Abstract
Background
The formation of a bolus of food is critical for proper feeding function, and there is substantial variation in the size and shape of a bolus prior to a swallow. Preterm infants exhibit decreased abilities to acquire and process food, but how that relates to their bolus size and shape is unknown. Here, we test two hypotheses: (1) that bolus size and shape will differ between term and preterm infants, and (2) bolus size and shape will change longitudinally through development in both term and preterm infants.
Methods
To test these hypotheses, we measured bolus size and shape in preterm and term infant pigs longitudinally through nursing using high-speed videofluoroscopy.
Results
Preterm infant pigs swallowed smaller volumes of milk. Although term infants increased the amount of milk per swallow as they aged, preterm infants did not. These changes in bolus volume were also correlated with changes in bolus shape; larger boluses became more elongate as they better filled the available anatomical space of the valleculae.
Conclusion
These results suggest that preterm birth reduces the ability of preterm pigs to increase bolus size as they grow, affecting development in this fragile population. These results highlight that studies on term infant feeding may not translate to preterm infants.
Introduction
For infant mammals, feeding involves (1) acquiring liquid from the nipple, (2) forming that food into a bolus while transporting it from the nipple to the back of the oropharynx, so that ultimately, they can (3) propel that bolus from the oropharynx into the esophagus via a swallow (1,2). As food acquisition, transport, and swallowing are linked both spatially and temporally, dysfunction during any of these behaviors can result in feeding difficulties. For example, although the trigger to swallow can be related to the volume of the bolus in the valleculae, sensorimotor feedback of how much milk is acquired during feeding also influences the trigger of the swallow (1). Additionally, without proper bolus formation and transport via movements of the tongue and pharynx, the liquid entering the pharynx may not trigger a swallow reflex (3,4). Despite the importance of bolus formation, extensive variation exists in both the size and shape of a bolus of milk during infant suckling (5). This variation occurs among boluses within an individual, among individuals, and among different neurologically compromised groups (5,6).
The size and shape of a bolus of liquid immediately prior to a swallow are a function of anatomy and physiology, both which change through early, pre-weaning maturation. As infants grow, and require more nutrition, they often increase the volume they consume per swallow (4). The space of the valleculae, which holds the bolus, also will change over time. However, there are tradeoffs associated with increasing bolus volume, as a larger or differently shaped bolus can result in increased aspiration and thus require an improved neurological control of swallowing (5–8). This suggests a strong potential for differences to exist in bolus size and shape between neurologically compromised and healthy populations, as well as between younger and older infants.
One population that is especially susceptible to feeding dysfunction are preterm infants. Up to 80% of infants born prematurely experience oral feeding difficulties, and problems feeding and coordinating that behavior with breathing are among the most common reasons for hospitalization and even death in preterm patients (8–11). These difficulties can arise due to problems during food acquisition, transport or swallowing. For example, preterm infants exhibit problems latching and sucking (12–14), decreased ability to acquire milk from the nipple and propel it into the esophagus (7,15), and exhibit worse suck-swallow and swallow-breathe coordination (4,16).
Although we know that preterm infants exhibit reduced performance among many feeding behaviors, and we understand the clinical outcomes of poor performance, our understanding of the mechanisms driving those performance differences is limited. One potential mechanism that could relate to reduced feeding performance in preterm infants is their decreased ability to form a bolus, as clinically, poor bolus formation is thought to relate to increased aspiration (4,15). Furthermore, neurologically compromised populations such as preterm patients and those with Parkinson’s disease are more likely to aspirate at lower bolus volumes (17). The decreased neuromuscular coordination in preterm infants may therefore result in a decreased ability to efficiently form a bolus of similar size and shape to term counterparts during feeding.
Preterm human infants are a fragile population that are difficult to study, and fluorographic studies, which are necessary to measure bolus formation, size and shape are tightly regulated. Because of this, clinical studies often cannot be performed longitudinally through development, and comparisons between healthy and dysphagic infants are limited. We used a validated animal model of preterm infants (pigs) to quantify the differences in bolus shape and size between term and preterm infants, and how those differences change longitudinally through maturation. We tested three hypotheses: (1) that there would be a change in bolus size and shape as animals mature, (2) that there would be differences in bolus shape and size between term and preterm infants, and (3) that there would be an interaction between gestational age at delivery (term or preterm) and longitudinal development of bolus properties.
Methods
Animal housing and care
Experimental Sus scrofa (Yorkshire/Landrace, Shoup Farms, Wooster, OH) used in experiments were acquired via Cesarean section either at term (2 litters, 8 pigs) or 6–8 days preterm (1 litter, N = 4 pigs, 107–109 days of gestation; human equivalent 30–32 weeks gestation, (18)). Surgical delivery of each litter, including term infants, ensured that differences in feeding among litters and due to gestational age of birth are precisely controlled and the same for term and preterm infants.
Detailed methods for the C-section can be found in Ballester et al. (19), but in brief, sows were sedated (Telazol, 5ml IM), placed on a surgical table and anesthetized with isoflourane before the C-section was performed using standard aseptic technique. An incision in the uterus of the sow was made to deliver individual neonatal pigs. Once neonates reached a stable state of breathing, they were placed in a warmed incubator set at 30°C (Dräger medical Isolette Infant Incubator C2000, Telford, PA), with strong breathers intermixed with those with slow breathing to encourage spontaneous ventilation. Neonates were fed colostrum within two hours of birth, followed by infant pig formula (Solustart Pig Milk Replacement, Land o’ Lakes, Arden Mills, MN) from a bottle fitted with a specially designed nipple. Infants were monitored 24 hours a day until a veterinarian determined they were strong enough to be left alone, at which point care followed validated and standard care for infant pigs (19–22). All animal care and surgical procedures were approved by the NEOMED IACUC (#17–04-071).
Data acquisition
We collected data on swallowing performance when pigs were seven days old (2–3 months human equivalent), the youngest age where pigs have developed suitable levels of thermoregulatory ability to be transported to the videofluorscopy suite, and seventeen days old (6–9 months human equivalent (23)), an age just prior to weaning, where pigs are highly efficient at consuming milk. Pigs were fed infant formula mixed with barium to visualize milk through a fluoroscope (GE94000 C-Arm, 85kV, 4MA) that digitally recorded images at 100fps using a high-speed camera (XC1M digital camera, XCitex, Cambridge, MA). Pigs were fed ad libitium during data collection, and we collected at least twenty swallows per pig per age.
Data processing
For each feeding sequence, we identified the first set of swallows that occurred without break following the first 5 seconds of feeding, which occurs at a faster rate than the rest of a feeding sequence (24). Swallows were identified by the frame at which the bolus was accumulated in the supraglottic space prior to passing the epiglottis (19). All individuals identifying swallows were trained on swallow identification using single-blind procedures until inter and intra-rater reliability reached at least 95%. We collected a total of 504 swallows (N = 234 preterm, 270 term).
Data Design
For all analyses for both size and shape, we tested for differences between two independent variables: different ages (younger/older) and birth status (preterm/term), as well as the potential interaction between age and birth status. We also included individual as a random effect to account for variation between individuals in the dataset. Specifics for each set of analyses are detailed below.
Measurement of Bolus Size
Following swallow identification, the frame before swallow initiation was isolated for analysis, following published protocols (5). In this frame, the bolus was outlined using the free select tool in ImageJ (25), on a touch screen tablet with a stylus (Surface Pro 2, Microsoft Corporation, Redwood, WA). Milk in the pyriform recesses was not outlined, as the amount of milk in the recesses is quite variable within pigs and also makes up a relatively small amount of total volume of the bolus per swallow (5). To control for differences in the size of the image of the pigs, all images were scaled to mm2. We used the outlined bolus in conjunction with the scale to measure the raw bolus area for each swallow using ImageJ (25). To control for differences in head position, we identified two points on the hard palate for each swallow and used these points to create a rotation matrix to align each image.
Statistical analysis of bolus size
Bolus areas were standardized by the square of palate length to control for differences in pig size between treatments and throughout growth during ontogeny. Differences in bolus area were evaluated using linear mixed models with gestational age, age at time of recording, and their interaction as fixed effects, and pig as a random effect (BolusArea ~ gestational age + Age + gestational * Age + (1|Pig); lme4 (26)) in R (v. 3.5.0, www.r-project.org), where gestational age indicates whether an individual was delivered term or preterm, and age indicates the animal’s age at the time of feeding and analysis. To test for significance of main effects (age and gestational age), p-values were obtained using the anova() function in R. Because each factor has only two levels (younger/older and preterm/term), significance of a main factor, together with the least-square means indicates significant differences. If the interaction term was significant, we performed Tukey’s post-hoc corrections to identify which treatment groups (age-birth combination) were significantly different (R package emmeans).
Calculation of bolus shape
To analyze bolus shape, we used standard methods developed for analyzing shape of morphologic features (5). We first rotated all boluses to the same orientation using custom MATLAB code (Mathworks, Natick, MA). These rotated outlines were processed using elliptical Fourier decomposition through the R package Momocs (Bonhomme et al., 2014), so that each outline was represented by an equal number of unique Fourier coefficients, following published methods for analyzing bolus shape (5).
Data reduction of bolus shape data
Fourier coefficient vectors were analyzed using principal component analysis, a standard methodology for analyzing shape (27), to reduce the dimensionality of the multivariate dataset. Principal components analysis produces a new set of axes from the original high dimensionality multivariate data that are independent of each other and align with the maximum covariation among the original variables, facilitating statistical interpretation of the original multivariate data. The first four principal components accounted for over 95% of the variation in outline shape, and no principal component (PC) past PC 4 accounted for more than one percent of the variation.
Statistical analyses of shape variation
The effect of postnatal age, degree of prematurity, and individual variation on principal components one through four were analyzed using multivariate analysis of variances (MANOVA), testing for all main effects and interactions. In analyses with significant differences in the interaction term, we performed Tukey’s post-hoc corrections to identify differences within the interaction term.
Statistical analysis of the relationship between shape and size
The relationship between principle components scores and standardized bolus area was tested by linear regression of principle components one through four against standard bolus area. As principal component one was strongly correlated with standardized bolus area and accounted for 75% of the variation in the sample, a univariate mixed model ANOVA was performed on PC1 scores with individual as a random factor and age, gestational age, and the interaction as factors. Pairwise posthoc tests were used to determine specific group differences when interactions were significant.
Results
Bolus size
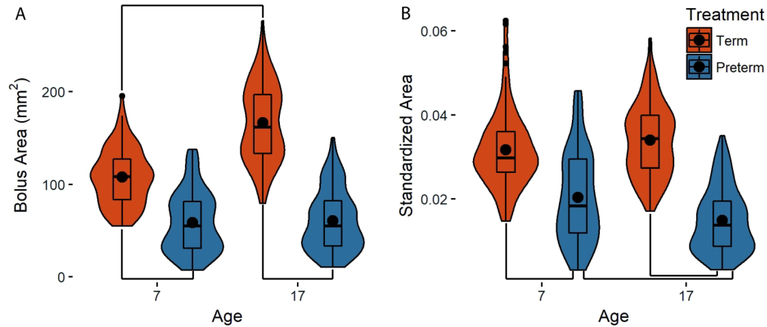
We found significant effects of age, gestational age, and their interaction (p<0.001) on bolus size during swallowing for absolute size, as well as standardized size which was corrected for body size. Term infants swallowed absolutely larger boluses at both day seven (term: 111.3 ± 7.5mm2, preterm: 56.3 ± 10.8mm2) and day 17 (term: 164.1 ± 7.3mm2, preterm: 59.7 ± 10.6mm2, Fig. 1A, Table 1). Furthermore, Tukey’s post hoc analyses revealed that although term infants increase the size of the bolus with age, preterm infants do not (Table 1). In our size-standardized analyses, we found that term pigs swallowed larger standardized bolus areas than preterm pigs at both day seven and 17, but because preterm infants did not increase the size of their bolus with age, their bolus sizes relative to body size were larger on seven than day (Fig. S1B, Table 1). In contrast, term infants increased their bolus size in proportion with their increase in body size, and we found no change in the standardized area of the bolus between day seven and 17 (Fig. S1B, Table 1).
Figure 1.
Size of the bolus in term (orange) and preterm (blue) pigs at day seven and 17. (A) Raw area (B) Bolus area standardized by the square of the length of the palate. Black dots: means for each group; lines between groups: statistically significant differences as identified with post-hoc analyses; width of each plot: distribution of the data along the y-axis.
Table 1.
Tukey’s post-hoc results for both raw and standardized bolus areas.
| Raw area p | Standardized area p | |
|---|---|---|
| Term seven - Preterm seven | 0.01 | 0.01 |
| Term seven - Term 17 | <0.001 | 0.67 |
| Term seven - Preterm 17 | 0.01* | 0.01* |
| Preterm seven - Term 17 | <0.001* | 0.01* |
| Preterm seven - Preterm 17 | 0.83 | <0.001 |
| Term 17 - Preterm 17 | <0.001 | <0.001 |
Indicates statistically significant, but not biologically relevant results.
Bolus shape
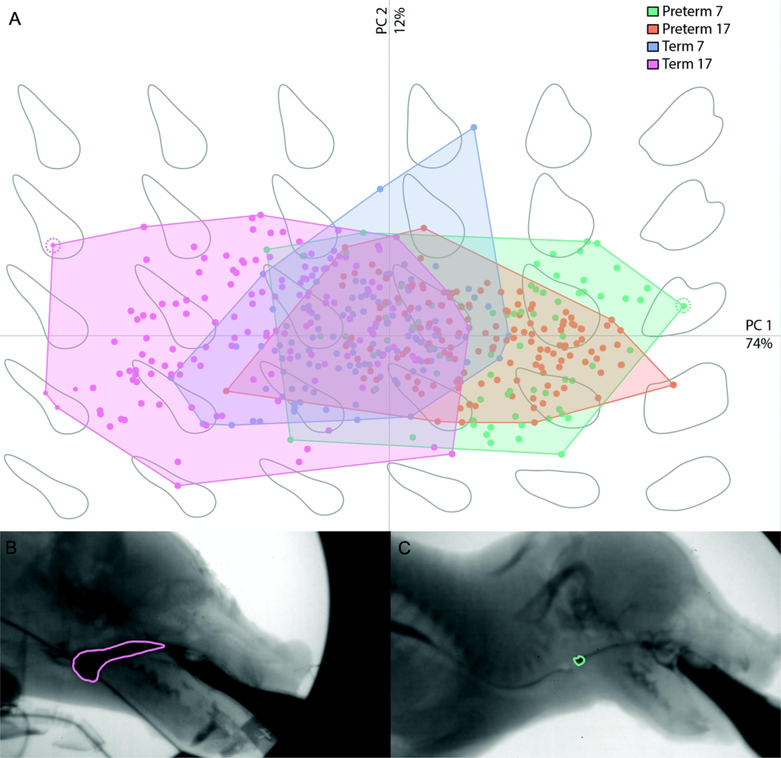
PCA analyses on bolus shape identified substantial differences between preterm and term infants at both ages along PC 1(73.9% variation; Fig. 2). We found no effect of age or birth age on PC2 (11.7% variation), PC3 (8.2% variation) or PC4 (2.3% variation). Furthermore, our MANOVA results found that birth age was the primary source of variation in bolus shape (Table S1), and no main effects exhibited high eigenvalues for any variable but PC1 (Table 2). We thus focused all further analyses on PC1, and found a birth age*age interaction for bolus shape (p <0.001).
Figure 2.
Bolus shape across PC1 (x-axis, 74% variation) and PC2 (y-axis, 12% variation) in term and preterm infant pigs (A) with examples of a bolus with a negative PC1 loading (B, pink outline) and a positive PC1 loading (C, green outline). Boluses for (B) and (C) are indicated by the color matched dotted line surrounding a point in (A). Preterm seven: green; preterm 17: orange; term seven: blue; term 17: pink.
Table 2.
Manova Eigen values for the four largest Principal components
| PC1 | PC2 | PC3 | PC4 | |
|---|---|---|---|---|
| Age | 0.22 | <0.001 | <0.001 | <0.001 |
| Birth age | 3.44 | <0.001 | <0.001 | |
| Individual | 1.39 | 0.05 | 0.03 | 0.01 |
| Age:Birth age | 0.12 | <0.001 | <0.001 | <0.001 |
| Age:Individual | 0.34 | 0.01 | 0.01 | 0.003 |
PC1: 73.9% variation. PC2: 11.7% variation. PC3: 8.2% variation. PC4: 2.3% variation
Bolded values indicate eigen values > 1.
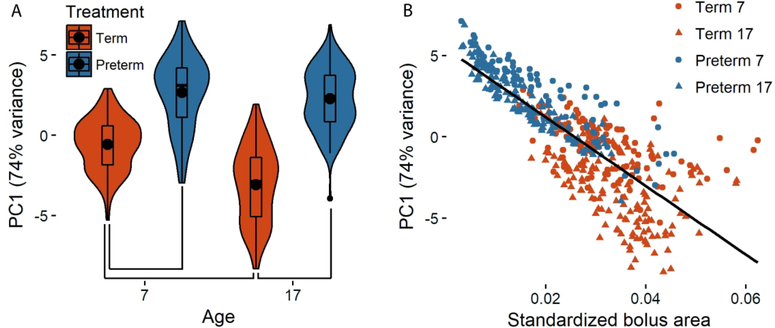
PC1 primarily distinguished cranially elongated, caudally rounded boluses from more ellipsoid or round boluses. In this context, a bolus with a negative loading along PC1 extends about midway into the oral cavity cranially with the caudal margin being defined by the line from the soft palate to the epiglottis and valleculae (Fig. 2B). In contrast, a bolus with a positive loading along PC1 indicates a bolus that only fills the vallecular floor, and does not extend into the oral cavity or contact the soft palate. Preterm infant pigs were characterized by rounder bolus shapes that did not change along PC1 as they aged (Preterm seven vs 17: Tukey’s p = 0.2), whereas term infant pigs were characterized by negative loads on PC1 compared to preterm pigs (term seven vs preterm seven Tukey’s p = 0.01; term 17 vs preterm 17 Tukey’s p = <0.001) and utilized a more elongate shaped bolus as they got older (term seven vs term 17 Tukey’s p <0.001, Fig. 2B, Fig. 3A). We found a significant correlation between bolus size and bolus shape (PC1), and as bolus shape became more elongate, it also became larger (p < 0.001, r2 = 0.65, Fig. 3B).
Figure 3.
Preterm and term infants differ in bolus shape along PC1 (74% variation) at both ages. Although term infants exhibit changes along PC1 as they mature, preterm infant bolus shape does not change (A). There is also a tight correlation between bolus size and bolus shape (B), whereby larger boluses utilized by term infants, especially at 17 days of age are more strongly negatively loaded than the smaller boluses used by preterm infants. Black dots in (A) indicate means for each group, with lines between groups indicating statistically significant differences as identified with post-hoc analyses, and the width of each plot represents the distribution of the data along the y-axis. Circles in (B) indicate seven day old infants; triangles indicate 17 day old infants; orange: term infants, blue: preterm infants.
Discussion
We found support for all three of our hypotheses. We found that preterm and term pigs exhibited marked differences in bolus size and bolus shape, and that there was an effect of maturation on bolus size and shape, especially within term infant pigs. As term pigs grew, the size of their bolus increased and their bolus shape changed, whereas preterm pigs did not swallow larger boluses as they themselves grew, and the shape of their bolus did not change through ontogeny despite them swallowing at similar rates to terms (16). Together, these results highlight the fact that preterm infants face a fundamentally different set of challenges than term infants, both in their feeding physiology, and in the development of their feeding performance.
The correlation between bolus size and bolus shape
The strong correlation between bolus size and bolus shape suggests that as animals take larger swallows, the shape of their bolus changes to reflect filling of the available anatomical space (Fig 3B). Pressure dynamics within the oropharynx play an important role in the formation and processing of a bolus, and the decreased pressure generation exhibited by both pig and human preterm infants during feeding suggests that they not only struggle to acquire and move milk (4), but also that their ability to form the bolus to fill the anatomical space within the pharynx is less than term infants. This is especially critical, as without proper bolus formation, liquid draining into the pharynx may not trigger the swallow reflex, which has can increase the risk of aspiration (15). This is especially pertinent for preterm infants that display reduced swallow-breathe coordination (16).
The maturation of bolus size and shape
As infants mature, bolus size typically increases (4). This increase has been suggested to be indicative of improvements in the swallowing process with maturity. Similar to research in human populations, we found the term pigs had larger boluses at day 17 than at day seven (4), and that the area of their bolus increased in proportion to their overall growth (Fig 1). This suggests that there is maturation in the swallowing process in term pigs, and that with time, they develop increased ability to process larger volumes of food at once. Furthermore, term pigs exhibited substantial development in their ability to fill the vallecular space prior to swallowing, as evidenced by the elongate shapes bolus shapes prior to the swallow in 17 day old, but not seven day old pigs (Fig. S1). This suggests that as term infant pigs matured, they met the increased metabolic demands of being larger by filling their vallecular space to a greater degree, allowing them to swallow greater quantities of milk per swallow.
In contrast, preterm infants did not swallow relatively larger boluses as they aged, and the shape of their bolus prior to the swallow did not change as they matured. Instead, preterm infants always had small, round boluses that did not extend as far into the oral cavity, and the caudal margin of the preterm bolus does not trace the soft palate-epiglottis-valleculae boundary as seen in older term pigs. Previous work has also shown that preterm infants acquire less milk per suck (13,14), and unlike term infants, they do not get better at coordinating swallowing with breathing as they age (16). The lack of development in preterm infant feeding suggests that preterm infants not only differ from term infants in their food acquisition and processing, but also in their swallow physiology. Although preterm infants could be fundamentally worse at processing food in order to swallow, one alternative explanation could be that they are compensating for poor airway protection by swallowing a smaller sized bolus, as smaller boluses are correlated with increased airway protection (5,6). However, neurologically compromised populations such as preterm infants have been shown to aspirate at lower bolus volumes compared to healthy populations (17). This suggests that aspiration frequency may be similar between preterm and term infants, even though bolus sizes are smaller in preterm infants.
Limitations of the study
One limitation of our study is that we used two-dimensional images as proxies for three-dimensional bolus properties (such as volume and shape). Thus, our results are limited in their insights into how the fluid dynamics of swallowing differs between term and preterm infants. Furthermore, we have not quantified tongue kinematics or muscle use and our results thus agree with the literature, rather than provide evidence to support the hypothesis that tongue kinematics and suction generation are worse in preterm infants (13,28,29). Additionally, we did not directly measure aspiration frequency, and although aspiration at a given volume is generally greater in preterms than in terms (8,11), and future research should pursue this possibility. Finally, although this study uses a validated animal model for infant feeding (30), how bolus size and shape mature in human infants is unknown. Instead, this research highlights the importance of studying bolus size and shape through maturation in future clinical work.
Role of muscular maturation of pharynx and esophagus in bolus properties
Although little is known about the maturation of bolus size and shape in term and preterm human infants, our results do have implications for clinical research on pediatric dysphagia. Bolus size and shape may be impacted by both the oral and pharyngeal phases of feeding, as noted above, but also might relate to the mechanics, requirements and constraints of the esophageal phase of feeding. For example, both human and pig preterm infants have decreased esophageal motility compared with term infants (31,32), and human preterm infants exhibit decreased pharyngeal contractions within bursts and decreased pharyngeal activity and contraction frequency (33). This is especially relevant because clinical work suggests that the pharyngeal swallow reflex itself does not show maturational changes in preterm infants, but preterm infants do show longitudinal maturation in esophageal sphincter relaxation reflexes (34). The smaller boluses in preterm infant pigs could therefore be due to decreased pharyngeal motility and activity in preterm infant pigs. This in turn suggests that a smaller bolus in preterm infants could be necessary to effectively move the bolus through the esophagus, a possibility which merits further study.
Conclusion
By using a longitudinal study design, this study adds to the growing body of work that suggests that there is a fundamental impact of preterm birth on feeding physiology (16), and that this fragile population faces a fundamentally different suite of problems than term infants as they mature that must be accounted for in making clinical decisions about their care and interventions.
Supplementary Material
Acknowledgements
We would like to thank K. Wu, E. Catchpole, C. Lewis, J. Irizarry, and K. McGrattan for their assistance caring for piglets and collecting data, as well as S. Dannemiller for his expertise in delivering and caring for the piglets.
Financial support: This project was funded by NIH R01 HD088561 to R.Z.G.
Footnotes
Competing interests: The authors declare no competing interests.
Category of study: Basic science
Ethics: Animal care and procedures were approved by NEOMED Institutional Animal Care and use Committee (#17-04-071).
References
- 1.German RZ, Crompton AW, Owerkowicz T, Thexton AJ. Volume and rate of milk delivery as determinants of swallowing in an infant model animal (Sus scrofia). Dysphagia 2004;19(3):147–54. [DOI] [PubMed] [Google Scholar]
- 2.Thexton AJ, Crompton AW, Owerkowicz T, German RZ. Correlation between intraoral pressures and tongue movements in the suckling pig. Arch Oral Biol 2004;49(7):567–75. [DOI] [PubMed] [Google Scholar]
- 3.Kawasaki M, Ogura JH. LXXIV Interdependence of deglutition with respiration. Ann Otol Rhinol Laryngol 1968;77(5):906–13. [DOI] [PubMed] [Google Scholar]
- 4.Lau C, Smith EO, Schanler RJ. Coordination of suck-swallow and swallow respiration in preterm infants. Acta Paediatr [Internet] 2003;92(6):721–7. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Citation&list_uids=12856985 [PubMed] [Google Scholar]
- 5.Gould FDH, Yglesias B, Ohlemacher J, German RZ. Pre-pharyngeal Swallow Effects of Recurrent Laryngeal Nerve Lesion on Bolus Shape and Airway Protection in an Infant Pig Model. Dysphagia [Internet] 2017;32(3):362–73. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27873091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding P, Fung GS, Lin M, Holman SD, German RZ. The effect of bilateral superior laryngeal nerve lesion on swallowing: a novel method to quantitate aspirated volume and pharyngeal threshold in videofluoroscopy. Dysphagia [Internet] 2015;30(1):47–56. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Citation&list_uids=25270532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rommel N, van Wijk M, Boets B, et al. Development of pharyngo-esophageal physiology during swallowing in the preterm infant. Neurogastroenterol Motil [Internet] 2011;23(10):e401–8. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Citation&list_uids=21827583 [DOI] [PubMed] [Google Scholar]
- 8.Jadcherla S Dysphagia in the high-risk infant: Potential factors and mechanisms. Am J Clin Nutr 2016;103(2):622S–628S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawdon JM, Beauregard N, Slattery J, Kennedy G. Identification of neonates at risk for developing feeding problems in infancy. Developmental Medicine & Child Neurology. Dev Med Child Neurol 2000;42(4):235–9. [DOI] [PubMed] [Google Scholar]
- 10.Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, Buekens P. Maternal stress and preterm birth. Am J Epidemiol 2003;157(1):14–24. [DOI] [PubMed] [Google Scholar]
- 11.Bryant-Waugh R, Markham L, Kreipe RE, Walsh BT. Feeding and eating disorders in childhood. Int J Eat Disord 2010;43(2):98–111. [DOI] [PubMed] [Google Scholar]
- 12.Lau C, People M. Oral feeding in low birth weight infants [Internet]. J. Pediatr 1997;130(4):561–9. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed4&NEWS=N&AN=1997355948 [DOI] [PubMed] [Google Scholar]
- 13.Lau C, Alagugurusamy R, Schanler RJ, Smith EO, Shulman RJ. Characterization of the developmental stages of sucking in preterm infants during bottle feeding. Acta Paediatr [Internet] 2000;89(7):846–52. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Citation&list_uids=10943969 [PubMed] [Google Scholar]
- 14.Gewolb IH, Vice FL, Schweitzer-Kenney EL, Taciak VL, Bosma JF. Developmental patterns of rhythmic suck and swallow in preterm infants. Dev Med Child Neurol 2001;43(1):22–7. [DOI] [PubMed] [Google Scholar]
- 15.Lau C Development of suck and swallow mechanisms in infants. Ann Nutr Metab 2015;66(suppl 5):7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayerl CJ, Gould FDH, Bond LE, Stricklen BM, Buddington RK, German RZ. Preterm birth disrupts the development of feeding and breathing coordination. J Appl Physiol 2019;126:1681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belo LR, Gomes NAC, Coriolano MDGWDS, et al. The relationship between limit of dysphagia and average volume per swallow in patients with Parkinson’s disease. Dysphagia 2014;29(4):419–24. [DOI] [PubMed] [Google Scholar]
- 18.Eiby YA, Wright LL, Kalanjati VP, et al. A pig model of the preterm neonate: anthropometric and physiological characteristics. PLoS One 2013;8(7):e68763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ballester A, Gould F, Bond L, et al. Maturation of the coordination between respiration and deglutition with and without recurrent laryngeal nerve lesion in an animal model. Dysphagia [Internet] 2018;33(5):627–35. Available from: 10.1007/s00455-018-9881-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.German RZ, Crompton AW, Thexton AJ. The coordination and interaction between respiration and deglutition in young pigs. J Comp Physiol 1998;182:539–47. [DOI] [PubMed] [Google Scholar]
- 21.German RZ, Crompton AW, Thexton AJ. Integration of the reflex pharyngeal swallow into rhythmic oral activity in a neurologically intact pig model. J Neurophysiol 2009;102(2):1017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thexton AJ, Crompton AW, German RZ. Electromyographic activity during the reflex pharyngeal swallow in the pig: Doty and Bosma (1956) revisited. J Appl Physiol [Internet] 2007;102(2):587–600. Available from: http://jap.physiology.org/cgi/doi/10.1152/japplphysiol.00456.2006 [DOI] [PubMed] [Google Scholar]
- 23.Sangild PT, Schmidt M, Elnif J, Björnvad CR, Weström BR, Buddington RK. Prenatal development of gastrointestinal function in the pig and the effects of fetal esophageal obstruction. Pediatr Res 2002;52(3):416–24. [DOI] [PubMed] [Google Scholar]
- 24.Gierbolini-Norat EM, Holman SD, Ding P, Bakshi S, German RZ. Variation in the Timing and Frequency of Sucking and Swallowing over an Entire Feeding Session in the Infant Pig Sus scrofa. Dysphagia [Internet] 2014;29:1–8. Available from: 10.1007/s00455-014-9532-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods [Internet] 2012;9(7):671–5. Available from: http://www.nature.com/doifinder/10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw [Internet] 2015;67(1):1–48. Available from: http://arxiv.org/abs/1406.5823 [Google Scholar]
- 27.Iwata H, Ukai Y. SHAPE: A computer program for quantitative evaluation of biological shapes based on elliptic fourier descriptors. J Hered [Internet] 2002;93(5):384–5. Available from: http://lbm.ab.a.utokyo.ac.jp/~iwata/shape/manual.pdf%5Cnhttp://jhered.oupjournals.org/cgi/doi/10.1093/jhered/93.5.384 [DOI] [PubMed] [Google Scholar]
- 28.Amaizu N, Shulman RJ, Schanler RJ, Lau C. Maturation of oral feeding skills in preterm infants. Acta Paediatr 2008;97(1):61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grassi A, Sgherri G, Chorna O, et al. Early intervention to improve sucking in preterm newborns. Adv Neonatal Care [Internet] 2018;0(0):1 Available from: http://insights.ovid.com/crossref?an=00149525-900000000-99775 [DOI] [PubMed] [Google Scholar]
- 30.German RZ, Crompton AW, Gould FD, Thexton AJ. Animal models for dysphagia studies: what have we learnt so far. Dysphagia [Internet] 2017;32(1):73–7. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28132098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasch S, Sangild PT, Gregersen H, Schmidt M, Omari T, Lau C. The preterm piglet - a model in the study of oesophageal development in preterm neonates. Acta Paediatr [Internet] 2010;99(2):201–8. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Citation&list_uids=19878132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staiano A, Boccia G, Salvia G, Zappulli D, Clouse RE. Development of esophageal peristalsis in preterm and term neonates. Gastroenterology 2007;132:1718–25. [DOI] [PubMed] [Google Scholar]
- 33.Prabhakar V, Hasenstab KA, Osborn E, Wei L, Jadcherla SR. Pharyngeal contractile and regulatory characteristics are distinct during nutritive oral stimulus in preterm-born infants: Implications for clinical and research applications. Neurogastroenterol Motil 2019;31:E13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jadcherla SR, Shubert TR, Gulati IK, Jensen PS, Wei L, Shaker R. Upper and lower esophageal sphincter kinetics are modified during maturation: effect of pharyngeal stimulus in premature infants. Pediatr Res 2015;77(1):99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.