Abstract
Background
Although many studies have determined that PD-L1 expression by immunohistochemistry can be somewhat predictive of a response to checkpoint inhibitor the impact of specific genomic changes and smoking history in the context of PD-L1 expression is limited. This single-center study examined clinical and genomic factors beyond STK11 and EGFR in patients with advanced non-small cell lung cancer (NSCLC) to determine which patients benefit from therapy with immune checkpoint inhibitors (ICIs).
Methods
Clinical and genomic features of patients with NSCLC treated with immunotherapy were compiled into a database. Genomic information collected included gene mutations via next generation sequencing, tumor mutation burden (TMB), and PD-L1 tumor proportional scores.
Results
A total of 131 patients with advanced NSCLC treated with ICIs were examined. Race was not associated with response. A positive response to immunotherapy was associated with smoke year increase (P=0.042). KRAS mutation and MYC amplification were associated with a positive response to immunotherapy while EGFR, RB1, and NF1 mutations were associated with a lack of response. KRAS mutation (P=0.007) and high TMB (P=0.070) were positively associated with smoking history. EGFR mutation was negatively associated with smoking history (P=0.002) . In multivariate analysis controlling for age and smoking history, MYC amplification continued to be the only predictive genomic marker with a trend toward response to therapy (P=0.092) beyond the smoking history.
Conclusions
Among the clinical and genomic factors examined in this study, smoking status is the most predictive of response to ICIs. Only MYC amplification continued to predict a trend toward response to immunotherapy when controlling for smoking history. Other genomic predictors such as EGFR and KRAS simply reflect their association with smoking. Detailed smoking history and MYC amplification alone can predict response to ICI.
Keywords: Immunotherapy, lung cancer, tobacco
Introduction
Immune checkpoint inhibitors (ICIs) are emerging as an alternative to traditional chemotherapy for several cancers including non-small cell lung cancer (NSCLC) (1-6). Programmed death receptor (PD-1) inhibitors such as nivolumab and pembrolizumab in particular demonstrate remarkable results in certain patients with increased survival and a favorable safety profile (7,8). Despite these promising results, the overall response rate for second-line treatment with ICIs is about 20%, highlighting the need for clinically practical predictors of response (1-3).
Most trials demonstrated improved response rates in tumors with increased expression of programmed death-ligand (PD-L1), specifically in treatment with pembrolizumab for tumors with PD-L1 expression ≥50% (1,3,9). Still, PD-L1 expression has several limitations as a biomarker, such as assay variability (10,11). Recent studies appear to show that higher tumor mutation burden (TMB) correlates with response to therapy as well, independent of PD-L1 expression (12). These biomarkers may predict response for some patients, but it appears that certain subgroups are less apt to benefit from immunotherapy, such as NSCLC with EGFR mutations or ALK rearrangements despite high PD-L1 expression in some of these tumors (1,13). Thus, the need for a clinically available predictor of response to ICIs remains extremely important.
As most patients with advanced lung cancer undergo genomic testing, in particular next-generation sequencing (NGS), and clinical data is readily obtainable (such as smoking history) we set to examine which genomic and clinical characteristics are predictive of response to immunotherapy in advanced NSCLC. We examined clinical characteristics including sex, age, and detailed smoking status and extensive NGS of targeted exomes in addition to PD-L1 expression, and TMB to determine what factors are correlated with response.
Methods
Patient population
Patients with NSCLC at UH Cleveland Medical Center are compiled into an IRB approved institutional database (N=3,169) that is continuously maintained and updated. From this database patients with advanced stage IV disease were identified to yield a total of 987 patients. Other inclusion criteria included patients treated with either pembrolizumab or nivolumab and age greater than 18. We collected data on age, sex, race, smoking status, histological subtype, and somatic genomic information.
Smoking status
Smoking status is defined as current smoker for patients smoking at the time of diagnosis or a quit date within 12 months of diagnosis. Former smoker are those who quit at 12 months or greater prior to diagnosis. Never smoker is defined as less than 100 cigarettes over an individual’s lifetime. Smoking index (SI) is defined as pack years multiplied by years smoked to yield smoke-years.
PD-L1 expression and genomic testing
Clarient Diagnostic Services are used to determine PD-L1 expression at our institution. Genomic information was gathered from the Foundation One sequencing platform, which utilizes next generation sequencing to interrogate 315 genes as well as introns of 28 genes involved in rearrangements (as previously described).
Statistical analysis
Chi-square tests were used to determine associations between response to immunotherapy and variables such as gene mutations and smoking status. The association between response and continuous variables (smoking quit time, smoke years, pack years, PD-L1 expression, and TMB) was estimated using logistic regression. The response rate and 95% confidence internals were estimated using Wilson’s method. All statistical tests were two-sided and P≤0.05 was considered statistically significant. However P values of ≤0.1 were considered as a trend.
Results
Patient characteristics
A total of 131 patients met the inclusion criteria. In regards to the specific immunotherapy agent used, 108 were treated with single agent nivolumab while 23 were treated with single agent pembrolizumab. Thirty-three patients underwent PD-L1 testing, which was determined using Calrient Diagnostic Services. Eighty-three patients underwent genomic testing with Foundation One next generation sequencing. Baseline characteristics including sex, race, smoking status, and tumor pathology are described in Table 1.
Table 1. Demographic characteristics of the patient cohort and their association with response to immunotherapy.
| Factor | No. of patients (%) or median (range) | P value |
|---|---|---|
| Sex | ||
| Women | 73 (55.7) | 0.858 |
| Men | 58 (44.3) | |
| Age, y | 62 (29, 88) | |
| Race (40 missing) | ||
| White | 49 (53.9) | 0.722 |
| Black | 41 (45.1) | |
| Other | 1 (1.1) | |
| Smoking | ||
| Never | 9 (6.9) | 0.097 |
| Former/current | 122 (93.1) | |
| Pathology (2 missing) | ||
| Adenocarcinoma | 91 (70.5) | 0.442 |
| Squamous | 32 (24.8) | |
| Other | 6 (4.7) |
Response rate
The overall response rate to immunotherapy is 22.1% (29 of 131). There is no significant difference between response rates for nivolumab vs. pembrolizumab (20.4% vs. 30.4%; P=0.192). Sex and race are not associated with response (P=0.853 and 0.722, respectively). Increasing patient age is associated with positive response to immunotherapy [odds ratio (OR) 1.05; 95% confidence interval (CI), 1.01–1.09; P=0.019].
Only 9 patients in the cohort were never smokers while 39 were current smokers and 83 former smokers. 0 of the 9 never smokers responded to immunotherapy. In comparison, a current or former smoking status showed a trend with response to immunotherapy (0% vs. 23.8%; P=0.097). Univariate logistic regression demonstrates a positive association to immunotherapy response with smoke year increase (OR 1.03; 95% CI, 1–1.06; P=0.042). Quit-time (OR 1; 95% CI, 0.97–1.03; P=0.091) showed a trend with response by univariate logistic regression.
The majority of pathology was either adenocarcinoma or squamous cell carcinoma (123 of 131; 94%); neither of which is associated with response to immunotherapy. PD-L1 expression by univariate logistic regression (per percent increase) is not associated with response to immunotherapy (OR 1.01; 95% CI, 0.99–1.03; P=0.423).
Association of tumor mutation and response to immunotherapy
Of 131 patients, 83 had genomic data available. Gene mutations represented in less than 4 patients were eliminated yielding 29 gene mutations. Only those with a P≤0.2 are reported here. Via this analysis, 5 gene mutations were identified: EGFR, KRAS, MYC amplification, NF1, and RB1. MYC amplification and KRAS mutation are associated with a positive response to immunotherapy while EGFR, NF1, and RB1 mutations are associated with a lack of response as summarized in Figure 1. Examination of TMB by univariate logistic regression does not demonstrate an association with response (OR 0.99; 95% CI, 0.91–1.07; P=0.717).
Figure 1.
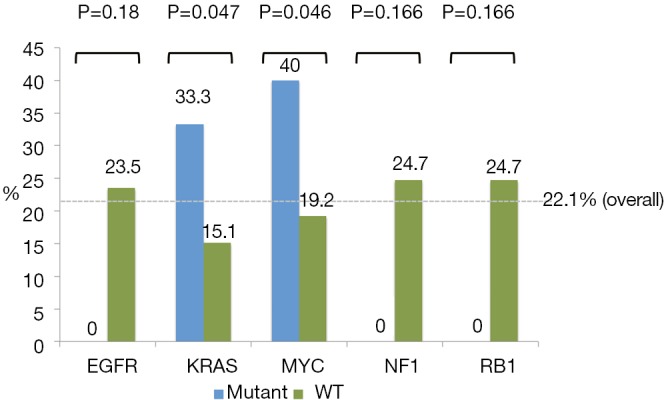
Response rates shown by gene mutation/wild-type (WT) with the corresponding P values.
Incorporation of clinical and genomic factors for predicting response
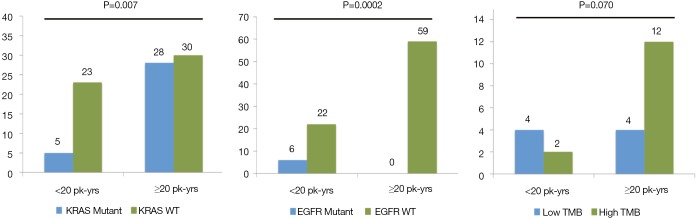
The relationship between smoking history and genomic factors was further examined by dividing smoking history into 2 groups, <20 vs. ≥20 pack-years. Those with KRAS mutations compared to those without a mutation were more likely to have a high smoking history (84.9% vs. 56.6%; P=0.007). Patients with EGFR mutations were more likely to have a low smoking history compared to those without a mutation (100% vs. 27.2%; P=0.002). Additionally those with a TMB ≥10 were more likely to have a high smoking history when comparing to those with a TMB <10 (85.7% vs. 50%; P=0.070) (Figure 2). Using logistical regression TMB did not increase with pack/year history of smoking (P=0.157) (Figure 3).
Figure 2.
KRAS mutant/WT, EGFR mutant/WT, and low/high TMB subdivided by smoking history demonstrating the relationship being smoking and the respective category.
Figure 3.
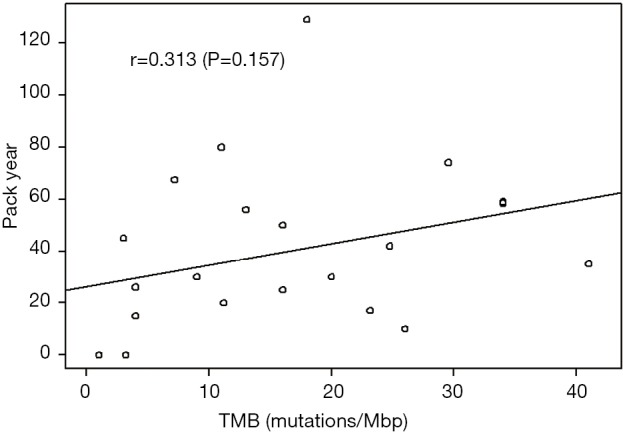
Plot tumor mutational burden (TMB) versus pack/year smoking rate.
Multivariable logistic regression using clinical, PD-L1 expression, and genomic data demonstrated that only SI and MYC amplification have an independent effect on response. It also demonstrates that MYC amplification continues to predict response to immunotherapy after controlling for the effects of age and smoking history (OR 3.85; 95% CI, 0.8–18.4; P=0.092). In this analysis, age, per year increase, is no longer significant at predicting response (OR 1.04; 95% CI, 0.98–1.1; P=0.17). Conversely, SI is predictive of response to immunotherapy per 1000 SI increase (OR 1.04; 95% CI, 1–1.08; P=0.048).
Discussion
This study demonstrates that increased smoking history and certain genomic characteristics are important factors in predicting response to ICIs in patients with advanced NSCLC, particularly when considered in combination.
Our single institution program captures a very detailed smoking history not available in the context of clinical trials and highlights the importance of this work. Clinical trial details include capturing never smokers/former smokers and current smokers but do not provide details on the amount and the duration the patients smoked. For example in a systematic review of the effects of tobacco smoking and PD-L1 inhibitors no mention is made of pack/years smoked (14). Both KRAS mutation and MYC amplification are associated with a response to immunotherapy in our study. In contrast to EGFR-positive tumors, KRAS mutation is strongly associated with a high smoking history in our cohort, which has also been demonstrated in a previous study (15). KRAS mutation did not predict response in multivariate analysis when accounting for smoking history and age. TMB is not a predictive biomarker in this study, likely due to lack of power, but more importantly high TMB is associated with a high smoking history and the use of a detailed smoking history may predict the TMB status of a patient with lung cancer. Examined together, these results suggest that smoking history is the most important factor predicting response to immunotherapy. As smoking history is the only independent factor (outside of MYC amplification as described below) to predict response, it is likely that TMB and PD-L1 changes are simply a reflection of patients smoking habits and provide no additional information beyond smoking details.
Unlike the other mutations, multivariate analysis shows that MYC amplification remains marginally significant in predicting response when controlling for patient age and smoking status with an OR of 3.85. Furthermore unlike KRAS mutation and TMB that follow the smoking pattern, MYC amplification does not correlate with smoking status and may explain why MYC amplification is an independent predictive marker of response. MYC is known to influence immune effector cells as well as regulatory cytokines, and its overexpression leads to tumor evasion of the immune response (16). This may explain why NSCLC with MYC amplification is particularly responsive to ICIs.
To our knowledge, this is the first study to show that Caucasian vs. African American race is not predictive of response to immunotherapy. Likewise, pathology of the tumor, predominantly adenocarcinoma and squamous carcinoma, is not associated with response to treatment.
It has been shown in subgroup analysis of previous studies and other investigations that EGFR-mutant tumors are less likely to respond to immunotherapy (1,13,17). This finding is supported in our study with 0 of 6 EGFR-mutants responding to immunotherapy. Similarly, 6 patients had RB1-mutant tumors and 6 had NF1-mutatnt tumors, of which 0 responded to immunotherapy for both groups. It has been shown that RB1 mutation correlates with a shorter disease-free survival in early stage adenocarcinoma (18). Our group also recently demonstrated that RB1 mutation is associated with worse prognosis in advanced NSCLC (19). To our knowledge, NF1 mutation has been suggested as a potential target in NSCLC, but has not been associated with prognosis (20). EGFR-positive tumors are associated with a negative or low smoking history in NSCLC, which could account for the lower response rates seen in these tumors. One prevailing theory is that these tumors represent a lower neoantigen and TMB, and thus do not respond well to immunotherapy (21). This hypothesis is supported by our study, as the EGFR-positive tumors were strongly associated with a lower smoking history. Although RB1 and NF1 mutations are not associated with smoking history, they may also confer decreased immunogenicity through a separate mechanism.
A limitation of this study is lack of power. The cohort examined in this study contains 131 patients, of which a subset lacked TMB and PD-L1 data. Another weakness in our trial is the risk of multiple testing and risk of false positive results. Nevertheless the strength and uniqueness of our dataset is the detailed smoking history not available in the setting of clinical trials.
This single-institution study demonstrates that smoking history is perhaps the most important factor at predicting response to ICIs in advanced NSCLC. The other commonly use predictive factors including TMB, PD-L1 expression and KRAS mutations are simply a reflection of smoking history. Obtaining a detailed smoking history is a much more cost effective strategy. Furthermore, MYC amplification is a potential predictive biomarker independent of smoking history.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by institutional review board of University Hospitals Cleveland Medical Center (Protocol DBR0014).
Data Sharing Statement: No additional data available.
Footnotes
Conflicts of Interest: A Dowlati—consulting or advisory role: Takeda, Abbvie, Seattle Genetics, Astra Zeneca, Bristol Myers Squibb; research funding: Loxo, Bayer, Incuron, Takeda, Regeneron, Tesaro, Amgen, Seattle Genetics, Symphogen, Abbvie, Ipsen. The other authors have no conflicts of interest to declare.
References
- 1.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Non-squamous Non-small Cell Lung Cancer. N Engl J Med 2015;373:1627-39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the Treatment of Non-Small-Cell Lung Cancer. N Engl J Med 2015;372:2018-28. 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 4.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. 10.1016/S0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- 5.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 6.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 7.Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2004-12. 10.1200/JCO.2014.58.3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carbone DP, Reck M, Paz-Ares L, et al. First-line Nivolumab in stage IV or recurrent non-small-cell lung Cancer. N Engl J Med 2017;376:2415-26. 10.1056/NEJMoa1613493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brahmer J, Rodríguez-Abreu D, Robinson A, et al. OA 17.06 Updated Analysis of KEYNOTE-024: Pembrolizumab vs Platinum-Based Chemotherapy for Advanced NSCLC With PD-L1 TPS ≥50%. J Thorac Oncol 2017;12:S1793-4. 10.1016/j.jtho.2017.09.431 [DOI] [Google Scholar]
- 10.McLaughlin J, Han G, Schalper KA, et al. Quantitative Assessment of the Heterogeneity of PD-L1 Expression in Non-Small-Cell Lung Cancer. JAMA Oncol 2016;2:46-54. 10.1001/jamaoncol.2015.3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rimm DL, Han G, Taube JM, et al. A Prospective, Multi-institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non-Small Cell Lung Cancer. JAMA Oncol 2017;3:1051-8. 10.1001/jamaoncol.2017.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizvi H, Sanchez-Vega F, La K, et al. Molecular Determinants of Response to Anti-Programmed Cell Death (PD)-1 and Anti-Programmed Death-Ligand 1 (PD-L1) Blockade in Patients With Non-Small-Cell Lung Cancer Profiled With Targeted Next-Generation Sequencing. J Clin Oncol 2018;36:633-41. 10.1200/JCO.2017.75.3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gainor JF, Shaw AT, Sequist LV, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer (NSCLC): A Retrospective Analysis. Clin Cancer Res 2016;22:4585. 10.1158/1078-0432.CCR-15-3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norum J, Niedler C. Tobacco smoking and cessation and PD-L1 inhibitors in non-small cell lung cancer (NSCLC): a review of the literature. ESMO Open 2018;3:e000406. 10.1136/esmoopen-2018-000406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng TL, Liu Y, Dimou A, et al. Predictive value of oncogenic driver subtype, programmed death-1 ligand (PD-L1) score, and smoking status on the efficacy of PD-1/PD-L1 inhibitors in patients with oncogene-driven non-small cell lung cancer. Cancer 2019;125:1038-49. 10.1002/cncr.31871 [DOI] [PubMed] [Google Scholar]
- 16.Casey SC, Baylot V, Felsher DW. The MYC oncogene is a global regulator of the immune response. Blood 2018;131:2007-15. 10.1182/blood-2017-11-742577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi S, Kim HR, Sung CO, et al. Genomic Alterations in the RB Pathway Indicate Prognostic Outcomes of Early-Stage Lung Adenocarcinoma. Clin Cancer Res 2015;21:2613-23. 10.1158/1078-0432.CCR-14-0519 [DOI] [PubMed] [Google Scholar]
- 19.Bhateja P, Chiu M, Wildey G, et al. Retinoblastoma mutation predicts poor outcomes in advanced non-small cell lung cancer. Cancer Med 2019;8:1459-66. 10.1002/cam4.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redig AJ, Capelletti M, Dahlberg SE, et al. Clinical and molecular characteristics of NF1 mutant lung cancer. Clin Cancer Res 2016;22:3148-56. 10.1158/1078-0432.CCR-15-2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]