Abstract
The aim of the study is to investigate the role of ATP-binding cassette subfamily B member 7 (ABCB7) in correlation with the progression of Parkinson’s disease. Initially, the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) was used to develop a mouse model of mild and severe forms of Parkinson’s disease. Histology, immunohistology, and Western blotting were used to investigate the role of ABCB7 in disease progression. Mice injected with MPTP, at doses of 18 and 30 mg/kg for 10 and 15 consecutive days, respectively, developed mild and severe forms of Parkinson’s disease, respectively. Motor dysfunction is accessed through pole test in which, mild and severe forms of Parkinson’s disease developed mice takes 1.7 and 3.3 times more time to reach the floor than the control mice. Similarly, in rotarod test, the progression of Parkinson’s disease is evident with the progressive loss of motor stability. Histologically, the progression of Parkinson’s disease is evident with formation of cell aggregates in mild form; with the formation of more Lewy body structure and tissue hardening in a severe form of Parkinson’s disease. Immunohistochemistry showed gradual upregulation of ABCB7 in the cellular cytoplasm in mild stage Parkinson’s disease, while significant overexpression of ABCB7 was observed in the severe forms. Western blotting results confirmed 1.6- and 2.9-fold overexpression of ABCB7 in mild and severe forms of Parkinson’s disease, respectively. Collectively, the results showed that ABCB7 was present during Parkinson’s disease progression. However, upregulation of ABCB7 increased the cytoplasm level of the iron–sulfur complex, which negatively regulated the iron-dependent protein and can be used to determine the progression of Parkinson’s disease.
Keywords: Parkinson’s disease, MPTP, ABCB7, Substantia nigra, Iron–sulfur complex
Introduction
Parkinson’s disease is a neurodegenerative disease that affects memory function, and is characterized by bradykinesia, tremor, rigidity, and postural instability (Zhang et al. 2016). Patients also experience sleep disorders, sensory disturbances, and autonomic dysfunction (Zhang et al. 2016). Depletion of dopamine, as well as reduction of dopamine receptors in the cortex and medial temporal lobe, is the primary causes of Parkinson’s disease (Christopher et al. 2013; Lisman and Grace 2005). In addition, Parkinson’s disease patients show memory deficits due to impaired coordination between memory retrieval and execution (Brønnick et al. 2011; Anderson and Green 2001). The incidence of brain disease is increasing each year with the aging of the population; therefore, efficient therapeutic interventions are important (Schlossmacher et al. 2017).
In recent studies on nonmotor symptoms associated with Parkinson’s disease, impaired iron metabolism was suggested to be responsible for restless leg syndrome (Piao et al. 2017). Parkinson’s disease patients show excessive iron deposition in the substantia nigra, which also leads to neurodegeneration (Salazar et al. 2008; Sofic et al. 1988) and thus promotes the progression of Parkinson’s disease. Transferrin is an iron metabolism-related protein that is abundantly expressed in the brain of Parkinson’s disease patients (Mariani et al. 2013). The mechanism underlying iron deposition, and the correlation thereof with the onset and progression of Parkinson’s disease, remains unclear. Iron-regulatory protein 1 (IRP-1) and IRP-2 are key regulators of iron homeostasis, and are important in maintaining cytoplasmic iron levels (Muñoz et al. 2016). In addition, IRP-1 and IRP-2 regulate mitochondrial ATP-binding cassette subfamily B member 7 (ABCB7), which is involved in the biogenesis of cytosolic iron–sulfur clusters (Pondarré et al. 2006). In the present study, the ABCB7 protein expression was investigated in different pathological stages of Parkinson’s disease.
Materials and methods
Mouse model of Parkinson’s disease
Three-month-old male C57BL/6 mice (n = 15) were used to develop an experimental model of Parkinson’s disease. The mice were maintained in a cage at 22 ± 1 °C under a 12-h light and 12-h dark cycle. The use of animals and the experimental protocols were approved by the institutional ethical and animal care committee. The mice were divided into three groups (n = 5 animals each) that were maintained separately. Mice in the first group (control) were intraperitoneally injected with saline solution for 15 consecutive days. To establish mild and severe forms of Parkinson’s disease, a standard neurotoxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), was used as described previously (Liu et al. 2018). To establish mild stage Parkinson’s disease, a second group of mice were intraperitoneally injected with MPTP (18 mg/kg) for 10 consecutive days, followed by injection with physiological saline only over the next 5 days. The mice in the final group were injected with MPTP (30 mg/kg) once daily for 15 days. Following a complete rest of 5 days (days 16–20), the mice were sacrificed for analysis.
Pole test
The pole test is a simple behavioral test used to evaluate the degree of bradykinesia (Ogawa et al. 1985). Before starting the experiment, the mice were acclimated to the experimental setup for 3 days. A 50-cm high vertical wooden pole with a base stand was positioned inside the working cage. We ensured that the pole surface was not slippery, i.e., was sufficiently rough. The mice were placed at the top of the pole; after orienting themselves, they climbed down toward the floor. The time to reach the floor was recorded, and the experiment was repeated twice at 15-min intervals.
Rotarod test
Motor coordination and endurance were analyzed using the rotarod test, as described previously (Dunham and Miya 1957). Before starting the experiment on day 20, mice were trained at 7, 10, and 15 rpm on days 17–19. The length of time that each mouse remained on the rotarod was recorded. Three trials were performed at 15-min intervals, and the average time on the rotarod was calculated.
Histology and immunohistochemistry
For the dissection of the brain tissue for histology, the intact brain without any tissue damage is obtained by perfused with 4% paraformaldehyde. Initially, the mice are anesthetized using ketamine/xylazine mixture (90/16 mg/kg) to reach surgical plane of anesthesia and perfused with 10 mM PBS and later with 4% paraformaldehyde (in 0.1 M phosphate buffer, pH 7.4) through vascular network of heart. Following perfusion technique, the whole brain is dissected out, sliced into smaller pieces, and fixed with 4% paraformaldehyde for 48 h. After fixation, the tissue sections were subjected to the following steps: gradual dehydration using isopropyl alcohol, tissue clearing with xylene, and wax infiltration; sections were then formed into blocks for histological sectioning. For immunohistochemistry, sections were initially treated with trypsin to unmask the antigens and epitopes, followed by blocking in 4% bovine serum albumin (BSA). Next, the sections were incubated with anti-ABCB7 antibody (ab151992; Abcam, Shanghai, China; 1:500 ratio) or anti-phosphorylated α-synuclein antibody (Abcam (ab51253), Shanghai, China; 1:1000 ratio) for 8 h at 4 °C in 4% BSA solution. After washing with Tris-buffered saline with Tween-20 (TBST), the sections were further incubated with horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at room temperature. After washing thrice with TBST, the sections were developed using 3,3′-diaminobenzidine (DAB) solution (1% DAB and 4 drops of 10 N HCl) by incubating for 15 min in the dark. For positive control experiments, mice liver tissue was treated with both primary and secondary antibody and later developed. For negative control, the mice brain tissue is treated only with secondary antibody and developed further with DAB solution.
Western blotting
Following brain removal, the substantia nigra was carefully separated from the rest of the tissue. After washing with ice-cold 1× phosphate-buffered saline (PBS), the tissue was transferred to an Eppendorf tube and subjected to protein extraction using a total protein extraction kit (Beyotime, Nanjing, China). Following protein quantification using the Lowry method, each well was loaded with 60 µg of protein sample and separated using 12% SDS-PAGE gel. The separated proteins in the gel were transferred to polyvinylidene difluoride (PVDF) membranes. After blocking (using 5% milk powder in 1 × TBST), the target protein in the PVDF membrane was probed using anti-ABCB7 antibody (Abcam (ab151992), Shanghai, China; 1:500 ratio) and the signals were detected using HRP-conjugated secondary antibody (Abcam (ab6721), Shanghai, China; 1:2000 ratio; diluted with 1 × TBST). The results are documented using Gel documentation system, and the band intensity was analyzed using ImageJ software.
Statistical analysis
To enhance the accuracy, three trials were performed, and the averaged data were used in the statistical analysis. The statistical data were analyzed using SPSS software (version 25.0; IBM Corp., Armonk, NY, USA). The groups were compared using ANOVA test, followed by Tukey’s post hoc test for multiple data comparisons. A P value < 0.05 was considered statistically significant. The results are presented as means ± standard deviation (SD).
Results
Pole and rotarod results showed that MPTP-injected mice developed Parkinson’s disease
To induce mild stage Parkinson’s disease, the mice were intraperitoneally injected with neurotoxin MPTP (18 mg/kg) for 10 consecutive days, followed by saline injection for 5 days. Similarly, to induce severe forms of Parkinson’s disease, mice were injected with MPTP (30 mg/kg) for 15 consecutive days. Next, the MPTP-injected and control mice were subjected to the pole test. Mice injected with MPTP for 10 or 15 consecutive days showed gradual loss of motor function in pole test (Fig. 1). The control mice reached the floor very quickly (15.38 ± 1.21 s); however, the mild stage Parkinson’s disease mice took 1.7 times (26.61 ± 1.31 s) longer to reach the floor than control mice, while mice with severe forms of Parkinson’s disease took 3.3 times longer (52.24 ± 1.18 s). To further evaluate the motor activity in each group, the rotarod test was performed; the results are shown in Fig. 2. The control mice exhibited better stability, remaining on the rotating rod for a longer period of time (213 ± 2.18 s) compared with mild (166 ± 2.63 s) and severe forms (119 ± 2.62 s) of Parkinson’s disease mice.
Fig. 1.
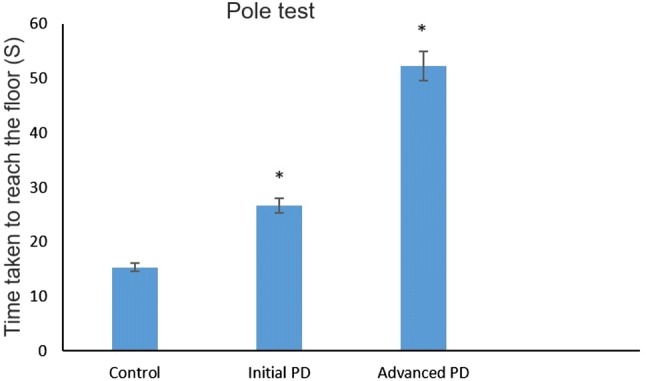
Pole test for assessing motor dysfunction: mild and severe forms of Parkinson’s disease mice and controls performed the pole test. The time for mice to reach the floor was recorded in seconds. The control mice take only 15.38 ± 1.21 s to reach the floor on the other hand mild and severe forms of Parkinson’s disease developed mice takes 26.61 ± 1.31 s and 52.24 ± 1.18 s respectively to reach the floor. The data are presented as means ± standard deviation (SD). P < 0.05 was considered statistically significant. PK Parkinson’s disease
Fig. 2.
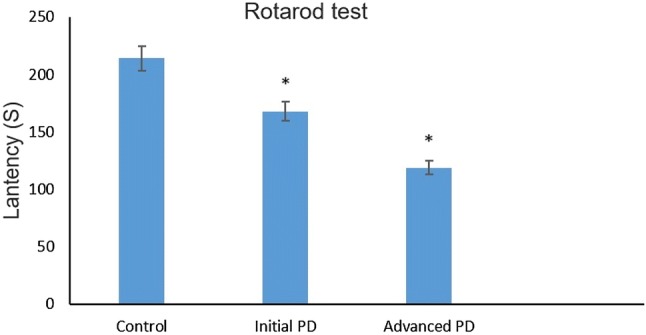
Rotarod test for assessing motor coordination: The time spent on the rotarod was considered indicative of motor ability. The control mice have the ability to grip on the rotarod for a longer time (213 ± 2.18 s) when compared with mild (166 ± 2.63 s) and severe forms (119 ± 2.62 s) of Parkinson’s disease developed mice. The graphs are plotted with time in seconds (y axis) and different mice groups (x axis). The data are presented as means ± SD. *P < 0.05 vs. control
Histopathological changes confirmed the establishment of mild and severe forms of Parkinson’s disease in mice
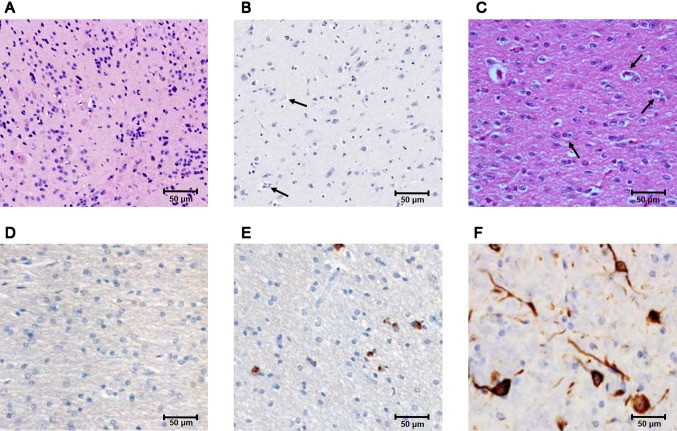
After the pole and rotarod tests, the key features of Parkinson’s disease were investigated based on the histopathological changes that occurred in the control, mild, and severe forms of Parkinson’s disease mice. In control mice, the substantia nigra showed a well-defined and uniform arrangement of neuronal cells (Fig. 3a). Mice injected with MPTP (18 mg/kg) for 10 consecutive days showed cell aggregates and abnormal cells, indicative of mild stage Parkinson’s disease (Fig. 3B). Conversely, mice injected with MPTP (30 mg/kg) for 15 consecutive days showed the formation of Lewy bodies and hardened tissue (Fig. 3c). For further evaluation, the brain tissue of substantia nigra is stained with anti-phosphorylated α-synuclein antibody. In the control tissue, no positive signals are observed against phosphorylated α-synuclein antibody (Fig. 3d), but in mild stages of Parkinson disease condition, it show signal around Lewy body structure that occurs limited in number (Fig. 3e). In severe forms of Parkinson disease, the Lewy bodies structure increase in number and show progressive bulge in size which are detected using phosphorylated α-synuclein antibody (Fig. 3f).
Fig. 3.
Histopathological analysis of the substantia nigra of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-injected mice: a control mice show normal neuronal structure with well-defined, uniform arrangement of neuronal cells. b Mice with mild stage Parkinson’s disease show neuronal damage and the formation of Lewy bodies. c Mice with severe forms of Parkinson’s disease show a complicated tissue structure characterized by tissue hardness and more Lewy bodies. d Control mice tissue shows no positive signal for phosphorylated α-synuclein antibody. e Mild stage of Parkinson disease shows few Lewy bodies structure that are stained with phosphorylated α-synuclein antibody. f Severe forms of Parkinson disease shows more Lewy bodies structures that are stained with phosphorylated α-synuclein antibody. Arrow denotes Lewy bodies. H&E stain. Scale bar, 50 µm
Abnormal ABCB7 expression in the different pathological stages of Parkinson’s disease
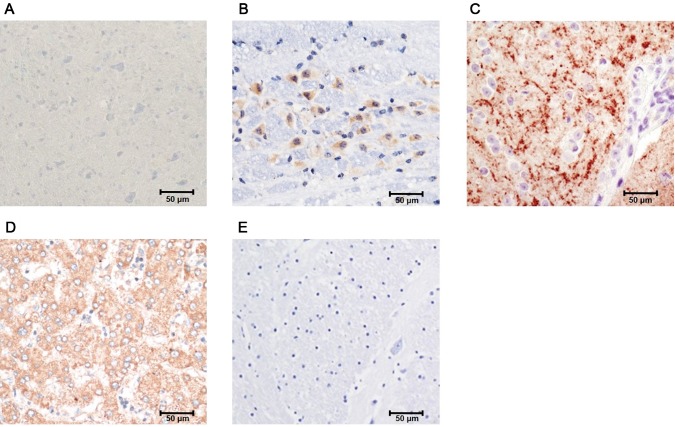
To identify and locate ABCB7 expression in the control and mild and severe forms of Parkinson’s disease mice, immunohistochemistry was performed. The ABCB7 protein expression pattern in the substantia nigra is shown in Fig. 4. In control mice, ABCB7 was mildly expressed (Fig. 4a); however, the expression gradually increased in mice with mild stage Parkinson’s disease (Fig. 4b). The ABCB7 expression was typically observed around abnormal cells in mice with mild stage Parkinson’s disease (Fig. 4b). The substantia nigra of mice with severe forms of Parkinson’s disease showed abnormally high ABCB7 protein expression (Fig. 4c) which is directly related with abnormal iron accumulation. The expression of ABCB7 protein occurs more prominently in cytoplasm and appears in most of the cells of substantia nigra (Fig. 4c). In order to determine the specificity of the antibody used, the mice liver and brain tissue are subjected to positive and negative control experiments as described in “Materials and methods”. For positive control experiments, mouse liver tissue against anti-ABCB7 antibody shows more prominent signals (Fig. 4d). To determine nonspecific signals, negative control experiments were carried out using mouse brain tissue without primary antibody incubation, and it shows no nonspecific signals (Fig. 4e).
Fig. 4.
Immunohistochemistry with ATP-binding cassette subfamily B member 7 (ABCB7) antibody: a control mice tissue with optimal ABCB7 expression. b Mice with mild stage Parkinson’s disease show gradual overexpression of ABCB7 protein. c Mice with severe forms of Parkinson’s disease show high ABCB7 expression. d Positive control; mouse liver tissue positive for ABCB7. e Negative control; mouse brain tissue negative for ABCB7. H&E stain. Scale bar, 50 µm
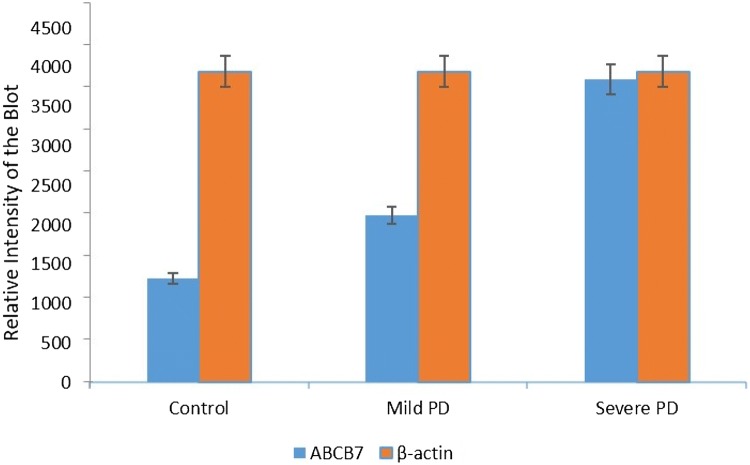
To investigate the differential expression of ABCB7, Western blotting was performed (Fig. 5). In control mice, ABCB7 was marginally expressed in substantia nigra tissue samples. In mild stage Parkinson’s disease, ABCB7 expression was 1.6-fold higher when compared with the control group (Fig. 6). Notably, in severe forms of Parkinson’s disease, ABCB7 expression was 2.9-fold higher when compared with the control (Fig. 6). For enhancing experimental accuracy, the samples are analyzed from three different individual mice, and the results obtained are normalized to internal loading control, β-actin.
Fig. 5.
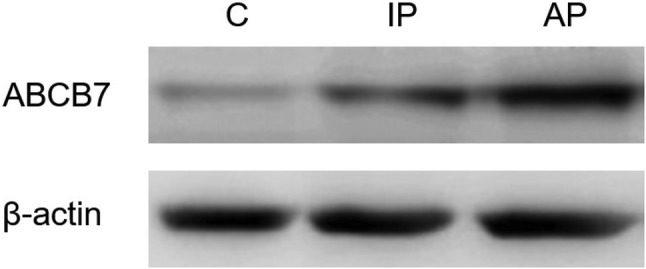
ABCB7 expression pattern analyzed using Western blotting: lane 1 shows the minimal ABCB7 expression seen in control tissue. Lane 2 shows a significant increase in ABCB7 expression in mild stage Parkinson’s disease. Lane 3 shows that ABCB7 was overexpressed in severe forms of Parkinson’s disease. β-Actin was used as a loading control
Fig. 6.
Quantification of ABCB7 expression in mild and severe forms of Parkinson’s disease: the expression of ABCB7 was quantified based on the band intensity and represented in bar diagram. The experiments are repeated for three times, and the results are represented as mean ± SD. The data were considered statistically significant when P < 0.05. The results are normalized to loading control, β-actin
Discussion
Proteins performing many important biological processes, such as cellular respiration, nitrogen fixation, DNA synthesis, DNA repair, oxygen transport, and neurotransmitter synthesis, require iron as a cofactor (Paul et al. 2017), which forms iron–sulfur clusters (Lill 2009) and regulates the expression of numerous genes (Mettert and Kiley 2015). Abnormal distribution of iron within the mitochondria, and protein iron–sulfur deficits due to abnormal regulation of iron, are also responsible for the development of neurodegenerative diseases, such as Parkinson’s disease and Friedreich ataxia (Isaya 2014). The neurotoxin MPTP used in the present study specifically targets dopaminergic neurons and activates cell death by inhibiting mitochondrial complex I in the substantia nigra (Meredith et al. 2008).
In the present study, we preferred to use three-month-old male C57BL/6 mice because as many of the previous studies uses 2–3 months old mice to get the precise results over old age mice (Liu et al. 2018; Zhang et al. 2017). In addition, we follow different time frame and different dose of MPTP injection, which are essential to establish mild and severe forms of Parkinson disease condition. The 5-day physiological saline injection following 10 days of MPTP injection in the second group of mice is to mimic the similar stress of injection which is continuously followed for 15 days in group three mice. The different concentrations of MPTP (18 mg/kg for 10 consecutive days and 30 mg/kg for 15 consecutive days) resulted in mild and severe forms of Parkinson’s disease, respectively. The dose of MPTP used for inducing the mild and severe forms of Parkinson disease was primarily determined using previous studies (Liu et al. 2018; Zhang et al. 2017) and customized to certain extent for the present study. Abnormal motor function in mice can be detected using the pole and rotarod tests (Abramow-Newerly et al. 2006). In the mice in the present study, as the concentration of MPTP increased, motor coordination decreased, based on the pole and rotarod test results. In mild stage Parkinson’s disease, cell aggregates form gradually due to insoluble ubiquitin and α-synuclein formation (Meredith et al. 2004). Tissue hardness was observed in mice with severe forms of Parkinson’s disease as similar to a previous report in which chronic Parkinson’s disease was characterized by reduced viscoelasticity of the substantia nigra (Hain et al. 2016).
In our studies, we observed the expression of ABCB7 shows steady overexpression pattern as Parkinson disease progress. The expression of ABCB7 appears more prominent in cellular cytoplasm and specifically in severe forms of Parkinson disease most of the cells present in substantia nigra are showing positive signals for ABCB7 expression. ABCB7 in the mitochondrial membrane plays a crucial role in the biosynthesis of cytosolic Fe–S proteins (Pondarré et al. 2006). In a previous study, silencing of ABCB7 led to iron overload in the mitochondria, indicating iron deficiency in the cellular cytoplasmic environment (Cavadini et al. 2006). Based on the previous results and those of this study, ABCB7 is gradually overexpressed as Parkinson’s disease progresses, indicating that ABCB7 function remains undisturbed. However, the overexpression led to increased accumulation of iron–sulfur complex in the cytoplasm, which may negatively regulate many genes involved in the initiation and progression of Parkinson’s disease.
Conclusions
In this study, we successfully established the mild (18 mg/kg MPTP injection for 10 days) and severe (30 mg/kg MPTP injection for 15 days) forms of Parkinson’s disease using different concentrations of MPTP. Injected mice showed dose-dependent degeneration of the substantia nigra. ABCB7 was gradually upregulated as Parkinson’s disease progressed, indicating abnormal iron–sulfur clusters in the cytoplasmic environment; this may lead to negative regulation of many genes involved in the development of Parkinson’s disease.
Author contributions
HC, YB: study design; WT: collected the samples; HC, YB: laboratory work; All authors: contributed to the manuscript, read, and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors of this work declare that they have no conflict of interest.
Ethical statement
Animal used in the experiments and protocols followed were approved by Institutional ethical and animal care committee.
References
- Abramow-Newerly W, Lipina T, Abramow-Newerly M, et al. Methods to rapidly and accurately screen a large number of ENU mutagenized mice for abnormal motor phenotypes. Amyotroph Lateral Scler. 2006;7:112–118. doi: 10.1080/14660820500443000. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Green C. Suppressing unwanted memories by executive control. Nature. 2001;410:366. doi: 10.1038/35066572. [DOI] [PubMed] [Google Scholar]
- Brønnick K, Alves G, Aarsland D, et al. Verbal memory in drug-naive, newly diagnosed Parkinson’s disease. The retrieval deficit hypothesis revisited. Neuropsychology. 2011;25:114. doi: 10.1037/a0020857. [DOI] [PubMed] [Google Scholar]
- Cavadini P, Biasiotto G, Poli M, et al. RNA silencing of the mitochondrial ABCB7 transporter in HeLa cells causes an iron-deficient phenotype with mitochondrial iron overload. Blood. 2006;109:3552–3559. doi: 10.1182/blood-2006-08-041632. [DOI] [PubMed] [Google Scholar]
- Christopher L, Marras C, Duff-Canning S, et al. Combined insular and striatal dopamine dysfunction are associated with executive deficits in Parkinson’s disease with mild cognitive impairment. Brain. 2013;137:565–575. doi: 10.1093/brain/awt337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham NW, Miya TS. A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc. 1957;46:208–209. doi: 10.1002/jps.3030460322. [DOI] [PubMed] [Google Scholar]
- Hain EG, Klein C, Munder T, et al. Dopaminergic neurodegeneration in the mouse is associated with decrease of viscoelasticity of substantia nigra tissue. PLoS ONE. 2016;11:e0161179. doi: 10.1371/journal.pone.0161179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaya G. Mitochondrial iron–sulfur cluster dysfunction in neurodegenerative disease. Front Pharmacol. 2014;5:29. doi: 10.3389/fphar.2014.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill R. Function and biogenesis of iron–sulphur proteins. Nature. 2009;460:831. doi: 10.1038/nature08301. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Liu H, Chen S, Guo C, et al. Astragalus polysaccharide protects neurons and stabilizes mitochondrial in a mouse model of Parkinson disease. Med Sci Monit Int Med J Exp Clin Res. 2018;24:5192. doi: 10.12659/MSM.908021. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mariani S, Ventriglia M, Simonelli I, et al. Fe and Cu do not differ in Parkinson’s disease: a replication study plus meta-analysis. Neurobiol Aging. 2013;34:632–633. doi: 10.1016/j.neurobiolaging.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Halliday GM, Totterdell S. A critical review of the development and importance of proteinaceous aggregates in animal models of Parkinson’s disease: new insights into Lewy body formation. Parkinsonism Relat Disord. 2004;10:191–202. doi: 10.1016/j.parkreldis.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Totterdell S, Potashkin JA, Surmeier DJ. Modeling PD pathogenesis in mice: advantages of a chronic MPTP protocol. Parkinsonism Relat Disord. 2008;14:S112–S115. doi: 10.1016/j.parkreldis.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettert EL, Kiley PJ. Fe–S proteins that regulate gene expression. Biochim Biophys Acta (BBA) Mol Cell Res. 2015;1853:1284–1293. doi: 10.1016/j.bbamcr.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz Y, Carrasco CM, Campos JD, et al. Parkinson’s disease: the mitochondria–iron link. Parkinsons Dis. 2016 doi: 10.1155/2016/7049108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa N, Hirose Y, Ohara S, et al. A simple quantitative bradykinesia test in MPTP-treated mice. Res Commun Chem Pathol Pharmacol. 1985;50:435–441. [PubMed] [Google Scholar]
- Paul BT, Manz DH, Torti FM, Torti SV. Mitochondria and Iron: current questions. Expert Rev Hematol. 2017;10:65–79. doi: 10.1080/17474086.2016.1268047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao Y-S, Lian T-H, Hu Y, et al. Restless legs syndrome in Parkinson disease: clinical characteristics, abnormal iron metabolism and altered neurotransmitters. Sci Rep. 2017;7:10547. doi: 10.1038/s41598-017-10593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pondarré C, Antiochos BB, Campagna DR, et al. The mitochondrial ATP-binding cassette transporter Abcb7 is essential in mice and participates in cytosolic iron–sulfur cluster biogenesis. Hum Mol Genet. 2006;15:953–964. doi: 10.1093/hmg/ddl012. [DOI] [PubMed] [Google Scholar]
- Salazar J, Mena N, Hunot S, et al. Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson’s disease. Proc Natl Acad Sci. 2008;105:18578–18583. doi: 10.1073/pnas.0804373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossmacher MG, Tomlinson JJ, Santos G, et al. Modelling idiopathic Parkinson disease as a complex illness can inform incidence rate in healthy adults: the PREDIGT score. Eur J Neurosci. 2017;45:175–191. doi: 10.1111/ejn.13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofic E, Riederer P, Heinsen H, et al. Increased iron (III) and total iron content in post mortem substantia nigra of parkinsonian brain. J Neural Transm. 1988;74:199–205. doi: 10.1007/BF01244786. [DOI] [PubMed] [Google Scholar]
- Zhang T, Yu S, Guo P, et al. Nonmotor symptoms in patients with Parkinson disease: a cross-sectional observational study. Medicine (Baltimore) 2016;95:e5400. doi: 10.1097/MD.0000000000005400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Heng Y, Mou Z, et al. Reassessment of subacute MPTP-treated mice as animal model of Parkinson’s disease. Acta Pharmacol Sin. 2017;38:1317–1328. doi: 10.1038/aps.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]