Abstract
Background
The relationship between lung function decline and eosinophils and neutrophils has important therapeutic implications among asthmatics, but it has rarely been studied in large cohort studies.
Objective
The aim is to study the relationship between blood eosinophils and neutrophils and FEV1 decline in a long-term follow-up of a population-based adult asthma cohort.
Methods
In 2012–2014, an adult asthma cohort was invited to a follow-up including spirometry, blood sampling, and structured interviews, and n = 892 participated (55% women, mean age 59 y, 32–92 y). Blood eosinophils, neutrophils and FEV 1 decline were analyzed both as continuous variables and divided into categories with different cut-offs. Regression models adjusted for smoking, exposure to vapors, gas, dust, or fumes (VGDF), use of inhaled and oral corticosteroids, and other possible confounders were utilized to analyze the relationship between eosinophils and neutrophils at follow-up and FEV1 decline.
Results
The mean follow-up time was 18 years, and the mean FEV 1 decline was 27 ml/year. The annual FEV1 decline was related to higher levels of both blood eosinophils and neutrophils at follow-up, but only the association with eosinophils remained when adjusted for confounders. Further, the association between FEV1 decline and eosinophils was stronger among those using ICS. With EOS <0.3 × 109/L as reference, a more rapid decline in FEV1 was independently related to EOS ≥0.4 × 109/L in adjusted analyses.
Conclusions and clinical relevance
Besides emphasizing the importance of smoking cessation and reduction of other harmful exposures, our real-world results indicate that there is an independent relationship between blood eosinophils and FEV1 decline among adults with asthma.
Keywords: Asthma, FEV1, Eosinophils, Neutrophils, Cohort
Abbreviations: ANOVA, Analysis of variance; ATS, American Thoracic Society; BMI, Body mass index; ECRHS, European Community Respiratory Health Survey; EOS, Eosinophils; ERS, European Respiratory Society; FEV1, Forced Expiratory Volume in 1 s; FEV1pp, FEV1 percent of predicted; FVC, Forced Expiratory Volume; GLI, Global Lung function Initiative; ICS, Inhaled corticosteroids; IgE, Immunoglobulin E; L, Liters; Ml, Milliliters; N, Number; NEU, Neutrophils; OCS, Oral corticosteroids; OLIN, Obstructive Lung Disease in Northern Sweden; OLS, Ordinary Least Squares; VGDF, Vapors, gas, dust or fumes
Introduction
It is well recognized that accelerated lung function decline is associated with adverse health outcomes including mortality.1 Impaired lung function is common among asthma patients, and a population-based study from the late 1990s showed that over 15 years, subjects with asthma had a larger mean annual FEV1 decline than subjects without asthma.2 In contrast, a more recent study from the European Community Respiratory Health Survey (ECRHS) found that the annual FEV1 decline was quite similar in adults with and without asthma.3 Although not all asthmatics experience accelerated FEV1 decline, it infers an increased burden of disease and mortality risk on those affected.
Large longitudinal population-based studies on lung function decline among asthmatics are uncommon, and most knowledge is currently based on cross-sectional results and on patients recruited from health care. Previous studies have shown associations between impaired lung function and smoking,2,4 occupational exposures,5,6 low initial FEV1,7 bronchial hyper-reactivity,7,8 atopy,8 age,2 duration and severity of disease,7,9 and airway inflammation.10, 11, 12
Several studies have shown associations between severe asthma and eosinophil and/or neutrophil counts in induced sputum,13 and reducing particularly eosinophilic inflammation is of importance in the management of asthma. However, there is no consensus on how to define increased levels of eosinophils or neutrophils, and the knowledge about cut-offs that associate with accelerated lung function decline among asthmatics is limited. Further, although we know that higher levels of sputum eosinophils and neutrophils are commonly associated with airway obstruction in asthma,12,14 the knowledge on the association with longitudinal changes in FEV1 is limited. And importantly, the relationship between eosinophil and neutrophil counts in blood and FEV1 decline is even less studied.
Therefore, we aimed to study the relationship between blood eosinophils and neutrophils and FEV1 decline in a long-term follow-up of a population-based adult asthma cohort.
Methods
The asthma cohort
The asthma cohort (n = 2055) consists of adults with asthma in northern Sweden identified at clinical examinations of population samples performed between 1986 and 2001 within the Obstructive Lung Disease in Northern Sweden (OLIN) studies. All subjects reporting physician-diagnosed asthma, those reporting ever having had asthma, and also those with a medical history of asthma along with physiologically verified bronchial variability or asthma medication were included, as previously described.15
In 2012–2014, all subjects in the asthma cohort still living in Norrbotten (n = 1425) were invited to a follow-up including a detailed structured interview, spirometry, blood sampling for total IgE, and blood cell counts, in which 1006 (71%) participated.15,16 The current study includes subjects with data on both lung function measurements at baseline and follow-up, as well as on blood cell counts (n = 892).
Spirometry and FEV1 decline
At study entry, spirometry was performed according to guidelines of the European Respiratory Society (ERS) and American Thoracic Society (ATS),17,18 with a Mijnhardt Vicatest 5 dry volume spirometer. At the 2012–14 follow-up, spirometry was performed according to the 2005 ERS/ATS guidelines17 using a Jaeger Masterscope pneumotach spirometer, as previously described in detail.15,16 Lung function decline was assessed as annual change in pre-bronchodilator FEV1 in ml (FEV1ml) and FEV1 % of predicted19 (FEV1pp), respectively, between the first examination (in 1986–2001) and the follow-up. The annual change was calculated as the value at follow-up minus the value at the first examination divided by the number of years in-between. The annual change in FEV1pp was also divided by quartiles and rapid decline in FEV1 was defined as the lowest (most accelerated or rapid) 25% of the n = 892 values.
Blood eosinophils and neutrophils
At follow-up, the absolute levels of eosinophil (EOS) and neutrophil (NEU) counts in blood were assessed both as continuous variables, and categorized into groups: EOS<0.3, 0.3≤EOS<0.4 and EOS≥0.4 × 109/L, and NEU<4.0, 4.0≤NEU<5.0 and NEU≥5.0 × 109/L, respectively.
Statistical analyses
IBM SPSS Statistics 25.0 (Armonk, NY) was used for statistical analyses. In bivariate analyses, the Chi-square test or Mantel-Haenszel test for trend was used to test for differences in proportions and the T-test or ANOVA, as appropriate, were used to test for differences in means. The Spearman rho correlation coefficient was used to evaluate correlations between FEV1 decline and EOS and NEU. P-values <0.05 were considered statistically significant. In adjusted regression models, EOS, NEU, age and body mass index (BMI) at follow-up, sex, number of pack-years, exposure to vapors, gas, dust, or fumes (VGDF) at work, number of years of follow-up, allergic sensitization, FEV1 reversibility at study entry, FEV1 <80% at study entry, and inhaled (ICS) and oral corticosteroid (OCS) use at follow-up and/or at study entry were included as independent variables (see Supplementary variable definitions for more information). Linear regression (OLS) was performed with annual FEV1 decline as dependent variable and with EOS and NEU as continuous independent variables. Logistic regression models were performed with rapid decline in FEV1pp (the most rapid 25%) as outcome variable (non-rapid as reference), and with EOS and NEU included as categorical covariates.
Several sensitivity analyses are briefly presented in the results section, with more thorough information available as Supplementary material. Adjusted logistic regression analyses were performed with the outcome variable based on different definitions of rapid decline in FEV1. These definitions were based on annual decline in FEV1 assessed in ml and as Z-scores based on the OLIN and Global Lung function Initiative (GLI) reference values, respectively. These sensitivity analyses also include log-transformations of EOS and NEU and a comparison of rapid decline definitions divided by tertiles, quartiles, quintiles, sextiles, and septiles.
Results
Characteristics and mean annual FEV1 decline
Among the n = 892 asthmatics, the mean age at follow-up was 58.5 y (min-max 32–92 y), and 55% were women. While men were more often former smokers, women tended to be more often current smokers, both at study entry and at follow-up. Further, men had more pack years of smoking and had been more frequently exposed to vapors, gas, dust or fumes (VGDF) at work. At follow-up, a substantially larger proportion was using ICS compared to at study entry (Table 1), and ICS users had higher levels of eosinophils (Table 2). The mean annual FEV1 decline among all subjects was −27ml and −0.07 units in FEV1pp, respectively, and was higher among men, −32 ml (−0.18pp), than among women, −24 ml (0.02pp), (p < 0.001 for both FEV1ml and FEV1pp by sex), and among ever-smokers, −30 ml (−0.16pp), than among never-smokers, −25 ml (0.02pp) (p < 0.001 for both FEV1ml and FEV1pp by smoking habits), displayed divided by pack-years of smoking in Table 3.
Table 1.
Characteristics at study entry and at follow-up, by sex and among all subjects.
| Characteristics | At study entry (1986–2001) |
At follow-up (2012–2014) |
||||
|---|---|---|---|---|---|---|
| By sex |
By sex |
|||||
| Women N = 495 |
Men N = 397 |
All N = 892 |
Women N = 495 |
Men N = 397 |
All N = 892 |
|
| Mean (SD) pre-bronchodilator FEV1 in Liters | 2.84 (0.55) | 3.73 (0.82) | 3.24 (0.81) | 2.40 (0.63) | 3.12 (0.84) | 2.72 (0.81) |
| Mean (SD) pre-bronchodilator FEV1% of predicted | 90.2 (12.5) | 86.6 (14.9) | 88.6 (13.8) | 90.3 (15.4) | 83.1 (16.4) | 87.1 (16.2) |
| Mean (SD) number of years of follow-up | 18.2 (4.2) | 18.7 (4.4) | 18.4 (4.3) | |||
| Mean (SD) age (years) | 39.6 (11.7) | 40.6 (11.1) | 40.0 (11.5) | 57.9 (12.5) | 59.3 (11.7) | 58.5 (12.2) |
| Min-max age (years) | 19–70 | 19–69 | 19–70 | 33–92 | 32–92 | 32–92 |
| Mean (SD) BMI | 25.1 (4.6) | 26.1 (3.4) | 25.6 (4.1) | 28.2 (5.4) | 28.9 (4.4) | 28.5 (5.0) |
| Family history of asthma | 44.8% | 36.8% | 41.3% | |||
| Allergic sensitization | N/A | 26.8% | 42.9% | 33.9% | ||
| OCS use last 12 months | N/A | 2.2% | 1.3% | 1.7% | ||
| ICS use last 12 months | 10.9% | 12.8% | 11.8% | 48.1% | 38.3% | 43.7% |
| Never-smoker | 47.1% | 43.1% | 45.3% | 50.3% | 46.3% | 48.5% |
| Former smoker | 25.1% | 31.7% | 28.0% | 36.4% | 43.3% | 39.5% |
| Current smoker | 27.9% | 25.2% | 26.7% | 13.3% | 10.3% | 12.0% |
| Mean (SD) number of pack years of smokinga | N/A | 14.3 (14.2) | 21.3 (17.3) | 17.5 (16.0) | ||
| VGDF exposure | N/A | 21.0% | 62.9% | 39.7% | ||
Data presented as column % unless otherwise stated, Allergic sensitization = Positive on Phadiatop (>0.35 kU/L), SD = Standard deviation.
VGDF = vapors, gas, dust or fumes, L = liters, pp = %of predicted, BMI = body Mass Index.
N/A = Data not available, ICS = Inhaled corticosteroid, OCS = Oral corticosteroid.
Among former and current smokers
Table 2.
Lung function and proportions with blood eosinophils (EOS) < 0.3, 0.3 ≤ EOS<0.4, and EOS≥0.4 × 109/L, respectively, by the use of inhaled corticosteroids (ICS) at follow-up.
| ICS use at follow-up |
p-valuea | |||
|---|---|---|---|---|
| ICS naive | ICS users | |||
| n = 502 | n = 390 | |||
| Blood eosinophils at follow-up | ||||
| EOS<0.3 | n (%) | 394 (78.5) | 255 (65.4) | |
| 0.3 ≤ EOS<0.4 | n (%) | 52 (10.4%) | 62 (15.9) | |
| EOS≥0.4 | n (%) | 56 (11.2) | 73 (18.7) | <0.001 |
| Lung function | ||||
| FEV1 % of predicted at baseline | mean (SD) | 91.0 (12.3) | 85.6 (14.9) | <0.001 |
| FEV1 % of predicted at follow-up | mean (SD) | 89.1 (14.9) | 84.4 (17.5) | <0.001 |
| Annual FEV1 decline in % of predicted | mean (SD) | −0.09 (0.55) | −0.05 (0.69) | 0.384 |
| Annual FEV1 decline in ml | mean (SD) | −28.2 (19.2) | −26.5 (23.7) | 0.239 |
chi-square p-value for difference in proportions or T-test p-value for difference in means between groups based on ICS use at follow-up. EOS = blood eosinophils at follow-up, ICS = Inhaled corticosteroids at follow-up. Bold values indicate statistical significance
Table 3.
Mean cell counts in blood (109/L) at follow-up and mean annual decline in FEV1 by smoking habits.
| Mean cell count in blood |
Mean annual decline |
||||
|---|---|---|---|---|---|
| Eosinophils | Neutrophils | FEV1pp | FEV1ml | ||
| Never-smoker (N = 433) | Mean (SD) | 0.21 (0.17) | 3.57 (1.25) | 0.02 (0.58) | −24.6 (20.8) |
| <10 PY (N = 170) | Mean (SD) | 0.21 (0.16) | 3.71 (1.39) | −0.01 (0.58) | −25.3 (20.3) |
| 10 ≤ PY < 20 (N = 124) | Mean (SD) | 0.23 (0.17) | 3.91 (1.44) | −0.16 (0.65) | −28.9 (21.7) |
| 20 ≤ PY < 30 (N = 86) | Mean (SD) | 0.22 (0.15) | 4.21 (1.70) | −0.28 (0.65) | −34.4 (22.1) |
| PY ≥ 30 (N = 79) | Mean (SD) | 0.22 (0.15) | 4.37 (1.48) | −0.33 (0.62) | −37.7 (19.7) |
| ANOVA | P-value | 0.639 | <0.001 | <0.001 | <0.001 |
PY = Packyears of smoking at follow-up, FEV1pp = FEV1 percent (%) of predicted, SD = Standard deviation, Bold values indicate statistical significance.
A mean annual decline in FEV1pp of e.g. −0.33 implicates an average decrease of 1 unit in FEV1% pf predicted over three years, e.g. from 73% to 72%. A mean annual decline in FEV1ml of e.g. −33 ml implicates an average decrease of about 100 units in FEV1ml over three years, e.g. from 3200 ml to 3100 ml
Relationship between blood eosinophils and annual FEV1 decline
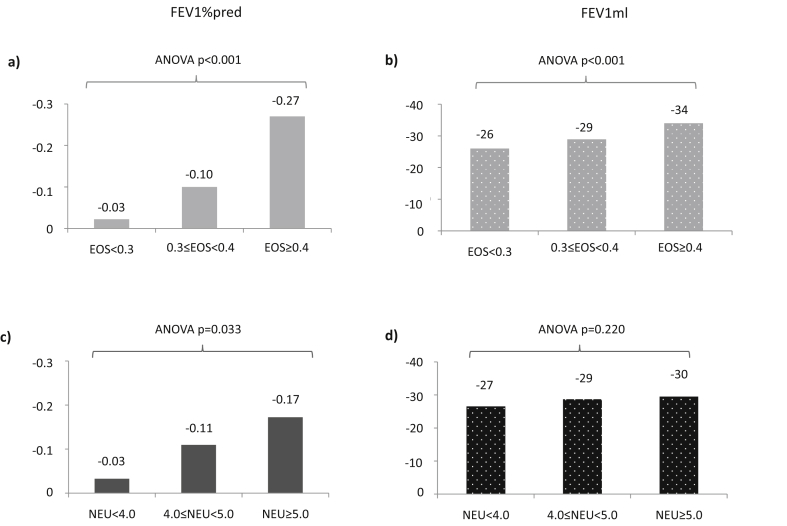
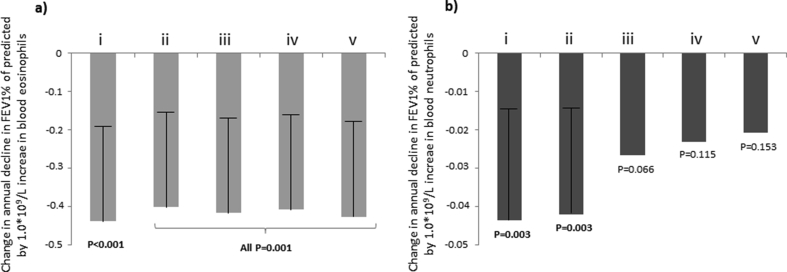
The mean annual FEV1 decline, both in terms of FEV1pp and FEV1ml, was significantly larger among subjects with higher levels of EOS (Fig. 1a and b). The Spearman rho correlation coefficient for association between annual FEV1pp decline and EOS was −0.123 (p < 0.001), with stronger association when only including those using ICS at follow-up, i.e. −0.203 (p < 0.001). The association between EOS and FEV1 decline remained significant also in linear regression models adjusted for NEU and possible confounders (Fig. 2a and Supplementary Figure E1).
Fig. 1.
a–d. Mean annual change in FEV1% of predicted (FEV1%) and FEV1 in ml (FEV1ml) within categories of blood eosinophils (a and b) and neutrophils (c and d) at follow-up, respectively. P-values from ANOVA for test of differences between groups
Fig. 2.
a–b. Mean annual change in FEV1% of predicted by one unit change in absolute levels (1.0∗109/L) of blood eosinophils (2a) and neutrophils (2b) at follow-up, respectively, assessed by B-coefficients with upper 95% CI and corresponding p-values from linear regression models. (i) = Unadjusted, (ii) = Adjusted for eosinophils (in Fig. 2b) and neutrophils (in Fig. 2a) in blood, number of years of follow-up, age, height and sex, (iii) = Adjusted for the same as (ii) and also for number of packyears of smoking and exposure to vapors, gas, dust or fumes at work, (iv) = Adjusted for the same as (iii) but also for BMI and allergic sensitization, (v) = Adjusted for the same as (iv) but also for ICS use and OCS use at study entry and/or follow-up, FEV1<80% of predicted at study entry, and significant FEV1 reversibility at study entry
Relationship between blood neutrophils and annual FEV1 decline
The mean annual decline in FEV1pp was significantly larger among subjects with higher levels of NEU, while the mean annual decline in FEV1ml was not (Fig. 1c and d). The Spearman rho correlation coefficients for the association between annual FEV1pp decline and NEU was −0.113 (p = 0.001), and −0.127 (p = 0.012) when only including those using ICS at follow-up. There was, however, no significant association between NEU and FEV1 decline when adjusted for EOS, smoking, exposure to VGDF, and other possible confounders in the linear regression models (Fig. 2b and Supplementary Figure E2).
Blood eosinophils and neutrophils in relation to rapid decline in FEV1
Among subjects with EOS <0.3, 0.3 ≤ EOS < 0.4, and EOS ≥0.4, respectively, 21.4%, 28.9%, and 39.5% (p < 0.001) had rapid FEV1 decline. Among subjects with NEU <4.0, 4.0 ≤ NEU < 5.0, and NEU ≥5.0, respectively, 22.0%, 30.2%, and 29.8% (p = 0.014) had rapid FEV1 decline.
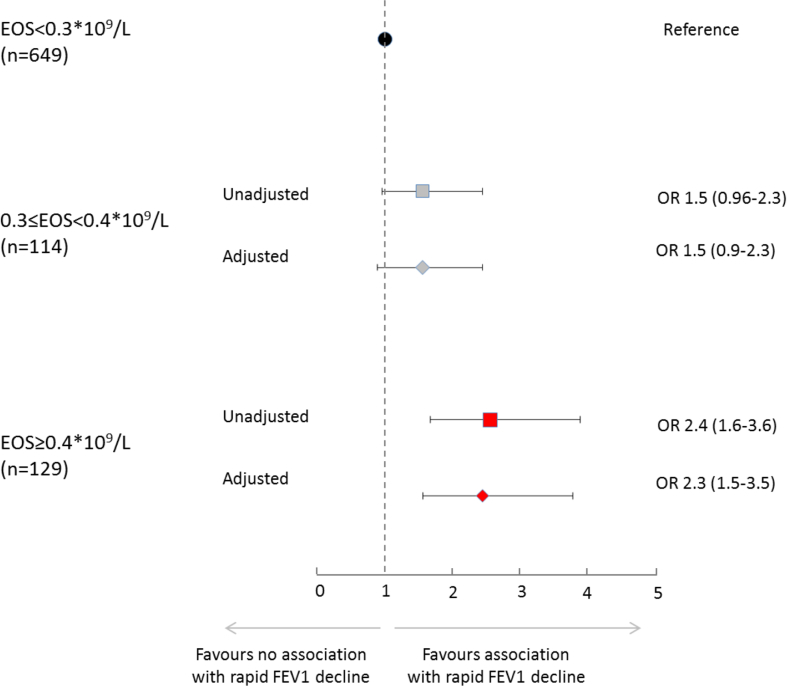
With subjects having EOS <0.3 as reference category, the odds ratios for rapid decline in FEV1 were 1.5 (95%CI 0.9–2.3) for subjects with 0.3 ≤ EOS < 0.4, and 2.3 (95%CI 1.5–3.5) for subjects with EOS ≥0.4, when adjusted for categories of NEU and possible confounders (Fig. 3). In the same logistic regression model, the odds ratios for rapid decline in FEV1 were 1.3 (95%CI 0.9–2.0) for subjects with 4.0 ≤ NEU < 5.0, and 1.2 (95%CI 0.7–1.8) for subjects with NEU ≥5.0, with subjects having NEU <4.0 as reference.
Fig. 3.
Unadjusted and adjusted associations between categories of blood eosinophils at follow-up and rapid decline in FEV1 (annual decline of −0.44 units or more in FEV1 % of predicted), expressed as odds ratios (OR) with 95% CI from logistic regression models (EOS<0.3∗109/L as reference). The outcome variable was rapid decline in FEV1% of predicted defined as the lowest (most rapid) 25% of all values (non-rapid decline as reference). Adjusted = adjusted for categories of neutrophils in blood, number of years of follow-up, age, height, sex, number of pack years of smoking, BMI, allergic sensitization, exposure to vapors, gas, dust or fumes at work, ICS use and OCS use at study entry and/or follow-up, FEV1<80% of predicted at study entry, and categories of significant FEV1 reversibility at study entry in logistic regression models.
Sensitivity analyses
Linear regression analyses stratified by smoking habits confirmed the main findings of an association between EOS and annual FEV1 decline among both never-smokers and ever-smokers, and the lack of association with NEU when adjusted (Supplemental Tables E1 and E2). Linear regression analyses stratified by sex revealed that, when adjusted, annual FEV1 decline was associated with EOS but not with NEU among women, while the opposite was observed among men (Supplemental Tables E3 and E4). Linear regression analyses with EOS and NEU entered into the model as log-transformed continuous independent variables confirmed the main findings (Supplemental Table E5).
The sensitivity analyses all confirmed the association with EOS≥0.4, and some results were significant also for 0.3 ≤ EOS < 0.4, while none of the NEU categories yielded statistically significant odds ratios in adjusted analyses (Supplemental Tables E6-E9). Further, analyses of the EOS cut-off of 0.150 did not yield significant associations with annual FEV1 decline (Supplemental Table E10).
Discussion
The main results of this study were that FEV1 decline was related to both eosinophils and neutrophils in blood in this adult population-based asthma cohort study, but only blood eosinophils remained independently associated with FEV1 decline also when taking age, sex, smoking, BMI, corticosteroid use, initial FEV1 level, and other factors possibly related to FEV1 into account. Further, blood eosinophil counts ≥0.4∗109/L was consistently and independently associated with larger decline in FEV1.
In the ECRHS starting in 1991–1993,3 the FEV1 decline was 29 ml/year over 9 years among n = 2116 subjects 20–56 years of age with asthma, results almost identical to our results of 27 ml/year. In 2 older studies, the decline was slightly higher.2,20 In the Australian Busselton Health Study performed between 1966 and 1995,20 the annual FEV1 decline among n = 1301 never-smoking adults with asthma was 28 ml/year among women and 40 ml/year among men, and in the Danish Copenhagen City Heart Study,2 the mean FEV1 decline was 38 ml/year among n = 1095 adults with asthma followed from 1976 to 1994. The less decline in more recent studies may be related to improvements in the treatment of asthma. However, neither of these two large population-based studies analyzed eosinophilic or neutrophilic inflammation in relation to FEV1 decline.
Our results of 27 ml annual FEV1 decline are also quite similar to those from smaller patient-based studies: 16.1–21.5 ml among n = 122 asthma patients on ICS treatment,21 31.5 ml among frequent exacerbators vs 15 ml/year among asthmatics not defined as frequent exacerbators,8 36 ml/year (0.34 pp/year) among those with <10packyears and 54 ml/year (0.75 pp/year) among those with ≥ 10 packyears among n = 203 Finnish asthmatics,4 and 51 ml in a study of n = 71 Dutch asthmatics completing a 2-year randomized controlled intervention study with bronchodilators.8 Blood eosinophils are related to severe asthma exacerbations,22 and it is possible that severe exacerbations contribute to excess FEV1 decline also in our study. Unfortunately, exacerbation data was lacking, why this could not be explored.
Airway eosinophilia is frequently observed in asthma but it is neither necessary nor sufficient for the development of the disease. However, the presence of eosinophils in sputum and blood can identify responders to inhaled or oral corticosteroids,23, 24, 25 and they are an important marker in the management of asthma. The ICS users also had higher levels of eosinophils, and lower FEV1 already at baseline. Interestingly, the association between FEV1 decline and blood eosinophils was stronger among those using ICS, results possibly due to confounding by indication, as prescriptions of ICS are more common in patients with a more severe asthma compared with those having mild asthma.26 It is well known from a large amount of clinical trials and also from population based studies27,28 that ICS use can prevent lung function decline in asthma patients. Thus, our results may underestimate the association between increased levels of EOS and decline in lung function.
Studies on random population-samples analyzing the relationship between lung function decline and blood cell counts have been lacking, but the association between eosinophils in blood and FEV1 decline among asthmatics was recently investigated in the Dunedin birth cohort29 which has been studied repeatedly from age 21 to 38. They found blood eosinophils significantly associated with decline in both the FEV1/FVC ratio and FEV1pp both among subjects with and without asthma,29 independent of smoking, which is well in line with our results. Neutrophil counts were, however, not analyzed in this study.
Although eosinophilia has been related to impaired lung function, all asthma patients with eosinophilia will not experience excess FEV1 decline,10,12 results that suggest complexity. Among n = 87 severe asthma patients in the UK with <10 pack-years of smoking and a mean FEV1 annual decline of 25.7 ml/year, it was actually the over-time variability in sputum eosinophilia rather than the baseline or follow-up eosinophil levels which associated with excess FEV1 decline.12 In contrast, one recent study on asthma patients showed that it was low (EOS <0.21 × 109/L) rather than high baseline eosinophils that associated with excess FEV1 decline,30 and yet another study on n = 141 patients with adult onset asthma showed that neither blood nor sputum eosinophilia associated with accelerated FEV1 decline.31 Thus, it is not clear how eosinophilic inflammation affects lung function decline and more and larger studies seem required to disentangle this relationship among asthmatics, where our large population-based asthma cohort contributes with important information.
There is evidence that neutrophils in a large proportion of patients with neutrophilic asthma can be in an activated state, thus likely to cause tissue damage,32 and that these patients may also be unresponsive to corticosteroids.33 Correspondingly, we have previously shown that the levels of blood neutrophils are elevated among those with severe asthma in our cohort.34 Neutrophilic asthma may represent a phenotype associated with exposures such as cigarette smoking32,35 and excess lung function decline,4 which also would be in line with previous results showing that smoking is a risk factor for the development of chronic airway obstruction among asthmatics.16 In our study, men were much more frequently exposed to both smoking and VGDF than women. In the sex-stratified analyses, the relationship with FEV1 decline was consistently related to eosinophils among women. In contrast, neutrophils were related to lung function decline among men also after adjustment for confounders, which despite our efforts to account for exposures may be due to residual confounding caused by their more dominant and extensive exposure pattern. In summary, it seems that the relationship between lung function decline and eosinophils among asthmatics is much less related to external factors such as smoking and exposure to VGDF compared to the relationship between lung function decline and neutrophils. However, blood neutrophils are no precise predictors of sputum neutrophils,35 why there may exist associations between neutrophilic airway inflammation and FEV1 decline not detected in our study.
Different relevant cut-offs for defining increased eosinophils and neutrophils have been proposed, but so far there is no consensus. We found that subjects with blood eosinophil counts ≥0.4 × 109/L consistently had an elevated risk for a larger decline in FEV1, in line with the results from the Dunedin study.29 Further, subjects with eosinophils in blood between 0.3 and 0.4 × 109/L also had a significantly increased risk for a larger FEV1 decline in our sensitivity analyses. Different cut-offs have also been analyzed for associations between eosinophils and asthma exacerbations, e.g. >0.15, >0.30, and >0.50 × 109/L,36 and different cut-offs for increased levels have been defined also for neutrophils.32 Further, blood eosinophils can predict sputum eosinophilia among asthmatics35,37 while blood neutrophils are more poorly related to sputum neutrophils,35 and interpreting and comparing different cut-offs in blood and sputum may be difficult. Results also based on induced sputum would have been valuable in the current study in order to assess inflammation in the target organs, i.e. the lungs. However, in contrast to analyses of induced sputum, analyses of cell counts in peripheral blood are suitable for large-scale studies due to methodological and cost-related matters, and as at least regarding eosinophils the correlation between blood and induced sputum is high.35,37
It has long been argued that airway inflammation is associated with airway re-modelling with thickening of the epithelium and sub-epithelial structures including the smooth muscles, all contributing to obstruction of the airway.38 The normal rate of lung function decline may be related to loss of elastic recoil in the lung and weaker diaphragm, as well as the inevitable life-long effects of exposure to pollutions and airway infections caused by e.g. viruses. Thus, the mechanism behind lung function decline due to ageing of the lung is multifactorial.
Regarding limitations of our study, we have no data on blood cell counts at baseline or during the follow-up period, why we cannot assess variability over time in e.g. eosinophils which previously has been associated with FEV1 decline when analyzed in sputum.12 Another possible limitation of this study is that as the FEV1 decline estimates were based on pre-bronchodilator values, the reversible obstruction aspect was not evaluated regarding the estimates of decline. However, analyses in the subsample with available post-bronchodilator values showed almost identical results (Supplementary material). An acute allergic inflammation at any time point could possibly be associated with both transient FEV1 decline and increased blood eosinophilia, however, as the individuals were not allowed to have a recent asthma exacerbation at the time of the examination, this is most probably not a source of bias. Also, different types of spirometers were used at study entry and at follow-up, which could affect the estimates of decline. However, this will affect all subjects in a similar way and thus probably not bias the associations with blood cell counts. With regards to strengths of this study, it is based on a large well-characterized population-based asthma cohort,15 enabling stratified analyses with sufficient power, and has a long follow-up time period. Our results on associations between FEV1 decline and eosinophils were clear and consistent across several different definitions. Further, the similarity of the mean annual FEV1 decline in our study with results from the few other large population-based studies on adults with asthma2,3,20 strengthens the external validity of our results.
Finally, in contrast to the current study, most previous large population studies on the association between FEV1 and blood cell counts are cross-sectional. It should be acknowledged that the long follow-up period probably reduces the effect of the day-by-day variability in FEV1 on the estimates of decline. Thus, this study provides novel data on progressing lung function changes among adults with asthma. Although baseline blood cell counts would have been of great value to assess prognostic associations, we still argue that the presented real-world data on a consistent relationship between FEV1 decline and blood eosinophils adds important input to the current body of evidence. Importantly, further research on asthma among adults is warranted, as we still have not seen an end to the increasing prevalence.39
In conclusion, this long-term follow-up of a population-based cohort of adults with asthma shows an association between FEV1 decline and blood eosinophils, independent of other factors and exposures. In contrast, the association between FEV1 decline and blood neutrophils was related to exposures such as smoking and vapors, gas, dust, or fumes at the work place. Besides emphasizing the importance of smoking cessation and reducing other harmful exposures, our results of a relationship between blood eosinophils and FEV1 decline among adults with asthma highlight both possibilities and need for other interventions.
Ethics approval and consent to participate
All participants provided written informed consent, and ethical approval was achieved from the Regional Ethical Review Board at Umeå University (Dnr 2011-106-31M).
Consent for publication
Not applicable.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
Supported by grants from The Swedish Heart & Lung Foundation, The Swedish Research Council, a regional agreement between Umeå University and Västerbotten County Council (ALF), Norrbotten County Council, the Swedish Asthma-Allergy Foundation, and VISARE NORR Fund: Northern county councils Regional federation. Additional support was provided by ThermoFisher, Uppsala, Sweden.
Authors’ contributions
BL and ER designed the methodology for the longitudinal design of the asthma cohort. ER and HB designed the current study, interpreted the data, and drafted the manuscript. HB performed the statistical analyses. All authors contributed with interpretation of data, revised the manuscript critically for important intellectual content, and approved the final version to be submitted. All authors are accountable for all aspects of the work and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of Competing Interest
Dr. Backman reports personal fees from Boehringer Ingelheim and AstraZeneca outside the submitted work, Dr. Lindberg reports personal fees from Boehringer Ingelheim, AstraZeneca, Novartis and Active Care outside the submitted work, Dr. Lundbäck reports grants from AstraZeneca, grants from GSK, personal fees from AstraZeneca, personal fees from GSK, and personal fees from Novartis, all outside the submitted work, Dr, Sandström, Dr Hedman and Dr Jansson have nothing to disclose, Dr Stridsman reports personal fees from Novartis and Astra Zeneca, outside the submitted work, and Dr. Rönmark reports grants from Astra Zeneca, grants from GlaxoSmithCline, outside the submitted work.
Acknowledgements
We would especially like to acknowledge the participants in the study. Also the research staff within the OLIN-studies is acknowledged for their excellent work.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2020.100110.
Contributor Information
Helena Backman, Email: helena.backman@norrbotten.se.
Anne Lindberg, Email: anne.lindberg@algmed.se.
Linnea Hedman, Email: linnea.hedman@norrbotten.se.
Caroline Stridsman, Email: caroline.stridsman@norrbotten.se.
Sven-Arne Jansson, Email: sven-arne.jansson@umu.se.
Thomas Sandström, Email: thomas.sandstrom@umu.se.
Bo Lundbäck, Email: bo.lundback@gu.se.
Eva Rönmark, Email: eva.ronmark@norrbotten.se.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Hole D.J., Watt G.C., Davey-Smith G., Hart C.L., Gillis C.R., Hawthorne V.M. Impaired lung function and mortality risk in men and women: findings from the renfrew and paisley prospective population study. BMJ. 1996;313(7059):711–715. doi: 10.1136/bmj.313.7059.711. discussion 715-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lange P., Parner J., Vestbo J., Schnohr P., Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339(17):1194–1200. doi: 10.1056/NEJM199810223391703. [DOI] [PubMed] [Google Scholar]
- 3.Aanerud M., Carsin A.E., Sunyer J. Interaction between asthma and smoking increases the risk of adult airway obstruction. Eur Respir J. 2015;45(3):635–643. doi: 10.1183/09031936.00055514. [DOI] [PubMed] [Google Scholar]
- 4.Tommola M., Ilmarinen P., Tuomisto L.E. The effect of smoking on lung function: a clinical study of adult-onset asthma. Eur Respir J. 2016;48(5):1298–1306. doi: 10.1183/13993003.00850-2016. [DOI] [PubMed] [Google Scholar]
- 5.Anees W., Moore V.C., Burge P.S. FEV1 decline in occupational asthma. Thorax. 2006;61(9):751–755. doi: 10.1136/thx.2005.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sikkeland L.I., Johnsen H.L., Riste T.B. Sputum neutrophils are elevated in smelter workers, and systemic neutrophils are associated with rapid decline in FEV1. Occup Environ Med. 2016;73(7):459–466. doi: 10.1136/oemed-2015-103083. [DOI] [PubMed] [Google Scholar]
- 7.Vonk J.M., Jongepier H., Panhuysen C.I., Schouten J.P., Bleecker E.R., Postma D.S. Risk factors associated with the presence of irreversible airflow limitation and reduced transfer coefficient in patients with asthma after 26 years of follow up. Thorax. 2003;58(4):322–327. doi: 10.1136/thorax.58.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Schayck C.P., Dompeling E., Van Herwaarden C.L., Wever A.M., Van Weel C. Interacting effects of atopy and bronchial hyperresponsiveness on the annual decline in lung function and the exacerbation rate in asthma. Am Rev Respir Dis. 1991;144(6):1297–1301. doi: 10.1164/ajrccm/144.6.1297. [DOI] [PubMed] [Google Scholar]
- 9.Brown P.J., Greville H.W., Finucane K.E. Asthma and irreversible airflow obstruction. Thorax. 1984;39(2):131–136. doi: 10.1136/thx.39.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai T.R., Vonk J.M., Postma D.S., Boezen H.M. Severe exacerbations predict excess lung function decline in asthma. Eur Respir J. 2007;30(3):452–456. doi: 10.1183/09031936.00165106. [DOI] [PubMed] [Google Scholar]
- 11.Contoli M., Baraldo S., Marku B. Fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease: 5-year follow-up. J Allergy Clin Immunol. 2010;125(4):830–837. doi: 10.1016/j.jaci.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Newby C., Agbetile J., Hargadon B. Lung function decline and variable airway inflammatory pattern: longitudinal analysis of severe asthma. J Allergy Clin Immunol. 2014;134(2):287–294. doi: 10.1016/j.jaci.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Moore W.C., Hastie A.T., Li X. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2014;133(6):1557–1563. doi: 10.1016/j.jaci.2013.10.011. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broekema M., Volbeda F., Timens W. Airway eosinophilia in remission and progression of asthma: accumulation with a fast decline of FEV(1) Respir Med. 2010;104(9):1254–1262. doi: 10.1016/j.rmed.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 15.Backman H., Hedman L., Stridsman C. A population-based cohort of adults with asthma: mortality and participation in a long-term follow-up. Eur Clin Respir J. 2017;4(1):1334508. doi: 10.1080/20018525.2017.1334508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Backman H., Jansson S.A., Stridsman C. Chronic airway obstruction in a population-based adult asthma cohort: prevalence, incidence and prognostic factors. Respir Med. 2018;138:115–122. doi: 10.1016/j.rmed.2018.03.036. [DOI] [PubMed] [Google Scholar]
- 17.Miller M.R., Hankinson J., Brusasco V. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 18.Standardization of spirometry, 1994 update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 19.Backman H., Lindberg A., Oden A. Reference values for spirometry - report from the obstructive lung disease in northern Sweden studies. Eur Clin Respir J. 2015;2:26375. doi: 10.3402/ecrj.v2.26375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James A.L., Palmer L.J., Kicic E. Decline in lung function in the busselton health study: the effects of asthma and cigarette smoking. Am J Respir Crit Care Med. 2005;171(2):109–114. doi: 10.1164/rccm.200402-230OC. [DOI] [PubMed] [Google Scholar]
- 21.Dijkstra A., Vonk J.M., Jongepier H. Lung function decline in asthma: association with inhaled corticosteroids, smoking and sex. Thorax. 2006;61(2):105–110. doi: 10.1136/thx.2004.039271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeiger R.S., Schatz M., Li Q. High blood eosinophil count is a risk factor for future asthma exacerbations in adult persistent asthma. J Allergy Clin Immunol Pract. 2014;2(6):741–750. doi: 10.1016/j.jaip.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Meijer R.J., Postma D.S., Kauffman H.F., Arends L.R., Koeter G.H., Kerstjens H.A. Accuracy of eosinophils and eosinophil cationic protein to predict steroid improvement in asthma. Clin Exp Allergy. 2002;32(7):1096–1103. doi: 10.1046/j.1365-2222.2002.01412.x. [DOI] [PubMed] [Google Scholar]
- 24.Berthon B.S., Gibson P.G., Wood L.G., MacDonald-Wicks L.K., Baines K.J. A sputum gene expression signature predicts oral corticosteroid response in asthma. Eur Respir J. 2017;49(6) doi: 10.1183/13993003.00180-2017. 00180-2017. [DOI] [PubMed] [Google Scholar]
- 25.Demarche S.F., Schleich F.N., Henket M.A., Paulus V.A., Van Hees T.J., Louis R.E. Effectiveness of inhaled corticosteroids in real life on clinical outcomes, sputum cells and systemic inflammation in asthmatics: a retrospective cohort study in a secondary care centre. BMJ Open. 2017;7(11) doi: 10.1136/bmjopen-2017-018186. e018186-2017-018186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W., Marra C.A., Lynd L.D., FitzGerald J.M., Zafari Z., Sadatsafavi M. The natural history of severe asthma and influences of early risk factors: a population-based cohort study. Thorax. 2016;71(3):267–275. doi: 10.1136/thoraxjnl-2015-207530. [DOI] [PubMed] [Google Scholar]
- 27.Lange P., Scharling H., Ulrik C.S., Vestbo J. Inhaled corticosteroids and decline of lung function in community residents with asthma. Thorax. 2006;61(2):100–104. doi: 10.1136/thx.2004.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Byrne P., Fabbri L.M., Pavord I.D., Papi A., Petruzzelli S., Lange P. Asthma progression and mortality: the role of inhaled corticosteroids. Eur Respir J. 2019;54(1) doi: 10.1183/13993003.00491-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hancox R.J., Pavord I.D., Sears M.R. Associations between blood eosinophils and decline in lung function among adults with and without asthma. Eur Respir J. 2018;51(4) doi: 10.1183/13993003.02536-2017. 02536-2017. [DOI] [PubMed] [Google Scholar]
- 30.Semprini R., Williams M., Semprini A. Type 2 biomarkers and prediction of future exacerbations and lung function decline in adult asthma. J Allergy Clin Immunol Pract. 2018;6(6):1982–1988. doi: 10.1016/j.jaip.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Coumou H., Westerhof G.A., de Nijs S.B., Zwinderman A.H., Bel E.H. Predictors of accelerated decline in lung function in adult-onset asthma. Eur Respir J. 2018;51(2):1785–2017. doi: 10.1183/13993003.01785-2017. [DOI] [PubMed] [Google Scholar]
- 32.Simpson J.L., Scott R.J., Boyle M.J., Gibson P.G. Differential proteolytic enzyme activity in eosinophilic and neutrophilic asthma. Am J Respir Crit Care Med. 2005;172(5):559–565. doi: 10.1164/rccm.200503-369OC. [DOI] [PubMed] [Google Scholar]
- 33.Holgate S.T., Holloway J., Wilson S. Understanding the pathophysiology of severe asthma to generate new therapeutic opportunities. J Allergy Clin Immunol. 2006;117(3):496–506. doi: 10.1016/j.jaci.2006.01.039. quiz 507. [DOI] [PubMed] [Google Scholar]
- 34.Backman H., Jansson S.A., Stridsman C. Severe asthma - a population study perspective. Clin Exp Allergy. 2019;49(6):819–828. doi: 10.1111/cea.13378. [DOI] [PubMed] [Google Scholar]
- 35.Schleich F.N., Manise M., Sele J., Henket M., Seidel L., Louis R. Distribution of sputum cellular phenotype in a large asthma cohort: predicting factors for eosinophilic vs neutrophilic inflammation. BMC Pulm Med. 2013;13 doi: 10.1186/1471-2466-13-11. 11-2466-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ortega H.G., Liu M.C., Pavord I.D. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X.Y., Simpson J.L., Powell H. Full blood count parameters for the detection of asthma inflammatory phenotypes. Clin Exp Allergy. 2014;44(9):1137–1145. doi: 10.1111/cea.12345. [DOI] [PubMed] [Google Scholar]
- 38.Saglani S., Lloyd C.M. Novel concepts in airway inflammation and remodelling in asthma. Eur Respir J. 2015;46(6):1796–1804. doi: 10.1183/13993003.01196-2014. [DOI] [PubMed] [Google Scholar]
- 39.Backman H., Hedman L., Jansson S.A., Lindberg A., Lundbäck B., Rönmark E. Prevalence trends in respiratory symptoms and asthma in relation to smoking - two cross-sectional studies ten years apart among adults in northern Sweden. World Allergy Organ J. 2014 Jan 2;7(1):1. doi: 10.1186/1939-4551-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.