Abstract
Background
This was an observational study of Japanese participants who underwent low-dose computed tomographic (LDCT) lung cancer screening between February 2004 and March 2012, to evaluate the lung cancers in never-smokers and smokers.
Methods
The study population consisted of a total of 12,114 subjects [never-smokers, 6,021 (49.70%); smokers with <30 pack-years of smoking, 3,785 (31.24%); smokers with ≥30 pack-years of smoking, 2,305 (19.03%); unknown smoking status, 3 (0.02%)]. The odds ratio (OR) of lung cancer detection according to the smoking status adjusted for age and gender was evaluated.
Results
A total of 152 lung cancers were diagnosed in 133 patients [never-smokers, 66 (49.6%); smokers with <30 pack-years of smoking, 31 (23.3%); smokers with ≥30 pack-years of smoking, 36 (27.1%)]; therefore, 72.9% of lung cancer patients did not meet the National Lung Screening Trial (NLST) criterion of smokers with ≥30 pack-years of smoking. The OR of lung cancer detection in smokers with ≥30 pack-years of smoking was higher than that in the never-smokers (OR =1.71, 95% CI: 1.04–2.82, P=0.03) and that in smokers with <30 pack-years of smoking (OR =1.71, 95% CI: 1.04–2.80, P=0.03), while the OR of lung cancer detection in smokers with <30 pack-years of smoking was the same as that in the never-smokers (OR =1.00, 95% CI: 0.62–1.61, P=0.99).
Conclusions
Although the OR of lung cancer detection in smokers with ≥30 pack-years of smoking was higher than that in the never-smokers and smokers with <30 pack-years of smoking, approximately 70% of lung cancer patients might be missed if we only adopted the NLST criterion of smokers with ≥30 pack-years of smoking. Therefore, never-smokers and smokers with <30 pack-years of smoking should be included in the target population for LDCT lung cancer screening in Japan.
Keywords: Low-dose computerized tomography, lung cancer screening, never-smoker, adenocarcinoma
Introduction
Lung cancer has become the leading cause of death from cancer in the Japanese population, with 74,120 deaths from lung cancer [men, 53,002 (71.5%); women, 21,118 (28.5%)] reported in 2017 (1). In Japan, lung cancer screening is presently conducted by plain chest radiography as population-based screening, since three of four case-control studies revealed a statistically significant reduction of the risk of death from lung cancer (2) and low-dose computed tomographic (LDCT) lung cancer screening has been conducted as an opportunistic screening method since 1993 (3). LDCT lung cancer screening was started in 1993 as a part of the Anti-Lung Cancer Association (ALCA) project conducted by the Tokyo Health Service Association. Between 1996 and 1998, a population-based lung cancer screening trial with mobile CT was conducted in Nagano prefecture (4,5). In 1998, the Hitachi Health Care Center introduced lung cancer screening using LDCT at annual health examinations of individuals belonging to the Hitachi Employee’s Health Insurance Group (6). The final results of the Nagano project (7) and the interim results of the ALCA project (8) and Hitachi project (9) have been reported.
In regard to the incidence of lung cancer in never-smokers reported from Japanese observational studies, one study of CT screening for lung cancer conducted in Nagano prefecture reported that 51.7% of all lung cancers developed in never-smokers (5). Another study of CT screening for lung cancer in Hitachi city reported that 57.5% of all lung cancers developed in never-smokers (6). In both studies, the percentage of never-smokers among the patients with lung cancer detected by CT screening was higher than 50%. In Japan, according to the cancer statistics for 2009, death from lung cancer in never-smokers was estimated to be the fifth most common cause of death in men and third most common cause of death in women, reflecting a relatively high rate (31% of all lung cancers in men and 80% of all lung cancers in women) of occurrence of lung cancers not related to smoking (10).
The purpose of this observational study of Japanese subjects who underwent LDCT lung cancer screening at the Research Center for Cancer Prevention and Screening (RCCPS) within the National Cancer Center between February 2004 and March 2012, was to evaluate the lung cancers in never-smokers and smokers.
Methods
Ethics statement
This study was performed with the approval of the institutional review board of the National Cancer Center (No. 2005-32). Written informed consent was obtained from each of the participants.
Participants
The criteria for participating in the cancer screening research conducted at the RCCPS were as follows. First, the Japanese participants had to be 40 years or older. Second, the participants should not have been diagnosed as having cancer at any other facility within one year prior to the cancer screening performed at the RCCPS. Third, the participants should not have been receiving treatment for cancer.
A document explaining the contents of the cancer screening and a questionnaire were mailed in advance to the participants. The document included the contents of the two-day cancer screening, an explanation of the benefits and disadvantages of the cancer screening, and a signing page to confirm consent. Just prior to the start of the first day of cancer screening, well-trained persons in charge explained, face-to-face, the contents, benefits, and disadvantages of the cancer screening to each of the participants. In order to participate in this cancer screening study, the participants had to sign the consent form as an indication of consent.
All the participants were required to complete a questionnaire that included questions to determine the smoking status at the time of the baseline screening, and status of exposure to second-hand smoke; information on the pack-years of smoking and status of exposure to second-hand smoke entered in the questionnaire were confirmed, face-to-face, by well-trained persons in charge, just prior to the start of the first day of cancer screening.
All the participants were self-referred and paid for the screening by themselves.
Cancer screening at the RCCPS
The actual cancer screening performed at the RCCPS required 2 days to complete. The contents of the screening during the first day were as follows: confirmation of the responses in the questionnaires by interviewers, measurements of the height, body weight and blood pressure, blood pooling for future genomic analyses (17 mL of blood collected from each participant), urine pooling (3 mL urine specimen collected from each participant), three-day-pooled sputum cytology, LDCT lung cancer screening, and abdominal ultrasonography. For women, cervical cytology, mammography, breast ultrasonography, and MRI of the pelvis were also included as additional screening examinations. The examinations on the second day included upper gastrointestinal endoscopy and colonoscopy. PET/CT screening was performed as an optional examination. Participants were recommended to undergo the same screening procedures again five years after the baseline screening, with the option to undergo additional annual screening examinations according to the wishes of the participants.
Low-dose CT lung cancer screening
The protocol for the LDCT screening is described elsewhere (11); the scanning conditions were 120 kVp and 15 mAs. Between February 2004 and November 2011, the CT images were reconstructed using sections obtained at 5-mm intervals and sections obtained at 2-mm intervals; after December 2011, the CT images were reconstructed using sections obtained at 5-mm intervals and 1-mm intervals.
Nodule management protocol
Information regarding any detected non-calcified nodules (NCNs), i.e., including consistency of the nodule (pure ground-glass nodule (GGN), part-solid nodule, or solid nodule) (12,13), and the maximal diameter and perpendicular diameter of the nodule, was recorded in the nodule database.
The nodule management protocol is described elsewhere (11); briefly, a positive result of screening was defined as detection of a nodule ≥5 mm in diameter; participants with nodules <5 mm in diameter detected at the baseline were recommended to undergo annual screening at 1 and 2 years later to confirm the stability of the nodule, participants with any solid nodules ≥5 but <10 mm in diameter were recommended to undergo follow-up as outpatients at 3–12-month intervals for at least 2 years, participants with any GGNs ≥5 but <15 mm in diameter were recommended to undergo follow-up as outpatients at 3–12-month intervals for at least 5 years, and participants with solid nodules ≥10 mm or any GGNs ≥15 mm in diameter were recommended to undergo further work-up, such as positron emission tomographic imaging, fiberoptic bronchoscopic examination, needle biopsy, and/or surgery.
Lung cancer patients
In the present study, lung cancer patients diagnosed at the National Cancer Center Hospital (Tokyo) and the National Cancer Center Hospital East (Kashiwa, Chiba) between February 2, 2004, and March 31, 2012, were included. In addition, two lung cancer patients who underwent surgery at the National Cancer Center Hospital in May 2012 were included in the present study, because the surgeries had already been planned before March 31, 2012.
Evaluation of the lung cancers detected by CT screening
The lung cancer patients were evaluated according to their smoking status (smokers vs. never-smokers or smokers with ≥30 pack-years of smoking vs. smokers with <30 pack-years of smoking vs. never-smokers), to compare the National Lung Screening Trial (NLST) criterion (smokers with ≥30 pack-years of smoking) (14), and gender. Never-smokers were defined as individuals who had smoked <100 cigarettes during their lifetime, based on their responses to the questionnaire. The 7th edition of the TNM staging system (15) was used for the present study. Resected lung adenocarcinomas were classified according to the International Association for the Study of Lung Cancer–American Thoracic Society–European Respiratory Society classification of lung adenocarcinoma (16).
Patients with adenocarcinoma were evaluated based on the following classification: group A [adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA)] and group B (invasive adenocarcinoma); in the case of patients with multiple adenocarcinomas, the most invasive histology was adopted for this analysis.
The statuses of exposure to second-hand smoke in the never-smokers were determined based on their responses to the questionnaire at three ages of the subjects (around 10 years old, around 30 years old, and at the time of the baseline screening), as follows: almost never; several times a month; several times a week; daily. Second-hand smoke exposure was defined as exposure for at least one hour. The subjects were classified according to the status of exposure to second-hand smoke as follows: group 1 (almost no exposure at any of the three chronological ages), group 2 (daily exposure at around 30 years old and/or at the time of the baseline screening), and group 3 (exposure several times a month at any of the three chronological ages, but not fulfilling the criterion for classification into group 2).
Treatment of the lung cancers detected by CT screening was retrospectively documented.
Statistical analysis
Calculation of the means and medians, the chi-squared test, the Mann Whitney U test, the Kruskal-Wallis test, and the Kaplan-Meier analysis were performed using statistical software (JMP version 13; SAS Institute, Cary, NC). The chi-squared test was used to compare the categorical variables. The Mann Whitney U test was used to compare the differences in the continuous variables among two groups, and the Kruskal-Wallis test was used to compare the differences in the continuous variables among three groups. In regard to the Kaplan-Meier method, survival time was calculated from the date of detection until the date of death from lung cancer or the date of the last follow-up, or March 31, 2017, whichever came first; differences among groups were determined using the log-rank test. The odds ratios (OR) of lung cancer detection adjusted for age and gender were estimated using an unconditional logistic regression model (SAS version 9.3; SAS Institute, Cary, NC). P values of less than 0.05 were considered as indicative of statistical significance.
Results
Characteristics of the participants
The study population comprised 12,114 subjects who underwent screening between February 2, 2004, and March 31, 2012. The numbers of participants classified according to the gender, smoking status, age group, and presence/absence of pulmonary nodules are shown in Table 1. Among the 12,114 participants, 6,090 participants (50.3%) were smokers, 6,021 participants (49.7%) were never-smokers, and in 3 participants, the smoking status was unknown. The mean age of the participants in the smoker group was 58.2±8.9 years and that in the never-smoker group was 57.9±9.2 years (P=0.3582). Of the 12,114 participants, 7,294 (60.2%) were men, and 4,820 participants (39.8%) were women. The mean age of the male participants was 58.5±9.1 years and that of the female participants was 57.4±9.0 years (P<0.0001). NCNs ≥5 mm in maximal diameter were detected in 42.6% of the participants (5,155 of the 12,114 participants) and NCNs <5 mm in maximal diameter were detected in 32.4% of the participants (3,924 of the 12,114 participants).
Table 1. Numbers of participants classified according to the smoking status, gender, age group, and presence/absence of pulmonary nodules at the baseline CT screening between February 2004 and March 2012.
| Smoking status | Subgroup according to the gender and smoking status | Age group | Number of participants | Total | ||
|---|---|---|---|---|---|---|
| With nodules ≥5 mm | With nodules <5 mm | No nodules | ||||
| Never-smoker, n=6,021 (49.7) | Never-smoker women, n=3,875 | 40–49 | 257 (34.1) | 261 (34.6) | 236 (31.3) | 754 (100.0) |
| 50–59 | 535 (41.7) | 439 (34.2) | 308 (24.0) | 1,282 (100.0) | ||
| 60–69 | 712 (46.9) | 494 (32.5) | 312 (20.6) | 1,518 (100.0) | ||
| ≥70 | 154 (48.0) | 115 (35.8) | 52 (16.2) | 321 (100.0) | ||
| Never-smoker men, n=2,146 | 40–49 | 145 (30.0) | 164 (33.9) | 175 (36.2) | 484 (100.0) | |
| 50–59 | 246 (36.6) | 240 (35.7) | 186 (27.7) | 672 (100.0) | ||
| 60–69 | 345 (44.2) | 254 (32.5) | 182 (23.3) | 781 (100.0) | ||
| ≥70 | 121 (57.9) | 61 (29.2) | 27 (12.9) | 209 (100.0) | ||
| Subtotal | 2,515 (41.8) | 2,028 (33.7) | 1,478 (24.5) | 6,021 (100.0) | ||
| Smoker, n=6,090 (50.3) | Women with <30 pack-years of smoking, n=796 | 40–49 | 82 (29.8) | 99 (36.0) | 94 (34.2) | 275 (100.0) |
| 50–59 | 119 (38.8) | 109 (35.5) | 79 (25.7) | 307 (100.0) | ||
| 60–69 | 87 (47.5) | 57 (31.1) | 39 (21.3) | 183 (100.0) | ||
| ≥70 | 17 (54.8) | 7 (22.6) | 7 (22.6) | 31 (100.0) | ||
| Men with <30 pack-years of smoking, n=2,989 | 40–49 | 209 (31.6) | 234 (35.3) | 219 (33.1) | 662 (100.0) | |
| 50–59 | 444 (43.4) | 291 (28.4) | 288 (28.2) | 1,023 (100.0) | ||
| 60–69 | 463 (43.9) | 329 (31.2) | 262 (24.9) | 1,054 (100.0) | ||
| ≥70 | 139 (55.6) | 73 (29.2) | 38 (15.2) | 250 (100.0) | ||
| Women ≥30 pack-years of smoking, n=149 | 40–49 | 6 (40.0) | 6 (40.0) | 3 (20.0) | 15 (100.0) | |
| 50–59 | 29 (46.0) | 14 (22.2) | 20 (31.7) | 63 (100.0) | ||
| 60–69 | 25 (43.9) | 21 (36.8) | 11 (19.3) | 57 (100.0) | ||
| ≥70 | 8 (57.1) | 4 (28.6) | 2 (14.3) | 14 (100.0) | ||
| Men with ≥30 pack-years of smoking, n=2,156 | 40–49 | 49 (38.9) | 40 (31.7) | 37 (29.4) | 126 (100.0) | |
| 50–59 | 331 (44.0) | 240 (31.9) | 182 (24.2) | 753 (100.0) | ||
| 60–69 | 504 (49.1) | 298 (29.0) | 225 (21.9) | 1,027 (100.0) | ||
| ≥70 | 126 (50.4) | 74 (29.6) | 50 (20.0) | 250 (100.0) | ||
| Subtotal | 2,638 (43.3) | 1,896 (31.1) | 1,556 (25.6) | 6,090 (100.0) | ||
| Unknown | Men with unknown smoking status, n=3 | 40–49 | 0 (0.0) | 0 (0.0) | 1 (100.0) | 1 (100.0) |
| 60–69 | 2 (100.0) | 0 (0.0) | 0 (0.0) | 2 (100.0) | ||
| Total | 5,155 (42.6) | 3,924 (32.4) | 3,035 (25.1) | 12,114 (100.0) | ||
Data presented as number (percentage).
Characteristics of the lung cancer patients
A total of 152 lung cancers were diagnosed in 133 patients (Table 2). Of the 152 lung cancers, 135 (88.8%) were adenocarcinomas, and 130 (85.5%) were clinical stage IA at detection. Furthermore, 140 out of the 152 lung cancers (92.1%) were treated by surgery alone. Nine patients had multiple lung cancers (2 lung cancers, n=4; 3 lung cancers, n=2; 4 lung cancers, n=2; 6 lung cancers, n=1). Of the 133 patients with lung cancer, 66 (49.6%) were never-smokers, 31 (23.3%) were smokers with <30 pack-years of smoking, and 36 (27.1%) were smokers with ≥30 pack-years of smoking. Therefore, 72.9% of lung cancer patients were never-smokers or smokers with <30 pack-years of smoking, and did not meet the NLST criterion of smokers with ≥30 pack-years of smoking. One hundred and fifty-one lung cancers in 132 patients were detected by CT examination, and one lung cancer in one patient was an interval case with symptoms (53-year-old woman with small cell carcinoma, clinical stage IIIA, T2aN2M0). Lung cancer in smokers (men, 55; women, 12) was detected predominantly in men, while that in never-smokers (women, 50; men, 16) was detected predominantly in women (P<0.0001). The mean age of the participants who were diagnosed as having lung cancer was 62.7±8.4 years in the smoker group and 60.6±8.3 years in the never-smoker group (P=0.2708). Among the 133 patients with lung cancer, 71 (53.4%) were men and 62 (46.6%) were women. The mean age of the participants diagnosed as having lung cancer was 63.6±7.8 years in the men and 59.5±8.5 years in the women (P=0.0041).
Table 2. Lung cancers according to the smoking status.
| Characteristics | Never-smoker | Pack-years <30 | Pack-years ≥30 | Total |
|---|---|---|---|---|
| No. of patients [no. of lesions§] | ||||
| Men | 16 [18] | 21 [22] | 34 [39] | 71 [79] |
| Women | 50 [59] | 10 [12] | 2 [2] | 62 [73] |
| Maximal diameter of the lesions (cm)* | 1.5±0.8 | 1.3±0.8 | 1.4±0.8 | 1.4±0.8 |
| No. of lesions | 77 (100.0) | 34 (100.0) | 41 (100.0) | 152 (100.0) |
| Consistency of the lesions [n=152], n (%) | ||||
| Pure GGN | 14 (18.2) | 7 (20.6) | 11 (26.8) | 32 (21.1) |
| Part-solid | 49 (63.6) | 19 (55.9) | 11 (26.8) | 79 (52.0) |
| Solid | 12 (15.6) | 8 (23.5) | 16 (39.0) | 36 (23.7) |
| Others¶ | 2 (2.6) | 0 (0.0) | 3 (7.3) | 5 (3.3) |
| Histology [n=152], n (%) | ||||
| Adenocarcinoma | 75 (97.4) | 30 (88.2) | 30† (73.2) | 135† (88.8) |
| Squamous cell carcinoma | 0 (0.0) | 0 (0.0) | 8 (19.5) | 8 (5.3) |
| Small cell carcinoma | 0 (0.0) | 1 (2.9) | 2†† (4.9) | 3†† (2.0) |
| Adenosquamous carcinoma | 0 (0.0) | 1 (2.9) | 0 (0.0) | 1 (0.7) |
| Carcinoid | 2 (2.6) | 0 (0.0) | 0 (0.0) | 2 (1.3) |
| NSCLC | 0 (0.0) | 1 (2.9) | 0 (0.0) | 1 (0.7) |
| Atypical cell** | 0 (0.0) | 1 (2.9) | 1 (2.4) | 2 (1.3) |
| Stage¶¶ [n=152], n (%) | ||||
| IA | 70 (90.9) | 29 (85.3) | 31 (75.6) | 130 (85.5) |
| IB | 4 (5.2) | 4 (11.8) | 4 (9.8) | 12 (7.9) |
| IIA | 0 (0.0) | 0 (0.0) | 1 (2.4) | 1 (0.7) |
| IIB | 0 (0.0) | 1 (2.9) | 0 (0.0) | 1 (0.7) |
| IIIA | 0 (0.0) | 0 (0.0) | 3 (7.3) | 3 (2.0) |
| IIIB | 2 (2.6) | 0 (0.0) | 1 (2.4) | 3 (2.0) |
| IV | 1 (1.3) | 0 (0.0) | 1 (2.4) | 2 (1.3) |
| First-line treatment [n=152], n (%) | ||||
| Surgery only | 76 (98.7) | 30 (88.2) | 34 (82.9) | 140 (92.1) |
| Surgery and radiotherapy | 1 (1.3) | 0 (0.0) | 0 (0.0) | 1 (0.7) |
| Surgery and chemotherapy | 0 (0.0) | 1 (2.9) | 1 (2.4) | 2 (1.3) |
| Chemotherapy and radiotherapy | 0 (0.0) | 0 (0.0) | 3 (7.3) | 3 (2.0) |
| Radiotherapy | 0 (0.0) | 2 (5.9) | 1 (2.4) | 3 (2.0) |
| Ablation‡ | 0 (0.0) | 1 (2.9) | 0 (0.0) | 1 (0.7) |
| N/A | 0 (0.0) | 0 (0.0) | 2 (4.9) | 2 (1.3) |
§, lesions: nodules, masses, and other than nodules or masses; *, mean ± SD; ¶, five lesions other than nodules or masses were diagnosed as lung cancers: a funicular-like opacity (n=1), a heterogeneous opacity in pulmonary fibrosis (n=1), hilar enlargement (n=1), a pneumonia-like opacity (n=1), and a cyst with a thick wall (n=1); †, one adenocarcinoma was diagnosed cytologically using fiberoptic bronchoscopic examination; ††, one cancer was an interval case; **, cytologic diagnosis using fiberoptic bronchoscopic examination; ¶¶, 7th TNM classification (the results based on the 8th edition of TNM staging system are shown in Table S1); ‡, this patient had double primary adenocarcinomas; one was resected and the other was treated by ablation in accordance with the patient’s wish; N/A, data were not available due to transfer of the patients to other hospitals. GGN, ground-glass nodule; NSCLC, non-small cell lung cancer.
Diagnosis of adenocarcinoma was made in 120 patients by histopathological evaluation (n=119) or cytological evaluation (n=1). Seven patients had multiple adenocarcinomas (2 adenocarcinomas, n=2; 3 adenocarcinomas, n=2; 4 adenocarcinomas, n=3). Among the 65 never-smokers in the 119 cases of adenocarcinoma diagnosed by histopathology, there were 42 cases of group A adenocarcinoma (AIS, 20; MIA, 22) and 23 cases of group B adenocarcinoma (invasive adenocarcinoma); on the other hand, among the remaining 54 smokers in this group, there were 29 cases of group A adenocarcinoma (AIS, 10; MIA, 19) and 25 cases of group B adenocarcinoma (P=0.2270); thus, there was no statistically significant difference in the number of invasive adenocarcinoma cases detected between the never-smokers and smokers.
The mean diameter of the lung cancers at the time of detection was not statistically significantly different between the smokers and never-smokers (P value for each pair of smoking statuses >0.05). Five lesions other than nodules or masses, as shown below, were diagnosed as lung cancers: a funicular-like opacity (n=1), a heterogeneous opacity in pulmonary fibrosis (n=1), hilar enlargement (n=1), a pneumonia-like opacity (n=1), and a cyst with a thick wall (n=1).
None of the lung cancers diagnosed between February 2004 and March 2012 was detected by sputum cytology.
Detection rates of lung cancer
The cumulative detection rate of lung cancer patients by CT was 1.1% (Table 3); the detection rate of lung cancer patients was 1.1% in never-smokers and 1.1% in smokers. According to the smoking status, lung cancer was diagnosed in 1.3% of women and 0.7% of men who were never-smokers, in 1.3% of women and 0.7% of men with <30 pack-years of smoking, and in 0.7% of women and 1.6% of men with ≥30 pack-years of smoking.
Table 3. Detection rate of lung cancer patients according to the smoking status, gender, and age.
| Smoking status (%) | Subgroup according to the gender and smoking status | Age group | Number of participants (%) | Number of lung cancer patients (%) | Detection rate by age group (%) | Subtotal of lung cancer patients (%) | Detection rate by subgroup of smoking status (%) | Detection rate by smoking status (never-smoker and smoker) (%) |
|---|---|---|---|---|---|---|---|---|
| Never-smoker, n=6,021 (49.7) | Never-smoker women (n=3,875) | 40–49 | 754 (6.2) | 5 (3.8) | 0.7 | 50 (37.9) | 1.3 (50/3,875) | Detection rate in never-smokers 1.1 (66/6,021) |
| 50–59 | 1,282 (10.6) | 19 (14.4) | 1.5 | |||||
| 60–69 | 1,518 (12.5) | 23 (17.4) | 1.5 | |||||
| ≥70 | 321 (2.6) | 3 (2.3) | 0.9 | |||||
| Never-smoker men (n=2,146) | 40–49 | 484 (4.0) | 1 (0.8) | 0.2 | 16 (12.1) | 0.7 (16/2,146) | ||
| 50–59 | 672 (5.5) | 3 (2.3) | 0.4 | |||||
| 60–69 | 781 (6.4) | 7 (5.3) | 0.9 | |||||
| ≥70 | 209 (1.7) | 5 (3.8) | 2.6 | |||||
| Smoker, n=6,090 (50.3) | Women with <30 pack-years of smoking (n=796) | 40–49 | 275 (2.3) | 2 (1.5) | 0.7 | 10 (7.6) | 1.3 (10/796) | Detection rate in smokers 1.1 (66/6,090)† |
| 50–59 | 307 (2.5) | 3 (2.3) | 1.0 | |||||
| 60–69 | 183 (1.5) | 2 (1.5) | 1.1 | |||||
| ≥70 | 31 (0.3) | 3 (2.3) | 9.7 | |||||
| Men with <30 pack-years of smoking (n=2,989) | 40–49 | 662 (5.5) | 0 | 0.0 | 21 (15.9) | 0.7 (21/2,989) | ||
| 50–59 | 1,023 (8.4) | 7 (5.3) | 0.7 | |||||
| 60–69 | 1,054 (8.7) | 8 (6.1) | 0.8 | |||||
| ≥70 | 250 (2.1) | 6 (4.5) | 2.4 | |||||
| Women ≥30 pack-years of smoking (n=149) | 40–49 | 15 (0.1) | 0 | 0.0 | 1† (0.8) | 0.7 (1/149)† | ||
| 50–59 | 63 (0.5) | 1† (0.8) | 1.6 | |||||
| 60–69 | 57 (0.5) | 0 | 0.0 | |||||
| ≥70 | 14 (0.12) | 0 | 0.0 | |||||
| Men with ≥30 pack-years of smoking (n=2,156) | 40–49 | 126 (1.0) | 1 (0.8) | 0.8 | 34 (25.8) | 1.6 (34/2,156) | ||
| 50–59 | 753 (6.2) | 10 (7.6) | 1.3 | |||||
| 60–69 | 1,027 (8.5) | 20 (15.2) | 1.9 | |||||
| ≥70 | 250 (2.1) | 3 (2.3) | 1.3 | |||||
| Unknown | Men with unknown smoking status (n=3) | 40–49 | 1 (0.0) | 0 | 0.0 | 0 | ||
| 60–69 | 2 (0.0) | 0 | 0.0 | |||||
| Total | 12,114 (100.0) | 132† (100.0) | 1.1 | 132† (100.0) | ||||
†, an interval case was excluded.
The detection rates of lung cancer patients at the baseline and at the repeat CT examinations were 1.0% (122 out of 12,114 participants) and 0.04% (10 out of a total of 24,844 repeat CT examinations in 8,237 participants), respectively. Of the 10 lung cancers detected by the repeat CT examinations, 8 were detected in participants with ≥30 pack-years of smoking and 2 were detected in participants with <30 pack-years of smoking. One interval lung cancer developed in a participant with ≥30 pack-years of smoking.
Multivariable odds ratio of lung cancer detection
According to the ORs adjusted for age and gender, the OR of lung cancer detection in smokers (smokers here refer to a group of smokers with <30 pack-years or ≥30 pack-years of smoking) was almost the same as that in the never-smokers (OR =1.24, 95% CI: 0.82–1.87, P=0.3); on the other hand, the OR of lung cancer detection in smokers with ≥30 pack-years of smoking was higher than that in the never-smokers (OR =1.71, 95% CI: 1.04–2.82, P=0.03), while the OR of lung cancer detection in smokers with <30 pack-years of smoking was the same as that in the never-smokers (OR =1.00, 95% CI: 0.62–1.61, P=0.99) (Table 4).
Table 4. Multivariable odds ratio of lung cancer detection*.
| Reference | Categorization by the smoking status | Odds ratio | 95% CI | P value |
|---|---|---|---|---|
| Never-smokers | Smokers¶ | 1.24 | 0.82–1.87 | 0.3 |
| Smokers with <30 pack-years of smoking | 1.00 | 0.62–1.61 | 0.99 | |
| Smokers with ≥30 pack-years of smoking | 1.71 | 1.04–2.82 | 0.03 | |
| Smokers with <30 pack-years of smoking | Never-smokers | 0.99 | 0.61–1.60 | 0.99 |
| Smokers with ≥30 pack-years of smoking | 1.71 | 1.04–2.80 | 0.03 |
*, results were adjusted for age and gender; ¶, smokers here refer to smokers with <30 pack-years or ≥30 pack-years of smoking.
Even though smokers with <30 pack-years of smoking were used as reference, the OR of lung cancer detection in smokers with ≥30 pack-years of smoking was higher than that in the smokers with <30 pack-years of smoking (OR =1.71, 95% CI: 1.04–2.80, P=0.03)
Second-hand smoke exposure in never-smoker lung cancer patients
Of the 133 patients with lung cancer, 66 were never-smokers (women, n=50; men, n=16). Among the 50 women, 17 (34%) were classified according to the exposure status to second-hand smoke into group 1 (almost no exposure to second-hand smoke at any of the three chronological ages), 17 (34%) were classified into group 2 (daily exposure to second-hand smoke at around 30 years and/or at the time of the baseline screening), and 16 (32%) were classified as group 3 (exposure to second-hand smoke at least several times a month at any of the three chronological ages, but not fulfilling the exposure criterion for classification into group 2); among the 16 men, 3 (19%) were classified into group 1, 6 (38%) into group 2, and 7 (44%) into group 3. In total, 23 of the 66 never-smokers (35%) had a history of daily second-hand smoke exposure during adulthood, and 20 of the 66 never-smokers (30%) had no history of second-hand smoke exposure; the remaining 23 of the 66 never-smokers (35%) had a history of intermediate second-hand smoke exposure.
Cancer-specific survival curves
Significant difference was observed in the cancer-specific survival curves between the participants detected as having lung cancer in the never-smoker and smoker groups (log-rank test, P<0.05) (Figure 1). The 5-year and 10-year survival rates in the never-smoker lung cancer patients were 96.8% (95% CI: 88.2–99.2%) and 96.8% (95% CI: 88.2–99.2%), respectively, whereas those in the smoker lung cancer patients were 90.4% (95% CI: 80.1–95.6%) and 77.4% (95% CI: 60.2–88.5%), respectively. The median follow-up period was 7.5 years (IQR, 5.6 to 9.1 years) in the never-smoker lung cancer patients and 6.6 years (IQR, 4.7 to 8.7 years) in the smoker lung cancer patients.
Figure 1.
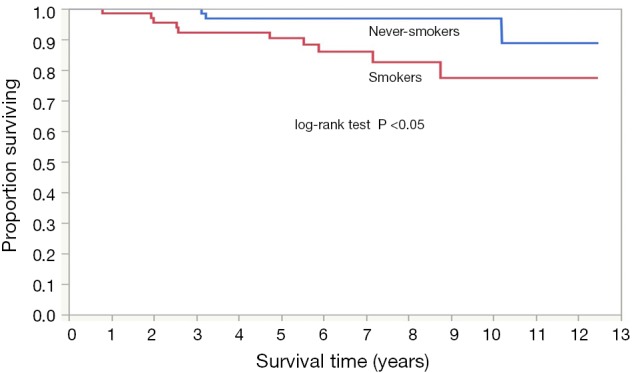
Cancer-specific survival of never-smokers (blue line) versus smokers (red line).
Discussion
Between February 2, 2004, and March 31, 2012, a total of 152 lung cancers in 133 patients were diagnosed by LDCT lung cancer screening at the RCCPS; 151 lung cancers in 132 patients were detected by CT examination, and one lung cancer in one patient was an interval case. Of all the lung cancers, 85.5% were clinical stage IA at detection and 88.8% were adenocarcinomas; 49.6% of the lung cancer patients were never-smokers, and 30% of the never-smoker lung cancer patients had no history of second-hand smoke exposure. The cumulative detection rate of lung cancer patients by CT examination was 1.1%. Although the OR of lung cancer detection in smokers with ≥30 pack-years of smoking was higher than that in the never-smokers and smokers with <30 pack-years of smoking, approximately 70% of lung cancer patients might be missed we only adopted the NLST criterion of smokers with ≥30 pack-years of smoking.
The rate of detection of NCNs could depend on the slice thickness of the CT images, size criteria for positive screening results, and/or the study population. A thinner slice thickness of CT is associated with a smaller partial volume effect, resulting in an increased likelihood of detection of NCNs by screening CT. The percentage of participants with NCNs detected by LDCT lung cancer screening with 10-mm-thick CT images in the Early Lung Cancer Action Project was 23% (17). CT screening with 5-mm-thick CT images at the Mayo Clinic showed that 51% of the participants had NCNs (18). The Pan-Canadian Early Detection of Lung Cancer Study (the PanCan study), which used 1.25-mm-thick section CT images, reported that 74% of the participants had NCNs (19). Table 1 shows that the rate of detection of NCNs in the present study was 74.9% [(5,155+3,942)/12,114], almost the same as that in the PanCan study.
In regard to the findings of observational studies conducted in Japan, one study of CT lung cancer screening conducted in Nagano prefecture reported that 88% of the lung cancers were clinical stage IA at detection, and 85% of the lung cancers were adenocarcinomas (5). Another study of CT lung cancer screening in Hitachi city reported that 77.5% of the cancers were clinical stage IA at detection, and 97.5% were adenocarcinomas (6). While the percentages of cases that were clinical stage IA at detection and percentage of adenocarcinomas in the NLST were 40%, and 46.8% (TNM staging system, 6th edition), respectively (20) the corresponding figures in the NELSON trial were 62.4%, and 56.9%, (TNM staging system, 7th edition) respectively (21). Thus, the percentages of clinical stage IA cases at detection and adenocarcinoma among the lung cancers detected by CT screening were higher in Japanese studies than those reported from Western studies. On the contrary, a study from China showed that the percentage of cases with clinical stage IA disease (TNM staging system, 8th edition) at detection and the percentage of cases with adenocarcinoma were 80.4%, and 92.2%, respectively (22), similar to the results reported from Japanese studies.
A Korean study showed that the percentages of cases with invasive adenocarcinoma among patients with adenocarcinoma were almost the same in the never-smoker patients (67.3%) and smoker patients (70.2%) (23). Our study also showed no statistically significant difference in the number of invasive adenocarcinoma cases detected between the never-smokers and smokers. The Korean study showed that the cumulative detection rate of lung cancer was 0.45% in never-smokers and 0.86% in smokers (past and present) (P<0.001) (23). However, our results show that the cumulative detection rate of lung cancer patients did not differ significantly between the never-smokers and smokers (i.e., 1.1% in both groups).
One of the entry criteria for the NLST was that participants should be smokers with ≥30 pack-years of smoking (14). Although the OR of lung cancer detection in smokers with ≥30 pack-years of smoking was higher than that in the never-smokers and smokers with <30 pack-years of smoking in this study population, if the target population for LDCT lung cancer screening in Japan was limited to only smokers with ≥30 pack-years of smoking (the NLST criterion), some people with early lung cancer might be missed, because based on our results, approximately 70% of lung cancer patients were never-smokers or smokers with <30 pack-years of smoking. Currently, the Japanese randomized trial for evaluating the efficacy of low-dose thoracic CT screening for lung cancer (JECS study) is under way, to assess the degree of reduction in lung cancer mortality in never-smokers and smokers with <30 pack-years of smoking afforded by LDCT lung cancer screening (24).
Global statistics in 2002 estimated that 15% of lung cancers in men and 53% in women are not attributable to smoking, accounting for 25% of all lung cancer cases worldwide (25). If considered as a separate category, lung cancer in never-smokers would rank as the seventh most common cause of cancer death worldwide (26). One study from the US reported an increasing proportion of non-small cell lung cancer (NSCLC) patients who had never smoked in a large and diverse patient population examined between 1990 and 2013, with a proportion of 8.0% between 1990 and 1995 and of 14.9% between 2011 to 2013 (27); another study from the UK reported a doubling in the annual incidence of never-smokers diagnosed as having NSCLC between 2008 and 2014, increasing from 13% to 28%; 67.7% of the patients were women, and the most common histological type was adenocarcinoma (28); these data suggest that the actual incidence of lung cancer in never-smokers is increasing. One study reported that 53% of patients with lung cancer had never smoked in Taiwan and that nearly 60% of patients with lung cancer who had never smoked had stage IV disease, which was similar to that observed among smokers with lung cancer, based on the national Taiwan Cancer Registry data, which contains data of all cancer cases in Taiwan recorded in a uniform format since 1979 (29). Therefore, not only smokers, but also never-smokers should be included in the target population for LDCT lung cancer screening.
Second-hand smoke exposure in the home during adulthood is known to result in a statistically significant increase in the risk of lung cancer (30). In the present study, 35% of never-smoker lung cancer patients had a history of daily second-hand smoke exposure during adulthood; however, on the other hand, 30% of never-smoker lung cancer patients had no history of second-hand smoke exposure. One study reported that level changes of particulate matter 2.5 (PM2.5), one of the risk factors for lung cancer other than smoking and second-hand smoke exposure, can affect the lung adenocarcinoma incidence and patient survival (29). Investigation, in the future, of lung cancer risk factors other than smoking and second-hand smoke exposure is warranted.
The present study had several limitations. First, the present LDCT screening study was not population-based; instead, the participants in this study were self-referred and paid for the screening by themselves. Therefore, a self-selection bias existed and could have affected the results of the present study. Second, lung cancers detected at other facilities after baseline CT screening were not included in the present study because sufficient information was not available. Such exclusion might have led to an underestimation of the detection rate of lung cancers in this observational study. However, further follow-up of pure GGNs and part-solid nodules has been under way in the National Cancer Center Hospital and the National Cancer Center Hospital East since March, 2012; an update of the cumulative detection rate might be possible in the future. Third, the multivariable odds ratio of lung cancer detection might be influenced by unmeasured confounding variables, because we showed the results based only on adjustment for age and gender. Further evaluation is warranted. Fourth, we could not use a volumetry-based nodule management algorithm, similar to that included in the NELSON trial protocol (31). The implementation of a volumetry-based nodule management algorithm should be discussed in future LDCT screening programs in Japan. Finally, the results of genetic analyses of the resected adenocarcinomas were not analyzed in the present study. Risk factors for lung cancer other than smoking and second-hand smoke exposure are currently being investigated intensively (32-39); however, detailed discussion about genetic factors is beyond the scope of the present study.
In conclusion, LDCT lung cancer screening was performed as part of cancer screening research at the RCCPS, not only in smokers, but also in never-smokers; approximately 90% of the detected lung cancers were adenocarcinomas; the number of invasive adenocarcinomas detected was not statistically significantly different between the smokers and never-smokers. Although the OR of lung cancer detection in smokers with ≥30 pack-years of smoking was higher than that in the never-smokers and smokers with <30 pack-years of smoking, approximately 70% of lung cancer patients might be missed if we only adopted the NLST criterion of smokers with ≥30 pack-years of smoking. Therefore, never-smokers and smokers with <30 pack-years of smoking should be included in the target population for LDCT lung cancer screening in Japan. Further study of LDCT lung cancer screening in never-smokers is warranted.
Table S1. The results based on the 8th edition of TNM staging system.
| Characteristics | Never-smoker | Pack-years <30 | Pack-years ≥30 | Total |
|---|---|---|---|---|
| Number of lesions stage [n=152] | 77 (100.0) | 34 (100.0) | 41 (100.0) | 152 (100.0) |
| 0 | 26 (33.8) | 9 (26.5) | 3 (7.3) | 38 (25.0) |
| IA1 | 37 (48.1) | 17 (50.0) | 20 (48.8) | 74 (48.7) |
| IA2 | 6 (7.8) | 3 (8.8) | 7 (17.1) | 16 (10.5) |
| IA3 | 3 (3.9) | 1 (2.9) | 3 (7.3) | 7 (4.6) |
| IB | 2 (2.6) | 3 (8.8) | 2 (4.9) | 7 (4.6) |
| IIB | 0 (0.0) | 1 (2.9) | 2 (4.9) | 3 (2.0) |
| IIIA | 1 (1.3) | 0 (0.0) | 2 (4.9) | 3 (2.0) |
| IIIB | 1 (1.3) | 0 (0.0) | 1 (2.4) | 2 (1.3) |
| IVA | 1 (1.3) | 0 (0.0) | 1 (2.4) | 2 (1.3) |
Data presented as number (percentage).
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This research was supported in part by a Grant-in-Aid from the Third-term Comprehensive Cancer Control Strategy sponsored by the Ministry of Health, Labour and Welfare, Tokyo, Japan (16-016, 19-1, 21-5-1, 22-019), the National Cancer Center Research and Development Fund, Tokyo, Japan (23-A-25, 23-A-48, 30-A-16).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was performed with the approval of the institutional review board of the National Cancer Center (No. 2005-32). Written informed consent was obtained from each of the participants.
Footnotes
Peer Review File: Available at http://dx.doi.org/10.21037/tlcr.2020.01.13.
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Cancer mortality from Vital Statistics in Japan (1958-2017). Vital Statistics in Japan, tabulated by Cancer Information Service, National Cancer Center, Japan. Accessed Aug 12, 2019. Available online: http://ganjoho.jp/en/professional/statistics/table_download.html
- 2.Sagawa M, Nakayama T, Tsukada H, et al. The efficacy of lung cancer screening conducted in 1990s: four case-control studies in Japan. Lung Cancer 2003;41:29-36. 10.1016/S0169-5002(03)00197-1 [DOI] [PubMed] [Google Scholar]
- 3.Kaneko M, Eguchi K, Ohmatsu H, et al. Peripheral lung cancer: screening and detection with low-dose spiral CT versus radiography. Radiology 1996;201:798-802. 10.1148/radiology.201.3.8939234 [DOI] [PubMed] [Google Scholar]
- 4.Sone S, Takashima S, Li F, et al. Mass screening for lung cancer with mobile spiral computed tomography scanner. Lancet 1998;351:1242-45. 10.1016/S0140-6736(97)08229-9 [DOI] [PubMed] [Google Scholar]
- 5.Sone S, Li F, Yang ZG, et al. Results of three-year mass screening programme for lung cancer using mobile low-dose spiral computed tomography scanner. Brit J Cancer 2001;84:25-32. 10.1054/bjoc.2000.1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nawa T, Nakagawa T, Kusano S, et al. Lung cancer screening using low-dose spiral CT: results of baseline and 1-year follow-up studies. Chest 2002;122:15-20. 10.1378/chest.122.1.15 [DOI] [PubMed] [Google Scholar]
- 7.Sone S, Nakayama T, Honda T, et al. Long-term follow-up study of a population-based 1996-1998 mass screening programme for lung cancer using mobile low-dose spiral computed tomography. Lung Cancer 2007;58:329-41. 10.1016/j.lungcan.2007.06.022 [DOI] [PubMed] [Google Scholar]
- 8.Sobue T, Moriyama N, Kaneko M, et al. Screening for lung cancer with low-dose helical computed tomography: Anti-Lung Cancer Association Project. J Clin Oncol 2002;20:911-20. 10.1200/JCO.20.4.911 [DOI] [PubMed] [Google Scholar]
- 9.Nawa T, Nakagawa T, Mizoue T, et al. A decrease in lung cancer mortality following the introduction of low-dose chest CT screening in Hitachi, Japan. Lung Cancer 2012;78:225-8. 10.1016/j.lungcan.2012.09.012 [DOI] [PubMed] [Google Scholar]
- 10.Suda K, Tomizawa K, Yatabe Y, et al. Lung cancers unrelated to smoking: characterized by single oncogene addiction? Int J Clin Oncol 2011;16:294-305. 10.1007/s10147-011-0262-y [DOI] [PubMed] [Google Scholar]
- 11.Kakinuma R, Muramatsu Y, Kusumoto M, et al. Solitary Pure Ground-Glass Nodules 5 mm or Smaller: Frequency of Growth. Radiology 2015;276:873-82. 10.1148/radiol.2015141071 [DOI] [PubMed] [Google Scholar]
- 12.Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 2013;266:304-17. 10.1148/radiol.12120628 [DOI] [PubMed] [Google Scholar]
- 13.MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology 2017;284:228-43. 10.1148/radiol.2017161659 [DOI] [PubMed] [Google Scholar]
- 14.National Lung Screening Trial Research Team , Aberle DR, Berg CD, et al. The National Lung Screening Trial: overview and study design. Radiology 2011;258:243-53. 10.1148/radiol.10091808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. 10.1097/JTO.0b013e31812f3c1a [DOI] [PubMed] [Google Scholar]
- 16.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. 10.1097/JTO.0b013e318206a221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999;354:99-105. 10.1016/S0140-6736(99)06093-6 [DOI] [PubMed] [Google Scholar]
- 18.Swensen SJ. CT screening for lung cancer. AJR Am J Roentgenol 2002;179:833-6. 10.2214/ajr.179.4.1790833 [DOI] [PubMed] [Google Scholar]
- 19.McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013;369:910-9. 10.1056/NEJMoa1214726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Lung Screening Trial Research Team , Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yousaf-Khan U, van der Aalst C, de Jong PA, et al. Final screening round of the NELSON lung cancer screening trial: the effect of a 2.5-year screening interval. Thorax 2017;72:48-56. 10.1136/thoraxjnl-2016-208655 [DOI] [PubMed] [Google Scholar]
- 22.Yang W, Qian F, Teng J, et al. Community-based lung cancer screening with low-dose CT in China: Results of the baseline screening. Lung Cancer 2018;117:20-6. 10.1016/j.lungcan.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 23.Kang HR, Cho JY, Lee SH, et al. Role of low-dose computerized tomography in lung cancer screening among never-smokers. J Thorac Oncol 2019;14:436-44. 10.1016/j.jtho.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 24.Sagawa M, Nakayama T, Tanaka M, et al. A randomized controlled trial on the efficacy of thoracic CT screening for lung cancer in non-smokers and smokers of <30 pack-years aged 50–64 years (JECS Study): research design. Jpn J Clin Oncol 2012;42:1219-21. 10.1093/jjco/hys157 [DOI] [PubMed] [Google Scholar]
- 25.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. 10.3322/canjclin.55.2.74 [DOI] [PubMed] [Google Scholar]
- 26.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers- a different disease. Nat Rev Cancer 2007;7:778-90. 10.1038/nrc2190 [DOI] [PubMed] [Google Scholar]
- 27.Pelosof L, Ahn C, Gao A, et al. Proportion of never-smoker non-small cell lung cancer patients at three diverse institutions. J Natl Cancer Inst 2017;109:djw295. 10.1093/jnci/djw295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cufari ME, Proli C, De Sousa P, et al. Increasing frequency of non-smoking lung cancer: presentation of patients with early disease to a tertiary institution in the UK. Eur J Cancer 2017;84:55-9. 10.1016/j.ejca.2017.06.031 [DOI] [PubMed] [Google Scholar]
- 29.Tseng CH, Tsuang BJ, Chiang CJ, et al. The relationship between air pollution and lung cancer in nonsmokers in Taiwan. J Thorac Oncol 2019;14:784-92. 10.1016/j.jtho.2018.12.033 [DOI] [PubMed] [Google Scholar]
- 30.Hori M, Tanaka H, Wakai K, et al. Secondhand smoke exposure and risk of lung cancer in Japan: a systematic review and meta-analysis of epidemiologic studies. Jpn J Clin Oncol 2016;46:942-51. 10.1093/jjco/hyw091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009;361: 2221-9. 10.1056/NEJMoa0906085 [DOI] [PubMed] [Google Scholar]
- 32.Kohno T, Kakinuma R, Iwasaki M, et al. Association of CYP19A1 polymorphisms with risks for atypical adenomatous hyperplasia and bronchioloalveolar carcinoma in the lungs. Carcinogenesis 2010;31:1794-99. 10.1093/carcin/bgq159 [DOI] [PubMed] [Google Scholar]
- 33.Ha SY, Choi SJ, Cho JH, et al. Lung cancer in never-smoker Asian females is driven by oncogenic mutations, most often involving EGFR. Oncotarget 2015;6:5465-74. 10.18632/oncotarget.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seow WJ, Matsuo K, Hsiung CA, et al. Association between GWAS-identified lung adenocarcinoma susceptibility loci and EGFR mutations in never-smoking Asian women, and comparison with findings from Western populations. Hum Mol Genet 2017;26:454-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dias M, Linhas R, Campainha S, et al. Lung cancer in never-smokers - what are the differences? Acta Oncol 2017;56:931-5. 10.1080/0284186X.2017.1287944 [DOI] [PubMed] [Google Scholar]
- 36.Han L, Lee CK, Pang H, et al. Genetic predisposition to lung adenocarcinoma among never-smoking Chinese with different epidermal growth factor receptor mutation status. Lung Cancer 2017;114:79-89. 10.1016/j.lungcan.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 37.Korpanty GJ, Kamel-Reid S, Pintilie M. Lung cancer in never smokers from the Princess Margaret Cancer Centre. Oncotarget 2018;9:22559-70. 10.18632/oncotarget.25176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo W, Tian P, Wang Y, et al. Characteristics of genomic alterations of lung adenocarcinoma in young never-smokers. Int J Cancer 2018;143:1696-705. 10.1002/ijc.31542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JJ, Park S, Park H, et al. Tracing oncogene rearrangements in the mutational history of lung adenocarcinoma. Cell 2019;177:1842-1857.e21. 10.1016/j.cell.2019.05.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


