Nash D, Galea S, Goodwin R D. Trends in depression prevalence in the USA from 2005 to 2015: widening disparities in vulnerable groups. Psychol Med. 2018;48(08):1308–1315. doi: 10.1017/S0033291717002781. [DOI] [PubMed] [Google Scholar]
- 30.Oh S, Salas-Wright C P, Vaughn M G. Trends in depression among low-income mothers in the United States, 2005-2015. J Affect Disord. 2018;235:72–75. doi: 10.1016/j.jad.2018.04.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


