Abstract
Objective
Linagliptin, a dipeptidyl peptidase-4 inhibitor, demonstrated cardiovascular and renal safety in type 2 diabetes mellitus (T2DM) patients with established cardiovascular disease (CVD) with albuminuria and/or kidney disease in the multinational CARMELINA® trial. We investigated the effects of linagliptin in Asian patients in CARMELINA®.
Methods
T2DM patients with HbA1c 6.5–10.0% and established CVD with urinary albumin-to-creatinine ratio (UACR) > 30 mg/g, and/or prevalent kidney disease (estimated glomerular filtration rate [eGFR] 15–< 45 ml/min/1.73 m2 or ≥ 45–75 with UACR > 200 mg/g), were randomized to linagliptin or placebo added to usual care. The primary endpoint was time to first occurrence of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke (3-point MACE).
Results
Of the 6979 patients, 555 (8.0%) were Asians living in Asia. During a median follow-up of 2.2 years, 3-point MACE occurred in 29/272 (10.7%) and 33/283 (11.7%) of linagliptin and placebo patients, respectively (hazard ratio [HR] 0.90; 95% confidence interval [CI] 0.55–1.48), consistent with the overall population (HR 1.02; 95% CI 0.89–1.17; P value for treatment-by-region interaction: 0.3349). Similar neutrality in Asian patients was seen for other cardiorenal events including the secondary kidney endpoint of death from renal failure, progression to end-stage kidney disease, or ≥ 40% eGFR decrease (HR 0.96; 95% CI 0.58–1.59). Linagliptin was associated with a nominal decrease in the risk of hospitalization for heart failure (HR 0.47; 95% CI 0.24–0.95). Overall in Asian patients, linagliptin had an adverse event rate similar to placebo, consistent with the overall population.
Conclusions
Linagliptin showed cardiovascular and renal safety in Asian patients with T2DM and established CVD with albuminuria and/or kidney disease.
Electronic supplementary material
The online version of this article (10.1007/s13340-019-00412-x) contains supplementary material, which is available to authorized users.
Keywords: Diabetes mellitus, type 2; Cardiovascular diseases; Renal insufficiency, chronic; Prescription drugs
Introduction
Type 2 diabetes mellitus (T2DM) is one of the leading causes of cardiovascular disease (CVD) in Asia [1]. The prevalence of T2DM in Asia has increased rapidly in recent years, largely because of increases in obesity driven by lifestyle changes associated with rapid economic development [2–6]. In 2017, it was estimated that 241 million of the 425 million people globally with diabetes lived in the Western Pacific and Southeast Asia regions, with China and India together having 195 million people with diabetes [7]. Compared with their white counterparts, Asians with T2DM generally have lower body mass index (BMI) and reduced β-cell function (especially East Asians), with many patients requiring early insulin treatment [2, 3]. Kidney complications are also more common in Asians with T2DM than in white patients [2, 3, 8]. Notably, the rate of increase in the number of patients with end-stage kidney disease (ESKD) requiring dialysis is higher in Asia than other regions [9].
Linagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor indicated for improving glycemic control in adults with T2DM. Unlike most other DPP-4 inhibitors, linagliptin is excreted by non-renal pathways and consequently does not require dose adjustment in patients with chronic kidney disease [10]. The glucose-lowering efficacy, safety, and tolerability of linagliptin in people with T2DM has been demonstrated in several pivotal clinical trials, including dedicated studies in patients from Asia [11–19].
In a multinational, placebo-controlled, cardiovascular safety trial in T2DM patients with established CVD with albuminuria and/or kidney disease (CARMELINA®), linagliptin added to standard of care did not increase the risk of major adverse cardiovascular events (MACE), as assessed by the composite endpoint of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke (3-point MACE) [20]. Linagliptin also demonstrated long-term kidney safety, with no increased risk for the key secondary composite endpoint of death due to renal failure, progression to ESKD, or decrease of ≥ 40% in estimated glomerular filtration rate (eGFR) [20]. Furthermore, the risk of hospitalization for heart failure was not increased with linagliptin [21].
In addition to patients from other regions of the world, the CARMELINA® trial also enrolled patients from several Asian countries (Japan, China, South Korea, Taiwan and Malaysia). Here, we report a subgroup analysis of CARMELINA® investigating the cardiovascular and kidney safety of linagliptin in Asian participants living in these Asian countries.
Materials and methods
Study design
The design of the CARMELINA® trial has been described in detail [20, 22]. Briefly, it was a randomized, double-blind, placebo-controlled, event-driven clinical trial conducted in 27 countries between August 2013 and January 2018 in accordance with the principles of the Declaration of Helsinki and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization, after approval by local authorities (ClinicalTrials.gov Identifier, NCT01897532).
T2DM patients aged ≥ 18 years (≥ 20 years in Japan) with glycated hemoglobin (HbA1c) levels of 6.5–10.0% and BMI ≤ 45 kg/m2 were eligible for inclusion. Patients also had to have either established CVD (including history of myocardial infarction or stroke > 2 or > 3 months before screening, respectively, and/or presence of coronary, carotid or peripheral artery disease) together with albuminuria (urinary albumin-to-creatinine ratio [UACR] > 30 mg/g or equivalent), or established kidney disease as defined by an eGFR of 15–< 45 ml/min/1.73 m2 or eGFR ≥ 45–75 ml/min/1.73 m2 with UACR > 200 mg/g or equivalent. Participants could have been receiving either no glucose-lowering background therapy or any therapy except DPP-4 inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, or sodium-glucose co-transporter-2 (SGLT2) inhibitors.
Participants were randomized to receive once-daily oral treatment with linagliptin 5 mg (the licensed dose) or placebo. The primary endpoint was 3-point MACE. The key secondary endpoint was a composite kidney outcome comprising death due to renal failure, progression to ESKD, or sustained decrease in eGFR of ≥ 40%. Other prespecified cardiovascular and mortality endpoints included the 4-point MACE composite outcome (3-point MACE plus hospitalization for unstable angina), hospitalization for heart failure, and all-cause mortality. Additional prespecified endpoints included the following: death due to renal failure or progression to ESKD; albuminuria progression (change from normoalbuminuria [UACR < 30 mg/g] to microalbuminuria [UACR 30–300 mg/g] or macroalbuminuria [UACR > 300 mg/g], or from microalbuminuria to macroalbuminuria); a composite microvascular outcome that included albuminuria progression, sustained ESKD, sustained decrease in eGFR of ≥ 50%, death due to renal failure, and major ocular events; and change from baseline in HbA1c and UACR. All-cause hospitalization was a post hoc endpoint.
All cardiovascular and kidney outcomes were centrally adjudicated by clinical events committees masked to treatment assignment. Adverse events were reported by investigators and classified using the Medical Dictionary for Regulatory Activities Version 20.1.
The trial was designed to continue until at least 611 participants had an adjudication-confirmed 3-point MACE event. Assuming a hazard ratio (HR) of 1.0, this provided 90% power to demonstrate non-inferiority of linagliptin versus placebo with the prespecified non-inferiority margin of 1.3 at a one-sided α-level of 2.5%.
Subgroup analysis of patients from Asia
The CARMELINA® trial included study sites in Japan, China, South Korea, Taiwan, and Malaysia. The participants were asked to self-identify their race, including Asian. For the analysis reported here, we evaluated outcomes in participants from Asian study sites who self-identified as being of Asian race. We also evaluated outcomes in the subgroup of East Asian participants (those from Japan, China, South Korea, and Taiwan who self-identified as Asian).
We evaluated clinical outcomes, changes in metabolic, clinical and laboratory parameters, and the incidence of adverse events for all randomized patients treated with at least one dose of study drug (the treated set). For clinical outcomes, treatment group differences were assessed using Cox proportional hazards models with terms for treatment group, geographic region, and treatment group by region interaction, except for outcomes that included hospitalization for heart failure, for which the models additionally included a term for history of heart failure. Changes from baseline in HbA1c, body weight, serum cholesterol, blood pressure, and UACR were evaluated within the Asian subset with either descriptive statistics or using mixed models for repeated measures that included terms for treatment, baseline value, week, treatment-by-week interaction and baseline value-by-week interaction. Adverse events were summarized within the Asian subset with descriptive statistics.
Results
Baseline characteristics
Of the 6979 patients treated in the CARMELINA® trial, 555 participants in Asian countries identified themselves as of Asian race, comprising 8.0% of the overall study population. At baseline, the clinical and demographic characteristics of the Asian participants were generally similar between treatment groups (Table 1). Overall, the population had a mean (SD) age of 65.3 (9.3) years, was borderline obese by Asian standards [mean (SD) BMI 27.0 (4.3) kg/m2], and had long-standing T2DM [mean (SD) duration: 14.3 (9.2) years] and moderate hyperglycemia [mean (SD) HbA1c: 7.8% (0.97)]. Nearly all patients (95.0%) were receiving background glucose-lowering treatment, with more than half (54.1%) on insulin.
Table 1.
Baseline demographic and clinical characteristics of Asian patients
| Characteristic | Linagliptin (n = 272) | Placebo (n = 283) |
|---|---|---|
| Age, years | 65.1 ± 9.22 | 65.5 ± 9.40 |
| Sex, n (%) | ||
| Male | 201 (73.9) | 199 (70.3) |
| Female | 71 (26.1) | 84 (29.7) |
| Race, n (%) | ||
| Asian | 272 (100.0) | 283 (100.0) |
| Smoking status, n (%) | ||
| Never smoker | 139 (51.1) | 138 (48.8) |
| Ex-smoker | 97 (35.7) | 97 (34.3) |
| Current smoker | 36 (13.2) | 48 (17.0) |
| History of heart failure, n (%) | 34 (12.5) | 21 (7.4) |
| Ischemic heart disease, n (%) | 160 (58.8) | 158 (55.8) |
| History of hypertension, n (%) | 249 (91.5) | 261 (92.2) |
| Atrial fibrillation, n (%) | 21 (7.7) | 19 (6.7) |
| eGFR (MDRD), ml/min/1.73 m2 | 52.6 ± 23.9 | 50.9 ± 23.4 |
| eGFR (MDRD), n (%) | ||
| ≥ 90 ml/min/1.73 m2 | 19 (7.0) | 22 (7.8) |
| ≥ 60– < 90 ml/min/1.73 m2 | 76 (27.9) | 70 (24.7) |
| ≥ 45 − <60 ml/min/1.73 m2 | 52 (19.1) | 50 (17.7) |
| ≥ 30 − <45 ml/min/1.73 m2 | 83 (30.5) | 85 (30.0) |
| < 30 ml/min/1.73 m2 | 42 (15.4) | 56 (19.8) |
| UACR, mg/g, median (25th − 75th percentile) | 299 (95–1420) | 256 (60–1120) |
| UACR, n (%) | ||
| < 30 mg/g | 19 (7.0) | 39 (13.8) |
| 30 − 300 mg/g | 118 (43.4) | 110 (38.9) |
| > 300 mg/g | 135 (49.6) | 134 (47.3) |
| BMI, kg/m2 | 27.2 ± l4.3 | 26.8 ± 4.3 |
| HbA1c, % | 7.80 ± 0.98 | 7.81 ± 0.97 |
| Fasting plasma glucose, mg/dla | 142.4 ± 46.4 | 140.0 ± 46.5 |
| Diabetes duration, years | 14.98 ± 9.63 | 13.70 ± 8.82 |
| Systolic blood pressure, mm Hg | 140.5 ± 17.9 | 139.6 ± 18.2 |
| Diastolic blood pressure, mm Hg | 76.6 ± 11.6 | 76.2 ± 11.1 |
| Heart rate, beats per minuteb | 68.2 ± 12.3 | 67.6 ± 10.7 |
| Total cholesterol, mg/dlc | 170.0 ± 49.3 | 169.2 ± 36.7 |
| LDL cholesterol, mg/dlc | 88.5 ± 41.0 | 88.3 ± 31.6 |
| HDL cholesterol, mg/dlc | 48.6 ± 12.9 | 50.1 ± 14.7 |
| Triglycerides, mg/dlc | 170.3 ± 104.3 | 157.5 ± 92.5 |
| Glucose-lowering therapy, n (%) | 257 (94.5) | 270 (95.4) |
| Insulin | 147 (54.0) | 153 (54.1) |
| Metformin | 137 (50.4) | 134 (47.3) |
| Sulfonylureas | 98 (36.0) | 105 (37.1) |
| Number of background glucose-lowering therapies, n (%) | ||
| 1 | 105 (38.6) | 116 (41.0) |
| 2 | 116 (42.6) | 113 (39.9) |
| 3 | 32 (11.8) | 30 (10.6) |
| ≥ 4 | 4 (1.5) | 6 (2.1) |
| Antihypertensives, n (%) | 267 (98.2) | 268 (94.7) |
| ACE inhibitors or ARBs | 224 (82.4) | 206 (72.8) |
| Calcium antagonists | 167 (61.4) | 171 (60.4) |
| β-Blockers | 143 (52.6) | 133 (47.0) |
| Diuretics | 98 (36.0) | 94 (33.2) |
| Aspirin, n (%) | 169 (62.1) | 175 (61.8) |
| Statins, n (%) | 231 (84.9) | 224 (79.2) |
Data are mean ± SD for patients treated with ≥ 1 dose of study medication unless otherwise specified
ACE angiotensin-converting enzyme, ARB angiotensin receptor blocker, BMI body mass index, eGFR estimated glomerular filtration rate, HbA1c glycated hemoglobin, HDL high-density lipoprotein, LDL low-density lipoprotein, MDRD Modification of Diet in Renal Disease study equation, SD standard deviation, UACR urinary albumin-to-creatinine ratio
aData missing for 1 patient (linagliptin: n = 1)
bData missing for 17 patients (linagliptin: n = 11; placebo: n = 6)
cData missing for 7 patients (linagliptin: n = 4; placebo: n = 3)
A total of 57.3% of patients had ischemic heart disease at baseline. More patients randomized to linagliptin (12.5%) had a history of heart failure than those randomized to placebo (7.4%); the overall incidence was 9.9%. Mean (SD) systolic and diastolic blood pressure was 140.0 (18.1) and 76.4 (11.4) mm Hg, respectively, and 91.9% of patients had hypertension. A total of 96.4% of patients were taking antihypertensives, including 77.5% taking angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (linagliptin: 82.4%; placebo: 72.8%). Mean (SD) eGFR was 51.7 (23.6) ml/min/1.73 m2 and median UACR was 269 mg/g (linagliptin: 299 mg/g; placebo: 256 mg/g). At baseline, 368 (66.3%) patients had eGFR < 60 ml/min/1.73 m2, 228 (41.1%) had microalbuminuria, and 269 (48.5%) had macroalbuminuria.
The median duration of treatment and observation was 2.0 years and 2.2 years, respectively.
Cardiovascular and mortality outcomes
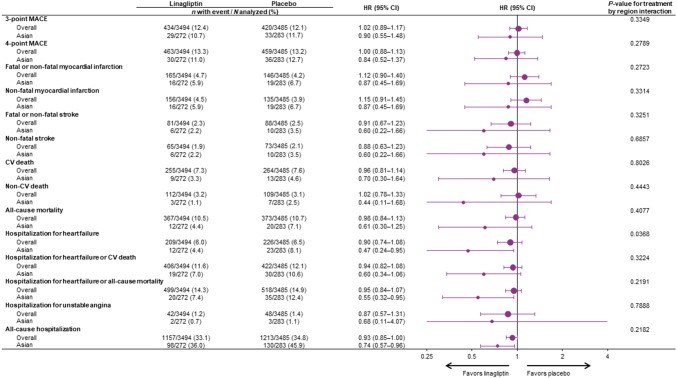
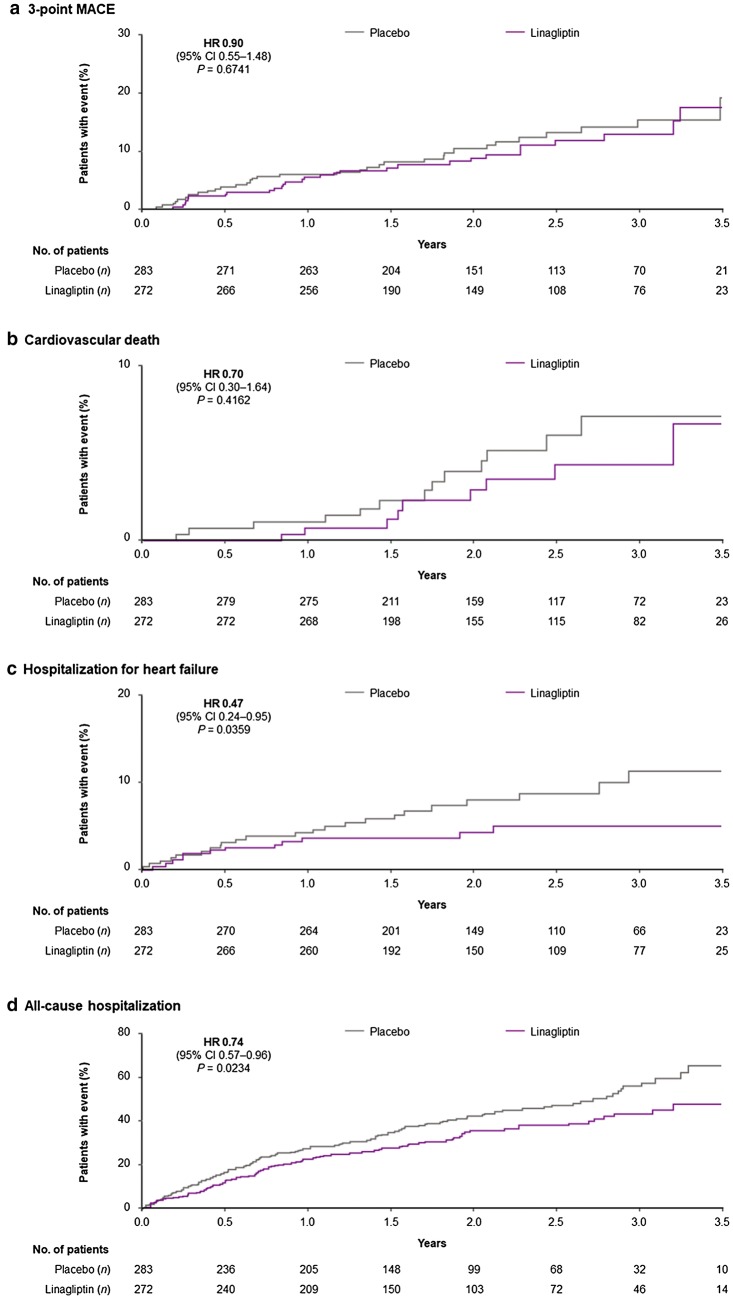
In Asian patients, the primary outcome of 3-point MACE occurred in 29 patients (10.7%) randomized to linagliptin and 33 (11.7%) randomized to placebo (HR 0.90; 95% CI 0.55–1.48) (Fig. 1). The neutral effect of linagliptin on risk of 3-point MACE was consistent with the overall trial population (HR 1.02; 95% CI 0.89–1.17; P value for treatment-by-region interaction: 0.3349). The 4-point MACE endpoint occurred in 30 (11.0%) linagliptin patients and 36 (12.7%) placebo patients (HR 0.84; 95% CI 0.52–1.37). Again, this was consistent with the overall population (HR 1.00; 95% CI 0.88–1.13; P value for treatment-by-region interaction: 0.2789) (Fig. 1).
Fig. 1.
Cardiovascular outcomes and mortality in overall trial population and Asian patients. CI confidence interval, CV cardiovascular, HR hazard ratio, MACE major adverse cardiovascular events
Furthermore, linagliptin did not increase the risk for any of the individual components of 3-point MACE or 4-point MACE (Fig. 1). For cardiovascular death, the HR was 0.70 (95% CI 0.30–1.64) in Asian patients, compared with 0.96 (95% CI 0.81–1.14) in the overall population. For non-fatal myocardial infarction, the HR was 0.87 (95% CI 0.45–1.69) in Asian patients compared with 1.15 (95% CI 0.91–1.45) for the overall population, whereas for non-fatal stroke the HR was 0.60 (95% CI 0.22–1.66) and 0.88 (95% CI 0.63–1.23) in Asian and overall patients, respectively. For all these endpoints, the P values for treatment-by-region interaction were not significant (Fig. 1).
Death due to any cause (all-cause mortality) occurred in 12 (4.4%) and 20 (7.1%) of Asian patients treated with linagliptin or placebo, respectively (HR 0.61; 95% CI 0.30–1.25), consistent with the neutral effect of linagliptin treatment on all-cause mortality in the overall trial population (HR 0.98; 95% CI 0.84–1.13; P value for treatment-by-region interaction: 0.4077).
In Asian patients, linagliptin treatment was associated with a nominally reduced risk of hospitalization for heart failure (HR 0.47; 95% CI 0.24–0.95; P value for treatment-by-region interaction: 0.0368), the composite of hospitalization for heart failure or all-cause mortality (HR 0.55; 95% CI 0.32–0.95; P value for treatment-by-region interaction: 0.2191), and all-cause hospitalization (HR 0.74; 95% CI 0.57–0.96; P value for treatment-by-region interaction: 0.2182).
Figure 2 shows the time to first event for 3-point MACE, cardiovascular death, hospitalization for heart failure, and all-cause hospitalization.
Fig. 2.
Time to first cardiovascular event or hospitalization in Asian patients. Two-sided P values. CI confidence interval, HR hazard ratio, MACE major adverse cardiovascular events (cardiovascular death, non-fatal myocardial infarction, non-fatal stroke)
In the 379 patients from East Asian countries, the incidence of adverse cardiovascular events was comparable to the wider Asian cohort as well as the overall trial population (Supplementary Table S1). For example, the incidence rate of 3-point MACE per 1000 patient-years was 42.1 and 56.2 with linagliptin and placebo, respectively, in East Asian patients, compared with 49.3 and 54.8 with linagliptin and placebo, respectively, in the wider Asian cohort, and 57.7 and 56.3 with linagliptin and placebo, respectively, in the overall trial population.
Kidney outcomes
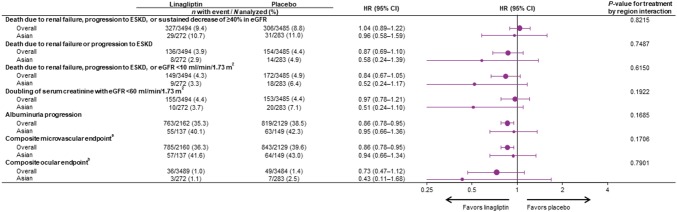
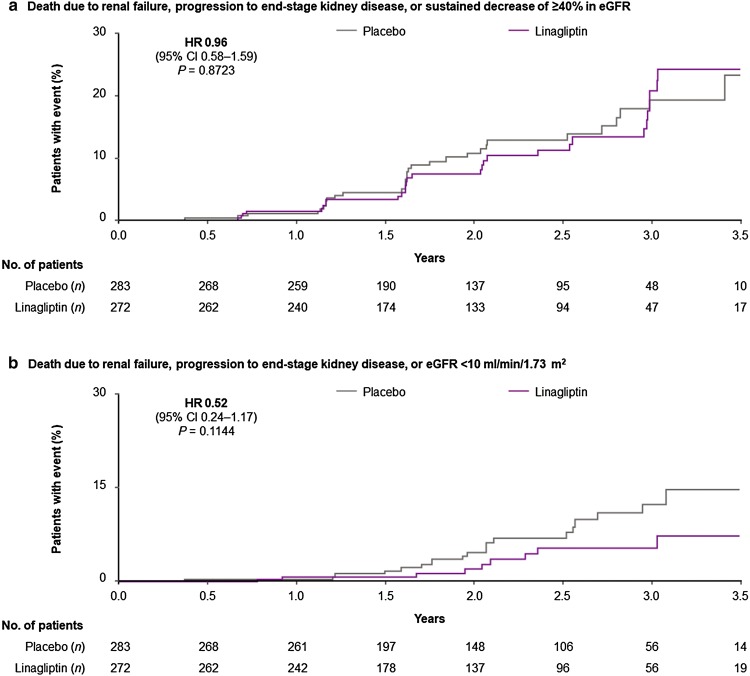
Death due to renal failure, progression to ESKD, or ≥ 40% decrease in eGFR (the key secondary endpoint) occurred in 29 (10.7%) and 31 (11.0%) Asian patients in the linagliptin and placebo groups, respectively (HR 0.96; 95% CI 0.58–1.59), consistent with the neutral treatment effect in the overall population (P value for treatment-by-region interaction: 0.8215) (Fig. 3). Time to first event of this key secondary endpoint and the additional post hoc composite endpoint of death due to renal failure, progression to ESKD, or eGFR < 10 ml/min/1.73 m2 are shown in Fig. 4. In the overall trial population, albuminuria progression occurred significantly less frequently in the linagliptin group than the placebo group. Consistent with this, fewer Asian patients experienced albuminuria progression with linagliptin (40.1%) than with placebo (42.3%) (P value for treatment-by-region interaction: 0.1685) (Fig. 3). Also consistent with its treatment effect in the overall population, linagliptin did not increase the risk of other kidney outcomes in Asian patients, including the composite of death due to renal failure or progression to ESKD, the composite microvascular outcome, or the composite ocular outcome (Fig. 3).
Fig. 3.
Kidney and microvascular outcomes in overall trial population and Asian patients. aDeath due to renal failure, sustained ESKD, sustained decrease of ≥ 50% in eGFR, albuminuria progression, use of retinal photocoagulation or intravitreal injections of an anti-VEGF therapy for diabetic retinopathy, vitreous hemorrhage, or diabetes-related blindness; bRetinal laser coagulation therapy or intravitreal injection(s) of an anti-VEGF therapy for diabetic retinopathy, vitreous hemorrhage, or diabetes-related blindness. CI confidence interval; eGFR estimated glomerular filtration rate, ESKD end-stage kidney disease, HR hazard ratio, VEGF vascular endothelial growth factor
Fig. 4.
Time to first kidney event in Asian patients. Two-sided P values. CI confidence interval, eGFR estimated glomerular filtration rate, HR hazard ratio
In the 379 patients from East Asian countries, the incidence of adverse kidney events was comparable to the wider Asian cohort as well as the overall trial population (Supplementary Table S1). For example, the incidence rate of the key secondary composite kidney endpoint per 1000 patient-years was 41.6 and 32.9 with linagliptin and placebo, respectively, in East Asian patients, compared with 54.2 and 55.7 with linagliptin and placebo, respectively, in the wider Asian cohort, and 48.9 and 46.6 with linagliptin and placebo, respectively, in the overall trial population.
Glycemic control
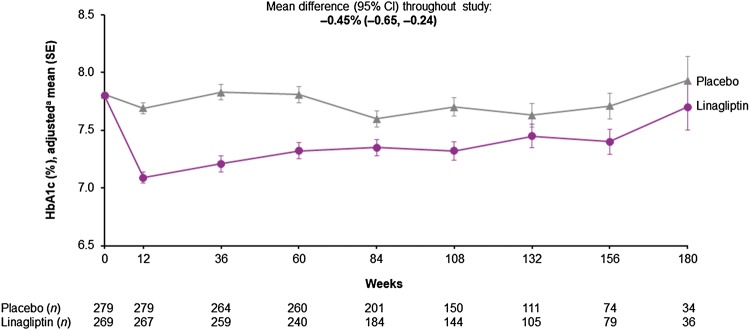
After 12 weeks of treatment of Asian patients, the adjusted mean difference in HbA1c level with linagliptin compared with placebo was − 0.60% (95% CI − 0.73 to − 0.47). The weighted average mean difference over the full study duration was − 0.45% (95% CI − 0.65 to − 0.24) in favor of linagliptin (Fig. 5). Over the course of the study, fewer patients in the linagliptin group (30.5%) received additional glucose-lowering therapies compared with the placebo group (35.7%), including insulin (16.5% and 20.1%, respectively) (Supplementary Table S2). There was no significant difference between treatment groups in the time to first initiation of any glucose-lowering medication; however, there was a trend towards delayed initiation or dose increase of insulin in the linagliptin group (Supplementary Fig. S1).
Fig. 5.
HbA1c over time in Asian patients. aBaseline values are descriptive; post-baseline data from mixed model for repeated measures. CI confidence interval, HbA1c glycated hemoglobin, SE standard error
Cardiovascular risk factors and medications
There was no difference in change over time in body weight, cholesterol levels, or blood pressure with linagliptin compared with placebo (Supplementary Fig. S2). Over the course of the study, slightly fewer patients in the linagliptin group received new antihypertensives or statins compared with the placebo group (Supplementary Table S3). These findings are consistent with the overall trial population [20].
Safety and tolerability
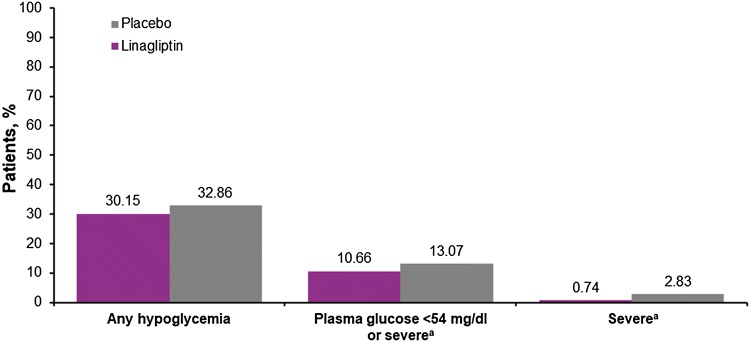
The adverse event profile of linagliptin in Asian patients (Table 2) was consistent with the overall population [20]. Fewer linagliptin-treated Asian patients (77.9%) had an adverse event compared with placebo-treated Asian patients (84.8%). Similarly, fewer linagliptin patients had a serious adverse event (39.0% vs 46.3%) or adverse event leading to the discontinuation of study drug (7.0% vs 8.1%). The incidence of hypoglycemia was also slightly lower with linagliptin than placebo, including severe episodes (Fig. 6).
Table 2.
Adverse events in Asian patients
| Adverse eventa | Linagliptin (n = 272) | Placebo (n = 283) |
|---|---|---|
| Any adverse event | 212 (77.9) | 240 (84.8) |
| Serious adverse event | 106 (39.0) | 131 (46.3) |
| Adverse event leading to discontinuation | 19 (7.0) | 23 (8.1) |
| Renal and urinary disordersb | 16 (5.9) | 13 (4.6) |
| Constipationc | 15 (5.5) | 19 (6.7) |
| Hypersensitivity reactionsd | 11 (4.0) | 16 (5.7) |
| Cancere | 5 (1.8) | 7 (2.5) |
| Colon cancer | 1 (0.4) | 0 |
| Pancreatic cancerf | 0 | 1 (0.4) |
| Gastric cancer | 0 | 0 |
| Pancreatitis (adjudication-confirmed) | 0 | 0 |
| Pemphigoid | 0 | 0 |
| Skin lesionsg | 0 | 0 |
Data are n (%) of patients treated with ≥ 1 dose of study medication
MedDRA Version 20.1 was used to code adverse events
BIcMQ Boehringer Ingelheim customized MedDRA Query, MedDRA Medical Dictionary for Regulatory Activities, SMQ standardized MedDRA query
aMedDRA preferred term unless otherwise specified
bBased on the narrow SMQ “acute renal failure”
cBased on the preferred terms “Constipation” and “Infrequent bowel movements”
dBased on the narrow SMQ “hypersensitivity”
eBased on SMQs “Malignant Tumors” and “Tumors of unspecified malignancy”
fBased on narrow BIcMQ “Pancreatic cancer”
gBased on the narrow SMQ “Severe cutaneous adverse reactions”
Fig. 6.
Incidence of hypoglycemia in Asian patients. aRequiring the assistance of another person to actively administer carbohydrate, glucagon or other resuscitative actions
Individual adverse events were also balanced between treatment groups in Asian patients. Cancer was reported in 1.8% of patients in the linagliptin group and 2.5% of the placebo group. There was one case of pancreatic cancer in the placebo group and none in the linagliptin group. No cases of pemphigoid, pancreatitis, or skin lesions were reported in Asian patients (Table 2), in contrast to the overall trial population where there were numerical imbalances for pemphigoid (linagliptin: n = 7 [0.2%]; placebo: n = 0), adjudication-confirmed acute pancreatitis (linagliptin: n = 9 [0.3%]; placebo: n = 5 [0.1%]), and skin lesions (linagliptin: n = 5 [0.2%]; placebo: n = 1 [< 0.1%)]) [20].
Discussion
This subgroup analysis of the multinational CARMELINA® trial indicates that linagliptin did not increase the risk of MACE in Asian T2DM patients with established CVD with albuminuria and/or kidney disease. Furthermore, linagliptin did not increase the risk of clinically relevant kidney complications or heart failure. These findings are consistent with the effects of linagliptin in the overall study population of CARMELINA® [20, 21].
The baseline clinical and demographic characteristics of Asian patients who participated in CARMELINA® show that they were at high risk for adverse cardiovascular and kidney events, as designed for the overall study. Compared with the overall trial population, notable differences in the Asian cohort included lower mean BMI (31 and 27 kg/m2, respectively) and worse albuminuria (UACR 160 and 269 mg/g, respectively). There were also substantially fewer Asian patients with heart failure at baseline (9.9% vs 27.0% in the overall study population).
In 2008, the US Food and Drug Administration introduced the requirement that new glucose-lowering drugs must demonstrate cardiovascular safety; since then, more than 15 large, multinational, cardiovascular outcomes trials have been conducted [23, 24]. However, none of these have been performed exclusively in Asian populations and few subgroup analyses of Asian patients from these studies have been reported. The SAVOR-TIMI 53, EXAMINE, and TECOS cardiovascular outcomes trials of saxagliptin [25], alogliptin [26], and sitagliptin [27], respectively, demonstrated that these DPP-4 inhibitors do not increase the risk for MACE; cardiovascular outcomes in the Asian patients from these studies have not been reported. A similar cardiovascular outcomes study of omarigliptin, a once-weekly DPP-4 inhibitor marketed mainly in Japan, was terminated early but an informal analysis suggested no increased cardiovascular risk [28]. No robust data on cardiovascular safety are available for other DPP-4 inhibitors marketed mostly in Asia such as anagliptin, evogliptin, gemigliptin, teneligliptin, and trelagliptin. Our analysis of CARMELINA® provides reassuring data on the cardiovascular and kidney safety of linagliptin in Asian patients. Although linagliptin did not reduce the risk of cardiovascular events, consistent with its effects in the overall population, these individuals were receiving a high standard of care for CVD—as stipulated by the study protocol—with most taking antihypertensives, aspirin, and statins. Of note, however, the SGLT2 inhibitor empagliflozin was shown to reduce the risk of cardiovascular events in Asian patients in a subgroup analysis of the EMPA-REG OUTCOME trial, which had a similarly high standard of care for CVD [29]. Given the cardiovascular risk reductions seen with empagliflozin and other SGLT2 inhibitors, as well as certain GLP-1 receptor agonists, the joint guidelines from the American Diabetes Association and the European Association for the Study of Diabetes now recommend that additional glucose-lowering therapy after metformin in T2DM patients with established CVD should be with such drugs that have cardiovascular benefit [30, 31]. Nevertheless, glucose-lowering treatments with proven cardiovascular safety are still needed, as most patients require multiple glucose-lowering drugs to achieve glycemic control. DPP-4 inhibitors are recognized by Chinese, Japanese, and Indian clinical practice guidelines as effective glucose-lowering drugs that do not increase risk for hypoglycemia [32–34]. Our study provides evidence on the cardiovascular and kidney safety of linagliptin in Asian patients.
The SAVOR-TIMI 53 trial showed a significantly increased risk of hospitalization for heart failure with saxagliptin [25]. Since then, there has been concern surrounding a potential class risk for heart failure with DPP-4 inhibitors. Our analysis of CARMELINA® is reassuring in this regard as linagliptin was not associated with an increased risk for heart failure in the overall population and, similarly, there was a nominally reduced risk of hospitalization for heart failure in Asian patients. Of note is the higher rate of heart failure in the placebo group in Asia compared to the placebo group of the overall study population, which could be related to certain risk factors for heart failure being more prevalent in the Asian subgroup (e.g., lower eGFR, more males and higher use of thiazolidinediones). The heterogeneity across regions in incidence of heart failure with linagliptin compared to placebo (treatment-by-region interaction P value of 0.0368) is, therefore, interesting, with a nominally lower HR in patients from Asia. However, given the many subgroup analyses conducted, this finding should not be over-interpreted, and further investigations would be needed to investigate whether there is a true treatment effect—if established, this could conceivably relate to the influence of pharmacogenetic factors [35] or the effect of adiposity differences on DPP-4 activity [36], and thus levels of its substrate peptides regulating cardiovascular functions.
CARMELINA® is unique among the cardiovascular outcomes trials of DPP-4 inhibitors in that it was also designed to evaluate kidney outcomes, and thus included a substantial number of patients with diabetic kidney disease who typically are excluded from such studies. The results indicate that linagliptin did not increase the risk of kidney complications in Asian patients, consistent with the findings in the overall population. However, as in the parent study, no benefit of linagliptin in reducing hard kidney outcomes was observed, despite the body of evidence from animal studies and small clinical trials suggesting that this DPP-4 inhibitor and other members of the drug class might have pleiotropic kidney benefits [37]. As chronic kidney disease is a slowly progressive condition, it is possible that the study was too short for any benefit on renal outcomes to manifest. Nevertheless, it is reassuring to note the trends toward risk reductions for certain composite kidney endpoints, such as death due to renal failure or progression of ESKD, and death due to renal failure, progression of ESKD, or eGFR decreasing to < 10 ml/min/1.73 m2. Experimental data for linagliptin highlight that effects on tubulointerstitial fibrosis may be one aspect by which linagliptin might confer kidney effects [37]. Thus, it may be speculated that patients at more advanced stages of kidney disease (e.g., lower GFR) are a potential population that may warrant further studies with hard kidney outcomes.
A modest benefit of linagliptin in reducing progression of albuminuria (HR 0.86 [95% CI 0.78, 0.95]) was seen in the overall trial population of CARMELINA® [20]. The treatment-by-region interaction P value of 0.1685 suggests that the overall HR of 0.86 is applicable to all regions, including Asia. This finding is consistent with the modest albuminuria-lowering effects seen in previous studies of linagliptin [38] and other DPP-4 inhibitors [39, 40], but its clinical relevance has yet to be determined. Improved glycemic control may also have contributed to albuminuria lowering, as there was a small mean HbA1c difference between the linagliptin and placebo groups, despite the trial being designed to achieve glycemic equipoise between treatment groups (investigators were encouraged to optimize background glucose-lowering medication in both groups according to local guidelines).
The safety and tolerability profile of linagliptin in Asian patients in CARMELINA® was consistent with the overall trial population [20] as well as previous shorter clinical trials investigating its glycemic efficacy and safety in Asian patients [11–19]. No new safety signals were identified in this study. Although there were a small number of cases of pancreatitis and pemphigoid in the overall trial population that were imbalanced between treatment groups [20], none of these occurred in Asian patients.
The strength of our study is that it provides evidence on the cardiorenal safety of linagliptin in Asian patients from a large, robustly controlled, randomized clinical trial. However, it is subject to the usual limitations of subgroup analyses. The overall trial was not powered to detect differences in treatment effects in subgroups. Furthermore, the trial duration may have been too short for long-term cardiovascular and/or kidney benefit to manifest.
Conclusion
Linagliptin demonstrated cardiovascular and renal safety in Asian patients with T2DM in the CARMELINA® trial, consistent with its effects in the overall study population.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The CARMELINA® trial was funded by the Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance. Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Giles Brooke, PhD, CMPP, of Elevate Scientific Solutions during the preparation of this manuscript.
Compliance with ethical standards
Conflicts of interest
N.I. has received honoraria from Kowa company; research funding from Mitsubishi Tanabe, Daiichi Sankyo and AstraZeneca; and subsidies/donations from Takeda, MSD, Ono, Sanofi, Japan Tobacco Inc., Mitsubishi Tanabe, Novartis, Boehringer Ingelheim, Kyowa Kirin, Astellas, Daiichi Sankyo, Kissei Pharmaceutical, Dainippon Pharma, Sanwa kagaku, Eli Lilly, Novo Nordisk, Teijin Pharma, and Taisho-Toyama Pharma. W.Y. has attended advisory boards for Novo Nordisk; received investigator-initiated trial research funds from AstraZeneca; been a speaker for Novo Nordisk, Bayer, Sanofi Aventis, Merck Sharp & Dohme China, AstraZeneca, Eli Lilly, Boehringer Ingelheim, and Servier; and received honorarium and travel support as an advisory board member from Merck & Co., Inc. H.W. has received honoraria from Eli Lilly, Mitsubishi Tanabe Pharma, Sanofi, Takeda Pharmaceutical Company, Novartis Pharma, Nippon Boehringer Ingelheim, Daiichi Sankyo, Ono Pharmaceutical, Astellas Pharma, FUJIFILM Pharma, Terumo Corporation, MSD; research funding from Eli Lilly, Novartis Pharma, Sanwa kagaku; subsidies/donations from Mitsubishi Tanabe Pharma, Kissei Pharmaceutical, Nippon Boehringer Ingelheim, Novartis Pharma, Sumitomo Dainippon Pharma, Sanofi, MSD, Pfizer Japan, Astellas Pharma, Takeda Pharmaceutical Company, Novo Nordisk, Teijin Pharma; and departmental endowments from Takeda Pharmaceutical Company, MSD, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Ono Pharmaceutical, Kowa company, and Sanwa kagaku. L.J. has received consulting and lecture fees from Eli Lilly and Company, Bristol-Myers Squibb, Novartis, Novo Nordisk, Merck, Bayer, MSD, Takeda, Sanofi, Roche, Boehringer Ingelheim, and AstraZeneca; and research support from Roche, Sanofi, MSD, AstraZeneca, Novartis, and Bristol-Myers Squibb. S.S., E.P., T.O., O.E.J., J.T.G., and M.v.E are employees of Boehringer Ingelheim. J.R. has served on scientific advisory boards and received honoraria or consulting fees from Eli Lilly, Sanofi, Novo Nordisk, Janssen, AstraZeneca, Boehringer Ingelheim, and Intarcia; he has also received grants/research support from Merck, Pfizer, Sanofi, Novo Nordisk, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Genentech, Janssen, Lexicon, Boehringer Ingelheim, and Intarcia. V.P. has received research support from the Australian National Health and Medical Research Council (Project and Program Grant); served on steering committees for trials supported by AbbVie, Boehringer Ingelheim, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Novo Nordisk, Pfizer, Retrophin, and Tricida; and served on advisory boards, spoken at scientific meetings, or both for AbbVie, Astellas Pharma, AstraZeneca, Bayer, Baxter, Bristol-Myers Squibb, Boehringer Ingelheim, Durect Corporation, Eli Lilly, Gilead Sciences, GlaxoSmithKline, Janssen, Merck, Novartis, Novo Nordisk, Pfizer, Pharmalink, Relypsa, Roche, Sanofi, Servier, and Vitae. He has a policy of having honoraria paid to his employer. C.W. has received fees for advisory services to Boehringer Ingelheim and MSD as well as honoraria for lecturing from AstraZeneca, Eli Lilly and Sanofi. M.E.C. has received fees for advisory services and honoraria from Boehringer Ingelheim, Sanofi, Servier, Bayer, Astra Zeneca, Reata, MundiPharma and MSD and a grant from NovoNordisk. J.H.A. has received personal fees from Abbvie, Bristol-Myers Squibb, CSL Behring, Janssen Pharmaceutics, Novo Nordisk, Pfizer, Portola, and Teikoku; and institutional research support from Boehringer Ingelheim, Bristol-Myers Squibb, Cryolife, CSL Behring, Tenax Therapeutics, and VoluMetrix. I.K. has received honororia from Astellas Pharma Inc., MSD K.K., Edwards Laboratories Corporation, Otsuka Pharmaceutical Co. Ltd, Kowa Company, Ltd., Daiichi Sankyo Company, Limited, Taisho Pharma Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd, Takeda Pharmaceutical Company Limited, Mitsubishi Tanabe Pharma Corporation, Teijin Pharma Limited, Toa Eiyo Ltd, Bayer Yakuhin, Ltd, Terumo Corporation, Nipro Corporation; research funding from Ono Pharmaceutical Co., Ltd; and subsidies/donations from Actelion Pharmaceuticals Japan Ltd. Astellas Pharma Inc., Amgen Astellas BioPharma K.K., AstraZeneca K.K., MSD K.K., Shionogi & Co., Ltd, Daiichi Sankyo Company, Limited, Takeda Pharmacentical Company Limited, Toa Eiyo Ltd, Nippon Boehringer Ingelheim Co., Ltd, Bayer Yakuhin, Ltd, and Pfizer Japan Inc. M.N. is an advisor for KHK, Astellas, GSK, Daiichi-Sankyo, Mitsubishi-Tanabe, JT, Boehringer-Ingelheim; has received honoraria from KHK, Astellas, AstraZeneca, GSK, Daiichi-Sankyo, Mitubishi-Tanabe, Chugai, Torii, JT; manuscript fees from KHK; research funding from JT, KHK; and subsidies/donations from KHK, Astellas, Ono, Daiichi-Sankyo, Takeda, Mitsubishi-Tanabe, Chugai, Torii, MSD, Otsuka, Dainippon-Sumitomo, JT, and Boehringer Ingelheim.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and/or with the Helsinki Declaration of 1964 and later versions.
Human rights statement
This research involves human participants. This report was limited to the Asian population from the CARMELINA® trial (ClinicalTrials.gov Identifier, NCT01897532). The study protocol was approved by the institutional review board or independent ethics committee from each site (approval numbers: not applicable) and all patients provided written informed consent before entering the trial. Full details of the approval process are provided in previous publications [20, 22].
Informed consent
Informed consent or substitute for it was obtained from all patients for being included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hata J, Kiyohara Y. Epidemiology of stroke and coronary artery disease in Asia. Circ J. 2013;77(8):1923–1932. doi: 10.1253/circj.CJ-13-0786. [DOI] [PubMed] [Google Scholar]
- 2.Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301(20):2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 3.Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64–91. doi: 10.1111/nyas.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nanditha A, Ma RC, Ramachandran A, Snehalatha C, Chan JC, Chia KS, Shaw JE, Zimmet PZ. Diabetes in Asia and the Pacific: implications for the global epidemic. Diabetes Care. 2016;39(3):472–485. doi: 10.2337/dc15-1536. [DOI] [PubMed] [Google Scholar]
- 5.Ramachandran A, Snehalatha C, Ma RC. Diabetes in South-East Asia: an update. Diabetes Res Clin Pract. 2014;103(2):231–237. doi: 10.1016/j.diabres.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Lu J, Bi Y, Ning G. Curbing the obesity epidemic in China. Lancet Diabetes Endocrinol. 2016;4(6):470–471. doi: 10.1016/S2213-8587(16)30007-9. [DOI] [PubMed] [Google Scholar]
- 7.International Diabetes Federation . IDF Diabetes Atlas. 8. Brussels: International Diabetes Federation; 2017. [PubMed] [Google Scholar]
- 8.Gujral UP, Pradeepa R, Weber MB, Narayan KM, Mohan V. Type 2 diabetes in South Asians: similarities and differences with white Caucasian and other populations. Ann N Y Acad Sci. 2013;1281:51–63. doi: 10.1111/j.1749-6632.2012.06838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prasad N, Jha V. Hemodialysis in Asia. Kidney Dis (Basel) 2015;1(3):165–177. doi: 10.1159/000441816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graefe-Mody U, Retlich S, Friedrich C. Clinical pharmacokinetics and pharmacodynamics of linagliptin. Clin Pharmacokinet. 2012;51(7):411–427. doi: 10.2165/11630900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Araki E, Kawamori R, Inagaki N, Watada H, Hayashi N, Horie Y, Sarashina A, Thiemann S, von Eynatten M, Dugi K, Woerle HJ. Long-term safety of linagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Obes Metab. 2013;15(4):364–371. doi: 10.1111/dom.12039. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Ning G, Wang C, Gong Y, Patel S, Zhang C, Izumoto T, Woerle HJ, Wang W. Efficacy and safety of linagliptin monotherapy in Asian patients with inadequately controlled type 2 diabetes mellitus: a multinational, 24-week, randomized, clinical trial. J Diabetes Investig. 2015;6(6):692–698. doi: 10.1111/jdi.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inagaki N, Watada H, Murai M, Kagimura T, Gong Y, Patel S, Woerle HJ. Linagliptin provides effective, well-tolerated add-on therapy to pre-existing oral antidiabetic therapy over 1 year in Japanese patients with type 2 diabetes. Diabetes Obes Metab. 2013;15(9):833–843. doi: 10.1111/dom.12110. [DOI] [PubMed] [Google Scholar]
- 14.Kawamori R, Inagaki N, Araki E, Watada H, Hayashi N, Horie Y, Sarashina A, Gong Y, von Eynatten M, Woerle HJ, Dugi KA. Linagliptin monotherapy provides superior glycaemic control versus placebo or voglibose with comparable safety in Japanese patients with type 2 diabetes: a randomized, placebo and active comparator-controlled, double-blind study. Diabetes Obes Metab. 2012;14(4):348–357. doi: 10.1111/j.1463-1326.2011.01545.x. [DOI] [PubMed] [Google Scholar]
- 15.Ma RC, Del Prato S, Gallwitz B, Shivane VK, Lewis-D’Agostino D, Bailes Z, Patel S, Lee J, von Eynatten M, Di Domenico M, Ross SA. Oral glucose lowering with linagliptin and metformin compared with linagliptin alone as initial treatment in Asian patients with newly diagnosed type 2 diabetes and marked hyperglycemia: subgroup analysis of a randomized clinical trial. J Diabetes Investig. 2018;9(3):579–586. doi: 10.1111/jdi.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Yang J, Yang G, Gong Y, Patel S, Zhang C, Izumoto T, Ning G. Efficacy and safety of linagliptin in Asian patients with type 2 diabetes mellitus inadequately controlled by metformin: a multinational 24-week, randomized clinical trial. J Diabetes. 2016;8(2):229–237. doi: 10.1111/1753-0407.12284. [DOI] [PubMed] [Google Scholar]
- 17.Wu W, Li Y, Chen X, Lin D, Xiang S, Shen F, Gu X. Effect of linagliptin on glycemic control in Chinese patients with newly-diagnosed, drug-naive type 2 diabetes mellitus: a randomized controlled trial. Med Sci Monit. 2015;21:2678–2684. doi: 10.12659/MSM.894026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng Z, Choi DS, Mohan V, Emser A, Siddiqui K, Gong Y, Patel S, Woerle HJ. Efficacy and safety of linagliptin as monotherapy or add-on treatment in Asian patients with suboptimal glycemic control: a pooled analysis. Curr Med Res Opin. 2015;31(1):99–106. doi: 10.1185/03007995.2014.964856. [DOI] [PubMed] [Google Scholar]
- 19.Zeng Z, Yang JK, Tong N, Yan S, Zhang X, Gong Y, Woerle HJ. Efficacy and safety of linagliptin added to metformin and sulphonylurea in Chinese patients with type 2 diabetes: a sub-analysis of data from a randomised clinical trial. Curr Med Res Opin. 2013;29(8):921–929. doi: 10.1185/03007995.2013.805123. [DOI] [PubMed] [Google Scholar]
- 20.Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, Alexander JH, Pencina M, Toto RD, Wanner C, Zinman B, Woerle HJ, Baanstra D, Pfarr E, Schnaidt S, Meinicke T, George JT, von Eynatten M, McGuire DK. CARMELINA investigators. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321(1):69–79. doi: 10.1001/jama.2018.18269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGuire DK, Alexander JH, Johansen OE, Perkovic V, Rosenstock J, Cooper ME, Wanner C, Kahn SE, Toto RD, Zinman B, Baanstra D, Pfarr E, Schnaidt S, Meinicke T, George JT, von Eynatten M, Marx N. CARMELINA investigators. Linagliptin effects on heart failure and related outcomes in individuals with type 2 diabetes mellitus at high cardiovascular and renal risk in CARMELINA. Circulation. 2019;139(3):351–361. doi: 10.1161/CIRCULATIONAHA.118.038352. [DOI] [PubMed] [Google Scholar]
- 22.Rosenstock J, Perkovic V, Alexander JH, Cooper ME, Marx N, Pencina MJ, Toto RD, Wanner C, Zinman B, Baanstra D, Pfarr E, Mattheus M, Broedl UC, Woerle HJ, George JT, von Eynatten M, McGuire DK. CARMELINA investigators. Rationale, design, and baseline characteristics of the CArdiovascular safety and Renal Microvascular outcomE study with LINAgliptin (CARMELINA®): a randomized, double-blind, placebo-controlled clinical trial in patients with type 2 diabetes and high cardio-renal risk. Cardiovasc Diabetol. 2018;17(1):39. doi: 10.1186/s12933-018-0682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Home P. Cardiovascular outcome trials of glucose-lowering medications: an update. Diabetologia. 2019;62(3):357–369. doi: 10.1007/s00125-018-4801-1. [DOI] [PubMed] [Google Scholar]
- 24.Marx N, McGuire DK, Perkovic V, Woerle HJ, Broedl UC, von Eynatten M, George JT, Rosenstock J. Composite primary end points in cardiovascular outcomes trials involving type 2 diabetes patients: should unstable angina be included in the primary end point? Diabetes Care. 2017;40(9):1144–1151. doi: 10.2337/dc17-0068. [DOI] [PubMed] [Google Scholar]
- 25.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I. SAVOR-TIMI steering committee investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 26.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F. EXAMINE investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 27.Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf F, Peterson ED, Holman RR. TECOS study group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 28.Gantz I, Chen M, Suryawanshi S, Ntabadde C, Shah S, O’Neill EA, Engel SS, Kaufman KD, Lai E. A randomized, placebo-controlled study of the cardiovascular safety of the once-weekly DPP-4 inhibitor omarigliptin in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2017;16(1):112. doi: 10.1186/s12933-017-0593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaku K, Lee J, Mattheus M, Kaspers S, George J, Woerle HJ. EMPA-REG OUTCOME investigators. Empagliflozin and cardiovascular outcomes in Asian patients with type 2 diabetes and established cardiovascular disease—results from EMPA-REG OUTCOME®. Circ J. 2017;81(2):227–234. doi: 10.1253/circj.CJ-16-1148. [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes Association 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S90–S102. doi: 10.2337/dc19-S009. [DOI] [PubMed] [Google Scholar]
- 31.Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41(12):2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haneda M, Noda M, Origasa H, Noto H, Yabe D, Fujita Y, Goto A, Kondo T, Araki E. Japanese clinical practice guideline for diabetes 2016. J Diabetes Investig. 2018;9(3):657–697. doi: 10.1111/jdi.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weng J, Ji L, Jia W, Lu J, Zhou Z, Zou D, Zhu D, Chen L, Chen L, Guo L, Guo X, Ji Q, Li Q, Li X, Liu J, Ran X, Shan Z, Shi L, Song G, Yang L, Yang Y, Yang W. Chinese diabetes society. Standards of care for type 2 diabetes in China. Diabetes Metab Res Rev. 2016;32(5):442–458. doi: 10.1002/dmrr.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bajaj S. RSSDI clinical practice recommendations for the management of type 2 diabetes mellitus 2017. Int J Diabetes Dev Ctries. 2018;38(Suppl 1):1–115. doi: 10.1007/s13410-018-0604-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zimdahl H, Ittrich C, Graefe-Mody U, Boehm BO, Mark M, Woerle HJ, Dugi KA. Influence of TCF7L2 gene variants on the therapeutic response to the dipeptidylpeptidase-4 inhibitor linagliptin. Diabetologia. 2014;57(9):1869–1875. doi: 10.1007/s00125-014-3276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirino Y, Sei M, Kawazoe K, Minakuchi K, Sato Y. Plasma dipeptidyl peptidase 4 activity correlates with body mass index and the plasma adiponectin concentration in healthy young people. Endocr J. 2012;59(10):949–953. doi: 10.1507/endocrj.EJ12-0158. [DOI] [PubMed] [Google Scholar]
- 37.Kanasaki K. The role of renal dipeptidyl peptidase-4 in kidney disease: renal effects of dipeptidyl peptidase-4 inhibitors with a focus on linagliptin. Clin Sci (Lond) 2018;132(4):489–507. doi: 10.1042/CS20180031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Groop PH, Cooper ME, Perkovic V, Hocher B, Kanasaki K, Haneda M, Schernthaner G, Sharma K, Stanton RC, Toto R, Cescutti J, Gordat M, Meinicke T, Koitka-Weber A, Thiemann S, von Eynatten M. Linagliptin and its effects on hyperglycaemia and albuminuria in patients with type 2 diabetes and renal dysfunction: the randomized MARLINA-T2D trial. Diabetes Obes Metab. 2017;19(11):1610–1619. doi: 10.1111/dom.13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mosenzon O, Leibowitz G, Bhatt DL, Cahn A, Hirshberg B, Wei C, Im K, Rozenberg A, Yanuv I, Stahre C, Ray KK, Iqbal N, Braunwald E, Scirica BM, Raz I. Effect of saxagliptin on renal outcomes in the SAVOR-TIMI 53 trial. Diabetes Care. 2017;40(1):69–76. doi: 10.2337/dc16-0621. [DOI] [PubMed] [Google Scholar]
- 40.Cornel JH, Bakris GL, Stevens SR, Alvarsson M, Bax WA, Chuang LM, Engel SS, Lopes RD, McGuire DK, Riefflin A, Rodbard HW, Sinay I, Tankova T, Wainstein J, Peterson ED, Holman RR. TECOS study group. Effect of sitagliptin on kidney function and respective cardiovascular outcomes in type 2 diabetes: outcomes from TECOS. Diabetes Care. 2016;39(12):2304–2310. doi: 10.2337/dc16-1415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.