Abstract
Obesity is accompanied by a systemic chronic low-grade inflammation as well as dysfunctions of several innate and adaptive immune cells. Recent findings emphasize an impaired functionality and phenotype of natural killer (NK) cells under obese conditions. This review provides a detailed overview on research related to overweight and obesity with a particular focus on NK cells. We discuss obesity-associated alterations in subsets, distribution, phenotype, cytotoxicity, cytokine secretion, and signaling cascades of NK cells investigated in vitro as well as in animal and human studies. In addition, we provide recent insights into the effects of physical activity and obesity-associated nutritional factors as well as the reduction of body weight and fat mass on NK cell functions of obese individuals. Finally, we highlight the impact of impaired NK cell physiology on obesity-associated diseases, focusing on the elevated susceptibility for viral infections and increased risk for cancer development and impaired treatment response.
Keywords: NK cells, obesity, adipokines, inflammation, cancer, prevention, immunosurveillance
Introduction
Overweight and obesity are defined as abnormal or excessive fat accumulation that is commonly classified by the body mass index (BMI), determined by dividing the body weight in kilograms by the square of the height in meters (kg/m2) of an individual (1). Over the past decades, the global prevalence of overweight and obesity in children and adolescents has increased dramatically in industrialized as well as in developing countries. In 2016 the World Health Organization (WHO) classified 124 million children (7%) and 650 million adults (13%) as obese worldwide (1). Overweight and obesity are commonly caused by an excess energy consumption and a decreased physical activity, but also by sleep restriction and a greater uptake of endocrine disruptors as well as social, genetic, and epigenetic factors contribute to the increasing prevalence of obesity (2–5). The escalating global epidemic of overweight and obesity is a major public health and economic problem, as excess body weight is associated with almost 4 million deaths and 120 million disability-adjusted life-years (6). In detail, overweight and obesity are significant risk factors for a number of chronic disorders, including cardiovascular diseases and type 2 diabetes mellitus as well as renal, musculoskeletal, and psychological disorders (7–10). Additionally, the susceptibility to infections and the incidence for several cancer types, like esophageal, renal, endometrial, liver, pancreatic, prostate, postmenopausal breast, and colorectal cancer, are increased in obesity (11–14). Until now, the pathophysiological processes for the increased cancer risk in obesity remain unresolved. Besides altered adipokine levels, increased secretion of growth factors and steroid hormones, oxidative stress, an altered microbiome, and low-grade inflammation have been discussed to influence cancer development in obese individuals (15–17). The excessive fat accumulation promotes a state of a systemic chronic low-grade inflammation, a condition characterized by an increased secretion of pro-inflammatory factors caused by the augmented number of adipocytes and immune cells in adipose tissue (18). Furthermore, obesity is associated with alterations in the functionality of several immune cells, like macrophages, B and T lymphocytes, mast cells, and natural killer (NK) cells (19–22).
NK Cells on the Edge of Innate and Adaptive Immunity
NK cells are large granular lymphocytes that mediate rapid innate immunity against viruses, bacteria, parasites, and tumor cells without prior sensitization. NK cells primarily develop and mature in the bone marrow, migrate through the bloodstream, and seed into secondary lymphoid organs, like thymus, spleen, and lymph nodes as well as in other peripheral tissues, like lung, liver, kidney, uterus, and the gastrointestinal tract (23). In blood, NK cells represent 1–6% of all leukocytes and comprise up to 15% of human peripheral blood mononuclear cells (PBMCs). Human NK cells have been defined by the expression of adhesion molecule CD56 and by the absence of the T cell marker CD3. Based on their expression level of CD56 and the Fcγ receptor CD16, NK cells are subdivided into different subpopulations. The two major and classically defined subsets are the CD56dimCD16bright NK cells and the CD56brightCD16dim/neg NK cells. CD56dimCD16bright NK cells represent about 90% of all NK cells and are predominantly found in peripheral blood (24). They are mostly responsible for cytotoxic activity and target cell killing but are also a source of pro-inflammatory cytokines and chemokines (25, 26). CD56brightCD16dim/neg NK cells represent up to 10% of peripheral blood NK cells, are most frequently found in lymph nodes, and can be subdivided according to their CD16 expression into CD56brightCD16dim NK cells and CD56brightCD16neg NK cells (27–29). They mainly play an immunoregulatory role by abundantly producing cytokines, but can also become cytotoxic after activation, for example, by interleukins (24, 28, 30–33). Two developmental models of the two major NK cell subsets CD56dim and CD56bright NK cells are discussed. On the one hand, it has been proposed that CD56bright CD16dim/neg NK cells are precursors for CD56dimCD16bright NK cells but can also result from activated CD56dimCD16bright, which upregulated CD56 and lost CD16 (34). On the other hand, it is discussed that both NK cell subsets have their origin in different hematopoietic lineages (28, 35).
In mice, commonly used surface markers to identify NK cells are NKp46, CD49b (DX5), and CD161 (NK1.1) gated on CD3-negative cells. In some mouse strains, such as BALB/c, NK cells are identified only by CD49b and NKp46, as these strains have allelic variants of the NK1.1 and do not react with the anti-NK1.1 antibody PK136 (36–39). In contrast to human NK cells, the surface density of CD27 and CD11b can be used to subdivide murine NK cells into four subsets that define different levels of maturation: CD11blowCD27low, CD11blowCD27high, CD11bhighCD27high, and CD11bhighCD27low (40, 41). These four different subsets correspond to the stages of their maturation and were described to be associated with a progressive acquisition of NK cell functionality. Thus, CD11bhighCD27high NK cells exhibit the highest levels of both cytokine production and cytotoxicity (40, 42). Moreover, studies of Crinier et al. indicated a correspondence between the murine CD11b+CD27− subset with the human CD56dim subset and the murine CD11b−CD27+ subset with the human CD56bright subset of NK cells (43).
In rats, two major NK cell subsets have been described, based on the expression of the Ly49s3 and NKR-P1B cell receptor (44, 45). Both NK cell subsets are potent in cytotoxicity and cytokine secretion, but show differences in expression of other surface NK cell receptors and proliferative capacity (46).
Recently, NK cells have been classified as one group of innate lymphoid cells (ILCs) developing from a common innate lymphoid progenitor on the bases of their transcription factor profile (47). Conventional NK cells circulate in the blood stream; however, some NK cell subsets are also known to be tissue-resident and have been identified in various tissues like uterus, skin, kidney, salivary glands, and adipose tissue (48, 49). Tissue-resident NK cells differ in the equipment of phenotyping markers, transcription factors, and cytokine secretion and may contribute therefore locally to the immunosurveillance of cancer (48–50).
NK cells mediate their cytotoxicity against target cells via two mechanisms: secretion of cytotoxic molecules or by death receptor-mediated apoptosis. NK cells can directly kill target cells by secreting a large number of cytolytic granules containing granzymes and perforins in a rapid and efficient manner. In addition, NK cell cytotoxicity can be mediated via activation of death cell receptors on target cells via binding of death ligands, e.g., FasL (Fas ligand) and TRAIL (tumor necrosis factor-related apoptosis-inducing ligand), expressed on NK cell surface, resulting in classical caspase-dependent apoptosis. Interestingly, these killing pathways are not mutually exclusive, but rather, NK cells are able to switch their strategies over the course of an immune response, and the same NK cell is able to perform serial killing (51, 52). Furthermore, NK cells are able to induce cell lysis via antibody-dependent, cell-mediated cytotoxicity, in which FcγIII receptors on NK cells recognize the Fc portion of the IgG antibody on target cells (53). Besides their cytolytic function, activated NK cells are able to produce a number of cytokines and chemokines, like TNF (tumor necrosis factor)-α, IFN (interferon)-γ, or GM-CSF (granulocyte macrophage colony-stimulating factor), in order to co-stimulate other cells of the immune system (54, 55).
The activity of NK cells is regulated by the balanced expression of activating and inhibitory surface receptors triggering a cascade of events inside the cells. In humans, the NK cell receptor repertoire essentially includes three major receptor families: human killer immunoglobulin-like receptors (KIRs) as well as natural cytotoxicity receptors (NCRs) and C-type lectin-like receptors. Most relevant activating NK cell receptors include the NCRs NKp30, NKp44 and NKp46, and the natural killer group (NKG) 2D receptor as well as DNAX accessory molecule-1 (DNAM-1) and the short-tail members of KIRs (56–58). Inhibitory NK receptors comprise the long-tail members of the KIR family, NKG2A and the killer cell lectin-like receptor subfamily G (KLRG) 1 (58, 59). Although several NK cell receptors, like NKp46 and NKG2D, are expressed in both species, the NK cell receptor repertoire in mice differs from humans. In particular, the counterpart for human KIRs is represented by the lectin-like Ly49 receptor family in mice (58). In addition, mice lack the expression of NKp44 and NKp30; however, NKp30 is an unexpressed pseudogene in mice (60, 61). Although some ligands for activating and inhibitory receptors are known today, the family of ligands is not yet fully elucidated (62).
The Influence of Adipokines on NK Cell Functionality in vitro and in vivo
Besides the storage of energy, adipose tissue has been identified as a highly active endocrine organ secreting numerous adipokines, like leptin, adiponectin, resistin, estrogens, and interleukin (IL)-6. These adipokines do not only function locally in adipose tissue, but have very distant effects on other organs, like liver, muscle, and brain, and therefore play an important role in the regulation of energy metabolism, influence reproduction, or cardiovascular function (63). The dysregulated secretion of adipokines by an excessive growth of adipose tissue in obesity has been shown to contribute to the pathogenesis of obesity-related diseases, like type 2 diabetes, cardiovascular events, and non-alcoholic fatty liver disease (64). Adipokines also affect the functionality of several immune cells. Besides T and B lymphocytes, macrophages, mast cells, and eosinophils, NK cell physiology is influenced by hormones and cytokines secreted by adipose tissue (65).
Studies on Leptin
Leptin is the best-characterized adipokine whose circulating plasma concentration correlates with the amount of adipose tissue and therefore is increased in obesity (66). Investigations demonstrated that the leptin receptor (obesity receptor, ObR) is expressed on splenic and peripheral blood NK cells of rats as well as on human hepatic and peripheral blood NK cells (67–76) (Table 1). Although leptin receptor expression levels on NK cells are rather dim, functional data upon leptin treatment performed by different laboratories demonstrate a functional role for this receptor on NK cells (69–73, 77).
Table 1.
Expression of adipokine receptors on human and rodent NK cells.
| Adipokine | Receptor and alternative names | Species | Detection of receptor expression in cell lines/ tissues | Percentage of surface receptor expression level [% of NK cells] | Obesity-related changes in receptor expression level | References |
|---|---|---|---|---|---|---|
| Leptin | Leptin receptor (LEP-R) Obesity receptor (ObR) CD295 |
Human | NK-92 cells | Ø | Ø | (67, 68) |
| YT cells | Ø | Ø | (68) | |||
| Peripheral blood NK cells | Very dim–dim (0–9%) | ow/ob = nw | (69–73, 77) | |||
| Hepatic NK cells | Ø | ob = nw | (74) | |||
| Rodent (rat) | Peripheral blood NK cells | Ø | ob > nw | (75) | ||
| Splenic NK cells | Ø | ob = nw | (75, 76) | |||
| Adiponectin | Adiponectin receptor 1/2 (AdipoR1/2) Progestin and adipoQ receptor 1/2 (PAQR1/2) |
Human | NKL/NK-92/KHYG cells | Ø | Ø | (78) |
| Peripheral blood NK cells | Intermediate–bright (20–90%) | ob = nw (AdipoR1); ob < nw (AdipoR2) |
(70, 79, 80) | |||
| Rodent (murine) | Splenic NK cells | Intermediate (15–17%) | Ø | (79, 81) | ||
| Interleukin-6 | IL-6 receptor (IL-6R, IL-6Ra) CD126 |
Human | NK-92 cells | Ø | Ø | (82) |
| Peripheral blood NK cells | Dim (5%) | Ø | (83) | |||
| Splenic NK cells | Dim (6%) | Ø | (83) | |||
| Tonsil NK cells | Intermediate (15%) | Ø | (83) | |||
| Rodent (murine) | Peripheral blood NK cells | Intermediate (8–25%) | ob > nw | (84) | ||
| Hepatic NK cells | Dim (8–12%) | ob = nw | (84) | |||
| Perigonadal adipose tissue | Intermediate (5–15%) | ob > nw | (84) | |||
| Splenic NK cells | Intermediate (15–40%) | Ø | (85, 86) | |||
| Estrogens | Estrogen receptor α/β (ESR1/2) |
Human | Peripheral blood NK cells | Ø | Ø | (87) |
| Rodent (murine) |
Splenic NK cells | Bright | Ø | (88) | ||
| Uterine natural killer cells | Bright | Ø | (89) |
AdipoR, adiponectin receptor; CD, cluster of differentiation; ESR, estrogen receptor; IL-6R, interleukin-6 receptor; NK cell, natural killer cell; nw, normal weight; ob, obese; ObR, obesity receptor (leptin receptor); ow, overweight; PAQR, progestin and adipoQ receptor; Ø, no data. Alternative receptor names are presented in italics.
Leptin was shown to influence proliferation as well as migration of NK cells (68, 90, 91). Conflicting data exist about the impact of leptin on NK cell cytotoxicity and cytokine secretion. Several studies demonstrated that leptin increases the cytolytic activity of NK cells (67–69, 90, 92). All these studies have been performed using the human myelogenous leukemia line K652 or the murine lymphoma cell line YAC-1 as target cells for cytotoxicity assays. Interestingly, data using other target cells, like colon or breast cancer cells, revealed evidence for a decreased cytotoxicity of NK cells upon leptin treatment (67, 93). Therefore, the influence of leptin on NK cell cytolysis may depend on the tumor target cells, most likely based on their differential NK cell ligand profile. In addition to effects on NK cell cytotoxicity, leptin administration leads to conflicting results in terms of expression and secretion of cytokines, surface receptors, and enzymes as well as the cell metabolism of NK cells. On the one hand, some studies demonstrated a stimulating leptin effect on the metabolic activity, mRNA expression or release of perforin, granzyme A, IFN-γ, TRAIL, FasL, and leptin receptors of NK cells (67–69, 94). On the other hand, leptin had been shown to decrease IFN-γ secretion, mRNA expression of the activating NK cell receptors NKG2D and NKp46, and the activation marker CD69 as well as the FasL and TRAIL mRNA expression (67, 69, 93). Other studies did not find leptin-related effects on granzyme and perforin secretion nor degranulation marker CD107a, IFN-γ, or FasL mRNA expression (68, 69). These contradictions may have resulted from the usage of different leptin concentrations, incubation times, measurement of mRNA expression, or secreted proteins as well as the use of different NK cell lines or primary NK cells. Interestingly, our own studies demonstrated a leptin-induced reduction of IFN-γ secretion in primary human NK cells but not in NK-92 cells. In addition, the data point toward a decreased basal lytic activity against target cells in NK-92 cells compared to primary NK cells (93). Although NK cell lines, like NK-92, are well-established human models for investigations on NK cell functionality and exhibit phenotypical and functional characteristics of primary NK cells, these data underline the limited transferability of findings obtained using immortalized cell lines to conclude the mode of action of primary NK cells (93, 95, 96). Furthermore, results showed that the leptin effects on NK cells are more pronounced using higher leptin concentrations for incubation experiments (67, 93). These findings indicate that leptin negatively affects NK cell activity primarily in pathologically elevated leptin concentrations as determined in obese individuals.
Besides the described in vitro investigations of leptin on NK cells, the impact of this adipokine has also been examined in and ex vivo. The administration of leptin in wild-type rats and mice led to an increased number of NKs in blood, spleen, and liver (75, 90, 97, 98). Interestingly, elevated NK cell numbers as well as a higher NK cell killing activity after administration of pathophysiologically high leptin concentrations was solely observed in normal-weight animals, but not in obese animals (75). In addition, investigations on human primary NK cells demonstrated that treatment with high leptin concentrations increased the IFN-γ production in NK cells isolated from normal-weight individuals, but not from obese individuals (72). In contrast, leptin in physiological concentrations decreased the cytotoxicity of NK cells isolated from normal-weight but not from obese human subjects (99). These data suggest that NK cells of obese individuals are resistant to regulatory effects of leptin. Leptin mediates its cellular effects primarily via the leptin receptor-linked activation of JAKs (Janus kinases) and STATs (signal transducers and activators of transcription), which was also confirmed for NK cells (68, 100). Studies on rat and human PBMCs reported an altered post-receptor signaling in obesity, as the leptin-induced activation of JAK/STAT signaling components were diminished in obese individuals compared to normal weight subjects (69, 75). A central leptin resistance in obesity is widely accepted; therefore, a peripheral leptin resistance with an abrogated JAK/STAT signaling pathway is likely and would explain the reduced biological activity after leptin challenge in NK cells of obese individuals (69, 72, 75, 101). Furthermore, an animal study in leptin receptor deficient db/db mice demonstrated that the number of NK cells was decreased in blood, spleen, liver, and lung (92). In addition, the cytotoxicity as well as the expression of the activation marker CD69 of NK cells isolated from mice lacking the leptin receptor was declined compared to NK cells isolated from wild-type mice (92). These findings suggest that leptin is involved in NK cell development, activation, and function (92).
To conclude, the reported effects for leptin on NK cells are varying, as also summarized in Table 2. Therefore, several technical or biological requirements like a pure leptin preparation, an elimination of indirect effects, and the exclusion of a second receptor need to be fulfilled in order to interpret the data correctly.
Table 2.
Effects of adipokine treatment on human and rodent natural killer cells.
| NK cell parameter | Leptin | Adiponectin | Interleukin-6 | Estrogen | ||||
|---|---|---|---|---|---|---|---|---|
| Human | Rodent | Human | Rodent | Human | Rodent | Human | Rodent | |
| Number | ↔ | ↑↑ (rat) ↑↑ (murine) |
Ø | ↓↓ (murine) | Ø | Ø | Ø | ↑ (murine) |
| Cytotoxicity | ↓↓↓/↑↑↑ | ↑ (rat) ↑↑↑ (murine) |
↑/↔ | ↓↓↓ (murine) | ↓↓↓↓↓/↑↑↑ | ↓ (murine) | ↓ | ↓↓↓↓↓↓↓↓ (murine) |
| Expression of activating receptors | ↓↓/↑↑/↔ | ↑ (murine) | Ø | ↓/↑ (murine) | ↑/↔ | Ø | Ø | ↓↑ (murine) |
| Expression of inhibiting receptors | Ø | Ø | Ø | Ø | ↔ | Ø | Ø | ↑ (murine) |
| Migration | Ø | Ø | Ø | Ø | Ø | ↓ (murine) | ↓ | ↓ (murine) |
| Proliferation | ↓/↑/↔↔↔ | ↑ (murine) | Ø | Ø | Ø | Ø | Ø | ↓ (murine) |
| Expression of granule components | ||||||||
| Perforin | ↓/↑↑/↔↔ | Ø | Ø | Ø | ↓↓ | Ø | Ø | Ø |
| Granzymes | ↑/↔↔ | Ø | Ø | Ø | ↓↓ | Ø | Ø | ↓ (murine) |
| Cytokine secretion | ||||||||
| IFN-γ | ↓↓/↑↑/↔ | Ø | ↓ | ↓ (murine) | ↓/↑ | Ø | Ø | ↓/↑ (murine) |
| TNF-α | Ø | Ø | Ø | Ø | Ø | Ø | Ø | ↔ (murine) |
| IL-17 | Ø | Ø | Ø | Ø | Ø | ↑ (murine) | Ø | Ø |
| Maturation | Ø | Ø | Ø | ↑ (murine) | Ø | Ø | Ø | Ø |
| Metabolic activity | ↑ | Ø | Ø | Ø | Ø | Ø | ↓ | Ø |
IFN-γ, interferon γ; NK cell, natural killer cell; TNF-α, tumor necrosis factor α; ↔, no change; ↑, increase; ↓, decrease; Ø, no data. Numbers of the arrow show the number of references.
Insights to Adiponectin
Aside from leptin, adiponectin is also a well-characterized adipokine. In contrast to leptin, plasma levels of adiponectin are reduced in obese compared to normal-weight individuals (102). Only a few studies exist investigating the influence of adiponectin on NK cell physiology. Data revealed evidence for the expression of the adiponectin receptors 1 and 2 (AdipoR1 and 2) on murine and human NK cells with expression levels of 15–90%, suggesting physiological effects of this adipokine on these immune cells (70, 78–81) (Table 1). The predominant expression of adiponectin receptors on the CD56dimCD16bright NK cells and lower expression rate in CD56brightCD16dim/neg NK cells indicate that adiponectin primarily influences the cytotoxic NK cell subset (79). Several results were found concerning the impact of adiponectin on NK cell function. Adiponectin incubation of IL-2-stimulated murine NK cells isolated from splenic tissue resulted in a reduced cytotoxicity, IFN-γ secretion, and expression of FasL and TRAIL (81, 103). Furthermore, adiponectin knockout mice exhibit a decreased CD107a and NKG2D receptor expression as well as a reduced frequency of CD11bhighCD27high NK cell subset—the most potent NK cell subset in mice—compared to wild-type mice (79). Accordingly, results of studies on human NK cells demonstrated that adiponectin was shown to increase CD107a expression and cytotoxicity of NK cells isolated from normal-weight humans (99), indicating a benefit of this adipokine on NK cell function. In line with the findings on leptin, adiponectin failed to enhance NK cell activity in obese humans (99). Moreover, studies on obese individuals showed that the expression of the adiponectin receptor 2 is reduced and that low plasma adiponectin concentrations correlated with a diminished cytolytic activity of NK cells from obese individuals (70, 81).
Taken together, data on adiponectin effects on NK cells are conflicting. Therefore, future studies on normal-weight compared to obese individuals could help to clarify these discrepancies.
Effects of Interleukin-6
Interleukin-6 plasma concentrations are typically increased in obese individuals (104). This cytokine is one of the major inflammatory mediators in obesity. In addition to effects on macrophage polarization, IL-6 was demonstrated to influence NK cells directly (105). IL-6 receptor (IL-6R) expression was detected in murine blood, hepatic, adipose tissue, and splenic NK cells as well as in the human NK-92 cell line and human blood, splenic, and tonsil NK cells (82–86) (Table 1). Interestingly, the expression of the IL-6 receptor was increased in obese mice compared to normal-weight mice in blood and hepatic NK cells (84). In addition, a distinct IL-6 receptor-positive NK cell population was described to be a specific mediator of metaflammation (metabolic inflammatory state) and insulin resistance in obesity. In detail, an IL-6/Stat3-dependent formation on these NK cells seems to account to obesity-associated pathologies (84). Several investigations on NK cell physiology demonstrated a decreased cytotoxicity and IFN-γ secretion as well as perforin and granzyme expression of human NK cells induced by IL-6 treatment (106–110). However, conflicting results exist with an IL-6-mediated increase of cytotoxicity, cytokine secretion, and expression of activating receptors of NK cells in human and mice (85, 106, 111–114). In addition, expression levels of inhibiting or activating NK cell receptors were observed to be unchanged by IL-6 treatment (106). Of note, physical activity suppresses subcutaneous B16 melanoma tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution in mice, suggesting a rather activating effect of IL-6 on NK cells (86). Future studies are necessary to evaluate possible dose-dependent differences as well as effects of IL-6 administration in vivo in lean and obese subjects to specify the meaning of an obesity-induced increase of IL-6 concentration on NK cell physiology.
Studies on Estrogens
In addition to other sex steroid hormones, high BMI is strongly associated with increased estrogen levels, especially in postmenopausal women (115). This is at least in part caused by the obesity-associated adipose tissue inflammation that leads to a stimulation of aromatase activity—the key enzyme of estrogen biosynthesis.
Estrogen receptors (ESR) have been detected in murine uterine and splenic NK cells as well as in human peripheral blood NK cells (87–89) (Table 1). Strikingly and in contrast to other adipokines, research outcomes on murine and human NK cells all point to an inhibiting effect of estrogen on NK cell functionality. Thus, estrogens reduced the cytotoxicity, expression of activating receptors, migration, proliferation, metabolic activity, granzyme expression, and IFN-γ secretion of NK cells, whereas estrogens increased the expression of the inhibiting receptor CD94 (88, 116–124). Only one publication demonstrated an increase of IFN-γ protein levels on NK cells after estrogen treatment, whereby in the same study, gene expression of IFN-γ was reduced by estrogens (117). As most of the investigations have been performed on mice, future studies on estrogen receptor expression as well as estrogen effects on human NK cells with respect to obesity are desirable. Estrogens are widely used for hormone replacement or fertility therapy. As elevated circulating estrogen levels are highly associated with increased risk of breast cancer in obese postmenopausal women, more detailed studies are urgently needed to elucidate the role of estrogens on NK cells. Furthermore, cancer-protecting effects of the hormone would be of high clinical interest (125).
Insights about expression of adipokine receptors on NK cells as well as obesity-associated changes in receptor expression levels are presented in Table 1. Data about the influence of leptin, adiponectin, IL-6, and estrogen on number, functionality, receptor expression, and proliferation of NK cells are summarized in Table 2.
Besides investigations about the effect of single adipokines, some studies investigated the effect of an incubation of NK cells with an adipocyte-conditioned media (ACM) to simulate a physiologically mixture of components secreted by adipocytes. Results demonstrated a reduced cytotoxicity of ACM-treated human NK cells against prostate cancer cells. This effect has been shown to be primarily mediated via the IL-6 and leptin content in the ACM (126). Furthermore, ACM-treatment of NK cells resulted in a decrease of granzyme- and TRAIL-positive NK cells, but an increase of IFN-γ production, although the effects of ACM seem to be species-specific and depending on the different phases of adipogenesis the ACM was harvested (69, 77).
In summary, although many conflicting data were gained in the past, obesity-related changes of adipokine concentrations leading to an altered NK cell physiology are still obvious (Tables 1, 2). For certain, more data are needed to strengthen particular findings and studies focusing on effects of other specific adipokines. Experiments using a mixture of obesity-related metabolites on NK cells, more resembling the in vivo situation, would help to elucidate their role on NK cell activity.
THE Impact of Systemic Obesity on NK Cells in Animal Studies
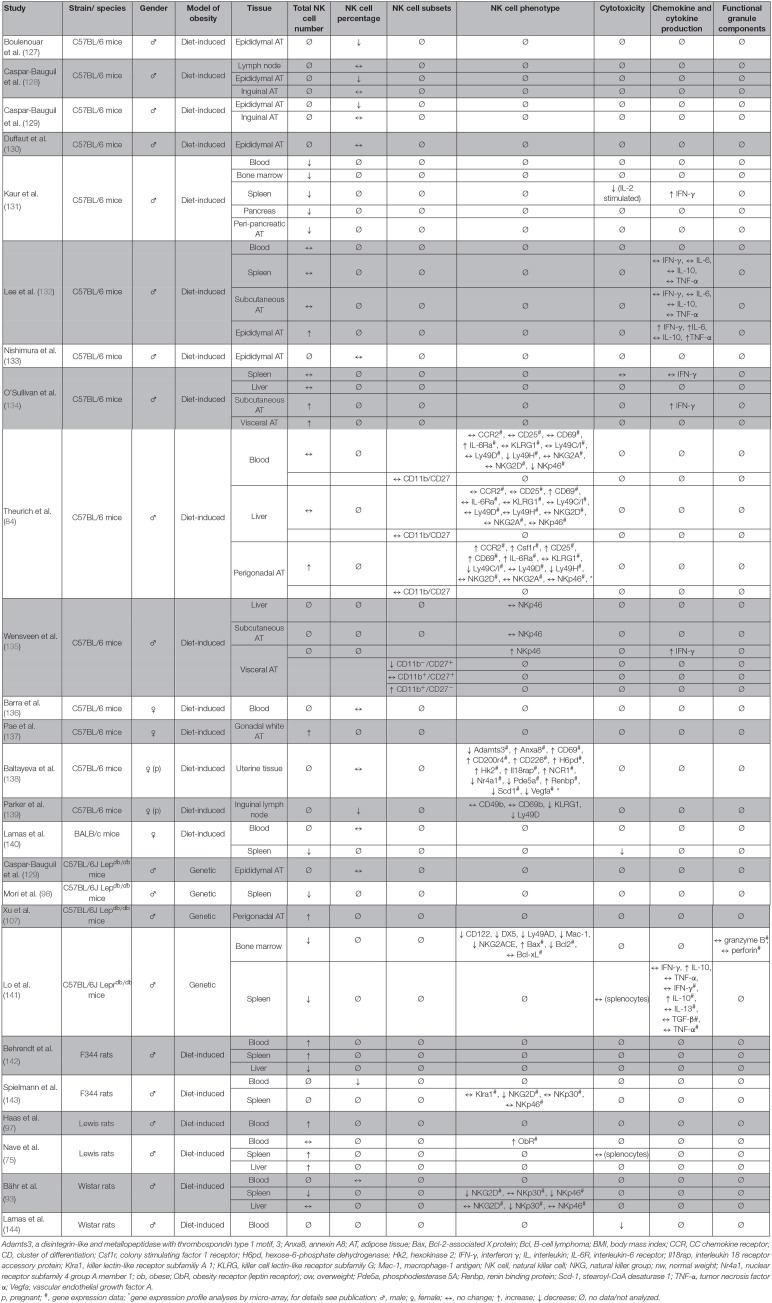
Several studies previously investigated the impact of obesity on NK cells (Table 3). Data exist about differences in NK cell numbers in peripheral blood and organs, although the results are conflicting. Numerous studies demonstrated a decrease of the NK cell amount in blood as well as in spleen, lung, and liver of obese rodents (93, 98, 131, 140–143). In contrast, other reports exist showing either no changes or an increase of the NK cell number in blood and different tissues of obese individuals (75, 93, 97, 131, 132, 136, 138, 142). These discrepancies may be explained by species- or strain-dependent metabolic characteristics or by differences in developmental, degradation, or migration status of NK cells. In addition, compartment-specific NK cell distribution had been described, with an increased NK cell number in blood and spleen, but a decreased amount in the liver tissue of obese rats compared to their lean littermates (142). Moreover, heterogeneous methods of measurement may have led to different results. The amount of NK cells in blood or tissue is usually specified as “total NK cell number” or “NK cell frequency as percentage of leukocytes or PBMCs.” As obesity is also known to be associated with alterations in the number of other blood cell types, obesity-related changes in NK cell frequency may additionally be a result of a shift of numbers of other immune cell types (145). Therefore, the indication of absolute NK cell numbers would help to make data obtained in different studies and under different conditions comparable. To our knowledge, only two studies regarding the proportion of CD11b/CD27 NK cell subsets in obese rodents exist until now. One study indicated no differences in blood, liver, and perigonadal adipose tissue in obese mice compared to lean animals (84). However, another showed an increase of the CD11b+/CD27− subpopulation and a decrease of the CD11b−/CD27+ subpopulation in visceral adipose tissue (135) (Table 3).
Table 3.
Overview of animal studies investigating the effect of obesity on number, subsets, phenotype, and functional parameters of NK cells in different tissues.
Adamts3, a disintegrin-like and metallopeptidase with thrombospondin type 1 motif, 3; Anxa8, annexin A8; AT, adipose tissue; Bax, Bcl-2-associated X protein; Bcl, B-cell lymphoma; BMI, body mass index; CCR, CC chemokine receptor; CD, cluster of differentiation; Csf1r, colony stimulating factor 1 receptor; H6pd, hexose-6-phosphate dehydrogenase; Hk2, hexokinase 2; IFN-γ, interferon γ; IL, interleukin; IL-6R, interleukin-6 receptor; Il18rap, interleukin 18 receptor accessory protein; Klra1, killer lectin-like receptor subfamiliy A 1; KLRG, killer cell lectin-like receptor subfamily G; Mac-1, macrophage-1 antigen; NK cell, natural killer cell; NKG, natural killer group; nw, normal weight; Nr4a1, nuclear receptor subfamily 4 group A member 1; ob, obese; ObR, obesity receptor (leptin receptor); ow, overweight; Pde5a, phosphodiesterase 5A; Renbp, renin binding protein; Scd-1, stearoyl-CoA desaturase 1; TNF-α, tumor necrosis factor α; Vegfa, vascular endothelial growth factor A.
p, pregnant;
#, gene expression data;
* gene expression profile analyses by micro-array, for details see publication; ♂, male; ♀, female; ↔, no change; ↑, increase; ↓ decrease; Ø, no data/not analyzed.
However, in addition to changes in NK cell number, the expression of the activating NK cell receptors NKp46 and NKG2D in splenic tissue and NKp30 in liver tissue was reduced in diet-induced obese rats, indicating an inhibited activation status of NK cells in obesity (93, 143). Moreover, NK cells of obese rodents exhibit a reduced cytotoxicity against tumor cells compared to NK cells isolated from normal weight animals (13, 131, 140, 144). Interestingly, a study using adoptive transfer of NK cells demonstrated that NK cell functionality is dependent on the surrounding metabolic environment as the impaired phenotype of NK cells from obese rats could be ameliorated by transfer of NK cells into normal weight rats. These data implicated for the first time that the altered NK cell phenotype in obesity can be improved by generating the physiological metabolic environment of normal weight individuals (76).
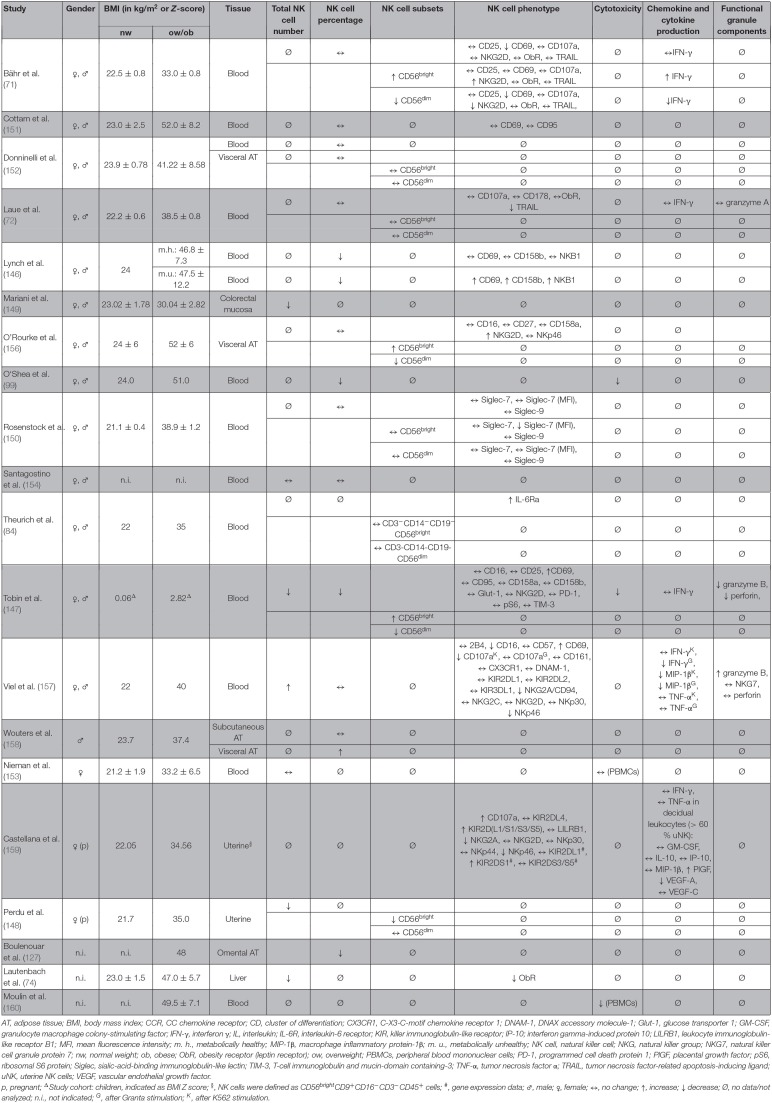
Effects of Systemic Obesity on Human NK Cells
In accordance to the results in mice and rats, obesity was shown to be associated with a decreased NK cell number in blood, liver, uterine, or colon tissue in humans (74, 99, 146–149), whereas several other studies did not observe any differences in the NK cell number comparing normal-weight and obese humans (71, 72, 84, 150–155) (Table 4). These discrepancies may be caused by the use of different markers and methods to identify NK cells and subsets in blood and different tissues and individual parameters in the study population, like gender, BMI, ethnicity, and nutritional or metabolic variances. Regarding functional parameters, obesity is associated with various alterations in NK cell phenotype. Data demonstrated that the expression of the functional marker TRAIL and the activating NK cell receptor NKp46 is reduced in obese individuals (72, 157). In contrast, studies reported a highly activated status of NK cells from obese humans with an increase of CD69, NKp46, and programmed cell death protein (PD)-1 expression as well as a decrease of the inhibiting NKG2A/CD94 complex and CD16 expression, which is known to be downregulated in activated NK cells (147, 157, 161). Despite the increased activation status, NK cells showed a decreased secretion of the cytokines granzyme B and perforin as well as the macrophage inflammatory protein (MIP)-1β after challenge with blood cancer cell lines in obese humans (147, 157). Furthermore, the degranulation capacity as well as the cytotoxicity against malignant cells is significantly impaired in obesity (99, 147, 157, 160). It has been discussed that the highly activated NK cells of obese individuals become exhausted much faster when exposed to target cells compared to NK cells of normal weight subjects, leading to an impaired capability to defend tumor or virus-infected cells (147, 157).
Table 4.
Overview of human studies investigating the effect of obesity on number, subsets, phenotype, and functional parameters of NK cells in different tissues.
AT, adipose tissue; BMI, body mass index; CCR, CC chemokine receptor; CD, cluster of differentiation; CX3CR1, C-X3-C-motif chemokine receptor 1; DNAM-1, DNAX accessory molecule-1; Glut-1, glucose transporter 1; GM-CSF, granulocyte macrophage colony-stimulating factor; IFN-γ, interferon γ; IL, interleukin; IL-6R, interleukin-6 receptor; KIR, killer immunoglobulin-like receptor; IP-10; interferon gamma-induced protein 10; LILRB1, leukocyte immunoglobulin-like receptor B1; MFI, mean fluorescence intensity; m. h., metabolically healthy; MIP-1β, macrophage inflammatory protein-1β; m. u., metabolically unhealthy; NK cell, natural killer cell; NKG, natural killer group; NKG7, natural killer cell granule protein 7; nw, normal weight; ob, obese; ObR, obesity receptor (leptin receptor); ow, overweight; PBMCs, peripheral blood mononuclear cells; PD-1, programmed cell death protein 1; PlGF, placental growth factor; pS6, ribosomal S6 protein; Siglec, sialic-acid-binding immunoglobulin-like lectin; TIM-3, T-cell immunoglobulin and mucin-domain containing-3; TNF-α, tumor necrosis factor α; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; uNK, uterine NK cells; VEGF, vascular endothelial growth factor.
p, pregnant;
ΔStudy cohort: children, indicated as BMI Z score; §, NK cells were defined as CD56brightCD9+CD16−CD3−CD45+ cells; #, gene expression data; ♂, male; ♀, female; ↔, no change; ↑, increase; ↓ decrease; Ø, no data/not analyzed; n.i., not indicated; G, after Granta stimulation; K, after K562 stimulation.
Aside from the characterization of total NK cells, recent studies investigated the impact of obesity on the CD56dimCD16bright and the CD56brightCD16dim/neg NK cell subsets. Whereas, several studies did not find any differences on the CD56dim/CD56bright ratio comparing NK cells isolated from normal-weight and obese humans (72, 150, 152, 156), results of two studies revealed evidence for a decrease of CD56dimCD16bright NK cells and an increase of CD56brightCD16dim/neg NK cells in obesity (71, 147). As previous studies observed that the CD56dimCD16bright NK cell subset can convert into the CD56brightCD16dim subset under the influence of specific cytokines and hormones, it had been assumed that obesity-related metabolites induce a conversion of CD56dimCD16bright in CD56brightCD16dim/neg NK cells in obese individuals (71, 162–164). Analyses of the subset-specific NK cell phenotype also demonstrated a decrease in NKG2D-positive and CD69-positive CD56dimCD16bright NK cells (71). In addition, data on NK cell cytokine production demonstrated a decreased expression of IFN-γ-positive CD56dimCD16bright NK cells in obese subjects, indicating an downregulation of the immunoregulatory effect of this NK cell subset in obesity (71). The lower CD56dimCD16bright /CD56bright CD16dim/neg ratio as well as the reduced expression the activation marker CD69 and the activating receptor in the cytotoxic CD56dimCD16bright NK cell subset under obese conditions may be one cause for the impaired cytotoxicity of NK cells against virus-infected and tumor cells and therefore contributes to the higher cancer risk and susceptibility for infections in obese subjects.
Investigations on children have revealed an impaired NK cell physiology even in childhood obesity. Obese children had a reduced NK cell frequency, which negatively correlated with BMI and insulin resistance. In addition, NK cells of obese children were highly activated and metabolically stressed but had significantly lower capabilities for target cell lyses and cytokine secretion compared to NK cells of normal-weight children (147). These data provided the first evidence for obesity-mediated alterations in NK cell physiology in childhood obesity and that this may contribute to the increased risk of obese children for developing infections and cancer diseases in adulthood.
Interestingly, some studies indicated that some of the impaired functional parameters are normalized in ex-obese individuals, as the CD69 expression and granzyme B secretion was decreased and IFN-γ production was increased after the loss of body weight and fat mass in obese adults (73, 157). In accordance with results of animal studies, these data indicate that the altered NK cell phenotype is dependent on the metabolic environment and can be reactivated by weight loss also in humans.
As obesity gets more and more in scientific and clinical focus, a subgroup of metabolically healthy obese individuals have been classified and is constantly more precisely defined (165, 166). Lynch et al. demonstrated that obese patients with an unhealthy metabolic profile exhibit less circulating NK cells as well as an altered NK cell phenotype compared to metabolically healthy patients. In detail, metabolically unhealthy obese patients had an increased expression of the activation marker CD69 and the inhibition markers NKB1 and CD158b on NK cells, compared to healthy obese individuals (146). This suggests that being unhealthy obese contributes to obesity-related complications and diseases through the negative influence on immune cells.
Moreover, in recent years, a group of so-called “normal-weight obese” individuals has been distinguished. This group is defined by a normal body weight and BMI, but an elevated body fat percentage, comparable to that of obese individuals. There are already studies showing similar associations of normal weight obesity with metabolic syndrome and insulin resistance and obesity related co-morbidities (167–169). Less is known about the impact of normal weight obesity on immune cells and the resulting risk for cancer burden.
Tissue-Resident NK Cells in Adipose Tissue of Obese Individuals
Obesity is not only accompanied by an adipocyte hypertrophy and hyperplasia but also with an increase of infiltration of immune cells. Numbers of macrophages, T cells, and NK cells increase in adipose tissue and therefore indicate a chronic low-grade inflammation of the fat tissue itself and also systemically. Thus, in recent years, the characterization of NK cells in distinct adipose tissue depots became of scientific interest. Generally, NK cells represent about 13% of all leukocytes in visceral adipose tissue (135). Characterization of NK cell subpopulations in the stromal vascular fraction of human visceral adipose tissue of lean healthy subjects demonstrated an accumulation of CD56brightCD16dim/neg NK cells (about 30% of total NK cells, compared to 10% in peripheral blood) and a lower frequency of CD56dimCD16bright NK cells (about 70% of total NK cells, compared to 90% in peripheral blood) compared to the NK cell subset distribution in the peripheral blood (152).
Several studies reported an increase of the NK cell number in the adipose tissue of leptin-deficient and diet-induced obese mice as well as in the adipose tissue of obese humans (84, 107, 132, 134, 135, 137, 158). This elevated NK cell number is primarily induced by an increased proliferation of tissue-resident NK cells and to a lesser extent by recruitment from the periphery (134, 135). Interestingly, data indicate that the obesity-associated change in NK cell numbers especially occurs in the metabolically active visceral adipose tissue, but not in subcutaneous adipose tissue (132, 135, 158). Nevertheless, in contrast, few studies also demonstrate a decrease of NK cell numbers in adipose tissue of obese mice or humans or could not observe any differences in NK cell numbers comparing adipose tissue of normal weight and obese individuals (128–130, 133). These discrepancies may result from different feeding periods or composition of high-fat diets, definition of NK cells in flow cytometry analyses, or the inconsistent measurement of total NK cell number or NK cell frequency.
Analyzing the functionality of adipose tissue-resident NK cells, animal studies demonstrated an increased secretion of IL-6, IFN-γ, and TNF-α by NK cells isolated from visceral and subcutaneous adipose tissue of obese mice (132, 134, 135). In subcutaneous adipose tissue of obese individuals, a shift from the cytotoxic CD56dim NK cell subset to the cytokine secreting CD56bright NK cell subpopulation could be observed (155). This might be one explanation for the increased cytokine secretion of adipose tissue NK cells in obesity. Interestingly, analyses of the stromal vascular fraction of omental adipose tissue from obese and ex-obese patients showed a vanished CD56bright subset and, in contrast to peripheral blood, an increase of the CD16 expression within the CD56dim subset (127). Moreover, obesity drove an upregulation of ligands of the activating receptor NKp46 on adipocytes of obese mice, which stimulated the proliferation and IFN-γ secretion of NK cells and, consequently, the polarization from the anti-inflammatory M2 macrophages to the pro-inflammatory M1 macrophages (135). In addition, systemic NK cell ablation in mice decreased the infiltration of macrophages in visceral adipose tissue (156). Therefore, NK cells have been discussed to contribute to the adipose tissue inflammation including the polarization of pro-inflammatory macrophages in obesity (132, 134, 135). Interestingly, a reduction of body weight and body fat mass by caloric restriction was associated with a decreased NK cell number in inguinal adipose tissue of diet-induced obese mice, leading to the assumption that weight loss may prevent adipose tissue inflammation in obese individuals (170).
Investigating the expression of activating NK cell receptors, studies on human subcutaneous adipose tissue NK cells demonstrated that the expression of the NKp30 and NKp44 receptors were decreased in obese subjects, whereas the NKG2D expression levels were not affected by obesity (155). These data indicate a reduced cytolytic activity of NK cells in subcutaneous adipose tissue of obese subjects, which might contribute to the higher susceptibility to infections and increased cancer risk under obese conditions. Future investigations focusing on the cytotoxic activity of adipose tissue NK cells of normal weight and obese individuals against tumor cells are necessary to complement the knowledge about the impact of NK cells in different adipose tissues. Moreover, the effect of body weight and fat mass reduction as well as physical activity on adipose tissue NK cells and inflammatory parameters would be of high interest.
The Link Between NK Cells and the Development of Obesity-Associated Inflammation and Insulin-Resistance
Changes in NK cell phenotype has also been shown in obese patients with comorbidities, especially type 2 diabetic patients. Romero et al. demonstrated a reduced frequency of the activating NK cell receptor KIR2DS4 in obese diabetic patients compared to normal-weight diabetic patients (171). Moreover, NK cells of obese patients with new onset type 2 diabetes mellitus exhibited an increase of NKG2D expression and degranulation capacity as well as a reduced NKG2A and KIR2DL3 expression in peripheral blood compared to normal-weight control patients (172). Besides the assumption that metabolic changes, like chronic low-grade inflammation, in obesity lead to an impaired NK cell physiology, recent studies demonstrated on the other hand that an altered NK cell function may contribute to the development of inflammatory processes and disturbances of glucose metabolism. Mice fed a high-fat diet revealed a significantly higher accumulation of pro-inflammatory M1 macrophages in adipose tissue compared to normal-weight mice, which was prevented by NK cell depletion in these animals (127, 132, 135). These results indicate that NK cells induce adipose tissue inflammation by enhancing the macrophage polarization, which was mediated via upregulation of NKp46 ligands on adipocytes and, consequently, a stimulation of IFN-γ secretion by NK cells (132, 134, 135). In addition, results of these studies reported that NK cell-depleted mice as well as mice lacking the NKp46 receptor fed with a high-fat diet failed to develop hyperinsulinemia and exhibited an improved glucose tolerance and insulin sensitivity (132, 134, 135). Investigations of Theurich et al. revealed that obesity is associated with an increase of an IL-6/Stat3-dependent expansion of a distinct IL-6Ra- and Csf1r (colony stimulating factor 1 receptor)-expressing NK cell subpopulation in mice and humans, which are important mediators of metaflammation and insulin resistance (84).
In summary, these data suggest that alterations in NK cell physiology may also contribute to obesity-associated pathogenesis of insulin resistance and type 2 diabetes mellitus.
The Impact of Obesity-Related Altered NK Cell Functionality in the Defense of Infection and Tumor Development
Little information exists about the influence of obesity-associated disturbances of NK cell physiology on the high susceptibility for infections and increased cancer risk in obese individuals.
So far, only limited studies investigated the impact of obesity on NK cell functionality during infections. An animal study from Smith et al. demonstrated a reduced cytotoxicity of NK cells isolated from obese mice infected with influenza virus, combined by an increased mortality rate compared to normal-weight animals with influenza infection (13). In humans, a relation of the high incidence of H1N1 infections and disturbed NK cell functions in obese individuals is proposed (173). Latest data also provided evidence for a decreased NK cell number in mice orally infected with Salmonella Typhimurium, which also may contribute to an impaired susceptibility for infections in obese individuals (174). Interestingly, it has been proposed that selective therapeutic drugs might get trapped in adipose tissue in the treatment of HIV infections and, furthermore, immune cells, including NK cells, could be retained in adipose tissue, blocking their immunosurveillance elsewhere in the body (175).
Several studies addressed obesity-related alterations in NK cell functionality during tumor development. Animal studies on diet-induced rats injected with a metastatic breast cancer cell line reported a reduced NK cell number in the blood as well as a decrease of NK cell-tumor cell interactions in the lung, compared to lean animals. Furthermore, obese rats showed reduced NKG2D expression levels in the spleen combined with an enhanced number of lung metastasis (143). Accordingly, obese mice injected with breast cancer cells revealed a lower NK cell number in blood and spleen as well as a reduced NK cell cytotoxicity against lymphoma cells, compared to their lean littermates (140).
In addition to investigations on breast cancer, several lines of evidence show an impact of altered NK cell functionality in obese individuals and colon cancer development. Studies in rats with chemically induced colon cancer demonstrated a decreased number of NK cells in liver, spleen, and colon tumors as well as reduced expression levels of activating NK cell receptors in splenic tissue (NKG2D and NKp46) and liver tissue (NKp30) of diet-induced obese animals. This was associated with an increased quantity, size, and weight of colon tumors in obese rats, compared to normal-weight animals (93). In accordance, results of human studies reported a reduced NK cell number in normal colon tissue of obese patients compared to normal-weight patients, which may contribute to an increased risk for the development of colon cancer in obesity (149). Moreover, analyses of human NKG2D genotypes indicated that subjects with a NKG2D genotype associated with a high NK cell activity exhibit a decreased risk to develop colon cancer (176). In contrast, no differences in the number or subset distribution of NK cells had been observed in blood or adipose tissue of patients with colorectal cancer (152).
Besides investigations in the context of breast and colon cancer, animal studies on leptin-deficient obese (ob/ob) mice injected with melanoma cells showed a significant decreased splenic NK cell number in obese mice compared to normal weight wild-type control mice. Interestingly, this effect was reversible by leptin administration (98). A recent study demonstrated associations between obesity-related alterations in NK cell physiology and pancreatic cancer development. Obese mice with KRAS mutation had a decreased NK cell number in blood, spleen, and bone marrow as well as a reduced NK cell cytotoxicity, compared to their normal weight littermates at the pre-malignant stage of pancreatic tumorigenesis. This has been discussed to contribute to the enhanced progression of cancer from a dysplastic stage to invasive cancer in obese individuals (131).
Additionally, the migration of NK cells under obese conditions was in the focus. Among leukocytes, NK cells are the most active migrating cells (177). A study from our group investigated the time-dependent influence of different leptin concentrations on the cellular morphology of NK-92 cells (91). The study revealed an impact of physiological concentrations of leptin on the filopodia length and of the co-localization of cofilin and F-actin, suggesting an increased motility of NK cells and a possible support of immune defense against tumor cells.
The latest studies have demonstrated that obesity is associated with a peroxisome proliferator-activated receptor (PPAR)-driven lipid accumulation in NK cells, leading to metabolic dysfunctions, like inhibition of perforin, IFN-γ, and granzyme B secretion as well as target cell lysis (178). These lipid-induced metabolic defects of NK cells were shown to be associated with a loss of antitumor response (178).
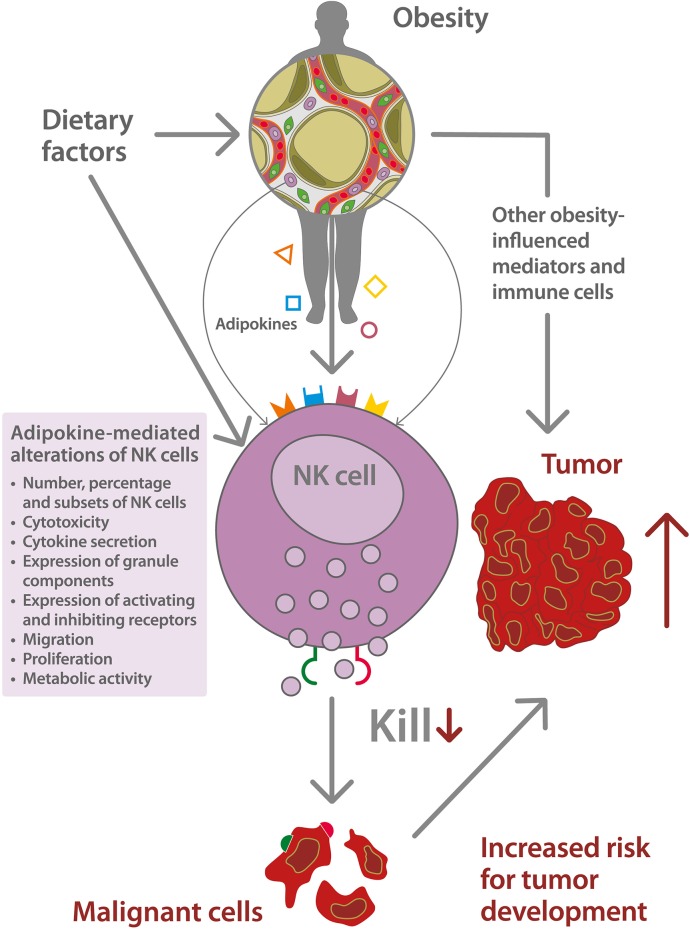
A schematic depiction of the relationship between obesity-associated alterations of NK cells, and the increased risk for tumor development is displayed in Figure 1.
Figure 1.
Schematic depiction of the impact of natural killer (NK) cell dysfunction on the increased risk for tumor development in obesity. Dietary factors, like high energy intake or high intake of saturated fatty acids, promote the development of overweight and obesity and mediate direct effects on NK cell function. Obesity is associated with an excessive growth of adipose tissue and a dysregulated secretion of adipokines, like leptin ( ), adiponectin (
), adiponectin ( ), interleukin-6 (
), interleukin-6 ( ), estrogens (
), estrogens ( ), and others. The obesity-related metabolic environment leads to an altered NK cell functionality including a dysbalance of the expression of activating and inhibiting NK cell receptors as well as NK cell receptor ligands (
), and others. The obesity-related metabolic environment leads to an altered NK cell functionality including a dysbalance of the expression of activating and inhibiting NK cell receptors as well as NK cell receptor ligands ( ,
,  ) on tumor cells. This NK cell dysfunction is associated with a decreased killing capacity of malignant cells and an increased risk for tumor development.
) on tumor cells. This NK cell dysfunction is associated with a decreased killing capacity of malignant cells and an increased risk for tumor development.
NK Cell-Based Immunotherapies in Cancer Treatment
A large number of scientific investigations and clinical studies have demonstrated that NK cells have a great potential for cancer immunotherapy (179). Besides the application of NK cell stimulating cytokines, the treatment of cancer patients with antibodies targeting inhibitory NK cell receptors or agonists of activating NK cell receptors were used to increase the number and functionality of NK cells. Very recently, a review by Nersesian et al. demonstrated the success of NK cell-based therapy in patients with ovarian cancer; however, aspects of obesity were not touched (180). In addition, NK cell adoptive transfer therapies for cancer patients using allogenic blood NK cells, stem cell-derived NK cells, or different NK cell lines exhibited promising effects in the treatment of several cancer types, whereas only limited clinical benefits were observed in cancer patients who received an adoptive transfer of autologous NK cells (181–183). Moreover, the use of genetically modified NK cells that express a chimeric antigen receptor (CAR) led to an optimized therapeutic potential of adoptive NK cell transfer therapies (184). Despite the large number of pre-clinical and clinical studies on NK cell-based immunotherapies, no data exist addressing a possible influence on therapeutic effects in the case of obese NK cell donors or obese recipients. With the knowledge from animal studies that an obese metabolic environment alters NK cell functionality and phenotype, an impaired therapeutic effect of adoptive NK cell transfer therapies in obese patients or the use of NK cells isolated from obese individuals for immunotherapies might be assumed (76).
Reactivation of NK Cells by Body Weight Reduction and Physical Activity
In addition to findings on altered NK cell physiology in obesity, a number of studies exist investigating obesity-preventing aspects, like body weight reduction by caloric restriction or bariatric surgery as well as physical activity on NK cells.
Investigations analyzing the effects of caloric restriction on NK cells demonstrated no changes or a decreased NK cell number after the dietary intervention in obese and non-obese mice and humans (170, 185–187). However, NK cell cytotoxicity in obese rats and humans as well as in non-obese humans has been shown to be increased after energy restriction or low-fat diet (144, 188, 189). In addition, caloric restriction was associated with a higher expression of the activation marker CD69 and B220 as well as an increased secretion of TNF-α and GM-CSF in non-obese mice (185). Although the majority of data indicate a stimulating effect of caloric restriction on NK cell functionality, a few studies reported contrary results with a reduced killing activity, an impaired maturation, and IFN-γ production of NK cells after caloric restriction in non-obese mice or obese woman (185, 187). Interestingly, life-long weight stability and prevention of weight cycling has been shown to be associated with a higher lytic activity of NK cells against tumor cells, providing evidence that frequent intentional weight loss may negatively influence NK cell function (190).
Aside from energy restriction via conventional dietary intervention, the loss of body weight and fat mass 6 months after bariatric surgery in obese patients clearly increased the cytotoxicity and cytokine production of NK cells (160). In contrast, another study demonstrated a reduced expression of the activation marker CD69 and the Fas antigen CD95 in obese patients 12 months after gastric bypass surgery (151). These contrary findings may result from different surgery methods, the intensity of weight loss, or the post-operative time the samples were analyzed.
Physical activity is one of the preventive strategies to inhibit the development of overweight and obesity and is the most effective intervention to reduce body weight and fat mass as well as to decrease the risk for secondary diseases in obese individuals. In the last 30 years, numerous studies demonstrated an increased number and cytotoxicity of NK cells during and after a moderate and intensive training program in mice and humans (86, 136, 170, 191–202). These effects seem to be independent of the type of the physical activity and have been shown for walking, running, marathon, race cycling, or aerobics. In addition, in these studies, activation of NK cells has been demonstrated by acute training programs as well as by experimental settings with chronic exercise. Most of the studies initially reported an acute stimulating effect of physical activity on NK cells during or right after the training session, followed by a recurrence back to basal levels after minutes or few hours (191–193, 197, 203). The increase of the NK cell numbers under exercise conditions has been proposed to be induced by a rapid mobilization of tissue-resident NK cells into the circulation, possibly caused by the increase of released cytokines, like IL-6, IL-7, or IL-15, from muscle cells (204–208). In addition to NK cell number and cytotoxicity, NK cell subset distribution is affected by exercise. Whereas, two studies demonstrated an increase in the CD56dimCD16bright NK cell subset, other studies demonstrated a higher frequency of the CD56brightCD16dim/neg NK cell subpopulation (86, 203, 209, 210). However, physical activity increased the expression of the activating NK cell receptors CD69 and NKG2D and the degranulation marker CD107a as well as the IFN-γ, perforin, and granzyme B content of NK cells (73, 196, 203, 211). Furthermore, the expression of the inhibitory NK cell receptor KLRG1 was reduced after exercise training (203). Taken together, the data provide convincing evidence for a NK cell stimulating effect of physical activity in normal-weight and obese individuals.
Until now, only two studies exist investigating the impact of exercise on NK cell physiology and the association with tumor development. A murine experiment demonstrated an increased NK cell infiltration in subcutaneous adipose and lung tumor tissues associated with an inhibited tumor growth in mice after voluntary wheel-running (86). This NK cell infiltration in the tumor tissue was proposed to be induced by the enhanced IL-6 release after physical activity. Furthermore, exercise led to an NK cell-activating milieu in the tumor tissue by increasing the expression of NK cell-activating ligands as well as a more pronounced release of stimulatory cytokines and chemoattractant chemokines (86). In addition, high-intensity interval training led to an increased number and activation status of blood NK cells and a reduced lung tumor burden in obese mice (136). These findings suggest that the enhanced risk for tumor development in obesity might be reduced by an exercise-induced activation of NK cells.
Conclusion and Outlook
In conclusion, the herein discussed data clearly demonstrate an association of impaired NK cell functionality with obesity. Among other factors, altered NK cell biology contributes to an increased cancer risk and a more severe cancer outcome in obese individuals (99). Future research is urgently needed to elucidate conflicting results in the field and to break down the most relevant alterations in NK cell functions. Obesity-related changes in concentrations and signaling cascades of adipokines might be primarily responsible for the alterations of NK cells. Nevertheless, in the future, other endocrine and metabolic factors associated with obesity and their influence on NK cells should be studied in more detail. Even though, available data are heterogeneous, obesity-related changes in activating NK cell receptors NKG2D and NKp46 were found in various studies. As obesity is one of the most important preventable risk factors for the development of cancer, more investigations on the potency of weight loss and physical activity as therapeutic interventions for cancer should be carried out. Pioneering work shows a positive feedback loop of physical activity and fat mass reduction on NK cell functionality that most likely control cancer development. Furthermore, NK cell therapies are promising components of modern immunotherapies to treat cancer. NK cell donor BMI will certainly have an impact on the success rate of the therapeutic intervention. Thus, studies elucidating the role of donor BMI on NK cell therapy outcome are of high interest.
NK cells are sensitive to obesity-related changes on multiple yet not fully elucidated levels and significantly shape infection and cancer outcome.
Author Contributions
IB and JS took the lead in writing the manuscript. DQ and HK contributed to the writing of the manuscript and provided critical feedback for the manuscript. HK supervised the underlying projects of the working group.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- ACM
adipocyte-conditioned media
- Adamts3
a disintegrin-like and metallopeptidase with thrombospondin type 1 motif, 3
- AdipoR
adiponectin receptor
- Anxa8
annexin A8
- AT
adipose tissue
- Bax
Bcl-2-associated X protein
- Bcl
B-cell lymphoma
- BMI
body mass index
- CCR
CC chemokine receptor
- Csf1r
colony stimulating factor 1 receptor
- CX3CR1
C-X3-C-motif chemokine receptor 1
- DNAM-1
DNAX accessory molecule-1
- ESR
estrogen receptor
- FasL
Fas ligand
- IFN-γ
interferon
- GM-CSF
granulocyte macrophage colony-stimulating factor
- H6pd
hexose-6-phosphate dehydrogenase
- Hk2
hexokinase 2
- IL
interleukinIL-6R; interleukin-6 receptor
- Il18rap
interleukin 18 receptor accessory protein
- IP-10
interferon gamma-induced protein 10
- JAK
Janus kinase
- KIR
killer immunoglobulin-like receptor
- Klra1
killer lectin-like receptor subfamiliy A 1
- KLRG
killer cell lectin-like receptor subfamily G
- LEP-R
leptin receptor
- LILRB1
leukocyte immunoglobulin-like receptor B1
- Mac-1
macrophage-1 antigen
- MFI
mean fluorescence intensity
- m.h.
metabolically healthy
- MIP-1β
macrophage inflammatory protein-1β
- m. u.
metabolically unhealthy
- NK cell
natural killer cell
- NKG
natural killer group
- NKG7
natural killer cell granule protein 7
- Nr4a1
nuclear receptor subfamily 4 group A member 1
- Nw
normal weight
- Ob
obese
- ObR
obesity receptor (leptin receptor)
- Ow
overweight
- PAQR
progestin and adipoQ receptor
- PBMCs
peripheral blood mononuclear cells
- PD-1
programmed cell death protein-1
- Pde5a
phosphodiesterase 5A
- PlGF
placental growth factor
- PPAR
peroxisome proliferator-activated receptor
- pS6
ribosomal S6 protein
- Renbp
renin binding protein
- Scd-1
stearoyl-CoA desaturase 1
- Siglec
sialic-acid-binding immunoglobulin-like lectin
- STAT
signal transducer and activator of transcription
- TGF-β
transforming growth factor
- TIM-3
T-cell immunoglobulin and mucin-domain containing-3
- TNF-α
tumor necrosis factor
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
- uNK
uterine NK cells
- VEGF
vascular endothelial growth factor
- Vegfa
vascular endothelial growth factor A.
Footnotes
Funding. This work was funded by the Wilhelm-Roux grant of the Medical Faculty of the Martin Luther University Halle-Wittenberg.
References
- 1.WHO Obesity and Overweight (WHO fact sheet N 311.). Geneva, Switzerland World Health Organisation: (2019). [Google Scholar]
- 2.Wright SM, Aronne LJ. Causes of obesity. Abdom Imaging. (2012) 37:730–2. 10.1007/s00261-012-9862-x [DOI] [PubMed] [Google Scholar]
- 3.Janesick A, Blumberg B. Endocrine disrupting chemicals and the developmental programming of adipogenesis and obesity. Birth Defects Res C Embryo Today. (2011) 93:34–50. 10.1002/bdrc.20197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Dijk SJ, Molloy PL, Varinli H, Morrison JL, Muhlhausler BS. Epigenetics and human obesity. Int J Obes. (2015) 39:85–97. 10.1038/ijo.2014.34 [DOI] [PubMed] [Google Scholar]
- 5.Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. (2007) 357:370–9. 10.1056/NEJMsa066082 [DOI] [PubMed] [Google Scholar]
- 6.Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. (2017) 377:13–27. 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. (2011) 377:1085–95. 10.1016/S0140-6736(11)60105-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elsayed EF, Sarnak MJ, Tighiouart H, Griffith JL, Kurth T, Salem DN, et al. Waist-to-hip ratio, body mass index, and subsequent kidney disease and death. Am J Kidney Dis. (2008) 52:29–38. 10.1053/j.ajkd.2008.02.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romain AJ, Marleau J, Baillot A. Impact of obesity and mood disorders on physical comorbidities, psychological well-being, health behaviours and use of health services. J Affect Disord. (2018) 225:381–8. 10.1016/j.jad.2017.08.065 [DOI] [PubMed] [Google Scholar]
- 10.Mitsuhashi K, Hashimoto Y, Tanaka M, Toda H, Matsumoto S, Ushigome E, et al. Combined effect of body mass index and waist-height ratio on incident diabetes; a population based cohort study. J Clin Biochem Nutr. (2017) 61:118–22. 10.3164/jcbn.16-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer–viewpoint of the IARC working group. N Engl J Med. (2016) 375:794–8. 10.1056/NEJMsr1606602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louie JK, Acosta M, Samuel MC, Schechter R, Vugia DJ, Harriman K, et al. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1). Clin Infect Dis. (2011) 52:301–12. 10.1093/cid/ciq152 [DOI] [PubMed] [Google Scholar]
- 13.Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. (2007) 137:1236–43. 10.1093/jn/137.5.1236 [DOI] [PubMed] [Google Scholar]
- 14.World Cancer Research Fund CUP Summary Report. London, UK: (2017). [Google Scholar]
- 15.Berger NA. Obesity and cancer pathogenesis. Ann N Y Acad Sci. (2014) 1311:57–76. 10.1111/nyas.12416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Himbert C, Delphan M, Scherer D, Bowers LW, Hursting S, Ulrich CM. Signals from the adipose microenvironment and the obesity-cancer link-asystematic review. Cancer Prev Res. (2017) 10:494–506. 10.1158/1940-6207.CAPR-16-0322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ackerman SE, Blackburn OA, Marchildon F, Cohen P. Insights into the link between obesity and cancer. Curr Obes Rep. (2017) 6:195–203. 10.1007/s13679-017-0263-x [DOI] [PubMed] [Google Scholar]
- 18.Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J Endocrinol. (2014) 222:R113–27. 10.1530/JOE-14-0283 [DOI] [PubMed] [Google Scholar]
- 19.Gerriets VA, MacIver NJ. Role of T cells in malnutrition and obesity. Front Immunol. (2014) 5:379. 10.3389/fimmu.2014.00379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castoldi A, Naffah de Souza C, Câmara NO, Moraes-Vieira PM. The macrophage switch in obesity development. Front Immunol. (2015) 6:637. 10.3389/fimmu.2015.00637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosaraju R, Guesdon W, Crouch MJ, Teague HL, Sullivan EM, Karlsson EA, et al. B cell activity is impaired in human and mouse obesity and is responsive to an essential fatty acid upon murine influenza infection. J Immunol. (2017) 198:4738–52. 10.4049/jimmunol.1601031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zelechowska P, Agier J, Kozłowska E, Brzezinska-Błaszczyk E. Mast cells participate in chronic low-grade inflammation within adipose tissue. Obes Rev. (2018) 19:686–97. 10.1111/obr.12670 [DOI] [PubMed] [Google Scholar]
- 23.Abel AM, Yang C, Thakar MS, Malarkannan S. Natural killer cells: development, maturation, and clinical utilization. Front Immunol. (2018) 9:1869. 10.3389/fimmu.2018.01869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. (2001) 22:633–40. 10.1016/S1471-4906(01)02060-9 [DOI] [PubMed] [Google Scholar]
- 25.Juelke K, Killig M, Luetke-Eversloh M, Parente E, Gruen J, Morandi B, et al. CD62L expression identifies a unique subset of polyfunctional CD56dim NK cells. Blood. (2010) 116:1299–307. 10.1182/blood-2009-11-253286 [DOI] [PubMed] [Google Scholar]
- 26.Fauriat C, Long EO, Ljunggren H-G, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. (2010) 115:2167–76. 10.1182/blood-2009-08-238469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chambers AM, Lupo KB, Matosevic S. Tumor microenvironment-induced immunometabolic reprogramming of natural killer cells. Front Immunol. (2018) 9:2517. 10.3389/fimmu.2018.02517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michel T, Poli A, Cuapio A, Briquemont B, Iserentant G, Ollert M, et al. Human CD56bright NK cells: an update. J Immunol. (2016) 196:2923–31. 10.4049/jimmunol.1502570 [DOI] [PubMed] [Google Scholar]
- 29.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. (2003) 101:3052–7. 10.1182/blood-2002-09-2876 [DOI] [PubMed] [Google Scholar]
- 30.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. (2006) 12:1065–74. 10.1038/nm1452 [DOI] [PubMed] [Google Scholar]
- 31.Bruno A, Focaccetti C, Pagani A, Imperatori AS, Spagnoletti M, Rotolo N, et al. The proangiogenic phenotype of natural killer cells in patients with non-small cell lung cancer. Neoplasia. (2013) 15:133–42. 10.1593/neo.121758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner JA, Rosario M, Romee R, Berrien-Elliott MM, Schneider SE, Leong JW, et al. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J Clin Invest. (2017) 127:4042–58. 10.1172/JCI90387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi E, Kuranaga N, Satoh K, Habu Y, Shinomiya N, Asano T, et al. Induction of CD16+ CD56bright NK cells with antitumour cytotoxicity not only from CD16- CD56bright NK cells but also from CD16- CD56dim NK cells. Scand J Immunol. (2007) 65:126–38. 10.1111/j.1365-3083.2006.01883.x [DOI] [PubMed] [Google Scholar]
- 34.Chan A, Hong D-L, Atzberger A, Kollnberger S, Filer AD, Buckley CD, et al. CD56bright human NK cells differentiate into CD56dim cells: role of contact with peripheral fibroblasts. J Immunol. (2007) 179:89–94. 10.4049/jimmunol.179.1.89 [DOI] [PubMed] [Google Scholar]
- 35.Luetke-Eversloh M, Killig M, Romagnani C. Signatures of human NK cell development and terminal differentiation. Front Immunol. (2013) 4:499. 10.3389/fimmu.2013.00499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goh W, Huntington ND. Regulation of murine natural killer cell development. Front Immunol. (2017) 8:130. 10.3389/fimmu.2017.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walzer T, Bléry M, Chaix J, Fuseri N, Chasson L, Robbins SH, et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc Natl Acad Sci USA. (2007) 104:3384–9. 10.1073/pnas.0609692104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walzer T, Jaeger S, Chaix J, Vivier E. Natural killer cells: from CD3(-)NKp46(+) to post-genomics meta-analyses. Curr Opin Immunol. (2007) 19:365–72. 10.1016/j.coi.2007.04.004 [DOI] [PubMed] [Google Scholar]
- 39.Meinhardt K, Kroeger I, Abendroth A, Müller S, Mackensen A, Ullrich E. Influence of NK cell magnetic bead isolation methods on phenotype and function of murine NK cells. J Immunol Methods. (2012) 378:1–10. 10.1016/j.jim.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 40.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. (2009) 113:5488–96. 10.1182/blood-2008-10-187179 [DOI] [PubMed] [Google Scholar]
- 41.Hayakawa Y, Andrews DM, Smyth MJ. Subset analysis of human and mouse mature NK cells. Methods Mol Biol. (2010) 612:27–38. 10.1007/978-1-60761-362-6_3 [DOI] [PubMed] [Google Scholar]
- 42.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. (2006) 176:1517–24. 10.4049/jimmunol.176.3.1517 [DOI] [PubMed] [Google Scholar]
- 43.Crinier A, Milpied P, Escalière B, Piperoglou C, Galluso J, Balsamo A, et al. High-Dimensional single-cell analysis identifies organ-Specific signatures and conserved NK cell subsets in humans and mice. Immunity. (2018) 49:971–86.e5. 10.1016/j.immuni.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kveberg L, Bäck CJ, Dai K-Z, Inngjerdingen M, Rolstad B, Ryan JC, et al. The novel inhibitory NKR-P1C receptor and Ly49s3 identify two complementary, functionally distinct NK cell subsets in rats. J Immunol. (2006) 176:4133–40. 10.4049/jimmunol.176.7.4133 [DOI] [PubMed] [Google Scholar]
- 45.Inngjerdingen M, Kveberg L, Naper C, Vaage JT. Natural killer cell subsets in man and rodents. Tissue Antigens. (2011) 78:81–8. 10.1111/j.1399-0039.2011.01714.x [DOI] [PubMed] [Google Scholar]
- 46.Kveberg L, Jiménez-Royo P, Naper C, Rolstad B, Butcher GW, Vaage JT, et al. Two complementary rat NK cell subsets, Ly49s3+ and NKR-P1B+, differ in phenotypic characteristics and responsiveness to cytokines. J Leukoc Biol. (2010) 88:87–93. 10.1189/jlb.0110039 [DOI] [PubMed] [Google Scholar]
- 47.Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells: 10 years on. Cell. (2018) 174:1054–66. 10.1016/j.cell.2018.07.017 [DOI] [PubMed] [Google Scholar]
- 48.Sun H, Sun C, Xiao W, Sun R. Tissue-resident lymphocytes: from adaptive to innate immunity. Cell Mol Immunol. (2019) 16:205–15. 10.1038/s41423-018-0192-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Björkström NK, Ljunggren H-G, Michaëlsson J. Emerging insights into natural killer cells in human peripheral tissues. Nat Rev Immunol. (2016) 16:310–20. 10.1038/nri.2016.34 [DOI] [PubMed] [Google Scholar]
- 50.Spits H, Bernink JH, Lanier L. NK cells and type 1 innate lymphoid cells: partners in host defense. Nat Immunol. (2016) 17:758–64. 10.1038/ni.3482 [DOI] [PubMed] [Google Scholar]
- 51.Prager I, Liesche C, van Ooijen H, Urlaub D, Verron Q, Sandström N, et al. NK cells switch from granzyme B to death receptor-mediated cytotoxicity during serial killing. J Exp Med. (2019) 216:2113–27. 10.1084/jem.20181454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhat R, Watzl C. Serial killing of tumor cells by human natural killer cells–enhancement by therapeutic antibodies. PLoS ONE. (2007) 2:e326. 10.1371/journal.pone.0000326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caligiuri MA. Human natural killer cells. Blood. (2008) 112:461–9. 10.1182/blood-2007-09-077438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Rev. (2006) 20:123–37. 10.1016/j.blre.2005.10.001 [DOI] [PubMed] [Google Scholar]
- 55.Stewart CA, Vivier E, Colonna M. Strategies of natural killer cell recognition and signaling. Curr Top Microbiol Immunol. (2006) 298:1–21. 10.1007/3-540-27743-9_1 [DOI] [PubMed] [Google Scholar]
- 56.Zingoni A, Molfetta R, Fionda C, Soriani A, Paolini R, Cippitelli M, et al. NKG2D and its ligands: One for All, All for One. Front Immunol. (2018) 9:476 10.3389/fimmu.2018.00476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barrow AD, Martin CJ, Colonna M. The natural cytotoxicity receptors in health and disease. Front Immunol. (2019) 10:909. 10.3389/fimmu.2019.00909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pende D, Falco M, Vitale M, Cantoni C, Vitale C, Munari E, et al. Killer Ig-like receptors (KIRs): their role in NK cell modulation and developments leading to their clinical exploitation. Front Immunol. (2019) 10:1179. 10.3389/fimmu.2019.01179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol. (2011) 89:216–24. 10.1038/icb.2010.78 [DOI] [PubMed] [Google Scholar]
- 60.Hollyoake M, Campbell RD, Aguado B. NKp30 (NCR3) is a pseudogene in 12 inbred and wild mouse strains, but an expressed gene in Mus caroli. Mol Biol Evol. (2005) 22:1661–72. 10.1093/molbev/msi162 [DOI] [PubMed] [Google Scholar]
- 61.Biassoni R, Cantoni C, Marras D, Giron-Michel J, Falco M, Moretta L, et al. Human natural killer cell receptors: insights into their molecular function and structure. J Cell Mol Med. (2003) 7:376–87. 10.1111/j.1582-4934.2003.tb00240.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pazina T, Shemesh A, Brusilovsky M, Porgador A, Campbell KS. Regulation of the functions of natural cytotoxicity receptors by interactions with diverse ligands and alterations in splice variant expression. Front Immunol. (2017) 8:369. 10.3389/fimmu.2017.00369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao H. Adipocytokines in obesity and metabolic disease. J Endocrinol. (2014) 220:T47–59. 10.1530/JOE-13-0339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jung UJ, Choi M-S. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. (2014) 15:6184–223. 10.3390/ijms15046184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cildir G, Akincilar SC, Tergaonkar V. Chronic adipose tissue inflammation: all immune cells on the stage. Trends Mol Med. (2013) 19:487–500. 10.1016/j.molmed.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 66.Shah NR, Braverman ER. Measuring adiposity in patients: the utility of body mass index (BMI), percent body fat, and leptin. PLoS ONE. (2012) 7:e33308. 10.1371/journal.pone.0033308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lamas B, Goncalves-Mendes N, Nachat-Kappes R, Rossary A, Caldefie-Chezet F, Vasson M-P, et al. Leptin modulates dose-dependently the metabolic and cytolytic activities of NK-92 cells. J Cell Physiol. (2013) 228:1202–9. 10.1002/jcp.24273 [DOI] [PubMed] [Google Scholar]
- 68.Zhao Y, Sun R, You L, Gao C, Tian Z. Expression of leptin receptors and response to leptin stimulation of human natural killer cell lines. Biochem Biophys Res Commun. (2003) 300:247–52. 10.1016/S0006-291X(02)02838-3 [DOI] [PubMed] [Google Scholar]
- 69.Wrann CD, Laue T, Hübner L, Kuhlmann S, Jacobs R, Goudeva L, et al. Short-term and long-term leptin exposure differentially affect human natural killer cell immune functions. Am J Physiol Endocrinol Metab. (2012) 302:E108–16. 10.1152/ajpendo.00057.2011 [DOI] [PubMed] [Google Scholar]
- 70.Keustermans G, van der Heijden LB, Boer B, Scholman R, Nuboer R, Pasterkamp G, et al. Differential adipokine receptor expression on circulating leukocyte subsets in lean and obese children. PLoS ONE. (2017) 12:e0187068. 10.1371/journal.pone.0187068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bähr I, Jahn J, Zipprich A, Pahlow I, Spielmann J, Kielstein H. Impaired natural killer cell subset phenotypes in human obesity. Immunol Res. (2018) 66:234–44. 10.1007/s12026-018-8989-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laue T, Wrann CD, Hoffmann-Castendiek B, Pietsch D, Hubner L, Kielstein H. Altered NK cell function in obese healthy humans. BMC Obes. (2015) 2:1. 10.1186/s40608-014-0033-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jahn J, Spielau M, Brandsch C, Stangl GI, Delank K-S, Bähr I, et al. Decreased NK cell functions in obesity can be reactivated by fat mass reduction. Obesity. (2015) 23:2233–41. 10.1002/oby.21229 [DOI] [PubMed] [Google Scholar]
- 74.Lautenbach A, Breitmeier D, Kuhlmann S, Nave H. Human obesity reduces the number of hepatic leptin receptor (ob-R) expressing NK cells. Endocr Res. (2011) 36:158–66. 10.3109/07435800.2011.580442 [DOI] [PubMed] [Google Scholar]
- 75.Nave H, Mueller G, Siegmund B, Jacobs R, Stroh T, Schueler U, et al. Resistance of ianus kinase-2 dependent leptin signaling in natural killer (NK) cells: a novel mechanism of NK cell dysfunction in diet-induced obesity. Endocrinology. (2008) 149:3370–8. 10.1210/en.2007-1516 [DOI] [PubMed] [Google Scholar]
- 76.Lautenbach A, Wrann CD, Jacobs R, Müller G, Brabant G, Nave H. Altered phenotype of NK cells from obese rats can be normalized by transfer into lean animals. Obesity. (2009) 17:1848–55. 10.1038/oby.2009.140 [DOI] [PubMed] [Google Scholar]
- 77.Huebner L, Engeli S, Wrann CD, Goudeva L, Laue T, Kielstein H. Human NK cell subset functions are differentially affected by adipokines. PLoS ONE. (2013) 8:e75703. 10.1371/journal.pone.0075703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jasinski-Bergner S, Büttner M, Quandt D, Seliger B, Kielstein H. Adiponectin and its receptors are differentially expressed in human tissues and cell lines of distinct origin. Obes Facts. (2017) 10:569–83. 10.1159/000481732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilk S, Jenke A, Stehr J, Yang C-A, Bauer S, Goldner K, et al. Adiponectin modulates NK-cell function. Eur J Immunol. (2013) 43:1024–33. 10.1002/eji.201242382 [DOI] [PubMed] [Google Scholar]
- 80.Pang TT, Narendran P. The distribution of adiponectin receptors on human peripheral blood mononuclear cells. Ann N Y Acad Sci. (2008) 1150:143–5. 10.1196/annals.1447.021 [DOI] [PubMed] [Google Scholar]
- 81.Kim K-Y, Kim JK, Han SH, Lim J-S, Kim KI, Cho DH, et al. Adiponectin is a negative regulator of NK cell cytotoxicity. J Immunol. (2006) 176:5958–64. 10.4049/jimmunol.176.10.5958 [DOI] [PubMed] [Google Scholar]
- 82.Böttger E, Grangeiro de Carvalho E, Meese S, Kun JF, Esen M. Expression of interleukin-6 family receptors in NK92 cells is regulated by cytokines and not through direct interaction with plasmodium falciparum-infected erythrocytes. J Interferon Cytokine Res. (2013) 33:65–71. 10.1089/jir.2012.0094 [DOI] [PubMed] [Google Scholar]
- 83.Oberg H-H, Wesch D, Grüssel S, Rose-John S, Kabelitz D. Differential expression of CD126 and CD130 mediates different STAT-3 phosphorylation in CD4+CD25- and CD25high regulatory T cells. Int Immunol. (2006) 18:555–63. 10.1093/intimm/dxh396 [DOI] [PubMed] [Google Scholar]
- 84.Theurich S, Tsaousidou E, Hanssen R, Lempradl AM, Mauer J, Timper K, et al. IL-6/Stat3-dependent induction of a distinct, obesity-associated NK cell subpopulation deteriorates energy and glucose homeostasis. Cell Metab. (2017) 26:171–84.e6. 10.1016/j.cmet.2017.05.018 [DOI] [PubMed] [Google Scholar]
- 85.Passos ST, Silver JS, O'Hara AC, Sehy D, Stumhofer JS, Hunter CA. IL-6 promotes NK cell production of IL-17 during toxoplasmosis. J Immunol. (2010) 184:1776–83. 10.4049/jimmunol.0901843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. (2016) 23:554–62. 10.1016/j.cmet.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 87.Laffont S, Rouquié N, Azar P, Seillet C, Plumas J, Aspord C, et al. X-Chromosome complement and estrogen receptor signaling independently contribute to the enhanced TLR7-mediated IFN-α production of plasmacytoid dendritic cells from women. J Immunol. (2014) 193:5444–52. 10.4049/jimmunol.1303400 [DOI] [PubMed] [Google Scholar]
- 88.Curran EM, Berghaus LJ, Vernetti NJ, Saporita AJ, Lubahn DB, Estes DM. Natural killer cells express estrogen receptor-alpha and estrogen receptor-beta and can respond to estrogen via a non-estrogen receptor-alpha-mediated pathway. Cell Immunol. (2001) 214:12–20. 10.1006/cimm.2002.1886 [DOI] [PubMed] [Google Scholar]
- 89.van den Heuvel M, McBey BA, Hahnel AC, Croy BA. An analysis of the uterine lymphocyte-derived hybridoma cell line GWM 1-2 for expression of receptors for estrogen, progesterone and interleukin 2. J Reprod Immunol. (1996) 31:37–50. 10.1016/0165-0378(96)00966-7 [DOI] [PubMed] [Google Scholar]
- 90.Elinav E, Abd-Elnabi A, Pappo O, Bernstein I, Klein A, Engelhardt D, et al. Suppression of hepatocellular carcinoma growth in mice via leptin, is associated with inhibition of tumor cell growth and natural killer cell activation. J Hepatol. (2006) 44:529–36. 10.1016/j.jhep.2005.08.013 [DOI] [PubMed] [Google Scholar]
- 91.Oswald J, Büttner M, Jasinsky-Bergner S, Jacobs R, Rosenstock P, Kielstein H. Leptin affects filopodia and cofilin in NK-92 cells in a dose- and time-dependent manner. Eur J Histochem. (2018) 62:2848. 10.4081/ejh.2018.2848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tian Z, Sun R, Wei H, Gao B. Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem Biophys Res Commun. (2002) 298:297–302. 10.1016/S0006-291X(02)02462-2 [DOI] [PubMed] [Google Scholar]
- 93.Bähr I, Goritz V, Doberstein H, Hiller GG, Rosenstock P, Jahn J, et al. Diet-induced obesity is associated with an impaired NK cell function and an increased colon cancer incidence. J Nutr Metab. (2017) 2017:4297025. 10.1155/2017/4297025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shirshev SV, Nekrasova IV, Gorbunova OL, Orlova EG. Hormonal regulation of NK cell cytotoxic activity. Dokl Biol Sci. (2017) 472:28–30. 10.1134/S0012496617010021 [DOI] [PubMed] [Google Scholar]
- 95.Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. (1994) 8:652–8. [PubMed] [Google Scholar]
- 96.Klingemann H, Boissel L, Toneguzzo F. Natural killer cells for immunotherapy - advantages of the NK-92 cell line over blood NK cells. Front Immunol. (2016) 7:91. 10.3389/fimmu.2016.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Haas P, Straub RH, Bedoui S, Nave H. Peripheral but not central leptin treatment increases numbers of circulating NK cells, granulocytes and specific monocyte subpopulations in non-endotoxaemic lean and obese LEW-rats. Regul Pept. (2008) 151:26–34. 10.1016/j.regpep.2008.05.004 [DOI] [PubMed] [Google Scholar]
- 98.Mori A, Sakurai H, Choo M-K, Obi R, Koizumi K, Yoshida C, et al. Severe pulmonary metastasis in obese and diabetic mice. Int J Cancer. (2006) 119:2760–7. 10.1002/ijc.22248 [DOI] [PubMed] [Google Scholar]
- 99.O'Shea D, Cawood TJ, O'Farrelly C, Lynch L. Natural killer cells in obesity: impaired function and increased susceptibility to the effects of cigarette smoke. PLoS ONE. (2010) 5:e8660. 10.1371/journal.pone.0008660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frühbeck G. Intracellular signalling pathways activated by leptin. Biochem J. (2006) 393(Pt 1):7–20. 10.1042/BJ20051578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Enriori PJ, Evans AE, Sinnayah P, Cowley MA. Leptin resistance and obesity. Obesity. (2006) 14(Suppl. 5):254S−8S. 10.1038/oby.2006.319 [DOI] [PubMed] [Google Scholar]
- 102.Matsubara M, Maruoka S, Katayose S. Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur J Endocrinol. (2002) 147:173–80. 10.1530/eje.0.1470173 [DOI] [PubMed] [Google Scholar]
- 103.Han S, Jeong AL, Lee S, Park JS, Kim KD, Choi I, et al. Adiponectin deficiency suppresses lymphoma growth in mice by modulating NK cells, CD8 T cells, and myeloid-derived suppressor cells. J Immunol. (2013) 190:4877–86. 10.4049/jimmunol.1202487 [DOI] [PubMed] [Google Scholar]
- 104.Roytblat L, Rachinsky M, Fisher A, Greemberg L, Shapira Y, Douvdevani A, et al. Raised interleukin-6 levels in obese patients. Obes Res. (2000) 8:673–5. 10.1038/oby.2000.86 [DOI] [PubMed] [Google Scholar]
- 105.Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. (2014) 15:423–30. 10.1038/ni.2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cifaldi L, Prencipe G, Caiello I, Bracaglia C, Locatelli F, Benedetti F de, et al. Inhibition of natural killer cell cytotoxicity by interleukin-6: implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheumatol. (2015) 67:3037–46. 10.1002/art.39295 [DOI] [PubMed] [Google Scholar]
- 107.Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ, Ferrante AW. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. (2013) 18:816–30. 10.1016/j.cmet.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kang Y-J, Jeung IC, Park A, Park Y-J, Jung H, Kim T-D, et al. An increased level of IL-6 suppresses NK cell activity in peritoneal fluid of patients with endometriosis via regulation of SHP-2 expression. Hum Reprod. (2014) 29:2176–89. 10.1093/humrep/deu172 [DOI] [PubMed] [Google Scholar]
- 109.Teruel A, Romero M, Cacalano NA, Head C, Jewett A. Potential contribution of naïve immune effectors to oral tumor resistance: role in synergistic induction of VEGF, IL-6, and IL-8 secretion. Cancer Immunol Immunother. (2008) 57:359–66. 10.1007/s00262-007-0375-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vredevoe DL, Widawski M, Fonarow GC, Hamilton M, Martínez-Maza O, Gage JR. Interleukin-6 (IL-6) expression and natural killer (NK) cell dysfunction and anergy in heart failure. Am J Cardiol. (2004) 93:1007–11. 10.1016/j.amjcard.2003.12.054 [DOI] [PubMed] [Google Scholar]
- 111.Tseng H-C, Arasteh A, Paranjpe A, Teruel A, Yang W, Behel A, et al. Increased lysis of stem cells but not their differentiated cells by natural killer cells; de-differentiation or reprogramming activates NK cells. PLoS ONE. (2010) 5:e11590. 10.1371/journal.pone.0011590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lisheng L, Zhengyan C, Zhigang T. Regulatory effects of human recombinant IL-6 on natural killer cell activity of human fetal spleens. Chin J Cancer Res. (1991) 3:35–8. 10.1007/BF02672087 [DOI] [Google Scholar]
- 113.Luger TA, Krutmann J, Kirnbauer R, Urbanski A, Schwarz T, Klappacher G, et al. IFN-beta 2/IL-6 augments the activity of human natural killer cells. J Immunol. (1989) 143:1206–9. [PubMed] [Google Scholar]
- 114.Rabinowich H, Sedlmayr P, Herberman RB, Whiteside TL. Response of human NK cells to IL-6 alterations of the cell surface phenotype, adhesion to fibronectin and laminin, and tumor necrosis factor-alpha/beta secretion. J Immunol. (1993) 150:4844–55. [PubMed] [Google Scholar]
- 115.Lukanova A, Lundin E, Zeleniuch-Jacquotte A, Muti P, Mure A, Rinaldi S, et al. Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: a cross-sectional study in healthy women. Eur J Endocrinol. (2004) 150:161–71. 10.1530/eje.0.1500161 [DOI] [PubMed] [Google Scholar]
- 116.Nilsson N, Carlsten H. Estrogen induces suppression of natural killer cell cytotoxicity and augmentation of polyclonal B cell activation. Cell Immunol. (1994) 158:131–9. 10.1006/cimm.1994.1262 [DOI] [PubMed] [Google Scholar]
- 117.Hao S, Zhao J, Zhou J, Zhao S, Hu Y, Hou Y. Modulation of 17beta-estradiol on the number and cytotoxicity of NK cells in vivo related to MCM and activating receptors. Int Immunopharmacol. (2007) 7:1765–75. 10.1016/j.intimp.2007.09.017 [DOI] [PubMed] [Google Scholar]
- 118.Seaman WE, Gindhart TD. Effect of estrogen on natural killer cells. Arthritis Rheum. (1979) 22:1234–40. 10.1002/art.1780221110 [DOI] [PubMed] [Google Scholar]
- 119.Hao S, Li P, Zhao J, Hu Y, Hou Y. 17beta-estradiol suppresses cytotoxicity and proliferative capacity of murine splenic NK1.1+ cells. Cell Mol Immunol. (2008) 5:357–64. 10.1038/cmi.2008.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ferguson MM, McDonald FG. Oestrogen as an inhibitor of human NK cell cytolysis. FEBS Lett. (1985) 191:145–8. 10.1016/0014-5793(85)81011-5 [DOI] [PubMed] [Google Scholar]
- 121.Hou J, Zheng WF. Effect of sex hormones on NK and ADCC activity of mice. Int J Immunopharmacol. (1988) 10:15–22. 10.1016/0192-0561(88)90145-2 [DOI] [PubMed] [Google Scholar]
- 122.Baral E, Nagy E, Berczi I. Modulation of natural killer cell-mediated cytotoxicity by tamoxifen and estradiol. Cancer. (1995) 75:591–9. [DOI] [PubMed] [Google Scholar]
- 123.Gibson DA, Greaves E, Critchley HO, Saunders PT. Estrogen-dependent regulation of human uterine natural killer cells promotes vascular remodelling via secretion of CCL2. Hum Reprod. (2015) 30:1290–301. 10.1093/humrep/dev067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Albrecht AE, Hartmann BW, Scholten C, Huber JC, Kalinowska W, Zielinski CC. Effect of estrogen replacement therapy on natural killer cell activity in postmenopausal women. Maturitas. (1996) 25:217–22. 10.1016/S0378-5122(96)01063-8 [DOI] [PubMed] [Google Scholar]
- 125.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. (2004) 4:579–91. 10.1038/nrc1408 [DOI] [PubMed] [Google Scholar]
- 126.Xu L, Shen M, Chen X, Zhu R, Yang D-R, Tsai Y, et al. Adipocytes affect castration-resistant prostate cancer cells to develop the resistance to cytotoxic action of NK cells with alterations of PD-L1/NKG2D ligand levels in tumor cells. Prostate. (2018) 78:353–64. 10.1002/pros.23479 [DOI] [PubMed] [Google Scholar]
- 127.Boulenouar S, Michelet X, Duquette D, Alvarez D, Hogan AE, Dold C, et al. Adipose type one innate lymphoid cells regulate macrophage homeostasis through targeted cytotoxicity. Immunity. (2017) 46:273–86. 10.1016/j.immuni.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 128.Caspar-Bauguil S, Cousin B, Galinier A, Segafredo C, Nibbelink M, André M, et al. Adipose tissues as an ancestral immune organ: site-specific change in obesity. FEBS Lett. (2005) 579:3487–92. 10.1016/j.febslet.2005.05.031 [DOI] [PubMed] [Google Scholar]
- 129.Caspar-Bauguil S, Cousin B, André M, Nibbelink M, Galinier A, Periquet B, et al. Weight-dependent changes of immune system in adipose tissue: importance of leptin. Exp Cell Res. (2006) 312:2195–202. 10.1016/j.yexcr.2006.03.023 [DOI] [PubMed] [Google Scholar]
- 130.Duffaut C, Galitzky J, Lafontan M, Bouloumié A. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem Biophys Res Commun. (2009) 384:482–5. 10.1016/j.bbrc.2009.05.002 [DOI] [PubMed] [Google Scholar]
- 131.Kaur K, Chang H-H, Topchyan P, Cook JM, Barkhordarian A, Eibl G, et al. Deficiencies in natural killer cell numbers, expansion, and function at the pre-neoplastic stage of pancreatic cancer by kras mutation in the pancreas of obese mice. Front Immunol. (2018) 9:1229. 10.3389/fimmu.2018.01229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee B-C, Kim M-S, Pae M, Yamamoto Y, Eberlé D, Shimada T, et al. Adipose natural killer cells regulate adipose tissue macrophages to promote insulin resistance in obesity. Cell Metab. (2016) 23:685–98. 10.1016/j.cmet.2016.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. (2009) 15:914–20. 10.1038/nm.1964 [DOI] [PubMed] [Google Scholar]
- 134.O'Sullivan TE, Rapp M, Fan X, Weizman O-E, Bhardwaj P, Adams NM, et al. Adipose-resident group 1 innate lymphoid cells promote obesity-associated insulin resistance. Immunity. (2016) 45:428–41. 10.1016/j.immuni.2016.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wensveen FM, Jelenčić V, Valentić S, Šestan M, Wensveen TT, Theurich S, et al. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat Immunol. (2015) 16:376–85. 10.1038/ni.3120 [DOI] [PubMed] [Google Scholar]
- 136.Barra NG, Fan IY, Gillen JB, Chew M, Marcinko K, Steinberg GR, et al. High intensity interval training increases natural killer cell number and function in obese breast cancer-challenged mice and obese women. J Cancer Prev. (2017) 22:260–6. 10.15430/JCP.2017.22.4.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pae M, Baek Y, Lee S, Wu D. Loss of ovarian function in association with a high-fat diet promotes insulin resistance and disturbs adipose tissue immune homeostasis. J Nutr Biochem. (2018) 57:93–102. 10.1016/j.jnutbio.2018.03.011 [DOI] [PubMed] [Google Scholar]
- 138.Baltayeva J, Konwar C, Castellana B, Mara DL, Christians JK, Beristain AG. Obesogenic diet exposure alters uterine natural killer cell biology and impairs vasculature remodeling in mice†. Biol Reprod. (2020) 102:63–75. 10.1093/biolre/ioz163 [DOI] [PubMed] [Google Scholar]
- 139.Parker VJ, Solano ME, Arck PC, Douglas AJ. Diet-induced obesity may affect the uterine immune environment in early-mid pregnancy, reducing NK-cell activity and potentially compromising uterine vascularization. Int J Obes. (2014) 38:766–74. 10.1038/ijo.2013.164 [DOI] [PubMed] [Google Scholar]
- 140.Lamas B, Nachat-Kappes R, Goncalves-Mendes N, Mishellany F, Rossary A, Vasson M-P, et al. Dietary fat without body weight gain increases in vivo MCF-7 human breast cancer cell growth and decreases natural killer cell cytotoxicity. Mol Carcinog. (2015) 54:58–71. 10.1002/mc.22074 [DOI] [PubMed] [Google Scholar]
- 141.Lo CK, Lam QL, Yang M, Ko K-H, Sun L, Ma R, et al. Leptin signaling protects NK cells from apoptosis during development in mouse bone marrow. Cell Mol Immunol. (2009) 6:353–60. 10.1038/cmi.2009.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Behrendt P, Buchenauer T, Horn R, Brabant G, Jacobs R, Bode F, et al. Diet-induced obesity, exogenous leptin-, and MADB106 tumor cell challenge affect tissue leukocyte distribution and serum levels of cytokines in F344 rats. Endocrine. (2010) 38:104–12. 10.1007/s12020-010-9358-9 [DOI] [PubMed] [Google Scholar]
- 143.Spielmann J, Hanke J, Knauf D, Ben-Eliyahu S, Jacobs R, Stangl GI, et al. Significantly enhanced lung metastasis and reduced organ NK cell functions in diet-induced obese rats. BMC Obes. (2017) 4:24. 10.1186/s40608-017-0161-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lamas O, Martínez JA, Marti A. Energy restriction restores the impaired immune response in overweight (cafeteria) rats. J Nutr Biochem. (2004) 15:418–25. 10.1016/j.jnutbio.2004.02.003 [DOI] [PubMed] [Google Scholar]
- 145.Alwarawrah Y, Kiernan K, MacIver NJ. Changes in nutritional status impact immune cell metabolism and function. Front Immunol. (2018) 9:1055. 10.3389/fimmu.2018.01055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lynch LA, O'Connell JM, Kwasnik AK, Cawood TJ, O'Farrelly C, O'Shea DB. Are natural killer cells protecting the metabolically healthy obese patient? Obesity. (2009) 17:601–5. 10.1038/oby.2008.565 [DOI] [PubMed] [Google Scholar]
- 147.Tobin LM, Mavinkurve M, Carolan E, Kinlen D, O'Brien EC, Little MA, et al. NK cells in childhood obesity are activated, metabolically stressed, and functionally deficient. JCI Insight. (2017) 2:94939. 10.1172/jci.insight.94939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Perdu S, Castellana B, Kim Y, Chan K, DeLuca L, Beristain AG. Maternal obesity drives functional alterations in uterine NK cells. JCI Insight. (2016) 1:e85560. 10.1172/jci.insight.85560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mariani F, Sena P, Magnani G, Mancini S, Palumbo C, Ponz de Leon M, et al. PLZF expression during colorectal cancer development and in normal colorectal mucosa according to body size, as marker of colorectal cancer risk. Sci World J. (2013) 2013:630869. 10.1155/2013/630869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rosenstock P, Horstkorte R, Gnanapragassam VS, Harth J, Kielstein H. Siglec-7 expression is reduced on a natural killer (NK) cell subset of obese humans. Immunol Res. (2017) 65:1017–24. 10.1007/s12026-017-8942-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cottam DR, Schaefer PA, Shaftan GW, Angus LD. Dysfunctional immune-privilege in morbid obesity: implications and effect of gastric bypass surgery. Obes Surg. (2003) 13:49–57. 10.1381/096089203321136584 [DOI] [PubMed] [Google Scholar]
- 152.Donninelli G, Del Cornò M, Pierdominici M, Scazzocchio B, Varì R, Varano B, et al. Distinct blood and visceral adipose tissue regulatory T cell and innate lymphocyte profiles characterize obesity and colorectal cancer. Front Immunol. (2017) 8:643. 10.3389/fimmu.2017.00643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nieman DC, Henson DA, Nehlsen-Cannarella SL, Ekkens M, Utter AC, Butterworth DE, et al. Influence of obesity on immune function. J Am Diet Assoc. (1999) 99:294–9. 10.1016/S0002-8223(99)00077-2 [DOI] [PubMed] [Google Scholar]
- 154.Santagostino A, Garbaccio G, Pistorio A, Bolis V, Camisasca G, Pagliaro P, et al. An Italian national multicenter study for the definition of reference ranges for normal values of peripheral blood lymphocyte subsets in healthy adults. Haematologica. (1999) 84:499–504. [PubMed] [Google Scholar]
- 155.Shoae-Hassani A, Behfar M, Mortazavi-Tabatabaei SA, Ai J, Mohseni R, Hamidieh AA. Natural killer cells from the subcutaneous adipose tissue underexpress the NKp30 and NKp44 in obese persons and are less active against major histocompatibility complex class i non-expressing neoplastic cells. Front Immunol. (2017) 8:1486. 10.3389/fimmu.2017.01486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.O'Rourke RW, Gaston GD, Meyer KA, White AE, Marks DL. Adipose tissue NK cells manifest an activated phenotype in human obesity. Metab Clin Exp. (2013) 62:1557–61. 10.1016/j.metabol.2013.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Viel S, Besson L, Charrier E, Marcais A, Disse E, Bienvenu J, et al. Alteration of Natural killer cell phenotype and function in obese individuals. Clin Immunol. (2016) 177:12–7. 10.1016/j.clim.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 158.Wouters K, Gaens K, Bijnen M, Verboven K, Jocken J, Wetzels S, et al. Circulating classical monocytes are associated with CD11c+ macrophages in human visceral adipose tissue. Sci Rep. (2017) 7:42665. 10.1038/srep42665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Castellana B, Perdu S, Kim Y, Chan K, Atif J, Marziali M, et al. Maternal obesity alters uterine NK activity through a functional KIR2DL1/S1 imbalance. Immunol Cell Biol. (2018) 10.1101/167213 [DOI] [PubMed] [Google Scholar]
- 160.Moulin CM, Marguti I, Peron JP, Halpern A, Rizzo LV. Bariatric surgery reverses natural killer (NK) cell activity and NK-related cytokine synthesis impairment induced by morbid obesity. Obes Surg. (2011) 21:112–8. 10.1007/s11695-010-0250-8 [DOI] [PubMed] [Google Scholar]
- 161.Grzywacz B, Kataria N, Verneris MR. CD56(dim)CD16(+) NK cells downregulate CD16 following target cell induced activation of matrix metalloproteinases. Leukemia. (2007) 21:356–9. 10.1038/sj.leu.2404499 [DOI] [PubMed] [Google Scholar]
- 162.Dowell AC, Oldham KA, Bhatt RI, Lee SP, Searle PF. Long-term proliferation of functional human NK cells, with conversion of CD56(dim) NK cells to a CD56 (bright) phenotype, induced by carcinoma cells co-expressing 4-1BBL and IL-12. Cancer Immunol Immunother. (2012) 61:615–28. 10.1007/s00262-011-1122-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lukassen HG, Joosten I, van Cranenbroek B, van Lierop MJ, Bulten J, Braat DD, et al. Hormonal stimulation for IVF treatment positively affects the CD56bright/CD56dim NK cell ratio of the endometrium during the window of implantation. Mol Hum Reprod. (2004) 10:513–20. 10.1093/molehr/gah067 [DOI] [PubMed] [Google Scholar]
- 164.Meier U-C, Owen RE, Taylor E, Worth A, Naoumov N, Willberg C, et al. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol. (2005) 79:12365–74. 10.1128/JVI.79.19.12365-12374.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sims EA. Are there persons who are obese, but metabolically healthy? Metab Clin Exp. (2001) 50:1499–504. 10.1053/meta.2001.27213 [DOI] [PubMed] [Google Scholar]
- 166.Jung CH, Lee MJ, Hwang JY, Jang JE, Leem J, Yang DH, et al. Association of metabolically healthy obesity with subclinical coronary atherosclerosis in a Korean population. Obesity. (2014) 22:2613–20. 10.1002/oby.20883 [DOI] [PubMed] [Google Scholar]
- 167.Madeira FB, Silva AA, Veloso HF, Goldani MZ, Kac G, Cardoso VC, et al. Normal weight obesity is associated with metabolic syndrome and insulin resistance in young adults from a middle-income country. PLoS ONE. (2013) 8:e60673. 10.1371/journal.pone.0060673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, et al. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J. (2010) 31:737–46. 10.1093/eurheartj/ehp487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Franco LP, Morais CC, Cominetti C. Normal-weight obesity syndrome: diagnosis, prevalence, and clinical implications. Nutr Rev. (2016) 74:558–70. 10.1093/nutrit/nuw019 [DOI] [PubMed] [Google Scholar]
- 170.Wasinski F, Bacurau RF, Moraes MR, Haro AS, Moraes-Vieira PM, Estrela GR, et al. Exercise and caloric restriction alter the immune system of mice submitted to a high-fat diet. Mediators Inflamm. (2013) 2013:395672. 10.1155/2013/395672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Romero V, Zúñiga J, Azocar J, Clavijo OP, Terreros D, Kidwai H, et al. Genetic interactions of KIR and G1M immunoglobulin allotypes differ in obese from non-obese individuals with type 2 diabetes. Mol Immunol. (2008) 45:3857–62. 10.1016/j.molimm.2008.06.004 [DOI] [PubMed] [Google Scholar]
- 172.Guo H, Xu B, Gao L, Sun X, Qu X, Li X, et al. High frequency of activated natural killer and natural killer T-cells in patients with new onset of type 2 diabetes mellitus. Exp Biol Med. (2012) 237:556–62. 10.1258/ebm.2012.011272 [DOI] [PubMed] [Google Scholar]
- 173.Nave H, Beutel G, Kielstein JT. Obesity-related immunodeficiency in patients with pandemic influenza H1N1. Lancet Infect Dis. (2011) 11:14–5. 10.1016/S1473-3099(10)70304-2 [DOI] [PubMed] [Google Scholar]
- 174.Ramírez-Orozco RE, Franco Robles E, Pérez Vázquez V, Ramírez Emiliano J, Hernández Luna MA, López Briones S. Diet-induced obese mice exhibit altered immune responses to early Salmonella Typhimurium oral infection. J Microbiol. (2018) 56:673–82. 10.1007/s12275-018-8083-6 [DOI] [PubMed] [Google Scholar]
- 175.Couturier J, Lewis DE. HIV persistence in adipose tissue reservoirs. Curr HIV AIDS Rep. (2018) 15:60–71. 10.1007/s11904-018-0378-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Furue H, Matsuo K, Kumimoto H, Hiraki A, Suzuki T, Yatabe Y, et al. Decreased risk of colorectal cancer with the high natural killer cell activity NKG2D genotype in Japanese. Carcinogenesis. (2008) 29:316–20. 10.1093/carcin/bgm260 [DOI] [PubMed] [Google Scholar]
- 177.Lang K, Drell TL, Niggemann B, Zänker KS, Entschladen F. Neurotransmitters regulate the migration and cytotoxicity in natural killer cells. Immunol Lett. (2003) 90:165–72. 10.1016/j.imlet.2003.09.004 [DOI] [PubMed] [Google Scholar]
- 178.Michelet X, Dyck L, Hogan A, Loftus RM, Duquette D, Wei K, et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat Immunol. (2018) 19:1330–40. 10.1038/s41590-018-0251-7 [DOI] [PubMed] [Google Scholar]
- 179.Boudreau JE, Hsu KC. Natural killer cell education and the response to infection and cancer therapy: stay tuned. Trends Immunol. (2018) 39:222–39. 10.1016/j.it.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nersesian S, Glazebrook H, Toulany J, Grantham SR, Boudreau JE. Naturally killing the silent killer: nk cell-based immunotherapy for ovarian cancer. Front Immunol. (2019) 10:1782. 10.3389/fimmu.2019.01782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fang F, Xiao W, Tian Z. NK cell-based immunotherapy for cancer. Semin Immunol. (2017) 31:37–54. 10.1016/j.smim.2017.07.009 [DOI] [PubMed] [Google Scholar]
- 182.Hu LJ, Zhao XY, Yu XX, Lv M, Han TT, Han W, et al. Quantity and quality reconstitution of NKG2A+ natural killer cells are associated with graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. (2019) 25:1–11. 10.1016/j.bbmt.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 183.Simonetta F, Alvarez M, Negrin RS. Natural killer cells in graft-versus-Host-Disease after allogeneic hematopoietic cell transplantation. Front Immunol. (2017) 8:465. 10.3389/fimmu.2017.00465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mehta RS, Rezvani K. Chimeric antigen receptor expressing natural killer cells for the immunotherapy of cancer. Front Immunol. (2018) 9:283. 10.3389/fimmu.2018.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Clinthorne JF, Beli E, Duriancik DM, Gardner EM. NK cell maturation and function in C57BL/6 mice are altered by caloric restriction. J Immunol. (2013) 190:712–22. 10.4049/jimmunol.1201837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kelley DS, Daudu PA, Branch LB, Johnson HL, Taylor PC, Mackey B. Energy restriction decreases number of circulating natural killer cells and serum levels of immunoglobulins in overweight women. Eur J Clin Nutr. (1994) 48:9–18. 10.1016/S0271-5317(98)00009-8 [DOI] [PubMed] [Google Scholar]
- 187.Scanga CB, Verde TJ, Paolone AM, Andersen RE, Wadden TA. Effects of weight loss and exercise training on natural killer cell activity in obese women. Med Sci Sports Exerc. (1998) 30:1666–71. 10.1097/00005768-199812000-00002 [DOI] [PubMed] [Google Scholar]
- 188.Barone J, Hebert JR, Reddy MM. Dietary fat and natural-killer-cell activity. Am J Clin Nutr. (1989) 50:861–7. 10.1093/ajcn/50.4.861 [DOI] [PubMed] [Google Scholar]
- 189.Wing EJ, Stanko RT, Winkelstein A, Adibi SA. Fasting-enhanced immune effector mechanisms in obese subjects. Am J Med. (1983) 75:91–6. 10.1016/0002-9343(83)91172-5 [DOI] [PubMed] [Google Scholar]
- 190.Shade ED, Ulrich CM, Wener MH, Wood B, Yasui Y, Lacroix K, et al. Frequent intentional weight loss is associated with lower natural killer cell cytotoxicity in postmenopausal women: possible long-term immune effects. J Am Diet Assoc. (2004) 104:903–12. 10.1016/j.jada.2004.03.018 [DOI] [PubMed] [Google Scholar]
- 191.Nieman DC, Miller AR, Henson DA, Warren BJ, Gusewitch G, Johnson RL, et al. Effects of high- vs moderate-intensity exercise on natural killer cell activity. Med Sci Sports Exerc. (1993) 25:1126–34. 10.1249/00005768-199310000-00008 [DOI] [PubMed] [Google Scholar]
- 192.Kappel M, Tvede N, Galbo H, Haahr PM, Kjaer M, Linstow M, et al. Evidence that the effect of physical exercise on NK cell activity is mediated by epinephrine. J Appl Physiol. (1991) 70:2530–4. 10.1152/jappl.1991.70.6.2530 [DOI] [PubMed] [Google Scholar]
- 193.Fiatarone MA, Morley JE, Bloom ET, Benton D, Solomon GF, Makinodan T. The effect of exercise on natural killer cell activity in young and old subjects. J Gerontol. (1989) 44:M37–45. 10.1093/geronj/44.2.M37 [DOI] [PubMed] [Google Scholar]
- 194.Crist DM, Mackinnon LT, Thompson RF, Atterbom HA, Egan PA. Physical exercise increases natural cellular-mediated tumor cytotoxicity in elderly women. Gerontology. (1989) 35:66–71. 10.1159/000213001 [DOI] [PubMed] [Google Scholar]
- 195.Brahmi Z, Csipo I, Bochan MR, Su B, Montel AH, Morse PA. Synergistic inhibition of human cell-mediated cytotoxicity by complement component antisera indicates that target cell lysis may result from an enzymatic cascade involving granzymes and perforin. Nat Immun. (1995) 14:271–85. [PubMed] [Google Scholar]
- 196.Moro-García MA, Fernández-García B, Echeverría A, Rodríguez-Alonso M, Suárez-García FM, Solano-Jaurrieta JJ, et al. Frequent participation in high volume exercise throughout life is associated with a more differentiated adaptive immune response. Brain Behav Immun. (2014) 39:61–74. 10.1016/j.bbi.2013.12.014 [DOI] [PubMed] [Google Scholar]
- 197.Berk LS, Nieman DC, Youngberg WS, Arabatzis K, Simpson-Westerberg M, Lee JW, et al. The effect of long endurance running on natural killer cells in marathoners. Med Sci Sports Exerc. (1990) 22:207–12. [PubMed] [Google Scholar]
- 198.Nieman DC, Nehlsen-Cannarella SL, Markoff PA, Balk-Lamberton AJ, Yang H, Chritton DB, et al. The effects of moderate exercise training on natural killer cells and acute upper respiratory tract infections. Int J Sports Med. (1990) 11:467–73. 10.1055/s-2007-1024839 [DOI] [PubMed] [Google Scholar]
- 199.Nieman DC, Buckley KS, Henson DA, Warren BJ, Suttles J, Ahle JC, et al. Immune function in marathon runners versus sedentary controls. Med Sci Sports Exerc. (1995) 27:986–92. 10.1249/00005768-199507000-00006 [DOI] [PubMed] [Google Scholar]
- 200.Pedersen BK, Tvede N, Christensen LD, Klarlund K, Kragbak S, Halkjr-Kristensen J. Natural killer cell activity in peripheral blood of highly trained and untrained persons. Int J Sports Med. (1989) 10:129–31. 10.1055/s-2007-1024888 [DOI] [PubMed] [Google Scholar]
- 201.Woods JA, Ceddia MA, Wolters BW, Evans JK, Lu Q, McAuley E. Effects of 6 months of moderate aerobic exercise training on immune function in the elderly. Mech Ageing Dev. (1999) 109:1–19. 10.1016/S0047-6374(99)00014-7 [DOI] [PubMed] [Google Scholar]
- 202.McFarlin BK, Flynn MG, Phillips MD, Stewart LK, Timmerman KL. Chronic resistance exercise training improves natural killer cell activity in older women. J Gerontol A Biol Sci Med Sci. (2005) 60:1315–8. 10.1093/gerona/60.10.1315 [DOI] [PubMed] [Google Scholar]
- 203.Wang J-S, Weng T-P. Hypoxic exercise training promotes antitumour cytotoxicity of natural killer cells in young men. Clin Sci. (2011) 121:343–53. 10.1042/CS20110032 [DOI] [PubMed] [Google Scholar]
- 204.Idorn M, Hojman P. Exercise-dependent regulation of NK cells in cancer protection. Trends Mol Med. (2016) 22:565–77. 10.1016/j.molmed.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 205.Millard A-L, Valli PV, Stussi G, Mueller NJ, Yung GP, Seebach JD. Brief exercise increases peripheral blood NK cell counts without immediate functional changes, but impairs their responses to ex vivo stimulation. Front Immunol. (2013) 4:125. 10.3389/fimmu.2013.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Schwindt CD, Zaldivar F, Wilson L, Leu S-Y, Wang-Rodriguez J, Mills PJ, et al. Do circulating leucocytes and lymphocyte subtypes increase in response to brief exercise in children with and without asthma? Br J Sports Med. (2007) 41:34–40. 10.1136/bjsm.2006.030205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gabriel H, Urhausen A, Kindermann W. Mobilization of circulating leucocyte and lymphocyte subpopulations during and after short, anaerobic exercise. Eur J Appl Physiol Occup Physiol. (1992) 65:164–70. 10.1007/BF00705075 [DOI] [PubMed] [Google Scholar]
- 208.Nielsen HB, Secher NH, Christensen NJ, Pedersen BK. Lymphocytes and NK cell activity during repeated bouts of maximal exercise. Am J Physiol. (1996) 271:R222–7. 10.1152/ajpregu.1996.271.1.R222 [DOI] [PubMed] [Google Scholar]
- 209.Kakanis MW, Peake J, Brenu EW, Simmonds M, Gray B, Hooper SL, et al. The open window of susceptibility to infection after acute exercise in healthy young male elite athletes. Exerc Immunol Rev. (2010) 16:119–37. 10.1016/j.jsams.2010.10.642 [DOI] [PubMed] [Google Scholar]
- 210.Suzui M, Kawai T, Kimura H, Takeda K, Yagita H, Okumura K, et al. Natural killer cell lytic activity and CD56(dim) and CD56(bright) cell distributions during and after intensive training. J Appl Physiol. (2004) 96:2167–73. 10.1152/japplphysiol.00513.2003 [DOI] [PubMed] [Google Scholar]
- 211.Zimmer P, Bloch W, Schenk A, Zopf EM, Hildebrandt U, Streckmann F, et al. Exercise-induced natural killer cell activation is driven by epigenetic modifications. Int J Sports Med. (2015) 36:510–5. 10.1055/s-0034-1398531 [DOI] [PubMed] [Google Scholar]