Abstract
Objective Microsurgical resection of cavernous sinus meningiomas (CSM) is associated with a high rate of incomplete resection, recurrence, and the risk for permanent, severe cranial nerve deficits. Stereotactic radiosurgery (SRS) has evolved as alternative treatment for primary and recurrent CSM. Here, we report about the long-term clinical and radiological follow-up (FU) of a unique cohort of patients with CSM treated with LINAC or Cyberknife based SRS.
Methods In this single-center retrospective analysis, we include all patients with CSM who underwent single fraction SRS between 1993 and 2016. Clinical and radiological tumor control were evaluated by the Kaplan–Meier method. Additionally, patient data were analyzed in terms of symptom control and incidence of side effects rated by the common terminology criteria for adverse events (CTCAE; v4.03).
Results 116 patients (female/male = 91/25; median age, 54 years; range, 33–82 years) were included. Mean tumor volume was 5.7 ± 3.3 cm 3 (range, 0.6–16.2 cm 3 ), the median marginal dose was 12.6 Gy applied to isodose levels of 75%. Median clinical FU was 55 months (range, 3–226 months). Tumor control was 98% after 2 and 5 years and 90% after 10 years. Twelve patients (10.3%) had permanent or transient radiation related toxicity (CTCAE I–III). An improvement of symptoms was observed in 26.7% of the symptomatic patients ( n = 20 of 75).
Conclusion SRS for CSM provides excellent long-term tumor and symptom control without considerable permanent side effects. Thus, SRS should be considered when counseling patients suffering from CSM.
Keywords: radiosurgery, meningioma, skull base, Cyberknife
Introduction
About 1% of all intracranial tumors are cavernous sinus meningiomas (CSM). 1 In general, CSM are regarded as benign skull base tumors with growth rates ranging between 0.02 and 0.24 cm 3 /year. 2 Following a review of Klinger et al, 3 only 34 to 77% of CSM show signs of growth within 4 years. Thus, observation seems a useful strategy while active treatment is usually only considered for cases with tumor growth and/or clinical symptoms. Due to the complex anatomy of the anterolateral skull base and the frequent involvement of cranial nerves (CN), surgical treatment of CSM has always been a challenge for neurosurgeons. Especially in cases where the tumor invades the cavernous sinus, complete resection is often impossible or associated with high morbidity and mortality and deterioration of quality of life. 3 4 Also, it became obvious in the recent decade that even an aggressive surgical approach cannot totally erase CSM in particular in cases with microscopic infiltration of neurovascular elements. 5
The innovations in radiotherapy and radiosurgery opened alternative approaches for the definitive or adjuvant treatment of CSM. Stereotactic radiosurgery (SRS) has the potential to induce long-term tumor control while maintaining neurovascular and CN function. 6 7 However, as SRS and surgery can be used in sequence or combination, the choice of the right treatment strategy and point in time for SRS are still part of a controversial debate. Here, we present the clinical and outcome data of patients with CSM treated with SRS by use of a modified linear accelerator (LINAC) or Cyberknife in a center with 25 years of experience in radiosurgery.
Material and Methods
Subjects and Posttherapeutic Evaluation
In this single-center retrospective analysis, patients with unilateral CSM who were treated with single-session SRS by use of a modified LINAC or by robotic radiosurgery with the Cyberknife between 1991 and 2016 were analyzed. The LINAC was used for radiosurgery until 2012. Since 2013 all patient were treated with the Cyberknife due to a change of the radiation system at our center. All meningiomas with contact to the cavernous sinus with intra- and/or extracavernous localization were included. Indications for SRS treatment were either radiologically determined tumor growth and/or clinical deterioration after surgery or after a period of observation. After therapy, clinical evaluations and magnetic resonance imaging (MRI) were normally performed at 6 months within the 1st year after radiosurgery, and annually thereafter in all patients.
Documented baseline data included patient characteristics (age, gender, and tumor volume) and relevant radiosurgical parameters (coverage, marginal dose, and isodose level). Clinical evaluation was performed by interviewing individual patients about symptoms and clinical condition at the follow-up (FU) visits. Symptom control after SRS was assumed in case of improved or stable clinical condition and absence of new complaints. For the evaluation of side effects, we collected reports of new or worsened symptoms after SRS. New symptom which occur simultaneously with significant tumor growth (section 2.2) were not considered as radiation induced toxicity. All symptoms were rated according to the CTCAE; (v4.03, pp. 51–55, chapter “Nervous system disorders”).
Tumor Control
For evaluation of radiological tumor control, the pretreatment MRI data were compared with the MRI images at last FU. Radiological tumor control was classified according to 8 such that the tumor regression was assumed in cases of more than 10% volume loss, tumor progression was diagnosed when the volume increased by > 10%, and the tumor was presumed to be stable when its volume changes did not exceed 10%. Postradiosurgery tumor volumes were calculated using OsiriX software (Pixmeo SARL, Switzerland). Evaluation of tumor volume was not feasible in some cases and FU images were only available on paper print especially until 2005. In these cases, the tumor size was estimated by multiplying its dimension in anteroposterior and mediolateral direction. Clinical tumor control was defined as freedom from planned or realized reintervention (e.g., repeated radiosurgery or microsurgery).
Radiosurgery with Modified LINAC
For radiosurgical treatment with the LINAC, the patient's head was immobilized under local anesthesia with a stereotactic frame (Riechert–Mundinger) followed by contrast enhanced high-resolution computed tomography (CT). The CT and a high-resolution MRI (routinely after 1996) obtained before treatment were registered using the software STP (STP 3.3 and 3.5, Howmedica Leibinger, Freiburg, Germany). Subsequently, the tumor and the adjacent critical structures (e.g., brainstem, optic pathway, and trigeminal nerve) were outlined by a neurosurgeon experienced in stereotactic radiosurgery, and a treatment plan was generated by a medical physicist. The used dose tolerance limit for the optic pathway was a maximum of 8 Gy referring to Grimm et al. 9 The final irradiation plan was reviewed and signed in an interdisciplinary consensus meeting between the stereotactic neurosurgeon, a radiation oncologist also experienced in SRS, and the medical physicist. Subsequently, the radiosurgical treatment was performed by using a LINAC (SL25, ELEKTA, 6 MV photon beams) equipped with tertiary changeable collimators with diameters ranging from 4.5 to 45 mm and a noncoplanar rotational scheme (6–10 arcs ranging from 20–160 or 200–340 degrees), as previously described by Ruge et al. 10 Dose conformation was achieved by use of multiple isocenter technique. 11
Radiosurgery with Cyberknife
Prior to Cyberknife treatment, 12 a high-resolution contrast-enhanced CT was acquired and merged with a high-resolution contrast-enhanced MRI with T1- and T2-weighted images. Cyberknife treatment planning was performed with the software Multiplan v4.5 using sequential optimization for nonisocentric irradiations with circular collimators. 13 The planning procedure and review process immobilized on the Cyberknife table (Accuray, Sunnyvale, California, U.S.A.) with a custom-made aquaplast mask. Six-dimensional skull tracking was applied. Usually Cyberknife treatment was performed in an out-patient setting and in a single session.
Imaging Techniques for SRS
Before 1996, the tumor was outlined only on stereotactic CT images because the early version of the planning software (STP2.0) did not allow registration of the MRI to the planning CT. However, the available MRI information was used qualitatively to support tumor delineation. Since 1996, the tumor was routinely outlined on MRI (Phillips, MR-Scanner 1.5 or 3 Tesla), which was obtained prior to SRS and integrated into stereotactic CT (1 mm slice thickness, Phillips 8-slice or 16-slice multidetector CT). Since the year 2008, a standardized MRI protocol comprising a set of four MRI (3 Tesla) modalities was used that included two T1-weighted contrast-enhanced sequences with 2 mm (T1 turbo field echo [TFE] three-dimensional [3D]) and 1.2 mm (T1 fast field echo [FFE] 3D) slice thickness, and two T2-weighted sequences with slice thickness of 2 mm (T2 turbo spin echo [TSE]) and 1 mm (T2 driven equilibrium [DRIVE] 3D). Before 2008, usually only T1 TFE 3D and T2 TSE MRI (1.5 Tesla) images were obtained.
Statistical Analysis
Descriptive summaries were prepared for the patients' demographics. The Wilcoxon's rank-sum test was used to compare clinical parameters between groups. A Kaplan–Meier analysis was used to evaluate tumor control and other time-related censored endpoints. Factors affecting tumor and symptom control were analyzed by a Cox's proportional hazards model. The following variables were tested: age, gender, tumor volume (TV), comorbidities, radiation dose to the tumor margin and used radiation system. A p -value of < 0.05 was considered as statistically significant. The statistical analysis was performed using the software Graphpad PRZM 7.0 and SPSS 25.0.
Results
Patients Demographics
We identified 116 patients of whom 94 had LINAC and 22 had Cyberknife based SRS between 1993 and 2016 (LINAC female:male = 70:24, Cyberknife female:male = 21:1). The median age was 54 years (LINAC, 53 years; range, 33–81 years, Cyberknife, 55 years; range, 37–82 years). The main indication for applying SRS was tumor growth either after surgery or after observation ( n = 93, 80.2%). Other indications were deterioration of symptoms subsequent to first diagnosis by MRI ( n = 19, 16.4%) or patient's preference ( n = 4, 3.4%). MRI FU was available in all patients. Median radiological FU time was 54 months (LINAC, 66 months; range, 3–266 months, Cyberknife, 30 months; range, 5–63 months), see Table 1 .
Table 1. Clinical characteristics and treatment of patients.
| All | LINAC (1993–2012) | CK (2013–2016) | |
|---|---|---|---|
| Patient characteristics | |||
| Total no. of patients | 116 | 94 | 22 |
| Gender (M:F) | 25:91 | 24:70 | 1:21 |
| Recurrent CSM | 20 (17%) | 18 (19.1%) | 2 (9%) |
| Age (y) | 54 (33;82) | 53 (33;81) | 55 (37;82) |
| Tumor volume (cm 3 ) a | 5.7 ± 3.3 (0.6; 16.2) | 5.9 ± 3.5 (0.7; 16.2) | 5.0 ± 2.6 (0.6; 10.7) |
| Radiological FU (mo) | 54 (3; 266) | 66 (3; 266) | 30 (5; 63) |
| Clinical FU (mo) | 55 (3; 266) | 69 (3; 266) | 30 (5; 63) |
| Radiation parameters | |||
| Marginal dose (Gy) | 12.6 (11; 18) | 12.5 (10; 18) | 13 (12; 13) |
| Isodose (%) | 75 (47; 86) | 65 (30; 86) | 80 (67; 80) |
| Coverage (%) a | 98.3 ± 1.4 (94; 100) | 97.7 ± 1.3 (94; 99) | 99.2 ± 1 (96; 100) |
| CI | – | – | 1.35 (1.25–1.56) |
Abbreviations: CI, confidence interval; CK, cyberknife; CSM, cavernous sinus meningiomas; F, female; FU, follow-up; GK, gammaknife; LINAC, linear accelerator; M, male; PFS, progression free survival.
Data are presented as mean (standard deviation).
Note : Data are presented as median value with range in brackets.
Radiation Specific Parameters
Median radiation dose prescribed on the tumor margin was 12.6 Gy (LINAC, 12.5 Gy; range, 10–18 Gy; Cyberknife, 13 Gy; range, 12–13 Gy) at a median isodose line of 75% (LINAC, 65%; range, 30–85%; Cyberknife, 80%; range, 67–80%). The median coverage was 98.3% and showed significant difference ( p < 0.0001) between LINAC (97.7%; range, 94–99%) and Cyberknife (99.2%; range, 96–100%), see Table 1 .
Tumor Specific Parameters
The mean tumor volume amounted to 5.7 ± 3.3 mL (median, 5.05 mL; range, 0.6–16.2mL). Forty-one patients underwent partial neurosurgical resection (LINAC: n = 37, Cyberknife: n = 4) and of which n = 21 (18%) received adjuvant SRS and n = 20 (17%) had SRS for recurrence after surgery. Tumor histology was unknown in 65% ( n = 75) of the collective. In 33% ( n = 39) Meningioma WHO I (World Health Organization, grade I) was diagnosed and in 1.7% ( n = 2), atypical Meningioma WHO II was present. The median time to SRS after surgery was 12 months (range, 2–144 months).
Local Tumor Control
The following changes in tumor size after SRS were observed: enlargement ( n = 5, 4.3%), regression ( n = 25, 21.6%), and stable tumor size ( n = 86, 74.1%). Such, radiological tumor control was observed in 111 cases (95.6%). The actuarial radiological tumor control rate was 98% at 2 years, 98% at 5 years, and 90% at 10 years. The decision for retreatment was made individually and based on the extent of tumor growth ( n = 5) and/or deterioration of symptoms ( n = 1). Microsurgical resection was performed in five cases and palliative treatment in one case. The crude rate for clinical tumor control (no need for retreatment) was 95% ( n = 110). The actuarial clinical tumor control rates were 99% at 2 years, 98% at 5 years, and 89% at 10 years.
We did not find any factors impacting significantly on clinical or radiological tumor control ( p > 0.05 for all factors). Noteworthy, also the applied radiosurgery system had no significant impact on local tumor control ( Fig. 1A , LINAC versus Cyberknife, p ≥ 0.39; confidence interval [CI]: 0.03–3.8; HR: 0.35).
Fig. 1.
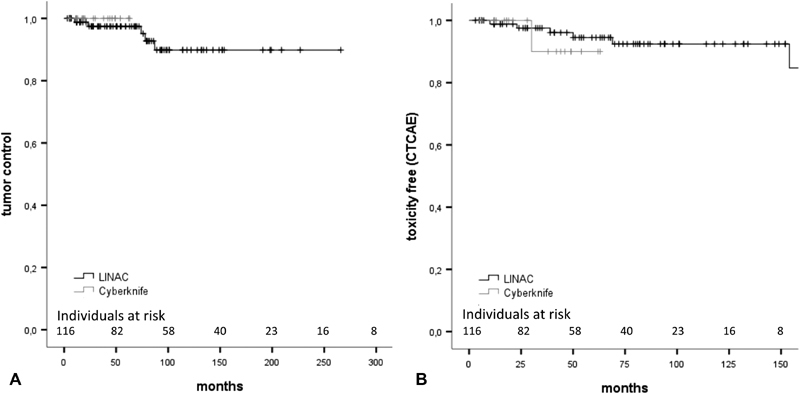
( A ) Kaplan–Meier analysis of clinical tumor control. The used radiation system had no significant impact on tumor control and ( B) on freedom of toxicity (CTCAE). CTCAE, common terminology criteria for adverse events; LINAC, linear accelerator.
Symptom Control
At presentation for radiosurgery, 41 (35.3%) were asymptomatic. The other 75 patients had symptoms prior to SRS ( Fig. 2 ) of which the most frequent were: diplopia in 32.8% ( n = 38), any trigeminal nerve dysfunction ( n = 19, 16.4%), visual disturbances ( n = 12, 10.3%), and epilepsy ( n = 4, 3.4%). Other unspecific symptoms were headache ( n = 6, 5.2%), vertigo ( n = 5, 4.3%), and imbalance ( n = 5, 4.3%).
Fig. 2.
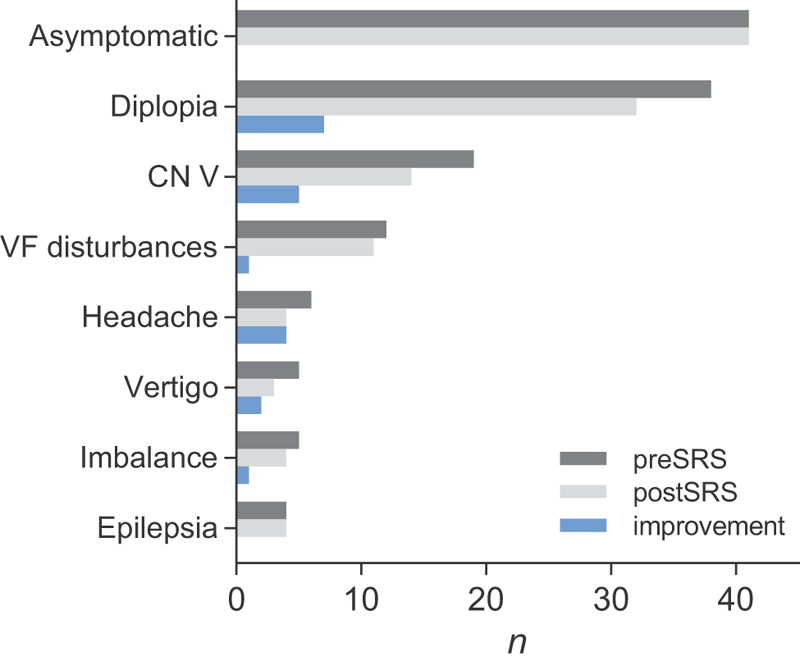
Comparison of symptoms before and after SRS. In 64.7% ( n = 75) of the collective symptoms were present prior SRS. An improvement was observed in 26.7% ( n = 20). CN, cranial nerve; SRS, stereotactic radiosurgery; VF, visual field.
Following SRS, a deterioration of symptoms was found in 6.7% ( n = 5 of 75) while an improvement was observed in 26.7% ( n = 20). Most frequently, cases with improved diplopia ( n = 7) and trigeminal nerve disorder ( n = 5) were found ( Fig. 2 ). Overall, the crude rate of symptom control was 90% ( n = 104). Actuarial symptom control was 97% at 1 year, 93% at 2 years, 88% at 5 years, and 86% at 10 years. We did not find any factors influencing significantly on symptom control ( p > 0.05 in any case).
Evaluation of Radiation Related Toxicity
Overall, 12 patients (10.3%) had permanent or transient radiation related toxicity. In eight patients (6.9%), permanent CTCAE grade 2 or greater toxicity was observed, including two grade 3 toxicities. Toxicities included headache, epilepsy, trigeminal nerve disorder, diplopia, and visual field (VF) disturbances ( Table 2 ). One case of epilepsy and one of diplopia were of grade 3. Median time to toxicity was 19 months (range, 5–67 months). Three cases of trigeminal nerve disorder and one case of headache resolved within the first 3 months after SRS. One trigeminal nerve disorder was treated with surgery and resulted in poor outcome. Headaches were treated with steroids and nonsteroidal anti-inflammatory drugs, seizures were treated with levetiracetam and steroids and trigeminal nerve disorders with steroids only.
Table 2. Overview of permanent ( n = 8) and transient ( n = 4) side-effects after SRS using CTCAE criteria .
| CTCAE grading | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|
| Epilepsia, n = 2 | 0 | 1 | 1 |
| Headache, n = 3 | 0 | 3 | 0 |
| CN V disorder, n = 5 | 3 | 2 | 0 |
| Diplopia, n = 1 | 0 | 0 | 1 |
| VF disturbance, n = 1 | 0 | 1 | 0 |
Abbreviations: CN, cranial nerve; CTCAE, common terminology criteria for adverse events; SRS, stereotactic radiosurgery; VF, visual field.
Note : In our series, CTCAE criteria were grade 1 (mild symptoms, asymptomatic, or mild symptoms without impact on daily life)), grade 2 (moderate, minimal, local, or noninvasive intervention indicated; limiting age-appropriate instrumental activities of daily life (ADL)), and grade 3 (severe or medically significant, but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care ADL). We did not find any adverse events matching grade 4 (life-threatening consequences; urgent intervention indicated) or grade 5 (death related to AE).
Cox's regression analysis of factors influencing the development of permanent CTCAE toxicity revealed no significant statistical impact of any factor ( p > 0.05 in all cases). Also, type of radiosurgical treatment (LINAC or Cyberknife) had no statistical impact on toxicity (hazard = 1.04; p > 0.94 [95% CI: 0.94–1.07]; Fig. 1B ). In addition, no significant impact of CTCAE toxicity grade on local tumor control was observed.
Discussion
Although CSM is considered a benign tumor, it can cause significant morbidity. Also, it can impact life expectancy especially in aggressively growing tumors where the average life expectancy amounts to only 2 years. 14 Therefore, tumor control should be the main treatment goal. As some authors point out, approximately 23% of the meningiomas (mainly the calcified ones) do not grow 6 and such, there is also good reason for an observation strategy at first diagnosis. However, in cases of growing tumor, active treatment strategies are recommended that comprise surgery, radiosurgery, and fractionated radiotherapy.
Surgery
The anatomical localization of CSM includes intracavernous extent, extracavernous extent, and mixed types. 3 Thus, some tumors are less preferable for surgery than others. Especially in cases of intracavernous tumor extension, a high risk for morbidity and even mortality is reported. In a synopsis of series ranging from 1994 to 2004, 4 the resection rate ranged between 20 and 81%.Tumor control was 87 to 90% but the overall complication rate including CN deficits amounted between 9 and 33%. Meanwhile, aggressive resection of CSMs is considered obsolete due to several reports that stress the procedure-related neurological morbidity. 3 4 6 15 Apart from the higher risk for morbidity, complete resection does not naturally result in effective tumor control. For instance, in a current series of Nanda et al, 5 complete resection has no effect on tumor recurrence. These authors found a better outcome with SRS adjuvant to surgery than with surgery alone. These findings are supported by other authors as well 3 6 and go in line with our results. Aggressive intracavernous surgery should probably be reserved for selected situations (e.g., recurrences resistant to all other treatment attempts, pre-existing severe deficits) and should be considered as “salvage therapy.” 16
Radiosurgery
In contrast, the general goal of SRS is to achieve local tumor control while avoiding early and late clinical deterioration. Also the present analysis aimed to clarify whether SRS could achieve these goals. Therefore, an extensive literature research was conducted that included studies which met the following criteria: (1) must exclusively address SRS, (2) must have a median FU of at least 24 months, and (3) include clinical outcome evaluation. Series that included patients treated before 1995 were excluded to increase comparability to our series. The literature search was conducted using PubMed and the search terms were “cavernous sinus meningioma” and “radiosurgery.” This approach yielded approximately 465 articles, which were then screened by title and abstract. We finally included 24 series with a total of 2,302 patients in the comparison ( Table 3 ).
Table 3. Characteristics of retrospective single-center series in the treatment of CSM.
| Year | n | Author and centre | SRS system | Prior surgery | SRS alone | FU (mo) | PFS 5 y (%) | PFS 10 y (%) | Deterioration of symptoms after SRS (%) | Improvement of symptoms after SRS (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1997 | 18 | Kurita et al, Tokyo 19 | GK | 15 | 3 | 35 a | 85.7 | – | 5.9 | – |
| 1998 | 24 | Chang et al, Stanford 20 | LINAC | – | 24 | 46 a | 100 (2 y) | – | 0 | 42 |
| 1999 | 88 | Morita et al, Minnesota 21 | GK | 49 | 39 | 34 b | 95 | – | 9 | 17 |
| 2000 | 92 | Roche et al, Marseille 22 | GK | 30 | 62 | 31 b | 92.8 | – | 0 | 43 |
| 2001 | 40 | Shin et al, Tokyo 23 | GK | – | 40 | 42 b | 86.4 (3 y) | 82.3 | 2.5 | 20 |
| 2002 | 159 | Lee et al, Pittsburgh 24 | GK | 76 | 83 | 39 a | 93.1 | 93.1 | 6.7 | 29 |
| 2002 | 156 | Nicolato et al, Verona 25 | GK | 75 | 81 | 49 b | 96.5 | – | 1 | 60 |
| 2002 | 138 | Nicolato et al, Verona 26 | GK | 70 | 68 | 48 b | 96 | – | 1 | 61 |
| 2002 | 42 | Spiegelmann, Hashomer 27 | LINAC | – | 42 | 36 b | 97.5 (7 y) | – | 7.1 | − |
| 2003 | 43 | Iwai et al, Osaka 28 | GK | 25 | 18 | 49 a | 92 | – | – | 28.6 |
| 2005 | 49 | Pollock and Stafford Rochester 29 | GK | – | 49 | 58 a | 80 (7 y) | – | 10 | 26 |
| 2007 | 123 | Franzin et al, Milan 30 | GK | 41 | 82 | 36 b | 90.5 | – | – | 31.1 |
| 2007 | 115 | Hasegawa et al, Komaki 31 | GK | 66 | 49 | 62 b | 94 | 92 | 4 | 46 |
| 2009 | 55 | Kimball et al, Gainesville 32 | LINAC | – | 55 | 50 b | 100 | 98 | 3.5 | 65 |
| 2010 | 100 | Skeie et al, Bergen 14 | GK | 60 | 40 | 82 a | 94.2 | 91.6 | 6 | 21 |
| 2010 | 117 | Spiegelmann, Hashomer 33 | LINAC | 35 | 82 | 67 a | 98 | – | 4 | 39 |
| 2011 | 88 | Dos Santos et al, Madrid 34 | LINAC | 41 | 47 | 87 a | 92.5 | 82.5 | 12.5 | 51.1 |
| 2012 | 19 | Hayashi et al, Tokyo 35 | GK | 5 | 14 | 55 a | 100 | – | 0 | – |
| 2013 | 115 | Pollock et al, Minnesota 36 | GK | 46 | 69 | 89 b | 99 | 93 | 12 | 36.5 |
| 2013 | 272 | Kano et al, Pittsburgh 18 | GK | 99 | 173 | 62 b | 94 | 86 | 11 | 36.8 |
| 2014 | 32 | Correa et al, Sao Paolo 37 | LINAC | 18 | 14 | 73 a | 100 | 95.7 | – | – |
| 2015 | 62 | Hafez et al, Cairo 38 | GK | 11 | 51 | 36 a | 95 | – | 5 | 47 |
| 2017 | 166 | Azar et al, Tehran 39 | GK | 44 | 122 | 32 a | 90 | – | 9.6 | 40.2 |
| 2018 | 189 | Cohen-Inbhar, Charlottesville 8 | GK | 84 | 105 | 71 b | 95.7 | 95.7 | – | – |
| 2018 | 116 | Our series, Cologne | LINAC CK |
41 | 75 |
54
b
68 a |
97.5 | 90 | 6.7 | 26.7 |
Abbreviations: FU, follow-up; LINAC, linear accelerator; SRS, stereotactic radiosurgery.
Values are reflecting means.
Values are reflecting medians.
Note : the number of patients with complete follow-up data are given in the n section.
Our series is the first to report about the results of a homogenous collective of patients with unilateral CSM treated with LINAC or Cyberknife radiosurgery. Thus, there is still a lack of comparable studies. The tumor control rates in the present study were well in the range of those in pre-existing Gammaknife (GK) series were the PFS ranged from 85.7 up to 100% after 5 years and to 83.3 up to 98% after 10 years. However, our study provides one of the largest series on this entity and the FU interval of our study is one of the longest reported in literature especially with regard to the LINAC treated cohort (median FU, 66 months). From our data we can also conclude that the different techniques of SRS do not differ significantly in terms of tumor control, preservation of clinical condition, or side-effects.
In our analysis, a large group of patients were treated with SRS for CSM after previous surgery which is a common indication also reported in the existing literature ( Table 3 ). Previous surgery was not a factor for a higher rate of toxicity or for deterioration of symptoms, although some authors 15 do report about a significantly higher rate of neurological morbidity, in such cases of combined treatment. Also, the current guideline on the use of SRS in CSM 7 gives no clearly recommendation with regard to application of SRS alone or in combination with surgery. As we did not find any significant impact of the timing of SRS after prior surgery on clinical outcome, this factor also warrants further investigation. 7
Compared with the collected literature, our series is unique in that the evaluation of toxicity of SRS was classified according to the CTCAE criteria. Overall, we found a low rate of permanent adverse events which fits well into the range reported in literature ( Table 3 ). Due to the proximity of CSM to the oculomotor and trigeminal nerves and the optic apparatus, deterioration of these nerve functions demands special attention. Whereas dose tolerance limits for the optic system are well-defined, 17 clear recommendations for the maximal tolerated radiation dose of the other CNs traversing the CS are not available. In our series, there was only one case (0.86%) of new diplopia after SRS. A similar incidence (less than 1%) is reported by Lee et al. 7 Another patient in our series developed a slight deterioration of the VF but this was probably caused by local tumor progression resulting in retreatment.
Besides toxicity, almost 30% of the symptomatic patients improved after SRS which is consistent to the figures from the current literature ( Table 3 ). The most frequent improvements were observed in oculomotor dysfunction. Also Kano et al 18 observed improvement rates of oculomotor function after SRS of 20% at 1 year and up to 39% at 5 years. They report that patients with primary SRS had significantly higher improvement rates of pre-existing CN symptoms than patients with primary microsurgery ( p = 0.001). We could not reproduce these observations in our cohort, but in general these results support the view that SRS provides an excellent means for long-term symptom improvement and local tumor control in primary or recurrent CSM.
Conclusion
The present study provides further level III evidence that SRS for primary or recurrent CSM achieves a high level of local tumor control and is able to control or improve symptoms in the majority of the patients while toxicity rates are low. This view is in concordance with current guidelines 7 and other selected series of SRS. Such SRS should be part of any counseling for patients suffering from CSM.
Footnotes
Conflict of Interest None.
References
- 1.Radhakrishnan K, Mokri B, Parisi J E, O'Fallon W M, Sunku J, Kurland L T. The trends in incidence of primary brain tumors in the population of Rochester, Minnesota. Ann Neurol. 1995;37(01):67–73. doi: 10.1002/ana.410370113. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura M, Roser F, Michel J, Jacobs C, Samii M.The natural history of incidental meningiomas Neurosurgery 2003530162–70., discussion 70–71 [DOI] [PubMed] [Google Scholar]
- 3.Klinger D R, Flores B C, Lewis J J, Barnett S L. The treatment of cavernous sinus meningiomas: evolution of a modern approach. Neurosurg Focus. 2013;35(06):E8. doi: 10.3171/2013.9.FOCUS13345. [DOI] [PubMed] [Google Scholar]
- 4.Sindou M, Wydh E, Jouanneau E, Nebbal M, Lieutaud T. Long-term follow-up of meningiomas of the cavernous sinus after surgical treatment alone. J Neurosurg. 2007;107(05):937–944. doi: 10.3171/JNS-07/11/0937. [DOI] [PubMed] [Google Scholar]
- 5.Nanda A, Thakur J D, Sonig A, Missios S. Microsurgical resectability, outcomes, and tumor control in meningiomas occupying the cavernous sinus. J Neurosurg. 2016;125(02):378–392. doi: 10.3171/2015.3.JNS142494. [DOI] [PubMed] [Google Scholar]
- 6.Fariselli L, Biroli A, Signorelli A, Broggi M, Marchetti M, Biroli F. The cavernous sinus meningiomas' dilemma: Surgery or stereotactic radiosurgery? Rep Pract Oncol Radiother. 2016;21(04):379–385. doi: 10.1016/j.rpor.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee C C, Trifiletti D M, Sahgal A et al. Stereotactic radiosurgery for benign (World Health Organization Grade I) cavernous sinus meningiomas-International Stereotactic Radiosurgery Society (ISRS) practice guideline: a systematic review. Neurosurgery. 2018;83(06):1128–1142. doi: 10.1093/neuros/nyy009. [DOI] [PubMed] [Google Scholar]
- 8.Cohen-Inbar O, Tata A, Moosa S, Lee C C, Sheehan J P. Stereotactic radiosurgery in the treatment of parasellar meningiomas: long-term volumetric evaluation. J Neurosurg. 2018;128(02):362–372. doi: 10.3171/2016.11.JNS161402. [DOI] [PubMed] [Google Scholar]
- 9.Grimm J, LaCouture T, Croce R, Yeo I, Zhu Y, Xue J. Dose tolerance limits and dose volume histogram evaluation for stereotactic body radiotherapy. J Appl Clin Med Phys. 2011;12(02):3368. doi: 10.1120/jacmp.v12i2.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruge M I, Kocher M, Maarouf M et al. Comparison of stereotactic brachytherapy (125 iodine seeds) with stereotactic radiosurgery (LINAC) for the treatment of singular cerebral metastases. Strahlenther Onkol. 2011;187(01):7–14. doi: 10.1007/s00066-010-2168-4. [DOI] [PubMed] [Google Scholar]
- 11.Treuer U, Treuer H, Hoevels M, Müller R P, Sturm V. Computerized optimization of multiple isocentres in stereotactic convergent beam irradiation. Phys Med Biol. 1998;43(01):49–64. doi: 10.1088/0031-9155/43/1/004. [DOI] [PubMed] [Google Scholar]
- 12.Kilby W, Dooley J R, Kuduvalli G, Sayeh S, Maurer C R., Jr The CyberKnife robotic radiosurgery system in 2010. Technol Cancer Res Treat. 2010;9(05):433–452. doi: 10.1177/153303461000900502. [DOI] [PubMed] [Google Scholar]
- 13.Treuer H, Hoevels M, Luyken K et al. Intracranial stereotactic radiosurgery with an adapted linear accelerator vs. robotic radiosurgery: comparison of dosimetric treatment plan quality. et al. Strahlenther Onkol. 2015;191(06):470–476. doi: 10.1007/s00066-014-0786-y. [DOI] [PubMed] [Google Scholar]
- 14.Skeie B S, Enger P O, Skeie G O, Thorsen F, Pedersen P H.Gamma knife surgery of meningiomas involving the cavernous sinus: long-term follow-up of 100 patients Neurosurgery 20106604661–668., discussion 668–669 [DOI] [PubMed] [Google Scholar]
- 15.Sughrue M E, Rutkowski M J, Aranda D, Barani I J, McDermott M W, Parsa A T. Factors affecting outcome following treatment of patients with cavernous sinus meningiomas. J Neurosurg. 2010;113(05):1087–1092. doi: 10.3171/2010.3.JNS091807. [DOI] [PubMed] [Google Scholar]
- 16.Couldwell W T, MacDonald J D, Taussky P. Complete resection of the cavernous sinus-indications and technique. World Neurosurg. 2014;82(06):1264–1270. doi: 10.1016/j.wneu.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Leber K A, Berglöff J, Pendl G. Dose-response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg. 1998;88(01):43–50. doi: 10.3171/jns.1998.88.1.0043. [DOI] [PubMed] [Google Scholar]
- 18.Kano H, Park K J, Kondziolka D et al. Does prior microsurgery improve or worsen the outcomes of stereotactic radiosurgery for cavernous sinus meningiomas? Neurosurgery. 2013;73(03):401–410. doi: 10.1227/01.neu.0000431471.64289.3d. [DOI] [PubMed] [Google Scholar]
- 19.Kurita H, Sasaki T, Kawamoto S et al. Role of radiosurgery in the management of cavernous sinus meningiomas. Acta Neurol Scand. 1997;96(05):297–304. doi: 10.1111/j.1600-0404.1997.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 20.Chang S D, Adler J R, Jr, Martin D P. LINAC radiosurgery for cavernous sinus meningiomas. Stereotact Funct Neurosurg. 1998;71(01):43–50. doi: 10.1159/000029647. [DOI] [PubMed] [Google Scholar]
- 21.Morita A, Coffey R J, Foote R L, Schiff D, Gorman D. Risk of injury to cranial nerves after gamma knife radiosurgery for skull base meningiomas: experience in 88 patients. J Neurosurg. 1999;90(01):42–49. doi: 10.3171/jns.1999.90.1.0042. [DOI] [PubMed] [Google Scholar]
- 22.Roche P H, Régis J, Dufour H et al. Gamma knife radiosurgery in the management of cavernous sinus meningiomas. J Neurosurg. 2000;93 03:68–73. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 23.Shin M, Kurita H, Sasaki T et al. Analysis of treatment outcome after stereotactic radiosurgery for cavernous sinus meningiomas. J Neurosurg. 2001;95(03):435–439. doi: 10.3171/jns.2001.95.3.0435. [DOI] [PubMed] [Google Scholar]
- 24.Lee J Y, Niranjan A, McInerney J, Kondziolka D, Flickinger J C, Lunsford L D. Stereotactic radiosurgery providing long-term tumor control of cavernous sinus meningiomas. J Neurosurg. 2002;97(01):65–72. doi: 10.3171/jns.2002.97.1.0065. [DOI] [PubMed] [Google Scholar]
- 25.Nicolato A, Foroni R, Alessandrini F, Bricolo A, Gerosa M.Radiosurgical treatment of cavernous sinus meningiomas: experience with 122 treated patients Neurosurgery 200251051153–1159., discussion 1159–1161 [DOI] [PubMed] [Google Scholar]
- 26.Nicolato A, Foroni R, Alessandrini F, Maluta S, Bricolo A, Gerosa M. The role of Gamma Knife radiosurgery in the management of cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys. 2002;53(04):992–1000. doi: 10.1016/s0360-3016(02)02802-x. [DOI] [PubMed] [Google Scholar]
- 27.Spiegelmann R, Nissim O, Menhel J, Alezra D, Pfeffer M R.Linear accelerator radiosurgery for meningiomas in and around the cavernous sinus Neurosurgery 200251061373–1379., discussion 1379–1380 [PubMed] [Google Scholar]
- 28.Iwai Y, Yamanaka K, Ishiguro T.Gamma knife radiosurgery for the treatment of cavernous sinus meningiomas Neurosurgery 20035203517–524., discussion 523–524 [DOI] [PubMed] [Google Scholar]
- 29.Pollock B E, Stafford S L. Results of stereotactic radiosurgery for patients with imaging defined cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys. 2005;62(05):1427–1431. doi: 10.1016/j.ijrobp.2004.12.067. [DOI] [PubMed] [Google Scholar]
- 30.Franzin A, Vimercati A, Medone M et al. Neuroophthalmological evaluation after Gamma Knife surgery for cavernous sinus meningiomas. Neurosurg Focus. 2007;23(06):E10. doi: 10.3171/FOC-07/12/E10. [DOI] [PubMed] [Google Scholar]
- 31.Hasegawa T, Kida Y, Yoshimoto M, Koike J, Iizuka H, Ishii D. Long-term outcomes of Gamma Knife surgery for cavernous sinus meningioma. J Neurosurg. 2007;107(04):745–751. doi: 10.3171/JNS-07/10/0745. [DOI] [PubMed] [Google Scholar]
- 32.Kimball M M, Friedman W A, Foote K D, Bova F J, Chi Y Y. Linear accelerator radiosurgery for cavernous sinus meningiomas. Stereotact Funct Neurosurg. 2009;87(02):120–127. doi: 10.1159/000204910. [DOI] [PubMed] [Google Scholar]
- 33.Spiegelmann R, Cohen Z R, Nissim O, Alezra D, Pfeffer R. Cavernous sinus meningiomas: a large LINAC radiosurgery series. J Neurooncol. 2010;98(02):195–202. doi: 10.1007/s11060-010-0173-1. [DOI] [PubMed] [Google Scholar]
- 34.dos Santos M A, de Salcedo J B, Gutiérrez Diaz J A et al. Long-term outcomes of stereotactic radiosurgery for treatment of cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys. 2011;81(05):1436–1441. doi: 10.1016/j.ijrobp.2010.07.2002. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi M, Chernov M, Tamura N et al. Gamma knife radiosurgery for benign cavernous sinus tumors: treatment concept and outcomes in 120 cases. Neurol Med Chir (Tokyo) 2012;52(10):714–723. doi: 10.2176/nmc.52.714. [DOI] [PubMed] [Google Scholar]
- 36.Pollock B E, Stafford S L, Link M J, Garces Y I, Foote R L. Single-fraction radiosurgery of benign cavernous sinus meningiomas. J Neurosurg. 2013;119(03):675–682. doi: 10.3171/2013.5.JNS13206. [DOI] [PubMed] [Google Scholar]
- 37.Correa S F, Marta G N, Teixeira M J. Neurosymptomatic carvenous sinus meningioma: a 15-years experience with fractionated stereotactic radiotherapy and radiosurgery. Radiat Oncol. 2014;9:27. doi: 10.1186/1748-717X-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hafez R F, Morgan M S, Fahmy O M. Stereotactic Gamma Knife surgery safety and efficacy in the management of symptomatic benign confined cavernous sinus meningioma. Acta Neurochir (Wien) 2015;157(09):1559–1564. doi: 10.1007/s00701-015-2509-2. [DOI] [PubMed] [Google Scholar]
- 39.Azar M, Kazemi F, Jahanbakhshi A et al. Gamma Knife Radiosurgery for Cavernous Sinus Meningiomas: Analysis of Outcome in 166 Patients. Stereotact Funct Neurosurg. 2017;95(04):259–267. doi: 10.1159/000478024. [DOI] [PubMed] [Google Scholar]


