Abstract
Glucagon-like peptide-1 receptor agonists (GLP-1RA) are effective agents for achieving glycemic control. Oral semaglutide is the first oral formulation of a GLP-1RA to be approved in the USA. This agent may lead to earlier initiation of GLP-1RA therapy in the type 2 diabetes continuum of care, and represents a valuable treatment option for patients with a preference for oral therapy. The efficacy and safety of oral semaglutide was assessed in the PIONEER clinical trial program, which included 9543 patients (1293 Japanese). The program included 10 trials, two of which were conducted specifically in Japan. Across the whole program, oral semaglutide was shown to be effective in helping patients achieve glycemic control and reducing body weight. The highest approved dose of oral semaglutide (14 mg) reduced glycated hemoglobin significantly more than placebo, empagliflozin, dulaglutide, and sitagliptin, and was non-inferior to liraglutide. Superior reductions in body weight were also observed with oral semaglutide 14 mg compared with placebo, sitagliptin, and liraglutide, and similar body weight reductions were seen vs. empagliflozin. In all the PIONEER trials, oral semaglutide was well tolerated; there were no unexpected safety concerns and the safety profile was consistent with other GLP-1RAs. Oral semaglutide also demonstrated a favorable cardiovascular safety profile, and significant reductions in cardiovascular death and all-cause mortality vs. placebo in the PIONEER 6 trial. Oral semaglutide, therefore, represents an effective treatment option, that may lead to earlier initiation of GLP-1RA therapy in the diabetes treatment landscape.
Keywords: Oral semaglutide, Glucagon-like peptide-1 receptor agonist, Empagliflozin, Sitagliptin, Dulaglutide, Liraglutide, Cardiovascular outcomes
Introduction
Timely and adequate glycemic control is needed to reduce the risk of diabetes-related complications in patients with type 2 diabetes [1–4]. However, many patients do not achieve adequate glucose lowering with the currently available treatment options [5–8]. As a result, in Japan as well as other countries, there is a need for additional effective treatment options to help more patients achieve good glycemic control.
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) can provide effective glycemic control [9, 10]. Moreover, these agents appear to be more effective at reducing glycated hemoglobin (HbA1c) in studies investigating Japanese patients [11, 12] than in studies in global populations [13, 14]. In addition to providing effective glycemic control, GLP-1RAs are associated with a low risk of hypoglycemia and can promote weight reduction [9, 10] and several GLP-1RAs have also shown a beneficial effect on cardiovascular outcomes [15–18]. In fact, the cardiovascular benefit of some GLP-1RAs is now recognized in guidelines [10, 19, 20], although cardiovascular outcomes trials have not yet been specifically conducted in Japanese patients. Consequently, these agents represent a valuable therapeutic approach for patients with type 2 diabetes.
GLP-1RAs are currently available as once-daily, twice-daily, or once-weekly subcutaneous injections. An oral formulation of a GLP-1RA represents a useful option to help improve acceptance and adherence compared with injectable formulations in those patients with a preference for oral therapy, and it may lead to earlier initiation of these agents in the continuum of the disease. Here the development of a novel oral formulation of a GLP-1RA, oral semaglutide, is reviewed.
Development of oral semaglutide
Semaglutide was designed as a potent, long-acting GLP-1 analog that could be administered by subcutaneous injection once-weekly, rather than subcutaneous injection once-daily, to improve convenience. It has also demonstrated superior efficacy compared with other GLP-1RAs [21–23]. Semaglutide has 94% sequence homology with native GLP-1 [24] and three key structural differences that confer improved albumin affinity and resistance to dipeptidyl peptidase-4 degradation. These differences prolong the half-life of semaglutide to approximately 1 week, without compromising GLP-1 receptor binding [25]. Once-weekly subcutaneous semaglutide has been shown to be effective in improving glycemic control, reducing body weight [11, 13, 21, 26], and reducing the risk of cardiovascular events [15].
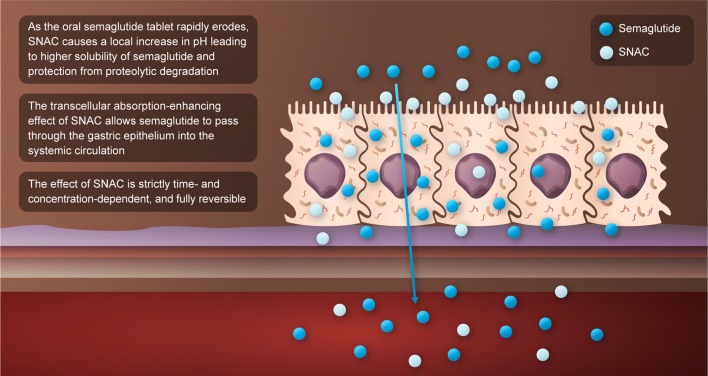
Oral delivery of protein-based drugs, like semaglutide, is limited by extensive degradation by proteolytic enzymes in the gastrointestinal tract and poor absorption across the gastrointestinal epithelium [27]. To achieve adequate bioavailability of semaglutide after oral administration, oral semaglutide has been co-formulated with 300 mg of the absorption enhancer, sodium N-(8-[2-hydroxybenzoyl] amino) caprylate (SNAC), a small fatty acid derivative that protects semaglutide against enzymatic degradation via a local pH buffering effect, and promotes absorption of semaglutide across the gastric epithelium in a concentration-dependent manner by effects on transcellular pathways, which are transient and fully reversible (Fig. 1). This absorption of semaglutide is highly localized and depends on the spatial proximity of semaglutide and SNAC [28]. The long half-life of semaglutide of approximately 1 week helps maintain exposure in the event of any variation in day-to-day absorption of the oral formulation.
Fig. 1.
Mode of action of oral semaglutide. SNAC, sodium N-(8-[2-hydroxybenzoyl] amino) caprylate
Dosing conditions
The dosing conditions for oral semaglutide are based on results from clinical pharmacology trials in healthy subjects. In these trials, food was shown to adversely impact the absorption of oral semaglutide and sufficient exposure was only achieved when oral semaglutide was administered in a fasting state [29]. In addition, the systemic exposure and time to maximum concentration for oral semaglutide increased with longer post-dose fasting periods; in subjects randomized to 15, 30, 60, or 120 min post-dose fasting, semaglutide exposure was significantly lower with a post-dose fasting period of 15 min compared with 30 min, but there was no significant difference between 30- and 60-min post-dose fasting [30]. Systemic absorption of oral semaglutide occurred early and semaglutide exposure was unaffected whether the volume of water used for administration of the tablet was 50 mL or 120 mL [30]. However, a scintigraphic study indicated that the oral semaglutide tablet erosion was slower and the exposure to semaglutide was greater when administering the tablet with 50 mL compared with 240 mL of water [31].
Based on these findings, across phase 3a clinical trials patients were instructed to take oral semaglutide in the morning in a fasting state, with up to half a glass of water (approximately 120 mL [~ 4 oz]), and to wait at least 30 min before eating, drinking, or taking any other oral medication. These dosing conditions result in clinically relevant semaglutide exposure, as validated in the confirmatory phase 3a trials reported here, and are expected to be acceptable to most patients in a real-world setting.
Clinical pharmacology
Oral semaglutide has been shown to be suitable for once-daily dosing in healthy volunteers and patients with type 2 diabetes [32]. In addition, several clinical pharmacology studies have been conducted to better understand how the exposure of semaglutide following oral administration is influenced by comorbidities or other medication, and how oral semaglutide might impact the exposure of concomitant medications.
Hepatic or renal impairment may influence the pharmacokinetics of medications and since these conditions may occur in patients with diabetes, their effect on the pharmacokinetics of oral semaglutide were assessed. In patients with renal or hepatic impairment no apparent effect was observed on the pharmacokinetics and tolerability of oral semaglutide, suggesting that dose adjustment is not necessary in these special populations [33, 34].
For a product that is absorbed in the stomach, and which may be sensitive to the local gastric environment, it was also important to assess the potential impact of upper gastrointestinal disease on exposure to oral semaglutide. In a study in patients with type 2 diabetes and with or without upper gastrointestinal disease, no significant difference in semaglutide exposure was observed following administration of oral semaglutide [35]. Similarly, it was important to assess the potential impact of medications that may alter gastric pH on the absorption of oral semaglutide. A drug–drug interaction study with the proton pump inhibitor, omeprazole, in healthy subjects noted that there was a slight non-statistically significant increase in semaglutide exposure when oral semaglutide was administered with omeprazole at the time of maximum anti-secretory effect, but this was not considered clinically relevant [36].
The effect of oral semaglutide on exposure to various medications commonly used by patients with type 2 diabetes has also been studied. When co-administered, oral semaglutide had no clinically relevant effect on the exposure of lisinopril, warfarin, and digoxin in healthy subjects [37]. Co-administration with oral semaglutide resulted in small changes in exposure to metformin [37], furosemide, and rosuvastatin [38], but these are not expected to be clinically relevant [37, 38]. Similarly, co-administration of oral semaglutide with levothyroxine resulted in an increase in thyroxine exposure [39]. Monitoring of thyroid parameters should be considered when treating patients with concomitant oral semaglutide and levothyroxine. Oral semaglutide did not affect the exposure of ethinylestradiol and levonorgestrel [40].
Phase 2 dose-finding study
A multinational, phase 2, randomized trial assessed the dose–response relationship of five doses (ranging from 2.5 to 40 mg) of once-daily oral semaglutide compared with placebo and once-weekly subcutaneous semaglutide in 632 patients with type 2 diabetes uncontrolled using diet and exercise alone or a stable dose of metformin [41]. Mean HbA1c levels decreased from baseline to week 26 in a dose-dependent manner (from − 0.7 to − 1.9%) with oral semaglutide, and these reductions were significantly greater compared with placebo (− 0.3%). The decreases in HbA1c achieved with the two highest doses of once-daily oral semaglutide (20 and 40 mg) were similar to those achieved with subcutaneous semaglutide 1 mg once-weekly. Reductions in body weight were also greater with oral semaglutide vs. placebo, and significant for oral semaglutide doses of 10 mg once-daily or more. As expected for a GLP-1RA, the most frequent adverse events were mild-to-moderate gastrointestinal adverse events. Nausea events appeared to occur less frequently when patients were initiated at a lower dose (2.5 mg) vs. a higher dose (5 mg) of oral semaglutide.
Based on the findings of the phase 2 trial, three dose levels were selected for the phase 3a program, which were expected to have the optimal benefit–risk profile: 3, 7, and 14 mg once-daily. In each trial, oral semaglutide treatment was initiated with the lowest dose and a 4-week dose escalation was used to reduce the risk of gastrointestinal adverse events.
Phase 3 PIONEER program
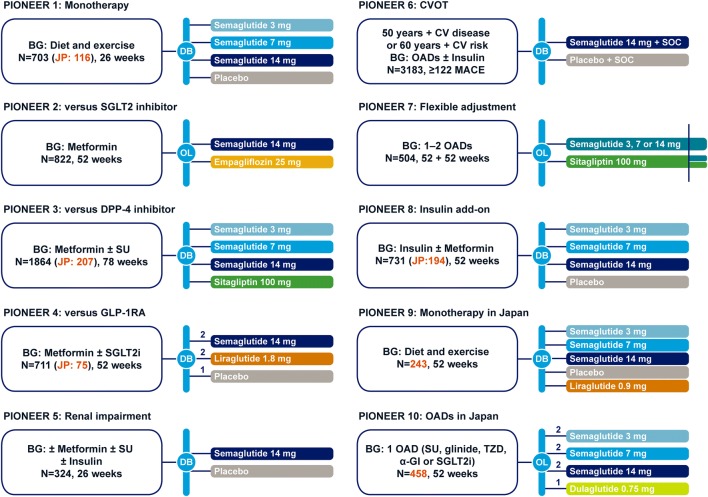
The PIONEER program consisted of 10 trials, including a pre-approval cardiovascular outcomes trial, and was designed to evaluate the efficacy and safety of oral semaglutide following treatment in a large and broad population of patients with type 2 diabetes (Fig. 2). The program included eight global trials, four of which included Japanese patients, and two trials conducted in Japan alone. All trials were initiated in 2016 with the main treatment periods completing in 2018.
Fig. 2.
PIONEER clinical trial program. Time to primary endpoint: 26 weeks for PIONEER 1, 2, 3, 4, 5, and 8 trials. BG, background medication; CV, cardiovascular; CVOT, cardiovascular outcomes trial; DB, double-blind; DPP-4i, dipeptidyl peptidase-4 inhibitor; α-GI, α-glucosidase inhibitor; GLP-1RA, glucagon-like peptide-1 receptor agonist; JP, Japan; MACE, major adverse cardiovascular event; OAD, oral anti-diabetes drug; OL, open-label; PIONEER, peptide innovation for early diabetes treatment; SGLT2i, sodium− glucose cotransporter-2 inhibitor; SOC, standard of care; SU, sulfonylurea; TZD, thiazolidinedione
A total of 9543 patients (including 1293 Japanese patients) were enrolled across the program, including those with early and advanced disease, different background treatments (drug-naïve, add-on to metformin and other oral glucose-lowering drugs, add-on to insulin), and different comparators (placebo, empagliflozin, sitagliptin, liraglutide, and/or dulaglutide). Two trials specifically recruited patients with type 2 diabetes and complications (renal impairment in PIONEER 5 and high cardiovascular risk in PIONEER 6, the cardiovascular outcomes trial). For the majority of the PIONEER studies, the primary endpoint was the change in HbA1c at 26 weeks. However, the primary endpoint differed for PIONEER 7 (patients achieving HbA1c target of < 7.0% at week 52), PIONEER 6 (time to first major adverse cardiovascular event [MACE]) and PIONEER 10 (number of treatment-emergent adverse events up to week 57). Change in body weight was the confirmatory secondary endpoint in PIONEER 1–5, 7, 8, and 9 trials.
The PIONEER studies employed estimands to understand the treatment effects of oral semaglutide. An estimand is a concept introduced in regulatory guidance from the International Council for Harmonisation in 2014 and revised in 2017, and reflects what is to be estimated to address the scientific question of interest posed by a clinical trial [42, 43]. Estimands prespecify how intercurrent events will be handled, as well as describing the population and endpoint of interest, and population level summary, to align with the study objectives and allow better interpretation of treatment effects and how they may vary under different conditions. In the PIONEER program, two different scientific questions related to the efficacy objectives were addressed through the definition of two estimands: ‘treatment policy’ and ‘trial product’. The treatment policy estimand evaluates the treatment effect for all randomized patients regardless of trial product discontinuation and/or addition of or switch to another glucose-lowering drug and reflects the intention-to-treat principle. The trial product estimand addresses the treatment effect for patients who continued on trial product without the use of rescue medication. The use of estimands in the PIONEER program is explained in more detail by Aroda et al. [44]. All data reported below refer to the treatment policy estimand unless otherwise stated. The treatment policy estimand was the primary estimand in all PIONEER trials except for PIONEER 9, for which the trial product estimand was the primary estimand as per agreement with the Japanese Pharmaceuticals and Medical Devices Agency.
PIONEER 1
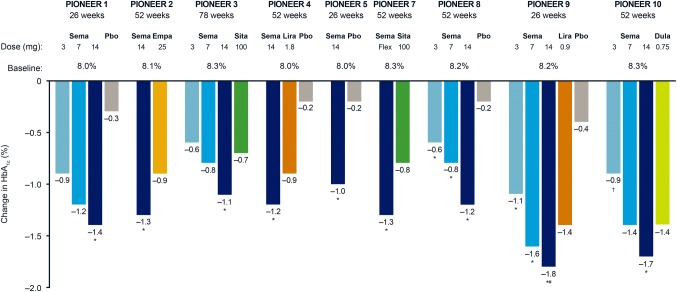
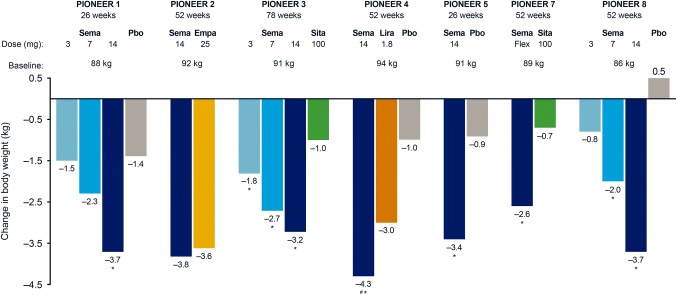
PIONEER 1 was a 26-week, multinational trial in which 703 patients (including 116 from Japan) with type 2 diabetes uncontrolled with diet and exercise were randomized to double-blind monotherapy with oral semaglutide 3, 7, or 14 mg, or placebo [45]. In PIONEER 1, all three doses of oral semaglutide resulted in clinically meaningful and superior reductions in HbA1c compared with placebo (placebo-adjusted estimated treatment differences ranging from 0.6 to 1.2%) (Fig. 3). Oral semaglutide helped 55.1% to 76.9% of patients achieve HbA1c < 7%. The highest dose of oral semaglutide (14 mg) provided superior reductions in body weight compared with placebo (placebo-adjusted estimated treatment difference of 2.3 kg) (Fig. 4) and significantly more patients achieved body weight loss ≥ 5% with oral semaglutide 7 and 14 mg (26.9% and 41.3%, respectively) vs. placebo (14.9%). The efficacy results for the trial product estimand were generally consistent with that observed for the treatment policy estimand.
Fig. 3.
Change in HbA1c from baseline with oral semaglutide versus comparators in PIONEER trials. All data are for the treatment policy estimand at the end of treatment, except PIONEER 9, which are at week 26 (of a 52-week trial). The primary estimand for PIONEER 9 was the trial product estimand. *Statistically significant estimated treatment difference (p < 0.05) in favor of oral semaglutide versus competitor or placebo; #Statistically significant estimated treatment difference (p < 0.05) in favor of oral semaglutide versus competitor and placebo; †Statistically significant estimated treatment difference (p < 0.05) in favor of competitor versus oral semaglutide. Dula, dulaglutide; empa, empagliflozin; HbA1c, glycated haemoglobin; lira, liraglutide; pbo, placebo; PIONEER, peptide innovation for early diabetes treatment; sema, semaglutide; sita, sitagliptin
Fig. 4.
Change in body weight from baseline with oral semaglutide versus comparators in PIONEER trials. All data are for the treatment policy estimand at the end of treatment. Body weight changes have not previously been published for PIONEER 9 or PIONEER 10 and so are excluded. *Statistically significant estimated treatment difference (p < 0.05) in favor of oral semaglutide versus competitor or placebo; #Statistically significant difference (p < 0.05) in favor of oral semaglutide versus competitor and placebo. Empa, empagliflozin; lira, liraglutide; pbo, placebo; PIONEER, peptide innovation for early diabetes treatment; sema, semaglutide; sita, sitagliptin
Oral semaglutide was well tolerated with a safety profile consistent with subcutaneous semaglutide and the known adverse effects of GLP-1RAs. The most frequent adverse events were gastrointestinal disorders, which were mostly transient, mild-to-moderate in severity, and occurred mostly during the dose-escalation period. The proportion of patients with at least one severe or blood− glucose-confirmed (< 56 mg/dL [3.1 mmol/L]) symptomatic hypoglycemic episode event was low (2.9%, 1.1%, and 0.6% with oral semaglutide 3, 7, and 14 mg, respectively, and 0.6% with placebo).
PIONEER 2
The efficacy and safety of oral semaglutide 14 mg was compared with the sodium− glucose cotransporter protein 2 inhibitor empagliflozin 25 mg in 822 patients with type 2 diabetes who were uncontrolled on metformin in the 52-week, multinational, randomized, open-label PIONEER 2 trial [46]. Oral semaglutide provided superior reductions in HbA1c vs. empagliflozin at week 26 (estimated treatment difference of 0.4%) and this effect was sustained at the end of treatment at week 52 (Fig. 3). Body weight loss with oral semaglutide was not superior to empagliflozin at week 26 and not significant at week 52 (Fig. 4). However, for the trial product estimand, which reflects the treatment effect without the confounding influence of rescue medication and treatment discontinuations, body weight loss with oral semaglutide (− 4.7 kg) was significantly greater than with empagliflozin (− 3.8 kg) at week 52.
Oral semaglutide was well tolerated with the proportion of patients reporting adverse events similar to empagliflozin. There were fewer serious adverse events with oral semaglutide than empagliflozin.
PIONEER 3
PIONEER 3 was a 78-week, multinational, randomized, double-blind trial that compared the long-term efficacy, safety, and tolerability of oral semaglutide 3, 7, or 14 mg with the dipeptidyl peptidase-4 inhibitor, sitagliptin, in 1864 patients (including 207 patients from Japan) with type 2 diabetes uncontrolled on metformin with or without a sulfonylurea [47]. Oral semaglutide 7 and 14 mg resulted in superior HbA1c reductions vs. sitagliptin at week 26 (estimated treatment differences of − 0.3% and − 0.5%, respectively), with this effect maintained to week 78 for the 14 mg dose (Fig. 3). Proportions of patients achieving HbA1c < 7.0% were significantly greater with oral semaglutide 7 and 14 mg than with sitagliptin at weeks 26 (42% [7 mg] and 55% [14 mg] vs. 32% [sitagliptin]) and 78 (37% [7 mg] and 44% [14 mg] vs. 29% [sitagliptin]). Oral semaglutide reduced body weight vs. sitagliptin at week 26, with superiority confirmed for the 7 and 14 mg doses (estimated treatment differences − 1.6 and − 2.5 kg, respectively). Superiority was not tested for the 3 mg dose in accordance with the hierarchical testing strategy. The effect of all three doses of oral semaglutide on body weight over sitagliptin was conserved at end of treatment (week 78) (Fig. 4). In general, efficacy results by the treatment policy estimand were mostly consistent with the trial product estimand.
The overall proportion of patients reporting adverse events was similar across treatment groups and the most frequent adverse events were mild-to-moderate gastrointestinal events and infections and infestations. Thus, the long-term safety profile of oral semaglutide was consistent with what is expected for the GLP-1RA class.
PIONEER 4
PIONEER 4, was a 52-week, randomized (2:2:1), controlled, double-blind trial that assessed the efficacy and safety of oral semaglutide 14 mg compared with subcutaneous liraglutide 1.8 mg or placebo in 711 patients (75 patients from Japan) with type 2 diabetes on a stable dose of metformin with or without a sodium− glucose cotransporter protein 2 inhibitor [48]. Oral semaglutide 14 mg was non-inferior to liraglutide 1.8 mg in reducing HbA1c from baseline (estimated treatment difference of − 0.1%) and superior to placebo (estimated treatment difference of − 1.1%) at week 26. At 52 weeks, oral semaglutide provided significantly greater reductions in HbA1c than liraglutide 1.8 mg (estimated treatment difference of − 0.3%) or placebo (estimated treatment difference of − 1.0%) (Fig. 3). Superior reductions in body weight were seen for oral semaglutide vs. liraglutide 1.8 mg (estimated treatment difference of − 1.2 kg) and placebo at week 26 (estimated treatment difference of − 3.8 kg), with this effect conserved at week 52 (Fig. 4). Efficacy results for the trial product estimand were broadly consistent with the treatment policy estimand.
Safety and tolerability of oral semaglutide were consistent with subcutaneous liraglutide, with the most frequent adverse events being gastrointestinal events that were generally of mild-to-moderate severity.
PIONEER 5
Type 2 diabetes is commonly associated with renal impairment, which can restrict treatment options. The PIONEER 5 trial, therefore, evaluated the efficacy and safety of oral semaglutide 14 mg vs. placebo once-daily in patients with type 2 diabetes and moderate renal impairment [49]. A total of 324 patients on stable doses of metformin and/or sulfonylurea and/or insulin were randomized to 52 weeks of treatment with oral semaglutide 14 mg or placebo. Superior reductions in HbA1c (estimated treatment difference of − 0.8%) (Fig. 3) and body weight (estimated treatment difference of − 2.5 kg) (Fig. 4) were seen with oral semaglutide vs. placebo at 26 weeks. Oral semaglutide resulted in 58% of patients achieving HbA1c < 7.0% and 36% achieving a body weight loss of ≥ 5%. The odds of achieving HbA1c < 7.0% and body weight loss ≥ 5% were significantly greater with oral semaglutide than with placebo.
More patients in the oral semaglutide group than placebo group had adverse events, and discontinued treatment as a result. The most common adverse events with oral semaglutide were gastrointestinal, and mainly mild-to-moderate nausea events. The overall safety profile, including renal safety, was consistent with that seen for other GLP-1RAs, and few blood− glucose-confirmed symptomatic hypoglycemic episodes occurred (nine [6%] with oral semaglutide vs. three [2%] with placebo), none of which were severe.
PIONEER 7
PIONEER 7 was a randomized, open-label, 52-week trial that compared the efficacy and safety of flexible dose adjustments with oral semaglutide 3, 7, or 14 mg once-daily vs. sitagliptin 100 mg once-daily in 504 patients with type 2 diabetes inadequately controlled on 1–2 oral glucose-lowering agents [50]. Oral semaglutide dose adjustment was performed at week 8, and every 8 weeks thereafter, based on pre-specified HbA1c and tolerability criteria. Among those who remained on-treatment with flexible dosing of oral semaglutide at week 52, 9%, 30%, and 59% were receiving oral semaglutide 3, 7, and 14 mg, respectively. More patients achieved HbA1c < 7.0% with oral semaglutide flexible dose adjustments (58%) compared with placebo (25%), and oral semaglutide was superior to sitagliptin for the odds of patients achieving HbA1c < 7% at week 52. Significantly greater reductions in HbA1c and body weight were seen with oral semaglutide flexible dosing vs. sitagliptin at week 52 (estimated treatment difference in HbA1c of − 0.5% and body weight of − 1.9 kg) (Figs. 3, 4). Efficacy results for the trial product estimand were broadly consistent with the treatment policy estimand.
The number of adverse events and proportion of patients who had adverse events were higher in the oral semaglutide group than in the sitagliptin group, with the most frequently reported being gastrointestinal events, most commonly nausea and diarrhea that were predominantly mild-to-moderate in severity and of short duration.
PIONEER 8
PIONEER 8 evaluated the efficacy and safety of three doses of oral semaglutide once-daily vs. placebo added to insulin treatment, with or without metformin, in 731 patients (194 patients from Japan) with type 2 diabetes [51]. A 20% reduction in total daily insulin dosage was recommended at randomization and maintained to week 8. The treatment period was then split into two insulin dosing stages: a capped insulin period during which total daily insulin dosage was not to exceed the dosage at randomization (weeks 8–26), followed by a period during which total daily insulin dosage was freely adjustable at the discretion of the investigator (weeks 26–52). Superior HbA1c reductions were seen with all doses of oral semaglutide vs. placebo at week 26 (placebo-adjusted estimated treatment differences ranging from − 0.6 to − 1.3%) and these were maintained at week 52 (Fig. 3). This occurred in the context of total daily insulin dose being significantly reduced from baseline with oral semaglutide vs. placebo at week 26 (except 3 mg) and week 52. Body weight was also significantly reduced with oral semaglutide compared with placebo at week 26 (placebo-adjusted estimated treatment differences ranging from − 0.9 to − 3.3 kg), and also at week 52 (Fig. 4).
Comparable proportions of patients experienced at least one adverse event across treatment groups. Consistent with other GLP-1RAs, gastrointestinal disorders, specifically nausea, were the most frequent adverse events with oral semaglutide. The incidence of severe or blood− glucose-confirmed symptomatic hypoglycemia with oral semaglutide was similar to that observed with placebo.
PIONEER 9 and PIONEER 10
Both the PIONEER 9 and 10 trials were conducted solely in Japan. PIONEER 9 was a 52-week, phase 2/3a, randomized, controlled trial to assess the efficacy, dose response, and safety of oral semaglutide monotherapy (3, 7, or 14 mg) compared with placebo and liraglutide 0.9 mg in 243 Japanese patients with type 2 diabetes uncontrolled on diet and exercise or one oral glucose-lowering drug (washed out during run-in) [52]. The estimated changes from baseline in HbA1c were significantly greater with oral semaglutide 3, 7, and 14 mg compared with placebo at week 26 (estimated treatment differences ranging from − 1.1 to − 1.7%), and were significantly greater with oral semaglutide 14 mg compared with liraglutide 0.9 mg (estimated treatment difference of − 0.3%), for the trial product estimand. Glycemic efficacy results for the treatment policy estimand were consistent with the trial product estimand (Fig. 3). The safety profile of oral semaglutide was consistent with the GLP-1RA class.
PIONEER 10 was a 52-week, randomized, open-label, safety trial that compared the safety and efficacy of oral semaglutide 3, 7, and 14 mg vs. dulaglutide 0.75 mg in 458 patients on one background glucose-lowering drug [53]. The primary endpoint was the number of adverse events. Oral semaglutide 3, 7, and 14 mg doses were well tolerated over 52 weeks, with similar overall rates of adverse events as dulaglutide 0.75 mg, the most frequent being mild gastrointestinal events. Using the treatment policy estimand, reductions in HbA1c with oral semaglutide 7 mg were similar to those observed with dulaglutide 0.75 mg (estimated treatment difference of − 0.1%), and the 14 mg dose of oral semaglutide reduced HbA1c more than dulaglutide (estimated treatment difference of − 0.4%) at week 26, with consistent results reported at week 52 (Fig. 3). For the trial product estimand, glycemic efficacy results were consistent with the treatment policy estimand.
PIONEER 6
Cardiovascular safety of once-daily oral semaglutide was assessed in PIONEER 6, an event-driven cardiovascular outcomes trial [54]. A total of 3183 patients with type 2 diabetes at high cardiovascular risk (age of ≥ 50 years with established cardiovascular and/or chronic kidney disease, or aged ≥ 60 years with cardiovascular risk factors only) were randomized to double-blind treatment with oral semaglutide or placebo added on to standard of care. The trial completed after the accumulated occurrence of 137 primary MACEs (cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke) and a median follow-up time of 16 months.
The primary endpoint was achieved, with oral semaglutide demonstrating non-inferiority (p < 0.001) of MACE compared with placebo. Encouragingly, oral semaglutide resulted in a 21% reduction in MACE, but this did not reach statistical significance due to the relatively few events. Significant reductions in cardiovascular death and all-cause mortality with oral semaglutide vs. placebo were also observed. Oral semaglutide reduced HbA1c from baseline to the end of the trial by 1.0%, compared with 0.3% with placebo. The mean change in body weight from baseline to the end of study was − 4.2 kg with oral semaglutide and − 0.8 kg with placebo. No unexpected adverse events were identified with oral semaglutide and tolerability was as expected for a GLP-1RA. Recent European Society of Cardiology guidelines recommend semaglutide to reduce cardiovascular events in patients with cardiovascular disease or those at high risk and do not distinguish between the oral and subcutaneous formulations [20].
Conclusions
Oral semaglutide is the first GLP-1RA to be approved for oral administration; approval was granted in the USA in September 2019, with additional regulatory submissions in Japan, Europe, and elsewhere. In the PIONEER program, oral semaglutide was administered in the morning in a fasted state, with up to half a glass of water (120 mL [~ 4 oz]), and waiting 30 min or longer before consuming food, drink, or other oral medications. These dosing conditions were validated in the PIONEER clinical trials and are expected to be acceptable to most patients [50].
The PIONEER clinical trial program included several studies that enrolled Japanese patients. Across the whole program, oral semaglutide 14 mg was shown to reduce HbA1c significantly more than placebo, empagliflozin, and sitagliptin, and was non-inferior to liraglutide. In the Japanese PIONEER trials, oral semaglutide 14 mg demonstrated greater reductions in HbA1c vs. liraglutide 0.9 mg or dulaglutide 0.75 mg, and the 7 mg dose reduced HbA1c to a similar extent as dulaglutide 0.75 mg. Superior reductions in body weight were also observed when oral semaglutide 14 mg was compared with placebo, sitagliptin, and liraglutide; similar body weight reductions were seen vs. empagliflozin. The beneficial effect of oral semaglutide on achieving glycemic control and reducing body weight vs. sitagliptin was observed even when it was administered with flexible dose adjustments, reflecting a real-world dose setting. Results were also generally consistent whether evaluated according to the treatment policy estimand (regardless of trial product discontinuation or rescue medication use) or the trial product estimand (patient continuing on trial product and without rescue medication).
In all the PIONEER trials, oral semaglutide was well tolerated, with an adverse event profile consistent with other GLP-1RAs delivered by subcutaneous administration. There were no unexpected safety concerns in the individual trials and the safety profile of oral semaglutide appeared to be acceptable for use in patients with moderate renal impairment. In Japanese patients, oral semaglutide was also well tolerated, with comparable numbers of adverse events observed with oral semaglutide vs. dulaglutide and a safety profile consistent with injectable GLP-1RAs. Oral semaglutide demonstrated a favorable cardiovascular safety profile and significant reduction in cardiovascular death and all-cause mortality vs. placebo, both in addition to standard care, in the PIONEER 6 trial.
Many patients with type 2 diabetes prefer oral treatment to injectable therapies. Oral semaglutide, therefore, represents an effective treatment option, which may lead to earlier initiation of GLP-1RA therapy in the diabetes treatment continuum of care.
Acknowledgements
The author thanks Morten Donsmark and Tine Bækdal (both Novo Nordisk A/S, Søborg, Denmark), and Kazushiro Fujiwara, and Eirik Quamme Bergan (both Novo Nordisk Pharma Ltd, Tokyo, Japan) for reviewing the manuscript, and to Andy Bond of Spirit Medical Communications Group Ltd for medical writing and editorial assistance (funded by Novo Nordisk A/S, Søborg, Denmark).
Compliance with ethical standards
Conflict of interest
Mads Frederik Rasmussen is an employee of and owns shares in Novo Nordisk A/S. This article does not contain any studies with human or animal subjects performed by the author.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.United Kingdom Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 2.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 3.Haneda M, Noda M, Origasa H, Noto H, Yabe D, Fujita Y, Goto A, Kondo T, Araki E. Japanese clinical practice guideline for diabetes 2016. Diabetol Int. 2018;9(1):1–45. doi: 10.1007/s13340-018-0345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laiteerapong N, Ham SA, Gao Y, Moffet HH, Liu JY, Huang ES, Karter AJ. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (The Diabetes & Aging Study) Diabetes Care. 2019;42(3):416–426. doi: 10.2337/dc17-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokoyama H, Oishi M, Takamura H, Yamasaki K, Shirabe SI, Uchida D, Sugimoto H, Kurihara Y, Araki SI, Maegawa H. Large-scale survey of rates of achieving targets for blood glucose, blood pressure, and lipids and prevalence of complications in type 2 diabetes (JDDM 40) BMJ Open Diabetes Res Care. 2016;4(1):e000294. doi: 10.1136/bmjdrc-2016-000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diabetes Clinical Data Management Study Group. Basic tabulation materials (in Japanese). http://jddm.jp/data/index-2017.html. Accessed 31 Aug 2019.
- 7.Jeon JY, Kim DJ, Ko SH, Kwon HS, Lim S, Choi SH, Kim CS, An JH, Kim NH, Won JC, Kim JH, Cha BY, Song KH, Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association Current status of glycemic control of patients with diabetes in Korea: the fifth Korea national health and nutrition examination survey. Diabetes Metab J. 2014;38(3):197–203. doi: 10.4093/dmj.2014.38.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carls G, Huynh J, Tuttle E, Yee J, Edelman SV. Achievement of glycated hemoglobin goals in the US remains unchanged through 2014. Diabetes Ther. 2017;8(4):863–873. doi: 10.1007/s13300-017-0280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Htike ZZ, Zaccardi F, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: a systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab. 2017;19(4):524–536. doi: 10.1111/dom.12849. [DOI] [PubMed] [Google Scholar]
- 10.Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2018;61(12):2461–2498. doi: 10.1007/s00125-018-4729-5. [DOI] [PubMed] [Google Scholar]
- 11.Seino Y, Terauchi Y, Osonoi T, Yabe D, Abe N, Nishida T, Zacho J, Kaneko S. Safety and efficacy of semaglutide once weekly vs sitagliptin once daily, both as monotherapy in Japanese people with type 2 diabetes. Diabetes Obes Metab. 2018;20(2):378–388. doi: 10.1111/dom.13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyagawa J, Odawara M, Takamura T, Iwamoto N, Takita Y, Imaoka T. Once-weekly glucagon-like peptide-1 receptor agonist dulaglutide is non-inferior to once-daily liraglutide and superior to placebo in Japanese patients with type 2 diabetes: a 26-week randomized phase III study. Diabetes Obes Metab. 2015;17(10):974–983. doi: 10.1111/dom.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorli C, Harashima SI, Tsoukas GM, Unger J, Karsbøl JD, Hansen T, Bain SC. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(4):251–260. doi: 10.1016/S2213-8587(17)30013-X. [DOI] [PubMed] [Google Scholar]
- 14.Umpierrez G, Tofe Povedano S, Perez Manghi F, Shurzinske L, Pechtner V, et al. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3) Diabetes Care. 2014;37(8):2168–2176. doi: 10.2337/dc13-2759. [DOI] [PubMed] [Google Scholar]
- 15.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T, SUSTAIN-6 Investigators Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 16.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB, LEADER Steering Committee; LEADER Trial Investigators Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernandez AF, Green JB, Janmohamed S, D’Agostino RB, Sr, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC, Thorpe KM, McMurray JJV, Del Prato S, Harmony Outcomes committees and investigators Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 18.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, Xavier D, Atisso CM, Dyal L, Hall S, Rao-Melacini P, Wong G, Avezum A, Basile J, Chung N, Conget I, Cushman WC, Franek E, Hancu N, Hanefeld M, Holt S, Jansky P, Keltai M, Lanas F, Leiter LA, Lopez-Jaramillo P, Cardona Munoz EG, Pirags V, Pogosova N, Raubenheimer PJ, Shaw JE, Sheu WH, Temelkova-Kurktschiev T, REWIND Investigators Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 19.Das SR, Everett BM, Birtcher KK, Brown JM, Cefalu WT, Januzzi JL, Jr, Kalyani RR, Kosiborod M, Magwire ML, Morris PB, Sperling LS. ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the American College of Cardiology Task Force on expert consensus decision pathways. J Am Coll Cardiol. 2018;72(24):3200–3223. doi: 10.1016/j.jacc.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa-Uva M, Valensi P, Wheeler DC, ESC Scientific Document Group ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD) Eur Heart J. 2019 doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 21.Ahmann AJ, Capehorn M, Charpentier G, Dotta F, Henkel E, Lingvay I, Holst AG, Annett MP, Aroda VR. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN3): a 56-week, open-label, randomized clinical trial. Diabetes Care. 2018;41(2):258–266. doi: 10.2337/dc17-0417. [DOI] [PubMed] [Google Scholar]
- 22.Pratley RE, Aroda VR, Lingvay I, Lüdemann J, Andreassen C, Navarria A, Viljoen A, SUSTAIN 7 investigators Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275–286. doi: 10.1016/S2213-8587(18)30024-X. [DOI] [PubMed] [Google Scholar]
- 23.Capehorn MS, Catarig AM, Furberg JK, Janez A, Price HC, Tadayon S, Vergès B, Marre M. Efficacy and safety of once-weekly semaglutide 1.0 mg vs once-daily liraglutide 1.2 mg as add-on to 1–3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10) Diabetes Metab. 2019 doi: 10.1016/j.diabet.2019.101117. [DOI] [PubMed] [Google Scholar]
- 24.Kapitza C, Nosek L, Jensen L, Hartvig H, Jensen CB, Flint A. Semaglutide, a once-weekly human GLP-1 analog, does not reduce the bioavailability of the combined oral contraceptive, ethinylestradiol/levonorgestrel. J Clin Pharmacol. 2015;55(5):497–504. doi: 10.1002/jcph.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau J, Bloch P, Schäffer L, Pettersson I, Spetzler J, Kofoed J, Madsen K, Knudsen LB, McGuire J, Steensgaard DB, Strauss HM, Gram DX, Knudsen SM, Nielsen FS, Thygesen P, Reedtz-Runge S, Kruse T. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J Med Chem. 2015;58(18):7370–7380. doi: 10.1021/acs.jmedchem.5b00726. [DOI] [PubMed] [Google Scholar]
- 26.Ahrén B, Masmiquel L, Kumar H, Sargin M, Karsbøl JD, Jacobsen SH, Chow F. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341–354. doi: 10.1016/S2213-8587(17)30092-X. [DOI] [PubMed] [Google Scholar]
- 27.Mahato RI, Narang AS, Thoma L, Miller DD. Emerging trends in oral delivery of peptide and protein drugs. Crit Rev Ther Drug Carrier Syst. 2003;20(2–3):153–214. doi: 10.1615/critrevtherdrugcarriersyst.v20.i23.30. [DOI] [PubMed] [Google Scholar]
- 28.Buckley ST, Bækdal TA, Vegge A, Maarbjerg SJ, Pyke C, Ahnfelt-Rønne J, Madsen KG, Schéele SG, Alanentalo T, Kirk RK, Pedersen BL, Skyggebjerg RB, Benie AJ, Strauss HM, Wahlund PO, Bjerregaard S, Farkas E, Fekete C, Søndergaard FL, Borregaard J, Hartoft-Nielsen ML, Knudsen LB. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci Transl Med. 2018;10(467):eaar7047. doi: 10.1126/scitranslmed.aar7047. [DOI] [PubMed] [Google Scholar]
- 29.Maarbjerg SJ, Borregaard J, Breitschaft A, Donsmark M, Sondergaard Evaluation of the effect of food on the pharmacokinetics of oral semaglutide. Diabetologia. 2017;60(Suppl 1):S69. [Google Scholar]
- 30.Donsmark M, Borregaard J, Breitschaft A, Bækdal TA, Søndergaard FL. Evaluation of the effects of water volume with dosing and post-dose fasting period on pharmacokinetics of oral semaglutide. Diabetologia. 2017;60(Suppl 1):S363–S364. [Google Scholar]
- 31.Connor A, Donsmark M, Hartoft-Nielsen M-L, Søndergaard FL, Bækdal TA. A pharmacoscintigraphic study of the relationship between tablet erosion and pharmacokinetics of oral semaglutide. Diabetologia. 2017;60(Suppl 1):S361. doi: 10.1002/cpdd.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Granhall C, Donsmark M, Blicher TM, Golor G, Søndergaard FL, Thomsen M, Bækdal TA. Safety and pharmacokinetics of single and multiple ascending doses of the novel oral human GLP-1 analogue, oral semaglutide, in healthy subjects and subjects with type 2 diabetes. Clin Pharmacokinet. 2019;58:781–791. doi: 10.1007/s40262-018-0728-4. [DOI] [PubMed] [Google Scholar]
- 33.Baekdal TA, Thomsen M, Kupčová V, Hansen CW, Anderson TW. Pharmacokinetics, safety, and tolerability of oral semaglutide in subjects with hepatic impairment. J Clin Pharmacol. 2018;58(10):1314–1323. doi: 10.1002/jcph.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Granhall C, Søndergaard FL, Thomsen M, Anderson TW. Pharmacokinetics, safety and tolerability of oral semaglutide in subjects with renal impairment. Clin Pharmacokinet. 2018;57:1571–1580. doi: 10.1007/s40262-018-0649-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meier JJ, Granhall C, Hoevelmann U, Navaria M, Plum-Moerschel L, Ramesh C, Tannapfel A, Kapitza C. Effect of upper gastrointestinal disease on the pharmacokinetics of oral semaglutide in subjects with type 2 diabetes. Diabetes. 2019;68(Suppl 1):1013-P. doi: 10.1111/dom.14632. [DOI] [PubMed] [Google Scholar]
- 36.Bækdal TA, Breitschaft A, Navarria A, Hansen CW. A randomized study investigating the effect of omeprazole on the pharmacokinetics of oral semaglutide. Expert Opin Drug Metab Toxicol. 2018;14:869–877. doi: 10.1080/17425255.2018.1488965. [DOI] [PubMed] [Google Scholar]
- 37.Bækdal TA, Borregaard J, Hansen CW, Thomsen M, Anderson TW. Effect of oral semaglutide on the pharmacokinetics of lisinopril, warfarin, digoxin, and metformin in healthy subjects. Clin Pharmacokinet. 2019;58(9):1193–1203. doi: 10.1007/s40262-019-00756-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bækdal TA, Albayaty M, Manigandan E, Anderson TW, Skibsted S. A trial to investigate the effect of oral semaglutide on the pharmacokinetics of furosemide and rosuvastatin in healthy subjects. Diabetologia. 2018;61(Suppl 1):S346. [Google Scholar]
- 39.Hauge C, Breitschaft A, Hartoft-Nielsen M-L, Jensen S, Bækdal T. A drug-drug interaction trial of oral semaglutide with levothyroxine and multiple coadministered tablets. J Endo Soc. 2019;3(Suppl 1):SAT-140. [Google Scholar]
- 40.Jordy AB, Breitschaft A, Christiansen E, Granhall C, Hansen CW, Houshmand-Oregaard A, Bækdal TA. Oral semaglutide does not affect the bioavailability of the combined oral contraceptive ethinylestradiol/levonorgestrel. Diabetologia. 2018;61(1):S346. [Google Scholar]
- 41.Davies M, Pieber TR, Hartoft-Nielsen ML, Hansen OKH, Jabbour S, Rosenstock J. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a Randomized Clinical Trial. JAMA. 2017;318(15):1460–1470. doi: 10.1001/jama.2017.14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.International Council for Harmonisation (ICH) of Technical Requirements for Pharmaceuticals for Human Use. ICH Concept Paper E9 (R1): Addendum to Statistical Principles for Clinical Trials on Choosing Appropriate Estimands and Defining Sensitivity Analyses in Clinical Trials, 2014. https://database.ich.org/sites/default/files/E9-R1_EWG_Concept_Paper.pdf. Accessed 30 Oct 2019.
- 43.International Council for Harmonisation (ICH) of Technical Requirements for Pharmaceuticals for Human Use. ICH Harmonised Guideline E9 (R1): Estimands and Sensitivity Analysis in Clinical Trials, 2017. https://database.ich.org/sites/default/files/E9-R1_EWG_Draft_Guideline.pdf. Accessed 30 Oct 2019.
- 44.Aroda VR, Saugstrup T, Buse JB, Donsmark M, Zacho J, Davies MJ. Incorporating and interpreting regulatory guidance on estimands in diabetes clinical trials: the PIONEER 1 randomized clinical trial as an example. Diabetes Obes Metab. 2019;21(10):2203–2210. doi: 10.1111/dom.13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aroda VR, Rosenstock J, Terauchi Y, Altuntas Y, Lalic NM, Morales Villegas EC, Jeppesen OK, Christiansen E, Hertz CL, Haluzík M, PIONEER 1 investigators PIONEER 1: randomized clinical trial comparing the efficacy and safety of oral semaglutide monotherapy with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42(9):1724–1732. doi: 10.2337/dc19-0749. [DOI] [PubMed] [Google Scholar]
- 46.Rodbard HW, Rosenstock J, Canani LH, Deerochanawong C, Gumprecht J, Lindberg SØ, Lingvay I, Søndergaard AL, Treppendahl MB, Montanya E, PIONEER 2 Investigators Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019 doi: 10.2337/dc19-0883. [DOI] [PubMed] [Google Scholar]
- 47.Rosenstock J, Allison D, Birkenfeld AL, Blicher TM, Deenadayalan S, Jacobsen JB, Serusclat P, Violante R, Watada H, Davies M, PIONEER 3 Investigators Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 Randomized Clinical Trial. JAMA. 2019;321(15):1466–1480. doi: 10.1001/jama.2019.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M, Pedersen KB, Saugstrup T, Meier JJ, PIONEER 4 investigators Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394(10192):39–50. doi: 10.1016/S0140-6736(19)31271-1. [DOI] [PubMed] [Google Scholar]
- 49.Mosenzon O, Blicher TM, Rosenlund S, Eriksson JW, Heller S, Hels OH, Pratley R, Sathyapalan T, Desouza C, PIONEER 5 Investigators Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):515–527. doi: 10.1016/S2213-8587(19)30192-5. [DOI] [PubMed] [Google Scholar]
- 50.Pieber TR, Bode B, Mertens A, Cho YM, Christiansen E, Hertz CL, Wallenstein SOR, Buse JB, PIONEER 7 investigators Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):528–539. doi: 10.1016/S2213-8587(19)30194-9. [DOI] [PubMed] [Google Scholar]
- 51.Zinman B, Aroda VR, Buse JB, Cariou B, Harris SB, Hoff ST, Pedersen KB, Tarp-Johansen MJ, Araki E, PIONEER 8 Investigators Efficacy, safety, and tolerability of oral semaglutide versus placebo added to insulin with or without metformin in patients with type 2 diabetes: the PIONEER 8 trial. Diabetes Care. 2019 doi: 10.2337/dc19-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamada Y, Katagiri H, Hamamoto Y, Christensen SB, Navarria AM, Nishijima K, Seino Y. Efficacy and safety of oral semaglutide monotherapy vs placebo or liraglutide in Japanese T2D patients: PIONEER 9 trial. J Diabetes Invest. 2019;10(Suppl 1):30. [Google Scholar]
- 53.Yabe D, Nakamura J, Kaneto H, Christensen SB, Navarria AM, Gislum M, Inagaki N. Safety and efficacy of oral semaglutide vs dulaglutide in Japanese T2D patients: the PIONEER 10 trial. J Diabetes Invest. 2019;10(Suppl 1):30. [Google Scholar]
- 54.Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, Tack CJ, Thomsen M, Vilsbøll T, Warren ML, Bain SC, PIONEER 6 Investigators Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]