Abstract
Background
Second cancer is the leading cause of death in lymphoma survivors, with lung cancer representing the most common solid tumor. Limited information exists about the treatment and prognosis of second lung cancer following lymphoma. Herein, we evaluated the outcome and prognostic factors of Lung Cancer in Lymphoma Survivors (the LuCiLyS study) to improve the patient selection for lung cancer treatment.
Methods
This is a retrospective multicentre study including consecutive patients treated for lymphoma disease that subsequently developed non-small cell lung cancer (NSCLC). Data regarding lymphoma including age, symptoms, histology, disease stage, treatment received and lymphoma status at the time of lung cancer diagnosis, and data on lung carcinoma as age, smoking history, latency from lymphoma, symptoms, histology, disease stage, treatment received, and survival were evaluated to identify the significant prognostic factors for overall survival.
Results
Our study population included 164 patients, 145 of which underwent lung cancer resection. The median overall survival was 63 (range, 58–85) months, and the 5-year survival rate 54%. At univariable analysis no-active lymphoma (HR: 2.19; P=0.0152); early lymphoma stage (HR: 1.95; P=0.01); adenocarcinoma histology (HR: 0.59; P=0.0421); early lung cancer stage (HR: 3.18; P<0.0001); incidental diagnosis of lung cancer (HR: 1.71; P<0.0001); and lung cancer resection (HR: 2.79; P<0.0001) were favorable prognostic factors. At multivariable analysis, no-active lymphoma (HR: 2.68; P=0.004); early lung cancer stage (HR: 2.37; P<0.0001); incidental diagnosis of lung cancer (HR: 2.00; P<0.0001); and lung cancer resection (HR: 2.07; P<0.0001) remained favorable prognostic factors. Patients with non-active lymphoma (n=146) versus those with active lymphoma (n=18) at lung cancer diagnosis presented better median survival (64 vs. 37 months; HR: 2.4; P=0.02), but median lung cancer specific survival showed no significant difference (27 vs. 19 months; HR: 0.3; P=0.17).
Conclusions
The presence and/or a history of lymphoma should not be a contraindication to resection of lung cancer. Inclusion of lymphoma survivors in a lung cancer-screening program may lead to early detection of lung cancer, and improve the survival.
Keywords: Lymphoma, second primary tumor, lung cancer
Introduction
Developments in modern chemotherapy and radiotherapy have increased the survival rate of lymphoma patients. The cure rate for patients with early stage Hodgkin lymphoma (HL) is generally ≥90%, and the 5-year survival rate (5-YSR) for patients with non-HL ranges from 26% to 73%, depending on the stage and the aggressiveness of the disease (1). However, lymphoma survivors develop second primary malignancies with a higher rate than people of the same age, and sex in the general population (2). Established risk factors for lung cancer include radiotherapy, and alkylating-agent chemotherapy as well as smoking (3,4). During prolonged follow-up after initial treatment of lymphoma, the mortality of lung cancer overcomes that of lymphoma, and it represents the main cause of death in lymphoma survivors (5). Nevertheless, only few studies (1,6-8) have evaluated the outcome of lymphoma survivors who developed lung cancer as second primary malignancy. Thus, it is still unclear whether standard treatment options for lung cancer are limited or not by lymphoma.
In this study, we evaluated the outcome and prognostic factors of Lung Cancer in Lymphoma Survivors (the LuCiLyS study) in order to improve the patient selection for lung cancer treatment.
Methods
Study design
This is a retrospective multicenter study. Consecutive patients who developed non-small cell lung cancer (NSCLC) as second primary cancer after lymphoma diagnosis, were identified from data base or medical records of each participating institution. A minimum latency of 2 months between lymphoma and diagnosis of NSCLC was required, which is the standard latency generally adopted to exclude synchronous primary cancers (6-8). We excluded from the analysis: (I) patients with a different histological diagnosis from NSCLC (i.e., neuroendocrine, carcinoid, small cell carcinoma, oat cell carcinoma, sarcoma, and mesothelioma histology) as the natural history, as treatment, and/or outcome of these histologic subtypes differ from those of NSCLC; (II) patients with simultaneous diagnosis of lung carcinoma and lymphoma; (III) patients diagnosed with an additional second malignancy before and after their diagnosis of NSCLC, as it was difficult to understand whether previous lymphoma or second malignancy affected lung cancer outcome; (IV) patients with incomplete follow-up.
The end-points of the paper were to evaluate post-operative morbidity and mortality of lung cancer resection and prognostic risk factors of overall survival. The study design was approved by Local Ethics Committees of University of Campania Luigi Vanvitelli (approvation code: 228/19), coordinating center of the study, and then approved by each participating center. All patients gave a written informed consent for the surgical treatment and were aware that all information could be used anonymously for scientific purpose only.
Data abstraction
The following data were recorded for each patient: gender, smoking history, age at diagnosis of lymphoma and at diagnosis of NSCLC, stage, and histology of lymphoma; treatment given for lymphoma including radiotherapy, chemotherapy or combined radio-chemotherapy, histology and stage of NSCLC re-classified based on the latest, 8th edition of TNM staging system, treatments given for lung carcinoma including surgery, chemotherapy, radiotherapy or combined chemo-radiotherapy. For patients undergoing lung cancer resection, we also evaluated the type of resection and post-operative morbidity and mortality. Operative mortality was defined as any death within 30 days of operation or prior to discharge.
Survival
End points of the analysis included overall survival, and lung cancer specific survival. Overall survival was measured from the date of surgery until death. Patients without events were censored at the time of last follow up. Lung cancer specific survival was calculated from the date of surgery until death due to lung cancer.
Statistical analysis
Data were reported as mean ± standard deviation (SD), or median and interquartile range for continuous variables or as number and percentages for categorical variables. The chi-square test and Fisher’s exact test were performed to evaluate significant differences of proportions or percentages between two groups. Particularly Fisher’s exact test was used where the chi-square test was not appropriate. Significant difference between two means was evaluated with Student’s t-test. Survival curves were calculated by the Kaplan-Meier method, and differences assessed by log-rank test. Univariate analysis was performed and the hazard ratio (HR) with confidence interval at 95% were evaluated to compare two conditions or states, in this way we identified the prognostic factors influencing the overall survival. The variables reaching statistically significant difference entered into a multivariate regression analysis with forward selection and backward elimination, using death as endpoint (dependent variable). A P<0.05 was considered statistically significant. We used MedCalc statistical software (Version 12.3, Broekstraat 52; 9030 Mariakerke; Belgium) for all statistical analyses
Results
One hundred and seventy-nine patients with lung cancer diagnosis and a history of lymphoma were retrospectively identified; of these, 15 patients were excluded due to a different histological diagnosis from NSCLC (n=7), presence of additional second malignancy before diagnosis of NSCLC (n=3), or incomplete follow-up (n=5). Thus, our study population included 164 patients.
Lymphoma data
Data are summarized in Table 1. The mean age at lymphoma diagnosis was 49±15 years. The majority of patients were male (n=123/164; 75%) and smokers (n=155/164; 95%), presented no lymphoma B symptoms (n=84/164; 51%), and had stage I (n=63/164; 38%) disease. The most common type of lymphoma was NHD (n=143/164; 87%), and follicular cell lymphoma was the most represented histological subtype (n=69/143; 48%). If considering lymphoma treatment, 74 (45%) patients received radiotherapy (RT), 54 (33%) chemotherapy (CT), 27 (16%) CT associated with RT, and 9 (5%) patients a combined treatment with RT, CT and surgery including splenectomy (n=6), gastrectomy (n=1), or intestinal resection (n=1).
Table 1. Characteristics of lymphoma (n=164).
| Variables | Number [%] |
|---|---|
| Gender (male/female) | 123 [75]/41 [25] |
| Smokers | 130 [79]/34 [21] |
| Age at lymphoma diagnosis (years) | 49±15 |
| ≤29 | 26 [16] |
| 30–39 | 39 [24] |
| 40–49 | 71 [43] |
| ≥50 | 28 [17] |
| Lymphoma B symptoms | |
| No | 84 [51] |
| Yes | 45 [27] |
| Unknown | 35 [22] |
| Lymphoma stage | |
| I | 63 [38] |
| II | 46 [28] |
| III | 39 [24] |
| IV | 16 [10] |
| Lymphoma classification | |
| Hodgkin disease | 21 [13] |
| Non-Hodgkin disease | 143 [87] |
| Follicular cell lymphoma | 69 [42] |
| Diffuse large B-cell lymphoma | 44 [27] |
| T-cell lymphoma | 2 [1] |
| Mantle cell lymphoma | 10 [6] |
| MALT lymphoma | 14 [9] |
| Burkitt lymphoma | 4 [2] |
| Treatment of lymphoma | |
| RT alone | 74 [45] |
| CT alone | 54 [33] |
| RT + CT | 27 [16] |
| RT + CT + surgery | 9 [5] |
CT, chemotherapy; RT, radiotherapy; MALT, mucosa-associated lymphoid tissue.
Lung cancer data
Data are summarized in Table 2. The mean age at lung cancer diagnosis was 57±9.7 years, and most patients were smokers (142/164; 87%). The mean latency between diagnosis of lymphoma and that of lung cancer was 8.8±2.1 years, with 79% of patients who developed lung cancer >5 years following lymphoma diagnosis (peak of incidence between 5 and 10 years: 55%). In most cases (n=134; 82%) patients were asymptomatic, and the lung cancer detection was made incidentally on chest X-RAY or chest computed tomography scan performed during lymphoma follow-up, lung cancer screening program, and preoperative screening for other surgical procedures (i.e., inguinal hernia, cataract etc.). The 30 (18%) remaining patients presented with signs and symptoms related to lung cancer. The most common histological types of lung cancer were adenocarcinoma (n=110/164; 67%). One hundred and six (65%) patients presented stage I, 29 (18%) stage II, 18 (11%) stage III, and 11 (7%) stage IV disease. Lung cancer resection was performed in 145 patients out of 164 (88%) including lobectomy (n=114; 79%), sublobar resection (n=21; 14%), bilobectomy (n=7; 5%); and pneumonectomy (n=3; 2%) followed by adjuvant chemotherapy and/or RT, if indicated. The remaining 19 (12%) patients underwent CT and/or RT due to the advanced stage of the tumor or unfit clinical condition.
Table 2. Characteristics of lung cancer (n=164).
| Variables | Total population (n=164) | Active lymphoma (n=18) | No active lymphoma (n=146) | P value |
|---|---|---|---|---|
| Age at lung cancer diagnosis | 57±9.7 | 55±9.7 | 57±8.3 | 0.63 |
| ≤59 years | 68 [41] | 7 [39] | 61 [42] | 0.77 |
| 60 to 69 years | 65 [40] | 9 [50] | 56 [38] | 0.37 |
| ≥70 years | 31 [19] | 2 [11] | 29 [20] | 0.76 |
| Latency between lymphoma and lung cancer diagnosis | 8.8±2.1 | 8.3±3.1 | 8.8±4.1 | 0.67 |
| ≤5 years | 34 [21] | 2 [11] | 32 [22] | 0.6 |
| >5 to 10 years | 90 [55] | 10 [56] | 80 [55] | 0.99 |
| >10 to 15 years | 30 [18] | 4 [22] | 26 [18] | 0.7 |
| >15 years | 10 [6] | 2 [11] | 8 [5] | 0.43 |
| Pre-operative morbidity | 81 [49] | 7 [39] | 74 [51] | 0.31 |
| COPD | 19 [12] | 2 [11] | 17 [11.6] | |
| Cardiac disease | 20 [12] | 2 [11] | 18 [12.3] | |
| Vascular disease | 10 [6] | 1 [6] | 9 [6] | |
| Diabetes | 15 [9] | 1 [6] | 14 [10] | |
| Renal failure | 3 [2] | – | 3 [2] | |
| Others | 14 [9] | 1 [6] | 13 [9] | |
| Symptoms at time of lung cancer diagnosis | ||||
| No symptoms | 134 [82] | 15 [83] | 119 [82] | 0.9 |
| Symptoms* | 30 [18] | 3 [17] | 27 [18] | 0.79 |
| Cough | 9 [5] | 3 [2] | 6 [4] | |
| Pneumothorax | 1 [0.6] | – | 1 [0.6] | |
| Hemoptysis | 5 [3] | – | 5 [3] | |
| Pleural effusion | 3 [2] | – | 3 [2] | |
| Chest pain | 10 [6] | 1 [6] | 10 [7] | |
| Dyspnea | 13 [8] | 1 [6] | 13 [9] | |
| Pneumonia | 2 [1] | – | 2 [1] | |
| Histology of lung cancer | ||||
| Adenocarcinoma | 110 [67] | 11 [61] | 99 [68] | 0.52 |
| Squamous cell carcinoma | 42 [26] | 5 [28] | 37 [25] | 0.87 |
| Large cell carcinoma | 12 [7] | 2 [11] | 10 [7] | 0.59 |
| Stage disease (8th edition) | ||||
| IA1 | 40 [24] | 5 [28] | 35 [24] | 0.77 |
| IA2 | 29 [18] | 2 [11] | 27 [18] | 0.4 |
| IA3 | 21 [13] | 2 [11] | 19 [13] | 0.76 |
| IB | 16 [10] | 1 [6] | 15 [10] | 0.47 |
| IIA | 6 [3] | 2 [11] | 4 [3] | 0.07 |
| IIB | 23 [14] | 3 [17] | 20 [14] | 0.79 |
| IIIA | 15 [9] | 2 [11] | 13 [9] | 0.83 |
| IIIB | 3 [2] | 0 | 3 [2] | 0.54 |
| IV | 11 [7] | 1 [6] | 10 [7] | 0.83 |
| Treatment of lung cancer | ||||
| Surgical resection | 145 [88] | 15 [83] | 130 [89] | 0.41 |
| Lobectomy | 114 [78] | 12 [80] | 102 [70] | 0.68 |
| Wedge resection | 10 [7] | 1 [1] | 9 [6] | 0.83 |
| Segmentectomy | 11 [8] | 1 [1] | 10 [7] | 0.76 |
| Bilobectomy | 7 [5] | 1 [1] | 6 [4] | 0.88 |
| Pneumonectomy | 3 [2] | 0 | 3 [2] | 0.54 |
| No resection | 19 [22] | 3 [17] | 16 [11] | 0.54 |
*, some patients presented more than one symptom.
Post-operative morbidity and mortality, long-term follow-up
Data are summarized in Table 3. Twenty-three out of 145 patients (16%) patients presented post-operative complications including air-leaks (n=10), atelectasis needing bronchoscopic aspiration (n=8), atrial fibrillation (n=4), and infection of surgical site (n=1). One patient (0.6%) died 25 days after pneumonectomy for acute respiratory distress syndrome (ARDS).
Table 3. Post-operative morbidity, post-operative mortality, cause of death.
| Variables | Total population (n=164) | Active lymphoma (n=18) | No active lymphoma (n=146) | P value |
|---|---|---|---|---|
| Post-operative morbidity | 23 [14] | 2 [11] | 21 [14] | 0.12 |
| Air leaks | 10 [6] | 1 [6] | 9 [6] | |
| Atelectasis | 8 [5] | 1 [6] | 7 [5] | |
| Atrial fibrillation | 4 [3] | – | 4 [3] | |
| Infection of surgical site | 1 [1] | – | 1 [1] | |
| Post-operative mortality | 1 [1] | – | 1 [0.7] | 0.72 |
| Cause of death | 68 [41] | 11 [61] | 57 [39] | 0.08 |
| Recurrence or metastasis lung cancer | 31 [19] | 3 [17] | 28 [19] | 0.74 |
| Recurrence or metastasis lymphoma | 12 [7] | 4 [22] | 8 [5.5] | 0.01 |
| Other medical conditions | 25 [15] | 4 [22] | 21 [14] | 0.43 |
| Myocardial ischemia | 13 [8] | 2 [11] | 11 [8] | |
| Respiratory failure | 7 [4] | 1 [6] | 6 [4] | |
| Cerebral Ictus | 5 [3] | 1 [6] | 4 [3] |
During the follow-up, 68 (41%) patients died due to recurrence or metastasis from lung cancer (n=31; 20%) or lymphoma (n=12; 7.3%), Acute Myocardial Infarction (n=13; 8%), respiratory failure (n=7; 4%); and cerebrovascular accident (n=5; 3%).
Lymphoma status
At the time of lung cancer diagnosis, 146 (89%) out of 164 patients were in complete clinical remission, while the remaining 18 (11%) patients presented active lymphoma. As summarized in Table 2, the comparison of active lymphoma versus non active lymphoma group showed no significant difference regarding the different lung cancer variables: age at lung cancer diagnosis (55±9.7 vs. 57±8.3 years; P=0.63), latency between lymphoma and lung cancer diagnosis (8.3±3.1 vs. 8.8±4.1 years; P=0.67); incidental diagnosis (83% vs. 72%; P=0.90); pre-operative morbidity (38% vs. 51%, P=0.31), histology and stage disease, lung cancer resection (83% vs. 89%; P=0.41); post-operative morbidity (11% vs. 14%; P=0.12) and post-operative mortality (0 vs. 0.6%; P=0.72). “Active lymphoma” had a higher rate of death due to lymphoma recurrence or metastasis than that of “no active” lymphoma group (22% vs. 5.4%, P=0.01).
Survival
The median overall survival after the diagnosis of lung cancer was 63 (range, 58–85) months, and the 5-YSR was 54% (Figure 1).
Figure 1.
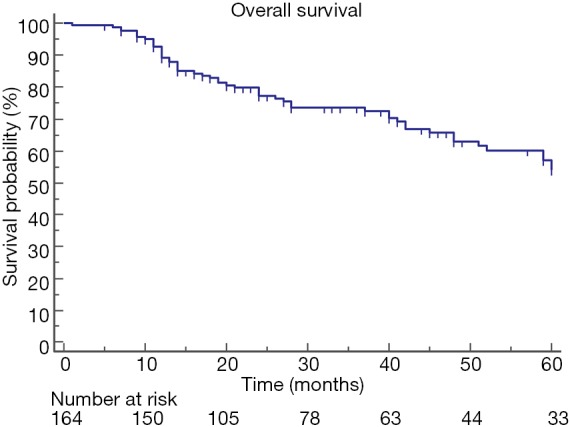
The median overall survival after diagnosis of lung cancer was 63 (range, 58–85) months.
Lymphoma characteristics
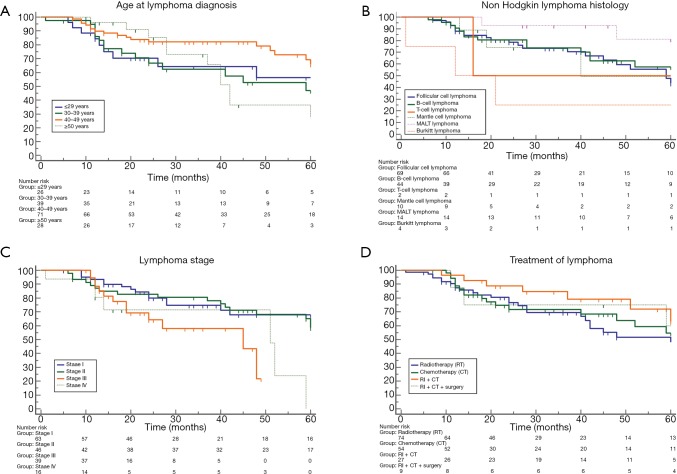
Median overall survival of patients with age ≤29; 30–39; 40–49; ≥50 years was 63 (range, 16–73); 59 (range, 24–64); 67 (range, 60–85); 42 (range, 28–72); 63 (range, 59–85) months, respectively. No significant difference was found among patient groups stratified according to age at lymphoma diagnosis (P=0.20; Figure 2A). Survival of HL patients compared to that of NHL patients showed no significant difference [67 (range, 24–85) vs. 61 (range, 51–85) months; HR: 1.1 (0.59–2.42); P=0.65]; among NHL patients the comparison of survival among patient groups stratified according to different histology also showed no significant difference (P=0.11, Figure 2B). The overall survival for patients with stage I; stage II; stage III; and stage IV lymphoma were 67 (range, 61–75); 72 (range, 59–85); 45 (range, 19–48); 51 (range, 14–59) months, respectively; no significant difference was found among patient groups stratified according to lymphoma stage (P=0.08; Figure 2C). The overall survival for patients undergoing radiotherapy (RT); chemotherapy (CT); RT plus CT; RT + CT + surgery were: 60 (range, 41–85); 61 (range, 41–85); 72 (range, 51–75); 61 (range, 11–76) months, respectively. No significant difference was found among patient groups stratified according to lymphoma treatment (P=0.56; Figure 2D).
Figure 2.
No significant difference was found among patients groups stratified according to age at lymphoma diagnosis (P=0.20; Part A); Non-Hodgkin lymphoma histology (P=0.11, Part B); lymphoma stage (P=0.08; Part C); and lymphoma treatment (P=0.56; Part D).
Lung cancer characteristics
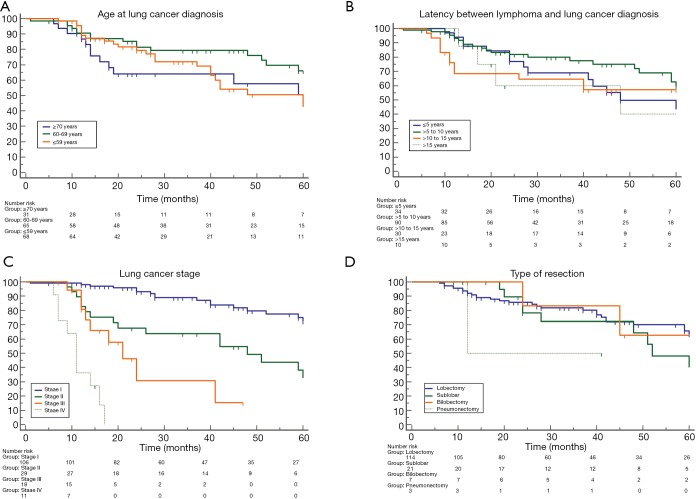
The median overall survival for patients with age ≤59; 60–69; and ≥70 years was: 63 (range, 18–73); 67 (range, 59–85); and 60 (range, 40–85) months, respectively; and no significant difference was found among patients stratified according to age at lung cancer diagnosis (P=0.17; Figure 3A). The median overall survival for patients with latency between lymphoma and lung cancer diagnosis ≤5; >5 to 10; >10 to 15; and >15 years was: 48 (range, 28–75); 67 (range, 59–85); 61 (range, 12–63), 48 (range, 13–79) respectively; no significant difference was found among patients stratified according to different latency (P=0.07; Figure 3B). Patients whose lung tumors were detected incidentally had a significantly better survival than that of symptomatic patients [67 (range, 63–85) vs. 28 (range, 18–45) months; HR: 5.3 (2.76 to 10.3); P<0.0001]. The overall survival for patients with stage I; stage II; stage III; and stage IV disease was: 76 (range, 64–85); 48 (range, 20–75); 21 (range, 13–41); 11 (range, 7–17) months, respectively. Significant difference was found by the comparison of patients stratified according to different stage disease (P<0.0001; Figure 3C). The overall survival for patients with adenocarcinoma, squamous cell carcinoma, and large cell carcinoma was: 61 (range, 50–81); 51 (range, 25–61); 43 (range, 20–59) months, respectively; no significant difference was found comparing patients stratified according to different histology (P=0.11). Patients undergoing lung cancer resection had better survival compared to that of non-surgical patients [64 (range, 60–85) vs. 14 (range, 11–18) months; HR: 1.7 (2.8–4.7); P<0.0001]. No significant difference was found among patients stratified according to the different type of resections (P=0.23; Figure 3D).
Figure 3.
No significant difference was found among patients stratified according to age at lung cancer diagnosis (P=0.17; Part A); latency between lymphoma and lung cancer diagnosis (P=0.07; Part B); and different type of resections (P=0.23; Part D), while significant difference was found among patients stratified according to lung cancer stage (P<0.0001; Part C).
Lymphoma status
Patients with lymphoma in remission presented a significantly better overall survival than those with active lymphoma [64 (range, 59–85) vs. 37 (range, 13–75) months; HR: 2.4 (1.09–5.49); P=0.02; Figure 4A]. The comparison of lung cancer specific survival showed no significant difference between no active lymphoma and active lymphoma patients [27 (range, 18–85) vs. 19 (range, 13–37) months; HR: 0.3 (0.05–1.70); P=0.17; Figure 4B].
Figure 4.
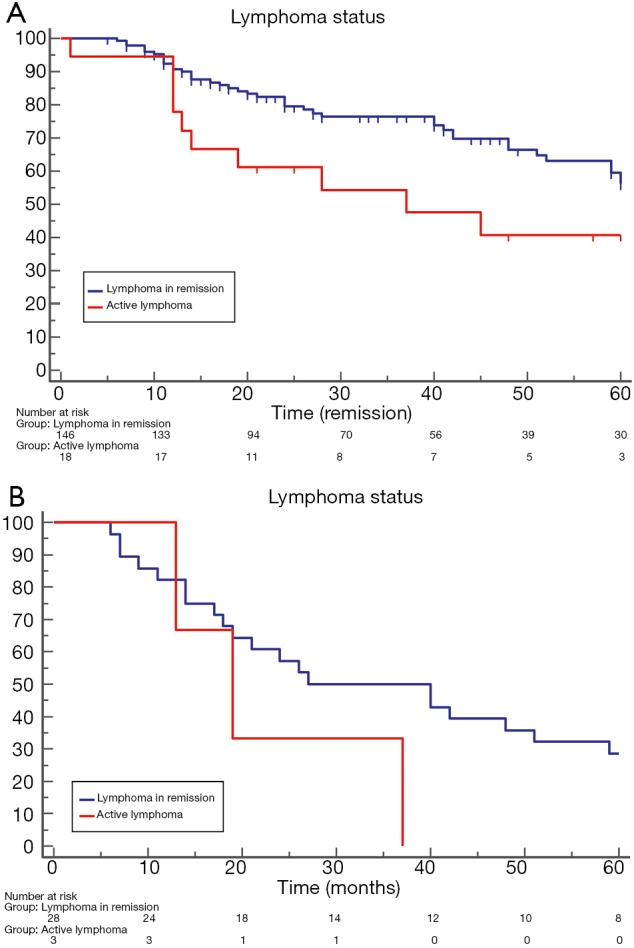
Patients with lymphoma in remission compared with those with active lymphoma presented significant better survival [64 (range, 59–85) vs. 37 (range, 13–75) months; HR: 2.4; P=0.02; Part A], but lung cancer specific survival showed no significant difference [27 (range, 18–85) vs. 19 (range, 13–37) months; HR: 0.3; P=0.17; Part B].
Univariable and multivariable analysis for overall survival
Data are summarized in Table 4. At univariable analysis no-active lymphoma (HR: 2.19; P=0.0152); early lymphoma stage (HR: 1.95; P=0.01); adenocarcinoma histology (HR: 0.59; P=0.0421); early stage of lung cancer (HR: 3.18; P<0.0001); incidental diagnosis of lung cancer (HR: 1.71; P<0.0001); and lung cancer resection (HR: 2.79; P<0.0001) were favorable prognostic factors. At multivariable analysis, no-active lymphoma (HR: 2.68; P=0.0043); early stage of lung cancer (HR: 2.37; P<0.0001); incidental diagnosis of lung cancer (HR: 2.00; P<0.0001); and lung cancer resection (HR: 2.07; P<0.0001) were confirmed to be favorable prognostic factors.
Table 4. Univariable and Multivariable analysis (dependent variable: overall survival).
| Covariates | Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | HR | 95% CI | P value | Coefficient | HR | 95% CI | P value | ||
| Sex (male vs. female) | 0.42 | 1.52 | 0.81–2.84 | 0.19 | |||||
| No active lymphoma (yes vs. not) | 0.79 | 2.19 | 1.16–4.14 | 0.0152 | 1.00 | 2.68 | 1.36–5.26 | 0.0043 | |
| Age at Lymphoma diagnosis (≤39 vs. ≥40 years) | −0.03 | 0.97 | 0.74–1.29 | 0.85 | |||||
| Type of lymphoma (HL vs. NHL) | 0.56 | 0.34 | 0.63–1.69 | 0.76 | |||||
| Stage of lymphoma (stage I–II vs. stage III–IV) | 1.59 | 1.95 | 1.68–2.03 | 0.01 | 0.67 | 0.98 | 0.97–2.96 | 0.78 | |
| Treatment of lymphoma (RT alone vs. others) | −0.19 | 0.83 | 0.63–1.09 | 0.18 | |||||
| Age at NSCLC diagnosis (≤59 vs. ≥60 years) | 0.21 | 1.24 | 0.87–1.76 | 0.24 | |||||
| Incidental diagnosis of lung cancer (yes vs. not) | 0.54 | 1.71 | 1.41–2.08 | <0.0001 | 0.69 | 2.00 | 1.48–2.70 | <0.0001 | |
| Histology of NSCLC (adenocarcinoma vs. others) | −0.52 | 0.59 | 0.36–0.98 | 0.0421 | 0.19 | 1.20 | 0.67–2.15 | 0.53 | |
| NSCLC stage (stage I–II vs. stage III–IV) | 1.16 | 3.18 | 2.28–4.24 | <0.0001 | 0.86 | 2.37 | 1.60–3.51 | <0.0001 | |
| Lung cancer resection (yes vs. not) | −2.45 | 2.79 | 2.98–3.65 | <0.0001 | 0.86 | 2.07 | 1.51–2.75 | <0.0001 | |
HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; RT, radiotherapy; NSCLC, non-small cell lung cancer.
Discussion
The increased risk of lung cancer among lymphoma survivors has been well described in several papers and meta-analyses (2-6), but limited information exists about the prognosis and treatment outcome of lung cancer. Milano et al. (7,8) and Kim et al. (9) evaluated survival of lymphoma patients who developed NSCLC, and matched them with non-lymphoma patients with de novo NSCLC. Both authors (7-9) found that lymphoma patients faced worse survival than did non-lymphoma patients. These findings supported the hypothesis that previous lymphoma adversely affect the survival and outcome of lung cancer treatment, but it is still unclear which lymphoma survivors with lung cancer could benefit from lung cancer resection. To investigate this issue, we evaluated the outcome and prognostic factors of lung cancer in lymphoma survivors in order to improve the patient selection for lung cancer treatment
First, we found that the safety of surgery was not impacted by lymphoma, as the latter disease did not significantly increase post-operative morbidity and mortality rate. Twenty-three (16%) patients presented post-operative complications including air-leaks (7%), atelectasis needing bronchoscopic aspiration (5%), atrial fibrillation (3%), and infection of surgical site (1%) while only one patient (0.6%) died of ARDS 25 days after pneumonectomy. These complications seemed to be correlated to the underlying patient’s diseases as chronic obstructive pulmonary disease (COPD), diabetes, cardiac disease, and extent of lung resection rather than to previous lymphoma. Furthermore, no patients presented pneumonia that was the most common complication reported by other authors (10,11) in lymphoma survivors undergoing lung cancer resection, most likely due to the lymphoma related immunodeficiency. In line with our data, Kim et al. (9) found no significant difference in postoperative morbidity and mortality between lymphoma patients and no-lymphoma patients after lung cancer resection. However, the authors (9) reported a mortality rate of 6% that was three times higher after standard lobectomy compared to matched no-lymphoma lung cancer patients. Therefore, these patients need more aggressive postoperative monitoring, especially in case of comorbid condition and extended resection such as pneumonectomy.
Second, we observed in our series a median overall survival of 63 months (mean, 54.8±2.5 months) that was better than what reported by other authors. Schoenfeld et al. (1) showed a median survival time of 10.3 months even because two thirds of the patients (67%) presented an advanced stage disease, (stage III or stage IV). Laurie et al. (12) reported a median survival of 5.1 months, and no patient lived longer than 10.5 months. Complete resection was achieved in 5 cases and only 2 of which (25%) presented stage I disease. The remaining 14 patients did not undergo a complete resection, and showed a median survival of 3.0 months. De Giacomo et al. (11) reported a 5-YSR of 20% and only 40% of patients had an early stage lung cancer. Das et al. (13) found a median survival of 9 months. Most patients (85%) presented at advanced disease stage (III or stage IV), and 40% underwent surgery. Yet, some patients presented different histology from NSCLC (i.e., small-cell lung cancer and mesothelioma). Riquet et al. (14) reported a 5-YSR of 38.1% (median 34 months), and 5 of 36 (14%) patients had pN2 disease. Thus, the higher percentage of patients with stage I lung cancer, as well as the higher rate of patients undergoing surgery, and the exclusion of those with different histology from NSCLC could explain our different results. This is further confirmed by Kim et al. (9) who reported a median overall survival duration of 53 months. All patients underwent surgery, and 70% presented Stage I disease.
Third, we found that early lung cancer stage, no-active lymphoma status at the time of lung cancer diagnosis, lung cancer resection, and incidental diagnosis of lung cancer were favorable predictors for overall survival. Incidental diagnosis of lung cancer allowed to make diagnosis of cancer at an early stage with the possibility of surgical treatment. Similarly, in Schoenfeld’s series (1) the subgroup of HL patients (n=12) with subsequent incidentally diagnosed lung malignancy presented a significant longer survival time (median 48 months after initial diagnosis) than that of patients who presented with symptomatic lung disease. Surgery was the only therapeutic chance for these patients, because definitive thoracic radiotherapy would be precluded in most patients by prior mantle-field radiation therapy. This observation was also confirmed by Laurie et al. (12) who found that only patients who underwent complete resection of their lung carcinoma were long-term survivors. Active lymphoma adversely affected overall survival, but no significant difference was found in lung cancer specific survival between active lymphoma and no active lymphoma patients. These results may be explained since patients with active lymphoma died of lymphoma recurrence or metastasis in a significantly higher rate than patients with no-active lymphoma (P=0.01), while the mortality rate due to lung cancer recurrence or metastasis was similar between the two sub-groups (P=0.74). Our data were also confirmed by Kim et al. (9) who found no significant difference regarding lung cancer specific survival between active lymphoma patients and no-active lymphoma patients, despite active lymphoma, also in our series, was shown to be an adverse significant prognostic factor at multivariable analysis. Thus, patients with active lymphoma could achieve reasonable survival after lung cancer resection, but they should be carefully selected for surgery (i.e., early stage lung cancer, treatable NHL, and adequate cardio-respiratory function). Differently from other authors (7-9), we found that previous radiotherapy for lymphoma, different histological types of lymphoma, and older age did not significantly affect the overall survival. Milano et al. (7,8) found that different histology sub-types and radiotherapy for lymphoma significantly influenced survival. However, the authors (7,8) evaluated only patients with HL, and in their series previous RT for lymphoma significantly limited the possibility of radiotherapy treatment for NSCLC. Conversely, in our series including patients with NH and NHL, RT for lymphoma did not have a significant impact on survival, as only one of the 10 patients scheduled for RT did not receive full dose radiation due to previous irradiation for lymphoma. Kim et al. (9) found that older age was an adverse prognostic factor in patients with lymphoma and lung cancer. In our series, 22 of 31 patients (71%) older than 70 years had an early stage tumor, probably because they were more likely to undergo diagnostic workups for related symptoms than younger patients. This was also observed in Milano’s studies (7,8). Fifteen of these patients (72%) underwent lung cancer resection, and in 11 (73%) cases a sublobar resection rather than a more extended resection was carried out. This strategy allowed to reduce the risk of post-operative morbidity, without significantly affecting the oncological results (15,16).
Fourth, the increased survival found in our patients with incidentally diagnosed lung cancer supported the usefulness of lung cancer screening in lymphoma survivors. The National Lung Screening Trial (NLST) found that CT screening reduced lung cancer deaths of 20% in high risk population (17). Based on these data, the National Comprehensive Cancer Network (NCCN) categorized patients with prior lymphoma as a high-risk group only if they are more than 50 years old and have a smoking history (18). It was recommended that those patients should have annual low-dose helical CT scan for 2 years (category 1) and should be considered for an annual screening if they are clinically fit for definitive treatment. However, Ibrahim et al. (4) in a recent meta-analysis supported these recommendations should be reconsidered for several reasons. The NLST study (17) did not include patients with prior lymphoma, and only 0.1% of patients were younger than 55 years of age. Moreover, the median age at the diagnosis of second lung cancer in Ibrahim et al. (4) meta-analysis was 45.0 years, and the rate of developing second lung cancer remained high after 15 years of follow-up. Our results seemed to confirm this hypothesis as the incidence of lung cancer reached its peak at 5–10 years following primary lymphoma treatment (55%), and remained elevated after 10 years of follow-up (24%). Thus, we also support the need for continued follow-up of these patients, also considering that screening for breast cancers in young women treated for HL has been recommended to start ~8 to 10 years after HL treatment (19). Therefore, we believe that lymphoma survivors should receive a closer follow-up than the general population. Following treatment of lymphoma, survivors tend to switch to health-care providers and often lose contact with their oncologists. Thus, it is essential that all physicians should promote appropriate investigations to screen second carcinomas, especially primary lung carcinomas in smokers and former smokers, keeping in mind that latency time to the development of further malignancies could be long. Smoking cessation and abstinence are likely to have the greatest impact on reducing the mortality from lung carcinoma in this population. Ibrahim’s meta-analysis (1) showed that approximately 80% of patients with second lung cancer had a history of smoking, and these data are in line with ours and many reported series. Travis et al. (20) showed that in 24% of lymphoma survivors, incidence of second lung cancer could be attributable to smoking alone, while other studies (21,22) found that the risk of developing second lung cancer was positively associated with increasing number of pack-years smoked.
Study limitations
Our study presents several limitations that do not allow to draw definitive conclusions. Due to the retrospective and the multicenter nature of the study, there was not a standardized treatment for lymphoma (i.e., different protocol and regimen of chemotherapy and radiotherapy) and for NSCLC (i.e., different type of resection), but the treatment depended on the era of diagnosis, and the choice of physicians in the treating center. In some cases, there was a lack of detailed information about the site of radiotherapy and/or tobacco history (i.e., number of pack-year), while other variables as weight loss and performance status were not considered.
Conclusions
The history of lymphoma or an active lymphoma should not be considered a contraindication to surgical treatment of lung cancer in selected patients who present with early stage lung cancer and are clinically fit for surgery. The increased survival observed in patients with an incidentally diagnosed lung cancer supports the usefulness of lung cancer screening in lymphoma survivors to detect lung cancer at an early stage. Due to the limitations of a retrospective analysis, our results should be furtherly validated by a prospective randomized study.
Acknowledgments
Funding: This study was supported by the funding of “VALERE” research program from University of Campania Luigi Vanvitelli.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study design was approved by Local Ethics Committees of University of Campania Luigi Vanvitelli (approvation code: 228/19), coordinating center of the study, and then approved by each participating center. All patients gave a written informed consent for the surgical treatment and were aware that all information could be used anonymously for scientific purpose only.
Data Sharing Statement: No additional data available.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Schoenfeld JD, Mauch PM, Das P, et al. Lung malignancies after Hodgkin lymphoma: disease characteristics, detection methods and clinical outcome. Ann Oncol 2012;23:1813-8. 10.1093/annonc/mdr551 [DOI] [PubMed] [Google Scholar]
- 2.Okines A, Thomson CS, Radstone CR, et al. Second primary malignancies after treatment for malignant lymphoma. Br J Cancer 2005;93:418-24. 10.1038/sj.bjc.6602731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franklin J, Pluetschow A, Paus M, et al. Second malignancy risk associated with treatment of Hodgkin’s lymphoma: meta-analysis of the randomised trials. Ann Oncol 2006;17:1749-60. 10.1093/annonc/mdl302 [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim EM, Kazkaz GA, Abouelkhair KM, et al. Increased risk of second lung cancer in Hodgkin's lymphoma survivors: a meta-analysis. Lung 2013;191:117-34. 10.1007/s00408-012-9418-4 [DOI] [PubMed] [Google Scholar]
- 5.Castellino SM, Geiger AM, Mertens AC, et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood 2011;117:1806-16. 10.1182/blood-2010-04-278796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng AK, Bernardo MP, Weller E, et al. Long-term survival and competing causes of death in patients with early-stage Hodgkin's disease treated at age 50 or younger. J Clin Oncol 2002;20:2101-8. 10.1200/JCO.2002.08.021 [DOI] [PubMed] [Google Scholar]
- 7.Milano MT, Li H, Constine LS, et al. Variables affecting survival after second primary lung cancer: A population-based study of 187 Hodgkin's lymphoma patients. J Thorac Dis 2012;4:22-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milano MT, Li H, Gail MH, et al. Long-term survival among patients with Hodgkin's lymphoma who developed breast cancer: a population-based study. J Clin Oncol 2010;28:5088-96. 10.1200/JCO.2010.29.5683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim MP, Correa AM, Swisher SG, et al. Non-small cell lung cancer resection in lymphoma patients. Ann Thorac Surg 2010;90:210-6. 10.1016/j.athoracsur.2010.02.114 [DOI] [PubMed] [Google Scholar]
- 10.Provencio M, España P, Salas C, et al. Hodgkin's disease: correlation between causes of death at autopsy and clinical diagnosis. Ann Oncol 2000;11:59-64. 10.1023/A:1008396826812 [DOI] [PubMed] [Google Scholar]
- 11.De Giacomo T, Martelli M, Venuta F., et al. Lung cancer after treatment for non-Hodgkin lymphoma. Minerva Chir 2006;61:467-71. [PubMed] [Google Scholar]
- 12.Laurie SA, Kris MG, Portlock CS, et al. The clinical course of nonsmall cell lung carcinoma in survivors of Hodgkin disease. Cancer 2002;95:119-26. 10.1002/cncr.10628 [DOI] [PubMed] [Google Scholar]
- 13.Das P, Ng AK, Stevenson MA, et al. Clinical course of thoracic cancers in Hodgkin's disease survivors. Ann Oncol 2005;16:793-7. 10.1093/annonc/mdi155 [DOI] [PubMed] [Google Scholar]
- 14.Riquet M, Arame A, Foucault C. Does presence of hematologic malignancy change our approach to non-small cell lung cancer? Ann Thorac Surg 2011;91:1652-3. 10.1016/j.athoracsur.2010.12.027 [DOI] [PubMed] [Google Scholar]
- 15.Fiorelli A, Caronia FP, Daddi N, et al. Sublobar resection versus lobectomy for stage I non-small cell lung cancer: an appropriate choice in elderly patients? Surg Today 2016;46:1370-82. 10.1007/s00595-016-1314-8 [DOI] [PubMed] [Google Scholar]
- 16.Fiorelli A, Vicidomini G, Mazzella A, et al. The influence of body mass index and weight loss on outcome of elderly patients undergoing lung cancer resection. Thorac Cardiovasc Surg 2014;62:578-87. 10.1055/s-0034-1373733 [DOI] [PubMed] [Google Scholar]
- 17.National Lung Screening Trial Research Team Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood DE, Kazerooni EA, Baum SL, et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:412-41. 10.6004/jnccn.2018.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henderson TO, Amsterdam A, Bhatia S, et al. Systematic review: surveillance for breast cancer in women treated with chest radiation for childhood, adolescent, or young adult cancer. Ann Intern Med 2010;152:444-55. 10.7326/0003-4819-152-7-201004060-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Travis LB, Gospodarowicz M, Curtis RE, et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin’s disease. J Natl Cancer Inst 2002;94:182-92. 10.1093/jnci/94.3.182 [DOI] [PubMed] [Google Scholar]
- 21.Metayer C, Lynch CF, Clarke EA, et al. Second cancers among long-term survivors of Hodgkin’s disease diagnosed in childhood and adolescence. J Clin Oncol 2000;18:2435-43. 10.1200/JCO.2000.18.12.2435 [DOI] [PubMed] [Google Scholar]
- 22.van Leeuwen FE, Klokman WJ, Stovall M, et al. Roles of radiotherapy and smoking in lung cancer following Hodgkin’s disease. J Natl Cancer Inst 1995;87:1530-7. 10.1093/jnci/87.20.1530 [DOI] [PubMed] [Google Scholar]